Abstract
Introduction:
Early discontinuation of aspirin after percutaneous coronary intervention (PCI) reduces bleeding risk, while inflammation-targeted strategies may offer additional benefit in patients with acute coronary syndrome (ACS). Residual inflammatory risk—reflected by persistently elevated high-sensitivity C-reactive protein (hs-CRP) levels—remains a significant contributor to adverse cardiovascular outcomes despite guideline-directed therapy. Colchicine has emerged as a potential anti-inflammatory agent in this context, but its optimal use remains uncertain.
Methods and analysis:
MACT (Mono Antiplatelet and Colchicine Therapy) II is an investigator-initiated, prospective, multicenter, single-arm study designed to evaluate the safety and efficacy of an aspirin-free, inflammation-guided treatment strategy in 490 patients with troponin-positive ACS or ST-segment elevation myocardial infarction undergoing PCI with a sirolimus-eluting stent. Aspirin is discontinued on the day after PCI, and ticagrelor is continued at the standard dose. Colchicine (0.6 mg once daily) is initiated within 24 h after PCI. At 1 month, colchicine is continued or discontinued based on hs-CRP levels. The primary outcome is the 12-month incidence of net adverse clinical events, a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unplanned urgent revascularization, and major bleeding (Bleeding Academic Research Consortium type 3 or 5). Secondary outcomes include longitudinal hs-CRP trends, platelet reactivity, and adverse drug reactions.
Conclusions:
MACT II evaluates an aspirin-free, inflammation-guided treatment strategy in patients with ACS undergoing PCI. Colchicine therapy is initiated early during the acute phase of ACS and continued or discontinued based on inflammatory response as measured by hs-CRP. By tailoring treatment duration in this manner, the trial aims to reduce both ischemic and bleeding risks while avoiding unnecessary drug exposure. The findings may inform personalized anti-inflammatory strategies in contemporary clinical practice.
Trial registration:
ClinicalTrials.gov Identifier: NCT06543082.
Introduction
Acute coronary syndrome (ACS) remains a leading cause of morbidity and mortality worldwide. The cornerstone of treatment following percutaneous coronary intervention (PCI) is dual antiplatelet therapy (DAPT), typically combining aspirin with a P2Y12 inhibitor to reduce thrombotic events (1, 2). However, prolonged DAPT significantly increases bleeding risks, highlighting the need for strategies that maintain antithrombotic efficacy while minimizing bleeding complications. Recent trials, including TWILIGHT, TICO, and ULTIMATE-DAPT (3–5), demonstrated that transitioning from DAPT to P2Y12 inhibitor monotherapy after a brief period effectively reduces bleeding without compromising ischemic protection. Despite this progress, patients remain at substantial risk for recurrent cardiovascular events, underscoring the need to address factors beyond platelet activation—particularly residual inflammation, which critically contributes to long-term cardiovascular risk.
Inflammation plays a central role in ACS pathogenesis, driving plaque rupture, thrombus formation, and subsequent ischemic complications. Persistently elevated high-sensitivity C-reactive protein (hs-CRP), a marker of systemic inflammation, indicates ongoing inflammatory risk even after clinical stabilization (6). Residual inflammatory risk, reflected by persistently elevated hs-CRP (≥2 mg/L) after PCI, independently predicts recurrent cardiovascular events such as myocardial infarction and stroke (7, 8). Consequently, targeting residual inflammation may offer an additional therapeutic benefit when layered onto modern antiplatelet strategies. Colchicine, a potent anti-inflammatory agent, has emerged as an effective option to reduce residual inflammation and improve cardiovascular outcomes. Landmark studies such as COLCOT and LoDoCo2 have shown that colchicine significantly reduces recurrent events in patients with recent myocardial infarction or stable coronary syndromes (9, 10). Moreover, substituting colchicine for aspirin may further lower bleeding risk, potentially offering a safer and more effective long-term treatment strategy following PCI.
