Abstract
Objective:
To explore the current status and characteristics of upper gastrointestinal bleeding (UGIB) in patients with acute myocardial infarction complicated by cardiogenic shock (AMICS) following emergency percutaneous coronary intervention (PCI), and to develop and validate a predictive model based on baseline risk factors at the time of admission.
Methods:
We selected patients diagnosed with AMICS who underwent emergency PCI. Patients were categorized into the non-bleeding group and the bleeding group based on the occurrence of UGIB during hospitalization. Logistic regression analysis was employed to construct a predictive model for UGIB based on baseline risk factors at admission.
Results:
A total of 253 patients were included in the study, of whom 58 experienced UGIB, resulting in an incidence rate of 22.9%. Univariate analysis indicated that the levels of uric acid, lactate, and alanine aminotransferase (ALT) were higher in the bleeding group compared to the non-bleeding group. Conversely, the estimated glomerular filtration rate (eGFR), left ventricular ejection fraction (LVEF), and albumin were lower in the bleeding group. Additionally, the bleeding group had a higher stage of American Society for Cardiovascular Angiography and Interventions-Cardiogenic Shock(SCAI-CS). The least absolute shrinkage and selection operator (LASSO) regression identified 5 non-zero coefficient variables, and the variance inflation factor (VIF) test excluded the collinearity relationships among these 5 variables. Continuous variables were converted into categorical variables and assigned as follows: albumin was categorized based on whether below 35 g/L, indicating hypoproteinemia; eGFR was categorized based on whether below 45 ml/(min·1.73 m2), indicating moderate to severe renal function decline;The receiver operating characteristic curve (ROC) curve was employed to identify the node with the highest Youden index, which determines the cut-off value: For lactate, the corresponding value is 6.95 mmol/L, rounded to 7 mmol/L. For LVEF, the corresponding value is 35.5%, rounded to 36%.Multivariate logistic regression analysis identified 3 risk factors for UGIB following AMICS emergency PCI: baseline SCAI-CS stage D + E, baseline eGFR < 45 ml/(min·1.73 m2), and baseline LVEF < 36% (P < 0.05). The predictive model based on multivariate logistic regression results, demonstrated good fit according to the Hosmer-Lemeshow test (χ² = 6.968, P = 0.324). The model achieved an AUC of 0.768 [95% CI (0.700, 0.837)] in predicting UGIB in patients undergoing emergency PCI for AMICS, with a sensitivity of 87.9% [95% CI (0.700, 0.837)] and a specificity of 52.3% [95% CI (27.8%, 61.2%)]. The DeLong test indicated that the AUC of the predictive model was superior to that of any individual indicator (P < 0.05), and the DCA curve confirmed the model's clinical utility. Recent Prognosis: The mortality rate during hospitalization in the bleeding group was significantly higher than that in the non-bleeding group (63.8% vs. 29.7%, P < 0.05). Additionally, the duration of hospital stays for surviving patients in the bleeding group was significantly longer than that in the non-bleeding group [19 (14,37) vs. 12 (9,16), (P < 0.001)].
Conclusion:
High baseline SCAI-CS stage, poor kidney function, and low LVEF are independent risk factors for UGIB during hospitalization. The constructed predictive model demonstrates high predictive efficacy.
1 Introduction
Cardiogenic shock (CS) is a clinical syndrome characterized by inadequate perfusion of terminal organs due to cardiac pump failure. It represents the most severe form of acute heart failure. Approximately 10% of patients with acute ST-segment elevation myocardial infarction (STEMI) develop CS, with an associated early mortality rate of 40%–50% (1, 2). In patients with acute coronary syndrome (ACS) complicated by CS, emergency percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) can improve survival, regardless of symptom onset timing (3–6). Although intra-aortic balloon pump (IABP) and extracorporeal membrane oxygenation (ECMO) have not demonstrated survival benefits in patients with acute myocardial infarction complicated by cardiogenic shock (AMICS), they remain valuable for hemodynamic stabilization and facilitating PCI. In particular, for patients with ACS complicated by mechanical complications, percutaneous mechanical circulatory support (pMCS) can serve as a bridge to recovery or provide durable support (7–14). Our hospital is equipped with a excellent Chest Pain Center and a specialized critical heart team, enabling us to perform emergency coronary revascularization and to proficiently utilize pMCS for AMICS patients in accordance with current guidelines.
Although patients with AMICS undergo early revascularization, the early mortality rate remains high. According to reference (15), 54% of these patients die from cardiogenic causes, 24% from brain injury, 20% from multi-organ dysfunction, and an additional 2% from other causes such as sepsis, respiratory failure, and bleeding.
Therefore, patients with AMICS urgently require management of various complications while maintaining hemodynamic stability. Bleeding is a common complication in AMICS, which can be classified based on the bleeding site into puncture site bleeding and non-puncture site bleeding. Puncture site bleeding is frequently associated with local vascular conditions, puncture techniques, and the use of pMCS.
Upper gastrointestinal bleeding (UGIB) following emergency PCI for AMICS complicates treatment. Significant bleeding can exacerbate hemodynamic instability and necessitate reduction or cessation of antithrombotic therapy. Bleeding-induced activation of the coagulation system and transfusions may lead to hypercoagulability, increasing the risk of reinfarction and embolism. Currently, there are no standardized guidelines for managing UGIB in the context of acute myocardial infarction (AMI). Clinical strategies primarily involve balancing antithrombotic and hemostatic interventions based on relevant scoring systems, such as the DAPT score and BARC bleeding classification. However, these scores are mainly designed for long-term management and are less applicable during the acute phase of AMICS, with limited research addressing UGIB in this setting.
This study aims to analyze the incidence, clinical characteristics, prognosis, and risk factors of UGIB in patients after AMICS undergoing emergency PCI during hospitalization. Additionally, a predictive model will be developed and validated based on baseline admission risk factors. The findings are expected to enhance understanding of this condition, facilitate early identification of high-risk patients, enable timely preventive measures, and ultimately reduce bleeding-related adverse outcomes.
2 Methods
2.1 Study design and study subjects
This study is a single-center, retrospective cohort analysis involving 253 patients treated at TEDA International Cardiovascular Hospital, all of whom were diagnosed with AMICS and underwent emergency PCI between January 2018 and December 2024. Patients were categorized into two groups based on the occurrence of UGIB during hospitalization: the non-bleeding group (n = 195) and the bleeding group (n = 58).
- 1.
Patients diagnosed with AMICS who underwent emergency PCI at the TEDA International Cardiovascular Hospital. The emergency PCI procedures included percutaneous coronary artery endovascular surgery, coronary stent implantation, and coronary thrombosis aspiration.
- 2.
Diagnostic criteria for CS: Systolic blood pressure (SBP) ≤90 mmHg (1 mmHg = 0.133 kPa) or a mean arterial pressure (MAP) drop ≥30 mmHg; a sustained SBP decrease of ≥60 mmHg for at least 30 min.Cardiac index (CI) ≤2.2 L/(min·m
2); pulmonary capillary wedge pressure ≥ 15 mmHg; Signs of organ hypoperfusion include cyanosis, limb chills, and decreased urine output < [0.5 ml/(kg·h)]. CS stage was based on the standard of the American Society for Cardiovascular Angiography and Interventions (SCAI) (
16):
- -
Stage A (At Risk): Patients without symptoms or signs of CS but at risk.
- -
Stage B (Beginning): Patients with relatively low blood pressure and no signs of inadequate perfusion.
- -
Stage C (Classic): Patients exhibiting low perfusion requiring initial intervention (inotropes, pharmacologic support, or mechanical circulatory support) after volume resuscitation to restore perfusion.
- -
Stage D (Deteriorating): Patients whose initial stabilization is unstable, necessitating further escalation of treatment.
- -
Stage E (Extremis): Patients with refractory circulatory failure, often in cardiac arrest or requiring multiple concurrent interventions, including ECMO-assisted cardiopulmonary resuscitation.
- -
- 3.
UGIB: Based on the “Expert Suggestions for the Prevention and Treatment of Stress Ulcers” (2018 Edition) (
17) and the “Expert Consensus for the Emergency Diagnosis and Treatment of Acute Upper Gastrointestinal Hemorrhage” (2020 Edition) (
18), UGIB is defined as follows:
- -
Clinical manifestations include vomiting, melena, tarry stool, or gastric tube drainage of brown or bloody gastric contents, with or without symptoms such as dizziness, palpitations, oliguria, pallor of skin and mucous membranes, tachycardia, hypotension, and altered consciousness. Additionally, gastric juice, vomit, or fecal samples may test positive for occult blood.
