- 1Department of Cardiovascular Medicine, Liyang Hospital of Traditional Chinese Medicine, Liyang, Jiangsu, China
- 2Department of Operating Room, Liyang People’s Hospital, Liyang, Jiangsu, China
Objective: The aim of this study was to investigate the effects of exercise rehabilitation training on left ventricular ejection fraction (LVEF) and quality of life in patients after percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI).
Methods: This study included 180 patients with AMI who underwent PCI between January 2022 and February 2024, and the patients were divided into control group (n = 90) and exercise group (n = 90) according to the randomized numeric table method. The control group was given routine care, and the exercise group was given rehabilitation exercise training on the basis of the control group. The rehabilitation exercise training lasted for 3 months, covering the inpatient rehabilitation period and the post-discharge rehabilitation period, with low-intensity bed and bedside activities as the main focus during the inpatient period, and aerobic exercise combined with strength and flexibility training as the main focus after discharge. The primary endpoint was LVEF at 3 months. Key secondary endpoints included exercise capacity [6-minute walk distance (6MWD)], quality of life [36-Item Short Form Health Survey (SF-36)], biomarkers of inflammation/oxidative stress, and adverse cardiovascular events.
Results: Baseline characteristics were similar between groups (P > 0.05). After 3 months, LVEF improved in both groups, with a greater increase in the exercise group (between-group P ≤ 0.01). Exercise rehabilitation also improved 6MWD and SF-36 domains and reduced adverse cardiovascular events [8/90 (8.9%) vs. 18/90 (20.0%); risk ratio 0.44; 95% confidence interval (CI) 0.20–0.97; P = 0.034].
Conclusion: Adding exercise rehabilitation to usual care after PCI for AMI improved LVEF and key clinical outcomes. These findings support routine integration of structured exercise rehabilitation in post-PCI care.
Introduction
Acute Myocardial Infarction (AMI) is a common and serious coronary artery disease characterized by stenosis or occlusion of coronary arteries further leading to hypoxia and ischemia of myocardial tissue (1). In recent years, its morbidity and mortality are at a high level globally with the changes in lifestyle and the acceleration of population aging (2). Despite significant advances in medical technology in recent years, Percutaneous Coronary Intervention (PCI) has become one of the mainstays of treatment for AMI by virtue of its significant advantage of being able to rapidly unblock infarct-related vessels and restore myocardial perfusion in a timely manner (3, 4).
However, PCI is not the end point of treating AMI, PCI can only temporarily relieve the mechanical stenosis or occlusion of coronary arteries, and the physiopathological process of atherosclerosis in patients does not terminate after PCI. In addition, the sudden onset of AMI can bring a double blow to the patient's body and psychology, and the decline in cardiac function, decreased exercise endurance, and malignant arrhythmia can occur after the operation, which affects the patient's life quality and prognosis (5–7). With the continuous development of medical science and technology, people have a more profound knowledge of cardiovascular diseases and gradually become dissatisfied with the traditional clinical treatment, and it may be difficult to fully meet the rehabilitation needs of patients relying only on medication and routine care (6, 8). Cardiac rehabilitation is based on measures designed to help patients minimize recovery time after a cardiac event and maximize physical, social, and psychological performance, aiming to promote health behaviors and improve patient health and outcomes (9, 10).
Physiologically, the heart can adapt to chronic exercise to meet the body's increased oxygen demand, a process known as “remodeling” (11, 12). A growing number of studies have shown that exercise rehabilitation training, as an effective adjunctive therapy, has a significant role in improving cardiac function, psychological status, and quality of life in patients with cardiovascular disease (13). For example, a meta-analysis of the effects of exercise training on left ventricular remodeling after myocardial infarction showed that exercise training had a beneficial effect on left ventricular remodeling in clinically stable postmyocardial infarction patients, with the greatest benefit when training was initiated early after myocardial infarction (starting at 1 week) and continued for more than 3 months (14). Early exercise-based rehabilitation is effective in improving cardiorespiratory fitness, health-related quality of life and functional capacity after AMI (15, 16).
Based on the above background, this study aims to systematically assess the effects of exercise rehabilitation on cardiac function and quality of life in patients with AMI after PCI, to further explore the underlying mechanisms, and to provide a scientific basis for clinical practice. Specifically, we will compare the differences between exercise rehabilitation and conventional care in improving patients' cardiac function and quality of life through a randomized controlled trial design, with the aim of providing new ideas for optimizing postoperative rehabilitation strategies for AMI patients.
Materials and methods
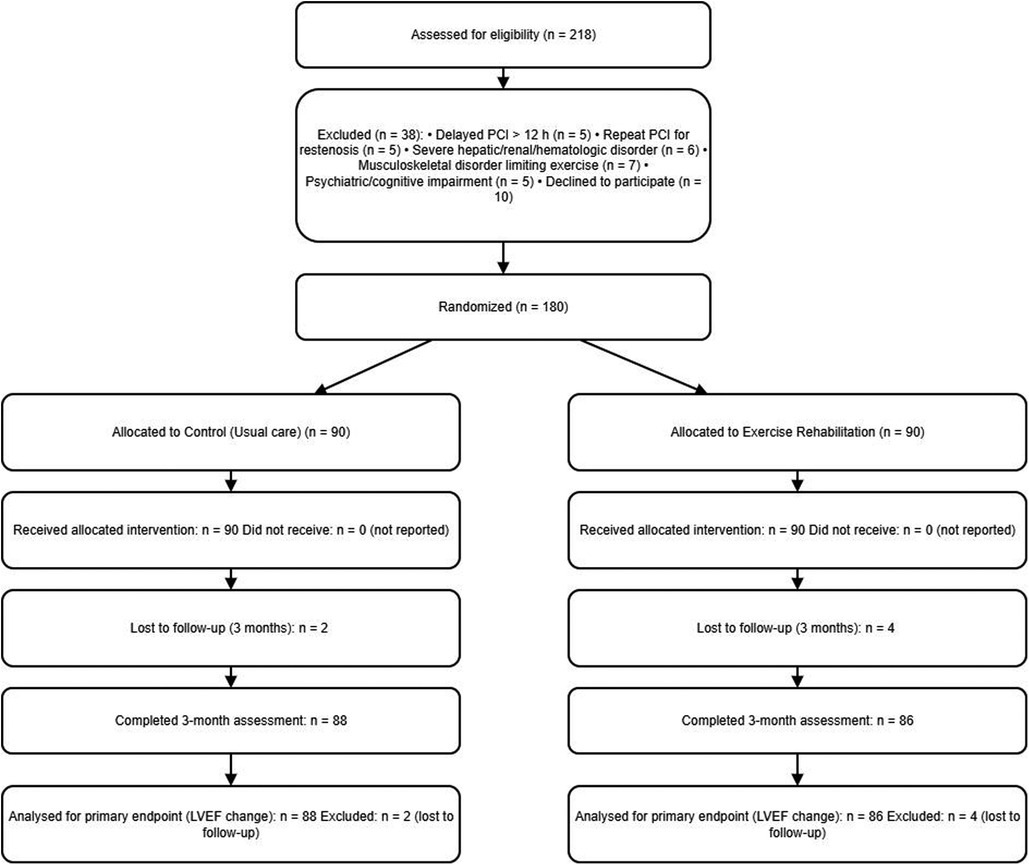
The study flowchart is provided as Figure 1.
