- 1Internal Medicine Department, Pham Ngoc Thach University of Medicine, Ho Chi Minh, Vietnam
- 2Tam Anh Hospital, Ho Chi Minh, Vietnam
Introduction: Coronary artery disease (CAD) remains a leading cause of morbidity and mortality worldwide. Lipoprotein (a) [Lp(a)] has emerged as an independent risk factor for CAD, but its role in predicting coronary severity in Vietnamese populations remains unclear.
Objectives: To evaluate the value of Lp(a) in predicting the severity of coronary artery stenosis in chronic CAD.
Materials and methods: This cross-sectional study was conducted at Tam Anh General Hospital from June 2024 to June 2025, including 138 patients diagnosed with chronic CAD. Demographic, clinical, laboratory, and coronary angiographic data were collected. CAD severity was assessed using the Gensini score. Logistic regression and ROC analysis were employed to evaluate the predicting value of Lp(a).
Results: Severe CAD (Gensini score >40) was present in 31.9% of the cohort. Patients with Lp(a) ≥30 mg/dL exhibited a significantly higher prevalence of severe CAD (72.5% vs. 8.0%). Lp(a) levels correlated strongly with the Gensini score. The optimal cut-off for predicting severe CAD was 30.6 mg/dL (AUC = 0.869). Multivariate analysis confirmed Lp(a) as an independent predictor.
Conclusions: Lp(a) ≥30 mg/dL is strongly associated with severe coronary artery stenosis. Lp(a) is a valuable independent predictor of CAD severity and may serve as an essential tool for risk stratification in clinical practice.
Introduction
Coronary artery disease (CAD) is a major global health concern and a leading cause of morbidity and mortality worldwide (1–3), particularly in low- and developing countries. Vietnam is witnessing a growing burden of CAD due to rapid economic development and changing lifestyles. Traditional risk factors such as hypertension, diabetes, dyslipidemia, smoking, and sedentary behavior are prevalent, but novel markers like Lipoprotein (a) [Lp(a)] are emerging as significant contributors to cardiovascular risk (4–6).
Lp(a) is structurally similar to low-density lipoprotein (LDL) but possesses an additional apolipoprotein (a) component, enhancing its pro-atherogenic and pro-thrombotic properties (5, 7–11). Elevated Lp(a) levels have been consistently linked to increased risks of myocardial infarction, stroke, and peripheral artery disease. Despite substantial evidence from Western populations, data regarding the role of Lp(a) in Southeast Asian and specifically Vietnamese populations remain limited (12–15).
This study aimed to investigate the association between Lp(a) levels and CAD severity in Vietnamese patients with chronic CAD using the Gensini scoring system, providing region-specific insights that may inform clinical practice.
Materials and methods
This was a cross-sectional study conducted at Tam Anh General Hospital, Ho Chi Minh City, Vietnam, between June 2024 and June 2025. The study population included patients aged 18 years or older with chronic coronary artery disease (CAD) diagnosed according to the 2019 ESC guidelines, who underwent invasive coronary angiography and had available Lipoprotein(a) [Lp(a)] measurements. Patients with congenital coronary anomalies, end-stage renal disease, active malignancy, pregnancy, or breastfeeding were excluded. Consecutive eligible patients during the study period were enrolled to minimize selection bias. Demographic, clinical, and laboratory characteristics—including comorbidities, lipid profiles, and Lp(a) levels—were collected, along with angiographic findings. The primary outcome was the association between Lp(a) concentration and CAD severity, which was assessed using the Gensini score. Lp(a) was measured by immunoturbidimetric assay (Beckman Coulter AU680 analyzer). Statistical analyses included descriptive statistics, Mann–Whitney U test, chi-square test, Spearman correlation, receiver operating characteristic (ROC) analysis, and logistic regression modeling. A significance level of p < 0.05 was considered statistically significant.
Results
Study population
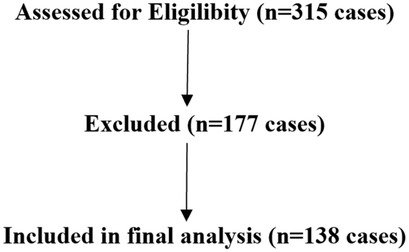
A total of 315 patients were analyzed. There were 177 patients were excluded included: stage V CKD (n = 42), active malignancy (n = 31), congenital anomalies (n = 7), pregnancy/breastfeeding (n = 3), no Lp(a) data (n = 94) (Figure 1).
