Abstract
Introduction:
This meta-analysis was designed to compare the safety and efficacy of percutaneous endovascular aortic repair (PEVAR) with endovascular aortic repair by cutdown access (CEVAR) in the treatment of TBAD.
Materials and methods:
Four databases (PubMed, Embase, Web of Science, and Cochrane Library) were carefully queried for articles comparing PEVAR and CEVAR in patients with TBAD. The search was performed from the foundation of the databases until March 31, 2025.
Results:
Totally 24 studies were included in this meta-analysis. The meta-analysis included a group of 28,263 patients diagnosed with TBAD, with 14,534 patients undergoing PEVAR and 13,729 patients undergoing CEVAR. In comparison to CEVAR, PEVAR resulted in a reduced hospital length of stay (MD = −2.16 days, 95% CI: −3.05 to −1.27, P < 0.00001), decreased operative time (MD = −40.87 min, 95% CI: −49.72 to −32.02, P < 0.00001), shorter postoperative duration (MD = −1.01 days, 95% CI: −1.56 to −0.45, P = 0.0004), diminished incidence of groin infection (OR = 0.44, 95% CI: 0.30 to 0.65, P < 0.0001), lower occurrence of heart-related complications (OR = 0.76, 95% CI: 0.59 to 1.00, P = 0.05), and reduced incidence of lymphocele (OR = 0.49, 95% CI: 0.24 to 0.98, P = 0.04), but a higher incidence of surgical suture failure (OR = 2.61, 95% CI: 1.52 to 4.50, P = 0.0005) and pseudoaneurysm (OR = 2.64, 95% CI: 1.09 to 6.41, P = 0.03). No statistically significant differences were observed between the two groups concerning estimated blood loss, ICU admissions, hematoma, acute kidney injury, lower extremity revascularization, ischemic colitis, and deep venous thrombosis.
Conclusions:
Compared to CEVAR, PEVAR was associated with a shorter hospital stay, reduced operative time, quicker postoperative recovery, lower rates of groin infections, fewer cardiac complications, and a diminished occurrence of lymphocele; however, it exhibited a higher incidence of pseudoaneurysm and an increased rate of surgical suture failure. PEVAR was a safe and effective method for the treatment of TBAD.
Systematic Review Registration:
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD420251110307, PROSPERO CRD420251110307.
1 Introduction
Aortic dissection is the predominant acute aortic syndrome (1). Aortic dissection affects four to five individuals per 100,000 each year, rendering it the most prevalent aortic-specific emergency, with 20%–30% of patients succumbing prior to hospital transfer (2). It is typically categorized into two categories based on anatomical characteristics: Stanford type B dissections commence distal to the ostium of the left subclavian artery, whereas Stanford type A dissections involve the ascending aorta and the aortic arch (3). Due to advancements in interventional therapy techniques, the evolution of related medical equipment, and the consolidation of surgical expertise, endovascular repair utilizing covered stents has become the predominant treatment for aortic dissection. This technique is preferred for its less invasive characteristics and positive prognosis, especially in cases of Stanford type B aortic dissection, where it has emerged as the favored surgical treatment (4).
Surgeons frequently select the femoral artery as the ideal access point for endovascular repair of dissection. Two independent surgical techniques exist: one use a typical incision to carefully dissect the layers of the femoral artery. Nonetheless, the CEVAR procedure requires a surgical incision of roughly 5 cm in the inguinal region, and is influenced by factors such as the postoperative environment, care, and alterations to the incision dressing, resulting in a relatively heightened risk of infection (5). The alternative procedure entails percutaneous puncture to access the femoral artery, which has increasingly been the dominant way in recent years, despite contemporary breakthroughs in medical and surgical interventions (6). The endovascular repair of abdominal and/or thoracic aortic disease, coupled with the progression of endograft technology that facilitates lower profiles and smaller access sheaths, has prompted the majority of vascular surgeons to adopt an endovascular-first strategy over an open-first approach. Technological advancements in the field have expanded the criteria of suitable aortic anatomy for secure endovascular repair (7). Totally PEVARP was first introduced in 1999 (8), and referred to the practice of CFA cannulation site closure using a “preclose” technique with a Perclose ProGlide® (Abbott Vascular) closure device.Since that time, this technique has been widely adopted (9). PEVAR is technically viable, alleviates patient discomfort, reduces operative duration, shortens hospital stays, diminishes complication rates, and decreases the ambulation period associated with the percutaneous method (10, 11). However, percutaneous access may result in injury to the femoral artery, potentially causing hematoma, pseudoaneurysm development, and arterial dissection (12). In addition, a financial drawback of percutaneous access has been noted due to PEVAR's dependence on expensive closure devices (6).
Therefore, Controversy persists on which of the two techniques possesses a definite benefit. A prior meta-analysis demonstrated no statistically significant differences between the two surgical techniques regarding overall hospitalization expenditures, length of hospital stay, postoperative hematoma, femoral artery stenosis or occlusion, and other problems (13). Moreover, the elevated puncture site complicates bleeding management using puncture suture methods, resulting in a greater frequency of postoperative pseudoaneurysms compared to incision surgery (14). In recent years, a growing number of clinical research articles have been published comparing the two surgical techniques (15, 16). Consequently, it is essential to perform an updated meta-analysis to assess the comparative advantages of the two surgical techniques. This meta-analysis was designed to compare the safety and efficacy of PEVAR with CEVAR in the treatment of TBAD.
2 Materials and methods
2.1 Search strategy
This meta-analysis followed the 2020 guidelines established by the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA).The study had been formally registered at PROSPERO with the registration number CRD420251110307. A comprehensive search was performed in four databases, including PubMed, Embase, Web of Science, and the Cochrane Library, to collect literature published up until March 31, 2025. The search methodology adhered to the PICOS principle and utilised a combination of MeSH terms and unrestricted text phrases. The search strategy employed consisted of combining the keywords “Percutaneous”, “Cutdown”, “Aortic aneurysm repair”, and “Type B aortic dissection”. Supplementary Data Sheet 1 offered a thorough summary of the search record. In addition, we conducted a thorough manual examination of the bibliographies of the identified papers, as well as pertinent reviews and meta-analyses, in order to uncover any studies that fit the criteria for inclusion.
2.2 Inclusion and exclusion criteria
The criteria for inclusion were as follows: (1) patients diagnosed with type B aortic dissection; (2) patients in the intervention group received percutaneous endovascular aneurysm repair; (3) patients in the controlled group received endovascular aortic repair by cutdown access; (4) at least one of the following outcomes was reported: hospital length of stay, operative time, estimated blood loss, stay of postoperative, patients requiring ICU stay, surgical suture failure, pseudoaneurysm, hematoma, groin infection, heart-related complications, lymphocele, acute kidney injury, lower extremity revascularization, ischemic colitis and deep venous thrombosis; (5) Study types: randomised controlled trials (RCTs), retrospective studies or prospective study.
The exclusion criteria were as follows: (1) other types of articles, such as case reports, protocols, letters, editorials, comments, reviews, meta-analyses; (2) not relevant; (3) failed to obtain full-text; (4) data cannot be extracted; (5) duplicate patient cohort. If there were studies conducted in the same location and population at different times, only the largest sample size or most recent studies would be included.
2.3 Selection of studies
The literature selection procedure, which included the elimination of duplicate entries, was carried out using EndNote (Version 20; Clarivate Analytics). The initial search was conducted by two autonomous reviewers. The redundant items were removed, and the titles and abstracts were evaluated to determine their relevance. Subsequently, each study was classified as either included or omitted. The settlement was reached through consensus. In the event that the parties involved are unable to come to a mutual agreement, a third reviewer assumes the function of a mediator.
