Abstract
Background:
Pericardial tamponade is a rare but life-threatening complication of catheter ablation for ventricular arrhythmias. While overt perforation—often steam-pop related—is classically implicated, alternative bleeding mechanisms are less well defined.
Case:
A 66-year-old man underwent left ventricular (LV) ablation for frequent idiopathic premature ventricular beats (34.3%). RF energy (30–40 W, ≤90 s) at the LV anterior wall markedly suppressed ectopy, followed by abrupt chest discomfort and hypotension. Fluoroscopy suggested pericardial effusion; emergent pericardiocentesis yielded 150 ml of bright red, arterialized blood with rapid hemodynamic recovery. Biventricular angiography showed no contrast extravasation, and serial echocardiography confirmed no recurrent effusion. The patient stabilized with indwelling drainage and was discharged uneventfully.
Conclusion:
Tamponade during LV ablation may occur without overt perforation. Prompt recognition and targeted drainage can be definitive when bleeding is self-limited.
Background
Pericardial tamponade is one of the complications of ventricular radiofrequency ablation. In pericardial tamponade, blood compresses the cardiac chambers, leading to hemodynamic impairment, circulatory shock, cardiac arrest, and death (1). Early detection and diagnosis of pericardial tamponade are critical for effective management of these patients. Cardiac perforation leading to tamponade is a common cause of pericardial tamponade during radiofrequency ablation, with an incidence of approximately 1%–2% (2). Most perforations are caused by steam pop phenomenon, which refers to barotrauma resulting from gas production due to excessive heating during catheter ablation or complications associated with transseptal perforation. However, radiofrequency ablation-related pericardial tamponade can also occur without explicit cardiac perforation, and may be characterized by bleeding resulting from minor injuries to the ventricular wall and/or epicardial small vessels. Here, we present a rare case of pericardial tamponade during radiofrequency ablation for idiopathic left ventricular premature beats without definite cardiac perforation and with spontaneous hemostasis.
Case report
The patient was a 66-year-old male referred to our medical institution due to palpitations. He was diagnosed with idiopathic ventricular premature beats (VPBs) via ambulatory electrocardiogram (ECG) recording, with a VPB burden of 34.3% (26,949/78,629). The patient had no history of organic heart disease, diabetes mellitus, chronic kidney disease, or coronary artery disease. Medical therapy for VPB control was limited, so catheter radiofrequency ablation for VPBs was performed. Preoperative ECG morphology of VPBs suggested the origin of premature beats was the left ventricle (LV) (Figure 1). The VPBs showed a right bundle branch block (RBBB) morphology with transition starting at V2, suggesting a possible origin from the LV apex. The preoperative cardiac ultrasound and left ventricular myocardial contrast echocardiography did not indicate any abnormalities in the left heart structure (Supplementary Figures SA,B).
Figure 1