Study population
218 AMI patients admitted to our cardiology department who underwent PCI from January 2022 to February 2024 were collected. Eligible patients provided written informed consent and were randomized within 24–48 h after PCI once hemodynamic stability was confirmed (no recurrent chest pain or ongoing ischemia, no vasoactive support, Killip class ≤ II). Randomization occurred before any rehabilitation exercise. Follow-up and outcome ascertainment began at randomization. Eligible, clinically stable patients were randomized 1:1 to usual care (control) or exercise rehabilitation in addition to usual care. The study was approved by the Ethics Committee of our hospital and complied with the Declaration of Helsinki.
Inclusion and exclusion criteria
Inclusion criteria: 1. in accordance with the Guidelines for the Diagnosis and Treatment of Acute ST-segment Elevation Myocardial Infarction and confirmed by electrocardiogram (ECG); 2. Successful primary PCI within 12 h of symptom onset; 3. Clinically stable within 24–48 h post-PCI (no recurrent chest pain or ongoing ischemia, no vasoactive/inotropic support, Killip class ≤ II). voluntarily participating in this study and signing an informed consent form, and being able to cooperate with the training and regular review after discharge from the hospital.
Exclusion criteria: 1. Non–ST-elevation myocardial infarction (NSTEMI) or unstable angina as the index presentation; 2. Repeat PCI before randomization (in-hospital re-intervention after the index AMI PCI) or in-stent restenosis (ISR)–related AMI as the index event requiring target-lesion revascularization; 3. Severe arrhythmia and undergoing heart transplantation, heart valve replacement, pacemaker implantation and other surgeries; 4. Severe comorbid systemic diseases, defined as: Liver dysfunction: alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 3× upper limit of normal (ULN), or total bilirubin > 2× ULN; Renal dysfunction: estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; Hematopoietic disorders: hemoglobin < 9 g/dL, platelets < 100 × 10⁹/L, or leukocytes < 3.0 × 10⁹/L; Severe psychiatric illness: active psychosis, major depression, or cognitive impairment that limits adherence to rehabilitation; 5. Musculoskeletal disorders (fracture, osteoarthritis, synovitis, or other conditions) limiting safe participation in exercise; 6 Combined with psychiatric diseases, cognitive dysfunction. Functional disorders.
Primary endpoint
Change in left ventricular ejection fraction (LVEF, %) from baseline (post-PCI, pre-randomization) to 3 months, assessed by transthoracic echocardiography following standard recommendations.
Secondary endpoints
Fractional shortening (FS), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), 6-minute walk distance (6MWD), 36-Item Short Form Health Survey domain scores (SF-36); inflammatory markers—high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), soluble intercellular adhesion molecule-1 (sICAM-1); oxidative stress markers—superoxide dismutase (SOD), total antioxidant capacity (T-AOC), catalase (CAT), malondialdehyde (MDA), and a 3-month composite of adverse cardiovascular events.
Sample size estimation
The trial was powered for the primary endpoint, change in left ventricular ejection fraction (LVEF) from baseline (post-PCI, pre-randomization) to 3 months. Based on prior exercise-based cardiac rehabilitation literature and local variability, we assumed a between-group difference in mean LVEF change of Δ = 4 percentage points with a standard deviation of change σ = 8. Using a two-sided α = 0.05% and 90% power, the required sample size per group for a parallel-group comparison is:
which with , , σ = 8, and Δ = 4 gives n ≈ 84 per group (total ≈ 168). Allowing for ∼5%–7% attrition, we pre-specified a target of 90 per group (total 180) and enrolled accordingly.
Treatment
Nursing measures in the control group: patients in the control group were given conventional drug therapy, including antiplatelet aggregation (e.g., aspirin combined with clopidogrel or tegretol), statin lipid modulation (e.g., atorvastatin, rasuvastatin), β-blockers (e.g., metoprolol, bisoprolol), angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin II receptor antagonists (ARBs) (e.g., enalapril, valsartan) and other drugs to improve myocardial ischemia, prevent thrombosis, and control blood pressure and blood lipids. At the same time, routine nursing interventions were carried out, including close monitoring of vital signs, dietary guidance (low-salt, low-fat, low-sugar diet), rest and activity guidance (absolute bed rest during the acute stage, gradually increasing the amount of activity), psychological care, etc. Routine health education was also carried out to inform patients of disease-related knowledge, drug intake methods and precautions.
Exercise group rehabilitation exercise training program: The exercise group, on the basis of treatment and care in the control group, implemented a 3-month exercise rehabilitation training program according to the different characteristics of the hospitalization rehabilitation period and the post-discharge rehabilitation period. The specific program is as follows:
1. Inpatient rehabilitation period: patients’ postoperative conditions are relatively unstable and their bodies are relatively weak, so the main purpose of the rehabilitation exercise at this stage is to promote the initial recovery of body functions, enhance the patients’ ability to adapt to exercise, and at the same time ensure the safety of exercise.
2. Exercise type: bed activities and bedside activities are the main activities. Bedside activities include passive and active activities of the limbs, such as flexion and extension of the joints of the limbs, slow flexion and extension of each joint for 5∼10 times, 3∼4 groups per day, which can promote the blood circulation of the limbs and prevent muscle atrophy and joint stiffness; deep breathing training, the patient takes a comfortable position, slowly inhale to make the abdomen bulge, and then slowly exhale, each training for 5∼10 min, 3∼4 times per day, which can help to improve the pulmonary This will help improve the ventilation function of the lungs. Bedside activities start from sitting up training, the patient gradually sits up from the lying position with the assistance of medical and nursing staff, and then conducts bedside standing training after adapting to it, and the time for standing up starts from 5∼10 min each time, and is gradually prolonged according to the patient's tolerance situation, and is conducted 2∼3 times a day.
Exercise intensity
During the inpatient phase, exercise intensity was maintained at a low level to ensure safety and gradual recovery. Intensity was prescribed according to the patient's tolerance, targeting an increase of ≤20 beats/min from resting heart rate, corresponding approximately to ≤40% of heart rate reserve (HRR), 2–3 METs, or an RPE of 9–11 on the Borg 6–20 scale, as recommended by the AHA and ESC guidelines for early cardiac rehabilitation. Exercise was terminated if palpitations, chest tightness, or excessive fatigue occurred, and vital signs were continuously monitored.
Exercise time
The total duration of each exercise should be controlled at 10∼15 min, and can be gradually increased to about 20 min with the improvement of the patient's physical condition. The exercise process is divided into preparatory activities (2∼3 min, such as simple body relaxation activities), formal exercise (5∼12 min) and relaxation activities (3∼5 min, such as slow deep breathing and muscle relaxation exercises).
Exercise frequency
2∼3 times of exercise training per day, and the number of training sessions can be increased appropriately if tolerated by the patient's body.
3. Post-discharge rehabilitation period: the patient's body functions have been recovered to some extent, and at this stage, on the basis of ensuring safety, the intensity and difficulty of exercise are gradually increased to further improve cardiopulmonary function and exercise endurance, and to promote the recovery of cardiac function and the enhancement of quality of life.
