- 1Department of Emergency, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 2Department of Medical, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 3Department of Radiology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Background: The role of ultra-rapid β-blockers in sepsis-associated cardiac dysfunction remains controversial, with conflicting evidence regarding mortality benefits and safety concerns in hemodynamically unstable patients.
Methods: This study retrieved relevant reports on randomized controlled trials of ultra-rapid β-blockers conducted for adult patients with sepsis-associated cardiac dysfunction, up to and including the date of May 30, 2025, from the databases of PubMed, Web of Science, Cochrane Library and Embase. Primary outcomes were 28-day mortality and adverse events; secondary outcomes included heart rate control and mean arterial pressure (MAP) at 48 h. Random-effects models calculated risk ratios (RR) or standardized mean differences (SMD) with 95% confidence intervals (CI). Heterogeneity was assessed using I² statistics.
Results: Eight studies reported 28-day mortality, showing no significant reduction with ultra-rapid β-blockers (RR, 0.84, 95% CI: 0.67–1.06; P = 0.15; I² = 54%). Safety data from four studies indicated no increased adverse events (RR, 1.04, 95% CI: 0.82–1.33; P = 0.72; I² = 0%). Paradoxically, ultra-rapid β-blockers were associated with worse heart rate control (RR, 1.51, 95% CI: 1.00–2.29; P = 0.05). MAP at 48 h showed no intergroup difference (SMD, −0.85, 95% CI: −2.24–0.54).
Conclusion: ultra-rapid β-blockers demonstrate an acceptable safety profile without compromising hemodynamic stability but fail to reduce 28-day mortality in sepsis-associated cardiac dysfunction patients. The inferior heart rate control suggests potential physiological incompatibility in this population. Precision targeting based on adrenergic activity and cardiac phenotyping warrants investigation.
Introduction
Sepsis, one of the leading causes of death in critically ill patients worldwide, is characterized by life-threatening organ dysfunction triggered by an uncontrolled host response to infection (1, 2). When sepsis involves the circulatory system, it can induce or exacerbate cardiac dysfunction (i.e., sepsis-associated cardiac dysfunction, refers to newly emerging reversible heart failure during the course of sepsis, characterized by a decrease in left ventricular ejection fraction (LVEF < 50%) or elevated cardiac injury markers (cTnI > 0.4 ng/ml), and must exclude patients with chronic heart failure), creating a critical state with complex pathophysiological mechanisms and a very poor clinical prognosis (3, 4). These patients not only face a high risk of death due to sepsis itself (in-hospital mortality can be as high as 40%–60%) but are also caught in a vicious circle of hemodynamic collapse due to the rapid deterioration of cardiac function. The severity of sepsis is twofold: on the one hand, sepsis releases a storm of inflammatory mediators and cytokines that can directly inhibit myocardial contractility and impair ventricular function, leading to “septic cardiomyopathy” (5); on the other hand, although the compensatory activation of the sympathetic nervous system (SNS) can temporarily maintain perfusion, the continuous excessive catecholamine release can cause tachycardia, a dramatic increase in myocardial oxygen consumption, calcium regulation disorders, and direct cardiotoxicity, which can accelerate cardiac failure and significantly increase the risk of multi-organ failure and death (6, 7).
The treatment of sepsis-associated cardiac dysfunction is highly urgent and complex. Conventional supportive therapies (e.g., fluid resuscitation, vasoactive drugs) can partially correct the hemodynamic disturbances but are often ineffective in controlling the vicious cycle of sympathetic overactivation (8, 9). β-blockers, as a class of negative inotropic and negative frequency drugs, are necessary because of their potential dual pathological interventions: by antagonizing β1 receptors, reducing persistently elevated heart rate, decreasing myocardial oxygen consumption, and shorten the ventricular filling time; in addition, they may also alleviate adrenergic-mediated myocardial cell damage, calcium overload, and metabolic disorders, thereby protecting myocardial function and potentially improving long-term prognosis (10, 11); However, when patients are in a state of metabolic hyperactivity and high stress, the use of ultra-rapid β-blockers may cause hypotension or mask signs of hypoperfusion by inhibiting compensatory cardiac output. These potential risks raise questions about the safety of their clinical application (12).
In view of this, this systematic review and Meta-analysis aims to comprehensively integrate the existing clinical research evidence and quantitatively assess the efficacy and safety of ultra-rapid β-blockers in the treatment of sepsis-associated cardiac dysfunction patients. The results of the study will provide a key evidence-based basis for clinical development of individualized treatment strategies and design of high-quality prospective trials.
Methods
Search strategy
The search was performed using the terms “sepsis” or “heart failure” or “cardiac insufficiency” or “β-blocker” up to 30 May 2025 in the Cochrane Library, Web of science and Embase databases. Duplicate items were excluded from the search results. The reference lists of articles were also reviewed for this study. Two researchers independently reviewed the titles, keywords, abstracts, and full text of all identified articles to retain those that met the screening criteria. Any doubts about the inclusion of articles were resolved by a third researcher after discussion and consensus. The detailed database search strategy is provided in Supplementary Table S1.
