Abstract
Background:
Cardiotoxicity is a major concern in cancer survivors, potentially compromising treatment efficacy, quality of life and long-term survival. With increasing survival rates, the need for effective cardioprotective strategies has become paramount.
Objective:
This narrative review evaluates current pharmacological, non-pharmacological, and emerging strategies for preventing cancer therapy-related cardiac dysfunction (CTR-CD), emphasizing recent advances, their clinical applicability and research gaps.
Methods:
We conducted a narrative review based on a non-systematic search of PubMed/MEDLINE, Scopus, and Web of Science up to June 2025, focusing on clinical trials, meta-analyses, guideline recommendations, and key observational studies relevant to CTR-CD prevention.
Results:
Among pharmacological approaches, renin-angiotensin-aldosterone system inhibitors (RAASi) and beta-blockers modestly preserve left ventricular ejection fraction (LVEF), though benefits on hard outcomes remain unproven. Dexrazoxane is the only FDA-approved agent and shows robust protection in anthracycline-treated patients. Statins and metformin demonstrate promising but still investigational cardioprotective effects, while sodium-glucose cotransporter-2 inhibitors (SGLT2i) show encouraging pilot data. Non-pharmacological strategies—including structured exercise, mediterranean diet, nutritional support and aggressive control of risk factors—are guideline-endorsed, although most evidence relies on surrogate endpoints. Emerging tools such as telemedicine, artificial intelligence and omics sciences offer innovative opportunities for personalized prevention but require multicenter validation.
Conclusion:
An integrated, multidisciplinary approach combining both pharmacological and non-pharmacological strategies is essential to effectively prevent cardiotoxicity in cancer patients. Current evidence supports dexrazoxane, risk factor control and selective use of RAASi or beta-blocker in high-risk patients. Exercise and nutrition provide functional and quality of life benefits, while several novel strategies remain exploratory. Future large-scale, multicenter, randomized trial are needed to harmonize international guidelines and define the most effective, sustainable prevention models across diverse patient populations.
1 Introduction
In recent years, cancer incidence has shown variable trends depending on tumour site. A stable or slightly increasing trend has been observed for breast and prostate cancers, while a significant reduction has been recorded for lung cancer, largely due to prevention policies and anti-smoking campaigns (1). In Europe, epidemiological data partially reflect trends reported in American registries, with more pronounced increases observed in cancers associated with obesity and smoking (2). In the United States, the cancer mortality rate has steadily declined since 1991, reaching an overall reduction of up to 31% by 2018 (1). Similarly, in Europe, cancer-related mortality is generally decreasing thanks to effective prevention strategies, early diagnosis, and increasingly innovative treatments (2). This progress has led to a significant increase in 5-year survival rates (3), resulting in a growing population of cancer survivors (4). However, the survival benefits are offset by the risk of long-term toxicities, particularly cardiovascular toxicity, which represents one of the most serious complications of oncological treatments (5, 6).
Figure 1
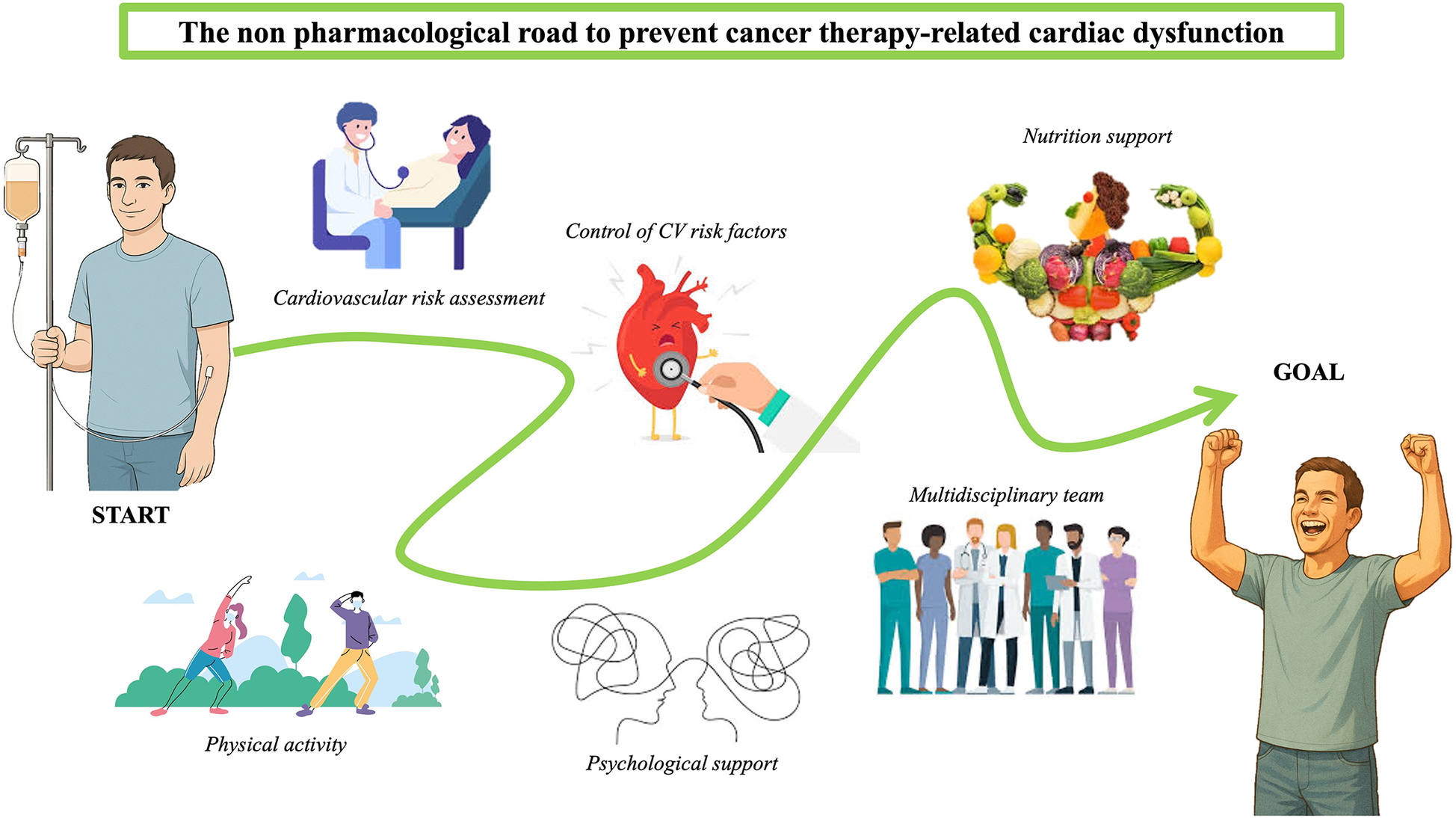
Non-pharmacological strategies to prevent CTR-CD, a schematic image.
Cardiovascular toxicity can significantly affect patients’ quality of life and, in some cases, become a leading cause of morbidity and mortality, especially in the elderly population (7, 8). This complex interaction has underscored the need for shared European cardio-oncology guidelines that encourage an integrated approach between oncologists and cardiologists in managing cardiovascular risk in cancer patients, to prevent and mitigate treatment-related toxicities (9). To this end, initial comprehensive risk assessment—including cardiovascular risk stratification—of cancer patients is crucial (10), along with monitoring during therapy to detect early signs of cardiovascular toxicity and implementing prompt pharmacological and non-pharmacological strategies (9, 10).
The aim of this review is to examine the current state of the art of both pharmacological and non-pharmacological strategies for the prevention of chemotherapy-induced cardiotoxicity, with particular emphasis on the most recent evidence.
2 Chemotherapy-related cardiac dysfunction
Chemotherapy-related cardiac dysfunction (CTR-CD) is one of the most feared complications of cancer therapy, with an estimated incidence of up to 10% in cancer patients (11). However, this figure may be underestimated, as subclinical forms detectable only via biomarkers or advanced imaging techniques may also be present. CTR-CD may occur during or after cancer therapy and can negatively impact treatment efficacy, quality of life, and overall survival (12–14) This dysfunction is heterogeneous, resulting from both direct (dose-dependent or dose-independent) and indirect mechanisms, and may be either reversible or permanent (11, 15). Although the pathogenesis is not yet fully elucidated, proposed mechanisms include oxidative stress, mitochondrial dysfunction, and irreversible cardiomyocyte damage (15, 16). Recent evidence also suggests a protective role of the endothelium in preserving myocardial integrity (17).
European guidelines have proposed a universal definition of CTR-CD, distinguishing between symptomatic and asymptomatic forms (18). Each form can present with varying degrees of severity: mild, moderate, or severe (very severe in the case of symptomatic CTR-CD) (13). Symptomatic forms are characterised by signs and symptoms of heart failure, while asymptomatic (subclinical) forms are detected using laboratory tests (troponin, BNP/NT-proBNP) or advanced imaging (echocardiography or cardiac magnetic resonance). Early identification of subclinical toxicity is of paramount importance, as it is a significant predictor of subsequent overt cardiac dysfunction, allowing for a reduction in the risk of progression to more severe clinical forms and improving both quality of life and survival (12, 19). Timely detection enables treatment protocols to be adapted or modified, avoiding unnecessary interruptions and optimizing antitumour efficacy (15).
Accordingly, the use of sensitive diagnostic tools capable of identifying functional and structural changes before clinical manifestations occur is essential. Biomarkers (troponin and BNP) facilitate early detection of myocardial injury (15).
Advanced imaging techniques such as speckle-tracking echocardiography (global longitudinal strain or GLS) and cardiac magnetic resonance (CMR) with parametric mapping can detect structural and functional changes before a significant drop in ejection fraction is observed (20, 21). Indeed, the biplane 2D ejection fraction method has several significant limitations: its reduction is only evident in advanced stages of dysfunction, when the damage may already be substantial and potentially irreversible; it is highly operator-dependent and subject to the variable loading conditions seen in oncology patients due to therapy and its gastrointestinal side effects (22). GLS helps overcome some of these limitations, although it remains sensitive to blood pressure and afterload variations, which may lead to false positives or negatives (23, 24). Myocardial work (MW), which incorporates systolic blood pressure, reduces this dependency and provides a more accurate assessment of myocardial function. Several studies, although with conflicting results, have shown that MW indices [Global Work Index [GWI], Global Constructive Work [GCW], and Global Work Efficiency [GWE]] change early during chemotherapy and may help differentiate between true myocardial dysfunction and blood pressure-related variations (23, 24). The combined use of MW and GLS, rather than their isolated application, improves diagnostic accuracy compared to the use of traditional parameters alone (24).
Finally, three-dimensional ejection fraction (3D-EF) reduces inter-operator variability and improves the diagnostic accuracy of this method; however, recent studies suggest it is less sensitive than GLS in detecting early subclinical dysfunction (25, 26). The implementation of monitoring strategies based on early detection of subclinical damage—through regular surveillance during and after cancer therapy—therefore represents a key element for timely intervention with pharmacological and non-pharmacological strategies to protect the hearts of cancer patients (12).
3 Pharmacological strategies
3.1 Renin-angiotensin-aldosterone system inhibitors (RAASi)
Renin-angiotensin-aldosterone system inhibitors (RAASi) have been widely studied for their potential cardioprotective effects in patient undergoing cancer therapy. The strongest evidence concerns anthracycline-induced cardiotoxicity, while data for other setting (e.g., trastuzumab, immune checkpoint inhibitors) are more limited. The biological rationale is strong, given their role in counteracting oxidative stress, fibrosis and ventricular remodelling. Clinical trials have tested angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs) and mineralocorticoid receptor antagonists (MRAs), with varying degrees of success (27, 28).
