Abstract
Background:
In severe aortic stenosis (AS), relief of afterload excess would be expected to improve left ventricular ejection fraction. However, the response of LVEF to transcatheter aortic valve replacement (TAVR) is variable, with some patients even demonstrating a decline. The mechanisms underlying this phenomenon are incompletely characterized. Accordingly, we investigated changes in systolic function in the near-term postoperative period following TAVR.
Methods:
We studied consecutive patients with severe AS referred for TAVR without identifiable perioperative sources of negative inotropy or ventricular dyssynchrony. Preoperative and postoperative day one echocardiograms were compared with respect to hemodynamics, LV geometry, LVEF, and midwall fractional shortening (FSmw). Contractility was assessed by comparing observed FSmw values to those predicted based on the stress-shortening relation of healthy controls.
Results:
Thirty-six patients were included (61% women; mean age 77 years; mean Society of Thoracic Surgeons mortality risk score 3.6%). Following TAVR, there was a precipitous decline in circumferential end-systolic wall stress from 122 ± 47 to 74 ± 32 kdyn/cm2 (p < 0.001) and a slight increase in LVEF. Surprisingly, however, there was also an increase in the percentage of patients with depressed contractility from 22% (8) to 78% (28) (p < 0.001). Heart rate and ventricular volumes remained unchanged.
Conclusions:
Contractility declined in the near-term postoperative period following TAVR. We interpret this finding to suggest that contractility is augmented by high afterload in severe AS and declines in parallel with afterload reduction. We speculate that autoregulatory mechanisms triggered by high valvular resistance support LVEF in severe AS and rapidly abate following TAVR.
Introduction
In severe aortic stenosis (AS), left ventricular systolic function is maintained through the development of concentric hypertrophy or remodeling (1, 2), wherein the parallel addition of sarcomeres increases wall thickness relative to chamber size (3). This process mitigates the adverse effect of excess end-systolic wall stress caused by high valvular resistance (4). It is generally assumed that reduced LVEF in severe AS without coronary artery disease results from afterload excess (4). However, data from studies employing midwall stress-shortening relations suggest some patients with severe AS also have abnormalities in LV contractility (5). Such reduced contractility appears to result from replacement fibrosis and microvascular dysfunction (6, 7).
Transcatheter aortic valve replacement (TAVR) represents a unique opportunity to study LV mechanics in severe AS due to the immediate drop in afterload and because the confounding effects of thoracotomy and cardioplegia-related myocardial depression are obviated. While pooled results from several large trials suggest an inverse relationship between baseline LVEF and the magnitude of LVEF improvement following TAVR (8–21), recent work has demonstrated a lack of significant improvement or even a decline in LVEF following TAVR in patients with baseline LVEF >60% (22). This occurred despite a decrease in afterload as assessed by both wall stress and valvuloarterial impedance. Barring significant changes in preload, which is thought to only have a modest impact on LVEF at the extremes (23), these findings suggest contractility decline. However, given the 1-year interval between TAVR and repeat echocardiography in that study, it is difficult to discount a reduction in contractility through earlier and faster regression of myocyte hypertrophy than interstitial fibrosis (24, 25). While subsequent work demonstrated similar findings in the immediate postoperative period (26), the use of LVEF, even when indexed to wall stress, fails to account for LV concentric remodeling, which inherently confounds the assessment of contractility (1, 27).
This study aimed to characterize the near-term, isolated effect of TAVR on LV contractility in the absence of confounding effects from loading conditions, geometric abnormalities, and common perioperative sources of negative inotropy encountered in this population.
Methods
Patient selection
This was a single-center retrospective cohort study. Patients were considered for inclusion if they had severe AS as defined by contemporary guidelines (28), underwent TAVR at UMass Memorial Medical Center between 1 January 2021 and 29 September 2021, and had preoperative echocardiograms available for review if performed at another institution. The following exclusion criteria were sequentially applied: previous transcatheter or surgical aortic valve replacement; concomitant non-AS severe valvular disease; myocardial infarction (MI), coronary artery bypass grafting, or percutaneous coronary intervention (PCI) within 8 weeks of TAVR or between pre- and postoperative echocardiograms; unrevascularized hemodynamically significant coronary artery disease (defined as vessel diameter reduction ≥80% or fractional flow reserve <0.8 on coronary angiography); initiation or dose escalation of negative inotropic agents between pre- and postoperative echocardiograms; new conduction abnormality or pacemaker dependence between pre- and postoperative echocardiograms; incomplete clinical data; and inadequate echocardiogram image quality. The UMass Chan Institutional Review Board approved the study and waived the requirement for informed consent due to the retrospective design and minimal potential harm to subjects.
Outcomes
The primary endpoint was a change in LV contractility based on the stress-shortening relation, as described below. The secondary endpoints included changes in LVEF, circumferential end-systolic wall stress (cESS), valvuloarterial impedance (Zva), and mean AV gradient (AVG).
