Abstract
Introduction:
Atherosclerosis (AS) is a chronic inflammatory metabolic disease strongly associated with risk factors, including hypertension, hyperlipidemia, hyperglycemia, and hyperuricemia. AS serves as the pathological foundation for numerous cardiovascular diseases (CVDs), and it remains a major threat to global health. However, the underlying mechanisms driving AS development are incompletely understood. Elucidating the pathogenesis and key influencing factors of AS is critical for identifying novel preventive strategies and therapeutic approaches.
Methods:
We searched PubMed and Web of Science for relevant studies. We selected relevant English research articles published between 2012 and 2024. Afterward,we analyzed and summarized the pharmacological effects and molecular mechanisms of these Chinese medicines.
Results:
Through our search and exclusion criteria, a total of 116 preclinical studies and 6 clinical research articles were found.
Discussion:
Traditional Chinese medicine (TCM), with over 2000 years of clinical application, offers a rich source of potential interventions. Integrating modern medical technologies allows for the reevaluation of TCM from a natural compound perspective. This review comprehensively summarizes the mechanisms by which single herbal medicines (SHMs) and their derived natural compounds (NCs) exert effects against AS on the basis of preclinical evidence and analysis of seven selected double-blind, randomized, placebo-controlled clinical trials (RCTs).
1 Introduction
Atherosclerosis (AS) is a primary pathological driver of cardiovascular diseases (CVDs) worldwide. The pathological features of atherosclerosis include endothelial cell dysfunction, lipoprotein deposition, inflammatory cell differentiation, vascular smooth muscle cell proliferation and death, etc. (1, 2). Moreover, CVDs are a significant cause of morbidity and mortality, especially in China, on the basis of the outcomes of ANNUAL REPORT ON CARDIOVASCULAR HEALTH AND DISEASES IN China (2023) (3).
For AS, artificially synthesized drugs, such as statins, nicotine, angiotensin receptor blockers, antioxidants, antiplatelets and anticoagulants, are commonly used in therapy. However, their toxic side effects limit their clinical use to some extent. While statins and antiplatelet therapies can lower lipid levels and inhibit thrombosis, they struggle to comprehensively target multiple pathological pathways, such as those involved in endothelial repair and inflammation regulation. Aspirin (4), although widely used in antiplatelet therapy to suppress thrombosis, has limited efficacy in preventing plaque progression and vascular events in asymptomatic carotid atherosclerosis patients. The long-term use of these drugs also has significant side effects, including hepatotoxicity and gastrointestinal bleeding.
In this context, Chinese medicine for the treatment of AS is gradually gaining widespread attention because of its advantages of being safe at specific doses, effective, less toxic, and inexpensive, and exploring efficient and safe Chinese medicine has become a new direction for the treatment of AS.
The Chinese medicines mentioned here include single herbal medicines (SHMs) and the natural compounds (NCs) derived from them. In addition to natural compounds, herbs have also been used for more than 2000 years. Modern medicine has also helped us understand TCM from the perspective of natural compounds. In this review, we searched PubMed and Web of Science for relevant studies. We selected relevant English research articles published between 2012 and 2024. Afterward, we analyzed and summarized the pharmacological effects and molecular mechanisms of these Chinese medicines.
2 Methods
2.1 Literature searches
We searched PubMed and Web of Science for relevant English research articles published between 2012 and 2024. There were no restrictions on the study type during the search process. The databases were searched via the following terms: [“traditional Chinese medicine (TCM)” OR “Chinese medicine” OR “herbal medicine” AND “atherosclerosis”]. To ensure the systematic integrity of the literature retrieval, each database's interface was used to retrieve subject words combined with keywords and free words. We selected all preclinical studies, both in vivo and in vitro, on the antiatherosclerotic mechanism of NC and HM and identified all proven targets. We also evaluated and summarized the retrieved RCTs (Figure 1).
Figure 1
Study flow diagram.
2.2 Criteria for inclusion and exclusion
According to discussions between researchers and the advice of experts, the following inclusion and exclusion criteria were developed to identify relevant studies.
(1) Types of participants or models: Atherosclerosis. (2) Types of interventions: single herbal medicines or the natural compounds. (3) Types of comparisons: no restrictions. (4) Types of outcomes: no restrictions. (5) Types of research design: animal experiments, cell experiments, RCT, cohort studies, case-control studies, case series, case reports.
2.2.1 Exclusion criteria
(1) Duplicates. (2) Review articles. (3) Unvalidated network pharmacology.
We searched PubMed and Web of Science for relevant English research articles published between 2012 and 2024. The databases were searched via the following terms: [“traditional Chinese medicine (TCM)” OR “Chinese medicine” OR “herbal medicine” AND “atherosclerosis”]. To ensure the systematic integrity of the literature retrieval, each database's interface was used to retrieve subject words combined with keywords and free words. We selected all preclinical studies, both in vivo and in vitro, on the antiatherosclerotic mechanism of NC and HM and identified all proven targets. We also evaluated and summarized the retrieved RCTs (Figure 1).
3 Results
3.1 Mechanisms of Chinese medicine for treating AS
Through our search and exclusion criteria, a total of 116 preclinical studies were found, and the validated targets were summarized (Table 1, Figure 2).
Table 1
| Name of Chinese Medicine | Type of Chinese Medicine | Applied model | Type of model | Reported targets | ||
|---|---|---|---|---|---|---|
| NC | SHM | Animal | Cell | |||
| Cryptotanshinone (5) | + | HUVECs | + | TNF-alpha, sICAM-1, sVCAM-1, MCP-1 | ||
| Paeonol (6) | + | HUVECs | + | LOX-1, Bcl-2, caspase-3, p38 MAPK | ||
| ginsenoside Rb2 (7) | + | HUVECs | + | Smad3, NF-kappa B | ||
| Clematichinenoside AR (8) | + | RAW264.7 | + | ABCA1, ABCG1, NLRP3 | ||
| Notoginsenoside R1 (9) | + | HUVECs | + | MGP, MCP-1, ICAM-1, JNK, NF-kappa B | ||
| Evodiamine (10) | + | VSMCs | + | PPAR gamma | ||
| Afrocyclamin A (11) | + | VSMCs | + | PCNA, p38 MAPK, TNF-alpha, IL-1 beta, IL-6, VCAM-1, MDA, ET-1, SOD, GSH, NO | ||
| Saikosaponin-a (12) | + | THP-1 | + | DLR-1, CD36, ATP, PPAR gamma, PI3K, AKT, NF-kappa B, NLRP3 | ||
| Icariin (13) | + | HUVECs | + | ICAM-1, VCAM-1, E-selectin, | ||
| Gypenoside | + | THP-1 | + | LC3-II, p62, Srit1, FOXO1 | ||
| Paeonol (14) | + | HUVECs | + | p53, acetyl H3K14, H4K16, Sirtl | ||
| Salvianolic Acid B (15) | + | RAW 264.7 | + | HO-1, NO, iNOS | ||
| (2S)-naringenin (16) | + | VSMCs | + | PDGF-R beta, ERK1/2, PI3 K, Akt, PKB, PLC gamma 1 | ||
| Paeonol (17) | + | HUVECs | + | Beclin-1, LC3II | ||
| Curcumin (18) | + | THP-1 | + | ABCA1, AMPK, SIRT1, LXR alpha | ||
| Rutaecarpine (19) | + | THP-1 | + | Cx37, Cx43 | ||
| HUVECs | + | |||||
| Protocatechuic aldehyde (20) | + | VSMCs | + | PI3K, Akt, MAPK | ||
| 13-Methylberberine (21) | + | HUVECs | + | ROS, NLRP3 | ||
| Celastrol (22) | + | VSMCs | + | ABCA1, LXR alpha | ||
| Triptolide (23) | + | HUVECs | + | NF-kappa B p65 | ||
| Gypenoside (24) | + | ApoE−/− mice | + | PI3K, Akt, Bad | ||
| Hydroxysafflor yellow A (25) | + | VSMCs | + | Akt, Heme oxygenase-1 | ||
| Resveratrol (26) | + | HUVECs | + | VEGF, KDR, VEGF receptor-2 | ||
| Leech peptide HE-D (27) | + | RAW264.7 | + | IKK alpha, IKK gamma, TRAF6, TLR4, TRAF5, NF-Kappa B, iNOS, TNF-alpha, Arg-1, IL-10 | ||
| Total flavonoids from Dracocephalum moldavica (28) | + | VSMCs | + | PCNA, NF-kappa B p65, ICAM-1, VCAM-1 | ||
| Protocatechuic Aldehyde (29) | + | HUVECs | + | Caspase-3 | ||
| Tanshindiol C (30) | + | RAW264.7 | + | Nrf2, Sirt1, Prdx1, ABCA1 | ||
| Tetramethylpyrazine and Paeoniflorin (31) | + | HUVECs | + | CD31, VEGF, VEGFR2, Notch1, Jagged1, Hes1 | ||
| Lycium (32) | + | VSMCs | + | PI3K, Akt, miR-145 | ||
| Shikonin (33) | + | VSMCs | + | NF-kappa B, PI3K, cyclin D1, cyclin E, Bcl 2, Bax; caspase-3, caspase-9 | ||
| Hyperoside (34) | + | VSMCs | + | LOX-1, ERK | ||
| Danshenol A (35) | + | HUVECs | + | ROS, NOX4, IKK beta, I kappa B alpha, NF-kappa B p65, TNF-alpha, ICAM-1 | ||
| Total Saponins of Aralia elata (36) | + | HUVECs | + | TNF-alpha, NF-kappa B, IL-6, MCP-1, and VCAM-1, PI3K, Akt, Bcl-2, Bax | ||
| THP-1 | + | |||||
| Icariin (37) | + | Wistar rats | + | IL-6, TNF-alpha, SOD, MDA, p-p38 MAPK | ||
| Tanshinone IIA (38) | + | SD Rats | + | CYP7A1, LDL-R, SREBP2, LCAT, ABCA1, CD36 | ||
| THP-1 | + | |||||
| Tetrahydroxystilbene glucoside (39) | + | SD Rats | + | Calreticulin, Vimentin, HSP 70, lipocortin 1, Apo A-I | ||
| Baicalin (40) | + | SD Rats | + | TBARS, SOD, GSH-Px | ||
| Composition of Ophiopogon polysaccharide, Notoginseng total saponins and Rhizoma Coptidis alkaloids (41) | + | New Zealand white rabbits | + | p-JNK, Caspase-3, Bcl-2, RAGE, AGEs | ||
| Ling Zhi 8 Protein (42) | + | New Zealand white rabbits | + | IL-1 beta | ||
| Ganoderma lucidum triterpenoids | + | Japanese big-ear white rabbits | + | LOX-1, ROS, MDA, NF-kappa B p65, Notch1, DLL4, TNF-alpha | ||
| Polysaccharides (43) | + | HUVECs | + | |||
| THP-1 | + | |||||
| Scutellarin (44) | + | New Zealand white rabbits | + | SOD1, Nox4, ROS | ||
| SD Rats | + | |||||
| HUVECs | + | |||||
| Leonurine (45) | + | New Zealand white rabbits | + | PECAM-1, sVCAM-1, sICAM-1, IL-6, TNF-alpha, MCP-1, iNOS, MMP-9, CAT, SOD-1, GPx | ||
| Catalpol (46) | + | ApoE−/− mice | + | ER alpha, ROS, iNOS, eNOS, CRP, IL-1 beta, TNF-α, IL-10, CD11b | ||
| J774A-1 | + | |||||
| Tanshinone IIA (47) | + | ApoE−/− mice | + | KLF4, miR-375 | ||
| Coptisine (48) | + | ApoE−/− mice | + | IL-6, IL-1 beta, TNF-alpha, NF-kappa B p65, VCAM-1, ICAM-1, p-p38, p-JNK1/2 | ||