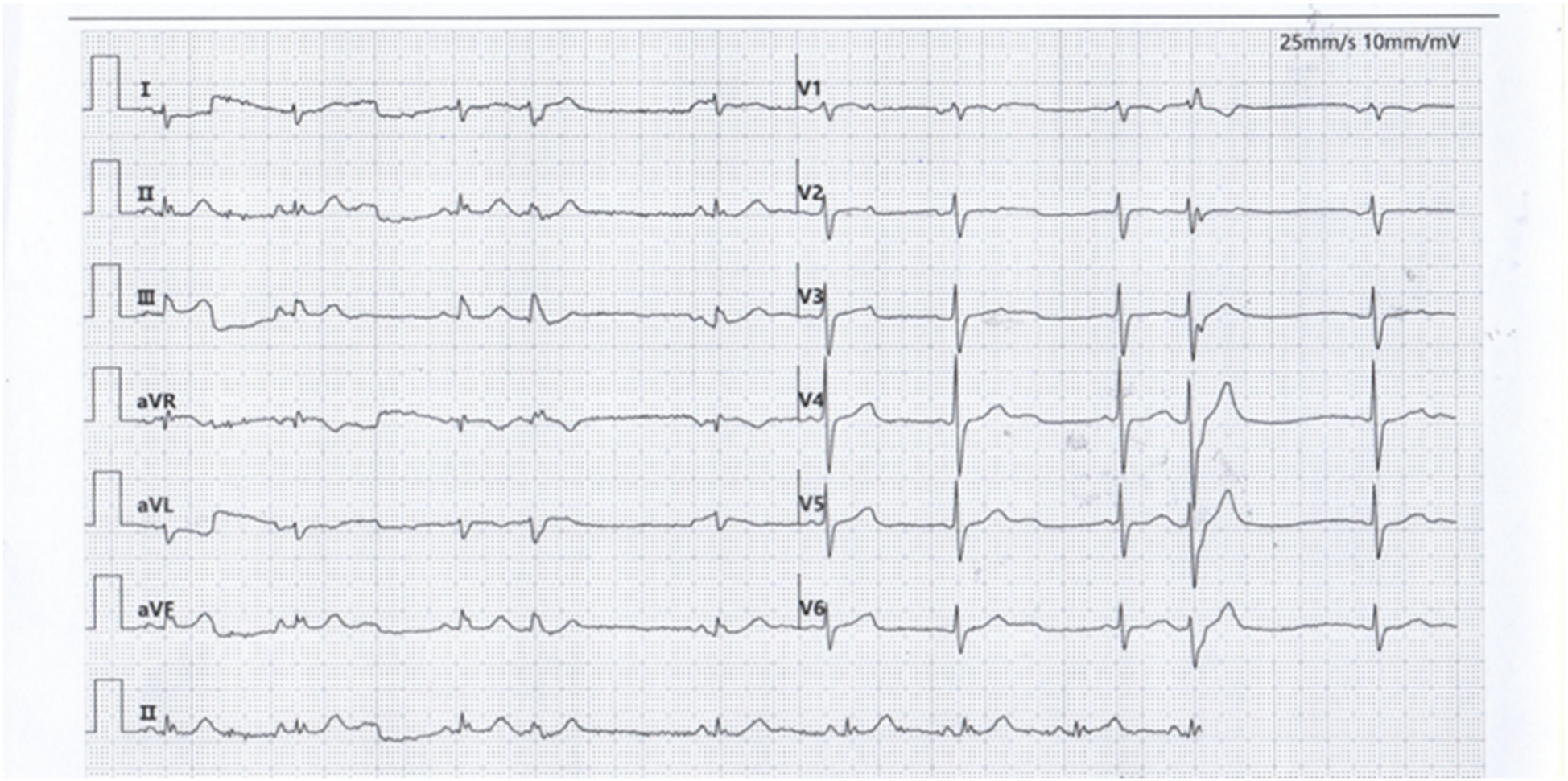
Premature ventricular contraction surface electrocardiogram.
After obtaining written informed consent from the patient, electrophysiological examination was performed, with continuous invasive arterial blood pressure monitoring via an arterial access. Under local anesthesia, the left femoral vein was punctured for his bundle electrode placement. Subsequently, the right femoral artery was punctured. Then intravenous heparin 5,000 IU was administered at the beginning of the procedure. A TCSE saline-irrigated tip catheter was advanced retrogradely through the aorta into the left ventricle for mapping and ablation using the Ensite three-dimensional mapping system. The earliest activation site of ventricular premature beats was identified at the apical portion of the left ventricular anterior wall (see target map), where radiofrequency (RF) energy was applied to terminate the premature beats.
Target map
With the radiofrequency ablation power set at 30–40 W and the target temperature at 55 °C, the radiofrequency transmission lasted up to 90 s (Figure 2). During the ablation process, steam pop was not perceived, and there were no sudden changes in ablation parameters such as impedance、power or pressure values (Figure 2). After the termination of radiofrequency, the ventricular premature beats were significantly reduced. During the observation period after ablation (not immediately after), the patient suddenly complained of chest tightness, shortness of breath, and chest pain, and the blood pressure dropped to 102/65 mmHg (baseline blood pressure value: 149/78 mmHg). x-ray fluoroscopy observation showed a translucent shadow around the heart in the pericardial cavity (Figure 3), and the patient was suspected of having pericardial tamponade. Pericardial puncture and drainage were immediately performed. After slowly withdrawing 150 ml of bright red blood, no more blood was withdrawn, and the patient's blood pressure gradually recovered and tended to be stable. The ACT value immediately before pericardiocentesis was 126 s. Protamine was not administered and no additional heparin was administered due to the short duration of ablation. The unexpectedly low ACT at the time of pericardiocentesis may reflect partial heparin metabolism and consumption. Blood gas analysis of the red blood cells drawn from the pericardium suggested arterial blood. Under fluoroscopy, the translucent zone around the heart was smaller than before. In order to further clarify the perforation site, angiography was performed in the left and right ventricles(Supplementary Videos S1–S4), and it was found that the contrast agent did not extravasate outside the cardiac cavity (Figures 4A,B). Bedside transthoracic echocardiography showed that the pericardial effusion did not further increase. After indwelling the pericardial drainage tube, the patient was returned to the cardiac care unit for further observation. After 1 day of continuous drainage, no effusion was drained, and continuous review of echocardiography showed no obvious effusion (Supplementary Figures C,D). The patient was discharged after the drainage tube was removed.
