Abstract
Background:
Mineralocorticoid receptor over-activation drives maladaptive myocardial fibrosis, vascular inflammation and renal sodium retention across the entire spectrum of left ventricular ejection fraction (LVEF). While steroidal MRAs have convincingly reduced hospitalizations and mortality in patients with heart failure and reduced EF (HFrEF), evidence remains fragmented for heart failure with mildly reduced (HFmrEF) or preserved EF (HFpEF), and no head-to-head data distinguish steroidal from non-steroidal agents. This study aimed to evaluate the effect of MRAs in patients with HF across the range of ejection fraction.
Methods:
Searched PubMed, Web of Science, Wanfang and Cochrane library (1 Jan 1987–10 Sep 2024) for randomized clinical trials (RCT) assessing MRAs (finerenone, spironolactone, eplerenone) in HFpEF, HFmrEF or HF. The primary endpoint was composite cardiovascular (CV) outcomes. Secondary endpoints included CV mortality, overall HF exacerbation events, safety, and adverse events. A meta-analysis was conducted using hazard ratios (HR), confidence intervals (CI), and relative risks (RR) to synthesize the findings.
Results:
In the analysis of nine RCTs, MRAs were associated with a 23% reduction in CV composite outcomes (RR: 0.77, 95% CI: 0.72–0.83, P < 0.00001), a 23% reduction in HF hospitalization risk (HR: 0.77, 95% CI: 0.70–0.84, P < 0.00001), and a 22% reduction in all-cause mortality (HR: 0.78, 95% CI: 0.72–0.85, P < 0.00001) in HFrEF patients, compared to a 17% reduction in CV composite events (HR: 0.85, 95% CI: 0.78–0.93, P = 0.0004), a 20% reduction in HF hospitalization risk (HR: 0.80, 95% CI: 0.73–0.89, P < 0.00001), and an 8% reduction in all-cause mortality (HR: 0.91, 95% CI: 0.85–0.99, P = 0.02) in HFmrEF/HFpEF patients. However, CV mortality was not significantly reduced in HFmrEF/HFpEF patients (HR: 0.92, 95% CI: 0.82–1.02, P = 0.13), but was reduced by 23% in HFrEF patients (HR: 0.77, 95% CI: 0.70–0.83, P < 0.00001). The incidence of any serious adverse events was similar between the MRA and placebo groups. The incidence of hyperkalemia was significantly higher in the MRA group (RR: 2.19, 95% CI: 1.97–2.43, P < 0.00001).
Conclusions:
MRAs should be considered for patients with HFrEF due to their substantial benefits. In HFmrEF or HFpEF, MRAs may confer benefit, though the effect is modest and hyperkalemia risk is higher, mandating close potassium monitoring.
Systematic Review Registration:
PROSPERO CRD42022304966.
Introduction
Mineralocorticoid receptor antagonists (MRAs) are a cornerstone in the management of heart failure (HF), particularly in patients with heart failure with reduced ejection fraction (HFrEF) (1). In 2023, the European Society of Cardiology (ESC) issued a focused update to its 2021 guidelines on acute and chronic heart failure, adding a class IIb recommendation for MRAs in patients with mildly reduced (HFmrEF) or preserved (HFpEF) ejection fraction. Crucially, the document declines to endorse any specific MRA for the HFpEF cohort, underscoring prevailing clinical caution in drug selection for this phenotype (2). In September 2024, FINEARTS showed finerenone significantly reduced the composite of worsening HF events and cardiovascular (CV) death, providing robust evidence for its use in HFmrEF and HFpEF (3).
Spironolactone and eplerenone are recommended for HFrEF to reduce mortality and hospitalization. They have shown significant efficacy in reducing CV death and HF hospitalizations in HFrEF patients (4, 5). Finerenone, a newer nonsteroidal MRA, has demonstrated efficacy in reducing CV mortality and hospitalizations in HFpEF and HFmrEF, with a lower risk of hyperkalemia compared to steroidal MRAs (6). Both steroidal and nonsteroidal MRAs are included in HF management guidelines, but their use is often limited by the risk of hyperkalemia and renal impairment (7).
Most studies have concentrated on HFrEF, leaving a gap in data for HFmrEF and HFpEF. Currently, robust evidence supports steroidal MRAs like spironolactone and eplerenone for HFrEF management, though they carry risks of hyperkalemia and sex hormone-related side effects. Non-steroidal MRA finerenone offers higher receptor selectivity and a potentially safer profile, with its heart failure evidence mainly from diabetic nephropathy trials. In July 2025, finerenone was FDA-approved for HFmrEF and HFpEF, and Japanese guidelines have a Class IIa recommendation for its use in these conditions (8). The efficacy of MRAs in these subtypes remains less clear and inconsistent across studies (4, 9). There is a lack of direct comparative studies between steroidal and nonsteroidal MRAs, particularly in patients with renal insufficiency and other comorbidities (10). The risk of hyperkalemia and renal function deterioration is a significant concern, especially in patients with chronic kidney disease (CKD), which limits the broader application of MRAs (9, 11).
We conducted a comprehensive meta-analysis to evaluate the efficacy and safety of MRAs in HF patients across the EF spectrum, including HFrEF, HFmrEF, and HFpEF.
Methods
Study design
This systematic review and meta-analysis was conducted to evaluate the efficacy and safety of steroidal and non-steroidal MRAs in patients with HFrEF, HFmrEF or HFpEF. The study adhered to a predefined protocol (PROSPERO: CRD 42022304966) and followed the guidelines outlined in the PRISMA statement for conducting this meta-analysis (Table 1). This meta-analysis focuses on symptomatic HF patients, including HFrEF: HF with LVEF ≤ 40%; HFmrEF: HF with LVEF 41%–49%; HFpEF: HF with LVEF ≥ 50% (12).
Search strategy, selection criteria, and data extraction
A systematic search was performed in PubMed, Web of Science, WANGFANG and Cochrane library from January 1, 1987 through September 10, 2024. The search strategy incorporated the terms “finerenone”, “spironolactone”, “eplerenone”, “HFpEF”, “HFmrEF,” and “heart failure” to identify relevant studies.
Eligible for this analysis were randomized clinical trials (RCT) designed to appraise the therapeutic efficacy of pharmacological agents recommended for the management of HFrEF, HFmrEF and HFpEF. The pharmacotherapies of interest in this study comprised spironolactone, eplerenone, and finerenone. We confined our search to trials involving adult subjects, 18 years of age or older, diagnosed with HFmrEF or HFpEF, as defined by the EF surpassing 40%. The stringent criteria for participant selection were implemented to guarantee the pertinence of our study cohort and to facilitate the extrapolation of our results to the broader demographic of adults grappling with these cardiac affections. Patients were excluded if they had an estimated glomerular filtration rate (eGFR) < 25 ml/min/1.73 m2, serum potassium >5.0 mmol/L, recent MRA use within 30 days, or a history of specific cardiomyopathies (e.g., dilated, peripartum, chemotherapy-induced, or amyloidosis cardiomyopathies), as well as HF symptoms from non-cardiac causes.
Two investigators (Y.Z. and Y-C.B.) conducted a thorough screening of titles and abstracts from the identified citations to ascertain eligibility. Subsequently, full texts of selected citations were independently reviewed by the same two investigators. Any discrepancies were resolved by consensus. Two authors extracted data from studies, cross-checking for accuracy.
Outcomes
The primary endpoint was a composite measure of CV mortality and HF events, encompassing CV death, hospitalizations due to HF, resuscitated cardiac arrests, initial hospitalizations for HF, and all episodes of worsening HF. The secondary outcomes included all-cause mortality, CV death, and hospitalization for HF. Safety outcomes were examined in patients who received at least one dose of randomly assigned treatment. The safety outcomes included serum creatinine ≥2.5 mg/dl, a decrease of more than 20% in eGFR, hypokalemia or hyperkalemia (serum potassium <3.5 mmol/L or >5.5 mmol/L), and systolic blood pressure <100 mm Hg.
