Abstract
Objective:
To identify the risk factors of prolonged mechanical ventilation after acute type A aortic dissection (ATAAD) surgery.
Methods:
466 patients undergoing ATAAD surgery from 2016.01 to 2021.01were studied retrospectively. Risk factors of delayed extubation (>48 h of ventilation) were identified using univariate and multivariate logistic regression. LASSO regression was used for variable selection. The ICU stay, hospital stay, complications, and 30-day mortality were analyzed.
Results:
Among the patients, 72% experienced delayed extubation. Risk factors included age (OR 1.042, 95% CI 1.005–1.083, P = 0.031), postoperative serum creatinine (Scr, OR 1.006, 95% CI 1.003–1.010, P < 0.001), and acute lung injury (ALI, OR 5.725, 95% CI 2.777–12.148, P < 0.001). Delayed extubation was associated with increased ICU stays, hospital stays, and complications, including MODS and higher mortality.
Conclusions:
Age, postoperative serum creatinine, and acute lung injury are significant risk factors for prolonged ventilation. Early identification and management of these factors may improve postoperative outcomes.
Introduction
Acute type A aortic dissection (ATAAD) is a life-threatening and urgent condition affecting the large blood vessels, which can have severe consequences if the dissection leads to rupture (1). Aortic dissection happens when damage to the aorta's inner layer leads to blood flowing between the aortic wall layers, causing them to separate (2). Aortic dissection can be fatal because it causes insufficient blood flow and the possibility of aorta or heart rupture. Emergency surgery is required for Stanford type A dissection, which progresses quickly and has a poor prognosis. Due to the complexity of aortic dissection, even if surgery allows the patient to survive temporarily, the occurrence of some later complications can still pose a life-threatening risk to the patient, such as acute kidney injury, lung injury, and hypoxemia (3). Following cardiac surgery, mechanical ventilation is commonly applied as a postoperative measure to decrease both the myocardial oxygen consumption and the workload associated with spontaneous ventilation. Due to factors such as varying degrees of lung dysfunction (4, 5), gas exchange abnormalities, poor pulmonary mechanics and extracorporeal circulation (6), patients with ATAAD are prone to postoperative hypoxemia and pulmonary complications, which affects the duration of mechanical ventilation (7, 8). Prolonged ventilator support due to respiratory failure is a frequent and serious complication in patients undergoing ATAAD which also accounts for a significant number of in-hospital deaths (9). Therefore, identifying, preventing, and managing the duration of mechanical ventilation in ATAAD patients is crucial.
Methods
Study population
The consecutive patients who underwent ATAAD from January 2016 to December 2021 were studied retrospectively in a single center. The Patients meet the following criteria: TAAD confirmed by aortic enhanced CT who underwent surgical treatment, onset of disease within 14 days, and age above 18 years. Patients who had traumatic aortic dissection, experienced aoritc dissection, experienced aortic dissection during pregnancy, onset of acute type A aortic dissection (ATAAD) beyond 14 days prior to admission, experienced fatalities during the surgery or were under 18 years of age, were excluded from the study.
This study adhered to the ethical principles outlined in the Declaration of Helsinki and received approval from the Ethics Committee of Guangdong Provincial People's Hospital (KY2023- 187-01). Given the observational and retrospective nature of the study, the requirement for informed consent was waived by the Ethics Committee of Guangdong Provincial People's Hospital (KY2023- 187-01).
Baseline data were collected, which included demographics, comorbidities, preoperative laboratory values, and surgical procedure documentation. The demographic information included age, gender, body mass index (BMI), history of smoking and drinking; comorbidities including atria fibrillation, hypertension, hyperlipidemia, diabetes mellitus, chronic kidney disease, COPD; laboratory data contained RBC, WBC, PLT, Ddimer, glucose, AST, ALT, LDL, Tirglyceride and Scr.
Data definition
Delayed extubation was defined as the total duration of mechanical ventilation exceeding 48 h after surgery. However, there is no universally accepted definition for delayed extubation after ATAAD surgery (7, 10). Hence, we adopted this particular definition based on previous studies and our institutional expertise. The secondary endpoints encompassed duration of ICU and hospital stays, various complications and mortality. Early mortality was categorized as deaths that occurred within 30 days following the hospitalization period subsequent to a surgical procedure. Stroke was charaterized as a prolonged central neurological deficit lasting beyond 72 h. The term “acute hepatic injury” was characterized by a notable increase in alanine aminotransferase levels surpassing tenfold the upper boundary of the standard range. Renal failure is defined as either the initiation of dialysis or a rise in serum creatinine levels to over 2.0 mg/dL, accompanied by a twofold increase compared to the most recent preoperative creatinine level. Multiorgan dysfunction syndrome (MODS) was characterized by the impairment of a minimum of two organ systems.
Surgical procedure
The procedures were performed using a median sternotomy approach and total cardiopulmonary bypass (CPB) with antegrade selective cerebral perfusion (SCP). Cannulation of the right axillary artery and femoral artery was utilized for CPB. During the cooling phase, the ascending aorta was clamped, followed by a longitudinal opening of the proximal ascending aorta. Antegrade perfusion of cold blood cardioplegic solution was directly infused into the coronary ostia during this period. Aortic root procedures were performed concurrently in this cooling phase. Circulatory arrest was initiated at a nasopharyngeal temperature of 20–25.8 degrees Celsius. Unilateral selective cerebral perfusion (SCP) was established via the right axillary artery following crossclamping of the brachiocephalic arteries. Crossclamping was also performed on the left carotid and left subclavian arteries. Rewarming started after completion of surgical operation.
Statistical analysis
The statistical analysis in this study utilized SPSS software (version 26.0) and R software (version 4.0.3). Descriptive statistics were conducted to analyze both categorical and continuous variables. Categorical variables were reported as n (%) while continuous variables were reported as mean ± standard deviation or median (interquartile range). The Chisquare or Fisher exact test was used to compare categorical variables, the Mann–Whitney U test was used for non-normal variables, and the Student's T test was used for normally distributed variables.