Criteria for study selection
The inclusion criteria were defined as follows: i. Study type: randomized controlled trial (RCT). ii. Study population: patients diagnosed with sepsis and presenting with at least one of the following conditions: definite reduction in left ventricular ejection fractions (LVEF); significantly elevated cardiac biomarkers (BNP/NT-proBNP, Troponin); persistent, uncontrollable sinus tachycardia (heart rate >110–120 bpm unresponsive to volume resuscitation and basal therapy) or tachyarrhythmias that require treatment (13). Although the search terms include traditional terms such as “heart failure,” the studies ultimately included in the analysis all meet the modern definition of sepsis-associated heart failure (sepsis-induced + acute onset + abnormal objective indicators), which is fundamentally different from traditional chronic heart failure. iii. Intervention: β-blocker therapeutic intervention. iv. Outcome Indicators: a. Primary Outcome: 28-day mortality, incidence of adverse events. b. Secondary outcomes: heart rate control effect, mean arterial pressure (MAP). v. Language of articles: Only English and Chinese articles were included.
Exclusion criteria were as follows: i. Duplicate literature studies, systematic evaluations, reviews and case reports. ii. Animal studies, studies in children or adolescents (<18 years old). iii. Non-β-blockers. iv. Studies with incomplete data or unclear methodology were excluded.
Data extraction
Two researchers independently reviewed titles and abstracts to identify studies relevant to the article topic. Two independent reviewers fully downloaded and assessed the eligible literature. Two independent reviewers extracted data from the included studies. Extracted data included 1. authors; 2. year of publication; 3. participants (age, gender, sample size); 4. intervention characteristics; and 6. study outcomes. Any discrepancies that arose during the process were resolved by consensus among the assessors, with a third assessor consulted if necessary.
Risk of bias assessment