Building upon these concepts, the MACT pilot study evaluated the feasibility of replacing aspirin with colchicine, combined with ticagrelor or prasugrel monotherapy, in ACS patients after PCI (11). In that study, a fixed 3-month colchicine regimen was well tolerated, maintained effective platelet inhibition, and significantly reduced residual inflammation, suggesting clinical feasibility for a colchicine-based, aspirin-free approach (11). The MACT II trial was designed to further investigate this approach in a larger, multicenter setting. A key feature of the study is the use of hs-CRP-guided colchicine therapy, in which continuation of treatment is based on the persistence of systemic inflammation (hs-CRP ≥2 mg/L) at 1 month post-PCI. This strategy enables individualized, inflammation-guided therapy that may optimize risk-benefit balance by selectively extending treatment in patients with ongoing inflammatory activity.
We hypothesize that an hs-CRP–guided, aspirin-free strategy combining ticagrelor monotherapy and colchicine will effectively reduce both ischemic and bleeding events in ACS patients undergoing PCI. The MACT II trial aims to evaluate the efficacy and safety of this personalized anti-inflammatory approach, with serial hs-CRP measurements during follow-up providing insights into the inflammatory response.
Methods and analysis
Study design
The MACT II trial is an investigator-initiated, prospective, multicenter, single-arm clinical trial designed to evaluate the efficacy and safety of ticagrelor monotherapy combined with low-dose colchicine in ACS patients undergoing PCI. Patients diagnosed with troponin-positive non-ST-elevation ACS or ST-segment elevation myocardial infarction (STEMI) undergoing PCI with ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro; Biotronik AG) are eligible for enrollment. After obtaining informed consent and confirming eligibility, aspirin therapy is discontinued the day after PCI, while ticagrelor (90 mg twice daily) is continued. Simultaneously, low-dose colchicine (0.6 mg daily) is initiated. Colchicine treatment duration is guided by hs-CRP measurements at one month after PCI: patients with hs-CRP ≥ 2 mg/L continue colchicine therapy for the study duration, while those with hs-CRP <2 mg/L discontinue colchicine at one month. A schematic overview illustrating patient flow, including enrollment, interventions, and follow-up timelines, is provided in Figure 1.
Figure 1
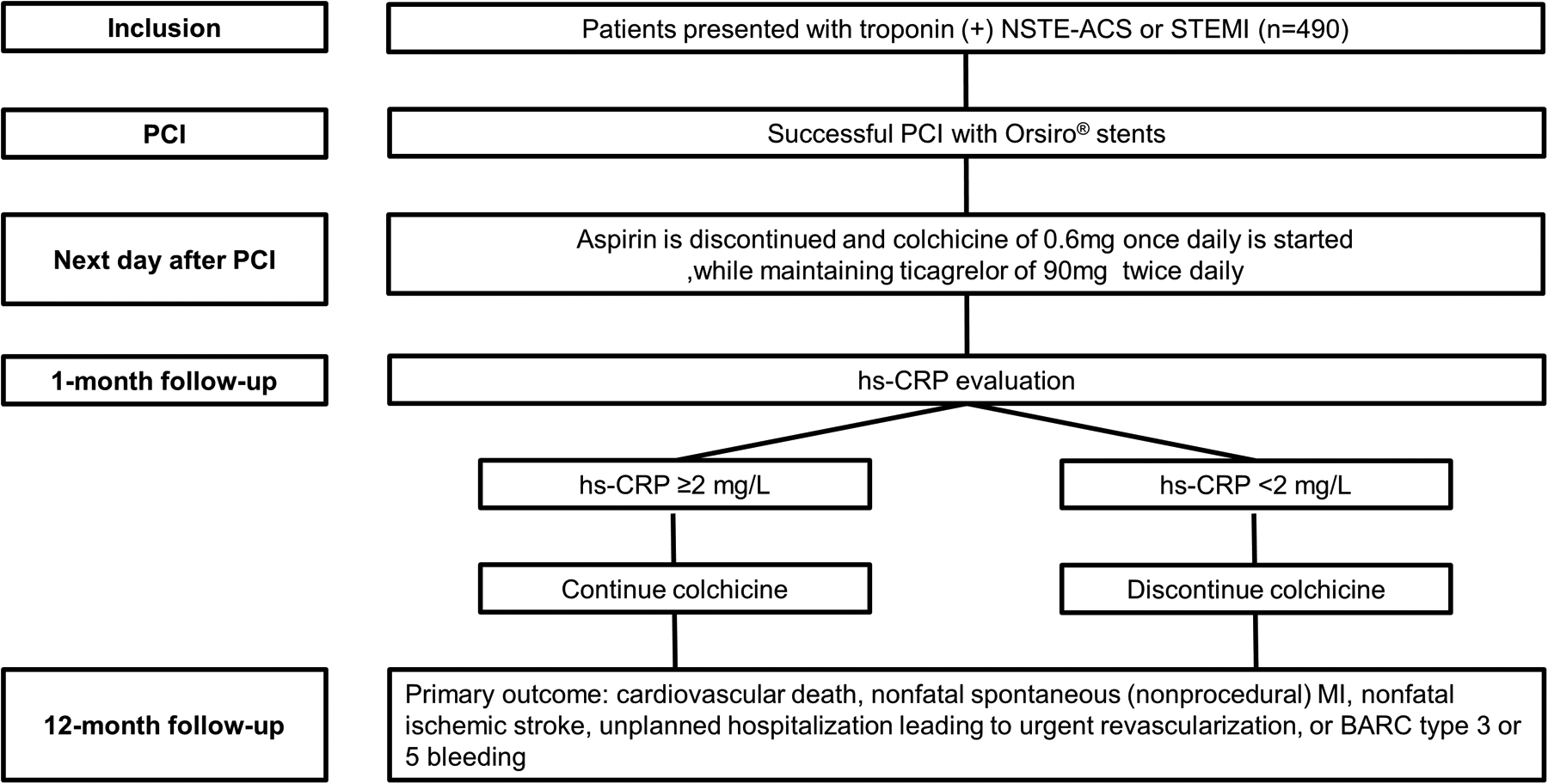
Study flow diagram. BARC, bleeding academic research consortium; hs-CRP, high-sensitivity C-reactive protein; MI, myocardial infarction; NSTE-ACS, non-ST-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Study population