2.4 Data extraction
Two independent reviewers extracted data. The extracted data included: (1) basic characteristics of studies included: author, nationality, year of publication; (2) baseline characteristics of study subjects: age, sample size, access sites; (3) outcome indicators: hospital length of stay, operative time, estimated blood loss, stay of postoperative, patients requiring ICU stay, surgical suture failure, pseudoaneurysm, hematoma, groin infection, heart-related complications, lymphocele, acute kidney injury, lower extremity revascularization, ischemic colitis, deep venous thrombosis.
2.5 Quality assessment
Two independent reviewers assessed the quality assessment of the studies that were included. In this study, we utilised the Newcastle-Ottawa Scale (NOS) (17) to assess the quality of retrospective or prospective cohort studies, including eight domains: (1) representativeness of the exposed cohort; (2) selection of the non-exposed cohort; (3) ascertainment of exposure; (4) demonstration that outcome of interest was not present at start of study; (5) comparability of cohorts on the basis of the design or analysis; (6) assessment of outcome; (7) was follow-up long enough for outcomes to occur; (8) adequacy of follow-up of cohorts. The score on this scale is considered high quality research if it was 7 stars or above. We evaluated the RCTs in this study using the risk of bias assessment tool implemented by the Cochrane Collaboration (18). The assessment encompassed seven essential domains: (1) randomization technique; (2) allocation concealment; (3) blinding of participants and treatment administrators; (4) blinding of outcome assessments; (5) completeness of outcome data; (6) selective outcome reporting; and (7) additional bias sources. All studies were evaluated as being at risk in each of the seven domains. Each study was categorized as high-quality, medium-quality, or low-quality based on the evaluation results according to the specified criteria.
2.6 Statistical analysis
The results were analysed using Review Manager 5.3, a software developed by the Cochrane Collaboration in Oxford, UK. The continuous variables were compared using the weighted mean difference (WMD) and a 95% confidence interval (CI). The odds ratio (OR) was used to compare binary variables, along with a 95% CI. The medians and interquartile ranges of continuous data were converted to the mean and standard deviation. The Cochrane's Q test and the I2 index were used to evaluate the statistical heterogeneity among the studies included. The Cochrane Q p value and I2 statistic were used to evaluate the heterogeneity of each meta-analysis. A fixed-effect model (FEM) was used for low heterogeneity (I2 < 50%), and a random-effect model (REM) was used for high heterogeneity (I2 ≥ 50%) when analyzing pooled data. A p-value below 0.05 was considered to have statistical significance. Funnel plots were used to evaluate the publication bias.
3 Results
3.1 Search results
Figure 1 illustrated the process of selecting and incorporating articles. A grand total of 1,211 publications were acquired from a combination of four databases and additional manual records. After applying the predetermined criteria for inclusion and exclusion, a total of 24 articles (6, 7, 10, 11, 15, 16, 19–38) were selected for the final meta-analysis.
Figure 1
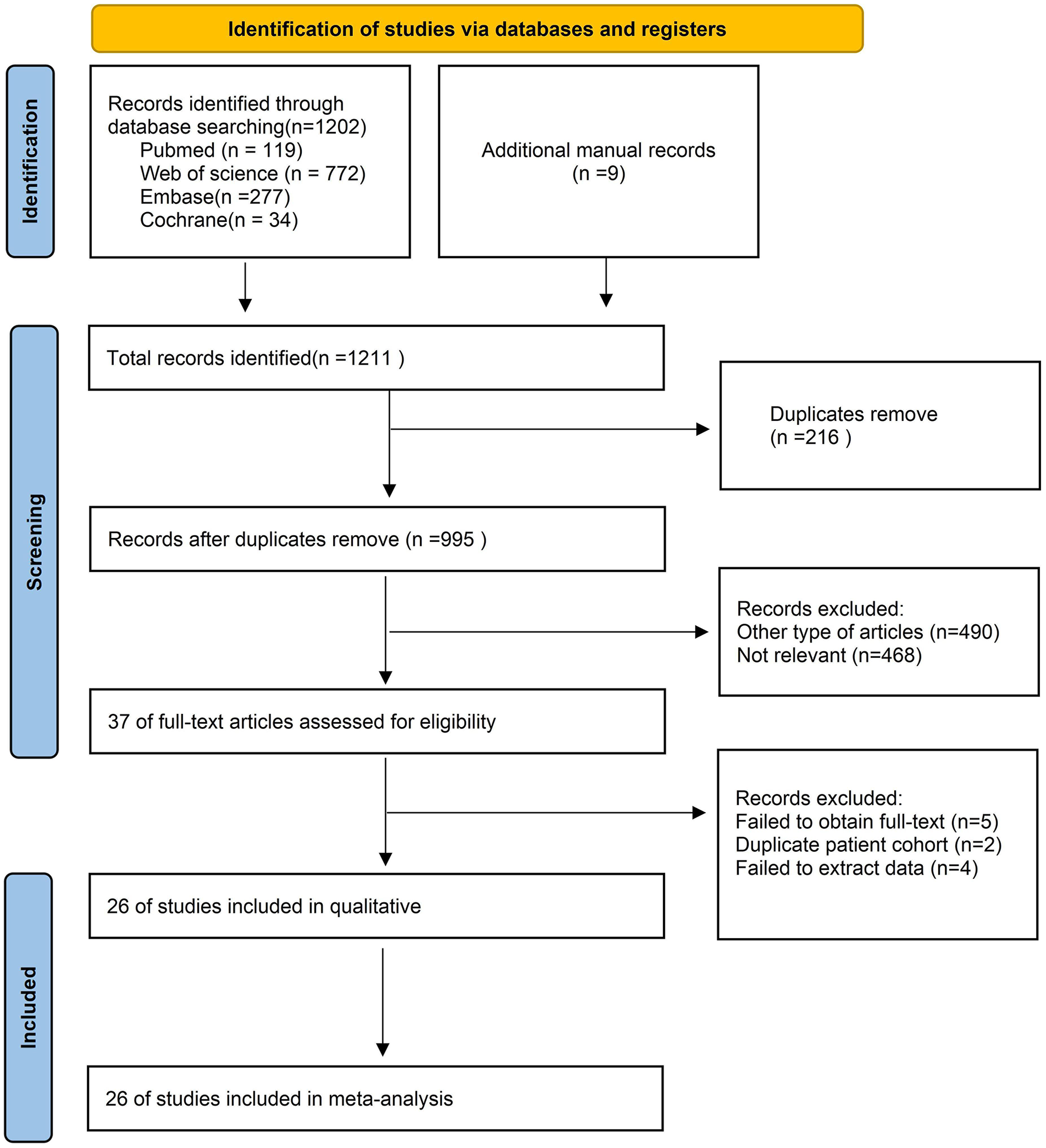
Flow chart of literature search strategies.
3.2 Characteristics of included studies and patients
The meta-analysis included a total of 24 studies (6, 7, 10, 11, 15, 16, 19–38), which consisted of 4 RCTs, 2 prospective studies and 18 retrospective studies. The meta-analysis included a combined total of 28,263 participants, with 14,534 patients undergoing PEVAR and 13,729 patients undergoing CEVAR. The studies were undertaken in several nations, including the USA, Japan, China, Turkey, Netherlands, Austria, Saudi Arabia, Finland. Table 1 contained detailed characteristics of included studies and patients.
