Abstract
Myocardial scarring is a hallmark of hypertrophic cardiomyopathy (HCM) and a major driver of adverse outcomes, including sudden cardiac death and heart failure progression. The fibrotic substrate in HCM is complex, encompassing both replacement and interstitial fibrosis, often accompanied by myocardial disarray. Advanced cardiovascular imaging enables detailed scar characterization, which is crucial for risk stratification and personalized management. Cardiovascular magnetic resonance (CMR) is the gold standard for non-invasive fibrosis assessment. Techniques such as late gadolinium enhancement, myocardial mapping of T1 and T2 relaxation properties, and diffusion tensor imaging provide complementary insights into scar burden and architecture. Cardiac computed tomography (CT) is an emerging modality with increasing clinical relevance. Delayed iodine enhancement and CT-derived extracellular volume mapping offer a valuable alternative for scar assessment, particularly when CMR is contraindicated. This review highlights the role of multimodality imaging in assessing myocardial scar in HCM, with a focus on CMR and CT, and explores their clinical implications.
Myocardial scarring in hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a prevalent inherited myocardial disease, affecting approximately 1 in 500 individuals in the general population (1, 2). It is characterized by left ventricular (LV) hypertrophy that occurs in the absence of systemic or cardiac conditions capable of inducing hemodynamic overload (3, 4). The hypertrophic myocardium in HCM exhibits a wide spectrum of structural alterations, both at the macroscopic and microscopic levels (5). In at least one-third of the myocardium, cardiomyocytes are hypertrophic and disorganized, displaying structural abnormalities in both shape and alignment, which is a phenomenon collectively referred to as myocardial disarray. While most pronounced in hypertrophic segments, disarray may also affect regions with normal wall thickness (5). The extracellular matrix in HCM is typically expanded and rich in glycogen, with widespread interstitial fibrosis that, in advanced stages, can lead to fibrotic replacement and the formation of myocardial scars (6). Additionally, microvascular abnormalities are a frequent histological finding, including medial hypertrophy, disorganized elastic fibres, and endothelial hyperplasia of the intramyocardial coronary arteries. These changes contribute to vessel wall thickening and luminal narrowing, leading to microvascular dysfunction and impaired perfusion reserve. The downstream consequences are myocardial ischemia, myocyte necrosis, and replacement fibrosis (6–9). The combination of myocyte disarray, extracellular matrix expansion, and microvascular dysfunction results in complex structural remodelling, with fibrosis -either interstitial or replacement- representing a pathological hallmark of the disease.
Myocardial scarring has been implicated in the most threatening outcomes of HCM, namely sudden cardiac death (SCD) and adverse LV remodelling (10). SCD is one of the most unpredictable and devastating complication of HCM, and it may be the initial manifestation of the disease. It occurs at an estimated annual incidence of 0.7% in unselected HCM cohorts (11) and disproportionately affects younger individuals, with a cumulative 5-year risk of approximately 8%–10% in paediatric patients (12). Ventricular arrhythmias, particularly ventricular tachycardia (VT) and fibrillation (VF) are the primary mechanisms underlying SCD (13), and myocardial scarring serves as a critical substrate for re-entrant circuits (14). Re-entry arrhythmias require the presence of a conduction barrier (anatomical or functional), two pathways with differing conduction velocities, a unidirectional block, and sufficient excitable myocardium. When these conditions are satisfied, electrical impulses may circulate continuously along a slow conduction pathway, reactivating previously recovered myocardium and leading to sustained VT (15). Dense fibrotic regions and anatomical structures act as non-conductive barriers, while myocardial disarray and interstitial fibrosis create a non-uniform conduction environment that promotes anisotropy and slowed conduction (16–18). Localized ischemia due to microvascular dysfunction contributes to electrical instability by creating zones of partial depolarization and further slowing of conduction, fostering arrhythmogenesis (18–21).
Adverse LV remodelling is another major disease progression pattern, occurring in approximately 15%–20% of HCM patients (22–24). It is defined by the superimposition of unfavourable structural changes upon the classic HCM phenotype (22). These changes include reduced LV ejection fraction (25), wall thinning (26), moderate-to-severe diastolic dysfunction (27, 28), marked left atrial enlargement (29), significant microvascular dysfunction (30), new-onset atrial fibrillation (31), spontaneous resolution of LV outflow tract obstruction (26, 32), and formation of LV apical aneurysms (33). Each of these features has been individually associated with poor outcomes in HCM cohorts. From a pathophysiological standpoint, adverse remodelling appears to result from a combination of chronic microvascular ischemia, cellular energy depletion, and myocyte apoptosis, ultimately leading to progressive myocyte loss and fibrotic replacement (24, 30, 34–38). The clinical manifestations of remodelling vary considerably, ranging from mild functional impairment to advanced heart failure (HF). End-stage HCM, the most extreme form, develops in approximately 5% of patients and is characterized by extensive fibrosis with either a hypokinetic-dilated phenotype, when systolic dysfunction predominates, or a hypokinetic-restrictive phenotype, marked by a small, stiff LV and severe diastolic impairment (4, 6, 25, 26, 35).
Together, SCD and HF, alongside thromboembolic complications, constitute the principal contributors to HCM-related mortality (25). Importantly, these outcomes are directly or indirectly related to the development of myocardial scar, which is a central prognostic marker. Consequently, the assessment of myocardial fibrosis has gained increasing importance in risk stratification and disease management.
Advances in cardiovascular imaging have greatly enhanced our ability to noninvasively characterize myocardial fibrosis in HCM. A multimodality approach including cardiovascular magnetic resonance (CMR) and cardiac computed tomography (CT) allows for comprehensive assessment of the extent, distribution, and nature of myocardial scarring, thereby informing prevention strategies and individualized risk stratification.
Cardiovascular magnetic resonance
CMR is the gold-standard non-invasive imaging modality for the assessment of myocardial scar (39, 40). Owing to its excellent spatial resolution and superior tissue contrast, CMR enables comprehensive characterization of both replacement and interstitial fibrosis. In recent years, significant technological advancements have expanded the capabilities of CMR, allowing for an even more detailed evaluation of myocardial scarring and its prognostic implications.
This section will explore the main CMR techniques employed for scar characterization in HCM, outlining their technical principles, summarizing the current evidence in the literature, and discussing their clinical relevance.
Late gadolinium enhancement
Late gadolinium enhancement (LGE) is the most established CMR technique for detecting replacement myocardial fibrosis (39). It involves the administration of a paramagnetic extracellular contrast agent (gadolinium), followed by the acquisition of T1-weighted images approximately 10–20 min after injection. This technique exploits the different kinetics of gadolinium distribution in healthy myocardium vs. scar tissue. Gadolinium accumulates in regions with expanded extracellular space due to necrosis or fibrosis, appearing as hyperintense areas on late-phase imaging, with signal intensity varying according to scar architecture (39).
LGE can be evaluated both qualitatively and quantitatively relative to total myocardial mass (39). Early investigations of LGE as a prognostic marker in HCM focused on total scar burden, which emerged as a strong predictor of SCD, adverse remodelling, and HF hospitalizations in several prospective unselected cohorts (16, 41–47). The largest study to date assessing the role of LGE in predicting SCD in HCM showed a significant increase in SCD events among patients with a high scar burden (≥15% of total myocardial mass), whereas no meaningful increase in SCD risk was observed in those with minimal LGE (1%–5%) compared to patients without detectable scar (16). Based on these findings, the American College of Cardiology and American Heart Association (ACC/AHA) included a scar burden threshold of 15% as a risk factor to be considered, particularly in patients lacking conventional clinical risk factors or when ICD implantation is uncertain (2). However, the use of scar burden alone has limited sensitivity, as numerous adverse events have been documented in patients with LGE ≤15% (7, 44, 45).
