Introduction
Regular exercise training and physical activity are linked to several health benefits, particularly at the cardiovascular (CV) level, enhancing quality of life, lowering the incidence of cardiovascular diseases, and preventing and controlling a number of risk factors (1, 2). Despite the overwhelming evidence of its many positive effects, exercise can cause serious clinical complications, including sudden cardiac death (SCD), in people with certain risk factors and cardiovascular disease (CVD). These complications can affect a wide range of people who appear to be healthy, from young athletes to seasoned athletes (3). Through preparticipation screening (PPS), cardiac disorders and abnormalities in electrophysiology of heart that could cause lethal arrhythmias during exercise can be identified. Athletes should be evaluated on a regular basis because SCD may be the initial sign of a hidden illness in asymptomatic people. The purpose of competitive cardiovascular screening prior to participation is not only to check for underlying cardiovascular abnormalities that could raise their risk of sudden cardiac death (SCD) while participating in sports but also highlights the importance of moving the assessment of CVD risk factors beyond the traditional focus to a more holistic and individualized approach like lifestyle modification, restriction from sports activity, and, when appropriate, medical therapy or implantation of a defibrillator (4–6).
Preparticipation screening is crucial for reducing sports-related complications, but some conditions may not be detectable. To increase survival after sudden cardiac arrest, bystanders must be capable of performing resuscitation of the heart particularly using defibrillators. Time between collapse and defibrillation determines survival (7).
The field of sports cardiology is constantly changing, with new research opportunities and some contentious issues. As in other medical fields, for instance, the use of digital tools, such as machine learning shows promise and may aid in better screening. There may be multiple options available to improve risk assessment, diagnosis, and monitoring of cardiovascular health of an athlete by integrating these tools (8, 9).
Causes of sudden cardiac death in athletes
According to previous research, the incidence of SCD was 6.64 per 100,000 person-years for older athletes over 35 and 0.47–1.21 per 100,000 person-years for young competitive athletes. In addition to age, other factors like gender, sports tenacity, and level of competition appear to influence the prevalence of SCD in athletes. More than 80% of SCD causes in athletes have a cardiovascular origin, primarily from inherited cardiac conditions in young athletes and coronary artery disease (CAD) in older athletes. Cardiomyopathies continue to be significant causes of inherited conditions, but recent studies have shown an increasing prevalence of cases involving hearts with normal physiology, that indicate sudden arrhythmia leading to death, dysautonomia and primary arrhythmia are the most likely underlying aetiology (10–12).
The significance of autopsy in SCD cases should be emphasised because autopsy imaging is still insufficiently reliable to identify certain cardiac diseases, and death certification by itself can be erroneous. Improved risk stratification and the development of preventative measures for vulnerable population, are crucial because genetic conditions account for a large portion of SCD cases in younger athletes (13). Molecular autopsy could be a crucial step in the assessment of SCD patients. Heat stroke, cardiopulmonary disease, and performance-enhancing substances are examples of environmental factors that are associated with SCD in athletes and should be taken into account (14, 15).
Various cardiovascular screening techniques for athletes ahead of participation
History and clinical evaluation
A thorough 12-lead electrocardiography (ECG), physical examination and medical history should be the main focus of pre-participation screening (PPS) for young athletes. Symptoms like palpitations, dizziness, syncope, or exertional chest pain should be considered warning signs. One crucial piece of advice is to teach athletes not to train when they have a fever or before fully recovering from an acute illness. The main goals of the physical examination are to rule out systolic and diastolic heart murmurs high blood pressure, radiofemoral delay, arrythmia, any musculoskeletal and ocular symptoms that could indicate Marfan syndrome. In many nations, the history and physical examination is still the most effective way to screen athletes, but there are some serious drawbacks. There is a great deal of variation in the providers' capacity to use cardiac auscultation to diagnose cardiac conditions during the physical examination portion of the test (16, 17).
Electrocardiography (ECG)
The main evidence in favour of ECG use comes from a small number of prospective studies in which the SCD rate was significantly lowered from 3.6% to 0.4% through systematic ECG screening. The sensitivity and specificity are increased by ECG as opposed to just a clinical history and physical examination. As ECG screening guidelines have evolved, the International Criteria now have significantly lower false positive rates, ranging from 1.3% to 6.8%, depending on the population under study. It is crucial to distinguish between pathological and physiological conditions in order to accurately identify athletes who are more vulnerable. One crucial step in ensuring high quality and lowering false positive results is the analysis and proper application of ECG criteria in athletes. Over the past twenty years, the standards for interpreting an athlete's ECG have been refined and shared (18).
