- 1Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Chongqing Health College, Chongqing, China
Objective: To systematically evaluate in-hospital mortality risk prediction models for patients with acute coronary syndrome (ACS) and provide valuable insights and references for the construction, application, and optimization of these models.
Methods: A comprehensive search was conducted in five databases, including CNKI, Wanfang, PubMed, Web of Science, and Embase, from inception to November 2024. Researchers screened the literature, extracted relevant data, and assessed the quality of the prediction models using the Prediction Model Risk of Bias Assessment Tool (PROBAST). Extracted data included study design, data sources, outcome definitions, sample size, predictive factors, model development, and performance.
Results: A total of 18 studies involving 44 prediction models were included. The area under the receiver operating characteristic curve (AUC) or C-index of these models ranged from 0.79 to 0.96. Overall, the included prediction models demonstrated a high risk of bias, primarily due to issues such as unreported missing data, methodological flaws in model construction, and a lack of model performance evaluation.
Conclusion: The construction of in-hospital mortality risk prediction models for patients with ACS is still in the developmental stage. Future development and validation of prediction models should adhere to the PROBAST and TRIPOD guidelines to establish models with strong predictive performance and high generalizability.
Systematic Review Registration: PROSPERO CRD42024567755.
1 Background
Acute coronary syndrome (ACS) is a severe cardiovascular condition that encompasses three clinical types: ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UAP). Globally, more than 7 million people are diagnosed with ACS each year. In 2023, approximately 50% of cardiovascular-related deaths were attributable to this condition, underscoring ACS as one of the leading causes of mortality worldwide (1, 2). During hospitalization, more than 5% of ACS patients experience in-hospital mortality, with certain subgroups showing mortality rates as high as 26.7%. Long-term follow-up studies indicate mortality rates reaching up to 26.5%. In contemporary cohorts of STEMI patients, in-hospital mortality exceeds 50% (3, 4). Additionally, a Swiss study reported that in-hospital mortality rates significantly increase when ACS is accompanied by multivessel disease, with no observed improvement in this trend over time (5). Therefore, accurately identifying the in-hospital mortality risk in ACS patients is crucial for developing effective treatment strategies. In recent years, numerous prediction models for in-hospital mortality risk in ACS patients have emerged (6). Among them, the Global Registry of Acute Coronary Events (GRACE) score and the Thrombolysis in Myocardial Infarction (TIMI) score are the most recommended and widely used risk assessment tools in clinical practice guidelines. Additionally, the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) risk model has demonstrated excellent performance in predicting in-hospital mortality (7). However, a systematic evaluation of the quality and applicability of these models in different clinical settings remains lacking. This study aims to systematically review and evaluate existing in-hospital mortality risk prediction models developed for ACS patients, providing valuable references for scholars in the construction, optimization, and validation of such models. The findings of this study will offer critical scientific support for clinical practice and future research.
2 Methods
The study protocol was registered on PROSPERO (Registration Number: CRD42024567755).
2.1 Search strategy
To ensure a comprehensive literature search and account for the broad dissemination of relevant studies, both Chinese and English databases were searched. The databases included CNKI, Wanfang, PubMed, Embase, and Web of Science, with the search period extending from the inception of each database to November 2024. A combination of subject terms and free-text terms was used in both Chinese and English searches. The main Chinese and English search terms are acute coronary syndrome (ACS), including its various variant forms such as acute coronary syndromes (plural form), coronary syndrome, acute (inverted form), and coronary syndromes, acute (inverted plural form); patients with acute coronary syndrome; risk, risk assessment, relative risk; death, cardiac death, in-hospital death, mortality rate, in-hospital mortality rate, risk of death; prediction, early warning, influencing factors, impact factor; risk prediction, model, tool and score. The detailed search strategy is provided in the supplementary material. To further enhance the comprehensiveness and accuracy of the literature collection, manual searching and a snowballing method were employed to supplement the references and citations of the included studies.
2.2 Inclusion and exclusion criteria
Inclusion Criteria: (1) Population: Patients with acute coronary syndrome (ACS), including ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UAP). (2) Study Focus: Construction or validation of in-hospital mortality risk prediction models for ACS patients. (3)Study Design: Observational studies.
Exclusion Criteria: (1) Non-English and non-Chinese publications. (2) Duplicate studies and articles with inaccessible full texts. (3) Publications in the form of abstracts, conference notices, reviews, or meta-analyses. (4) Studies that only analyzed predictive factors for in-hospital mortality in ACS patients without constructing a prediction model.
2.3 Literature screening and data extraction
Two researchers independently screened the literature by reviewing the titles and abstracts according to the inclusion and exclusion criteria. In cases of disagreement, discussions or consultation with a third party were conducted to reach a consensus. After excluding irrelevant studies, the full texts of the remaining articles were thoroughly reviewed to determine the final included studies. Data extraction was guided by the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS) checklist (8). Extracted data included: Publication Date, Study Design, Country, Data Source, Sample Size, Candidate Variables, Modeling Methods, Variable Selection Methods, Number of Models, Model Performance, Model Validation Methods, Model Presentation Format.
2.4 Risk of bias and applicability assessment
Two researchers independently assessed the risk of bias and applicability of the included studies using the Prediction Model Risk Of Bias Assessment Tool (PROBAST) (9). In case of disagreement, a third party's opinion was sought.
The risk of bias assessment covered four domains: participants, predictors, outcomes, and analysis, comprising a total of 20 specific questions. Following the “shortest plank theory,” each domain was evaluated as follows: Low Risk:If all items were marked as “probably yes” or “yes.” High Risk: If any item was marked as “no” or “probably no.” Unclear Risk: If insufficient information was provided for any item. For the overall risk of bias, a study was classified as “low risk” only if all four domains were rated as “low risk.” If any domain was rated as “high risk,” the overall bias risk was deemed “high.” If any domain was rated as “unclear,” the overall risk of bias was also classified as “unclear.” Applicability was evaluated across three domains: study population, predictors, and outcomes. The assessment method was consistent with the risk of bias evaluation, using the same criteria for low, high, and unclear applicability.
2.5 Data synthesis
A descriptive analysis method will be used to summarize the basic characteristics of the included studies and the constructed prediction models.
3 Results
3.1 Literature screening process and results
A total of 5,232 relevant studies were identified through database searches and other resources. After removing 741 duplicate records, 4,491 articles remained. Initial screening of titles and abstracts resulted in 168 studies for full-text review. Following a detailed assessment, 19 studies were deemed eligible, and finally, 18 studies were included in the descriptive analysis. The screening process is shown in Figure 1.
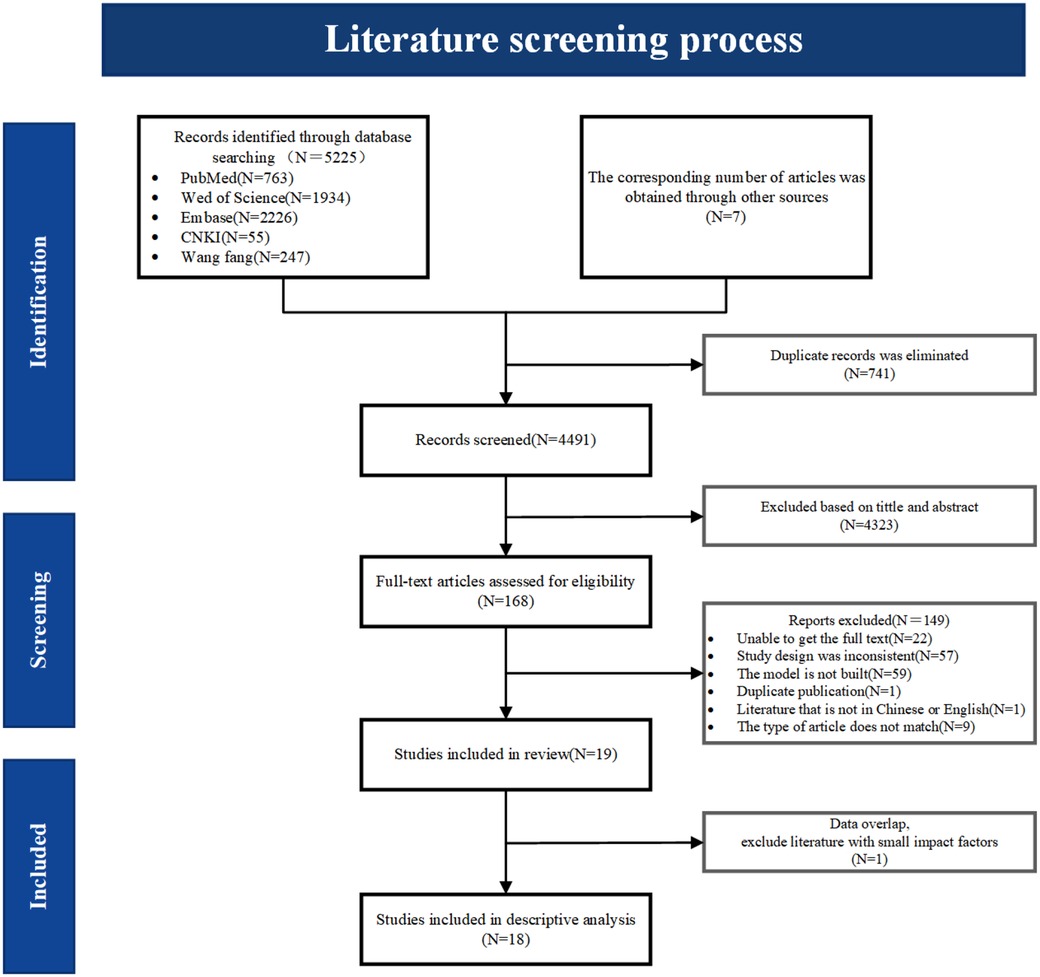
Figure 1. Literature screening process diagram. During the data analysis, two included literatures showed overlapping data in terms of modeling methods, data time, and screening variables. To avoid bias in the analysis results, the one with a lower impact factor was excluded.