The study population includes patients presenting with ACS, defined as troponin-positive unstable angina/non-ST-elevation myocardial infarction (NSTEMI), or STEMI, who undergo successful PCI with ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro; Biotronik AG). Patients with cardiac arrest or cardiogenic shock, those requiring anticoagulation therapy, or lesions associated with stent failure (stent restenosis or thrombosis) are excluded. The trial aims to enroll a total of 490 patients, who must meet all eligibility criteria, detailed in Table 1.
Table 1
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients aged ≥19 years | Age <19 years |
| Diagnosis of troponin-positive acute coronary syndrome or ST-segment elevation myocardial infarction | Cardiac arrest or cardiogenic shock |
| Successful percutaneous coronary intervention with ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro; Biotronik AG) | Requirement for anticoagulation therapy |
| Provision of signed informed consent | Lesions with stent failure (stent restenosis or thrombosis) |
| Renal impairment (creatinine clearance <50 mL/min) | |
| Severe liver disease (Child-Pugh class B or C) | |
| Concomitant use or requirement of strong CYP3A4 inhibitors or P-glycoprotein inhibitors | |
| Hematologic abnormalities: myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, aplastic anemia | |
| Severe gastrointestinal disorders | |
| Pregnant, lactating, or women of childbearing potential not using contraception | |
| Current malignancy or malignancy within the past 5 years | |
| Life expectancy <5 years | |
| Known hypersensitivity or contraindication to colchicine or ticagrelor |
Eligibility criteria.
Study procedure
Following successful PCI with ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro; Biotronik AG), eligible patients are enrolled after providing written informed consent. Aspirin is discontinued on the day after PCI, and ticagrelor (90 mg twice daily) is continued as the maintenance antiplatelet agent. For patients who received a different P2Y12 inhibitor (e.g., clopidogrel) prior to PCI, a loading dose of ticagrelor (180 mg) is administered on the day after PCI, followed by the standard maintenance dose (12).
Colchicine (0.6 mg once daily) is initiated within 24 h after PCI. At one month, hs-CRP levels are measured using a standardized, high-sensitivity immunoturbidimetric assay to guide treatment duration. Patients with hs-CRP ≥ 2 mg/L continue colchicine therapy until 12 months, while those with hs-CRP < 2 mg/L discontinue colchicine at one month.