The reviewers used the revised Cochrane Risk of Bias tool for randomized trials to assess the risk of bias (RoB 2.0) (14). This study evaluated the risk of bias based on aspects such as bias during the randomization process, deviation from the expected intervention measures, the situation of missing result data, the measurement of results, and the selection of intervention subjects. It classified the risk of bias into four levels: low, possibly low, possibly high, or high. The disagreement was resolved through discussion, and in case of necessity, a third party would make the final decision.
Statistical analysis
The Mantel-Haenszel random effects model was used for the statistical analysis of binary classification results, and the inverse variance random effects model was used for the statistical analysis of continuous results. The risk ratio (RR) or standardized mean difference (SMD) was presented in the form of point estimates, along with a 95% confidence interval and P value. For data presented in the form of median and interquartile range, the median and interquartile range were converted to mean and standard deviation to obtain the combined RR and SMD. The Mantel-Haenszel x2 test and I2 statistic (the proportion of total variation explained by heterogeneity) were used to investigate statistical heterogeneity (15). All analyses were completed using RevMan 5.1.6.
Results
Study selection and study characteristics
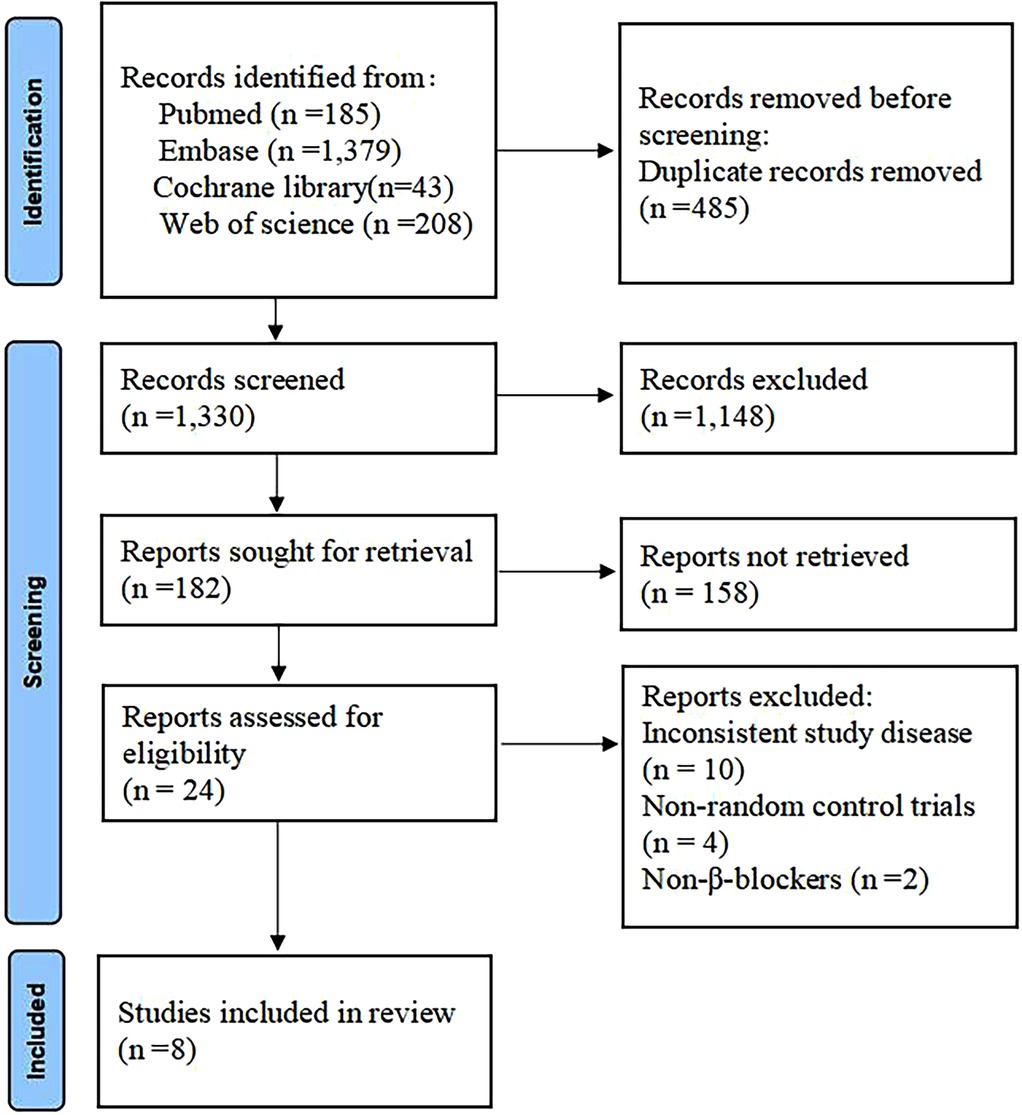
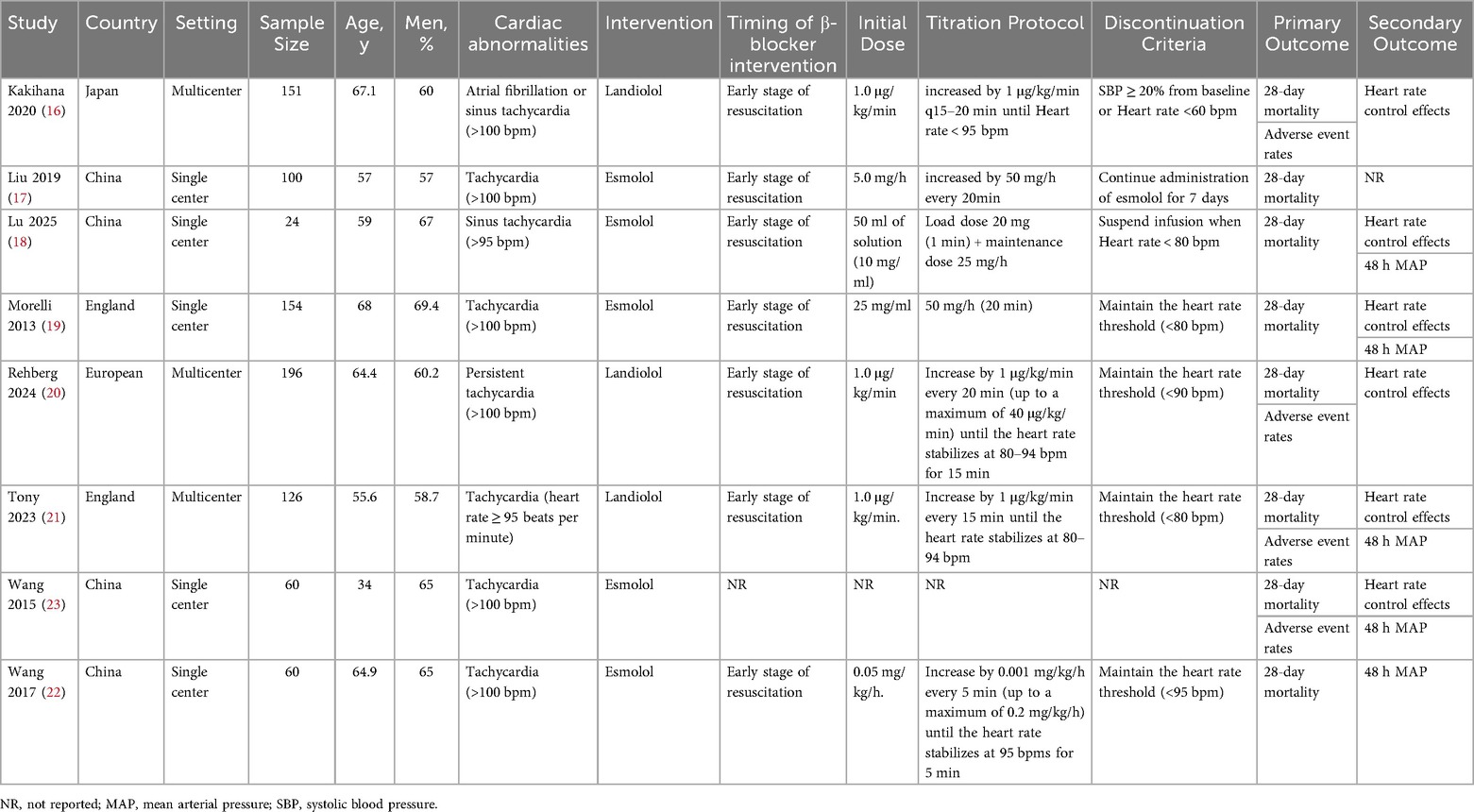
A total of 1,815 articles were retrieved through the database search (Figure 1). Of these, 485 duplicates were removed, and 1,148 records were excluded due to title and abstract incompatibility. Ultimately, a total of eight studies were included in this meta-analysis, with a combined sample size of 871 patients (16–23). Table 1 presents the basic characteristics of the included studies. The sample size ranged from 24–196. All eight trials reported 28-day mortality, six reported the effect of heart rate control, five investigated hemodynamic parameters, and only four reported the incidence of adverse events. Four trials were single-center RCTs in China, two were single-center and multicenter RCTs in the United Kingdom, one trial was a multicenter RCT in Japan, and one trial was conducted in multicenter RCT conducted in Europe. The mean/median age of the participants was reported to be between 34 and 68 years, with 57%–67% of them being male.