Exercise type
Aerobic exercise, combined with appropriate amount of strength training and flexibility training. Aerobic exercise includes walking, choosing a place with fresh air and flat ground, walking at a moderate pace (about 60∼80 steps per minute) and gradually transitioning to fast walking (about 100∼120 steps per minute); jogging, starting to try when the patient's ability to walk is good and there is no discomfort, paying attention to maintaining a smooth rhythm and breathing; cycling, choosing to ride outdoors or use indoor fitness bike. Riding speed is adjusted according to the patient's own condition. Strength training using elastic bands or small weight dumbbells, such as arm flexion and extension, shoulder abduction for the upper limbs, leg extension for the lower limbs, leg lifting and other exercises, each action is performed 8∼12 times, each interval of 1∼2 min, each time 2∼3 groups, in order to enhance muscle strength and improve the body's metabolic rate. Flexibility training through simple stretching movements, such as neck, waist and leg stretching, hold each movement for 15∼30 s, perform 2∼3 groups to improve joint mobility and prevent sports injuries. Exercise intensity: The heart rate reserve (HRR) method combined with conscious exertion rating (RPE) was used to determine the exercise intensity. Exercise intensity was initially set at 40%–60% of HRR, which corresponded to an RPE score of 11–13 (slightly strenuous), and then gradually increased to 60%–80% of HRR, with an RPE score of 13–15 (strenuous), as the patient's exercise endurance improved. For example, a 50-year-old patient with a maximal heart rate of approximately 170 beats per minute (220 - age), a resting heart rate of 70 beats per minute, and a heart rate reserve of 100 beats per minute would have a target heart rate range of (100 × 40% + 70) = 110 beats per minute to (100 × 60% + 70) = 130 beats per minute during initial exercise.
Exercise duration
The total duration of each exercise is 30∼45 min, including 5∼10 min of warm-up exercises (e.g., slow walking, joint mobilization exercises), 20∼30 min of formal exercise (a combination of aerobic exercise, strength training, and flexibility training), and 5∼10 min of relaxation exercises (e.g., slow walking, stretching of muscles).
Exercise frequency
Exercise frequency is 3–5 times per week to ensure that patients have enough rest time for recovery. During exercise, patients should carry first aid medication, such as nitroglycerin, and inform their families of the exercise precautions so that they can deal with any discomfort in time. At the same time, patients are advised to go to the hospital for regular review, and according to the review results, the professional physician will adjust the exercise rehabilitation program in a timely manner.
Data collection
Patients' age, gender, Body Mass Index (BMI), smoking history, alcohol consumption history, comorbidities (hypertension, hyperlipidemia, diabetes mellitus), infarct locations (anterior wall, inferior wall, posterior-inferior wall, lateral wall), cardiac function class (I∼III), number of stents (≤1, ≥2) were collected. wall), cardiac function class (I-III), and number of stents (≤1, ≥2).
Clinical assessment
Cardiac function, exercise tolerance, inflammatory factor levels, oxidative stress indicators, and quality of life were compared between the two groups before and 3 months after the intervention. And count the adverse cardiovascular events such as angina pectoris, re-infarction, heart failure, arrhythmia and so on.
Cardiac function indexes
Vivid-7Demision color echocardiography from GE was used to measure the cardiac function of coronary artery disease patients in the two groups, including LVEF, LVEDD, LVESD, and left ventricular short-axis shortening.
Exercise endurance indexes
Patients' exercise endurance was rated by using the 6 min walking test, patients walked on a flat, unobstructed corridor in the room, and the distance they were able to walk in 6MWD.
Inflammatory factor indexes
Before and 3 months after the intervention, patients were collected 5 mL of fasting venous blood in the early morning, centrifuged at 3,000 r/min for 10 min, serum was separated and stored in the refrigerator at - 80℃ for measurement. The serum was separated and stored at −80℃ in a refrigerator for measurement. hs-CRP, IL-6, TNF-α, and soluble intercellular adhesion molecule-1 (sICAM-1) were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturers' instructions (hs-CRP kit E-EL-H5134, Wuhan Eliot Biotechnology; IL-6 PI325, TNF-α PT518, and sICAM-1 PI498, Shanghai Biyuntian Biotechnology). All operations were performed in strict accordance with the instructions.
Indicators of oxidative stress
The SOD Activity Test Kit (S0101S), T-AOC Test Kit (A015-1-2), CAT Assay Kit (A007-1-1) were used to determine the activity of SOD, T-AOC, and CAT in serum. A malondialdehyde (MDA) assay kit (A003-1-1) was used to determine the serum MDA content. The SOD kit was purchased from Shanghai Biyuntian Biotechnology Co., Ltd, and the T-AOC, CAT and MDA kits were purchased from Nanjing Jiancheng Bioengineering Institute. All the operations were carried out in accordance with the instructions.
Quality of life assessment
The quality of life of patients was assessed by using the Short Form Health Survey (SF-36), which contains the following scales: General health, Physical functioning, Role physical, Bodily pain, Role emotional, Vitality and Vitality. emotional, Vitality, Social functioning and Mental health. Each dimension is scored on a scale ranging from 0 to 100, with higher scores indicating better quality of life (17).
Incidence of adverse cardiovascular events
The adverse cardiovascular events such as angina pectoris, re-infarction, heart failure, arrhythmia and so on were counted in both groups.
Statistical analysis
Data were statistically analyzed and graphed using GraphPad Prism 9.5.0 software (GraphPad Software Inc., San Diego, CA, USA). The Shapiro–Wilk test was used to test for normal distribution, and measures that conformed to normal distribution were expressed as mean ± standard deviation, with independent samples t-tests for comparisons between the two groups, and paired samples t-tests for comparisons between pre- and post-intervention within groups. Measurement information that did not conform to normal distribution was expressed as quartiles, Mann–Whitney U test was used for comparison between two groups, and Wilcoxon Signed Rank Test was used for pre- and post-intervention comparisons. Count data were expressed as number of cases and percentages, and comparisons between groups were made using the chi-square test. p was a two-sided test, and differences were considered statistically significant at p < 0.05.
Results
Comparison of baseline data between the two groups
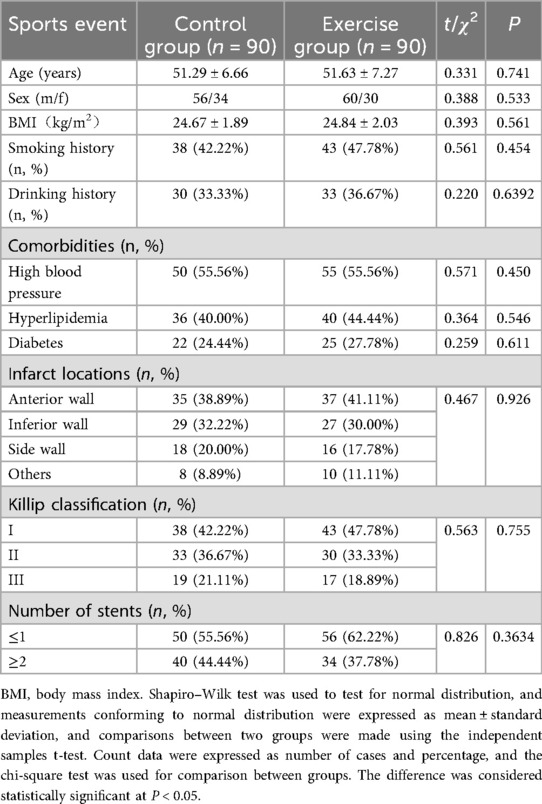
One hundred and eighty patients with AMI who were admitted at our hospital and underwent PCI from 12 January 2022 to February 2024 were selected for the study. A total of 218 patients were screened after PCI during the study period; 38 were excluded (delayed PCI >12 h after symptom onset, n = 5; repeat PCI for restenosis, n = 5; severe hepatic/renal dysfunction or hematologic disorder, n = 6; fractures/osteoarthritis or other musculoskeletal disease, n = 7; psychiatric disorder or cognitive impairment, n = 5; declined participation, n = 10). One hundred eighty (180) patients were randomized and allocated equally to the control group (n = 90) and the exercise group (n = 90). The control group was given routine care, and the exercise group was given rehabilitation exercise training on the basis of the control group. Of 180 randomized participants, 6 were lost to follow-up by 3 months (2/90 control; 4/90 exercise). Thus, 174/180 (96.7%) completed 3-month assessments. There was no significant difference between the two groups in terms of age, gender, Body Mass Index (BMI), smoking history, drinking history, family history of coronary artery disease, comorbidities (hypertension, hyperlipidemia, diabetes mellitus), Infarct locations, cardiac function classification and the number of stents put in, etc. (all P > 0.05), see Table 1.
