Abstract
Objective:
To evaluate the clinical impact of ultrasound-guided venous cannulation positioning during the initiation of venous-arterial extracorporeal membrane oxygenation (VA-ECMO).
Methods:
This retrospective study included 48 patients who received bedside VA-ECMO support between June 2019 and August 2024. Patients were divided into an ultrasound-guided group (UG, n = 23) and conventional body surface landmark group (BSL, n = 25). Clinical outcomes, cannula positioning accuracy, complications, infection markers, and prognosis were compared. A subgroup analysis was performed in patients who did not undergo cardiopulmonary resuscitation (non-CPR).
Results:
Compared to BSL group, patients in the UG group had significantly higher rates of optimal venous cannula positioning (p < 0.01), lower incidence of unstable flow and pulmonary edema, and shorter aortic valve closure time, infection markers (WBC, PCT) were also significantly lower in the UG group (p < 0.05). In the non-CPR subgroup, the UG group had shorter ECMO duration, hospital stay, and dual antibiotic therapy duration (all p < 0.05), with non-significant trends toward better survival.
Conclusion:
Ultrasound-guided venous cannulation improves cannula positioning accuracy, reduces early complications, and may enhance clinical outcomes, particularly in non-CPR patients. Routine use of ultrasound guidance is thus recommended in bedside VA-ECMO procedures.
Introduction
Extracorporeal membrane oxygenation (ECMO) has emerged as a pivotal life-support modality for patients experiencing severe cardiac and/or respiratory failure (1–3) Since its inception in the 1960s, ECMO has evolved from a perioperative adjunct in cardiac surgery to a widely adopted rescue therapy in various critical care scenarios, including acute respiratory distress syndrome (ARDS), cardiogenic shock (CS), and cardiac arrest (4–8). Its utility was particularly underscored during the COVID-19 pandemic, where ECMO served as a vital intervention in managing patients with refractory respiratory failure (9), and veno-venous ECMO (VV-ECMO) saw the biggest expansion, and the VA also, both modalities being directed to their respective indications. With ongoing advancements in medical technology and rising clinical demand, the application of ECMO, especially venous-arterial ECMO (VA-ECMO), which offers both cardiac and respiratory support, continues to expand globally (10–13).
Figure 1
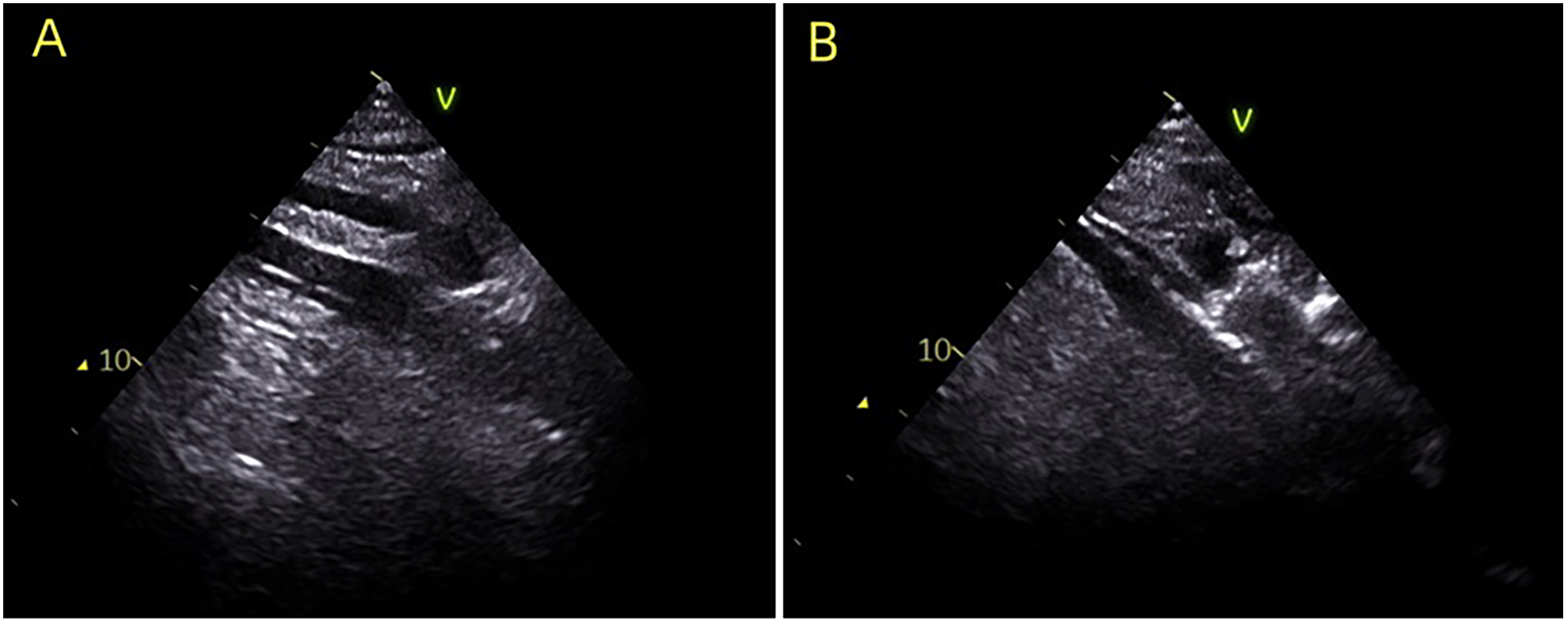
During the establishment of ECMO, ultrasound guidance was utilized to ensure accurate placement of the venous cannula in the ideal position. (A) Indicates the guide wire positioned in the right atrium; (B) illustrates the venous cannula guided into the right atrium.