Given the heterogeneity and number of available studies, the main randomized and controlled trials are summarized in Table 1.
Table 1
| Study (year) | Population | Intervention | Primary endpoint | Main results | Clinical implications |
|---|---|---|---|---|---|
| Cardinale (2006) | 114 pts, anthracyclines | Enalapril vs. control | Systolic dysfunction | Complete prevention of LVEF decline with enalapril | Proof-of-concept for early ACEi therapy |
| ICOS-ONE (2018) | 273 pts, anthracyclines | Enalapril “troponine-guided” vs. prophylactic | LVEF decline | Both strategies effective; troponin-guided more practical | Supports biomarker-driven approach |
| OVERCOME (2013) | 90 pts, hematologic cancers | Enalapril + Carvedilol vs. placebo | LVEF + MACE | Preserved LVEF, fewer major events | Combination therapy appears protective |
| PROACT (2025) | 400 pts, high-dose anthracyclines | Enalapril + SOC vs. SOC | CTR-CD | No significant difference | Questions routine prophylactic use |
| SAFE (2025) | 190 pts, breast cancer | Ramipril vs. placebo | LVEF + troponin | Attenuated LVEF decline and subclinical injury | Suggests ramipril as an alternative option |
| PRADA (2016) | 120 pts, early breast cancer | Candesartan +/- Metoprolol | LVEF (MRI) | Candesartan attenuated LVEF decline; metoprolol neutral | Robust support for ARBs use |
| Akpek (2015) | 83 pts, anthracyclines | Spironolactone vs. control | LVEF + diastolic function | Preserved systolic and diastolic function | MRAs promising in small RCTs |
| Meta-analyses (2015–2025) | 500–1,000 pts across RCTs | ACEi/ARB +/- MRAs | LVEF, HF events | Consistent modest preservation of LVEF; no reduction in HF incidence | Benefit limited to surrogate outcomes |
Key clinical trial of RAASi inhibitors in the prevention of chemotherapy-related cardiac dysfunction.
SOC, standard of care; LVEF, left ventricular ejection fraction; RAASi, rening-angiotensin-aldosterone system inhibitors; ACEi, angiotensin-converting enzyme inhibitors; ARBs, angiontensin II receptor blockers; MRAs, mineralocorticoid receptor antagonists; MRI, magnetic resonance imaging; RCT, randomized clinical trials; MACE, major adverse cardiac events; CTR-CD, cancer therapy related cardiac dysfunction.
Table 2
| Component | Definition | Score | |
|---|---|---|---|
| H | History | Prior history of cardiovascular disease (e.g., CAD, HF, arrhythmia) | 3 |
| F | Function | Abnormal cardiac function (e.g., reduced LVEF, diastolic dysfunction, LVH) | 2–3 |
| A | Biomarkers (assessment) | Elevated | 2–3 |
| I | imaging | Abnormal findings on baseline imaging | 2 |
| C | Co-morbidities | Hypertension, dyslipidemia, diabetes, renal dysfunction, etc | 1–2 |
| O | Oncologic therapy-related risk | Type of cancer therapy (e.g., anthracyclines, HER2 inhibitors, radiation, etc) | 1–3 |
| S | Stage or summary | Total score determines risk category: low, moderate, high or very high | 0–3 = Low4–6 = Moderate 7–9 = High ≥ 10 = Very High |
HFA-ICOS score.
CAD, coronary artery disease; HF, heart failure; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy.
Table 3
| Key features | Aerobic Training | Resistance Training |
|---|---|---|
| Main goal | Improve cardiovascular health, VO2-peak and functional capacity | Preserve/increase muscle mass and strength; counteract fatigue and sarcopenia |
| Initial assessment | Cardiovascular and cancer therapy-related cardiotoxicity risk stratification | Estime maximal strength (with maximum repetition RM or via Brzycki formula) |
| Training type | Moderate-intensity continuous training (MICT) or High-intensity interval training (HIIT) | Strength, Hypertrophy or Endurance |
| Recommended frequency | 3–5 days/week | 2–4 days/week depending on goal (strength, hypertrophy or endurance) |
| Session duration | 30–60 min | Varies with reps; 30–45 min/session |
| Total weekly time |
|
Dependent on program; minimum 2–4 sessions/week |
| Precautions | Avoid overexertion if impaired cardiac function | Start with low loads in frail patients |
| Expected benefits | ↑ VO₂peak, ↓ cardiovascular risk, ↑ functional capacity, ↓ fatigue, ↑ survival | ↑ strength and lean mass, ↓ sarcopenia, ↑ metabolic health, ↑ treatment tolerance |
A schematic visualization of different type of physical activity in cardio-oncology.
Overall, ACE inhibitors, particularly enalapril, have been the most studied. Early RCTs such as Cardinale (2006), ICOS-ONE (2018) and OVERCOME (2013) suggested that enalapril could effectively prevent LVEF decline and, in combination with carvedilol, reduce major cardiac events. However, the more recent PROACT trial (2025), the largest to date, failed to demonstrate benefit in high-risk patients on high-dose anthracyclines, thus tempering enthusiasm for routine use. The SAFE trial (2025) with ramipril provided additional signals of benefit in breast cancer patients, though further validation is required (29–33).
Evidence for ARBs is less robust but supportive. The PRADA trial (2016) demonstrated that candesartan attenuated LVEF decline assessed with cardiac MRI (magnetic resonance imaging), while smaller studies with telmisartan reported preservation of diastolic function (34, 35).
MRAs, particularly spironolactone, also showed preservation of systolic and diastolic function in small trials and appear highly effective in network meta-analyses, though large-scale confirmation is lacking (36).
Given the variability across individual trials, meta-analyses offer a more consistent and comprehensive assessment of the cardioprotective effects of RAAS inhibitors in patients undergoing anthracycline-based chemotherapy. Overall, these analyses (Yun 2015, Dong 2020, Caspani 2021, Harmouch 2025) consistently indicated that RAASi modestly preserve LVEF during anthracycline therapy. However, these benefits have not translated into significant reductions in overt heart failure or mortality, highlighting the gap between surrogate endpoints and clinically meaningful outcomes (37–40).
Beyond anthracyclines, exploratory data suggest a potential role of RAASi in other contexts. Observational studies have reported lower rates of dysfunction with trastuzumab (Moey 2019) and improved outcomes with immune checkpoint inhibitors (Chiang 2023). Perioperative RAASi exposure has also been linked with reduced long-term mortality after oncologic surgery (Oh 2022) (41–43).
Although various studies have demonstrated cardioprotective benefits from all three classes of RAASi—ACEi, ARBs, and MRAs—some network meta-analyses have aimed to directly compare their relative efficacy to identify the most effective agents for preventing chemotherapy-induced cardiotoxicity. Among these, several analyses consistently highlight enalapril and spironolactone as the most promising options. Ali Mir et al. and Xinye Li et al. found that spironolactone produced the greatest improvement in LVEF, followed by enalapril. Additionally, spironolactone significantly reduced troponin levels, while enalapril was associated with the largest drop in BNP and the lowest risk of clinical heart failure (44, 45).
The role of angiotensin receptor-neprilysin inhibitors (ARNI) in preventing CTR-CD in an emerging area of interest, particularly for patients receiving anthracyclines. Preclinical studies in animal models have shown that ARNI (sacubitril/valsartan) can prevent anthracycline-induced myocardial dysfunction by reducing oxidative stress, mitochondrial dysfunction and inflammatory response and these effects were more pronounced than with ARBs o ACEi alone (46, 47). Human data, however, remain limited (48). The ongoing PRADAII trial is specifically evaluating ARNI for prevention of cardiac dysfunction in breast cancer patients receiving chemotherapy (49).
Current official recommendations, as outlined in the 2022 European cardio-oncology guidelines (13), support the use of RAAS inhibitors for primary prevention of cardiotoxicity with a class IIa indication. This recommendation specifically applies to patients at high or very high risk of developing cardiac dysfunction who are undergoing treatment with anthracyclines, anti-HER2 agents, or other potentially cardiotoxic therapies. While the ESC guidelines represent the most comprehensive and detailed framework, recommendations from other major societies provide complementary perspectives that increase global relevance.
The ASCO clinical practice guidelines (2017) primarily addresses long-term survivorship, emphasizing baseline cardiovascular risk assessment and periodic monitoring with troponin and echocardiography in high-risk patients. Preventive pharmacological therapy is mentioned, but without strong class recommendations, reflecting the limited evidence base at that time (12). The AHA scientific statements (2019–2023) integrate cardio-oncology into broader heart failure prevention strategies, highlighting aggressive control of traditional cardiovascular risk factors. Pharmacological prophylaxis with RAAS inhibitors or beta-blockers is described as reasonable in high-risk patients, but without universal endorsement (50–52). The ESMO consensus recommendations (2020), on the other hand, adopt a more oncology-driven perspectives, stressing multidisciplinary collaboration and continuity of cancer treatment. While acknowledging the potential role of ACE inhibitors and beta-blockers, ESMO does not provide formal class of recommendations, instead focusing on structured monitoring multidisciplinary strategies (10). Taken together, these guidelines converge on the importance of baseline cardiovascular risk stratification, close surveillance of high-risk patients and multidisciplinary management, but diverge in the strength of recommendations for prophylactic pharmacotherapy. The ESC uniquely provides formal class IIa recommendations for RAASi and beta-blockers in high and very-high risk patients, whereas ASCO and AHA remain more cautious, and ESMO prioritize monitoring and oncological treatment continuity.
This heterogeneity underscores the need for harmonized global guidance and highlights the importance of ongoing larger and better-powered randomized trials in higher-risk populations, which are essential to refine the role of pharmacological prevention in cardio-oncology.
3.2 Beta-blockers
Although the use of beta-blockers (BBs) to prevent anthracycline-induced cardiotoxicity has been widely investigated, the evidence remains heterogeneous, with conflicting results across trials. An editorial contextualized this variability, noting that studies had important design limitations, and highlighted the CECCY trial as the largest placebo-controlled RCT evaluating carvedilol for primary prevention (53, 54). In CECCY, carvedilol did not reduce the incidence of LVEF decline ≥10%, but was associated with significantly lower troponin I levels and reduced diastolic dysfunction, suggesting subclinical benefit. This is corroborated by a recent meta-analysis by Attar et al., which included 17 RCTs (n = 1,291), and found that BBs attenuated the decline in LVEF after chemotherapy, although this did not translate into a statistically significant reduction in the incidence of cancer therapy–related cardiac dysfunction (55). Among included trials, CECCY was the largest and showed only modest clinical benefits. A troponin-guided strategy was further evaluated using carvedilol combined with candesartan in high-risk patients but found no significant protection against LVEF decline (56).
Recently, the rationale and design of the ongoing CARDIOTOX trial reinforce the need for large-scale studies powered for clinical outcomes. With over 1,000 planned participants, CARDIOTOX aims to clarify whether carvedilol prevents meaningful cardiovascular events, including heart failure, arrhythmias, and LVEF decline in anthracycline-treated patients (57).
3.3 SGLT2 inhibitors
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) are a well-established class of drugs initially developed for glycemic control in diabetes, but their clinical use has expanded remarkably following the impressive evidence demonstrating benefits in patients with heart failure. However, their role in the prevention of cancer therapy-related cardiac dysfunction remains less defined and is currently under investigation in recent studies. As reviewed by Dhabour et al., there is strong biological plausibility that SGLT2i may counteract anthracycline-induced cardiotoxicity by attenuating oxidative stress, inflammation, and fibrosis, mechanisms shared with conventional heart failure pathology (58, 59).