3.2 Basic characteristics of included studies
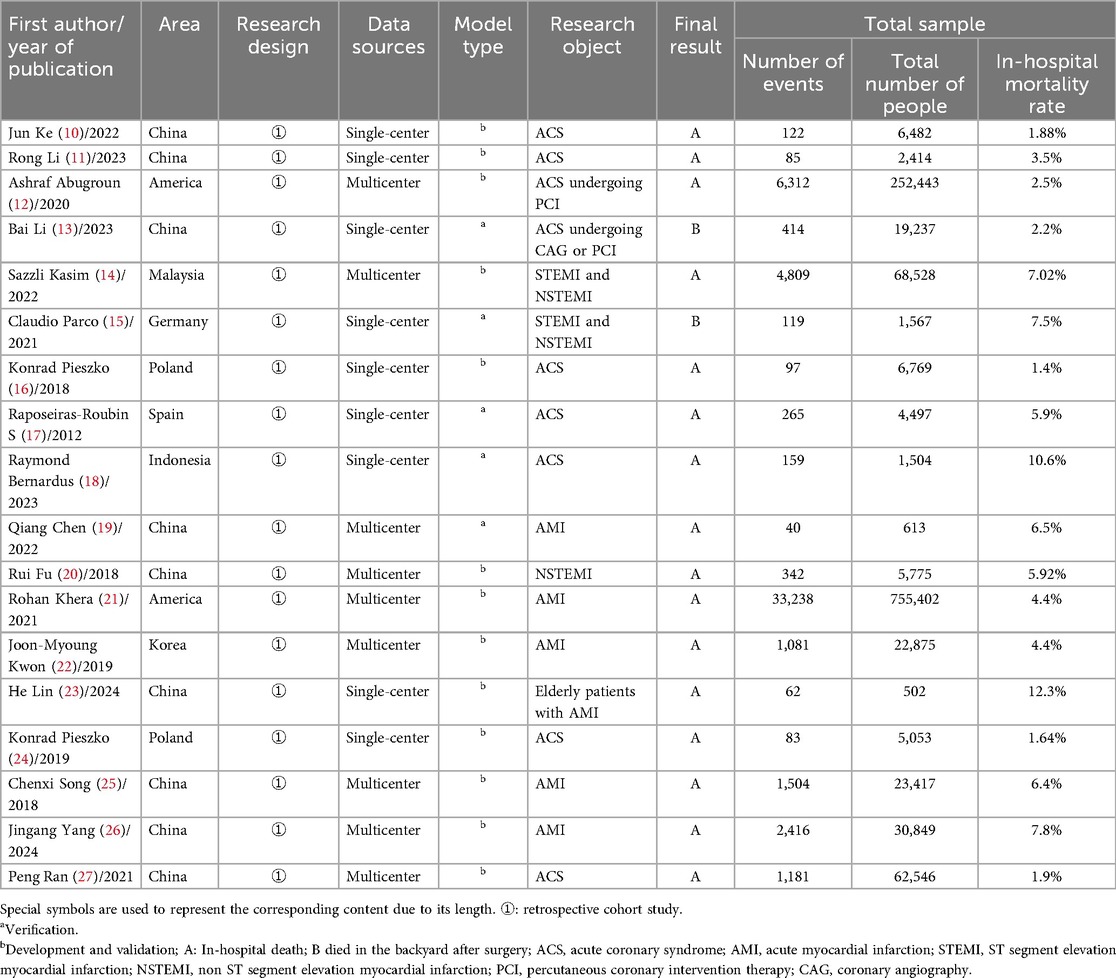
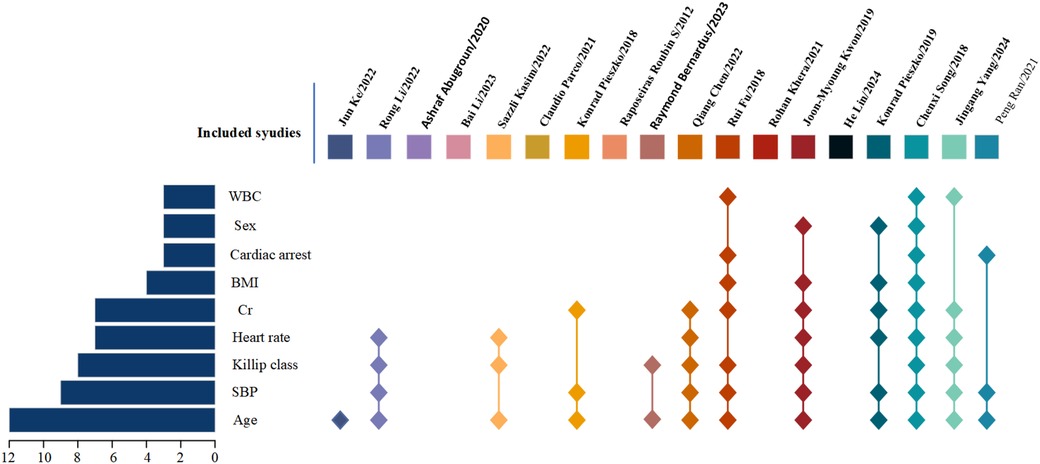
This study conducted a comprehensive analysis of 18 relevant studies (10–27). It was found that 67% (12/18) of the in-hospital risk prediction models for acute coronary syndrome (ACS) were published within the past five years (10–15, 18, 19, 21, 23, 26, 27). These studies primarily originated from China (n = 9) (9, 10, 11, 13, 19, 20, 23, 25–27), the United States (n = 2) (12, 21), and Poland (n = 2) (16, 24). All included studies were retrospective cohort studies. In terms of data sources, half of the studies were based on single-center data (10, 11, 13, 15–18, 23, 24), while the other half utilized multi-center data (12, 14, 19–22, 25–27). All studies reported the sample sizes required for model development, with sample sizes ranging from 502 to 755,402. The in-hospital mortality rates varied between 1.88% and 12.3%. For details, please refer to Table 1.