| Berberine | + | ApoE−/− mice | + | IL-1 beta, TNF-alpha, NF-kappa B p65, i-NOS, ICAM-1, IL-6 | ||
| 8-cetylberberine (49) | + | |||||
| Kaempferol (50) | + | ApoE−/− mice | + | GPER, PI3K, AKT, Nrf2, ROS | ||
| Alisa B 23-acetate (51) | + | Ldlr−/− mice | + | CYP7A1, CYP8B1, SHP, FXR, BSEP | ||
| Celastrol (52) | + | ApoE−/− mice | + | LOX-1, ROS, iNOS, NO, TNF-alpha, IL-6 | ||
| RAW264.7 | + | |||||
| Isoflavones from Semen Sojae Preparatum (53) | + | ApoE−/− mice | + | ET-1, LDH, SOD, MDA, 2, HO-1, NQO1, | ||
| Ganoderma Triterpenoids (54) | + | BALB/cC mice | + | VCAM-1, TNF-alpha, IL-6, MCP-1, ET-1 | ||
| HUVECs | + | |||||
| Artesunate (55) | + | ApoE−/− mice | + | TNF-alpha, IL-6, IL-8, MCP-1 | ||
| HUVECs | + | |||||
| Baicalin | + | HUVECs | + | CAMs, ROS, NF-kappa B | ||
| Baicalein | + | |||||
| Wogonin (56) | + | |||||
| Gastrodin (57) | + | C57BL/6J mice | + | TNF-alpha, IL-1 beta, IL-6, IL-8, IL-10, VCAM-1, | ||
| VSMCs | + | |||||
| ShenLian (58) | + | THP-1 | + | alpha-SMA, TGF-beta, TGF-beta, iNOS, JAK2, STAT3, SOCS3, Smad2/3 | ||
| RAW264.7 | + | |||||
| ApoE−/− mice | + | |||||
| Baicalin | + | ApoE−/− mice | + | CD11, CD83, CD80, CD86, TNFα, IL-12 | ||
| Geniposide (59) | + | |||||
| Baicalin (60) | + | ApoE−/− mice | + | VCAM-1, MCP-1, IL-6 | ||
| Cryptotanshinone (61) | + | ApoE−/− mice | + | LOX-1, MMP-9, ROS, NOX4, VCAM-1, ICAM-1, NF-kappa B, IL-1 beta, IL-6, IL-17A, IFN-gamma, TNF-alpha | ||
| HUVECs | + | |||||
| Berberine (62) | + | ApoE−/− mice | + | TNF-alpha, IL-6, IL-1 beta, IFN-gamma, MCP, MIP, Ampk, Nf-kappa B | ||
| Homoplantaginin | + | ApoE−/− mice | + | ICAM-1, VCAM-1, ROS, ERK, NF-kappa B, Nrf2, HO-1 | ||
| Dihydrohomoplantagin (63) | + | HUVECs | + | |||
| Panax notoginseng saponins (64) | + | ApoE−/− mice | + | VEGF, NOX4, CD34 | ||
| Astragaloside IV (65) | + | ApoE−/− mice | + | PAC-1, CD40l, CXCR4, SDF-1, | ||
| Myricitrin (66) | + | ApoE−/− mice | + | ROS, NO, p53, caspase-3, MAPK, Bl-2, Bax, eNOS, MDA, 4-HNE, SOD, CAT, ERK1/2, ERK1/2, JNK | ||
| HUVECs | + | |||||
| Thonningianin A (67) | + | ApoE−/− mice | + | Ca2+, AMPK, IL-1, ROS, NLRP3 | ||
| HUVECs | + | |||||
| Alisol B 23-acetate (68) | + | ApoE−/− mice | + | MHC II, CD80, CD86, IL12, IFN-gamma, IL-10, TGF-beta 1 | ||
| BMDCs | + | |||||
| Celosins (69) | + | ApoE−/− mice | LC3, CD36, SR-A1, ABCA1, ABCG1, BCL-1 | |||
| Patchouli alcohol (70) | + | ApoE−/− mice | Mac2, MCP-1, iNOS, IL-1 beta, IL-6, CXCL9, CXCL11 | |||
| Alisol A (71) | + | ApoE−/− mice | + | ICAM-1, IL-6, MMP-9, PPAR alpha, PPAR delta, NF-kappa B, I kappa B alpha | ||
| HepG2 | + | |||||
| Artesunate (72) | + | ApoE−/− mice | + | iNOS, TNF-alpha, IL-4, IL-6, IL-1 beta, CD80, CD206, TGF-beta 1, HIF-1 alpha, P65, NF-kappa B | ||
| RAW 264.7 | + | |||||
| Icariin (73) | + | ApoE−/− mice | + | CX3CR1, CX3CL1 | ||
| RAW 264.7 | + | |||||
| Total flavonoids of Engelhardia roxburghiana Wall (74) | + | ApoE−/− mice | + | IL-1 beta, AKT, p-AKT, mTOR, p-mTOR, Beclin-1, LC3-II, p62 | ||
| THP-1 | + | |||||
| Lactones from Ligusticum chuanxiong Hort (75) | + | ApoE−/− mice | + | ICAM-1, VCAM-1, TNF-alpha, NF-kappa B, CD31, MCP-1 | ||
| HUVECs | + | |||||
| Tanshinone IIA (76) | + | ApoE−/− mice | + | CD40, G-CSF, IFN-gamma, IL-1 beta, IL-6, MCP-1, MIP-3 alpha, TNF-alpha, VEGF, CCL-2, CD40, MMP-2, miR-146b, miR-155 | ||
| Dioscin (77) | + | Ldlr−/− mice | + | MDA, GSH, MDA and GSH, ROS, NOX4, P22phox, I kappa 3, p-p65, n-p65, ICAM-1, VCAM-1, caspase-3, caspase-9, bcl-2, PGC-1 alpha, ER alpha, ER beta, I kappa B, caspase-3, caspase-9, bcl-2, LC3 | ||
| HAECs | + | |||||
| Ginsenoside Rg1-Notoginsenoside R1-Protocatechuic aldehyde (78) | + | ApoE−/− mice | + | ET-1, eNOS, TXA2, PGI2, Piezo1, PI3 K, Akt, FAK | ||
| HUVECs | + | |||||
| Curcumin analog L3 (79) | + | ICR mice | + | GSH, SOD, GPx, MDA, GST, CAT ROS, LOX-1, NO, TNOS, iNOS | ||
| Tanshinone IIA Sodium sulfonate (80) | + | ApoE−/− mice | + | ROS, MDA, TNF-alpha, IL-6, ICAM-1, VCAM-1, CLIC1 | ||
| HUVECs | + | |||||
| Dendrobium catenatum Lindl (81) | + | Zebrafish | + | ROS, SOD, MDA, GSH, GSSG, NO, ET-1, PGI2, ICAM-1, VCAM-1 | ||
| EA.hy926 | + | |||||
| Guang Chen Pi (82) | + | RAW264.7 | + | SRA1, CD36, PPAR gamma, LXR alpha, SRB1, ABCG1, p38 MAPK, ERK1/2, JNK1/2, NF-kappa B p65, IKK alpha/beta. | ||
| Danshen (83) | + | HUVECs | + | VCAM-1, ICAM-1, CD40, IL-6, IL-8, MCP-1 | ||
| Ophiopogonis Radix (84) | + | VSMCs | + | ROS, NO, ICAM-1, VCAM-1 | ||
| Crataegus pinnatifida Bge (85) | + | SD Rats | + | vWF, VCAM-1, ICAM-1, t-PA, 6-keto-PGF1 alpha, PAI-1, TXB2 | ||
| HUVECs | + | |||||
| Crataegus pinnatifida var. major (86) | + | SD Rats | + | IL-1 beta, IL-8, NO, ET, 6-keto-PGF (1 alpha), TXB2, IL-18, CRP | ||
| Usnea diffracta (87) | + | SD Rats | + | TLR5, MyD88, NF-kappa B, IL-10 | ||
| Ganoderma lucidum spore (88) | + | Japanese rabbits | + | LXR alpha, CYP7A1, ABCA1/G1, ABCG5/G8 | ||
| Schisandrae chinensis fructus (89) | + | SD Rats | + | Nrf-2, HO-1, ET-1,6-keto-PGF (1 alpha), TXB2 | ||
| Cynanchum wilfordii (90) | + | ApoE−/− mice | + | ET-1, VCAM-1, ICAM-1, E-selectin | ||
| Eucommia leaf (91) | + | ApoE−/− mice | + | IL-1, TNF-alpha | ||
| Danshen (92) | + | ApoE−/− mice | + | LC3, p62, caspase-3, caspase-9, IL-6 | ||
| RAW 264.7 | + | |||||
| HUVECs | + | |||||
| Polygoni Multiflori Radix Praeparata | + | ApoE−/− mice | + | IL-6, TNF-alpha, VCAM-1, MCP-1, ICAM-1, CCRA | ||
| 2,3,5,4′-Tetrahydroxy-stilbene-2-O-beta-D-glucoside (TSG) (93) | + | |||||
| Xiaoxianggou (94) | + | ApoE−/− mice | + | miR-203, Ets-2 | ||
| Leech (95) | + | ApoE−/− mice | + | ICAM-1, MCP-1, NF-kappa B | ||
| EA.hy926 | + | |||||
| Zanthoxylum nitidum var. tomentosum Toddalolactone (96) | + | ICR mice | + | PAI-1, CCL4, | ||
| Total red ginseng saponin extracts (TGS) (97) | + | |||||
| Bear bile powder (98) | + | ApoE−/− mice | + | IL-2, IL-6, TNF-alpha, IFN-gamma, MDA, GSH, SOD, ROS, miR-20, miR-21, miR-126, miR-155 | ||
| Alisa B 23-acetate (AB23A) (51) | + | Ldlr−/− mice | + | FXR, AB23A, CYP7A1, CYP8B1, SHP, CDCA, BSEP | ||
| L02 cells | + | |||||
| Isoliquiritigenin (99) | + | HUVECs | + | SIRT6, TNF-α, NLRP3 | ||
| Geniposide (100) | + | ApoE−/− mice | + | CXCL14 | ||
| RAW 264.7 | + | |||||
| Guang Chen Pi (82) | + | RAW 264.7 | + | SRA1, CD36, PPARγ, LXRα, SRB1, ABCG1, p38 MAPK, ERK1/2, JNK1/2, NF-κB p65, IKKα/β | ||
| Chinese agarwood (101) | + | ApoE−/− mice | + | ER stress, P-JNK, PPARγ, CD36 | ||
| Salvia miltiorrhiza | + | ApoE−/− mice | + | COX-2, TNF-α, NF-κB | ||
| Tanshinone IIA (102) | + | HUVECs | + | |||
| Quercetin (103) | + | ApoE−/− mice | + | FGF2 | ||
| VSMCs | + | |||||
| Andrographolide (104) | + | RAW 264.7 | + | NF-κB, CEBPB, PPARG | ||
| Dihydrotanshinone I (105) | + | RAW 264.7 | + | NF-α, IL-1β, and MCP-1 | ||
| Baicalein (106) | + | HUVECs | + | AKT1, MAPK1, PIK3CA, JUN, TP53, SRC, EGFR, ESR1 | ||
| Tanshinone IIA (107) | + | LDLR(−/−) mice | + | MFGE8, CX3CR1, MERTK, AXL, TYRO3 | ||
| RAW 264.7 | + | |||||
| Ginkgetin (108) | + | HepG2 | + | CDK2 | ||
| Puerarin and Tanshinone IIA (109) | + | ApoE−/− mice | + | HIF-1α, IL-1β | ||
| HUVECs | + | |||||
| THP-1 | + | |||||
| Carthamus tinctorius L. (110) | + | ApoE−/− mice | + | IL-1β, IL-6, CXCL12, miR166a-3p, ICAM-1, VCAM-1 | ||
| HUVECs | + | |||||
| Evodiamine (111) | + | LDLR−/− mice | + | PCNA, MMP2, Myh11, Acta2, Nrf2, TNF-α, IL-1β, PI3K, Akt | ||
| MOVAS | + | |||||
| Diterpenoids (112) | + | RAW264.7 | + | ABCA1/G1, LXRα, PPARγ | ||
| Tribulus terrestris L. (113) | + | ApoE−/− mice | + | α-SMA, OPN, Akt, MAPKs, MEK, ERK, JNK | ||
| A7r5 | + | |||||
| Naringenin and quercetin (114) | + | RAW264.7 | + | VEGFA, MMP9, IL-1β | ||
| Triptolide (115) | + | HUVECs | + | IκBα, NF-kappa B | ||
| Cryptotanshinone, isoeugenol, tanshinone IIA (116) | + | SD rats | + | Akt, p-Akt, ERK1/2, p-ERK1/2, cSrc, p-cSrc, RhoA | ||
| Ganoderma lucidum spore powder (117) | + | LDLR(−/−) mice | + | ABCA1/G1, RUNX2 | ||
| Salvianolic acid A (118) | + | ApoE−/− mice | + | PKR, PKM2 | ||
| Rhodiola rosea polysaccharides (119) | + | Balb/c mice | + | PI3K, AKT, GSK-3β | ||
The mechanisms of Chinese medicine for treating AS.
Figure 2
Target-Chinese medicine component association network.
The validation targets were enriched in GO biological processes, and a total of 207 terms were enriched, of which 30 terms were significantly enriched (Figure 3). Among them, the most obviously enriched pathways were the AGE-RAGE signaling pathway in diabetic complications, prion diseases, the longevity regulating pathway in multiple species, EGFR tyrosine kinase inhibitor resistance, and the adipocytokine signaling pathway. Through the description of these five terms by KEGG, we can learn that the main functions of these terms are related to inflammation, glucose metabolism, lipid metabolism, autophagy, and apoptosis, which often profoundly affect the generation and development of AS. The AGE-RAGE signaling pathway in diabetic complications activates several intracellular signaling pathways that can lead to NF-κB activity. NF-κB promotes the expression of a series of proinflammatory cytokines and various atherosclerosis-related genes, such as VCAM-1, VEGF, and RAGE. In addition, the AGE-RAGE signaling pathway is involved in cell proliferation and apoptosis in diabetic patients. Pathways of action in prion diseases include oxidative stress, the regulatory activation of complement, the corticosteroid response and endoplasmic reticulum stress. In longevity-regulating pathways, multiple species are involved in antioxidative stress, energy metabolism, DNA damage repair, glucose metabolism, autophagy, and other processes, thus resisting molecular, cellular, and organ damage; preventing the loss of various functions of human tissues; and reducing the risk of disease and death. EGFR can effectively interfere with the growth, differentiation, and proliferation of vascular cells. The adipocytokine signaling pathway is an important regulatory pathway for energy intake and metabolic rates. This function is due mainly to JAK kinase activity, STAT3 phosphorylation, and activation of AMPK to inhibit endogenous glucose production. In addition, JNK, mTOR, and IKK have also been shown to be involved (Figure 4).