Table 1
| Author | Year | Recruitment period | Country | Type of study | Age (Mean ± SD) | Male/female | Patients/access sites | BMI | |
|---|---|---|---|---|---|---|---|---|---|
| Taku (26) | 2015 | 2009–2013 | Japan | Retrospective study | PEVAR | 75.5 ± 7.6 | 46/6 | 50/100 | 23.2 ± 2.8 |
| CEVAR | 75.3 ± 7.9 | 80/16 | 96/192 | 22.7 ± 3.7 | |||||
| Peter (10) | 2020 | 2005–2013 | USA | Retrospective study | PEVAR | NR | NR | 49/49 | NR |
| CEVAR | NR | NR | 24/24 | NR | |||||
| Lin (23) | 2018 | 2011–2016 | China | Retrospective study | PEVAR | 58.27 ± 11.60 | 51/9 | 60/99 | NR |
| CEVAR | 61.11 ± 12.78 | 43/12 | 55/84 | NR | |||||
| Sahin (19) | 2020 | 2014–2020 | Turkey | Retrospective study | PEVAR | 58.7 ± 9.9 | 45/11 | 56/56 | 26.4 ± 5.9 |
| CEVAR | 57.2 ± 11.8 | 29/11 | 40/40 | 26.8 ± 6.1 | |||||
| Christopher (20) | 2017 | 2008–2014 | USA | Retrospective study | PEVAR | 75.2 ± 0.85 | 88/14 | 102/204 | 28.4 ± 1.26 |
| CEVAR | 74.6 ± 0.89 | 83/15 | 98/182 | 27.8 ± 0.57 | |||||
| Dipankar (21) | 2017 | 2012–2015 | USA | Retrospective study | PEVAR | 73.3 ± 9.5 | 115/17 | 132/264 | 27.1 ± 5.0 |
| CEVAR | 72.1 ± 8.2 | 45/6 | 51/102 | 27.9 ± 4.5 | |||||
| Dominique (22) | 2015 | 2011–2013 | Netherlands | prospective study | PEVAR | 74 | 81/1,027 | 1,108/2,177 | NR |
| CEVAR | 74 | 81/2,923 | 3,004/5,632 | NR | |||||
| Miriam (24) | 2018 | 2016–2017 | Austria | RCT | PEVAR | 74.04 | 43/7 | 50/50 | NR |
| CEVAR | 74.04 | 43/7 | 50/50 | NR | |||||
| Akbulut (27) | 2022 | 2013–2020 | Turkey | Retrospective study | PEVAR | 70.5 ± 8 | 57/8 | 65/65 | NR |
| CEVAR | 68.9 ± 7.4 | 78/9 | 87/87 | NR | |||||
| Weesam (28) | 2012 | 2000–2009 | USA | Retrospective study | PEVAR | 78 | 0/31 | 31/24 | NR |
| CEVAR | 75.5 | 0/112 | 112/154 | NR | |||||
| Altoijry (16) | 2023 | 2015–2022 | Saudi Arabia | Retrospective study | PEVAR | 62.0 ± 18.5 | 31/4 | 35/35 | 27.7 ± 6.6 |
| CEVAR | 57.3 ± 20.6 | 23/1 | 24/24 | 29.4 ± 10.5 | |||||
| Baxter (7) | 2021 | 2010–2021 | USA | Retrospective study | PEVAR | 72.1 ± 10.2 | 1,687/330 | 2,017/2,017 | 27.8 ± 5.5 |
| CEVAR | 71.4 ± 10.5 | 2,002/444 | 2,446/2,446 | 27.3 ± 5.1 | |||||
| Etezadi (30) | 2011 | 2003–2010 | USA | Retrospective study | PEVAR | 75 ± 8.2 | 58/12 | 70/85 | 26.9 ± 3.9 |
| CEVAR | 76.3 ± 7.7 | 326/49 | 375/557 | 27.2 ± 4.6 | |||||
| Hahl (15) | 2023 | 2005–2013 | Finland | Retrospective study | PEVAR | 76.3 | 223/34 | 257/257 | NR |
| CEVAR | 77.9 | 166/20 | 186/ 186 | NR | |||||
| Kauvar (32) | 2016 | 2011–2013 | USA | Retrospective study | PEVAR | 74 ± 9 | 1,264/269 | 1,533/1,533 | 28.3 ± 5.9 |
| CEVAR | 73 ± 9 | 1,285/304 | 1,589/1,589 | 28.5 ± 6.0 | |||||
| Siracuse (34) | 2018 | 2014–2017 | USA | Retrospective study | PEVAR | 73.0 ± 8.8 | 6,926/1,414 | 8,340/8,340 | 28.2 ± 5.9 |
| CEVAR | 73.5 ± 8.8 | 2,701/694 | 4,747/4,747 | 27.9 ± 6.0 | |||||
| Tay (36) | 2022 | 2015–2021 | USA | Retrospective study | PEVAR | 70 ± 8 | 18/3 | 21/42 | 30 ± 7 |
| CEVAR | 75 ± 10 | 12/2 | 14/28 | 30 ± 6 | |||||
| WU (38) | 2022 | 2017–2021 | China | Retrospective study | PEVAR | 59 | 141/37 | 178/178 | NR |
| CEVAR | 57 | 97/20 | 117/117 | NR | |||||
| Howell (31) | 2002 | 2001–2004 | USA | prospective study | PEVAR | 73 ± 7.1 | ND | 30/60 | NR |
| CEVAR | ND | ND | 96/96 | NR | |||||
| Torsello (6) | 2003 | 2002–2002 | USA | RCT | PEVAR | 74.5 ± 10.4 | 14/1 | 15/25 | NR |
| CEVAR | 71 ± 9.6 | 15/0 | 15/30 | NR | |||||
| Vierhout (11) | 2019 | 2014–2016 | Netherlands | RCT | PEVAR | 72.6 ± 8.1 | 67/6 | 73/137 | 27.5 ± 3.6 |
| CEVAR | 72.4 ± 6.2 | 56/8 | 64/137 | 27.2 ± 3.7 | |||||
| Thurston (37) | 2019 | 2009–2016 | USA | Retrospective study | PEVAR | 74.0 ± 10.1 | 39/11 | 50/100 | NR |
| CEVAR | 72.4 ± 7.5 | 38/12 | 50/100 | NR | |||||
| Smith (35) | 2009 | 2005–2007 | USA | Retrospective study | PEVAR | 72 ± 10 | 19/2 | 22/38 | 27.4 ± 5 |
| CEVAR | 71 ± 8 | 21/1 | 22/50 | 27.4 ± 6 | |||||
| Nelson (33) | 2014 | 2010–2012 | USA | RCT | PEVAR | 74 ± 11 | 91/10 | 101/101 | 28.4 ± 4.3 |
| CEVAR | 73 ± 8.8 | 45/5 | 50/50 | 28 ± 4.7 | |||||
Characteristics of included studies and patients.
PEVAR, percutaneous endovascular aortic repair; CEVAR, endovascular aortic repair by cutdown access; NR, not reported.
3.3 Quality assessment
The Newcastle-Ottawa Scale was employed to assess the quality of the listed studies. Among the 20 studies, three studies had a rating of 9 points, twelve studies received a rating of 8 points, and nine studies received a rating of 7 points. Table 2 presented a detailed analysis of the quality assessment conducted by NOS and Jadad (39). Cochrane Risk of Bias Tool evaluation indicated that the included trials were of high quality. Three trials produced a sufficient random sequence, four studies reported appropriate allocation concealment, four studies clearly implemented participant blinding, four studies reported outcome assessor blinding, four studies provided complete outcome data, four studies did not engage in selective reporting, and one studies did not exhibit any other bias (Figure 2).