- -
The above symptoms are absent, except for positive stool occult blood or gastric juice tests. After multidisciplinary evaluation, bleeding from the oral cavity, nasopharynx, or other sites is excluded. In cases where bleeding is due to hemorrhoids, mucus, blood-stained stool, pus, gastroenteritis, or other causes, a decrease in hemoglobin concentration of ≥20 g/L is observed for unknown reasons.
- -
- 4.
Grading the Severity of UGIB according to the GUSTO Bleeding Classification Criteria (
19):
- -
Severe bleeding: Life-threatening or severe bleeding.
- -
Moderate bleeding: Bleeding requiring blood transfusion but not causing hemodynamic instability.
- -
Mild bleeding: Bleeding that does not meet the criteria for severe or moderate bleeding.
- -
- 1.
Patients with esophageal or gastric varices;
- 2.
Patients with a tendency to bleed due to coagulation dysfunction, platelet abnormalities, or abnormal platelet counts upon admission;
- 3.
Patients exhibiting symptoms of gastrointestinal bleeding, such as hematemesis or melena, or with a recent history of untreated gastrointestinal bleeding diseases.
This study was approved by our ethics committee.
2.2 Study protocol
-
1.
Patients diagnosed with AMICS will receive liquid resuscitation, vasoactive agents, mechanical ventilation, pMCS, and other supportive therapies tailored to their clinical condition to maintain vital signs.
-
2.
Preoperative oral loading of dual antiplatelet agents—aspirin 300 mg and clopidogrel 300 mg or ticagrelor 180 mg—is recommended. Postoperative antiplatelet therapy includes aspirin 100 mg once daily and either clopidogrel 75 mg once daily or ticagrelor 90 mg twice daily. The antiplatelet regimen should be adjusted according to the patient's bleeding risk. Routine administration of statins is advised for lipid regulation, along with other secondary prevention strategies for coronary heart disease.
-
3.
Coronary angiography and PCI are performed using standard techniques. The PCI strategy is determined by the operator based on the patient's condition and is followed by transfer to the cardiac intensive care unit post-procedure.
-
4.
Proton pump inhibitor(PPI) prophylaxis was administered post-PCI upon transfer to the CCU, with no preoperative PPI use; this timing was determined by the hospital's emergency PCI pathway, which prioritizes rapid revascularization over prophylactic medications. Routine PPI prophylaxis: Following PCI, all patients were transferred to the CCU and subsequently administered oral Pantoprazole 40 mg once daily. PPI adjustment for UGIB: In cases where patients developed UGIB, the oral PPI was promptly switched to intravenous Pantoprazole at a dosage of 80 mg daily.
-
5.
Patients with UGIB should undergo an assessment of bleeding severity. In cases of moderate or severe bleeding, antiplatelet drugs should be promptly discontinued. For mild bleeding, therapy may be temporarily halted or limited to a single antiplatelet agent, with adjustments made based on clinical changes. All patients with UGIB should receive symptomatic treatment, which includes fasting, gastrointestinal decompression, and the use of PPIs to inhibit gastric acid secretion and protect the gastric mucosa. Hemostatic agents may be administered orally or via a gastric tube, depending on the patient's condition.
2.3 Observation indicators
-
1.
Collect baseline data for both groups of patients upon hospital admission. This data should include gender, age, body mass index (BMI), medical history, personal history, estimated glomerular filtration rate (eGFR), total leukocyte count, hemoglobin levels, platelet count, C-reactive protein, alanine aminotransferase (ALT), baseline systolic blood pressure, baseline heart rate, D-dimer levels, lactic acid, uric acid, total cholesterol, triglycerides, low-density lipoprotein cholesterol, left ventricular ejection fraction (LVEF), plasma B-type natriuretic peptide (BNP), results from coronary angiography, and targeted blood vessels for PCI.
-
2.
Prognosis: Document the in-hospital mortality rates and the duration of hospitalization for patients who survive in both groups.
2.4 Data cleaning
The data contains fewer than 5% missing values, which will be addressed through imputation. For continuous variables exhibiting a normal distribution, the mean will be used for imputation; otherwise, the median will be applied. For categorical variables, the most frequent value will be utilized for imputation.
2.5 Statistical methods
Data analysis was conducted using SPSS version 27.0 and R version 4.5.1. Continuous variables that followed a normal distribution were expressed as mean ± standard deviation (SD), and comparisons between groups were performed using the t-test. Variables that did not follow a normal distribution were presented as medians (P25, P75), and the Mann–Whitney U test was utilized for inter-group comparisons. Categorical data were described using frequencies (n) and percentages (%), and comparisons were made using the chi-square test or Fisher's exact test.Variables that demonstrated statistically significant differences in univariate analysis were subjected to least absolute shrinkage and selection operator(LASSO) regression to identify key predictors. These selected variables were then assessed using the variance inflation factor (VIF) test to address multicollinearity issues. Finally, the refined set of variables underwent multivariate logistic regression analysis. The model's predictive efficacy and fit were evaluated using the receiver operating characteristic (ROC) curve and the Hosmer-Lemeshow goodness-of-fit test. The area under the curve (AUC) was compared using the DeLong test. Decision curve analysis (DCA) was performed to assess the clinical utility of the model. The length of hospital stay was compared between the two groups. P < 0.05 was considered statistically significant. All tests were two-sided, with a significance level set at α = 0.05. A prognostic flow diagram is presented in Figure 1.
Figure 1
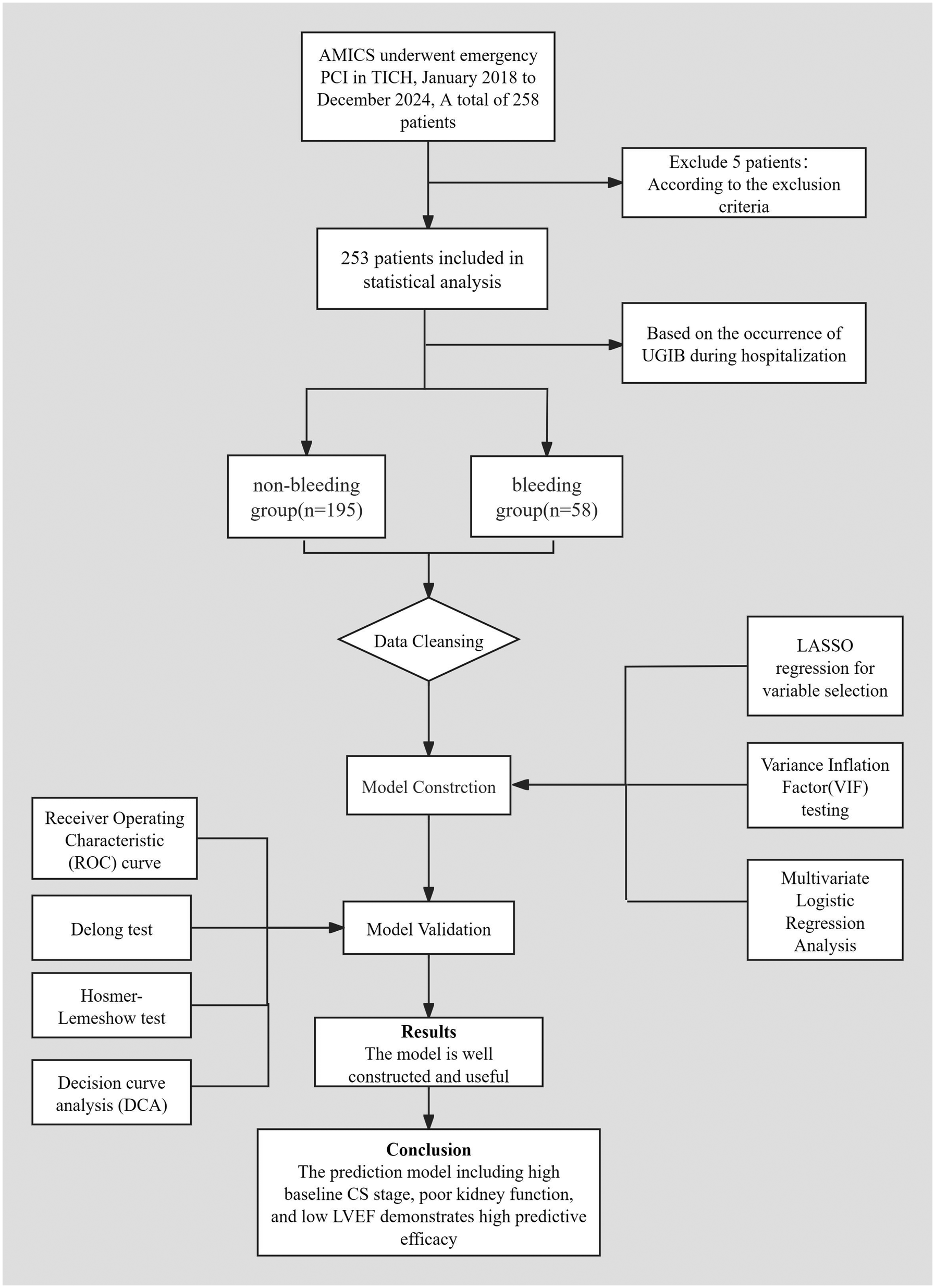
Prognostic flow diagram.
