Abstract
Background:
Reversible cerebral vasoconstriction syndrome (RCVS) is a rare but severe neurovascular complication following cardiac transplantation. Its diagnosis is often complicated by atypical presentations and by neuroimaging limitations, as transplant-related devices can hinder timely magnetic resonance (MR) imaging.
Case presentation:
A 59-year-old woman developed a severe headache 9 days post-cardiac transplantation. Neurological examination revealed left lower quadrantanopia. A head Computed Tomography (CT) scan showed a subdural hematoma, along with a subcortical and convexity subarachnoid hemorrhage in the right parieto-occipital lobe. Initial CT angiography showed only focal arterial stenosis. On day 10, she developed left hemineglect. After removal of transplant-related devices, MR imaging showed a watershed infarct and MR angiography revealed multiple vasospasms.
Diagnostic challenges:
The diagnostic challenge was the delayed onset of diffuse vasospasms, the confirmation of which was precluded by the initial contraindication to MR imaging.
Management:
The patient was diagnosed with RCVS. Management included withdrawal of tacrolimus and initiation of oral verapamil and prasugrel. This led to rapid clinical and radiological improvement.
Conclusion:
This case highlights that RCVS can present with a subdural hematoma before the onset of delayed vasospasm after cardiac transplantation. Repeated cerebrovascular evaluations are crucial for timely diagnosis and management in post-transplant patients with unexplained headaches or intracranial hemorrhage.
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS), a cerebrovascular syndrome characterized by severe headaches and reversible constriction of cerebral arteries, has been reported as a rare complication of cardiac transplantation (1, 2). The etiology of this condition is recognized as multifactorial, often resulting from a convergence of factors including calcineurin inhibitor (CNI) toxicity, labile hypertension, and the physiological stress of surgery (1). These triggers overlap with those of posterior reversible encephalopathy syndrome (PRES), and the two disorders can occasionally coexist (2, 3). The diagnosis of RCVS is inherently complex, as patients may present with atypical hemorrhagic or ischemic strokes, and the typical multiple vasospasms are frequently not apparent in the early stages of the disease (4). This problem is further complicated after cardiac transplantation by devices that hinder timely magnetic resonance (MR) imaging. We describe a case of RCVS presenting with a subdural hematoma before the onset of delayed vasospasm after cardiac transplantation, adding to the literature by highlighting this atypical presentation and its associated diagnostic challenges.
Case description
A 59-year-old woman with cardiomyopathy due to Duchenne muscular dystrophy underwent cardiac transplantation. Her pre-transplant history included muscular dystrophy-related weakness but no history of hypertension, migraine, or other significant cardiovascular comorbidities. Her perioperative immunosuppression regimen consisted of tacrolimus, mycophenolate mofetil, and prednisolone. She had been taking aspirin for donor-transmitted coronary atherosclerosis. Her postoperative course was complicated by labile hypertension, with systolic pressures ranging from 140 to 180 mmHg. On day nine, she developed a headache. Neurological examination revealed left lower quadrantanopia. A head Computed Tomography (CT) scan showed a subdural hematoma with a maximum thickness of 1 cm, along with a subcortical and convexity subarachnoid hemorrhage in the right parieto-occipital lobe (Figures 1a,b). CT angiography revealed stenosis confined to the left middle cerebral artery, though it was unclear whether this represented vasospasm or mere stenosis (Figures 1c,d). MR imaging was not immediately available due to the presence of transplant-related devices. Discontinuation of aspirin did not prevent the development of left hemineglect on the 10th day. After the transplant-related devices were removed, cranial MR images on the 10th day revealed a watershed infarct in the right parietal lobe (Figures 2a,b), and MR angiography demonstrated diffuse, multifocal moderate vasospasms affecting the bilateral anterior, middle, and posterior cerebral arteries (Figure 2c). She was diagnosed with RCVS. Management included immediate withdrawal of tacrolimus and initiation of oral verapamil. Following the identification of the watershed infarct, an antiplatelet agent was considered for secondary stroke prevention. Prasugrel was chosen over other P2Y12 inhibitors because its antiplatelet effect is not influenced by CYP2C19 loss-of-function variants, which are prevalent in Asian populations (5). This regimen led to an improvement in her neurologic symptoms within 24 h, and follow-up MR angiography on day 18 showed improvement in the vasospasm (Figure 2d). Her immunosuppressive regimen was adjusted to a combination of prednisolone, everolimus, and mycophenolate mofetil, and then gradually tapered. She was discharged home on day 49. At a 6-month follow-up visit, she remained neurologically stable with a persistent, isolated left lower quadrantanopia but was independent in her activities of daily living (modified Rankin Scale score of 1). Follow-up cranial magnetic resonance imaging at 6 months revealed no new infarction or hemorrhage and no recurrence of vasospasm.