Table 2
| Author, year | Selection | Comparability | Outcome | Total scores | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness | Selection of non-exposure | Ascertainment of exposure | Outcome not present at start | Comparability on most important factors | Comparability on other risk factors | Assessment of outcome | Adequate follow-up time | Complete follow-up | ||
| Taku, 2015 (26) | * | – | * | * | * | * | * | * | – | 7 |
| Peter, 2020 (10) | * | * | – | * | * | * | * | * | – | 7 |
| Lin, 2018 (23) | * | * | * | * | * | * | * | * | * | 9 |
| Sahin, 2020 (19) | * | – | * | * | * | – | * | * | * | 7 |
| Christopher, 2017 (20) | * | * | – | * | * | * | * | * | – | 7 |
| Dipankar, 2017 (21) | * | * | – | * | * | * | * | * | * | 8 |
| Dominique, 2015 (22) | * | * | * | * | * | * | * | * | * | 9 |
| Akbulut, 2022 (27) | * | – | * | * | * | * | * | * | * | 8 |
| Weesam, 2012 (28) | * | * | * | * | * | * | * | – | – | 7 |
| Altoijry, 2023 (16) | * | * | * | * | * | * | * | * | – | 8 |
| Baxter, 2021 (7) | * | * | – | * | * | * | * | * | – | 7 |
| Etezadi, 2011 (30) | * | * | * | * | * | * | * | * | – | 8 |
| Hahl, 2023 (15) | * | * | * | * | * | * | * | – | – | 7 |
| Kauvar, 2016 (32) | * | * | * | * | * | * | * | * | – | 8 |
| Siracuse, 2018 (34) | * | * | * | * | * | * | * | * | * | 9 |
| Tay, 2022 (36) | * | * | * | * | * | * | * | * | – | 8 |
| WU, 2022 (38) | * | - | * | * | * | * | * | * | * | 8 |
| Howell, 2002 (31) | * | * | * | * | * | * | * | * | – | 8 |
| Thurston, 2019 (37) | * | – | * | * | * | * | * | * | – | 7 |
| Smith, 2009 (35) | * | * | * | * | * | * | * | * | – | 8 |
Quality assessment according to the Newcastle-Ottawa scale.
"*"indicates criterion met; “–"indicates significant of criterion not.
Figure 2
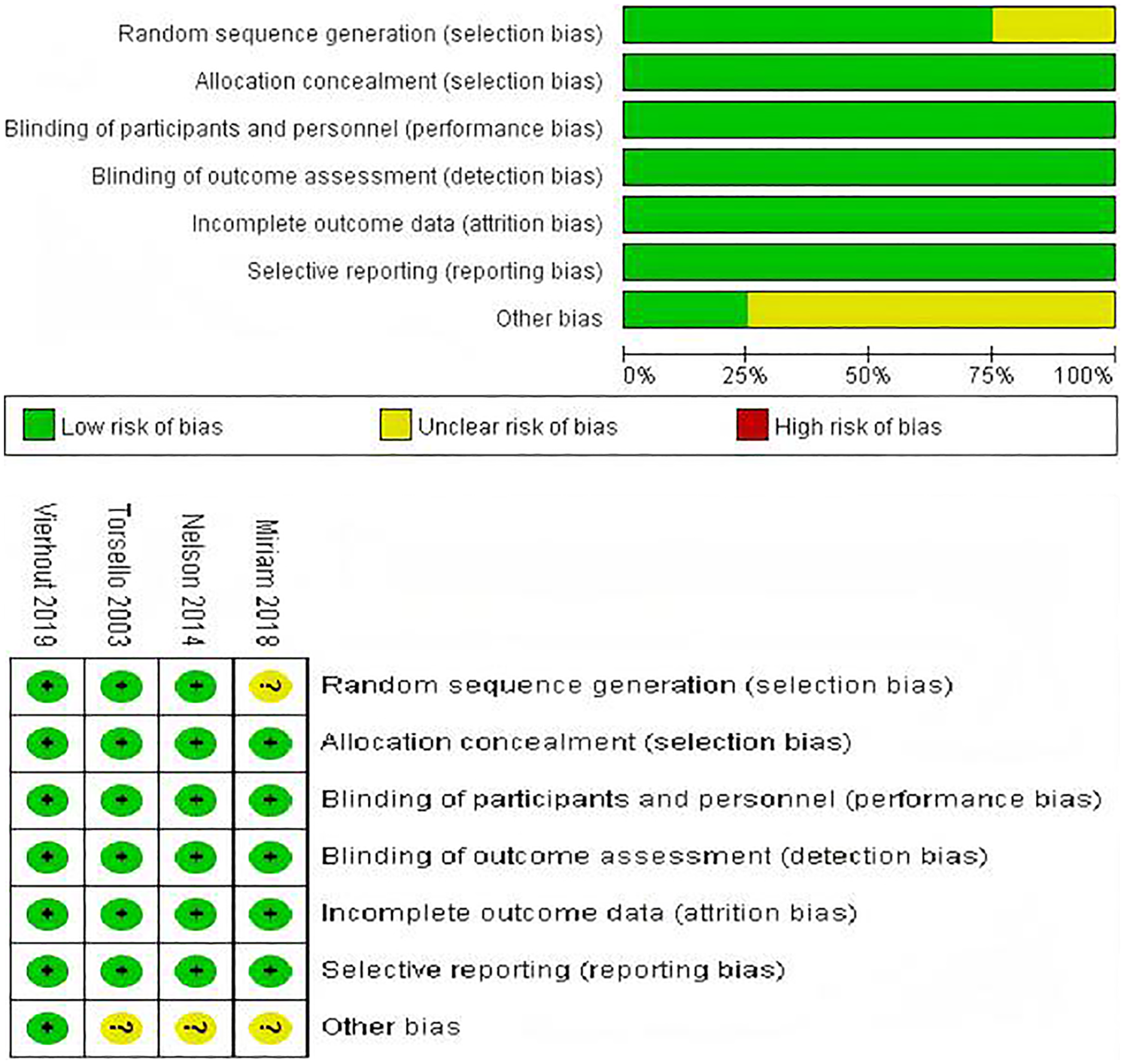
Risk of bias assessment for the included RCTs.
3.4 Outcomes
The results of the meta-analysis were summarized in Table 3.
Table 3
| Outcomes | Sample size | No. of studies | Heterogeneity | Overall effect size | 95% CI of overall effect | P Value | ||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P Value | |||||||
| Hospital length of stay | PEVAR | 2,729 | 9 | 92 | <0.00001 | WMD = −2.16 | −3.05 to −1.27 | <0.00001 |
| CEVAR | 3,056 | |||||||
| Operative time | PEVAR | 10,449 | 11 | 95 | <0.00001 | WMD = −40.87 | −49.72 to −32.02 | <0.00001 |
| CEVAR | 6,906 | |||||||
| Estimated blood loss | PEVAR | 8,811 | 6 | 100 | <0.00001 | WMD = −128.55 | −277.51 to 20.40 | 0.09 |
| CEVAR | 5,163 | |||||||
| Stay of postoperative | PEVAR | 10,035 | 4 | 96 | <0.00001 | WMD = −1.01 | −1.56 to −0.45 | 0.0004 |
| CEVAR | 6,489 | |||||||
| Patients requiring ICU stay | PEVAR | 269 | 3 | 74 | 0.02 | OR = 0.26 | 0.08∼0.86 | 0.03 |
| CEVAR | 173 | |||||||
| Surgical suture failure | PEVAR | 1,731 | 10 | 48 | 0.04 | OR = 2.61 | 1.52∼4.50 | 0.0005 |
| CEVAR | 1,539 | |||||||
| Pseudoaneurysm | PEVAR | 2,643 | 7 | 0 | 0.53 | OR = −2.64 | 1.09∼6.41 | 0.03 |
| CEVAR | 3,567 | |||||||
| Hematoma | PEVAR | 2,804 | 12 | 0 | 0.46 | OR = 0.81 | 0.49∼1.32 | 0.39 |
| CEVAR | 3,734 | |||||||
| Groin infection | PEVAR | 12,678 | 18 | 3 | 0.42 | OR = 0.44 | 0.30∼0.65 | <0.0001 |
| CEVAR | 11,878 | |||||||
| Heart-related complications | PEVAR | 12,153 | 7 | 0 | 0.80 | OR = 0.76 | 0.59∼1.00 | 0.05 |
| CEVAR | 10,316 | |||||||
| Lymphocele | PEVAR | 518 | 7 | 40 | 0.12 | OR = 0.49 | 0.24∼0.98 | 0.04 |
| CEVAR | 444 | |||||||
| Acute kidney injury | PEVAR | 2,944 | 6 | 0 | 0.79 | OR = 0.88 | 0.53∼1.47 | 0.64 |
| CEVAR | 4,732 | |||||||
| Lower extremity revascularization | PEVAR | 12,039 | 11 | 0 | 0.82 | OR = 1.17 | 0.86∼1.58 | 0.33 |
| CEVAR | 10,625 | |||||||
| Ischemic colitis | PEVAR | 1,178 | 3 | 0 | 0.43 | OR = 1.02 | 0.42∼2.49 | 0.96 |
| CEVAR | 3,042 | |||||||
| Deep venous thrombosis | PEVAR | 2,807 | 5 | 63 | 0.03 | OR = 1.14 | 0.34∼3.84 | 0.84 |
| CEVAR | 5,219 | |||||||
Results of the meta-analysis.
