Abstract
Background:
Previous studies on aortic valve disease have mainly focused on the left ventricle, but increasing evidence suggests that left atrial strain also has prognostic value in significant aortic valve disease.
Objective:
To systematically evaluate the prognostic value of left atrial strain in significant aortic valve disease.
Methods:
Multiple electronic databases were searched for studies evaluating significant aortic stenosis (AS) or aortic regurgitation (AR) using peak left atrial longitudinal strain (PALS) from the inception of each database to 1 February 2025. There were no language or regional restrictions. The primary endpoint was a composite outcome comprising all-cause mortality, hospitalization for heart failure, aortic valve replacement, pulmonary hypertension, and postoperative new-onset atrial fibrillation.
Results:
A total of 25 studies were included, involving 7,195 patients, with 2,039 (28%) patients experiencing primary endpoint events. The PALS was lower in the positive group (EVENT+) compared to the negative group (EVENT−) (SMD = −1.03, 95% CI [−1.22, −0.84], p < 0.05). For each unit increase in PALS, the risk of the primary endpoint event decreased by 7% (HR = 0.93, 95% CI [0.91, 0.96], p < 0.001). PALS exhibited consistent incremental predictive value in both the AR and AS cohorts, although the strength of its effect and the underlying mechanisms varied between groups.
Conclusion:
PALS is an independent predictor of adverse cardiovascular events in patients with significant aortic valve disease. PALS has certain value in the prognosis of significant aortic valve disease.
Systematic Review Registration:
[www.crd.york.ac.uk/prospero/], identifier [CRD 42024623883].
Introduction
According to multiple studies, aortic valve disease accounts for 61% of all deaths from valvular heart disease. Aortic valve disease includes conditions such as aortic stenosis (AS) and aortic regurgitation (AR), which are closely associated with aging and chronic cardiovascular disease. Among these, aortic stenosis (AS) is the most prevalent valvular heart disease in developed countries, currently affecting approximately 9 million individuals worldwide, and its incidence continues to rise in parallel with population aging and the growing burden of atherosclerosis. In 2019, AS caused approximately 127,000 deaths worldwide, with related losses amounting to 1.8 million disability-adjusted life years. AR is associated with diastolic rather than systolic hypertension, and its incidence has also increased in developed countries (1). Previous studies on significant valvular heart disease have primarily focused on the left ventricle (LV), with relatively little research conducted on the left atrium (LA). However, LA is a bridge connecting the systemic circulation and pulmonary circulation, closely related to cardiopulmonary function and affecting the patient's symptoms. Moreover, in recent years, an increasing number of studies have suggested that left atrial strain (LAS), particularly peak atrial longitudinal strain (PALS), has important prognostic value in significant aortic valve disease (2). However, studies on the prognostic value of PALS in aortic valve disease, particularly in AR, are mostly single-center, with small sample sizes and controversial results. Currently, there is a lack of meta-analyses on the prognostic value of PALS in significant aortic valve disease. Therefore, this study aims to explore the prognostic value of PALS in significant aortic valve disease through systematic evaluation and meta-analysis.
Methods
Search strategy and study inclusion
This study was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD 42024623883).
A systematic search in PubMed, Cochrane, Web of Science, Embase, Ovid, and CNKI databases was performed between their inception to 1 February 2025. Key words including: atrial function, atrial deformation, atrial longitudinal strain, aortic stenosis, aorta insufficiency, aorta regurgitation and aortic incompetence. Additionally, the reference lists of all included articles were hand-searched to identify additional eligible studies, and no language restrictions were imposed during the entire search process. The specific search strategies for each database are detailed in Supplementary Table S1. Two investigators independently performed the two-stage screening process: first, titles and abstracts were screened, and then full-text articles were reviewed for eligibility (NC and JW). Disagreements were resolved by a third author (WHG) to reach a consensus.
Inclusion criteria: (1) The study included patients aged ≥18 years diagnosed with moderate-to-severe or severe AS or AR by echocardiography. (2) PALS was measured using speckle tracking echocardiography (STE). (3) Prospective or retrospective studies in which the composite endpoint was defined as all-cause mortality, hospitalization for heart disease, aortic valve replacement, pulmonary hypertension, and postoperative new-onset atrial fibrillation (AF).
Exclusion criteria: (1) Studies that employed cardiac magnetic resonance imaging for PALS quantification were excluded to avoid heterogeneity arising from methodological disparities. (2) Case reports, reviews, letters, and editorials were also excluded. (3) In instances where overlapping datasets yielded multiple publications, only the most recent or methodologically complete report was retained.
Data extraction
The two authors (NC and JW) independently extracted the data and summarized them in a data extraction file. Any discrepancies were resolved through consensus or consultation with the third author (WHG). For missing data in eligible studies, researchers attempted to obtain it by contacting the original article authors via email. The quality of the selected trials was evaluated using the Cochrane risk of bias assessment tool from seven aspects.
Statistical analysis
The data of continuous variables were pooled to calculate the standardized mean difference (SMD) and 95% confidence interval (CI), while the data of binary variables were pooled to calculate the hazard ratio (HR) and 95% CI, to assess their prognostic value for the primary endpoint. The I2 statistic was used to evaluate statistical heterogeneity among studies. If significant heterogeneity was detected (e.g., I2 > 50%), a random—effects model was used to construct a forest plot to display the overall effect. A subgroup analysis was conducted to identify the effect of PALS on the prognosis of patients with severe AS and severe AR. Incremental prognostic value of PALS beyond traditional risk models was assessed using incremental discrimination analysis (C-statistic improvement) and the NRI. Sensitivity analyses were performed to exclude the effects of small-sample studies and different subgroup conditions on the overall pooled estimates. Funnel plots were used to assess publication bias. Statistical analysis was performed using Review Manager 5.3 (Cochrane Collaboration, Oxford), and a p-value <0.05 was considered statistically significant.
Results
Study inclusion
A total of 2,890 records were retrieved according to the search strategy, and 2,534 trials remained after excluding duplicates. Thirty-nine trials remained after initial screening of titles and abstracts. Seven articles that did not report information such as HR values, 5 articles that lacked reporting of PALS, and 2 non-positive and negative event-controlled studies were excluded after detailed reading of the full text. This resulted in the inclusion of 25 studies with a total of 7,195 patients (2–26). The screening process and results are shown in Figure 1.
Figure 1
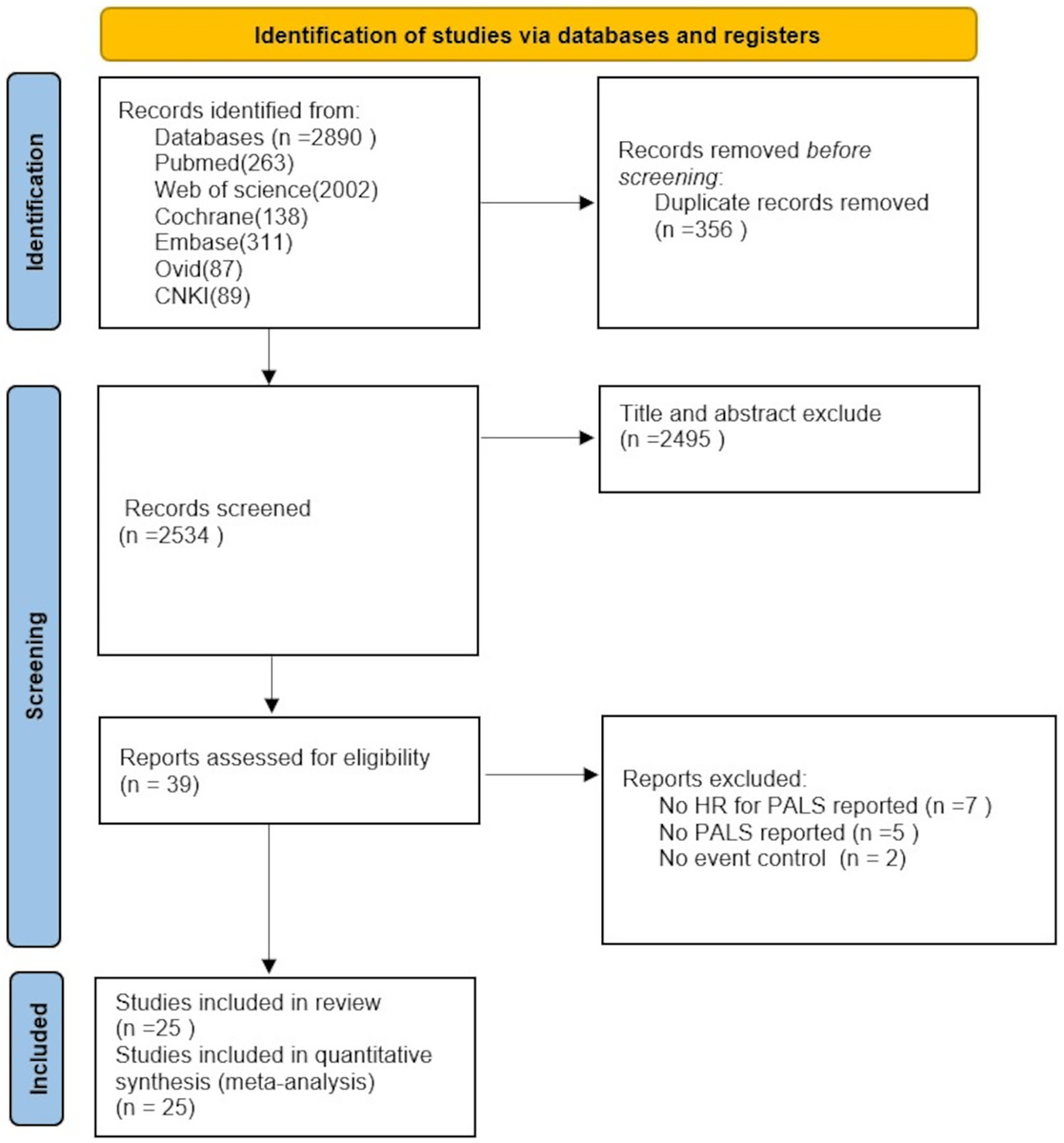
Flowchart of PRISMA for literature screening.