In HCM, LGE patterns demonstrate substantial heterogeneity in their extent, intensity, and distribution (48). A large prospective registry provided novel insights into this variability, showing that distinct LGE patterns are closely associated with specific morphological subtypes and sarcomere mutation status (49). This heterogeneity reflects the complex histopathological nature of HCM fibrosis, which differs from post-ischemic scars by comprising diffuse fibrosis interspersed with viable myocytes, rather than dense, localized subendocardial fibrosis within a specific coronary territory (7, 50–52). Such structural complexity creates a highly arrhythmogenic substrate, ideal for rapid-rate re-entrant VTs (15). These arrhythmias may progress to polymorphic VT or VF, driven by the distinct properties of the HCM myocardium—marked by sarcomeric disarray and heterogeneous, anisotropic conduction (53). CMR studies have demonstrated that areas of mild-to-intermediate LGE enhancement are more strongly associated with ventricular arrhythmias than intensely enhanced regions (54, 55). Moreover, in low- and intermediate-risk HCM patients, quantitative assessment of LGE heterogeneity (such as signal dispersion) has been shown to independently predict major arrhythmic events, even beyond total scar burden (48).
These findings support the notion that a qualitative evaluation of scar architecture, particularly LGE signal heterogeneity, may offer deeper insights into arrhythmic risk. However, conventional 2D LGE imaging has limitations in capturing the full complexity of the three-dimensional scar structure (48). To address this, advanced post-processing software have been developed to improve scar characterization (56, 57). These tools segment signal intensity at the pixel level to differentiate dense core fibrosis from diffuse border zone (BZ) fibrosis and reconstruct the data into 3D images (Figure 1). They identify corridors of BZ tissue surrounded by dense scars or anatomical barriers, which connect regions of viable myocardium, referred to as border zone channels (BZCs) (56). Functionally, BZCs represent slow-conducting pathways composed of excitable myocardium insulated by non-conductive fibrotic tissue serving as substrates for re-entrant VTs (56, 58). Recent CMR data demonstrated that the presence of BZCs is a strong independent predictor of ICD interventions for VT/VF in high-risk HCM patients (59).
Figure 1
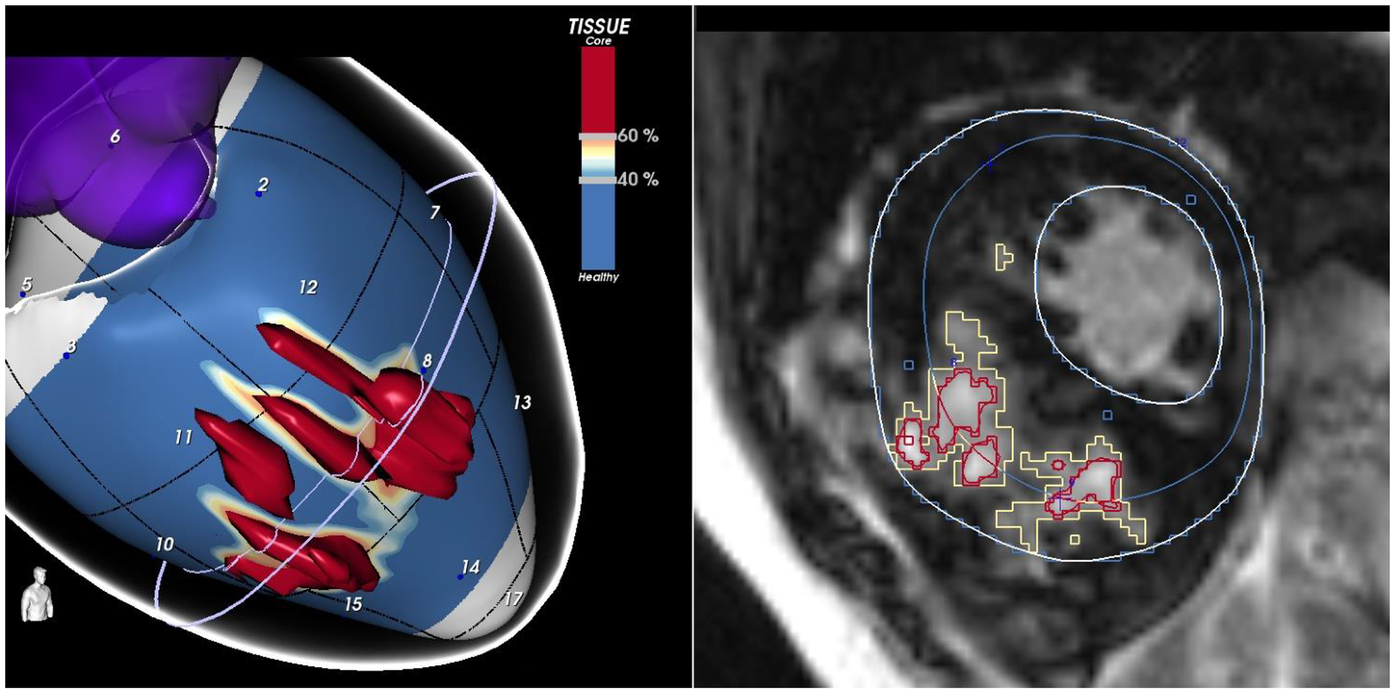
The figure shows a short-axis view of a CMR of a patient with HCM, highlighting an extensive scar involving the interventricular septum. LGE-CMR images were post-processed using ADAS 3D (Galgo Medical, Barcelona, Spain), creating nine concentric surface layers spanning from the endocardium to the epicardium of the left ventricular wall thickness, resulting in a 3D shell for each layer. Color-coded pixel signal intensity (PSI) maps were projected onto each shell. Hyper-enhanced areas were classified as the core zone, borderline zone (BZ), or healthy tissue using thresholds of 40 ± 5% and 60 ± 5% of the maximum PSI. The scar-dense core is coded in red, BZ is coded in orange and white, and healthy myocardium is coded in blue.
Myocardial mapping
Tissue characterization through myocardial mapping is a relatively recent advancement in CMR. By applying specific imaging sequences, this technique enables quantitative measurement of the longitudinal (T1) and transverse (T2 or T2*) relaxation times of each myocardial voxel (60). These values are then visually represented as color-coded parametric maps. This allows the detection of microstructural and biochemical myocardial tissue properties that are invisible to the naked eye, including the quantification of extracellular volume (ECV) (60). Both in vivo and in vitro studies have shown that ECV and T1 mapping are reliable surrogate markers of diffuse myocardial fibrosis, reflecting extracellular space expansion due to interstitial collagen deposition (61). By contrast, T2 mapping is a marker of myocardial oedema and inflammation (62).
A recent study in a large cohort of HCM patients demonstrated that interstitial fibrosis assessed via ECV and native T1 values was independently associated with cardiac death and provided incremental prognostic value beyond traditional clinical risk markers (63). Additional studies have further confirmed the adverse prognostic impact of interstitial fibrosis, linking it to unfavourable clinical features such as adverse remodelling (64), left ventricular diastolic dysfunction (65), and ventricular arrhythmias (66). Notably, interstitial fibrosis detected by T1 mapping and ECV is often present in myocardial segments without LGE and retains significant independent prognostic value (67). These findings suggest that interstitial fibrosis plays a central and independent role in the prognosis of patients with HCM.
A recent single-centre observational study in a large cohort of patients demonstrated that cardiovascular death or appropriate implantable cardioverter-defibrillator therapies occurred more frequently in individuals with elevated myocardial T2 values. These findings suggest a role of myocardial oedema in the pathophysiology of major adverse events and highlight T2 mapping as an additional parameter with potential value for prognostic stratification in HCM (68).