Establishing effective VA-ECMO support hinges on the accurate placement of cannulas, particularly the venous cannula, which is essential for maintaining adequate drainage and ensuring stable extracorporeal flow. Misplacement can lead to serious consequences: cannulas positioned too shallowly within the inferior vena cava may adhere to vessel walls and compromise drainage, while those inserted too deeply into the right atrium or superior vena cava may cause atrial or vascular trauma, potentially resulting in catastrophic hemorrhage (5, 14–16). The optimal position for venous cannulation is in the mid-right atrium, balancing efficient drainage with reduced procedural risk. While digital subtraction angiography (DSA) offers ideal visualization for cannula positioning, its availability is often limited, particularly during emergent bedside procedures. When time permits, cannulation is performed under DSA guidance; if time does not allow, bedside implantation is performed. Ultrasound guidance has been increasingly adopted in critical care procedures for its real-time visualization and bedside applicability.
At the bedside, arterial and venous punctures are almost always performed under ultrasound guidance. However, not all patients had their venous cannula tip advanced into the mid–right atrium under ultrasound guidance. However, despite its theoretical advantages, evidence supporting its routine use for guiding venous cannulation into the middle of the right atrium during VA-ECMO initiation remains sparse, and comparative studies are lacking. In order to clarify the clinical impact of this latter step, we conducted the present study.
In this study, we retrospectively analyzed VA-ECMO cases performed at our institution to evaluate the clinical utility of ultrasound-guided venous cannulation. We aimed to determine whether ultrasound guidance improves cannulation accuracy, enhances flow stability, and reduces complications compared to traditional anatomical landmark-based methods.
Methods
Study design and population
We conducted a retrospective analysis of 48 patients (36 males, 12 females; age range 32–78 years; mean age 57.52 ± 14.31 years) who underwent bedside VA-ECMO support in the Emergency Department of Xiamen Cardiovascular Hospital, Xiamen University, between June 2019 and August 2024. Inclusion criteria were: (1) clinical diagnosis of cardiogenic shock, electrical storm, or cardiac arrest; and (2) initiation of VA-ECMO support via peripheral femoral cannulation at the bedside. Exclusion criteria were: (1) cannulation performed under digital subtraction angiography (DSA) guidance in a catheterization laboratory; or (2) early termination of ECMO for non-medical reasons. The protocol was approved by the Institutional Ethics Committee (2025-4).
Grouping and cannulation procedure
Patients were categorized into two groups based on the method of venous cannulation positioning: an ultrasound-guided group (UG, n = 23) and a body surface landmark group (BSL, n = 25). As shown in Figure 1, in the UG group, real-time ultrasound guidance using a phased-array cardiac probe was employed to track guidewire advancement into the right atrium, and the cannula was subsequently adjusted to ensure placement in the mid-right atrium. In contrast, the BSL group underwent cannulation based solely on anatomical surface landmarks, with guidewire insertion and cannula depth estimated by external measurements, without the aid of imaging guidance.
Subgroup analysis
To further evaluate the effect of ultrasound guidance on infectious complications and prognosis, a subgroup analysis was performed among patients who did not undergo cardiopulmonary resuscitation (non-CPR subgroup). These patients were further stratified into ultrasound-guided (UG-NCPR) and body surface landmark (BSL-NCPR) subgroups. Comparative analyses were conducted to assess differences in infection-related biomarkers and outcome indicators, aiming to elucidate the potential impact of ultrasound-guided cannulation in the non-CPR patients.
ECMO cannulation and support protocol
All patients received ECMO support using the Maquet (Germany) peripheral cannulation system. Cannulation was performed via the femoral artery and femoral vein. We routinely used ultrasound to verify guidewire position in both the femoral artery and vein before cannulation. The arterial cannula was advanced into the mid-abdominal aorta, and the venous cannula was positioned either at the mid-right atrium (UG group) or at an empirically determined depth (BSL group). The initial ECMO flow rate was set at 50 mL/kg/min. Systemic anticoagulation was maintained with heparin, adjusted according to activated clotting time (ACT) monitoring. All patients receiving mechanical ventilation were managed using synchronized intermittent mandatory ventilation (SIMV). In patients requiring intra-aortic balloon pump (IABP) support, counter-pulsation was maintained at a 1:1 ratio.
Clinical data collection
Baseline clinical data were collected for all patients, including demographic characteristics, medical history, primary diagnosis, time to ECMO initiation, pre-ECMO arterial blood gas values, electrocardiographic findings, and transthoracic echocardiographic measurements. Post-ECMO parameters recorded within the first 24 h included fluid infusion volume, ECMO flow stability, presence of pulmonary edema on chest radiographs, central venous pressure, follow-up arterial blood gas and echocardiographic data, duration of ECMO support, use of additional mechanical circulatory devices (e.g., intra-aortic balloon pump), laboratory biochemical indices, and occurrence of ECMO-related complications.