Characteristics of the study population
Table 1 summarizes the characteristics of the 25 studies included in the systematic evaluation. All studies were published between 2014 and 2025. Among them, 11 studies were single-center or multicenter prospective cohort studies. Regarding the patient population, 4 studies reported only AR, 20 studies reported only AS, and 1 study reported both AS and AR. The mean age range of patients was 49–82 years, and 56% were male. Of the 11 studies reporting comorbidities, the most common diseases were hypertension (68.1%) and diabetes (27.5%). Among the reported studies, mean baseline PALS ranged from 12.4 ± 6.9% to 39.4 ± 17.4%. The composite endpoints of all-cause death, cardiac hospitalization, postoperative new-onset AF, and pulmonary hypertension were seen in 2013 (27.5%) patients during follow-up from 0.1 to 57 months.
Table 1
| Publication | Design | Sample size | Group | Age (years) | Male (%) | PALS (%) | Primary endpoint | Follow-up, month | Events, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pessoa-Amorim et al. (13) | Prosp | 114 | AS | 74 ± 8.6 | 76 (67) | 25.5 ± 10.9 | New onset of AF | 0.10 (0.03–0.13) | 36 (32) |
| Anastasius et al. (17) | Prosp | 109 | AS | 81 ± 7.3 | 53 (49) | 18 ± 14 | HF hospitalization, ACD | 12 | 8 (7.3) |
| Benfari et al. (25) | Prosp | 67 | AS | 72 ± 8 | 23 (34) | 23 ± 7 | ACD, HF hospitalization, ischemic disease or AF onset | 26.30 ± 8.20 | 8 (12) |
| Butcher et al. (2) | Retro | 601 | AS | 81 (76–85) | 318 (53) | NR | ACD | 40 (26–58) | 258 (43) |
| Cameli et al. (24) | Prosp | 76 | AS | 66.5 ± 12.1 | 42 (69) | 33.6 ± 9.5 | POAF | 0.06 (0.03–0.10) | 15 (19.7) |
| 71.5 ± 10.1 | 10 (67) | 22.5 ± 7.1 | |||||||
| Parasca et al. (15) | Prosp | 132 | AS | 76.6 ± 7.5 | 56 (42) | 12.4 ± 6.9 | ACD and rehospitalization | 29.60 (4.50–36.50) | 38 (28.7) |
| Galli et al. (23) | Retro | 128 | AS | 78.9 ± 9.1 | 73 (57) | 18.4 ± 7.9 | HF, cardiac-related hospitalizations, ACD | 14 (9–20) | 38 (30) |
| Imanishi et al. (21) | Retro | 40 | AS | 73 ± 9 | 13 (65) | 18.7 ± 3.0 | HF Symptoms | 0 | 20 (50) |
| 78 ± 6 | 5 (25) | 13.0 ± 2.3 | |||||||
| Jenner et al. (20) | Prosp | 85 | AR | 54 (46–63) | 56 (86) | 26.3 ± 6.7 | Diastolic dysfunction grade ≥ 2, LVEF < 50%, or LVEDVI above the gender-specific normal range | 12 | 27 (41.5) |
| Controls | 59 (49–68) | 11 (55) | 25.7 ± 6.0 | 0 | |||||
| Lai et al. (19) | Retro | 352 | AR | 59 ± 17 | 284 (80) | 39.4 ± 17.4 | ACD | 56.40 (21.60–108) | 68 (19.3) |
| Lee et al. (18) | Retro | 712 | AS | 78 ± 12 | 337 (47) | 24.4 ± 13.4 | ACD, MACE | 18 (12.36–26.50) | 93 (13) |
| Martín et al. (22) | Retro | 126 | AR | 70.1 ± 17.2 | 75 (59.5) | 34.0 ± 12.7 | HF hospitalization, cardiovascular mortality, AVR | 34.10 (16.50–48.10) | 25 (19.8) |
| Meimoun et al. (16) | Prosp | 117 | AS | 77 ± 10 | 51 (50) | 20 ± 8 | HF hospitalization, ACD | 25 | 53 (52) |
| Controls | 74 ± 6 | 8 (53) | 32 ± 3 | 0 | |||||
| Salas-Pacheco et al. (11) | Cross-sectional | 72 | AS&AR | 55.1 ± 17.6 | 41 (56.9) | 26.6 (17.7–35.3) | Pulmonary hypertension | NR | 34 (47) |
| Pernigo et al. (14) | Prosp | 60 | AS | 69.3 ± 8.1 | 50 | 14.8 ± 2.8 | Postoperative AF | 0.17 | 26 (43.3) |
| 73.6 ± 7.6 | 50 | 14.6 ± 3.4 | |||||||
| Van Roeder et al. (5) | Retro | 606 | AS | 80 (77–84) | 282 (606) | 13.0 (8.4–18.3) | ACD, HF hospitalization | 12 | 94 (15.5) |
| Sabatino et al. (12) | Retro | 100 | AS | 80.7 ± 5.3 | 36 (55) | NR | Cardiovascular mortality, HF hospitalization | 31 | 35 (35) |
| 82 ± 5.4 | 16 (46) | NR | |||||||
| Sonaglioni et al. (10) | Retro | 186 | AS | 71.9 ± 12.7 | 115 (61.8) | 24.9 ± 8.3 | AVR, CV hospitalization, ACD | 27.60 ± 22.80 | 63 (34) |
| Springhetti et al. (9) | Retro | 467 | AS | 80.6 ± 8.2 | 237 (50.7) | 20.0 ± 9.3 | ACD, HF hospitalization | 19.20 (12.50–24.40) | 96 (21) |
| Stolz et al. (8) | Retro | 1,888 | AS | 81.0 ± 7.8 | 1,052 (56) | 16.5 ± 9.4 | ACD | 36 | 557 (29.5) |
| Tan et al. (26) | Prosp | 173 | AS | 69 ± 11 | 95 (54.9) | 27.2 (22.3–32.2) | ACD, HF hospitalization, NYHA ≥ III, ACS, syncope | 32.40 (16.80–55.20) | 66 (38) |
| Tan et al. (7) | Prosp | 220 | AR | 49 (36–56) | 175 (79.5) | 27.05 (22.40–30.84) | ACD, HF hospitalization, AVR | 12 (24.50–62.30) | 46 (20.9) |
| Thellier et al. (6) | Retro | 387 | AS | 76 (75–77) | 181 (47) | 24 (17–33) | ACD | 57 (37–83) | 158 (41) |
| Weber et al. (4) | Retro | 150 | AS | 82 ± 8 | 63 (42) | 21.96 ± 9.4 | New onset of AF, HF hospitalization, ACD | 5.7 (0.70–24.20) | 37 (25) |
| Wedin et al. (3) | Prosp | 227 | AS | 65.3 ± 9.2 | 87 (65.4) | 22.2 ± 10.8 | POAF, TIA or stroke | 44.7 | POAF64 (48.1); TIA/stroke21(16) |
| 71.2 ± 7.1 | 53 (56.4) | 28.1 ± 9.4 | 52 | POAF50(53.1); TIA/stroke5(5) |
Study characteristics.