A large retrospective analysis of over 17,000 propensity-matched diabetic patients undergoing potentially cardiotoxic chemotherapy was recently conducted (60). Interestingly, SGLT2i use was independently associated with a significant reduction in CTR-CD, heart failure events, and all-cause mortality. The EMPACARD-PILOT trial prospectively tested empagliflozin in high-risk breast cancer patients receiving anthracyclines (61). Compared to controls, those treated with empagliflozin had a markedly lower incidence of CTR-CD (6.5% vs. 35.5%, p = 0.005), with preserved LVEF and GLS at follow-up, despite no significant difference in NT-proBNP or troponin elevation. These findings, though promising, call for larger randomized trials to confirm the cardioprotective effect of SGLT2i in this setting.
In conclusion, SGLT2i appears to be safe and may reduce cardiovascular events in patient with cancer treated with anthracycline (62–65).
3.4 Other cardioprotective agents
A robust body of evidence, including meta-analyses and clinical trials, supports the use of statins for the primary prevention of cardiovascular disease (CVD), with a generally favorable balance of efficacy and safety. Meta-analyses consistently show that statins significantly reduce the risk of major cardiovascular events and CVD disease mortality in primary prevention population (66–68). Additionally, statins are associated with a small increased risk of adverse effects such as self-reported muscle symptoms, liver dysfunction and renal insufficiency, but not with clinically confirmed muscle disorders or diabetes (69).
Statins are generally well-tolerated in the oncology setting, with no significant increase in adverse events reported. Their cardioprotective effects are attributed to pleiotropic actions: reducing oxidative stress, inflammation, fibrosis and endothelial dysfunction—all mechanisms central to cancer-therapy induced cardiotoxicity (70, 71).
Multiple recent meta-analyses and RCTs demonstrate that statin therapy is associated with a significantly lower risk of CTR-CD and heart failure in cancer patients, reducing the incidence of anthracycline-induced CTR-CD by about 50%, with a smaller decline in LVEF compared to controls, a lower risk of heart failure and effective also in the trastuzumab-only population (72–74). However, some RCT found no significant difference in LVEF decline or CTR-CD incidence, highlighting, once again, heterogeneity in results and the need for further large-scale trials (73).
Metformin, a first-line antidiabetic agent, has attracted attention in cardio-oncology because of its potential pleiotropic cardioprotective effects beyond glycemic control. Mechanistically, metformin activates AMP-activated protein kinase (AMPK), which reduces oxidative stress, improves mitochondrial function and attenuates apoptosis. These pathways overlap with those implicated in anthracycline-induced cardiotoxicity, providing a strong biological rationale for its use (75, 76).
Clinical evidence, however, is limited and influenced by study design, cancer type and patient population. Observational studies in diabetic cancer patients have reported lower rates of cardiotoxicity, heart failure events and mortality in those receiving metformin compared with non-users. Some retrospective analyses also suggest improved cancer-related outcomes, likely due to metformin's anti-proliferative and insulin-sensitizing properties. However, prospective randomized controlled trials specifically designed to assess metformin's cardioprotective effect in non-diabetic cancer patients are lacking (75, 77–79).
From a guideline's perspective, ESC and ESMO mention metformin as a promising but experimental option, without formal class of recommendation grading. In summary, metformin is biologically plausible and supported by encouraging preclinical and observational evidence, but its role in routine cardioprotection remain investigational. Ongoing and future prospective trials are essential to clarify whether metformin should be considered beyond diabetic population, potentially as a repurposed therapy for anthracycline-induced cardiotoxicity (10, 13).
Dexrazoxane is currently the only FDA-approved drug for the prevention of anthracycline-induced cardiotoxicity. Its mechanism of action is primarily related to iron chelation, which reduces the formation of anthracycline-iron complexes responsible for oxidative stress and free radical-mediated myocardial injury. In addition, dexrazoxane interferes with topoisomerease IIBeta inhibition in cardiomyocytes, thereby mitigating DNA damage and cell death. Clinical evidence of its use is robust, especially in pediatric oncology. Multiple randomized trials have demonstrated that dexrazoxane significantly reduces the incidence of left ventricular dysfunction, troponin elevation and the risk of heart failure, though concerns about potential interference with chemotherapy efficacy and the risk of secondary malignancies initially limited its widespread adoption. More recent analyses, however, have not substantiated these concerns, and international consensus now supports its safe use in carefully selected populations (80–83).
The ESC cardio-oncology guidelines endorse dexrazoxane as a class IIa recommendation in patients expected to receive high cumulative dose of anthracyclines (> 250–300 mg/mq of doxorubicin equivalent) or those at very high-risk of cardiotoxicity. Similarly, the ASCO guidelines acknowledge its cardioprotective role but highlight the need to balance benefit with oncologic efficacy. Moreover, the ESMO consensus recommends its use in patients requiring high-dose anthracyclines when alternative regimens are not feasibile (10, 12, 13).
4 Non-pharmacological strategies
Non-pharmacological strategies represent an essential complement to pharmacological cardioprotection in cancer patients. These strategies include behavioural interventions, lifestyle modifications, psychological support, and a multidisciplinary approach aimed at improving quality of life and reducing overall cardiovascular risk.
Unlike drug-based approaches, the supporting evidence for lifestyle-oriented strategies—such as exercise and nutrition—is largely driven by improvements in functional parameters and quality of life, rather than by robust reductions in hard clinical endpoints such as heart failure or mortality. Most available trials are small, heterogeneous and rely on surrogate outcomes (e.g., LVEF, GLS, VO2 peak or peak oxygen consumption), which limits the certainty of evidence. An overview of non-pharmacological approaches to prevent cancer therapy-related cardiac dysfunction is shown in Figure 1.
Nevertheless, both the ESC 2022 cardio-oncology guidelines and consensus documents from ASCO, AHA and ESMO emphasize the importance of integrating exercise and nutritional support into survivorship care (10, 12, 13). These strategies therefore play a pivotal role in multidisciplinary cardio-oncology, even if their formal class-of-recommendation grading is less strong than for pharmacological agents such as RAASi or dexrazoxane.
4.1 Cardiovascular risk assessment
Accurate cardiovascular risk assessment through a detailed medical history, physical examination and risk stratification tools is the cornerstone of cardio-oncology, enabling tailored surveillance and prevention strategies.
Among these tools, the Heart Failure Association–International Cardio-Oncology Society (HFA-ICOS) score is currently the most widely adopted and validated. It integrates patient-related factors (age, comorbidities), therapy-related risk (type and dose of anticancer treatments) and cardiac biomarkers, stratifying patients into low, moderate, high and very high-risk categories. The Heart Failure Association–International Cardio-Oncology Society (HFA-ICOS) score is summarized in Table 2. Prospective studies have shown that HFA-ICOS predicts the incidence of anthracycline-induced cardiotoxocity and guides follow-up intensity (13). Timely use of this score is crucial for planning both pharmacological and non-pharmacological preventive strategies (84). However, its derivation mainly from European cohorts may limit generalizability to non-European populations, pediatric settings and patients receiving newer agents such as immune checkpoint inhibitors.
Other models, including SCORE2 or AH-HA, have been explored but lack formal validation in oncology cohorts. Overall, the robustness of available score is moderate: they provide a practical framework for clinical use yet rely on surrogate endpoints and observational validation rather than randomized evidence (85–88).
4.2 Control of cardiovascular risk factors
Aggressive management of modifiable cardiovascular risk factors (hypertension, obesity, dyslipidemia, diabetes, and smoking) is a low-cost, high-yield strategy to reduce CTR-CD. Numerous studies have demonstrated that the presence of these factors significantly increases the risk of developing cardiac damage during cancer therapies (89–94). Addressing these factors remains a primary preventive strategy.
Contemporary guidelines strongly emphasize this approach. The 2024 ESC hypertension guidelines recommend a blood pressure target of SBP 120–129 mmHg and DBP 70–79 mmHg if tolerated (otherwise ALARA or as low as reasonably achievable) in high-risk patients with clinical hypertension, including those with cancer. The 2019 ESC/EAS lipid guidelines advise initiating statins in patients with a 10-yeas ASCVD risk ≥7.5% and observational data suggest that statins attenuate CTR-CD. Similarly, AHA scientific statement and the ASCO guidelines endorse aggressive control of risk factors as a primary prevention strategy (12, 50, 52, 95, 96).
Despite the strong consensus, real-world adherence remains suboptimal. Registry data show that hypertension and dyslipidemia are often undertreated in cancer patients, leading to preventable cardiovascular complications (97, 98). Incorporating structured cardio-oncology rehabilitation and early referral to cardiology could bridge this gap.
Evidence quality for risk factor control is high for overall cardiovascular benefit, but indirect for CTR-CD prevention, highlighting the need for oncology-specific implementation studies.
4.3 Physical activity and rehabilitation
Exercise is one of the most consistently recommended non-pharmacological strategies. Randomized controlled trials, including the ONCORE trial, and several meta-analyses suggest that aerobic and combined aerobic-resistance training modestly improve LVEF, GLS, VO2 peak and diastolic function in patient receiving anthracyclines. However, sample sizes are small, follow-up is short and clinical outcomes such as heart failure hospitalization or survival are rarely captured (99, 100).
A recent study by our group (101) showed that regular physical activity is associated with reduced cardiovascular and overall mortality, improved cardiorespiratory fitness, and decreased symptoms of chemotherapy-induced cardiotoxicity. Exercise also promotes favorable cardiac remodeling, improves endothelial function, and reduces oxidative stress, contributing to better quality of life in cancer patients. A schematic overview of different types of physical activity used in cardio-oncology is shown in Table 3.
Systematic reviews grade the certainty of evidence as low-to-moderate for surrogate endpoints and for low for clinical outcomes. Heterogeneity in exercise prescription (intensity, timing, delivery) further limits generalizability. Nevertheless, safety is consistently demonstrated, and adherence is feasible with supervised programs (100, 102).
Guidelines reflect these nuances: the ESC provide a classe I recommendation for exercise in cancer survivors as part of cardiovascular prevention, while AHA and ASCO statements support supervised exercise but without strong class grading for CTR-CD prevention. Future priorities include large multicenter RCTs with standardized exercise protocols, integration into cardiac rehabilitation frameworks and evaluation of long-term outcomes beyond functional parameters (10, 12, 13).
In summary, exercise is safe and beneficial for functional endpoins and quality of life, but robust evidence for hard outcomes is still lacking.
4.4 Nutrition and nutritional support
Nutrition interventions are essential for holistic cardioprotection, though direct evidence for CTR-CD prevention remains limited. Preclinical studies and small human trials suggest that adherence to a Mediterranean diet and supplementation with antioxidants (e.g., coenzyme Q10, zinc, selenium, polyphenols) may attenuate anthracycline-induced oxidative stress and reduce biomarker release (103–105). However, results are inconsistent, and the overall certainty of evidence is very low for specific supplements (103).
In contrast, the role of nutritional screening and support is supported by more solid evidence. Malnutrition and sarcopenia predict poor treatment tolerance, increased toxicity and higher cardiovascular risk in both pediatric and adult oncology populations (106, 107). Early involvement of dietitians and individualized support has been shown to improve outcomes and quality of life, with moderate evidence for clinical benefit (108, 109).