3.3 Basic information on prediction model construction
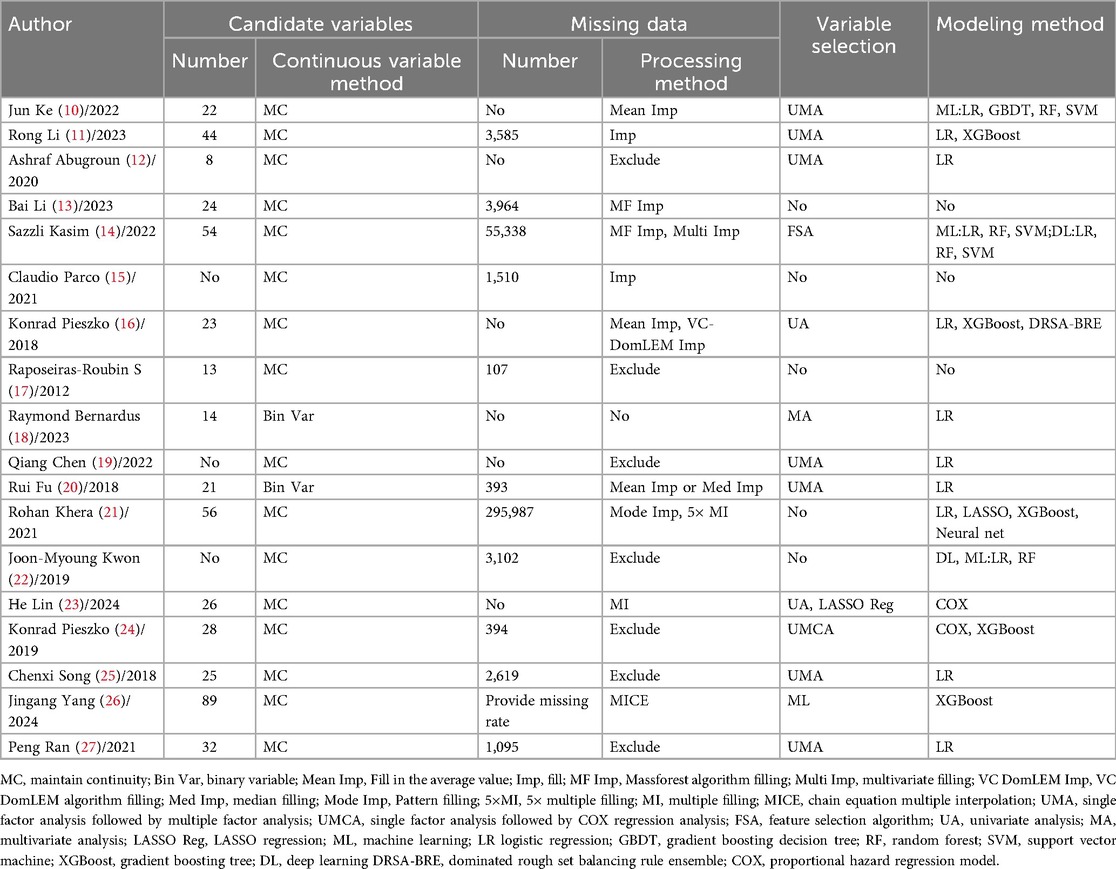
The number of candidate variables varied widely among studies, ranging from 8 to 89. When handling continuous variables, the majority of the studies retained their continuous nature, with only two studies converting continuous variables into binary categories (18, 20). For missing data, common approaches included exclusion and imputation. The variable selection process typically followed a stepwise procedure, starting with univariate analysis and subsequently proceeding to multivariate analysis. In terms of modeling methods, most studies employed traditional regression analysis to construct models, while others integrated machine learning or deep learning techniques. For specifics, please refer to Table 2.
3.4 Characteristics of prediction models
3.4.1 Predictive performance of models
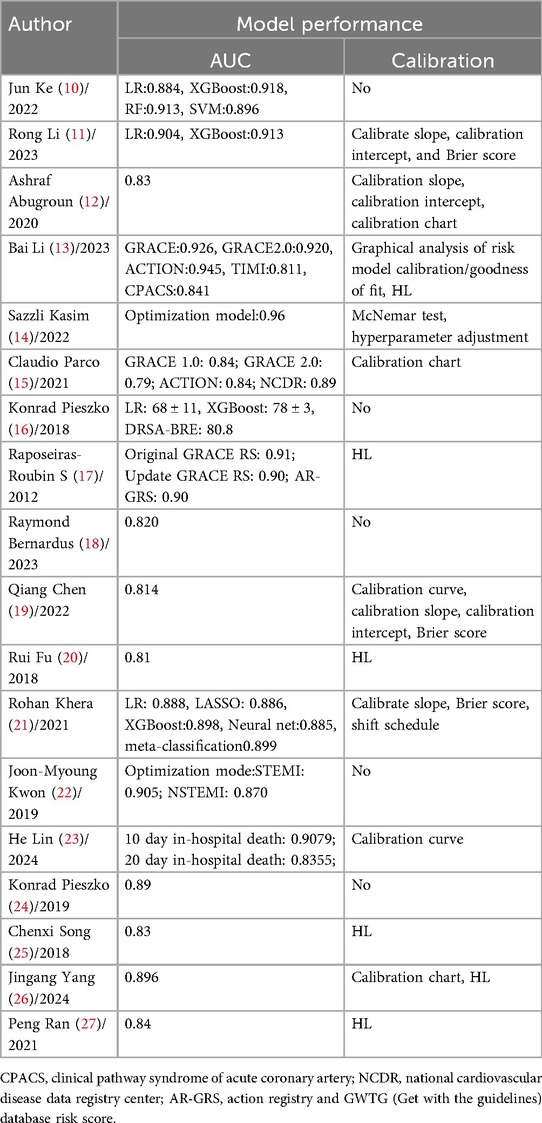
All included studies reported discrimination metrics, including the area under the curve (AUC) or C-index, ranging from 0.79 to 0.96. These values indicate that most prediction models demonstrated at least moderate accuracy and good discriminatory ability. In terms of model calibration, the most commonly used test method was the Hosmer-Lemeshow (HL) goodness-of-fit test, which was employed in six studies (13, 17, 20, 25–27), followed by the calibration slope, used in four studies (11, 12, 19, 21). Additionally, five studies did not provide calibration information (10, 16, 18, 22, 24), while others utilized calibration plots, calibration curves, calibration intercepts, nomograms, or the Brier score either individually or in combination.
The results indicated good calibration performance. For details, please refer to Table 3.
3.4.2 Predictive factors in the models
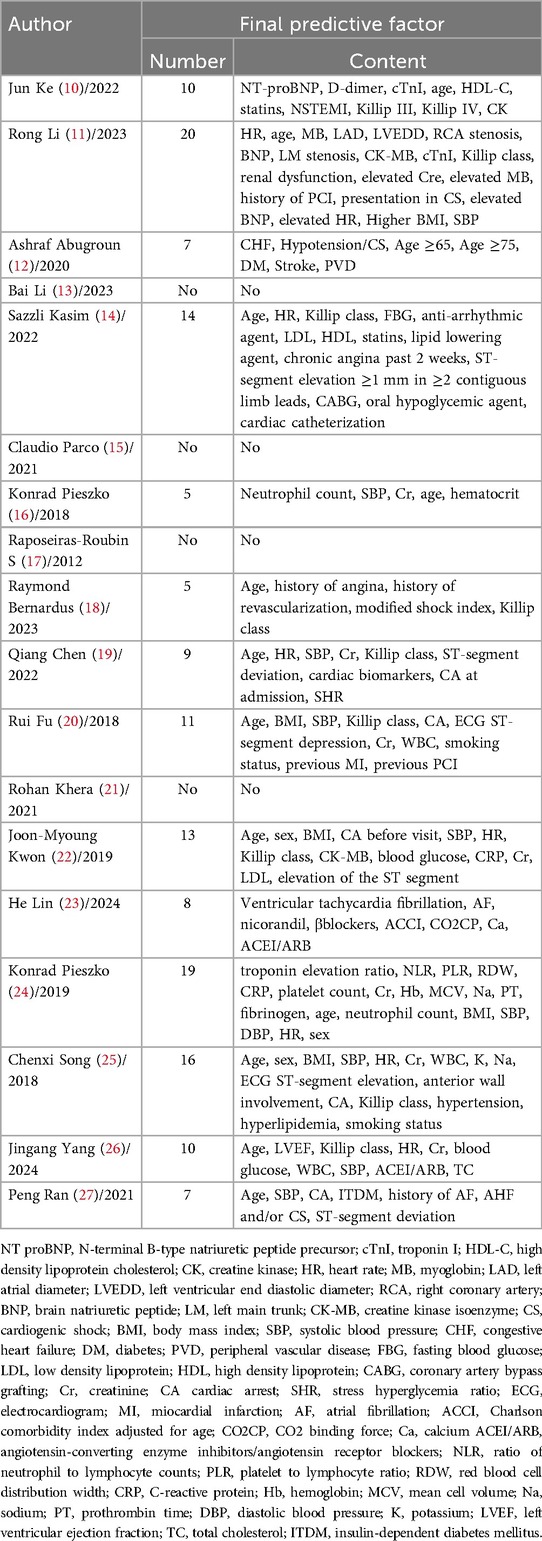
The number of predictive factors included in the models ranged from 5 to 20. However, four studies (13, 15, 17, 21) did not provide detailed information about the final predictive factors included in their models. Among the top nine predictors most frequently included in the models, age (n = 12), systolic blood pressure (n = 9), Killip classification (n = 8), heart rate (n = 7), creatinine (n = 7), body mass index (n = 4), cardiac arrest (n = 3), sex (n = 3), and white blood cell count (n = 3) were the most prominent. For details, please refer to Table 4 and Figure 2.