Comparison of cardiac function between two groups of patients
We compared the cardiac function of patients in the two groups, and the results are shown in Figure 2. The cardiac function indexes of patients in the two groups before the intervention were not statistically significant (both P > 0.05); the LVEF and FS of patients in the exercise group were significantly higher than those before the intervention, and the LVEDD and LVESD were significantly lower than those before the intervention (both P < 0.001); moreover, the LVEF and FS of patients in the exercise group were significantly higher than those in the control group after the intervention. In addition, LVEF and FS were significantly higher in the exercise group than in the control group after the intervention, and LVEDD and LVESD were significantly lower than in the control group, with statistically significant differences (all P < 0.001). It is suggested that exercise rehabilitation training can more effectively improve the cardiac functional status of AMI patients after PCI.
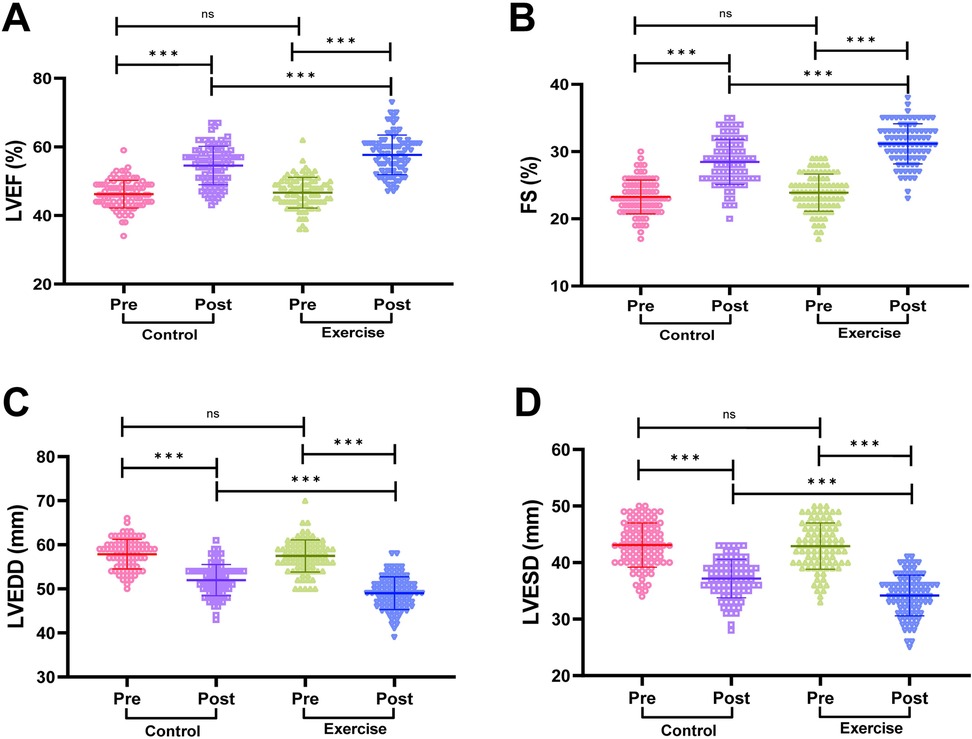
Figure 2. Comparison of cardiac function between the two groups. (A) Left ventricular ejection fraction (LVEF). (B) Left ventricular end-diastolic dimension (LVEDD). (C) Left ventricular end-systolic dimension (LVESD). (D) Fractional shortening (FS). Notes: The Shapiro-Wilk test was used to assess normality. Normally distributed data are expressed as mean ± standard deviation. Between-group comparisons used independent samples t-tests; within-group pre/post comparisons used paired t-tests. Significance levels: ns (P > 0.05), **P < 0.001.
Comparison of exercise endurance indexes between two groups of patients
We compared the exercise endurance of the two groups of patients before and after the intervention by 6MWD. The results are shown in Figure 3: Before the intervention, there was no significant difference in 6MWD between the two groups of patients (P > 0.05). After the intervention, the 6MWD of patients in both groups was significantly higher than that before the intervention (P < 0.001). In addition, the 6MWD of the exercise group was significantly higher than that of the control group after intervention (P < 0.001). It is suggested that exercise rehabilitation training has a significant effect on enhancing the exercise endurance of AMI patients after PCI.
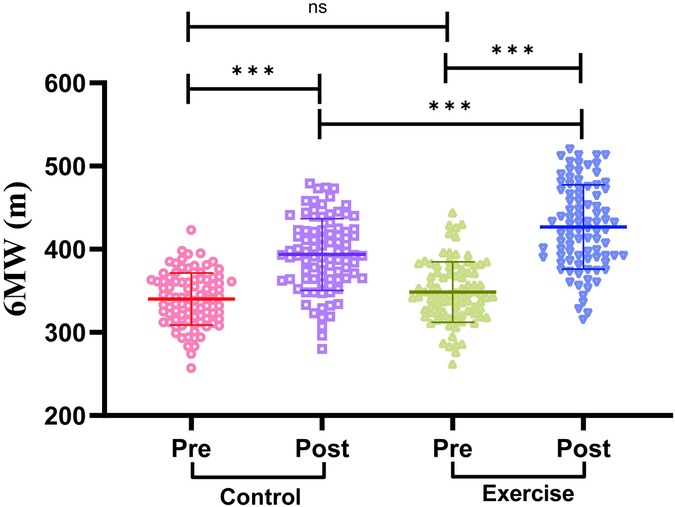
Figure 3. Comparison of exercise endurance indexes between two groups of patients. 6MWD, 6-minute walking distance. The Shapiro–Wilk test was used to test for normal distribution. Measures that conformed to normal distribution were expressed as mean ± standard deviation, and comparisons between the two groups were made using the independent samples t-test, while comparisons between the pre-intervention and post-intervention periods within the groups were made using the paired samples t-test. Differences were considered statistically significant at P < 0.05. ns indicates P > 0.05 and *** indicates P < 0.001.