Guidelines from ESC and ESMO recommend systematic malnutrition screening and personalized nutritional counseling but stop short of endorsing any particular dietary supplement for CTR-CD prevention. ASCO and AHA also emphasize weight control, diabetes prevention and general cardiometabolic health as key targets during survivorship (10, 12, 13).
In summary, nutrition is indispensable for global cardiovascular and oncologic outcomes, but its role in specific CTR-CD prevention remains exploratory. Stronger evidence supports structured nutritional assessment and support rather than isolated supplement use.
4.5 Psychological support and mind-body techniques
Psychological support and stress management strategies (such as yoga, mindfulness, and meditation) have shown promise in improving the quality of life and psychological well-being of cancer patients. Recent studies indicate that meditation, when incorporated into cardiac rehabilitation programmes, reduces anxiety, depression, and stress in patients with coronary artery disease compared with standard care (110–113).
In oncology, particularly among women with breast cancer, mindfulness and loving-kindness practices have been shown to alleviate pain, fatigue, and anxiety, with potential benefits for heart rate modulation (114, 115).
Couple-based meditation interventions, including online programmes, have demonstrated positive effects on quality of life and symptom management for both cancer patients and their partners (116).
A recent randomized study found that regular Buddhist walking meditation may mitigate anthracycline-related cardiotoxicity, improving vascular function and quality of life compared with controls (117).
However, robust evidence of a direct effect of psychological support on cardiotoxicity prevention is currently lacking (118). Nevertheless, psychological support is recommended as an integral part of a multidisciplinary approach, facilitating adherence to follow-up, symptom management, and emotional processing, with potential indirect cardiovascular benefits.
4.6 Multidisciplinary approach
Continuous communication between cardiologists and oncologists is essential for managing cardiovascular risk in cancer patients. This integrated approach enables personalized prevention, monitoring, and treatment strategies, ultimately improving patient prognosis (119–121).
Balancing the benefits of cancer therapies against cardiovascular risks and promoting joint education between cardiologists and oncologists are fundamental (122–124).
The creation of dedicated cardio-oncology units is recommended to ensure comprehensive and coordinated care for cancer patients, bridging existing gaps in clinical practice (125, 126).
5 Future prospectives
Current research in the field of cardio-oncology prevention is increasingly focused on developing innovative strategies to enhance diagnostic accuracy and enable earlier identification of patients at risk of cancer therapy–related cardiac dysfunction (CTR-CD). These approaches aim to detect subclinical cardiac damage and to stratify individual risk in a more personalized way, with the ultimate goal of integrating precision medicine more effectively into routine clinical practice.
To further strengthen the clinical applicability of these approaches, there is a growing need for well-designed comparative clinical trials aimed not only at evaluating the efficacy of pharmacological and non-pharmacological preventive strategies, but also at determining which of these approaches—or what combination thereof—offers the most effective and sustainable protection in specific patient populations.
5.1 Medical genetics
A field of growing interest is pharmacogenomics and the study of genetic polymorphisms. Several studies have identified genetic variants, such as single nucleotide polymorphisms (SNPs), that increase individual susceptibility to the cardiotoxic effects of cancer therapies, particularly anthracyclines and HER2 inhibitors. For example, polymorphisms in the CYBA, RAC2, CYP3A5, ABCC1, ABCC2, and HER2 genes have been associated with an increased risk of cardiac toxicity. Integrating pharmacogenomics into clinical practice could allow for a more precise individual risk stratification compared to traditional risk factors, enabling the identification of more susceptible patients and the implementation of personalized prevention strategies, such as closer cardiological monitoring or early use of cardioprotective drugs. The use of genetic testing and polygenic risk scores represents a promising frontier in cardio-oncology, with the potential to make cancer therapies safer and more targeted. However, the clinical validation of these genetic markers is still ongoing, and further studies are needed to define their actual impact on the prevention of CTR-CD (127–134).
Medical genetics therefore offers promising tools for personalized prevention of cardiotoxicity, but their routine application—considering costs and turnaround times—requires careful patient selection to identify those who could truly benefit (135–139).
5.2 Omics sciences
Another emerging tool is represented by omics sciences, particularly metabolomics, which can identify early biomarkers of cardiotoxicity induced by both chemotherapy and radiotherapy. Recent studies have demonstrated that the analysis of plasma metabolic profiles can detect alterations associated with cardiac damage before the clinical onset of symptoms. In particular, in thoracic radiotherapy, changes in steroid hormone and vitamin E metabolism have been linked to an increased risk of cardiotoxicity (140–142).
In vitro, exposure to common chemotherapeutic agents (such as anthracyclines or 5-fluorouracil) has shown metabolic changes, with an increase in metabolites associated with inflammation and oxidative stress (143, 144).
Furthermore, a clinical study conducted by the Cleveland Clinic on patients treated with chemotherapy identified 13 plasma proteins and 14 metabolites associated with the development of left ventricular dysfunction assessed via echocardiography (145).
The integrated omics approach (genomics, proteomics, transcriptomics, and metabolomics) offers new opportunities for the early identification of biomarkers and for understanding the molecular mechanisms underlying cardiotoxicity, overcoming the limitations of traditional markers that only change after significant damage has occurred (146, 147).
Metabolic profiling enables a more sensitive and earlier characterization of cardiac injury phenotypes, supporting precision medicine strategies for surveillance and prevention (148).
However, research in this field is still under development, and further studies are needed to validate and standardize the metabolite panels to be used in clinical practice (146, 148).
5.3 Telemedicine
Telemedicine is emerging as a promising tool for the prevention and management of cardiovascular toxicity in cancer patients. Recent studies suggest that telemonitoring programs, such as the ON-CARDIO model, can facilitate the early detection of cardiac complications related to cancer therapies, especially in certain patient groups, such as those with colorectal cancer, although the reasons for this selection need further clarification. This model involves continuous telemonitoring of parameters such as ECG, blood pressure, and biomarkers, aiming for the timely detection of arrhythmias and cardiac dysfunction during therapy (149).
American and European guidelines emphasize the importance of a multidisciplinary and personalized approach to the cancer patient, which also includes the use of digital technologies for continuous surveillance (9, 10, 118, 150).
A recent systematic review has shown that telemedicine, particularly when integrated with remote monitoring and specialist consultations, can reduce mortality and hospitalizations for cardiovascular causes in patients with heart failure, suggesting potential benefits in cardio-oncology as well (151).
However, specific research on the effectiveness of telemedicine in preventing cardiovascular toxicity in cancer patients is still in its early stages, and further studies are needed to confirm these results (152).
5.4 Artificial intelligence in cardio-oncology
Artificial intelligence (AI), thanks to its ability to integrate and analyze large amounts of clinical, instrumental, and imaging data, is revolutionizing the prevention of cardiotoxicity in cancer patients, providing advanced tools for risk stratification, early diagnosis, and monitoring of complications related to cancer therapies. Machine learning and deep learning models, such as neural networks and random forest algorithms, applied to clinical data, echocardiographic parameters, and electrocardiograms (ECGs), have shown good predictive capability for the risk of cardiac dysfunction and heart failure in patients treated with anthracyclines and trastuzumab, allowing for early identification of at-risk individuals and potentially guiding personalized prevention strategies (153–158).
The application of AI to ECG analysis can detect subclinical alterations of left ventricular function with diagnostic performance comparable to echocardiography, enabling risk stratification even before the initiation of therapy (155–157). In addition, two recent large-scale, retrospective cohort studies applied validated AI models to ECG to predict and stratify the risk of CTR-CD in cancer patients receiving anthracyclines or trastuzumab (157, 159). While these are not randomized clinical trials, they represent robust, real-world clinical evidence supporting AI's role in CTR-CD prevention and early detection.
Moreover, integrating AI with advanced imaging data (echocardiography, cardiac magnetic resonance, PET) and multi-omics biomarkers allows for an even more precise and personalized cardiovascular risk assessment, facilitating early diagnosis and monitoring of complications (160–164).
However, these tools must be validated and standardized in multicenter studies and integrated into routine clinical practice to ensure equitable access and reliable results. Additionally, it is essential to adequately train healthcare professionals in the use of these technologies (165–167).
A 2024 systematic review and meta-analysis evaluated the efficacy of AI in automating cardiothoracic ratio measurement on chest x-ray, which is relevant for screening and monitoring cardiac dysfunction, including CTR-CD. This meta-analysis included 14 studies with over 70,000 images, demonstrating that AI models are highly accured (pooled AUC 0.959) and efficient compared to manual methods (168).
There are a high-quality meta-analysis and multiple large-scale clinical studies supporting the use of AI for the prevention and detection of CTR-CD, though randomized clinical trials are still lacking.
6 Critical appraisal and evidence gaps
Despite the growing body of literature on cardiotoxicity prevention, several limitations temper the strength of current evidence. First, most pharmacological trials are relatively small, single-center studies with short follow-up, often relying on surrogate endpoints such as LVEF or GLS rather than hard outcomes like heart failure, hospitalization or mortality. Even meta-analyses, though more robust, are influenced by heterogeneity in study design, patient populations and definitions of cardiotoxicity. As a result, while agents such as RAAS inhibitors, beta-blockers, statins and dexrazoxane demonstrate consistent preservation of LVEF, their impact on long-term clinical outcomes remains uncertain.
For non-pharmacological interventions, evidence quality is further constrained. Exercise and nutrition strategies are supported by safety data and improvements in functional parameters, but most trials remain underpowered, heterogeneous in protocols, and focused on surrogate markers. This limits generalizability and prevents strong class-of-recommendation grading in guidelines.
Emerging tools such as artificial intelligence, telemedicine and omics sciences show considerable promise, yet are still in exploratory phases. Most studies are retrospective or based on pilot cohorts, underscoring the urgent need for multicentered, prospective validation before widespread clinical adoption.
Finally, while current guidelines converge on the importance of cardiovascular risk factor management, they diverge in the strength of recommendations for prophylactic pharmacotherapy. This reflects a broader gap in harmonized, global consensus and highlights the need for large-scale, international randomized trials with longer follow-up.
In summary, the field is rapidly evolving but still characterized by significant evidence gaps. Addressing these limitations will be essential to move from surrogate-based preventive strategies to interventions that meaningfully improve survival and quality of life in cancer patients.
7 Conclusions
Cardiotoxicity remain a central challenge in modern oncology, significantly affecting prognosis and survivorship. Strongest evidence supports dexrazoxane, RAASi and beta-blockers in selected high-risk patients, as well as strict control of cardiovascular risk factors, which together represent the cornerstone of current preventive strategies. Exercise and nutritional interventions are safe and improve functional capacity and quality of life, although their impact on hard cardiovascular outcomes is less well established.
Promising approaches—including statin, metformin, SGLT2i and emerging technologies such as AI, telemedicine and omics sciences—expand the preventive armamentarium but remain investigational.
International guidelines converge on baseline risk stratification and multidisciplinary management yet diverge in the strength of recommendation for prophylactic pharmacotherapy. This underscores the urgent need for harmonized global guidance supported by multicenter randomized trials with longer follow-up, focusing on clinically meaningful endpoints.
In conclusion, cardio-oncology prevention must evolve towards an integrated, evidence-based and globally applicable model, combining validated pharmacological therapies, lifestyle interventions and innovative technologies to optimize survival and quality of life for cancer patients and survivors.