We utilized clinical data to analyze the risk factors of delayed extubation in patients following ATAAD administration. Univariate analysis was performed to identify variables that could potentially be associated with delayed extubation in the cohort. We employed the least absolute shrinkage and selection operator (LASSO) method to identify the most informative predictive features from the primary dataset. This method is particularly useful for dimensionality reduction in high-dimensional data. By utilizing a LASSO binary logistic regression model, we were able to narrow down the pool of clinicopathologic variables in the cohort to a subset of potential predictors. When the penalization coefficient lambda (λ) is high, the estimated regression parameters remain unaffected; however, as the coefficients decrease, certain coefficients may shrink to zero. To determine the optimal λ in the LASSO model, we performed 10-fold cross-validation using minimum criteria and applied the one standard error of the minimum criteria (the 1-SE criterion). Variables were included in the multivariate logistic regression analysis.
Results
Baseline characteristics
During a 5-year period in our institution, a total of 715 patients underwent type A acute aortic dissection repair surgeries. However, we excluded 249 patients, leaving us with 466 patients for the analysis (Figure 1). Out of the 466 patients included in the analysis, delayed extubation occurred in 336 patients (72%) after repair of acute type A aortic dissection. The average age of the entire cohort was 51.69 years, with males comprising 83.69% of the subjects. The average body mass index was 24.91 kg/m2, and 16.38% of the patients had a history of smoking. Furthermore, variables such as hypertension was observed in over 50% of the study population. Diabetes mellitus, chronic kidney disease, smoking and COPD were observed in less than 20% of the study population (Table 1).
Figure 1
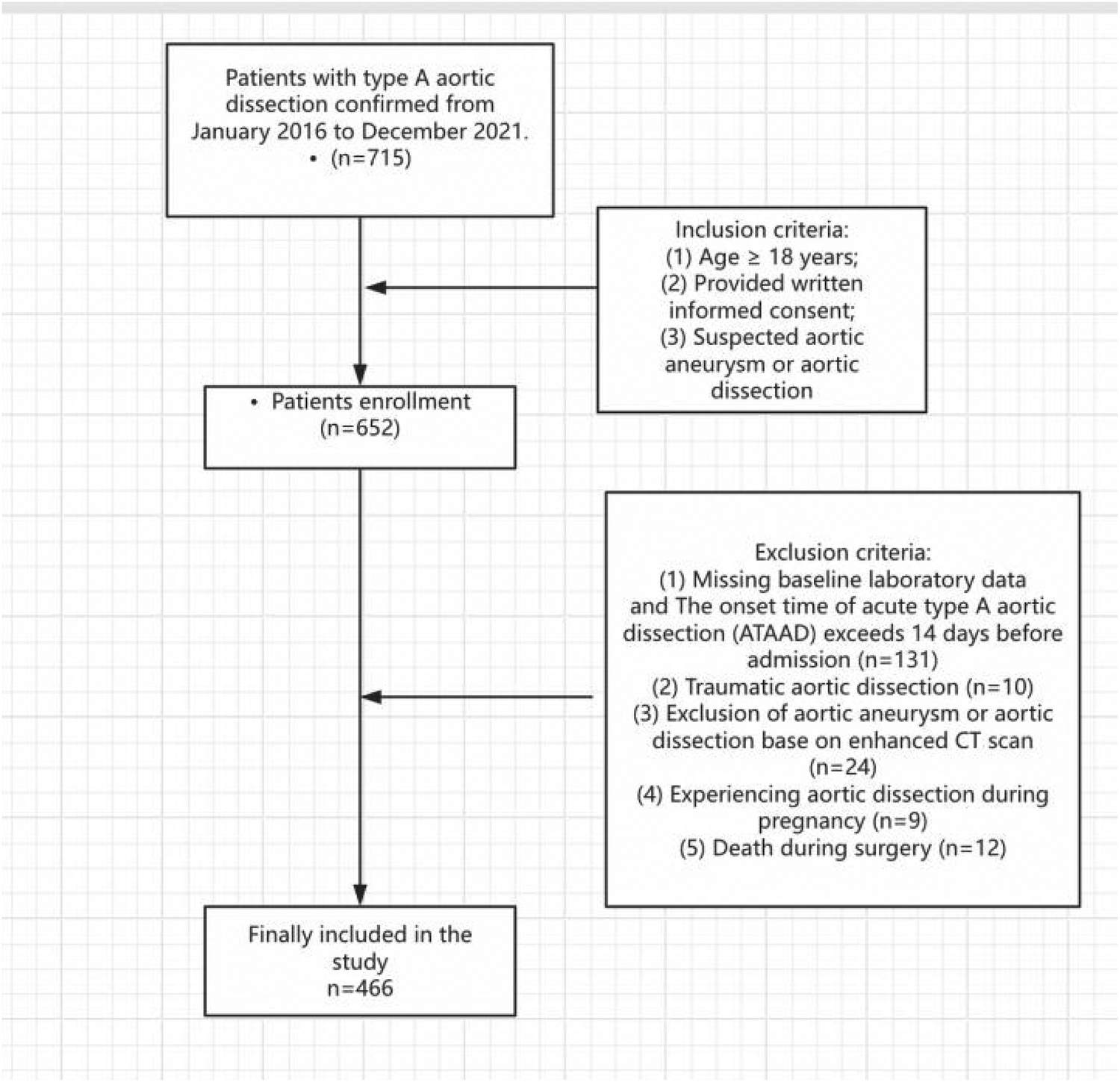
Flow diagram of patient inclusion and exclusion according to STROBE guidelines.
