- Department of Cardiology, The Second Clinical Medical College of North Sichuan Medical College, Nanchong, China
Background: Malnutrition frequently complicates heart failure (HF), interacting with systemic inflammation, metabolic dysregulation, and immune dysfunction to accelerate disease progression. The Controlling Nutritional Status (CONUT) score, derived from objective laboratory parameters (serum albumin, total cholesterol, lymphocyte count), quantifies nutritional derangements and has emerged as a promising tool for HF risk stratification and prognostic prediction. However, accumulating evidence requires systematic synthesis to establish its clinical validity.
Methods: A comprehensive literature search was conducted in databases including PubMed, Embase, the Cochrane Library and Web of Science, covering all available records up to January 27, 2025, to identify research examining the association between the CONUT score and HF outcomes.
Results: The analysis included 28 cohort studies. Pooled data demonstrated a significant correlation between elevated CONUT scores and higher rates of all-cause mortality (HR = 1.57, 95% CI 1.35–1.83; P < 0.00001). Despite substantial heterogeneity, sequential exclusion sensitivity analyses confirmed the robustness of this association, with recalculated estimates consistently showing overlapping confidence intervals across all analytical scenarios.
Conclusion: Based on the definition of the CONUT score, malnutrition remains a significant factor associated with overall mortality risk in individuals diagnosed with heart failure, even after controlling for potential confounders. Utilizing the CONUT score for nutritional assessment enables clinicians to detect patients who are more likely to experience unfavorable clinical outcomes.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251023217, PROSPERO CRD420251023217.
1 Introduction
Heart failure constitutes the final phase in the progression of various cardiovascular diseases. Despite significant advances in heart failure management, its incidence continues to rise (1). Epidemiological studies indicate that heart failure affects an estimated 64.3 million individuals globally, with a prevalence of 4%–11% among individuals aged ≥65 (2). As reported by the Global Burden of Disease Study, the age-standardized mortality rate attributed to heart failure increased by 38.3% between 1990 and 2017, and the condition accounted for approximately 3 million deaths globally in 2019 (3). This growing disease burden is closely linked to population aging, improved survival rates of coronary heart disease, and the growing prevalence of metabolic disorders. Nutritional deficiencies are widespread in heart failure patients: 24% of chronic heart failure (CHF) patients exhibit hypoalbuminemia (<3.5 mg/dl), and 68% experience muscle wasting (4). However, current heart failure guidelines offer limited guidance on managing nutritional disorders (5). Research suggests that the pathogenesis of heart failure is associated with novel inflammation-related circulating biomarkers (6). Malnutrition accelerates disease progression by promoting muscle catabolism, immune dysfunction, and exacerbated inflammatory responses (5).
The CONUT Score integrates three objective biochemical parameters-serum albumin, total cholesterol, and peripheral blood lymphocyte count (Table 1), to provide a multidimensional quantitative assessment system for nutritional status. Scores on the CONUT scale span from 0–12, with elevated values signifying poorer nutritional status. Recent clinical research has shown a significant link between elevated CONUT scores and adverse clinical outcomes among individuals with heart failure (HF). For example, Kato et al., in a multicenter cohort analysis (7), found that individuals with a CONUT score ≥5 had a 2.80-fold increased risk of all-cause mortality compared to controls (HR = 2.80, 95% CI 1.92–4.08). Similarly, Zhao et al. found (8) that a higher CONUT score at admission independently predicted poor prognosis in individuals with systolic heart failure (HR = 1.79, 95% CI 1.37–2.32). The underlying mechanisms may involve malnutrition-induced multifaceted pathophysiological alterations. However, systematic evidence supporting the prognostic value of the CONUT score in HF remains limited and warrants further synthesis.