Statements
Author contributions
MM: Conceptualization, Writing – original draft, Writing – review & editing. LF: Conceptualization, Writing – original draft, Writing – review & editing. NC: Conceptualization, Writing – original draft, Writing – review & editing. MDd: Writing – review & editing. MDs: Writing – review & editing. CC: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT (OpenAI, version GPT-5, 2025) was employed exclusively to enhance the manuscript's language for native-level fluency and to assist in the creation of figure sections.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Siegel RL Miller KD Fuchs HE Jemal A . Cancer statistics, 2022. CA Cancer J Clin. (2022) 72(1):7–33. 10.3322/caac.21708
2.
Karim-Kos HE de Vries E Soerjomataram I Lemmens V Siesling S Coebergh JWW . Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. (2008) 44(10):1345–89. 10.1016/j.ejca.2007.12.015
3.
Kang MJ Jung KW Bang SH Choi SH Park EH Hwa Yun E et al Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. Cancer Res Treat. (2023) 55(2):385–99. 10.4143/crt.2023.447
4.
Shelton J Zotow E Smith L Johnson SA Thomson CS Ahmad A et al 25 year trends in cancer incidence and mortality among adults aged 35–69 years in the UK, 1993–2018: retrospective secondary analysis. The BMJ. (2024) 384:e076962. 10.1136/bmj-2023-076962
5.
Wu C Lin D Ma F Jiang F Wang Y . New progress in elucidating the relationship between cancer therapy and cardiovascular toxicity. Biosci Trends. (2021) 15(4):211–8. 10.5582/bst.2021.01278
6.
Gutierrez C Rajendram P Pastores SM . Toxicities associated with immunotherapy and approach to cardiotoxicity with novel cancer therapies. Crit Care Clin. (2021) 37(1):47–67. 10.1016/j.ccc.2020.08.003
7.
Chen M Xue J Wang M Yang J Chen T . Cardiovascular complications of pan-cancer therapies: the need for cardio-oncology. Cancers (Basel). (2023) 15(11):3055. 10.3390/cancers15113055
8.
Khouri MG Douglas PS Mackey JR Martin M Scott JM Scherrer-Crosbie M et al Cancer therapy-induced cardiac toxicity in early breast cancer addressing the unresolved issues. Circulation. (2012) 126(23):2749–63. 10.1161/CIRCULATIONAHA.112.100560
9.
Leong DP Waliany S Abdel-Qadir H Atkins KM Neilan TG Lang NN et al Cardiovascular considerations during cancer therapy. JACC Cardio Oncol. (2024) 6(6):815–34. 10.1016/j.jaccao.2024.06.005
10.
Curigliano G Lenihan D Fradley M Ganatra S Barac A Blaes A et al Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. (2020) 2(2):171–90. 10.1016/j.annonc.2019.10.023
11.
Perez IE Taveras Alam S Hernandez GA Sancassani R . Cancer therapy-related cardiac dysfunction: an overview for the clinician. Clin Med Insights Cardiol. (2019) 13:1–11. 10.1177/1179546819866445
12.
Armenian SH Lacchetti C Barac A Carver J Constine LS Denduluri N et al Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2017) 35 8(8):893–911. 10.1200/JCO.2016.70.5400
13.
Lyon AR López-Fernánde T Couch LS Asteggiano R Aznar MC Bergler-Klei J et al 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J. (2022) 43(41):4229–361. 10.1093/eurheartj/ehac244
14.
Mecinaj A Gulati G Ree AH Gravdehaug B Røsjø H Steine K et al Impact of the ESC cardio-oncology guidelines biomarker criteria on incidence of cancer therapy–related cardiac dysfunction. JACC CardioOncol. (2024) 6(1):83–95. 10.1016/j.jaccao.2023.10.008
15.
Theofilis P Vlachakis PK Oikonomou E Drakopoulou M Karakasis P Apostolos A et al Cancer therapy-related cardiac dysfunction: a review of current trends in epidemiology, diagnosis, and treatment. Biomedicines. (2024) 12(12):2914. 10.3390/biomedicines12122914
16.
Bloom MW Hamo CE Cardinale D Ky B Nohria A Baer L et al Cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. (2016) 9(1):e002661. 10.1161/CIRCHEARTFAILURE.115.002661
17.
Ching C Gustafson D Thavendiranathan P Fish JE . Cancer therapy-related cardiac dysfunction: is endothelial dysfunction at the heart of the matter?Clin Sci. (2021) 135(12):1487–503. 10.1042/CS20210059
18.
Haj-Yehia E Michel L Mincu RI Rassaf T Totzeck M . Prevention of cancer-therapy related cardiac dysfunction. Curr Heart Fail Rep. (2025) 22(1):9. 10.1007/s11897-025-00697-x
19.
Manrique CR Park M Tiwari N Plana JC Garcia MJ . Diagnostic strategies for early recognition of cancer therapeutics–related cardiac dysfunction. Clin Med Insights Cardiol. (2017) 11:2017. 10.1177/1179546817697983
20.
Oikonomou EK Kokkinidis DG Kampaktsis PN Amir EA Marwick TH Gupta D et al Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. (2019) 4(10):1007–18. 10.1001/jamacardio.2019.2952
21.
Akbalaeva BA Shulzhenko LV Pershukov IV Raiimbek uulu N Batyraliev TA Gurovich OV et al Speckle-Tracking echocardiography in assessment of breast cancer therapy-related subclinical cardiac dysfunction. Innov Med Kuban. (2024) 9(2):8–15. 10.35401/2541-9897-2024-9-2-8-15
22.
Sławiński G Hawryszko M Liżewska-Springer A Nabiałek-Trojanowska I Lewicka E . Global longitudinal strain in cardio-oncology: a review. Cancers (Basel). (2023) 15(3):986. 10.3390/cancers15030986
23.
Di Lisi D Manno G Madaudo C Filorizzo C Intravaia RCM Galassi AR et al Chemotherapy-related cardiac dysfunction: the usefulness of myocardial work indices. Int J Cardiovasc Imaging. (2023) 39(10):1845–53. 10.1007/s10554-023-02897-9
24.
Kosmala W Negishi T Thavendiranathan P Penicka M De Blois J Murbræch K et al Incremental value of myocardial work over global longitudinal strain in the surveillance for cancer-treatment-related cardiac dysfunction: a case–control study. J Clin Med. (2022) 11(4):912. 10.3390/jcm11040912
25.
Oliveira C Coutinho R Flores R Medeiros P Pires C Mané F et al The predictive role of speckle-tracking and left ventricular ejection fraction estimation using 2D and 3D echocardiography in the detection of chemotherapy related cardiotoxicity. Eur Heart J. (2021) 42(Supplement_1). 10.1093/eurheartj/ehab724.2859
26.
Esmaeilzadeh M Urzua Fresno CM Somerset E Shalmon T Amir E Fan CPS et al A combined echocardiography approach for the diagnosis of cancer therapy–related cardiac dysfunction in women with early-stage breast cancer. JAMA Cardiol. (2022) 7(3):330–40. 10.1001/jamacardio.2021.5881
27.
Pawlonka J Buchalska B Buczma K Borzuta H Kamińska K Cudnoch-Jędrzejewska A . Targeting the renin–angiotensin–aldosterone system (RAAS) for cardiovascular protection and enhanced oncological outcomes: review. Curr Treat Options Oncol. (2024) 25(11):1406–27. 10.1007/s11864-024-01270-9
28.
Li J Bin Lee ARY Tariq A Lau G Yau CE Tan LL et al Comparing renin-angiotensin-aldosterone blockade regimens for long-term chemotherapy-related cardiac dysfunction: a network meta-analysis. Cardiovasc Drugs Ther. (2025) 39(1):171–86. 10.1007/s10557-023-07457-w
29.
Cardinale D Colombo A Sandri MT Lamantia G Colombo N Civelli M et al Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. (2006) 114(23):2474–81. 10.1161/CIRCULATIONAHA.106.635144
30.
Cardinale D Ciceri F Latini R Franzosi MG Sandri MT Civelli M et al Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the international CardioOncology society-one trial. Eur J Cancer. (2018) 94:126–37. 10.1016/j.ejca.2018.02.005
31.
Bosch X Rovira M Sitges M Domènech A Ortiz-Pérez JT de Caralt TM et al Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left ventricular dysfunction with enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of malignant hEmopathies). J Am Coll Cardiol. (2013) 61(23):2355–62. 10.1016/j.jacc.2013.02.072
32.
Meattini I Becherini C Martella F Del Bene MR Saieva C Bacci C et al Cardioprotection in patients with anthracycline-treated breast cancer: final analysis from the 2×2 randomized, placebo-controlled, double-blind SAFE trial. ESMO Open. (2025) 10(6):105116. 10.1016/j.esmoop.2025.105116
33.
Austin D Maier RH Akhter N Sayari M Ogundimu E Maddox JM et al Preventing cardiac damage in patients treated for breast cancer and lymphoma: the PROACT clinical trial. JACC CardioOncol. (2024) 6(5):684–96. 10.1016/j.jaccao.2024.07.010
34.
Cadeddu C Piras A Mantovani G Deidda M Dessì M Madeddu C et al Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. (2010) 160(3):487.e1–7. 10.1016/j.ahj.2010.05.037
35.
Gulati G Heck SL Ree AH Hoffmann P Schulz-Menger J Fagerland MW et al Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. (2016) 37(21):1671–80. 10.1093/eurheartj/ehw022
36.
Akpek M Ozdogru I Sahin O Inanc M Dogan A Yazici C et al Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail. (2015) 17(1):81–9. 10.1002/ejhf.196
37.
Yun S Vincelette ND Abraham I . Cardioprotective role of β-blockers and angiotensin antagonists in early-onset anthracyclines-induced cardiotoxicity in adult patients: a systematic review and meta-analysis. Postgrad Med J. (2015) 91(1081):627–33. 10.1136/postgradmedj-2015-133535
38.
Dong H Yao L Wang M Wang M Li X Sun X et al Can ACEI/ARB prevent the cardiotoxicity caused by chemotherapy in early-stage breast cancer?-a meta-analysis of randomized controlled trials. Transl Cancer Res. (2020) 9(11):7034–43. 10.21037/tcr-20-1869
39.
Caspani F Tralongo AC Campiotti L Asteggiano R Guasti L Squizzato A . Prevention of anthracycline-induced cardiotoxicity: a systematic review and meta-analysis. Intern Emerg Med. (2021) 16(2):477–86. 10.1007/s11739-020-02508-8
40.
Harmouch W Thakker R Dang A Etewa AM Suthar K Hayek S et al ACEi and ARBs as primary prevention of cancer therapy-related cardiomyopathy in patients undergoing chemotherapy with anthracyclines: a systematic review and meta-analysis. Cardiol Ther. (2025) 14(2):141–59. 10.1007/s40119-025-00401-z
41.
Chiang CH Wang SS Chang YC Chiang CH Chen CY Chen YJ et al The effect of renin-angiotensin-aldosterone system inhibitors on outcomes of patients treated with immune checkpoint inhibitors: a retrospective cohort study. Clin Oncol (R Coll Radiol). (2023) 35(7):446–53. 10.1016/j.clon.2023.02.014
42.
Moey MYY Liles DK Carabello BA . Concomitant use of renin-angiotensin-aldosterone system inhibitors prevent trastuzumab-induced cardiotoxicity in HER2+breast cancer patients: an institutional retrospective study. CardioOncology. (2019) 5:9. 10.1186/s40959-019-0043-8
43.
Oh AR Park J Lee JH Min JJ Gook J Jang JN et al The use of renin angiotensin aldosterone system inhibitors may be associated with decreased mortality after cancer surgery. Sci Rep. (2022) 12(1):1–9. 10.1038/s41598-022-10759-y
44.