As such, mapping techniques have provided deeper insights into the role of interstitial fibrosis in HCM, particularly enhancing risk stratification in patients without LGE, traditionally considered low-risk (64, 69) However, combining mapping with LGE assessment has proven more effective than using either method separately, supporting the idea that LGE and mapping provide distinct but complementary information for a comprehensive evaluation of myocardial scar architecture (70).
Finally, a key advantage of tissue characterization by mapping is the ability to detect myocardial fibrosis sensitively without the need for contrast administration. Malek et al. demonstrated that automated machine learning models based on native (pre-contrast) T1 mapping can accurately identify myocardial fibrosis and closely align with LGE in HCM patients (71).
Perfusion CMR
First-pass perfusion CMR during vasodilator stress is a well-established technique for detecting myocardial ischemia due to either obstructive epicardial coronary artery disease or microvascular dysfunction (72). Images are acquired during the first pass of an intravenous gadolinium bolus under pharmacological stress (most commonly adenosine or regadenoson). Perfusion can be evaluated qualitatively by visual inspection or quantitatively through the calculation of absolute myocardial blood flow (MBF, ml/min/g) and myocardial perfusion reserve (MPR, stress-to-rest MBF ratio) (73).
In HCM, microvascular dysfunction typically manifests as subendocardial perfusion defects with a near-circumferential distribution, often extending beyond a single coronary territory, and is associated with reduced MBF and MPR (74, 75). Several studies have demonstrated that inducible ischemia identified by CMR perfusion, whether assessed qualitatively or quantitatively, correlates with a greater burden of LGE, underscoring the central role of microvascular dysfunction in the development of replacement fibrosis (47, 76, 77). Furthermore, perfusion abnormalities have been associated with adverse clinical features, including ventricular arrhythmias and the formation of apical aneurysms, even independently of the overall fibrosis burden (76–79).
Diffusion tensor CMR
Diffusion Tensor Imaging (DTI) is an advanced CMR technique that enables visualization of myocardial microstructure by mapping the three-dimensional diffusion of water molecules within the myocardium (80). By calculating fractional anisotropy (FA), cardiac DTI quantifies the directional variability of water diffusion: FA values approaching zero reflect isotropic diffusion (random, unrestricted movement), while values closer to one represent anisotropic diffusion (preferential movement along a single direction). Consequently, high FA values are observed in voxels with coherently aligned myocytes, whereas low FA values indicate disorganized or misaligned fibres due to myocardial disarray (18, 81).
DTI is currently the only non-invasive imaging modality capable of identifying myocardial disarray, thus offering significant diagnostic and prognostic potential in HCM (82–86). A recent study demonstrated that myocardial disarray detected via cardiac DTI independently correlates with ventricular arrhythmic risk, regardless of the degree of fibrosis or hypertrophy, suggesting a direct pro-arrhythmic role (18).
In addition, diffusion-weighted imaging (DWI), a simplified precursor of DTI, has emerged as a feasible alternative to native T1 mapping and ECV for the identification of interstitial fibrosis in HCM. A recent study demonstrated that the mean apparent diffusion coefficient (ADC) measured by DWI can identify areas of LGE with sensitivity and specificity comparable to that of T1 mapping and ECV quantification (87).
Despite its potential, DTI is still constrained by major technical challenges. Image acquisition is highly vulnerable to motion artifacts and requires complex, non-standardized protocols, while signal-to-noise ratio and reproducibility across vendors remain suboptimal (88, 89). Furthermore, the lack of universally accepted reference values for DTI-derived metrics, such as fractional anisotropy, limits clinical interpretation (90). As a result, DTI remains largely confined to research settings, and significant methodological refinements are needed before its integration into routine risk stratification in HCM.
Radiomics
Radiomics is an emerging field in cardiovascular imaging that focuses on the extraction and analysis of high-dimensional quantitative features from medical images, effectively transforming visual data into mineable information (91, 92). The core principle of radiomics is that biomedical images contain a wealth of information that remains invisible to the human eye and is not captured through traditional qualitative interpretation (93). Radiomics seeks to uncover these hidden insights and derive novel biomarkers to improve diagnostic and prognostic accuracy, while reducing observer bias and subjectivity (94). Technically, radiomic analysis consists of several interdependent steps: image acquisition, raw data reconstruction, image pre-processing, segmentation, feature extraction, feature selection, and predictive model construction (95).
As previously discussed, scar heterogeneity in HCM is a critical prognostic factor that goes beyond simple quantitative assessment. Visual evaluation of scar heterogeneity, however, is often challenging and subject to considerable interobserver variability. Radiomics offers a promising solution by enabling a more objective and reproducible analysis. Recent studies in large, unselected cohorts of HCM patients have shown that LGE-based radiomic features reflecting scar heterogeneity are significant predictors of SCD and provide incremental prognostic value beyond current clinical risk models (96–98).
In addition to assessing LGE texture, radiomics has broader potential applications in scar characterization, including integration with myocardial mapping techniques (99–101). However, translation into clinical practice remains limited. Radiomic features are highly sensitive to variations in acquisition, segmentation, and pre-processing, raising concerns about reproducibility and generalizability (99, 102). The absence of standardized pipelines or validated multicenter datasets precludes routine use. At present, radiomics should be regarded as a promising but experimental approach, with clinical adoption contingent on methodological standardization and large-scale prospective validation.
Serial CMR for monitoring scar evolution
Myocardial fibrosis in HCM is not a static phenomenon but a dynamic process that progresses over time, driving adverse ventricular remodeling and increasing arrhythmic risk (103). Longitudinal studies have shown that both the extent and heterogeneity of LGE frequently increase during follow-up, with progression rates influenced by clinical phenotype and patient profile (46, 47, 104). Importantly, fibrosis progression has been independently associated with higher rates of ventricular arrhythmias, sudden cardiac death, and the transition to end-stage remodeling, underscoring the prognostic significance of serial scar assessment (47).
In this context, repeat CMR examinations provide a unique opportunity to monitor scar evolution and refine risk stratification over time, particularly in patients who may not initially demonstrate extensive fibrosis. Serial CMR may be especially warranted in younger individuals, in those with intermediate risk profiles, or when clinical status changes. CMR thus emerges as a sensitive longitudinal biomarker of disease progression, enabling timely adjustment of preventive and therapeutic strategies in HCM.
Computed tomography
Cardiac CT has shown growing potential in the assessment of myocardial fibrosis in recent years, driven by continuous improvements in spatial resolution and advanced post-processing techniques (105). As a result, CT has emerged as a viable alternative to CMR, particularly in patients for whom CMR is contraindicated or not available.
Delayed iodine enhancement
Delayed Iodine Enhancement (DIE) represents the CT-based approach to direct assessment of myocardial fibrosis (106). This technique is conceptually analogous to LGE in CMR: gadolinium- and iodine-based contrast agents share similar kinetics, accumulating in regions with expanded extracellular space. Following the administration of iodinated contrast, late-phase images (typically acquired 5–10 min post-injection) allow for the identification of myocardial areas with altered contrast kinetics, indicative of scar tissue (107).
In HCM, several preliminary studies have shown that DIE enables the detection of late enhancement patterns topographically similar to those seen with CMR, predominantly located in the interventricular septum and at the ventricular insertion points (108). Comparative studies have confirmed this topographic concordance between DIE-CT and LGE-CMR, suggesting good sensitivity of the technique, especially in the presence of extensive or transmural scarring (109, 110).