Although numerous observational studies indicate a potential link between the CONUT score and adverse prognosis in heart failure patients, its effectiveness as a predictive tool still lacks robust evidence-based support. This meta-analysis therefore aims to investigate the relationship between the CONUT score and clinical outcomes in heart failure patients, thereby providing a foundation for improved clinical management and risk stratification.
2 Methods
This meta-analysis was performed following the guidelines of the PRISMA statement and was prospectively registered in the PROSPERO database (CRD420251023217). Ethical approval was not required (9).
2.1 Literature search
We conducted comprehensive literature searches in PubMed, Embase, the Cochrane Library, and Web of Science for studies published from the inception of each database to January 27, 2025. The major search terms used in PubMed were as follows: “Heart Failure”, “Cardiac Failure”, “Congestive Heart Failure”, “Myocardial Failure”, “Controlling Nutritional Status score” and “CONUT” (Supplementary Table S1).
2.2 Study selection
To be considered for inclusion, studies had to fulfill the following selection criteria: Population: (1) Individuals (aged ≥18 years) with a confirmed diagnosis of heart failure; (2) All types of heart failure (HFrEF, HFmrEF, HFpEF); (3) Regardless of the New York Heart Association (NYHA) functional class.
Exposure: (4) Patients stratified into high and low CONUT score groups based on predefined cutoff values.
Outcomes: All-cause mortality, cardiovascular mortality, heart failure readmission rate and the composite outcomes of all-cause mortality or heart failure readmission; (5) Studies providing hazard ratio (HR) and 95% confidence interval (CI) data, either directly reported or calculable from available data; (6) Studies published in full-text form.
Studies were excluded based on the criteria listed below: (1) Review articles, case reports, conference abstracts, commentaries, and letters; (2) Studies not providing sufficient data to calculate HRs and 95% CIs; (3) Studies that did not report survival data; (4) Duplicate publications or those with overlapping data.
2.3 Data extraction and quality assessment
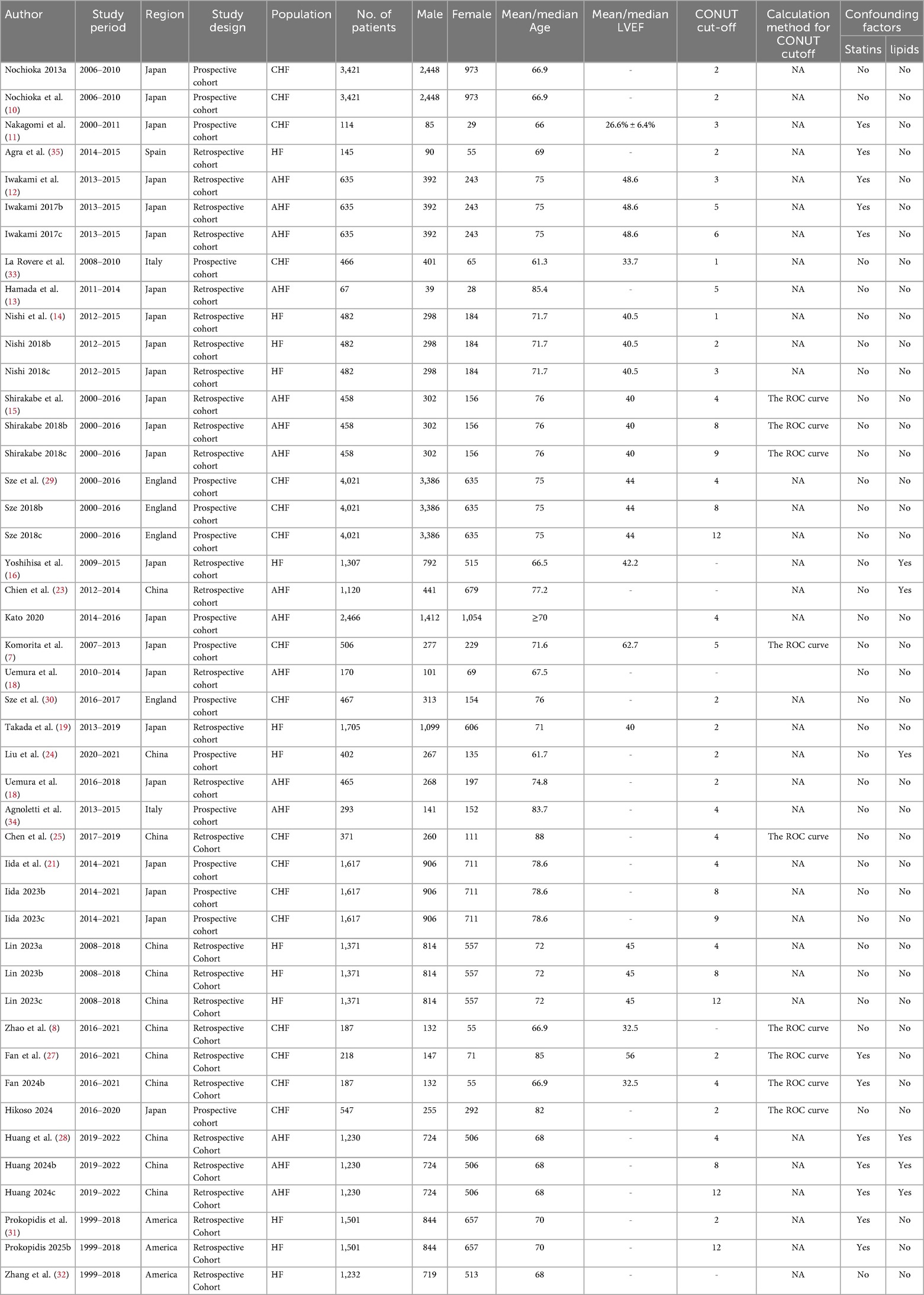
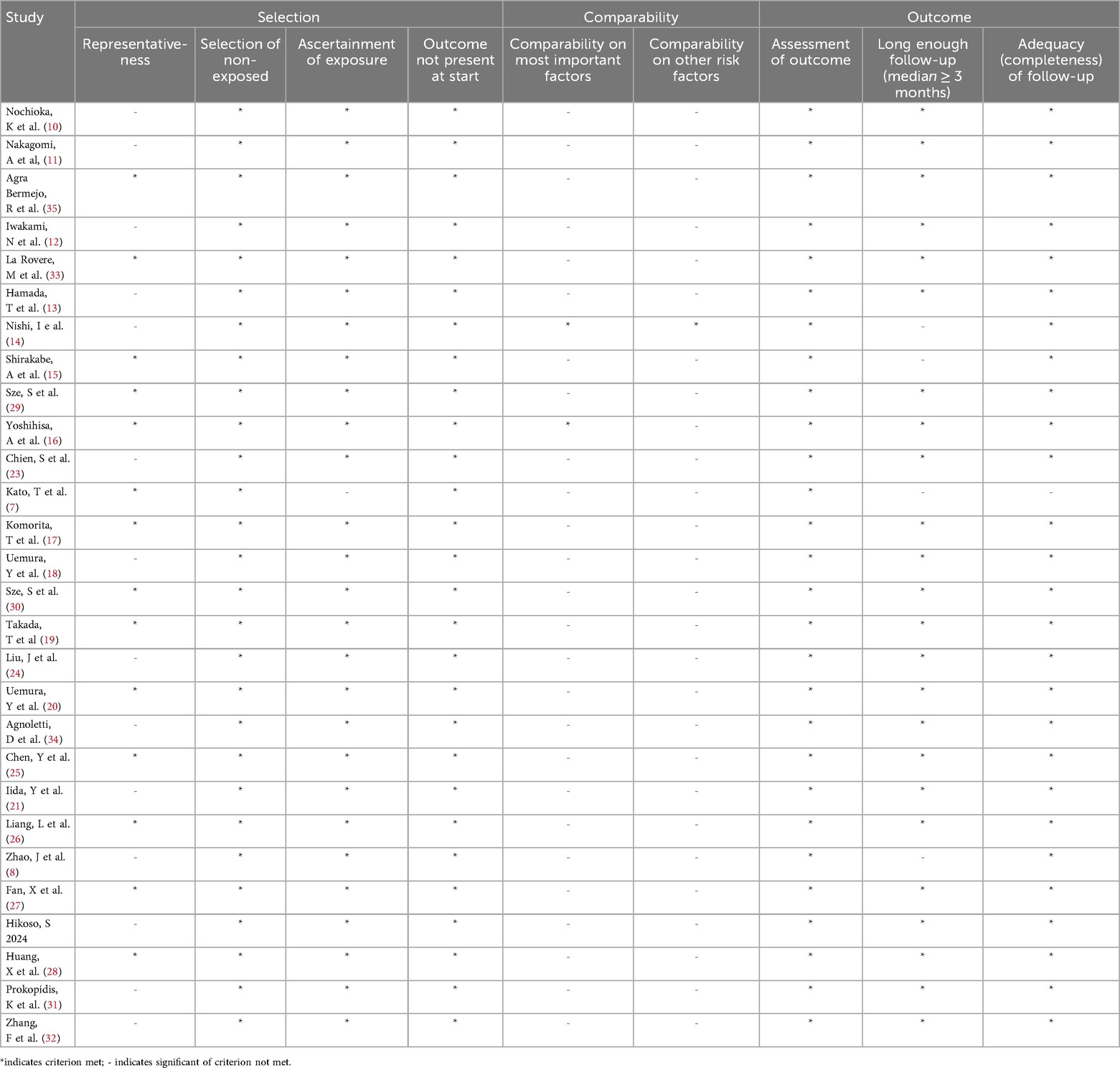
Two researchers, YY and ZPY, independently collected the data. Any inconsistencies were resolved through discussion with all co-authors. The extracted variables included the first author's name, year of publication, country or region of the study, study design, number of participants, patient age, patient type, LVEF, cut-off CONUT score, SMD and HRs (95% CIs) for outcomes (Table 2). The quality of the studies incorporated into the meta-analysis was assessed using the Newcastle-Ottawa Scale (NOS), which considers three domains for assessment: selection, comparability, and outcomes, with a maximum score of nine points. Studies scoring between 7 and 9 were classified as high quality (Table 3).
2.4 Statistical analysis
The statistical analyses were performed using RevMan 5.4 and STATA 15. HRs or standardized mean differences (SMD) were employed as effect sizes for categorical and continuous variables, respectively, with corresponding 95% CIs calculated. We evaluated heterogeneity using Cochran's Q test and I² statistic. A P-value below 0.10 or an I² above 50% indicated significant heterogeneity, prompting the use of a random-effects model for the meta-analysis. Statistical significance was set at a two-sided P-value of less than 0.05. Publication bias was evaluated using Egger's test alongside a visual inspection of funnel plots. Sensitivity analyses were conducted to assess the stability of the meta-analytic results and to investigate possible contributors to heterogeneity. Subgroup analyses were conducted according to study design, age, geographic region, heart failure subtype, and CONUT score cut-off values to investigate clinical and methodological heterogeneity.