Table 1
| n | All patients(n = 466) | No delayed extubation (n = 130) | Delayed extubation (n = 336) | p |
|---|---|---|---|---|
| Age, mean(±SD) | 51.689 ± 10.535 | 49.131 ± 10.723 | 52.679 ± 10.291 | 0.00 |
| BMI, mean(±SD) | 24.914 ± 3.846 | 24.383 ± 3.873 | 25.156 ± 3.809 | 0.15 |
| Male, n (%) | 390 (83.691) | 109 (83.846) | 281 (83.631) | 0.96 |
| Medical history | ||||
| Atria_fibrillation, n (%) | 11 (2.439) | 5 (3.937) | 6 (1.852) | 0.20 |
| Hypertension, n (%) | 303 (65.161) | 76 (58.462) | 227 (67.761) | 0.06 |
| Hyperlipidemia, n (%) | 205 (44.469) | 65 (50.388) | 140 (42.169) | 0.11 |
| Diabetes mellitus, n (%) | 17 (3.656) | 2 (1.538) | 15 (4.478) | 0.13 |
| Chronic kidney disease, n (%) | 19 (4.086) | 4 (3.077) | 15 (4.478) | 0.49 |
| Smoking, n (%) | 76 (16.379) | 24 (18.462) | 52 (15.569) | 0.45 |
| COPD, n (%) | 53 (11.422) | 18 (13.953) | 35 (10.448) | 0.29 |
| Preoperative laboratory data | ||||
| RBC, mean (±SD) | 143.557 ± 82.659 | 147.862 ± 81.793 | 141.887 ± 82.932 | 0.49 |
| WBC, mean (±SD) | 194.548 ± 118.016 | 190.392 ± 117.897 | 196.161 ± 118.022 | 0.64 |
| PLT, mean (±SD) | 150.183 ± 88.079 | 154.100 ± 82.157 | 148.663 ± 90.227 | 0.55 |
| Ddimer, mean (±SD) | 103.099 ± 82.115 | 115.069 ± 79.379 | 98.454 ± 82.686 | 0.05 |
| Glucose, mean (±SD) | 180.194 ± 104.778 | 175.354 ± 102.983 | 182.072 ± 105.406 | 0.54 |
| AST, mean (±SD) | 73.908 ± 37.630 | 73.077 ± 38.298 | 74.230 ± 37.362 | 0.77 |
| ALT, mean (±SD) | 63.170 ± 31.249 | 58.462 ± 31.006 | 64.997 ± 31.152 | 0.04 |
| LDL, mean (±SD) | 65.584 ± 22.335 | 65.760 ± 25.542 | 65.515 ± 20.948 | 0.92 |
| Tirglyceride, mean (±SD) | 57.148 ± 20.186 | 58.674 ± 22.086 | 56.552 ± 19.360 | 0.31 |
| Scrpre, mean (±SD) | 216.699 ± 126.065 | 213.569 ± 128.216 | 217.913 ± 125.199 | 0.74 |
Preoperative data.
RBC, red blood cell; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLT, platelets; Scr, serum creatinine; COPD, chronic obstructive pulmonary disease.
Intraoperative and postoperative conditions
Intraoperative conditions was showen in Table 2. Delayed extubation group had longer peration time, CPB time and cross clamp time. But no statistically significant differences were observed between the two groups in terms of other variables. Early Mortality, stroke, CRRT, reexploration for bleeding delayed chest closure, MODS, ECMO, acute lung injury, acute liver injury ccurred more frequently in the delayed extubation group. Patients with delayed extubation had a higher RBC count, WBC count, PLT count, Ddimer count and Scr postoperatively. Delayed extubation group had longer hospital stay and ICU stay day (Tables 3, 4).
Table 2
| n | All patients(n = 466) | No delayed extubation (n = 130) | Delayed extubation (n = 336) | p |
|---|---|---|---|---|
| Cannulation, n (%) | 0.197 | |||
| Axillary artery | 281 (60.954) | 77 (60.630) | 204 (61.078) | |
| Femoral artery | 24 (5.206) | 9 (7.087) | 15 (4.491) | |
| Axillary + femoral artery | 132 (28.633) | 31 (24.409) | 101 (30.240) | |
| Others | 24 (5.206) | 10 (7.874) | 14 (4.192) | |
| Bentall procedure without valve conduit, n (%) | 23 (27.711) | 9 (37.500) | 14 (23.729) | 0.20 |
| Wheats, n (%) | 9 (10.345) | 2 (8.333) | 7 (11.111) | 0.70 |
| Cabrol, n (%) | 59 (56.731) | 14 (45.161) | 45 (61.644) | 0.12 |
| CABG, n (%) | 22 (100.000) | 4 (100.0 00) | 18 (100.000) | 1.000 |
| Operation time, hour, mean (±SD) | 7.500 ± 1.539 | 7.184 ± 1.511 | 7.621 ± 1.533 | 0.01 |
| CPB time, min, mean (±SD) | 245.467 ± 68.058 | 230.177 ± 73.962 | 251.400 ± 64.657 | 0.00 |
| ACC time min, mean (±SD) | 132.144 ± 42.620 | 124.354 ± 44.615 | 135.167 ± 41.427 | 0.01 |
| DHCA tempnasal, mean (±SD) | 23.222 ± 2.604 | 23.219 ± 2.854 | 23.223 ± 2.500 | 0.99 |
| DHCAtime, mean (±SD) | 39.487 ± 18.493 | 40.238 ± 17.626 | 39.195 ± 18.811 | 0.586 |
| RBC transfusion, U, mean (±SD) | 3.183 ± 3.529 | 2.995 ± 3.220 | 3.255 ± 3.639 | 0.52 |
| Plasma transfusion, mL, mean (±SD) | 232.468 ± 261.013 | 210.748 ± 249.956 | 240.827 ± 264.671 | 0.31 |
| Platele transfusion, U, mean (±SD) | 1.941 ± 3.108 | 1.680 ± 2.286 | 2.041 ± 3.366 | 0.31 |
Intraoperative conditions.
CPB, cardiopulmonary bypass; ACC, aortic cross clamp; CABG, coronary artery bypass graft; DHCA, deep hypothermic circulatory arrest.