However, DIE has several intrinsic limitations. CT offers lower contrast resolution compared to CMR, making it more challenging to detect subtle or patchy fibrosis. Moreover, fibrosis quantification is less standardized, although some studies have proposed attenuation thresholds in Hounsfield Units (HU) and semi-quantitative approaches (111, 112). Additional considerations include radiation exposure and the need for relatively high doses of iodinated contrast, which may be a concern particularly in younger patients or those with impaired renal function (106).
Despite these limitations, DIE-CT is an extremely valuable technique in patients who are not eligible for CMR or as a complementary tool when concurrent anatomical and coronary assessment is required (110). An interesting study in a small cohort of high-risk HCM patients with ICDs demonstrated that the myocardial fibrosis burden assessed by CT predicted ventricular fibrillation and ventricular tachycardia events (113). These findings were corroborated in an unselected group of HCM patients without coronary artery disease, where the presence of CT-detected scar was associated with higher rate of major adverse cardiovascular events (114). Larger, prospective studies are needed to validate the prognostic value of DIE in HCM.
CT mapping
Contrast-enhanced CT techniques allow for the quantitative estimation of ECV. Technically, the principle is analogous to that of CMR, relying on HU attenuation measured before and after contrast administration (115). More recent technological advancements have further evolved CT mapping. Dual-energy CT or spectral CT systems acquire images at different energy levels, enabling the reconstruction of quantitative iodine distribution maps within the myocardium. Notably, these techniques do not require a pre-contrast scan, thereby reducing radiation exposure (116–118).
Several studies have demonstrated a good correlation between CT-derived and CMR-derived ECV in non-HCM patient cohorts (119–121), and Bandula et al. validated this approach histologically (122).
CT mapping potentially overcomes some limitations of conventional DIE, such as the subjectivity of visual thresholding for enhancement detection, particularly when integrated with emerging radiomics and deep learning post-processing techniques (115). However, clinical experience with CT mapping in HCM remains limited. To date, only one study has investigated the prognostic role of CT-derived ECV in a high-risk cohort of HCM patients with ICDs, revealing no significant association with the incidence of ventricular arrhythmias (123).
Overall, iodine mapping represents a promising frontier for the quantitative assessment of myocardial fibrosis in HCM. Further studies are needed to explore its clinical utility. On the other hand, widespread clinical adoption may be hindered by limited availability of dual-energy systems, increased post-processing complexity, and the need for optimized acquisition protocols.
Conclusions
Myocardial scarring is a hallmark of HCM and a major driver of adverse outcomes, including SCD and HF progression. The fibrotic substrate in HCM is complex, encompassing both replacement and interstitial fibrosis, often accompanied by myocardial disarray. CMR is the gold-standard non-invasive imaging modality for myocardial scar evaluation. Indeed, techniques such as LGE, myocardial mapping, and DTI provide complementary insights into scar burden and architecture. CT is an emerging modality with increasing clinical relevance. DIE and CT-derived ECV mapping offer a valuable alternative for scar assessment, particularly when CMR is contraindicated.
Table 1 summarizes the applications of CMR and CT techniques in scar assessment.
Table 1
| Modality | Imaging technique | Scar characterization | Clinical significance |
|---|---|---|---|
| Cardiac magnetic resonance (CMR) | Late gadolinium enhancement (LGE) | Qualitative and quantitative assessment of replacement fibrosis | Strong prognostic marker for ventricular arrhythmias, sudden cardiac death and adverse remodelling (16) |
| Myocardial mapping (native T1 and ECV) | Detection of interstitial fibrosis not visible with LGE technique | Marker of poor prognosis; useful for risk stratification in low-risk patients and when gadolinium is contraindicated (63) | |
| Diffusion tensor imaging (DTI) | Assessment of myocardial disarray | Independent arrhythmic prognostic role (18) | |
| Radiomics | Assessment of scar heterogeneity using multi-step automated quantitative analysis | Additional arrhythmic prognostic value beyond qualitative visual assessment of LGE (96) | |
| Cardiac computer tomography (CT) | Delayed iodine enhancement (DIE) | Qualitative assessment of replacement fibrosis | Marker of poor prognosis. Useful alternative when MRI is contraindicated (113) |
| CT myocardial mapping | Assessment of interstitial fibrosis | Emerging tool not yet specifically studied in HCM patients |
Applications of CMR and CT techniques in scar assessment.
Cardiac CT and CMR offer complementary strengths in the multimodality imaging assessment of myocardial scar, each contributing synergistic information that enhances risk stratification and may inform individualized preventive strategies.
Statements
Author contributions
MS: Writing – review & editing, Conceptualization, Writing – original draft, Methodology, Investigation. CP: Writing – review & editing, Writing – original draft. LS: Writing – review & editing, Writing – original draft. MM: Writing – review & editing, Writing – original draft. CL: Writing – review & editing, Writing – original draft. GC: Writing – review & editing, Writing – original draft. GF: Writing – review & editing, Writing – original draft. BM: Writing – review & editing, Writing – original draft. DP: Writing – original draft, Writing – review & editing. AS: Writing – review & editing, Writing – original draft. AI: Writing – review & editing, Writing – original draft. EB: Writing – review & editing, Writing – original draft. AB: Writing – original draft, Writing – review & editing. PF: Writing – review & editing, Supervision, Writing – original draft, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Matteo Sclafani has been supported by a research grant provided by the DigiCardiopaTh PhD program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Maron BJ Ommen SR Semsarian C Spirito P Olivotto I Maron MS . Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. (2014) 64:83–99. 10.1016/j.jacc.2014.05.003
2.
Ommen SR Mital S Burke MA Day SM Deswal A Elliott P et al 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2020) 76:e159–240. 10.1016/j.jacc.2020.08.045
3.
Russo D Sclafani M Tini G Musumeci B Arcari L Limite L et al Prognostic implications of different clinical profiles in hypertrophic cardiomyopathy. Minerva Cardiol Angiol. (2022) 70:189–206. 10.23736/S2724-5683.21.05752-5
4.
Musumeci B Tini G Biagini E Merlo M Calore C Ammirati E et al Clinical characteristics and outcome of end stage hypertrophic cardiomyopathy: role of age and heart failure phenotypes. Int J Cardiol. (2024) 400:131784. 10.1016/j.ijcard.2024.131784
5.
Hughes SE . The pathology of hypertrophic cardiomyopathy. Histopathology. (2004) 44:412–27. 10.1111/j.1365-2559.2004.01835.x
6.
Olivotto I Girolami F Nistri S Rossi A Rega L Garbini F et al The many faces of hypertrophic cardiomyopathy: from developmental biology to clinical practice. J Cardiovasc Transl Res. (2009) 2:349–67. 10.1007/s12265-009-9137-2
7.
Foà A Agostini V Rapezzi C Olivotto I Corti B Potena L et al Histopathological comparison of intramural coronary artery remodeling and myocardial fibrosis in obstructive versus end-stage hypertrophic cardiomyopathy. Int J Cardiol. (2019) 291:77–82. 10.1016/j.ijcard.2019.03.060
8.
Schwartzkopff B Mundhenke M Strauer BE . Alterations of the architecture of subendocardial arterioles in patients with hypertrophic cardiomyopathy and impaired coronary vasodilator reserve: a possible cause for myocardial ischemia. J Am Coll Cardiol. (1998) 31:1089–96. 10.1016/S0735-1097(98)00036-9
9.
Maron B Wolfson J Epstein S Roberts W . Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. (1986) 8:545–57. 10.1016/s0735-1097(86)80181-4
10.
Maron BJ . Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. (2018) 379:655–68. 10.1056/nejmra1710575
11.
Efthimiadis GK Zegkos T Meditskou S Stavros H . Perspectives on sudden death prevention in hypertrophic cardiomyopathy. Cardiol Rev. (2014) 22:210–6. 10.1097/CRD.0000000000000017
12.