Comparison of inflammatory factor levels between the two groups of patients
We detected the serum levels of hs-CRP, IL-6, TNF-α and sICAM-1 by ELISA, and the results are shown in Figure 4. Before the intervention, there was no statistically significant difference in the serum levels of hs-CRP, IL-6, TNF-α and sICAM-1 in the two groups of patients (all P > 0.05). The serum levels of hs-CRP, IL-6, TNF-α and sICAM-1 were significantly lower in both groups after intervention (all P < 0.001), and the serum levels of hs-CRP, IL-6, TNF-α and sICAM-1 in the exercise group were significantly lower than those in the control group after intervention (all P < 0.01). It is suggested that exercise rehabilitation training can effectively reduce the level of inflammatory factors in AMI patients after PCI, so as to better regulate the inflammatory response in patients.
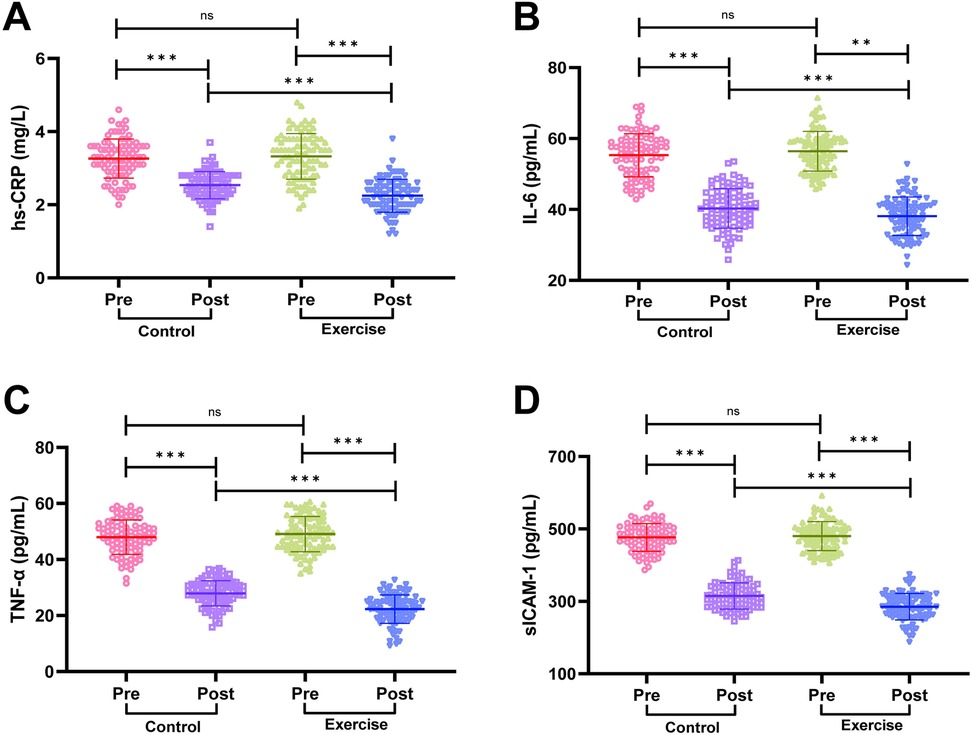
Figure 4. Comparison of inflammatory factor levels between the two groups. (A) High-sensitivity C-reactive protein (hs-CRP). (B) Interleukin-6 (IL-6). (C) Tumor necrosis factor-α (TNF-α). (D) Soluble intercellular adhesion molecule-1 (sICAM-1). Notes: Normality was tested with the Shapiro-Wilk test. Normally distributed data are shown as mean ± SD; between-group comparisons used independent t-tests. Significance levels: ns (P > 0.05), **P < 0.01, **P < 0.001.
Comparison of oxidative stress indicators between the two groups of patients
We measured serum SOD, T-AOC, CAT activity and MDA content by the kit, and the results are shown in Figure 5. Before the intervention, there was no statistically significant difference in serum SOD, T-AOC, CAT activity and MDA content in the two groups of patients (P > 0.05). After the intervention, serum SOD, T-AOC and CAT activities in the patients of the exercise group were significantly higher than before the intervention, while MDA content was significantly lower than before the intervention (P < 0.001); intergroup comparison revealed that SOD, T-AOC and CAT activities in the patients of the exercise group were significantly higher than that of the control group after the intervention, and MDA content was significantly lower than that of the control group (P < 0.001), suggesting that exercise rehabilitation training can significantly improve the oxidative stress status of AMI patients after PCI.
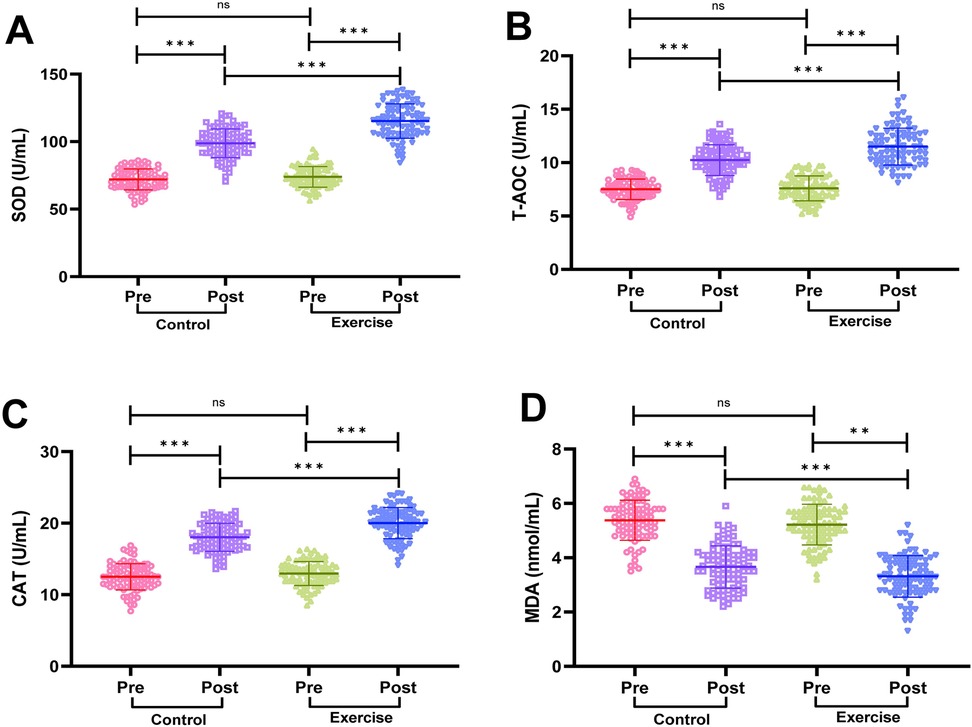
Figure 5. Comparison of oxidative stress indicators between the two groups. (A) Superoxide dismutase (SOD). (B) Total antioxidant capacity (T-AOC). (C) Catalase (CAT). (D) Malondialdehyde (MDA). Notes: Shapiro-Wilk test confirmed normality for parametric data (mean ± SD). Between-group comparisons used independent t-tests. Significance levels: ns (P > 0.05), **P < 0.01, **P < 0.001.
Comparison of quality-of-life scores before and after intervention in both groups
We used the SF-36 Concise Health Questionnaire to evaluate patients' quality of life in terms of General health, Physical functioning, Role physical, Bodily pain, Role emotional, Vitality, Social functioning and Mental health aspects to evaluate the patients' quality of life, and it was found (Table 2) that before the intervention, the difference between the SF-36 scores of each dimension between the two groups was not statistically significant (all P > 0.05); after three months of intervention, the SF-36 scores of each dimension of the two groups were significantly higher (all P < 0.001); moreover, the scores of each dimension of the quality of life of patients in the exercise group were significantly higher than those of the control group after the intervention (all P < 0.05). It is suggested that exercise rehabilitation training can improve the quality of life of AMI patients after PCI.