Mir A Badi Y Bugazia S Nourelden AZ Fathallah AH Ragab KM et al Efficacy and safety of cardioprotective drugs in chemotherapy-induced cardiotoxicity: an updated systematic review & network meta-analysis. CardioOncology. (2023) 9(1):10. 10.1186/s40959-023-00159-0
45.
Li X Li Y Zhang T Xiong X Liu N Pang B et al Role of cardioprotective agents on chemotherapy-induced heart failure: a systematic review and network meta-analysis of randomized controlled trials. Pharmacol Res. (2020) 151:104577. 10.1016/j.phrs.2019.104577
46.
Sobiborowicz-Sadowska AM Kamińska K Cudnoch-Jędrzejewska A . Neprilysin inhibition in the prevention of anthracycline-induced cardiotoxicity. Cancers (Basel). (2023) 15(1):312. 10.3390/cancers15010312
47.
Kim D Jang G Hwang J Wei X Kim H Son J et al Combined therapy of low-dose angiotensin receptor–neprilysin inhibitor and sodium–glucose cotransporter-2 inhibitor prevents doxorubicin-induced cardiac dysfunction in rodent model with minimal adverse effects. Pharmaceutics. (2022) 14(12):2629. 10.3390/pharmaceutics14122629
48.
Hsu YL Lee CH Chung WP Lee WH Lee KT Tsai JH et al Primary cardioprotective effect of sacubitril/valsartan in breast cancer patients receiving adjuvant therapy. Eur J Heart Fail. (2025). 10.1002/ejhf.3758
49.
Mecinaj A Gulati G Heck S Holte E Fagerland M Larsen A et al Rationale and design of the PRevention of cArdiac dysfunction during adjuvant breast cancer therapy (PRADA II) trial: a randomized, placebo-controlled, multicenter trial. Cardiooncology. (2021) 7(1):33. 10.1186/s40959-021-00115-w
50.
Gilchrist SC Barac A Ades PA Alfano CM Franklin BA Jones LW et al Cardio-Oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. (2019) 139(21):E997–1012. 10.1161/CIR.0000000000000679
51.
Brown SA . Preventive cardio-oncology: the time has Come. Front Cardiovasc Med. (2020) 6:187. 10.3389/fcvm.2019.00187
52.
Addison D Branch M Baik AH Fradley MG Okwuosa T Reding KW et al Equity in cardio-oncology care and research: a scientific statement from the American Heart Association. Circulation. (2023) 148(3):297–308. 10.1161/CIR.0000000000001158
53.
Asnani A . Beta-blockers for primary prevention of anthracycline cardiotoxicity: not quite ready for prime time. J Am Coll Cardiol. (2018) 71(20):2291–2. 10.1016/j.jacc.2018.03.461
54.
Avila MS Ayub-Ferreira SM de Barros Wanderley MR das Dores Cruz F Gonçalves Brandão SM Rigaud VOC et al Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. (2018) 71(20):2281–90. 10.1016/j.jacc.2018.02.049
55.
Attar A Behnagh AK Hosseini M Amanollahi F Shafiekhani P Kabir A . Beta-Blockers for primary prevention of anthracycline-induced cardiac toxicity: an updated meta-analysis of randomized clinical trials. Cardiovasc Ther. (2022) 2022:8367444. 10.1155/2022/8367444
56.
Henriksen PA Hall P MacPherson IR Joshi SS Singh T Maclean M et al Multicenter, prospective, randomized controlled trial of high-sensitivity cardiac troponin I-guided combination angiotensin receptor blockade and Beta-blocker therapy to prevent anthracycline cardiotoxicity: the cardiac CARE trial. Circulation. (2023) 148(21):1680–90. 10.1161/CIRCULATIONAHA.123.064274
57.
Costa IBS da S Furtado RHM Drager LF de Barros E Silva PGM de Melo MDT Araruna P et al Effects of carvedilol on the prevention of cardiotoxicity induced by anthracyclines: design and rationale of the CARDIOTOX trial. Am Heart J. (2025) 285:1–11. 10.1016/j.ahj.2025.02.014
58.
Dabour MS George MY Daniel MR Blaes AH Zordoky BN . The cardioprotective and anticancer effects of SGLT2 inhibitors: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. (2024) 6(2):159–82. 10.1016/j.jaccao.2024.01.007
59.
Quagliariello V Di Mauro A Ferrara G Bruzzese F Palma G Luciano A et al Cardio–renal and systemic effects of SGLT2i dapagliflozin on short-term anthracycline and HER-2-blocking agent therapy-induced cardiotoxicity. Antioxidants. (2025) 14(5):612. 10.3390/antiox14050612
60.
Bhatti AW Patel R Dani SS Khadke S Makwana B Lessey C et al SGLT2i and primary prevention of cancer therapy-related cardiac dysfunction in patients with diabetes. JACC CardioOncol. (2024) 6(6):863–75. 10.1016/j.jaccao.2024.08.001
61.
Daniele AJ Gregorietti V Costa D López-Fernández T . Use of EMPAgliflozin in the prevention of CARDiotoxicity: the EMPACARD - PILOT trial. CardioOncology. (2024) 10(1):58. 10.1186/s40959-024-00260-y
62.
Dicembrini I Nreu B Mannucci E Monami M . Sodium-glucose co-transporter-2 (SGLT-2) inhibitors and cancer: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. (2019) 21(8):1871–7. 10.1111/dom.13745
63.
Abdel-Qadir H Carrasco R Austin PC Chen Y Zhou L Fang J et al The association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in anthracycline-treated patients with cancer. JACC CardioOncol. (2023) 5(3):318–28. 10.1016/j.jaccao.2023.03.011
64.
Gongora CA Drobni ZD Quinaglia Araujo Costa Silva T Zafar A Gong J Zlotoff DA et al Sodium-Glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. JACC Heart Fail. (2022) 10(8):559–67. 10.1016/j.jchf.2022.03.006
65.
Fath AR Aglan M Aglan A Chilton RJ Trakhtenbroit A Al-Shammary OA et al Cardioprotective potential of sodium-glucose cotransporter-2 inhibitors in patients with cancer treated with anthracyclines: an observational study. Am J Cardiol. (2024) 222:175–82. 10.1016/j.amjcard.2024.04.032
66.
Yourman LC Cenzer IS Boscardin WJ Nguyen BT Smith AK Schonberg MA et al Evaluation of time to benefit of statins for the primary prevention of cardiovascular events in adults aged 50 to 75 years: a meta-analysis. JAMA Intern Med. (2021) 181(2):179–85. 10.1001/jamainternmed.2020.6084
67.
Armitage J Baigent C Barnes E John Betteridge D Blackwell L Blazing M et al Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. (2019) 393:407–15. 10.1016/S0140-6736(18)31942-1
68.
Yebyo HG Aschmann HE Kaufmann M Puhan MA . Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: a systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am Heart J. (2019) 210:18–28. 10.1016/j.ahj.2018.12.007
69.
Cai T Abel L Langford O Monaghan G Aronson JK Stevens RJ et al Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. Br Med J. (2021) 374:1537. 10.1136/bmj.n1537
70.
Contaldi C D’Aniello C Panico D Zito A Calabrò P Di Lorenzo E et al Cancer-therapy-related cardiac dysfunction: latest advances in prevention and treatment. Life. (2025) 15:471. 10.3390/life15030471
71.
Jiang R Lou L Shi W Chen Y Fu Z Liu S et al Statins in mitigating anticancer treatment-related cardiovascular disease. Int J Mol Sci. (2024) 25(18):10177. 10.3390/ijms251810177
72.
Felix N Nogueira PC Silva IM Costa TA Campello CA Stecca C et al Cardio-protective effects of statins in patients undergoing anthracycline-based chemotherapy: an updated meta-analysis of randomized controlled trials. Eur J Intern Med. (2024) 126:43–8. 10.1016/j.ejim.2024.04.007
73.
Thavendiranathan P Houbois C Marwick TH Kei T Saha S Runeckles K et al Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur Heart J Cardiovasc Pharmacother. (2023) 9(6):515–25. 10.1093/ehjcvp/pvad031
74.
Abdel-Qadir H Bobrowski D Zhou L Austin PC Calvillo-Argüelles O Amir E et al Statin exposure and risk of heart failure after anthracycline-or trastuzumab-based chemotherapy for early breast cancer: a propensity score‒matched cohort study. J Am Heart Assoc. (2021) 10(2):1–12. 10.1161/JAHA.119.018393
75.
Yu H Zhong X Gao P Shi J Wu Z Guo Z et al The potential effect of metformin on cancer: an Umbrella review. Front Endocrinol (Lausanne). (2019) 10:617. 10.3389/fendo.2019.00617
76.
Morales DR Morris AD . Metformin in cancer treatment and prevention. Annu Rev Med. (2015) 66:17–29. 10.1146/annurev-med-062613-093128
77.
Kordes S Pollak MN Zwinderman AH Mathôt RA Weterman MJ Beeker A et al Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. (2015) 16(7):839–47. 10.1016/S1470-2045(15)00027-3
78.
Wink KCJ Belderbos JSA Dieleman EMT Rossi M Rasch CRN Damhuis RAM et al Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother Oncol. (2016) 118(3):453–9. 10.1016/j.radonc.2016.01.012
79.
O’Connor L Bailey-Whyte M Bhattacharya M Butera G Hardell KNL Seidenberg AB et al Association of metformin use and cancer incidence: a systematic review and meta-analysis. J Natl Cancer Inst. (2024) 116(4):518–29. 10.1093/jnci/djae021
80.
Chow EJ Aggarwal S Doody DR Aplenc R Armenian SH Baker KS et al Dexrazoxane and long-term heart function in survivors of childhood cancer. J Clin Oncol. (2023) 41(12):2248–57. 10.1200/JCO.22.02423
81.
Macedo AVS Hajjar LA Lyon AR Nascimento BR Putzu A Rossi L et al Efficacy of dexrazoxane in preventing anthracycline cardiotoxicity in breast cancer. JACC CardioOncol. (2019) 1(1):68–79. 10.1016/j.jaccao.2019.08.003
82.
Swain SM Vici P . The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J Cancer Res Clin Oncol. (2004) 130(1):1–7. 10.1007/s00432-003-0498-7
83.
Zheng H Zhan H . Dexrazoxane makes doxorubicin-induced heart failure a rare event in sarcoma patients receiving high cumulative doses. Cardio-Oncology. (2025) 11(1):29. 10.1186/s40959-025-00323-8
84.
Rivero-Santana B Saldaña-García J Caro-Codón J Zamora P Moliner P Martínez Monzonis A et al Anthracycline-induced cardiovascular toxicity: validation of the heart failure association and international cardio-oncology society risk score. Eur Heart J. (2025) 46(3):273–84. 10.1093/eurheartj/ehae496
85.
Caro-Codón J López-Fernández T Álvarez-Ortega C Zamora Auñón P Rodríguez IR Prieto PG et al Cardiovascular risk factors during cancer treatment. Prevalence and prognostic relevance: insights from the CARDIOTOX registry. Eur J Prev Cardiol. (2022) 29(6):859–68. 10.1093/eurjpc/zwaa034
86.
Tong J Senechal I Ramalingam S Lyon AR . Risk assessment prior to cardiotoxic anticancer therapies in 7 steps. Br J Hosp Med. (2025) 86(1):1–21. 10.12968/hmed.2024.0632
87.
Weaver KE Dressler EVM Lee SC Foraker RE Smith S Klepin HD et al Acceptability of the AH-HA cardiovascular health assessment tool among oncology providers and post-treatment cancer survivors in community oncology. JCO Oncol Pract. (2023) 19(11_suppl):423–423. 10.1200/OP.2023.19.11_suppl.423
88.