Figure 3
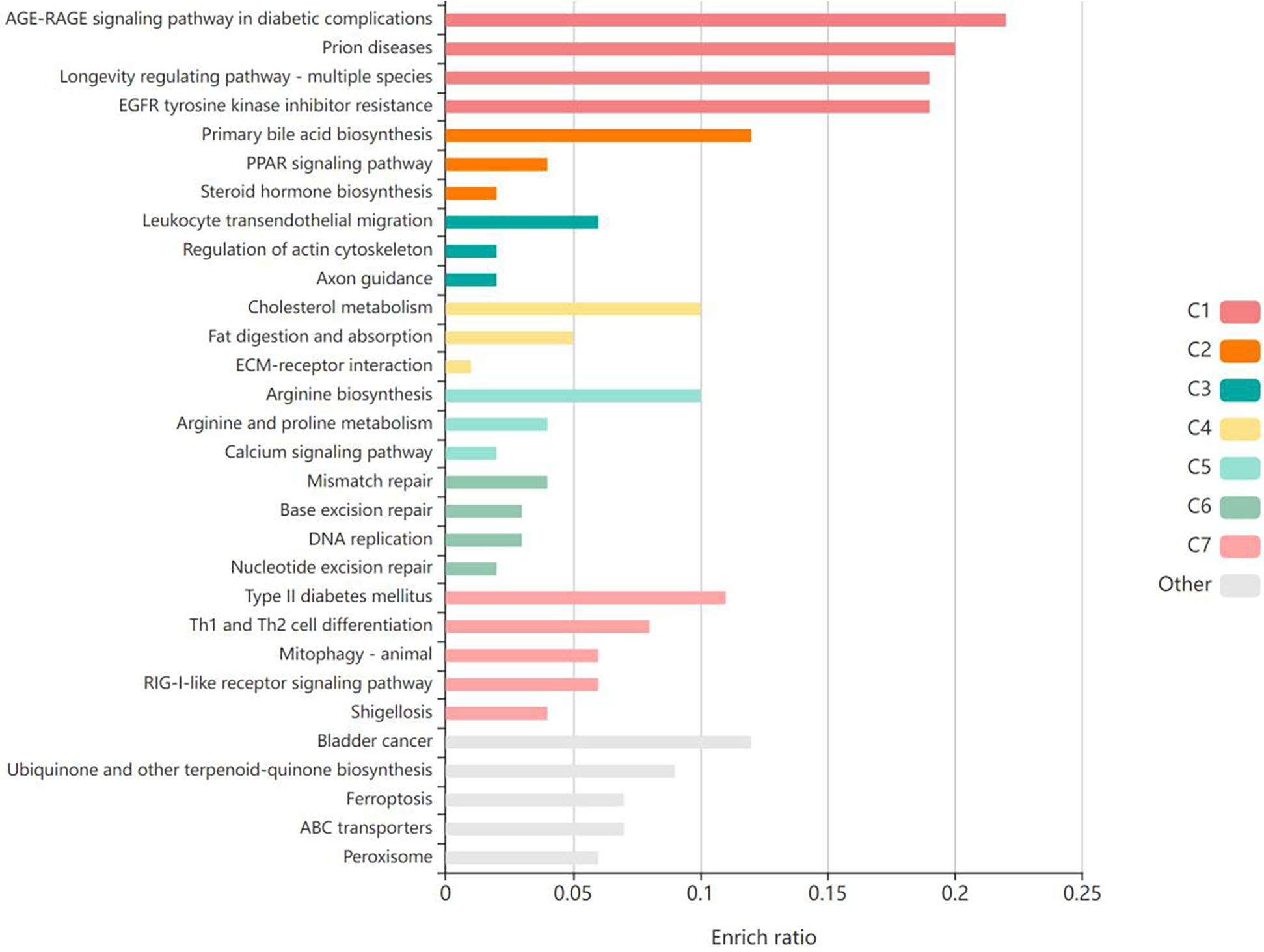
The validation targets were enriched in GO biological processes, and the top 30 terms were significantly enriched. Produced by KOBAS (http://kobas.cbi.pku.edu.cn/).
Figure 4
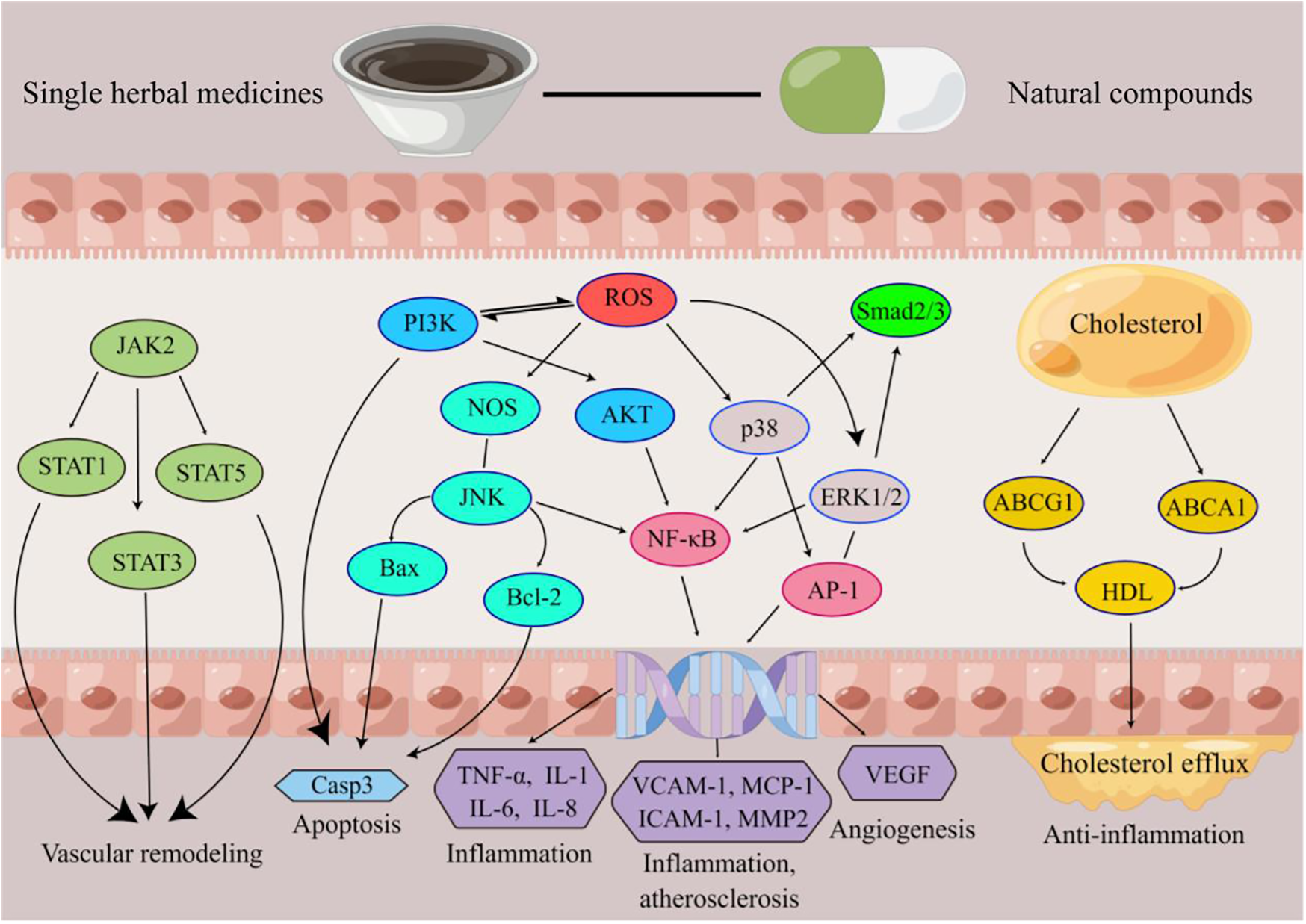
Schematic diagram of the mechanism of action of Chinese medicine for AS. Produced by Figdraw (https://www.figdraw.com).
3.1.1 Inflammation
Inflammation is the most significant risk factor for AS. The development of vascular inflammation is mainly due to the expression of various effector proteins, including adhesion molecules [e.g., intercellular adhesion molecule-1 (ICAM-1) and VCAM-1 (VCAM-1)] and chemokines [e.g., IL-1, IL-8, and monocyte chemoattractant protein-1 (MCP-1)]. Abnormal expression of these proteins leads to vascular endothelial cell injury and dysfunction.
In the area of anti-inflammation, numerous studies have demonstrated the superior efficacy of Chinese medicine. The anti-inflammatory properties of cryptotanshinone may prevent and treat cytokine-induced atherosclerosis (5). The mechanism of action of lactones from Lilusticum chuanxiong Hort. lactones may be related to the inhibition of NF-κB expression (75); this mechanism is very similar to that of coptisine, but coptisine also acts through the MAPK signaling pathway (48). Many other medicines, such as leonurine from Leonuri (45), polygoni multiflori radix and TSG (93), and Danshen (83), have demonstrated antiatherosclerotic effects associated with inflammation.
3.1.2 Lipids and glucose
Hyperlipidemia is also an important risk factor for AS and is characterized by elevated serum levels of total cholesterol (TC), triglycerides (TG), and LDL-C. For hyperlipidemia, statins are currently the mainstay of treatment. However, owing to the side effects of statins, such as liver damage, the search for new alternative drugs remains an important research direction. High glucose can interfere with the endothelial cell cycle, increase endothelial cell injury, delay endothelial cell repair, and cause excessive cell death. In addition, high glucose can lead to structural destruction of the vascular endothelium through oxidative stress, the production of large amounts of advanced glycation end products (AGEs), and damage to endothelial progenitor cells. Damage to endothelial cells and disruption of their structure impair their normal function, which in turn leads to AS.
Some natural lipid-lowering compounds, such as icariin (37), baicalin (40), Ganodermalucidum triterpenoids and polysaccharides (43), coptisine (48), astragaloside IV (65), and disocin (120), are also useful. Many Chinese medicines, such as resveratrol (26), ophiopogon polysaccharide, notoginseng total saponins, Rhizoma coptidis alkaloids (41), baicalin, baicalein, and wogonin (56), which have significant therapeutic effects on hyperglycemia, diabetic complications, and atherosclerosis, have excellent hypoglycemic effects. However, they do not have the same mechanism of action. The AMPK/ACC pathway, Akt/GSK-3β pathway, and VEGF/KDR pathway are involved. The AMPK/ACC pathway is a key influencer of adipogenesis, the Akt/GSK-3β pathway is a key influencer of glycogen synthesis, the VEGF/KDR pathway ameliorates caveolae-mediated hyperpermeability induced by high glucose, and the endothelial hyperpermeability induced by hyperglycemia is an important prerequisite for the development of AS. There are also medicines, such as Rhodiola rosea polysaccharides (121), that have excellent modulatory effects on blood glucose and blood lipids. Because the mechanism of action is not described in the article on R. rosea polysaccharides, we have not cited it in the table. However, other studies have shown that Rhodiola rosea polysaccharides can increase hepatic glycogen synthesis and improve hepatic glycogen metabolism in diabetic mice by regulating the PI3K/AKT/GSK3β pathway in the liver. Those study confirmed the hypoglycemic and hypolipidemic effects of this natural compound and its great industrial value.
3.1.3 Endothelial cells (ECs)
The vascular endothelium is the continuous cellular lining of the cardiovascular system and is a key link in the development of numerous vascular problems, such as vascular tone, thrombosis, VSMC proliferation, leukocyte adhesion, and vascular inflammation. In recent years, endothelial function impairment has been found to be the primary and earliest link in the development of AS. The earliest manifestation of AS is impaired diastolic function when there are no microscopically visible lesions in the endothelium or vascular wall, and the only changes are decreased release of nitric oxide (NO), increased peroxides, and increased oxygen ions, which are only signs of functional damage. Additionally, as a mechanical barrier between the vessel wall and plasma molecules, the endothelium plays a crucial role in maintaining vascular status, and endothelial cell dysfunction (ECD) is a major contributing factor to the progression of atherosclerotic plaques in the early stages of AS (122). The recruitment of inflammatory cells, which in turn leads to an inflammatory response in the vasculature, is an important factor leading to endothelial cell damage and dysfunction. Fortunately, many Chinese medicines have therapeutic effects on vascular damage and related inflammatory conditions.
Many Chinese medicines can treat ECD. The therapeutic effect of ginsenoside Rb2 is achieved by targeting miR-216a (7). 13-Methylberberine has significant hypolipidemic and anti-inflammatory activities. In addition, this function was achieved by inducing autophagy in HUVECs and inhibiting the activation of NLRP3 inflammatory vesicles, thus exerting cytoprotective effects in a model of H2O2-induced injury in HUVECs (21). Triptolide may inhibit the inflammatory response of endothelial cells by inhibiting NF-κ B activation (23). The PI3K/Akt signaling pathway plays a key role in total saponins of aralia elat of promoting cell survival and anti-inflammatory responses (36). A key component of the therapeutic effect of ganoderma lucidum triterpenoids and polysaccharides is the regulation of the Notch1 and DLL4 pathways (43). The therapeutic effect of kaempferol is associated with activation of the GPER-mediated PI3K/AKT/Nrf2 pathway (50). Other medicines are dendrobium ling zhi 8 (42), ganoderma triterpenoids (54), artesunate (55), disocin (77), catenatum lindl (81), cynanchum wilfordii (90), all of which have similar functions, although the mechanism of action varies. The therapeutic effects of salvianolic acid B (15), scutellarin (44), tanshinone IIA sodium sulfonate (80), and extract of ophiopogonis radix (84) were reflected in the effects on ROS, NO, eNOS, and MDA expression levels. Among them, the investigators found that scutellarin acted in a more diverse manner, with scutellarin restoring mRNA and protein expression of SOD1 and Nox4 in cellular experiments.