Methodological quality
Risk of bias was evaluated in accordance with the Cochrane Collaboration's tool for assessing risk of bias in RCT trials (13). This tool evaluates the presence of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks of confounding. Publication bias was assessed using Begg's adjusted rank correlation test.
Statistical analysis
Meta-analysis was performed using Review Manager software (version 5.4.1) and STATA 12.0 software. Continuous data were analyzed using mean differences or standardized mean differences with 95% confidence intervals (CIs). Recalculation of the pooled effect estimates using the original metric used by the meta-analysis study authors [hazard ratio (HR), and risk ratio (RR) with 95% CI]. Heterogeneity was assessed using the I2 test. A random-effects model was applied if significant heterogeneity was detected (I2 > 50%). When heterogeneity is high, we conduct subgroup analyses or sensitivity analyses. Otherwise, a fixed-effects model was utilized. Weighted mean differences (WMD) with 95% CIs were used to evaluate outcomes. Funnel plot analysis was performed to assess publication bias. Statistical significance was set at P < 0.05.
Results
Study selection
Per the flowchart, the system search generated 10,963 records from databases (Cochrane Library, PubMed, Web of Science, and WAMFANG). After removing duplicates, 7,374 records remained. Following exclusions of 4,954 records including reviews, letters, and comments, 1,328 non-process trials, and 705 records lacking endpoint definitions, 387 records were selected for full-text review. After excluding studies with incompatible outcome metrics, low-quality studies, and data conversion issues, 9 studies were ultimately included in the meta-analysis (Figure 1).
Figure 1
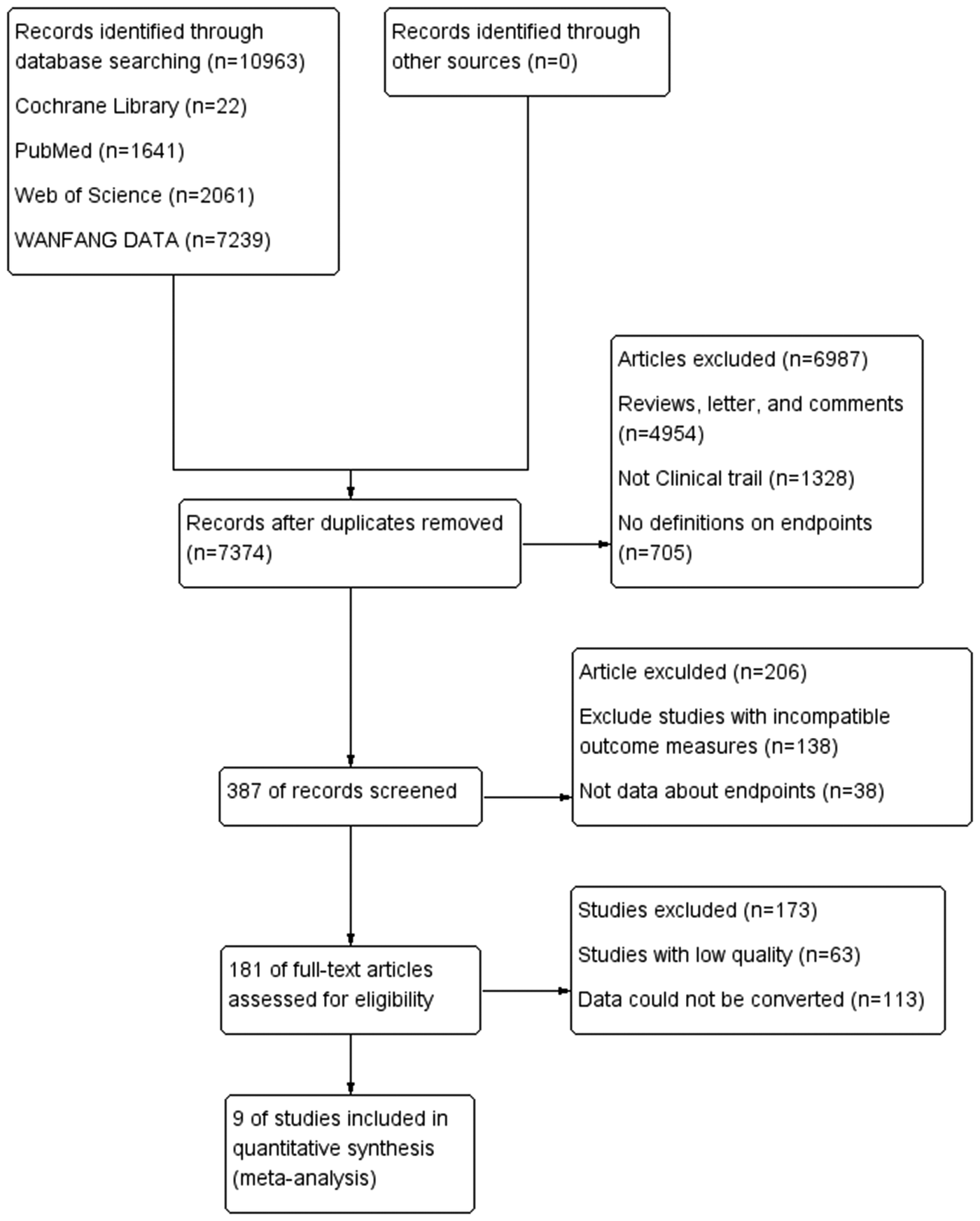
Study flow diagram.
This study included a total of nine RCTs: RALES (The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure) (1), which evaluated spironolactone; EPHESUS (Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction) (14), which assessed eplerenone; EMPHASIS-HF (Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms) (15), also focusing on eplerenone; Aldo-DHF (Effect of Spironolactone on Diastolic Function and Exercise Capacity in Patients With Heart Failure With Preserved Ejection Fraction) (16), which examined spironolactone; TOPCAT (Spironolactone for Heart Failure with Preserved Ejection Fraction) (17), again evaluating spironolactone; FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) (18) and FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) (19), FINEARTS-HF (Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction) (3), and ARTS (The minerAlocorticoid Receptor Antagonist Tolerability Study) (20), all assessing finerenone. These trials collectively involved 33,128 participants, comprising 67.4% males and 32.6% females (Table 1) (Supplementary Table S2).
Table 1
| Study Year |
Number (%)a Age (years) |
Included patients | Follow-up | MRAs | Outcome |
|---|---|---|---|---|---|
| RALES 1999 |
1,663 (27) 65 ± 11 |
HFrEF, LVEF ≤ 35%, K ≤ 5 mmol/L | 12 months | Spironolactone 25 mg/days for 8 weeks → 50 mg/days |
CV composite events (CV death or HHF or aborted cardiac arrest), CV death, HHF |
| EPHESUS 2003 |
6,642 (29) 64 ± 11 |
HFpEF, LVEF ≤ 40%, K ≤ 5 mmol/L |
16 months | Eplerenone 25 mg/days for 4 weeks → 50 mg/days |
CV composite events (CV death or HHF), CV death, HHF |
| EMPHASIS-HF 2011 |
2,737 (22) 68.7 ± 7.7 |
HFpEF, LVEF < 35%, K ≤ 5 mmol/L | 21 months | Eplerenone 25 mg/days for 4 weeks → 50 mg/days |
CV composite events (CV death or FHHF), CV death, HHF |
| Aldo-DHF 2013 |
422 (52) 67 ± 8 |
HFpEF, LVEF ≥ 50%, K ≤ 5 mmol/L | 11.6 months | Spironolactone 25 mg/days |
Cardiac hospitalization, Noncardiac hospitalization, Worsening coronary heart disease, New or worsening anemia |
| TOPCAT 2014 |
3,445 (52) 68 ± 9 |
HFpEF, LVEF ≥ 45%, K ≤ 5 mmol/L | 3.3 years | Spironolactone 15 mg/days → 45 mg/days during 4 months |
CV composite events (CV death or HHF), CV death, HHF |
| FIGARO-DKD 2022 |
7,352 (31) 65 ± 9 |
T2DM, CKD, LVEF ≥ 40%, K ≤ 5 mmol/L | 3.4 years | Finerenone 10 or 20 mg/days |
CV composite events (CV death or HHF), CV death, HHF |
| FIDELIO-DKD 2020 |
5,674 (30) 65 ± 9 |
T2DM, CKD, LVEF ≥ 40%, K ≤ 5 mmol/L | 2.6 years | Finerenone 10 or 20 mg/days |
CV composite events (CV death or HHF), CV death, HHF |
| FINEARTS-HF2024 | 6,001 (46) 72 ± 9 |
HFpEF, HFmrEF, LVEF ≥ 40% K ≤ 5 mmol/L |
32 months | Finerenone 20 or 40 mg/days |
CV composite events (CV death or Total worsening heart failure events), CV death, HHF |
| ARTS-HF2013 | 458 (21) 71 ± 8 |
HFrEF, CKD, LVEF ≤ 40%, K ≤ 5 mmol/L | 4 weeks | Finerenone 2.5, 5 or 10 mg/days | Change in serum potassium concentration, safety, tolerability, and renal effects |
Baseline characteristics of patients in each MRAs trials.
