Abstract
Cardiometabolic diseases—including type 2 diabetes, cardiovascular disease, and metabolic dysfunction–associated steatotic liver disease—are increasingly driven by near-continuous after-meal exposure to glucose and lipid surges that traditional fasting tests often miss. This review prioritizes human studies from 2020 to 2025 and uses earlier work only as foundational anchors; non-English reports were excluded and preclinical findings are cited solely for mechanistic context. Evidence converges on six processes that amplify risk within hours after eating: impaired insulin signaling, delayed clearance of dietary lipids, mitochondrial and oxidative stress, loss of endothelial nitric oxide, inflammasome-mediated inflammation, and microbiome–hormone interactions. Dynamic, after-meal markers and simple composites such as the triglyceride–glucose index outperform fasting measures for identifying risk and guiding care. Practical strategies to shorten the “damage window” include Mediterranean-style meals with low glycemic index swaps and unsaturated fats, earlier distribution of daily energy and early time-restricted eating, a small pre-meal protein portion, and brief post-meal walking. Fast-acting medicines—glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide receptor agonists, rapid-acting insulin analogues, sodium–glucose cotransporter 2 inhibitors taken before meals, and proprotein convertase subtilisin/kexin type 9 inhibitors—further blunt peaks, while continuous glucose monitoring with algorithmic feedback enables timing-aware, person-specific adjustments. A tiered workflow—screen, stratify, and personalize—reframes prevention and treatment around after-meal physiology, with particular relevance to settings where resources are limited.
1 Introduction
Chronic noncommunicable diseases (CNCDs) now account for 41 million deaths each year, roughly 71 percent of all global mortality—and have overtaken infectious illnesses as the leading public-health threat (1). Within that broad category, cardiometabolic conditions—type 2 diabetes (T2D), cardiovascular disease (CVD) and metabolic-dysfunction-associated steatotic liver disease (MASLD, formerly NAFLD)—are rising fastest (2). T2D prevalence in sub-Saharan Africa has jumped from four million cases in 1980 to 23.6 million in 2021 and is projected to exceed 54 million by 2045 (3). MASLD affects roughly one adult in four worldwide (4, 5), while CVD alone claims 17.9 million lives annually, most of them in low- and middle-income regions (6).
Decades of epidemiology and mechanistic work converge on a common upstream driver: modern eating patterns characterized by frequent snacking on energy-dense, highly refined foods. This dietary behavior shortens fasting intervals and maintains most individuals in a near-continuous postprandial state—typically involving four to ten eating occasions per day with minimal overnight respite (7). These repeated surges of glucose and triglyceride-rich lipoproteins (TRLs) disrupt circadian clocks, overload mitochondrial redox systems, and activate innate-immune pathways, thereby accelerating atherogenesis, β-cell failure and hepatic steatosis (8, 9). Critically, this shift represents a departure from evolutionary eating patterns, where extended fasting periods allowed metabolic recovery and cellular repair processes that are now chronically interrupted.
The postprandial window now stretches well beyond half of every 24 h cycle; in many individuals it exceeds sixteen hours (10). Prolonged exposure to elevated glucose and lipid concentrations fuels low-grade systemic inflammation, a process termed “metaflammation”, which is central to the pathogenesis of T2D, CVD and MASLD (11, 12). The term postprandial dysmetabolism denotes the triad of hyperglycemia, hypertriglyceridemia, and hyperinsulinemia that follows each meal in susceptible individuals (13). When that triad is amplified by poor diet quality and increased meal frequency, oxidative stress, endothelial dysfunction and chronic inflammation ensue (14–16). Importantly, these metabolic perturbations can occur while fasting markers remain normal, highlighting a critical blind spot in current diagnostic approaches.
Prospective cohort studies demonstrate that the height and duration of post-meal glucose and triglyceride peaks predict carotid-intima thickening and future cardiovascular events even when fasting markers remain within normal ranges (17, 18). This finding challenges the traditional paradigm of metabolic assessment and underscores the clinical relevance of postprandial monitoring. Because the gut, liver, muscle, adipose tissue and pancreas coordinate postprandial homeostasis through complex inter-organ crosstalk, disturbances in any single organ rapidly propagate across the entire metabolic network (10, 17, 18). Despite this evidence, preventive care continues to rely predominantly on fasting glucose or low-density-lipoprotein cholesterol (LDL-C) measurements, leaving a substantial portion of cardiometabolic risk undetected and unaddressed—particularly in resource-limited settings where pharmacotherapy access is constrained and health-system capacity is limited (19–23).
This review applies a contemporary lens (2020–2025) reflecting methodological and clinical inflection points—widespread continuous glucose monitoring (CGM), standardized assays for TRL, multi-omics workflows, and the clinical introduction of glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide (GLP-1/GIP) co-agonists—while selectively incorporating pre-2020 “foundational” contributions limited to seminal meta-analyses, consensus statements, pivotal randomized trials, or first-in-field mechanistic studies. Primary evidence prioritizes human adult studies indexed in Scopus (randomized controlled trials, controlled feeding/postprandial challenge studies over 0–6 h, and prospective cohorts), with inclusion contingent on clear test-meal composition, defined sampling windows, and assay standardization. Preclinical studies (animal or cell preparations) are cited only for mechanistic context, to probe causal links impractical or unethical to test in humans, and to nominate druggable targets relevant to the postprandial state [e.g., NADPH-oxidase (NOX)–endothelial nitric oxide synthase (eNOS) coupling, Yes-associated protein/TEA domain transcription factor (YAP/TEAD) signaling, calciprotein particle–driven pathways]. Such findings are not used to claim clinical efficacy, estimate effect sizes, or define clinical endpoints and are explicitly flagged in-text as “preclinical”, with model (mouse/rat) and exposure type (dietary, genetic, pharmacological) specified. We excluded non-English publications and did not treat narrative reviews as primary evidence; when cited, such reviews provided historical framing or methodological context only. Foundational citations are flagged in-text and collated in Supplementary Table S1 with rationale and study type (meta-analysis, pivotal RCT, first-in-field).
The aim of this narrative review is to move the spotlight from static fasting metrics to the dynamic metabolic stresses that arise after every meal, offering clinicians, researchers, and policymakers a practical roadmap for earlier detection, tailored intervention, and, ultimately, more effective prevention of CNCD-related morbidity and mortality.
This review synthesizes evidence on five inter-related domains of postprandial dysmetabolism: (i) the molecular and physiological pathways that precipitate metabolic dysfunction following nutrient intake; (ii) fasting-state surrogates and dynamic biomarkers that reveal these otherwise occult perturbations; (iii) dietary, behavioral, and pharmacological interventions that can mitigate postprandial stress; (iv) emerging technologies for real-time monitoring and personalized therapeutic targeting; and (v) implementation strategies for translating these advances into clinical practice, particularly in diverse populations and resource-variable settings.
2 Mechanistic drivers of postprandial dysmetabolism
2.1 Conceptual framework and temporal dynamics
Postprandial dysmetabolism is a time-dependent systems disturbance with min-to-hours fluctuations in glucose and lipids and hours-to-days adaptations in redox/circadian and gut–hormone axes. It reflects the convergence of nutrient overload, redox imbalance, and circadian misalignment across six interconnected nodes—from rapid glucose handling (min) to lipid clearance (peaks approximately 4–6 h) and microbiome–endocrine shifts (hours–days). Epidemiologic and clinical evidence links this state to endothelial injury and higher cardiovascular risk in people with and without T2D, supporting assay/intervention timing by temporal bands (operational definitions in Supplementary Table S1) (24, 25).
2.2 Substrate-specific metabolic overload (0–2 h post-meal)
Excess glucose engages canonical insulin signaling [insulin receptor substrate (IRS)—phosphoinositide 3-kinase (PI3K)—protein kinase B (Akt)] to drive glucose transporter type 4 (GLUT4) translocation in skeletal muscle and adipose tissue; impaired signaling delays vesicle delivery and prolongs hyperglycemia (26). Preclinical data indicate that SHIP2 (“SKIP” in rodents) limits phosphatidylinositol-3,4,5-trisphosphate (PIP3)/Akt signaling and that glucolipotoxic stress induces IRS-1 serine phosphorylation, dampening PI3K/Akt activity and GLUT4 trafficking (27–29). In parallel, intestinal chylomicron export can exceed lipoprotein lipase (LPL) capacity, leaving triglyceride-rich remnants that typically peak approximately 4–6 h (and may persist longer) after a mixed meal (30). The combined substrate surplus elevates mitochondrial reactive oxygen species (ROS) [reverse electron transport (RET) at Complex I; high potential at Complex III] within approximately 60–180 min, taxing antioxidant defenses (mechanistic/preclinical) (31, 32).
2.3 Vascular and inflammatory cascade (1–6 h post-meal)
The oxidative burst plus remnant lipoproteins activates the endothelium, lowers bioavailable nitric oxide (NO) (eNOS uncoupling; NOX/xanthine oxidase), and upregulates intercellular adhesion molecule 1/vascular cell adhesion molecule-1 (ICAM-1/VCAM-1). Innate sensors (Toll-like and NOD-like receptors) promote NOD-, LRR-, and pyrin-domain–containing protein 3 (NLRP3) inflammasome assembly, raising interleukin 1β (IL-1β) and interleukin 6 (IL-6); obesity amplifies these inputs via adipose-derived cytokines and lipotoxic mediators, reinforcing a feed-forward loop (33–35).
2.4 Microbiome-Endocrine integration (hours to days)
Dysbiosis reshapes the bile-acid pool via microbial bile-salt hydrolase (BSH) activity, modulating farnesoid X receptor/Takeda G-protein-coupled receptor 5 (FXR/TGR5) signaling (preclinical), and microbiota-derived bile acids/short-chain fatty acids (SCFAs) can influence L-cell GLP-1 secretion, helping explain variability in subsequent postprandial responses (foundational preclinical listed in Supplementary Table S1; contemporary human/preclinical syntheses) (36–38).
2.5 Clinical relevance and paradigm implications
Together, these nodes explain why the height and duration of post-meal peaks predict carotid-intima thickening and incident cardiovascular events independent of fasting markers (39, 40). With this theoretical framework, postprandial metabolism can be rapidly identified for targeted intervention; Figure 1 represents the interconnected network that links high nutrient intake with endothelial damage, insulin insensitivity, and hepatic lipid accumulation.
Figure 1
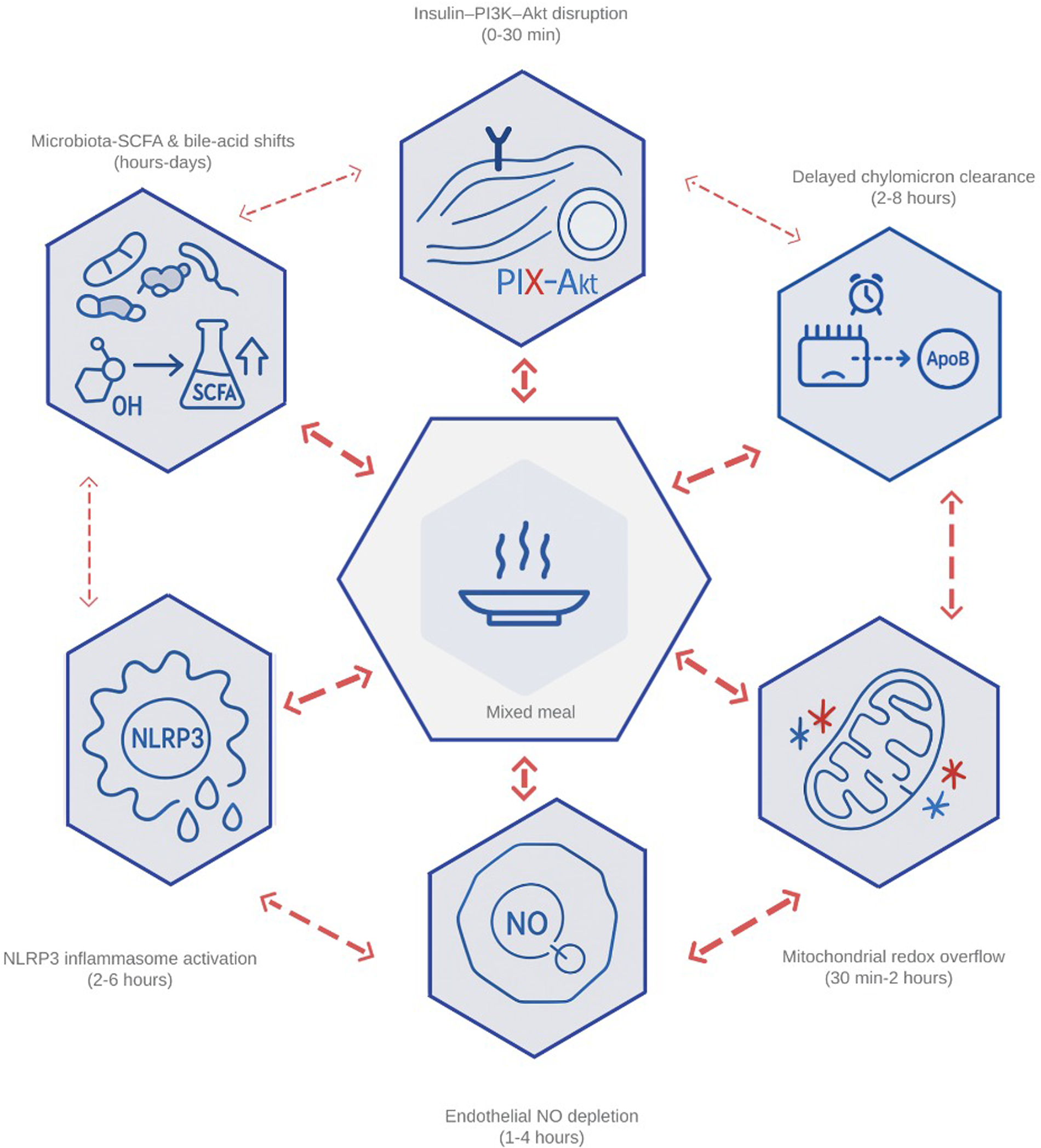
Integrated network of the six primary drivers of postprandial dysmetabolism. The diagram illustrates how (1) impaired insulin–PI3K–Akt signaling, (2) delayed clearance of chylomicron-derived remnants, (3) mitochondrial redox overflow, (4) endothelial nitric-oxide depletion, (5) inflammasome-driven cytokine release, and (6) microbiota-mediated shifts in bile-acid and short-chain-fatty-acid profiles interact within min after a mixed meal. Bidirectional arrows highlight feed-forward loops—ROS amplifying endothelial activation, remnant lipids fueling NLRP3 assembly, and butyrate modulating GLP-1—that transform transient surges into chronic cardiometabolic stress. PI3K, phosphoinositide-3-kinase; Akt, protein kinase B; ROS, reactive oxygen species; NLRP3, NOD-, LRR-, and pyrin-domain–containing protein 3 (inflammasome); GLP-1, glucagon-like peptide 1; SCFA, short-chain fatty acid; NO, nitric oxide.
2.6 Glucose metabolism and insulin resistance
2.6.1 Normal postprandial insulin signaling
In metabolically healthy adults, a meal elicits insulin secretion within 5–10 min. Circulating insulin binds to the insulin receptor (IR) in target tissues, resulting in autophosphorylation of the receptor as well as phosphorylation of IRS-1/2. IRS-1/2 recruits PI3K, generating PIP3 that recruits Akt to the membrane, where phosphoinositide-dependent kinase 1 and mammalian target of rapamycin Complex 2 (mTORC2) activate Akt (39, 40).
2.6.2 Glucose uptake and metabolic integration
Akt phosphorylation of AS160 relieves Rab-GTPase restraint and drives GLUT4 vesicle fusion with the plasma membrane, enabling rapid glucose uptake. In parallel, Akt inhibits glycogen-synthase-kinase-3β to promote glycogen synthesis and—via mTORC1—supports protein synthesis and cell growth. Energy-sensing by adenosine monophosphate-activated protein kinase (AMPK) complements this program by enhancing GLUT4 trafficking and fatty-acid oxidation when the adenosine monophosphate/adenosine triphosphate (AMP/ATP) ratio rises, sustaining postprandial metabolic flexibility (41, 42).
2.6.3 Temporal dynamics and individual variation
Nonetheless, postprandial glucose clearance varies substantially with age, fitness, genetics, and meal timing. In PREDICT 1, glycemic responses to identical meals showed large between-person differences with strong person-specific predictability (r = 0.77) (mixed-risk adults; n ≈ 1,100; standardized test meals approximately 500–900 kcal with varied macronutrient composition; capillary glucose/CGM sampling 0–4 h) (43). Variants at circadian loci (MTNR1B rs10830963, CRY2 rs12419690) relate to diurnal glycemic control (UK Biobank adults; n ≈ 420,000; random serum glucose linked to time-of-day; replication Estonian Biobank n approximately 100,000; 24 h cosinor modeling; not a meal test) (44). This heterogeneity underscores limits of one-size-fits-all diagnostics and supports personalized postprandial monitoring, including CGM-guided dietary interventions that outperform standard advice in randomized trials (45, 46).
2.6.4 Inflammatory disruption of insulin sensitivity
Repeated exposure to saturated fats and refined carbohydrates activates pro-inflammatory kinases— IκB kinase beta (IKKβ) and c-Jun N-terminal kinase 1 (JNK-1)—that serine-phosphorylate IRS-1, impairing tyrosine phosphorylation and PI3K recruitment (preclinical) (39, 40). This molecular injury contributes to selective insulin resistance, where metabolic signaling decreases while inflammatory/lipogenic pathways remain active.