Clinical follow-up visits are scheduled at 1, 3, 6, 9, and 12 months post-PCI. At each visit, data are collected on symptoms, adverse events, medication adherence, and concomitant medications. Laboratory tests—including hematologic, renal and liver function, lipid profile, cardiac biomarkers, and hs-CRP—are performed at baseline and at 1, 6, and 12 months post-PCI. Platelet function testing using VerifyNow or Thromboelastography is recommended at 1 and 12 months.
Study outcomes
The primary efficacy outcome is net adverse clinical events, defined as a composite of cardiovascular death, nonfatal spontaneous myocardial infarction, nonfatal ischemic stroke, unplanned hospitalization requiring urgent revascularization, and major bleeding, assessed at 12 months post-PCI. The primary safety outcome is the incidence of stent thrombosis within 12 months, classified as definite, probable, or possible according to the Academic Research Consortium definitions (13).
Cardiovascular death includes fatal events of cardiac or vascular origin, as well as unwitnessed or unknown-cause deaths (13). Spontaneous myocardial infarction is defined by a rise and/or fall in cardiac troponin, with at least one value above the 99th percentile upper reference limit, accompanied by evidence of ischemia (symptoms, ECG changes, or imaging findings) (14). Ischemic stroke is defined as a new focal neurological deficit lasting ≥24 h with imaging confirmation (13). Urgent revascularization refers to unplanned PCI or coronary artery bypass surgery performed during hospitalization for recurrent or worsening ischemic symptoms (15). Major bleeding is defined as type 3 or 5 according to the Bleeding Academic Research Consortium criteria, including bleeding requiring transfusion, surgical intervention, or resulting in death (16).
Secondary outcomes include each component of the primary outcome, as well as measures of inflammatory status, platelet reactivity, thrombogenicity, and drug safety over 12 months post-PCI. High residual inflammation is assessed by the proportion of patients with hs-CRP ≥2 mg/L at 1, 6, and 12 months (7), along with changes in hs-CRP following colchicine discontinuation. High and low residual platelet reactivity, defined as platelet reaction units >208 and <85 respectively (17), and thrombogenicity are evaluated at 1 and 12 months (18). Adverse drug reactions to colchicine are assessed at 1, 3, 6, 9, and 12 months.
All clinical outcomes are adjudicated by an independent Clinical Events Committee blinded to treatment exposure and hs-CRP status. In addition, safety monitoring is conducted by an independent Data and Safety Monitoring Board, which regularly reviews adverse events and overall study conduct to ensure participant safety and study integrity.
Sample size calculation
The sample size for the MACT II trial was determined based on the expected incidence of net adverse clinical events at 12 months post-PCI. Based on previous data from the TICO trial, the event rate in a comparable population receiving standard therapy was estimated at 8.7% (4). Informed by findings from the MACT pilot study (11), the anticipated event rate in the investigational group—ticagrelor monotherapy combined with colchicine—was projected to be 4.0%. To detect this difference with 80% power and a one-sided alpha of 2.5%, a total of 419 patients were required. Allowing for an estimated 15% loss to follow-up, the final target sample size was set at 490 patients.
Statistical analysis
The cumulative incidence of the primary outcome will be estimated using the Kaplan–Meier method. Secondary outcomes involving continuous variables, such as hs-CRP measured at 1, 6, and 12 months, will be analyzed using repeated-measures ANOVA or the Friedman test, as appropriate. For comparisons between two time points, paired t-tests or Wilcoxon signed-rank tests will be applied based on data distribution. Repeated categorical variables will be analyzed using McNemar's test or Cochran's Q test.
For the predefined subgroup analysis comparing patients with and without high residual inflammation (hs-CRP ≥ 2 mg/L vs. <2 mg/L at 1 month), continuous variables will be compared using independent samples t-tests or the Mann–Whitney U test, and categorical variables using the chi-square test or Fisher's exact test. Kaplan–Meier curves and the log-rank test will be used for time-to-event comparisons, and Cox proportional hazards models will estimate hazard ratios with 95% confidence intervals. To identify predictors of high residual inflammation despite colchicine therapy, logistic regression analysis will be performed using baseline clinical, procedural, and laboratory variables. Both univariable and multivariable models will be constructed.