Risk of bias
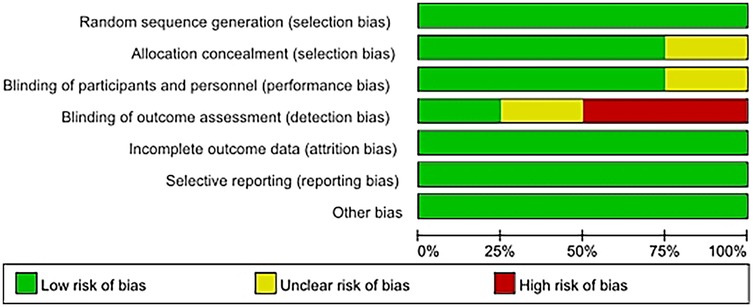
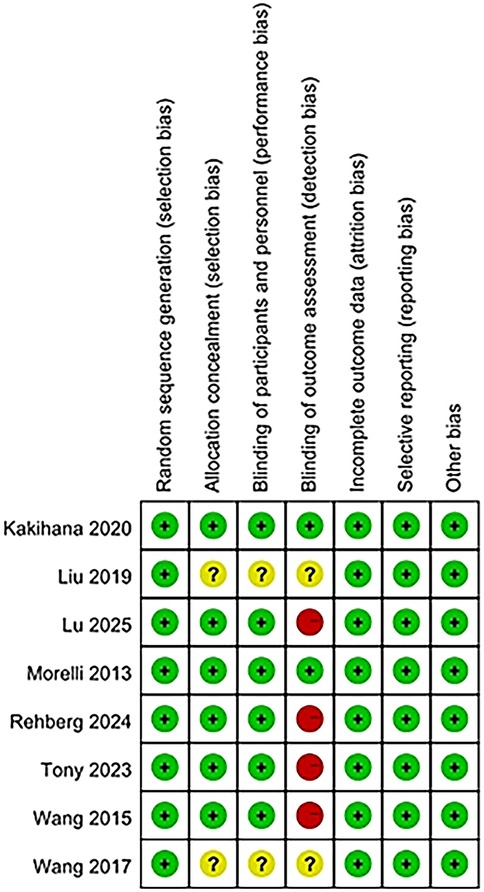
The risk of bias for eight studies was evaluated, and detailed information can be found in the bias risk summary table (Figure 2) and the bias risk chart (Figure 3). Six studies had a low risk of bias in terms of allocation concealment (selection bias); while two other studies could not be evaluated due to the lack of relevant information. Six studies were at low risk of blinding of participants and staff (performance bias). Two other studies could not be evaluated regarding the blinding of participants and personnel. Four studies had a high risk of bias in terms of outcome assessment blinding, while two studies had a low risk of bias in this regard, and two other studies could not be evaluated regarding the outcome assessment blinding situation due to the lack of relevant information. Regarding other biases, the risk was low for all eight studies.