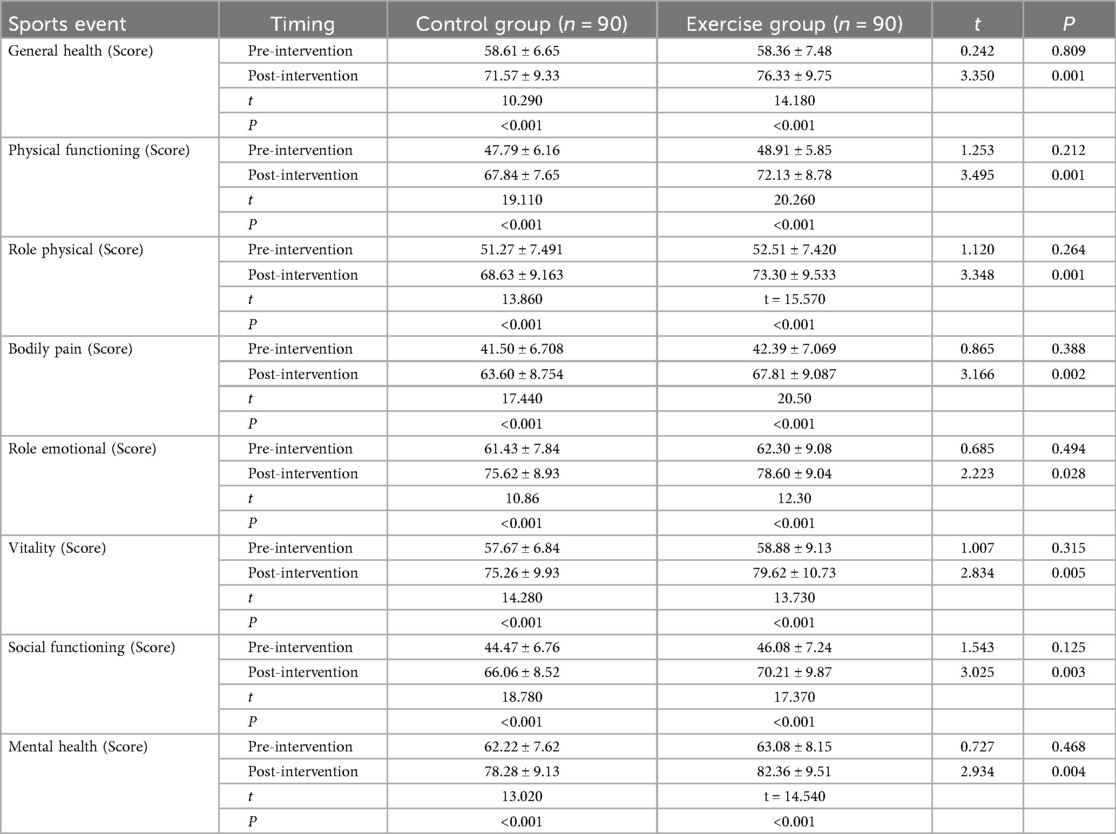
Table 2. Comparison of quality of life scores before and after intervention in the two groups of patients.
The Shapiro–Wilk test was used to test for normal distribution, and measures that conformed to normal distribution were expressed as mean ± standard deviation, and independent samples t-tests were used to compare the two groups, and paired t-tests were used before and after the intervention. Differences were considered statistically significant at P < 0.05.
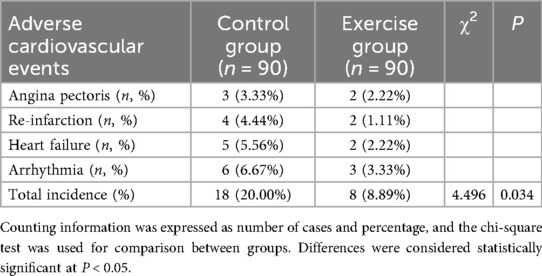
Comparison of adverse cardiovascular events between the two groups
The occurrence of adverse cardiovascular events (angina pectoris, re-infarction, heart failure, arrhythmia, etc.) during the intervention period was recorded in the two groups of patients, and the results showed that (Table 3): In the control group, there were three cases of angina pectoris, four cases of re-infarction, five cases of heart failure, and six cases of arrhythmia (total incidence 20.0%). In the exercise group, there were two cases of angina pectoris, two cases of re-infarction, two cases of heart failure, and three cases of arrhythmia (total incidence 8.9%). The overall incidence of adverse cardiovascular events was therefore significantly lower in the exercise group than in the control group (χ2 = 4.496, P = 0.034). The relative risk (RR) was 0.44, indicating that exercise rehabilitation reduced the risk of adverse cardiovascular events by 56%. The absolute risk reduction (ARR) was 11.1%, meaning that for every 100 patients receiving exercise rehabilitation, approximately 11 adverse events could be prevented. These findings suggest that exercise rehabilitation training after PCI for acute myocardial infarction significantly reduces the risk of subsequent adverse cardiovascular events.
Discussion
PCI can help the stenotic coronary arteries reopen, prompting the blood supply of myocardial tissues to return to normal and reduce ischemia, but routine postoperative care does not eliminate the risk factors of the disease, and patients are prone to anxiety, depression, and other adverse emotions due to restricted activities and other problems during bed rest, which is not conducive to the prognosis (18, 19). This study focused on the effects of exercise rehabilitation training on AMI patients after PCI, and the results showed that exercise rehabilitation training was effective in improving patients' cardiac function, exercise endurance, inflammatory factor levels, oxidative stress status, quality of life, and reducing the incidence of adverse cardiovascular events.
In terms of improving cardiac function, patients in the exercise group had significantly higher LVEF and FS, and significantly lower LVEDD and LVESD. This result is consistent with previous studies, which reported that exercise rehabilitation training can promote cardiac left ventricular remodeling, during which cardiac oxygen demand increases, and in order to satisfy the demand, myocardial contractility is enhanced, and cardiac structure and function are gradually optimized, thus enhancing cardiac function (14, 20, 21). Exercise also acts directly on the vascular system, improving endothelial and coronary smooth muscle function, enhancing coronary vasodilatation, providing a more adequate blood supply to the myocardium, and further supporting cardiac function recovery (22, 23).
For the improvement of exercise endurance, 6MWD was significantly increased in the exercise group. Low-intensity activities during inpatient rehabilitation helped patients adapt to exercise, and aerobic exercise, strength training and flexibility training after discharge gradually improved cardiopulmonary function and muscle strength. Aerobic exercise enhances cardiorespiratory endurance, strength training improves muscle metabolic rate and contraction capacity, and flexibility training ensures the fluidity and safety of exercise, and the three synergistic effects result in a significant increase in patients' exercise endurance (24, 25). Regular aerobic exercise can enhance cardiorespiratory function, improve the body's ability to take up, transport and utilize oxygen, while improving skeletal muscle metabolism, enhancing muscle strength and endurance, enabling patients to tolerate longer and more intense exercise (26).