Operational definitions
-
•
Optimal venous cannula position: Defined radiographically as the cannula tip located in the middle third of the right atrium following the establishment of circulatory support.
-
•
ECMO flow stability: refers to stable ECMO machine operation without cannula vibration or marked fluctuations in flow.
-
•
Severe pulmonary edema: Diagnosed when bilateral chest radiographs showed pulmonary infiltrates occupying more than one-third of both lung fields.
-
•
Successful weaning: Defined as hemodynamic stability maintained for at least 48 h after ECMO discontinuation, without the need for newly initiated mechanical circulatory support (continued use of pre-existing IABP was not classified as new support).
Statistical analysis
Statistical analyses were performed using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) and compared between groups using independent samples t-tests. Categorical variables were presented as percentages and analyzed using Fisher's exact tests. A two-tailed p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
As shown in Table 1, there were no statistically significant differences between the ultrasound-guided (UG) group and the body surface landmark (BSL) group with respect to age, sex, ECMO time, primary diagnosis, timing of mechanical ventilation, or other baseline clinical parameters (all p > 0.05), indicating that the clinical basic conditions of the two groups were comparable.
Table 1
| Characteristics | UG (n = 23) | BSL (n = 25) | χ 2/T value | p value |
|---|---|---|---|---|
| Gender | 2.254 | 0.133 | ||
| Male (%) | 15 (65.22%) | 21 (84.00%) | ||
| Female (%) | 8 (34.78%) | 4 (16.00%) | ||
| Age(years old) | 62.26 ± 15.34 | 53.16 ± 17.79 | 1.890 | 0.065 |
| ECMO time | 1.268 | 0.279 | ||
| Normal working hours | 12 (52.17%) | 14 (56.00%) | ||
| Off working hours | 11 (47.83%) | 11 (44.00%) | ||
| Primary disease | ||||
| AMI (%) | 15 (65.22%) | 14 (56.00%) | 0.483 | 0.566 |
| Myocarditis (%) | 6 (26.09%) | 3 (12.00%) | 0.832 | 0.279 |
| Aortic valve stenosis (%) | 0 (0.00%) | 1 (4.00%) | 1.106 | 0.999 |
| Others (%) | 2 (8.70%) | 7 (28.00%) | 0.973 | 0.140 |
| The condition of patient before ECMO | ||||
| ECPR (%) | 9 (39.13%) | 15 (60.00%) | 0.877 | 0.248 |
| Hypotensive shock (%) | 13 (56.52%) | 9 (36.00%) | 0.912 | 0.246 |
| Electric storm (%) | 1 (4.35%) | 1 (4.00%) | 1.201 | 0.999 |
| Lactic acid value before ECMO (mmol/L) | 13.00 ± 6.62 | 11.69 ± 5.31 | 0.785 | 0.452 |
| pH value before ECMO | 7.11 ± 0.35 | 7.11 ± 0.32 | −0.091 | 0.928 |
| The duration for establishing ECMO (min) | 30.43 ± 10.87 | 35.75 ± 11.17 | −1.671 | 0.102 |
| The inner diameter of the venous cannula (F) | 20.13 ± 1.01 | 19.64 ± 0.95 | 1.728 | 0.091 |
| The inner diameter of the arterial cannula (F) | 16.13 ± 1.01 | 16.04 ± 1.17 | 0.285 | 0.777 |
Comparison of baseline data between the two groups.
UG, the ultrasound-guided group, BSL, the body surface landmark group; AMI, acute myocardial infarction; ECPR, extracorporeal cardiopulmonary resuscitation.
Clinical parameters during ECMO support
As shown in Table 2, post-ECMO chest radiographs confirmed optimal venous cannula positioning in 21 patients (91.30%) in the UG group, compared to only 8 patients (32.00%) in the BSL group, a statistically significant difference (p < 0.05). Furthermore, the UG group demonstrated fewer episodes of flow instability, lower cumulative fluid infusion volumes within 24 h post-ECMO initiation, and a lower incidence of severe pulmonary edema (all p < 0.05).
Table 2
| Measurements | UG (n = 23) | BSL (n = 25) | χ2/T value | p value |
|---|---|---|---|---|
| Optimal venous cannula positioning (n, %) | 21 (91.30%) | 8 (32.00%) | 20.340 | 0.001 |
| Unstable flow (n, %) | 10 (43.48%) | 18 (72.00%) | 4.009 | 0.045 |
| Fluid infusion volumes (L) | 3.99 ± 1.26 | 5.01 ± 1.72 | −2.338 | 0.024 |
| Severe pulmonary edema (n, %) | 8 (34.78%) | 19 (76.00%) | 8.270 | 0.004 |
| LVEF (%) | 21.00 ± 14.58 | 17.24 ± 10.71 | 1.024 | 0.311 |
| Left ventricular diameter (mm) | 45.57 ± 9.38 | 45.48 ± 6.24 | 0.037 | 0.970 |
| Aortic closure (n, %) | 12 (52.17%) | 17 (68.00%) | 1.255 | 0.263 |
Comparison of clinical data between the two groups during the support period 24 h post-ECMO.