Primary outcomes
28-day mortality rate
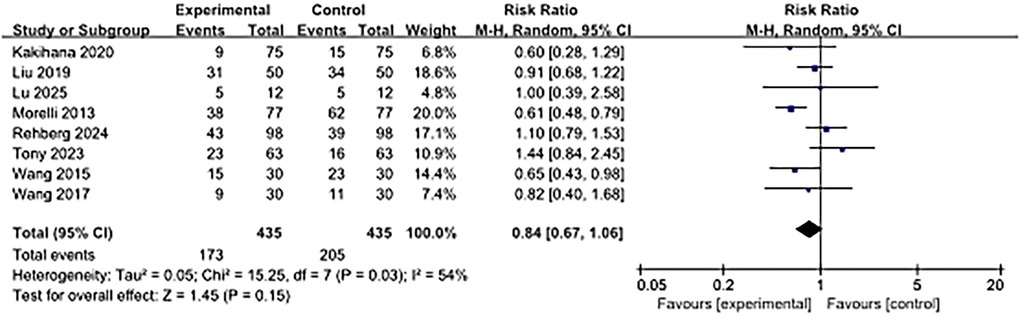
As shown in Figure 4, 28-day mortality was reported in eight studies. The use of ultra-rapid β-blockers in patients with sepsis-associated cardiac dysfunction did not show a significant association with lower 28-day mortality (RR, 0.84; 95% CI: 0.67–1.06; P = 0.15). Moderate heterogeneity was observed (I2 = 54%).
The incidence of adverse events in patients
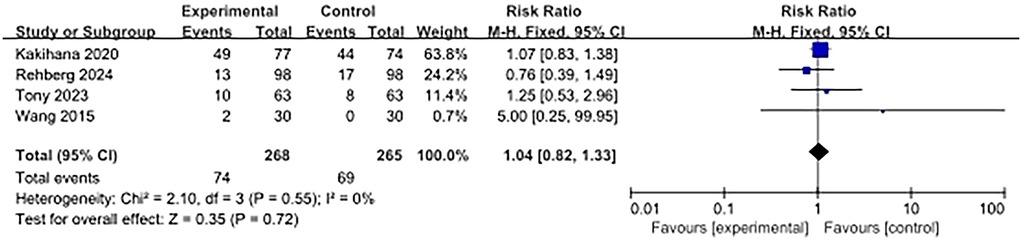
Four studies reported adverse events. The use of ultra-rapid β-blockers in patients with sepsis-associated cardiac dysfunction did not show a significant association with the occurrence of adverse events (RR, 1.04; 95% CI: 0.82–1.33; P = 0.72). No heterogeneity was observed (I2 = 0%) (Figure 5).
Secondary outcomes
Heart rate control effect
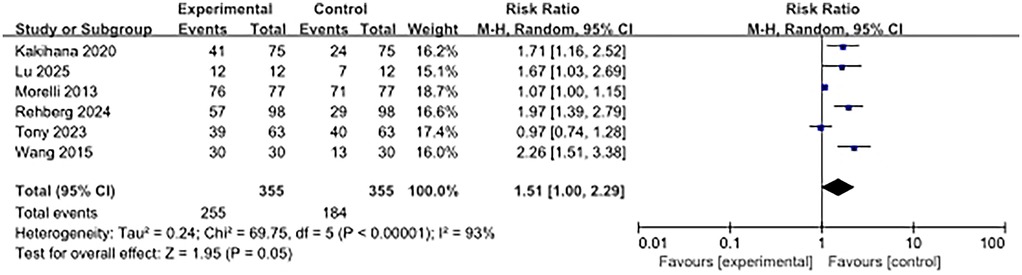
Six articles examined the heart rate control effect. The results showed that the heart rate control effect of the beta-blocker group was significantly worse than that of the control group (RR, 1.51; 95% CI, 1.00–2.29; P = 0.05). High heterogeneity was observed (I2 = 93%) (Figure 6).
Mean arterial pressure
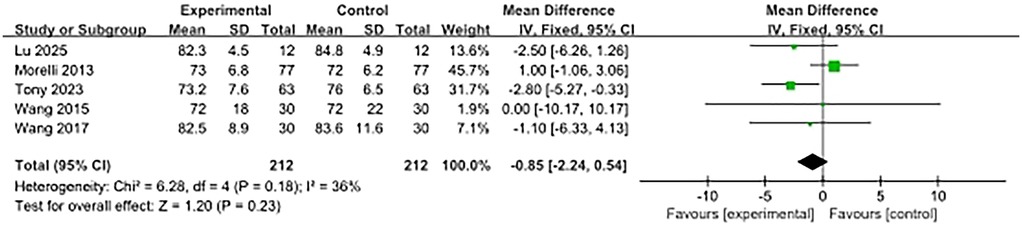
Five studies reported on MAP at 48 h after enrolment, and there was no significant difference between the β-blocker group and the control group (SMD, −0.85; 95% CI, −2.24–0.54). Moderate heterogeneity was observed (I2 = 36%) (Figure 7).
Publication bias
In this study, the publication bias of the primary outcome measure (28-day mortality rate) was evaluated by visually inspecting the symmetry of the funnel plot. The results indicated that the funnel plot was symmetrical, and there was no possibility of publication bias (Supplementary Figure S1).
Discussion
This meta-analysis provides a comprehensive evaluation of ultrashort-acting β-blockers (esmolol/landiolol) in sepsis-associated cardiac dysfunction, revealing critical insights into their hemodynamic effects and clinical outcomes. The findings challenge conventional assumptions while highlighting the complexity of adrenergic modulation in critical illness.