Inflammatory response plays an important role in the development of cardiovascular disease (27). Studies have shown that exercise rehabilitation significantly reduces the levels of inflammatory factors, including hs-CRP, IL-6, TNF-α, and sICAM-1, in AMI patients after PCI. Exercise rehabilitation modulates the inflammatory response through a variety of mechanisms: exercise activates anti-inflammatory signaling pathways, such as inducing the release of anti-inflammatory cytokines (e.g., IL-10), and by inhibiting TNF-α and stimulating IL- 1ra exerting a direct anti-inflammatory effect (28); exercise helps to restore the homeostasis of the immune system and reduces the occurrence of excessive inflammatory responses (29, 30); moreover, by improving the function of the vascular endothelium, exercise can reduce the damage of inflammatory factors to the vascular wall, thus reducing the risk of atherosclerosis (31, 32).
Oxidative stress is a common pathophysiological phenomenon in AMI patients after surgery, which may lead to cardiomyocyte damage and dysfunction (33, 34). In this study, we found that exercise rehabilitation can effectively alleviate oxidative stress in AMI patients after PCI. Through exercise, the body can produce adaptive changes and enhance the antioxidant defense system, thereby reducing the generation of free radicals and the oxidative damage they trigger. This helps to restore the stability of the intra- and extracellular environment, reduce the damage of oxidative stress to cardiomyocytes, and thus protect cardiomyocytes and vascular endothelial cells and maintain the normal function of the cardiovascular system (35).
The enhancement of quality of life by exercise rehabilitation training is a direct reflection of its combined effect. By improving cardiac function and exercise endurance, and reducing inflammation and oxidative stress, exercise rehabilitation training positively affects patients at both the physiological and psychological levels. From the physiological point of view, the enhancement of cardiac function and exercise endurance significantly improves the mobility of patients, and at the same time effectively relieves somatic symptoms, enabling patients to cope with various needs in daily life more easily (15). From a psychological perspective, exercise prompts the body to secrete neurotransmitters such as endorphins, which improves the patients' emotional state, enhances psychological resilience, and enhances self-perception and social adaptability (36). This all-encompassing psychological adjustment not only improved patients' social functioning, but also significantly improved mental health.
The role of exercise rehabilitation training in reducing the incidence of adverse cardiovascular events should also not be overlooked. Exercise improves cardiac function, regulates the levels of inflammatory factors and oxidative stress, stabilizes coronary atherosclerotic plaques, and reduces the risk of plaque rupture and thrombosis (37, 38); exercise also lowers blood pressure and lipids, controls body weight, and improves glycemic metabolism, minimizing the risk factors for cardiovascular disease and reducing the risk of adverse cardiovascular events (39, 40).
Despite the remarkable results of this study, some limitations remain. Participants and staff were not blinded, which may introduce performance bias; however, objective outcomes and blinded assessors minimized this risk. Such partial blinding is standard in exercise-based rehabilitation trials. The sample was only from one hospital, which may have geographical and population limitations and affect the generalizability of the results; the study period of 3 months is relatively short, and the long-term effects need to be further observed. Although the baseline data of the two groups of patients were comparable, confounding factors such as dietary structure, medication adherence, and lifestyle after discharge were not strictly controlled or tracked; future studies may consider expanding the sample size, strictly controlling for confounding factors and extending the follow-up time to further validate the long-term efficacy of exercise rehabilitation. In addition, the specific regulatory mechanisms of exercise rehabilitation on cardiac function, inflammatory factors and oxidative stress can also be explored in depth by combining with molecular biology techniques.
In conclusion, exercise rehabilitation can significantly improve several physiological function indexes, including cardiac function, inflammatory factor level and oxidative stress status, in AMI patients after PCI, and at the same time significantly improve the quality of life of patients. This suggests that this method has potential value in early postoperative rehabilitation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Liyang Hospital of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JZ: Writing – review & editing, Software, Methodology. LC: Writing – original draft, Formal analysis, Data curation. WZ: Methodology, Resources, Writing – original draft. ZC: Data curation, Writing – original draft, Resources. CH: Writing – original draft, Validation. YZ: Supervision, Writing – original draft, Resources. YS: Visualization, Project administration, Writing – original draft. JS: Writing – review & editing, Conceptualization, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin YH, Tsai WC, Chiu CC, Chi NY, Liu YH, Huang TC, et al. The beneficial effect of the SGLT2 inhibitor dapagliflozin in alleviating acute myocardial infarction-induced cardiomyocyte injury by increasing the sirtuin family SIRT1/SIRT3 and cascade signaling. Int J Mol Sci. (2024) 25(15):8541. doi: 10.3390/ijms25158541
2. Liu YH, Tsai WC, Chi NY, Chang CT, Lee WH, Chu CY, et al. The association between percentage of mean arterial pressure and long-term mortality in acute myocardial infarction patients: an observational cohort study. Int J Med Sci. (2024) 21(11):2119–26. doi: 10.7150/ijms.95430
3. Pu X, Huang H, Zhao X, Liu F, Leng Y, Deng Y, et al. Improving lower limb function and frailty in frail older patients with acute myocardial infarction after percutaneous coronary intervention: a randomized controlled study of neuromuscular electrical stimulation. Clin Interv Aging. (2024) 19:1163–76. doi: 10.2147/CIA.S460805
4. Saito Y, Tateishi K, Kanda M, Shiko Y, Kawasaki Y, Kobayashi Y, et al. Volume-outcome relationships for percutaneous coronary intervention in acute myocardial infarction. J Am Heart Assoc. (2022) 11(6):e023805. doi: 10.1161/JAHA.121.023805
5. Liu X, Zou Y, Huang D, Lu H. Effect of evidence-based nursing combined with exercise rehabilitation in patients with acute myocardial infarction after percutaneous coronary intervention. Am J Transl Res. (2022) 14(10):7424–33.36398266
6. Qu B, Hou Q, Men X, Zhai X, Jiang T, Wang R. Research and application of KABP nursing model in cardiac rehabilitation of patients with acute myocardial infarction after PCI. Am J Transl Res. (2021) 13(4):3022–33.34017470
7. Mansilla-Chacón M, Gómez-Urquiza JL, Martos-Cabrera MB, Albendín-García L, Romero-Béjar JL, Cañadas-De La Fuente GA, et al. Effects of supervised cardiac rehabilitation programmes on quality of life among myocardial infarction patients: a systematic review and meta-analysis. J Cardiovasc Dev Dis. (2021) 8(12):166. doi: 10.3390/jcdd8120166