Figure 2
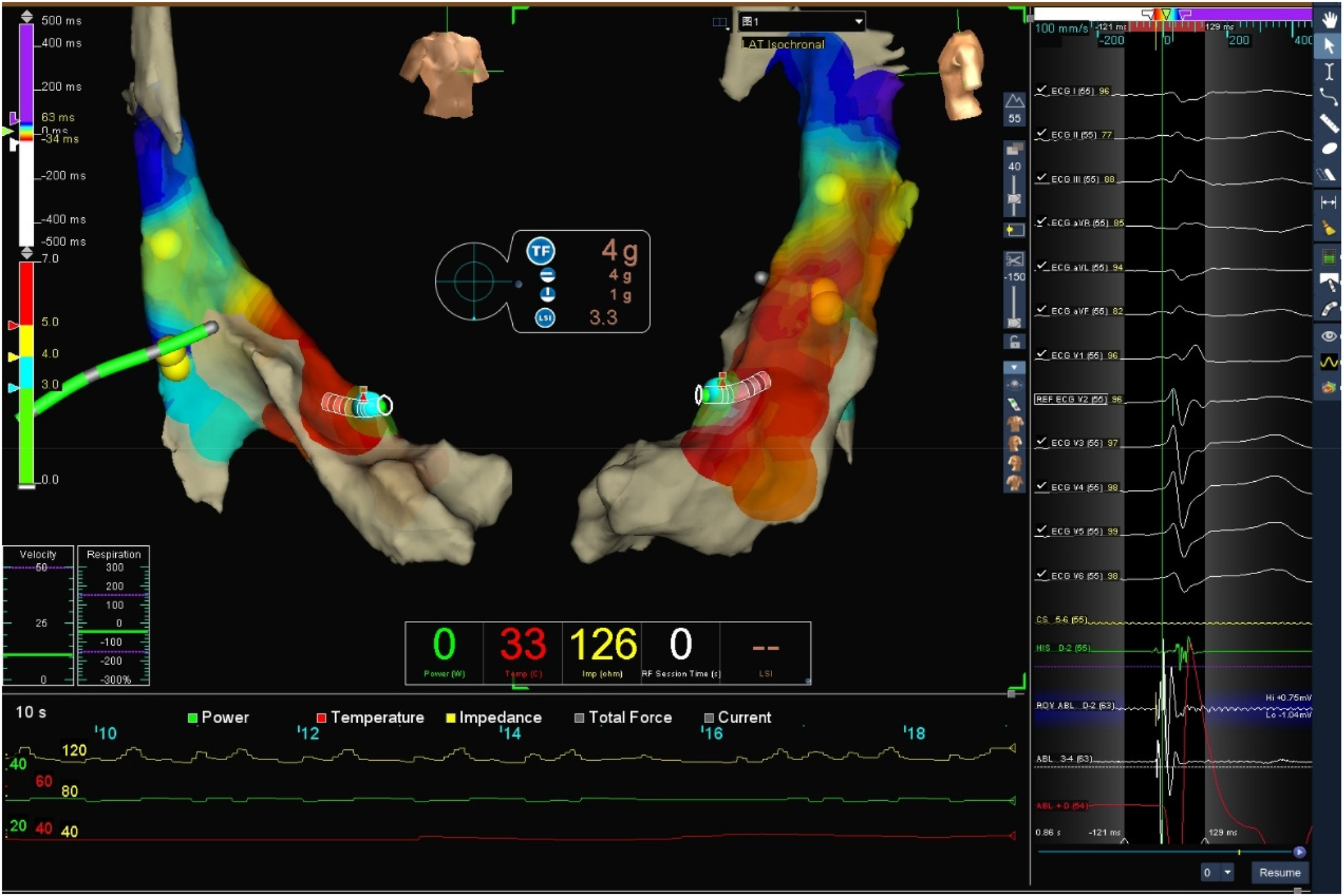
Target three-dimensional mapping image and ablation parameter curve graph: the green curve represents power, red represents temperature, and yellow represents impedance.