3.4.3 Model validation and presentation methods
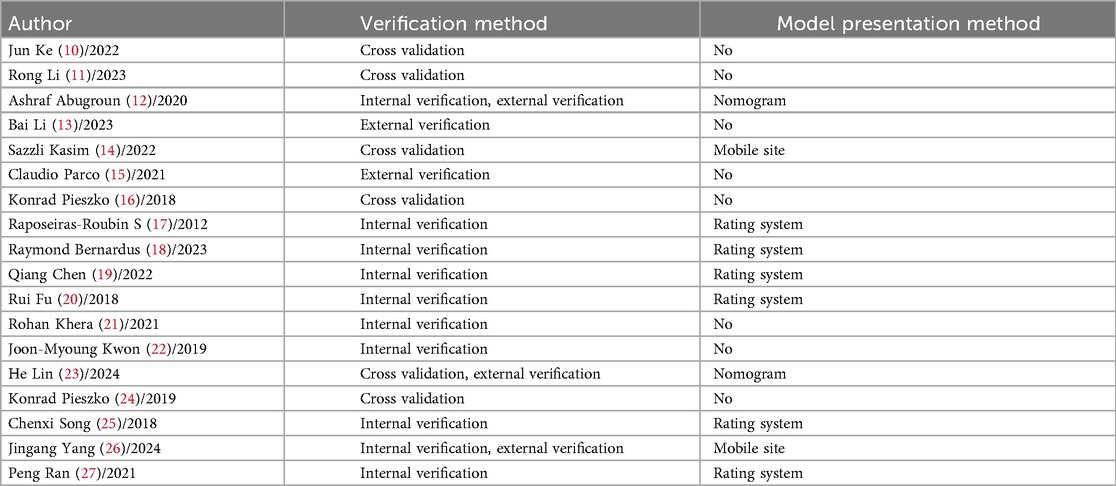
Among the studies validating predictive models, 13 studies (10, 11, 14, 16, 17–22, 24, 25, 27) utilized only internal validation methods, 2 study (15) employed only external validation, and 3 studies (12, 14, 23, 26) combined both internal and external validation. Regarding the presentation methods of the models, 6 studies (17–20, 25, 27) chose to display them through scoring systems, 2 studies (14, 26) opted for mobile websites, 2 studies (12, 23) used nomograms, while the remaining 8 studies (10, 11, 13, 15, 16, 21, 22, 24) did not specify the presentation methods of their models. For details, please refer to Table 5.
3.5 Risk of bias and applicability assessment results
3.5.1 Risk of bias domains
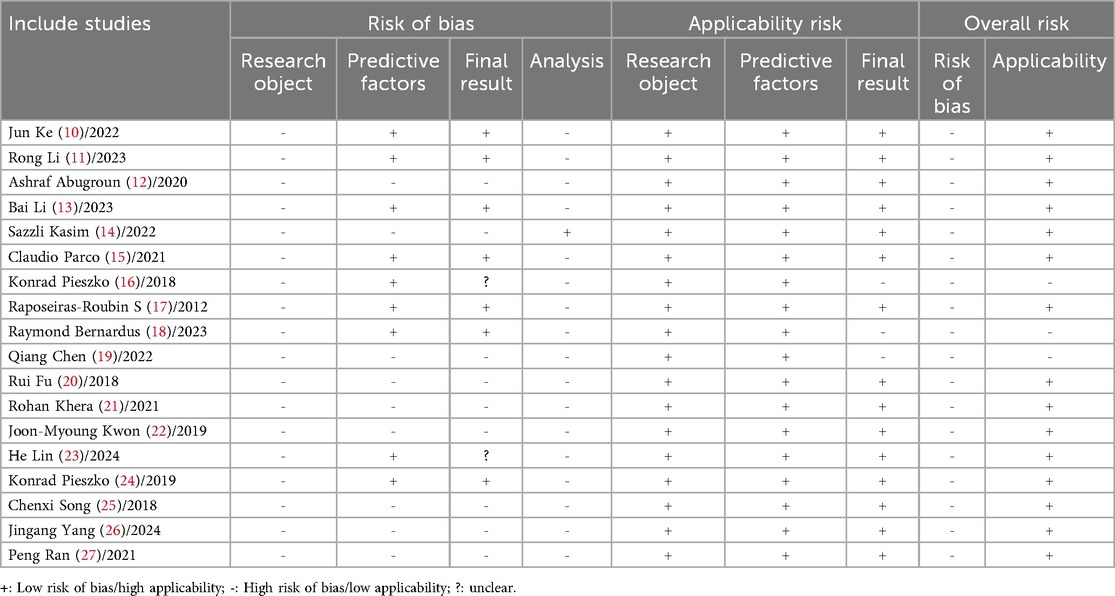
The overall risk of bias was high across all domains. In the participants domain, all studies (10–27) were identified as having a high risk of bias, primarily because the studies relied on retrospective cohort data, which depended on historical records. This led to issues such as missing data, recording errors, or inconsistencies. The selection of participants may not have been representative, and it was challenging to control for all confounding factors, resulting in potential information bias, selection bias, and confounding bias. In the predictors domain, 9 studies (12, 14, 19–22, 25–27) were assessed as having a high risk of bias, mainly due to the lack of uniformity in the definition and measurement of predictors. Data were derived from multi-center studies, where differences in patient characteristics, medical standards, and data collection methods across centers could introduce selection bias and performance bias. In the outcome domain, the same 9 studies (12, 14, 19–22, 25–27) were also considered to have a high risk of bias, primarily because of the multi-center nature of the data sources and inconsistencies in the definition and measurement of outcomes. The lack of standardization and the complexity of statistical analyses increased the likelihood of confounding bias. Two studies (16, 23) were rated as “unclear” because they did not specify the definition of outcomes. In the analysis domain, 1 study (14) was rated as having a low risk of bias, while 17 studies (10–13, 15–27) were rated as having a high risk of bias. Specific issues included: 6 studies (10, 11, 16, 19, 23, 24) had an events per variable (EPV) of <20; 2 studies (18, 20) converted continuous variables into binary categories; 3 studies (24, 26, 27) did not include all participants in the statistical analysis; 7 studies (12, 17, 19, 22, 25, 27) directly excluded missing values; 10 studies (10–12, 16, 19, 20, 23–25, 27) selected predictors based solely on univariate analysis, which was considered inappropriate; 9 studies (10, 16–18, 20, 22, 24, 25, 27) had incomplete evaluation of the models; 14 studies (10, 11, 13, 15–18, 20–25, 27) were overly optimistic in assessing model fit. These inappropriate experimental designs and data processing methods inevitably introduced varying degrees of bias risk. For details, please refer to Table 6.
3.5.2 Applicability assessment
In terms of applicability, 15 studies (10–15, 17, 20–27) demonstrated good overall applicability, while 3 studies (16, 18, 19) showed poor applicability. In the selection of study participants, all studies established inclusion and exclusion criteria that aligned with the principles of this review, demonstrating good applicability. Regarding the selection of predictors, all studies adhered to the inclusion principles of this review, also indicating good applicability. However, in the outcome domain, 3 studies (16, 18, 19) did not report specific definitions of the outcomes, resulting in poor applicability. For details, please refer to Table 6.