3.1.4 Macrophages
As a chronic inflammatory response recognized by the medical research community, macrophages play an important role in the development of AS. Macrophages can phagocytose inflammatory cells and actively participate in cholesterol accumulation, on the other hand, anti-inflammatory macrophages contribute to tissue repair and plaque stabilization.
Many Chinese medicines have been shown to inhibit foam cell formation and cholesterol accumulation in RAW264.7 macrophages, but the mechanisms by which they exert anti-AS effects vary. Clematichinenoside AR (8) acts through upregulation of ABCA1/ABCG1, gypenoside (123) acts by enhancing Sirt1-FOXO1-mediated autophagic flux, tanshindiol C (30) acts by activating the Prdx1/ABCA1 signaling pathway, celastrol (52) acts by inhibiting the production of LOX-1 and ROS. Additionally, Guang Chen Pi (82), Danshen (92) had the same effect. The functions of saikosaponins-a (12), curcumin (18) in promoting cholesterol efflux from macrophage-derived foam cells were similarly demonstrated on THP-1 macrophages.
Some Chinese medicines achieve their anti-AS effects by regulating the different polarization patterns of M1-type macrophages and M2-type macrophages. Leech peptide HE-D (27), catalpol (46), tanshinone IIA (47), artesunate (72) inhibit M1 subtype macrophage polarization, while providing enhanced M2 macrophage polarization. Some representative mechanisms of action are leech peptide HE-D (27) which can act by decreasing the expression of IKK α and IKK γ in the NF-κ B signaling pathway, catalpol (46) which acts by increasing the expression of ER α, tanshinone IIA (47) which acts by inhibiting miR-375 and activating KLF4, and artesunate (72) which acts by regulating HIF-1 α and NF- κB signaling pathways.
In addition to in vitro experiments, numerous in vivo experiments have provided evidence that Chinese medicines affect macrophages. Berberine (62) reduces macrophage activation as well as cytokine release, and celosins (69) inhibit lipid phagocytosis by macrophages while increasing intracellular autophagy levels through increases in LC3 and Beclin-1. Eucommia leaf extract (91) can modulate the function of macrophages and reduce the number of AS lesions.
3.1.5 Vascular smooth muscle cells
VSMCs are located in the middle membrane of the vessel wall. In response to growth factors and proinflammatory factors secreted by foam cells, VSMCs proliferate and migrate to the middle membrane of the vessel and participate in plaque formation together with foam cells and macrophages.
Numerous studies have shown that different Chinese medicines can significantly inhibit the proliferation and migration of VSMCs and promote their apoptosis, although their mechanisms of action are different. Evodiamine (10) works by upregulating PPAR, afrocyclamine A (11) acts through the p38 MAPK signaling pathway, (2S)-naringenin from Typha angustata (16) acts by blocking G(0)/G(1), protocatechuic aldehyde (20) acts through antioxidants, celastrol (22) acts by activating LXR α and upregulating ABCA1, hydroxysafflor yellow A (25) acts through the akt signaling pathway, total flavonoids from dracocephalum moldavica (28) act by reducing the expression of VCAM-1, ICAM-1, NF-κ B p65, PCNA, and Lycium barbarum polysaccharide (32)s act through the PI3K/Akt signaling pathway, and shikonin (33) has a wide range of regulatory effects on the NF-kappa B, Bax, Bcl-2, cyclin D1, cyclin E, caspase-3, caspase-9, and PI3K signaling pathways, with hyperoside (34) acting through the LOX-1-ERK pathway.
3.1.6 Platelets
In the treatment of AS, antiplatelet drugs are receiving increasing attention, and their proper use can prevent platelet aggregation and thrombosis. Recent studies have shown that platelets act as “inflammatory cells”, and their activation releases some inflammatory mediators that are directly involved in the formation and development of atherosclerosis and are closely related to plaque instability. We focus on the role of Chinese medicines in preventing platelet aggregation and thrombosis. Protocatechuic aldehyde (20) has been shown to inhibit platelet aggregation and prevent thrombosis in studies, whereas leonurine (45) acts by reducing the expression of platelet-endothelial cell adhesion molecule-1 (PECAM-1) in the aorta. Astragaloside IV (65) not only reduces platelet aggregation but also inhibits the expression of CXCR4, CD40l, and PAC-1 on the platelet surface. Baicalin (124) also has significant antiplatelet aggregation effects, but the exact mechanism has not been investigated in depth by the authors.
3.1.7 Dendritic cells
In a high-fat environment, abnormal lipid metabolism in dendritic cells (DCs) leads to abnormal immune function, promotes the development of immune inflammatory responses, and facilitates the development of AS. Baicalin and geniposide (59) can reduce atherosclerotic lesions and modulate bone marrow dendritic cells, and alisol B 23-acetate (68) promotes cholesterol efflux from DCs and reduces the expression of CD80, CD86, and MHC II and the production of the inflammatory cytokines IFN-γ and IL-12.
3.2 Clinical studies
3.2.1 Research overview
Although there are few clinical studies on Chinese medicines for the treatment of AS, we still found 6 relevant clinical research articles in addition to a large number of in vivo and in vitro studies in the course of our systematic review. These six articles focus on studies that address AS or AS-related high-risk factors, such as inflammation, lipids, and glucose. We then conducted an in-depth evaluation of these six articles. All studies were designed with at least one experimental group and one control group. Patients in the experimental group were treated with one or more single herbal medicines or natural compounds. Patients in the control group received only placebo or conventional treatment, which included exercise and lifestyle modification. All six included studies randomized patient groups, and the method of random assignment had a low impact on trial outcomes and was rated as “low risk”. Five studies had no risk of bias in allocation concealment and were rated as “low risk”, whereas one study did not explicitly mention allocation concealment and was rated as “unclear”. Five studies specified that double-blinding was used during the study to avoid implementation bias and detection bias and were rated as “low risk”, whereas one study did not mention blinding according to the article description and was rated as “high risk” for implementation bias and detection bias. Six studies were rated as “low risk” for the presence or absence of complete reporting of data-complete outcome indicators. All six studies were rated as “low risk” for selective reporting. No other risk of bias was found in any of the six studies, and they were rated as “low risk” (Figures 5, 6).
Figure 5
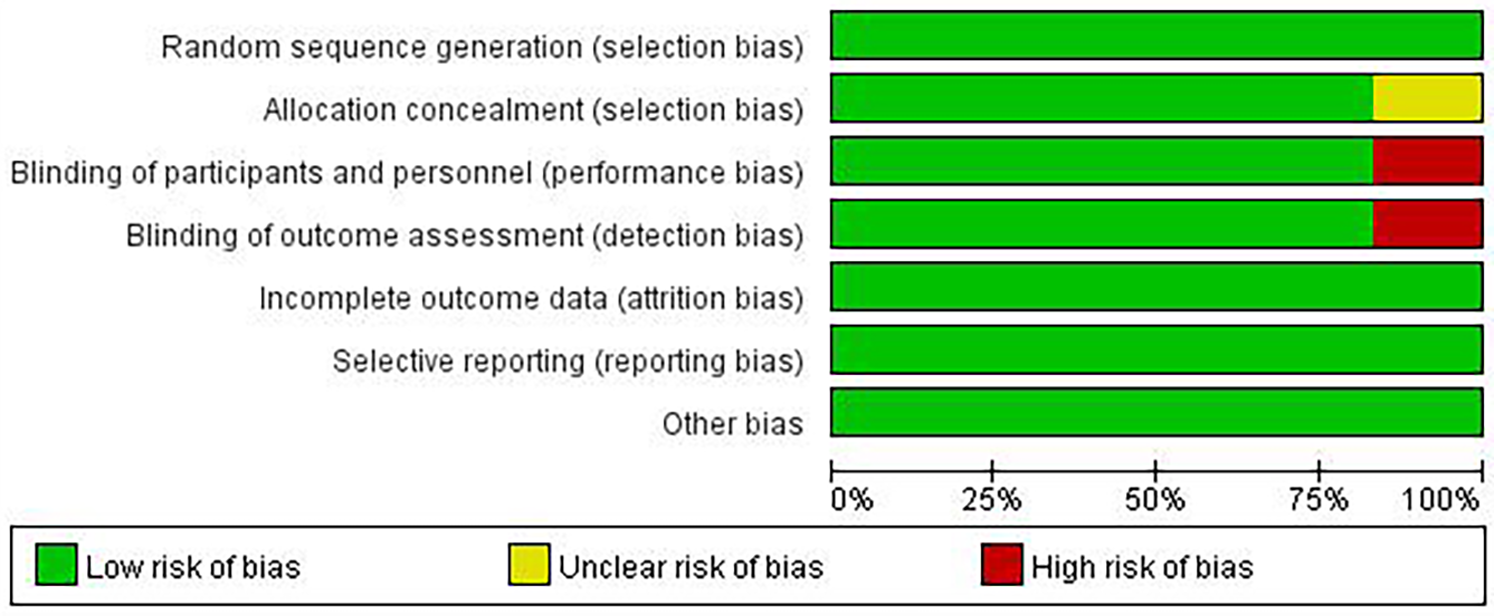
Risk of bias summary.
Figure 6
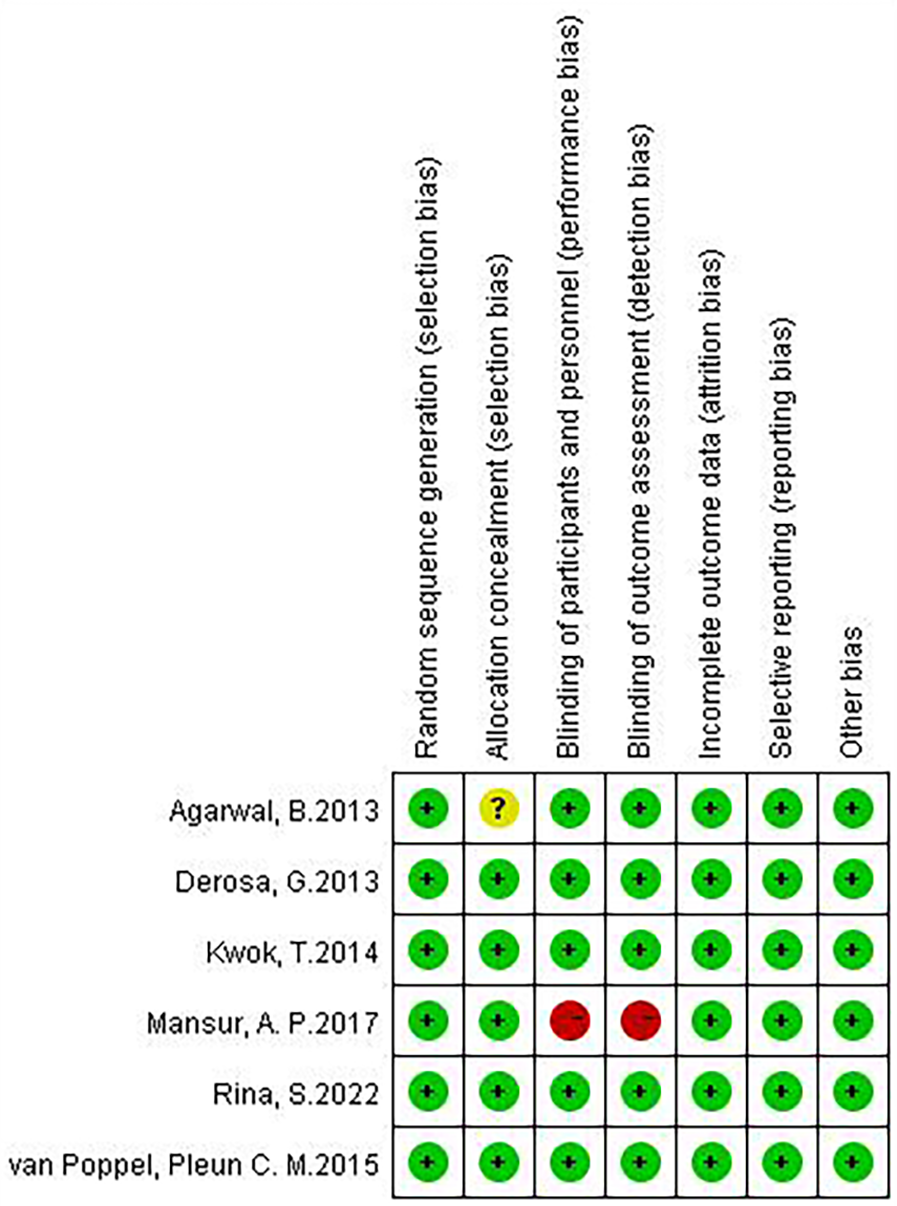
Risk of bias graph.
