Abstract
The Taussig-Bing anomaly is a rare form of double outlet right ventricle (DORV) characterized by complex physiology, often requiring multiple surgeries. This report describes the use of the VenusP-Valve System ina 36-year-old patient with Taussig-Bing anomaly, transposition of the great arteries (TGA), and prior surgical interventions including Blalock–Taussig shunt, arterial switch, and ventricular septal defect (VSD) closure. The patient presented with atrial fibrillation, baseline clinical signs of right-sided heart failure (jugular venous distension, edema, holosystolic murmur), New York Heart Association (NYHA) III symptoms, severe tricuspid regurgitation (TR), residual VSD, and significant pulmonary stenosis. Right-heart catheterization revealed an extremely high baseline Qp/Qs of 10.4, derived by the Fick method with oxygen consumption estimation and mixed venous sampling; this value was interpreted as artifact-influenced, and normalized to ∼2 after medical optimization. After multidisciplinary discussion, a transcatheter pulmonary valve replacement (TPVR) using the VenusP-Valve System was successfully performed. At 30 days, the patient improved to NYHA II with no major complications, reduction of TR to moderate, and stable residual VSD shunt (Qp/Qs ∼2). This case highlights the feasibility and clinical utility of VenusP-Valve in managing complex congenital heart disease, particularly in enlarged, irregular, patch-augmented right ventricular outflow tracts (RVOTs) not suitable for balloon-expandable or hourglass-anchoring valves.
Introduction
The Taussig-Bing anomaly, a variant of DORV, involves both great arteries originating from the right ventricle with a subpulmonary VSD (1). Surgical correction typically includes arterial switch and VSD closure, but patients may require multiple interventions (2). Post-surgical RVOT anatomy is often enlarged, irregular, or patch-augmented, posing challenges for subsequent valve replacement. This report presents a transcatheter approach using the VenusP-Valve System in a patient with TGA and DORV.
Case presentation
A 36-year-old male with known Taussig-Bing anomaly underwent initial palliation with a Blalock–Taussig shunt, followed by arterial switch and attempted VSD closure (Figure 1). He presented with chronic atrial fibrillation, jugular venous distension, peripheral edema, resting O₂ saturation of 92%, and NYHA class III dyspnea. CT and transoesophageal echocardiography (TOE) revealed severe TR, residual VSD, thickened neo-pulmonary trunk, and pulmonary stenosis. CT confirmed an enlarged, irregular, patch-augmented RVOT, favoring VenusP sizing. Precise numeric diameters were not archived, which is acknowledged as a limitation.
Figure 1
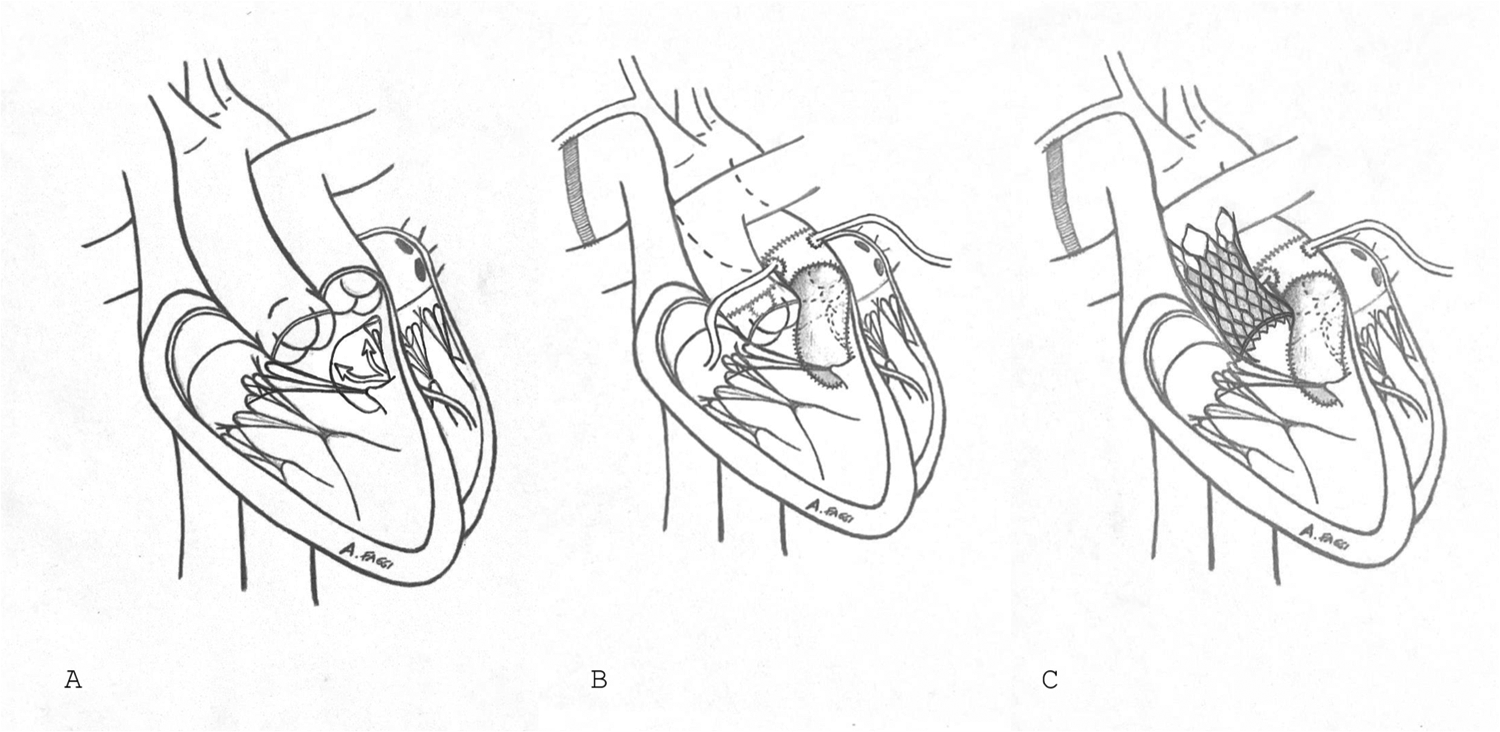
Visual summary. (A) Double outlet right ventricle with trasposition of great vessels. (B) Correction of great vessels transposition after Blalock–Taussig shunt. (C) Impantation of VenusP valve into pulmonary valve.