Despite these advancements, physicians still need to receive training in athletes' ECG interpretation. Left ventricular hypertrophy, isolated right bundle branch block, nonpathological T wave inversion (TWI), J waves, and nonpathological rhythm variations are common findings that are misinterpreted. ECG screening opponents frequently point to the high expense of ECGs, high false-positive rates that result in unnecessary secondary testing, a lack of generalised proficiency in interpreting an athlete's ECG, and the incapacity to identify critical conditions like congenital anomalous coronary arteries as reasons why universal screening is not necessary. Other ECG findings, such as ST-segment depression, low QRS voltage, premature beats, premature ventricular contractions (PVC) and QRS fragmentation may also have clinical significance, according to recent evidence. To fully comprehend its clinical utility, more investigation is required (19, 20).
Echocardiography
Transthoracic echocardiograms (TTE) can increase the precision of PPS by identifying certain conditions linked to SCD that would go undetected by a standard PPS, such as mitral valve prolapse, aortic bicuspid valve, and abnormal coronary artery origin. There is a fee associated with incorporating TTE into a PPS. It makes the PPS more complicated, places a significant financial strain on society, necessitates more extensive follow-up and initial PPS logistics, is operator dependent, and requires training, expertise, and echocardiography experience (21).
Even though a single TTE performed during adolescence will rule out congenital cardiac conditions, it might not detect cardiomyopathies, which might not manifest phenotypically until later. This point emphasises the necessity of reevaluating these athletes in the future, but it is still unclear when the TTE should be repeated. It should be made clear what the ideal age is for the first PPS, how often it occurs, and whether it should change depending on the sport. There is still a lack of knowledge regarding the role of TTE in athletes' PPS, the best protocol to follow, and when to reevaluate. Apart from FIFA, no significant professional or athletic organisation currently advises routine multimodality imaging as part of cardiovascular screening. However, a number of screening initiatives, academic institutions, national teams, and professional teams have incorporated imaging into their standard screening procedure (22, 23).
Exercise testing
For many years, asymptomatic people with elevated cardiovascular risk factors who plan to begin regular exercise training have been screened using exercise testing (ET). Given its accessibility, affordability, and status as one of the important functional investigations, ET is highly valuable for assessing veteran athletes with cardiovascular risk factors as well as sedentary individuals planning to start vigorous exercise. Given the increased incidence of coronary artery disease, CV screening in athletes must focus on this condition. ET's diagnostic precision in asymptomatic people without CV risk factors is questionable, though. Due to susceptible plaques that could burst and cause sudden cardiac arrest during exercise, ET may not detect small stenosis even though it may be sensitive in detecting obstructive CAD (24, 25).