3.2.2 Study results
The findings of these clinical studies reaffirm that Chinese medicine is stable and reliable for both AS and AS-related high-risk factors. A double-blind, randomized, placebo-controlled clinical study of RESV (400 mg of trans-resveratrol, 400 mg of grape skin extract and 100 mg of quercetin) revealed that RESV significantly reduced the mRNA expression levels of ICAM, VCAM and IL-8, as did the levels of inflammatory markers, such as plasma IFN-γ and insulin (125). A double-blind, randomized, placebo-controlled clinical study of berberine revealed that berberine reduced total cholesterol, triglyceride and LDL cholesterol and increased HDL cholesterol (126). A randomized, double-blind, placebo-controlled clinical study of Danshen (Salvia miltiorrhiza) and Gegen (Radix puerariae) (D&G) revealed that D&G significantly reduced LDL, cholesterol, and total cholesterol levels, as well as carotid intima-media thickness (IMT) (127). A multicenter, double-blind, double-model, positive-controlled, parallel-randomized controlled clinical study of Ginkgo biloba extract (GBE50) revealed that the TCM symptom pattern score was greater overall than the control group was. The TCM symptom pattern score was the main observation of this study (128). However, two studies have also shown that the effects of Chinese medicine are not consistent. A randomized, parallel, prospective clinical study revealed that resveratrol increased serum concentrations of Sirt1 but had no significant effect on metabolic pathways and that the drug increased total cholesterol and apolipoprotein B concentrations and HOMA-IR scores in subjects during the study (129). A randomized, placebo-controlled, double-blind crossover study of Salvia miltiorrhiza root water extract (Danshen) revealed that the drug mildly elevated LDL-C levels in subjects but had no effect on multiple other risk indicators for AS (130). The actual effect of Chinese medicine in clinical use is clearly not stable, and the current research on the causes of this instability is insufficient (Table 2).
Table 2
| Name of Chinese Medicine | Type of Chinese Medicine | Number of patients | Patient Information | Results | |
|---|---|---|---|---|---|
| NC | HM | ||||
| Trans-resveratrol, grapeskin extract, quercetin (125) | + | 44 | Exclusion criteria: age <18 years; history of significant general illness; history of drug or supplement use that may alter metabolism or cardiovascular physiology; and use of grape-related supplements within 1 year. | Two subjects in the experimental group and one subject in the placebo group reported minor gastrointestinal side effects. For the experimental group, mRNA expression of ICAM, VCAM and IL-8 was significantly reduced (p < 0. 05), and biomarkers, such as plasma IFN-γ and insulin, also appeared to be reduced. | |
| Derberine (126) | + | 144 | Caucasian subjects | Treatment with berberine for 3 months reduced total cholesterol, triglycerides and LDL cholesterol and increased HDL cholesterol compared to placebo. | |
| Danshen (Salvia miltiorrhiza) and Gegen (Radix puerariae) (127) | + | 165 | 165 women aged 47–65 years who had been menopausal for more than 12 months were included in the study. | There were no significant changes in blood pressure and general biochemical parameters in both groups and serum LDL, cholesterol and total cholesterol were significantly lower in the experimental group. Carotid intima-media thickness (IMT) decreased by 1.52% from baseline in the experimental group, while it decreased by only 1.13% in the placebo-treated group. Twelve adverse events were reported (6 in the placebo group and 6 in the experimental group), but none were directly related to the study's herbal formulation. | |
| Ginkgo biloba extract (GBE50) (128) | + | 404 | 404 patients with cerebral atherosclerotic dizziness (blood stasis symptom pattern) from 10 hospitals in China | The overall efficiency of the experimental group was higher than that of the control group after 6 weeks of treatment (TCM symptom pattern score). The difference in the incidence of adverse reactions between the experimental group and the control group was not statistically significant. One drug-related adverse event occurred in the experimental group, showing mild elevation of ALT, AST and GGT, which was relieved after discontinuation of concomitant drugs (statins). One serious adverse event occurred in the control group. | |
| Resveratrol (129) | + | 48 | Twenty-four women with 1-year spontaneous amenorrhea and 24 men between the ages of 55 and 65 years. | Resveratrol increased the concentration of Sirt1, with insignificant effects on various metabolic pathways. In contrast, total cholesterol and apolipoprotein B concentrations and HOMA-IR scores increased after treatment. | |
| Danshen (130) | + | 20 | Subjects with hyperlipidemia and hypertension between the ages of 40 and 70 years. Patients on antihypertensive medication were included as eligible when the average of three office blood pressure recordings (sphygmomanometer, sitting position, at least 5 min apart) showed a systolic blood pressure >140 mmHg and/or a diastolic blood pressure >90 mmHg. Subjects with fasting LDL cholesterol >3.5 mmol/L and/or triglycerides >1.7 mmol/L. Excluded subjects were those with triglycerides >8 mmol/L and/or LDL-cholesterol >5 mmol/L or systolic blood pressure >180 mmHg and/or diastolic blood pressure >110 mmHg. | Mild increase in LDL-cholesterol after 4 weeks of Danshen (water-extract) treatment, no change in various other risk indicators. | |
Clinical study of Chinese medicine for AS.
The incidence of adverse events in these clinical studies was 0%–10% (125–130). Two RESV (400 mg trans-resveratrol, 400 mg grapeskin extract, and 100 mg quercetin) subjects reported mild gastrointestinal side effects (125). Regarding the study of “GBE50”, one drug-related adverse event occurred in the experimental group, but had no relation with traditional Chinese medicine (128), No adverse reactions were reported in other clinical studies.
4 Discussion
This review systematically integrates preclinical and clinical evidence to demonstrate that single herbal medicines (SHMs) and their derived natural compounds (NCs) exert anti-atherosclerotic (AS) effects through multifaceted mechanisms targeting key pathological drivers of AS. As summarized in Figure 7, Chinese medicines act on critical cellular components (e.g., endothelial cells, macrophages, vascular smooth muscle cells) and molecular pathways (e.g., NF-κB,MAPK, PI3K/Akt), underscoring their multi-target, multi-pathway regulatory characteristics. Preclinical studies highlight that TCM targets a spectrum of molecular nodes, Clinical trials further validate these effects. Collectively, these findings establish TCM as a promising therapeutic strategy for AS by concurrently targeting multiple pathological pathways.
Figure 7
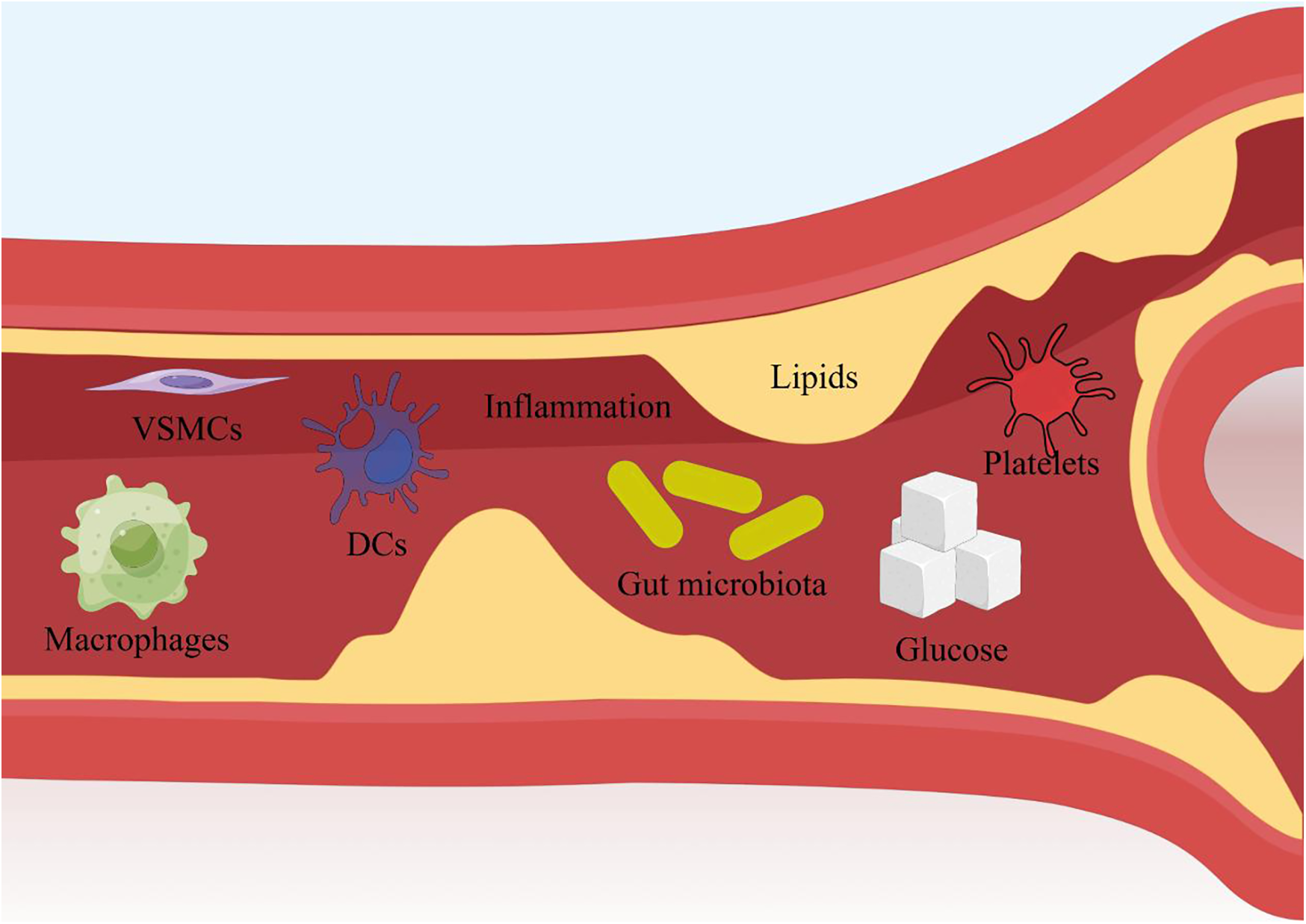
The main methods of Chinese medicine for treating AS. Produced by Figdraw (https://www.figdraw.com).
Despite promising preclinical and clinical data, three critical challenges hinder TCM's translation into robust AS therapies. The first is efficacy instability. Clinical studies report inconsistent outcomes. For example, resveratrol failed to improve metabolic pathways and elevated total cholesterol in some trials. Such variability may arise from heterogeneous study designs (e.g., dosage, duration), population differences, or unstandardized TCM preparations. The lack of deep mechanistic exploration for these discrepancies limits our ability to optimize clinical application. The second is the mechanistic blind spots. While preclinical studies have identified numerous targets and pathways, a systematic understanding of how TCM components coordinate to exert holistic effects remains elusive. Most research focuses on single-target or single-pathway analyses, neglecting network-level interactions. Additionally, the dynamic temporal and spatial regulation of TCM in vivo—such as bioavailability, metabolite profiling, and tissue-specific effects—requires further clarification. Finally, Chinese medicine has good safety at specific doses, but potential risks need to be paid attention toIf. Although clinical trials report low adverse event rates (0%–10%), potential risks are underexplored. Basic research rarely investigates long-term toxicity, drug-drug interactions, or dose-dependent side effects. This gap raises concerns about long-term clinical use.
In order to address these challenges, improve the efficacy stability and safety of TCM in treating AS, and clarify the blind spots at the mechanism level, we propose that future research can focus on the following directions. To enhance therapeutic consistency, we can implement modern techniques such as high-performance liquid chromatography (HPLC) fingerprint analysis and biomarker quantification to standardize quality control of traditional Chinese medicinal materials, thereby minimizing fluctuations in active ingredient concentrations. By designing multi-center randomized double-blind controlled trials (RCTs) stratified by patient groups (classified by AS severity and comorbidity types), we can precisely identify subgroups with optimal therapeutic outcomes for TCM treatments. Concurrently, pharmacokinetic-pharmacodynamic (PK-PD) models are employed to optimize dosing regimens, enabling precise therapeutic regulation. To clarify the blind spots of mechanisms, through integrate multi-omics (genomics, proteomics, metabolomics) to map TCM's global regulatory networks, identifying key hubs and crosstalk between pathways. Adopt advanced Models, utilize organ-on-a-chip or humanized mouse models to simulate AS progression in vivo, enabling real-time tracking of TCM's spatiotemporal effects. To strengthen the safety evaluation, we can conduct long-term (6–12 months) toxicity studies in relevant animal models, focusing on liver, kidney, and gastrointestinal systems. Establish standardized adverse event reporting protocols in TCM-AS trials, with specific attention to rare but severe reactions (e.g., hepatotoxicity). Drug interaction assessment is also required, evaluate potential interactions between TCM and conventional AS drugs (e.g., statins) to prevent adverse synergies or antagonisms.
In summary, while traditional Chinese medicine has historically addressed AS-related symptoms without explicit terminology, modern research confirms its scientific basis for targeting AS pathogenesis. However, translating this potential into clinical practice requires rigorous, evidence-driven efforts to resolve efficacy instability, decode mechanisms, and ensure safety-steps that will ultimately validate and optimize Chinese medicine as a key player in AS prevention and treatment. In conclusion, although the modern name of AS has never been explicitly proposed in traditional Chinese medicine owing to the limitations of diagnostic techniques and equipment, it has consciously or unconsciously provided ideas and inspiration for how to treat AS in the modern context in the clinical practice of traditional Chinese medicine for thousands of years. Many single herbal medicines and natural compounds have indeed proven effective in treating AS. However, much work is still needed to improve how to make efficient use of these valuable experiences.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
CT: Writing – original draft, Project administration, Methodology, Visualization, Conceptualization. BL: Writing – original draft, Investigation. YZ: Methodology, Writing – original draft. ML: Writing – original draft, Resources. WZ: Validation, Writing – original draft. JL: Writing – original draft, Data curation. HL: Investigation, Writing – original draft. ZX: Writing – review & editing. YW: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Chongqing Bayu Qihuang Scholars Support Programme (No grant numbers), Chongqing Yingcai Master Teacher Programme (grant numbers: CQYC20220203145). Postdoctoral Innovation Talents Support Program of the Daping Hospital of Army Medical University (grant numbers: ZXBSH038). Youth Doctoral Incubation Program of the Second Affiliated Hospital of Army Medical University (grant numbers: 2024YQB007).