Soh C Marwick T . Comparison of cardiovascular disease risk assessment tools for heart failure prediction among cancer patients. Eur Heart J. (2024) 45(Supplement_1):ehae666.2707. 10.1093/eurheartj/ehae666.2707
89.
Cho H Lee S Sim SH Park IH Lee KS Kwak MH et al Cumulative incidence of chemotherapy-induced cardiotoxicity during a 2-year follow-up period in breast cancer patients. Breast Cancer Res Treat. (2020) 182(2):333–43. 10.1007/s10549-020-05703-5
90.
Guenancia C Lefebvre A Cardinale D Yu AF Ladoire S Ghiringhelli F et al Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol. (2016) 34(26):3157–65. 10.1200/JCO.2016.67.4846
91.
Hauwanga WN McBenedict B Amadi ES Dohadwala TK Johnny C Asaju F et al A systematic review of the cardiotoxic effects of targeted therapies in oncology. Cureus. (2024) 16(8):e66258. 10.7759/cureus.66258
92.
Kobat H Elkonaissi I Foreman E Davidson M Idaikkadar P O’Brien M et al Smoking, diabetes Mellitus, and previous cardiovascular disease as predictors of anticancer treatment-induced cardiotoxicity in non-small-cell lung cancer: a real-world study. Clin Lung Cancer. (2024) 25(1):e35–42. 10.1016/j.cllc.2023.09.007
93.
Kourek C Touloupaki M Rempakos A Loritis K Tsougkos E Paraskevaidis I et al Cardioprotective strategies from cardiotoxicity in cancer patients: a comprehensive review. J Cardiovasc Dev Dis. (2022) 9(8):259. 10.3390/jcdd9080259
94.
Li C Ngorsuraches S Chou C Chen L Qian J . Risk factors of fluoropyrimidine induced cardiotoxicity among cancer patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. (2021) 162:103346. 10.1016/j.critrevonc.2021.103346
95.
Mach F Baigent C Catapano AL Koskinas KC Casula M Badimon L et al 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European atherosclerosis society (EAS). Eur Heart J. (2020) 41(1):111–88. 10.1093/eurheartj/ehz455
96.
McCarthy CP Bruno RM Brouwers S Canavan MD Ceconi C Christodorescu RM et al 2024 ESC guidelines for the management of elevated blood pressure and hypertension: developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European stroke organisation (ESO). Eur Heart J. (2024) 45(38):3912–4018. 10.1093/eurheartj/ehae178
97.
Chow EJ Chen Y Armstrong GT Baldwin LM Cai CR Gibson TM et al Underdiagnosis and undertreatment of modifiable cardiovascular risk factors among survivors of childhood cancer. J Am Heart Assoc. (2022) 11(12):e024735. 10.1161/JAHA.121.024735
98.
Cohen JB Geara AS Hogan JJ Townsend RR . Hypertension in cancer patients and survivors: epidemiology, diagnosis, and management. JACC CardioOncol. (2019) 1(2):238–51. 10.1016/j.jaccao.2019.11.009
99.
Díaz-Balboa E Peña-Gil C Rodríguez-Romero B Cuesta-Vargas AI Lado-Baleato O Martínez-Monzonís A et al Exercise-based cardio-oncology rehabilitation for cardiotoxicity prevention during breast cancer chemotherapy: the ONCORE randomized controlled trial. Prog Cardiovasc Dis. (2024) 85:74–81. 10.1016/j.pcad.2024.02.002
100.
Murray J Bennett H Bezak E Perry R . The role of exercise in the prevention of cancer therapy-related cardiac dysfunction in breast cancer patients undergoing chemotherapy: systematic review. Eur J Prev Cardiol. (2022) 29(3):463–72. 10.1093/eurjpc/zwab006
101.
Campana N Fazzini L Donisi C Nava A Migliari M Deidda M et al Exercise prescription in cardio-oncology. J Clin Med. (2025) 14(11):3724. 10.3390/jcm14113724
102.
Wilson RL Christopher CN Yang EH Barac A Adams SC Scott JM et al Incorporating exercise training into cardio-oncology care: selecting: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. (2023) 5(5):553–69. 10.1016/j.jaccao.2023.08.008
103.
Alizadehasl A Malekzadeh Moghani M Mirzaei H Keshvari M Fadaei F Cramer H et al Cardioprotective diet to prevent anthracycline-induced cardiotoxicity in patients with breast cancer: a randomized open-label controlled trial. J Integr Complement Med. (2024) 30(10):995–1001. 10.1089/jicm.2023.0777
104.
Stephenson E Mclaughlin M Bray JW Saxton JM Vince RV . Nutrition modulation of cardiotoxicity in breast cancer: a scoping review. Nutrients. (2024) 16(21):3777. 10.3390/nu16213777
105.
Zhang XY Yang KL Li Y Zhao Y Jiang KW Wang Q et al Can dietary nutrients prevent cancer chemotherapy-induced cardiotoxicity? An evidence mapping of human studies and animal models. Front Cardiovasc Med. (2022) 9:921609. 10.3389/fcvm.2022.921609
106.
Guida F Masetti R Andreozzi L Zama D Fabi M Meli M et al The role of nutrition in primary and secondary prevention of cardiovascular damage in childhood cancer survivors. Nutrients. (2022) 14(16):3279. 10.3390/nu14163279
107.
Ravasco P . Nutrition in cancer patients. J Clin Med. (2019) 8(8):1211. 10.3390/jcm8081211
108.
Callegari IOM Viviani H Frias E Oliveira A De Melo M . Dietary intervention and physical exercise in prevention and treatment of pharmacological cardiotoxicity during cancer. Concilium. (2023) 23(17):761–85. 10.53660/CLM-2176-23Q17
109.
Sadigova T . Advances in cardio-oncology: the emerging role of Sglt2 inhibitors in cardioprotection. Am J Biomed Life Sci. (2024) 12(6):93–7. 10.11648/j.ajbls.20241206.11
110.
Monteiro AV Pinto R Pires ML Borges M Pinto F Zuzarte P et al Meditation as a stress management strategy in cardiac rehabilitation for coronary artery disease patients: a randomized controlled trial. Eur J Prev Cardiol. (2023) 30(Supplement_1):i312. 10.1093/eurjpc/zwad125.236
111.
Rao A DiGiacomo M Phillips JL Newton PJ Zecchin R Denniss AR et al Integrating MEditatioN inTO heaRt disease (the MENTOR study): phase II randomised controlled feasibility study protocol. Collegian. (2022) 29(3):414–22. 10.1016/j.colegn.2021.09.002
112.
Rao A Zecchin R Newton PJ Read SA Phillips JL Digiacomo M et al Feasibility of integrating MEditatioN inTO heaRt disease (the MENTOR study). J Cardiovasc Nurs. (2023) 38(5):492–510. 10.1097/JCN.0000000000000997
113.
Ott MJ Norris RL Bauer-Wu SM . Mindfulness meditation for oncology patients: a discussion and critical review. Integr Cancer Ther. (2006) 5(2):108–98. 10.1177/1534735406288083
114.
Boyle CC Stanton AL Ganz PA Crespi CM Bower JE . Improvements in emotion regulation following mindfulness meditation: effects on depressive symptoms and perceived stress in younger breast cancer survivors. J Consult Clin Psychol. (2017) 85(4):397–402. 10.1037/ccp0000186
115.
Wren AA Shelby RA Soo MS Huysmans Z Jarosz JA Keefe FJ . Preliminary efficacy of a lovingkindness meditation intervention for patients undergoing biopsy and breast cancer surgery: a randomized controlled pilot study. Support Care Cancer. (2019) 27(9):3583–92. 10.1007/s00520-019-4657-z
116.
Milbury K Weathers SP Durrani S Li Y Whisenant M Li J et al Online couple-based meditation intervention for patients with primary or metastatic brain tumors and their partners: results of a pilot randomized controlled trial. J Pain Symptom Manage. (2020) 59(6):1260–7. 10.1016/j.jpainsymman.2020.02.004
117.
Siripanya S Parinyanitikul N Tanaka H Suksom D . Home-based buddhist walking meditation mitigates cardiotoxicity of anthracycline chemotherapy in breast cancer patients: a randomized controlled trial. J Integr Complement Med. (2023) 29(9):562–73. 10.1089/jicm.2022.0778
118.
Alexandre J Cautela J Ederhy S Damaj GL Salem JE Barlesi F et al Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. J Am Heart Assoc. (2020) 9(18):18403. 10.1161/JAHA.120.018403
119.
Bisceglia I Canale ML Silvestris N Gallucci G Camerini A Inno A et al Cancer survivorship at heart: a multidisciplinary cardio-oncology roadmap for healthcare professionals. Front Cardiovasc Med. (2023) 10:1223660. 10.3389/fcvm.2023.1223660
120.
Di Lisi D Cadeddu Dessalvi C Zito C Madaudo C Manganaro R Mercurio V et al Management of cancer patients at high and very-high risk of cardiotoxicity: main questions and answers. Curr Probl Cardiol. (2024) 49(3):102229. 10.1016/j.cpcardiol.2023.102229
121.
López-Fernández T Farmakis D Ameri P Asteggiano R de Azambuja E Aznar M et al European Society of cardiology core curriculum for cardio-oncology. Eur J Heart Fail. (2024) 26(4):754–71. 10.1002/ejhf.3102
122.
Chianca M Fabiani I Del Franco A Grigoratos C Aimo A Panichella G et al Management and treatment of cardiotoxicity due to anticancer drugs: 10 questions and answers. Eur J Prev Cardiol. (2022) 29(17):2163–72. 10.1093/eurjpc/zwac170
123.
Fabiani I Chianca M Aimo A Emdin M Dent S Fedele A et al Use of new and emerging cancer drugs: what the cardiologist needs to know. Eur Heart J. (2024) 45(22):1971–87. 10.1093/eurheartj/ehae161
124.
Anez LP Haase T Harst L Ibrahim K Christoph M Koesters M et al Multidisciplinary strategies for cardiotoxicity management in ambulatory care: exploring practices, insights, and prospects for advancement. Eur Heart J. (2024) 45(Supplement_1):ehae666.3146. 10.1093/eurheartj/ehae666.3146
125.
Peng J Rushton M Johnson C Brezden-Masley C Sulpher J Chiu MG et al An international survey of healthcare providers’ knowledge of cardiac complications of cancer treatments. Cardio-oncology. (2019) 5(1):12. 10.1186/s40959-019-0049-2
126.
Barros-Gomes S Herrmann J Mulvagh SL Lerman A Lin G Villarraga HR . Rationale for setting up a cardio-oncology unit: our experience at mayo clinic. Cardio-oncology. (2016) 2(1):5. 10.1186/s40959-016-0014-2
127.
Figueiral M Paldino A Fazzini L Pereira NL . Genetic biomarkers in heart failure: from gene panels to polygenic risk scores. Curr Heart Fail Rep. (2024) 21(6):554–69. 10.1007/s11897-024-00687-5
128.
Fazzini L Campana N Cossu S Deidda M Madaudo C Quagliariello V et al Genetic background in patients with cancer therapy-induced cardiomyopathy. J Clin Med. (2025) 14(4):1286. 10.3390/jcm14041286
129.
Kim Y Seidman JG Seidman CE . Genetics of cancer therapy-associated cardiotoxicity. J Mol Cell Cardiol. (2022) 167:85–91. 10.1016/j.yjmcc.2022.03.010
130.