Artificial intelligence (Ai) in sports cardiology
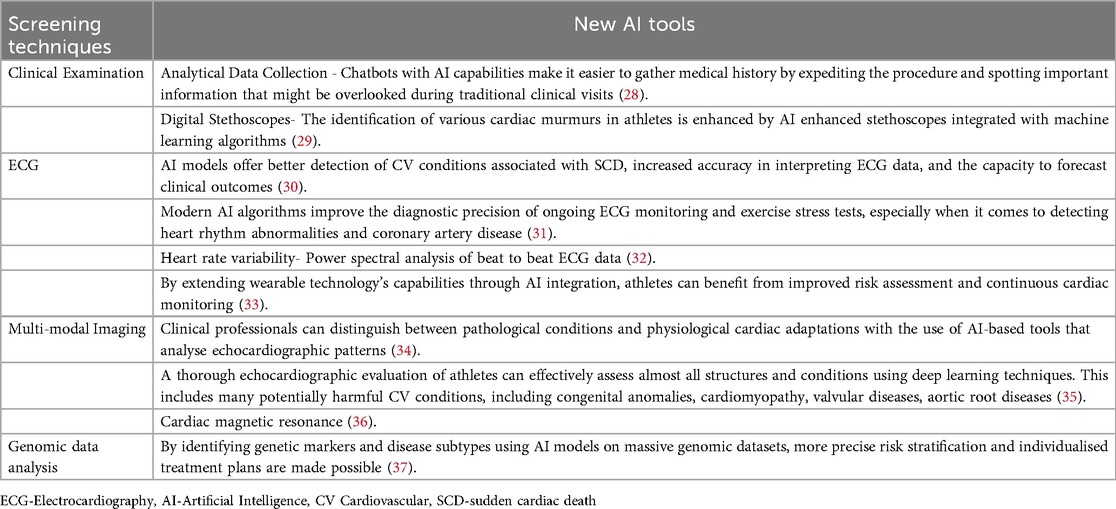
Modern imaging and ECG software has completely changed how accurately data is gathered during cardiac evaluations. When it comes to identifying asymptomatic cardiac abnormalities that could be dangerous during sports, these tools are invaluable. At the same time, sports medicine is changing due to the growing popularity of consumer wearable devices (CWDs), like heart rate monitors and activity trackers. CWDs primarily use photoplethysmography (PPG) for physiological measurements, and PPG is especially common in devices worn on the fingers and wrists. Wearable technology has great promise for widespread remote monitoring and early cardiac irregularity detection, improving preventive care by delivering an extensive spectrum of cardiopulmonary metrics straight to consumers. Training regimens and customised exercise recommendations for athletes with cardiac conditions can be created by integrating data from these devices to guide training intensity. Use of machine learning has also been implemented by using the ECG data in generation of heart rate variability (HRV) reports for diagnosing any autonomic dysfunction giving rise to arrythmia (26–31). As the current gold standard for assessing myocardial structure and tissue architecture, cardiac magnetic resonance imaging (CMR) is a well-established imaging modality for assessing the cardiovascular system in athletes. It can be difficult to accurately interpret CMR images, and mistakes can have serious repercussions, even for athletes. Accurate interpretation and increased efficiency are two benefits of integrating ML into CMR. The CMR process can be made simpler with the help of AI solutions that have been proposed to aid in image acquisition, reconstruction, and quality improvement. CMR is usually requested in case of diagnostic suspicion of structural heart disease, and it plays a fundamental role in athletes, especially when echocardiography is normal. Only afterwards it would be appropriate to mention the challenges of image interpretation and the potential role of AI in improving accuracy and efficiency (32, 33).
By strengthening risk assessment, preventing cardiac abnormalities early, and improving athlete treatment planning and monitoring, the incorporation of AI technologies holds the potential to completely transform sports cardiology. A promising avenue for converting conventional screening procedures into more sophisticated, accurate, and predictive medical interventions is provided by this innovation (34, 35). With a focus on their potential to improve PPS and lower the risk of SCD in athletes, Table 1 lists AI applications in sports cardiology.
Conclusion
Sports cardiology places a lot of emphasis on athlete screening, but there are still a number of issues and problems that need to be resolved in order to enhance the early detection of people who are at risk of sudden cardiac death. In this context, creating required prospective registries that systematically report all cases of SCD in athletes is essential. AI and digital technology integration in sports medicine may improve athletes' assessments, but since this field is developing, strong protocol frameworks will be essential to maximising the potential of these technologies.