The reduction in the 28-day mortality rate did not reach statistical significance. Although the point estimate suggested a 16% relative risk reduction, the confidence interval included the null value (RR = 1), indicating that the result may have been due to random variation. However, from a pathophysiological perspective, beta-blockers have a dual mechanism of action in sepsis: they provide potential cardiac protection by reducing myocardial oxygen demand and alleviating catecholamine toxicity, while also impairing compensatory mechanisms in distributive shock (24, 25). The observed moderate heterogeneity (I² = 54%) may be due to differences in patients' hemodynamic phenotypes (e.g., cardiogenic shock vs. vasodilatory shock). Although the current data exclude a clinically significant effect of a mortality reduction >33%, smaller effects may still exist and require validation with a larger sample size.
The adverse event profile with null heterogeneity is arguably the most significant finding. It confirms the hemodynamic safety margin of ultrashort-acting agents in this high-risk population (26). Their rapid offset enables precise titration, mitigating traditional concerns about ultra-rapid β-blockers in shock states (27). This pharmacodynamic advantage likely underpins the preserved MAP at 48 h, suggesting these agents can be administered without compromising perfusion pressure when hemodynamic monitoring is available. Contrary to mechanistic expectations, β-blocker use associated with inferior heart rate control. This counterintuitive result exposes fundamental knowledge gaps: In sepsis-associated cardiac dysfunction, tachycardia may be essential to maintain cardiac output in the context of reduced stroke volume (28, 29). Blunting this reflex without concomitant inotropic support could precipitate decompensation. Rapid drug discontinuation in studies with protocolized limited-duration infusions may trigger paradoxical tachycardia. Current titration protocols (often targeting HR < 100 bpm) might inadequately address the hyperadrenergic state of sepsis, leading to underdosing and apparent “failure” of rate control.
This study shares continuity with the meta-analyses conducted by Hasegawa D et al. and Perala A et al. in terms of the core research question, both focusing on the application value of ultra-rapid β-blockers in patients with sepsis and showing a consistent trend of benefit in terms of 28-day mortality outcomes (30, 31). However, previous studies only used 28-day mortality as a single outcome. This study expanded the evaluation framework to a three-dimensional system of “survival-function-safety,” adding heart rate control efficacy, hemodynamic stability, and adverse event incidence, addressing the clinical need for multi-dimensional efficacy and safety assessment; Second, this study exclusively included randomized controlled trials, reducing confounding bias by excluding observational studies, thereby providing high-level evidence support. In contrast, previous studies did not strictly restrict study types, leading to variations in evidence strength. This design optimization not only validated the core conclusions but also enhanced the clinical relevance of the findings through rigorous study type screening and expanded outcome dimensions.
Although the use of ultra-rapid β-blockers in sepsis has been explored in multiple randomized controlled trials, existing studies have the following limitations: First, most trials only report a single outcome (e.g., 28-day mortality) and lack systematic analysis of functional indicators such as heart rate control and hemodynamics; Second, different trials have inconsistent definitions of the “sepsis-associated cardiac dysfunction” subgroup, leading to fragmented evidence. This study systematically integrated eight high-quality randomized controlled trials to establish a three-dimensional outcome framework of “survival-function-safety” for the first time, consolidating dispersed single-trial data into a multidimensional evidence chain.
Interpretation of this study requires careful consideration of the following limitations: first, the limited number of original studies included (only 8 assessing mortality and 4 reporting adverse events) may reduce statistical power and increase the risk of type II error, especially for key outcomes such as 28-day mortality; second, significant clinical heterogeneity (I² = 54%) stemmed from differences in baseline patient characteristics (e.g., sepsis etiology, cardiac dysfunction severity, concomitant therapy) and inconsistent intervention regimens (β-blocker dose, titration rate, target heart rate), which were not adequately corrected for; finally, the lack of individual patient data limited the ability of subgroup analyses (e.g., distinguishing between preserved vs. reduced cardiac dysfunction with preserved ejection fraction) to identify populations of potential benefit. Furthermore, this analysis only confirms that ultra-rapid beta-blockers do not lower mean arterial pressure, but this does not equate to overall hemodynamic stability. Future studies need to systematically monitor changes in vasoactive drug requirements (such as norepinephrine equivalent doses), cardiac function parameters (cardiac index, stroke volume), and tissue perfusion indicators (lactate, ScvO₂) to comprehensively assess safety.