Demographics and clinical characteristics
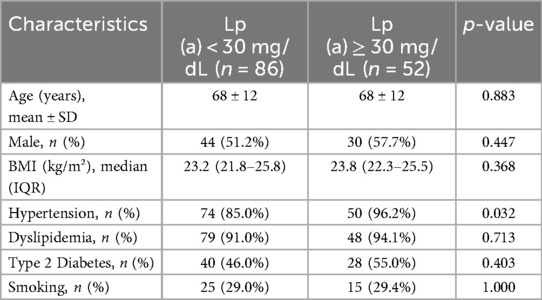
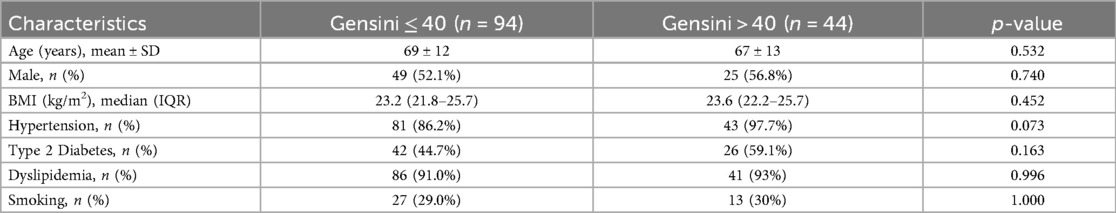
The mean age of the study population was 68 ± 12 years, and 74 patients (54%) were male.The baseline demographic and cardiovascular risk factors of the two groups are summarized in Table 1. There were no significant differences between the groups in age, gender distribution, body mass index (BMI), type 2 diabetes mellitus, dyslipidemia, or smoking status between patients with Lipoprotein (a) [Lp(a)] levels ≥30 mg/dL and those with Lp(a) levels <30 mg/dL. However, hypertension was significantly more prevalent in the high Lp(a) group (96.2% vs. 85.0%, p = 0.032).
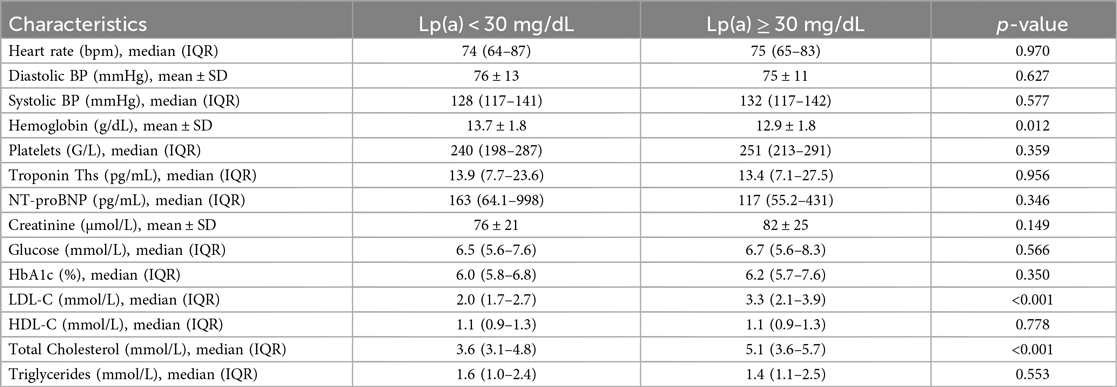
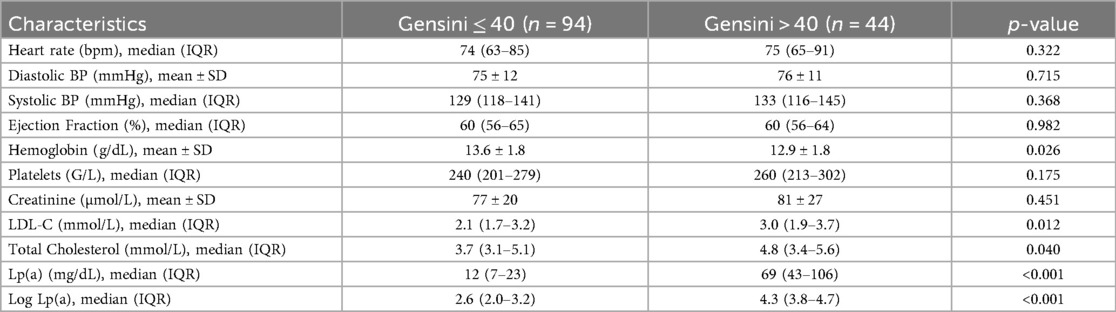
The clinical and laboratory characteristics are presented in Table 2. There were no significant differences between groups regarding heart rate, systolic and diastolic blood pressure, platelet count, creatinine, blood glucose, HbA1c, HDL-C, triglycerides, NT-proBNP, or Troponin Ths levels (all p > 0.05).