ACD, all cause death; AF, atrial fibrillation; AVR, aortic valve replacement; HF, heart failure; LVEF, left ventricular ejection fraction; LVEDVI, left ventricle end-diastolic volume index; MACE, major adverse cardiovascular events; NR, not reported; NYHA, New York Heart Association; PALS, peak atrial longitudinal strain; POAF, postoperative atrial fibrillation; TIA, transient ischemic attack.
Relationship between PALS and events
Thirteen studies reporting on PALS were included, involving a total of 2,622 patients, with 1,052 cases in the positive group (EVENT+) and 1,570 cases in the negative group (EVENT−). Heterogeneity analysis revealed that the included studies lacked good homogeneity (p < 0.05, I2 = 74%), so a random-effects model was used for analysis. The results showed that the difference in PALS between the positive group (EVENT+) and the negative group (EVENT−) reached statistical significance (SMD = −1.03, 95% CI [−1.22, −0.84], p < 0.05), indicating that there was a significant difference in PALS between the positive group (EVENT+) and the negative group (EVENT−) (Figure 2).
Figure 2
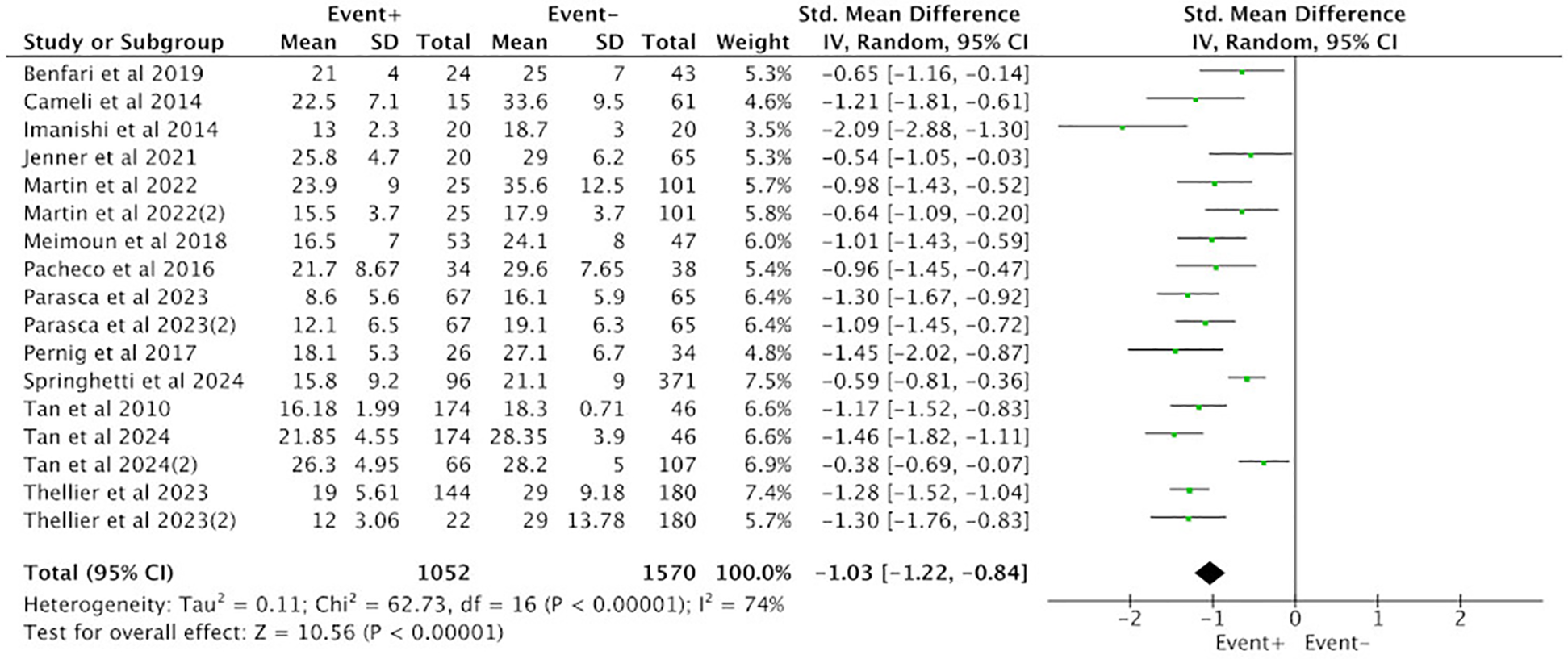
Difference in PALS between the positive group (EVENT+) and the negative group (EVENT−).
Subgroup analysis was conducted between the positive group (EVENT+) and the negative group (EVENT−) based on the type of aortic valve lesions, and the results were consistent with the overall findings. Among the 10 studies reporting PALS in the AS group, a total of 1,892 cases were included, with 614 cases in the positive group (EVENT+) and 1,278 cases in the negative group (EVENT−). Heterogeneity analysis revealed poor homogeneity among the included studies (p < 0.05, I2 = 79%), so a random-effects model was used for analysis. The results showed that the difference in PALS between the positive group (EVENT+) and the negative group (EVENT−) reached statistical significance (SMD = −1.20, 95% CI [−1.46, −0.95], p < 0.05), indicating a significant difference in PALS between the positive group (EVENT+) and the negative group (EVENT−) in the AS subgroup (Figure 3).
Figure 3
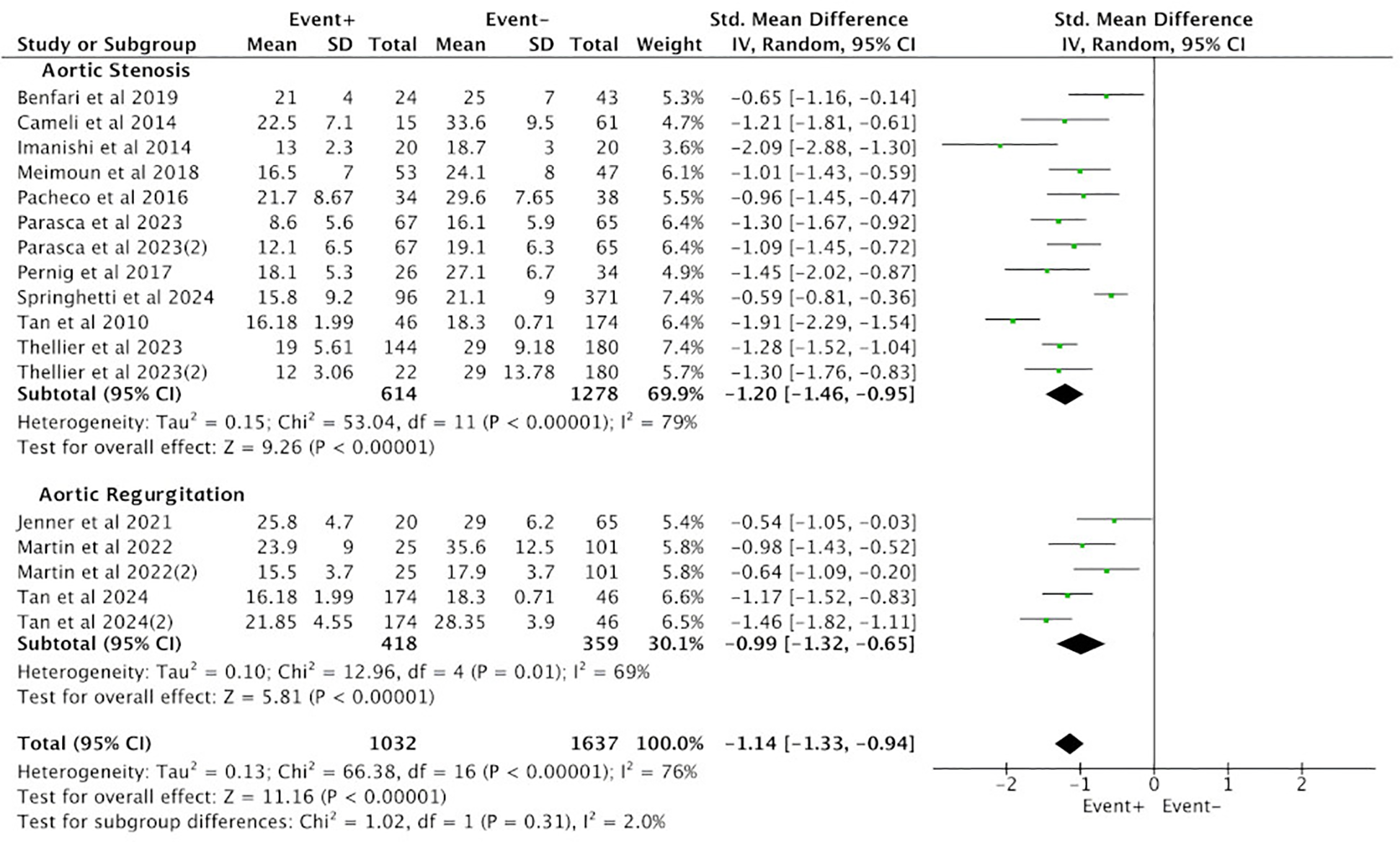
Subgroup analysis of difference in PALS between the positive group (EVENT+) and the negative group (EVENT−).