3.6 Key risk prediction models
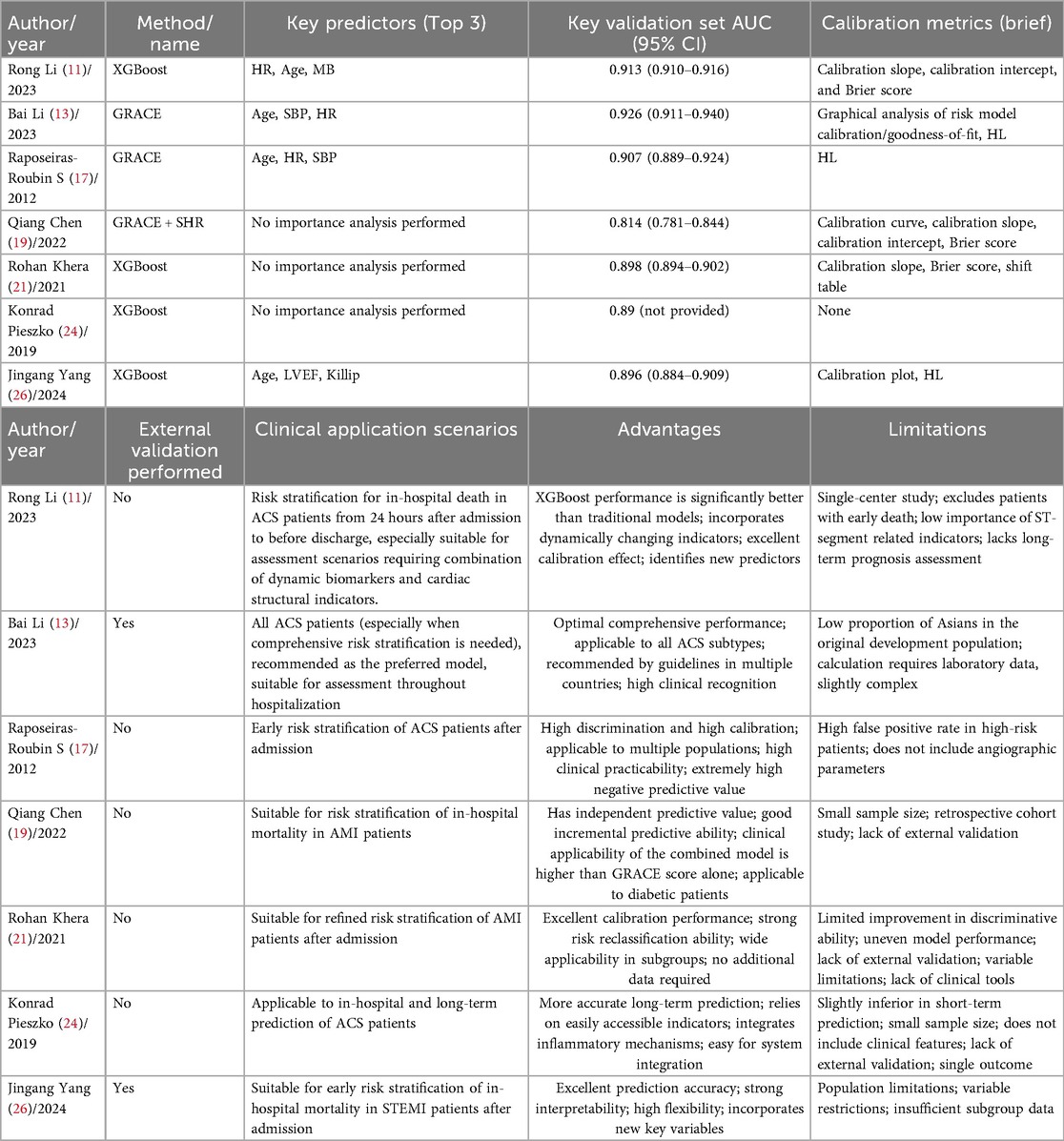
In the field of clinical research, to more accurately grasp disease risks and improve diagnosis and treatment outcomes, it is crucial to sort out and evaluate various clinical risk prediction models. We first select the optimal model from each study by synthesizing factors such as predictive performance, stability, and applicability, and then screen out the most common models based on the frequency of occurrence of model construction methods. This initiative aims to promote direct comparison between different models, provide them with high-quality objects and a clear scope, so as to eliminate interference, enhance the reliability of results, and facilitate clinical decision-making and the development of the field. Details are shown in Table 7.
4 Discussion
4.1 Existing prediction models have clinical significance
Patients with ACS face a relatively high risk of in-hospital death. Constructing an accurate and effective risk prediction system and formulating intervention strategies in advance are of great significance for improving patient prognosis. In-hospital death risk prediction models can identify high-risk populations at an early stage, thus gaining time for clinical intervention. This review included 44 prediction models from 18 studies for analysis. The results showed that their AUC or C-index ranged from 0.79 to 0.96, indicating good discriminative ability and prediction accuracy, which enables accurate identification of patients at high risk of in-hospital death. Moreover, most models are presented in the form of scoring systems, which are easy to operate, understand, and use, meeting the needs of efficient clinical decision-making.
Age, systolic blood pressure, Killip classification, heart rate, and creatinine are frequently included predictors. Multiple studies (28–33) have confirmed that these factors are strongly associated with in-hospital death, serving as the core basis for risk modeling. It is worth noting that the RURUS SURYAWAN score proposed by scholars such as Suryawan IG (34) is designed for patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. By quantifying clinical indicators to construct a scoring system, it achieves the stratification of 30-day death risk. From a practical perspective, it confirms the feasibility of the model construction path of “screening key factors—quantifying and assigning values—risk stratification” and also provides a reference for subgroup scenarios in the overall risk prediction of ACS.
In summary, in clinical practice, it is necessary to rely on existing prediction models, pay attention to the risk factors of in-hospital death, combine model scores with patients’ individual clinical characteristics, conduct dynamic assessments, and intervene in a timely manner to ensure patients’ in-hospital safety.
4.2 Data collection and processing impact prediction model performance
In this review, the in-hospital mortality rate of patients with ACS showed a significant variation (1.88%–12.3%), which is closely associated with inconsistent definitions of outcome indicators: 88% of the studies used “all-cause in-hospital mortality”, while 12% adopted “postoperative in-hospital mortality”. Such inconsistency severely impairs the cross-study comparability of models, leading to a lack of unified reference for prediction results and making it difficult to screen superior models. From the perspective of disease characteristics, ACS-related death is affected by multiple factors; focusing only on “postoperative in-hospital mortality” will miss non-surgical fatal events and fail to reflect the real risk. From the clinical practice perspective, it is unfavorable for evaluating the efficacy of conservative drug treatment and conducting objective comparisons of different treatment strategies. From the research value perspective, “all-cause in-hospital mortality” is more conducive to the promotion of research findings in different medical settings. Therefore, it is recommended that future studies on ACS in-hospital mortality prediction models uniformly take “all-cause in-hospital mortality” as the standardized outcome indicator, so as to facilitate global sharing of medical achievements and promote the advancement of ACS diagnosis and treatment.
All studies included in this review were retrospective cohort studies, involving extensive data collection and long-term follow-up. Such studies inherently face challenges with missing data, contributing to information bias. Additionally, the multicenter nature of the data sources led to inconsistent definitions and measurement standards for predictive factors, further increasing the risk of bias.
During the development of prediction models, the selection of predictive factors might not always be comprehensive, leading to potential information bias. For example, Konrad Pieszko and colleagues (24) utilized hospital electronic medical records for data collection and found that the data in medical records were often incomplete, complex, and disorganized. This introduced potential bias when extracting information for predictive factors. Particularly concerning was the presence of unstructured data stored in physicians’ notes, highlighting the importance of the expertise of the personnel designated to assess predictive factors. The performance of a model could vary significantly depending on whether experienced experts or inexperienced researchers handled this task.
Regarding sample size estimation, six studies (10, 11, 16, 19, 23, 24) did not meet the event per variable (EPV) principle, potentially leading to an overfitting risk in the models. Researchers must ensure a sufficient sample size to maintain model performance while recognizing that an excessively large sample size does not necessarily enhance model accuracy.
In terms of data preprocessing, 7 studies (12, 17, 19, 22, 25, 27) directly excluded missing data. This approach may bias the association between predictors and study outcomes, thereby constructing a biased model. Even if no bias occurs, it will still reduce the sample size and compromise information integrity, further decreasing the model's predictive accuracy. In the clinical data of ACS patients, variables such as laboratory indicators and comorbidities often have certain missing values. Simple exclusion or mean imputation can also lead to reduced sample size or data distortion, while multiple imputation can effectively retain sample information and reduce bias. To minimize the loss of valuable information during model development and evaluation, we should consider adopting advanced imputation techniques (e.g., multiple imputation) to appropriately account for the uncertainty of missing data, reduce bias, and improve model performance.
4.3 Variable selection affects prediction model performance
During the selection of predictive factors, 10 studies (10–12, 16, 19, 20, 23–25, 27) in this review used univariate analysis as the basis for variable selection. This approach might lead to improper selection of predictive factors because it overlooks interactions between variables and potential collinearity issues. When univariate modeling results in the omission of relevant variables, it introduces bias, causing overfitting and reducing the predictive accuracy of the model. Therefore, optimization during model development is crucial.
For instance, in the study by Jun Ke et al. (10), researchers initially performed univariate analysis to select the most appropriate variables for model development. To avoid overfitting and enhance model accuracy, they split the training dataset into a cross-validation scheme and adjusted the hyperparameters of each machine learning model to optimize cross-validation performance. The final model was then developed using the best hyperparameters to fit all training data.
