Abstract
We propose a novel clinical construct, the “Gulliver syndrome”, to describe the scenario in which multiple, mildly elevated cardiovascular risk factors (CVRFs) coexist within an individual and together result in a significantly heightened overall risk of cardiovascular disease (CVD). This accumulation of small deviations, often dismissed in clinical practice, can exert a synergistic impact on vascular health. Our aim is to formalize this underrecognized phenotype, which falls outside traditional diagnostic entities such as the metabolic syndrome, and to provide a framework that enables early recognition and management. We outline proposed diagnostic criteria, contrast this syndrome with related constructs, support its clinical relevance with emerging literature, and present a representative case. Ultimately, we advocate for this framework as a tool to overcome therapeutic inertia and encourage proactive, multifactorial interventions in primary and preventive care.
Introduction
A recurring theme in preventive cardiology is the tendency to underestimate the cumulative impact of multiple subclinical or borderline risk factor elevations. While clinical guidelines provide thresholds that delineate normal from pathological, the pathophysiology of atherosclerotic cardiovascular disease (ASCVD) is a continuum. Even modest elevations in blood pressure, lipids, or glycemia can interact in complex ways to accelerate endothelial dysfunction, inflammation, and plaque formation (1).
The “Gulliver syndrome” is named after Jonathan Swift's fictional character Lemuel Gulliver, who, though a giant among the Lilliputians, finds himself incapacitated by countless tiny ropes. Analogously, many patients are immobilized by multiple minor aberrations in cardiovascular risk factors (CVRFs), each one clinically unalarming, yet jointly capable of creating a high-risk internal milieu. These patients often remain untreated or undertreated due to the absence of any single alarming metric, a phenomenon termed “therapeutic inertia” (2).
We argue that current frameworks, such as the metabolic syndrome, fall short in capturing this phenotype. While the metabolic syndrome focuses on obesity and insulin resistance, the Gulliver syndrome emphasizes the cumulative burden of borderline CVRFs independent of overt obesity or glucose dysregulation. Moreover, recent literature has challenged the predictive value of some metabolic syndrome criteria, such as low high-density lipoprotein (HDL) cholesterol, as independent causal factors (3–5). This phenomenon is particularly relevant in patients with borderline or mildly elevated cardiovascular risk profiles, who often remain below treatment thresholds despite accumulating multiple non-optimal markers.
Proposed definition and diagnostic criteria
The Gulliver syndrome is characterized by the simultaneous presence of at least four mildly abnormal CVRFs, each falling within a “borderline” range but together elevating total cardiovascular risk.
Mandatory criteria (all four must be present):
- -
Waist circumference (WC): 90–101 cm in men or 80–87 cm in women [borderline abdominal adiposity; measured in a clinical setting under standardized conditions, values between optimal and diagnostic thresholds (≥102 cm in men, ≥88 cm in women), associated with increased cardiometabolic risk] (6–8).
- -
Systolic blood pressure (SBP): 120–139 mmHg and/or diastolic blood pressure (DBP): 80–89 mmHg [borderline blood pressure; measured in a clinical setting under standardized conditions, below diagnostic hypertension thresholds (≥140/90 mmHg), but above optimal levels] (9, 10).
- -
Fasting plasma glucose (FPG): 100–125 mg/dL [borderline glycemia; measured after ≥8 hours of fasting, below the diagnostic threshold for diabetes (≥126 mg/dL), but consistent with impaired fasting glucose] (11).
- -
Non-HDL cholesterol: 130–189 mg/dL [borderline-to-high range; associated with increased atherogenic burden without reaching the threshold for very high-risk dyslipidemia (≥190 mg/dL)] (8, 12–14).
Additional aggravating factors (non-essential but contributory):
- -
Sedentary lifestyle.
- -
Smoking.
- -
Alcohol consumption.
- -
Psychosocial stress.
- -
Family history of premature cardiovascular disease (CVD).
- -
Unhealthy diet.
- -
Short or disrupted sleep.
Although not formally included in the diagnostic core, these factors may act as amplifiers of cardiovascular risk and are therefore relevant in the Gulliver syndrome framework. These criteria are grounded in evidence linking each variable with elevated cardiovascular morbidity and mortality (even at sub-threshold levels) when present in combination (
15–
17).
Figure 1illustrates the proposed concept of Gulliver syndrome, highlighting the convergence of borderline CVRFs and their cumulative impact on overall cardiovascular risk.