UG, the ultrasound-guided group, BSL, the body surface landmark group; LVEF, Left ventricular ejection fraction.
Echocardiographic data collected 24 h after ECMO initiation showed no significant differences between the two groups in terms of left ventricular ejection fraction or end-diastolic dimension (p > 0.05). Although the proportion of patients exhibiting aortic valve closure did not differ significantly between groups (p > 0.05), the time of aortic valve closure was significantly shorter in the UG group compared to the BSL group (1,833.00 ± 288.70 min vs. 2,372.00 ± 379.08 min, p < 0.05), suggesting earlier cardiac unloading.
Inferior vena cava injury and infection markers
As summarized in Table 3, no cases of inferior vena cava (IVC) injury occurred in the UG group. In contrast, two IVC injuries were reported in the BSL group, both involving cannulation-related vascular trauma and bleeding. Additionally, peak white blood cell (WBC) counts and procalcitonin (PCT) levels were significantly lower in the UG group (p < 0.05), suggesting reduced inflammatory response. However, there were no significant differences between groups in the proportion of patients receiving dual antibiotic therapy or in the duration of such therapy (p > 0.05).
Table 3
| Measurements | UG (n = 23) | BSL (n = 25) | χ 2/T value | p value |
|---|---|---|---|---|
| Injury of the inferior vena cava (n, %) | 0 (0.00%) | 2 (8.00%) | 0.920 | 0.116 |
| The maximum value of WBC (*109/L) | 16.17 ± 5.01 | 25.32 ± 11.35 | −3.556 | 0.001 |
| The maximum value of PCT (ng/mL) | 11.10 ± 8.10 | 25.11 ± 13.49 | −4.315 | 0.001 |
| Cases using dual antibiotics (n, %) | 17 (73.91%) | 23 (92.00%) | 2.822 | 0.093 |
| Duration of using dual antibiotics (day) | 6.48 ± 5.98 | 7.08 ± 7.71 | −0.300 | 0.765 |
Comparison of indicators of inferior vena cava injury and infection between the two groups during hospitalization.
UG, the ultrasound-guided group, BSL, the body surface landmark group; WBC, white blood cell; PCT, procalcitonin.
Prognostic outcomes
Figure 2 illustrates the clinical outcomes for both groups. No significant differences were observed between the UG and BSL groups regarding total duration of ECMO support or hospital stay (p > 0.05). The differences of rates of successful weaning, hospital discharge, and one- and six-month between two groups survivalwere not significance (p > 0.05).