Table 3
| n | All patients (n = 466) | No delayed extubation (n = 130) | Delayed extubation (n = 336) | p |
|---|---|---|---|---|
| Early Mortality, n (%) | 39 (8.387) | 5 (3.876) | 34 (10.119) | 0.03 |
| Stroke, n (%) | 48 (10.300) | 7 (5.385) | 41 (12.202) | 0.03 |
| CRRT, n (%) | 120 (25.751) | 6 (4.615) | 114 (33.929) | <0.001 |
| Reexploration for bleeding delayed chest closure, n (%) | 46 (9.871) | 5 (3.846) | 41 (12.202) | 0.01 |
| MODS, n (%) | 147 (31.613) | 17 (13.178) | 130 (38.690) | <0.001 |
| ECMO, n (%) | 22 (4.721) | 2 (1.538) | 20 (5.952) | 0.04 |
| Acute lung injury, n (%) | 325 (69.742) | 53 (40.769) | 272 (80.952) | <0.00 |
| Acute Liver Injury, n (%) | 208 (44.635) | 45 (34.615) | 163 (48.512) | 0.01 |
| Postoperative laboratory data | ||||
| RBC, median [IQR] | 3.010 [2.600,3.550] | 3.180 [2.840,3.670] | 2.950 [2.510,3.450] | <0.001 |
| WBC, median [IQR] | 20.090 [16.710,24.060] | 19.140 [16.400,22.860] | 20.320 [17.190,24.830] | 0.019 |
| PLT, median [IQR] | 106.000 [65.000,143.000] | 131.000 [98.000,178.000] | 98.000 [56.000,129.000] | <0.001 |
| Ddimer, mean (±SD) | 9,891.473 ± 6,311.687 | 7,419.587 ± 5,647.908 | 10,870.899 ± 6,293.054 | <0.001 |
| Glucose, median [IQR] | 12.530 [10.010,15.490] | 11.470 [9.780,15.420] | 12.730 [10.270,15.520] | 0.118 |
| AST, mean (±SD) | 401.393 ± 1,615.408 | 234.496 ± 1,091.381 | 464.231 ± 1,768.942 | 0.176 |
| ALT, mean (±SD) | 271.063 ± 751.591 | 184.124 ± 599.790 | 304.641 ± 800.045 | 0.122 |
| Scr, median [IQR] | 186.920 [123.350,350.860] | 125.800 [93.000,173.200] | 240.200 [142.300,424.660] | <0.001 |
| ICU stay day, median [IQR] | 9.000 [6.000,20.000] | 5.000 [3.000,10.000] | 11.000 [8.000,24.000] | <0.001 |
| Hospital stay, day, median [IQR] | 21.000 [15.000,29.000] | 18.000 [14.000,25.000] | 22.000 [16.000,30.000] | <0.001 |
Postoperative conditions.
RBC, red blood cell; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PLT, platelets; Scr, serum creatinine; COPD, chronic obstructive pulmonary disease; MODS, multiple organ dysfunction syndromes; CRRT, continuous renal replacement therapy; ICU, intensive care unit.
Table 4
| Predictor | OR | 95%CI | P-value |
|---|---|---|---|
| Age | 1.033 | [1.013,1.053] | 0.001 |
| BMI | 1.055 | [0.982,1.133] | 0.145 |
| Male | 0.784 | [0.389,1.489] | 0.473 |
| Preoperative laboratory data | |||
| RBC | 0.999 | [0.997,1.002] | 0.484 |
| WBC | 1.000 | [0.999,1.002] | 0.636 |
| PLT | 0.999 | [0.997,1.002] | 0.550 |
| D-dimer | 0.998 | [0.995,1.000] | 0.051 |
| Glucose | 1.001 | [0.999,1.003] | 0.535 |
| AST | 1.001 | [0.995,1.006] | 0.767 |
| ALT | 1.007 | [1.000,1.013] | 0.044 |
| LDL | 1.000 | [0.990,1.009] | 0.916 |
| Tirglyceride | 0.995 | [0.985,1.005] | 0.312 |
| Scr | 1.000 | [0.999,1.002] | 0.739 |
| CPB time | 1.006 | [1.002,1.009] | 0.003 |
| ACC time | 1.006 | [1.001,1.012] | 0.015 |
| DHCA time | 0.997 | [0.986,1.008] | 0.585 |
| DHCA tempnasal | 1.001 | [0.926,1.081] | 0.990 |
| RBC transfusion, U | 1.021 | [0.958,1.089] | 0.517 |
| Plasma transfusion, mL | 1.000 | [1.000,1.001] | 0.312 |
| Platelets transfusion, U | 1.044 | [0.960,1.136] | 0.313 |
| Postoperative laboratory data | |||
| RBC | 0.998 | [0.992,1.004] | 0.520 |
| WBC | 1.053 | [1.017,1.091] | 0.003 |
| PLT | 0.991 | [0.988,0.995] | <0.001 |
| Ddimer | 1.000 | [1.000,1.000] | <0.001 |
| Glucose | 1.041 | [0.991,1.094] | 0.109 |
| AST | 1.000 | [1.000,1.000] | 0.213 |
| ALT | 1.000 | [1.000,1.001] | 0.141 |
| Scr | 1.006 | [1.004,1.009] | <0.001 |
| Blood loss, mL | 1.001 | [1.000,1.003] | 0.021 |
| Medical history | |||
| Atria_fibrillation | 0.460 | [0.138,1.536] | 0.207 |
| Hypertension | 1.493 | [0.984,2.267] | 0.060 |
| Diabetes mellitus | 3.000 | [0.676,13.305] | 0.148 |
| Hyperlipidemia | 0.718 | [0.477,1.080] | 0.112 |
| Chronic kidney disease | 1.477 | [0.481,4.535] | 0.496 |
| Smoking | 0.814 | [0.478,1.387] | 0.450 |
| COPD | 0.719 | [0.391,1.322] | 0.289 |
| Cannulation | |||
| Femoral vs. axillary | 0.629 | [0.264,1.497] | 0.295 |
| Femoral + axillary vs. axillary | 1.230 | [0.761,1.988] | 0.399 |
| Othera vs. axillary | 0.528 | [0.225,1.240] | 0.143 |
| Postoperative outcomes | |||
| Stroke | 3.203 | [1.226,8.372] | 0.018 |
| CRRT | 9.064 | [3.838,21.407] | <0.001 |
| Re-exploration for bleeding delayed chest closure | 3.106 | [1.187,8.129] | 0.021 |
| MODS | 4.161 | [2.354,7.357] | <0.001 |
| ECMO | 3.394 | [0.771,14.945] | 0.106 |
| Mediastinitis | 0.630 | [0.148,2.684] | 0.532 |
| Acute lung injury | 6.055 | [3.728,9.833] | <0.001 |
| Acute liver injury | 1.744 | [1.103,2.757] | 0.017 |
Univariable analysis of the risk factors for delayed extubation.