Marston NA Han L Olivotto I Day SM Ashley EA Michels M et al Clinical characteristics and outcomes in childhood-onset hypertrophic cardiomyopathy. Eur Heart J. (2021) 42:1988–96. 10.1093/eurheartj/ehab148
13.
Segev A Wasserstrum Y Arad M Larrañaga-Moreira JM Martinez-Veira C Barriales-Villa R . Ventricular arrhythmias in patients with hypertrophic cardiomyopathy: prevalence, distribution, predictors, and outcome. Hear Rhythm. (2023) 20:1385–92. 10.1016/j.hrthm.2023.06.015
14.
Proietti R Roux JF Verma A Alturki A Bernier ML Essebag V . A historical perspective on the role of functional lines of block in the re-entrant circuit of ventricular tachycardia. Pacing Clin Electrophysiol. (2016) 39:490–6. 10.1111/pace.12827
15.
Sclafani M Falasconi G Tini G Musumeci B Penela D Saglietto A et al Substrates of sudden cardiac death in hypertrophic cardiomyopathy. J Clin Med. (2025) 14:1331. 10.3390/jcm14041331
16.
Chan RH Maron BJ Olivotto I Pencina MJ Assenza GE Haas T et al Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. (2014) 130:484–95. 10.1161/CIRCULATIONAHA.113.007094
17.
Galati G Leone O Pasquale F Olivotto I Biagini E Grigioni F et al Histological and histometric characterization of myocardial fibrosis in end-stage hypertrophic cardiomyopathy. Circ Hear Fail. (2016) 9:1–10. 10.1161/CIRCHEARTFAILURE.116.003090
18.
Ariga R Tunnicliffe EM Manohar SG Mahmod M Raman B Piechnik SK et al Identification of myocardial disarray in patients with hypertrophic cardiomyopathy and ventricular arrhythmias. J Am Coll Cardiol. (2019) 73:2493–502. 10.1016/j.jacc.2019.02.065
19.
Krul SPJ Berger WR Smit NW Van Amersfoorth SCM Driessen AHG Van Boven WJ et al Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ Arrhythmia Electrophysiol. (2015) 8:288–95. 10.1161/CIRCEP.114.001752
20.
Burstein B Nattel S . Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. (2008) 51:802–9. 10.1016/j.jacc.2007.09.064
21.
Camelliti P Green CR LeGrice I Kohl P . Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. (2004) 94:828–35. 10.1161/01.RES.0000122382.19400.14
22.
Schinkel AFL Vriesendorp PA Sijbrands EJG Jordaens LJLM Ten Cate FJ Michels M . Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: systematic review and meta-analysis. Circ Hear Fail. (2012) 5:552–9. 10.1161/CIRCHEARTFAILURE.112.969626
23.
Migliore F Zorzi A Marra MP Basso C Corbetti F De Lazzari M et al Myocardial edema underlies dynamic T-wave inversion (Wellens’ ECG pattern) in patients with reversible left ventricular dysfunction. Hear Rhythm. (2011) 8:1629–34. 10.1016/j.hrthm.2011.04.035
24.
Musumeci B Tini G Russo D Sclafani M Cava F Tropea A et al Left ventricular remodeling in hypertrophic cardiomyopathy: an overview of current knowledge. J Clin Med. (2021) 10:1547. 10.3390/jcm10081547
25.
Olivotto I Maron BJ Appelbaum E Harrigan CJ Salton C Gibson CM et al Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. (2010) 106:261–7. 10.1016/j.amjcard.2010.03.020
26.
Maron BJ Spirito P . Implications of left ventricular remodeling in hypertrophic cardiomyopathy. Am J Cardiol. (1998) 81:1339–44. 10.1016/S0002-9149(98)00164-7
27.
Biagini E Spirito P Rocchi G Ferlito M Rosmini S Lai F et al Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol. (2009) 104:1727–31. 10.1016/j.amjcard.2009.07.057
28.
Melacini P Basso C Angelini A Calore C Bobbo F Tokajuk B et al Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J. (2010) 31:2111–23. 10.1093/eurheartj/ehq136
29.
Nistri S Olivotto I Betocchi S Losi MA Valsecchi G Pinamonti B et al Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian registry for hypertrophic cardiomyopathy). Am J Cardiol. (2006) 98:960–5. 10.1016/j.amjcard.2006.05.013
30.
Maron MS Olivotto I Maron BJ Prasad SK Cecchi F Udelson JE et al The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. (2009) 54:866–75. 10.1016/j.jacc.2009.04.072
31.
Olivotto I Cecchi F Casey SA Dolara A Traverse JH Maron BJ . Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. (2001) 104:2517–24. 10.1161/hc4601.097997
32.
Ciró E Maron BJ Bonow RO Cannon RO Epstein SE . Relation between marked changes in left ventricular outflow tract gradient and disease progression in hypertrophic cardiomyopathy. Am J Cardiol. (1984) 53:1103–9. 10.1016/0002-9149(84)90645-3
33.
Maron MS Finley JJ Bos JM Hauser TH Manning WJ Haas TS et al Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. (2008) 118:1541–9. 10.1161/CIRCULATIONAHA.108.781401
34.
Force T Bonow RO Houser SR Solaro RJ Hershberger RE Adhikari B et al Research priorities in hypertrophic cardiomyopathy: report of a working group of the national heart, lung, and blood institute. Circulation. (2010) 122:1130–3. 10.1161/CIRCULATIONAHA.110.950089
35.
Yacoub MH Olivotto I Cecchi F . “End-stage” hypertrophic cardiomyopathy: from mystery to model. Nat Clin Pract Cardiovasc Med. (2007) 4:232–3. 10.1038/ncpcardio0859
36.
Ashrafian H McKenna WJ Watkins H . Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res. (2011) 109:86–96. 10.1161/CIRCRESAHA.111.242974
37.
Doenst T Nguyen TD Abel ED . Cardiac metabolism in heart failure: implications beyond atp production. Circ Res. (2013) 113:709–24. 10.1161/CIRCRESAHA.113.300376
38.
Ferrantini C Belus A Piroddi N Scellini B Tesi C Poggesi C . Mechanical and energetic consequences of HCM-causing mutations. J Cardiovasc Transl Res. (2009) 2:441–51. 10.1007/s12265-009-9131-8
39.
Kellman P Arai AE . Cardiac imaging techniques for physicians: late enhancement. J Magn Reson Imaging. (2012) 36:529–42. 10.1002/jmri.23605
40.
Sclafani M Tini G Musumeci B Cianca A Maestrini V Cacciotti L et al Advanced cardiovascular magnetic resonance imaging in takotsubo syndrome : update on feature tracking and tissue mapping. Curr Cardiovasc Imaging Rep. (2024) 17(6):61–71. 10.1007/s12410-024-09593-9
41.
Bruder O Wagner A Jensen CJ Schneider S Ong P Kispert EM et al Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. (2010) 56:875–87. 10.1016/j.jacc.2010.05.007
42.
Kwon DH Smedira NG Rodriguez ER Tan C Setser R Thamilarasan M et al Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy. Correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. (2009) 54:242–9. 10.1016/j.jacc.2009.04.026
43.
Suk T Edwards C Hart H Christiansen JP . Myocardial scar detected by contrast-enhanced cardiac magnetic resonance imaging is associated with ventricular tachycardia in hypertrophic cardiomyopathy patients. Hear Lung Circ. (2008) 17:370–4. 10.1016/j.hlc.2008.03.080
44.
Mentias A Raeisi-Giglou P Smedira NG Feng K Sato K Wazni O et al Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J Am Coll Cardiol. (2018) 72:857–70. 10.1016/j.jacc.2018.05.060
45.