Figure 2
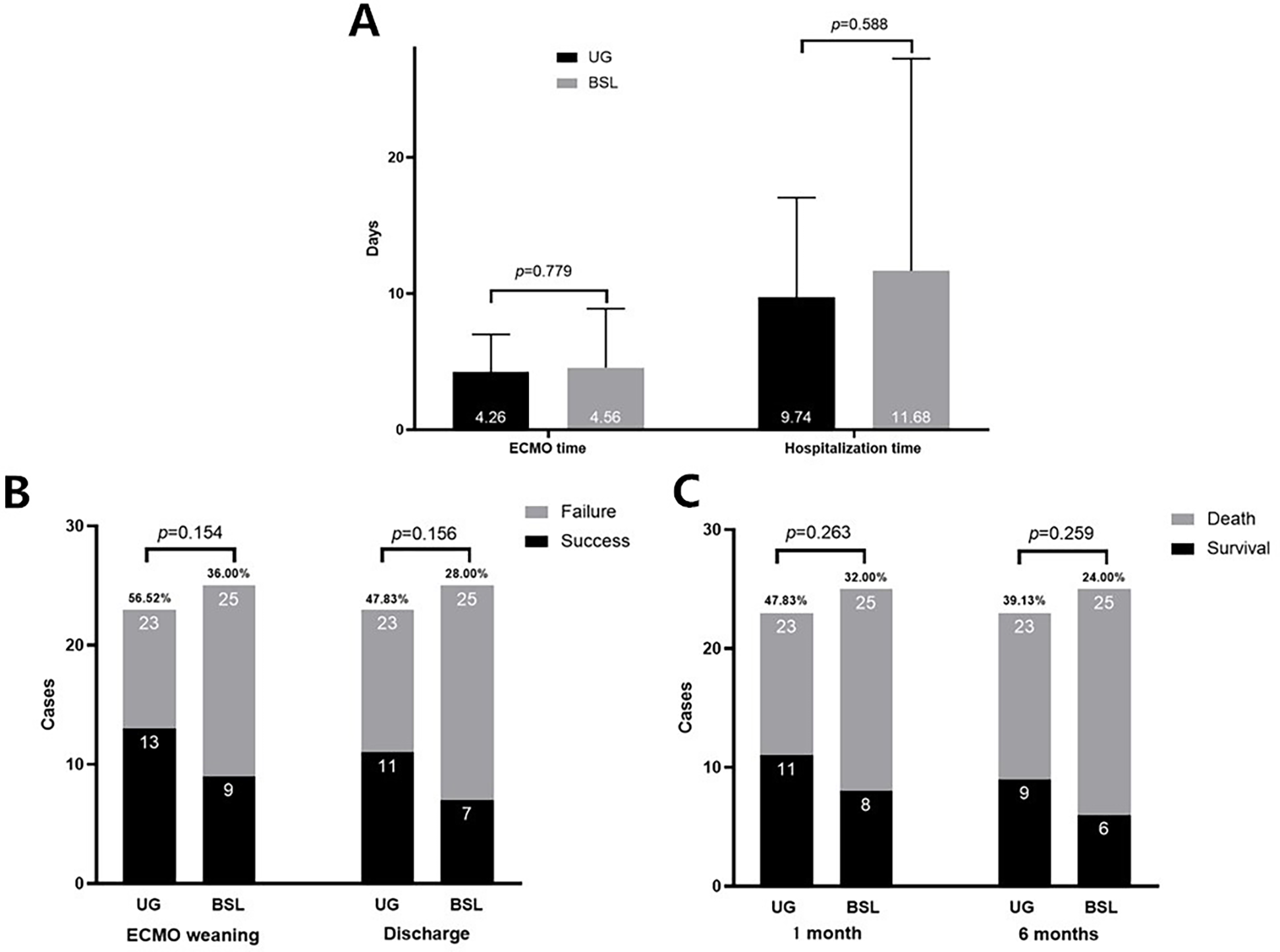
The comparison of clinical outcomes between two groups. (A) ECMO Support Duration and Hospital Stay: The duration of ECMO support and length of hospital stay were comparable between the two groups (p > 0.05). (B) ECMO Weaning Success and Hospital Discharge Rates: The differences between two groups were not significant (p > 0.05). (C) One-Month and Six-Month Survival Rates: The differences between two groups were not significant (p > 0.05).
Subgroup analysis in non-CPR patients
Considering the typically poor prognosis of patients requiring ECMO after cardiopulmonary resuscitation (CPR), a subgroup analysis was conducted among patients who did not undergo CPR (UG-NCPR vs. BSL-NCPR). This cohort included 14 patients in the UG-NCPR group and 10 patients in the BSL-NCPR group. As presented in Table 4, the UG-NCPR group had significantly lower peak WBC counts (p = 0.006) and PCT levels (p = 0.001), along with a significantly shorter duration of dual antibiotic therapy (p = 0.001).
Table 4
| Measurements | UG-NCPR (n = 14) | BSL-NCPR (n = 10) | χ 2/T value | p value |
|---|---|---|---|---|
| The maximum value of WBC (*109/L) | 15.71 ± 4.45 | 29.10 ± 15.69 | −3.049 | 0.006 |
| The maximum value of PCT (ng/mL) | 9.37 ± 8.25 | 31.08 ± 17.12 | −4.143 | 0.001 |
| Cases using dual antibiotics (n, %) | 9 (64.29%) | 8 (80.00%) | 0.697 | 0.404 |
| Time of using dual antibiotics (day) | 6.38 ± 3.32 | 12.50 ± 8.24 | −2.535 | 0.019 |
Comparison of indicators of infection between the two subgroups during hospitalization.
UG-NCPR, the ultrasound-guided group of none-CPR patients before ECMO, BSL-NCPR, the body surface landmark group of none-CPR patients before ECMO; WBC, white blood cell; PCT, procalcitonin.
Moreover, as shown in Figure 3, patients in the UG-NCPR group had shorter durations of ECMO support (p = 0.022) and hospital stay (p = 0.033) compared to those in the BSL-NCPR group. The difference of rates of successful weaning, discharge, and 1- and 6-month survival between two groups were were not significant (all p > 0.05).