Author contributions
AB: Writing – review & editing, Methodology, Supervision, Conceptualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza J, et al. Exercise benefits in cardiovascular disease: beyond attenuating traditional risk factors. Nat Rev Cardiol. (2018) 15:731–43. doi: 10.1038/s41569-018-0065-1
2. Banerjee A, Kumar S, Dasgupta S. Association of physical activity and stress reactivity with depression in elderly hypertensive population: a cross-sectional study from Eastern India. J Family Med Prim Care. (2023) 12(11):2635–9. doi: 10.4103/jfmpc.jfmpc_1072_23
3. Walker J, Calkins H, Nazarian S. Evaluation of cardiac arrhythmia among athletes. Am J Med. (2010) 123:1075–81. doi: 10.1016/j.amjmed.2010.05.008
4. Pelliccia A, Adami PE, Quattrini F, Squeo MR, Caselli S, Verdile L, et al. Are Olympic athletes free from cardiovascular diseases? Systematic investigation in 2352 participants from Athens 2004 to sochi 2014. Br J Sports Med. (2017) 51:238–43. doi: 10.1136/bjsports-2016-096961
5. Ben Halima A, Kobaa D, Ben Halima M, Ayachi S, Belkhiria M, Addala H. Assessment of premature ventricular beats in athletes. Ann Cardiol Angeiol (Paris). (2019) 68:175–80. doi: 10.1016/j.ancard.2018.10.013
6. Bjerregaard P. Premature beats in healthy subjects 40–79 years of age. Eur Heart J. (1982) 3:493–503. doi: 10.1093/oxfordjournals.eurheartj.a061344
7. Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. (2003) 42(11):1959–63. doi: 10.1016/j.jacc.2003.03.002
8. Sanchis-Gomar F, Pérez LM, Joyner MJ, Löllgen H, Lucia A. Endurance exercise and the heart: friend or foe? Sport Med. (2016) 46:459–66. doi: 10.1007/s40279-015-0434-4
9. Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes. JAMA. (2006) 296:1593–601. doi: 10.1001/jama.296.13.1593
10. Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: a state-of-the-art review. Br J Sports Med. (2014) 48:1185–92. doi: 10.1136/bjsports-2014-093872
11. Marijon E, Tafflet M, Celermajer DS, Dumas F, Perier MC, Mustafic H, et al. Sports-related sudden death in the general population. Circulation. (2011) 124:672–81. doi: 10.1161/CIRCULATIONAHA.110.008979
12. Dostal J, Hybska T, Saganelidze K, Pudil R, Stasek J. Autonomic dysfunction as a possible cause of sudden cardiac death in swimming sports. Front Cardiovasc Med. (2024) 11:1443214. doi: 10.3389/fcvm.2024.1443214
13. Graziano F, Schiavon M, Cipriani A, Savalla F, De Gaspari M, Bauce B, et al. Causes of sudden cardiac arrest and death and the diagnostic yield of sport preparticipation screening in children. Br J Sports Med. (2024) 58:255–61. doi: 10.1136/bjsports-2023-107357
14. Landry CH, Allan KS, Connelly KA, Cunningham K, Morrison LJ, Dorian P. Sudden cardiac arrest during participation in competitive sports. N Engl J Med. (2017) 377:1943–53. doi: 10.1056/NEJMoa1615710
15. Sollazzo F, Palmieri V, Gervasi SF, Cuccaro F, Modica G, Narducci ML, et al. Sudden cardiac death in athletes in Italy during 2019: internet-based epidemiological research. Medicina (B Aires). (2021) 57:1–9. doi: 10.3390/medicina57010061
16. Antonio P, Jonathan AD, Alessandro Z, Domenico C. Prevalence and clinical significance of low QRS voltages in healthy individuals, athletes, and patients with cardiomyopathy: implications for sports pre-participation cardiovascular screening. Eur J Prev Cardiol. (2024) 31:1106–14. doi: 10.1093/eurjpc/zwae027
17. Kim JH, Baggish AL, Levine BD, Ackerman MJ, Day SM, Dineen EH, et al. Clinical considerations for competitive sports participation for athletes with cardiovascular abnormalities: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. (2025) 85(10):1059–108. doi: 10.1016/j.jacc.2024.12.025
18. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. (2018) 39:1466–80. doi: 10.1093/eurheartj/ehw631
19. Zorzi A, Bettella N, Tatangelo M, Del Monte A, Vessella T, Poscolieri B, et al. Prevalence and clinical significance of isolated low QRS voltages in young athletes. Europace. (2022) 24(9):1484–95. doi: 10.1093/europace/euab330
20. Bratincsák A, Kimata C, Limm-Chan BN, Vincent KP, Williams MR, Perry JC. Electrocardiogram standards for children and young adults using Z-scores. Circ Arrhythm Electrophysiol. (2020) 13:e008253. doi: 10.1161/CIRCEP.119.008253