2.6.5 Tissue-specific insulin resistance
Consequences differ by tissue. Skeletal muscle (approximately 80 percent of postprandial glucose disposal) develops GLUT4 translocation defects that limit uptake (47–49); the liver maintains gluconeogenesis/glycogenolysis despite hyperinsulinemia, sustaining hyperglycemia; and adipose tissue insulin resistance augments lipolysis and circulating FFAs, further propagating insulin resistance across organs (50).
2.7 Postprandial lipemia, triglyceride clearance, and lipotoxicity
2.7.1 Normal postprandial triglyceride processing
After a mixed meal, dietary triglycerides are assembled into intestinal chylomicrons (CM) and reach the bloodstream via lymph within 30–60 min. Clearance depends on LPL and its endothelial anchor GPIHBP1 at capillaries of adipose tissue and skeletal muscle, enabling efficient intravascular hydrolysis and tissue uptake (7, 9, 51).
2.7.2 Insulin-mediated regulation of lipid clearance
Physiologic postprandial insulin acutely increases LPL activity (e.g., post-heparin LPL) and promotes LPL trans-endothelial positioning via GPIHBP1 (52–56). Structural features of GPIHBP1 that accelerate LPL capture and luminal presentation have been defined (preclinical/biophysical) (53, 54). In insulin-sensitive states, this coordination rapidly hydrolyzes CM triglycerides, yielding controlled rises in tissue FFAs and minimal TRL remnants, with most clearance completed by approximately 2–4 h (55, 56).
2.7.3 Pathological disruption of TRL metabolism
Insulin resistance lowers adipose LPL expression and raises endogenous LPL inhibitors— angiopoietin-like protein 3/angiopoietin-like protein 4 (ANGPTL3/ANGPTL4) and apolipoprotein C3 (APOC3)—slowing TRL hydrolysis and extending the lipemic phase from approximately 4–6 h to 8–12 h or longer, thereby sustaining exposure to atherogenic remnant particles (57–59).
2.7.4 Vascular consequences of remnant accumulation
Small TRL remnants penetrate and are retained within the arterial intima (60, 61). They can be taken up by intimal macrophages—promoting foam-cell formation—and amplify chemokine/cytokine production, leukocyte adhesion, and vascular inflammation. This remnant-driven process contributes to residual atherosclerotic cardiovascular disease (ASCVD) risk beyond LDL-C lowering (61, 62).
2.7.5 Hepatic lipid overload and MASLD progression
Elevated postprandial triglycerides create a “dual-TRL hit” to the liver: increased FFA influx (from impaired peripheral clearance and heightened lipolysis) fosters re-esterification and VLDL secretion, while CM remnants add lipid/cholesterol cargo. Together these inputs magnify dyslipidemia and drive hepatic steatosis and progression toward MASLD (63, 64).
2.7.6 Cellular lipotoxicity and metabolic dysfunction
Excess FFAs generate diacylglycerol and ceramides that activate novel protein kinase C (PKC) isoforms, disrupt IR/IRS phosphorylation, and impair GLUT4 translocation, producing metabolic inflexibility with reduced glucose uptake and sustained hyperglycemia (65, 66).
2.7.7 Inflammatory amplification and clinical biomarkers
Oxidized remnants and FFAs stimulate toll-like receptor 4 (TLR4), driving nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, upregulate NOX2/NOX4, and elevate IL-6 and tumor necrosis factor-alpha (TNF-α), while IL-1β can rise via inflammasome activation. In population settings, triglyceride incremental area under the curve (iAUC) > 5 mmol·h·L−1 associates with approximately 25 percent higher IL-6 within 4 h, supporting this metric as a prognostic marker of lipemic–inflammatory burden (67, 68). This environment decreases the bioavailability of endothelial NO and reinforces insulin resistance, closing the pathophysiological cycle (66, 69).
2.7.8 Targeted therapeutic approaches
APOC3 antisense/siRNA accelerate CM and VLDL clearance, lowering peak postprandial triglycerides by up to approximately 45 percent in controlled trials (70, 71). ANGPTL3 inhibition (and to a lesser extent ANGPTL4) relieves LPL suppression, enhancing triglycerides hydrolysis and reducing remnants (72). Fibroblast growth factor 21 analogs improve hepatic β-oxidation and lower VLDL output, showing promise for MASLD-associated dyslipidemia (73).
2.8 Oxidative and mitochondrial stress in the postprandial window
A meal is more than caloric delivery—it is an acute redox challenge. Min after absorption, mitochondrial and enzymatic sources of ROS stimulate and briefly overcome endogenous antioxidant defenses. In healthy individuals this transient “spark” is hormetic, fine-tuning insulin action and vascular tone; in insulin-resistant or metabolic-syndrome phenotypes, the ROS pulse is higher and longer, synergizing with hyperglycemia and chylomicronemia to oxidize lipids/proteins, quench endothelial NO, and activate inflammasome/kinase pathways, feeding forward into endothelial dysfunction and insulin resistance (74, 75). Clinically, high-fat mixed meals reduce brachial-artery flow-mediated dilation (FMD) by approximately 1 percentage point at 2–4 h, placing peak vascular impairment squarely in the 60–180 min postprandial window (76). A concise mapping of sequelae, mechanisms, timing and read-outs is provided in Table 1.
Table 1
| Consequence | Core mechanistic driver (concise) | Peak window post-meal (typical) | Primary read-outs (preferred) | Assay notes/standardization |
|---|---|---|---|---|
| Endothelial dysfunction | Superoxide reacts with NO to generate peroxynitrite; eNOS uncoupling from tetrahydrobiopterin depletion; NOX2/XO-derived ROS (77, 78). | 1–4 h | Brachial-artery FMD (% change vs. baseline); plasma nitrite/nitrate; sNOX2-dp (if available) | Control caffeine/smoking and cuff/segment; adjust for baseline diameter; typical FMD drop ≈1 percentage point at 2–4 h |
| Inflammasome/innate immune activation | ROS activate IKKβ/JNK; these kinases recruit and oligomerize the NLRP3, which then activates caspase-1 (79, 80). | 2–6 h | Plasma IL-1β and IL-18 | Exclude acute infection; standardize timing and pre-analytical handling; freeze–thaw affects cytokines |
| β-cell stress and loss | Persistent ROS oxidize ER chaperones, forcing prolonged unfolded-protein-response signaling through PERK and eIF2α; the downstream rise in CHOP and caspase-3 expression accelerates pancreatic β-cell apoptosis (81). | Hours–days (magnified with repeated loads) | Proinsulin-insulin ratio (clinical proxy) | No direct plasma marker of β-cell apoptosis; interpret with glucose/FFA |
Oxidative-stress sequelae and clinical read-outs.
NO, nitric oxide; eNOS, endothelial nitric-oxide synthase; NOX, NADPH-oxidase; FMD, flow-mediated dilation; ROS, reactive oxygen species; IKKβ, inhibitor-of-κB-kinase-β; JNK, c-Jun N-terminal kinase; NLRP3, NOD-, LRR- and pyrin-domain–containing protein 3; IL, interleukin; PERK, protein-kinase R–like ER kinase; eIF2α, eukaryotic initiation factor 2α; CHOP, C/EBP homologous protein (pro-apoptotic factor); FFA, free fatty acids.
Standardization details for platforms and pre-analytical handling are summarized in Supplementary Table S1.
Mechanistically, rapid substrate overflow raises the nicotinamide adenine dinucleotide (NADH:NAD+) and flavin adenine dinucleotide, reduced: flavin adenine dinucleotide (FADH₂:FAD) ratios, hyper-reduces CoQ, and favors RET at Complex I—an efficient in vivo superoxide source—while Complex III contributes under high membrane potential (82, 83). Parallel nutrient cues (acute hyperglycemia; TRL remnants) activate PKC—especially PKC-β—driving p47^phox translocation and NOX2/NOX4 activation; tetrahydrobiopterin depletion uncouples eNOS, and xanthine oxidase adds to ROS supply—together producing a convergent, multi-organ burst that typically peaks at 1–3 h (84–87).
In healthy muscle and endothelium, the postprandial ROS burst is normally self-limited by nuclear factor erythroid 2–related factor 2 (Nrf2)–driven induction of glutathione peroxidase, catalase, and heme oxygenase-1 (88–91). In metabolic-syndrome/MASLD phenotypes, Nrf2 tone and circulating antioxidants (e.g., bilirubin, paraoxonase-1) are diminished, shifting the balance toward peroxynitrite formation, LDL oxidation, and redox-sensitive inflammatory signaling (92–94). With repeated meals, unresolved redox stress extends beyond the 1–3 h window and engages β-cell unfolded-protein-response pathways [protein-kinase R–like ER kinase (PERK); eukaryotic initiation factor 2α eIF2α]), driving CHOP/caspase-3 and increasing vulnerability to apoptosis. There is no direct plasma marker of β-cell death; in practice, an elevated proinsulin:insulin ratio serves as a crude stress proxy (81).
Human translational data strengthen causality: reducing mitochondrial oxidants alleviates lipid-induced muscle insulin resistance, and postprandial metabolomics consistently show acylcarnitine signatures compatible with mitochondrial redox pressure and PDH inhibition during mixed-meal challenges (95, 96).
2.8.1 Translational clues from intervention trials
Superoxide reacts with endothelial NO to generate peroxynitrite; the associated NO loss aligns with an approximately 1 absolute percentage-point decrement in brachial-artery FMD at 2–4 h after a single high-fat meal (
76–
78). ROS also signal through IKKβ/JNK to promote NLRP3 inflammasome assembly, increasing IL-1β and IL-18 (
79,
80).
- –
Polyphenols boluses. Acute, meal-time polyphenols attenuate oxidative stress and can preserve endothelial function in standardized high-fat challenges (97). Examples include: (i) grape-seed extract taken 1 h pre-meal lowered oxide LDL and glucose exposure without changing insulin (metabolic-syndrome adults; n = 12; approximately 670 kcal mixed meal, approximately 40 percent fat/ approximately 50 percent carbohydrate; sampling 0–5 h) (98); (ii) grape-pomace extract co-ingested with a high-fat meal modulated oxidative-stress biomarkers with body mass index-stratified effects (healthy women; n = 18; 1,131 kcal high-fat meal, 66.7 percent fat; sampling 0–6 h) (99); and (iii) a high-flavanol cocoa beverage [150 mg (−)-epicatechin] co-ingested with a high-fat load preserved FMD during a standardized ischemia–reperfusion stress paradigm vs. a low-flavanol control (young healthy adults; n = 23; high-fat meal with 56.5 g fat; FMD assessed approximately 1.5–3.0 h post-meal) (100).
- –
Targeting NADPH-oxidases. Setanaxib (a selective NOX1/4 inhibitor) shows clinical signals in primary biliary cholangitis; postprandial vascular-endpoint trials (e.g., FMD, carotid-femoral pulse wave velocity) are still needed (primary biliary cholangitis; adults; randomized, placebo-controlled phase 2; n = 111; no test meal; 24 weeks; primary endpoint = %ΔGGT; secondary = ALP, liver stiffness, fatigue; vascular endpoints not assessed) (69).
- –
N-acetylcysteine (NAC). A single-blinded, placebo-controlled crossover in hypertensive adults showed that oral NAC (600 mg) reduced thiolated albumin (Thio-HSA) by approximately 25 percent at 60 min and i.v. NAC lowered it by approximately 69 percent at 30 min, with increased plasma antioxidant capacity—supporting rapid in vivo mercaptoalbumin regeneration relevant to the 0–3 h postprandial window (no meal challenge) (hypertensive adults; n = 6; oral or i.v. NAC; sampling 0–6 h) (84).
- –
Mitochondria-targeted antioxidants. During lipid/heparin infusion clamps (not a meal), intravenous mitoquinone increased insulin-stimulated leg glucose uptake and reduced ex vivo mitochondrial H₂O₂ emission in adult humans, directly linking mitochondrial oxidants to insulin resistance [adults; n = 10 (mitoquinone arm n = 9); 3 h intravenous lipid infusion + hyperinsulinemic–isoglycemic clamp; leg glucose uptake and muscle respirometry assessed approximately 30–120 min] (96).
Taken together, exaggerated ROS generation paired with an insufficient antioxidant response creates a modifiable hinge between nutrient overload and downstream vascular–metabolic injury. Curbing ROS production (e.g., NADPH-oxidase blockade, improved mitochondrial efficiency) or reinforcing endogenous defenses (Nrf2 activators, thiol donors, polyphenol-rich foods) may help re-establish the brief, adaptive nature of the postprandial redox signal (
74,
75).
Note. Dedicated postprandial RCTs with vascular endpoints are lacking; current evidence supports a mechanistic, rapid thiol-replenishing action of NAC that is plausibly relevant to the 0–3 h window (84).
2.9 Endothelial activation and vascular inflammation
The vascular endothelium is the first interface to encounter postprandial blood. Under physiological conditions it releases NO, maintains an antithrombotic surface, and regulates nutrient delivery. Within min of a mixed meal, concurrent exposure to glucose, CM remnants, FFA, gut-derived lipopolysaccharide (LPS), and a burst of ROS can shift this interface toward a pro-inflammatory, vasoconstrictive phenotype—a shift amplified in obesity, MASLD, and chronic kidney disease (
101,
102).
- –
Mineral stress and calciprotein particles (CPP). In chronic kidney disease, calcium–phosphate nanocrystals coated with fetuin-A circulate as CPPs. These colloids bind TLR4 on endothelial cells, activate NF-κB, upregulate VCAM-1/ICAM-1, and suppress eNOS phosphorylation; intravenous CPPs reproduce this injury pattern in ApoE-knockout mice (preclinical), underscoring systemic vasculotoxicity (103).
- –
Canonical cytokine signaling. TNF-α, IL-1β, and IL-6 converge on endothelial NOX2/NOX4, raising superoxide and uncoupling eNOS, thereby reducing bioavailable NO and impairing vasodilation. In meal tests, postprandial FMD falls within approximately 2–4 h (magnitude protocol-dependent) (104, 105).
- –
Renin–angiotensin–YAP/TEAD crosstalk. Angiotensin II activates YAP; nuclear YAP partners with TEAD factors to drive a VCAM-1 promoter, sustaining leukocyte adhesion. Verteporfin (YAP–TEAD disruptor) or endothelial YAP knockdown restores FMD and lowers VCAM-1 in mouse models (preclinical), highlighting a druggable redox-sensitive switch (106).
- –
Gut–vascular signaling. Metabolic endotoxemia (chronically elevated LPS from increased intestinal permeability) engages endothelial TLR4, boosts ROS, and further uncouples eNOS; TLR4 antagonism or antioxidant therapy rescues NO signaling and barrier integrity in cell and animal models (preclinical), linking dysbiosis to vascular dysfunction (107).
2.9.1 Clinical snapshots
-
–
Obesity: postprandial endothelial impairment is exaggerated; miR-485 mimics suppress NOX4, lower VCAM-1, and improve FMD in obese mice (preclinical) (108).
-
–
Human NOX2 signal: high-fat meals provoke a rapid NOX2-dependent ROS burst; intravenous NOX2 blockade or a polyphenol-rich beverage at mealtime preserves endothelial function despite lipid load (human/challenge) (78, 104).
Oxidative–inflammatory endothelial injury sits at the crossroads of mineral imbalance, intestinal dysbiosis, systemic cytokines, and classic cardiometabolic risk. Limiting ROS generation (e.g., NOX inhibitors, improved mitochondrial efficiency), disrupting maladaptive transcriptional responses (YAP, NF-κB), and reinforcing NO signaling may complement lipid- and blood-pressure–lowering strategies in restoring vascular health.
2.10 Inflammation and innate-immune activation
A mixed meal elicits a rapid innate-immune pulse (0–6 h): gut-derived LPS and other danger signals reach the portal circulation within 30–60 min, priming monocytes/macrophages. In metabolically healthy adults the surge resolves quickly; proinflammatory baseline, genetic liability, or frequent energy-dense meals amplify and prolong the response (
109,
110).
- –
Innate sensors and cytokine kinetics. The NLRP3 inflammasome is a key nutrient-danger hub: a phosphate-enriched breakfast doubles caspase-1 activity in human monocytes and elevates IL-1β/IL-6 with approximately 2 h peak that wanes by approximately 6 h (111). TLR4 is activated by saturated fatty acids and CM remnants, driving dependent upregulation of VCAM-1 and ICAM-1, mechanistically linking dyslipidemia to endothelial dysfunction (110). Outside the vasculature, a fat bolus triggers hypothalamic astrocyte swelling and microglial activation by approximately 4 h in mice (preclinical) (112).
- –
Inter-individual variation. Host phenotype shapes the wave's height/duration: older adults with cardiometabolic risk generate approximately 40 percent higher peaks in IL-1β, C-reactive protein (CRP), and soluble ICAM-1 than healthy peers (113). APOE ε4 carriers mount approximately 2× postprandial rises in CRP/endothelial-activation markers vs. ε3/ε3 (114). Monocyte-subset dynamics also differ: CD14++CD16++; cells persist at 4 h in older adults but contract by approximately 50 percent in younger adults—evidence of innate-immune “memory” with aging (115).
- –
Microbiome modulation and trained immunity. Lower butyrate output is associated with sharper IL-6 and glycosylated acute-phase reactants (GlycA) peaks, an effect magnified by variants in sodium-coupled monocarboxylate transporter 1 [solute carrier Family 5 Member 8 (SLC5A8) or free fatty acid receptors 2 and 3 (FFAR2/3)] (116). Beyond innate signals, a single high-fat/high-sugar challenge can remodel T-cell chromatin at NF-κB– signal transducer and activator of transcription motifs and increase IL-17A for ≥1 week, consistent with diet-induced trained immunity (117).
- –
Implications and levers. The amplified cytokine environment accelerates vascular injury, upregulates endothelial adhesion molecules, and drives insulin resistance. Practical levers include boosting butyrate (resistant starch, inulin-type fructans), tempering TLR4 signaling (marine omega-3 fatty acids), and inhibiting NLRP3/ROS sources (mitochondrial antioxidants or NOX2 inhibitors), alongside post-meal physical-activity “snacks”.