3 Results
3.1 Search results
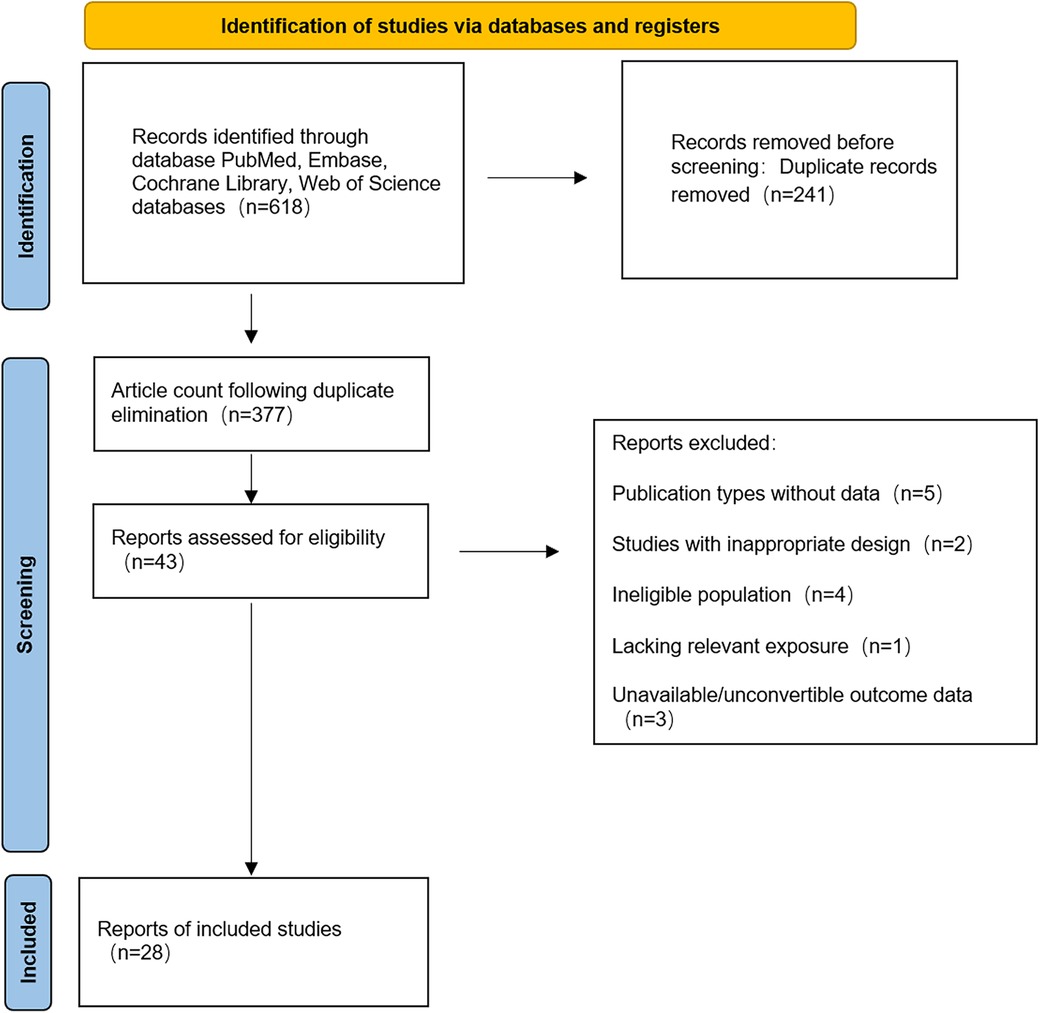
A total of 618 records were retrieved from PubMed (n = 166), EMBASE (n = 249), Cochrane Library (n = 7) and Web of Science (n = 196). Following the removal of duplicates using EndNote X9 (241 duplicates excluded), 377 articles remained for screening. Following the screening of titles and abstracts, 43 articles were selected for full-text review, with 28 studies (7, 10–22) ultimately meeting the inclusion criteria. The geographical distribution of the 28 eligible studies was as follows: Japan (7, 10–22) (n = 14), China (8, 23–28) (n = 7), United Kingdom (29, 30) (n = 2), United States (31, 32) (n = 2), Italy (33, 34) (n = 2), and Spain (35) (n = 1). The study designs comprised 17 retrospective cohort studies (8, 10, 13–17, 20–22, 24, 26–29) and 11 prospective cohort studies (7, 10, 11, 17, 21–23, 29, 30, 33–35). All included cohort research articles, published in English, appeared between 2013 and 2025. The retrieval flowchart is presented in Figure 1.
3.2 Study quality
Each of the 28 included studies received a score ranging from 6–8 on the NOS, indicating high methodological quality (Table 3).
3.3 Meta-analysis results
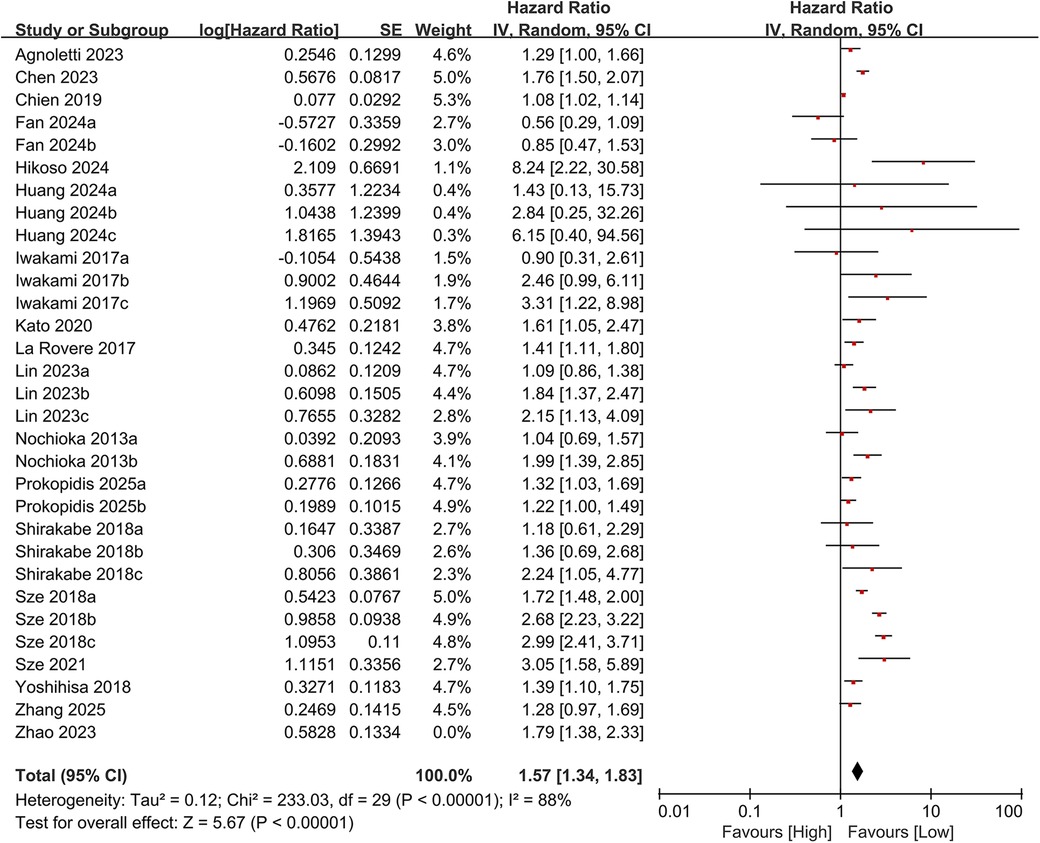
3.3.1 All-cause mortality
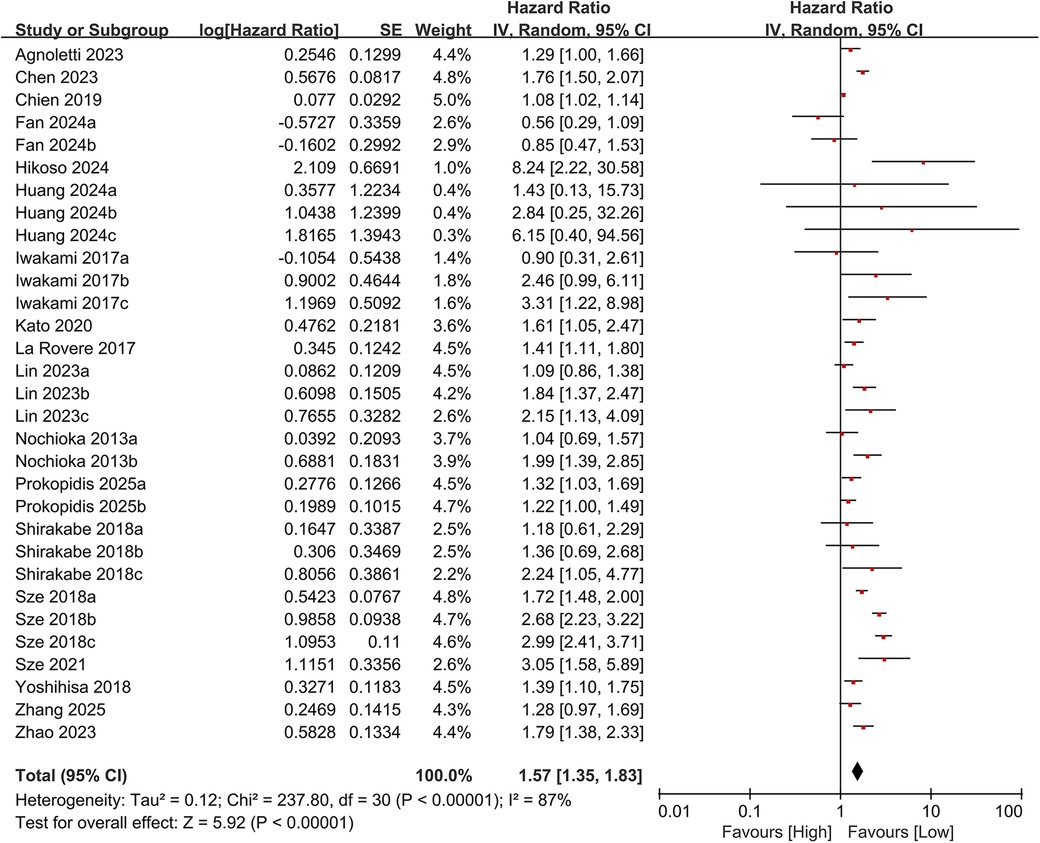
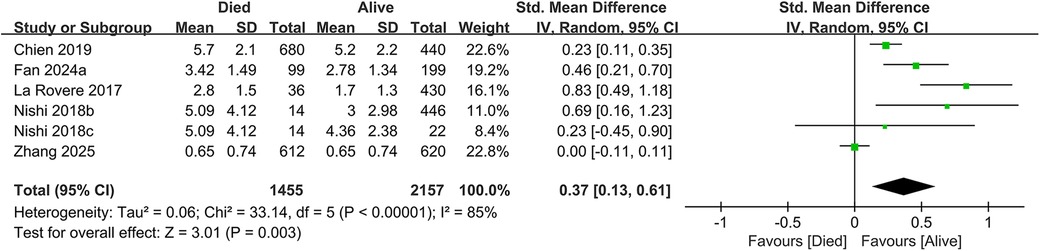
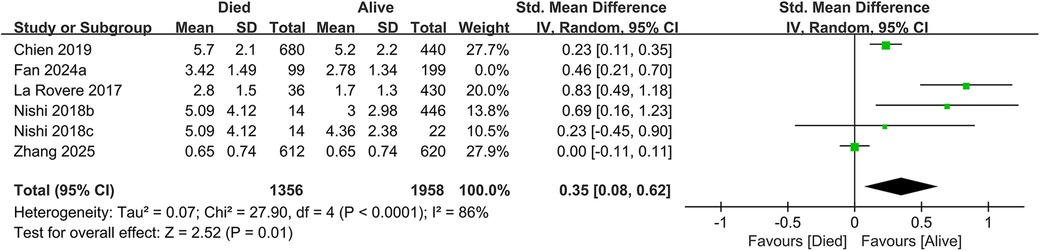
In total, 19 studies evaluated the link between CONUT scores and all-cause mortality. Of these, 17 studies analyzed CONUT as a categorical variable. The meta-analysis demonstrated that elevated CONUT scores were significantly correlated with a higher risk of all-cause mortality (HR = 1.57, 95% CI 1.35–1.83; P < 0.00001), with substantial heterogeneity across studies (P < 0.00001, I² = 87%; Figure 2). Sensitivity analyses were subsequently conducted to explore potential sources of heterogeneity. Sequential exclusion of individual studies yielded stable pooled HR estimates, ranging from 1.52–1.62, with all recalculated estimates maintaining overlapping confidence intervals with the original analysis, indicating the robustness of the primary findings (Figure 3). Notably, the high CONUT group demonstrated