Three studies in the AR group reported PALS, involving a total of 777 cases, with 418 cases in the positive group (EVENT+) and 359 cases in the negative group (EVENT−). Heterogeneity analysis revealed poor homogeneity among the included studies (p < 0.05, I2 = 69%), so a random-effects model was used for analysis. The results showed that the difference in PALS between the positive group (EVENT+) and the negative group (EVENT−) reached statistical significance (SMD = −0.99, 95% CI [−1.32, −0.65], p < 0.05), indicating a significant difference in PALS between the positive group (EVENT+) and the negative group (EVENT−) in the AR subgroup (Figure 3).
Meta-analysis of PALS on composite endpoint
A total of 17 studies reporting PALS were included in the analysis. Heterogeneity analysis revealed a lack of good homogeneity among the included studies (p < 0.05, I2 = 77%), so a random-effects model was used for analysis. The results indicated that, among the 17 studies reporting PALS, high PALS was associated with a statistically significant increased HR for the endpoint event compared with low PALS. Figure 4 illustrates the relationship between PALS and the incidence of endpoint events in a multivariate Cox regression model. Specifically, based on the adjusted HR from all 17 studies, each unit increase in PALS was associated with a 7% reduction in the risk of the primary endpoint event (HR = 0.93, 95% CI [0.91, 0.96], p < 0.001).
Figure 4
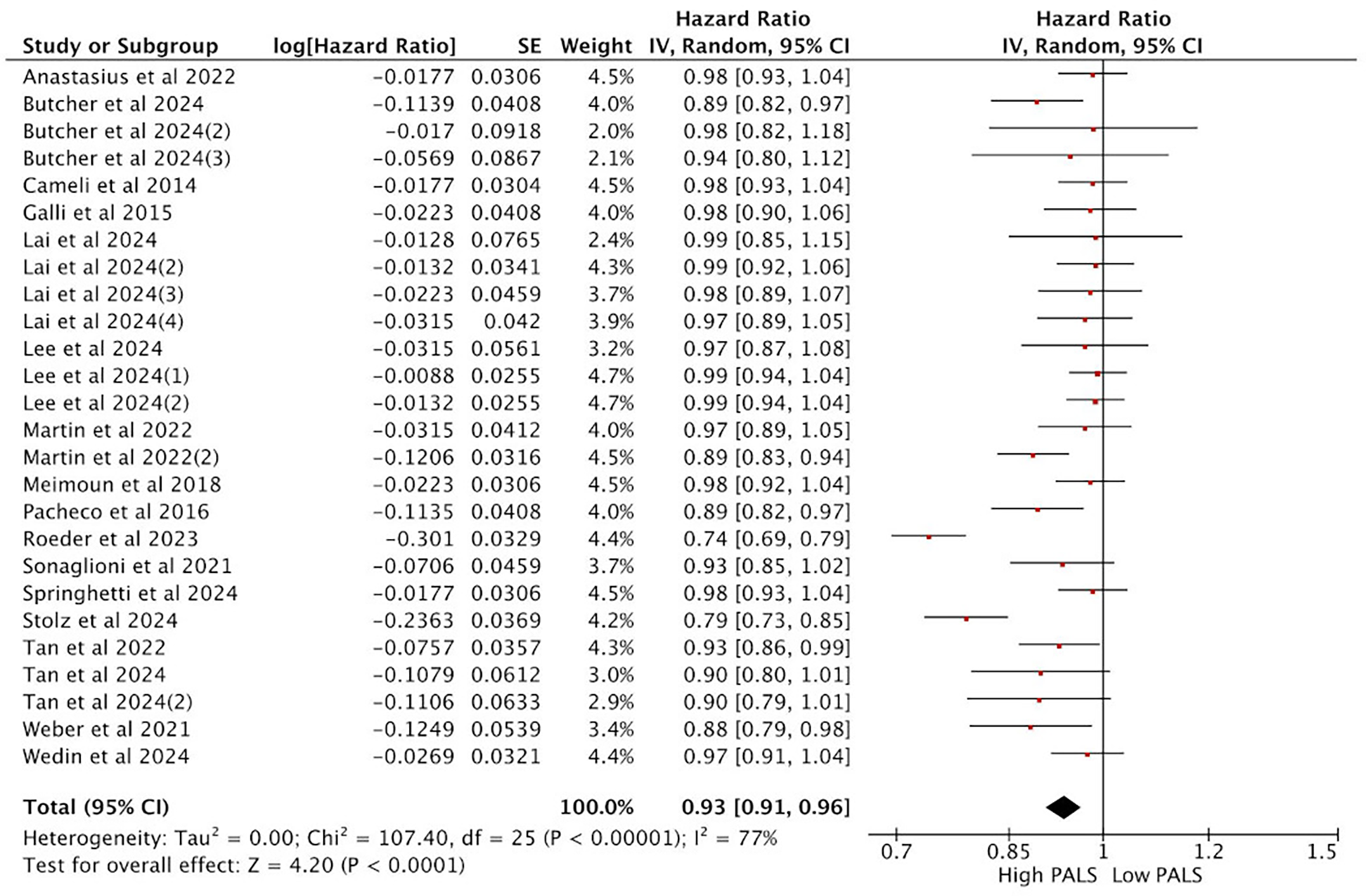
Meta-analysis of the relationship between PALS and composite endpoint.
Subgroup analysis of the relationship between PALS and composite endpoint
In subgroup analyses based on aortic valve lesion types, the pooled HR from the multi-variable Cox regression model was consistent with the results of the overall analysis. Among the 14 studies reporting PALS in AS, the pooled HR for predicting endpoint events was statistically significant between high PALS and low PALS. In the AS subgroup, each unit increase in PALS was associated with a 7% reduction in the risk of the primary endpoint (HR = 0.93, 95% CI [0.89, 0.97], p < 0.001) (Figure 5). In AR, 3 studies reported PALS, and the combined HR for high PALS compared with low PALS was statistically significant for predicting endpoint events. In the AR subgroup, each unit increase in PALS was associated with a 5% reduction in the risk of the primary endpoint (HR = 0.95, 95% CI [0.91, 0.98], p < 0.001) (Figure 5).