Todiere G Nugara C Gentile G Negri F Bianco F Falletta C et al Prognostic role of late gadolinium enhancement in patients with hypertrophic cardiomyopathy and low-to-intermediate sudden cardiac death risk score. Am J Cardiol. (2019) 124:1286–92. 10.1016/j.amjcard.2019.07.023
46.
Habib M Adler A Fardfini K Hoss S Hanneman K Rowin EJ et al Progression of myocardial fibrosis in hypertrophic cardiomyopathy: a cardiac magnetic resonance study. JACC Cardiovasc Imaging. (2021) 14:947–58. 10.1016/j.jcmg.2020.09.037
47.
Raman B Ariga R Spartera M Sivalokanathan S Chan K Dass S et al Progression of myocardial fibrosis in hypertrophic cardiomyopathy: mechanisms and clinical implications. Eur Heart J Cardiovasc Imaging. (2019) 20:157–67. 10.1093/ehjci/jey135
48.
Aquaro GD Grigoratos C Bracco A Proclemer A Todiere G Martini N et al Late gadolinium enhancement-dispersion mapping: a new magnetic resonance imaging technique to assess prognosis in patients with hypertrophic cardiomyopathy and low-intermediate 5-year risk of sudden death. Circ Cardiovasc Imaging. (2020) 13:E010489. 10.1161/CIRCIMAGING.120.010489
49.
Neubauer S Kolm P Ho CY Kwong RY Desai MY Dolman SF et al Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM registry. J Am Coll Cardiol. (2019) 74:2333–45. 10.1016/j.jacc.2019.08.1057
50.
Konno T Hayashi K Fujino N Nagata Y Hodatsu A Masuta E et al High sensitivity of late gadolinium enhancement for predicting microscopic myocardial scarring in biopsied specimens in hypertrophic cardiomyopathy. PLoS One. (2014) 9:13–6. 10.1371/journal.pone.0101465
51.
Moravsky G Ofek E Rakowski H Butany J Williams L Ralph-Edwards A et al Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging. (2013) 6:587–96. 10.1016/j.jcmg.2012.09.018
52.
De Bakker JMT Van Capelle FJL Janse MJ Wilde AAM Coronel R Becker AE et al Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiology and anatomic correlation. Circulation. (1988) 77:589–606. 10.1161/01.CIR.77.3.589
53.
Perez-alday EA Haq KT German DM Hamilton C Johnson K Phan F et al Mechanisms of arrhythmogenicity in hypertrophic cardiomyopathy : insight from non-invasive electrocardiographic imaging. Front Physiol. (2020) 11:1–13. 10.3389/fphys.2020.00344
54.
Appelbaum E Maron BJ Adabag S Hauser TH Lesser JR Haas TS et al Intermediate-signal-intensity late gadolinium enhancement predicts ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. (2012) 5:78–85. 10.1161/CIRCIMAGING.111.963819
55.
Aquaro GD Masci P Formisano F Barison A Strata E Pingitore A et al Usefulness of delayed enhancement by magnetic resonance imaging in hypertrophic cardiomyopathy as a marker of disease and its severity. Am J Cardiol. (2010) 105:392–7. 10.1016/j.amjcard.2009.09.045
56.
Jáuregui B Soto-Iglesias D Penela D Acosta J Fernández-Armenta J Linhart M et al Cardiovascular magnetic resonance determinants of ventricular arrhythmic events after myocardial infarction. Europace. (2022) 24:938–47. 10.1093/europace/euab275
57.
Acosta J Fernández-Armenta J Borràs R Anguera I Bisbal F Martí-Almor J et al Scar characterization to predict life-threatening arrhythmic events and sudden cardiac death in patients with cardiac resynchronization therapy: the GAUDI-CRT study. JACC Cardiovasc Imaging. (2018) 11:561–72. 10.1016/j.jcmg.2017.04.021
58.
Francia P Falasconi G Penela D Viveros D Alderete J Saglietto A et al Scar architecture affects the electrophysiological characteristics of induced ventricular arrhythmias in hypertrophic cardiomyopathy. Europace. (2024) 26:1–10. 10.1093/europace/euae050
59.
Francia P Ocaña-Franco P Cristiano E Falasconi G Adduci C Soto-Iglesias D et al Substrates of scar-related ventricular arrhythmia in patients with hypertrophic cardiomyopathy: a cardiac magnetic resonance study. JACC Cardiovasc Imaging. (2023) 16:1359–62. 10.1016/j.jcmg.2023.03.016
60.
Kellman P Hansen MS . T1-mapping in the heart : accuracy and precision. J Cardiovasc Magn Reson. (2014) 16:2. 10.1186/1532-429X-16-2
61.
Bakermans AJ Kouwenhoven M De Vos J De Vries DK Reckman YJ Boekholdt SM . A comparison of myocardial magnetic resonance extracellular volume mapping at 3T against histology of tissue collagen in severe aortic valve stenosis and obstructive hypertrophic cardiomyopathy. Magn Reson Mater Phys Biol Med. (2023) 36:701–9. 10.1007/s10334-023-01070-6
62.
O’Brien AT Gil KE Varghese J Simonetti OP Zareba KM . T2 mapping in myocardial disease: a comprehensive review. J Cardiovasc Magn Reson. (2022) 24:33. 10.1186/s12968-022-00866-0
63.
Wang J Zhang J Liu W Pu L Qi W Xu Y et al Prognostic value of myocardial T1 mapping for predicting adverse events in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. (2025) 18(3):e017174. 10.1161/CIRCIMAGING.124.017174
64.
Li Y Liu X Yang F Wang J Xu Y Fang T et al Prognostic value of myocardial extracellular volume fraction evaluation based on cardiac magnetic resonance T1 mapping with T1 long and short in hypertrophic cardiomyopathy European Society of Cardiology. Eur Radiol. (2021) 31:4557–67. 10.1007/s00330-020-07650-7
65.
Ellims AH Iles LM Ling L Hare JL Kaye DM Taylor AJ . Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson. (2012) 14:70. 10.1186/1532-429X-14-76
66.
McLELLAN AJ Ellims AH Prabhu S Voskoboinik A Iles LM Hare JL et al Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. (2016) 27:571–80. 10.1111/jce.12948
67.
Dass S Suttie JJ Piechnik SK Ferreira VM Holloway CJ Banerjee R et al Myocardial tissue characterization using magnetic resonance noncontrast T1 mapping in hypertrophic and dilated cardiomyopathy. Circulation. (2012) 5:726–33. 10.1161/CIRCIMAGING.112.976738
68.
Xu Z Wang J Cheng W Wan K Li W Pu L et al Incremental significance of myocardial oedema for prognosis in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2023) 24:876–84. 10.1093/ehjci/jead065
69.
Yu T Cai Z Yang Z Lin W Su Y Li J et al The value of myocardial fibrosis parameters derived from cardiac magnetic resonance imaging in risk stratification for patients with hypertrophic cardiomyopathy. Acad Radiol. (2023) 30:1962–78. 10.1016/j.acra.2022.12.026
70.
Hara RPO Prakosa A Binka E Lacy A Trayanova NA . Arrhythmia in hypertrophic cardiomyopathy: risk prediction using contrast enhanced MRI, T1 mapping, and personalized virtual heart technology. J Electrocardiol. (2022) 74:122–7. 10.1016/j.jelectrocard.2022.09.004.Arrhythmia
71.
Małek Ł Werys K Kłopotowski M Śpiewak M Miłosz-Wieczorek B Mazurkiewicz Ł et al Native T1-mapping for non-contrast assessment of myocardial fibrosis in patients with hypertrophic cardiomyopathy—comparison with late enhancement quantification. Magn Reson Imaging. (2015) 33:718–24. 10.1016/j.mri.2015.04.001
72.