3 Results
3.1 Univariate analysis
In this study, 253 AMICS patients undergoing emergency PCI were included. Among these, 58 patients experienced UGIB during hospitalization, resulting in an incidence rate of 22.9%. Of these, 33 cases (13.0%) were classified as mild bleeding, 22 cases (8.7%) as moderate bleeding requiring transfusion, and 3 cases (1.2%) as severe bleeding, which was life-threatening. All three patients with severe bleeding exhibited abnormal coagulation function and multiple bleeding sites.
The univariate analysis indicated that the levels of uric acid, lactate, and ALT were higher in the bleeding group compared to the non-bleeding group. Conversely, the levels of eGFR, LVEF, and albumin were lower in the bleeding group. Additionally, the bleeding group exhibited a higher stage of SCAI-CS. All differences were statistically significant (P < 0.05) (Table 1).
Table 1
| Items | Non-bleeding group | Bleeding group | t/χ2/Z value | P |
|---|---|---|---|---|
| (n = 195) | (n = 58) | |||
| Age (years) | 67 (57,77) | 68 (56,77) | −0.159 | 0.873 |
| Male [Case (%)] | 134 (68.7) | 43 (74.1) | 0.625 | 0.429 |
| BMI (kg/m2) | 24.61 (22.57,26.56) | 25.39 (22.64,27.3) | −0.941 | 0.347 |
| Past medical history and personal history | ||||
| Malignant tumor [case (%)] | 7 (3.6) | 0 (0) | ⋆ | 0.357 |
| History of stroke [case (%)] | 28 (14.4) | 9 (15.5) | 0.048 | 0.827 |
| Hypertension [case (%)] | 99 (50.8) | 30 (51.7) | 0.016 | 0.898 |
| Diabetes [case (%)] | 59 (30.3) | 20 (34.5) | 0.372 | 0.542 |
| Smoking history [cases (%)] | 103 (52.8) | 26 (44.8) | 1.143 | 0.285 |
| History of Peptic ulcer [case (%)] | 6 (3.1) | 2 (3.5) | 0.000 | 1.000 |
| Baseline SBP (mmHg) | 80 (70,90) | 78 (60.75,89.25) | −1.862 | 0.063 |
| Baseline heart rate (beats/min) | 81 (50,108) | 84.5 (50,104) | −0.077 | 0.939 |
| D-dimer (ng/ml) | 2.210 | 0.530 | ||
| <500 [case(%)] | 85 (43.6) | 25 (43.1) | ||
| 500–1,000 [case(%)] | 42 (21.5) | 8 (13.8) | ||
| 1,000–5,000 [case(%)] | 58 (29.7) | 21 (36.2) | ||
| >5,000 [case(%)] | 10 (5.1) | 4 (6.9) | ||
| eGFR [ml/(min·1.73 m2)] | 71.77 (54.18,91.77) | 59.28 (39.9,75.71) | −3.400 | <0.001 |
| Uric Acid (mmol/L) | 395 (323,464) | 464 (300,535) | −3.710 | <0.001 |
| WBC (*109/L) | 11.2 (9,15.2) | 12.1 (10.3,15.3) | −1.616 | 0.106 |
| HGB (g/L) | 137.7 ± 21.6 | 136.8 ± 24.0 | 0.27 | 0.787 |
| PLT (×109/L) | 238 (197,282) | 230 (171,276) | −1.478 | 0.139 |
| CRP (mg/L) | 7.8 (2.1,31.4) | 12.6 (2.8,48.8) | −1.295 | 0.195 |
| Lactate (mmol/L) | 4.6 (2.8,7.9) | 7.3 (3.9,15.3) | −3.724 | <0.001 |
| Plasma BNP (pg/ml) | 142 (34,559) | 204 (47,643) | −0.950 | 0.342 |
| ALT (U/L) | 90 (42,169) | 157 (90,256) | −4.249 | <0.001 |
| Total cholesterol (mmol/L) | 4.2 (3.6,4.8) | 4.1 (3.1,5.1) | −0.970 | 0.332 |
| Triglyceride (mmol/L) | 1.03 (0.72,1.49) | 1.12 (0.67,1.45) | −0.047 | 0.962 |
| LDL-C (mmol/L) | 2.60 (2.06,3.21) | 2.60 (1.88,3.53) | −0.0.128 | 0.898 |
| Albumin (g/L) | 37 (35,40) | 36 (33,39) | −2.773 | 0.006 |
| LVEF (%) | 42.4 ± 11.1 | 36.3 ± 13.7 | 3.461 | <0.001 |
| LM lesion [case (%)] | 22 (11.3) | 12 (20.7) | 3.401 | 0.065 |
| History of cardiac arrest [case (%)] | 69 (35.4) | 23 (39.7) | 0.352 | 0.553 |
| Anticoagulation [case (%)] | 32 (16.4) | 10 (17.2) | 0.022 | 0.881 |
| P2Y₁₂ receptor antagonist | 0.026 | 0.871 | ||
| Ticagrelor [case (%)] | 20 (34.5) | 65 (33.3) | ||
| SCAI-CS stage | 21.830 | <0.001 | ||
| Stage C [case (%)] | 127 (65.1) | 18 (31.0) | ||
| Stage D [case (%)] | 50 (25.6) | 27 (46.6) | ||
| Stage E [case (%)] | 18 (9.2) | 13 (22.4) | ||
Univariate analysis of UGIB in AMICS patients after emergency PCI during hospitalization.
Note: ⋆Fisher's exact test was used.
The comparison across the three SCAI-CS stages was performed using the Bonferroni correction (α = 0.05/3 ≈ 0.0167). The results indicated significant differences between stage C and stage D (χ² = 15.966, P < 0.001), and between stage C and stage E (χ² = 15.339, P < 0.001). No significant difference was observed between stages D and E (χ² = 0.447, P = 0.504). Consequently, stages D and E were combined into stage D + E for subsequent analysis.
A significant difference was found between stage C and the stage D + E (χ² = 21.239, P < 0.001).
3.2 Variable selection
The 7 variables identified as statistically significant through univariate analysis were subjected to LASSO regression for dimensionality reduction and initial variable screening. A 10-fold cross-validation was employed to determine the optimal λ value, which minimized the cross-validation error. Variables with non-zero regression coefficients corresponding to the optimal λ value were further analyzed statistically. The LASSO regression results indicated a minimum error value of 0.044. At this point, the variables with non-zero regression coefficients were lactate, eGFR, LVEF, SCAI-CS stage, and albumin (Figures 2, 3). A variance inflation factor (VIF) test was conducted on the aforementioned 5 variables, and the results indicated no multicollinearity issues among them (Figure 4).