significantly elevated risk compared to the low CONUT group. Six studies evaluated CONUT as a continuous variable, revealing a 37% mean increase in CONUT scores among deceased patients vs. survivors (SMD = 0.37, 95% CI 0.13–0.61; P = 0.003; Figure 4). Despite significant statistical heterogeneity (I² = 85%), sensitivity analyses confirmed the reliability of the CONUT score–mortality association, with pooled effect sizes remaining stable (range: 0.35–.47). All recalculated estimates exhibited overlapping confidence intervals with the original analysis, further supporting the robustness of the primary results (Figure 5).
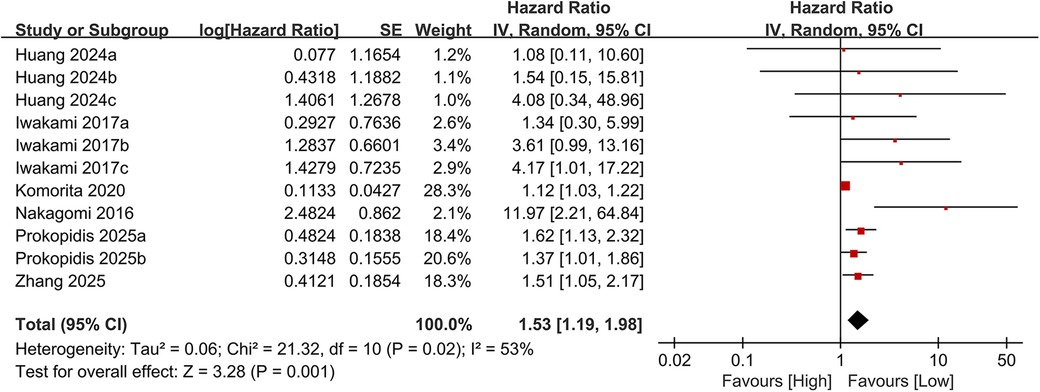
3.3.2 Cardiovascular mortality
Eleven studies examined the association between the CONUT score and cardiovascular mortality. When analyzed as a categorical variable, the meta-analysis revealed a significantly elevated risk of cardiovascular mortality in the high CONUT group (HR = 1.53, 95% CI 1.19–1.98; P = 0.001), with moderate heterogeneity across studies (P = 0.001, I² = 53%; Figure 6). Sensitivity analyses, based on the sequential exclusion of individual studies, confirmed the robustness of the observed relationship between CONUT scores and cardiovascular mortality (Figure 7). Despite methodological heterogeneity, all analytical scenarios consistently demonstrated that worsening nutritional status significantly increased the risk of cardiovascular mortality.
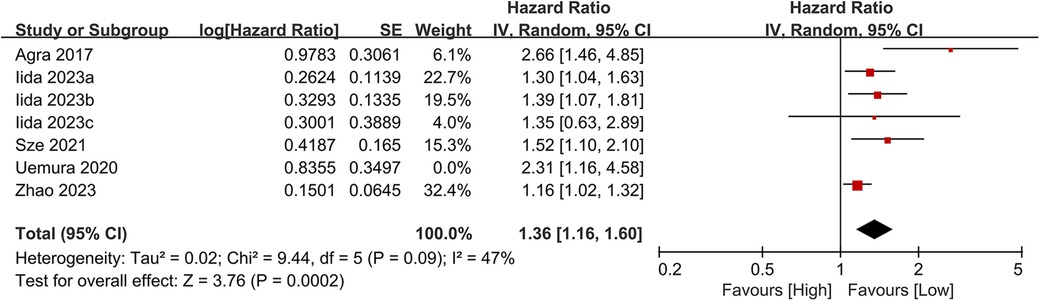
3.3.3 Heart failure readmission rate
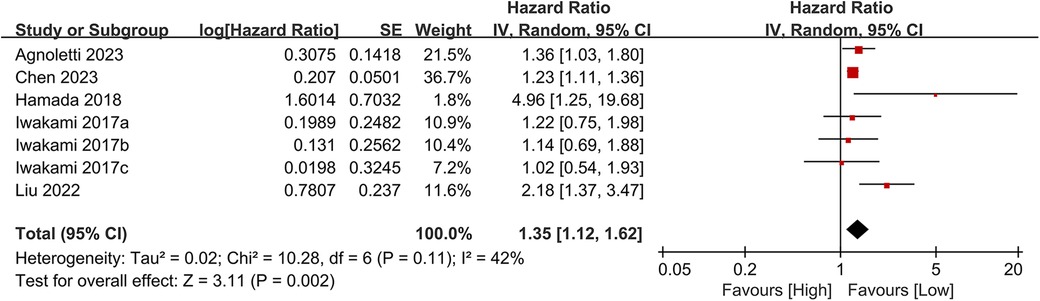
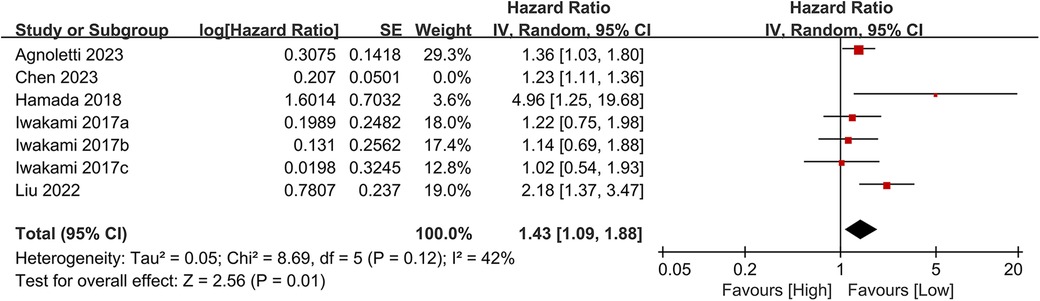
Seven studies examined the association between CONUT scores and heart failure readmission rates. The meta-analysis indicated a significant link between high CONUT scores and increased heart failure readmission risk (HR = 1.35, 95% CI 1.12–1.62; P = 0.02), with moderate heterogeneity across studies (P = 0.002, I² = 42%; Figure 8). To assess the stability of the results, sensitivity analyses were performed by sequentially excluding individual studies. All recalculated estimates showed overlapping confidence intervals with the original analysis, further confirming the robustness of the primary findings (Figure 9).