3.4.1 Hospital length of stay
The data was provided in 9 (7, 15, 16, 19, 25–27, 29, 36–38) out of the 24 articles included. The hospital length of stay in the PEVAR group was significantly shorter than that in the CEVAR group (MD = −2.16 days, 95% CI: −3.05 to −1.27, P < 0.00001, I2 = 92%) (Supplementary Figure S1).
3.4.2 Operative time
The data was provided in 11 (6, 19, 23, 25–27, 31–34, 36, 38) out of the 24 articles included. The Operative time in the PEVAR group was significantly shorter than that in the CEVAR group (MD = −40.87 min, 95% CI: −49.72 to −32.02, P < 0.00001, I2 = 95%) (Supplementary Figure S2).
3.4.3 Estimated blood loss
The data was provided in 6 (20, 23, 31, 33, 34, 38) out of the 24 articles included. There was no statistical significance in the amount of estimated blood loss between the two groups (MD = −128.55 mL, 95% CI: −277.51 to 20.40, P = 0.09, I2 = 100%) (Supplementary Figure S3).
3.4.4 Stay of postoperative
The data was provided in 4 (20, 23, 32, 34) out of the 24 articles included. The length of stay of postoperative in the PEVAR group was statistically shorter than that in the CEVAR group (MD = −1.01days, 95% CI: −1.56 to −0.45, P = 0.0004, I2 = 96%) (Supplementary Figure S4).
3.4.5 Patients requiring ICU stay
The data was provided in 3 (16, 20, 21, 25) out of the 24 articles included. There was no significant difference in the amount of patients requiring ICU stay between the two groups (OR = 0.26, 95% CI: 0.08 to 0.86, P = 0.0004, I2 = 96%) (Supplementary Figure S5).
3.4.6 Surgical suture failure
The data was provided in 10 (11, 15, 16, 19, 20, 23–26, 29, 33, 36) out of the 24 articles included. The incidence of surgical suture failure in the PEVAR group was significantly higher than that in the CEVAR group (OR = 2.61, 95% CI: 1.52 to 4.50, P = 0.0005, I2 = 48%) (Supplementary Figure S6).
3.4.7 Pseudoaneurysm
The data was provided in 7 (7, 11, 15, 19, 23, 28, 30) out of the 24 articles included. The incidence of pseudoaneurysm in the PEVAR group was significantly higher than that in the CEVAR group (OR = 2.64, 95% CI: 1.09 to 6.41, P = 0.03, I2 = 0%) (Supplementary Figure S7).
3.4.8 Hematoma
The data was provided in 12 (7, 11, 16, 19, 20, 23, 24, 28, 30, 35, 36, 38) out of the 24 articles included. There was no statistically significant difference in the incidence of hematoma between the two groups (OR = 0.81, 95% CI: 0.49 to 1.32, P = 0.39, I2 = 0%) (Supplementary Figure S8).
3.4.9 Groin infection
The data was provided in 9 (7, 10, 11, 15, 16, 19, 20, 22–24, 26–30, 34–36, 38) out of the 24 articles included. The incidence of groin infection in the PEVAR group was significantly lower than that in the CEVAR group (OR = 0.44, 95% CI: 0.30 to 0.65, P < 0.0001, I2 = 3%) (Supplementary Figure S9).
3.4.10 Heart-related complications
The data was provided in 7 (10, 20–22, 25, 29, 32, 34, 36) out of the 24 articles included. The incidence of heart-related complications in the PEVAR group was significantly lower than that in the CEVAR group (OR = 0.76, 95% CI: 0.59 to 1.00, P = 0.05, I2 = 0%) (Supplementary Figure S10).
3.4.11 Lymphocele
The data was provided in 7 (6, 10, 23, 26, 27, 33, 38) out of the 24 articles included. The incidence of lymphocele in the PEVAR group was significantly lower than that in the CEVAR group (OR = 0.49, 95% CI: 0.24 to 0.98, P = 0.04, I2 = 40%) (Supplementary Figure S11).
3.4.12 Acute kidney injury
The data was provided in 6 (10, 21, 22, 25, 32, 33, 36) out of the 24 articles included. There was no statistically significant difference in the incidence of acute kidney injury between the two groups (OR = 0.88, 95% CI: 0.53 to 1.47, P = 0.64, I2 = 0).(Supplementary Figure S12).
3.4.13 Lower extremity revascularization
The data was provided in 11 (7, 10, 20–25, 27, 29, 33, 34, 36) out of the 24 articles included. There was no statistically significant difference in the incidence of lower extremity revascularization between the two groups (OR = 1.17, 95% CI: 0.86 to 1.58, P = 0.33, I2 = 0) (Supplementary Figure S13).
3.4.14 Ischemic colitis
The data was provided in 3 (10, 22, 25, 29, 36) out of the 24 articles included. There was no significant difference in the incidence of ischemic colitis between the two groups (OR = 1.02, 95% CI: 0.42 to 2.49, P = 0.96, I2 = 0%) (Supplementary Figure S14).
3.4.15 Deep venous thrombosis
The data was provided in 5 (22, 23, 25, 30, 32, 36) out of the 24 articles included. There was no significant difference in the incidence of deep venous thrombosis between the two groups (OR = 1.14, 95% CI: 0.34 to 3.84, P = 0.84, I2 = 63%) (Supplementary Figure S15).
3.5 Publication bias
Funnel plots were used to evaluate publication bias. The bilaterally symmetrical funnel plot did not provide any apparent indications of publication bias with regards to Hospital length of stay (Supplementary Figure S16), operative time (Supplementary Figure S17), groin infection (Supplementary Figure S18), lower extremity revascularization (Supplementary Figure S19). The funnel plots for surgical suture failure (Supplementary Figure S20) and hematoma (Supplementary Figure S21) indicated that the studies included in the analysis had significant asymmetry.
3.6 Subgroup analysis regarding studies with long term follow up
A subgroup analysis regarding studies with long term follow up (at least 6 months) was performed (Table 4; Supplementary Figure S22–S31). Compared with CEVAR, PEVAR resulted in a reduced hospital length of stay, decreased operative time, and reduced incidence of groin infection and lymphocele. No statistically significant differences were observed between the two groups concerning hematoma, acute kidney injury, lower extremity revascularization, deep venous thrombosis, surgical suture failure and pseudoaneurysm.