Feature selection
The LASSO binary logistic regression model was utilized due to the insufficient sample size in this study, which did not meet the recommended number of events per variable. Out of all the relevant variables, a cohort-based analysis resulted in the reduction of 9 features to 6 potential predictors. The final model utilized the 6 variables with non-zero coefficients in the LASSO logistic regression model, namely Age, PLTpost, postoperative D-dimer level, MODS, Acute lung injury and Scrpost (Figures 2A,B). Subsequently, the aforementioned 6 variables were incorporated into a multivariable logistic regression analysis to identify the risk factors associated with delayed extubation. The analysis revealed that age, acute lung injury and Scrpost were significant risk factors for delayed extubation (Table 5).
Figure 2
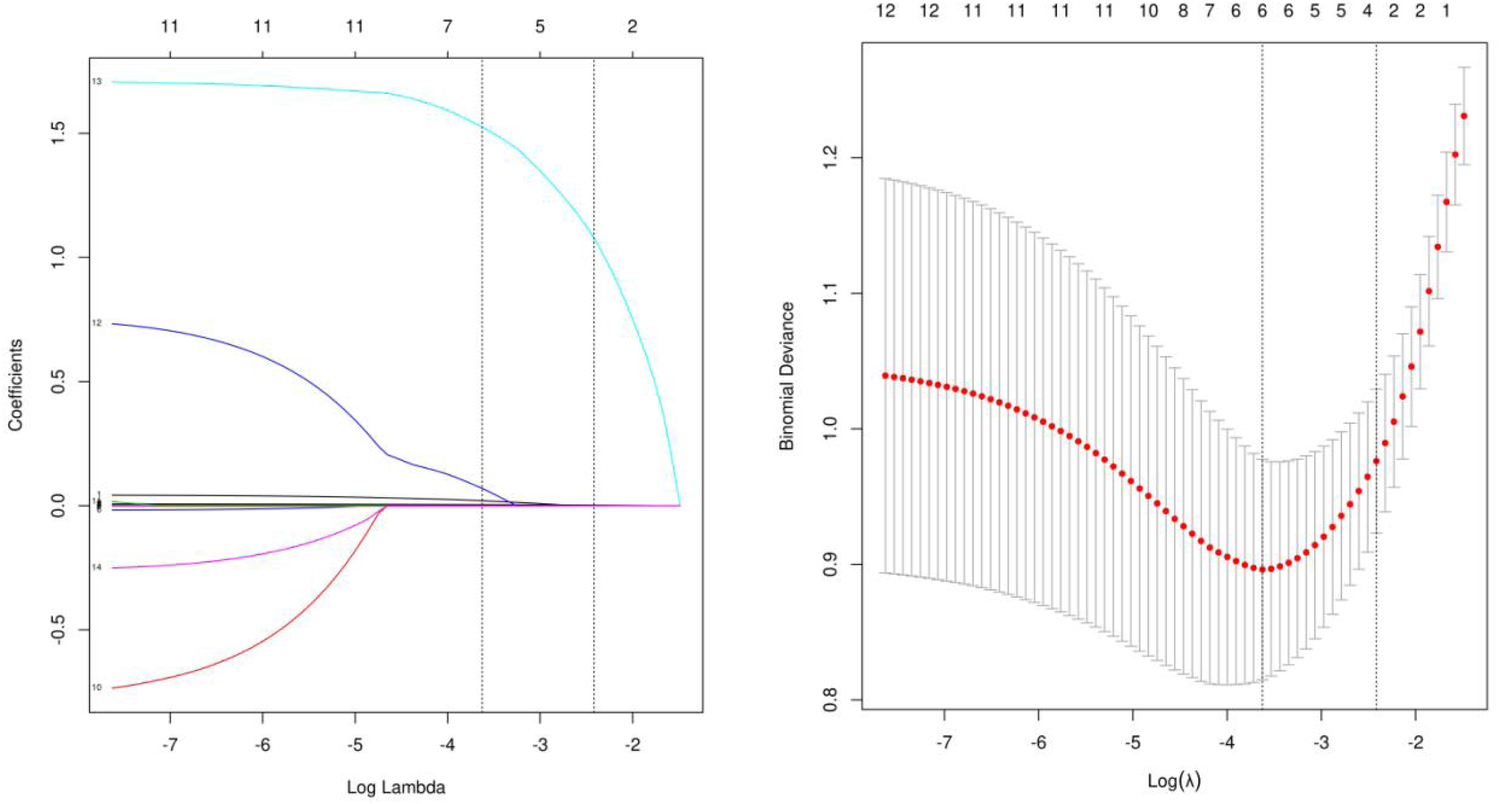
Demographic and clinical feature selection using lasso binary logistic regression model. (A) Tuning parameters λ were obtained through 10 times crossvalidation with the minimum standard, the partial likelihood deviance curve was drawn through Log (λ), and the minimum standard and 1-SE standard were used to draw the vertical dotted line, after 10 times cross-validation, the Log (λ) was determined to be 0.027 (1-SE standard) at the optimal value. (B) Lasso coefficient profiles of the 9 features. A coefficient profile plot was produced against the log (λ) sequence. The vertical line was drawn at the value selected using 10fold cross-validation, where optimal resulted in 6 features with non-zero coefficients.