3.3.4 Composite outcomes
Seven studies investigated the association between the CONUT score and the composite outcome of all-cause mortality or heart failure readmission. The meta-analysis demonstrated a significant association between elevated CONUT scores and increased risk of the composite outcome (HR = 1.41, 95% CI 1.19–1.68; P < 0.00001), with moderate heterogeneity across studies (P < 0.00001, I² = 51%; Figure 10). Sensitivity analyses were conducted to assess the stability of the results, involving the sequential exclusion of individual studies. All recalculated pooled estimates exhibited overlapping confidence intervals with the original analysis, confirming the robustness of the primary findings (Figure 11).
3.3.5 Publication bias
Egger's regression test was used to evaluate publication bias. Evidence of significant publication bias was found in the meta-analyses of all-cause mortality (categorical variable analysis), cardiovascular mortality, and the composite outcome of all-cause mortality or heart failure readmission (Egger's test: P = 0.022, P = 0.004, and P = 0.013). Visual inspection of the corresponding funnel plots revealed asymmetric distributions (Figures 12A–C). In contrast, Egger's test demonstrated no significant publication bias in the meta-analyses of all-cause mortality (continuous variable analysis) or heart failure readmission rates (P = 0.129 and P = 0.247, respectively). Their funnel plots exhibited approximate symmetry with evenly distributed data points, indicating a low risk of publication bias (Figures 12D,E). The consistency between funnel plot observations and Egger's test results further validated the reliability of these conclusions.
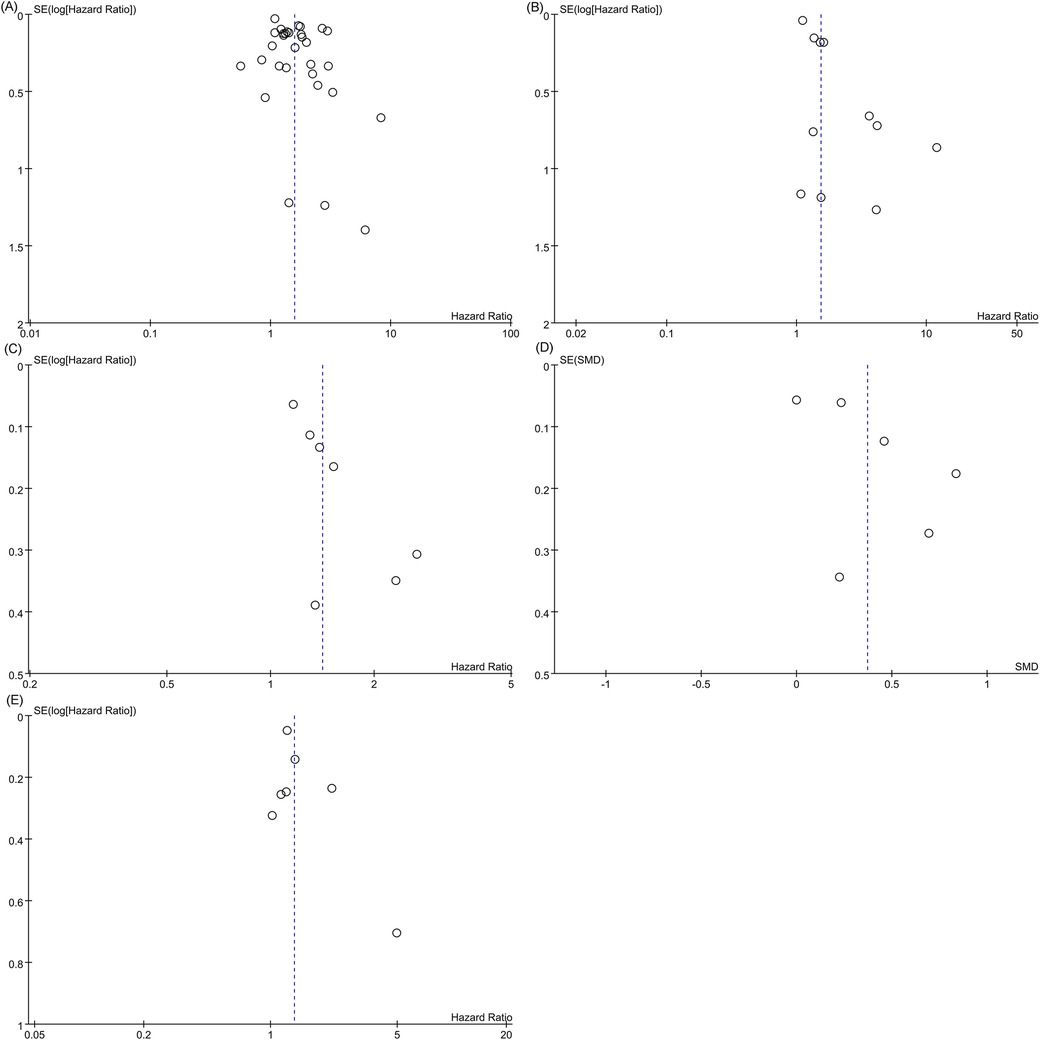
Figure 12. Funnel plot. (A) All-cause mortality (categorical variable); (B) Cardiovascular mortality; (C) Funnel plot for the composite outcome; (D) Funnel plot of all-cause mortality (continuous variable); (E) Funnel plot of heart failure readmission rate.