Figure 1
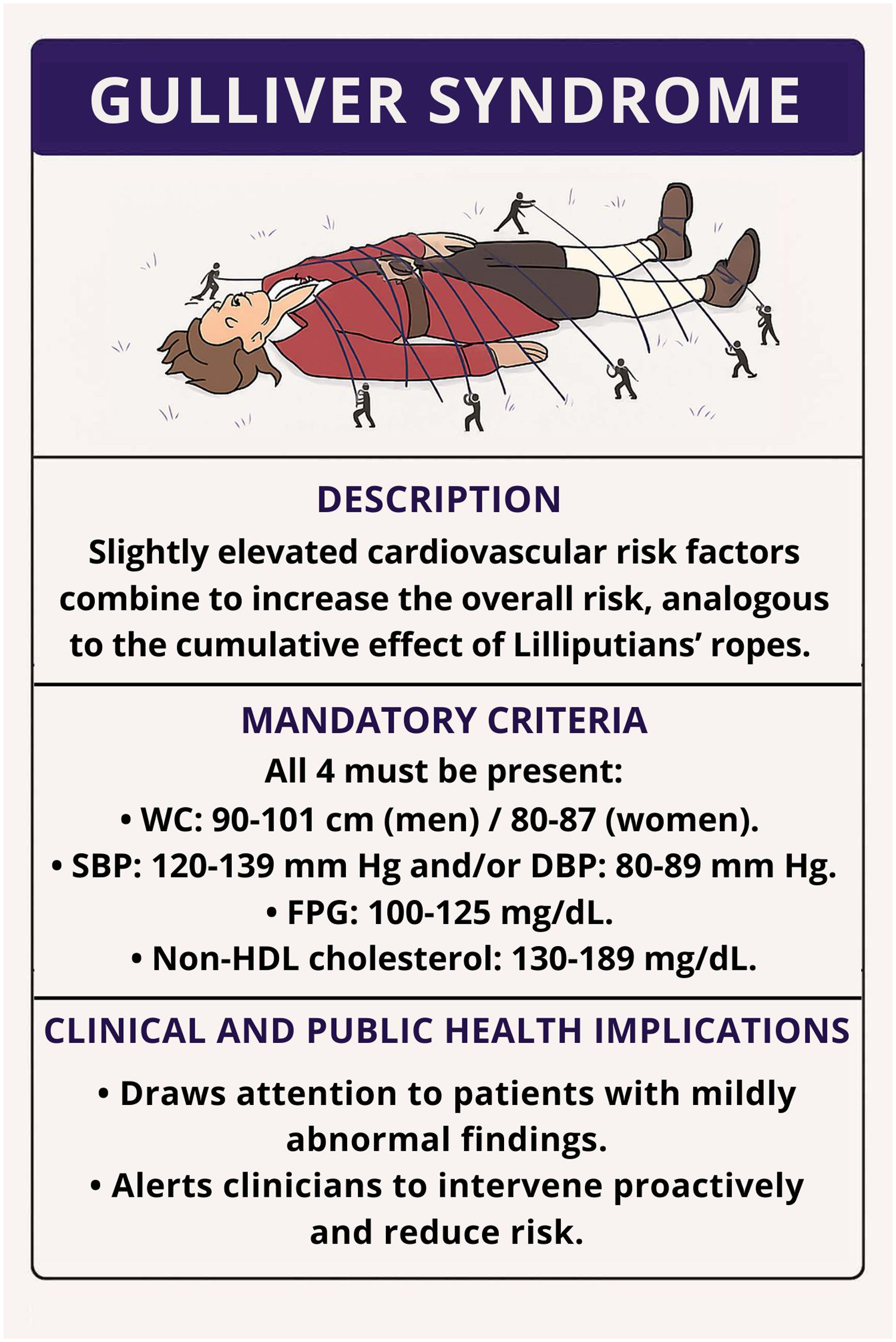
Graphical representation of the Gulliver syndrome. A patient's overall cardiovascular risk increases when multiple mildly elevated risk factors coexist. Each rope represents a single, subclinical abnormality (waist circumference, blood pressure, glucose, or non-high-density lipoprotein colesterol) that collectively restrain health, analogous to the ropes of the Lilliputians. DBP, diastolic blood pressure; FPG, Fasting plasma glucose; HDL, high-density lipoprotein; SBP, systolic blood pressure; WC, waist circumference.
Case illustration
Mr. A, a 52-year-old male software engineer, presents for an annual check-up. He is asymptomatic, non-smoking, and has no prior diagnosis of diabetes or hypertension. He reports minimal physical activity and frequent job-related stress. On physical examination and laboratory evaluation, the following findings were noted:
- -
WC: 96 cm.
- -
Blood pressure: 128/84 mmHg.
- -
FPG: 106 mg/dl.
- -
Total cholesterol: 220 mg/dl; HDL cholesterol: 48 mg/dl; Non-HDL cholesterol: 172 mg/dl.