Based on current evidence, ultra-rapid β-blockers for patients with sepsis-associated cardiac dysfunction, although demonstrating an acceptable safety profile (no significant increase in risk of adverse events, blood pressure stabilization at 48 h), failed to significantly reduce 28-day mortality and were associated with worse heart rate control. This paradox suggests that using heart rate alone as a therapeutic target may be insufficient or even harmful, especially in pathological states where compensatory tachycardia maintains cardiac output. Future studies should focus on precise patient selection (e.g., high sympathetic tone subgroups), optimizing ICU hemodynamic management (integrating cardiac output monitoring), and exploring combination therapeutic strategies (e.g., coadministration of positive inotropic medications) to reevaluate the balance of risk-benefit of ultra-rapid β-blockers in this complex population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
MZ: Conceptualization, Data curation, Formal analysis, Writing – original draft. JW: Conceptualization, Data curation, Formal analysis, Writing – original draft. PX: Data curation, Investigation, Methodology, Writing – review & editing. SG: Investigation, Methodology, Validation, Writing – review & editing. BC: Investigation, Methodology, Visualization, Writing – review & editing. ZH: Investigation, Methodology, Visualization, Writing – review & editing. GY: Funding acquisition, Investigation, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Zhaoyang Talent Plan Project of Guangdong Provincial Hospital of Chinese Medicine (Grant No. 2022KT1221). The title of the project is observation on the efficacy of qi-invigorating, blood-activating and detoxifying formula in preventing and treating heart dysfunction related to sepsis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1665466/full#supplementary-material
Abbreviations
MAP, mean arterial pressure; RR, risk ratios; SMD, standardized mean differences; CI, confidence intervals; SNS, sympathetic nervous system; RCT, randomized controlled trial; LVEF, left ventricular ejection fractions.
References
1. Srzić I, Nesek Adam V, Tunjić Pejak D. Sepsis definition: what’s new in the treatment guidelines. Acta Clin Croat. (2022) 61(Suppl 1):67–72. doi: 10.20471/acc.2022.61.s1.11
2. O'Brien JM Jr, Ali NA, Aberegg SK, Abraham E. Sepsis. Am J Med. (2007) 120(12):1012–22. doi: 10.1016/j.amjmed.2007.01.035
3. Carbone F, Liberale L, Preda A, Schindler TH, Montecucco F. Septic cardiomyopathy: from pathophysiology to the clinical setting. Cells. (2022) 11(18):2833. doi: 10.3390/cells11182833
4. L'Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG. Sepsis-induced cardiomyopathy: a comprehensive review. Curr Cardiol Rep. (2020) 22(5):35. doi: 10.1007/s11886-020-01277-2
5. Chong DLW, Sriskandan S. Pro-inflammatory mechanisms in sepsis. Contrib Microbiol. (2011) 17:86–107. doi: 10.1159/000324022
6. Joseph J, Gilbert EM. The sympathetic nervous system in chronic heart failure. Prog Cardiovasc Dis. (1998) 41(1 Suppl 1):9–16. doi: 10.1016/S0033-0620(98)80026-1
7. De Backer D, Foulon P. Minimizing catecholamines and optimizing perfusion. Crit Care. (2019) 23(Suppl 1):149. doi: 10.1186/s13054-019-2433-6
8. Arabi YM, Belley-Cote E, Carsetti A, De Backer D, Donadello K, Juffermans NP, et al. European society of intensive care medicine clinical practice guideline on fluid therapy in adult critically ill patients. Part 1: the choice of resuscitation fluids. Intensive Care Med. (2024) 50(6):813–31. doi: 10.1007/s00134-024-07369-9
9. Seymour CW, Rosengart MR. Septic shock: advances in diagnosis and treatment. Jama. (2015) 314(7):708–17. doi: 10.1001/jama.2015.7885
10. Jondeau G, Milleron O. Beta-blockers in acute heart failure: do they cause harm? JACC Heart Fail. (2015) 3(8):654–6. doi: 10.1016/j.jchf.2015.04.009
11. Sadaf A, Hasan B, Das JK, Colan S, Alvi N. Calcium channel blockers for preventing cardiomyopathy due to iron overload in people with transfusion-dependent beta thalassaemia. Cochrane Database Syst Rev. (2018) 7(7):Cd011626. doi: 10.1002/14651858.CD011626.pub3
12. de Oliveira MT Jr., Baptista R, Chavez-Leal SA, Bonatto MG. Heart failure management with β-blockers: can we do better? Curr Med Res Opin. (2024) 40(sup1):43–54. doi: 10.1080/03007995.2024.2318002
13. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European Society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure: endorsed by the Canadian heart failure society, heart failure association of India, cardiac society of Australia and New Zealand, and Chinese heart failure association. Eur J Heart Fail. (2021) 23(3):352–80. doi: 10.1016/j.cardfail.2021.01.022
14. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