3.3.6 Subgroup analysis
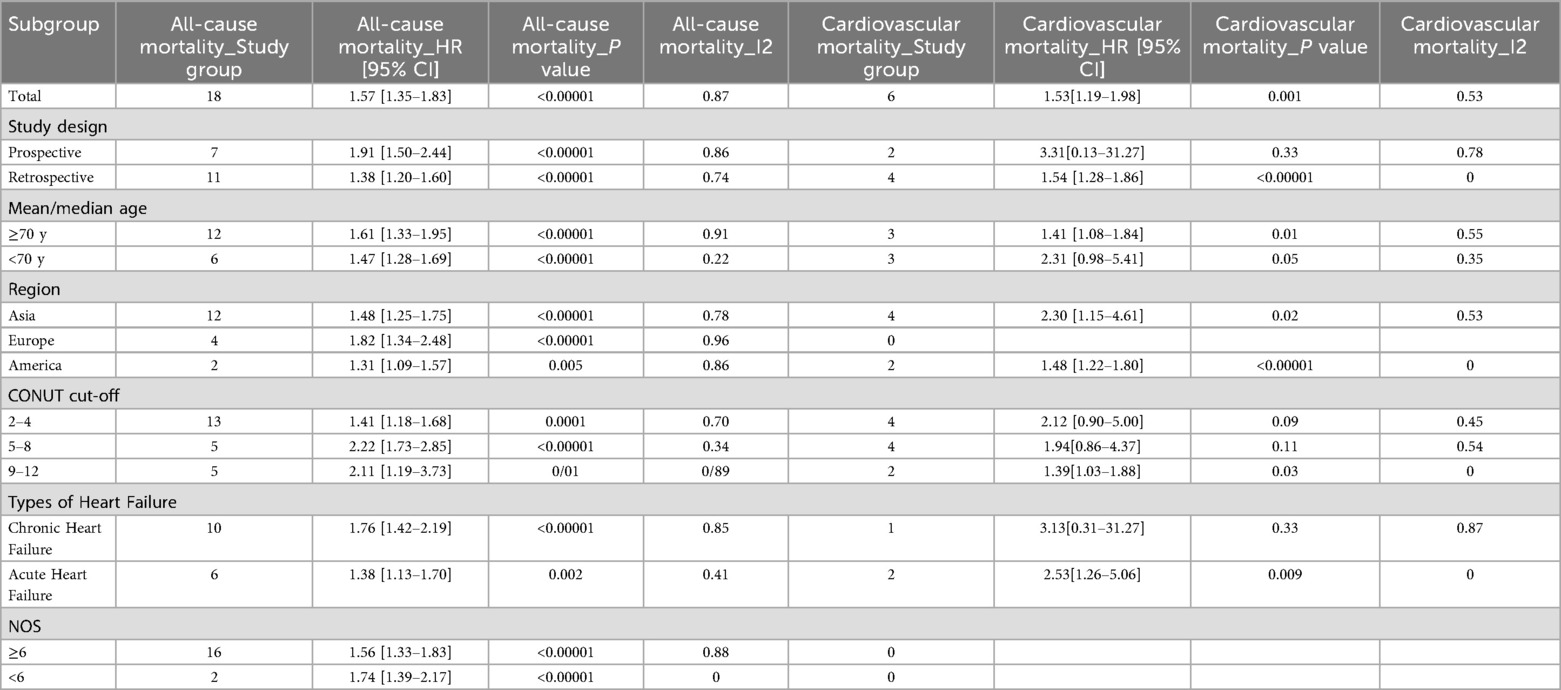
Subgroup analyses based on study design, age, region, CONUT cutoff value, heart failure type, and NOS score demonstrated that a higher CONUT score remained significantly associated with an increased risk of all-cause mortality across all predefined subgroups. The predictive value was consistent regardless of the specific optimal cutoff value applied. With respect to cardiovascular mortality, a significant association was observed only when the CONUT cutoff exceeded 9, suggesting that severe malnutrition may elevate the risk of cardiovascular death. Furthermore, in patients with acute heart failure (AHF), elevated CONUT scores were positively correlated with both all-cause and cardiovascular mortality. In contrast, among patients with chronic heart failure (CHF), the CONUT score remained a significant predictor of all-cause mortality, although its association with cardiovascular mortality did not reach statistical significance, In Asian and European populations, a higher CONUT score was associated with a significantly increased risk of all-cause mortality. Whereas in America, this association was not statistically significant, possibly due to the limited number of studies available (Table 4).
4 Discussion
The aim of this meta-analysis was to evaluate the prognostic value of the CONUT score in individuals with heart failure, drawing on data from 28 cohort studies encompassing a total of 26,984 participants. This meta-analysis showed that individuals with elevated CONUT scores faced significantly increased risks of all-cause mortality, cardiovascular mortality, and heart failure readmission. The underlying mechanisms may involve malnutrition-driven pathological cascades, including inflammatory activation, immune dysfunction, and metabolic dysregulation. For instance, hypoalbuminemia reflects protein-energy malnutrition, decreased total cholesterol indicates impaired lipid metabolism, and lymphocytopenia correlates with immune exhaustion-factors that synergistically contribute to accelerated cardiac decompensation.
The study by Li et al. (36) investigated the prognostic value of the CONUT score for all-cause mortality in patients with heart failure. Their results demonstrated that patients with CONUT scores ≥2 had a 1.92-fold increased risk of all-cause mortality compared to those with scores 0–1 (RR = 1.92, 95% CI 1.58–2.34). Subgroup analysis further revealed a 1.78-fold elevated mortality risk in the malnourished subgroup (RR = 1.78, 95% CI 1.29–2.46). Unlike the meta-analysis by Li et al., which highlighted the CONUT score's predictive value for all-cause mortality and identified a stronger association in CHF patients, our subgroup analyses revealed no statistically significant relation between CONUT and cardiovascular mortality in CHF populations (P > 0.05). This difference could be explained by the relatively smaller sample size in the CHF subgroup or the potential masking of the independent prognostic effect of nutritional status by overriding pathophysiological mechanisms in CHF, such as hemodynamic deterioration and neurohormonal activation. In addition, we performed subgroup analyses based on different optimal cutoff values of the CONUT score. Regardless of the specific cutoff value used, a higher CONUT score was consistently associated with a significantly increased risk of all-cause mortality. Regarding the prediction of cardiovascular mortality, a significant association was observed only when the cutoff value was set above 9, suggesting that severe malnutrition may increase the risk of cardiovascular death. Among the studies included in this meta-analysis, five used ROC analysis to determine the optimal CONUT cutoff value in patients with CHF, which were reported as 2, 2.5, and 4, respectively. One study identified a cutoff of 5 for patients with AHF. In older patients over 70 years of age, cutoff values ranging from 2–5 were shown to effectively predict clinical outcomes. Differences in nutritional-metabolic status and disease severity across patient phenotypes may account for the variation in optimal thresholds. Therefore, further studies targeting specific patient subgroups are warranted to validate phenotype-specific CONUT cutoff values.
In comparison to other nutritional screening and assessment tools, the CONUT score is calculated using objective laboratory parameters, including serum albumin levels and lymphocyte counts (37), providing a more standardized reflection of nutritional status with minimized subjective bias. For instance, the Subjective Global Assessment (SGA) primarily relies on clinicians' qualitative evaluations of dietary intake, weight changes, and gastrointestinal symptoms, which may introduce inter-rater variability (38). The validity of the CONUT score may be influenced by factors such as lipid-lowering medications and infections. Six studies adjusted for statin use in their multivariate Cox regression models, and four studies adjusted for blood lipid profiles. The results demonstrated that the CONUT score remained a significant predictor of both all-cause mortality and cardiovascular mortality even after adjusting for these and other important clinical confounders. However, significant heterogeneity exists in the CONUT cutoff values (range: 2–5 points) across current studies, highlighting the need for large-scale cohort studies to establish population-specific optimal thresholds. While the European Society for Clinical Nutrition and Metabolism (ESPEN) recommends a cutoff ≥4 points as diagnostic for malnutrition (37), this standard still requires validation across diverse ethnic and clinical populations. Further mechanistic studies using animal models are warranted to elucidate how malnutrition directly exacerbates myocardial injury-for example, investigating whether hypoalbuminemia potentiates cardiomyocyte death via autophagy dysregulation or other molecular pathways.
The precise mechanisms underlying the prognostic value of the CONUT score in HF remain incompletely elucidated. Serum albumin synthesis is influenced by nutritional intake and systemic inflammation (39). Several studies (40) have reported that hypoalbuminemia correlates with adverse clinical outcomes in HF patients, with inflammation and malnutrition posited as primary etiological contributors. Albumin exerts antioxidant and anti-inflammatory properties, and its deficiency may impair myocardial repair capacity, accelerating ventricular remodeling. Cholesterol levels, reflective of nutritional status, also impact prognosis. Extensive evidence confirms the association between hypercholesterolemia and coronary artery disease (CAD) progression and mortality risk (41). Data from the Framingham Study indicate that elevated serum cholesterol not only jeopardizes cardiovascular health but also serves as a significant risk factor for HF development (42). Pathophysiologically, hypocholesterolemia may signal two underlying states: (1) neurohormonal overactivation disrupting cholesterol metabolism, or (2) hypermetabolic stress exacerbating nutrient depletion. Furthermore, hypocholesterolemia is closely linked to malnutrition and cachexia-both strongly associated with poor HF outcomes (43). Inflammation is an important factor in HF pathogenesis and progression (44). Chronic inflammation and malnutrition in HF patients drive T-cell exhaustion, increasing susceptibility to infections (e.g., pneumonia, sepsis), which frequently trigger acute decompensation. Lymphocytopenia (<1,500/mm³) has been linked to an 82% higher mortality risk in HF patients, independent of ejection fraction (45). Malnutrition activates the nuclear factor kappa-B (NF-κB) pathway, promoting the release of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which induce myocardial fibrosis and microvascular dysfunction. Concurrently, metabolic disturbances (e.g., insulin resistance, lipid peroxidation) exacerbate mitochondrial dysfunction, leading to cardiomyocyte energy depletion and apoptosis (46). These interconnected mechanisms synergistically accelerate cardiac functional decline and adverse clinical outcomes.