Figure 5
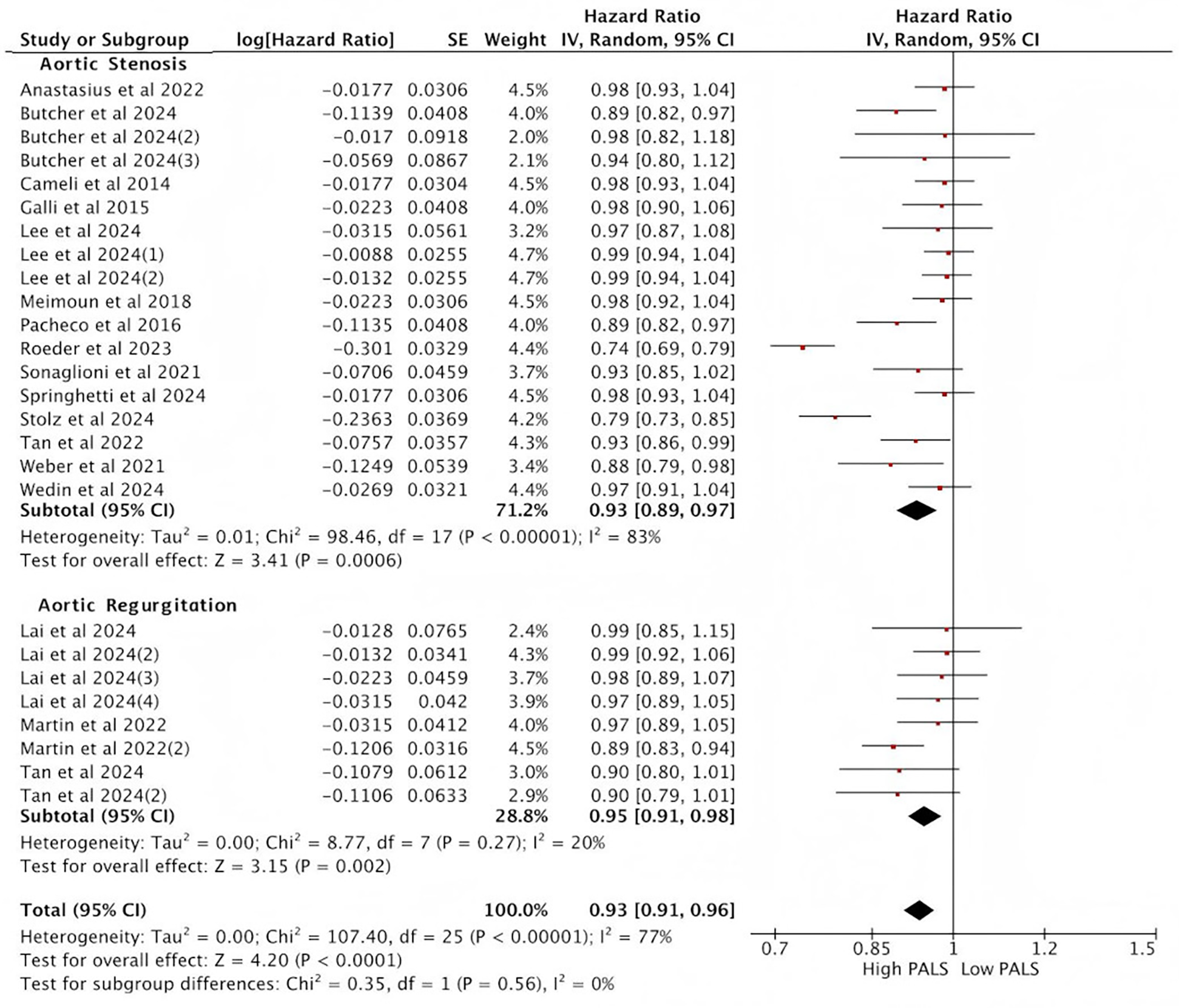
Subgroup analysis of the relationship between PALS and composite endpoint.
The addition of PALS to baseline risk models
The addition of PALS to baseline risk models contributed to the increase of the C-statistic or the NRI (Table 2). Two studies consistently demonstrated that adding PALS variables to the baseline model significantly improved risk prediction for patients with AR. Specifically, the C-statistic increased from 0.78 to 0.80 and from 0.81 to 0.82, respectively, indicating that PALS enhances the ability to identify high-risk AR patients. In patients with AS, three studies reached similar conclusions. After incorporating PALS into the baseline model, the C-statistic rose significantly from 0.73 to 0.82 and from 0.77 to 0.79, respectively. A large-scale study (n = 923) further quantified the reclassification benefit using the NRI, yielding an NRI of 0.224 (95% CI: 0.07–0.38). This indicates that PALS correctly reclassified 22.4% of patients into more appropriate risk categories, with the confidence interval excluding zero, confirming both statistical significance and clinical relevance.
Table 2
| Study | Subgroup | N | Baseline model | Baseline C-statistics | Baseline + PALS C-statistics | NRI (95% CI) |
|---|---|---|---|---|---|---|
| Jenner et al. (20) | AR | 80 | Age, Gender, LVEDVi, LVESVi, LVEF, LVGLS, Stroke work, DD grade, E/e′ | 0.78 | 0.80 | NR |
| Lai et al. (19) | AR | 352 | Age, CCI, Gender, LVGLS | 0.81 | 0.82 | NR |
| Benfari et al. (25) | AS | 67 | Age, Gender, BSA, HT, DM | 0.73 | 0.82 | NR |
| Thellier et al. (6) | AS | 387 | Age, Gender, BSA, BMI, SBP, DBP, HT, DM, CAD, CCI | 0.77 | 0.79 | NR |
| Lee et al. (18) | AS | 923 | Age, Sex, HT, AF, history of HF, IHD, COPD, hemoglobin, AVR as a time-dependent covariate | NR | NR | 0.224 (0.07–0.38) |
The addition of PALS to baseline risk models.
AF, atrial fibrillation; AVR, aortic valve replacement; BMI, body mass index; BSA, body surface area; CAD, coronary artery disease; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DBP, diastolic blood pressure; DD, diastolic dysfunction; HF, heart failure; HT, hypertension; IHD, ischemic heart disease; LVEDVi, left ventricular end diastolic volume index; LVESVi, left ventricular end-systolic volume index; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; NRI, net reclassification index; NR, not reported; SBP, systolic blood pressure.
In summary, PALS delivers robust incremental prognostic value in both AR and AS cohorts, yet its magnitude and underlying mechanisms differ between the two groups. In AR, the gain conferred by PALS is consistent but modest, likely because the baseline model already incorporates sensitive parameters such as LVGLS. Conversely, in AS, PALS contributes more markedly, especially when the baseline model's discriminative performance is suboptimal. Furthermore, the NRI analysis substantiates PALS's pivotal role in refining individualized risk stratification for AS patients. Therefore, PALS is indispensable in aortic valve disease: it reliably enhances risk discrimination for AR, while for AS it not only significantly improves model discrimination but—via a validated NRI—also demonstrates powerful reclassification capacity, underscoring its necessity to overcome the limitations of traditional risk models.
Sensitivity analysis
Given that studies with small sample sizes tend to have lower reliability, this study excluded studies with single-group sample sizes less than 25 and reanalyzed the data. The results showed that the difference in PALS between the positive group (EVENT+) and the negative group (EVENT−) reached statistical significance (SMD = −1.14, 95% CI [−1.39, −0.89], p < 0.05), indicating that the PALS scores between the positive group (EVENT+) and the negative group (EVENT−) remained significantly different (Figure 6A). Additionally, the combined HR for predicting endpoint events between high PALS and low PALS was statistically significant. Specifically, for each unit increase in PALS, the risk of the primary endpoint event decreased by 7% (HR = 0.92, 95% CI [0.87, 0.96], p < 0.001) (Figure 6B). Therefore, the results of this study passed the sensitivity analysis.
Figure 6
![Forest plots depicting meta-analysis results. Graph A shows standardized mean differences for eight studies, with a pooled effect size of -1.14 [−1.39, −0.89]. Graph B presents hazard ratios for fourteen studies, with a pooled value of 0.92 [0.87, 0.96]. Both include heterogeneity statistics and confidence intervals.](https://www.frontiersin.org/files/Articles/1667871/xml-images/fcvm-12-1667871-g006.webp)
Sensitivity analysis results (A) and (B).
Meta-regression analysis
In this study, meta-regression analysis was further employed to identify and quantify the sources of heterogeneity. By constructing statistical models, we explored whether the differences in effect sizes among studies were driven by specific covariates. The selection of indicators primarily focused on three core dimensions of heterogeneity: methodological heterogeneity, population characteristic heterogeneity, and clinical intervention heterogeneity. Indicators such as PALS cutoff value, age, hypertension, LVEF, and endpoint events (death) were selected respectively. As shown in the results of the meta-regression analysis in Table 3, the difference in the definition of the mortality endpoint is a source of heterogeneity that has statistical significance for the effect size of all-cause death, while indicators such as PALS cutoff value, age, and hypertension do not have statistical significance. Based on the meta-regression results, it can be preliminarily concluded that the inconsistency in the definition and criteria for endpoint events among studies is one of the causes of heterogeneity. Additionally, the failure to identify significant effects of other factors may be related to the limited number of studies included and insufficient statistical power.
Table 3
| Sources | Coefficient | SE | t | p value |
|---|---|---|---|---|
| AGE | 0.02 | 0.01 | 1.25 | 0.21 |
| PALS-Cutoff | −0.19 | 0.48 | −0.40 | 0.69 |
| Death | −0.66 | 0.37 | −1.77 | 0.08 |
| Hypertension | −0.01 | 0.01 | −1.20 | 0.23 |
| LVEF | −0.02 | 0.02 | −0.80 | 0.43 |
| Constant | 0.10 | 1.76 | 0.05 | 0.96 |
Results of meta-regression analysis.