Right-heart catheterization revealed Qp/Qs 10.4 by the Fick method, with artifact-related overestimation; after diuretic optimization, Qp/Qs normalized to ∼2.0. Trans-RVOT gradient and RV systolic pressures were available, whereas complete invasive pressures and oxygen saturations could not be retrieved retrospectively and are reported as a limitation.
TPVR was performed via right femoral vein using a 34 mm VenusP-Valve. A 26F sheath was advanced, a stiff wire and rail were positioned, anticoagulation maintained ACT >250 s, and the valve was deployed under fluoroscopic and echocardiographic guidance. No rapid pacing was used. Contrast and radiation doses were not archived. Valve positioning was verified with angiography and echocardiography. Deployment was successful without complications.
At 30 days, echocardiography showed no pulmonary stenosis, trivial pulmonary regurgitation, TR reduced to moderate, residual VSD shunt stable with Qp/Qs ∼2, and reduced RV systolic pressure. The patient improved to NYHA II, with relief of dyspnea and no arrhythmias.
Discussion
This case illustrates the complexity of managing congenital patients after arterial switch, where factors such as altered RVOT geometry, scarring from multiple surgeries, and coronary reimplantation must be considered. In this setting, choosing the appropriate valve platform is critical.
Comparative valve features are summarized in Table 1. Balloon-expandable valves such as Sapien 3 offer high radial force but require rigid prestented conduits (3–5). The Harmony valve, although self-expanding, anchors via an hourglass design and is suitable mainly for smoothly dilated, compliant RVOTs (6). The Melody valve, a balloon-expandable valve, is limited to narrow conduits and requires prestenting (7). In contrast, the VenusP-Valve provides strong radial force with dual flares, allowing anchoring in enlarged or irregular RVOTs without prestenting (8, 9).
Table 1
| Valve | Type | Radial force | Anchoring mechanism | Anatomical requirements | Prestenting | Regulatory status |
|---|---|---|---|---|---|---|
| VenusP-Valve | Self-expanding nitinol | High | Dual proximal & distal flares | Enlarged, irregular or patch-augmented RVOTs | Not required | CE-marked (Europe), under evaluation in US |
| Harmony Valve | Self-expanding nitinol | Moderate | Hourglass design with inflow/outflow flares | Compliant, smoothly dilated RVOTs | Not required | FDA-approved (US), CE-marked (Europe) |
| Sapien 3 | Balloon-expandable cobalt-chromium | High | Radial force against rigid prestented conduit | Requires rigid prestented or conduit landing zone | Required | FDA & CE-marked |
| Melody Valve | Balloon-expandable platinum–iridium | Moderate | Radial force in prestented conduit or homograft | Narrow conduits/homografts, limited size range | Required | FDA & CE-marked |
Comparative features of available transcatheter pulmonary valve platforms.
RVOT, right ventricular outflow tract; FDA, Food and Drug Administration; CE, Conformité Européenne.
Mechanistically, VenusP expands against the thickened RVOT, relieving obstruction and restoring laminar flow. Afterload reduction and improved RV geometry contributed to the observed decrease in TR. Residual VSD remained stable, supporting a staged management approach, as described in adult congenital practice (10).
Although not the first application of transcatheter pulmonary valves in this anatomy, this case adds to evidence supporting VenusP-Valve feasibility in complex congenital heart disease. It underscores the importance of individualized planning based on anatomy, valve characteristics, and surgical history.
Patient perspective
The patient reported significant improvement in symptoms and quality of life post-procedure.