This study presents some limitations. First, the overall quality of the evidence may be limited by the inclusion of some studies with relatively low methodological quality, as two studies scored below 6 on the Newcastle–Ottawa Scale (NOS). However, subgroup and sensitivity analyses based on NOS scores showed no significant change in heterogeneity, and the association between malnutrition and all-cause mortality remained statistically significant, which strengthens the robustness of our primary conclusion. Second, publication bias was detected for certain outcomes, possibly due to the inclusion of several small-sample studies and the absence of gray literature. This bias may have led to a slight overestimation of the predictive value of the CONUT score. Nevertheless, sensitivity and subgroup analyses indicated that the overall results are robust, supporting the clinical value of the CONUT score as a tool for assessing nutritional status and prognosis in patients with heart failure. Future high-quality, large-scale, prospective studies are needed to further validate its cutoff values and clinical applicability. Third, the included studies used different cutoff values for the CONUT score (e.g., ≥2 vs. ≥5), which may have introduced heterogeneity into the results. Fourth, this study did not assess the potential influence of nutritional indicators—such as acute infection, use of lipid-lowering agents, or SGLT2 inhibitors—on the CONUT score. Further research is warranted to explore the relationship between medication dosage and the individual components of the CONUT score. Fifth, due to limited data availability, original individual-level data from the included articles could not be obtained. Therefore, a detailed dose-response analysis quantifying the specific impact of each 1-point increase in the CONUT score on prognostic risk was not feasible. Future studies should consider conducting individual participant data meta-analyses or large-scale cohort studies to further explore this dose-response relationship, thereby addressing the current gaps and providing direction for subsequent research. Finally, as the majority of included studies were observational in design, a causal relationship between the CONUT score and prognosis cannot be established.
5 Conclusion
Based on the CONUT score definition, malnutrition is recognized as an effective predictor of all-cause mortality in HF patients after adjusting for confounders. Utilizing the CONUT score for nutritional assessment enables clinicians to identify HF patients at elevated risk for adverse clinical outcomes. Acknowledging these limitations, prospective, large-scale studies are warranted to further validate the prognostic relevance of the CONUT score across diverse HF populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YY: Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. S-PW: Data curation, Resources, Software, Validation, Writing – review & editing. YG: Methodology, Resources, Validation, Writing – review & editing. QY-Y: Resources, Software, Validation, Writing – review & editing. PY-Z: Formal analysis, Methodology, Software, Writing – review & editing. HY-W: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1665713/full#supplementary-material
References
1. Beghini A, Sammartino AM, Papp Z, von Haehling S, Biegus J, Ponikowski P, et al. 2024 update in heart failure. ESC Heart Fail. (2025) 12:8–42. doi: 10.1002/ehf2.14857
2. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118:3272–87. doi: 10.1093/cvr/cvac013
3. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
4. Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. (2004) 43:1439–44. doi: 10.1016/j.jacc.2003.11.039
5. Driggin E, Cohen LP, Gallagher D, Karmally W, Maddox T, Hummel SL, et al. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J Am Coll Cardiol. (2022) 79:1623–35. doi: 10.1016/j.jacc.2022.02.025
6. Guo W, Zhao L, Huang W, Chen J, Zhong T, Yan S, et al. Sodium-glucose cotransporter 2 inhibitors, inflammation, and heart failure: a two-sample Mendelian randomization study. Cardiovasc Diabetol. (2024) 23:118. doi: 10.1186/s12933-024-02210-5
7. Kato T, Yaku H, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, et al. Association with controlling nutritional Status (CONUT) score and in-hospital mortality and infection in acute heart failure. Sci Rep. (2020) 10:3320. doi: 10.1038/s41598-020-60404-9
8. Zhao J, Xie W, Ye S, Zhang S, Shi W, Cui M, et al. The clinical value of the controlling nutritional status score for predicting prognosis in systolic heart failure cases in the vulnerable phase. Front Nutr. (2023) 10:1084107. doi: 10.3389/fnut.2023.1084107
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
10. Nochioka K, Sakata Y, Takahashi J, Miyata S, Miura M, Takada T, et al. Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: a report from the CHART-2 study. Circ J. (2013) 77:2318–26. doi: 10.1253/circj.CJ-13-0127
11. Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. (2016) 23:713–27. doi: 10.5551/jat.31526
12. Iwakami N, Nagai T, Furukawa TA, Sugano Y, Honda S, Okada A, et al. Prognostic value of malnutrition assessed by controlling nutritional status score for long-term mortality in patients with acute heart failure. Int J Cardiol. (2017) 230:529–36. doi: 10.1016/j.ijcard.2016.12.064
13. Hamada T, Kubo T, Yamasaki N, Kitaoka H. Predictive factors of rehospitalization for worsening heart failure and cardiac death within 1 year in octogenarians hospitalized for heart failure. Geriatr Gerontol Int. (2018) 18:101–7. doi: 10.1111/ggi.13148
14. Nishi I, Seo Y, Hamada-Harimura Y, Sato K, Sai S, Yamamoto M, et al. Utility of nutritional screening in predicting short-term prognosis of heart failure patients. Int Heart J. (2018) 59:354–60. doi: 10.1536/ihj.17-073
15. Shirakabe A, Hata N, Kobayashi N, Okazaki H, Matsushita M, Shibata Y, et al. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the prognostic nutritional Index (PNI) and controlling nutritional Status (CONUT) score. Heart Vessels. (2018) 33:134–44. doi: 10.1007/s00380-017-1034-z
16. Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart. (2018) 5:e000730. doi: 10.1136/openhrt-2017-000730
17. Komorita T, Yamamoto E, Sueta D, Tokitsu T, Fujisue K, Usuku H, et al. The controlling nutritional status score predicts outcomes of cardiovascular events in patients with heart failure with preserved ejection fraction. Int J Cardiol Heart Vasc. (2020) 29:100563. doi: 10.1016/j.ijcha.2020.100563
18. Uemura Y, Shibata R, Masuda A, Katsumi Y, Takemoto K, Koyasu M, et al. Utility of the nutritional screening in predicting adverse outcome of patients with overweight/obesity and acute heart failure. J Card Fail. (2020) 26:566–73. doi: 10.1016/j.cardfail.2020.02.005
19. Takada T, Jujo K, Inagaki K, Abe T, Kishihara M, Shirotani S, et al. Nutritional status during hospitalization is associated with the long-term prognosis of patients with heart failure. ESC Heart Fail. (2021) 8:5372–82. doi: 10.1002/ehf2.13629
20. Uemura Y, Shibata R, Miyagaki Y, Takemoto K, Ishikawa S, Murohara T, et al. A comparative study of three nutritional risk/screening indices for predicting cardiac events and physical functioning among patients with acute heart failure. Int Heart J. (2022) 63:541–9. doi: 10.1536/ihj.21-809
21. Iida Y, Kamiya K, Adachi T, Iwatsu K, Kamisaka K, Iritani N, et al. Prognostic impact of nutrition measures in patients with heart failure varies with coexisting physical frailty. ESC Heart Fail. (2023) 10:3364–72. doi: 10.1002/ehf2.14519
22. Kitao T, Hikoso S, Tamaki S, Seo M, Yano M, Hayashi T, et al. Post-discharge changes in nutritional status predict prognosis in patients with acute decompensated HFpEF from the PURSUIT-HFpEF registry. Heart Vessels. (2025) 40:577–91. doi: 10.1007/s00380-024-02499-y
23. Chien SC, Lo CI, Lin CF, Sung KT, Tsai JP, Huang WH, et al. Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Fail. (2019) 6:953–64. doi: 10.1002/ehf2.12501
24. Liu J, Liu J, Wang J, Yan Z, Liang Q, Wang X, et al. Prevalence and impact of malnutrition on readmission among hospitalized patients with heart failure in China. ESC Heart Fail. (2022) 9:4271–9. doi: 10.1002/ehf2.14152
25. Chen Y, Zheng H, He Y. Prognostic significance of controlling nutritional status in older adults with heart failure with preserved ejection fraction: a prospective comparative study with other objective nutritional indices. Aging Clin Exp Res. (2023) 35:1305–15. doi: 10.1007/s40520-023-02395-x
26. Liang L, Zhao X, Huang L, Tian P, Huang B, Feng J, et al. Prevalence and prognostic importance of malnutrition, as assessed by four different scoring systems, in elder patients with heart failure. Nutr Metab Cardiovasc Dis. (2023) 33:978–86. doi: 10.1016/j.numecd.2023.01.004
27. Fan X, He Q, Zhang K, Lan X, Li Y, Zhang H. Comparison of the value of four objective nutritional indices in assessing the long-term prognosis of elderly patients with heart failure with preserved ejection fraction. Rev Cardiovasc Med. (2024) 25:201. doi: 10.31083/j.rcm2506201
28. Huang X, Qiu J, Kuang M, Wang C, He S, Yu C, et al. Assessing the predictive value of the controlling nutritional status score on all-cause mortality during hospitalization in patients with acute decompensated heart failure: a retrospective cohort study from jiangxi, China. Front Nutr. (2024) 11:1392268. doi: 10.3389/fnut.2024.1392268
29. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass Index. JACC Heart Fail. (2018) 6:476–86. doi: 10.1016/j.jchf.2018.02.018
30. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr. (2021) 113:695–705. doi: 10.1093/ajcn/nqaa311
31. Prokopidis K, Chen Y, Liu Y, Zhong Z, Morwani-Mangnani J, Cuthbertson DJ, et al. Assessing the Link of Malnutrition with Diabetes and Mortality Risk in Heart Failure Patients. New Jersey, NJ: John Wiley & Sons Ltd on behalf of the European Society of Cardiology (2025).