15. Liu IM, Agresti A. Mantel-Haenszel-type inference for cumulative odds ratios with a stratified ordinal response. Biometrics. (1996) 52(4):1223–34. doi: 10.2307/2532838
16. Kakihana Y, Nishida O, Taniguchi T, Okajima M, Morimatsu H, Ogura H, et al. Efficacy and safety of landiolol, an ultra-short-acting β1-selective antagonist, for treatment of sepsis-related tachyarrhythmia (J-Land 3S): a multicentre, open-label, randomised controlled trial. Lancet Respir Med. (2020) 8(9):863–72. doi: 10.1016/S2213-2600(20)30037-0
17. Liu H, Ding XF, Zhang SG, Wang HX, Luo YG, Duan XG, et al. Effect of esmolol in septic shock patients with tachycardia: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. (2019) 99(17):1317–22. doi: 10.3760/cma.j.issn.0376-2491.2019
18. Lu S, Kattan E, Pan C, Shen J, Zhang T, Wang P, et al. Very early use of esmolol in hyperkinetic septic shock patients with persistent tachycardia: a randomized controlled pilot study. Shock. (2025) 63(6):870–7. doi: 10.1097/SHK.0000000000002576
19. Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. Jama. (2013) 310(16):1683–91. doi: 10.1001/jama.2013.278477
20. Rehberg S, Frank S, Černý V, Cihlář R, Borgstedt R, Biancofiore G, et al. Landiolol for heart rate control in patients with septic shock and persistent tachycardia. A multicenter randomized clinical trial (Landi-SEP). Intensive Care Med. (2024) 50(10):1622–34. doi: 10.1007/s00134-024-07587-1
21. Whitehouse T, Hossain A, Perkins GD, Gordon AC, Bion J, Young D, et al. Landiolol and organ failure in patients with septic shock: the STRESS-L randomized clinical trial. Jama. (2023) 330(17):1641–52. doi: 10.1001/jama.2023.20134
22. Wang S, Li M, Duan J, Yi L, Huang X, Chen D, et al. Effect of esmolol on hemodynamics and clinical outcomes in patients with septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2017) 29(5):390–5. doi: 10.3760/cma.j.issn.2095-4352.2017.05.002
23. Wang Z, Wu Q, Nie X, Guo J, Yang C. Combination therapy with milrinone and esmolol for heart protection in patients with severe sepsis: a prospective, randomized trial. Clin Drug Investig. (2015) 35(11):707–16. doi: 10.1007/s40261-015-0325-3
24. Knabb RM, Ely SW, Bacchus AN, Rubio R, Berne RM. Consistent parallel relationships among myocardial oxygen consumption, coronary blood flow, and pericardial infusate adenosine concentration with various interventions and beta-blockade in the dog. Circ Res. (1983) 53(1):33–41. doi: 10.1161/01.RES.53.1.33
25. Lançon JP, Leneuf P, Rerolle A, Caillard B. Changes in myocardial metabolism induced by drugs used during intensive care. Ann Fr Anesth Reanim. (1990) 9(1):31–41. doi: 10.1016/s0750-7658(05)80034-6
26. Barbier GH, Shettigar UR, Appunn DO. Clinical rationale for the use of an ultra-short acting beta-blocker: esmolol. Int J Clin Pharmacol Ther. (1995) 33(4):212–8.7620691
27. Blanski L, Lutz J, Laddu A. Esmolol, the first ultra-short-acting intravenous beta blocker for use in critically ill patients. Heart Lung. (1988) 17(1):80–9.3276651
28. Song J, Fang X, Zhou K, Bao H, Li L. Sepsis-induced cardiac dysfunction and pathogenetic mechanisms (review). Mol Med Rep. (2023) 28(6):1–12. doi: 10.3892/mmr.2023.13114
29. Lima MR, Silva D. Septic cardiomyopathy: a narrative review. Rev Port Cardiol. (2023) 42(5):471–81. doi: 10.1016/j.repc.2021.05.020
30. Perala A, Wishart AV, Hamouda RK, Elsaady E, Aslam MR, Khan S. Efficacy of β-blockers in decreasing mortality in sepsis and septic shock patients: a systematic review. Cureus. (2024) 16(8):e66888. doi: 10.7759/cureus.66888
31. Hasegawa D, Sato R, Prasitlumkum N, Nishida K, Takahashi K, Yatabe T, et al. Effect of ultrashort-acting β-blockers on mortality in patients with sepsis with persistent tachycardia despite initial resuscitation: a systematic review and meta-analysis of randomized controlled trials. Chest. (2021) 159(6):2289–300. doi: 10.1016/j.chest.2021.01.009
Keywords: ultra-rapid β-blockers, sepsis, cardiac dysfunction, healing efficacy, safety
Citation: Zheng M, Wang J, Xie P, Guo S, Chen B, He Z and Yao G (2025) Efficacy and safety of short-acting β-blockers in patients with sepsis-associated cardiac dysfunction: a systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 12:1665466. doi: 10.3389/fcvm.2025.1665466
Received: 14 July 2025; Accepted: 28 August 2025;
Published: 9 September 2025.
Edited by:
Sascha Treskatsch, Charité University Medicine Berlin, GermanyReviewed by:
Fabio Guarracino, Azienda Ospedaliero Universitaria Pisana, ItalyGötz Schmidt, University of Giessen, Germany
Copyright: © 2025 Zheng, Wang, Xie, Guo, Chen, He and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoyan Yao, eWFvZ3VveWFuNzlAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Min'an Zheng1,†
Min'an Zheng1,† Guoyan Yao
Guoyan Yao