Conclusion
VenusP-Valve TPVR is a feasible and effective option in high-risk congenital heart disease cases. Its deployment avoids high-risk re-sternotomy and supports continued expansion of percutaneous options in complex anatomies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by IRB—IRCCS San Raffaele Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FG: Validation, Writing – review & editing, Formal analysis, Writing – original draft. PD: Writing – original draft, Writing – review & editing. MS: Writing – review & editing, Writing – original draft. NB: Writing – original draft, Writing – review & editing. AF: Writing – review & editing, Writing – original draft. EA: Writing – original draft, Writing – review & editing. OA: Writing – original draft, Writing – review & editing. MC: Writing – original draft, Writing – review & editing. FM: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DORV, double outlet right ventricle; RVOT, right ventricular outflow tract; TGA, transposition of the great arteries; TOE, trans-oesophageal echocardiogram; TPVR, transcatheter pulmonary valve replacement; VSD, ventricular septal defect.
References
1.
Van Praagh R Van Praagh S . The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol. (1965) 16(3):406–25. 10.1016/0002-9149(65)90732-0. PMID: .
2.
Dearani JA Danielson GK Puga FJ Mair DD Schleck CD . Late results of the rastelli operation for transposition of the great arteries. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2001) 4:3–15. 10.1053/pcsu.2001.24649. PMID:
3.
Lim DS Kim D Aboulhosn J Levi D Fleming G Hainstock M et al Congenital pulmonic valve dysfunction treated with SAPIEN 3 transcatheter heart valve (from the COMPASSION S3 trial). Am J Cardiol. (2023) 190:102–9. 10.1016/j.amjcard.2022.12.010. PMID: .
4.
Kenny D Rhodes JF Fleming GA Kar S Zahn EM Vincent J et al 3-Year Outcomes of the edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position from the COMPASSION multicenter clinical trial. JACC Cardiovasc Interv. (2018) 11(19):1920–9. 10.1016/j.jcin.2018.06.001. PMID: .
5.
Dimas VV Babaliaros V Kim D Lim DS Morgan G Jones TK et al Multicenter pivotal study of the alterra adaptive prestent for the treatment of pulmonary regurgitation. JACC Cardiovasc Interv. (2024) 17(19):2287–97. 10.1016/j.jcin.2024.07.036. PMID: .
6.
Bergersen L Benson LN Gillespie MJ Cheatham SL Crean AM Hor KN et al Harmony feasibility trial: acute and short-term outcomes with a self-expanding transcatheter pulmonary valve. JACC Cardiovasc Interv. (2017) 10(17):1763–73. 10.1016/j.jcin.2017.05.034. PMID: .
7.
Jones TK McElhinney DB Vincent JA Hellenbrand WE Cheatham JP Berman DP et al Long-Term outcomes after melody transcatheter pulmonary valve replacement in the US investigational device exemption trial. Circ Cardiovasc Interv. (2022) 15(1):e010852. 10.1161/CIRCINTERVENTIONS.121.010852. PMCID: PMC8765216.
8.
Jin Q Long Y Zhang G Pan X Chen M Feng Y et al Five-year follow-up after percutaneous pulmonary valve implantation using the Venus P-valve system for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract. Catheter Cardiovasc Interv. (2024) 103(2):359–66. 10.1002/ccd.30916. PMID: .
9.
Patel ND Levi DS Cheatham JP Qureshi SA Shahanavaz S Zahn EM . Transcatheter pulmonary valve replacement: a review of current valve technologies. J Soc Cardiovasc Angiogr Interv. (2022) 1(6):100452. 10.1016/j.jscai.2022.100452. PMID: ; PMCID: PMC11307711
10.
Ran L Wang W Secchi F Xiang Y Shi W Huang W . Percutaneous pulmonary valve implantation in patients with right ventricular outflow tract dysfunction: a systematic review and meta-analysis. Ther Adv Chronic Dis. (2019) 10:2040622319857635. 10.1177/2040622319857635
Summary
Keywords
pulmonary valve, venusP valve, double outlet right ventricle, transposition of the great arteries, transcatheter valve implantation
Citation
Gramegna F, Denti P, Saccocci M, Buzzatti N, Faggi A, Agricola E, Alfieri O, Chessa M and Maisano F (2025) Transcatheter pulmonary valve replacement with the venusP-valve system in a patient with double outlet right ventricle and transposition of the great arteries a first-in-human case report. Front. Cardiovasc. Med. 12:1672008. doi: 10.3389/fcvm.2025.1672008
Received
23 July 2025
Accepted
14 October 2025
Published
05 December 2025
Volume
12 - 2025
Edited by
Eustaquio Maria Onorato, U.O. Cardiologia Universitaria, Ospedale Galeazzi—Sant’Ambrogio, GSD, Istituto di Ricovero e Cura a Carattere Scientifico (I.R.C.C.S.), Italy
Reviewed by
Vlasios Ninios, Interbalkan Medical Center, Greece
Abdurashid Mussayev, National Research Cardiac Surgery Center, Kazakhstan
Updates
Copyright
© 2025 Gramegna, Denti, Saccocci, Buzzatti, Faggi, Agricola, Alfieri, Chessa and Maisano.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Matteo Saccocci Saccocci.matteo@hsr.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.