32. Zhang F, Xie Y, Liu L, Liu H, Feng O, Li Y, et al. Association of immune nutrition indices with the risk of all-cause mortality and cardiovascular mortality in patients with heart failure in the NHANES (1999–2018). Rev Cardiovasc Med. (2025) 26:25055. doi: 10.31083/RCM25055
33. Rovere L, Maestri MT, Olmetti R, Paganini F, Riccardi V, Riccardi G, et al. Additional predictive value of nutritional status in the prognostic assessment of heart failure patients. Nutr Metab Cardiovasc Dis. (2017) 27:274–80. doi: 10.1016/j.numecd.2016.09.009
34. Agnoletti D, Arcaro G, Scaturro G, Turcato E, Grison E, Ferrari E, et al. Controlling nutritional status score predicts 2-year outcomes in elderly patients admitted for acute heart failure. Intern Emerg Med. (2023) 18:1031–9. doi: 10.1007/s11739-023-03230-x
35. Agra Bermejo RM, González Ferreiro R, Varela Román A, Gómez Otero I, Kreidieh O, Conde Sabarís P, et al. Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int J Cardiol. (2017) 230:108–14. doi: 10.1016/j.ijcard.2016.12.067
36. Li H, Zhou P, Zhao Y, Ni H, Luo X, Li J. Prediction of all-cause mortality with malnutrition assessed by controlling nutritional status score in patients with heart failure: a systematic review and meta-analysis. Public Health Nutr. (2022) 25:1799–806. doi: 10.1017/S1368980021002470
37. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
38. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Agreement and classification performance of malnutrition tools in patients with chronic heart failure. Curr Dev Nutr. (2020) 4:nzaa071. doi: 10.1093/cdn/nzaa071
39. Kuller LH, Eichner JE, Orchard TJ, Grandits GA, McCallum L, Tracy RP. The relation between serum albumin levels and risk of coronary heart disease in the multiple risk factor intervention trial. Am J Epidemiol. (1991) 134:1266–77. doi: 10.1093/oxfordjournals.aje.a116030
40. Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. (2008) 155:883–9. doi: 10.1016/j.ahj.2007.11.043
41. Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. (1990) 322:1700–7. doi: 10.1056/NEJM199006143222403
42. Fonarow GC, Horwich TB. Cholesterol and mortality in heart failure: the bad gone good? J Am Coll Cardiol. (2003) 42:1941–3. doi: 10.1016/j.jacc.2003.09.005
43. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. (1997) 349:1050–3. doi: 10.1016/S0140-6736(96)07015-8
44. Triposkiadis F, Xanthopoulos A, Butler J. Cardiovascular aging and heart failure: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:804–13. doi: 10.1016/j.jacc.2019.06.053
45. Majmundar M, Kansara T, Park H, Ibarra G, Marta Lenik J, Shah P, et al. Absolute lymphocyte count as a predictor of mortality and readmission in heart failure hospitalization. Int J Cardiol Heart Vasc. (2022) 39:100981. doi: 10.1016/j.ijcha.2022.100 35281758
Keywords: CONUT, heart failure, prognosis, malnutrition, all-cause mortality
Citation: Yuan Y, Wang S-P, Guan Y, Yang Q-Y, Zhong P-Y and Wang H-Y (2025) Association between controlling nutritional status score and the prognosis of patients with heart failure: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1665713. doi: 10.3389/fcvm.2025.1665713
Received: 14 July 2025; Accepted: 30 September 2025;
Published: 20 October 2025.
Edited by:
Otilia Tica, Emergency County Clinical Hospital of Oradea, RomaniaReviewed by:
Guanglin Cui, Huazhong University of Science and Technology, ChinaYu He, Capital Medical University, China
Copyright: © 2025 Yuan, Wang, Guan, Yang, Zhong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao-Yu Wang, MzYxNTM2NzEyQHFxLmNvbQ==
 Yuan Yuan
Yuan Yuan Su-Ping Wang
Su-Ping Wang Yi Guan
Yi Guan Peng-Yu Zhong
Peng-Yu Zhong Hao-Yu Wang
Hao-Yu Wang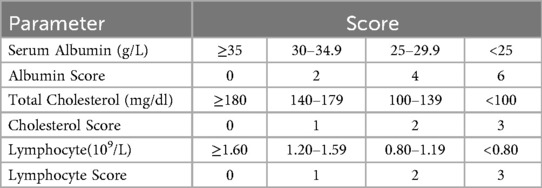
![Forest plot showing hazard ratios from multiple studies with 95% confidence intervals. Each study is listed with log hazard ratio, standard error, weight, and hazard ratio. The overall hazard ratio is 1.58 with confidence interval [1.16, 2.13]. Heterogeneity shows Tau-squared = 0.07, Chi-squared = 19.55, degrees of freedom = 9, P = 0.02, I-squared = 54%. The test for the overall effect is Z = 2.93, P = 0.003. The plot favors high on the right and low on the left, with values ranging from 0.02 to 50.](https://www.frontiersin.org/files/Articles/1665713/fcvm-12-1665713-HTML/image_m/fcvm-12-1665713-g007.jpg)
![Forest plot illustrating hazard ratios with 95% confidence intervals for various studies. Each study's point estimate is represented by a red square, with size proportional to its weight. Horizontal lines depict confidence intervals. The overall effect is shown as a diamond at 1.41 [1.19, 1.68]. Heterogeneity statistics include Tau-squared equals 0.02, Chi-squared equals 12.29, degrees of freedom equals 6, and I-squared equals 51 percent. The test for overall effect has a Z score of 3.96 with a P-value less than 0.0001.](https://www.frontiersin.org/files/Articles/1665713/fcvm-12-1665713-HTML/image_m/fcvm-12-1665713-g010.jpg)