Figure 3
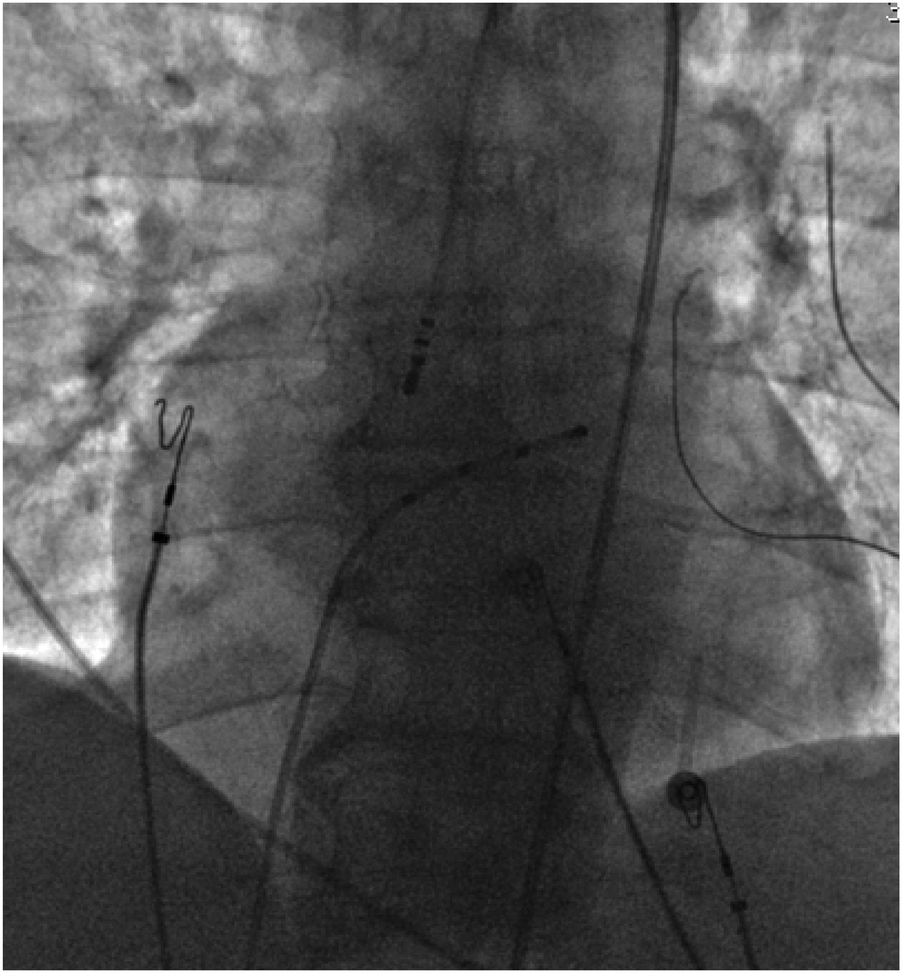
Pericardial effusion.