Despite each parameter being only mildly abnormal, his calculated 10-year Systematic Coronary Risk Evaluation 2 (SCORE2) cardiovascular risk places him in the moderate-to-high category. Yet no therapeutic intervention is initiated beyond generic lifestyle advice. This reflects a missed opportunity for early intervention and risk modification.
Theoretical basis and pathophysiological rationale
Evidence from longitudinal cohort studies demonstrates that cardiovascular risk accumulates progressively with incremental elevations in risk factors, even below diagnostic cut-offs. For instance, Vasan et al. (18) showed that individuals with prehypertension and borderline cholesterol levels had a significantly increased lifetime risk of coronary artery disease.
Furthermore, the concept of “residual risk” (i.e., the risk that remains even after managing a primary risk factor) underscores the need for multifactorial control. The Gulliver syndrome, by identifying patients with diffuse low-grade risk, may help target this residual burden earlier.
The pathophysiology supports this notion: even small elevations in glucose and blood pressure can impair endothelial function, increase arterial stiffness, and enhance oxidative stress. Subclinical inflammation (a hallmark of early atherogenesis) is fueled by such chronic metabolic perturbations (19–21).
Implications for clinical practice and policy
Therapeutic inertia, defined as the failure to initiate or intensify therapy when indicated, has been widely documented in hypertension, type 2 diabetes, and dyslipidemia (22–24). It is particularly prevalent in patients with borderline abnormalities, where no single value crosses the treatment threshold.
By defining and naming Gulliver syndrome, we provide a conceptual tool to prompt clinician awareness. This syndrome encourages the use of composite risk scores (e.g., SCORE2, ASCVD Risk Estimator) and favors the early use of lifestyle modification and, when appropriate, pharmacologic intervention. The polypill strategy is especially relevant for such patients (25).
Moreover, as cardiovascular prevention shifts toward precision medicine, identifying subtle but meaningful phenotypes like Gulliver syndrome could enhance individualized care pathways and inform public health strategies (Figure 1).
The American Heart Association's Life's Essential 8 framework, which includes metrics such as diet quality, physical activity, sleep health, and nicotine exposure (26), offers a holistic approach to cardiovascular health that complements our conceptualization of Gulliver syndrome. A recent meta-analysis has shown that a large proportion of adults score poorly across several Life's Essential 8 components (27), especially in domains such as diet and sleep, reinforcing the prevalence of diffuse risk accumulation. Recognizing individuals with poor composite metrics could support earlier identification of Gulliver syndrome candidates and reduce therapeutic inertia. Thus, while the Life's Essential 8 provides a population-level assessment tool, the Gulliver syndrome framework adds clinical granularity by identifying individuals who, despite lacking severe abnormalities, may benefit from targeted preventive intervention.
Conclusion
The Gulliver syndrome encapsulates a common but under-recognized clinical reality: the aggregation of mild, subclinical CVRFs that, if unaddressed, lead to significant morbidity and mortality. Formal recognition of this construct may reduce therapeutic inertia, enhance preventive strategies, and improve long-term outcomes. We call for empirical studies to quantify its prevalence and impact, and advocate for its inclusion in clinical education and decision-support tools.
Statements
Author contributions
JL-G: Writing – original draft. JA-H: Conceptualization, Validation, Writing – review & editing. JA-A: Conceptualization, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare Generative AI was used in the creation of this manuscript. Figure 1 was created using ChatGPT’s image generation tool (OpenAI). ChatGPT was used exclusively for image generation and did not participate in writing or interpretation.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction Note
A correction has been made to this article. Details can be found at: 10.3389/fcvm.2025.1696969.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Libby P . Inflammation in atherosclerosis. Nature. (2002) 420:868–74. 10.1038/nature01323
2.
Dixon DL Sharma G Sandesara PB Yang E Braun LT Mensah GA et al Therapeutic inertia in cardiovascular disease prevention. J Am Coll Cardiol. (2019) 74:1728–31. 10.1016/j.jacc.2019.08.014
3.
Barter P Gotto AM LaRosa JC Maroni J Szarek M Grundy SM et al HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. (2007) 357:1301–10. 10.1056/NEJMoa064278
4.
Madsen CM Varbo A Nordestgaard BG . Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. (2017) 38:2478–86. 10.1093/eurheartj/ehx163
5.
Voight BF Peloso GM Orho-Melander M Frikke-Schmidt R Barbalic M Jensen MK et al Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. (2012) 380:572–80. 10.1016/S0140-6736(12)60312-2
6.
International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome [Internet]. Brussels: IDF (2006). Available from:https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwidedefinitionof-the-metabolic-syndrome.html(Accessed Jul 29, 2025).
7.