However, patients with Lp(a) ≥ 30 mg/dL had significantly:
• Lower hemoglobin levels: 12.9 ± 1.8 g/dL vs. 13.7 ± 1.8 g/dL; p = 0.012
• Higher LDL-C levels: 3.3 mmol/L vs. 2.0 mmol/L; p < 0.001
• Higher total cholesterol levels: 5.1 mmol/L vs. 3.6 mmol/L; p < 0.001
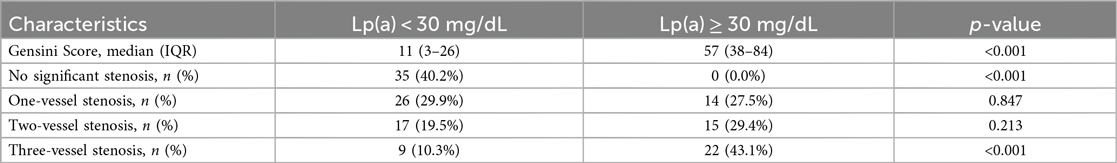
The median Gensini score was significantly higher in the Lp(a) ≥ 30 mg/dL group compared to the <30 mg/dL group [57 (IQR: 38–84) vs. 11 (IQR: 3–26); p < 0.001]. Severe multi-vessel disease (≥3 vessels with >50% stenosis) was observed in 43.1% of the Lp(a) ≥ 30 mg/dL group vs. 10.3% in the <30 mg/dl group (p < 0.001) (Table 3).
Comparison by CAD severity
When stratified by Gensini score, there were no significant differences in demographic characteristics or most clinical and laboratory parameters between the mild and severe stenosis groups, except that patients with severe stenosis had significantly lower hemoglobin (12.9 vs. 13.6 g/dL; p = 0.026) and higher LDL-C (3.0 vs. 2.1 mmol/L; p = 0.012) and total cholesterol levels (4.8 vs. 3.7 mmol/L; p = 0.040). Lp(a) levels were significantly higher in the severe group (69 vs. 12 mg/dL; p < 0.001) (Tables 4, 5).
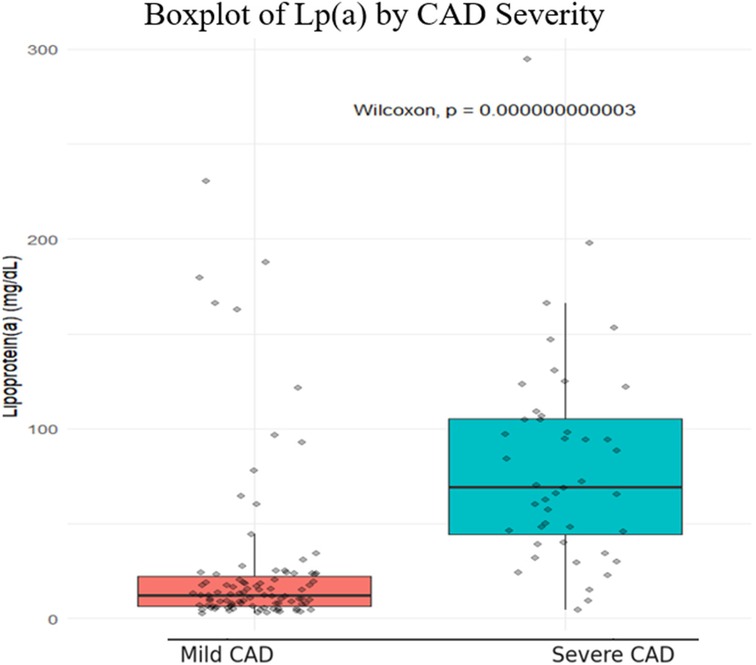
Distribution of Lp(a)
The boxplot showed that Lp(a) levels in the severe CAD group (Gensini > 40) were about six times higher than in the mild group (median 69 vs. 12 mg/dL), with a highly significant difference (p < 0.001) (Figure 2).
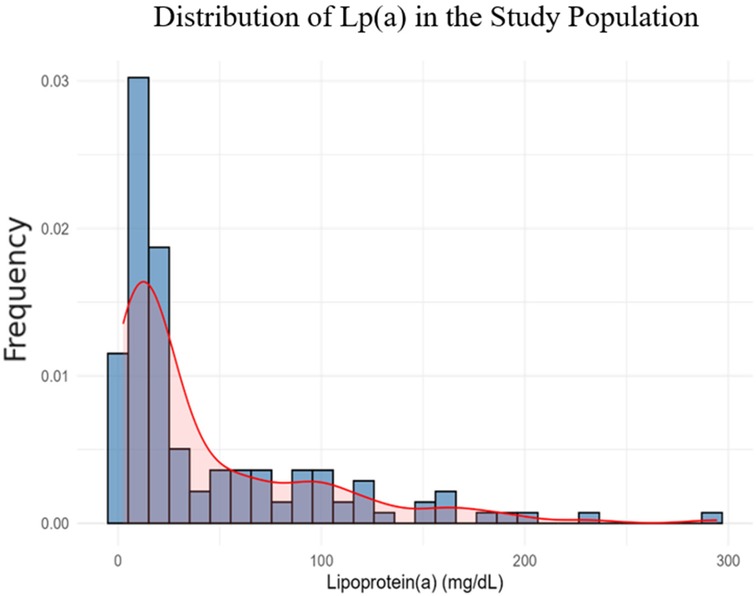
The distribution of Lp(a) was right-skewed (p < 0.001), with most values below 30 mg/dL. Therefore, we applied a natural logarithmic transformation to normalize the data and improve the reliability of subsequent statistical analyses (Figure 3).