Table 5
| Predictor | Odds Ratio | Lower | Upper | p |
|---|---|---|---|---|
| (Intercept) | 0.048 | 0.005 | 0.427 | 0.008 |
| Age | 1.042 | 1.005 | 1.083 | 0.031 |
| PLTpost | 0.995 | 0.99 | 1.0 | 0.055 |
| Scrpost | 1.006 | 1.003 | 1.01 | <0.001 |
| Acute lung injury | 5.725 | 2.777 | 12.148 | <0.001 |
Univariable analysis of the risk factors for delayed extubation.
Nomogram construction
A backwards selection logistic regression was performed to screen the aforementioned variables. And AUC was 0.86 (Figure 3B). Age, acute lung injury and Scrpost were identified as risk factors for delayed extubation (Table 5). These findings were utilized to develop a nomogram that can estimate the risk of delayed extubation in patients with aortic dissection during their hospitalization (Figures 3A,B). Figure 3C displayed the calibration curves of the nomogram. In the cohort, the calibration curves demonstrated nearly diagonal alignment, indicating that the nomogram had a good fit. The decision curve analysis revealed that our predictive model yielded greater clinical net benefits when compared to both the “intervention for all” and “no intervention” strategies (Figure 3D). The clinical impact curve also demonstrated that the model exhibited favorable predictive capacity and clinical usefulness (Figure 3E).
Figure 3
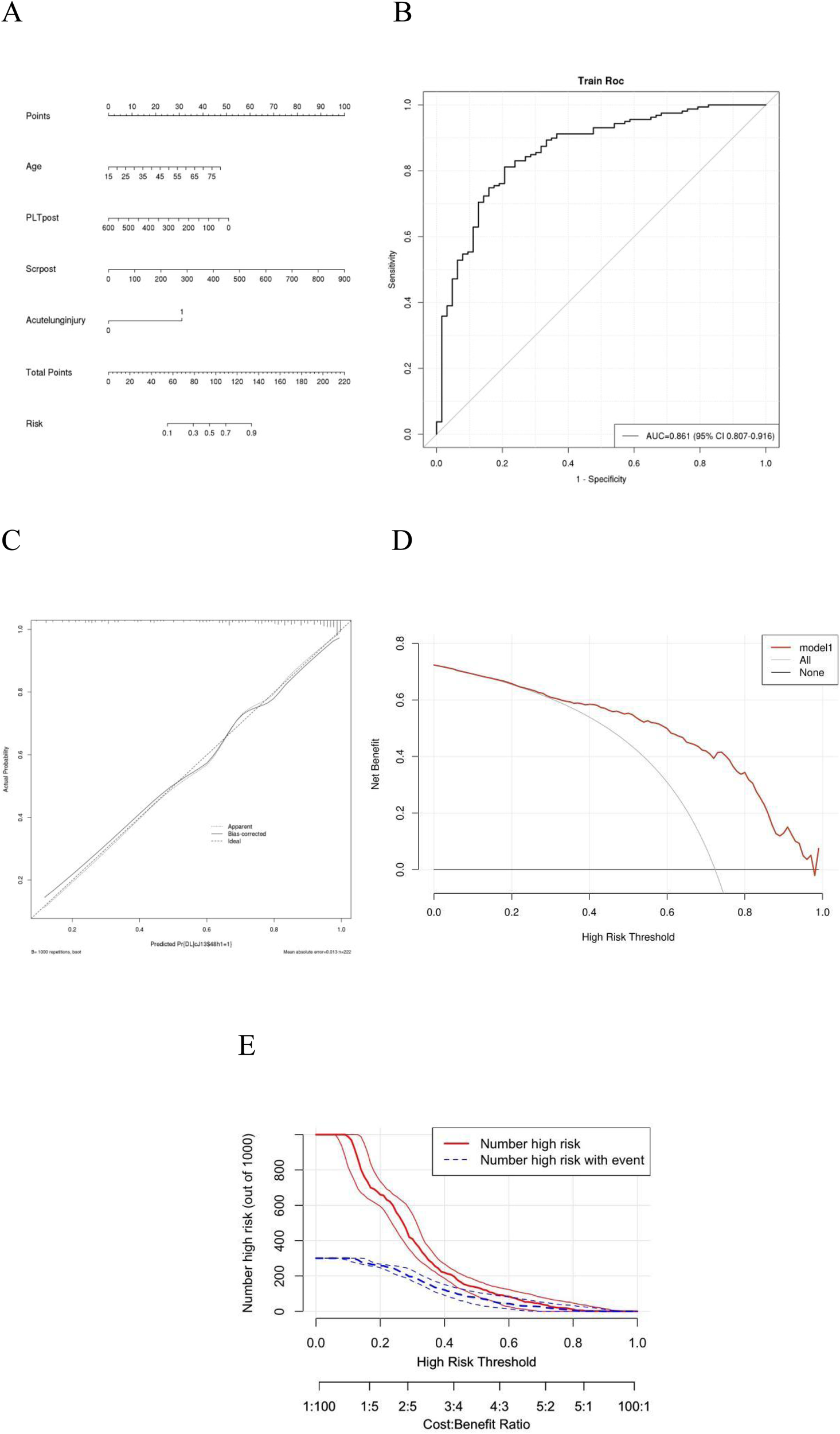
ROC analysis, calibration curves and DCA for delayed extubation. Plots (A) shows the nomogram analysis of delayed extubation. Plots (B) shows the ROC analysis of delayed extubation. Plots (C) Calibration curves of the prediction model. Calibration curves depicted the calibration of the model. The 45◦ line represents a perfect prediction, and the red line and green line represent the actual prediction and bootstrap adjusted curve respectively. Plots (D) show DCA of Model for delayed extubation. Plots (E) show clinical impact curve of Model for delayed extubation. AUC, area under the receiver operating characteristic curve; CI, confidence interval; DCA, decision curve analysis; ROC, receiver operating characteristic.