CV, cardiovascular; HF: heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HHF, heart failure hospitalization; FHHF, first hospitalization for heart failure; K, potassium; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; T2DM, type 2 diabetes mellitus.
Indicates proportion of female.
Outcomes
The impact of MRAs in patients with HFpEF and HFmrEF
The research analyzed four studies with 7,008 patients to assess the composite primary endpoints (3, 17–19). In the analysis of CV composite events, which encompassed the time to first hospitalization for HF or CV death, MRAs were associated with a significant reduction in risk compared with placebo in patients with HFpEF and HFmrEF. The pooled HR of 0.85 (95% CI: 0.78–0.93; P = 0.0004) indicated a 15% relative risk reduction without heterogeneity (I2 = 0%), suggesting consistent treatment effects across studies (Figure 2A). MRAs showed no significant impact on CV death (pooled HR: 0.92, 95% CI: 0.82–1.02, P = 0.13) with low heterogeneity (I2 = 0%) (Figure 2B). MRAs reduced the risk of hospitalization for HF by 20% (pooled HR: 0.80, 95% CI: 0.73–0.89, P < 0.00001) without heterogeneity (I2 = 0%) (Figure 2C). For all-cause mortality, the pooled analysis demonstrated a significant reduction with MRAs, with an HR of 0.91 (95% CI: 0.85–0.99; P = 0.02), indicating a 9% relative risk reduction without heterogeneity (Figure 2D).
Figure 2
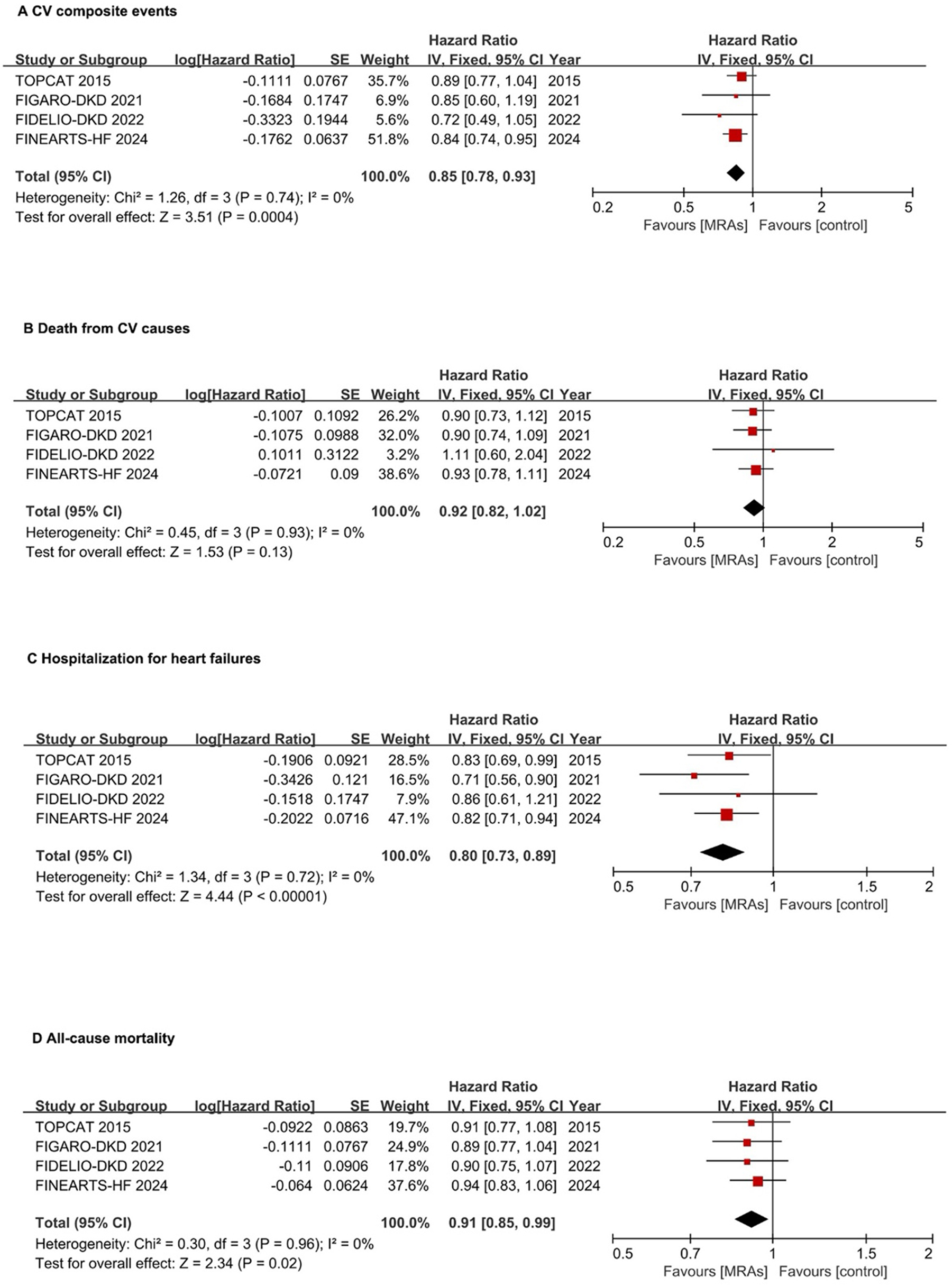
Forest plots assessing MRA effectiveness in hFpEF and hFmrEF patients. (A) CV composite events; (B) CV death; (C) Hospitalization for heart failures; (D) All-cause mortality. CI, confidence interval; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; IV, inverse variance; MRA, mineralocorticoid receptor antagonists.
Subgroup analysis
In the CV composite events, the non-steroidal MRA drug finerenone significantly reduced CV composite events by 17% (HR: 0.83, 95% CI: 0.74–0.93, P = 0.001). The steroidal MRA drug spironolactone showed no statistically significant reduction in CV composite events for HFmrEF/HFpEF patients (HR: 0.89, 95% CI: 0.77–1.04, P = 0.15) (Figure 3A). For the risk of the hospitalization for HF, the non-steroidal MRA drug finerenone significantly reduced HF hospitalizaiton events by 20% (HR: 0.80, 95% CI: 0.71–0.89, P < 0.0001). The steroidal MRA drug spironolactone showed statistically significant reduction in HF hospitalizaiton events (HR: 0.83, 95% CI: 0.69–0.99, P = 0.04) (Figure 3B).