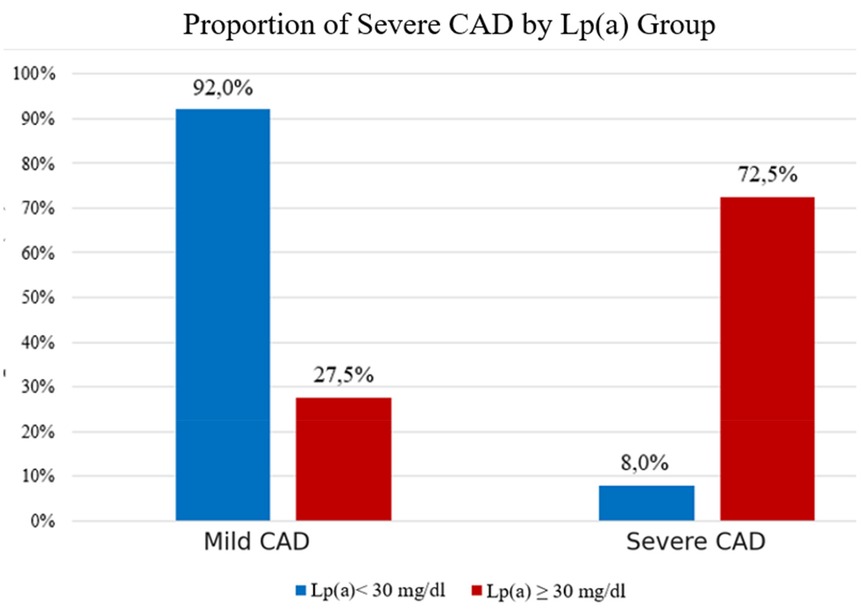
Proportion of severe CAD by Lp(a) group
The study showed that the prevalence of severe coronary stenosis was significantly higher in the Lp(a) ≥ 30 mg/dL group (72.5%) compared to the < 30 mg/dL group (8.0%) (p < 0.001). Conversely, mild stenosis was more common in the low Lp(a) group (92.0%). These findings confirm that elevated Lp(a) is a strong risk factor for severe coronary artery disease in this population (Figure 4).
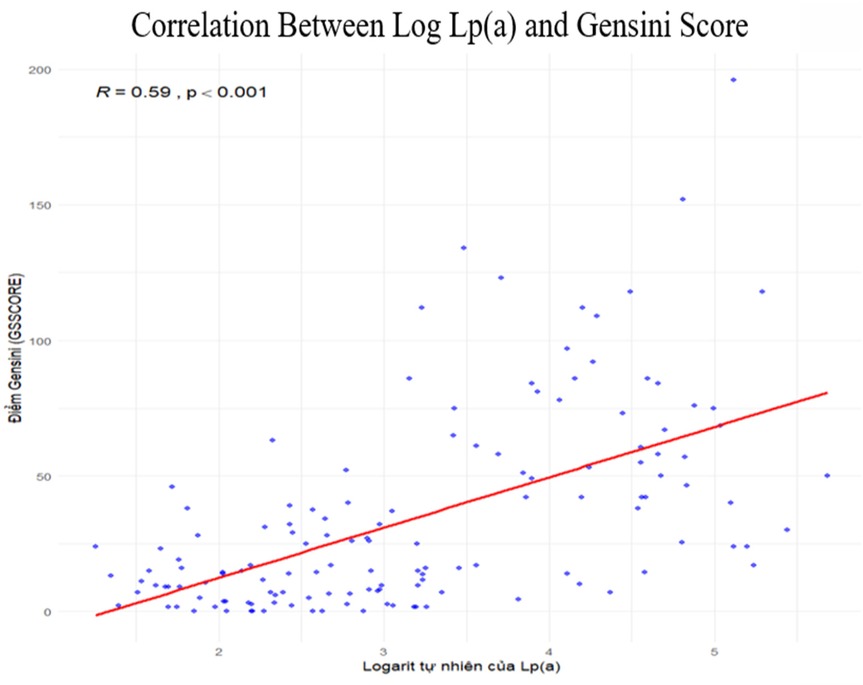
Correlation between Lp(a) and Gensini score
Spearman correlation analysis demonstrated a significant positive correlation between the natural logarithm of Lp(a) and the Gensini score (ρ = 0.59; p < 0.001). Higher Lp(a) levels were associated with more severe coronary stenosis, with a clear upward trend across the sample. No major outliers or abnormal distributions were observed, indicating a consistent relationship (Figure 5).