Figure 1
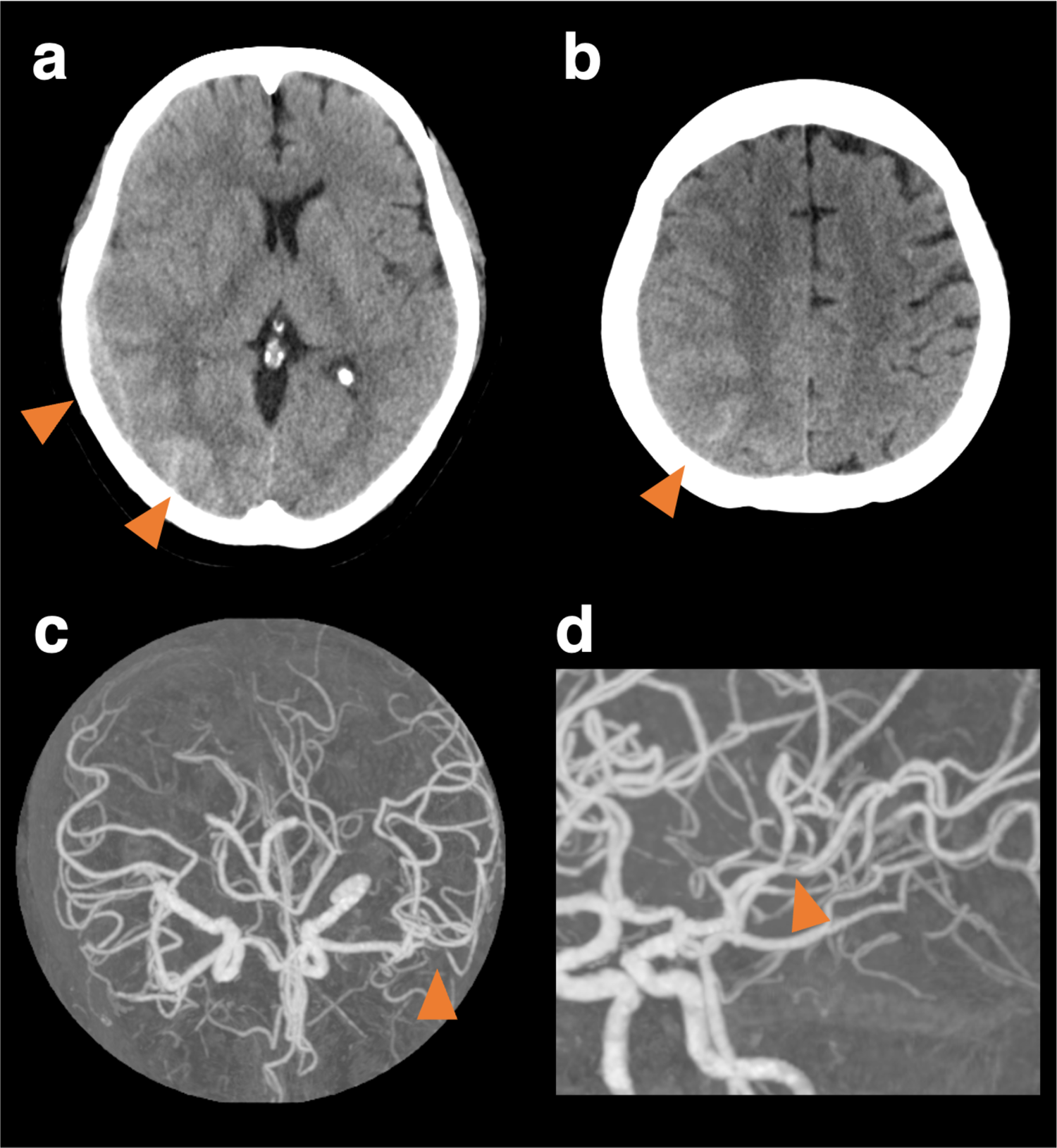
Cranial computed tomography and computed tomography angiography at the onset. Cranial computed tomography (CT) shows subcortical hemorrhage in the right posterior lobe, subdural, and convexity subarachnoid hemorrhage (a, b). CT angiography shows vasoconstriction limited to the left middle cerebral artery, though it was unclear whether it was a vasospasm or mere stenosis (c, d).