Figure 2
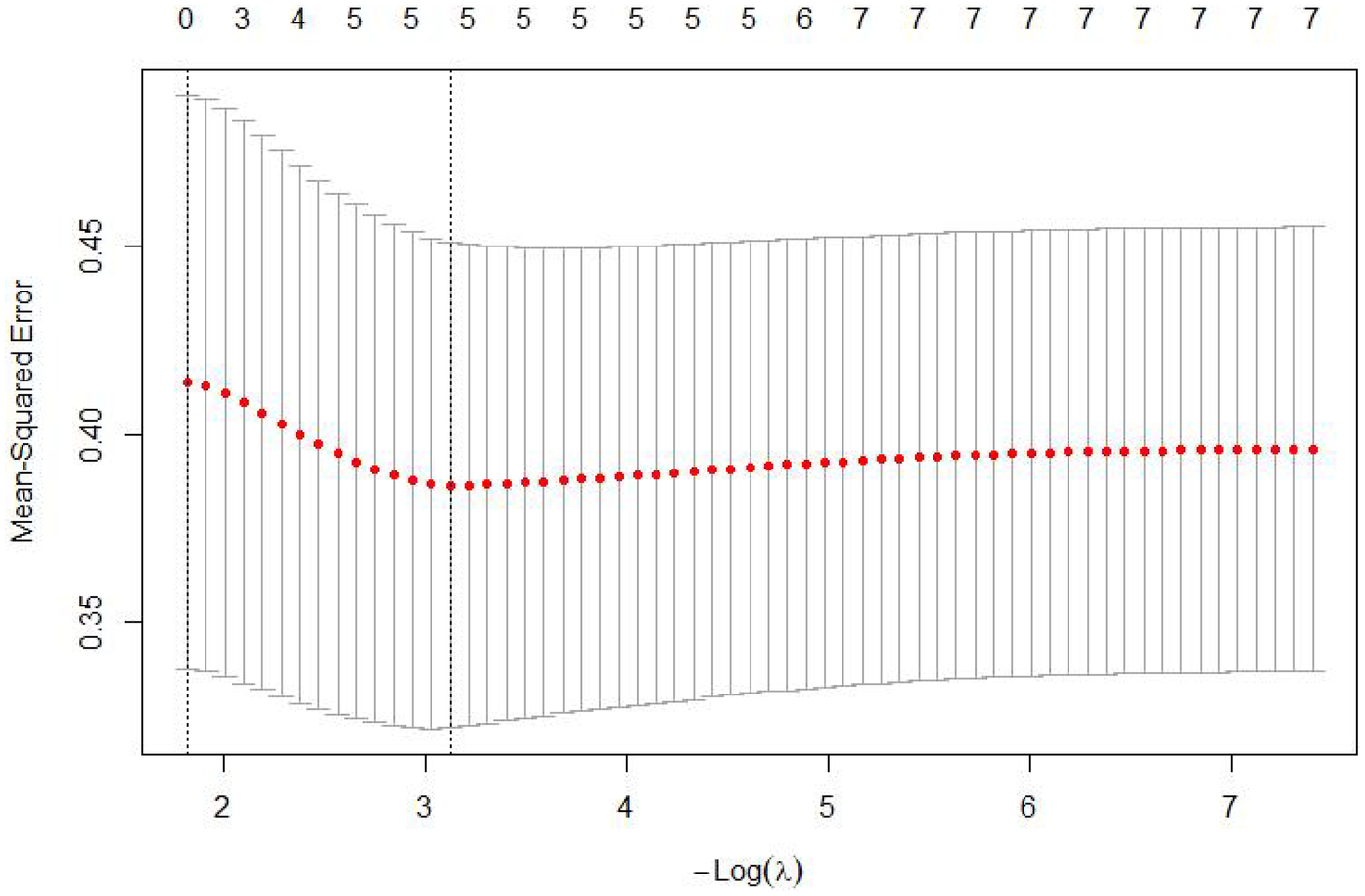
Cross-validation curve of LASSO regression.
Figure 3
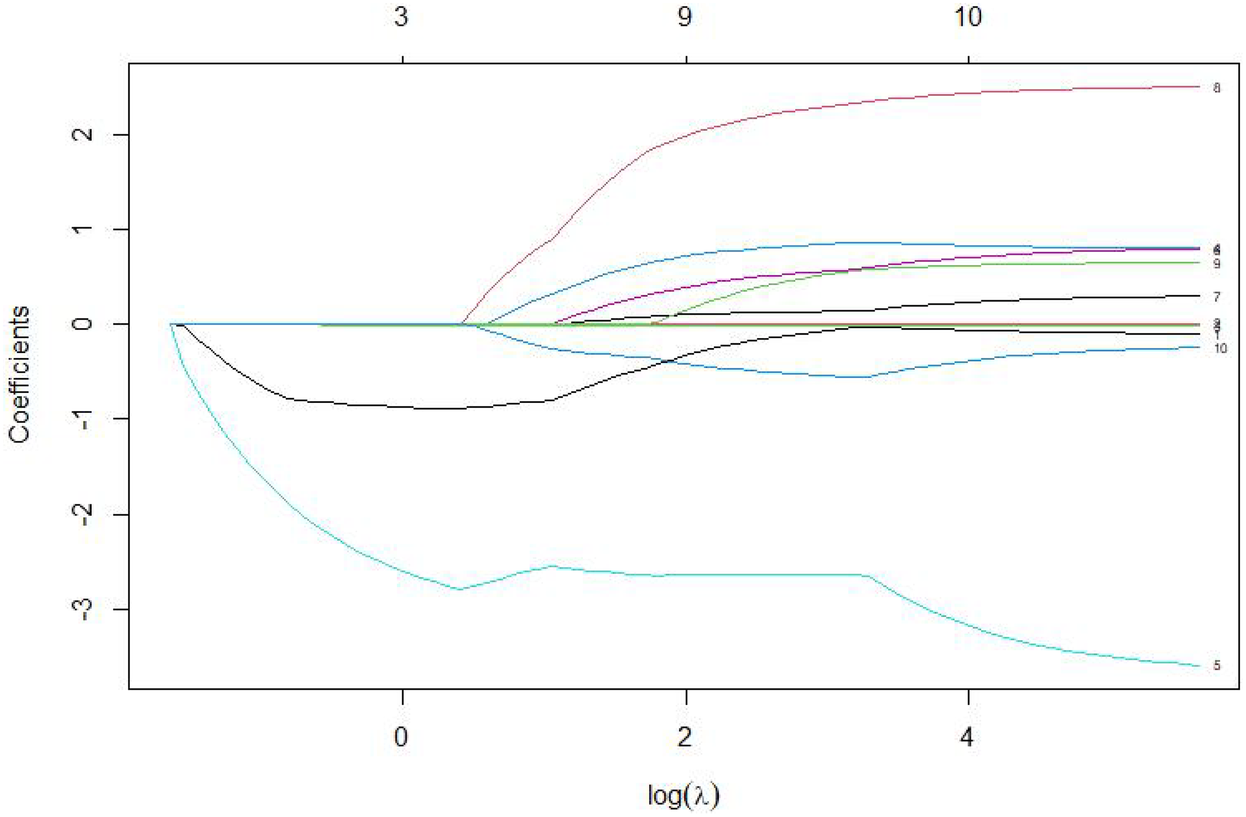
Coefficient path diagram of risk variables.
3.3 Variable assignment
Continuous variables were converted into categorical variables and assigned as follows: albumin was categorized based on whether below 35 g/L, indicating hypoproteinemia; eGFR was categorized based on whether below 45 ml/(min·1.73 m2), indicating moderate to severe renal function decline;The ROC curve was employed to identify the node with the highest Youden index, which determines the cut-off value: For lactate, the corresponding value is 6.95 mmol/L, rounded to 7 mmol/L. For LVEF, the corresponding value is 35.5%, rounded to 36%.
The specific assignments are as follows: SCAI-CS stage (C = 0, D + E = 1), lactate (<7 mmol/L = 0, ≥7 mmol/L = 1), LVEF (≥ 36% = 0, <36% = 1), hypoproteinemia (≥35 g/L = 0, <35 g/L = 1), and eGFR [≥45 ml/(min·1.73 m2) = 0, <45 ml/(min·1.73 m2) = 1].
3.4 Multivariate logistic analysis, construction and validation of a predictive model for UGIB in patients undergoing emergency PCI for AMICS
After conducting the univariate analysis and selecting variables, those with statistical significance were transformed into binary categorical variables and utilized as independent variables. The occurrence of UGIB after emergency PCI for AMICS was set as the dependent variable (no bleeding = 0, bleeding = 1). A binary multivariate logistic regression analysis was then conducted. The results identified the following as independent risk factors (P < 0.05): baseline SCAI-CS stage D + E, baseline eGFR < 45 ml/(min·1.73 m2), and baseline LVEF < 36% (Table 2).
Table 2
| Risk factors | Β value | Waldχ 2 value | OR value 95% CI | P value |
|---|---|---|---|---|
| SCAI-CS D + E | 1.023 | 8.028 | 2.781 (1.371,5.642) | 0.005 |
| eGFR < 45 ml/(min·1.73 m2) | 0.991 | 6.322 | 2.695 (1.244,5.836) | 0.012 |
| LVEF < 36% | 0.908 | 7.200 | 2.479 (1.227,4.810) | 0.007 |
Multivariate logistic analysis of UGIB in AMICS patients after emergency PCI during hospitalization.
The Hosmer-Lemeshow test yielded a χ² value of 6.968 with a P value of 0.324, indicating a good fit for the model. The ROC for predicting in-hospital UGIB indicated that for patients with baseline SCAI-CS stage D + E, baseline eGFR < 45 ml/(min·1.73 m2), baseline LVEF < 36%, and the combined indicator were 0.670 [95% CI (0.591, 0.750)], 0.654 [95% CI (0.503, 0.679)], 0.634 [95% CI (0.550, 0.718)], and 0.768 [95% CI (0.700, 0.837)], respectively (Figure 5). At the optimal cut-off for the combined indicator, the sensitivity was 87.9% [95% CI (0.700, 0.837)] and the specificity was 52.3% [95% CI (27.8%, 61.2%)].