Acknowledgments
We are grateful for the support of the Chongqing Bayu Qihuang Scholars Support Programme and the Chongqing Yingcai Master Teacher Programme.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AS, atherosclerosis; CVDs, cardiovascular diseases; TCM, traditional chinese medicine; SHMs, single herbal medicines; NCs, natural compounds; TC, total cholesterol; TG, triglycerides; AGEs, advanced glycation end products; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; AGEs, advanced glycation end products; NO, nitric oxide; ECD, endothelial cell dysfunction; PECAM-1, platelet-endothelial cell adhesion molecule-1; DCs, dendritic cells; D&G, Danshen (Salvia miltiorrhiza) and Gegen (Radix puerariae); IMT, intima-media thickness.
References
1.
Cheng J Huang H Chen Y Wu R . Nanomedicine for diagnosis and treatment of atherosclerosis. Adv Sci (Weinh). (2023) 10(36):e2304294. 10.1002/advs.202304294
2.
Libby P . The changing landscape of atherosclerosis. Nature. (2021) 592(7855):524–33. 10.1038/s41586-021-03392-8
3.
National Center for Cardiovascular Diseases C. China Cardiovascular Health and Disease Report 2023. Beijing: Peking Union Medical College Press (2024). p. 257.
4.
Anand SS Bosch J Eikelboom JW Connolly SJ Diaz R Widimsky P et al Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. (2018) 391(10117):219–29. 10.1016/s0140-6736(17)32409-1
5.
Ahmad Z Ng CT Fong LY Abu Bakar NA Hussain NHM Ang KP et al Cryptotanshinone inhibits TNF-alpha-induced early atherogenic events in vitro. J Physiol Sci. (2016) 66(3):213–20. 10.1007/s12576-015-0410-7
6.
Bao M-h Zhang Y-w Zhou H-h . Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-kappa B pathway. J Ethnopharmacol. (2013) 146(2):543–51. 10.1016/j.jep.2013.01.019
7.
Chen Y Wang S Yang S Li R Yang Y Chen Y et al Inhibitory role of ginsenoside Rb2 in endothelial senescence and inflammation mediated by microRNA-216a. Mol Med Rep. (2021) 23(6):415. 10.3892/mmr.2021.12054
8.
Diao Y . Clematichinenoside AR alleviates foam cell formation and the inflammatory response in Ox-LDL-induced RAW264.7 cells by activating autophagy. Inflammation. (2021) 44(2):758–68. 10.1007/s10753-020-01375-x
9.
Fu C Yin D Nie H Sun D . Notoginsenoside R1 protects HUVEC against oxidized low density lipoprotein (Ox-LDL)-induced atherogenic response via down-regulating miR-132. Cell Physiol Biochem. (2018) 51(4):1739–50. 10.1159/000495677
10.
Ge X Chen S Liu M Liang T Liu C . Evodiamine attenuates PDGF-BB-induced migration of rat vascular smooth muscle cells through activating PPAR gamma. Int J Mol Sci. (2015) 16(12):28180–93. 10.3390/ijms161226093
11.
Gu Y Xiao Z Wu J Guo M Lv P Dou N . Anti-atherosclerotic effect of afrocyclamin A against vascular smooth muscle cells is mediated via p38 MAPK signaling pathway. Cell J. (2021) 23(2):191–8. 10.22074/cellj.2021.7148
12.
He D Wang H Xu L Wang X Peng K Wang L et al Saikosaponin-a attenuates oxidized LDL uptake and prompts cholesterol efflux in THP-1 cells. J Cardiovasc Pharmacol. (2016) 67(6):510–8. 10.1097/fjc.0000000000000373
13.
Hu Y Liu K Yan M Zhang Y Wang Y Ren L . Effects and mechanisms of icariin on atherosclerosis. Int J Clin Exp Med. (2015) 8(3):3585–9.
14.
Jamal J Mustafa MR Wong P-F . Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J Ethnopharmacol. (2014) 154(2):428–36. 10.1016/j.jep.2014.04.025
15.
Joe Y Zheng M Kim HJ Kim S Uddin MJ Park C et al Salvianolic acid B exerts vasoprotective effects through the modulation of heme oxygenase-1 and arginase activities. J Pharmacol Exp Ther. (2012) 341(3):850–8. 10.1124/jpet.111.190736
16.
Lee J-J Yi H Kim I-S Kim Y Nguyen Xuan N Kim YH et al (2s)-Naringenin from Typha angustata inhibits vascular smooth muscle cell proliferation via a G(0)/G(1) arrest. J Ethnopharmacol. (2012) 139(3):873–8. 10.1016/j.jep.2011.12.038
17.
Li C Yang L Wu H Dai M . Paeonol inhibits oxidized low-density lipoprotein-induced vascular endothelial cells autophagy by upregulating the expression of miRNA-30a. Front Pharmacol. (2018) 9:95. 10.3389/fphar.2018.00095
18.
Lin X-l Liu M-H Hu H-J Feng H -r, Fan X-J Zou W-w et al Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXR alpha signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol. (2015) 34(9):561–72. 10.1089/dna.2015.2866
19.
Liu Y Fu Y-Q Peng W-J Yu Y-R Wu Y-S Yan H et al Rutaecarpine reverses the altered connexin expression pattern induced by oxidized low-density lipoprotein in monocytes. J Cardiovasc Pharmacol. (2016) 67(6):519–25. 10.1097/fjc.0000000000000372
20.
Moon CY Ku CR Cho YH Lee EJ . Protocatechuic aldehyde inhibits migration and proliferation of vascular smooth muscle cells and intravascular thrombosis. Biochem Biophys Res Commun. (2012) 423(1):116–21. 10.1016/j.bbrc.2012.05.092
21.
Peng Z Zhan H Shao Y Xiong Y Zeng L Zhang C et al 13-Methylberberine improves endothelial dysfunction by inhibiting NLRP3 inflammasome activation via autophagy induction in human umbilical vein endothelial cells. Chin Med. (2020) 15(1):8. 10.1186/s13020-020-0286-1
22.
Shi Y Jiang S Zhao T Gong Y Liao D Qin L . Celastrol suppresses lipid accumulation through LXR alpha/ABCA1 signaling pathway and autophagy in vascular smooth muscle cells. Biochem Biophys Res Commun. (2020) 532(3):466–74. 10.1016/j.bbrc.2020.08.076
23.
Song C Wang Y Cui L Yan F Shen S . Triptolide attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: involvement of NF-kappa B pathway. BMC Complement Altern Med. (2019) 19(1):198. 10.1186/s12906-019-2616-3
24.
Song N Jia L Cao H Ma Y Chen N Chen S et al Gypenoside inhibits endothelial cell apoptosis in atherosclerosis by modulating mitochondria through PI3K/Akt/Bad pathway. BioMed Res Int. (2020) 2020:2819658. 10.1155/2020/2819658
25.
Song Y Long L Zhang N Liu Y . Inhibitory effects of hydroxysafflor yellow A on PDGF-BB-induced proliferation and migration of vascular smooth muscle cells via mediating Akt signaling. Mol Med Rep. (2014) 10(3):1555–60. 10.3892/mmr.2014.2336
26.
Tian C Zhang R Ye X Zhang C Jin X Yamori Y et al Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway. Genes Nutr. (2013) 8(2):231–9. 10.1007/s12263-012-0319-1
27.
Wang K Cao Q Yang Q Wei Q Zhao J Wang Y et al Study on the regulatory effect of leech peptide HE-D on macrophages in atherosclerosis by transcriptome sequencing. J Ethnopharmacol. (2022) 294:115380. 10.1016/j.jep.2022.115380
28.
Xing J Peng K Cao W Lian X Wang Q Wang X . Effects of total flavonoids from Dracocephalum moldavica on the proliferation, migration, and adhesion molecule expression of rat vascular smooth muscle cells induced by TNF-alpha. Pharm Biol. (2013) 51(1):74–83. 10.3109/13880209.2012.711839
29.
Xing Y-L Zhou Z , Agula, ZhongZ-YMaY-JZhaoY-let alProtocatechuic aldehyde inhibits lipopolysaccharide-induced human umbilical vein endothelial cell apoptosis via regulation of caspase-3. Phytother Res. (2012) 26(9):1334–41. 10.1002/ptr.3720
30.
Yang Y Li X Peng L An L Sun N Hu X et al Tanshindiol C inhibits oxidized low-density lipoprotein induced macrophage foam cell formation via a peroxiredoxin 1 dependent pathway. Biochim Et Biophys Acta-Mol Basis of Dis. (2018) 1864(3):882–90. 10.1016/j.bbadis.2017.12.033
31.
Yuan R Shi W Xin Q Yang B Hoi MP Lee SM et al Tetramethylpyrazine and paeoniflorin inhibit oxidized LDL-induced angiogenesis in human umbilical vein endothelial cells via VEGF and notch pathways. Evid Based Complemen Altern Med. (2018) 2018:3082507. 10.1155/2018/3082507
32.
Zhang M Li F Pokharel S Ma T Wang X Wang Y et al Lycium barbarum polysaccharide protects against homocysteine-induced vascular smooth muscle cell proliferation and phenotypic transformation via PI3K/Akt pathway. J Mol Histol. (2020) 51(6):629–37. 10.1007/s10735-020-09909-1
33.
Zhang X Hu W Wu F Yuan X Hu J . Shikonin inhibits TNF-alpha-induced growth and invasion of rat aortic vascular smooth muscle cells. Can J Physiol Pharmacol. (2015) 93(8):615–24. 10.1139/cjpp-2014-0464
34.
Zhang Z Zhang D Du B Chen Z . Hyperoside inhibits the effects induced by oxidized low-density lipoprotein in vascular smooth muscle cells via oxLDL-LOX-1-ERK pathway. Mol Cell Biochem. (2017) 433(1-2):169–76. 10.1007/s11010-017-3025-x
35.
Zhao W Feng H Guo S Han Y Chen X . Danshenol A inhibits TNF-alpha-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci Rep. (2017) 7:12953. 10.1038/s41598-017-13072-1
36.
Zhou P Xie W Luo Y Lu S Dai Z Wang R et al Protective effects of total saponins of Aralia elata (Miq.) on endothelial cell injury induced by TNF-alpha via modulation of the PI3K/Akt and NF-kappa B signalling pathways. Int J Mol Sci. (2019) 20(1):36. 10.3390/ijms20010036
37.
Hu Y Sun B Liu K Yan M Zhang Y Miao C et al Icariin attenuates high-cholesterol diet induced atherosclerosis in rats by inhibition of inflammatory response and p38 MAPK signaling pathway. Inflammation. (2016) 39(1):228–36. 10.1007/s10753-015-0242-x
38.
Jia L-q Zhang N Xu Y Chen W-n Zhu M-l Song N et al Tanshinone IIA affects the HDL subfractions distribution not serum lipid levels: involving in intake and efflux of cholesterol. Arch Biochem Biophys. (2016) 592:50–9. 10.1016/j.abb.2016.01.001
39.
Yao W Fan W Huang C Zhong H Chen X Zhang W . Proteomic analysis for anti-atherosclerotic effect of tetrahydroxystilbene glucoside in rats. Biomed Pharmacother. (2013) 67(2):140–5. 10.1016/j.biopha.2012.10.007
40.
Waisundara VY Siu SY Hsu A Huang D Tan BK . Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci. (2011) 88(23–24):1016–25. 10.1016/j.lfs.2011.03.009
41.
Jin Z-h Gao P Liu Z-t Jin B Song G-y Xiang T-y . Composition of ophiopogon polysaccharide, notoginseng total saponins and rhizoma coptidis alkaloids inhibits the myocardial apoptosis on diabetic atherosclerosis rabbit. Chin J Integr Med. (2020) 26(5):353–60. 10.1007/s11655-018-3014-2
42.
Lee M-F Chiang C-H Lin S-J Song P-P Liu H-C Wu T-J et al Recombinant lactococcus lactis expressing ling Zhi 8 protein ameliorates nonalcoholic fatty liver and early atherogenesis in cholesterol-fed rabbits. BioMed Res Int. (2020) 2020:3495682. 10.1155/2020/3495682
43.
Li Y Tang J Gao H Xu Y Han Y Shang H et al Ganoderma lucidum triterpenoids and polysaccharides attenuate atherosclerotic plaque in high-fat diet rabbits. Nutr Metab Cardiovasc Di.. (2021) 31(6):1929–38. 10.1016/j.numecd.2021.03.023
44.
Mo J Yang R Li F Zhang X He B Zhang Y et al Scutellarin protects against vascular endothelial dysfunction and prevents atherosclerosis via antioxidation. Phytomedicine. (2018) 42:66–74. 10.1016/j.phymed.2018.03.021
45.