Kotecha T Martinez-Naharro A Boldrini M Knight D Hawkins P Kalra S et al Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. (2019) 12:1958–69. 10.1016/j.jcmg.2018.12.022
73.
Biglands JD Radjenovic A Ridgway JP . Cardiovascular magnetic resonance physics for clinicians: part II. J Cardiovasc Magn Reson. (2012) 14:1–40. 10.1186/1532-429X-14-66
74.
Sharka I Panichella G Grigoratos C Muca M De Gori C Keilberg P et al Myocardial perfusion imaging with cardiovascular magnetic resonance in nonischemic cardiomyopathies: an in-depth review of techniques and clinical applications. Med. (2025) 61:1–35. 10.3390/medicina61050875
75.
Tezuka D Kosuge H Terashima M Koyama N Kishida T Tada Y et al Myocardial perfusion reserve quantified by cardiac magnetic resonance imaging is associated with late gadolinium enhancement in hypertrophic cardiomyopathy. Heart Vessels. (2018) 33:513–20. 10.1007/s00380-017-1088-y
76.
Aguiar Rosa S Thomas B Fiarresga A Papoila AL Alves M Pereira R et al The impact of ischemia assessed by magnetic resonance on functional, arrhythmic, and imaging features of hypertrophic cardiomyopathy. Front Cardiovasc Med. (2021) 8:1–11. 10.3389/fcvm.2021.761860
77.
Chiribiri A Leuzzi S Conte MR Bongioanni S Bratis K Olivotti L et al Rest perfusion abnormalities in hypertrophic cardiomyopathy: correlation with myocardial fibrosis and risk factors for sudden cardiac death. Clin Radiol. (2015) 70:495–501. 10.1016/j.crad.2014.12.018
78.
Kim EK Lee SC Chang SA Jang SY Kim SM Park SJ et al Prevalence and clinical significance of cardiovascular magnetic resonance adenosine stress-induced myocardial perfusion defect in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. (2020) 22:30. 10.1186/s12968-020-00623-1
79.
Komuro J Iguchi N Utanohara Y Takayama M Umemura J Tomoike H . Prediction of serious adverse events of patients with hypertrophic cardiomyopathy by magnetic resonance. Int Heart J. (2021) 62:135–41. 10.1536/ihj.20-479
80.
Nielles-Vallespin S Scott A Ferreira P Khalique Z Pennell D Firmin D . Cardiac diffusion: technique and practical applications. J Magn Reson Imaging. (2020) 52:348–68. 10.1002/jmri.26912
81.
Khalique Z Scott AD Ferreira PF Vallespin SN Firmin DN Pennell DJ . Diffusion tensor cardiovascular magnetic resonance in hypertrophic cardiomyopathy: a comparison of motion—compensated spin echo and stimulated echo techniques. Magn Reson Mater Phys Biol Med. (2020) 33:331–42. 10.1007/s10334-019-00799-3
82.
Das A Chowdhary A Kelly C Teh I Stoeck CT Kozerke S et al Insight into myocardial microstructure of athletes and hypertrophic cardiomyopathy patients using diffusion tensor imaging. J Magn Reson Imaging. (2020) 53:73–82. 10.1002/jmri.27257
83.
Ferreira PF Kilner PJ Mcgill L Nielles-vallespin S Scott AD Ho SY et al In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. (2014) 16:87. 10.1186/s12968-014-0087-8
84.
Joy G Kelly CI Webber M Pierce I Teh I McGrath L et al Microstructural and microvascular phenotype of sarcomere mutation carriers and overt hypertrophic cardiomyopathy. Circulation. (2023) 148:808–18. 10.1161/CIRCULATIONAHA.123.063835
85.
Das A Kelly C Teh I Nguyen C Brown LAE Chowdhary A et al Phenotyping hypertrophic cardiomyopathy using cardiac diffusion magnetic resonance imaging : the relationship between microvascular dysfunction and microstructural changes. Eur Hear J Cardiovasc Imaging. (2022) 44:352–62. 10.1093/ehjci/jeab210
86.
Ashkir Z Samat AHA Ariga R Finnigan LEM Jermy S Akhtar MA et al Myocardial disarray and fibrosis across hypertrophic cardiomyopathy stages associate with ECG markers of arrhythmic risk. Eur Hear J Cardiovasc Imaging. (2025) 26:218. 10.1093/ehjci/jeae260
87.
Wu L-M Chen B-H Yao Q-Y Ou Y-R Wu R Jiang M et al Quantitative diffusion-weighted magnetic resonance imaging in the assessment of myocardial fibrosis in hypertrophic cardiomyopathy compared with T1 mapping. Int J Cardiovasc Imaging. (2016) 32:1289–97. 10.1007/s10554-016-0909-x
88.
Mazur W Krzyżak AT Hennel F . Diffusion-weighted imaging and diffusion tensor imaging of the heart in vivo: major developments. Postep Kardiol Interwencyjnej. (2022) 18:350–9. 10.5114/AIC.2022.121345
89.
Khalique Z Ferreira PF Scott AD Nielles-Vallespin S Firmin DN Pennell DJ . Diffusion tensor cardiovascular magnetic resonance imaging: a clinical perspective. JACC Cardiovasc Imaging. (2020) 13:1235–55. 10.1016/j.jcmg.2019.07.016
90.
MacGowan GA Parikh JD Hollingsworth KG . Diffusion tensor magnetic resonance imaging of the heart: looking into the layers of the myocardium. J Am Coll Cardiol. (2017) 69:677–8. 10.1016/j.jacc.2016.10.080
91.
Rizzo S Botta F Raimondi S Origgi D Fanciullo C Morganti AG et al Radiomics : the facts and the challenges of image analysis. Eur Radiol Exp. (2018) 2:36. 10.1186/s41747-018-0068-z
92.
Doran SJ Downey K Connor JPBO Koh D Orton MR . Radiomics in oncology : a practical guide. Radiographics. (2021) 41:1717–32. 10.1148/rg.2021210037
93.
Mayerhoefer ME Materka A Langs G Ida H Szczypi P Gibbs P et al Introduction to radiomics. J Nucl Med. (2020) 61:488–95. 10.2967/jnumed.118.222893
94.
Lambin P Leijenaar RTH Deist TM Peerlings J . Radiomics : the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. (2017) 14:749–62. 10.1038/nrclinonc.2017.141
95.
Baeßler B Engelhardt S Hekalo A Hennemuth A Hüllebrand M Laube A et al Perfect match: radiomics and artificial intelligence in cardiac imaging. Circ Cardiovasc Imaging. (2024) 17:e015490. 10.1161/CIRCIMAGING.123.015490
96.
Fahmy A Rowin E Jaafar N Chan R Rodriguez J Nakamori S et al Radiomics of late gadolinium enhancement reveals prognostic value of myocardial scar heterogeneity in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. (2024) 17:16–27. 10.1016/j.jcmg.2023.05.003
97.
Wang J Bravo L Zhang J Liu W Wan K Sun J et al Radiomics analysis derived from LGE-MRI predict sudden cardiac death in participants with hypertrophic cardiomyopathy. Front Cardiovasc Med. (2021) 8:1–11. 10.3389/fcvm.2021.766287
98.
Durmaz ES Karabacak M Ozkara BB Kargın OA Demir B Raimoglou D et al Machine learning and radiomics for ventricular tachyarrhythmia prediction in hypertrophic cardiomyopathy: insights from an MRI-based analysis. Acta Radiol. (2024) 65:1473–81. 10.1177/02841851241283041
99.