Figure 4
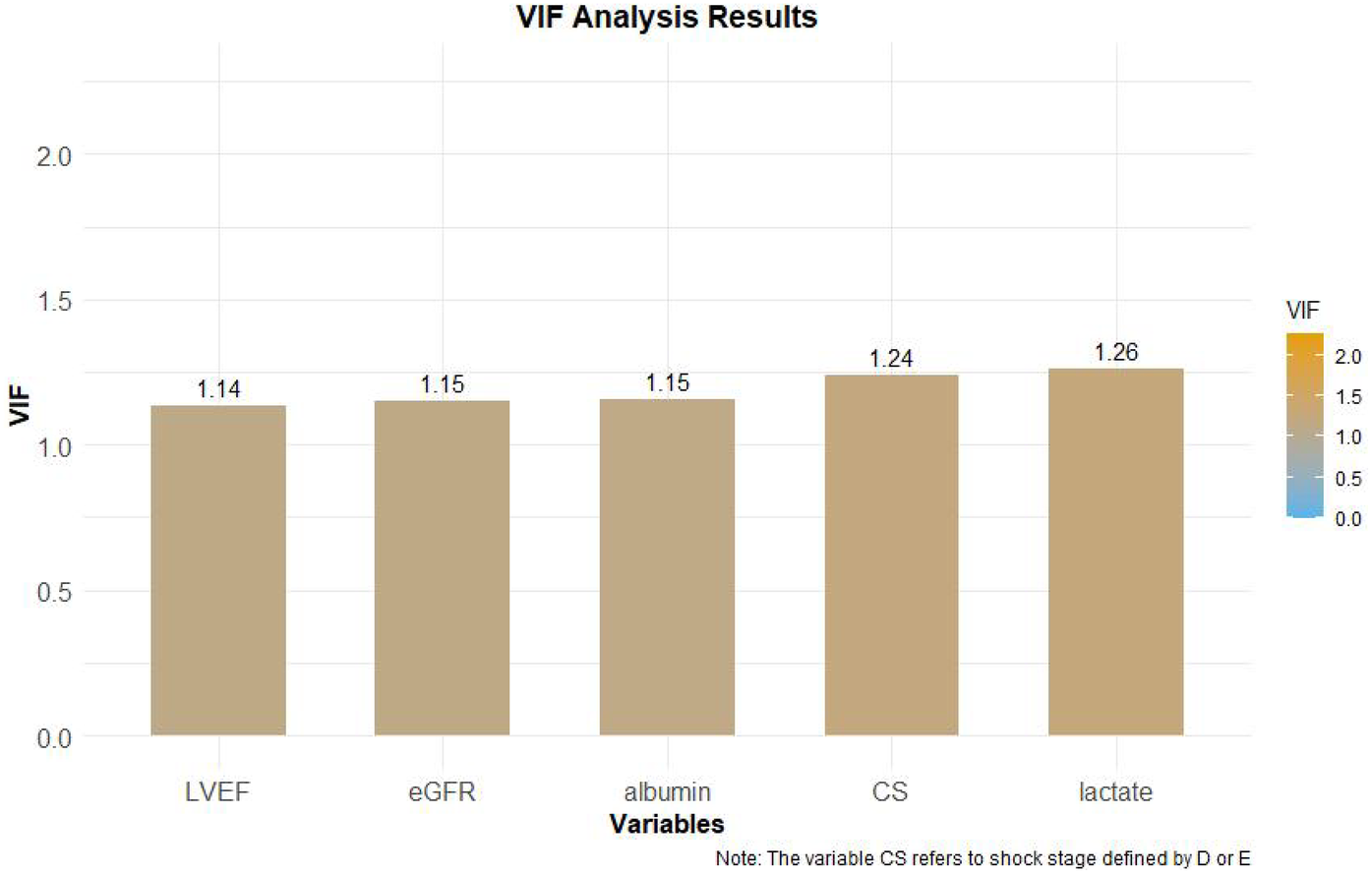
VIF test results.
The Delong test demonstrated that the AUC of the predictive model was significantly higher than that of any individual indicator (all P < 0.05), indicating superior diagnostic efficacy.
According to the DCA, this combined prediction model offers greater clinical utility when the risk threshold ranges from 0%–61% (Figure 6).
Figure 5
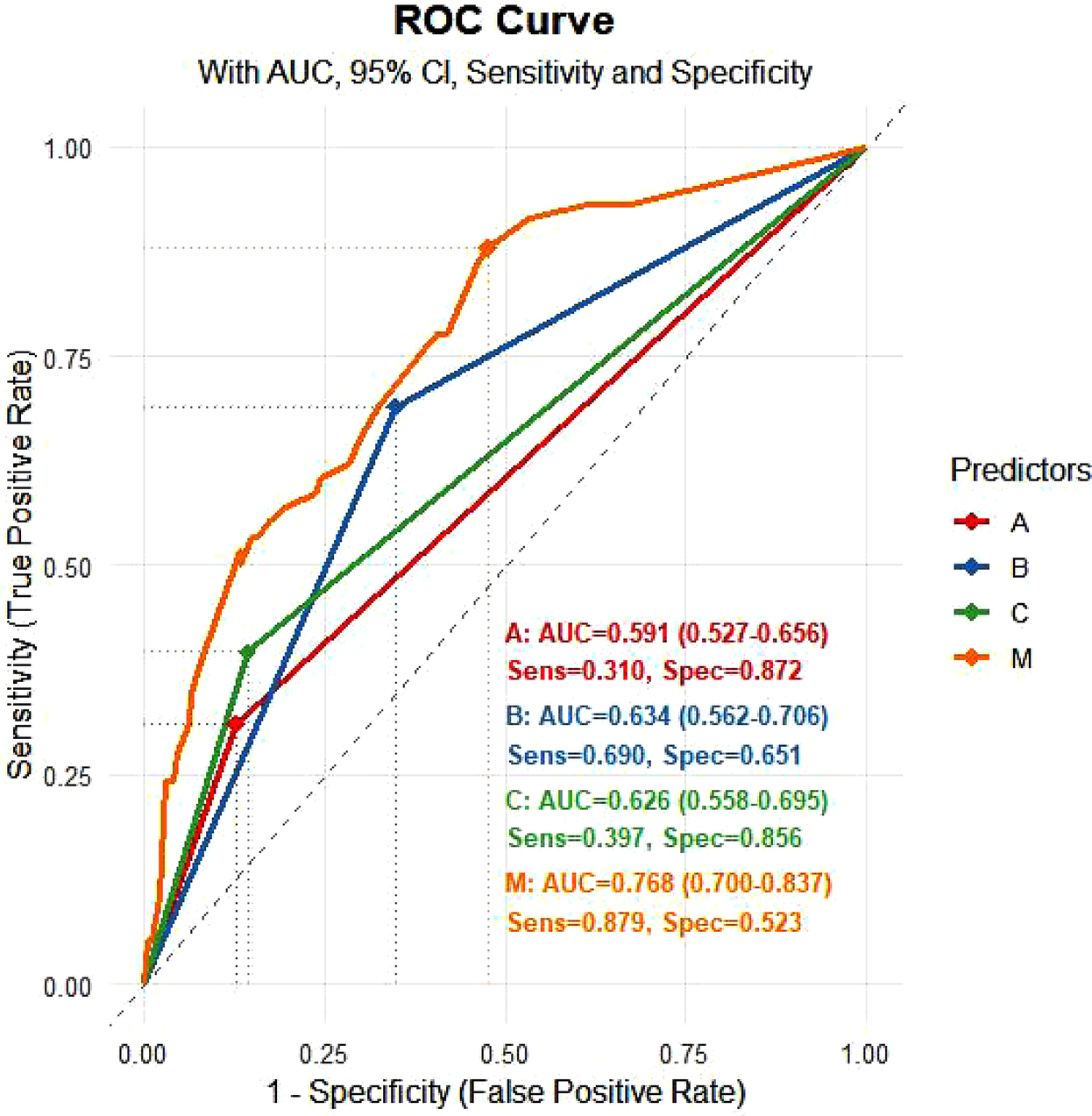
ROC curve for logistic regression (validation). (A): eGFR < 45 ml/(min·1.73 m²); (B): LVEF < 36%; (C): SCAI-CS D + E; M: prediction probability.