Zhang Y Guo W Wen Y Xiong Q Liu H Wu J et al SCM-198 attenuates early atherosclerotic lesions in hypercholesterolemic rabbits via modulation of the inflammatory and oxidative stress pathways. Atherosclerosis. (2012) 224(1):43–50. 10.1016/j.atherosclerosis.2012.06.066
46.
Chen Q Qi X Zhang W Zhang Y Bi Y Meng Q et al Catalpol inhibits macrophage polarization and prevents postmenopausal atherosclerosis through regulating estrogen receptor alpha. Front Pharmacol. (2021) 12:655081. 10.3389/fphar.2021.655081
47.
Chen W Li X Guo S Song N Wang J Jia L et al Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int Immunopharmacol. (2019) 70:486–97. 10.1016/j.intimp.2019.02.054
48.
Feng M Kong S-Z Wang Z-X He K Zou Z-Y Hu Y-R et al The protective effect of coptisine on experimental atherosclerosis ApoE(-/-) mice is mediated by MAPK/NF-kappa B-dependent pathway. Biomed Pharmacother. (2017) 93:721–9. 10.1016/j.biopha.2017.07.002
49.
Feng M Zou Z Zhou X Hu Y Ma H Xiao Y et al Comparative effect of berberine and its derivative 8-cetylberberine on attenuating atherosclerosis in ApoE(-/-) mice. Int Immunopharmacol. (2017) 43:195–202. 10.1016/j.intimp.2016.12.001
50.
Feng Z Wang C Yue J Meng Q Wu J Sun H . Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm Biol. (2021) 59(1):1104–14. 10.1080/13880209.2021.1961823
51.
Fu Y Feng H Ding X Meng Q-H Zhang S-R Li J et al Alisol B 23-acetate adjusts bile acid metabolisim via hepatic FXR-BSEP signaling activation to alleviate atherosclerosis. Phytomedicine. (2022) 101:154120. 10.1016/j.phymed.2022.154120
52.
Gu L Bai W Li S Zhang Y Han Y Gu Y et al Celastrol prevents atherosclerosis via inhibiting LOX-1 and oxidative stress. PLoS One. (2013) 8(6):e65477. 10.1371/journal.pone.0065477
53.
Guo J Ma J Cai K Chen H Xie K Xu B et al Isoflavones from semen sojae preparatum improve atherosclerosis and oxidative stress by modulating Nrf2 signaling pathway through estrogen-like effects. Evid-Based Complement Altern Med. (2022) 2022:4242099. 10.1155/2022/4242099
54.
Hsu P-L Lin Y-C Ni H Mo F-E . Ganoderma triterpenoids exert antiatherogenic effects in mice by alleviating disturbed flow-induced oxidative stress and inflammation. Oxid Med Cell Longevity. (2018) 2018:3491703. 10.1155/2018/3491703
55.
Jiang W Cen Y Song Y Li P Qin R Liu C et al Artesunate attenuated progression of atherosclerosis lesion formation alone or combined with rosuvastatin through inhibition of pro-inflammatory cytokines and pro-inflammatory chemokines. Phytomedicine. (2016) 23(11):1259–66. 10.1016/j.phymed.2016.06.004
56.
Ku S-K Bae J-S . Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. (2015) 48(9):519–24. 10.5483/BMBRep.2015.48.9.017
57.
Liu F-Y Wen J Hou J Zhang S-Q Sun C-B Zhou L-C et al Gastrodia remodels intestinal microflora to suppress inflammation in mice with early atherosclerosis. Int Immunopharmacol. (2021) 96:107758. 10.1016/j.intimp.2021.107758
58.
Liu L Li Q Yin J Zhao Z Sun L Ran Q et al Shenlian extract enhances TGF-beta functions in the macrophage-SMC unit and stabilizes atherosclerotic plaques. Front Pharmacol. (2021) 12:669730. 10.3389/fphar.2021.669730
59.
Liu L Liao P Wang B Fang X Li W Guan S . Oral administration of baicalin and geniposide induces regression of atherosclerosis via inhibiting dendritic cells in ApoE-knockout mice. Int Immunopharmacol. (2014) 20(1):197–204. 10.1016/j.intimp.2014.02.037
60.
Liu L Liao P Wang B Fang X Li W Guan S . Baicalin inhibits the expression of monocyte chemoattractant protein-1 and interleukin-6 in the kidneys of apolipoprotein E-knockout mice fed a high cholesterol diet. Mol Med Rep. (2015) 11(5):3976–80. 10.3892/mmr.2015.3186
61.
Liu Z Xu S Huang X Wang J Gao S Li H et al Cryptotanshinone, an orally bioactive herbal compound from Danshen, attenuates atherosclerosis in apolipoprotein E-deficient mice: role of lectin-like oxidized LDL receptor-1 (LOX-1). Br J Pharmacol. (2015) 172(23):5661–75. 10.1111/bph.13068
62.
Ma X Zhang T Luo Z Li X Lin M Li R et al Functional nano-vector boost anti-atherosclerosis efficacy of berberine in Apoe((-/-)) mice. Acta Pharm Sin B. (2020) 10(9):1769–83. 10.1016/j.apsb.2020.03.005
63.
Meng N Chen K Wang Y Hou J Chu W Xie S et al Dihydrohomoplantagin and homoplantaginin, Major flavonoid glycosides from salvia plebeia R. Br. inhibit oxLDL-induced endothelial cell injury and restrict atherosclerosis via activating Nrf2 anti-oxidation signal pathway. Molecules. (2022) 27(6):1990. 10.3390/molecules27061990
64.
Qiao Y Zhang P-j Lu X-t Sun W-w Liu G-l , Ren M et al Panax notoginseng saponins inhibits atherosclerotic plaque angiogenesis by down-regulating vascular endothelial growth factor and nicotinamide adenine dinucleotide phosphate oxidase subunit 4 expression. Chin J Integr Med. (2015) 21(4):259–65. 10.1007/s11655-014-1832-4
65.
Qin H Liu P Lin S . Effects of astragaloside IV on the SDF-1/CXCR4 expression in atherosclerosis of apoE(-/-) mice induced by hyperlipaemia. Evid-Based Complemen Altern Med. (2015) 2015:385154. 10.1155/2015/385154
66.
Sun G-b Qin M Ye J-x Pan R-l Meng X-b Wang M et al Inhibitory effects of myricitrin on oxidative stress-induced endothelial damage and early atherosclerosis in ApoE -/- mice. Toxicol Appl Pharmacol. (2013) 271(1):114–26. 10.1016/j.taap.2013.04.015
67.
Sun X Wu A Law BYK Liu C Zeng W Qiu ACL et al The active components derived from Penthorum chinense Pursh protect against oxidative-stress-induced vascular injury via autophagy induction. Free Radic Biol Med. (2020) 146:160–80. 10.1016/j.freeradbiomed.2019.10.417
68.
Sun Y Long J Chen W Sun Y Zhou L Zhang L et al Alisol B 23-acetate, a new promoter for cholesterol efflux from dendritic cells, alleviates dyslipidemia and inflammation in advanced atherosclerotic mice. Int Immunopharmacol. (2021) 99:107956. 10.1016/j.intimp.2021.107956
69.
Tang Y Wu H Shao B Wang Y Liu C Guo M. Celosins inhibit atherosclerosis in ApoE(-/-) mice and promote autophagy flow. J Ethnopharmacol. (2018) 215:74–82. 10.1016/j.jep.2017.12.031
70.
Wang H-T Wang Z-Z Wang Z-C Wang S-M Cai X-J Su G-H et al Patchouli alcohol attenuates experimental atherosclerosis via inhibiting macrophage infiltration and its inflammatory responses. Biomed Pharmacother. (2016) 83:930–5.10.1016/j.biopha.2016.08.005
71.
Wang K Zhang B Song D Xi J Hao W Yuan J et al Alisol A alleviates arterial plaque by activating AMPK/SIRT1 signaling pathway in apoE-deficient mice. Front Pharmacol. (2020) 11:580073. 10.3389/fphar.2020.580073
72.
Wang X Du H Li X . Artesunate attenuates atherosclerosis by inhibiting macrophage M1-like polarization and improving metabolism. Int Immunopharmacol. (2022) 102:108413. 10.1016/j.intimp.2021.108413
73.
Wang Y Wang Y-S Song S-L Liang H Ji A-G . Icariin inhibits atherosclerosis progress in Apoe null mice by downregulating CX3CR1 in macrophage. Biochem Biophys Res Commun. (2016) 470(4):845–50. 10.1016/j.bbrc.2016.01.118
74.
Wei J Huang L Li D He J Li Y He F et al Total flavonoids of engelhardia roxburghiana wall. Leaves alleviated foam cells formation through AKT/mTOR-mediated autophagy in the progression of atherosclerosis. Chem Biodiversity. (2021) 18(9):e2100308. 10.1002/cbdv.202100308
75.
Xiao Y Wang Y-C Li L-L Jin Y-C Sironi L Wang Y et al Lactones from ligusticum chuanxiong hort. Reduces atherosclerotic lesions in apoE-deficient mice via inhibiting over expression of NF-kB -dependent adhesion molecules. Fitoterapia. (2014) 95:240–6. 10.1016/j.fitote.2014.02.012
76.
Xuan Y Gao Y Huang H Wang X Cai Y Luan QX . Tanshinone IIA attenuates atherosclerosis in apolipoprotein E knockout mice infected with porphyromonas gingivalis. Inflammation. (2017) 40(5):1631–42. 10.1007/s10753-017-0603-8
77.
Yang Q Wang C Jin Y Ma X Xie T Wang J et al Disocin prevents postmenopausal atherosclerosis in ovariectomized LDLR-/- mice through a PGC-1 alpha/ER alpha pathway leading to promotion of autophagy and inhibition of oxidative stress, inflammation and apoptosis. Pharmacol Res. (2019) 148:104414. 10.1016/j.phrs.2019.104414
78.
Zhang L Li Y Ma X Liu J Wang X Zhang L et al Ginsenoside Rg1-notoginsenoside R1-protocatechuic aldehyde reduces atherosclerosis and attenuates low-shear stress-induced vascular endothelial cell dysfunction. Front Pharmacol. (2021) 11:588259. 10.3389/fphar.2020.588259
79.
Zheng B Yang L Wen C Huang X Xu C Lee K-H et al Curcumin analog L3 alleviates diabetic atherosclerosis by multiple effects. Eur J Pharmacol. (2016) 775:22–34. 10.1016/j.ejphar.2016.02.016
80.
Zhu J Xu Y Ren G Hu X Wang C Yang Z et al Tanshinone IIA sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. Eur J Pharmacol. (2017) 815:427–36. 10.1016/j.ejphar.2017.09.047
81.
Han J Dong J Zhang R Zhang X Chen M Fan X et al Dendrobium catenatum Lindl. Water extracts attenuate atherosclerosis. Mediat Inflamm. (2021) 2021:9951946.10.1155/2021/9951946
82.
Liang P-L Chen X-L Gong M-J Xu Y Tu H-S Zhang L et al Guang Chen Pi (the pericarp of Citrus reticulata Blanco's cultivars ‘Chachi’) inhibits macrophage-derived foam cell formation. J Ethnopharmacol. (2022) 293:115328. 10.1016/j.jep.2022.115328
83.
Stumpf C Fan Q Hintermann C Raaz D Kurfuerst I Losert S et al Anti-inflammatory effects of Danshen on human vascular endothelial cells in culture. Am J Chin Med. (2013) 41(5):1065–77. 10.1142/s0192415x13500729
84.
Tian Y Gong P Wu Y Chang S Xu J Yu B et al Screening and identification of potential active components in ophiopogonis radix against atherosclerosis by biospecific cell extraction. J Chromatogr B Analyt Technol Biomed Life Sci.. (2019) 1133:121817. 10.1016/j.jchromb.2019.121817
85.
Ding S Wang W Yin X Wang L Gong L Liao F et al The joint effect of a combination of components from the fruit of crataegus pinnatifida Bge. Var. major NE Br. and the root of salvia miltiorrhiza Bge. With exercises on swimming in focal cerebral infraction in rat. Front Physiol. (2020) 11:574535. 10.3389/fphys.2020.574535
86.
Zhang J Liang R Wang L Yan R Hou R Gao S et al Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major NEBr. Fruit on experimental atherosclerosis in rats. J Ethnopharmacol. (2013) 148(2):563–9. 10.1016/j.jep.2013.04.053
87.
Zhao X Zhu J Wang L Li Y Zhao T Chen X et al U. diffracta extract mitigates high fat diet and VD3-induced atherosclerosis and biochemical changes in the serum liver and aorta of rats. Biomed Pharmacother. (2019) 120:109446. 10.1016/j.biopha.2019.109446
88.
Lai P Cao X Xu Q Liu Y Li R Zhang J et al Ganoderma lucidumspore ethanol extract attenuates atherosclerosis by regulating lipid metabolism via upregulation of liver X receptor alpha. Pharm Biol. (2020) 58(1):760–70. 10.1080/13880209.2020.1798471
89.
Chen X Cao J Sun Y Dai Y Zhu J Zhang X et al Ethanol extract of Schisandrae chinensis fructus ameliorates the extent of experimentally induced atherosclerosis in rats by increasing antioxidant capacity and improving endothelial dysfunction. Pharm Biol. (2018) 56(1):612–9. 10.1080/13880209.2018.1523933
90.