Figure 3
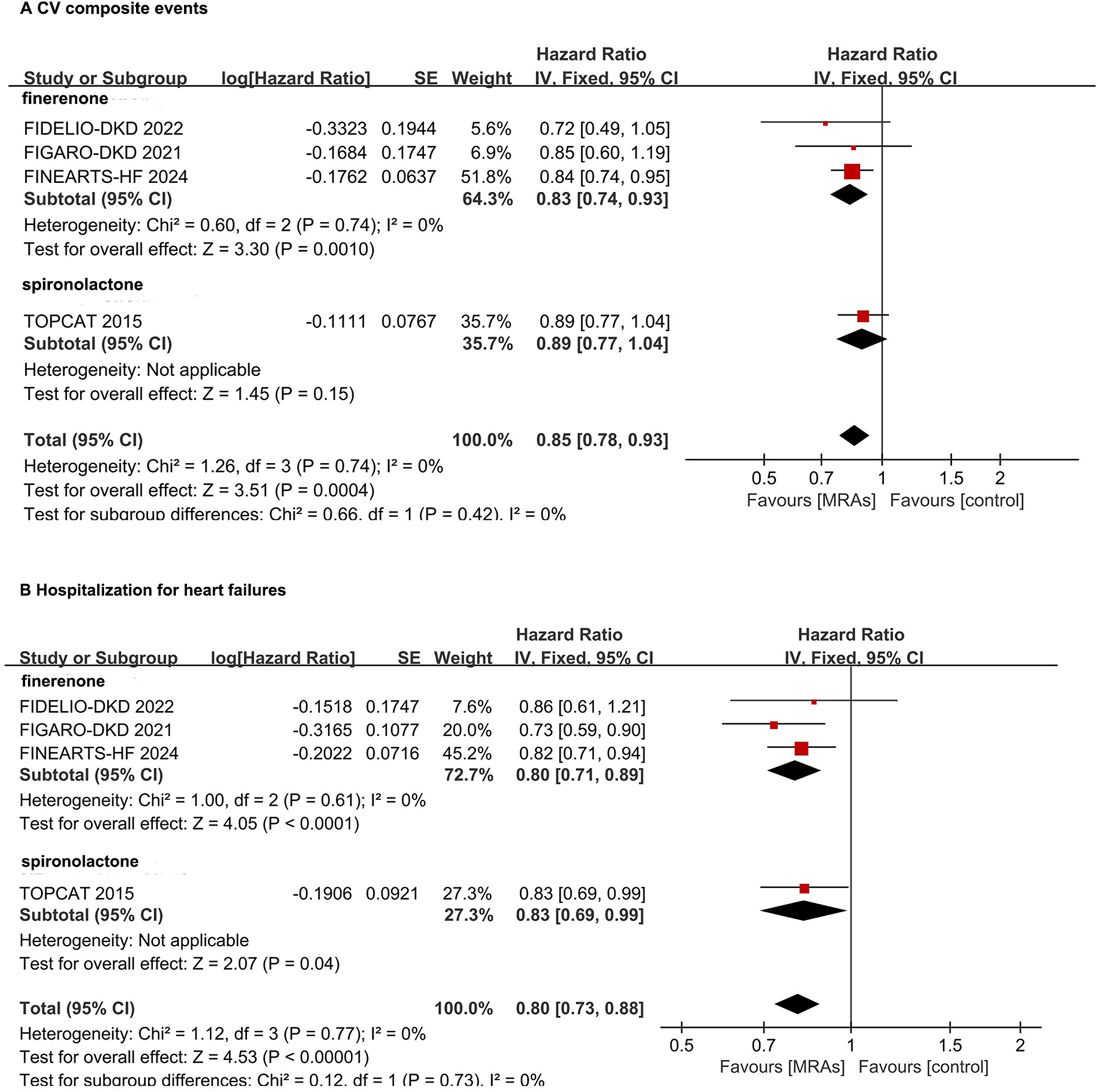
Subgroup analysis of non-steroidal MRA and steroidal MRA in hFpEF and hFmrEF patients. (A) CV composite events; (B) Hospitalization for heart failures. CI, confidence interval; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; IV, inverse variance; MRA, mineralocorticoid receptor antagonists.
The impact of MRAs in patients with HFrEF
In patients with HFrEF, MRAs reduced the risk of CV composite events (first HF hospitalization or CV mortality) by 23% (pooled HR: 0.77, 95% CI: 0.72–0.83, P < 0.00001). However, high heterogeneity was observed (I² = 87%, P = 0.0006) (Figure 4A).
Figure 4
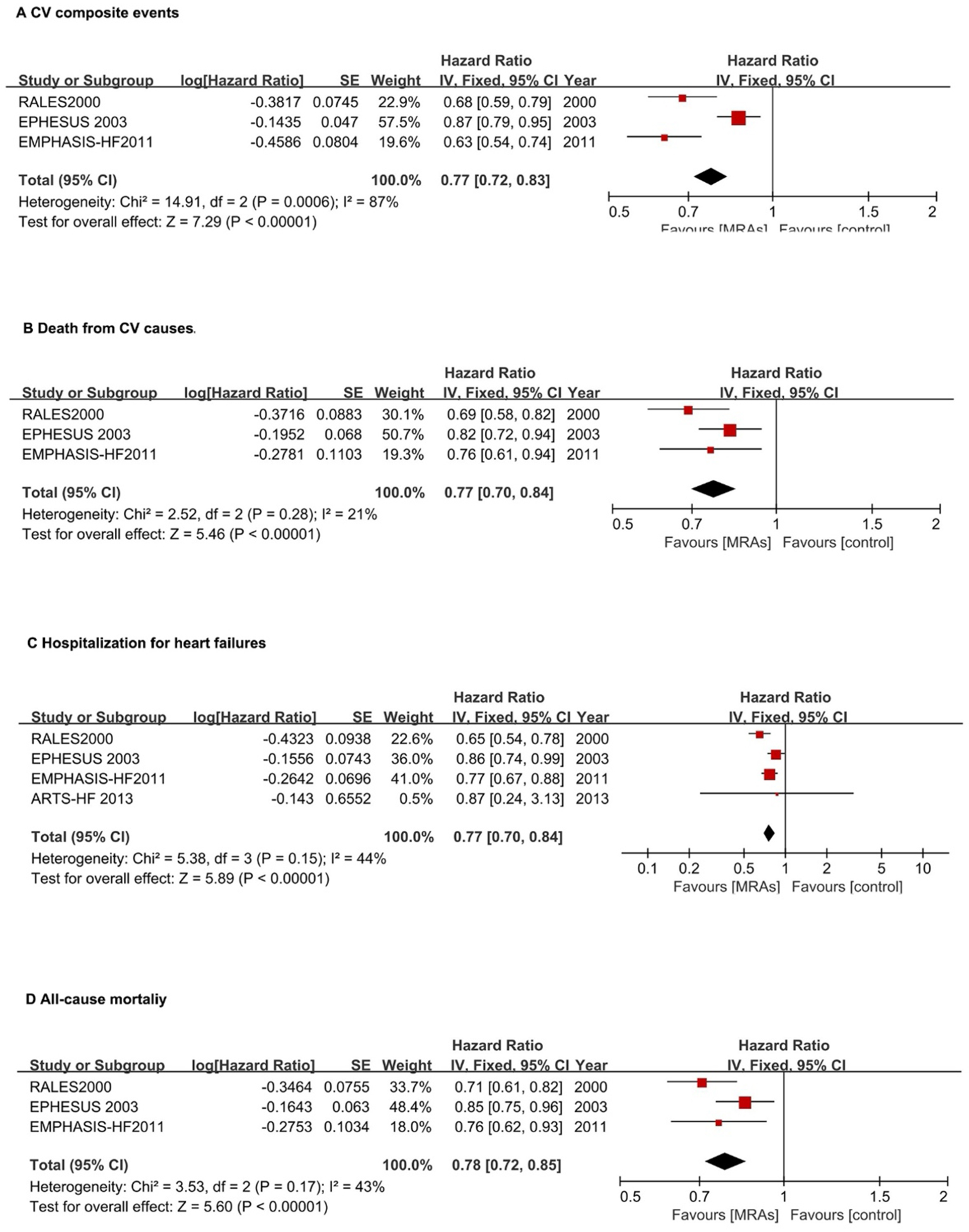
Forest plots assessing MRA effectiveness in hFrEF patients.(A) CV composite events; (B) CV death; (C) Hospitalization for heart failures; (D) All-cause mortality. CI, confidence interval; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, inverse variance; MRA, mineralocorticoid receptor antagonists.