2.11 Gut microbiota–derived signals in post-meal metabolism
During a mixed meal, host nutrients surge systemically while unabsorbed carbohydrate/protein reach the colon, where microbes generate SCFAs, secondary bile acids, and indoles that enter the portal vein near-synchronously with host substrates. Microbiome features (
α-diversity;
Bacteroides/Prevotella/Akkermansia) explain substantial between-person variance in postprandial glycemic and lipemic responses (systematic review of 36 trials; deep-phenotyping cohort
n= 1,098) (
118,
119).
- –
Bile acid–L-cell axis (preclinical—human association). High BSH activity rapidly deconjugates meal bile acids, increasing ligands for TGR5 on L-cells; in gnotobiotic mice, TGR5 blockade abrogates the GLP-1 surge and its glycemic benefit (preclinical) (120, 121). In humans, intestinal TGR5 messenger ribonucleic acid together with fecal BSH activity correlates with GLP-1 dynamics, explaining a meaningful fraction of 2-h GLP-1 iAUC variability (38, 67).
- –
TRL output and clearance (preclinical with human links). Antibiotic-treated mice show a approximately 35 percent fall in postprandial CM triglycerides paralleling reduced microsomal triglycerides and apoB-48 transcripts; Bacteroides thetaiotaomicron recolonization restores both expression and lipemia (preclinical). Indole-acetate suppresses ANGPTL4 via AhR, relieving the LPL brake and accelerating remnant clearance; conversely, microbial stimulation of GLP-2 upregulates enterocyte MTP/apoB-48, doubling CM output—effects blunted by a GLP-2R antagonist (preclinical) (38, 122).
- –
Endocannabinoid and neuro-immune loops (human/preclinical). In a randomized cross-over study, 2 h rises in N-acylethanolamines (e.g., anandamide, oleoylethanolamide) varied inversely with Fecalibacterium, with approximately 40 percent larger surges in metabolic syndrome (human) (123). Microbiota enriched in Enterobacteriaceae associate with sharper IL-6/IL-1β peaks and greater fullness after a Western-style meal, suggesting a gut–brain–immune loop (human association) (118). The lipid-lowering effect of endogenous GLP-1 depends on intact vagal afferents and is attenuated by acute fructose, implying neuroendocrine gating of CM handling (preclinical/physiology) (124).
- –
Microbiota enriched. In Enterobacteriaceae associate with sharper IL-6/IL-1β peaks and greater fullness after a Western-style meal, suggesting a gut–brain–immune loop (human association) (118). The lipid-lowering effect of endogenous GLP-1 depends on intact vagal afferents and is attenuated by acute fructose, implying neuroendocrine gating of CM handling (preclinical/physiology) (124).
Evidence type for each circuit is indicated above; key models/readouts are summarized in
Supplementary Table S1(human vs. preclinical).
2.12 Integrative modulators of postprandial metabolism
Post-meal fuel handling emerges from the intersection of cellular energy sensors, multi-organ nutrient sensing, the microbiome, circadian clocks, and adipose–endocrine–neural feedback. These axes determine whether calories are oxidized, stored, or routed to gluconeogenesis/lipogenesis—helping explain person-to-person heterogeneity in glycemic and lipemic excursions.
- 1)
Cellular energy sensors (AMPK–mTORC1– Sirtuin-1). When ATP falls, AMPK restrains mTORC1 and shifts flux toward fatty-acid oxidation/autophagy; higher NAD+/NADH activates sirtuin 1 (SIRT1), deacetylating PGC-1α/FOXO to support mitochondrial biogenesis and antioxidant defense. In the fed state, Akt re-engages mTORC1 to promote anabolism. Disrupting this AMPK–mTOR–SIRT1 switch accelerates steatosis, endothelial dysfunction, and insulin resistance (125, 126).
- 2)
Epithelial/host-context modulation. In the intestinal epithelium, selective repression of IRS–PI3K–Akt drives FOXO nuclear entry, tightens junctions, lowers paracellular permeability, and can lower systemic triglycerides/glucose; hyperactivation does the opposite (preclinical) (127). Host factors further rewire this axis: SARS-CoV-2 proteins perturb IRS adaptors and upregulate suppressor of cytokine signaling-3, blunting Akt and contributing to de novo insulin resistance (human mechanistic/observational), while estrogen receptor-α scaffolds IRS-1 to bolster Akt–mTORC2 (mechanistic; sex-difference context) (128, 129). With chronic hypoinsulinemia (type 1 diabetes), liver IRS-2 falls as muscle AMPK/SIRT1 compensates—an adaptive multi-omics “rewiring” (130).
- 3)
Multi-organ nutrient sensing (gut–brain–pancreas). Hypothalamic glucose-responsive neurons (GLUT2/ATP-sensitive potassium channel) and carnitine palmitoyltransferase 1C-positive neurons sense sugars and long-chain acyl-CoAs; L-cells convert luminal nutrient signals (SGLT-1; FFAR1/4; GPR119) into GLP-1/GIP/Peptide YY that reach the brainstem via vagal afferents. Vagotomy or acute fructose attenuates GLP-1–mediated suppression of CM triglycerides by approximately 35 percent, illustrating gut–brain control of postprandial lipemia (124). Microbial butyrate/indoles further tune this pathway (preclinical) (131).
- 4)
Circadian timing. Core clock genes (brain and muscle ARNT-Like (BMAL1), circadian locomotor output cycles kaput (CLOCK), Period (PER) and cryptochrome (CRY)) gate insulin sensitivity and substrate partitioning. Front-loading energy at breakfast advances clock phase and blunts glucose/ triglycerides excursions, whereas the same load at dinner does the opposite; “Big-Breakfast” RCTs show approximately 38 percent lower post-meal glucose and upregulated leukocyte CLOCK/BMAL1 (132–136). Diet-induced thermogenesis is higher mid-afternoon than late night (137). Hepatic clock disruption increases nocturnal glucose output; intestinal clocks modulate CM assembly, explaining higher night-lipemia in circadian misalignment; PER2-deficient β-cells lose first-phase insulin release (138–140).
- 5)
Adipose buffering and endocrine–neural feedback. In insulin-sensitive states, microvascular recruitment + LPL + GLUT4 trap dietary fat in adipose triglycerides stores. First-degree relatives of patients with T2D show approximately 40 percent smaller adipose blood-flow rises and approximately 35 percent greater non-esterified fatty acids (NEFA) spillover during mixed meals (141–143). LDL-receptor/CD36 density, visceral fat, and daily moderate to vigorous physical activity (MVPA) explain much of the spread in TAG iAUCs (144, 145). Circadian cues modulate adipose clocks (132, 146). Brown adipose tissue (BAT) activation via low-protein ketogenic diets or bile-acid signaling flattens triglycerides peaks and raises thermogenesis (human/rodent) (147). With aging, senescent visceral adipocytes (IL-6/TNF-α) amplify hyperglycemia; time-restricted eating or NAD+ boosters can blunt this signature (148).
- 6)
Neuro-endocrine crosstalk. Vagal afferents relay luminal glucose/lipid/stretch to the nucleus tractus solitarius; silencing delays satiation and the return of insulin/GLP-1 to baseline, while optogenetic GLP-1 cell activation triggers nodose firing within approximately 60 s (149, 150). Dopamine released in proportion to dietary glucose enhances GLP-1 signaling in adipose, suppressing lipolysis and limiting NEFA spillover (151). After bariatric surgery, muted glucagon counter-surges can produce late dumping hypoglycemia, revealing pancreas–brain vulnerability (152). Functional magnetic resonance imaging links oxyntomodulin/GIP to reward-circuit activity; their rapid post-meal rise tempers this signal—exaggerated by added sugars (153, 154). Chemogenetic data suggest the brain sets approximately 30 percent of basal glucose turnover, whereas the pancreas controls approximately 70 percent of postprandial disposal, underscoring gut–brain–pancreas control of iAUC spread (155, 156).
Derailments across these axes—AMPK–mTOR imbalance, mistimed meals, loss of butyrate-producing microbes, impaired adipose perfusion, or a sluggish incretin–vagal relay—tilt metabolism toward postprandial hyperglycemia and hypertriglyceridemia. Interventions that align feeding with circadian phase, expand SCFA production, activate GLP-1/GIP receptors, or deploy very-low-energy ketogenic therapy to boost BAT capacity (
130,
138,
157) are rational complements to calorie restriction and exercise, and fit an endocrine-centric MASLD prevention paradigm (
138).
3 Biomarkers and clinical assessment of postprandial dysmetabolism
A mixed-meal test or CGM best captures postprandial physiology but remains resource-intensive. In practice, clinicians use fasting surrogates that mirror post-meal dynamics. Among them, the triglyceride–glucose (TyG) index stands out for consistency, cost, and external validity across settings.
3.1 Traditional markers and TyG index
The TyG index is calculated from early-morning blood drawn by multiplying fasting triglycerides (milligrams per deciliter) by fasting glucose (milligrams per deciliter), dividing that product by two, and then taking the natural logarithm of the result (
158).
- –
Dynamic signal. Higher fasting TyG predicts steeper 2 h glucose and triglyceride rises on standardized meal tests—outperforming homeostatic model assessment for insulin resistance (HOMA-IR) (159).
- –
Outcomes. Across large cohorts, elevated TyG associates with faster carotid intima-media thickness (IMT) progression, higher incident CVD, ischemic stroke, and events in cancer survivors; in premature coronary artery disease (CAD), TyG ≥ 8.8 flagged approximately 75 percent higher 5-year major adverse cardiovascular event (159–163).
- –
Comparisons and special populations. Case–control work shows TyG (AUROC approximately 0.78) beats non-HDL-C and TG/HDL-C for angiographic stenosis; for MASLD, triglycerides/HDL-C slightly edges TyG (AUROC 0.82 vs. 0.80) (164, 165). In pediatrics, TyG > 8.2 detected abnormal glucose tolerance with approximately 82 percent sensitivity (166). Visceral adiposity (not total fat) drives the TyG–post-meal triacylglycerol (TAG) link, while ≥150 min/week MVPA halves the slope—supporting TyG as a modifiable risk indicator. Pairing TyG with meal-challenge or CGM traces yields a low-cost, high-yield view of postprandial burden (167). Operational details for TyG sampling/units are summarized in Supplementary Table S1 (TS1).
3.2 Emerging biomarkers: metabolomic, inflammatory & endothelial panels
-
–
Metabolomics (LC–MS). Mixed meals transiently raise saturated ceramides (C16:0, C18:0), the C18:0/C24:0 ratio, branched-chain α-keto acids, medium-chain acyl-carnitines, and indole-3-propionate. Prospective data and meta-reviews identify ceramide C18:0/C24:0—especially with TyG—as a strong composite predictor of ASCVD events and IR conversion. Run times are falling as ion-mobility separation and machine learning (ML)–assisted readouts shorten gradients and automate pattern recognition, with sub-30 min workflows reported in research settings (168, 169). Pre-analytical handling and panel composition are detailed in TS1.
-
–
Inflammation/innate immunity. GlycA (Nuclear Magnetic Resonance) integrates acute-phase glycoproteins; along with cluster of differentiation 163 (sCD163) and calprotectin, it outperforms high-sensitivity C-reactive protein (hs-CRP) for low-grade inflammation and predicts metabolic syndrome and coronary calcification. In severe dysmetabolism, neutrophil extracellular traps-derived cell-free DNA and IL-6 trans-signaling rise and track with carotid remodeling and impaired FMD (170, 171). Assay timing and stability notes appear in TS1.
-
–
Endothelial activation. Glycocalyx shedding yields soluble thrombomodulin (sTM) and von Willebrand factor (vWF); endothelial extracellular vesicles (ICAM-1+) and miRNAs (miR-126-3p, miR-210) correlate with IMT progression and FMD decline, and portend mortality in severe COVID-19, underscoring a shared redox–endothelial axis (172, 173).
-
–
Composite scores. Meta-analyses show TyG, TyG/waist, and triglycerides/HDL-C outperform LDL-C for detecting coronary disease, particularly in obesity/MASLD; adding vWF or miR-126 to TyG can push c-statistic > 0.80, rivaling costlier omics (174).
3.2.1 Implementation (pragmatic workflow)
-
•
Step 1—Screen with TyG and, where visceral adiposity is obvious, the triglyceride-to-HDL-cholesterol ratio.
-
•
Step 2—Stratify intermediate-risk patients with GlycA and endothelial-vesicle counts to unmask subclinical inflammation or glycocalyx injury.
-
•
Step 3—Personalize very-high-risk cases with ceramide/oxylipin panels to guide intensified lipid-lowering, antioxidant, or anti-inflammatory therapy (169, 170, 172).
Cutoffs, sample handling, and standardization are summarized in TS1.
3.3 Functional tests and dynamic indices—“Rate-of-Change” phenotyping
Static fasting values miss how fast systems absorb a meal-induced perturbation. Four protocols translate lability into time constants or impulse ratios clinicians can interpret:
- •
Cardiorespiratory coupling time constant linking heart rate to oxygen-consumption kinetics (τ_HR–V˙O₂). In a ramp-cycle test approximately 45 min post-breakfast, a time constant > approximately 60 s tracks upper-tertile TyG and predicts lower aerobic power at 12 months (175).
- •
Impulse-based Dynamical Strength Index (IB-DSI). A single countermovement jumps at approximately 2 h post-meal: impulse/maximum voluntary contraction ≤ 0.60 flags blunted neuromuscular recovery and co-segregates with higher ceramide C18:0/C24:0 and triglycerides peaks (176).
- •
Dynamic-Fit Index (DFI). Bayesian state-space fit to dense capillary glucose/lipid sampling; lower DFI (more error-corrections/min) precedes the first fasting-glucose rise by approximately 2 years (177).
- •
Diaphragm excursion on four-dimensional computed tomography (4-D CT). Failure to augment excursion by ≥10 percent after a meal associates with visceral adiposity, higher TyG, and heavier TAG iAUC (178).
3.4 Clinical relevance—why dynamic biomarkers matter
Post-meal signals anticipate hard outcomes years before fasting markers drift. Microbiome-informed ML models explain approximately 40 percent of variance in 2-h glucose iAUC, doubling glucose-only models; in PREDICT-1, this approach outperformed hemoglobin A1c and TyG for predicting conversion to impaired glucose tolerance (156, 157, 179). In T2D with CAD, TRL-TAG AUC > 5 mmol·h·L−1 forecasts microalbuminuria and hs-IL-6 increases within 12 months. Population data show non-fasting TAG 175 mg/dl beats the fasting 150 mg/dl cut-off for CVD risk (68, 142, 180–182). Palm-oil challenges that elevate ceramide d18:1/24:0 also raise VCAM-1 overnight; glycomics identify a fucose-rich, sialic-acid–poor N-glycan profile that flags incident T2D independent of glucose or TyG (183–185).
Taken together, postprandial biomarkers —whether they are kinetic (τ_HR–V˙O₂, IB-DSI, DFI), molecular (ceramides, GlycA), or microbial (butyrate-producing taxa)—capture
how resilientan individual is to a metabolic load. Their predictive value supports a tiered clinical strategy:
- •
Step 1 Screen with inexpensive composites (TyG, triglyceride-to-HDL-cholesterol ratio).
- •
Step 2 Stratify intermediate-risk patients using GlycA plus a simple functional test such as τ_HR–V˙O₂.
- •
Step 3 Personalize (ceramides/microbiome-guided diets). Shifting from static concentrations to rates of change enables earlier, targeted intervention—before vascular, renal, or β-cell damage accrues.
Across large cohorts, highest-vs.-lowest strata of two-hour post-meal glucose exposure, triglyceride-rich lipoprotein triacylglycerol exposure, the triglyceride–glucose index, the plasma ceramide C18:0/C24:0 ratio, and glycoprotein acetylation show consistent graded risk. TS1 lists assay methods, cut-offs, and timing windows for each biomarker.
4 Nutritional and lifestyle interventions
Restore a brief, adaptive postprandial response by: (i) lowering substrate surges (glucose/TRL-TAG), (ii) dampening oxidative–inflammatory signaling, and (iii) aligning timing with circadian biology. Dynamic triggers to escalate care are summarized at the end (see also Supplementary Table S1).
4.1 Mediterranean-style eating as a postprandial buffer
The Mediterranean dietary pattern—extra-virgin olive oil (EVOO), vegetables, legumes, whole grains, fish, and modest red-wine use—consistently lowers cardiometabolic events (186, 187) and blunts postprandial “turbulence”. In healthy men, a single Mediterranean-type meal preserved endothelial function and attenuated triglyceride excursions vs. a high–saturated-fat comparator (healthy men; n = 28; randomized crossover; Mediterranean-type meal vs. high–saturated-fat meal, 858–885 kcal, 51–57 g fat; FMD and lipids 0–4 h) (111). In overweight/obese older adults, a Mediterranean-like meal produced smaller TAG rises than a Western high-fat meal while IL-6 increased similarly across meals [overweight/obese older adults; n = 60; randomized crossover; isoenergetic meals approximately 1,000 kcal (approximately 4,200 kJ); sampling 0–5 h] (111).
Small, targeted adjustments amplify benefits:
- –
Gene–diet interaction. In coronary-artery patients carrying the minor G-allele at zinc finger protein 1 (ZPR1) rs964184, switching from low-fat to Mediterranean reduced post-meal TAG by approximately 0.31 mmol·L−1; non-carriers changed little (188).