Figure 4
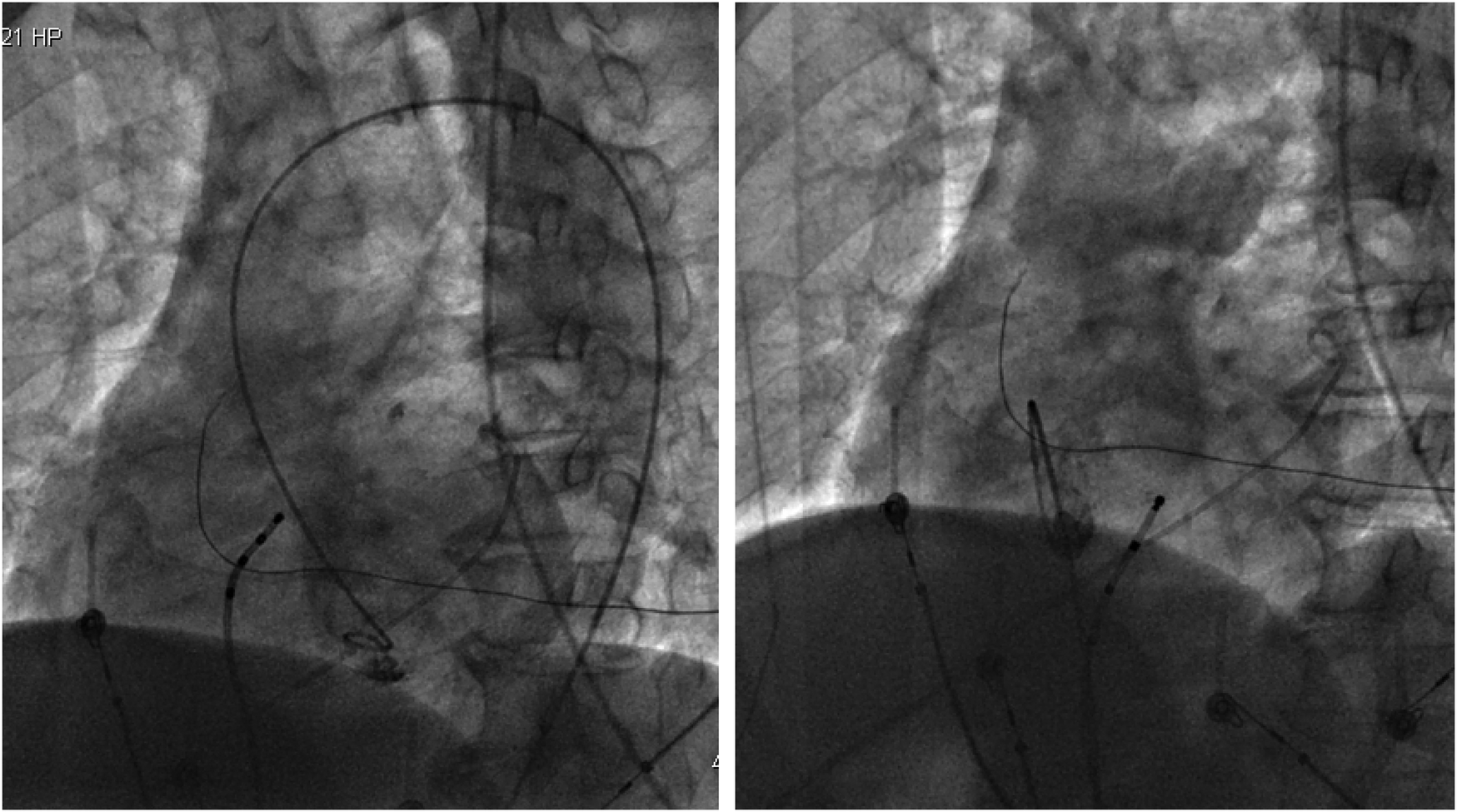
(Left) Left ventricular angiography showing no contrast agent extravasation into the pericardial cavity. (Right) Right ventricular angiography showing no contrast agent extravasation into the pericardium.
Discussion
Radiofrequency ablation (RFA) has long been an integral part of clinical practice and has become a critical therapeutic option for most clinically relevant arrhythmias. Achieving an optimal balance between the efficacy and safety of RFA is widely recognized as a significant challenge (3). Despite advances in the understanding of arrhythmias, accumulated experience with radiofrequency ablation (RFA), and the development of new ablation technologies such as mapping, cryoablation, and magnetic navigation, procedure-related acute complications have decreased (4–6), There remains a risk of complications after radiofrequency ablation (RFA).
Pericardial tamponade is one of the major complications of radiofrequency ablation (RFA). The incidence of pericardial tamponade in ventricular RFA is very low. Tokuda et al. statistically analyzed 1,152 RFA procedures for ventricular arrhythmias in a single center over 12 years and found that pericardial tamponade occurred in 11 cases, with an incidence of only 1% (7), but it carries high morbidity and mortality if unrecognized. Furthermore, tamponade can occur either intra-procedurally or as a delayed complication. Xu et al. reported a case of pericardial tamponade occurring 19 days after RFA for ventricular premature beats originating from the right ventricular outflow tract (8). Therefore, both operators and nursing staff need to be vigilant for the occurrence of pericardial tamponade during and after radiofrequency ablation (RFA).