Figure 6
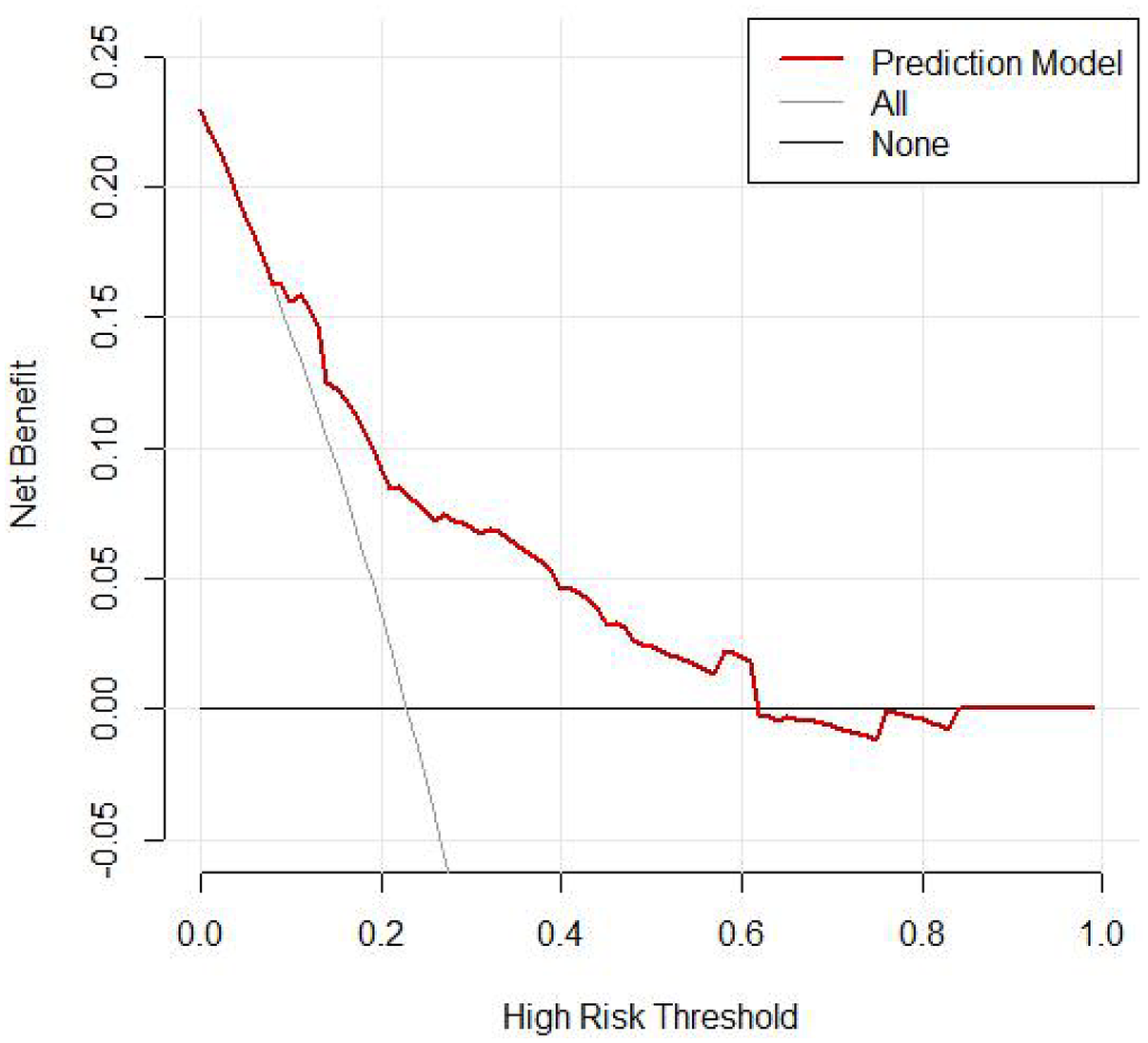
DCA curve for logistic regression (validation).
3.5 Short-term prognosis
The results indicated that the in-hospital mortality rate for patients in the bleeding group was significantly higher than that of the non-bleeding group (29.7% vs. 63.8%), with a statistically significant difference (P < 0.05). Additionally, the duration of hospital stay for surviving patients in the bleeding group was significantly longer than that of the non-bleeding group (Table 3), and this difference was also statistically significant (P < 0.05).
Table 3
| Items | Non-bleeding group | Bleeding group | Z value | P value |
|---|---|---|---|---|
| (n = 137) | (n = 21) | |||
| Hospital stays | 12 (9,16) | 19 (14,37) | −3.871 | <0.001 |
Comparison of length of hospital stay among patients who survived the hospital.
4 Discussion
4.1 The incidence of UGIB during hospitalization for AMICS after emergency PCI
Liang Zhong et al. (20) found that among the 5,868 AMI patients included in their study, gastrointestinal bleeding occurred during hospitalization. The study identified a history of diabetes, Killip class IV, and renal insufficiency as independent risk factors for gastrointestinal bleeding. A meta-analysis of risk factors for gastrointestinal bleeding in patients following PCI (21) included 16 studies involving 584,903 patients. The results indicated that age ≥60 years, a history of smoking, hypertension, renal insufficiency, a previous history of digestive tract disease, combined with CS, a diagnosis of ACS, a decline in platelet count, a decrease in baseline hemoglobin, and the use of glycoprotein IIb/IIIa inhibitors are significant risk factors for gastrointestinal bleeding in patients after PCI. Conversely, the use of PPIs was identified as a protective factor. Tian Xue et al. (22) analyzed 3,207 AMI patients at Anzhen Hospital, reporting an incidence of gastrointestinal bleeding during hospitalization of 6.4%. Their study identified combined with CS, a history of gastrointestinal bleeding, and elevated creatinine levels as risk factors for gastrointestinal bleeding in AMI patients.
Current research primarily focuses on the study of ACS and its association with gastrointestinal bleeding. These studies indicate that conditions such as hypotension and shock are significant risk factors for bleeding and are also correlated with patient prognosis. However, there is a scarcity of research on in-hospital UGIB in patients with AMICS following emergency PCI. This study specifically targets critically ill ACS patients and seeks to investigate the incidence, severity, and risk factors of UGIB in AMICS patients post-emergency PCI. It also aims to analyze the potential impact of UGIB on the prognosis of these patients. By focusing on this high-risk population, our research addresses a notable gap in existing studies regarding gastrointestinal bleeding in AMI patients. Our research shows that the incidence of UGIB during hospitalization for AMICS patients after emergency PCI was 22.9%, which is significantly higher than the reported incidence among AMI patients.
AMICS leads to a decrease in cardiac output, activation of the sympathetic nervous system, and stimulation of the renin-angiotensin-aldosterone system. These responses promote vascular constriction and cause intense contraction of gastrointestinal blood vessels, prioritizing perfusion to vital organs such as the heart and brain. The gastrointestinal mucosa is highly sensitive to ischemia and hypoxia; prolonged ischemia damages mucosal cells, impairs their function, and weakens the mucosal barrier. This enhances the susceptibility of the gastric mucosa to the corrosive effects of gastric acid and pepsin, leading to erosion, ulceration, and potentially bleeding in severe cases (23, 24). Additionally, reperfusion after PCI may exacerbate systemic oxidative stress, further damaging the gastrointestinal mucosa (25).
4.2 The predictive model and risk factors of UGIB for AMICS after emergency PCI
Our predictive model constructed for in-hospital UGIB for AMICS after emergency PCI identifies the following independent risk factors: baseline SCAI-CS stage D + E, baseline eGFR < 45 ml/(min·1.73 m2), and baseline LVEF < 36%.
Severe shock activates systemic inflammatory responses, increases gastric acid secretion, reduces mucosal defenses, and predisposes to stress ulcer formation (26). Patients with CS stage D or E, according to the SCAI classification, experience more severe systemic hypoperfusion and are more likely to receive higher doses of antithrombotic agents or pMCS. These stages are often associated with disseminated intravascular coagulation or platelet depletion, which elevate the bleeding risk (27).
An eGFR below 45 ml/(min·1.73 m2) signifies moderate to severe renal insufficiency, resulting in the accumulation of uremic toxins, impaired platelet aggregation, and adhesion functions, thereby increasing the risk of bleeding (28). Furthermore, renal dysfunction can affect the metabolism and clearance of antiplatelet and anticoagulant drugs, potentially causing drug accumulation and increased bleeding tendencies (29).
A severe reduction in LVEF results in a significant decrease in cardiac output and inadequate perfusion of systemic tissues, including the gastrointestinal tract. This condition can lead to mucosal ischemia, disruption of barrier function, and the formation of ulcers (30). Antiplatelet and anticoagulant therapies are necessary following AMI-PCI. However, a severe reduction in LVEF can prolong drug metabolism and increase cumulative exposure to these medications (31). Additionally, a significant reduction in LVEF is frequently linked to right heart failure. This can result in liver congestion, impaired liver function, and reduced production of coagulation factors—including II, VII, IX, and X—and platelets, thereby increasing the risk of bleeding (32). Research conducted by Jacob et al. (33) and others indicates that LVEF correlates with the SCAI-CS stage, with low LVEF being associated with a higher mortality rate.