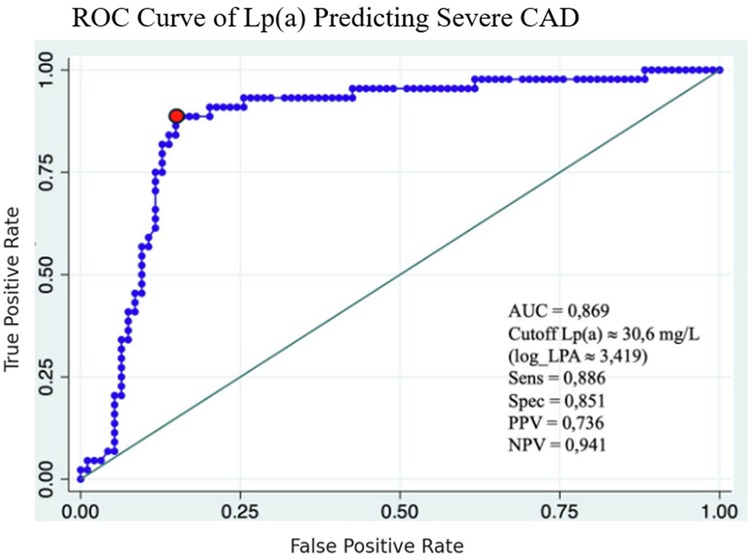
ROC curve analysis
The ROC analysis identified 30.6 mg/dL as the optimal cutoff for Lp(a) to predict severe coronary stenosis, with a sensitivity of 88.6% and specificity of 85.1%. The positive and negative predictive values at this cutoff were 73.6% and 94.1%, respectively, highlighting the clinical value of Lp(a) in assessing disease severity (Figure 6).
Logistic regression results
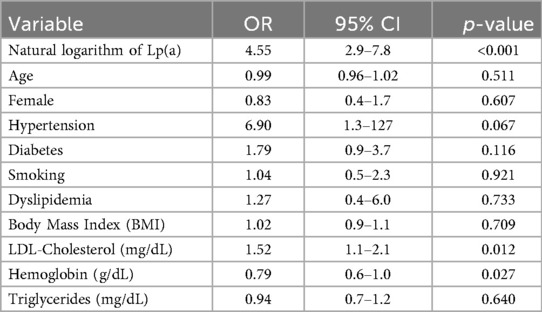
Univariate logistic regression showed that the natural logarithm of Lp(a) was strongly associated with severe coronary stenosis (OR = 4.55; 95% CI: 2.9–7.8; p < 0.001), with LDL-C (OR = 1.52; p = 0.012) and hemoglobin (OR = 0.79; p = 0.027) also showing significant associations. Lp(a) had the strongest predictive value. Hypertension and diabetes showed a trend but were not statistically significant. Other factors, including age, sex, smoking, dyslipidemia, BMI, and triglycerides, were not associated with disease severity. The wide confidence interval for hypertension suggests possible estimation bias due to limited sample size (Table 6).
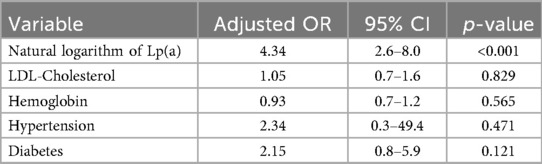
When performing multivariate logistic regression analysis, we selected variables with a p-value < 0.2 from the univariate logistic regression (including LDL-C, hemoglobin, hypertension, and diabetes). In the multivariate logistic regression, only the natural logarithm of Lipoprotein (a) [Lp(a)] remained a strong independent predictor of severe coronary stenosis (adjusted OR = 4.34, 95% CI: 2.6–8.0; p < 0.001). Although LDL-C and hemoglobin were significant in the univariate analysis, they lost significance after adjustment. Hypertension and diabetes were not independent predictors in either model (Table 7).