Additionally, when selecting variables, it is essential not only to rely on statistical significance but also to consider potential confounding factors and other independent variables comprehensively. Ashraf Abugoun et al. (12) illustrated this approach while optimizing the modified CHA2DS2-VASc score. In their exploratory study, they found that hypertension and vascular disease had minimal impact on predicting mortality in ACS patients without a history of stroke. Conversely, low blood pressure and shock were associated with the highest mortality, while female gender contributed insignificantly to the model. As a result, they replaced hypertension with low blood pressure and shock, reduced the score for a history of stroke to 1 point, and removed the female gender variable.
Notably, contemporary model development needs to enhance the scientific rigor and transparency of feature selection. Among relevant approaches, penalized regression, cross-validation, and feature selection algorithms represent the best practices for screening predictive variables. High-dimensional data easily leads to model overfitting. LASSO compresses the coefficients of redundant features through L1 regularization, enabling simultaneous modeling and feature screening. Cross-validation dynamically tests the generalization ability of the model, avoiding evaluation bias caused by a single data partition. Feature selection algorithms reduce dimensionality and computational consumption in advance, and can also complement and optimize LASSO. These three approaches form a modeling loop, balancing accuracy, generalization, and interpretability, and serve as the key to addressing complex data. Therefore, we should adopt advanced techniques such as penalized regression methods and automatic feature selection with cross-validation. When screening predictors, we need to balance the correlation of variables and the generalization ability of the model, avoid model bias caused by the limitations of univariate analysis, reduce the risk of overfitting through technical approaches, and improve the rigor of model construction.
In summary, although existing prediction models are clinically instructive, whether for the development and validation of existing models or the reconstruction of new models, the data sources and the selection methods of predictors should be considered before construction. For example, prospective cohort studies with good data representativeness can be used, and predictors can be screened through literature review combined with multivariate analysis.
4.4 Application of artificial intelligence in in-hospital mortality risk prediction models for acute coronary syndrome
Currently, the construction methods for in-hospital death risk prediction models in ACS are relatively singular. Most studies adopt Logistic regression for modeling, while some studies attempt to break through traditional limitations through machine learning and deep learning technologies. For example, the team of Rong Li (11) applied the XGBoost algorithm, which showed higher accuracy than traditional logistic regression in identifying the risk of in-hospital death in ACS patients. Studies have demonstrated that machine learning is efficient and highly adaptive in processing large volumes of data, discovering complex patterns, and achieving accurate predictions (35); on this basis, deep learning can further automatically extract features, solve more complex problems, and realize high-precision prediction and classification (36). After comparing multiple methods, Sazzli Kasim et al. (14) confirmed that the deep learning model (SVM selected var) is more effective in predicting in-hospital mortality of ACS. These achievements fully confirm the great potential of AI technology in the field of risk prediction. However, from the perspective of clinical practice, traditional scoring systems are still applied more frequently in real-world settings.
In this review, multiple studies (13, 16, 17, 19, 25, 27) indicate that the GRACE model still performs excellently in various aspects. It remains a reliable tool for ACS risk prediction in the foreseeable future and is currently the most suitable model for routine clinical use. This score was developed based on large-scale, unbiased multicenter registry data and validated by external datasets, thus showing excellent performance when applied to the general population. However, its prediction accuracy for patients undergoing PCI is suboptimal. Therefore, there is a need for updated risk scores adapted to current clinical practices to supplement the application of existing scoring systems.
Among the 8 studies (10, 11, 14, 16, 21, 22, 24, 26) included in this review, machine learning and deep learning technologies were applied either independently or in combination, and the constructed models showed excellent performance in predictive ability. Among them, only the model constructed by scholars such as Sazzli Kasim (14) has been integrated into routine clinical diagnosis and treatment processes. This model has been deployed on a risk calculator within the hospital's internal network, but the network is not open to the public as the research is still in the testing phase. Other machine learning-based methods have not yet been fully validated in clinical integration, and most remain in the stage of research and small-scale validation. The core obstacles lie in the universality of validation, interpretability, and compatibility with clinical workflows. For interpretability assessment, only 3 studies (11, 14, 26) employed the SHAP value method. The remaining studies merely reported model performance without clarifying the logic underlying predictive outcomes. Clinicians, however, need to understand this logic to trust the model; unexplainable “black-box models” may cause confusion in clinical decision-making, highlighting a severe lack of interpretability. In terms of reproducibility, only 3 studies (14, 24, 26) made model codes or detailed parameter settings publicly available. For the rest, incomplete methodological reporting rendered the model construction process irreproducible. In contrast, regression-based models exhibit significantly higher reproducibility due to their transparent parameters and simple calculation. Regarding clinical integration, only 1 study (14) conducted clinical applicability testing; the remaining studies only achieved performance validation at the data level. Clinical decision-making for ACS requires models to be “fast and convenient,” yet most current AI models fail to meet practical clinical needs, as they are time-consuming for computation and require professional software support. It is thus evident that compared with regression-based models, current AI models in the ACS field have obvious disadvantages in “interpretability, reproducibility, and clinical integration.” The translation of AI technology from research to clinical application still requires addressing key issues.
Firstly, the adaptability to clinical scenarios needs to be clarified. Traditional scoring systems, due to their simplicity of operation and mature clinical application, still have advantages in primary medical institutions or rapid emergency assessment. Although AI models have higher prediction accuracy, their operational complexity and the difficulty in interpreting results may affect clinical acceptance. The SPADAFORA L team (37) included 23,270 ACS patients and found that the impact of in-hospital bleeding (IHB) on 1-year prognosis varies among subgroups such as age, gender, and treatment pathways. This suggests that different models need further comparison in specific scenarios. For example, regarding the precise stratification of complex cases, whether the performance advantages of AI models can cover their application costs still requires more practical verification.
Secondly, the value of clinical intervention needs to be deepened. The SPADAFORA L team (37) also revealed that IHB, as one of the markers of the severity of ACS patients’ condition, suggests that risk prediction models should expand their dimensions, not limited to identifying death risks, but also include indicators such as bleeding risk and prognostic changes after intervention. However, existing AI models mostly remain in the stage of risk stratification and have not fully explored their guiding role in clinical decision-making. For example, whether dynamic risk assessment can be used to adjust the intensity of antithrombotic therapy, optimize monitoring frequency, and whether they can more effectively reduce the incidence of adverse events and improve patients’ long-term prognosis compared with traditional models, these still need in-depth research combined with clinical practice.
In general, artificial intelligence technology has provided new tools for ACS risk prediction and shown great potential. However, the full realization of its clinical value needs to focus on the verification of scenario adaptability and the exploration of intervention pathways, focusing on the closed-loop verification of “model-clinical scenario-patient outcome”. Through more real-world studies, the technology can be promoted from “accurate prediction” to “clinical practicality”, ultimately achieving effective supplementation and optimization of traditional models.
4.5 The applicability of prediction models requires further validation
Model validation is a critical step in assessing the performance and generalizability of prediction models, involving both internal and external validation. Internal validation estimates model performance by training and evaluating the model on the same dataset, which helps identify whether the model is overfitting or underfitting. In contrast, external validation evaluates the model on an independent dataset to assess its generalizability and extrapolation capability.
In this review, 13 studies (10, 11, 14, 16, 17–22, 24, 25, 27) conducted only internal validation, while 2 study (13, 15) performed external validation exclusively. Therefore, 72% of ACS in-hospital mortality prediction models may be overfitted due to the lack of external validation, resulting in insufficient clinical generalization. Unless a model undergoes external validation across multiple centers and diverse populations (e.g., cross-regional and cross-ethnic cohorts), it is not recommended for direct use in clinical decision-making; further validation is still required to support its clinical application. Many researchers acknowledged the limitation of lacking external validation and expressed concerns about the model's applicability to different regional populations.
For example, in the study by Peng Ran et al. (27), although the model was developed using a large dataset, it was limited to Chinese patients. The authors highlighted the need for further research to verify the model's performance in other populations and emphasized the necessity of external validation before widespread clinical adoption.
Overall, most studies on in-hospital mortality risk prediction models for ACS are single-center studies, lacking consideration for differences in applicability across diverse cultural and geographical environments.
Therefore, when constructing in-hospital mortality risk prediction models for acute coronary syndrome, researchers should integrate both internal and external validation. Techniques such as cross-validation, bootstrap resampling, and the “internal-external” approach can be employed for internal validation, while temporal validation, spatial validation, and domain validation methods can be utilized for external validation. Additionally, conducting multi-center studies can significantly enhance the generalizability of the prediction models.