Marfisi D Tessa C Marzi C Del Meglio J Linsalata S Borgheresi R et al Image resampling and discretization effect on the estimate of myocardial radiomic features from T1 and T2 mapping in hypertrophic cardiomyopathy. Sci Rep. (2022) 12:10186. 10.1038/s41598-022-13937-0
100.
Neisius U El-rewaidy H Kucukseymen S Tsao CW Mancio J Nakamori S et al Texture signatures of native myocardial T1 as novel imaging markers for identi fi cation of hypertrophic cardiomyopathy patients without scar. J Magn Reson Imaging. (2020) 52:906–19. 10.1002/jmri.27048
101.
Wang J Yang F Liu W Sun J Han Y Li D et al Radiomic analysis of native T1 mapping images discriminates between MYH7 cardiomyopathy. J Magn Reson Imaging. (2020) 52:1714–21. 10.1002/jmri.27209
102.
Marzi C Marfisi D Barucci A Del Meglio J Lilli A Vignali C et al Collinearity and dimensionality reduction in radiomics: effect of preprocessing parameters in hypertrophic cardiomyopathy magnetic resonance T1 and T2 mapping. Bioengineering. (2023) 10:80. 10.3390/bioengineering10010080
103.
Olivotto I Cecchi F Poggesi C Yacoub MH . Patterns of disease progression in hypertrophic cardiomyopathy an individualized approach to clinical staging. Circ Hear Fail. (2012) 5:535–46. 10.1161/CIRCHEARTFAILURE.112.967026
104.
Raja AA Farhad H Valente AM Couce JP Jefferies JL Bundgaard H et al Prevalence and progression of late gadolinium enhancement in children and adolescents with hypertrophic cardiomyopathy. Circulation. (2018) 138:782–92. 10.1161/CIRCULATIONAHA.117.032966
105.
Nakano Y Ichikawa T Kobayashi H Ishii M Kameda S Nakano E et al Delayed enhancement of ascites after iodine contrast administration assessed with dual-energy computed tomography: a case report. Tokai J Exp Clin Med. (2021) 46:132–6.
106.
Rodriguez-Granillo GA . Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: from bench to bedside. Cardiovasc Diagn Ther. (2017) 7:159–70. 10.21037/cdt.2017.03.16
107.
Ohta Y Kitao S Yunaga H Fujii S Mukai N Yamamoto K et al Myocardial delayed enhancement CT for the evaluation of heart failure: comparison to MRI. Radiology. (2018) 288:682–91. 10.1148/radiol.2018172523
108.
Shiozaki AA Senra T Santos G . Images in cardiovascular medicine myocardial delayed enhancement by computed tomography in hypertrophic cardiomyopathy. Circulation. (2007) 115:430–1. 10.1161/CIRCULATIONAHA.106.674911
109.
Zhao L Ma X Feuchtner GM Zhang C Fan Z . Quantification of myocardial delayed enhancement and wall thickness in hypertrophic cardiomyopathy: multidetector computed tomography versus magnetic resonance imaging. Eur J Radiol. (2014) 83:1778–85. 10.1016/j.ejrad.2014.05.035
110.
Zhao L Ma X Delano MC . Assessment of myocardial fibrosis and coronary arteries in hypertrophic cardiomyopathy using combined arterial and delayed enhanced CT: comparison with MR and coronary angiography. Eur Radiol. (2013) 23:1034–43. 10.1007/s00330-012-2674-0
111.
Szczykutowicz TP Viggiano B Rose S Pickhardt PJ Lubner MG . A metric for quantification of iodine contrast enhancement (Q-ICE) in computed tomography. J Comput Assist Tomogr. (2021) 45:870–6. 10.1097/RCT.0000000000001215
112.
Langer C Schaefer P Lutz M Eden M Hohnhorst M Harders H et al Myocardial fibrosis in hypertrophic cardiomyopathy: volumetric assessment of late enhancement provided by cardiac computed tomography. J Comput Assist Tomogr. (2015) 39:797–803. 10.1097/RCT.0000000000000272
113.
Shiozaki AA Senra T Arteaga E Martinelli Filho M Pita CG Ávila LFR et al Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr. (2013) 7:173–81. 10.1016/j.jcct.2013.04.002
114.
Takaoka H Funabashi N Uehara M Ozawa K Kobayashi Y . Successful prediction of MACE by myocardial fibrosis on CT in hypertrophic cardiomyopathy patients without obstructed coronary arteries. Int J Cardiol. (2015) 199:34–7. 10.1016/j.ijcard.2015.06.139
115.
Scully PR Bastarrika G Moon JC Treibel TA . Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep. (2018) 20:15. 10.1007/s11886-018-0961-3
116.
Graser A Johnson TRC Chandarana H Macari M . Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol. (2009) 19:13–23. 10.1007/s00330-008-1122-7
117.
Hong YJ Kim TK Hong D Park CH Yoo SJ Wickum ME et al Myocardial characterization using dual-energy CT in doxorubicin-induced DCM: comparison with CMR T1-mapping and histology in a rabbit model. JACC Cardiovasc Imaging. (2016) 9:836–45. 10.1016/j.jcmg.2015.12.018
118.
Lee H-J Im DJ Youn J-C Chang S Suh YJ Hong YJ et al Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology. (2016) 280:49–57. 10.1148/radiol.2016151289
119.
Nacif MS Kawel N Lee JJ Zavodni A Sibley CT Lima JAC . Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. (2012) 264:876–83. 10.1148/radiol.12112458
120.
Nacif MS Liu Y Yao J Liu S Sibley CT Summers RM et al 3D left ventricular extracellular volume fraction by low-radiation dose cardiac CT: assessment of interstitial myocardial fibrosis. J Cardiovasc Comput Tomogr. (2013) 7:51–7. 10.1016/j.jcct.2012.10.010
121.
Treibel TA Bandula S Fontana M White SK Gilbertson JA Herrey AS et al Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr. (2015) 9:585–92. 10.1016/j.jcct.2015.07.001
122.
Bandula S White SK Flett AS Lawrence D Pugliese F Ashworth MT et al Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology. (2013) 292:396–403. 10.1148/radiology.13130130
123.
Mirelis G Sa J Salguero-bodes R Filgueiras-rama D Gonza E Ferna R et al Myocardial extracellular volume is not associated with malignant ventricular arrhythmias in high-risk hypertrophic cardiomyopathy. Spanish J Cardiol. (2017) 70:933–40. 10.1016/j.rec.2017.01.026
Summary
Keywords
hypertrophic cardiomyopathy (HCM), cardiovascular magnetic resonance (CMR), cardiac computed tomography (CT), myocardial scar, late gadolinium enhancement
Citation
Sclafani M, Perrotti C, Salerno L, Muthukkattil ML, Lustri C, Cristofari G, Falasconi G, Musumeci MB, Penela D, Saglietto A, Iannaccone A, Barbato E, Berruezo A and Francia P (2025) Imaging myocardial scar in hypertrophic cardiomyopathy: advances in CMR and CT. Front. Cardiovasc. Med. 12:1649728. doi: 10.3389/fcvm.2025.1649728
Received
18 June 2025
Accepted
02 September 2025
Published
18 September 2025
Volume
12 - 2025
Edited by
Giovanni Quarta, Papa Giovanni XXIII Hospital, Italy
Reviewed by
Michael Jerosch-Herold, Brigham and Women's Hospital, Harvard Medical School, United States
Updates
Copyright
© 2025 Sclafani, Perrotti, Salerno, Muthukkattil, Lustri, Cristofari, Falasconi, Musumeci, Penela, Saglietto, Iannaccone, Barbato, Berruezo and Francia.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Pietro Francia pietro.francia@uniroma1.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.