Table 4
| Outcomes | Sample size | No. of studies | Heterogeneity | Overall effect size | 95% CI of overall effect | P Value | ||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P Value | |||||||
| Hospital length of stay | PEVAR | 2,360 | 4 | 96 | <0.00001 | WMD = −1.42 | −1.65 to −1.18 | <0.00001 |
| CEVAR | 2,732 | |||||||
| Operative time | PEVAR | 187 | 3 | 95 | <0.00001 | WMD = −40.42 | −50.24 to −30.60 | <0.00001 |
| CEVAR | 151 | |||||||
| Hematoma | PEVAR | 2,161 | 4 | 0 | 0.82 | OR = 0.41 | 0.15 to 1.11 | 0.08 |
| CEVAR | 3,066 | |||||||
| Surgical suture failure | PEVAR | 379 | 3 | 79 | 0.008 | OR = 1.90 | 0.18∼20.03 | 0.59 |
| CEVAR | 250 | |||||||
| Pseudoaneurysm | PEVAR | 2,359 | 3 | 55 | 0.11 | OR = 2.77 | 0.90∼8.48 | 0.07 |
| CEVAR | 3,188 | |||||||
| Groin infection | PEVAR | 2,483 | 6 | 37 | 0.16 | OR = 0.41 | 0.18∼0.90 | 0.03 |
| CEVAR | 3,339 | |||||||
| Lymphocele | PEVAR | 166 | 2 | 0 | 0.80 | OR = 0.11 | 0.01∼1.00 | 0.05 |
| CEVAR | 137 | |||||||
| Acute kidney injury | PEVAR | 122 | 2 | 0 | 0.83 | OR = 1.79 | 0.35∼9.33 | 0.49 |
| CEVAR | 64 | |||||||
| Lower extremityrevascularization | PEVAR | 2,204 | 4 | 0 | 0.42 | OR = 0.55 | 0.21∼1.46 | 0.23 |
| CEVAR | 2,596 | |||||||
| Deep venous thrombosis | PEVAR | 106 | 2 | 74 | 0.05 | OR = 0.65 | 0.11∼3.89 | 0.64 |
| CEVAR | 571 | |||||||
Meta-analysis results of studies with a follow-up period of six months or more.
3.7 Sensitivity analysis
Sensitivity analyses were performed for results with significant heterogeneity (Supplementary Figure S32–S38). Sensitivity analyses indicated that the results were stable regarding hospital length of stay, operative time, stay of postoperative and deep venous thrombosis, while the results were not stable regarding estimated blood loss and patients requiring ICU stay.
4 Discussion
4.1 A general interpretation of the results in the context of other evidence
This updated meta-analysis was conducted to compare the safety and efficacy of PEVAR with CEVAR in the treatment of TBAD. This meta-analysis demonstrated that PEVAR, in comparison to CEVAR, was associated with reduced hospital length of stay, decreased operative time, shorter postoperative duration, lower rates of groin infection, fewer heart-related complications, and a diminished incidence of lymphocele; however, it exhibited a higher occurrence of pseudoaneurysm and an increased rate of surgical suture failure. Besides, there was no significant difference between the two groups regarding estimated blood loss, patients requiring ICU stay, hematoma, acute kidney injury, lower extremity revascularization, ischemic colitis and deep venous thrombosis.
PEVAR was associated with reduced hospital stays, surgical durations, and postoperative recovery intervals, likely due to the smaller incision and the simplicity of executing PEVAR (40). The PEVAR group exhibited a reduced incidence of groin infections, lymphoceles, and cardiac complications. These enhanced outcomes to improvements in user experience and comfort with percutaneous access. Both open and percutaneous access methods entail hazards of infection. The open surgical method necessitates a wider incision, which may increase the risk of surgical site infections, and the carriage of Staphylococcus aureus may be significant (41). The percutaneous technique enables the execution of the same complex surgery in a far less invasive manner, resulting in less surgical site problems and utilizing local anesthesia. This may be highly beneficial in urgent situations where hemodynamic instability prevents the administration of general anesthesia (10). Furthermore, open femoral access has demonstrated the ability to generate substantial scar tissue, which may restrict future groin access for subsequent operations related to endoleak management or further graft maintenance (42).
The findings indicated that PEARV had certain drawbacks in comparison to CEVAR, primarily evidenced by an increased occurrence of surgical suture failure and pseudoaneurysm. Percutaneous access facilitates a smaller incision; nonetheless, the absence of control may result in access-related vascular injury, including stenosis or pseudoaneurysm. The device's failure to convert may result in increased blood loss and necessitate emergency surgery (42). In this meta-analysis, the success rate in the PEVAR group was approximately 95.0%. While it was 97.7% in the CEVAR group. This may be attributed to PEVAR's dependence on experience for identifying the puncture site, while CEVAR can be executed with direct visualization. Surgeons must complete training and practice to competently perform surgical procedures; hence, the risk of arterial puncture or vascular suture failures may increase during the early phases of this technology's use (33). The sheath's dimensions substantially affect the surgical procedure's success percentage. Previous research indicated that bigger sheath sizes correlated with an increased chance of closure failure, and a higher ratio of sheath size to CFA diameter was associated with an elevated risk of failure (43). Although the success rate of PEVAR is slightly lower than that of CEVAR, the safety profile of PEVAR is deemed acceptable, as unsuccessful cases can be promptly transitioned to CEVAR. It is essential to acknowledge that multiple factors can affect the success rate. Ultrasound guiding has been proposed to correlate with technical success in PEVAR (26). While there are no absolute contraindications for PEVAR, numerous studies have highlighted characteristics that may lead to procedural failure and need conversion to open femoral access. Consistent anatomic variables linked to PEVAR failure include femoral artery calcification exceeding 50% of vessel circumference and a femoral artery diameter of less than 5 mm (44, 45). Open femoral exposure should be contemplated in patients exhibiting these anatomical characteristics. Obesity and arterial calcification can influence the success rate; prior studies have indicated that obesity and significant arterial calcification are primary causes of hemostasis failure (45).
4.2 Any limitations of the evidence included in the review
First, the majority of the included studies were retrospective or prospective cohort studies, with a very small number of randomized controlled trials. This constrained the overall quality and robustness of the outcomes. Second, the funnel plots for surgical suture failure, hematoma and lymphocele indicated that the studies included in the analysis had significant asymmetry, which may result in an exaggeration of statistical significance (e.g., P values), heighten the likelihood of Type I error (false positive), and induce heterogeneity. Third, significant discrepancies in various factors across studies, such as patient baseline data, surgical techniques, urgical proficiency and equipment may potentially result in notable heterogeneity and compromise the validity of pooled outcome analyses. Besides, the included studies were marked by relatively brief follow-up durations, predominantly confined to a 30-day observation period, with just a minor fraction extending beyond one year. This time constraint may potentially influence the generalizability of our findings about long-term effects.
4.3 Any limitations of the review processes used
The literature search strategy, while thorough across PubMed, Embase, Web of Science, and Cochrane Library, may not have identified all pertinent studies due to discrepancies in database indexing or unpublished data, hence introducing potential selection bias. Moreover, statistical heterogeneity was pronounced for several aggregated outcomes, potentially because to substantial variations in multiple parameters among studies, including patient baseline characteristics, surgical methodologies, surgical expertise, and instrumentation. However, we were unable to conduct subgroup or meta-regression studies to investigate its sources due to our inadequate statistical proficiency and the lack of accurate outcome data. Besides, we failed to perform multivariate analysis or forest blot due to our inadequate statistical proficiency.