For CV mortality, MRAs reduced risk by 23% (pooled HR: 0.77, 95% CI: 0.70–0.84, P < 0.0001) with low heterogeneity (I² = 21%) (Figure 4B).
MRAs significantly reduced HF hospitalization risk by 23% (pooled HR: 0.77, 95% CI: 0.70–0.84, P < 0.00001) with moderate heterogeneity (I² = 44%) (Figure 4C). For all-cause mortality, MRAs reduced risk by 22% (HR: 0.78, 95% CI: 0.72–0.85; P < 0.00001) with moderate heterogeneity (I² = 43%) (Figure 4D).
For CV endpoints, a sensitivity analysis was executed. The exclusion of the EPHESUS trial culminated in a marked reduction in heterogeneity (I2 = 0). This outcome underscores the EPHESUS trial as the principal determinant of the heterogeneity (Figure 5).
Figure 5
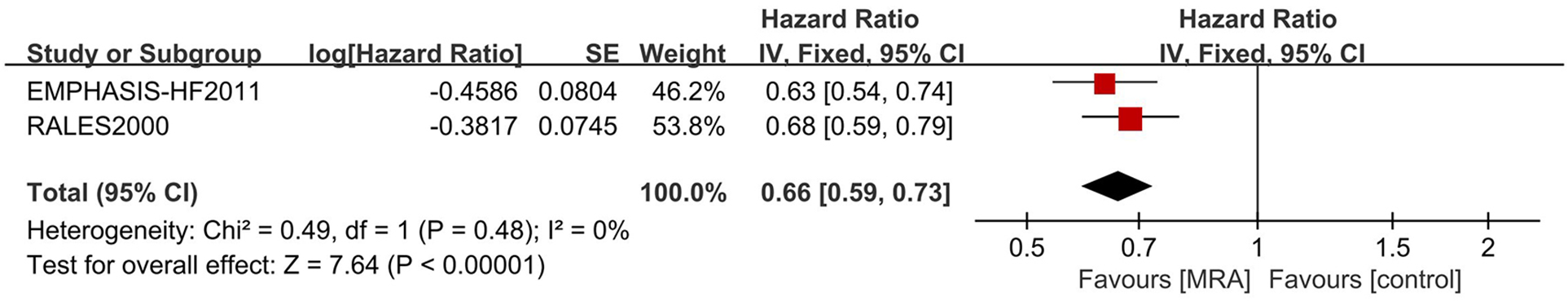
Sensitive analysis of MRA in hFrEF patients. IV, inverse variance; MRA, mineralocorticoid receptor antagonists.
Adverse events associated with MRAs
The RR for any serious adverse event with MRAs vs. placebo is 0.99 (95% CI: 0.97–1.01; P = 0.20) without heterogeneity (Figure 6A). MRAs significantly increase the risk of hyperkalemia, with an RR of 2.19 (95% CI: 1.98–2.44; P < 0.00001) with mild heterogeneity (I2 = 26%) (Figure 6B). The RR for hypokalemia with MRAs is significantly reduced to 0.56 (95% CI: 0.51–0.62; P < 0.00001) with high heterogeneity (I2 = 83%) (Figure 6C). The risk of hypotension is significantly increased with MRAs (RR: 1.35, 95% CI: 1.15–1.47, P < 0.00001) with moderate heterogeneity (I² = 68%) (Figure 6D). The risk of acute kidney injury is significantly increased with MRAs (RR: 1.30, 95% CI: 1.06–1.59, P = 0.01) with moderate heterogeneity (I² = 66%) (Figure 6E).
Figure 6
![Forest plots show meta-analysis results for different adverse events linked to Mineralocorticoid Receptor Antagonists (MRAs) versus placebo. (A) Serious adverse events show no significant risk difference, risk ratio 0.99 [0.97, 1.01]. (B) Hyperkalemia shows increased risk with MRAs, risk ratio 2.19 [1.98, 2.44]. (C) Hypokalemia shows reduced risk with MRAs, risk ratio 0.56 [0.51, 0.62]. (D) Hypotension shows increased risk with MRAs, risk ratio 1.35 [1.25, 1.47]. (E) Acute kidney injury shows slight increased risk with MRAs, risk ratio 1.30 [1.06, 1.59]. Each plot includes study subsets and the overall effect.](https://www.frontiersin.org/files/Articles/1667236/xml-images/fcvm-12-1667236-g006.webp)
Forest plots assessing MRA safety. (A) Any serious adverse event; (B) Hyperkalemia; (C) Hypokalemia; (D) Hypotension; (E) Acute kidney injury. MRA, mineralocorticoid receptor antagonists.
Subgroup analysis of hyperkalemia, MRAs revealed a higher risk of hyperkalemia across all MRA drugs, categorized into finerenone, spironolactone, and eplerenone. Notably, the spironolactone group exhibited significant statistical heterogeneity (I2 = 72%) (Supplementary Figure S1).
MRAs' differential impact on HFrEF vs. HFmrEF/HFpEF
For the CV endpoint, MRAs significantly lowered the HR to 0.85 (95% CI: 0.78–0.93) in the HFpEF/HFmrEF subgroup and to 0.77 (95% CI: 0.72–0.83) in the HFrEF subgroup, exhibiting considerable heterogeneity (I2 = 65.9%, P = 0.007). In terms of CV mortality, the HR for MRA was 0.92 (95% CI: 0.82–1.03) in the HFpEF/HFmrEF subgroup and 0.77 (95% CI: 0.70–0.84) in the HFrEF subgroup, with significant heterogeneity (I2 = 83.4%, P = 0.014). For heart failure hospitalization, the HR with MRA was 0.80 (95% CI: 0.72–0.88) in the HFpEF/HFmrEF subgroup and 0.77 (95% CI: 0.70–0.84) in the HFrEF subgroup, with no significant heterogeneity (I2 = 0%, P = 0.578). Regarding all-cause mortality, the HR for MRA was 0.91 (95% CI: 0.84–0.98) in the HFpEF/HFmrEF subgroup and 0.78 (95% CI: 0.72–0.85) in the HFrEF subgroup, showing significant heterogeneity (I2 = 86.1%, P = 0.007) (Figure 7).