A pre-specified analysis will compare patient-level data from the MACT II cohort with matched populations from the TICO trial who received either 12-month DAPT or 3-month DAPT followed by P2Y12 inhibitor monotherapy. This comparison is exploratory and intended for contextual interpretation rather than formal hypothesis testing. Propensity score matching will be used to balance baseline characteristics, and time-to-event outcomes will be analyzed using the same methods described above. Sensitivity analyses will include multivariable Cox regression and inverse probability weighting to adjust for potential confounders. While exploratory, this comparative analysis will provide important clinical context and generate hypotheses regarding the potential benefits or limitations of the aspirin-free, inflammation-guided strategy evaluated in MACT II, thereby informing the design and rationale of future randomized trials.
All analyses will be performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A two-sided p-value <0.05 will be considered statistically significant.
Predefined subgroup of patients and ancillary studies
A predefined subgroup analysis will compare clinical outcomes between patients with high residual inflammation (hs-CRP ≥2 mg/L at 1 month) and those without (hs-CRP <2 mg/L), to assess whether persistent inflammation after PCI is associated with an increased risk of adverse events despite colchicine therapy. In addition, clinical, procedural, and laboratory characteristics will be compared between the two groups to identify factors associated with an inadequate inflammatory response to colchicine.
Pre-specified patient-level comparisons will be conducted between the MACT II cohort and matched populations from the TICO trial who received either 12-month DAPT or 3-month DAPT followed by P2Y12 inhibitor monotherapy. This analysis aims to explore the relative efficacy and safety of colchicine-based therapy in the context of conventional antiplatelet strategies.
Status
As of the time of manuscript submission, a total of 83 patients have been enrolled in the MACT II trial. Patient recruitment and follow-up are ongoing at participating centers.
Discussion
The MACT II trial was designed to evaluate the efficacy and safety of an aspirin-free strategy combining ticagrelor monotherapy with low-dose colchicine in patients with ACS undergoing PCI. Building upon the findings of the MACT pilot study (11), which demonstrated the feasibility of colchicine use following early aspirin discontinuation, MACT II incorporates an hs-CRP-guided approach to personalize treatment duration. Patients with hs-CRP ≥2 mg/L at 1 month continue colchicine, while those with hs-CRP <2 mg/L discontinue therapy, allowing for inflammation-guided risk stratification. This single-arm, prospective, multicenter study aims to assess whether this strategy can effectively reduce both residual inflammatory risk and bleeding, while maintaining ischemic protection in a contemporary ACS population.
Early aspirin discontinuation after PCI has been shown to reduce major bleeding without increasing ischemic risk, as demonstrated in trials such as TWILIGHT and TICO (3, 4). These findings support the use of P2Y12 inhibitor monotherapy in selected patients, particularly those at high bleeding risk (1, 2). However, in the acute phase of ACS, heightened inflammation amplifies thrombogenicity, raising concerns about premature aspirin withdrawal. Studies using clopidogrel or low-dose prasugrel monotherapy—such as STOPDAPT-2 ACS and STOPDAPT-3 and—reported increased ischemic events (19, 20), underscoring the need for potent platelet inhibition during this vulnerable period. MACT II addresses this by combining early aspirin discontinuation with standard-dose ticagrelor, initiated immediately after PCI. This strategy aims to minimize bleeding while maintaining sufficient antithrombotic protection in the pro-inflammatory setting of ACS. The addition of colchicine may further counteract inflammation-driven thrombosis during this high-risk window (21).
Residual inflammatory risk after PCI is increasingly recognized as a key determinant of adverse outcomes in patients with ACS, even in the setting of effective antiplatelet therapy. Early anti-inflammatory intervention may therefore be essential in mitigating this risk during the vulnerable post-PCI phase. In the MACT pilot study, colchicine initiated shortly after PCI significantly reduced hs-CRP levels—from 6.1 mg/L (IQR: 2.6–15.9) at 24 h to 0.6 mg/L (IQR: 0.4–1.2) at 1 month (P < 0.001) (11). The proportion of patients with high residual inflammation (hs-CRP ≥ 2 mg/L) also declined from 81.8% to 11.8% (11). Comparative data further suggested that colchicine suppressed inflammation more effectively than aspirin (22). MACT II builds on these findings by applying an hs-CRP-guided approach to tailor colchicine duration—limiting therapy in patients with adequate early response while extending it in those with persistent inflammation. This strategy aims to optimize anti-inflammatory benefit, reduce unnecessary drug exposure, and minimize side effects such as diarrhea. As most patients are expected to discontinue colchicine at 1 month, the trial will also assess longitudinal hs-CRP trends to evaluate the durability of inflammatory control after early withdrawal.