4.4 Implications of the results for practice, policy, and future research
The increasing utilization of percutaneous endovascular aneurysm repair (PEVAR) signifies its revolutionary impact in vascular surgery. In contrast to the previous meta-analyses (13, 14), this meta-analysis included more studies published in recent years and finally incorporated a total of 24 high-quality studies, with more outcomes and more deep search, hence yielding more robust and reliable conclusions. This meta-analysis indicated that PEVAR, relative to CEVAR, was linked to a reduced hospital length of stay, decreased operational duration, shorter postoperative recovery, lower rates of groin infection, fewer cardiac problems, and a reduced incidence of lymphocele. These benefits correspond with contemporary value-based care models that prioritize minimally invasive methods and expedited recovery processes. Practitioners must weigh these advantages against CEVAR's reduced incidence of suture failure and pseudoaneurysm development, especially in patients with significant femoral calcification or obesity. The technical success rate of PEVAR highlights the significance of operator proficiency and patient selection criteria, including a femoral artery diameter exceeding 5 mm and low circumferential calcification. Future research should concentrate on enhancing the success rate of PEVAR surgery and mitigating the occurrence of pseudoaneurysm, including advancements in PEVAR equipment and improvement of doctors' surgical abilities.
In conclusion, relative to CEVAR, PEVAR was linked to a shorter hospital stay, reduced operative duration, briefer postoperative recovery, lower groin infection rates, fewer cardiac complications, and a decreased incidence of lymphocele; nevertheless, it demonstrated a higher prevalence of pseudoaneurysm and an elevated rate of surgical suture failure. PEVAR was a secure and efficacious approach for the management of TBAD.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
ZL: Writing – original draft, Data curation, Conceptualization, Project administration. ZH: Conceptualization, Formal analysis, Writing – original draft, Data curation. MW: Writing – original draft, Methodology, Investigation. LL: Writing – original draft, Investigation, Methodology. BC: Software, Writing – original draft, Supervision. SY: Supervision, Writing – original draft, Software. HL: Writing – original draft, Formal analysis. JQ: Writing – original draft, Validation. WL: Visualization, Writing – review & editing, Resources, Funding acquisition. XH: Writing – review & editing, Resources, Funding acquisition, Visualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Key Laboratory Construction Project of Guangxi Health Commission (ZPZH2020007) and the Scientific Research Foundation of Guangxi Health Commission (Z-B20240841).
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1673817/full#supplementary-material
Supplementary Data Sheet 1Comprehensive listing of the search results.
Supplementary Data Sheet 2Supplement Figures.
References
1.
Banceu CM Banceu DM Kauvar DS Popentiu A Voth V Liebrich M et al Acute aortic syndromes from diagnosis to treatment-a comprehensive review. J Clin Med. (2024) 13(5):16. 10.3390/jcm13051231
2.
Durham CA Cambria RP Wang LJ Ergul EA Aranson NJ Patel VI et al The natural history of medically managed acute type B aortic dissection. J Vasc Surg. (2015) 61(5):1192–8. 10.1016/j.jvs.2014.12.038
3.
Erbel R Aboyans V Boileau C Bossone E Di Bartolomeo R Eggebrecht H et al 2014 Esc guidelines on the diagnosis and treatment of aortic diseases. Kardiol Pol. (2014) 72(12):1169–252. 10.5603/kp.2014.0225
4.
Alves CM da Fonseca JH de Souza JA Kim HC Esher G Buffolo E . Endovascular treatment of type B aortic dissection: the challenge of late success. Ann Thorac Surg. (2009) 87(5):1360–5. 10.1016/j.athoracsur.2009.02.050
5.
Metcalfe MJ Brownrigg JRW Black SA Loosemore T Loftus IM Thompson MM . Unselected percutaneous access with large vessel closure for endovascular aortic surgery: experience and predictors of technical success. Eur J Vasc Endovasc Surg. (2012) 43(4):378–81. 10.1016/j.ejvs.2011.12.025
6.
Torsello GB Kasprzak B Klenk E Tessarek J Osada N Torsello GF . Endovascular suture versus cutdown for endovascular aneurysm repair: a prospective randomized pilot study. J Vasc Surg. (2003) 38(1):78–82. 10.1016/s0741-5214(02)75454-2
7.
Baxter RD Hansen SK Gable CE DiMaio JM Shutze WP Gable DR . Outcomes of open versus percutaneous access for patients enrolled in the great registry. Ann Vasc Surg. (2021) 70:370–7. 10.1016/j.avsg.2020.06.033
8.
Haas PC Krajcer Z Diethrich EB . Closure of large percutaneous access sites using the prostar Xl percutaneous vascular surgery device. J Endovasc Surg. (1999) 6(2):168–70. 10.1177/152660289900600209
9.
Dosluoglu HH Cherr GS Harris LM Dryjski ML . Total percutaneous endovascular repair of abdominal aortic aneurysms using perclose proglide closure devices. J Endovasc Ther. (2007) 14(2):184–8. 10.1177/152660280701400210
10.
DeVito P Jr Kimyaghalam A Shoukry S DeVito R Williams J Kumar E et al Comparing and correlating outcomes between open and percutaneous access in endovascular aneurysm repair in aortic aneurysms using a retrospective cohort study design. Int J Vasc Med. (2020) 2020:8823039. 10.1155/2020/8823039
11.
Vierhout BP Pol RA Ott MA Pierie MEN van Andringa de Kempenaer TMG Hissink RJ et al Randomized multicenter trial on percutaneous versus open access in endovascular aneurysm repair (piero). J Vasc Surg. (2019) 69(5):1429–36. 10.1016/j.jvs.2018.07.052
12.
Koreny M Riedmüller E Nikfardjam M Siostrzonek P Müllner M . Arterial puncture closing devices compared with standard manual compression after cardiac catheterization. Systematic review and meta-analysis. JAMA. (2004) 291(3):350–7. 10.1001/jama.291.3.350
13.
Vierhout BP Pol RA El Moumni M Zeebregts CJ . Editor’s choice - arteriotomy closure devices in evar, tevar, and tavr: a systematic review and meta-analysis of randomised clinical trials and cohort studies. Eur J Vasc Endovasc Surg. (2017) 54(1):104–15. 10.1016/j.ejvs.2017.03.015
14.
Bi G Wang Q Xiong G Chen J Luo D Deng J et al Is percutaneous access superior to cutdown access for endovascular abdominal aortic aneurysm repair? A meta-analysis. Vascular. (2022) 30(5):825–33. 10.1177/17085381211032765
15.
Hahl T Karvonen R Uurto I Protto S Suominen V . The safety and effectiveness of the prostar Xl closure device compared to open groin cutdown for endovascular aneurysm repair. Vasc Endovascular Surg. (2023) 57(8):848–55. 10.1177/15385744231180663
16.
Altoijry A Alsheikh S Alanezi T Aljabri B Aldossary MY Altuwaijri T et al Percutaneous versus cutdown access for endovascular aortic repair. Heart Surg Forum. (2023) 26(5):E455–e62. 10.59958/hsf.6665
17.
Wells GA Shea B O’Connell D Peterson J Welch V Losos M et al The Newcastle-Ottawa Scale (Nos) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2000).
18.
Higgins JP Altman DG Gøtzsche PC Jüni P Moher D Oxman AD et al The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed). (2011) 343:d5928. 10.1136/bmj.d5928
19.
Sahin AA Guner A Demir AR Uzun N Onan B Topel C et al Comparison between percutaneous and surgical femoral access for endovascular aortic repair in patients with type iii aortic dissection (preclose trial). Vascular. (2021) 29(4):616–23. 10.1177/1708538120965310
20.
Agrusa CJ Meltzer AJ Schneider DB Connolly PH . Safety and effectiveness of a “percutaneous-first” approach to endovascular aortic aneurysm repair. Ann Vasc Surg. (2017) 43:79–84. 10.1016/j.avsg.2017.02.004
21.
Mukherjee D Emery E Majeed R Heshmati K Hashemi H . A real-world experience comparison of percutaneous and open femoral exposure for endovascular abdominal aortic aneurysm repair in a tertiary medical center. Vasc Endovascular Surg. (2017) 51(5):269–73. 10.1177/1538574417702774
22.