- –
Carbohydrate quality. Within an isocaloric Mediterranean day, replacing refined starches with low-GI pulses and whole grains blunted postprandial glucose/insulin excursions during an 8-h mixed-meal tolerance test (high-cardiometabolic-risk adults; n = approximately 180; standardized breakfast and lunch; sampling 0–8 h) (189). In type 2 diabetes, two isocaloric “healthy” patterns (Mediterranean-multifactorial vs. MUFA-rich) elicited distinct postprandial lipid and lipoprotein-subfraction responses after standardized test meals (T2D adults; randomized; serial sampling over several hours) (189).
- –
Exercise synergy. Adding approximately 150 min/week of brisk walking to a Mediterranean prescription improved the lipoprotein subclass profile (lower fasting triglycerides and small dense LDL, with favorable shifts in VLDL/LDL subclasses) (metabolic-syndrome adults; n = 202; energy-reduced Mediterranean diet + physical-activity promotion vs. energy-unrestricted Mediterranean diet; fasting NMR profiling; no standardized test meal) (190).
- –
Fat-quality swap. Replacing saturated fat with monounsaturated fat shifted the postprandial metabolomic profile toward lower acylcarnitines and higher antioxidant-related signals compared with a saturated-fat pattern; low-fat, high-complex-carbohydrate (LFHCC) arms with/without omega-3 (n-3) showed distinct postprandial signatures as well (metabolic syndrome; n = 75; randomized, 12-week isoenergetic diets: high–saturated fat [HSFA] vs. high–monounsaturated fat [HMUFA] vs. LFHCC vs. LFHCC + n-3; standardized high-fat challenge; sampling 0–8 h [0, 4, 8 h]) (191).
- –
Timing matters. Early time-restricted variants (“Mediterranean breakfast front-loading”) further dampen TAG/glucose peaks and improve adipose clock-gene expression (132, 133).
Across diverse trials, head-to-head crossover work shows a Mediterranean day outperforms DASH for 4-h TAG (approximately −18 percent) and oxidized-LDL, and a 2024 meta-analysis of ≥18 randomized controlled trials confirms reductions in fasting and postprandial TAG across healthy, pre-diabetic, and T2D cohorts (
192). Practically, earlier eating with a Mediterranean first meal, low-GI pulses in place of refined starches, EVOO/marine ω-3 instead of saturates, and daily brisk walking magnify innate buffering. Response is not one-size-fits-all: ZPR1 rs964184 carriers show larger lipemic drops, whereas late chronotypes or habitual breakfast-skippers lose much of the gain. A 2024 umbrella review reporting parallel improvements in pre-diabetes conversion rates reinforces the pattern as a versatile, first-line, timing-aware prescription (
193,
194).
4.2 Meal-timing and chrononutrition—aligning food with the body clock
Crossover trials, CGM studies, and meta-analyses converge: front-loading energy in the morning and tapering evening carbohydrates blunts glycemic and lipemic excursions, whereas breakfast skipping or late high-GI dinners do the reverse (
195).
- –
Illustrative signals. Skipping breakfast increases lunchtime and dinnertime glycemic excursions in type 2 diabetes, accompanied by higher glucagon and lower iGLP-1 despite identical subsequent meals (T2D adults; n = 22; randomized crossover; breakfast vs. no breakfast with isocaloric lunch/dinner approximately 700 kcal; sampling 0–3 h) (196, 197). Shifting the main meal earlier—specifically, an early dinner at 18:00 vs. 21:00—lowers 24 h mean glucose and increases next-morning fat oxidation at identical energy intake (healthy adults; n = 12; randomized crossover; isocaloric day with dinner timing 18:00 vs. 21:00; 24 h CGM and next-morning indirect calorimetry) (198). Across randomized crossover trials, identical carbohydrate loads elicit higher evening than morning glycemic responses, with no consistent differences in insulinemia (adults with overweight/T2D; n = 8 crossover trials; standardized high-GI meals approximately 500–700 kcal; postprandial AUCs over approximately 2–3 h) (199).
- –
Chronotype matters. A randomized crossover stratified by chronotype showed that a high-GI dinner produced larger 2 h glucose excursions in late chronotypes, whereas early chronotypes had a comparatively attenuated evening response (healthy university students; n = 45; high-GI meal: cereal bar + cornflakes + milk + pretzel; breakfast 07:00 vs. dinner 20:00; CGM 0–3 h) (200). A complementary trial likewise found greater postprandial glycemia at dinner than at breakfast with identical high- vs. low-GI test meals (healthy older adults; n = 34 per protocol; high- or low-GI meals served at breakfast vs. dinner; capillary glucose 0–3 h) (201).
- –
Early time-restricted eating (eTRE). A short early window reduced 24 h mean glucose and glycemic variability and increased fat utilization without weight loss (overweight adults; n = 11; randomized 4-day crossover; eTRE 08:00–14:00 vs. 08:00–20:00; all meals provided; 24-h CGM; companion respiratory-chamber study) (202, 203). In a tightly controlled inpatient protocol, concentrating intake early in the day improved glycemic control and reduced glycemic variability under standardized conditions (healthy adults; n = 16; early vs. extended eating window as above; CGM 24 h; mixed-meal test 0–4 h) (203).
- –
Within-meal sequencing. In T2D, a small whey preload flattens early glycemia: 15 g whey taken 10 min before breakfast reduced the 0–240 min glucose iAUC and increased insulin/GLP-1 (T2D adults; n = 18; randomized crossover; 15 g whey 10 min pre-meal; standardized mixed-meal tolerance test; plasma sampling 0–4 h) (204). Evidence in type 1 diabetes is more heterogeneous but generally supports early-phase attenuation without worsening late hypoglycemia when modest doses are used; small crossover studies report blunted 0–120 min excursions with 10–20 g protein given 10–15 min before the meal, with dose and insulin strategy determining late effects (T1D adults; n approximately 10–30 across studies; 10–20 g protein 10–15 min pre-meal; capillary/CGM sampling 0–2–4 h) (205, 206). Across controlled-feeding studies, starting the meal with protein or fat (“protein-first/fat-first”) consistently lowers early postprandial glucose vs. carbohydrate-first, without raising triglycerides in the same window (mixed-risk adults; multiple small RCTs/crossovers; mixed meals typically approximately 500–900 kcal; sampling 0–2–4 h) (207).
4.3 Macronutrient manipulation—quality over quantity
Meta-analytic and crossover evidence (2020–2025) highlights three levers:
- –
Swap refined carbohydrates for Monounsaturated Fatty Acids/Polyunsaturated Fatty Acids (MUFA/PUFA). Replacing approximately 10 percent of carbohydrate with monounsaturated/polyunsaturated fat reduces postprandial glucose AUC by approximately 12 percent (adults with mixed risk; umbrella meta-analysis of approximately 27 RCTs; standardized test meals approximately 500–800 kcal; sampling 0–2/4 h) (208).
- –
Protein preload (“micro-pulses”). A small protein dose before the meal blunts the early glucose rise; approximately 20 g whey taken approximately 15 min pre-meal lowers glucose iAUC by approximately 12 percent (T2D/healthy adults; randomized crossover; mixed meals approximately 600–700 kcal; sampling 0–2 h) (209–211).
- –
Resistant starch (RS) and fermentable fiber. RS4 (phosphorylated wheat) acutely lowers incremental insulin iAUC and attenuates the second-meal glucose peak, while RS2 (potato) over weeks reduces fasting glucose and free fatty acids with modest, context-dependent postprandial improvements; a practical intake range is 15–30 g/day (overweight adults; RS4: n = 15; two standardized high-carbohydrate meals ∼600–800 kcal; sampling 0–180 min; RS2: n = 19; 12-week randomized crossover; standardized mixed-meal test ∼600–800 kcal; sampling 0–300 min) (212–214).
Shift refined-starch calories toward EVOO, nuts, and marine ω-3s; consider a 10–20 g protein preload before high-carb meals; and build RS-rich sides to boost butyrate and curb postprandial endotoxemia. Combine with Section
3.2timing tactics for drug-like smoothing without pharmacotherapy.
4.4 Dietary bioactives and polyphenols—rapid-response molecules
Plant-derived secondary metabolites can blunt oxidative, inflammatory, and metabolic surges within min; with sustained intake they also re-condition endothelial and Nrf2 defenses and remodel the microbiome.
- –
Catechins + chlorogenic acids (acute, dose–response). In two randomized studies in healthy men, co-ingestion of combined catechins/chlorogenic acids produced a graded reduction in early postprandial glycemia (150 and 300 mg vs. 0 mg), supporting a practical pre-meal “rapid-response” strategy (healthy men; randomized designs; cookie-/drink-based tolerance tests; capillary/plasma sampling up to approximately 2 h) (215).
- –
Anthocyanin-rich red raspberries. In adults with prediabetes/insulin resistance, test meals containing 0, 125, or 250 g red raspberries on separate days produced dose-dependent metabolite changes with improvements in postprandial glucose/insulin dynamics across the day (adults with prediabetes/insulin resistance; randomized crossover; three meals with 0/125/250 g frozen red raspberries; plasma metabolites and glycemia 0–8 h and again at 24 h) (216).
- –
Epigallocatechin gallate (EGCG) and Nrf2 pathway (mechanistic/kinetic support). A physiologically based kinetic model integrating human data predicts that colonic metabolites of EGCG (e.g., gallic acid, pyrogallol) can reach concentrations sufficient to activate Nrf2-regulated gene expression in vivo, providing a mechanistic rationale for antioxidant “pre-meal” strategies (model-based prediction; fasting and non-fasting scenarios evaluated) (217).
- –
Curcumin (longer-term). Meta-analysis of randomized trials shows curcumin supplementation (≈80–1,000 mg/day for ≥4 weeks) lowers fasting glucose and CRP and improves overall glycemic indices—consistent with attenuation of chronic postprandial stress across meals (mixed adult populations; multiple RCTs; no standardized test meal; outcomes over weeks to months) (218).
For acute control, an approximately 150–300 mg catechin/chlorogenic-acid mix taken with or shortly before a carbohydrate-rich meal can dampen early glycemic excursions (0–2 h). In carbohydrate-heavy contexts, adding anthocyanin-rich fruit portions (e.g., red raspberries) to the meal supports postprandial glucose handling across the subsequent 8–24 h. For sustained conditioning of redox and inflammatory tone, multi-week curcumin courses can complement dietary timing and macronutrient strategies (Sections 3.2–3.3) (
215).
4.5 surgical nutrition windows—pre-operative “Metabolic Priming”
Pre-operative nutritional status predicts wound healing, length of stay, and long-term outcomes after bariatric procedures. Two elements are consistently actionable:
- –
Micronutrient optimization. Many candidates present with subclinical iron, vitamin D, or thiamine deficits; routine screening and targeted repletion are recommended to minimize postoperative deficiency-related morbidity (e.g., fatigue, hair loss), although precise effect sizes for symptom reduction remain heterogeneous across studies (219, 220).
- –
Very-low-calorie diet (VLCD) and Enhanced Recovery After Surgery bundle. A 2–4-week protein-sparing VLCD reduces liver volume by about 16–17 percent and improves operative conditions; when embedded within an ERABS pathway, programs typically report shorter length of stay (approximately 1–2 days) and fewer overall complications. (Adults with severe obesity; VLCD 2–4 weeks; ERABS multimodal pathways) (221).
Treat the month before metabolic surgery as leverage—screen and replete micronutrients, implement a short VLCD to debulk hepatic fat while preserving lean mass, and apply ERABS protocols to temper inflammation and accelerate recovery (
222,
223).
4.6 Physical-activity “Snacks” & structured exercise —turning skeletal muscle into a second pancreas
Even brief muscle contractions stimulate GLUT4 translocation and LPL activation. Breaking up sitting with 2–5 min bouts of standing or light walking every 20–30 min lowers postprandial glucose and insulin vs. uninterrupted sitting (adults with and without T2D; k approximately 22 randomized trials; standardized mixed meals approximately 500–900 kcal; sampling 0–2–3–4 h) (224). In people with T2D, desk-work break protocols similarly reduce postprandial glycemia and several studies report concurrent decreases in postprandial triglycerides during standardized meal tests (T2D adults; systematic review of break-frequency interventions every approximately 20–30 min during mixed-meal challenges; sampling 0–2–4 h) (225). Timing also matters: walking performed after meals produces larger reductions in postprandial glucose than the same walking done before meals (adults with overweight/T2D; meta-analysis of randomized crossover trials; identical standardized meals approximately 500–700 kcal; sampling 0–2 h) (226, 227). For intensity, high-intensity interval exercise reduces postprandial glucose and insulin vs. control and can outperform matched-work moderate-intensity exercise (mixed-risk adults; multi-study meta-analysis; meal-based and glucose-load protocols; outcome windows 0–2–4 h) (228). In practice: (i) stand or stroll 2–3 min at least every 30 min; (ii) add a short, well-timed bout within the first 2 h after eating (e.g., approximately 10 min of moderate walking); and (iii) remember that timing often beats duration—activity placed soon after a meal yields a larger immediate metabolic payoff than a longer session done late at night (implementation guidance from contemporary reviews) (229–231). In practice, brief, well-timed bouts yield measurable acute benefits across diverse populations; Table 2 summarizes representative activity-snack prescriptions (2020–2025) and their immediate metabolic effects.
Table 2
| Study/population | Prescription—timing and structure | Principal acute metabolic effect(s) |
|---|---|---|
| Meta-analysis of randomized trials in adults with and without T2D (k ≈ 22; standardized mixed meals ≈500–900 kcal; sampling 0–2–3–4 h | Standing or light walking for 2–5 min every 20–30 min during a seated lab protocol (224). | Two-hour glucose iAUC decreased by ≈12% and insulin iAUC by ≈20% vs. uninterrupted sitting |
| Systematic review focused on adults with T2D (breaks during desk-type tasks; standardized mixed-meal challenges; sampling 0–2–4 h) | At least one brief standing or slow-step break about every 20–30 min while seated work continued (225). | Postprandial glucose iAUC fell by ≈15% and TAG iAUC by ≈10% compared with continuous sitting |
| Meta-analysis of randomized crossover trials comparing post-meal vs. pre-meal walking (adults with overweight/T2D; standardized meals ≈500–700 kcal; sampling 0–2 h) | Brisk walking (about 10–20 min) initiated within ≈30 min after meals vs. the same dose before meals (226). | Greater reduction in postprandial glucose when walking is performed after meals; supports timing-sensitive placement of short bouts |
Representative “activity-snack” prescriptions (2020–2025) and acute metabolic effects.
CGM, continuous-glucose monitoring; iAUC, incremental area under the curve; TAG, triacylglycerol.
5 Pharmacological and technological advances —shrinking the postprandial “Damage Window”
Over the last half-decade, the emphasis has shifted from fasting targets to how quickly therapies flatten post-meal spikes. In parallel, continuous glucose monitoring (CGM) and algorithmic feedback allow clinicians to match fast-acting tools to the meals that need them most.
5.1 Pharmacological approaches that act within two to four hours after a meal
-
–
Enteroendocrine mimetics and co-agonists (human evidence). Oral semaglutide lowers postprandial glucose exposure and attenuates TRL–TAG responses in phase-III settings (T2D; pooled phase-III meal-test substudies/post-hoc; standardized mixed meals approximately 500–700 kcal; sampling 0–4 h) (232). Tirzepatide (GLP-1/GIP) achieves comparable glucose control with additional reductions in TRL measures (T2D; SURPASS meal-test substudies/post-hoc; standardized mixed meals approximately 500–700 kcal; sampling 0–4 h) (233).
-
–
Adjunct glucose “shuttlers” (human evidence). Faster-aspart reaches systemic circulation earlier than conventional rapid analogs and improves early post-meal control with less late hypoglycemia in CGM cohorts (T1D/T2D; real-world CGM; ad-libitum meals; 0–4 h CGM windows) (232). A single pre-prandial dose of empagliflozin reduces the 0–2 h glucose excursion in randomized crossover designs (T2D adults; randomized crossover; 5–25 mg immediately pre-meal; standardized mixed meal approximately 500–700 kcal; sampling 0–2–4 h) (234).
-
–
Lipid-centric modulators (human evidence). PCSK9 inhibition reduces postprandial remnant/TRL exposure when added to background statins (T2D or mixed dyslipidemia; randomized add-on; standardized fat-tolerance tests; sampling 0–4–6 h) (235).
-
–
Bile-acid signaling (preclinical). The dual FXR/TGR5 agonist INT-767 lowers postprandial TAG in high-fat-diet models; translation to clinical endpoints is ongoing (preclinical; HFD mice; oral fat tolerance or mixed lipid challenges; sampling approximately 0–4–6 h) (236).
-
–
Gut-facing/dual-action tools (early human). LEAP-2 analogues (ghrelin antagonism) show acute appetite suppression with blunted glucose peaks in first-in-human testing (early human; single/short-course dosing; standardized liquid meal or OGTT; sampling approximately 0–2–4 h) (237). Endoscopic duodenal devices (e.g., mucosal resurfacing or sleeves) improve postprandial glucose/insulin dynamics in early studies (pilot human plus DIO-rat support; standardized mixed meal; sampling approximately 0–2 h) (238).
Table 3 (unchanged in structure) summarizes acute mechanisms, magnitude where reported in your sources, and development stage for agents with 0–4 h post-meal impact—strictly aligned with refs (232, 234–238).