Yang X Li G Guan M Bapat A Dai Q Zhong C et al Potential gene association studies of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. Front Cardiovasc Med. (2021):8. 10.3389/fcvm.2021.651269
131.
Zambelli A Della Porta MG Eleuteri E De Giuli L Catalano O Tondini C et al Predicting and preventing cardiotoxicity in the era of breast cancer targeted therapies. Novel molecular tools for clinical issues. Breast. (2011) 20(2):176–83. 10.1016/j.breast.2010.11.002
132.
Chong JH Chuah CTH Lee CG . Revolutionising cardio-oncology care with precision genomics. Int J Mol Sci. (2025) 26(5):2052. 10.3390/ijms26052052
133.
Yang X Li G Yang T Guan M An N Yang F et al Possible susceptibility genes for intervention against chemotherapy-induced cardiotoxicity. Oxid Med Cell Longev. (2020) 2020:4894625. 10.1155/2020/4894625
134.
Yeh ETH Chang HM . Oncocardiology-past, present, and future: a review. JAMA Cardiol. (2016) 19(9):1066–72. 10.1001/jamacardio.2016.2132
135.
Koldehoff A Danner M Civello D Rhiem K Stock S Müller D . Cost-effectiveness of targeted genetic testing for breast and ovarian cancer: a systematic review. Value Health. (2021) 24(2):303–12. 10.1016/j.jval.2020.09.016
136.
Payne K Gavan SP Wright SJ Thompson AJ . Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat Rev Genet. (2018) 19(4):235–46. 10.1038/nrg.2017.108
137.
Schwarze K Buchanan J Taylor JC Wordsworth S . Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature. Genet Med. (2018) 20(10):1122–30. 10.1038/gim.2017.247
138.
Weiner S Austin S Carr G Kidwell KM Resnicow K Stoffel EM et al Oncologist knowledge of cost of genetic testing. J Clin Oncol. (2022) 40(16_suppl):10591–10591. 10.1200/JCO.2022.40.16_suppl.10591
139.
Tocchetti CG Cadeddu C Di Lisi D Femminò S Madonna R Mele D et al From molecular mechanisms to clinical management of antineoplastic drug-induced cardiovascular toxicity: a translational overview. Antioxid Redox Signal. (2019) 30(18):2110–53. 10.1089/ars.2016.6930
140.
Fazzini L Caggiari L Deidda M Onnis C Saba L Mercuro G et al Metabolomic profiles on antiblastic cardiotoxicity: new perspectives for early diagnosis and cardioprotection. J Clin Med. (2022) 11(22):6745. 10.3390/jcm11226745
141.
Antoniadi K Thomaidis N Nihoyannopoulos P Toutouzas K Gikas E Kelaidi C et al Prognostic factors for cardiotoxicity among children with cancer: definition, causes, and diagnosis with omics technologies. Diagnostics. (2023) 13(11):1864. 10.3390/diagnostics13111864
142.
Unger K Li Y Yeh C Barac A Srichai MB Ballew EA et al Plasma metabolite biomarkers predictive of radiation induced cardiotoxicity. Radiother Oncol. (2020) 152:133–45. 10.1016/j.radonc.2020.04.018
143.
Dougherty BV Moore CJ Rawls KD Jenior ML Chun B Nagdas S et al Identifying metabolic adaptations characteristic of cardiotoxicity using paired transcriptomics and metabolomics data integrated with a computational model of heart metabolism. PLoS Comput Biol. (2024) 20(2):e1011919. 10.1371/journal.pcbi.1011919
144.
Kuang Z Kong M Yan N Ma X Wu M Li J . Precision cardio-oncology: update on omics-based diagnostic methods. Curr Treat Options Oncol. (2024) 25(5):679–701. 10.1007/s11864-024-01203-6
145.
Lal JC Fang MZ Hussain M Abraham A Tonegawa-Kuji R Hou Y et al Discovery of plasma proteins and metabolites associated with left ventricular cardiac dysfunction in pan-cancer patients. Cardiooncology. (2025) 11(1):17. 10.1186/s40959-025-00309-6
146.
Brown SA Sandhu N Herrmann J . Systems biology approaches to adverse drug effects: the example of cardio-oncology. Nat Rev Clin Oncol. (2015) 12(12):718–31. 10.1038/nrclinonc.2015.168
147.
Dreyfuss AD Bravo PE Koumenis C Ky B . Precision cardio-oncology. J Nucl Med. (2019) 60(4):443–50. 10.2967/jnumed.118.220137
148.
Lal H Kolaja KL Force T . Cancer genetics and the cardiotoxicity of the therapeutics. J Am Coll Cardiol. (2013) 61 3(3):267–74. 10.1016/j.jacc.2012.05.066
149.
Styczkiewicz K Mędrek S Styczkiewicz M Jankowski P Szmit S Stec S . Cardiooncological and telemedical care in patients with colon cancer - management optimisation (cartagina study). J Hypertens. (2018) 36(Supplement 1):e171–2. 10.1097/01.hjh.0000539470.74302.cd
150.
Viñas-Mendieta AE Gallardo-Grajeda A López-Fernández T . Cardio-oncology: chances and challenges. Basic Res Cardiol. (2025) 120:3–9. 10.1007/s00395-024-01080-y
151.
Kuan PX Chan WK Fern Ying DK Rahman MAA Peariasamy KM Lai NM et al Efficacy of telemedicine for the management of cardiovascular disease: a systematic review and meta-analysis. Lancet Digit Health. (2022) 4(9):676–91. 10.1016/S2589-7500(22)00124-8
152.
Salloum FN Tocchetti CG Ameri P Ardehali H Asnani A de Boer RA et al Priorities in cardio-oncology basic and translational science. JACC CardioOncol. (2023) 5(6):715–31. 10.1016/j.jaccao.2023.08.003
153.
Chang WT Liu CF Feng YH Liao CT Wang JJ Chen ZC et al An artificial intelligence approach for predicting cardiotoxicity in breast cancer patients receiving anthracycline. Arch Toxicol. (2022) 96(10):2731–7. 10.1007/s00204-022-03341-y
154.
Güntürkün F Akbilgic O Davis RL Armstrong GT Howell RM Jefferies JL et al Artificial intelligence assisted prediction of late onset cardiomyopathy among childhood cancer survivors. SSRN Electronic J. (2020). 10.2139/ssrn.3680602
155.
Jacobs JEJ Greason G Mangold KE Wildiers H Willems R Janssens S et al Artificial intelligence ECG as a novel screening tool to detect a newly abnormal left ventricular ejection fraction after anthracycline-based cancer therapy. Eur J Prev Cardiol. (2024) 31(5):560–6. 10.1093/eurjpc/zwad348
156.
Khera R Asnani AH Krive J Addison D Zhu H Vasbinder A et al Artificial intelligence to enhance precision medicine in cardio-oncology: a scientific statement from the American Heart Association. Circ Genom Precis Med. (2025) 18(2):e000097. 10.1161/HCG.0000000000000097
157.
Oikonomou EK Sangha V Dhingra LS Aminorroaya A Coppi A Krumholz HM et al Artificial intelligence-enhanced risk stratification of cancer therapeutics-related cardiac dysfunction using electrocardiographic images. Circ Cardiovasc Qual Outcomes. (2024) 18(1):11504. 10.1161/CIRCOUTCOMES.124.011504
158.
Scalia IG Pathangey G Abdelnabi M Ibrahim OH Abdelfattah FE Pietri MP et al Applications of artificial intelligence for the prediction and diagnosis of cancer therapy-related cardiac dysfunction in oncology patients. Cancers (Basel). (2025) 17(4):605. 10.3390/cancers17040605
159.
Yagi R Goto S Himeno Y Katsumata Y Hashimoto M Macrae CA et al Artificial intelligence-enabled prediction of chemotherapy-induced cardiotoxicity from baseline electrocardiograms. Nat Commun. (2024) 15(1):2536. 10.1038/s41467-024-45733-x
160.
Kwan JM Oikonomou EK Henry ML Sinusas AJ . Multimodality advanced cardiovascular and molecular imaging for early detection and monitoring of cancer therapy-associated cardiotoxicity and the role of artificial intelligence and big data. Front Cardiovasc Med. (2022) 9:829553. 10.3389/fcvm.2022.829553
161.
Madan N Lucas J Akhter N Collier P Cheng F Guha A et al Artificial intelligence and imaging: opportunities in cardio-oncology. Am Heart J Plus: Cardiology Res Pract. (2022) 15:100126. 10.1016/j.ahjo.2022.100126
162.
Ahmed B Abdelaziz D Abdelmajid B . Advancements in cardiotoxicity detection and assessment through artificial intelligence: a comprehensive review. 2024 4th International Conference on Innovative Research in Applied Science, Engineering and Technology (IRASET) (2024). p. 1–8
163.
Mushcab H Ramis MA AlRujaib A Eskandarani R Sunbul T AlOtaibi A et al Application of artificial intelligence in cardio-oncology imaging for cancer therapy–related cardiovascular toxicity: systematic review. JMIR Cancer. (2025) 11(1):e63964. 10.2196/63964
164.
Guha A Shah V Nahle T Singh S Kunhiraman HH Shehnaz F et al Artificial intelligence applications in cardio-oncology: a comprehensive review. Curr Cardiol Rep. (2025) 27(1):56. 10.1007/s11886-025-02215-w
165.
Martinez DSL Noseworthy PA Akbilgic O Herrmann J Ruddy KJ Hamid A et al Artificial intelligence opportunities in cardio-oncology: overview with spotlight on electrocardiography. Am Heart J Plus: Cardiology Res Pract. (2022) 15:100129. 10.1016/j.ahjo.2022.100129
166.
Zheng Y Chen Z Huang S Zhang N Wang Y Hong S et al Machine learning in cardio-oncology: new insights from an emerging discipline. Rev Cardiovasc Med. (2023) 24(10):296. 10.31083/j.rcm2410296
167.
Echefu G Shah R Sanchez Z Rickards J Brown SA . Artificial intelligence: applications in cardio-oncology and potential impact on racial disparities. Am Heart J Plus: Cardiology Res Pract. (2024) 48:100479. 10.1016/j.ahjo.2024.100479
168.
Kufel J Czogalik Ł Bielówka M Magiera M Mitręga A Dudek P et al Measurement of cardiothoracic ratio on chest x-rays using artificial intelligence—a systematic review and meta-analysis. J Clin Med. (2024) 13(16):4659. 10.3390/jcm13164659
Summary
Keywords
cardio-oncology, prevention, cardiotoxicity, cancer therapy-related cardiac dysfunction, pharmacological strategies, non-pharmacological strategies, artificial intelligente
Citation
Migliari M, Fazzini L, Campana N, Deidda M, Dessì M and Cadeddu Dessalvi C (2025) Current strategies for prevention of cancer therapy-related cardiotoxicity: pharmacological, non-pharmacological and emerging approaches. Front. Cardiovasc. Med. 12:1668308. doi: 10.3389/fcvm.2025.1668308
Received
17 July 2025
Accepted
25 September 2025
Published
23 October 2025
Volume
12 - 2025
Edited by
Prabhu Mathiyalagan, Benthos Prime Central, United States
Reviewed by
Ayman R. Fath, Creighton University, United States
Shimaa Hussein Kotb, Sphinx University, Egypt
Jieli Tong, Tan Tock Seng Hospital, Singapore
Updates
Copyright
© 2025 Migliari, Fazzini, Campana, Deidda, Dessì and Cadeddu Dessalvi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Christian Cadeddu Dessalvi cadedduc@unica.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.