Quality assessment
The Cochrane risk of bias assessment tool evaluated the quality of the included articles and found that most types of bias were low risk (green) in the trials, indicating that most studies were of good methodological quality, but there were some differences in certain aspects such as selection bias. All studies clearly defined their study subjects, patient groups, and outcomes (Figure 7, Supplementary Figure 1).
Figure 7
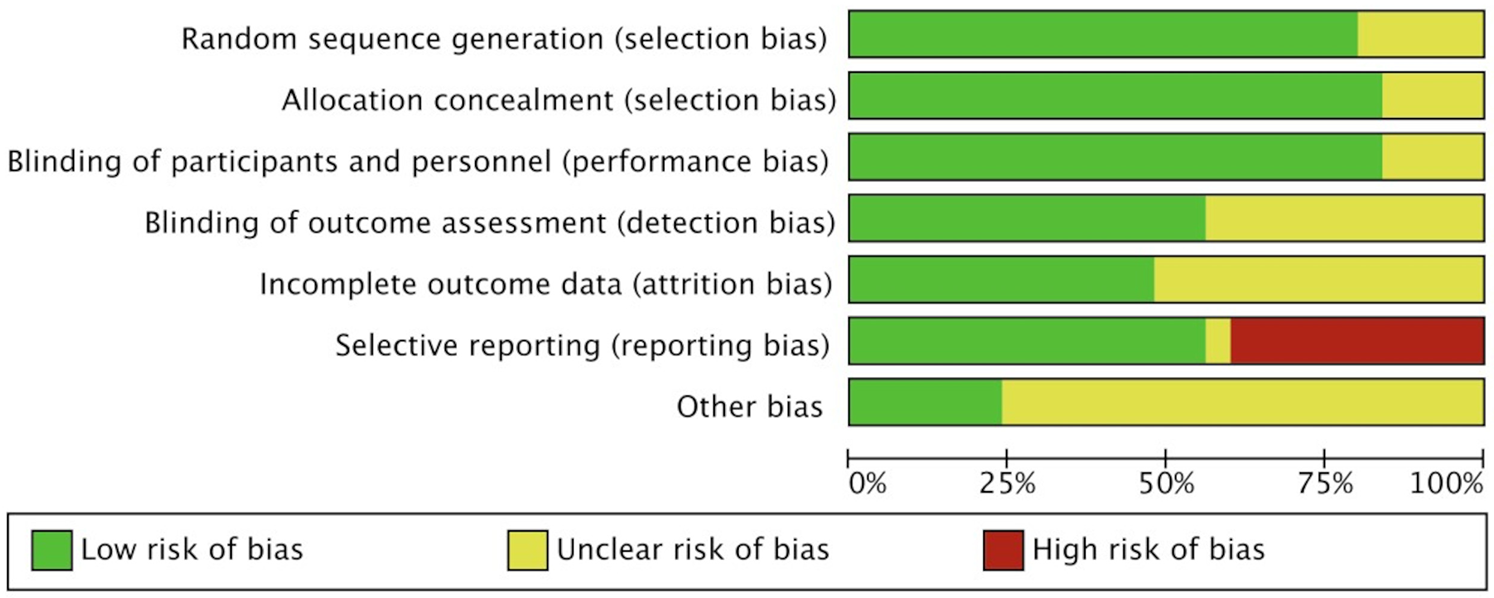
Cochrane risk of bias assessment tool for quality evaluation of included studies.
Despite the comprehensive assessment of the prognostic value of PALS in severe aortic valve disease through systematic review and meta-analysis in this study, there are still certain risks of bias and limitations in generalizability. According to the Cochrane Risk of Bias Assessment Tool (Figure 7), some studies had unclear or high risk of selection bias, mainly reflected in the incomplete reporting of patient inclusion criteria and baseline characteristics. Moreover, the majority of the included studies were single-center (20/25) and retrospective in design (15/25), which may introduce selection bias. In terms of geographical distribution, 21 studies were from European populations, with fewer studies from Asia and North America, limiting the generalizability of the results on a global scale. Table 4 summarizes the main limitations of this study and their potential impacts.
Table 4
| Type of limitation | Specific manifestations | Potential impacts |
|---|---|---|
| Retrospective design | 15 studies were retrospective cohort studies. | Selection bias may be introduced, affecting causal inference. |
| Single-center study | 20 studies had a single-center design. | The representativeness of the sample and the external validity are limited. |
| Geographical bias | 21 studies were based on European populations, with fewer studies from Asia and North America. | The findings may not be applicable to other ethnic groups and healthcare settings. |
| Selection bias | Patient enrollment process or baseline matching was not clearly reported in 12 studies. | The comparability between groups and the reliability of the results may be affected. |
| Variability in PALS measurement | Different ultrasound devices and software were used to measure PALS in the included studies. | Measurement heterogeneity |
Summary of limitations of the included studies.
Risk of bias
According to the funnel plots (Supplementary Figures S2–S5), the results of all analyses show that the variability of study effects increases with the increase in standard error, which is consistent with the greater variability and wider confidence intervals typically observed in studies with smaller sample sizes. Furthermore, all funnel plots exhibit a symmetrical distribution, suggesting that the likelihood of substantial bias in this study is low. Therefore, it is considered that the likelihood of reporting bias in this study is low.
Quality in prognostic studies (QUIPS)
To more systematically and specifically assess the methodological quality of the observational prognostic studies included in this analysis, QUIPS tool was further employed for evaluating the risk of bias in prognostic studies in this study. The QUIPS framework evaluates studies across six key domains: study participation, study attrition, prognostic factor measurement, outcome measurement, confounding, and statistical analysis and reporting. This approach allows for a more precise identification of potential sources of bias in observational prognostic studies, thereby enhancing the rigor and comprehensiveness of the quality assessment in this study.
The results in Table 5 showed that all studies had a low risk in the measurement of prognostic factors, outcome assessment, and statistical analysis, which ensured the reliability of the core conclusions. However, there were significant limitations in study participation (11.54% medium risk), study attrition (34.62% medium risk), and confounding control (34.62% medium risk). These limitations were primarily due to the fact that most of the included studies had a single-center retrospective design, which may lead to selection bias and affect the generalizability of the results. This is consistent with the previous assessment results of the Cochrane tool and provides a more detailed analysis of the sources of bias.
Table 5
| Bias | Low risk | Moderate risk | High risk |
|---|---|---|---|
| Study participation | 23 (88.46%) | 3 (11.54%) | 0 (0.00%) |
| Study attrition | 17 (65.38%) | 9 (34.62%) | 0 (0.00%) |
| Prognostic factor measurement | 26 (100.00%) | 0 (0.00%) | 0 (0.00%) |
| Outcome measurement | 26 (100.00%) | 0 (0.00%) | 0 (0.00%) |
| Study confounding | 17 (65.38%) | 9 (34.62%) | 0 (0.00%) |
| Statistical analysis and reporting | 26 (100.00%) | 0 (0.00%) | 0 (0.00%) |
Quality assessment results of prognostic studies based on the QUIPS framework.
Discussion
To the best of our knowledge, this meta-analysis is the first systematic collection and quantitative synthesis of reports on the prognostic value of PALS in significant aortic valve disease. An analysis of 25 studies involving 7,195 patients revealed the following findings: (1) PALS was lower in patients with endpoint events compared to those without endpoint events. (2) The risk of major endpoint events decreased with increasing PALS. PALS is an independent prognostic predictor in patients with severe aortic valve disease, and this holds true in both AS and AR patients. (3) PALS demonstrated robust incremental predictive value in both the AR and AS groups, yet its effect size and underlying mechanisms differed between the two cohorts. In summary, our findings emphasize the important clinical value of assessing LAS in patients with significant aortic valve disease.