Figure 7
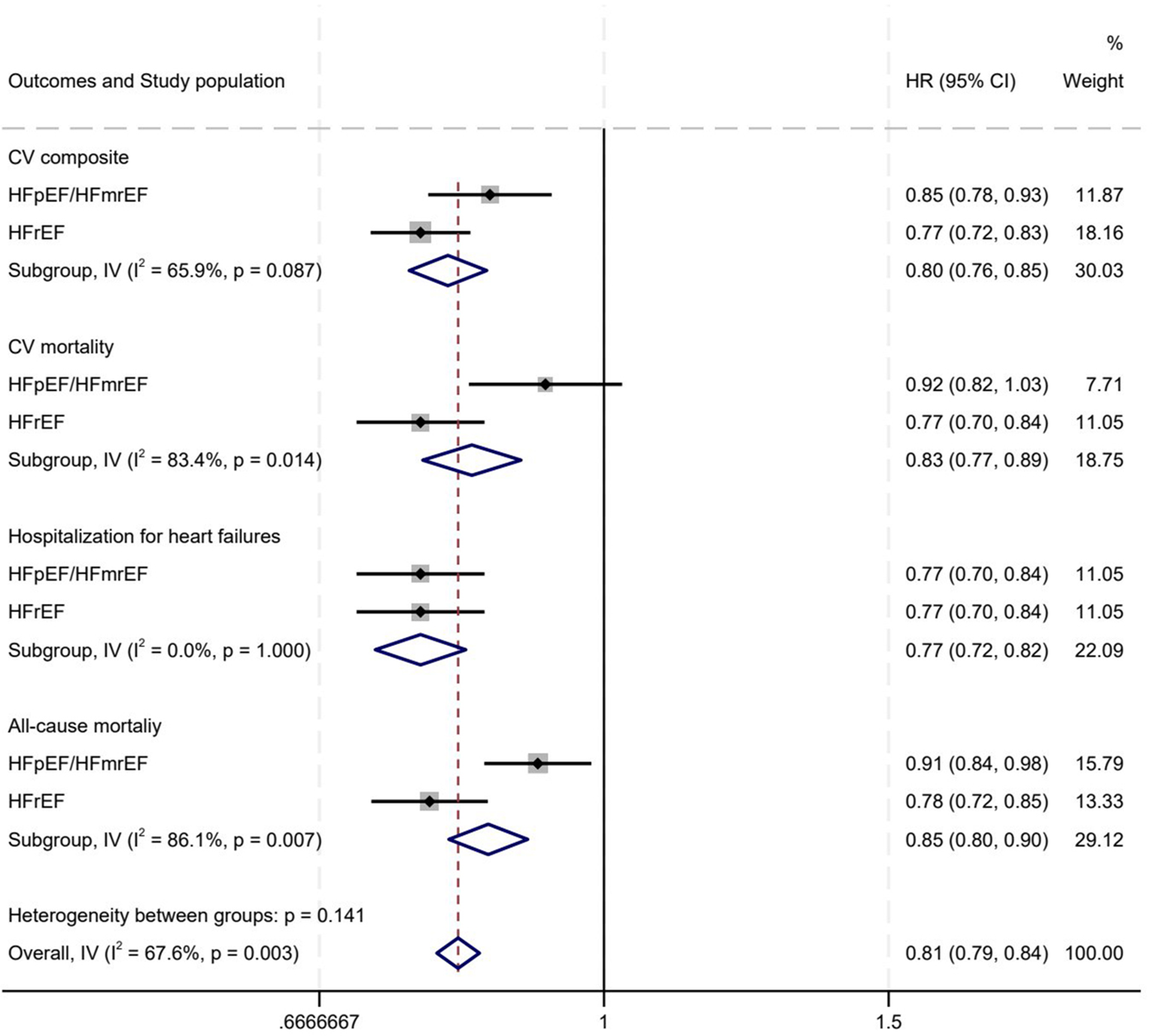
MRAs' differential impact on hFrEF vs. HFmrEF/HFpEF. (A) CV composite events; (B) CV death; (C) Hospitalization for heart failures; (D) All-cause mortality. HR, hazard ratio; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; IV, inverse variance; MRA, mineralocorticoid receptor antagonists.
Risk of bias and publication bias
The risk of bias assessment suggests that most trials included in the meta-analysis are at a low risk of bias across most domains, which enhances the credibility of the findings. However, the high risk of bias in TOPCAT study, particularly in allocation concealment and other bias, may affect the interpretation of its results and should be considered when assessing the overall evidence (Supplementary Figure S2). The forest plot analysis revealed a low likelihood of publication bias (Supplementary Figure S3–S5).
Discussion
Our analysis of MRA treatment in HFrEF patients corroborates findings from major studies such as RALES and EMPHASIS-HF, highlighting its substantial benefits in decreasing hospitalization rates and mortality across all EF spectum, with notable effects in HFrEF cases. This reinforces MRA's pivotal role as a therapeutic strategy in managing HF.
The mechanisms behind the varying efficacy of MRAs in HFpEF and HFrEF are multifaceted, involving both cardiac and extracardiac factors (21). HFpEF and HFrEF exhibit distinct pathophysiological mechanisms, which in turn influence the effectiveness of MRAs in these conditions (22, 23). While MRAs have shown clear efficacy in HFrEF, their role in HFpEF remains well-defined, largely clear due to the complex and heterogeneous nature of HFpEF (24). MRAs block the MR, curbing myocardial fibrosis and fluid retention. In HFrEF, characterized by heightened neurohormonal activity like renin-angiotensin-aldosterone system (RAAS) and sympathetic overdrive, MRAs are notably effective due to their impact on contractility and remodeling (25). HFpEF, often tied to conditions like obesity and diabetes, involves adipose tissue releasing inflammatory cytokines. This leads to coronary microvascular inflammation and diminished nitric oxide levels, causing myocardial stiffness and diastolic dysfunction. The complexity of HFpEF's pathophysiology means that MR blockade alone is insufficient to halt its progression effectively (26).
In HFrEF, ventricular function in HFrEF is characterized by impaired contraction, whereas in HFpEF, it is marked by impaired relaxation. This distinction is critical as it influences the efficacy of MRAs, which primarily target fluid retention and blood pressure regulation—mechanisms that are more relevant to systolic dysfunction observed in HFrEF (27). Furthermore, calcium handling differs between two conditions: HFrEF is associated with disrupted t-tubule structure and reduced Ca²+release, leading to impaired systolic function, whereas HFpEF maintains t-tubule structure but exhibits diastolic Ca²+ irregularities, resulting in increased myocardial stiffness. These differences may clarify why MRAs tend to be more effective in HFrEF (27). Additionally, systemic inflammation and comorbidities play a significant role in HFpEF, which is often associated with hypertension, diabetes, and obesity, which contribute to coronary microvascular dysfunction and myocardial hypertrophy—factors that are not directly targeted by MRAs. Such associations may explain the limited efficacy of MRAs in HFpEF compared to HFrEF (28). Vascular and metabolic factors significantly affect HFpEF by altering ventricular-vascular coupling and increasing arterial stiffness, primarily due to aging and comorbidities. Since these extracardiac factors are not the main targets of MRAs, the effectiveness of MRAs in treating HFpEF is further limited (29).
MRAs, including spironolactone and eplerenone, play a crucial role in managing CV diseases such as HF and hypertension. Despite therapeutic benefits, they are associated with side effects such as hyperkalemia, renal dysfunction, and hormonal disturbances, particularly with spironolactone. Addressing these adverse effects is vital for optimizing MRA therapy outcomes.
Hyperkalemia, a significant side effect of MRAs, poses a particular risk for patients with CKD or those concurrently using RAAS inhibitors (30). Subgroup analysis revealed the heterogeneity in hyperkalemia risk among spironolactone users correlates with study-specific dosages and patient demographics. As a first-generation, non-selective MRA, spironolactone leads to metabolic issues with varying impacts across age groups. Its main metabolite, canrenone, is a potent MR antagonist with a long half-life of 10–35 h, increasing the risk of hyperkalemia due to prolonged potassium retention. In the RALE study (1), targeting elderly patients (79 years old) with severe heart failure (NYHA Class IV), the highest spironolactone dosage (25–50 mg/day) was used, raising hyperkalemia risk. The TOPCAT study (17), focusing on HFpEF and hypertensive patients, used a moderate dosage (15–45 mg/day). The Aldo-DHF study (16), with a stable dosage (25 mg/day), involved patients with isolated HFpEF and preserved renal function.
Our analysis supports a tiered monitoring approach for hyperkalemia in HF patients, with a focus on initial screening and ongoing surveillance. Key groups for monitoring include those over 65, with estimated glomerular filtration rate (eGFR) < 45 ml/min, CKD, diabetes, or prior hyperkalemia. Initiate MRA therapy only if serum potassium is ≤5.0 mmol/L, with subsequent checks at 1–2 weeks, monthly, and annual eGFR and electrolyte assessments for CKD patients (31, 32). Adjust therapy based on potassium levels: continue MRA at 5.1–5.5 mmol/L with potassium supplement or NSAID discontinuation, potassium-restricted diet, and new binders; reduce MRA by 50% at 5.6–6.0 mmol/L with binders; cease MRA immediately if potassium exceeds 6.0 mmol/L, initiate urgent potassium-lowering measures, and restart at a lower dose once levels return to ≤5.0 mmol/L (33). Additionally, co-treatment with sodium-glucose cotransporter 2 (SGLT2) inhibitors may mitigate hyperkalemia risk due to their diuretic properties (30).