The classical mechanism of tamponade is overt perforation, frequently related to steam-pop phenomena, in which tissue barotrauma from rapid intramyocardial steam formation disrupts the ventricular wall. The “explosive phenomenon” explains how energy delivery during RFA disrupts the heart wall: when the temperature at the interface between the ablation electrode and cardiac tissue rapidly rises above the boiling point, blood evaporates, causing small explosions and audible popping sounds. Evaporation may occur within the tissue, leading to the formation of intra-tissue air bubbles. As ablation proceeds, these bubbles expand and erupt through the weakest pathway, tearing the tissue. Intracardiac echocardiography can be used to identify the site where bubble explosions emit popping sound signals, which is often the site of cardiac perforation (9). O J Eick et al. conducted experiments using porcine ventricular tissue and found that the likelihood of popping significantly increases when the ablation electrode and endocardial tissue surface are exposed to flowing fluid and there is poor electrode-tissue contact. In these scenarios, tissue temperatures may be much higher than those measured at the tip electrode and may reach 100 °C, leading to intramyocardial steam formation and popping phenomena (10).
In our case, however, several features argue against an overt perforation: the absence of steam pop, limited volume of hemopericardium (150 ml), spontaneous hemostasis without surgical repair, negative biventricular angiography, and absence of effusion recurrence on serial echocardiography. Three possible mechanisms should be considered:
- 1.
Classic perforation/steam pop—although not directly observed, this cannot be entirely excluded.
- 2.
Small apical perforation with spontaneous sealing—previous study reliably detected a small confined region of the normal apical myocardium with a thickness of <3 mm—the left ventricular thin point (11); a minute defect may have temporarily bled and then sealed under pericardial pressure.
- 3.
Thermal injury to epicardial or intramyocardial micro-vessels—heat-induced microvascular injury may represent a possible mechanism.
Alternative explanations, including structural abnormalities or coronary artery complications (spasm or dissection), also warrant consideration, although coronary angiography was not performed for safety reasons. This limitation, together with the absence of intracardiac echocardiography (ICE) or cardiac CT due to urgent and economic constraints, is acknowledged as a weakness of this report.
From a clinical perspective, several lessons emerge. First, early recognition is crucial: abrupt chest symptoms, fluoroscopic “halo” signs, and invasive pressure changes should prompt immediate suspicion of tamponade. Second, when drainage yields a small amount of blood with hemodynamic recovery and imaging excludes contrast leak, conservative management with indwelling catheter drainage can be sufficient. Third, careful modulation of ablation parameters in thin-walled ventricular regions may reduce the risk of microvascular rupture.
Traditional methods for identifying pericardial tamponade primarily involve clinical, radiological, and echocardiographic evaluations (12), fluoroscopy of the cardiac silhouette is very useful in radiofrequency ablation (RFA) (13), transthoracic echocardiography is the clinical diagnostic criterion for pericardial tamponade. Additionally, the application of new technologies helps accelerate diagnosis and treatment, van Roest MH et al. (14) used near-infrared spectroscopy (NIRS) to non-invasively monitor bilateral cerebral oxygen saturation. Before the patient's arterial blood pressure dropped, a sudden decrease in bilateral cerebral oxygen saturation was detected to identify pericardial tamponade, which was then diagnosed by transthoracic echocardiography, enabling early control of the complication. Finally, emerging pulsed field ablation (PFA) has shown a favorable safety profile with a lower risk of myocardial perforation and tamponade (15). As adoption increases, PFA may represent a safer alternative for selected patients.
Conclusion
In summary, this case broadens the mechanistic spectrum of RFA-related tamponade beyond classic perforation. Awareness of potential causes, coupled with prompt recognition and tailored management, may improve outcomes in similar scenarios.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Aerospace Center Hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CH: Conceptualization, Data curation, Writing – review & editing. JL: Data curation, Validation, Writing – original draft. CD: Supervision, Validation, Writing – review & editing. WZ: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1669648/full#supplementary-material
References
1.
Adler Y Ristić AD Imazio M Brucato A Pankuweit S Burazor I et al Cardiac tamponade. Nat Rev Dis Primers. (2023) 9(1):36. 10.1038/s41572-023-00446-1
2.
Cheung JW Yeo I Ip JE Thomas G Liu CF Markowitz SM et al Outcomes, costs, and 30-day readmissions after catheter ablation of myocardial infarct-associated ventricular tachycardia in the real world: nationwide readmissions database 2010 to 2015. Circ Arrhythm Electrophysiol. (2018) 11(11):e006754. 10.1161/CIRCEP.118.006754
3.