AS primarily leads to increased LV pressure overload, resulting in concentric hypertrophy; AR, however, not only causes increased LV volume overload but also increased LV pressure overload, leading to LV dilation and eccentric remodeling (27). Both conditions may result in increased LV wall tension, particularly AR, where sustained high wall tension activates inflammatory responses in the myocardium, ultimately causing structural and functional damage to the heart muscle. Theoretically, the LA and LV are anatomically connected and function as LV filling regulators during different cardiac cycles to maintain cardiac output. Therefore, persistently increased LV filling pressure is transmitted to the LA via the atrioventricular connection. The LA must then contract more actively to resist the elevated LV filling pressure, which may lead to loss of atrial wall compliance and impaired LA contraction function (7). Recent studies have shown that LA strain impairment occurs earlier than LA dilation, particularly in PALS, which has garnered attention as a potential marker of LV diastolic dysfunction. Less load-dependent and more sensitive than conventional indices, it also emerges as an independent predictor of all-cause mortality in the general population, surpassing LVGLS in prognostic accuracy (17). However, in recent years, research has mostly used PALS as an indicator of LV diastolic dysfunction. But in theory, since PALS occurs during LV systole, when the LV contracts and pulls the LA towards the apex, PALS theoretically also reflects the longitudinal contraction ability of the LV. Moreover, PALS can also reflect the elasticity and stiffness of the LA myocardium. Atrial walls are thin and sensitive to pressure and volume changes. Additionally, the LA is connected to the pulmonary veins and serves as a blood reservoir. If its function is impaired, it may more directly lead to symptoms such as chest tightness and shortness of breath, which are associated with endpoint events. In this study, PALS was significantly lower among patients who reached the primary endpoint than among those who did not, thereby corroborating our earlier hypothesis.
Current guidelines recommend intervention for patients with symptomatic or LV systolic dysfunction (LVEF < 50%) and significant AS. For patients with symptomatic or asymptomatic significant AR, aortic valve surgery should be performed if the LV end-systolic diameter is >50 mm or the LV end-diastolic diameter is >65 mm (28). As can be seen, the current timing of intervention for severe active valve disease primarily depends on LV parameters, with less attention given to LA. However, these recommendations stemmed from studies with limited sample sizes and relatively outdated data. Recent evidence indicated that the extent and pattern of LV remodeling were influenced by age and sex: women and elderly patients with AR tended to maintain comparatively smaller LV dimensions, yet most experienced adverse cardiovascular events before reaching guideline-recommended LV size thresholds. In contrast, PALS showed a similar trajectory across age and sex groups; therefore, using PALS to inform operative timing may be superior to relying on LV parameters alone (29). In the future, LV criteria could be supplemented in AR patients with gender/age bias, or PALS parameters could be combined with LV parameters to determine the timing of surgery. This study employed C-statistic and NRI to evaluate the incremental prognostic value of PALS over traditional risk models. PALS conferred robust incremental predictive power in both AR and AS cohorts. Among AR patients, PALS consistently enhanced the discriminatory capacity of the risk model, whereas among AS patients it not only markedly improved model discrimination but also demonstrated reclassification efficacy as validated by the NRI, underscoring its necessity in addressing the limitations of conventional risk models. Consequently, integrating PALS into clinical risk stratification and surgical-timing decisions is likely to confer greater benefit to patients.
Regarding the value of LAS in aortic valve disease, there have been no previous meta-analyses evaluating the prognostic value of LAS in aortic valve disease. Only one qualitative systematic review, which included 18 studies involving 2,660 patients, was conducted, and it focused solely on AS and did not include AR (30). However, recent studies have suggested that LAS may also have prognostic value in AR. This meta-analysis included a total of 25 articles involving 7,195 patients, of which 4 studies reported only on AR, 20 reported only on AS, and 1 reported on both AS and AR. Compared with previous systematic reviews, the number of articles included and the number of patients involved have increased. Moreover, this study not only investigated the value of LAS in AS but also in AR, so the results of this study are widely representative and applicable to patients with severe aortic valve disease. As far as we know, this has not been reported before. Lacy et al. conducted a systematic review of 4 studies involving 822 patients and concluded that patients with AS who experienced adverse events had significantly lower PALS compared to those without events. This is consistent with our findings, but we included 10 studies involving 2,669 patients (10 for AS and 3 for AR). Compared with previous studies, our study has improved in the number of articles included, the number of patients, and the types of diseases. More importantly, in the research on prognosis, previous studies only systematically reviewed the value of PALS in the prognosis of AS without conducting a quantitative analysis. In contrast, we performed a meta-analysis of 17 articles (14 for AS and 3 for AR) and reached a quantitative conclusion. This has greater clinical value. Therefore, our study has taken a step forward based on the previous research.
After conducting subgroup analyses stratified by the type of aortic valve disease, it was observed that for every one-unit increase in PALS, the risk of major adverse cardiovascular events was reduced by 7% in patients with severe AS, compared to a 5% reduction in those with severe AR. This suggests that changes in PALS have a more pronounced impact on the prognosis of patients with AS. This difference is largely attributable to the distinct pathophysiological mechanisms underlying these two conditions. AS mainly leads to increased LV pressure load, while AR can lead to increased LV volume load and pressure load, which in turn activates different intracellular signaling pathways and leads to different types of cardiomyocyte hypertrophy and fibrosis patterns (28). Also, the type of LV hypertrophy presented by the patients with AR, with a chronic development of eccentric remodelling, probably better accommodates the increase of cavity filling pressures (31). At molecular level, Holst et al. conducted proteomic analyses on patients with severe isolated AS and AR and found that the LV myocardial proteome differed between AR and AS patients. AS was associated with higher levels of extracellular region proteins related to hematopoiesis/angiogenesis and tissue healing. In contrast, AR was associated with higher levels of intracellular region proteins related to energy production and cellular metabolism, which may indicate a compensatory “high metabolic intracellular myocardial state” to meet the increased energy demands (32). This could be the reason why patients with AR have a stronger compensatory capacity compared to those with AS. However, due to the limited number of articles on AR included in this study, the conclusions regarding the prognostic value of PALS in severe AR are only exploratory. Further research is needed in the future to validate our hypotheses.
In sensitivity analyses, we excluded small-sample studies and adjusted grouping criteria, yet the findings remained fully consistent. PALS retained prognostic value in significant aortic valve disease and served as an effective prognostic indicator, providing clinicians with new reference criteria.
Limitation
This study is the first to comprehensively evaluate the prognostic value of PALS in significant aortic valve disease through systematic review and meta-analysis. However, this study also has certain limitations. First, the heterogeneity of the included studies. Meta-regression analysis was further employed to identify and quantify the sources of heterogeneity in this study. Meanwhile, subgroup analyses and sensitivity analyses were performed to analyze and eliminate the marked heterogeneity observed. Although heterogeneity still existed, the prognostic significance of PALS remained robust, indicating its broad applicability across diverse aortic valve disease populations. Currently, there are still certain challenges in the standardization of PALS cut-off points. Secondly, the number of studies on AR included in this study is limited, which means that the findings regarding the prognostic value of PALS in AR are only exploratory. This is also due to the fact that the application of PALS in AR has not yet been fully recognized, which indirectly reflects the value of this study. Finally, due to the limited number of included articles and insufficient data, it was not possible to determine a PALS cutoff value for predicting the prognosis of severe aortic valve disease. At present, there is no validated PALS threshold value available for clinical practice, and existing guidelines lack LA parameters for judging the timing of intervention. The possible reason is that the adoption of PALS in clinical practice will face many practical barriers, such as the need for training and the variability of software. At present, it is possible to consider combining PALS with LV parameters for risk stratification of aortic valve disease and selection of surgical timing. More large-sample studies are needed in the future to further investigate this area.
Conclusion
PALS is an independent predictor of adverse cardiovascular events in patients with significant aortic valve disease. PALS has certain value in the prognosis of significant aortic valve disease and can provide new reference indicators for clinicians.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
NC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. WG: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. JW: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction Note
A correction has been made to this article. Details can be found at: 10.3389/fcvm.2026.1787377.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1667871/full#supplementary-material
References
1.
Aluru JS Barsouk A Saginala K Rawla P Barsouk A . Valvular heart disease epidemiology. Med Sci. (2022) 10(2):32. 10.3390/medsci10020032
2.
Butcher SC Hirasawa K Meucci MC Stassen J Kuneman JH Pereira AR et al Prognostic implications and alterations in left atrial deformation following transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. (2024) 25(12):1638–48. 10.1093/ehjci/jeae170
3.
Wedin JO Rodin S Flachskampf FA Simonson OE Pallin J Hörsne Malmborg J et al Left atrial dysfunction in bicuspid aortic valve patients with severe aortic stenosis is associated with post-operative atrial fibrillation following aortic valve replacement. Eur Heart J Open. (2024) 4(2):oeae020. 10.1093/ehjopen/oeae020
4.
Weber J Bond K Flanagan J Passick M Petillo F Pollack S et al The prognostic value of left atrial global longitudinal strain and left atrial phasic volumes in patients undergoing transcatheter valve implantation for severe aortic stenosis. Cardiology. (2021) 146(4):489–500. 10.1159/000514665
5.