Figure 2
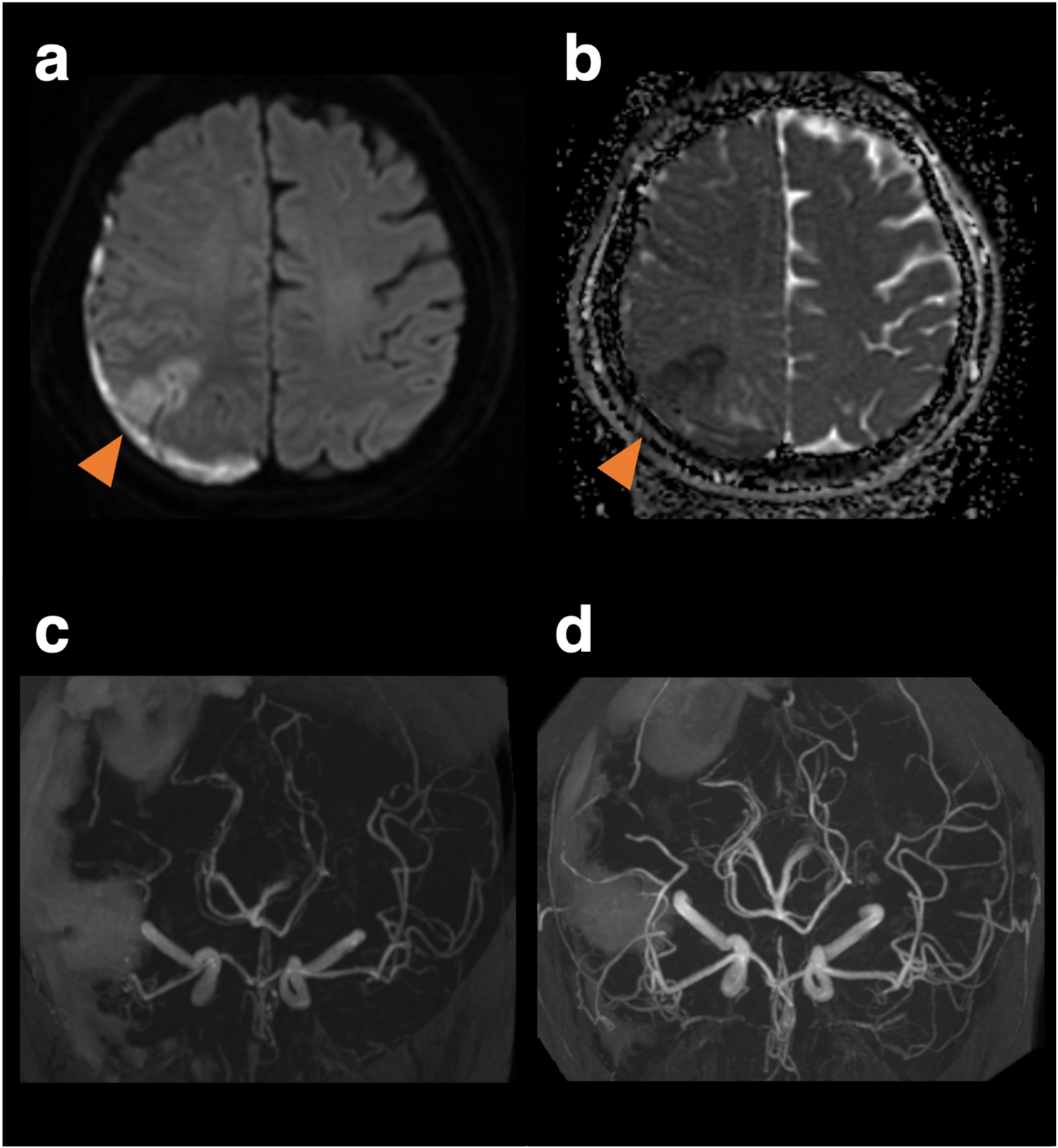
Magnetic resonance images and angiography on the 10th day when the patient developed left hemineglect, and magnetic resonance angiography on the 18th day of follow-up. Diffusion-weighted imaging (a) and apparent diffusion coefficient map (b) reveals a watershed infarct. Vasoconstriction peaked on day 10 (c) and improved on day 18 (d).