Table 3
| Class/agent(s) | Dominant acute post-meal effect | Key efficacy data (design & population) |
|---|---|---|
| Entero-endocrine mimetics/co-agonists Oral semaglutide -Tirzepatide |
Semaglutide lowers 4-h glucose iAUC and TRL-TAG iAUC; tirzepatide achieves comparable glucose control with additional TRL reductions (where reported) (233, 239). | Phase-III T2D programs with meal-test substudies/post-hoc analyses; standardized mixed meals ≈500–700 kcal; sampling ≈0–4 h. |
| Adjunct glucose “shuttlers” Faster-aspart—Empagliflozin (pre-meal) |
Faster-aspart reaches systemic circulation ≈10 min sooner than standard rapid analogs and reduces late hypoglycemia; single pre-prandial empagliflozin dose lowers early glucose excursion (232, 234). | Real-world CGM cohorts (T1D/T2D; ad-libitum meals; 0–4 h CGM windows) for faster-aspart. Randomized crossover (T2D adults; 5–25 mg immediately pre-meal; standardized mixed meal ≈500–700 kcal; sampling ≈0–2–4 h) for empagliflozin. |
| Lipid-centric modulators Alirocumab (anti-PCSK9) INT-767 (dual FXR/TGR5) | PCSK9 inhibition reduces remnant/TRL exposure post-prandially (human); INT-767 lowers TAG iAUC in HFD mice (preclinical) (235, 236). | Alirocumab: randomized add-on in T2D/mixed dyslipidemia; fat-tolerance/mixed-meal tests; sampling ≈0–4–6 h. INT-767: preclinical HFD mouse models; lipid challenge tests; sampling ≈0–4–6 h. |
| Gut-facing/dual-action tools LEAP-2 analog · Endoscopic duodenal sleeve | LEAP-2 analog blunts glucose peaks without hypoglycemia (early human); duodenal sleeve improves 2 h glucose/insulin responses (pilot human; DIO-rat support) (237, 238). | LEAP-2: first-in-human; standardized liquid meal/OGTT; sampling ≈0–2–4 h. Sleeve: DIO-rat plus pilot human; standardized mixed meal; sampling ≈0–2 h. |
Pharmacological agents that flatten the 0- to 4 h post-meal window: dominant acute mechanism, key efficacy data and development stage.
CGM, continuous-glucose monitoring; FIH, first-in-human; FXR, farnesoid X receptor; GIP, glucose-dependent insulinotropic polypeptide; HFD, high-fat diet; iAUC, incremental area-under-the-curve; PCSK9, pro-protein-convertase-subtilisin/kexin 9; TAG, triacylglycerol; TRL, triglyceride-rich lipoprotein; T2D, T2D; TGR5, takeda G-protein-coupled receptor 5.
5.2 Digital therapeutics and AI-assisted food coaching
CGM-guided, algorithm-predicted diets reduce time above range and blunt 0–2 h glucose rises in primary-care programs vs. general advice, with high adherence due to actionable, real-time nudges (45, 240). Integrating CGM into inpatient and outpatient workflows reduces glycemic variability and unmasks “silent” post-meal excursions that fasting tests miss (241–243). Personalized postprandial targeting menus informed by individual features (including microbiome signals) outperform standard patterns for several glycemic metrics in selected cohorts (155, 244).
5.3 Clinical implementation—linking postprandial control to liver health
In MASLD, attenuating post-meal glucose/TRL/oxidative surges is clinically relevant (
245–
247). A pragmatic sequence is:
- 1)
Screen with TyG ± non-fasting TAG or a simple TRL-TAG curve;
- 2)
prescribe a Mediterranean template with earlier energy distribution plus brief post-meal activity;
- 3)
if high postprandial burden persists, escalate with GLP-1/GIP co-agonists or PCSK9 inhibitors;
- 4)
repeat liver enzymes and a post-meal TAG assessment at approximately 12 weeks to adjust therapy
6 Conclusions and future directions
Postprandial metabolism is now recognized as a network of druggable nodes, extending from the gut lumen to the vascular wall. Three key targets are gaining traction: the enterohepatic bile acid loop, intracellular steroid and SUMO switches, and nutrient-sensing GPCRs. Promising agents already in development reduce mixed-meal triglycerides, reverse insulin resistance, and disrupt lipogenesis and late-phase hyperinsulinemia.
Importantly, these post-meal metabolic surges are not only cardiometabolic but also oncogenic triggers—fueling inflammation, insulin signaling, and epithelial dysplasia. Early shifts in glucose and triglyceride waves, impaired thermogenesis, and altered bile acid profiles are strong predictors of diabetes, fatty liver, and vascular damage—often before fasting markers change.
Advanced multi-omics, real-time wearables, and AI pipelines are transforming these insights into precision care. Emerging tools now outperform classical risk scores, identify distinct postprandial endotypes, and enable real-time interventions that significantly reduce glycemic exposure. As these technologies scale, equity-centered frameworks will be essential to ensure access, relevance, and impact across diverse populations.
Statements
Author contributions
CR-G: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Methodology. EC-F: Visualization, Writing – original draft, Writing – review & editing. BJ: Writing – original draft, Writing – review & editing. DS-R: Conceptualization, Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to Universidad UTE for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1655889/full#supplementary-material
References
1.
Pan American Health Organization (PAHO). Noncommunicable Diseases Progress Monitor 2025 [Internet]. (2025). Available online at:https://iris.paho.org/handle/10665.2/65519 (Accessed May 14, 2025).
2.
Chew NWS Pan XH Chong B Chandramouli C Muthiah M Lam CS . Type 2 diabetes Mellitus and cardiometabolic outcomes in metabolic dysfunction-associated steatotic liver disease population. Diabetes Res Clin Pract. (2024) 211:111652. 10.1016/j.diabres.2024.111652
3.
Cox D . Diabetes Is Rising in Africa. Could it lead to new breakthroughs? - WIRED [Internet]. (2025). Available online at:https://bit.ly/43S7Gdv (Accessed May 14, 2025).
4.
Liu B Jia Y Gu Z Li Y Zhou Y Cao Y . Metabolic dysfunction associated steatotic liver disease is associated with an increased risk of multiple respiratory system diseases. Sci Rep. (2025) 15(1):15937. 10.1038/s41598-025-96710-3
5.
Miao L Targher G Byrne CD Cao YY Zheng MH . Current status and suture trends of the global burden of MASLD. TEM. (2024) 35(8):697–707. 10.1016/j.tem.2024.02.007
6.
World Health Organization (WHO). Noncommunicable diseases [Internet]. (2024). Available online at:https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (Accessed May 14, 2025).
7.
Peters B Vahlhaus J Pivovarova-Ramich O . Meal timing and its role in obesity and associated diseases. Front Endocrinol. (2024) 15:1359772. 10.3389/fendo.2024.1359772
8.
Teo PS van Dam RM Whitton C Tan LWL Forde CG . Consumption of foods with higher energy intake rates is associated with greater energy intake, adiposity, and cardiovascular risk factors in adults. J Nutr. (2021) 151(2):370–8. 10.1093/jn/nxaa344
9.
Giosuè A Skantze V Hjorth T Hjort A Brunius C Giacco R et al Association of the glucose patterns after a single nonstandardized meal with the habitual diet composition and features of the daily glucose profile in individuals without diabetes. Am J Clin Nutr. (2024) 121(2):246–55. 10.1016/j.ajcnut.2024.11.028
10.
Sasso E Baticic L Sotosek V . Postprandial dysmetabolism and its medical implications. Life. (2023) 13(12):2317. 10.3390/life13122317
11.
Schönknecht YB Crommen S Stoffel-Wagner B Coenen M Fimmers R Stehle P et al Influence of a proinflammatory state on postprandial outcomes in elderly subjects with a risk phenotype for cardiometabolic diseases. Eur J Nutr. (2022) 61(6):3077–83. 10.1007/s00394-022-02870-7
12.
van de Vyver M . Immunology of chronic low-grade inflammation: relationship with metabolic function. J Endocrinol. (2023) 257(1):e220271. 10.1530/JOE-22-0271
13.
Maki KC Dicklin M Balakrishnan M Neff D . National Lipid Association. (2020). Postprandial Dysmetabolism: Understanding the Impact of Elevated Postprandial Glucose and Triglycerides with the Potential to Prevent or Intervene Early.
14.
Hiyoshi T Fujiwara M Yao Z . Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. J Biomed Res. (2017) 33(1):1–16. 10.7555/JBR.31.20160164
15.
Oh RC Trivette ET Westerfield KL . Management of hypertriglyceridemia: common questions and answers. Am Fam Physician. (2020) 102(6):347–54. Available online at:https://www.aafp.org/pubs/afp/issues/2020/0915/p347.html
16.
Lee SH Park SY Choi CS . Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2021) 46(1):15–37. 10.4093/dmj.2021.0280
17.
Jiang S Liu H Li C . Dietary regulation of oxidative stress in chronic metabolic diseases. Foods. (2021) 10(8):1854. 10.3390/foods10081854
18.
Sivri D Akdevelioğlu Y . Effect of fatty acids on glucose metabolism and type 2 diabetes. Nutr Rev. (2025) 83(5):897–907. 10.1093/nutrit/nuae165
19.
Eroglu T Capone F Schiattarella GG . The evolving landscape of cardiometabolic diseases. EBioMedicine. (2024) 109:105447. 10.1016/j.ebiom.2024.105447
20.
Theodorakis N Nikolaou M Krentz A . Cardiovascular–endocrine–metabolic medicine: proposing a new clinical sub-specialty amid the cardiometabolic pandemic. Biomolecules. (2025) 15(3):373. 10.3390/biom15030373
21.
Rahim NE Flood D Marcus ME Theilmann M Aung TN Agoudavi K et al Diabetes risk and provision of diabetes prevention activities in 44 low-income and middle-income countries: a cross-sectional analysis of nationally representative, individual-level survey data. Lancet Glob Health. (2023) 11(10):e1576–86. 10.1016/S2214-109X(23)00348-0
22.
Swinburn B Hovmand P Waterlander W Allender S . The global syndemic of obesity, undernutrition, and climate change. In: KopelmanPCatersonI, editors. Clinical Obesity in Adults and Children. 4th ed.Hoboken, NJ: Wiley (2022). p. 409–27.
23.
Joseph JJ Deedwania P Acharya T Aguilar D Bhatt DL Chyun DA et al Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation. (2022) 145(9):39–51. 10.1161/CIR.0000000000001040
24.
Pappas C Kandaraki EA Tsirona S Kountouras D Kassi G Diamanti-Kandarakis E . Postprandial dysmetabolism: too early or too late?Hormones. (2016) 15(3):321–44. 10.14310/horm.2002.1697
25.
Garber AJ . Postprandial dysmetabolism and the heart. Heart Fail Clin. (2012) 8(4):563–73. 10.1016/j.hfc.2012.06.004
26.
van Gerwen J Shun-Shion AS Fazakerley DJ . Insulin signaling and GLUT4 drafficking in insulin resistance. Biochem Soc Trans. (2023) 51(3):1057–69. 10.1042/BST20221066
27.
Robinson KA Buse MG . Mechanisms of high-glucose/insulin-mediated desensitization of acute insulin-stimulated glucose transport and Akt activation. Am J Physiol Endocrinol Metab. (2008) 294(5):E870–81. 10.1152/ajpendo.00644.2007
28.
Toejing P Khat-Udomkiri N Intakhad J Sirilun S Chaiyasut C Lailerd N . Putative mechanisms responsible for the antihyperglycemic action of Lactobacillus paracasei HII01 in experimental type 2 diabetic rats. Nutrients. (2020) 12(10):3015. 10.3390/nu12103015
29.
Ijuin T Takenawa T . Regulation of insulin signaling and glucose transporter 4 (GLUT4) exocytosis by phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, skeletal muscle, and kidney enriched inositol polyphosphate phosphatase (SKIP). J Biol Chem. (2012) 287(10):6991–9. 10.1074/jbc.M111.335539
30.
Zhang B-H Yin F Qiao Y-N Guo S-D . Triglyceride and triglyceride-rich lipoproteins in atherosclerosis. Front Mol Biosci. (2022) 9:909151. 10.3389/fmolb.2022.909151
31.
Bao Y Hu C Wang B Liu X Wu Q Xu D et al Mitochondrial reverse electron transport: mechanisms, pathophysiological roles, and therapeutic potential. Biology (Basel). (2025) 14(9):1140. 10.3390/biology14091140
32.
Mazat JP Devin A Ransac S . Modelling mitochondrial ROS production by the respiratory chain. Cell Mol Life Sci. (2020) 77(3):455–65. 10.1007/s00018-019-03381-1
33.
Reytor-González C Simancas-Racines D Román-Galeano NM Campuzano-Donoso M Carella AM Zambrano-Villacres R et al Obesity and breast cancer: exploring the nexus of chronic inflammation, metabolic dysregulation, and nutritional strategies. Food Agric Immunol. (2025) 36(1):2521270. 10.1080/09540105.2025.2521270
34.
Simancas-Racines D Reytor-González C Frias-Toral E Katsanos CS Hidalgo R . Weighty matters: unraveling the impact of obesity on colorectal cancer and nutritional interventions. Semin Cancer Biol. (2025) 114:29–40. 10.1016/j.semcancer.2025.06.004
35.
Reytor-González C Parise-Vasco JM González N Simancas-Racines A Zambrano-Villacres R Zambrano AK et al Obesity and periodontitis: a comprehensive review of their interconnected pathophysiology and clinical implications. Front Nutr. (2024) 7:11. 10.3389/fnut.2024.1440216
36.
Foley MH Walker ME Stewart AK O’Flaherty S Gentry EC Patel S et al Bile salt hydrolases shape the bile acid landscape and restrict clostridioides difficile growth in the murine gut. Nat Microbiol. (2023) 8(4):611–28. 10.1038/s41564-023-01337-7
37.
Parasar B Zhou H Xiao X Shi Q Brito IL Chang PV . Chemoproteomic profiling of gut microbiota-associated bile salt hydrolase activity. ACS Cent Sci. (2019) 5(5):867–73. 10.1021/acscentsci.9b00147
38.
Zeng Y Wu Y Zhang Q Xiao X . Crosstalk between glucagon-like peptide 1 and gut Microbiota in metabolic diseases. mBio. (2024) 15(1):e0203223. 10.1128/mbio.02032-23
39.
McGee SL Hargreaves M . Exercise performance and health: role of GLUT4. Free Radic Biol Med. (2024) 224:479–83. 10.1016/j.freeradbiomed.2024.09.004
40.
Chang Y-C Chan M-H Yang Y-F Li C-H Hsiao M . Glucose transporter 4: insulin response mastermind, glycolysis catalyst and treatment direction for cancer progression. Cancer Lett. (2023) 563:216179. 10.1016/j.canlet.2023.216179
41.
Huang X Liu G Guo J Su Z . The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. (2018) 14(11):1483–96. 10.7150/ijbs.27173
42.
Knudsen JR Persson KW Henriquez-Olguin C Li Z Di Leo N Hesselager SA et al Microtubule-Mediated GLUT4 trafficking is disrupted in insulin-resistant skeletal muscle. eLife. (2023) 12:e83338. 10.7554/eLife.83338
43.
Berry SE Valdes AM Drew DA Asnicar F Mazidi M Wolf J et al Human postprandial responses to food and potential for precision nutrition. Nat Med. (2020) 26(6):964–73. 10.1038/s41591-020-0934-0
44.
Sinnott-Armstrong N Strausz S Urpa L Abner E Valliere J , Estonian Biobank Research Team, et al. Genetic variants affect diurnal glucose levels throughout the day. bioRxiv [Preprint] 2024.07.22.604631 (2024).
45.
Ben-Yacov O Godneva A Rein M Shilo S Kolobkov D Koren N et al Personalized postprandial glucose response–targeting diet versus mediterranean diet for glycemic control in prediabetes. Diabetes Care. (2021) 44(9):1980–91. 10.2337/dc21-0162
46.
Ungersboeck M Tang X Neeff V Steele D Grimm P Fenech M . Personalised nutritional recommendations based on individual post-prandial glycaemic responses improve glycaemic metrics and PROMs in patients with type 2 diabetes: a real-world assessment. Nutrients. (2022) 14(10):2123. 10.3390/nu14102123
47.
Le TKC Dao XD Nguyen DV Luu DH Bui TMH Le TH et al Insulin signaling and its application. Front Endocrinol. (2023) 14:1226655. 10.3389/fendo.2023.1226655
48.
Bo T Gao L Yao Z Shao S Wang X Proud CG et al Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. (2024) 36(5):947–68. 10.1016/j.cmet.2024.04.006
49.
Pan Q Ai W Chen Y Kim DM Shen Z Yang W et al Reciprocal regulation of hepatic TGF-β1 and Foxo1 controls gluconeogenesis and energy expenditure. Diabetes. (2023) 72(9):1193–206. 10.2337/db23-0180
50.
Hou Y Tian P Song G Song A Liu D Wang Z et al Postprandial triglyceride-rich lipoproteins as predictors of carotid atherosclerosis in individuals with normal fasting lipid profiles: a prospective follow-up study. Front Endocrinol. (2025 Feb 25) 16:1502792. 10.3389/fendo.2025.1502792
51.
Wilson ML Lane KE Fadel A Dawson EA Moore E Mazidi M et al Effects of single low-carbohydrate, high-fat meal consumption on postprandial lipemia and markers of endothelial dysfunction: a systematic review of current evidence. Nutr Rev. (2025) 83(3):e1049–67. 10.1093/nutrit/nuae103
52.
Young SG Song W Yang Y Birrane G Jiang H Beigneux AP et al A protein of capillary endothelial cells, GPIHBP1, is crucial for plasma triglyceride metabolism. Proc Natl Acad Sci U S A. (2022) 119(36):e2211136119. 10.1073/pnas.2211136119
53.
Kristensen KK Leth-Espensen KZ Kumari A Grønnemose AL Lund-Winther A-M Young SG et al GPIHBP1 And ANGPTL4 utilize protein disorder to orchestrate order in plasma triglyceride metabolism and regulate compartmentalization of LPL activity. Front Cell Dev Biol. (2021) 9:702508. 10.3389/fcell.2021.702508
54.
Song W Beigneux AP Weston TA Chen K Yang Y Nguyen LP et al The lipoprotein lipase that is shuttled into capillaries by GPIHBP1 enters the glycocalyx where it mediates lipoprotein processing. Proc Natl Acad Sci U S A. (2023) 120(44):e2313825120. 10.1073/pnas.2313825120
55.