8. Wang R, Duan G, Xu H, Wu Y, Su Y, Li J, et al. Analysis on the effect of the rehabilitation intervention-centered targeted nursing model on the cardiac function recovery and negative emotions in patients with acute myocardial infarction. J Healthc Eng. (2022) 2022:1246092. doi: 10.1155/2022/1246092
9. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. (2019) 1(1):CD003331. doi: 10.1002/14651858.CD003331
10. Dibben G, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. (2021) 11(11):CD001800. doi: 10.1002/14651858.CD001800.pub4
11. Marino F, Scalise M, Cianflone E, Salerno L, Cappetta D, Salerno N, et al. Physical exercise and cardiac repair: the potential role of nitric oxide in boosting stem cell regenerative biology. Antioxidants (Basel). (2021) 10(7):1002. doi: 10.3390/antiox10071002
12. Schüttler D, Clauss S, Weckbach LT, Brunner S. Molecular mechanisms of cardiac remodeling and regeneration in physical exercise. Cells. (2019) 8(10):1128. doi: 10.3390/cells8101128
13. Yang X, Li Y, Ren X, Xiong X, Wu L, Li J, et al. Effects of exercise-based cardiac rehabilitation in patients after percutaneous coronary intervention: a meta-analysis of randomized controlled trials. Sci Rep. (2017) 7:44789. doi: 10.1038/srep44789
14. Haykowsky M, Scott J, Esch B, Schopflocher D, Myers J, Paterson I, et al. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials. (2011) 12:92. doi: 10.1186/1745-6215-12-92
15. Peixoto TC, Begot I, Bolzan DW, Machado L, Reis MS, Papa V, et al. Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction: a randomized controlled trial. Can J Cardiol. (2015) 31(3):308–13. doi: 10.1016/j.cjca.2014.11.014
16. Cai M, Wang L, Ren YL. Effect of exercise training on left ventricular remodeling in patients with myocardial infarction and possible mechanisms. World J Clin Cases. (2021) 9(22):6308–18. doi: 10.12998/wjcc.v9.i22.6308
17. Zhao Y, Yang Y, Chen L, Sun H, Xia J. Effects of the hospital-community-family trinity cardiac rehabilitation on patients with acute myocardial infarction after percutaneous coronary intervention: a randomized trial. Int J Nurs Sci. (2025) 12(2):161–8. doi: 10.1016/j.ijnss.2025.02.010
18. Yu H, Yang H. Effect of early home-based exercise for cardiac rehabilitation on the prognosis of patients with acute myocardial infarction after percutaneous coronary intervention. Am J Transl Res. (2021) 13(7):7839–47.34377261
19. Peng X, Zhang J, Wan L, Wang H, Zhang W. The effect of a seven-step rehabilitation training program on cardiac function and quality of life after percutaneous coronary intervention for acute myocardial infarction. Pak J Med Sci. (2022) 38(1):123–7. doi: 10.12669/pjms.38.1.4945
20. Zhang YM, Lu Y, Tang Y, Yang D, Wu HF, Bian ZP, et al. The effects of different initiation time of exercise training on left ventricular remodeling and cardiopulmonary rehabilitation in patients with left ventricular dysfunction after myocardial infarction. Disabil Rehabil. (2016) 38(3):268–76. doi: 10.3109/09638288.2015.1036174
21. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. (2008) 88(3):1009–86. doi: 10.1152/physrev.00045.2006
22. Bruning RS, Sturek M. Benefits of exercise training on coronary blood flow in coronary artery disease patients. Prog Cardiovasc Dis. (2015) 57(5):443–53. doi: 10.1016/j.pcad.2014.10.006
23. Sangha GS, Goergen CJ, Prior SJ, Ranadive SM, Clyne AM. Preclinical techniques to investigate exercise training in vascular pathophysiology. Am J Physiol Heart Circ Physiol. (2021) 320(4):H1566–600. doi: 10.1152/ajpheart.00719.2020
24. Squeo MR, Di Giacinto B, Perrone MA, Santini M, Sette ML, Fabrizi E, et al. Efficacy and safety of a combined aerobic, strength and flexibility exercise training program in patients with implantable cardiac devices. J Cardiovasc Dev Dis. (2022) 9(6):182. doi: 10.3390/jcdd9060182
25. Aguirre LE, Villareal DT. Physical exercise as therapy for frailty. Nestle Nutr Inst Workshop Ser. (2015) 83:83–92. doi: 10.1159/000382065
26. Araújo BTS, Barros AEVR, Nunes DTX, Remígio de Aguiar MI, Mastroianni VW, de Souza JAF, et al. Effects of continuous aerobic training associated with resistance training on maximal and submaximal exercise tolerance, fatigue, and quality of life of patients post-COVID-19. Physiother Res Int. (2023) 28(1):e1972. doi: 10.1002/pri.1972
27. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17(1):122. doi: 10.1186/s12933-018-0762-4
28. Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. (2017) 47(8):600–11. doi: 10.1111/eci.12781
29. Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. (2019) 25(11):1761–71. doi: 10.1038/s41591-019-0633-x
30. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. (2011) 11(9):607–15. doi: 10.1038/nri3041
31. Gao J, Pan X, Li G, Chatterjee E, Xiao J. Physical exercise protects against endothelial dysfunction in cardiovascular and metabolic diseases. J Cardiovasc Transl Res. (2022) 15(3):604–20. doi: 10.1007/s12265-021-10171-3
32. Glodzik J, Rewiuk K, Adamiak J, Marchewka J, Salakowski A, Mazur M, et al. Controlled aerobic training improves endothelial function and modifies vascular remodeling in healthy adults with high normal blood pressure. J Physiol Pharmacol. (2018) 69(5). doi: 10.26402/jpp.2018.5.04
33. Bugger H, Pfeil K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta Mol Basis Dis. (2020) 1866(7):165768. doi: 10.1016/j.bbadis.2020.165768
34. Kurian GA, Rajagopal R, Vedantham S, Rajesh M. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxid Med Cell Longev. (2016) 2016:1656450. doi: 10.1155/2016/1656450
35. Tofas T, Draganidis D, Deli CK, Georgakouli K, Fatouros IG, Jamurtas AZ. Exercise-induced regulation of redox Status in cardiovascular diseases: the role of exercise training and detraining. Antioxidants (Basel). (2019) 9(1):13. doi: 10.3390/antiox9010013
36. Alizadeh Pahlavani H. Possible role of exercise therapy on depression: effector neurotransmitters as key players. Behav Brain Res. (2024) 459:114791. doi: 10.1016/j.bbr.2023.114791
37. Chacon D, Fiani B. A review of mechanisms on the beneficial effect of exercise on atherosclerosis. Cureus. (2020) 12(11):e11641. doi: 10.7759/cureus.11641
38. Nystoriak MA, Bhatnagar A. Cardiovascular effects and benefits of exercise. Front Cardiovasc Med. (2018) 5:135. doi: 10.3389/fcvm.2018.00135
39. Dobrosielski DA. How can exercise reduce cardiovascular disease risk? A primer for the clinician. Pol Arch Intern Med. (2021) 131(10):16122. doi: 10.20452/pamw.16122
Keywords: exercise rehabilitation training, acute myocardial infarction, percutaneous coronary intervention, cardiac function, inflammatory factor, quality of life
Citation: Zhou J, Cai L, Zhang W, Chen Z, Huang C, Zheng Y, Shi Y and Shen J (2025) Effect of exercise rehabilitation training on cardiac function and quality of life after PCI for acute myocardial infarction. Front. Cardiovasc. Med. 12:1662376. doi: 10.3389/fcvm.2025.1662376
Received: 9 July 2025; Revised: 29 October 2025;
Accepted: 5 November 2025;
Published: 10 December 2025.
Edited by:
Chun Chin Chang, Taipei Veterans General Hospital, TaiwanReviewed by:
Yuki Katagiri, Sapporo Higashi Tokushukai Hospital, JapanPei Hsi Wu, Taipei Veterans General Hospital, Taiwan
Copyright: © 2025 Zhou, Cai, Zhang, Chen, Huang, Zheng, Shi and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfeng Shen, Y2xvdWQxNEAxMjYuY29t
†These authors share first authorship
 Jianru Zhou1,†
Jianru Zhou1,† Chunhui Huang
Chunhui Huang Jianfeng Shen
Jianfeng Shen