The three indicators involved in this study are SCAI-CS stage, eGFR, and LVEF, which are easy to obtain and are suitable for all medical institutions.
4.3 Recent prognosis of patients with UGIB following emergency PCI for AMICS
Gastrointestinal bleeding after PCI can negatively impact the prognosis of patients with AMI and increase the risk of MACEs during the early stage, including hospitalization and within 30 days post-discharge. The in-hospital mortality rate among patients with gastrointestinal bleeding post-AMI is significantly higher than that of patients without gastrointestinal bleeding (14.3% vs. 2.1%, P = 0.047). Elevated hospital mortality is independently associated with peak TnI levels, the presence of CS, and the use of mechanical ventilators (20). Wang Lei et al. (34) also identified gastrointestinal bleeding as a risk factor for in-hospital mortality following emergency PCI in patients with AMICS.
This study is similar to the abolve study,demonstrating that the in-hospital mortality rate among patients with UGIB following emergency PCI for AMICS was significantly higher compared to patients without bleeding (63.8% vs. 29.7%, P < 0.05). Additionally, surviving patients in the bleeding group experienced significantly longer hospitalization durations than those in the non-bleeding group (P < 0.05). These findings suggest that UGIB after emergency PCI for AMICS is associated with poor prognosis, prolonged hospital stays, and increased financial burden. Therefore, preventing upper gastrointestinal bleeding in high-risk patients is of critical importance.
4.4 Prevention of UGIB patients following AMICS emergency PCI
Although the aforementioned risk factors are immutable, a comprehensive understanding of these factors enables standardized risk stratification and scientific management, facilitating the development of personalized antithrombotic plans. While the predictive model's specificity is only 52.3%, for a severe complication such as “AMICS emergency PCI postoperative UGIB” which has an in-hospital mortality rate of 63.8%, the primary goal of the model is to “maximize the identification of high-risk individuals to avoid missed diagnoses and prevent deaths,” rather than to pursue “absolute high specificity to reduce false diagnoses.” The core value of the model is “identifying high-risk individuals” and providing early prevention. PPIs elevate gastric juice pH by inhibiting gastric acid secretion, thereby promoting blood clot formation, stabilizing existing clots, and accelerating lesion healing (35). In the early stages, intravenous administration of PPIs, H2 receptor antagonists, and gastric mucosal protectors should be implemented to reduce the likelihood of bleeding (36).Given the high mortality rate among patients with UGIB, to enhance clinical translatability, we have developed and validated a simplified bedside scoring system. For example, assigning 1 point for each risk factor (SCAI-CS D + E = 1, LVEF < 36% = 1, eGFR < 45 = 1) creates a 0–3 point scale, with a score of ≥2 corresponding to high risk.Once high-risk patients have been identified, intravenous proton pump inhibitor (PPI) preparations should be administered promptly to prevent UGIB.
5 Study strengths
Firstly, by addressing a gap in existing research, we developed a composite indicator that integrates three complementary dimensions: the SCAI-CS stage (shock severity), LVEF (cardiac function), and eGFR (renal function).Unlike previous studies that relied on single-domain predictors, such as isolated eGFR or LVEF, our indicator captures the synergistic effects of multi-organ dysfunction on UGIB risk. This approach aligns with the clinical reality that AMICS-related UGIB is rarely driven by a single factor. For instance, our sensitivity analysis demonstrated that excluding any one of the three components reduced the model's AUC by 8.2%–11.5%, confirming the added value of this multi-dimensional integration.Secondly, the model exhibits strong clinical relevance and practicality. It utilizes only routine, bedside-measurable indicators: SCAI-CS stage is assessed via standard clinical examination, LVEF through rapid emergency echocardiography (a standard test for AMICS patients), and eGFR via point-of-care serum creatinine testing. Consequently, a single cardiologist can apply our model within 30 min of patient admission, addressing the “time-sensitive” need for UGIB risk stratification in AMICS patients undergoing emergency PCI. Additionally, we performed three different internal validations, including the Hosmer-Lemeshow test, the ROC curve, and the DCA of the predictive model, all of which indicated that the model offers good clinical utility.
6 Limitations
First, it is a single-center, retrospective cohort study, which may induce selection bias and information bias. Second, the relatively small patient cohort may reduce the statistical power, affecting the stability and reliability of the results, and limiting in-depth analysis of complex factor interactions. The third and most critical point is that this study did not conduct external validation of the predictive model, which is the primary factor limiting the general applicability of the model. Without external validation, we cannot confirm whether our model would perform similarly in non-third-level hospitals, regions with different racial compositions (such as areas with a higher prevalence of Helicobacter pylori infection), or populations with more comorbidities (such as patients with chronic liver diseases)—all of which may alter the risk of UGIB and the performance of the model. Due to practical operational limitations, we were unable to collect independent external sample groups. We are well aware that this is a significant limitation, which weakens the clinical generalizability of the model.
7 Future research recommendations
Firstly, the top priority is to validate the model in external, multi-center cohorts, including non-tertiary hospitals and regions with diverse ethnic and demographic characteristics. Secondly, to improve the model's moderate specificity (52.3%), future research should explore incorporating additional predictors, such as the use anticoagulants pre-PCI, genetic markers, and biomarkers of mucosal damage. A larger sample size (target n = 1,000) will enable multivariate analysis to determine which additional predictors most effectively improve specificity without reducing sensitivity.
8 Conclusions
This study constructed a predictive model, which demonstrates that high baseline SCAI-CS stage, poor kidney function, and low LVEF are independent risk factors for UGIB during hospitalization. The constructed predictive model demonstrates high predictive efficacy.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by TEDA International Cardiovascular Hospital Research Project Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FD: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. YZ: Conceptualization, Data curation, Writing – review & editing, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. YZ: Formal analysis, Methodology, Validation, Writing – review & editing. YZ: Formal analysis, Project administration, Writing – review & editing. HL: Data curation, Project administration, Writing – review & editing. YS: Data curation, Methodology, Project administration, Visualization, Writing – review & editing. RJ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funded by Tianjin Key Medical Discipline Construction Project (Grant No.TJYXZDXK-3-035C); The hospital-level research project of TEDA International Cardiovascular Hospital (2024-TD-005); Tianjin - Science and Technology Project of the Health Commission of Binhai New Area Tianjin (2022BWKQ006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
UGIB, upper gastrointestinal bleeding; PCI, percutaneous coronary intervention; AMICS, acute myocardial infarction combined with cardiogenic shock; ROC, receiver operating characteristic; AUC, area under the curve; DCA, decision curve analysis; CS, cardiogenic shock; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction; ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; Pmcs, percutaneous mechanical circulatory support; SBP, systolic blood pressure; MAP, mean arterial pressure; BMI, body mass index; BNP, B-type natriuretic peptide; SCAI-CS, American society for cardiovascular angiography and interventions-cardiogenic shock.
References
1.
Joglar JA Chung MK Armbruster AL Benjamin EJ Chyou JY Cronin EM et al 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American college of ardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2024) 149:e1–e156. 10.1161/CIR.0000000000001193
2.
Thomsen AF Jacobsen PK Køber L Joergensen RM Huikuri HV Bloch Thomsen PE et al Risk of arrhythmias after myocardial infarction in patients with left ventricular systolic dysfunction according to mode of revascularization: a cardiac arrhythmias and risk stratification after myocardial infarction (CARISMA) substudy. EP: Europace. (2021) 23:616–23. 10.1093/europace/euaa273
3.
Scholz KH Maier SK Maier LS Lengenfelder B Jacobshagen C Jung J et al Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. (2018) 39:1065–74. 10.1093/eurheartj/ehy004
4.
Hochman JS Sleeper LA Webb JG Sanborn TA White HD Talley JD et al Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. (1999) 341:625–34. 10.1056/NEJM199908263410901
5.
Axelsson TA Mennander A Malmberg M Gunn J Jeppsson A Gudbjartsson T . Is emergency and salvage coronary artery bypass grafting justified? The Nordic emergency/ salvage coronary artery bypass grafting study. Eur J Cardiothorac Surg. (2016) 49:1451–6. 10.1093/ejcts/ezv388
6.
Liakopoulos OJ Schlachtenberger G Wendt D Choi YH Slottosch I Welp H et al Early clinical outcomes of surgical myocardial revascularization for acute coronary syndromes complicated by cardiogenic shock: a report from the North-Rhine-Westphalia surgical myocardial infarction registry. J Am Heart Assoc. (2019) 8:e012049. 10.1161/JAHA.119.012049
7.