Johnsson K Freitas E DeFilippis E Roust L Katsanos C . Relationships between body composition measurements and insulin-stimulated plasma lipoprotein lipase activity in individuals with varying degrees of body adiposity. Physiology. (2024) 39(S1):1015. 10.1152/physiol.2024.39.S1.1015
56.
Carpentier AC . 100th Anniversary of the discovery of insulin perspective: insulin and adipose tissue fatty acid metabolism. Am J Physiol Endocrinol Metab. (2021) 320(4):E653–70. 10.1152/ajpendo.00620.2020
57.
Li M Cui M Li G Liu Y Xu Y Eftekhar SP et al The pathophysiological associations between obesity, NAFLD, and atherosclerotic cardiovascular diseases. Horm Metab Res. (2024) 56(10):683–96. 10.1055/a-2266-1503
58.
Hou X Guan Y Tang Y Song A Zhao J Ren L et al A correlation study of the relationships between nonalcoholic fatty liver disease and Serum triglyceride concentration after an oral fat tolerance test. Lipids Health Dis. (2021) 20(1):54. 10.1186/s12944-021-01483-z
59.
Guan Y Hou X Tian P Ren L Tang Y Song A et al Elevated levels of apolipoprotein CIII increase the risk of postprandial hypertriglyceridemia. Front Endocrinol. (2021) 12:646185. 10.3389/fendo.2021.646185
60.
Ginsberg HN Packard CJ Chapman MJ Borén J Aguilar-Salinas CA Averna M et al Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—a consensus statement from the European atherosclerosis society. Eur Heart J. (2021) 42(47):4791–806. 10.1093/eurheartj/ehab551
61.
Tall AR Thomas DG Gonzalez-Cabodevilla AG Goldberg IJ . Addressing dyslipidemic risk beyond LDL-cholesterol. J Clin Invest. (2022) 132(1):e148559. 10.1172/JCI148559
62.
Baratta F Cocomello N Coronati M Ferro D Pastori D Angelico F et al Cholesterol remnants, triglyceride-rich lipoproteins and cardiovascular risk. Int J Mol Sci. (2023) 24(5):4268. 10.3390/ijms24054268
63.
Ghosh R Colon-Negron K Papa FR . Endoplasmic Reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes. Mol Metab. (2019) 27:S60–8. 10.1016/j.molmet.2019.06.012
64.
Kuchay MS Choudhary NS Ramos-Molina B . Pathophysiological underpinnings of metabolic dysfunction-associated steatotic liver disease. Am J Physiol Cell Physiol. (2025) 328(5):C1637–66. 10.1152/ajpcell.00951.2024
65.
Jani S Da Eira D Hadday I Bikopoulos G Mohasses A de Pinho RA et al Distinct mechanisms involving diacylglycerol, ceramides, and inflammation underlie insulin resistance in oxidative and glycolytic muscles from high fat-fed rats. Sci Rep. (2021) 11(1):19160. 10.1038/s41598-021-98819-7
66.
Ziolkowska S Binienda A Jabłkowski M Szemraj J Czarny P . The interplay between insulin resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int J Mol Sci. (2021) 22(20):11128. 10.3390/ijms222011128
67.
Tily H Patridge E Cai Y Gopu V Gline S Genkin M et al Gut microbiome activity contributes to prediction of individual variation in glycemic response in adults. Diabetes Ther. (2022) 13(1):89–111. 10.1007/s13300-021-01174-z
68.
Guo X Zhai Y Song C Mi Z Peng J Guo J et al Elevated postprandial triglyceride-rich lipoproteins in patients with diabetes and stable coronary artery disease correlated with early renal damage and systemic inflammation. Lipids Health Dis. (2023) 22(1):58. 10.1186/s12944-023-01820-4
69.
Invernizzi P Carbone M Jones D Levy C Little N Wiesel P et al Setanaxib, a first-in-class selective NADPH oxidase 1/4 inhibitor for primary biliary cholangitis: a randomized, placebo-controlled, phase 2 trial. Liver Int. (2023) 43(7):1507–22. 10.1111/liv.15596
70.
Bergmark BA Marston NA Prohaska TA Alexander VJ Zimerman A Moura FA et al Olezarsen in patients with hypertriglyceridemia at high cardiovascular risk: rationale and design of the Essence–TIMI 73b trial. Am Heart J. (2025) 286:116–24. 10.1016/j.ahj.2025.02.022
71.
Karwatowska-Prokopczuk E Lesogor A Yan J-H Hoenlinger A Margolskee A Li L et al Efficacy and safety of olezarsen in lowering apolipoprotein C-III and triglycerides in healthy Japanese Americans. Lipids Health Dis. (2024) 23(1):329. 10.1186/s12944-024-02297-5
72.
Rosenson RS Gaudet D Ballantyne CM Baum SJ Bergeron J Kershaw EE et al Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial. Nat Med. (2023) 29(3):729–37. 10.1038/s41591-023-02222-w
73.
Harrison SA Ruane PJ Freilich BL Neff G Patil R Behling CA et al Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. (2021) 27(7):1262–71. 10.1038/s41591-021-01425-3
74.
Jelleschitz J Kehm R Schnell V Brandt A Bergheim I Höhn A . Islet function during aging and senescence. Free Radic Biol Med. (2022) 192:19. 10.1016/j.freeradbiomed.2022.10.014
75.
He F Liu J Huang Y Chen L Rizi EP Zhang K et al Nutritional load in post-prandial oxidative stress and the pathogeneses of diabetes mellitus. npj Sci Food. (2024) 8(1):41. 10.1038/s41538-024-00282-x
76.
Fewkes JJ Kellow NJ Cowan SF Williamson G Dordevic AL . A single, high-fat meal adversely affects postprandial endothelial function: a systematic review and meta-analysis. Am J Clin Nutr. (2022) 116(3):699–729. 10.1093/ajcn/nqac153
77.
Bae J-H Bassenge E Kim K-B Kim Y-N Kim K-S Lee H-J et al Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. (2001) 155(2):517–23. 10.1016/S0021-9150(00)00601-8
78.
Beckman JS Koppenol WH . Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol. (1996) 271(5):C1424–37. 10.1152/ajpcell.1996.271.5.C1424
79.
Li Y Zhang H Liu M Guo W Yu L . Microglia NLRP3 inflammasomes activation involving diabetic neuroinflammation in diabetic mice and BV2 cells. Curr Pharm Des. (2021) 27(24):2802–16. 10.2174/1381612827666210716104606
80.
Schmacke NA O’Duill F Gaidt MM Szymanska I Kamper JM Schmid-Burgk JL et al IKKβ primes inflammasome formation by recruiting NLRP3 to the trans-Golgi network. Immunity. (2022) 55(12):2271–2284.e7. 10.1016/j.immuni.2022.10.021
81.
Karaskov E Scott C Zhang L Teodoro T Ravazzola M Volchuk A . Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic β-cell apoptosis. Endocrinology. (2006) 147(7):3398–407. 10.1210/en.2005-1494
82.
Brand MD . The sites and topology of mitochondrial superoxide production. Exp Gerontol. (2010) 45(7–8):466–72. 10.1016/j.exger.2010.01.003
83.
Chouchani ET Pell VR Gaude E Aksentijević D Sundier SY Robb EL et al Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. (2014) 515(7527):431–5. 10.1038/nature13909
84.
Altomare AA Brioschi M Eligini S Bonomi A Zoanni B Iezzi A et al N-acetylcysteine regenerates in vivo mercaptoalbumin. Antioxidants. (2022) 11(9):1758. 10.3390/antiox11091758
85.
Ihlemann N Rask-Madsen C Perner A Dominguez H Hermann T Køber L et al Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol. (2003) 285(2):H875–82. 10.1152/ajpheart.00008.2003
86.
Ceriello A Novials A Ortega E Canivell S La Sala L Pujadas G et al Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. (2013) 36(8):2346–50. 10.2337/dc12-2469
87.
Okoye CN Koren SA Wojtovich AP . Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. (2023) 67:102926. 10.1016/j.redox.2023.102926
88.
Lim S Won H Kim Y Jang M Jyothi KR Kim Y et al Antioxidant enzymes induced by repeated intake of excess energy in the form of high-fat, high-carbohydrate meals are not sufficient to block oxidative stress in healthy lean individuals. Br J Nutr. (2011) 106(10):1544–51. 10.1017/S0007114511002091
89.
Yubero-Serrano EM Gonzalez-Guardia L Rangel-Zuñiga O Delgado-Casado N Delgado-Lista J Perez-Martinez P et al Postprandial antioxidant gene expression is modified by Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age. (2013) 35(1):159–70. 10.1007/s11357-011-9331-4
90.
Sutherland WHF Walker RJ de Jong SA van Rij AM Phillips V Walker HL . Reduced postprandial serum paraoxonase activity after a meal rich in used cooking fat. ATVB. (1999) 19(5):1340–7. 10.1161/01.ATV.19.5.1340
91.
Lima-Oliveira G Salvagno GL Lippi G Gelati M Montagnana M Danese E et al Influence of a regular, standardized meal on clinical chemistry analytes. Ann Lab Med. (2012) 32(4):250–6. 10.3343/alm.2012.32.4.250
92.
Li N Hao L Li S Deng J Yu F Zhang J et al The NRF-2/HO-1 signaling pathway: a promising therapeutic target for metabolic dysfunction-associated steatotic liver disease. J Inflamm Res. (2024) 17:8061–83. 10.2147/JIR.S490418
93.
Durrington PN Bashir B Soran H . Paraoxonase 1 and atherosclerosis. Front Cardiovasc Med. (2023) 10:1065967. 10.3389/fcvm.2023.1065967
94.
Nikouei M Cheraghi M Ghaempanah F Kohneposhi P Saniee N Hemmatpour S et al The association between bilirubin levels, and the incidence of metabolic syndrome and diabetes Mellitus: a systematic review and meta-analysis of cohort studies. Clin Diabetes Endocrinol. (2024) 10(1):1. 10.1186/s40842-023-00159-0
95.
Anfinsen ÅM Myklebust VH Johannesen CO Christensen JJ Laupsa-Borge J Dierkes J et al Serum concentrations of lipids, ketones and acylcarnitines during the postprandial and fasting state: the postprandial metabolism (PoMet) study in healthy young adults. Br J Nutr. (2024) 132(7):851–61. 10.1017/S0007114524001934
96.
Fiorenza M Onslev J Henríquez-Olguín C Persson KW Hesselager SA Jensen TE et al Reducing the mitochondrial oxidative burden alleviates lipid-induced muscle insulin resistance in humans. Sci Adv. (2024) 10(44):eadq4461. 10.1126/sciadv.adq4461
97.
Natella F Belelli F Gentili V Ursini F Scaccini C . Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. Agric Food Chem. (2002) 50(26):7720–5. 10.1021/jf020346o
98.
Tadapaneni RK Krishnankutty S Alandete L Randolph JM Cheema M Park E et al Grape seed extract attenuates oxidative stress induced by high fat/carbohydrate meal in metabolic syndrome patients. FASEB J. (2012) 26(S1):626.14. 10.1096/fasebj.26.1_supplement.626.14
99.
Choleva M Matalliotaki E Antoniou S Asimomyti E Drouka A Stefani M et al Postprandial metabolic and oxidative stress responses to grape pomace extract in healthy normal and overweight/obese women: a randomized, double-blind, placebo-controlled crossover study. Nutrients. (2022) 15(1):156. 10.3390/nu15010156
100.
Baynham R Veldhuijzen van Zanten JJCS Rendeiro C . Cocoa flavanols rescue stress-induced declines in endothelial function after a high-fat meal, but do not affect cerebral oxygenation during stress in young, healthy adults. Food Funct. (2024) 15(23):11472–90. 10.1039/D4FO03834G
101.
Dri E Lampas E Lazaros G Lazarou E Theofilis P Tsioufis C et al Inflammatory mediators of endothelial dysfunction. Life. (2023) 13(6):1420. 10.3390/life13061420
102.
González-Villalva A Morales-Ricardes G Rojas-Lemus M Bizarro-Nevares P López-Valdez N Ustarroz-Cano M et al El endotelio sano y su disfunción en el riesgo cardiovascular. Rev Fac Med. (2023) 66(6):37–52. 10.22201/fm.24484865e.2023.66.6.07
103.
Shishkova DK Velikanova EA Bogdanov LA Sinitsky MY Kostyunin AE Tsepokina AV et al Calciprotein particles link disturbed mineral homeostasis with cardiovascular disease by causing endothelial dysfunction and vascular inflammation. Int J Mol Sci. (2021) 22(22):12458. 10.3390/ijms222212458
104.
Zhang J Tecson KM McCullough PA . Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. (2020) 21(3):315–9. 10.31083/j.rcm.2020.03.126
105.
Mogensen M Sahlin K Fernström M Glintborg D Vind BF Beck-Nielsen H et al Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. (2007) 56(6):1592–9. 10.2337/db06-0981
106.
Keppeler K Helmstädter J Kuester L Strohm L Ubbens H Bayo Jimenez MT et al Abstract 154: endothelial dysfunction in experimental celiac disease is mediated by gut-derived vascular inflammation and oxidative stress. ATVB. (2022) 42(Suppl_1):154. 10.1161/atvb.42.suppl_1.154
107.
Barton B Chavez D Connelly M Bohmke N Wolver S Siddiqui M et al Vascular dysfunction and inflammation in liver transplant recipients. Physiology. (2024) 39(S1):1471. 10.1152/physiol.2024.39.S1.1471
108.
Bestepe F Pal-Ghosh R Fritsche C Lakhotiya K Smolgovsky S Weston J et al 354-OR: attenuation of obesity-induced vascular inflammation by microRNA-485 in endothelial cells. Diabetes. (2023) 72(1):354-OR. 10.2337/db23-354-OR
109.
Ford AC Staudacher HM Talley NJ . Postprandial symptoms in disorders of gut-brain interaction and their potential as a treatment target. Gut. (2024) 73(7):1199–211. 10.1136/gutjnl-2023-331833
110.
Montserrat-de la Paz S del Carmen Naranjo M Lopez S del Carmen Millan-Linares M Rivas-Dominguez A Jaramillo-Carmona SM et al Immediate-release niacin and a monounsaturated fatty acid-rich meal on postprandial inflammation and monocyte characteristics in men with metabolic syndrome. Clin Nutr. (2023) 42(11):2138–50. 10.1016/j.clnu.2023.08.017
111.
Schönknecht YB Crommen S Stoffel-Wagner B Coenen M Fimmers R Holst JJ et al Acute effects of three different meal patterns on postprandial metabolism in older individuals with a risk phenotype for cardiometabolic diseases: a randomized controlled crossover trial. Mol Nutr Food Res. (2020) 64(9):e1901035. 10.1002/mnfr.201901035
112.
Snodgrass RG Jiang X Stephensen CB . Monocyte subsets display age-dependent alterations at fasting and undergo non-age-dependent changes following consumption of a meal. Immun Ageing. (2022) 19(1):41. 10.1186/s12979-022-00297-6
113.
Hulander E Bärebring L Winkvist A Gjertsson I Lindqvist HM . A randomized controlled cross-over trial investigating the acute inflammatory and metabolic response after meals based on red meat, fatty fish, or soy protein: the postprandial inflammation in rheumatoid arthritis (PIRA) trial. Eur J Nutr. (2024) 63(7):2631–42. 10.1007/s00394-024-03451-6
114.
Cansell C Stobbe K Sanchez C Le Thuc O Mosser C Ben-Fradj S et al Dietary fat exacerbates postprandial hypothalamic inflammation involving glial fibrillary acidic protein-positive cells and microglia in male mice. Glia. (2021) 69(1):42–60. 10.1002/glia.23882
115.
Kumar A Rivadienera D Delgoffe G Poholek A Kohan A . Postprandial changes to systemic metabolism imprint durable changes on T cell immune responses. J Immunol. (2024) 212(1):1397_4724. 10.4049/jimmunol.212.supp.1397.4724
116.
Nier A Ulrich C Volk C Wolffgang M-C Brandsch C Wensch-Dorendorf M et al Effects of a single phosphate-enriched test meal on inflammasome activity and postprandial inflammatory markers in healthy subjects. Eur J Nutr. (2024) 63(3):797–807. 10.1007/s00394-023-03306-6
117.
Nogal A Asnicar F Vijay A Kouraki A Visconti A Louca P et al Genetic and gut microbiome determinants of SCFA circulating and fecal levels, postprandial responses and links to chronic and acute inflammation. Gut Microbes. (2023) 15:2240050. 10.1080/19490976.2023.2240050
118.
Wilson ML Davies IG Waraksa W Khayyatzadeh SS Al-Asmakh M Mazidi M . The impact of microbial composition on postprandial glycaemia and lipidaemia: a systematic review of current evidence. Nutrients. (2021) 13(11):3887. 10.3390/nu13113887
119.
Zmora N . Harnessing the gut microbiota to promote metabolic health. Nutr Rev. (2020) 78(3):75–8. 10.1093/nutrit/nuaa076
120.
Asnicar F Berry SE Valdes AM Nguyen LH Piccinno G Drew DA et al Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. (2021) 27(2):321–32. 10.1038/s41591-020-01183-8
121.
Wang Q Lin H Shen C Zhang M Wang X Yuan M et al Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling. Gut Microbes. (2023) 15(2):2274124. 10.1080/19490976.2023.2274124
122.
Gu Z Tan H Pan L Zheng X Wang X Wang J et al 1203-P: multiomics imbalances are associated with glycemic control in patients with type 1 diabetes. Diabetes. (2024) 73(1):1203-P. 10.2337/db24-1203-P
123.
Crudele L Gadaleta RM Cariello M Moschetta A . Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine. (2023) 97:104821. 10.1016/j.ebiom.2023.104821
124.