Figure 3
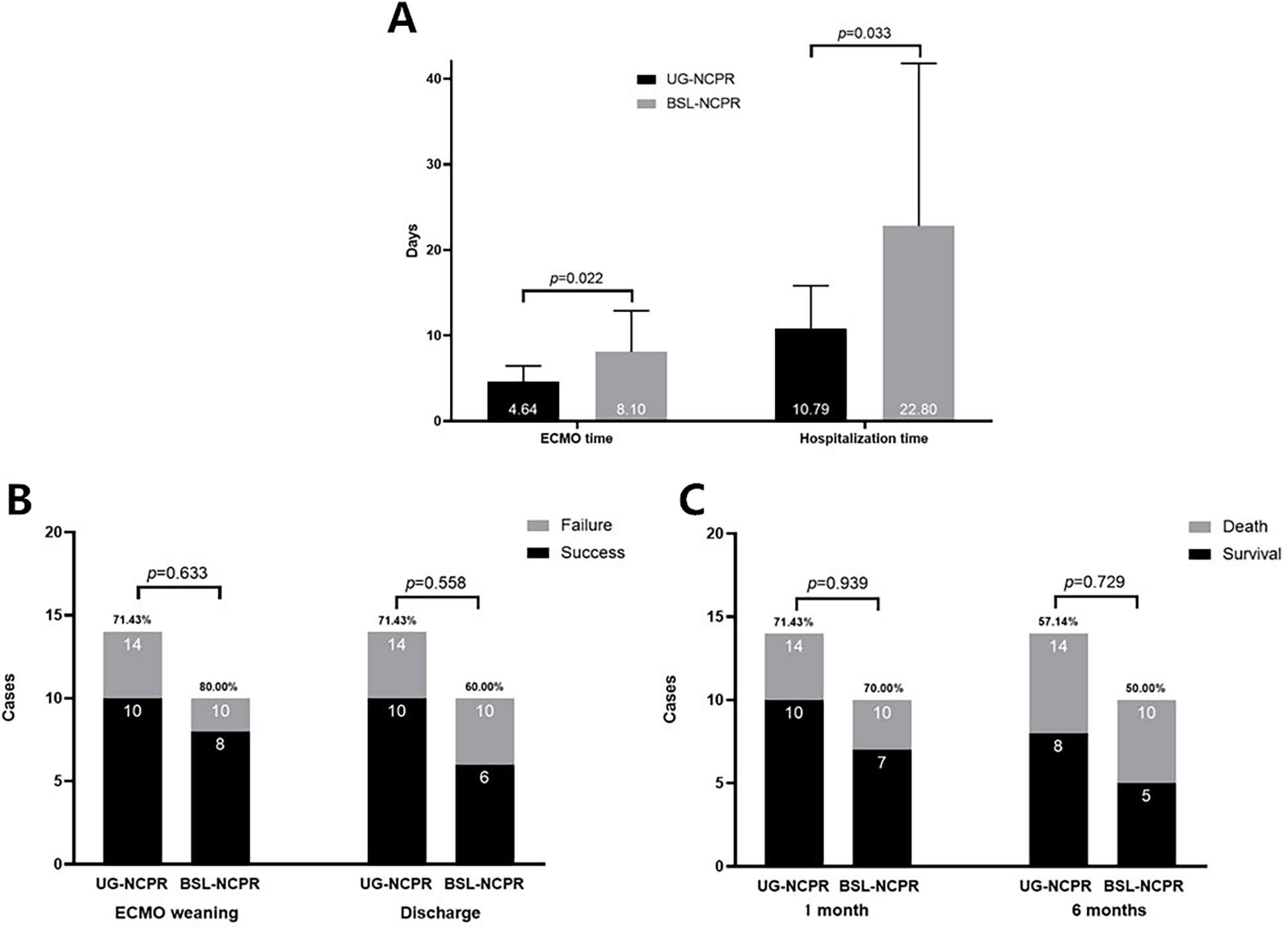
The comparison of clinical outcomes between the two sub-groups. (A) ECMO Support Duration and Hospital Stay: The duration of ECMO support and length of hospital stay of the UG-NCPR group were shorter than those of BSL-NCPR group (p < 0.05). (B) ECMO Weaning Success and Hospital Discharge Rates: The differences between two groups were not significant (p > 0.05). (C) One-Month and Six-Month Survival Rates: The differences between two groups were not significant (p > 0.05).
Discussion
ECMO remains a cornerstone in the management of patients with refractory cardiogenic shock. The establishment of stable and adequate extracorporeal flow is critical to its effectiveness. Excessive flow may increase left ventricular afterload, hindering myocardial recovery (8, 17–19), while insufficient flow results in inadequate perfusion and failure to resolve oxygen debt, thereby compromising therapeutic efficacy (2, 20). In addition to flow rate modulation, cannula positioning—particularly the venous cannula—plays a central role in maintaining effective ECMO performance. Cannulae with multiple side holes enhance drainage; however, when positioned too low within the inferior vena cava, suction-related adherence to the vessel wall may occur, reducing venous return. Conversely, placement within the mid-right atrium mitigates this risk and optimizes drainage dynamics.
In clinical practice, especially in urgent bedside settings, cannulation is often performed without fluoroscopic guidance. Traditional reliance on external anatomical landmarks lacks precision, increasing the risk of malposition. Although case reports have described the feasibility of ultrasound-assisted venous cannulation, comparative studies evaluating its efficacy against conventional methods remain limited (21–23). Our retrospective review over a five-year period provides evidence supporting the utility of ultrasound guidance in improving procedural accuracy and reducing complications.
In our study, baseline characteristics were comparable between the ultrasound-guided (UG) and body surface landmark (BSL) groups, confirming the reliability of between-group comparisons. Importantly, the time required for ECMO establishment did not differ significantly, indicating that real-time ultrasound did not delay cannulation. However, significantly more patients in the UG group achieved optimal cannula placement in the mid-right atrium, with a concomitant reduction in early fluid infusion volume. This suggests improved venous drainage and more efficient oxygen debt repayment, potentially minimizing the risk of North-South syndrome. In contrast, inadequate venous return may necessitate compensatory increases in flow, predisposing patients to left heart distension and delayed myocardial recovery.