Discussion
This cross-sectional study of 138 patients with chronic coronary artery disease (CAD) demonstrated a strong association between elevated Lipoprotein(a) [Lp(a)] levels and the severity of coronary artery stenosis. Patients with Lp(a) ≥ 30 mg/dL were significantly more likely to have severe CAD (72.5%) compared to those with Lp(a) < 30 mg/dL (8.0%). Among various factors analyzed, only the natural logarithm of Lp(a) remained an independent predictor of severe stenosis in both univariate and multivariate logistic regression, with an adjusted odds ratio of 4.34 (95% CI: 2.6–8.0, p < 0.001). These findings emphasize the strong predictive value of Lp(a) in assessing anatomical CAD burden. Among the 138 study participants, we recorded 74 males and 64 females, with males accounting for 53.6% (n = 74). This proportion is lower than that reported in international studies by David Leistner et al. (10) and Christoph Sinning et al. (16), where the male proportions were 70.1% and 77.4%, respectively, in European countries. In a 2024 study conducted in China by Yang et al. (17), the proportion of males was comparable to that in our study, accounting for 58.28%. In contrast, a domestic study by Thái Thị Phương Thảo et al. (18) reported a higher proportion of males (72.9%) compared to our findings. Overall, coronary artery disease (CAD) tends to develop earlier in men than in women (6, 19). In our study, the male-to-female ratio was nearly equal, reflecting a shifting epidemiological trend of CAD in the population of Ho Chi Minh City. When analyzing BMI data based on weight and height, we recorded a median BMI of 23.3 kg/m2 in our study, with an interquartile range of 22.1–25.7 kg/m2. The study conducted by Krzysztof Dyrbuś et al. (20), primarily involving a Turkish population, reported a significantly higher median BMI compared to ours, with a median value of 28.1 kg/m² (interquartile range 25.1–31.8 kg/m²). Regarding biochemical characteristics, our study recorded a mean serum creatinine concentration of 78 ± 23 µmol/L in the study population. In the group with lipoprotein(a) < 30 mg/dL, the mean creatinine level was 76.15 ± 20.79 µmol/L, while in the group with Lp(a) ≥ 30 mg/dL, it was 82.20 ± 25.06 µmol/L. There was no statistically significant difference between the two groups (p = 0.149). Our findings are consistent with those reported by He et al. (21).
Diagnostic performance of Lp(a) was further evaluated using receiver operating characteristic (ROC) analysis. An optimal cut-off value of 30.6 mg/dL yielded excellent results: an area under the curve (AUC) of 0.869, with sensitivity of 88.6% and specificity of 85.1%. These figures indicate a high discriminatory capacity for identifying patients with severe coronary stenosis. Interestingly, the AUC in our study was higher than that reported in several Western studies, suggesting possible ethnic or genetic influences on Lp(a) expression and cardiovascular risk profiles in Asian populations.
When compared to the international literature, our results are in agreement with major studies that also highlight the independent role of Lp(a) in coronary disease severity. For instance, Wang et al. found a similar association in a large cohort of patients with myocardial infarction (8), and the European LipidCardio study confirmed the value of Lp(a) using both Gensini and SYNTAX scores (10). Notably, our identified threshold (30.6 mg/dL) closely aligns with the ≥30 mg/dL level proposed by the European Atherosclerosis Society (EAS) as a clinically relevant cutoff (16, 22). These concordant findings add weight to current guideline recommendations that recognize Lp(a) as an important, genetically determined cardiovascular risk factor that warrants measurement in certain high-risk populations. Pare et al. reported that there is diversity in concentration and isoform distribution among different ethnic groups, and that the risk of cardiovascular events increases with higher lipoprotein(a) levels, especially in South Asian populations (23). A recent study by Cesaro et al. (24), conducted within the RELACS study on 975 European patients, also confirmed the association between elevated Lp(a) levels and the complexity of coronary artery disease as assessed by the SYNTAX-I and Gensini scores. Patients with Lp(a) ≥150 nmol/L had a significantly higher median Gensini score compared to those with lower levels (16.0 vs. 10.0; p < 0.01).
Despite its strengths, the study has limitations. The relatively small sample size may limit the generalizability of the results. Additionally, imbalances in subgroup distributions, particularly for traditional risk factors like hypertension or diabetes, may affect the precision of odds ratio estimates. Furthermore, as a cross-sectional study, it lacks longitudinal follow-up data to assess the impact of elevated Lp(a) on clinical outcomes such as myocardial infarction, revascularization, or cardiovascular mortality.
Nonetheless, the findings of this study offer important clinical implications. First, Lp(a) measurement may serve as a valuable tool for risk stratification in patients with chronic CAD, particularly in those with well-controlled LDL-C levels but persistent residual cardiovascular risk. Second, identifying patients with high Lp(a) could prompt consideration of more aggressive risk-reduction strategies, including lipoprotein apheresis, lifestyle modification, and future Lp(a)-lowering therapies currently in development. However, to our knowledge, this is the first study in Vietnam to systematically evaluate the association between Lp(a) and CAD severity using the Gensini score. Ethnic and genetic differences may influence Lp(a) expression and its impact on cardiovascular risk. Previous studies Enkhmaa et al. (25) and Leistner et al. (10) have shown significant ethnic variation in Lp(a)-related cardiovascular risk. Therefore, our findings provide region-specific insights that complement existing international evidence and highlight the need for further studies in Southeast Asian populations.