Recent trials investigating colchicine in ACS have yielded inconsistent results. The CLEAR SYNERGY (OASIS 9) trial, a large-scale, placebo-controlled study enrolling over 4,000 patients with STEMI, did not demonstrate a reduction in ischemic events and reported a higher rate of non-cardiovascular death in the colchicine group (23). Although CRP levels were significantly reduced at 3 months (2.98 ± 0.19 mg/L in the colchicine group vs. 4.27 ± 0.19 mg/L in the placebo group) (23), the residual inflammatory burden remained substantial. One possible contributing factor was the conduct of the trial during the COVID-19 pandemic, which may have increased baseline and follow-up systemic inflammation through subclinical infection or immune activation (24, 25). Furthermore, colchicine dosing was fixed throughout the study and not adapted based on individual inflammatory response, limiting the ability to target residual inflammatory risk more precisely. The persistently high residual inflammation despite treatment may, at least in part, explain the neutral clinical effect observed in the trial.
In contrast, the MACT II trial, launched before the publication of CLEAR SYNERGY, was independently designed to address residual inflammatory risk through a personalized approach. Colchicine duration is guided by hs-CRP levels at 1 month, allowing therapy to be extended only in patients with persistent inflammation while minimizing unnecessary treatment in those with early resolution. However, similar to CLEAR SYNERGY, colchicine dosing in MACT II remains fixed and is not adjusted according to the magnitude of residual inflammation. Given the persistently elevated CRP levels observed in a subset of patients despite therapy in prior studies, the appropriateness of a one-size-fits-all dosing strategy warrants further consideration. Notably, MACT II incorporates serial hs-CRP assessments, allowing longitudinal evaluation of inflammatory trends even among patients who fail to achieve an early response. These data may provide insight into the dynamics of residual inflammation. Future studies might explore dose adjustments or combination anti-inflammatory strategies to manage persistent inflammation more effectively and further enhance clinical outcomes.
This study has key limitations. First, MACT II is a single-arm, open-label trial without a randomized control group, limiting causal interpretation and safety comparisons with standard care. Planned patient-level comparisons with the TICO trial are exploratory and subject to confounding (4). Second, hs-CRP is measured while patients are still on colchicine, so early responders with low hs-CRP may have treatment withdrawn despite potential benefit; hs-CRP will also be measured at 6 and 12 months to assess longitudinal inflammatory response according to colchicine use. Third, comparisons between patients with and without ongoing inflammation are non-randomized, and outcome differences may reflect baseline risk rather than treatment effect. Fourth, hs-CRP alone may not fully capture vascular or plaque-specific inflammation; additional biomarkers could enhance interpretability. Fifth, the fixed colchicine dose (0.6 mg daily) does not account for bodyweight-related pharmacokinetic variability, which may affect exposure and tolerability. Finally, the use of a single stent platform with ticagrelor may limit generalizability.
The MACT II trial is a prospective, multicenter study designed to evaluate the safety and efficacy of an aspirin-free, inflammation-guided strategy combining ticagrelor monotherapy with low-dose colchicine in patients with ACS undergoing PCI. Early discontinuation of aspirin is expected to reduce bleeding risk, while substituting colchicine may offer additional protection against ischemic events through suppression of residual inflammation. By tailoring colchicine duration according to hs-CRP levels, the study aims to maximize clinical benefit while minimizing unnecessary drug exposure. Through early initiation, individualized treatment, and structured longitudinal biomarker assessment, MACT II seeks to address residual inflammatory risk during the vulnerable post-PCI phase. Although promising, the findings will require confirmation in randomized controlled trials with diverse patient populations to validate this precision inflammation modulation approach in contemporary ACS management.