Von Roeder M Maeder M Wahl V Kitamura M Rotta Detto Loria J Dumpies O et al Prognostic significance and clinical utility of left atrial reservoir strain in transcatheter aortic valve replacement. Eur Heart J Cardiovasc Imaging. (2024) 25(3):373–82. 10.1093/ehjci/jead268
6.
Thellier N Altes A Layec J Castel AL Delelis F Hubert T et al Impact of left atrial and diastolic ventricular dysfunction on mortality in patients with aortic stenosis. Arch Cardiovasc Dis. (2023) 16(3):126–35. 10.1016/j.acvd.2022.12.006
7.
Tan Y Li Y Deng W Zhang R Zhao R Abulipizi A et al Prognostic implications of left atrial strain in bicuspid aortic valve with chronic aortic regurgitation. J Am Heart Assoc. (2024) 13(6):e032770. 10.1161/JAHA.123.032770
8.
Stolz L Schmid S Steffen J Doldi PM Weckbach LT Stocker TJ et al Prognostic impact of left- and right-atrial strain in patients undergoing transcatheter aortic valve replacement. Eur Heart J Cardiovasc Imaging. (2025) 26(4):664–73. 10.1093/ehjci/jeae322
9.
Springhetti P Tomaselli M Benfari G Milazzo S Ciceri L Penso M et al Peak atrial longitudinal strain and risk stratification in moderate and severe aortic stenosis. Eur Heart J Cardiovasc Imaging. (2024) 25(7):947–57. 10.1093/ehjci/jeae040
10.
Sonaglioni A Nicolosi GL Rigamonti E Lombardo M . Incremental prognostic role of left atrial reservoir strain in asymptomatic patients with moderate aortic stenosis. Int J Cardiovasc Imaging. (2021) 37(6):1913–25. 10.1007/s10554-021-02175-6
11.
Salas-Pacheco JL Ávila-Vanzzini N Eugenia RM Arias-Godínez JA . Left atrium function by 2D speckle tracking in aortic valve disease. Echocardiography. (2016) 33(12):1828–34. 10.1111/echo.13368
12.
Sabatino J De Rosa S Leo I Strangio A La Bella S Sorrentino S . Early reduction of left atrial function predicts adverse clinical outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Open Heart. (2021) 8(2):e001685. 10.1136/openhrt-2021-001685
13.
Pessoa-Amorim G Mancio J Vouga L Ribeiro J Gama V Bettencourt N . Impaired left atrial strain as a predictor of new-onset atrial fibrillation after aortic valve replacement independently of left atrial size. Rev Esp Cardiol. (2018) 71(6):466–76. 10.1016/j.rec.2017.10.005
14.
Pernigo M Benfari G Geremia G Noni M Borio G Mazzali G et al Atrial function as an independent predictor of postoperative atrial fibrillation in patients undergoing aortic valve surgery for severe aortic stenosis. J Am Soc Echocardiogr. (2017) 30(10):956–65. 10.1016/j.echo.2017.07.001
15.
Parasca CA Calin A Cadil D Mateescu A Rosca M Botezatu SB et al Right ventricle to pulmonary artery coupling after transcatheter aortic valve implantation-determinant factors and prognostic impact. Front Cardiovasc Med. (2023) 10:1150039. 10.3389/fcvm.2023.1150039
16.
Meimoun P Djebali M Botoro T Djou Md U Bidounga H Elmkies F et al Left atrial strain and distensibility in relation to left ventricular dysfunction and prognosis in aortic stenosis. Echocardiography. (2019) 36(3):469–77. 10.1111/echo.14258
17.
Anastasius M Ro R Gavalas M Patel N Prandi FR Tang GHL et al The effect of TAVR on left ventricular and left atrial mechanics in patients with aortic stenosis. J Cardiovasc Dev Dis. (2022) 9(2):35. 10.3390/jcdd9020035
18.
Lee CY Tsai CM Chiang KC Huang CC Lin MS Hung CL et al Prognostic value of left ventricular and left atrial strain imaging in moderate to severe aortic stenosis: insights from an Asian population. Int J Cardiol. (2024) 407:132103. 10.1016/j.ijcard.2024.132103
19.
Lai KY Lee CY Chang YC Liu K Takeuchi M Yang LT et al Prognostic value of fully-automated left atrial strain in patients with asymptomatic chronic severe aortic regurgitation. Int J Cardiol. (2024) 416:132487. 10.1016/j.ijcard.2024.132487
20.
Jenner J Ilami A Petrini J Eriksson P Franco-Cereceda A Eriksson MJ et al Pre- and postoperative left atrial and ventricular volumetric and deformation analyses in severe aortic regurgitation. Cardiovasc Ultrasound. (2021) 19(1):14. 10.1186/s12947-021-00243-4
21.
Imanishi J Tanaka H Sawa T Motoji Y Miyoshi T Mochizuki Y et al Association of left atrial booster-pump function with heart failure symptoms in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Echocardiography. (2015) 32(5):758–67. 10.1111/echo.12733
22.
García Martín A Abellás Sequeiros M González Gómez AG Rincón Díaz LM Monteagudo Ruiz JM Hinojar Baydés R et al Prognostic value of diastolic function parameters in significant aortic regurgitation: the role of the left atrial strain. J Echocardiogr. (2022) 20(4):216–23. 10.1007/s12574-022-00577-6
23.
Galli E Fournet M Chabanne C Lelong B Leguerrier A Flecher E et al Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: a 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging. (2016) 17(5):533–41. 10.1093/ehjci/jev230
24.
Cameli M Lisi M Reccia R Bennati E Malandrino A Solari M et al Pre-operative left atrial strain predicts post-operative atrial fibrillation in patients undergoing aortic valve replacement for aortic stenosis. Int J Cardiovasc Imaging. (2014) 30(2):279–86. 10.1007/s10554-013-0323-6
25.
Benfari G Noni M Onorati F Cerrito LF Pernigo M Vinco G et al Effects of aortic valve replacement on left ventricular diastolic function in patients with aortic valve stenosis. Am J Cardiol. (2019) 124(3):409–15. 10.1016/j.amjcard.2019.04.046
26.
Tan ESJ Jin X Oon YY Chan SP Gong L Lunaria JB et al Prognostic value of left atrial strain in aortic stenosis: a competing risk analysis. J Am Soc Echocardiogr. (2023) 36(1):29–37. 10.1016/j.echo.2022.10.011
27.
Vollema EM Singh GK Prihadi EA Regeer MV Ewe SH Ng ACT et al Time course of left ventricular remodeling and mechanics after aortic valve surgery: aortic stenosis vs. aortic regurgitation. Eur Heart J Cardiovasc Imaging. (2019) 20(10):1105–11. 10.1093/ehjci/jez049
28.
Vahanian A Beyersdorf F Praz F Milojevic M Baldus S Bauersachs J et al 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2021) 43(7):561–632. 10.1093/eurheartj/ehab395
29.
Akintoye E Saijo Y Braghieri L Badwan O Patel H Dabbagh MM et al Impact of age and sex on left ventricular remodeling in patients with aortic regurgitation. J Am Coll Cardiol. (2023) 81(15):1474–87. 10.1016/j.jacc.2023.02.037
30.
Lacy SC Thomas JD Syed MA Kinno M . Prognostic value of left atrial strain in aortic stenosis: a systematic review. Echocardiography. (2024) 41(5):e15829. 10.1111/echo.15829
31.
Pires LT Rosa VEE Morais TC Bello JHSM Fernandes JRC de Santis A et al Postoperative myocardial fibrosis assessment in aortic valvular heart diseases-a cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. (2023) 24(7):851–62. 10.1093/ehjci/jead041
32.
Holst T Petersen J Ameling S Müller L Christ T Gedeon N et al Proteomic analysis in valvular cardiomyopathy: aortic regurgitation vs. aortic stenosis. Cells. (2023) 12:878. 10.3390/cells.12060878
Summary
Keywords
aortic stenosis, aortic regurgitation, left atrial strain, peak left atrial longitudinal strain, systematic review, meta-analysis
Citation
Chen N, Gu W and Wu J (2025) Prognostic value of left atrial strain in significant aortic valve disease: a systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1667871. doi: 10.3389/fcvm.2025.1667871
Received
17 July 2025
Accepted
01 September 2025
Published
16 September 2025
Corrected
10 February 2026
Volume
12 - 2025
Edited by
Theodor (Teddy) Fischlein, Klinikum Nürnberg, Germany
Reviewed by
Chiara Zocchi, Careggi University Hospital, Italy
Li Ying, China Medical University, China
Updates
Copyright
© 2025 Chen, Gu and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jun Wu wujun108@sina.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.