Lipoprotein(a) [Lp(a)] is structurally similar to low-density lipoprotein (LDL), but it possesses an additional apolipoprotein(a) component that confers unique biological properties. Apolipoprotein(a) is covalently bound to apolipoprotein B-100, and its structural resemblance to plasminogen contributes to impaired fibrinolysis, thereby promoting thrombosis. Moreover, Lp(a) carries oxidized phospholipids, which exert potent pro-inflammatory and pro-atherogenic effects. These mechanisms collectively accelerate atherosclerotic plaque development, progression, and instability, increasing the risk of myocardial infarction and other adverse cardiovascular events.
In addition to these biological mechanisms, ethnic variability in Lp(a) expression is increasingly recognized as an important determinant of cardiovascular risk. Studies have demonstrated that Lp(a) levels and their impact on atherosclerotic disease differ substantially across populations, with Southeast Asian cohorts showing distinct distributions compared with Western counterparts. This variability may be related to genetic factors, including differences in kringle IV type 2 repeat polymorphisms, as well as environmental influences. Our findings, therefore, provide region-specific insights into the role of Lp(a) in Vietnamese patients, complementing global evidence and underscoring the importance of context-specific data in cardiovascular risk assessment.
In our multivariate logistic regression, we adjusted for key covariates including LDL-C, hemoglobin, hypertension, and diabetes. Despite these adjustments, we acknowledge that residual confounding cannot be fully excluded. Important unmeasured factors such as systemic inflammation, variability in renal function, or socioeconomic determinants may still influence the observed association between Lp(a) and coronary artery disease severity. This limitation highlights the need for larger, multicenter studies with more comprehensive adjustment for potential confounders to validate and extend our findings in broader and more diverse Vietnamese populations.
In conclusion, this study supports existing international evidence and current guidelines that recognize Lp(a) as an important biomarker of atherosclerotic burden. With its strong association with severe coronary stenosis and high diagnostic performance, Lp(a) measurement should be considered in routine cardiovascular risk assessment, particularly in populations where traditional risk factors do not fully explain disease severity.
Conclusion
In conclusion, Lp(a) is a strong and independent predictor of severe coronary artery stenosis in patients with chronic coronary artery disease. A cut-off value of 30.6 mg/dL provides high sensitivity and specificity for identifying high-risk patients. These results support the clinical relevance of routine Lp(a) assessment in cardiovascular risk evaluation and may guide more personalized prevention and treatment strategies (Central Illusion).
![Receiver operating characteristic (ROC) curve illustrating the diagnostic performance of lipoprotein(a) [Lp(a)] in predicting severe coronary artery disease (CAD), with an area under the curve (AUC) of 0.869. The optimal cutoff value is approximately 30.6 mg/L, yielding a sensitivity of 0.886, specificity of 0.851, positive predictive value of 0.736, and negative predictive value of 0.941.](https://www.frontiersin.org/files/Articles/1669234/fcvm-12-1669234-HTML-r1/image_m/fcvm-12-1669234-g007.jpg)
Central Illustration. ROC Curve with AUC = 0.869, Lp(a) cutoff 30.6 mg/dL, sensitivity 88.6%, specificity 85.1%, correlation graph of log Lp(a) vs. Gensini score.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Pham Ngoc Thach University of Medicine ethics commmittee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HP: Supervision, Writing – review & editing. MH: Data curation, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2021) 42(34):3227–337. doi: 10.1093/eurheartj/ehab484
2. Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol. (2013) 61(11):1146–56. doi: 10.1016/j.jacc.2012.12.023
3. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. Jama. (2009) 302(4):412–23. doi: 10.1001/jama.2009.1063
4. Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. (2009) 361(26):2518–28. doi: 10.1056/NEJMoa0902604
5. Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler, Thromb, Vasc Biol. (2010) 30(12):2311–6. doi: 10.1161/atvbaha.108.179697
6. Xue Y, Jian S, Zhou W, Zhou Q, Xiang J, Zhu Y, et al. Associations of lipoprotein(a) with coronary atherosclerotic burden and all-cause mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Front Cardiovasc Med. (2021) 8:638679. doi: 10.3389/fcvm.2021.638679
7. Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. (2016) 57(8):1339–59. doi: 10.1194/jlr.R067314
8. Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. (2019) 40(33):2760–70. doi: 10.1093/eurheartj/ehy902
9. Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Circulation. (2014) 129(6):635–42. doi: 10.1161/circulationaha.113.004406
10. Leistner DM, Laguna-Fernandez A, Haghikia A, Abdelwahed YS, Schatz AS, Erbay A, et al. Impact of elevated lipoprotein(a) on coronary artery disease phenotype and severity. Eur J Prev Cardiol. (2024) 31(7):856–65. doi: 10.1093/eurjpc/zwae007
11. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. (2017) 69(6):692–711. doi: 10.1016/j.jacc.2016.11.042
12. Wang Y, Lv Q, Li Y, Chen S, Zhao L, Fu G, et al. Gensini score values for predicting periprocedural myocardial infarction: an observational study analysis. Medicine (Baltimore). (2022) 101(29):e29491. doi: 10.1097/md.0000000000029491
13. König M, Joshi S, Leistner DM, Landmesser U, Sinning D, Steinhagen-Thiessen E, et al. Cohort profile: role of lipoproteins in cardiovascular disease-the LipidCardio study. BMJ Open. (2019) 9(9):e030097. doi: 10.1136/bmjopen-2019-030097
14. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. (2016) 57(4):526–37. doi: 10.1194/jlr.R061648
15. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. Jama. (2009) 301(22):2331–9. doi: 10.1001/jama.2009.801
16. Sinning C, Lillpopp L, Appelbaum S, Ojeda F, Zeller T, Schnabel R, et al. Angiographic score assessment improves cardiovascular risk prediction: the clinical value of SYNTAX and Gensini application. Clin Res Cardiol. (2013) 102(7):495–503. doi: 10.1007/s00392-013-0555-4
17. Yang HH, Dou J, Guo RL, Gao J, Li HZ, Wang K, et al. Clinical diagnostic significance of combined measurement of lipoprotein(a) and neck circumference in patients with coronary heart disease. Int J Gen Med. (2024) 17:5015–27. doi: 10.2147/ijgm.S485570
18. Thái TPT, Trương TH, Trần HT. KHẢO SÁT NỒNG ĐỘ LIPOPROTEIN (A) Ở BỆNH NHÂN CÓ BỆNH ĐỘNG MẠCH VÀNH DO XƠ VỮA. Tạp chí Y học Việt Nam. (2023) 523(1):120–3. doi: 10.51298/vmj.v523i1.4424
19. Yurtseven E, Ural D, Gursoy E, Cunedioglu BO, Guler OU, Baysal K, et al. Is there a need for sex-tailored lipoprotein(a) cut-off values for coronary artery disease risk stratification? Clin Cardiol. (2024) 47(9):e70012. doi: 10.1002/clc.70012
20. Dyrbuś K, Mędrala Z, Konsek K, Nowowiejska-Wiewiora A, Trzeciak P, Skrzypek M, et al. Lipoprotein(a) and its impact on cardiovascular disease—the Polish perspective: design and first results of the Zabrze-Lipoprotein(a) registry. Arch Med Sci. (2024) 20(4):1069–76. doi: 10.5114/aoms/188294
21. He J, Lin Z, Song C, Yuan S, Bian X, Li B, et al. J-shaped association between apolipoprotein B and CV outcomes in statin-treated patients with chronic coronary syndrome. Rev Esp Cardiol (Engl Ed). (2025) 78(5):404–13. doi: 10.1016/j.rec.2024.08.004
22. Kronenberg F, Mora S, Stroes ES, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European atherosclerosis society consensus statement. Eur Heart J. (2022) 43(39):3925–46. doi: 10.1093/eurheartj/ehac361
23. Paré G, Çaku A, McQueen M, Anand SS, Enas E, Clarke R, et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation. (2019) 139(12):1472–82. doi: 10.1161/circulationaha.118.034311
24. Cesaro A, Acerbo V, Scialla F, Scherillo G, De Michele G, Panico D, et al. Role of lipoprotein(a) in cardiovascular diseases and premature acute coronary syndromes (RELACS study): impact of lipoprotein(a) levels on the premature coronary event and the severity of coronary artery disease. Nutr Metab Cardiovasc Dis. (2025) 35(5):103843. doi: 10.1016/j.numecd.2024.103843
Keywords: lipoprotein (a) [Lp (a)], gensini coronary score, predicting, coronary artery disease, coronary stenosis
Citation: Phan HT and Ho MTT (2025) Value of lipoprotein(a) in predicting severity of coronary artery stenosis in patients with chronic coronary artery disease: a cross-sectional study in Vietnam. Front. Cardiovasc. Med. 12:1669234. doi: 10.3389/fcvm.2025.1669234
Received: 19 July 2025; Accepted: 28 October 2025;
Published: 13 November 2025.
Edited by:
Konstantinos Tsarouhas, University Hospital of Larissa, GreeceReviewed by:
Ivica Bosnjak, Osijek Clinical Hospital Center, CroatiaBinaya Basyal, MedStar Washington Hospital Center, United States
Yipin Zhao, Fuwai Central China Cardiovascular Hospital, China
Copyright: © 2025 Phan and Ho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Thai Phan, cGhhbnRoYWloYW9AeWFob28uY29t
 Hao Thai Phan
Hao Thai Phan Mai Thi Tuyet Ho2
Mai Thi Tuyet Ho2