World Health Organization, editor. Obesity: preventing and managing the global epidemic: report of a WHO consultation (WHO technical report series).Geneva: World Health Organization (2000). p 253. 10.1093/eurheartj/ehz455
8.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. (2001) 285(19):2486–97. 10.1001/jama.285.19.2486
9.
Unger T Borghi C Charchar F Khan NA Poulter NR Prabhakaran D et al 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. (2020) 75(6):1334–57. 10.1161/HYPERTENSIONAHA.120.15026
10.
Jones DW Ferdinand KC Taler SJ Johnson HM Shimbo D Abdalla M et al 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2025) 10.1161/CIR.0000000000001356
11.
American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes-2024. Diabetes Care. (2024) 47(Supplement_1):S20–42. 10.2337/dc24-S002
12.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda (MD): National Institutes of Health (2001). NIH Publication No. 01-3670.
13.
Grundy SM Stone NJ Bailey AL Beam C Birtcher KK Blumenthal RS et al 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2019) 139(25):e1082–143. 10.1161/CIR.0000000000000625
14.
Mach F Baigent C Catapano AL Koskinas KC Casula M Badimon L et al 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41(1):111–88. 10.1093/eurheartj/ehz455
15.
Yusuf S Joseph P Rangarajan S Islam S Mente A Hystad P et al Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. (2020) 395:795–808. 10.1016/S0140-6736(19)32008-2
16.
Lloyd-Jones DM Larson MG Beiser A Levy D . Lifetime risk of developing coronary heart disease. Lancet. (1999) 353:89–92. 10.1016/S0140-6736(98)10279-9
17.
Kannel WB . Diabetes and cardiovascular disease: the framingham study. JAMA. (1979) 241:2035. 10.1001/jama.1979.03290450033020
18.
Vasan RS Larson MG Leip EP Evans JC O’Donnell CJ Kannel WB et al Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. (2001) 345:1291–7. 10.1056/NEJMoa003417
19.
Hansson GK . Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95. 10.1056/NEJMra043430
20.
Ross R . Atherosclerosis—an inflammatory disease. N Engl J Med. (1999) 340:115–26. 10.1056/NEJM199901143400207
21.
Tabas I Williams KJ Borén J . Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. (2007) 116:1832–44. 10.1161/CIRCULATIONAHA.106.676890
22.
Khunti K Gomes MB Pocock S Shestakova MV Pintat S Fenici P et al Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. (2018) 20:427–37. 10.1111/dom.13088
23.
Okonofua EC Simpson KN Jesri A Rehman SU Durkalski VL Egan BM . Therapeutic inertia is an impediment to achieving the healthy people 2010 blood pressure control goals. Hypertension. (2006) 47:345–51. 10.1161/01.HYP.0000200702.76436.4b
24.
Faggiano A Gualeni A Barbieri L Mureddu GF Venturini E Giallauria F et al Therapeutic inertia in dyslipidemia management for secondary cardiovascular prevention: results from the Italian ITACARE-P network. J Clin Med. (2025) 14:493. 10.3390/jcm14020493
25.
Salim H Musmar B Saifi M Ayyad M Ruzieh M Azar J et al The impact of polypill on adherence and cardiovascular outcomes:a comprehensive systematic review with meta-analysis. Curr Cardiol Rev. (2024) 20:e230124225968. 10.2174/011573403X283174240110025442
26.
Lloyd-Jones DM Allen NB Anderson CAM Black T Brewer LC Foraker RE et al Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. (2022) 146:e18–43. 10.1161/cir.0000000000001078
27.
López-Bueno R Núñez-Cortés R Calatayud J Salazar-Méndez J Petermann-Rocha F López-Gil JF et al Global prevalence of cardiovascular risk factors based on the Life’s Essential 8 score: an overview of systematic reviews and meta-analysis. Cardiovasc Res. (2024) 120(1):13–33. 10.1093/cvr/cvad176
Summary
Keywords
obesity, hypertension, diabetes mellitus, cholesterol, biomarkers
Citation
López-Gil JF, Abellán-Huerta J and Abellán-Alemán J (2025) The Gulliver syndrome: a conceptual framework to address therapeutic inertia in patients with borderline cardiovascular risk profiles. Front. Cardiovasc. Med. 12:1652447. doi: 10.3389/fcvm.2025.1652447
Received
23 June 2025
Accepted
21 July 2025
Published
14 August 2025
Corrected
06 November 2025
Volume
12 - 2025
Edited by
Mario Daidone, University of Palermo, Italy
Reviewed by
Pompilio Faggiano, Fondazione Poliambulanza Istituto Ospedaliero, Italy
Updates
Copyright
© 2025 López-Gil, Abellán-Huerta and Abellán-Alemán.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: José Francisco López-Gil josefranciscolopezgil@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.