Discussion
We reported a case of RCVS with a subdural hematoma before the onset of delayed vasospasm after cardiac transplantation. While RCVS is a known, albeit rare, complication post-transplantation, our case is distinguished by its initial presentation with a subdural hematoma, which is an uncommon manifestation of RCVS. Furthermore, the delayed onset of diffuse, multifocal vasospasms, which were not apparent on initial angiogram, highlights a diagnostic pitfall. This clinical course, combined with the diagnostic challenge posed by contraindications to immediate MR imaging due to transplant-related devices, adds a critical perspective to the existing literature on post-transplant neurovascular complications.
The pathophysiology of RCVS in the post-transplant setting is complex. Since tacrolimus is a known CNI trigger, the rapid clinical improvement following its withdrawal suggests it played a key role; however, this interpretation should be made with caution. The improvement may also be partly attributed to the natural, self-limiting course of RCVS or the therapeutic effect of the calcium channel blocker that was initiated. Therefore, it is most plausible that the clinical course was influenced by a convergence of these factors. Although specific biomarkers such as serum tacrolimus, endothelin-1, or catecholamine levels were not measured in our patient, CNI-induced endothelial dysfunction is a well-described mechanism, thought to involve an imbalance of vasoactive substances like reduced nitric oxide and increased endothelin-1 (6). The additional physiological stress from surgery and altered catecholamine dynamics from the denervated heart likely contributed to a vulnerable cerebrovascular state in this patient (7).
The clinical course of RCVS is varied, typically starting with a sudden, severe headache. While ischemic and hemorrhagic complications are common, subdural hematomas are a rarer manifestation (2%–4%) (8–11). Cerebral infarction often occurs days after the initial headache or hemorrhage, frequently affecting watershed zones, as seen in our patient (10). Vasospasm typically begins distally, and the characteristic multifocal involvement of major vessels may not be apparent on initial imaging, with vasospasm peaking on average around 16 days after onset (4).
Treatment for RCVS focuses on removing the offending agent and initiating calcium channel blocker. Given that CNIs are often essential for preventing graft rejection, a switch to an alternative agent, such as the mTOR inhibitor everolimus, is one of the reasonable strategies.
This case provides new information that could modify existing clinical practice by demonstrating that an initial angiogram does not rule out evolving RCVS in post-transplant patients. The primary implication for clinicians is that a low threshold for repeat vascular imaging, preferably within 3–7 days, is warranted for post-transplant patients who present with thunderclap headache, focal deficits, or intracranial hemorrhage. This proactive approach is particularly crucial for patients who exhibit an atypical clinical course, such as the co-occurrence of subdural hematoma and cerebral infarction seen in our case, even if initial findings are non-diagnostic.
Limitations
This study has several limitations. First, as a single case report, the findings cannot be generalized. Second, specific biomarkers that could have provided further insight into the pathophysiology, such as serum tacrolimus levels, endothelin-1, or catecholamines, were not measured. Third, the diagnosis and follow-up of vasospasm were based on MR angiography; digital subtraction angiography was not performed.
Future directions
This case underscores the need for further research to better define the incidence, risk factors, and optimal management strategies for RCVS in patients after cardiac transplantation. Future research could also focus on identifying non-invasive biomarkers to facilitate earlier diagnosis and on establishing standardized protocols for cerebrovascular monitoring in this high-risk population.