The UG group also experienced a lower incidence of moderate to severe pulmonary edema within 24 h post-cannulation, further supporting the hemodynamic advantages of accurate venous positioning. Infection-related markers, including peak leukocyte count and procalcitonin levels, were significantly lower in the UG group, suggesting a potential reduction in systemic inflammatory response (24, 25). While the overall cohort did not show significant differences in antibiotic exposure, subgroup analysis of patients who did not undergo cardiopulmonary resuscitation (CPR) revealed a significantly shorter duration of dual antibiotic therapy in the UG group. These findings imply that precise venous cannula positioning may improve pulmonary fluid management, reduce infection risk, and thereby decrease antibiotic burden.
Although no statistically significant differences in ECMO duration, hospital stay, or survival outcomes were observed in the overall cohort, the non-CPR subgroup demonstrated clinical advantages with ultrasound guidance. Specifically, patients in the UG-NCPR group had significantly shorter ECMO support and hospitalization durationsThese results are consistent with prior literature suggesting that ultrasound may enhance procedural safety and clinical outcomes during ECMO initiation.
This study has several limitations. Its retrospective, single-center design inherently introduces selection bias. Precise timing of cannulation procedures and measurement of anatomical parameters, such as inferior vena cava diameter, were not uniformly available, limiting the granularity of procedural comparisons. In this study, we used PCT to preliminarily assess the patients' lung infection status. The use of a single evaluation indicator might lead to biased results. Additionally, the small sample size may have restricted statistical power in detecting differences in long-term outcomes. Given the small sample size, selection bias may indeed account for some of the observed differences. Our intention was to provide preliminary insights that may inform clinical management, and we hope that future studies with larger sample sizes will provide more robust evidence.
Conclusion
Ultrasound-guided venous cannulation during VA-ECMO initiation enhances cannula positioning accuracy and contributes to more stable hemodynamic support, with a lower incidence of early pulmonary edema and reduced inflammatory markers. Among patients not undergoing CPR, ultrasound guidance was associated with reduced infection burden, shorter antibiotic exposure, and shorter ECMO support and hospitalization durations. These findings support the integration of bedside ultrasound into routine ECMO cannulation protocols, particularly in emergency settings.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of Xiamen Cardiovascular Hospital of Xiamen University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study was a retrospective one. All the techniques used in both groups were mature clinical routine techniques. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Writing – review & editing, Methodology, Software. CH: Writing – review & editing, Formal analysis, Investigation. WO: Writing – review & editing, Formal analysis, Investigation. ZL: Writing – review & editing, Project administration, Data curation. GS: Data curation, Project administration, Writing – review & editing. XZ: Formal analysis, Data curation, Writing – review & editing. XC: Methodology, Software, Visualization, Supervision, Writing – review & editing. BW: Visualization, Validation, Conceptualization, Writing – review & editing. GZ: Visualization, Funding acquisition, Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Natural Science Foundation of Fujian Province (2023J011676) and Xiamen Medical and Health Guidance Project (3502Z20224ZD1180).
Acknowledgments
The authors thank all donors and patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Springer A Dreher A Reimers J Kaiser L Bahlmann E van der Schalk H et al Prognostic influence of mechanical cardiopulmonary resuscitation on survival in patients with out-of-hospital cardiac arrest undergoing ECPR on VA-ECMO. Front Cardiovasc Med. (2023) 10:1266189. 10.3389/fcvm.2023.1266189
2.
Blumer V Kanwar MK Barnett CF Cowger JA Damluji AA Farr M et al Cardiogenic shock in older adults: a focus on age-associated risks and approach to management: a scientific statement from the American Heart Association. Circulation. (2024) 149:e1051–65. 10.1161/CIR.0000000000001214
3.
Vishram-Nielsen JKK Foroutan F Rizwan S Peck SS Bodack J Orchanian-Cheff A et al Patients with fulminant myocarditis supported with veno-arterial extracorporeal membrane oxygenation: a systematic review and meta-analysis of short-term mortality and impact of risk factors. Heart Fail Rev. (2023) 28:347–57. 10.1007/s10741-022-10277-z
4.
Richardson ASC Tonna JE Nanjayya V Nixon P Abrams DC Raman L et al Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. (2021) 67:221–8. 10.1097/MAT.0000000000001344
5.
Geller BJ Sinha SS Kapur NK Bakitas M Balsam LB Chikwe J et al Escalating and de-escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation. (2022) 146:e50–68. 10.1161/CIR.0000000000001076
6.
Tonna JE Boonstra PS MacLaren G Paden M Brodie D Anders M et al Extracorporeal life support organization registry international report 2022: 100,000 survivors. ASAIO J. (2024) 70:131–43. 10.1097/mat.0000000000002128
7.
Tan Z Su L Chen X He H Long Y . Relationship between the pre-ECMO and ECMO time and survival of severe COVID-19 patients: a systematic review and meta-analysis. J Clin Med. (2024) 13(3):868. 10.3390/jcm13030868
8.
Tsangaris A Alexy T Kalra R Kosmopoulos M Elliott A Bartos JA et al Overview of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support for the management of cardiogenic shock. Front Cardiovasc Med. (2021) 8:686558. 10.3389/fcvm.2021.686558
9.
Martínez-Martínez M Schmidt M Broman LM Roncon-Albuquerque R Jr Langouet E Campos I et al Survival and long-term functional Status of COVID-19 patients requiring prolonged extracorporeal membrane oxygenation support. Ann Am Thorac Soc. (2024) 21:449–55. 10.1513/AnnalsATS.202306-572OC
10.