4.6 The presentation and reporting of prediction models need further standardization
The reporting of prediction model results should adhere to the Transparent Reporting of a Multivariate Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement, ensuring that the report includes a complete model equation to enable reproducibility and independent external validation studies. Unfortunately, all studies included in this review lack transparency in their construction processes, with information gaps that affect the quality assessment of the literature. When evaluating prediction models, model calibration is a core indicator for measuring model reliability, whose importance is equivalent to or even greater than discriminative ability in clinical practice. Therefore, in addition to these two core indicators, comprehensive evaluation should be conducted from multiple aspects such as overall performance, reclassification, and clinical utility to improve the assessment of model performance. For example, consideration should be given to indicators including the model's sensitivity, specificity, accuracy, as well as the Hosmer-Leme show test and calibration curve that directly reflect calibration performance, while combining metrics like Decision Curve Analysis (DCA) and clinical impact curve. Notably, although DCA is highly valuable for evaluating the clinical utility of clinical prediction models, its adoption in practical research remains low. Three reasons account for this: first, traditional studies focus more on model predictive accuracy and insufficiently emphasize “clinical utility,” with inertial thinking leading to DCA being overlooked; second, DCA is not a universal indicator—it only applies to models for which “interventions are needed after outcome prediction,” resulting in limited application scope; third, compared with easily calculable indicators such as AUC and calibration curves, DCA is more complex to operate, requiring higher data standards and relying on professional programming software, which raises the threshold for researchers to use it.
Furthermore, there are challenges in applying machine learning and deep learning to model construction while adhering to TRIPOD. Since machine learning and deep learning models are often regarded as “black boxes,” their internal decision-making processes and interpretability of feature impacts are poor, which contradicts the transparency required by the TRIPOD statement. The TRIPOD statement does not provide detailed reporting guidelines for feature selection of input variables and feature engineering that improves and transforms raw data, leading to deficiencies in reporting—particularly the potential neglect of systematic assessment and reporting of calibration, which is a critical prerequisite for the application of models in clinical decision-making. When applying machine learning or deep learning algorithms, researchers should select appropriate visualization and interpretation tools to demonstrate the impact of each variable on outcomes, while ensuring the practical significance of model-predicted probabilities through rigorous calibration validation. For instance, when constructing a deep learning model, Rui Fu (20) and colleagues, despite being able to fit the model through individual weights, still found it difficult to interpret the deep learning model using methods such as variable importance or risk score-based decision-making. This highlights the need for further exploration in the field of interpretable deep learning, with optimization and validation of calibration as one of its core objectives. When using machine learning models, Jingang Yang (26) and colleagues utilized SHAP (SHapley Additive exPlanations) to explain how the predicted risk for individual patients is determined, revealed the complex relationships between predictors and outcomes embedded in the XGBoost model, and combined this with calibration assessment—greatly enhancing the clinical credibility of the model.
Therefore, when constructing risk prediction models, scholars should strictly follow the requirements of the TRIPOD document, attach importance to and standardize the assessment and reporting of model calibration, comprehensively improve the transparency of the construction process, and further optimize the application of artificial intelligence technology in prediction model construction.
5 Limitations of the study
This systematic review has several limitations: (1) The review included only Chinese and English literature and searched only five databases, potentially leading to literature omissions. (2) The included studies were predominantly conducted in Chinese regions, which may limit the generalizability of the findings to Western countries and other diverse populations. (3) This study only included Chinese and English literature, which may introduce language bias. Additionally, 50% of the study populations were Chinese, leading to an overrepresentation of models developed for the Chinese population and insufficient coverage of models for other regions. Consequently, the conclusions have low applicability to non-Chinese populations.
6 Conclusion
The construction of in-hospital mortality risk prediction models for acute coronary syndrome (ACS) is currently in a phase of rapid development. While many models demonstrate good predictive ability, there remain significant gaps in data analysis and processing methods. Many studies did not adhere to the TRIPOD reporting guidelines, lacked external validation, and were predominantly single-center studies, resulting in a high overall risk of bias and limited generalizability.
Looking forward, the development of ACS in-hospital mortality risk prediction models should follow the PROBAST standards to create models with strong predictive performance and broad applicability. Rigorous adherence to reporting and validation protocols will enhance the clinical utility and reliability of these models.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RJ: Data curation, Formal analysis, Investigation, Writing – original draft. JZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. YZ: Formal analysis, Investigation, Writing – original draft. TZ: Conceptualization, Data curation, Investigation, Writing – original draft. YW: Formal analysis, Supervision, Writing – review & editing. LW: Investigation, Supervision, Writing – review & editing. YY: Formal analysis, Project administration, Supervision, Validation, Writing – review & editing. CX: Formal analysis, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China Youth Science Foundation Project (82105049) and General Project of the University-College Joint Innovation Fund of Chengdu University of Traditional Chinese Medicine: Clinical-Basic (LH202402019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. (2022) 327(7):662–75. doi: 10.1001/jama.2022.0358
2. Ayayo SA, Kontopantelis E, Martin GP, Zghebi SS, Taxiarchi VP, Mamas MA. Temporal trends of in-hospital mortality and its determinants following percutaneous coronary intervention in patients with acute coronary syndrome in England and Wales: a population-based study between 2006 and 2021. Int J Cardiol. (2024) 412:132334. doi: 10.1016/j.ijcard.2024.132334
3. Zhang X, Wang X, Xu L, Liu J, Ren P, Wu H. The predictive value of machine learning for mortality risk in patients with acute coronary syndromes: a systematic review and meta-analysis. Eur J Med Res. (2023) 28(1):451. doi: 10.1186/s40001-023-01027-4
4. Nichol G, West A. Contemporary approaches to reducing morbidity and mortality in patients with acute coronary syndromes. J Am Coll Cardiol. (2022) 80(19):1799–801. doi: 10.1016/j.jacc.2022.09.009
5. Roffi M, Radovanovic D, Iglesias JF, Eberli FR, Urban P, Pedrazzini GB, et al. Multisite vascular disease in acute coronary syndromes: increased in-hospital mortality and no improvement over time. Eur Heart J Acute Cardiovasc Care. (2020) 9(7):748–57. doi: 10.1177/2048872618814708
6. El Amrawy AM, Abd El Salam SFED, Ayad SW, Sobhy MA, Awad AM. QTc interval prolongation impact on in-hospital mortality in acute coronary syndromes patients using artificial intelligence and machine learning. Egyptian Heart Journal. (2024) 76(1):149. doi: 10.1186/s43044-024-00581-4
7. Yu T, Tian C, Song J, He D, Sun Z, Sun Z. ACTION (acute coronary treatment and intervention outcomes network) registry-GWTG (get with the guidelines) risk score predicts long-term mortality in acute myocardial infarction. Oncotarget. (2017) 8(60):102559–72. doi: 10.18632/oncotarget.21741
8. Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. (2014) 11(10):e1001744. doi: 10.1371/journal.pmed.1001744
9. Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. (2019) 170(1):W1–W33. doi: 10.7326/M18-1377
10. Ke J, Chen Y, Wang X, Wu Z, Zhang Q, Lian Y, et al. Machine learning-based in-hospital mortality prediction models for patients with acute coronary syndrome. Am J Emerg Med. (2022) 53:127–34. doi: 10.1016/j.ajem.2021.12.070
11. Li R, Shen L, Ma W, Yan B, Chen W, Zhu J, et al. Use of machine learning models to predict in-hospital mortality in patients with acute coronary syndrome. Clin Cardiol. (2023) 46(2):184–94. doi: 10.1002/clc.23957
12. Abugroun A, Hassan A, Gaznabi S, Ayinde H, Subahi A, Samee M, et al. Modified CHA2DS2-VASc score predicts in-hospital mortality and procedural complications in acute coronary syndrome treated with percutaneous coronary intervention. Int J Cardiol Heart Vasc. (2020) 28:100532. doi: 10.1016/j.ijcha.2020.100532
13. Bai L, Li YM, Yang BS, Cheng YH, Zhang YK, Liao GZ, et al. Performance of the risk scores for predicting in-hospital mortality in patients with acute coronary syndrome in a Chinese cohort. Rev Cardiovasc Med. (2023) 24(12):356. doi: 10.31083/j.rcm2412356
14. Kasim S, Malek S, Song C, Wan Ahmad WA, Fong A, Ibrahim KS, et al. In-hospital mortality risk stratification of Asian ACS patients with artificial intelligence algorithm. PLoS One. (2022) 17(12):e0278944. doi: 10.1371/journal.pone.0278944
15. Parco C, Brockmeyer M, Kosejian L, Quade J, Tröstler J, Bader S, et al. Modern NCDR and ACTION risk models outperform the GRACE model for prediction of in-hospital mortality in acute coronary syndrome in a German cohort. Int J Cardiol. (2021) 329:28–35. doi: 10.1016/j.ijcard.2020.12.085
16. Pieszko K, Hiczkiewicz J, Budzianowski P, Rzezniczak J, Budzianowski J, Blaszczynski J, et al. Machine-learned models using hematological inflammation markers in the prediction of short-term acute coronary syndrome outcomes. J Transl Med. (2018) 16(1):334. doi: 10.1186/s12967-018-1702-5
17. Raposeiras-Roubín S, Abu-Assi E, Cabanas-Grandío P, Agra-Bermejo RM, Gestal-Romarí S, Pereira-López E, et al. Walking beyond the GRACE (global registry of acute coronary events) model in the death risk stratification during hospitalization in patients with acute coronary syndrome: what do the AR-G (ACTION [acute coronary treatment and intervention outcomes network] registry and GWTG [get with the guidelines] database), NCDR (national cardiovascular data registry), and EuroHeart risk scores provide? JACC Cardiovasc Interv. (2012) 5(11):1117–25. doi: 10.1016/j.jcin.2012.06.023
18. Bernardus R, Pramudyo M, Akbar MR. A revised PADMA scoring system for predicting in-hospital mortality in acute coronary syndrome patient. Int J Gen Med. (2023) 16:3747–56. doi: 10.2147/IJGM.S421913
19. Chen Q, Su H, Yu X, Chen Y, Ding X, Xiong B, et al. The stress hyperglycemia ratio improves the predictive ability of the GRACE score for in-hospital mortality in patients with acute myocardial infarction. Hellenic J Cardiol. (2023) 70:36–45. doi: 10.1016/j.hjc.2022.12.012
20. Fu R, Song C, Yang J, Wang Y, Li B, Xu H, et al. CAMI-NSTEMI Score - China acute myocardial infarction registry-derived novel tool to predict in-hospital death in non-ST segment elevation myocardial infarction patients. Circ J. (2018) 82(7):1884–91. doi: 10.1253/circj.CJ-17-1078
21. Khera R, Haimovich J, Hurley NC, McNamara R, Spertus JA, Desai N, et al. Use of machine learning models to predict death after acute myocardial infarction. JAMA Cardiol. (2021) 6(6):633–41. doi: 10.1001/jamacardio.2021.0122
22. Kwon JM, Jeon KH, Kim HM, Kim MJ, Lim S, Kim KH, et al. Deep-learning-based risk stratification for mortality of patients with acute myocardial infarction. PLoS One. (2019) 14(10):e0224502. doi: 10.1371/journal.pone.0224502
23. Lin H, Xi YB, Yang ZC, Tong ZJ, Jiang G, Gao J, et al. Optimizing prediction of in-hospital mortality in elderly patients with acute myocardial infarction: a nomogram approach using the age-adjusted charlson comorbidity Index score. J Am Heart Assoc. (2024) 13(14):e032589. doi: 10.1161/JAHA.123.032589
24. Pieszko K, Hiczkiewicz J, Budzianowski P, Budzianowski J, Rzezniczak J, Pieszko K, et al. Predicting long-term mortality after acute coronary syndrome using machine learning techniques and hematological markers. Dis Markers. (2019) 2019:9056402. doi: 10.1155/2019/9056402
25. Song C, Fu R, Dou K, Yang J, Xu H, Gao X, et al. The CAMI-score: a novel tool derived from CAMI registry to predict in-hospital death among acute myocardial infarction patients. Sci Rep. (2018) 8(1):9082. doi: 10.1038/s41598-018-26861-z
26. Yang J, Li Y, Li X, Tao S, Zhang Y, Chen T, et al. A machine learning model for predicting in-hospital mortality in Chinese patients with ST-segment elevation myocardial infarction: findings from the China myocardial infarction registry. J Med Internet Res. (2024) 26:e50067. doi: 10.2196/50067
27. Ran P, Yang JQ, Li J, Li G, Wang Y, Qiu J, et al. A risk score to predict in-hospital mortality in patients with acute coronary syndrome at early medical contact: results from the improving care for cardiovascular disease in China-acute coronary syndrome (CCC-ACS) project. Ann Transl Med. (2021) 9(2):167. doi: 10.21037/atm-21-31
28. Gashi MM, Gashi GBSL, Ahmeti HR, Degoricija V. Correlates of in-hospital mortality in patients with acute coronary syndrome in Kosovo. Acta Clin Croat. (2022) 61(1):19–29. doi: 10.20471/acc.2022.61.01.03
29. Pramudyo M, Yahya AF, Martanto E, Tiksnadi BB, Karwiky G, Rafidhinar R, et al. Predictors of in-hospital mortality in patients with acute coronary syndrome in Hasan Sadikin hospital, Bandung, Indonesia: a retrospective cohort study. Acta Med Indones. (2022) 54(3):379–88.36156467
30. Shariefuddin WWA, Pramudyo M, Martha JW. Shock index creatinine: a new predictor of mortality in acute coronary syndrome patients. BMC Cardiovasc Disord. (2024) 24(1):87. doi: 10.1186/s12872-024-03730-4
31. Jensen MT, Pereira M, Araujo C, Malmivaara A, Ferrieres J, Degano IR, et al. Heart rate at admission is a predictor of in-hospital mortality in patients with acute coronary syndromes: results from 58 European hospitals: the European hospital benchmarking by outcomes in acute coronary syndrome processes study. Eur Heart J Acute Cardiovasc Care. (2018) 7(2):149–57. doi: 10.1177/2048872616672077
32. Ahmed OE, Abohamr SI, Alharbi SA, Aldrewesh DA, Allihimy AS, Alkuraydis SA, et al. In-hospital mortality of acute coronary syndrome in elderly patients. Saudi Med J. (2019) 40(10):1003–7. doi: 10.15537/smj.2019.10.24583
33. Garadah TS, Thani KB, Sulibech L, Jaradat AA, Al Alawi ME, Amin H. Risk stratification and in hospital morality in patients presenting with acute coronary syndrome (ACS) in Bahrain. Open Cardiovasc Med J. (2018) 12:7–17. doi: 10.2174/1874192401812010007
34. Suryawan IGR, Oktaviono YH, Dharmadjati BB, Hernugrahanto AP, Alsagaff MY, Nugraha D, et al. RURUS SURYAWAN score: a novel scoring system to predict 30-day mortality for acute myocardial infarction undergoing primary percutaneous coronary intervention. J Clin Med. (2025) 14(5):1716. doi: 10.3390/jcm14051716
35. Martin-Morales A, Yamamoto M, Inoue M, Vu T, Dawadi R, Araki M. Predicting cardiovascular disease mortality: leveraging machine learning for comprehensive assessment of health and nutrition variables. Nutrients. (2023) 15(18):3937. doi: 10.3390/nu15183937
36. Bhagawati M, Paul S, Agarwal S, Protogeron A, Sfikakis PP, Kitas GD, et al. Cardiovascular disease/stroke risk stratification in deep learning framework: a review. Cardiovasc Diagn Ther. (2023) 13(3):557–98. doi: 10.21037/cdt-22-438
Keywords: models, acute coronary syndrome, in-hospital mortality, risk prediction, systematic review
Citation: Jian R, Zhang J, Zeng Y, Zhou T, Wu Y, Wu L, Yu Y and Xi C (2025) In-hospital mortality risk prediction models for patients with acute coronary syndrome: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1659184. doi: 10.3389/fcvm.2025.1659184
Received: 3 July 2025; Accepted: 16 September 2025;
Published: 31 October 2025.
Edited by:
Nicola Mumoli, ASST Valle Olona, ItalyReviewed by:
Nicola Pierucci, Sapienza University of Rome, ItalyRicardo Adrian Nugraha, Universitas Airlangga, Indonesia
Bodhayan Prasad, University of Glasgow, United Kingdom
Copyright: © 2025 Jian, Zhang, Zeng, Zhou, Wu, Wu, Yu and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Yu, eXV5YW5nQGNkdXRjbS5lZHUuY24=; Chongcheng Xi, eGljaG9uZ2NoZW5nQGJ1Y20uZWR1LmNu
 Rui Jian1
Rui Jian1 Jie Zhang
Jie Zhang Yuxiu Zeng
Yuxiu Zeng Chongcheng Xi
Chongcheng Xi