Buck DB Karthaus EG Soden PA Ultee KH van Herwaarden JA Moll FL et al Percutaneous versus femoral cutdown access for endovascular aneurysm repair. J Vasc Surg. (2015) 62(1):16–21. 10.1016/j.jvs.2015.01.058
23.
Yang L Liu J Li Y . Femoral artery closure versus surgical cutdown for endovascular aortic repair: a single-center experience. Med Sci Monit. (2018) 24:92–9. 10.12659/msm.905350
24.
Uhlmann ME Walter C Taher F Plimon M Falkensammer J Assadian A . Successful percutaneous access for endovascular aneurysm repair is significantly cheaper than femoral cutdown in a prospective randomized trial. J Vasc Surg. (2018) 68(2):384–91. 10.1016/j.jvs.2017.12.052
25.
Chen SL Kabutey NK Whealon MD Kuo IJ Fujitani RM . Comparison of percutaneous versus open femoral cutdown access for endovascular repair of ruptured abdominal aortic aneurysms. J Vasc Surg. (2017) 66(5):1364–70. 10.1016/j.jvs.2017.03.431
26.
Ichihashi T Ito T Kinoshita Y Suzuki T Ohte N . Safety and utility of total percutaneous endovascular aortic repair with a single perclose proglide closure device. J Vasc Surg. (2016) 63(3):585–8. 10.1016/j.jvs.2015.08.111
27.
Akbulut M Ak A Arslan Ö Akardere ÖF Karakoç AZ Gume S et al Comparison of percutaneous access and open femoral cutdown in elective endovascular aortic repair of abdominal aortic aneurysms. Turk Gogus Kalp Damar Cerrahisi Dergisi. (2022) 30(1):11–7. 10.5606/tgkdc.dergisi.2022.21898
28.
Al-Khatib WK Zayed MA Harris EJ Dalman RL Lee JT . Selective use of percutaneous endovascular aneurysm repair in women leads to fewer groin complications. Ann Vasc Surg. (2012) 26(4):476–82. 10.1016/j.avsg.2011.11.026
29.
Cheng TW Maithel SK Kabutey NK Fujitani RM Farber A Levin SR et al Access type for endovascular repair in ruptured abdominal aortic aneurysms does not affect Major morbidity or mortality. Ann Vasc Surg. (2021) 70:181–9. 10.1016/j.avsg.2020.07.004
30.
Etezadi V Katzen BT Naiem A Johar A Wong S Fuller J et al Percutaneous suture-mediated closure versus surgical arteriotomy in endovascular aortic aneurysm repair. J Vasc Interv Radiol. (2011) 22(2):142–7. 10.1016/j.jvir.2010.10.008
31.
Howell M Doughtery K Strickman N Krajcer Z . Percutaneous repair of abdominal aortic aneurysms using the aneurx stent graft and the percutaneous vascular surgery device. Catheter Cardiovasc Interv. (2002) 55(3):281–7. 10.1002/ccd.10072
32.
Kauvar DS Martin ED Givens MD . Thirty-day outcomes after elective percutaneous or open endovascular repair of abdominal aortic aneurysms. Ann Vasc Surg. (2016) 31:46–51. 10.1016/j.avsg.2015.10.009
33.
Nelson PR Kracjer Z Kansal N Rao V Bianchi C Hashemi H et al A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the pevar trial). J Vasc Surg. (2014) 59(5):1181–93. 10.1016/j.jvs.2013.10.101
34.
Siracuse JJ Farber A Kalish JA Jones DW Rybin D Doros G et al Comparison of access type on perioperative outcomes after endovascular aortic aneurysm repair. J Vasc Surg. (2018) 68(1):91–9. 10.1016/j.jvs.2017.10.075
35.
Smith ST Timaran CH Valentine RJ Rosero EB Clagett GP Arko FR . Percutaneous access for endovascular abdominal aortic aneurysm repair: can selection criteria be expanded?Ann Vasc Surg. (2009) 23(5):621–6. 10.1016/j.avsg.2008.09.002
36.
Tay S Zaghloul MS Shafqat M Yang C Desai KA De Silva G et al Totally percutaneous endovascular repair for ruptured abdominal aortic aneurysms. Front Surg. (2022) 9:1040929. 10.3389/fsurg.2022.1040929
37.
Thurston JS Camara A Alcasid N White SB Patel PJ Rossi PJ et al Outcomes and cost comparison of percutaneous endovascular aortic repair versus endovascular aortic repair with open femoral exposure. J Surg Res. (2019) 240:124–9. 10.1016/j.jss.2019.02.011
38.
Wu Q Jiang D Lv X Zhang J Huang R Qiu Z et al Comparison of early efficacy of the percutaneous presuture technique with the femoral artery incision technique in endovascular aortic repair under local anesthesia for uncomplicated type B aortic dissection. J Interv Cardiol. (2022) 2022:6550759. 10.1155/2022/6550759
39.
Jadad AR Moore RA Carroll D Jenkinson C Reynolds DJ Gavaghan DJ et al Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17(1):1–12. 10.1016/0197-2456(95)00134-4
40.
Dwivedi K Regi JM Cleveland TJ Turner D Kusuma D Thomas SM et al Long-Term evaluation of percutaneous groin access for evar. Cardiovasc Intervent Radiol. (2019) 42(1):28–33. 10.1007/s00270-018-2072-3
41.
Bode LG Kluytmans JA Wertheim HF Bogaers D Vandenbroucke-Grauls CM Roosendaal R et al Preventing surgical-site infections in nasal carriers of Staphylococcus Aureus. N Engl J Med. (2010) 362(1):9–17. 10.1056/NEJMoa0808939
42.
Jahnke T Schäfer JP Charalambous N Trentmann J Siggelkow M Hümme TH et al Total percutaneous endovascular aneurysm repair with the dual 6-F perclose-at preclosing technique: a case-control study. J Vasc Interv Radiol. (2009) 20(10):1292–8. 10.1016/j.jvir.2009.06.030
43.
Lee WA Brown MP Nelson PR Huber TS Seeger JM . Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the preclose technique. J Vasc Surg. (2008) 47(5):919–23. 10.1016/j.jvs.2007.12.029
44.
Mousa AY Campbell JE Broce M Abu-Halimah S Stone PA Hass SM et al Predictors of percutaneous access failure requiring open femoral surgical conversion during endovascular aortic aneurysm repair. J Vasc Surg. (2013) 58(5):1213–9. 10.1016/j.jvs.2013.04.065
45.
Manunga JM Gloviczki P Oderich GS Kalra M Duncan AA Fleming MD et al Femoral artery calcification as a determinant of success for percutaneous access for endovascular abdominal aortic aneurysm repair. J Vasc Surg. (2013) 58(5):1208–12. 10.1016/j.jvs.2013.05.028
Summary
Keywords
aortic dissection, endovascular aneurysm repair, percutaneous, cutdown, pseudoaneurysm, meta-analysis
Citation
Liang Z, Huang Z, Wu M, Lin L, Chen B, Yin S, Li H, Quan J, Liang W and Huang X (2025) Comparison of percutaneous vs. cutdown access for endovascular aortic repair in the treatment of type B aortic dissection: a meta-analysis. Front. Cardiovasc. Med. 12:1673817. doi: 10.3389/fcvm.2025.1673817
Received
26 July 2025
Revised
26 October 2025
Accepted
12 November 2025
Published
24 November 2025
Volume
12 - 2025
Edited by
Shahzad Raja, Harefield Hospital, United Kingdom
Reviewed by
Wang Qi, Shandong University, China
Mohamed Ahmed Mousa, Ain Shams University, Egypt
Updates
Copyright
© 2025 Liang, Huang, Wu, Lin, Chen, Yin, Li, Quan, Liang and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xiaolong Huang xiaolonghuang2024@126.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.