Tonna JE Cho SM . Extracorporeal cardiopulmonary resuscitation. Crit Care Med. (2024) 52:963–73. 10.1097/CCM.0000000000006185
11.
Bian Y Pan Y Zheng J Zheng W Qin L Zhou G et al Extracorporeal versus conventional cardiopulmonary resuscitation for in-hospital cardiac arrest: a propensity score matching cohort study. Crit Care Med. (2024) 52:e268–78. 10.1097/CCM.0000000000006223
12.
Kang JK Darby Z Bleck TP Whitman GJR Kim BS Cho SM . Post-cardiac arrest care in adult patients after extracorporeal cardiopulmonary resuscitation. Crit Care Med. (2024) 52:483–94. 10.1097/CCM.0000000000006102
13.
Diaz-Gomez JL . Should we explore transesophageal echocardiography during advanced cardiac life support to improve cardiopulmonary resuscitation quality and efficacy?Crit Care Med. (2024) 52:1487–90. 10.1097/CCM.0000000000006370
14.
Kowalewski M Malvindi PG Zieliński K Martucci G Słomka A Suwalski P et al Left ventricle unloading with veno-arterial extracorporeal membrane oxygenation for cardiogenic shock. Systematic review and meta-analysis. J Clin Med. (2020) 9(4):1039. 10.3390/jcm9041039
15.
Russo JJ Aleksova N Pitcher I Couture E Parlow S Faraz M et al Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. (2019) 73:654–62. 10.1016/j.jacc.2018.10.085
16.
van Diepen S . Routine unloading in patients treated with extracorporeal membrane oxygenation for cardiogenic shock: mixed outcomes set the stage for future trials. Circulation. (2020) 142:2107–9. 10.1161/CIRCULATIONAHA.120.050847
17.
Thevathasan T Füreder L Fechtner M Mørk SR Schrage B Westermann D et al Left-ventricular unloading with impella during refractory cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care Med. (2024) 52:464–74. 10.1097/ccm.0000000000006157
18.
Ling RR Low CJW Ramanathan K . Mechanical left-ventricular unloading in extracorporeal cardiopulmonary resuscitation: a state of clinical equipoise. Crit Care Med. (2024) 52:512–5. 10.1097/ccm.0000000000006179
19.
Lakatos BK Ladanyi Z Fabian A Ehrenberger R Turschl T Bagyura Z et al Non-invasive assessment of left ventricular contractility by myocardial work index in veno-arterial membrane oxygenation patients: rationale and design of the MIX-ECMO multicentre observational study. Front Cardiovasc Med. (2024) 11:1399874. 10.3389/fcvm.2024.1399874
20.
Gong FF Vaitenas I Malaisrie SC Maganti K . Mechanical complications of acute myocardial infarction: a review. JAMA Cardiol. (2021) 6:341–9. 10.1001/jamacardio.2020.3690
21.
Shah D Sen J . Mechanical circulatory support in cardiogenic shock: a narrative review. Cureus. (2024) 16:e69379. 10.7759/cureus.69379
22.
Bearl DW Jaquiss RDB Vesel TP . Indications and outcomes for temporary mechanical circulatory support in pediatric patients with cardiac failure. ASAIO J. (2019) 65:389–94. 10.1097/mat.0000000000000819
23.
Qureshi F Kumar S Yadav S Mohammed S Bhatia P . Point-of-care ultrasound (POCUS) to the rescue in VA-ECMO. J Ultrason. (2024) 24:20240013. 10.15557/jou.2024.0013
24.
Lee CC Kwa ALH Apisarnthanarak A Feng JY Gluck EH Ito A et al Procalcitonin (PCT)-guided antibiotic stewardship in Asia-Pacific countries: adaptation based on an expert consensus meeting. Clin Chem Lab Med. (2020) 58:1983–91. 10.1515/cclm-2019-1122
25.
Jankovic R Stojanovic M Bozov H Domi R Ivancan V Karisik M et al Procalcitonin guided antibiotic stewardship: a Balkan expert consensus statement. Acta Clin Croat. (2023) 62:36–44. 10.20471/acc.2023.62.01.05
Summary
Keywords
ECMO, ultrasound guidance, venous cannulation, hemodynamics, infection
Citation
Sun Y, Huang C, Ou W, Liu Z, Sun G, Zhang X, Chen X, Wang B and Zhang G (2025) Efficacy of ultrasound guided venous cannulation positioning during venous-arterial extracorporeal membrane oxygenation. Front. Cardiovasc. Med. 12:1656101. doi: 10.3389/fcvm.2025.1656101
Received
29 June 2025
Accepted
30 September 2025
Published
16 October 2025
Volume
12 - 2025
Edited by
Andrea Boccatonda, University of Bologna, Italy
Reviewed by
Mihai Strachinaru, Université libre de Bruxelles, Belgium
Jan-Steffen Pooth, University of Freiburg Medical Center, Germany
Updates
Copyright
© 2025 Sun, Huang, Ou, Liu, Sun, Zhang, Chen, Wang and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Guoming Zhang guomingheart@xmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.