Discussion
This study revealed a prevalence higher rate of delayed extubation in patients undergoing AADS, which was not similar to data that had been previously reported (9, 11, 12). We observed higher resource utilization and in-hospital mortality among patients with delayed extubation after AADS, as compared to those without delayed extubation. The in-hospital mortality rate for patients undergoing delayed extubation a AADS was 10.12%, which was less than the findings from previous studies on cardiac surgical patients (9, 13).
Our study examined the predictors of delayed extubation in patients with AADS and develop a nomogram model for predicting the complication. Based on clinical data from 466 patients who underwent AADS at a single institution, it was found that certain preoperative factors such as age, along with postoperative factors including age, SCr (Serum creatinine), and acute lung injury, were independently associated with delayed extubation following AADS. Our predictive model was developed based on these risk factors and subsequently validated, demonstrating excellent predictive performance and strong clinical utility. And the ACU was 0.86 therefore the prediction is relatively good.
It was observed that older age was associated with a higher risk of delayed extubation in our study population. However, the findings regarding the relationship between age and the probability of tracheostomy varied across different studies (9, 14, 15). We believe that this inconsistency could be attributed, at least in part, to variations in the disease populations under investigation. And we also found that there was no significant correlation between postoperative platelet count and delayed extubation, with a p-value of 0.055. Increasing the sample size may lead to different conclusions. Similarly, during univariate analysis, no correlation was found between preoperative platelet levels and delayed extubation. However, some other studies have found a correlation between preoperative platelet levels and delayed extubation (10). And the other found the transfusion of blood products had a significant correlation with the duration of delayed extubation. The incidence of delayed extubation was found to increase with the transfusion of packed red blood cells (pRBC) and platelet concentrate (PC), as well as with advancing age, across all three endpoints (15).
In our study, we found that postoperative creatinine has a good predictive role in delayed extubation, which was not discovered in previous research. However, increased serum cystatin C levels upon admission were identified as risk factors for delayed extubation in one research study (16). The researcher identified elevated leukocyte count, lower preoperative platelet count, and longer cardiopulmonary bypass (CPB) duration as risk factors for delayed extubation. However, this study only conducted univariate logistic regression without incorporating multivariate logistic regression (16). The impact of creatinine on aortic dissection has been extensively investigated in previous research. Earlier studies primarily examined creatinine as a predictive factor for postoperative renal injury (17), while more recent studies have discovered its potential as a reliable predictor of mortality (18–21). Creatinine, primarily eliminated via glomerular filtration, represents the metabolic product of muscle-produced creatine. Serum creatinine (SCr) remains unaffected by exogenous factors including diet and intense catabolism, as it is under homeostatic regulation through glomerular filtration (21). The association between SCr and delayed extubation was uncovered by our study, and further investigation is required to understand its underlying mechanism. The possible reasons are that decreased renal function leads to fluid retention and positive fluid balance, which increases pulmonary capillary hydrostatic pressure and results in interstitial and alveolar edema, thereby reducing gas exchange and prolonging the time to extubation (22). Additionally, renal dysfunction causes an imbalance in the immune response (with both immunosuppression and inflammation present), increasing the risk of hospital-acquired or pulmonary infections, and thus prolonging mechanical ventilation (23).
The study has some limitations, including a single centre study, relatively small dataset and a lack of long-term follow-up. We will continue with further investigation in a subsequent study. Significant differences in operation time, CPB time, and ACC time were noted between groups. These parameters are biologically relevant to PMV. It's possible that I didn't include these variables in my Lasso regression analysis. Perhaps with an expanded sample size in the future, these factors might be incorporated as well. I will address this in the limitations section.
In conclusion, Age, postoperative serum creatinine, and acute lung injury are significant risk factors for prolonged ventilation. Early identification and management of these factors may improve postoperative outcomes.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Guangdong Provincial People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GC: Writing – original draft, Formal analysis, Methodology, Data curation. ZheL: Writing – original draft, Methodology, Formal analysis. ZhoL: Formal analysis, Writing – original draft. QS: Formal analysis, Writing – original draft. YL: Investigation, Writing – original draft. TL: Writing – original draft. CZ: Writing – review & editing, Investigation, Data curation, Methodology, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Key Research and Development Program of China, with the project number 2023YFC2410305.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction Note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Nienaber CA Eagle KA . Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation. (2003) 108:628–35. 10.1161/01.CIR.0000087009.16755.E4
2.
Shi Q Mu X Zhang C Wang S Hong L Chen X . Risk factors for postoperative delirium in type A aortic dissection patients: a retrospective study. Med Sci Monit. (2019) 25:3692–9. 10.12659/MSM.913774
3.
Andersen ND Ganapathi AM Hanna JM Williams JB Gaca JG Hughes GC . Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol. (2014) 63(17):1796–803. 10.1016/j.jacc.2013.10.085
4.
Guo Z Yang Y Zhao M Zhang B Lu J Jin M et al Preoperative hypoxemia in patients with type A acute aortic dissection: a retrospective study on incidence, related factors and clinical significance. J Thorac Dis. (2019) 11(12):5390–7. 10.21037/jtd.2019.11.68
5.
Hasegawa Y Ishikawa S Ohtaki A Otani Y Takahashi T Sato Y et al Impaired lung oxygenation in acute aortic dissection. J Cardiovasc Surg (Torino). (1999) 40(2):191–5.
6.