Hoffman SS Alvares DC Adeli K . 477-P: vagal GLP-1–mediated reductions in postprandial lipids are sensitive to fructose feeding. Diabetes. (2022) 71(1):477–P. 10.2337/db22-477-P
125.
Keleher MR Erickson K Kechris K Yang IV Dabelea D Friedman JE et al Associations between the activity of placental nutrient-sensing pathways and neonatal and postnatal metabolic health: the ECHO healthy start cohort. Int J Obes. (2020) 44(11):2203–12. 10.1038/s41366-020-0574-y
126.
Tao Z Cheng Z . Hormonal regulation of metabolism—recent lessons learned from insulin and estrogen. Clin Sci. (2023) 137(6):415–34. 10.1042/CS20210519
127.
Strilbytska OM Semaniuk UV Storey KB Yurkevych IS Lushchak O . Insulin signaling in intestinal stem and progenitor cells as an important determinant of physiological and metabolic traits in Drosophila. Cells. (2020) 9(4):803. 10.3390/cells9040803
128.
Kelesidis T Mantzoros CS . Cross-talk between SARS-CoV-2 infection and the insulin/IGF signaling pathway: implications for metabolic diseases in COVID-19 and for post-acute sequelae of SARS-CoV-2 infection. Metab Clin Exp. (2022) 134:155267. 10.1016/j.metabol.2022.155267
129.
Lin KY Hsu HJ . Regulation of adult female germline stem cells by nutrient-responsive signaling. Curr Opin Insect Sci. (2020) 37:16–22. 10.1016/j.cois.2019.10.005
130.
Marroncini G Naldi L Martinelli S Amedei A . Gut–liver–pancreas axis crosstalk in health and disease: from the role of microbial metabolites to innovative Microbiota manipulating strategies. Biomedicines. (2024) 12(7):1398. 10.3390/biomedicines12071398
131.
Schertzer JD Lam TKT . Peripheral and central regulation of insulin by the intestine and microbiome. Am J Physiol Endocrinol Metab. (2021) 320(2):E234–9. 10.1152/ajpendo.00547.2020
132.
Jakubowicz D Rosenblum RC Wainstein J Twito O . Influence of fasting until noon (extended postabsorptive state) on clock gene mRNA expression and regulation of body weight and glucose metabolism. Int J Mol Sci. (2023) 24(8):7154. 10.3390/ijms24087154
133.
Tuvia N Pivovarova-Ramich O Murahovschi V Lück S Grudziecki A Ost A-C et al Insulin directly regulates the circadian clock in adipose tissue. Diabetes. (2021) 70(9):1985–99. 10.2337/db20-0910
134.
Dimitriadis GD Maratou E Kountouri A Board M Lambadiari V . Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: an integrative approach. Nutrients. (2021) 13(1):159. 10.3390/nu13010159
135.
Jakubowicz D Wainstein J Tsameret S Landau Z . Role of high energy breakfast “big breakfast diet” in clock gene regulation of postprandial hyperglycemia and weight loss in type 2 diabetes. Nutrients. (2021) 13(5):1558. 10.3390/nu13051558
136.
Flanagan A Bechtold DA Pot GK Johnston JD . Chrono-nutrition: from molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J Neurochem. (2021) 157(1):53–72. 10.1111/jnc.15246
137.
Kroon T Hagstedt T Alexandersson I Ferm A Petersson M Maurer S et al 193-OR: chronotherapy with the glucokinase activator AZD1656 greatly affects its ability to improve metabolism in obese zucker rats. Diabetes. (2022) 71(Supplement_1):193-OR. 10.2337/db22-193-OR
138.
Smith HA Betts JA . Nutrient timing and metabolic regulation. J Physiol. (2022) 600(6):1299–312. 10.1113/JP280756
139.
Guan D Lazar MA . Interconnections between circadian clocks and metabolism. J Clin Med. (2021) 131(15):e148278. 10.1172/JCI148278
140.
Brubaker PL Martchenko A . Metabolic homeostasis: it’s all in the timing. Endocrinology. (2022) 163(1):bqab199. 10.1210/endocr/bqab199
141.
Montastier É Ye RZ Noll C Bouffard L Fortin M Frisch F et al Increased postprandial nonesterified fatty acid efflux from adipose tissue in prediabetes is offset by enhanced dietary fatty acid adipose trapping. Am J Physiol Endocrinol Metab. (2021) 320(6):E1093–106. 10.1152/ajpendo.00619.2020
142.
Freudenberger S Keirns B Sciarrillo C Poindexter K Dixon M Hart S et al Body composition measures associated with postprandial triglyceride concentrations. Curr Dev Nutr. (2022) 6:441–7. 10.1093/cdn/nzac057.007
143.
Roberts-Thomson KM Hu D Russell RD Greenaway T Betik AC Parker L et al Impaired postprandial adipose tissue microvascular blood flow responses to a mixed-nutrient meal in first-degree relatives of adults with type 2 diabetes. Am J Physiol Endocrinol Metab. (2022) 323(5):E418–27. 10.1152/ajpendo.00109.2022
144.
Wilson SM Vella CA Miles MP . Impact of moderate-to-vigorous physical activity and visceral adiposity on postprandial triglycerides in metabolically at-risk adults. Med Sci Sports Exerc. (2022) 54(9S):357. 10.1249/01.mss.0000879516.87505.7a
145.
Cyr Y Bissonnette S Lamantia V Wassef H Loizon E Ngo Sock ET et al White adipose tissue surface expression of LDLR and CD36 is associated with risk factors for type 2 diabetes in adults with obesity. Obesity. (2020) 28(12):2357–67. 10.1002/oby.22985
146.
Fazeli PK Zhang Y O'Keefe J Pesaresi T Lun M Lawney B et al Prolonged fasting drives a program of metabolic inflammation in human adipose tissue. Mol Metab. (2020) 42:101082. 10.1016/j.molmet.2020.101082
147.
Munoz MD Zamudio A Mccann MA Liew CW . 1353-P: activation of brown adipose tissue by low-protein diet ameliorates hyperglycemia in lipodystrophic diabetic mice model. Diabetes. (2022) 71(Supplement_1):1353-P. 10.2337/db22-1353-P
148.
Chait A den Hartigh LJ . Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7(22):1322869. 10.3389/fcvm.2020.00022
149.
Tavares G Rosendo-Silva D Simões F Eickhoff H Marques D Sacramento JF et al Circulating dopamine is regulated by dietary glucose and controls glucagon-like 1 peptide action in white adipose tissue. Int J Mol Sci. (2023) 24(3):2464. 10.3390/ijms24032464
150.
Cabot L Erlenbeck-Dinkelmann J Fenselau H . Neural gut-to-brain communication for postprandial control of satiation and glucose metabolism. J Endocrinol. (2023) 258(3):e220320. 10.1530/JOE-22-0320
151.
Moris JM Heinold C Blades A Koh Y . Nutrient-based appetite regulation. J Obes Metab Syndr. (2022) 31(2):161–8. 10.7570/jomes22031
152.
Wagner R Kullmann S Hummel J Veit R Prystupa K Hosenfeld E et al 34-OR: postprandial glucagon action in the human brain. Diabetes. (2023) 72(1):34-OR. 10.2337/db23-34-OR
153.
Lkhagvasuren B Mee-inta O Zhao Z-W Hiramoto T Boldbaatar D Kuo Y-M . Pancreas-brain crosstalk. Front Neuroanat. (2021) 15:691777. 10.3389/fnana.2021.691777
154.
Diz-Chaves Y Herrera-Pérez S González-Matías LC Lamas JA Mallo F . Glucagon-like Peptide-1 (GLP-1) in the integration of neural and endocrine responses to stress. Nutrients. (2020) 12(11):3304. 10.3390/nu12113304
155.
Franks PW Berry S Valdes AM Drew DA Davies RJ Merino J et al 228-OR: decoding human postprandial responses to food and their potential for precision nutrition: the PREDICT 1 study. Diabetes. (2020) 69(1):228-OR. 10.2337/db20-228-OR
156.
Weinisch P Fiamoncini J Schranner D Raffler J Skurk T Rist MJ et al Dynamic patterns of postprandial metabolic responses to three dietary challenges. Front Nutr. (2022) 9:933526. 10.3389/fnut.2022.933526
157.
Penhaligan J Sequeira-Bisson IR Miles-Chan JL . The role of postprandial thermogenesis in the development of impaired glucose tolerance and type II diabetes. Am J Physiol Endocrinol Metab. (2023) 325(3):E171–9. 10.1152/ajpendo.00113.2023
158.
Simental-Mendía LE Guerrero-Romero F . The correct formula for the triglycerides and glucose index. Eur J Pediatr. (2020) 179(7):1171. 10.1007/s00431-020-03644-1
159.
Liu L Wu Z Zhuang Y Zhang Y Cui H Lu F et al Association of triglyceride–glucose index and traditional risk factors with cardiovascular disease among non-diabetic population: a 10-year prospective cohort study. Cardiovasc Diabetol. (2022) 21(1):256. 10.1186/s12933-022-01694-3
160.
Yu H Tao L Li Y-G Yang L Liu D Wang Y et al Association between triglyceride-glucose index trajectories and carotid atherosclerosis progression. Cardiovasc Diabetol. (2023) 22(1):130. 10.1186/s12933-023-01847-y
161.
Jung M-H Yi S-W An SJ Yi J-J Ihm S-H Han S et al Associations between the triglyceride-glucose index and cardiovascular disease in over 150,000 cancer survivors: a population-based cohort study. Cardiovasc Diabetol. (2022) 21(1):52. 10.1186/s12933-022-01490-z
162.
Sheng G Kuang M Yang R Zhong Y Zhang S Zou Y . Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of diabetes in a non-diabetic population. J Transl Med. (2022) 20(1):266. 10.1186/s12967-022-03470-z
163.
Wu Z Liu L Wang W Cui H Zhang Y Xu J et al Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: a retrospective cohort study. Cardiovasc Diabetol. (2022) 21(1):142. 10.1186/s12933-022-01576-8
164.
Mahdavi-Roshan M Mozafarihashjin M Shoaibinobarian N Ghorbani Z Salari A Savarrakhsh A et al Evaluating the use of novel atherogenicity indices and insulin resistance surrogate markers in predicting the risk of coronary artery disease: a case‒control investigation with comparison to traditional biomarkers. Lipids Health Dis. (2022) 21(1):126. 10.1186/s12944-022-01732-9
165.
Mirshafiei H Darroudi S Ghayour-Mobarhan M Esmaeili H AkbariRad M Mouhebati M et al Altered triglyceride glucose index and fasted serum triglyceride high-density lipoprotein cholesterol ratio predict incidence of cardiovascular disease in the Mashhad cohort study. BioFactors. (2022) 48(3):643–50. 10.1002/biof.1816
166.
Song S Son D-H Baik S-J Cho W-J Lee Y-J . Triglyceride glucose-waist circumference (TyG-WC) is a reliable marker to predict non-alcoholic fatty liver disease. Biomedicines. (2022) 10(9):2251. 10.3390/biomedicines10092251
167.
Wang X Feng B Huang Z Cai Z Yu X Chen Z et al Relationship of cumulative exposure to the triglyceride-glucose index with ischemic stroke: a 9-year prospective study in the Kailuan cohort. Cardiovasc Diabetol. (2022) 21(1):66. 10.1186/s12933-022-01510-y
168.
Sandhu A Rockel JS Lively S Kapoor M . Emerging molecular biomarkers in osteoarthritis pathology. Ther Adv Musculoskelet Dis. (2023) 15:1759720X231177116. 10.1177/1759720X231177116
169.
Ahmad A Imran M Ahsan H . Biomarkers as biomedical bioindicators: approaches and techniques for the detection, analysis, and validation of novel biomarkers of diseases. Pharmaceutics. (2023) 15(6):1630. 10.3390/pharmaceutics15061630
170.
Pritzker KPH . Blood-based biomarkers of chronic inflammation. Expert Rev Mol Diagn. (2023) 23(6):495–504. 10.1080/14737159.2023.2215928
171.
Medina-Leyte DJ Zepeda-García O Domínguez-Pérez M González-Garrido A Villarreal-Molina T Jacobo-Albavera L . Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci. (2021) 22(8):3850. 10.3390/ijms22083850
172.
Bauset C Gisbert-Ferrándiz L Cosín-Roger J . Metabolomics as a promising resource identifying potential biomarkers for inflammatory bowel disease. J Clin Med. (2021) 10(4):622. 10.3390/jcm10040622
173.
Chakrala T Prakash R Valdes C Pepine CJ Keeley EC . Circulating biomarkers in coronary microvascular dysfunction. J Am Heart Assoc. (2023) 12(12):e029341. 10.1161/JAHA.122.029341
174.
Rochette L . Emerging new biomarkers for cardiovascular disease. Int J Mol Sci. (2022) 23(6):3274. 10.3390/ijms23063274
175.
Mongin D Chabert C Uribe Caparros A Collado A Hermand E Hue O et al Validity of dynamical analysis to characterize heart rate and oxygen consumption during effort tests. Sci Rep. (2020) 10(1):12420. 10.1038/s41598-020-69218-1
176.
Haischer MH Krzyszkowski J Roche S Kipp K . Impulse-based dynamic strength index: considering time-dependent force expression. JSCR. (2021) 35(5):1177–81. 10.1519/JSC.0000000000004032
177.
McNeish D Wolf MG . Dynamic fit index cutoffs for confirmatory factor analysis models. Psychol Methods. (2023) 28(1):61–88. 10.1037/met0000425
178.
Uemura R Nagatani Y Hashimoto M Oshio Y Sonoda A Otani H et al Association of respiratory functional indices and smoking with pleural movement and mean lung density assessed using four-dimensional dynamic-ventilation computed tomography in smokers and patients with COPD. Int J Chron Obstruct Pulmon Dis. (2023) 18:327–39. 10.2147/COPD.S389075
179.
Søndertoft NB Vogt JK Arumugam M Kristensen M Gøbel RJ Fan Y et al The intestinal microbiome is a co-determinant of the postprandial plasma glucose response. PLoS One. (2020) 15(9):e0238648. 10.1371/journal.pone.0238648
180.
Loh X Sun L Allen JC Goh HJ Kong SC Huang W et al Gender differences in fasting and postprandial metabolic traits predictive of subclinical atherosclerosis in an asymptomatic Chinese population. Sci Rep. (2022) 12(1):16890. 10.1038/s41598-022-20714-6
181.
Krysa JA Ball GDC Vine DF Jetha M Proctor SD . ApoB-lipoprotein remnant dyslipidemia and high-fat meal intolerance is associated with markers of cardiometabolic risk in youth with obesity. Pediatr Obes. (2021) 16(5):e12745. 10.1111/ijpo.12745
182.
Williams RA Dring KJ Cooper SB Morris JG Sunderland C Nevill ME . Predictors of postprandial glycaemia, insulinaemia and insulin resistance in adolescents. Br J Nutr. (2021) 125(10):1101–10. 10.1017/S0007114520003505
183.
Michalski M-C Calzada C Cheillan D Moulin P Nazare J-A Pettazzoni M et al A meal rich in palm oil or butter modifies the sphingolipid profile of postprandial triglyceride-rich lipoproteins from type 2 diabetic patients. Curr Dev Nutr. (2022) 6:450. 10.1093/cdn/nzac057.016
184.
Keirns BH Sciarrillo CM Koemel NA Emerson SR . Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J Nutr Sci. (2021) 10:e75. 10.1017/jns.2021.73
185.
Enkaku A Chujo D Kamigishi M Inagawa S Sakai W Matsukoshi S et al 1551-P: association between short-term postprandial C-peptide Index recovery during hospitalization and future glycemic control in patients with type 2 diabetes. Diabetes. (2023) 72(1):1551-P. 10.2337/db23-1551-P
186.
Reytor-González C Zambrano AK Frias-Toral E Campuzano-Donoso M Simancas-Racines D . Mediterranean Diet and breast cancer: a narrative review. Medwave. (2025) 25(02):e3027. 10.5867/medwave.2025.02.3027
187.
Reytor-González C Zambrano AK Montalvan M Frias-Toral E Simancas-Racines A Simancas-Racines D . Adherence to the mediterranean diet and its association with gastric cancer: health benefits from a planeterranean perspective. J Transl Med. (2024) 22(1):483. 10.1186/s12967-024-05176-w
188.
Alcala-Diaz JF Arenas-de Larriva AP Torres-Peña JD Rodriguez-Cantalejo F Rangel-Zuñiga OA Yubero-Serrano EM et al A gene variation at the ZPR1 locus (rs964184) interacts with the type of diet to modulate postprandial triglycerides in patients with coronary artery disease: from the coronary diet intervention with olive oil and cardiovascular prevention study. Front Nutr. (2022) 9:885256. 10.3389/fnut.2022.885256
189.
Costabile G Salamone D Della Pepa G Vitale M Testa R Cipriano P et al Differential effects of two isocaloric healthy diets on postprandial lipid responses in individuals with type 2 diabetes. Nutrients. (2024) 16(3):333. 10.3390/nu16030333
190.
Candás-Estébanez B Fernández-Cidón B Corbella E Tebé C Fanlo-Maresma M Esteve-Luque V et al The impact of the Mediterranean diet and lifestyle intervention on lipoprotein subclass profiles among metabolic syndrome patients: findings of a randomized controlled trial. Int J Mol Sci. (2024) 25(2):1338. 10.3390/ijms25021338
191.
Mora-Ortiz M Yubero-Serrano E Priego-Capote F Gutierrez-Mariscal F Alcala-Diaz J Torres-Peña J et al Dietary lipid quantity and quality modulate the postprandial metabolomic profile in patients with metabolic syndrome. Nutrients. (2024) 16(24):4267. 10.3390/nu16244267
192.