Statements
Ethics statement
The studies involving humans were approved by CHA Bundang Medical Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JJ: Writing – review & editing, Writing – original draft. YS: Writing – review & editing, Writing – original draft. CK: Writing – review & editing. JB: Writing – review & editing. KY: Writing – review & editing. J-HL: Writing – review & editing. K-HJ: Writing – review & editing. SC: Writing – review & editing. H-JY: Writing – review & editing. JK: Writing – review & editing. BL: Writing – review & editing. SK: Writing – review & editing. S-HK: Writing – review & editing. JM: Writing – review & editing. YJ: Conceptualization, Supervision, Funding acquisition, Writing – review & editing. S-YL: Project administration, Writing – review & editing, Supervision, Methodology, Writing – original draft, Funding acquisition, Visualization, Software, Investigation, Validation, Conceptualization, Resources, Formal analysis, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Biotronik (Bülach, Switzerland). Biotronik had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors used ChatGPT (OpenAI) to assist in the drafting and refinement of the manuscript text for improved clarity and language. After using this tool, the authors reviewed and edited the content as needed and took full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Vrints C Andreotti F Koskinas KC Rossello X Adamo M Ainslie J et al 2024 ESC guidelines for the management of chronic coronary syndromes. Eur Heart J. (2024) 45(36):3415–537. 10.1093/eurheartj/ehae177
2.
Rao SV O'Donoghue ML Ruel M Rab T Tamis-Holland JE Alexander JH et al 2025 ACC/AHA/ACEP/NAEMSP/SCAI guideline for the management of patients with acute coronary syndromes: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2025) 85(22):2135–237. 10.1016/j.jacc.2024.11.009
3.
Mehran R Baber U Sharma SK Cohen DJ Angiolillo DJ Briguori C et al Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. (2019) 381(21):2032–42. 10.1056/NEJMoa1908419
4.
Kim BK Hong SJ Cho YH Yun KH Kim YH Suh Y et al Effect of ticagrelor monotherapy vs ticagrelor with aspirin on Major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. (2020) 323(23):2407–16. 10.1001/jama.2020.7580
5.
Ge Z Kan J Gao X Raza A Zhang JJ Mohydin BS et al Ticagrelor alone versus ticagrelor plus aspirin from month 1 to month 12 after percutaneous coronary intervention in patients with acute coronary syndromes (ULTIMATE-DAPT): a randomised, placebo-controlled, double-blind clinical trial. Lancet. (2024) 403(10439):1866–78. 10.1016/S0140-6736(24)00473-2
6.
Ridker PM Bhatt DL Pradhan AD Glynn RJ MacFadyen JG Nissen SE et al Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. (2023) 401(10384):1293–301. 10.1016/S0140-6736(23)00215-5
7.
Kalkman DN Aquino M Claessen BE Baber U Guedeney P Sorrentino S et al Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur Heart J. (2018) 39(46):4101–8. 10.1093/eurheartj/ehy633
8.
Guedeney P Claessen BE Kalkman DN Aquino M Sorrentino S Giustino G et al Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol. (2019) 73(19):2401–9. 10.1016/j.jacc.2019.01.077
9.
Tardif JC Kouz S Waters DD Bertrand OF Diaz R Maggioni AP et al Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381(26):2497–505. 10.1056/NEJMoa1912388
10.
Nidorf SM Fiolet ATL Mosterd A Eikelboom JW Schut A Opstal TSJ et al Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 383(19):1838–47. 10.1056/NEJMoa2021372
11.
Lee SY Jeong YH Yun KH Cho JY Gorog DA Angiolillo DJ et al P2y(12) inhibitor monotherapy combined with colchicine following PCI in ACS patients: the MACT pilot study. JACC Cardiovasc Interv. (2023) 16(15):1845–55. 10.1016/j.jcin.2023.05.035
12.
Angiolillo DJ Rollini F Storey RF Bhatt DL James S Schneider DJ et al International expert consensus on switching platelet P2Y(12) receptor-inhibiting therapies. Circulation. (2017) 136(20):1955–75. 10.1161/CIRCULATIONAHA.117.031164
13.
Cutlip DE Windecker S Mehran R Boam A Cohen DJ van Es GA et al Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115(17):2344–51. 10.1161/CIRCULATIONAHA.106.685313
14.