MRAs can trigger acute kidney function decline, notably in individuals with existing renal issues. It is essential to evaluate renal function before beginning MRA treatment and to perform ongoing monitoring. If significant impairment is detected, dosage adjustment or drug withdrawal may be warranted (34). Spironolactone, a steroidal MRA, may induce sex-related side effects including gynecomastia in men and menstrual irregularities in women, attributed to its anti-androgenic and progesterone-like activities (34). Switching to eplerenone, a more selective MRA with fewer hormonal side effects, can help mitigate these issues (34). Among patients with CKD, MRAs have demonstrated advantages in decreasing CV mortality and proteinuria; however, their application is frequently constrained due to the hyperkalemia risk (35). Developing non-steroidal MRAs holds promise for diminished side effects while preserving therapeutic efficacy, potentially broadening the clinical application of MRAs (34). Non-steroidal MRAs, distinct from steroidal MRAs that are progesterone derivatives, feature a unique chemical structure. This distinction endows non-steroidal MRAs with greater selectivity and affinity for the MR, minimizing off-target effects and enhancing safety (36). Furthermore, steroidal MRAs can lead to side effects like gynecomastia due to their impact on multiple nuclear receptors. Non-steroidal MRAs, with their higher selectivity, are less likely to cause such hormonal issues (37).
Finerenone, a non-steroidal MRA, exhibits a balanced effect on both the heart and kidneys, unlike spironolactone, which is more kidney-focused (38). This difference is complemented by finerenone's unique pharmacokinetic characteristics, including a shorter half-life and distinct metabolic pathways, which enhance its safety profile (39). Additionally, finerenone demonstrates significant anti-inflammatory and anti-fibrotic properties that are essential for cardiorenal health, with particular efficacy in mitigating renal inflammation and fibrosis more effectively than eplerenone (36). Compared to steroidal MRAs, non-steroidal MRAs like finerenone pose a lower risk of hyperkalemia, rendering them a safer option for patients with CKD and those concurrently using RAAS inhibitors (37). This advantage is further underscored by the effectiveness of non-steroidal MRAs in reducing cardiovascular and renal event rates among patients with CKD and type 2 diabetes. Notably, clinical trials have shown that finerenone significantly slows the progression of kidney disease and decreases cardiovascular events (39).
Furthermore, the potential of non-steroidal MRAs to provide synergistic benefits when used in combination with other therapies, such as SGLT2 inhibitors, is currently under investigation. Such combinations may further reduce the risk of hyperkalemia and strengthen cardiorenal protection, offering promising avenues for future treatment strategies (37, 40).
Non-steroidal MRAs present several advantages over steroidal MRAs, including enhanced safety and efficacy profiles. However, these agents are not without limitations. Although the risk of hyperkalemia is diminished, it remains a concern necessitating vigilant monitoring. Furthermore, the long-term effects and potential benefits of non-steroidal MRAs across a broader range of patient populations remain under investigation. As ongoing research unfolds, these agents may emerge as a pivotal component in the management of cardiorenal diseases, particularly for patients at elevated risk of adverse outcomes with conventional therapies.
Study strengths and limitations
This study marks the first comprehensive meta-analysis to evaluate the efficacy of MRAs in HF patients, spanning across HFrEF, HFmrEF, and HFpEF categories. Beyond the comparative analysis of MRAs within EF subgroups, we have also performed a direct “head-to-head” assessment of both the efficacy and safety profiles of steroidal vs. nonsteroidal MRAs concerning specific critical endpoints.
Several of our findings demonstrate notable heterogeneity. To ensure consistency, we conducted sensitivity and subgroup analyses on those results exhibiting substantial heterogeneity in order to identify its root causes. Among patients with HFrEF, the primary cardiovascular composite endpoint revealed significant heterogeneity. A sensitivity analysis that excluded the EPHESUS trial indicated no heterogeneity, thereby highlighting EPHESUS as a principal contributor. The EPHESUS trial's composite endpoint encompassed cardiovascular mortality or the first hospitalization for cardiovascular events, contrasting with EMPHASIS-HF, which included cardiovascular mortality or the first hospitalization for heart failure. The employment of spironolactone in the RALES trial suggests that differences in medication may also play a role.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YZ: Writing – original draft. Y-CB: Writing – original draft. L-XW: Data curation, Writing – original draft. H-JZ: Writing – original draft, Data curation. JS: Data curation, Writing – original draft. LS: Data curation, Writing – original draft. WD: Writing – original draft, Data curation. MD: Writing – original draft, Data curation. L-JW: Data curation, Writing – original draft. Q-YA: Supervision, Writing – review & editing. W-ZY: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely thank Professor Jiang Rong (Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine) for her invaluable guidance on this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1667236/full#supplementary-material
Supplementary Figure 1Subgroup analysis of hyperkalemia. MRA, mineralocorticoid receptor antagonists.
Supplementary Figure 2Risk of bias analysis for randomized control studies
Supplementary Figure 3Publication bias of HFpEF and HFmrEF study. ((A) CV composite events; (B) CV death; (C) Hospitalization for heart failures; (D) All-cause mortality. HFpEF, heart failure with preserved ejection fraction; HFmrEF: heart failure with mildly reduced ejection fraction.
Supplementary Figure 4Publication bias of HFpEF and HFmrEF study. ((A) CV composite events; (B) CV death; (C) Hospitalization for heart failures; (D) All-cause mortality. HFrEF: heart failure with reduced ejection fraction.
Supplementary Figure 5Publication bias of MRA safety. MRA, mineralocorticoid receptor antagonists. (A) Any serious adverse event; (B) Hyperkalemia; (C) Hypokalemia; (D) Hypotension; (E) Acute kidney injury.
References
1.
Pitt B Zannad F Remme WJ Cody R Castaigne A Perez A et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. (1999) 341:709–17. 10.1056/NEJM199909023411001
2.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Bohm M et al 2021 Esc guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the heart failure association (hfa) of the esc. Eur J Heart Fail. (2022) 24:4–131. 10.1002/ejhf.2333
3.
Solomon SD McMurray JJV Vaduganathan M Claggett B Jhund PS Desai AS et al Finerenone in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2024) 391(16):1475–85. 10.1056/NEJMoa2407107
4.
Jhund PS Talebi A Henderson AD Claggett BL Vaduganathan M Desai AS et al Mineralocorticoid receptor antagonists in heart failure: an individual patient level meta-analysis. Lancet. (2024) 404:1119–31. 10.1016/S0140-6736(24)01733-1
5.
Ferreira JP Pitt B Zannad F . Mineralocorticoid receptor antagonists in heart failure: an update. Circ Heart Fail. (2024) 17:e011629. 10.1161/CIRCHEARTFAILURE.124.011629
6.
Yang P Shen W Chen X Zhu D Xu X Wu T et al Comparative efficacy and safety of mineralocorticoid receptor antagonists in heart failure: a network meta-analysis of randomized controlled trials. Heart Fail Rev. (2019) 24:637–46. 10.1007/s10741-019-09790-5
7.
Pitt B . Heart failure: the role for mineralocorticoid receptor antagonists. Swiss Med Wkly. (2014) 144:w13959. 10.4414/smw.2014.13959
8.
Kitai T Kohsaka S Kato T Kato E Sato K Teramoto K et al JCS/JHFS 2025 guideline on diagnosis and treatment of heart failure. Circ J. (2025) 89:1278–444. 10.1253/circj.CJ-25-0002
9.
Khan MS Khan MS Moustafa A Anderson AS Mehta R Khan SS . Efficacy and safety of mineralocorticoid receptor antagonists in patients with heart failure and chronic kidney disease. Am J Cardiol. (2020) 125:643–50. 10.1016/j.amjcard.2019.11.014
10.