21. Migliore F, Zorzi A, Michieli P, Perazzolo Marra M, Siciliano M, Rigato I, et al. Prevalence of cardiomyopathy in Italian asymptomatic children with electrocardiographic T-wave inversion at preparticipation screening. Circulation. (2012) 125:529–38. doi: 10.1161/CIRCULATIONAHA.111.055673
22. Pentikäinen H, Toivo K, Kokko S, Alanko L, Heinonen OJ, Korpelainen R, et al. Resting electrocardiogram and blood pressure in young endurance and non-endurance athletes and non-athletes. J Athl Train. (2020) 56:484–90. doi: 10.4085/78-20
23. Galderisi M, Cardim N, D'Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the athlete’s heart: an expert consensus of the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:353. doi: 10.1093/ehjci/jeu323
24. Graziano F, Cozza E, Millin A, Gianni A, Mattesi G, Motta R. Prevalence and characteristics of coronary artery disease in master athletes with ST-segment depression or high-risk premature ventricular beats at pre-participation exercise testing. Eur Heart J Open. (2025) 5(4):oeaf090. doi: 10.1093/ehjopen/oeaf090
25. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. (2012) 54:380–6. doi: 10.1016/j.pcad.2012.01.006
26. Cho JY, Kim KH, Rink L, Hornsby K, Park H, Park JH, et al. University athletes and changes in cardiac geometry: insight from the 2015 Gwangju Summer Universiade. Eur Heart J Cardiovasc Imaging. (2019) 20:407–16. doi: 10.1093/ehjci/jey196
27. Van Rosendael AR, de Graaf MA, Scholte AJ. Cardiac arrest during vigorous exercise: coronary plaque rupture or myocardial ischaemia? Neth Heart J. (2015) 23(2):130–2. doi: 10.1007/s12471-014-0647-4
28. Kumar S, Banerjee A. Artificial intelligence-enabled smartwatch used for the detection of idiopathic ventricular tachycardia: a case report. Cureus. (2023) 15(7):e42054. doi: 10.7759/cureus.42054
29. Banerjee A. Artificial intelligence enabled mobile health technologies in arrhythmias-an opinion article on recent findings. Front Cardiovasc Med. (2025) 12:1548554. doi: 10.3389/fcvm.2025.1548554
30. Hagiwara Y, Fujita H, Oh SL, Tan JH, Tan S, Ciaccio R, et al. Computer-aided diagnosis of atrial fibrillation based on ECG signals: a review. Inf Sci. (2018) 467:99–114. doi: 10.1016/j.ins.2018.07.063
31. Mehta SS, Shete DA, Lingayat NS, Chouhan VS. K-means algorithm for the detection and delineation of QRS-complexes in electrocardiogram. IRBM. (2010) 31:48–54. doi: 10.1016/j.irbm.2009.10.001
32. Haque Y, Zawad RS, Rony CSA, Banna HA, Ghosh T, Kaiser MS, et al. State-of-the-art of stress prediction from heart rate variability using artificial intelligence. Cogn Comput. (2024) 16:455–81. doi: 10.1007/s12559-023-10200-0
33. Madani A, Arnaout R, Mofrad M, Arnaout R. Fast and accurate view classification of echocardiograms using deep learning. NPJ Digit Med. (2018) 1:1–8. doi: 10.1038/s41746-017-0013-1
34. Narula S, Shameer K, Omar AMS, Dudley JT, Sengupta PP. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. (2016) 68:2287–95. doi: 10.1016/j.jacc.2016.08.062
35. Siegersma K, Leiner T, Chew D, Appelman Y, Hofstra L, Verjans J. Artificial intelligence in cardiovascular imaging: state of the art and implications for the imaging cardiologist. Netherlands Heart J. (2019) 27:403–13. doi: 10.1007/s12471-019-01311-1
36. Banerjee A, Sarangi P, Kumar S. Medical Doctors’ perceptions of artificial intelligence (AI) in healthcare. Cureus. (2024) 16(9):e70508. doi: 10.7759/cureus.70508
Keywords: sports medicine, prescreening, cardiovascular evaluation, electrocardiography (ECG), sudden cardiac arrest (SCA)
Citation: Banerjee A (2025) Cardiovascular evaluation of athletes ahead of participation. Front. Cardiovasc. Med. 12:1666981. doi: 10.3389/fcvm.2025.1666981
Received: 16 July 2025; Accepted: 22 September 2025;
Published: 3 October 2025.
Edited by:
Francesca Graziano, University of Padova, ItalyReviewed by:
Amedeo De Antoni, University of Padova, ItalyCopyright: © 2025 Banerjee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arijita Banerjee, Yi5hcmlqaXRhQGdtYWlsLmNvbQ==; YXJpaml0YUBiY3JtcmMuaWl0a2dwLmFjLmlu
 Arijita Banerjee
Arijita Banerjee