Thygesen K Alpert JS Jaffe AS Chaitman BR Bax JJ Morrow DA et al Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138(20):e618–51. 10.1161/CIR.0000000000000617
15.
De Bruyne B Pijls NH Kalesan B Barbato E Tonino PA Piroth Z et al Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. (2012) 367(11):991–1001. 10.1056/NEJMoa1205361
16.
Mehran R Rao SV Bhatt DL Gibson CM Caixeta A Eikelboom J et al Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123(23):2736–47. 10.1161/CIRCULATIONAHA.110.009449
17.
Tantry US Bonello L Aradi D Price MJ Jeong YH Angiolillo DJ et al Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. (2013) 62(24):2261–73. 10.1016/j.jacc.2013.07.101
18.
Lee SH Kim HK Ahn JH Kang MG Kim KH Bae JS et al Prognostic impact of hypercoagulability and impaired fibrinolysis in acute myocardial infarction. Eur Heart J. (2023) 44(19):1718–28. 10.1093/eurheartj/ehad088
19.
Watanabe H Morimoto T Natsuaki M Yamamoto K Obayashi Y Ogita M et al Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. (2022) 7(4):407–17. 10.1001/jamacardio.2021.5244
20.
Natsuaki M Watanabe H Morimoto T Yamamoto K Obayashi Y Nishikawa R et al An aspirin-free versus dual antiplatelet strategy for coronary stenting: sTOPDAPT-3 randomized trial. Circulation. (2024) 149(8):585–600. 10.1161/CIRCULATIONAHA.123.066720
21.
Buckley LF Libby P . Colchicine’s role in cardiovascular disease management. Arterioscler Thromb Vasc Biol. (2024) 44(5):1031–41. 10.1161/ATVBAHA.124.319851
22.
Lee SY Cho JY Gorog DA Angiolillo DJ Yun KH Ahn JH et al Inflammation and platelet reactivity during adjunctive colchicine versus aspirin in patients with acute coronary syndrome treated with potent P2Y12 inhibitor. Front Med (Lausanne). (2024) 11:1349577. 10.3389/fmed.2024.1349577
23.
Jolly SS d'Entremont MA Lee SF Mian R Tyrwhitt J Kedev S et al Colchicine in acute myocardial infarction. N Engl J Med. (2025) 392(7):633–42. 10.1056/NEJMoa2405922
24.
Kwong JC Schwartz KL Campitelli MA Chung H Crowcroft NS Karnauchow T et al Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. (2018) 378(4):345–53. 10.1056/NEJMoa1702090
25.
Laudani C Abbate A Angiolillo DJ Galli M . CLEAR results, cloudy impact: colchicine’s neutral role in ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Pharmacother. (2025) 11(3):215–7. 10.1093/ehjcvp/pvaf011
Summary
Keywords
acute coronary syndrome, percutaneous coronary intervention, aspirin, colchicine, ticagrelor
Citation
Jang JY, Suh Y, Kim C, Byoun JT, Yun KH, Lee J-H, Jeon K-H, Cho S, Yoon H-J, Kim JW, Lee B, Kang SH, Kim S-H, Moon JY, Jang Y and Lee S-Y (2025) Efficacy and safety of mono antiplatelet therapy with colchicine in acute coronary syndrome patients following percutaneous coronary intervention: rationale and design of the MACT II trial. Front. Cardiovasc. Med. 12:1662392. doi: 10.3389/fcvm.2025.1662392
Received
09 July 2025
Accepted
14 October 2025
Published
27 October 2025
Volume
12 - 2025
Edited by
Xiaofeng Yang, Temple University, United States
Reviewed by
Rozen Grigorov, Medicinski universitet Varna Prof d-r Paraskev Stoanov Biblioteka, Bulgaria
Emmanuel De Cock, AZ Sint-Jan Brugge-Oostende AV, Belgium
Updates
Copyright
© 2025 Jang, Suh, Kim, Byoun, Yun, Lee, Jeon, Cho, Yoon, Kim, Lee, Kang, Kim, Moon, Jang and Lee.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Yangsoo Jang jangys1212@yuhs.ac Seung-Yul Lee seungyul79@gmail.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.