Frankenstein L Seide S Tager T Jensen K Frohlich H Clark AL et al Relative efficacy of spironolactone, eplerenone, and canrenone in patients with chronic heart failure (research): a systematic review and network meta-analysis of randomized controlled trials. Heart Fail Rev. (2020) 25:161–71. 10.1007/s10741-019-09832-y
11.
Matsumoto S Henderson AD Shen L Yang M Swedberg K Vaduganathan M et al Mineralocorticoid receptor antagonists in patients with heart failure and impaired renal function. J Am Coll Cardiol. (2024) 83:2426–36. 10.1016/j.jacc.2024.03.426
12.
Bozkurt B Coats AJS Tsutsui H Abdelhamid CM Adamopoulos S Albert N et al Universal definition and classification of heart failure: a report of the heart failure society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and writing committee of the universal definition of heart failure: endorsed by the Canadian Heart Failure Society, heart failure association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. (2021) 23:352–80. 10.1002/ejhf.2115
13.
Cumpston M Li T Page MJ Chandler J Welch VA Higgins JP et al Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. 10.1002/14651858.ED000142
14.
Pitt B Remme W Zannad F Neaton J Martinez F Roniker B et al Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. (2003) 348:1309–21. 10.1056/NEJMoa030207
15.
Zannad F McMurray JJ Krum H van Veldhuisen DJ Swedberg K Shi H et al Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. (2011) 364:11–21. 10.1056/NEJMoa1009492
16.
Edelmann F Wachter R Schmidt AG Kraigher-Krainer E Colantonio C Kamke W et al Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the aldo-dhf randomized controlled trial. JAMA. (2013) 309:781–91. 10.1001/jama.2013.905
17.
Pitt B Pfeffer MA Assmann SF Boineau R Anand IS Claggett B et al Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 370:1383–92. 10.1056/NEJMoa1313731
18.
Pitt B Filippatos G Agarwal R Anker SD Bakris GL Rossing P et al Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. (2021) 385:2252–63. 10.1056/NEJMoa2110956
19.
Bakris GL Agarwal R Anker SD Pitt B Ruilope LM Rossing P et al Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. (2020) 383:2219–29. 10.1056/NEJMoa2025845
20.
Pitt B Kober L Ponikowski P Gheorghiade M Filippatos G Krum H et al Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist bay 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. (2013) 34:2453–63. 10.1093/eurheartj/eht187
21.
Dunlay SM Roger VL Redfield MM . Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14:591–602. 10.1038/nrcardio.2017.65
22.
Dunlay SM Roger VL Killian JM Weston SA Schulte PJ Subramaniam AV et al Advanced heart failure epidemiology and outcomes: a population-based study. JACC Heart Fail. (2021) 9:722–32. 10.1016/j.jchf.2021.05.009
23.
Pfeffer MA Shah AM Borlaug BA . Heart failure with preserved ejection fraction in perspective. Circ Res. (2019) 124:1598–617. 10.1161/CIRCRESAHA.119.313572
24.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Bohm M et al Focused update of the 2021 esc guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2023) 2023(44):3627–39. 10.1093/eurheartj/ehad195
25.
Kourek C Briasoulis A Papamichail A Xanthopoulos A Tsougos E Farmakis D et al Beyond quadruple therapy and current therapeutic strategies in heart failure with reduced ejection fraction: medical therapies with potential to become part of the therapeutic armamentarium. Int J Mol Sci. (2024) 25:3113. 10.3390/ijms25063113
26.
Obokata M Reddy YNV Pislaru SV Melenovsky V Borlaug BA . Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. (2017) 136:6–19. 10.1161/CIRCULATIONAHA.116.026807
27.
Louch WE . A horse of a different colour: distinct mechanisms of hfpef and hfref. J Physiol. (2020) 598:5005–6. 10.1113/JP280691
28.
Solomon SD McMurray JJV Claggett B de Boer RA DeMets D Hernandez AF et al Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387:1089–98. 10.1056/NEJMoa2206286
29.
Ali D Callan N Ennis S Powell R McGuire S McGregor G et al Heart failure with preserved ejection fraction (hfpef) pathophysiology study (identify-hf): does increased arterial stiffness associate with hfpef, in addition to ageing and vascular effects of comorbidities? Rationale and design. BMJ Open. (2019) 9:e027984. 10.1136/bmjopen-2018-027984
30.
Huang B McDowell G Rao A Lip GYH . Mineralocorticoid receptor antagonist for chronic kidney disease, risk or benefit?J Hypertens. (2024) 42:396–8. 10.1097/HJH.0000000000003643
31.
Mancia G Kreutz R Brunstrom M Burnier M Grassi G Januszewicz A et al Esh guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the international society of hypertension (ish) and the European renal association (era). J Hypertens. (2023) 2023(41):1874–2071. 10.1097/HJH.0000000000003480
32.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Bohm M et al Esc guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 2021(42):3599–726. 10.1093/eurheartj/ehab368
33.
Ferreira JP Butler J Rossignol P Pitt B Anker SD Kosiborod M et al Abnormalities of potassium in heart failure: jacc state-of-the-art review. J Am Coll Cardiol. (2020) 75:2836–50. 10.1016/j.jacc.2020.04.021
34.
Lainscak M Pelliccia F Rosano G Vitale C Schiariti M Greco C et al Safety profile of mineralocorticoid receptor antagonists: spironolactone and eplerenone. Int J Cardiol. (2015) 200:25–9. 10.1016/j.ijcard.2015.05.127
35.
Baran W Krzeminska J Szlagor M Wronka M Mlynarska E Franczyk B et al Mineralocorticoid receptor antagonists-use in chronic kidney disease. Int J Mol Sci. (2021) 22:9995. 10.3390/ijms22189995
36.
Agarwal R Kolkhof P Bakris G Bauersachs J Haller H Wada T et al Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. (2021) 42:152–61. 10.1093/eurheartj/ehaa736
37.
Gregg LP Navaneethan SD . Steroidal or non-steroidal mras: should we still enable raasi use through k binders?Nephrol Dial Transplant. (2023) 38:1355–65. 10.1093/ndt/gfac284
38.
Kolkhof P Delbeck M Kretschmer A Steinke W Hartmann E Barfacker L et al Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. (2014) 64:69–78. 10.1097/FJC.0000000000000091
39.
Ashjian E Clarke M Pogue K . Pharmacotherapy considerations with finerenone in the treatment of chronic kidney disease associated with type 2 diabetes. Am J Health Syst Pharm. (2023) 80:1708–21. 10.1093/ajhp/zxad192
40.
He M Jiang R Fei S Cao JX Wang L Shi JY . Cardiac magnetic resonance imaging-derived septum swing index detects pulmonary hypertension: a diagnostic study. J Transl Int Med. (2023) 11:459–67. 10.2478/jtim-2023-0114
Summary
Keywords
mineralocorticoid receptor antagonists, heart failure and reduced ejection fraction, HF and mildly reduced ejection fraction, HF with preserved ejection fraction, hyperkalemia
Citation
Zhang Y, Bao Y-C, Wang L-X, Zhao H-J, Sun J, Sun L, Duan W, Du M, Wang L-J, An Q-Y and Yang W-Z (2025) Mineralocorticoid receptor antagonists in heart failure: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1667236. doi: 10.3389/fcvm.2025.1667236
Received
18 July 2025
Accepted
13 August 2025
Published
01 September 2025
Volume
12 - 2025
Edited by
Serafino Fazio, Federico II University Hospital, Italy
Reviewed by
Christos Ioannis Fragoulis, National and Kapodistrian University of Athens, Greece
Christopher El Mouhayyar, Massachusetts General Hospital and Harvard Medical School, United States
Updates
Copyright
© 2025 Zhang, Bao, Wang, Zhao, Sun, Sun, Duan, Du, Wang, An and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Wen-Ze Yang 13993749703@163.com Qin-Yan An anqinyan2008@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.