Apostolakis E Filos KS Koletsis E Dougenis D . Lung dysfunction following cardiopulmonary bypass. J Card Surg. (2010) 25:47–55. 10.1111/j.1540-8191.2009.00823.x
7.
Maisat W Siriratwarangkul S Charoensri A Wongkornrat W Lapmahapaisan S . Perioperative risk factors for delayed extubation after acute type A aortic dissection surgery. J Thorac Dis. (2020) 12:4796–804. 10.21037/jtd-20-742
8.
Fernandez-Zamora MD Gordillo-Brenes A Banderas-Bravo E Arboleda-Sanchez JA Hinojosa-Perez R Aguilar-Alonso E et al Prolonged mechanical ventilation as a predictor of mortality after cardiac surgery. Respir Care. (2018) 63(5):550–7. 10.4187/respcare.04915
9.
Wang D Wang S Song Y Wang H Zhang A Wu L et al Predictors and outcomes of postoperative tracheostomy in patients undergoing acute type A aortic dissection surgery. BMC Cardiovasc Disord. (2022) 22(1):94. 10.1186/s12872-022-02538-4
10.
Jin M Ma WG Liu S Zhu J Sun L Lu J et al Predictors of prolonged mechanical ventilation in adults after acute type-A aortic dissection repair. J Cardiothorac Vasc Anesth. (2017) 31(5):1580–7. 10.1053/j.jvca.2017.03.036
11.
Comas GM Leshnower BG Halkos ME Thourani VH Puskas JD Guyton RA et al Acute type A dissection: impact of antegrade cerebral perfusion under moderate hypothermia. Ann Thorac Surg. (2013) 96(6):2135–41. 10.1016/j.athoracsur.2013.06.085
12.
Hu Z Wang Z Chang J Zhang M Hu X Ren Z et al Application of prosthesis eversion method for ascending aorta replacement guarantees better clinical outcomes of type A acute aortic dissection surgery. J Thorac Dis. (2021) 13(2):533–40. 10.21037/jtd-20-2578
13.
Diaz-Castrillon CE Brown JA Navid F Serna-Gallegos D Yousef S Thoma F et al The impact of prolonged mechanical ventilation after acute type A aortic dissection repair. J Thorac Cardiovasc Surg. (2024) 167(5):1672–9.e2. 10.1016/j.jtcvs.2022.07.007
14.
Li CN Chen L Ge YP Zhu JM Liu YM Zheng J et al Risk factors for prolonged mechanical ventilation after total aortic arch replacement for acute DeBakey type I aortic dissection. Heart Lung Circ. (2014) 23(9):869–74. 10.1016/j.hlc.2014.03.022
15.
Xie Q Li C Zhong Y Luo C Guo R Liu Y et al Blood transfusion predicts prolonged mechanical ventilation in acute Stanford type A aortic dissection undergoing total aortic arch replacement. Front Cardiovasc Med. (2022) 9:832396. 10.3389/fcvm.2022.832396
16.
Ge M Wang Z Chen T Cheng Y Ye J Lu L et al Risk factors for and outcomes of prolonged mechanical ventilation in patients received DeBakey type I aortic dissection repairment. J Thorac Dis. (2021) 13:735–42. 10.21037/jtd-20-2736
17.
Helgason D Helgadottir S Ahlsson A Gunn J Hjortdal V Hansson EC et al Acute kidney injury after acute repair of type A aortic dissection. Ann Thorac Surg. (2021) 111(4):1292–8. 10.1016/j.athoracsur.2020.07.019
18.
Wei XB Wang Y Luo JF Hong WZ Su Z Zhang CX et al Utility of age, creatinine, and ejection fraction score in patients with type B aortic dissection undergoing thoracic endovascular aortic repair. Int J Cardiol. (2020) 303:69–73. 10.1016/j.ijcard.2019.09.076
19.
Bayram M Duman ZM Timur B Yasar E Ustunisik CT Kaplan MC et al Predictive value of age, creatinine, and ejection fraction (ACEF) scoring system for operative mortality in patients with Stanford type A aortic dissection. Indian J Thorac Cardiovasc Surg. (2023) 39:6–13. 10.1007/s12055-022-01431-1
20.
Mohamed MA Hamid KA . How an elevated creatinine level can deter the diagnosis of acute aortic dissection. Cureus. (2018) 10:e2057. 10.7759/cureus.2057
21.
Wu ZN Guan XL Xu SJ Wang XL Li HY Gong M et al Does preoperative serum creatinine affect the early surgical outcomes of acute Stanford type A aortic dissection? J Chin Med Assoc. (2020) 83(3):266–71. 10.1097/JCMA.0000000000000264
22.
Yuan Q Tang B Zhang C . Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct Target Ther. (2022) 7(1):182. 10.1038/s41392-022-01036-5
23.
Bonacchi G Rossi VA Garofalo M Mollace R Uccello G Pieragnoli P et al Pathophysiological link and treatment implication of heart failure and preserved ejection fraction in patients with chronic kidney disease. Biomedicines. (2024) 12(5):981. 10.3390/biomedicines12050981
Summary
Keywords
extubation, acute type A aortic dissection, risk factors, delay, mortality
Citation
Chen G, Li Z, Lin Z, Su Q, Ling Y, Li T and Zhou C (2025) Risk factors of prolonged mechanical ventilation after acute type A aortic dissection surgery: a single-center retrospective study. Front. Cardiovasc. Med. 12:1659336. doi: 10.3389/fcvm.2025.1659336
Received
03 July 2025
Accepted
20 October 2025
Published
13 November 2025
Corrected
21 November 2025
Volume
12 - 2025
Edited by
Gianni Angelini, University of Bristol, United Kingdom
Reviewed by
Christian Jörg Rustenbach, University of Tübingen, Germany
Xiaomao Long, People’s Hospital of Guangxi Zhuang Autonomous Region, China
Updates
Copyright
© 2025 Chen, Li, Lin, Su, Ling, Li and Zhou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Chengbin Zhou zcbwwww@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.