Møller JE Engstrøm T Jensen LO Eiskjær H Mangner N Polzin A et al Microaxial flow pump or standard care in infarct-related cardiogenic shock. N Engl J Med. (2024) 390:1382–93. 10.1056/NEJMoa2312572
8.
Ronco D Matteucci M Ravaux JM Marra S Torchio F Corazzari C et al Mechanical circulatory support as a bridge to definitive treatment in post-infarction ventricular septal rupture. J Am Coll Cardiol Intv. (2021) 14:1053–66. 10.1016/j.jcin.2021.02.046
9.
Elbadawi A Elgendy IY Mahmoud K Barakat AF Mentias A Mohamed AH et al Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. J Am Coll Cardiol Intv. (2019) 12:1825–36. 10.1016/j.jcin.2019.04.039
10.
Matteucci M Fina D Jiritano F Meani P Raffa GM Kowalewski M et al The use of extracorporeal membrane oxygenation in the setting of postinfarction mechanical complications: outcome analysis of the extracorporeal life support organization registry. Interact Cardiovasc Thorac Surg. (2020) 31:369–74. 10.1093/icvts/ivaa108
11.
Ostadal P Rokyta R Karasek J Kruger A Vondrakova D Janotka M et al Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO-CS randomized clinical trial. Circulation. (2023) 147:454–64. 10.1161/CIRCULATIONAHA.122.062949
12.
Thiele H Zeymer U Thelemann N Neumann FJ Hausleiter J Abdel-Wahab M et al Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. (2019) 139:395–403. 10.1161/CIRCULATIONAHA.118.038201
13.
Vlachakis PK Theofilis P Leontsinis I Drakopoulou M Karakasis P Oikonomou E et al Bridge to life: current landscape of temporary mechanical circulatory support in heart-failure-related cardiogenic shock. J Clin Med. (2024) 13(14):4120. 10.3390/jcm13144120
14.
Thiele H Zeymer U Akin I Behnes M Rassaf T Mahabadi AA et al Extracorporeal life support in infarct-related cardiogenic shock. N Engl J Med. (2023) 389:1286–97. 10.1056/NEJMoa2307227
15.
Witten Davodian L Larsen JK Povlsen AL Josiassen J Helgestad OK Udesen NL et al Timing and causes of death in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. (2022) 11(Supplement_1):zuac041–042. 10.1016/j.amjcard.2022.01.050
16.
Baran DA Grines CL Bailey S Burkhoff D Hall SA Henry TD et al SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American college of cardiology (ACC), the American heart association (AHA), the society of critical care medicine (SCCM), and the society of thoracic surgeons (STS) in April 2019. Catheter Cardiovasc Interv. (2019) 94(1):29–37. 10.1002/ccd.28329
17.
Yu B Yanqing L Xu R . Expert suggestions on prevention and treatment of stress ulcers (2018 edition). Chin J Med. (2018) 98(42):3392–5. 10.3760/cma.j.issn.0376-2491.2018.42.003
18.
Emergency Physicians Branch of the Chinese Medical Association. Expert consensus on emergency diagnosis and treatment of acute upper gastrointestinal bleeding. Chin Emerg Med. (2010) 30(4):289–93. 10.3969/j.issn.1002-1949.2010.04.001
19.
Gusto Investigators. An international randomized trial comparing four thrombolytic strategies for acutemyocardial infarction. N Engl J Med. (1993) 329(10):673–82. 10.1056/NEJM199309023291001
20.
Zhong L Quan X Dang P Tang M Yu H Guo F . Clinical characteristics and risk factors of in-hospital gastrointestinal bleeding in patients with acute myocardial infarction. Front Cardiovasc Med. (2022) 9:933597. 10.3389/fcvm.2022.933597
21.
Haihuan F Shuangyin C Siqi H Xiaoyu F Xiaobing W . Meta-analysis of risk factors for gastrointestinal bleeding in patients after PCI. Evid Based Nurs. (2024) 10(22):4011–8. 10.12102/j.issn.2095-8668.2024.22.004
22.
Xue T Xinyue X Hong L . Analysis of risk factors for in-hospital gastrointestinal bleeding in patients with acute myocardial infarction. J Cardiovasc Pulm Dis. (2023) 42(7):662–5. 10.3969/j.issn.1007-5062.2023.07.004
23.
Reynolds HR Hochman JS . Cardiogenic shock: current concepts and improving outcomes. Circulation. (2008) 117(5):686–97. 10.1161/CIRCULATIONAHA.106.613596
24.
Grenz A Clambey E Eltzschig HK . Hypoxia signaling during intestinal ischemia and inflammation. Curr Opin Crit Care. (2012) 18(2):178–85. 10.1097/MCC.0b013e3283514bd0
25.
Baran DA Grines CL Bailey S Burkhoff D Hall SA Henry TD et al SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American college of cardiology (ACC), the American heart association (AHA), the society of critical care medicine (SCCM), and the society of thoracic surgeons (STS) in April 2019. Catheter Cardiovasc Interv. (2019) 94(1):29–37. 10.1002/ccd.28329
26.
Heusch G . Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. (2020) 17(12):773–89. 10.1038/s41569-020-0403-y
27.
Cook D Deane A Lauzier F Zytaruk N Guyatt G Saunders L et al Stress ulcer prophylaxis during invasive mechanical ventilation. N Engl J Med. (2024) 391(1):9–20. 10.1056/NEJMoa2404245
28.
Lin Y Li C Waters D Kwok CS . Gastrointestinal bleeding in chronic kidney disease patients: a systematic review and meta-analysis. Renal Fail. (2023) 45(2):2276908. 10.1080/0886022X.2023.2276908
29.
Natale P Palmer SC Saglimbene VM Ruospo M Razavian M Craig JC et al Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. (2022) 2(2):CD008834. 10.1002/14651858.CD008834.pub4
30.
Thiele H Ohman EM de Waha-Thiele S Zeymer U Desch S . Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. (2019) 40(32):2671–83. 10.1093/eurheartj/ehz363
31.
Robert F S . More transparency for a therapeutic window in platelet P2Y12 inhibition?Eur Heart J. (2015) 36(27):1714–7. 10.1093/eurheartj/ehv137
32.
Kim JH Shah P Tantry US Gurbel PA . Coagulation abnormalities in heart failure:pathophysiology and therapeutic implications. Curr Heart Fail Rep. (2016) 13(6):319–28. 10.1007/s11897-016-0308-6
33.
Jentzer JC Wiley BM Anavekar NS Pislaru SV Mankad SV Bennett CE et al Noninvasive hemodynamic assessment of shock severity and mortality risk prediction in the cardiac intensive care unit. JACC Cardiovasc Imaging. (2021) 14(2):321–32. 10.1016/j.jcmg.2020.05.038
34.
Lei W Xiaohui Z Yue Z Wei C . Risk factors for in-hospital mortality after emergency PCI in patients with acute myocardial infarction complicated with cardiogenic shock. Occup Health Emerg Rescue. (2020) 38(1):15–9. 10.16369/j.oher.issn.1007-1326.2020.01.004
35.
Kanno T Yuan Y Tse F Howden CW Moayyedi P Leontiadis GI . Proton pump inhibitortreatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. Cochrane Database Syst Rev. (2022) 1(1):CD005415. 10.1002/14651858.CD005415.pub4
36.
Wang Y Ye Z Ge L Siemieniuk RA Wang X Wang Y et al Efficacy and safety of gastrointestinal bleeding prophylaxis in critically ill patients: systematic review and network meta-analysis. Br Med J. (2020) 368:l6744. 10.1136/bmj.l6744
Summary
Keywords
acute myocardial infarction, cardiogenic shock, upper gastrointestinal bleeding, predictive model, prognosis
Citation
Dong F, Zhou Y, Zhao Y, Zhang Y, Liang H, Song Y and Jing R (2025) A predictive model for upper gastrointestinal bleeding in patients with acute myocardial infarction complicated by cardiogenic shock during hospitalization. Front. Cardiovasc. Med. 12:1662067. doi: 10.3389/fcvm.2025.1662067
Received
08 July 2025
Accepted
17 September 2025
Published
02 October 2025
Volume
12 - 2025
Edited by
António Tralhão, Hospital de São José, ULS S. José, Portugal
Reviewed by
Ikhwan Rinaldi, RSUPN Dr. Cipto Mangunkusumo, Indonesia
Halil İbrahim Doru, Horasan State Hospital, Türkiye
Updates
Copyright
© 2025 Dong, Zhou, Zhao, Zhang, Liang, Song and Jing.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Rui Jing nkjr1979@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.