Papadaki A Nolen-Doerr E Mantzoros CS . The effect of the mediterranean diet on metabolic health: a systematic review and meta-analysis of controlled trials in adults. Nutrients. (2020) 12(11):3342. 10.3390/nu12113342
193.
Ruiz-Pozo VA Guevara-Ramírez P Paz-Cruz E Tamayo-Trujillo R Cadena-Ullauri S Frias-Toral E et al The role of the Mediterranean diet in prediabetes management and prevention: a review of molecular mechanisms and clinical outcomes. Food Agric Immunol. (2024) 35(1):2398042. 10.1080/09540105.2024.2398042
194.
Hernandez AV Marti KM Marti KE Weisman N Cardona M Biello DM et al Effect of mediterranean diets on cardiovascular risk factors and disease in overweight and obese adults: a systematic review and meta-analysis of randomized controlled trials. J Am Nutr Assoc. (2025) 44:387–404. 10.1080/27697061.2024.2440051
195.
Reytor-González C Simancas-Racines D Román-Galeano NM Annunziata G Galasso M Zambrano-Villacres R et al Chrononutrition and energy balance: how meal timing and circadian rhythms shape weight regulation and metabolic health. Nutrients. (2025) 17(13):2135. 10.3390/nu17132135
196.
Chauhan YV Hakke MD Sanamandra P Gada JV Misra S Rahate SS et al Glucagon, rather than glucagon-like Peptide 1, mediates higher post-lunch glucose excursions during breakfast skipping in Asian Indian patients with uncontrolled type 2 diabetes Mellitus. Indian J Endocrinol Metab. (2024) 28(6):645–52. 10.4103/ijem.ijem_295_24
197.
Jakubowicz D Wainstein J Ahren B Landau Z Bar-Dayan Y Froy O . Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care. (2015) 38(10):1820–6. 10.2337/dc15-0761
198.
Nakamura K Tajiri E Hatamoto Y Ando T Shimoda S Yoshimura E . Eating dinner early improves 24-h blood glucose levels and boosts lipid metabolism after breakfast the next day: a randomized cross-over trial. Nutrients. (2021) 13(7):2424. 10.3390/nu13072424
199.
de Almeida RS Marot LP Latorraca CDOC Oliveira RdÁ Crispim CA . Is evening carbohydrate intake in healthy individuals associated with higher postprandial glycemia and insulinemia when compared to morning intake? A systematic review and meta-analysis of randomized crossover studies. J Am Nutr Assoc. (2023) 42(4):349–60. 10.1080/07315724.2022.2043199
200.
Haldar S Egli L De Castro CA Tay SL Koh MXN Darimont C et al High or low glycemic index (GI) meals at dinner results in greater postprandial glycemia compared with breakfast: a randomized controlled trial. BMJ Open Diabetes Res Care. (2020) 8(1):e001099. 10.1136/bmjdrc-2019-001099
201.
Stutz B Krueger B Goletzke J Jankovic N Alexy U Herder C et al Glycemic response to meals with a high glycemic index differs between morning and evening: a randomized cross-over controlled trial among students with early or late chronotype. Eur J Nutr. (2024) 63(5):1593–604. 10.1007/s00394-024-03372-4
202.
Nasserifar S Bruno J Vanegas S Popp C Walker JM Aleman Diaz JO . THU272 early time restricted feeding as a weight neutral approach to improved glycemic variation. J Endocr Soc. (2023) 7(Supplement_1):bvad114.708. 10.1210/jendso/bvad114.708
203.
Dawson MA Cheung SN La Frano MR Nagpal R Berryman CE . Early time-restricted eating improves markers of cardiometabolic health but has no impact on intestinal nutrient absorption in healthy adults. Cell Rep Med. (2024) 5(1):101363. 10.1016/j.xcrm.2023.101363
204.
Smith K Taylor GS Walker M Brunsgaard LH Bowden Davies KA Stevenson EJ et al Pre-meal whey protein alters postprandial insulinemia by enhancing β-cell function and reducing insulin clearance in T2D. J Clin Endocrinol Metab. (2023) 108(8):e603–12. 10.1210/clinem/dgad069
205.
Li X Wainwright A Fio CZ Brodie S Alexander K McGill M et al Do the types of dietary carbohydrate and protein affect postprandial glycemia in type 1 diabetes? Nutrients. (2025) 17(11):1868. 10.3390/nu17111868
206.
Dao GM Kowalski GM Bruce CR O’Neal DN Smart CE Zaharieva DP et al The glycemic impact of protein ingestion in people with type 1 diabetes. Diabetes Care. (2025) 48(4):509–18. 10.2337/dci24-0096
207.
Kubota S Liu Y Iizuka K Kuwata H Seino Y Yabe D . A review of recent findings on meal sequence: an attractive dietary approach to prevention and management of type 2 diabetes. Nutrients. (2020) 12(9):2502. 10.3390/nu12092502
208.
Tian W Cao S Guan Y Zhang Z Liu Q Ju J et al The effects of low-carbohydrate diet on glucose and lipid metabolism in overweight or obese patients with T2DM: a meta-analysis of randomized controlled trials. Front Nutr. (2025) 11:1516086. 10.3389/fnut.2024.1516086
209.
Neeland IJ de Gregório LH Zagury R Ahrén B Neutel J Darimont C et al A randomized, placebo-controlled, single-center, crossover study to evaluate the effects of pre-meal whey protein microgel on post-prandial glucometabolic and amino acid response in people with type 2 diabetes and overweight or obesity. Metabolites. (2025) 15(1):61. 10.3390/metabo15010061
210.
Ueoka H Fukuba Y Yamaoka Endo M Kobayashi T Hamada H Kashima H . Effects of soy protein isolate and soy peptide preload on gastric emptying rate and postprandial glycemic control in healthy humans. J Physiol Anthropol. (2022) 41(1):25. 10.1186/s40101-022-00299-9
211.
Damman C Frias JP Lee ML Rikse L Lam WS Lai R-H et al 836-P: a prebiotic fiber blend improved postprandial glucose (PPG) and time in range (TIR) as evaluated by continuous glucose monitoring (CGM) in healthy subjects with normal glucose tolerance. Diabetes. (2022) 71(1):836-P. 10.2337/db22-836-P
212.
Rashed AA Saparuddin F Rathi D-NG Nasir NNM Lokman EF . Effects of resistant starch interventions on metabolic biomarkers in Pre-diabetes and diabetes adults. Front Nutr. (2022) 8:793414. 10.3389/fnut.2021.793414
213.
Sanders LM Dicklin MR Palacios OM Maki CE Wilcox ML Maki KC . Effects of potato resistant starch intake on insulin sensitivity, related metabolic markers and appetite ratings in men and women at risk for type 2 diabetes: a pilot cross-over randomised controlled trial. J Hum Nutr Diet. (2021) 34(1):94–105. 10.1111/jhn.12822
214.
Wolever TMS Maningat CC Seib PA Campbell JE Jenkins AL . Cross-linked phosphorylated RS4 wheat starch reduces glucose and insulin responses after 3 days of pre-feeding in healthy adults: an acute, double-blind, randomized controlled clinical trial. Int J Food Sci Nutr. (2023) 74(5):621–9. 10.1080/09637486.2023.2236809
215.
Yanagimoto A Matsui Y Yamaguchi T Saito S Hanada R Hibi M . Acute dose–response effectiveness of combined catechins and chlorogenic acids on postprandial glycemic responses in healthy men: results from two randomized studies. Nutrients. (2023) 15(3):777. 10.3390/nu15030777
216.
Zhang X Fan J Xiao D Edirisinghe I Burton-Freeman BM Sandhu AK . Pharmacokinetic evaluation of red raspberry (Poly)phenols from two doses and association with metabolic indices in adults with prediabetes and insulin resistance. J Agric Food Chem. (2021) 69(32):9238–48. 10.1021/acs.jafc.1c02404
217.
Liu C van Mil J Noorlander A Rietjens IMCM . Use of physiologically based kinetic modeling-based reverse dosimetry to predict In Vivo Nrf2 activation by EGCG and its colonic metabolites in humans. J Agric Food Chem. (2022) 70(43):14015–31. 10.1021/acs.jafc.2c04811
218.
Dehzad MJ Ghalandari H Nouri M Askarpour M . Effects of curcumin/turmeric supplementation on glycemic indices in adults: a grade-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Diabetes Metab Syndr. (2023) 17(10):102855. 10.1016/j.dsx.2023.102855
219.
Simancas-Racines D Frias-Toral E Campuzano-Donoso M Ramos-Sarmiento D Zambrano-Villacres R Reytor-González C et al Preoperative nutrition in bariatric surgery: a narrative review on enhancing surgical success and patient outcomes. Nutrients. (2025) 17(3):566. 10.3390/nu17030566
220.
Reytor-González C Frias-Toral E Nuñez-Vásquez C Parise-Vasco JM Zambrano-Villacres R Simancas-Racines D et al Preventing and managing pre- and postoperative micronutrient deficiencies: a vital component of long-term success in bariatric surgery. Nutrients. (2025) 17(5):741. 10.3390/nu17050741
221.
Simancas-Racines D Reytor-González C Parise-Vasco JM Angamarca-Iguago J Garcia-Velasquez E Cuzco-Macias AC et al Effectiveness and safety of preoperative nutritional interventions on surgical outcomes in patients undergoing metabolic and bariatric surgery: a systematic review and meta-analysis. Nutrients. (2025) 17(9):1533. 10.3390/nu17091533
222.
Pandolfini L Conti D Ballo P Rollo S Falsetto A Paroli GM et al Length of stay after colorectal surgery in Italy: the gap between “Fit For” and “Actual” discharge in a prospective cohort of 4529 cases. Perioper Med. (2025) 14(1):14. 10.1186/s13741-025-00492-1
223.
Bayat Z Govindarajan A Victor JC Kennedy ED . Impact of structured multicentre enhanced recovery after surgery (ERAS) protocol implementation on length of stay after colorectal surgery. BJS Open. (2024) 8(5):zrae094. 10.1093/bjsopen/zrae094
224.
Zhang X Zheng C Ho RST Miyashita M Wong SHS . The effects of accumulated versus continuous exercise on postprandial glycemia, insulin, and triglycerides in adults with or without diabetes: a systematic review and meta-analysis. Sports Med Open. (2022) 8(1):14. 10.1186/s40798-021-00401-y
225.
Dong Y Pan Y Zhang X He Q Chen S Du L et al Impact of prolonged sitting interruption on blood glucose, insulin and triacylglycerol in adults: a systematic review and meta-analysis. Appl Sci. (2024) 14(8):3201. 10.3390/app14083201
226.
Khalafi M Mojtahedi S Ostovar A Rosenkranz SK Korivi M . High-intensity interval exercise versus moderate-intensity continuous exercise on postprandial glucose and insulin responses: a systematic review and meta-analysis. Obes Rev. (2022) 23(8):e13459. 10.1111/obr.13459
227.
Kang J Fardman BM Ratamess NA Faigenbaum AD Bush JA . Efficacy of postprandial exercise in mitigating glycemic responses in overweight individuals and individuals with obesity and type 2 diabetes—a systematic review and meta-analysis. Nutrients. (2023) 15(20):4489. 10.3390/nu15204489
228.
Engeroff T Groneberg DA Wilke J . After dinner rest a while, after supper walk a mile? A systematic review with meta-analysis on the acute postprandial glycemic response to exercise before and after meal ingestion in healthy subjects and patients with impaired glucose tolerance. Sports Med. (2023) 53(4):849–69. 10.1007/s40279-022-01808-7
229.
Bellini A Scotto di Palumbo A Nicolò A Bazzucchi I Sacchetti M . Exercise prescription for postprandial glycemic management. Nutrients. (2024) 16(8):1170. 10.3390/nu16081170
230.
Buffey AJ Herring MP Langley CK Donnelly AE Carson BP . The acute effects of interrupting prolonged sitting time in adults with standing and light-intensity walking on biomarkers of cardiometabolic health in adults: a systematic review and meta-analysis. Sports Med. (2022) 52(8):1765–87. 10.1007/s40279-022-01649-4
231.
Homer AR Taylor FC Dempsey PC Wheeler MJ Sethi P Townsend MK et al Frequency of interruptions to sitting time: benefits for postprandial metabolism in type 2 diabetes. Diabetes Care. (2021) 44(6):1254–63. 10.2337/dc20-1410
232.
Gomez-Peralta F Valledor X Lopez-Picado A Abreu C Pujante Alarcón P Fernández-Rubio E et al 817-P: late postprandial hypoglycemia is reduced with ultrarapid insulin—an insulclock connected cap-based real-world study. Diabetes. (2023) 72(1):817-P. 10.2337/db23-817-P
233.
Piccirillo F Mastroberardino S Nusca A Frau L Guarino L Napoli N et al Novel antidiabetic agents and their effects on lipid profile: a single shot for several cardiovascular targets. Int J Mol Sci. (2023) 24(12):10164. 10.3390/ijms241210164
234.
Irvin MR Montasser ME Kind T Fan S Barupal DK Patki A et al Genomics of postprandial lipidomics in the genetics of lipid-lowering drugs and diet network study. Nutrients. (2021) 13(11):4000. 10.3390/nu13114000
235.
Liu C Chen J Chen H Zhang T He D Luo Q et al PCSK9 inhibition: from current advances to evolving future. Cells. (2022) 11(19):2972. 10.3390/cells11192972
236.
Burggraaf B Pouw NMC Arroyo SF van Vark-van der Zee LC van de Geijn GM Birnie E et al A placebo-controlled proof-of-concept study of alirocumab on postprandial lipids and vascular elasticity in insulin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. (2020) 22(5):807–16. 10.1111/dom.13960
237.
Liu D Li S . LEAP2: next game-changer of pharmacotherapy for overweight and obesity?Cell Rep Med. (2022) 3(4):100612. 10.1016/j.xcrm.2022.100612
238.
Nimgaonkar A Bryant C Habegger K Colbert K Carlson T Polomoscanik S et al 823-P: GLY-200, a pharmacologic duodenal exclusion therapy, improved metabolic parameters in the DIO rat. Diabetes. (2023) 72(1):823-P.
239.
Farr S Stankovic B Hoffman S Masoudpoor H Baker C Taher J et al Bile acid treatment and FXR agonism lower postprandial lipemia in mice. Am J Physiol Gastrointest Liver Physiol. (2020) 318(4):G682–93. 10.1152/ajpgi.00386.2018
240.
Chen R Chen G . Personalized nutrition for people with diabetes and at risk of diabetes has begun. J Future Foods. (2022) 2(3):193–202. 10.1016/j.jfutfo.2022.06.001
241.
Zhu T Kuang L Daniels J Herrero P Kezhi Li K Georgiou P . IoMT-enabled real-time blood glucose prediction with deep learning and edge computing. IEEE Internet Things J. (2023) 10(5):3706–19.
242.
Jarvis PRE Cardin JL Nisevich-Bede PM McCarter JP . Continuous glucose monitoring in a healthy population: understanding the post-prandial glycemic response in individuals without diabetes Mellitus. Metab Clin Exp. (2023) 146:155640. 10.1016/j.metabol.2023.155640
243.
Piersanti A Giurato F Göbl C Burattini L Tura A Morettini M . Software packages and tools for the analysis of continuous glucose monitoring data. DTT. (2023) 25(1):69–85. 10.1089/dia.2022.0237
244.
Ben-Yacov O Godneva A Rein M Shilo S Lotan-Pompan M Weinberger A et al Gut microbiome modulates the effects of a personalised postprandial-targeting (PPT) diet on cardiometabolic markers: a diet intervention in pre-diabetes. Gut. (2023) 72(8):1486–96. 10.1136/gutjnl-2022-329201
245.
Reytor-González C Annunziata G Campuzano-Donoso M Morales-López T Basantes-Tituaña C Fascì-Spurio F et al Endocrinologist’s crucial role in metabolic dysfunction-associated steatotic liver disease: a comprehensive review. Minerva Endocrinol. (2025) 50(2):209–26. 10.23736/S2724-6507.24.04314-8
246.
Simancas-Racines D Annunziata G Verde L Fascì-Spurio F Reytor-González C Muscogiuri G et al Nutritional strategies for battling obesity-linked liver disease: the role of medical nutritional therapy in metabolic dysfunction-associated steatotic liver disease (MASLD) management. Curr Obes Rep. (2025) 14(1):7. 10.1007/s13679-024-00597-6
247.
Reytor-González C Simancas-Racines D Campuzano-Donoso M Castellano Jimenez J Román-Galeano NM Sarno G et al Harnessing nutrition to combat MASLD: a comprehensive guide to food-based therapeutic strategies. Food Agric Immunol. (2025) 36(1):2496499.
Summary
Keywords
postprandial dysmetabolism, metaflammation, insulin resistance, cardiometabolic health, precision medicine, healthcare
Citation
Reytor-González C, Cevallos-Fernández E, Jácome B and Simancas-Racines D (2025) From meal to malfunction: exploring molecular pathways, biomarkers and interventions in postprandial cardiometabolic health. Front. Cardiovasc. Med. 12:1655889. doi: 10.3389/fcvm.2025.1655889
Received
28 June 2025
Accepted
23 September 2025
Published
29 October 2025
Volume
12 - 2025
Edited by
Tzortzis Nomikos, Harokopio University, Greece
Reviewed by
Elizaberh Fragopoulou, Harokopio University, Greece
Austin Angelotti, The Pennsylvania State University (PSU), United States
Updates
Copyright
© 2025 Reytor-González, Cevallos-Fernández, Jácome and Simancas-Racines.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Daniel Simancas-Racines dsimancas@ute.edu.ec
ORCID Claudia Reytor-González orcid.org/0009-0007-4234-5524 Emilia Cevallos-Fernández orcid.org/0009-0006-6784-1471 Belén Jácome orcid.org/0000-0002-5939-4660 Daniel Simancas-Racines orcid.org/0000-0002-3641-1501
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.