Choi DH Lee YJ Oh HC Cui YL Kim JS Kang DG et al Improved endothelial dysfunction by Cynanchum wilfordii in Apolipoprotein E−/− mice fed a high fat/cholesterol diet. J Med Food. (2012) 15(2):169–79. 10.1089/jmf.2010.1222
91.
Hashikawa-Hobara N Hashikawa N Sugiman N Hosoo S Hirata T Yamaguchi Y et al Oral administration of Eucommia ulmoides Oliv. leaves extract protects against atherosclerosis by improving macrophage function in ApoE knockout mice. J Food Sci. (2020) 85(11):4018–24. 10.1111/1750-3841.15461
92.
Ko M Oh GT Park J Kwon HJ . Extract of high hydrostatic pressure-treated danshen (Salvia miltiorrhiza) ameliorates atherosclerosis via autophagy induction. BMB Rep. (2020) 53(12):652–7. 10.5483/BMBRep.2020.53.12.184
93.
Li F Zhang T He Y Gu W Yang X Zhao R et al Inflammation inhibition and gut microbiota regulation by TSG to combat atherosclerosis in ApoE(-/-) mice. J Ethnopharmacol. (2020) 247:112232. 10.1016/j.jep.2019.112232
94.
Nie W Zhang X Yan H Li S Zhu W Fan F et al Xiaoxianggou attenuates atherosclerotic plaque formation in endogenous high Ang II ApoE(-/-) mice via the inhibition of miR-203 on the expression of Ets-2 in endothelial cells. Biomed Pharmacother. (2016) 82:173–9. 10.1016/j.biopha.2016.04.065
95.
Wang Y Zhao X Wang Y-S Song S-L Liang H Ji A-G . An extract from medical leech improve the function of endothelial cells in vitro and attenuates atherosclerosis in ApoE null mice by reducing macrophages in the lesions. Biochem Biophys Res Commun. (2014) 455(1-2):119–25. 10.1016/j.bbrc.2014.10.135
96.
Yu B Zhang G Jin L Zhang B Yan D Yang H et al Inhibition of PAI-1 activity by toddalolactone as a mechanism for promoting blood circulation and removing stasis by Chinese herb Zanthoxylum nitidum var. tomentosum . Front Pharmacol. (2017) 8:489. 10.3389/fphar.2017.00489
97.
Wang Y Wu J Zhu J Ding C Xu W Hao H et al Ginsenosides regulation of lysophosphatidylcholine profiles underlies the mechanism of Shengmai Yin in attenuating atherosclerosis. J Ethnopharmacol. (2021) 277:114223. 10.1016/j.jep.2021.114223
98.
Xiong M-q Jia C-l Cui J-g Ming B-b Zhu Y-l Wang W-j et al Anti-atherosclerotic effects of bear bile powder in shexiang tongxin dripping pill: a mechanism study. Zhongguo Zhong Xi Yi Jie He Za Zhi. (2015) 35(9):1083–9. CNKI:SUN:ZZXJ.0.2015-09-016
99.
He J Deng Y Ren L Jin Z Yang J Yao F et al Isoliquiritigenin from licorice flavonoids attenuates NLRP3-mediated pyroptosis by SIRT6 in vascular endothelial cells. J Ethnopharmacol. (2023) 303:115952. 10.1016/j.jep.2022.115952
100.
He P Wang H Cheng S Hu F Zhang L Chen W et al Geniposide ameliorates atherosclerosis by regulating macrophage polarization via perivascular adipocyte-derived CXCL14. J Ethnopharmacol. (2023) 314:116532. 10.1016/j.jep.2023.116532
101.
Ma J Wang L Zhao Y Gao Y Yin Z Zhao M et al 2-(2-Phenylethyl)chromone-enriched Extract of Chinese agarwood (Aquilaria sinensis) inhibits atherosclerosis progression through endoplasmic reticulum stress-mediated CD36 expression in macrophages. J Ethnopharmacol. (2024) 320:117411. 10.1016/j.jep.2023.117411
102.
Ma X Zhang L Gao F Jia W Li C . Salvia miltiorrhiza and tanshinone IIA reduce endothelial inflammation and atherosclerotic plaque formation through inhibiting COX-2. Biomed Pharmacother. (2023) 167:115501. 10.1016/j.biopha.2023.115501
103.
Shi R Liu Z Yin J Deng Y . Quercetin relieves coronary atherosclerosis via regulating fibroblast growth factor 2. Cell Mol Biol (Noisy-le-grand). (2023) 69(15):223–9. 10.14715/cmb/2023.69.15.38
104.
Shi S Ji X Shi J Shi S She F Zhang Q et al Andrographolide in atherosclerosis: integrating network pharmacology and in vitro pharmacological evaluation. Biosci Rep. (2022) 42(7):BSR20212812. 10.1042/bsr20212812
105.
Sun T Quan W Peng S Yang D Liu J He C et al Network pharmacology-based strategy combined with molecular docking and in vitro validation study to explore the underlying mechanism of Huo Luo Xiao Ling Dan in treating atherosclerosis. Drug Des Devel Ther. (2022) 16:1621–45. 10.2147/dddt.S357483
106.
Wang J Li J Hu M . Mechanism analysis of Buyang Huanwu decoction in treating atherosclerosis based on network pharmacology and in vitro experiments. Chem Biol Drug Des. (2024) 103(1):e14447. 10.1111/cbdd.14447
107.
Wang J Zhang Y Feng X Du M Li S Chang X et al Tanshinone IIA alleviates atherosclerosis in LDLR(-/-) mice by regulating efferocytosis of macrophages. Front Pharmacol. (2023) 14:1233709. 10.3389/fphar.2023.1233709
108.
Wang LT Huang H Chang YH Wang YQ Wang JD Cai ZH et al Biflavonoids from Ginkgo biloba leaves as a novel anti-atherosclerotic candidate: inhibition potency and mechanistic analysis. Phytomedicine. (2022) 102:154053. 10.1016/j.phymed.2022.154053
109.
Xu J Tian Z Li Z Du X Cui Y Wang J et al Puerarin-Tanshinone IIA suppresses atherosclerosis inflammatory plaque via targeting succinate/HIF-1α/IL-1β axis. J Ethnopharmacol. (2023) 317:116675. 10.1016/j.jep.2023.116675
110.
Yang R Lin F Wang W Dai G Ke X Wu G . Investigating the therapeutic effects and mechanisms of Carthamus tinctorius L.-derived nanovesicles in atherosclerosis treatment. Cell Commun Signal. (2024) 22(1):178. 10.1186/s12964-024-01561-6
111.
Zha Y Yang Y Zhou Y Ye B Li H Liang J . Dietary evodiamine inhibits atherosclerosis-associated changes in vascular smooth muscle cells. Int J Mol Sci. (2023) 24(7):6653. 10.3390/ijms24076653
112.
Zhang C Wu X Shi P Ma H Fang F Feng Q et al Diterpenoids inhibit ox-LDL-induced foam cell formation in RAW264.7 cells by promoting ABCA1 mediated cholesterol efflux. Front Pharmacol. (2023) 14:1066758. 10.3389/fphar.2023.1066758
113.
Zhang J Zhao WR Shi WT Tan JJ Zhang KY Tang JY et al Tribulus terrestris L. extract ameliorates atherosclerosis by inhibition of vascular smooth muscle cell proliferation in ApoE(-/-) mice and A7r5 cells via suppression of Akt/MEK/ERK signaling. J Ethnopharmacol. (2022) 297:115547. 10.1016/j.jep.2022.115547
114.
Zhang L Yang Z Li X Hua Y Fan G He F . Anti-atherosclerotic effects of naringenin and quercetin from Folium Artemisiae argyi by attenuating interleukin-1 beta (IL-1β)/ matrix metalloproteinase 9 (MMP9): network pharmacology-based analysis and validation. BMC Complement Med Ther. (2023) 23(1):378. 10.1186/s12906-023-04223-1
115.
Zhang S Xie S Gao Y Wang Y . Triptolide alleviates oxidized LDL-induced endothelial inflammation by attenuating the oxidative stress-mediated nuclear factor-kappa B pathway. Curr Ther Res Clin Exp. (2022) 97:100683. 10.1016/j.curtheres.2022.100683
116.
Zhang Y Xin G Zhou Q Yu X Feng L Wen A et al Elucidating the distinctive regulatory effects and mechanisms of active compounds in Salvia miltiorrhiza Bunge via network pharmacology: unveiling their roles in the modulation of platelet activation and thrombus formation. Toxicol Appl Pharmacol. (2024) 484:116871. 10.1016/j.taap.2024.116871
117.
Zheng G Zhao Y Li Z Hua Y Zhang J Miao Y et al GLSP and GLSP-derived triterpenes attenuate atherosclerosis and aortic calcification by stimulating ABCA1/G1-mediated macrophage cholesterol efflux and inactivating RUNX2-mediated VSMC osteogenesis. Theranostics. (2023) 13(4):1325–41. 10.7150/thno.80250
118.
Zhu J Chen H Le Y Guo J Liu Z Dou X et al Salvianolic acid A regulates pyroptosis of endothelial cells via directly targeting PKM2 and ameliorates diabetic atherosclerosis. Front Pharmacol. (2022) 13:1009229. 10.3389/fphar.2022.1009229
119.
Yesheng L Shujie Y Bin Z Xiaoxian Q . Effects of Rhodiola rosea L. polysaccharides on PI3K/AKT/GSK3βSignal pathway in diabetic mice. China Trad Chin Med Technol. (2018) 25(06):812–4. CNKI:SUN:TJYY.0.2018-06-012
120.
Zhang Y Gu Y Chen Y Huang Z Li M Jiang W et al Dingxin recipe IV attenuates atherosclerosis by regulating lipid metabolism through LXR-alpha/SREBP1 pathway and modulating the gut microbiota in ApoE(-/-) mice fed with HFD. J Ethnopharmacol. (2021) 266:113436. 10.1016/j.jep.2020.113436
121.
Mao Y . Hypoglycemic and hypolipidaemic activities of polysaccharides from Rhodiola rosea in KKAy mice. J Food Proc Preserv. (2017) 41(6):e13219. 10.1111/jfpp.13219
122.
Botts SR Fish JE Howe KL . Dysfunctional vascular endothelium as a driver of atherosclerosis: emerging insights into pathogenesis and treatment. Front Pharmacol. (2021) 12:787541. 10.3389/fphar.2021.787541
123.
Hui B Hou X Liu R Liu X-H Hu Z . Gypenoside inhibits ox-LDL uptake and foam cell formation through enhancing Sirt1-FOXO1 mediated autophagy flux restoration. Life Sci. (2021) 264:118721. 10.1016/j.lfs.2020.118721
124.
He XW Yu D Li WL et al Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed Pharmacother. (2016) 83:257–64. 10.1016/j.biopha.2016.06.046
125.
Agarwal B Campen MJ Channell MM Wherry SJ Varamini B Davis JG et al Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. Int J Cardiol. (2013) 166(1):246–8. 10.1016/j.ijcard.2012.09.027
126.
Derosa G D'Angelo A Bonaventura A Bianchi L Romano D Maffioli P . Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther. (2013) 13(4):475–82. 10.1517/14712598.2013.776037
127.
Kwok T Chung LP Lam C Ho S Kwok WC Fai CK et al A randomized placebo controlled trial of an innovative herbal formula in the prevention of atherosclerosis in postmenopausal women with borderline hypercholesterolemia. Complement Ther Med. (2014) 22(3):473–80. 10.1016/j.ctim.2014.03.010
128.
Sha R Tang L Du Y Wu S Shi H Zou H et al Effectiveness and safety of Ginkgo biloba extract (GBE50) in the treatment of dizziness caused by cerebral arteriosclerosis: a multi-center, double-blind, randomized controlled trial. J Trad Chin Med. (2022) 42(1):83–9. 10.19852/j.cnki.jtcm.20211214.001
129.
Mansur AP Roggerio A Goes MFS Avakian SD Leal DP Maranhão RC et al Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: a randomized trial. Int J Cardiol. (2017) 227:788–94. 10.1016/j.ijcard.2016.10.058
130.
van Poppel PC Breedveld P Abbink EJ Roelofs H van Heerde W Smits P et al Salvia miltiorrhiza root water-extract (danshen) has no beneficial effect on cardiovascular risk factors. A randomized double-blind cross-over trial. PLoS One. (2015) 10(7):e0128695. 10.1371/journal.pone.0128695
Summary
Keywords
Chinese medicine, atherosclerosis, inflammation, lipid metabolism, glucose metabolism
Citation
Tang C, Liu B, Zhang Y, Long M, Zheng W, Lu J, Li H, Xu Z and Wang Y (2025) Systematic review of Chinese medicine for the treatment of atherosclerosis. Front. Cardiovasc. Med. 12:1671008. doi: 10.3389/fcvm.2025.1671008
Received
22 July 2025
Accepted
13 October 2025
Published
03 November 2025
Volume
12 - 2025
Edited by
Tzong-Shyuan Lee, National Taiwan University, Taiwan
Reviewed by
Liangxing Tu, Jiangxi University of Traditional Chinese Medicine, China
Rahni Hossain, Walailak University, Thailand
Updates
Copyright
© 2025 Tang, Liu, Zhang, Long, Zheng, Lu, Li, Xu And Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Zihui Xu zihuixu@tmmu.edu.cn Yunqiao Wang yunqiaowang@tmmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.