Bhaskaran A Chik W Thomas S Kovoor P Thiagalingam A . A review of the safety aspects of radio frequency ablation. Int J Cardiol Heart Vasc. (2015) 8:147–53. 10.1016/j.ijcha.2015.04.011
4.
Bauernfeind T Akca F Schwagten B de Groot N Van Belle Y Valk S et al The magnetic navigation system allows safety and high efficacy for ablation of arrhythmias. Europace. (2011) 13(7):1015–21. 10.1093/europace/eur073
5.
Bella PD Baratto F Tsiachris D Trevisi N Vergara P Bisceglia C et al Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation. (2013) 127(13):1359–68. 10.1161/CIRCULATIONAHA.112.000872
6.
Banchs JE Patel P Naccarelli GV Gonzalez MD . Intracardiac echocardiography in complex cardiac catheter ablation procedures. J Interv Card Electrophysiol. (2010) 28(3):167–84. 10.1007/s10840-010-9474-8
7.
Tokuda M Kojodjojo P Epstein LM Koplan BA Michaud GF Tedrow UB et al Outcomes of cardiac perforation complicating catheter ablation of ventricular arrhythmias. Circ Arrhythm Electrophysiol. (2011) 4(5):660–6. 10.1161/CIRCEP.111.963413
8.
Xu X Meng X Ma F . Delayed cardiac tamponade following catheter ablation of frequent premature ventricular complexes: a case report. BMC Cardiovasc Disord. (2020) 20(1):359. 10.1186/s12872-020-01642-7
9.
Juneja R O'Callaghan P Rowland E . Tissue rupture and bubble formation during radiofrequency catheter ablation: “echoes of a pop”. Circulation. (2001) 103(9):1333–4. 10.1161/01.CIR.103.9.1333
10.
Eick OJ Gerritse B Schumacher B . Popping phenomena in temperature-controlled radiofrequency ablation: when and why do they occur. Pacing Clin Electrophysiol. (2000) 23(2):253–8. 10.1111/j.1540-8159.2000.tb00807.x
11.
Ferencik M Abbara S Hoffmann U Cury RC Brady TJ Achenbach S . Left ventricular thin-point detection using multidetector spiral computed tomography. Am J Cardiol. (2004) 93(7):949–51. 10.1016/j.amjcard.2003.12.033
12.
Holmes DR Jr Nishimura R Fountain R Turi ZG . Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC Cardiovasc Interv. (2009) 2(8):705–17. 10.1016/j.jcin.2009.04.019
13.
Huang XM Hu JQ Zhou F Qin YW Cao J Zhou BY et al Early diagnosis and rescue pericardiocentesis for acute cardiac tamponade during radiofrequency ablation for arrhythmias. Is fluoroscopy enough. Pacing Clin Electrophysiol. (2011) 34(1):9–14. 10.1111/j.1540-8159.2010.02938.x
14.
van Roest MH Vercauteren M Vrints C Sarkozy A . Early recognition of cardiac tamponade using cerebral oximetry during ventricular tachycardia ablation. Eur J Anaesthesiol. (2015) 32(8):581–2. 10.1097/EJA.0000000000000216
15.
Pierucci N Mariani MV Laviola D Silvetti G Cipollone P Vernile A et al Pulsed field energy in atrial fibrillation ablation: from physical principles to clinical applications. J Clin Med. (2024) 13(10):2980. 10.3390/jcm13102980
Summary
Keywords
pericardial tamponade, left ventricular premature, radiofrequency ablation, spontaneous hemostasis, pericardial puncture
Citation
He C, Lin J, Ding C and Zhang W (2025) Case Report: Pericardial tamponade during left ventricular radiofrequency ablation with spontaneous hemostasis. Front. Cardiovasc. Med. 12:1669648. doi: 10.3389/fcvm.2025.1669648
Received
20 July 2025
Accepted
15 October 2025
Published
29 October 2025
Volume
12 - 2025
Edited by
Manuel De Lazzari, University Hospital of Padua, Italy
Reviewed by
Giuseppe Giunta, Sapienza University of Rome, Italy
Christian Waechter, Philipps University of Marburg, Germany
Updates
Copyright
© 2025 He, Lin, Ding and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Wenchang Zhang tom-z@yeah.net
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.