Patient perspective
After my heart transplant, just as I was starting to recover, I suddenly experienced a severe headache and visual disturbances, which made me feel very anxious. At first, not knowing the cause was frightening, but thanks to thorough testing and clear explanations, I was able to receive an accurate diagnosis and appropriate treatment. I hope my experience can offer encouragement to others who may face health issues after transplantation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SE: Writing – review & editing, Conceptualization, Resources, Writing – original draft, Investigation, Formal analysis, Project administration, Data curation, Methodology. MI: Data curation, Writing – original draft, Conceptualization, Writing – review & editing, Visualization, Funding acquisition, Formal analysis. TH: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. SF: Data curation, Conceptualization, Writing – review & editing, Writing – original draft. YT: Writing – review & editing, Data curation, Conceptualization, Writing – original draft. MK: Data curation, Writing – review & editing, Conceptualization, Writing – original draft. KT: Data curation, Conceptualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Japan Agency for Medical Research and Development (grant nos. JP251k0221171, JP25lk0221186) and the Intramural Research Fund (24-B-6) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Chen S Hu J Xu L Brandon D Yu J Zhang J . Posterior reversible encephalopathy syndrome after transplantation: a review. Mol Neurobiol. (2016) 53:6897–909. 10.1007/s12035-015-9560-0
2.
Park CS Park JJ . Two rare neurologic complications after heart transplantation: posterior reversible encephalopathy syndrome, followed by reversible cerebral vasoconstriction syndrome. J Heart Lung Transplant. (2016) 35:940–3. 10.1016/j.healun.2016.01.1221
3.
Kaufmann J Buecke P Meinel T Beyeler M Scutelnic A Kaesmacher J et al Frequency of ischaemic stroke and intracranial haemorrhage in patients with reversible cerebral vasoconstriction syndrome (RCVS) and posterior reversible encephalopathy syndrome (PRES)—a systematic review. Eur J Neurol. (2024) 31:e16246. 10.1111/ene.16246
4.
Chen SP Fuh JL Wang SJ Chang FC Lirng JF Fang YC et al Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. (2010) 67:648–56. 10.1002/ana.21951
5.
Yoshimoto T Hattori Y Ishiyama H Nakaoku Y Ogata S Abe S et al CYP2C19 Polymorphism and clopidogrel efficacy in long-term outcomes of large-artery atherosclerotic stroke: the NCVC genome registry. JACC Asia. (2025):S2772-3747(25)00395-3. 10.1016/j.jacasi.2025.07.020
6.
Bajon A Sikora J Siwek M Wiecanowska J Miedziaszczyk M Idasiak-Piechocka I et al Neurotoxicity of calcineurin inhibitors. Ann Indian Acad Neurol. (2025) 28:505–11. 10.4103/aian.aian_614_24
7.
Masters RG Davies RA Keon WJ Walley VM Koshal A de Bold AJ . Neuroendocrine response to cardiac transplantation. Can J Cardiol. (1993) 9:609–17.
8.
Patel SD Topiwala K Saini V Patel N Pervez M Al-Mufti F et al Hemorrhagic reversible cerebral vasoconstriction syndrome: a retrospective observational study. J Neurol. (2021) 268:632–9. 10.1007/s00415-020-10193-y
9.
Ducros A Fiedler U Porcher R Boukobza M Stapf C Bousser MG . Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. (2010) 41:2505–11. 10.1161/STROKEAHA.109.572313
10.
Ducros A Boukobza M Porcher R Sarov M Valade D Bousser MG . The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. (2007) 130:3091–101. 10.1093/brain/awm256
11.
Topcuoglu MA Singhal AB . Hemorrhagic reversible cerebral vasoconstriction syndrome: features and mechanisms. Stroke. (2016) 47:1742–7. 10.1161/STROKEAHA.116.013136
Summary
Keywords
reversible cerebral vasoconstriction syndrome, cardiac transplantation, intracranial hemorrhage, ischemic stroke, subdural hematoma
Citation
Egashira S, Inoue M, Hada T, Fukushima S, Tsukamoto Y, Koga M and Toyoda K (2025) Reversible cerebral vasoconstriction syndrome with delayed vasospasms and subdural hematoma after cardiac transplantation: a case report. Front. Cardiovasc. Med. 12:1660432. doi: 10.3389/fcvm.2025.1660432
Received
06 July 2025
Accepted
16 October 2025
Published
28 October 2025
Volume
12 - 2025
Edited by
Leonardo Roever, Brazilian Evidence-Based Health Network, Brazil
Reviewed by
Odair Alves da Silva, Federal University of Pernambuco, Brazil
Amogh Verma, Rama Medical College Hospital and Research Centre, India
Updates
Copyright
© 2025 Egashira, Inoue, Hada, Fukushima, Tsukamoto, Koga and Toyoda.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Manabu Inoue gakinoue@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.