Echocardiography
All patients underwent comprehensive two-dimensional and Doppler echocardiography preoperatively and on postoperative day 1 according to American Society of Echocardiography standards (28). Valvuloarterial impedance was calculated as the sum of systolic blood pressure (SBP) and mean AVG divided by stroke volume indexed to body surface area. Left ventricular end-diastolic and end-systolic volumes (EDV and ESV) and LVEF were measured by the biplane disk summation method.
Two-dimensional measurements of septal thickness, LV internal diameter, and LV posterior wall thickness were obtained at the level of the mitral valve leaflet tips in the parasternal long-axis view at end-diastole and end-systole. Midwall fractional shortening and cESS were derived based on a two-shell model of the LV, assuming constant volumes of each shell throughout the cardiac cycle, as previously described (29, 30). Left ventricular mass was calculated using the Devereux equation (cube method) (31, 32).
Clinical data
Average blood pressure values were derived using readings taken ±2 h from the time of echocardiography to decrease the influence of outliers in afterload calculations. When available, arterial catheter data were used instead of sphygmomanometer measurements. Reported heart rate values were recorded during patients' parasternal long-axis views on echocardiograms. Demographic, anthropomorphic, and comorbidities data were abstracted from the electronic medical record.
Control cohort
A control cohort from a prior study (33) of midwall dynamics, comprised of 30 patients (43% women, mean age 74 ± 6 years) with normal echocardiograms, was used to derive a control stress-shortening relation through linear regression, defined as FSmw = 28.486 − 0.104 × cESS. The 95% confidence interval lower bound equation was FSmw = 22.702 − 0.106 × cESS.
Contractility calculations
Contractility was assessed by comparing observed to predicted FSmw based on the control stress-shortening relation. Abnormal contractility was defined as observed FSmw greater than two standard deviations below predicted.
Statistical analysis
Categorical variables are reported as percentages with absolute values and, when applicable, compared before and after TAVR using McNemar's test. Continuous variables are reported as means with standard deviations and compared before and after TAVR using the paired sample t-test if normally distributed or the Wilcoxon signed-rank test if not normally distributed. Normality was assessed by the Shapiro–Wilk test. P-values < 0.05 were considered statistically significant. The corresponding author had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Patient characteristics
Among the 100 patients eligible for inclusion, 4 were excluded due to prior surgical or transcatheter aortic valve replacement, 1 due to interval MI, 9 due to interval PCI, 4 due to unrevascularized coronary artery disease, 15 due to new conduction abnormality or pacemaker dependence, 5 due to initiation or uptitration of negative inotropic agents, 1 due to an intraprocedural complication (iatrogenic aorta–RV fistula), 9 due to incomplete clinical data, and 16 due to suboptimal image quality. Therefore, our cohort consists of 36 patients (61% women) with a mean age of 77 ± 9 years and a mean Society of Thoracic Surgeons (STS) mortality risk score of 3.6 ± 2.3%. Additional clinical characteristics are summarized in Table 1. The preoperative rhythm was sinus in 29 patients (81%), atrial fibrillation (AF) in 6 patients (17%), and unclear in 1 patient (3%). There were no missing data for any included patients.
Table 1
| Characteristic | N = 36 |
|---|---|
| Age (years) | 77 ± 9 |
| Women | 61% (22) |
| Height (inches) | 65 ± 4 |
| Weight (lbs) | 165 ± 47 |
| BSA (m2) | 1.83 ± 0.29 |
| BMI (kg/m2) | 27.8 ± 7.8 |
| STS score (%) | 3.6 ± 2.3 |
| Severe AS subtype | |
| HG | 75% (27) |
| Classical LFLG | 8% (3) |
| Paradoxical LFLG | 6% (2) |
| NFLG | 11% (4) |
| NYHA class | |
| II–IV | 89% (32) |
| II | 36% (13) |
| III | 44% (16) |
| IV | 8% (3) |
| Hypertension | 94% (34) |
| Diabetes mellitus | 25% (9) |
| Prior MI | 8% (3) |
| Prior PCI | 6% (2) |
| Prior CABG | 6% (2) |
| AF paroxysmal | 9% (3) |
| AF persistent | 31% (11) |
| Conduction defect | 14% (5) |
| Pacemaker pre-TAVR | 11% (4) |
| CVA | 8% (3) |
| TIA | 8% (3) |
| Chronic lung disease | 19% (7) |
| ESRD | 3% (1) |
| Valve type | |
| Balloon-expandable | 92% (33) |
| Self-expanding | 8% (3) |
Baseline demographics and comorbidities.
BSA, body surface area; BMI, body mass index; STS, Society of Thoracic Surgeons; HG, high gradient; LFLG, low flow, low gradient; NFLG, normal flow, low gradient; NYHA, New York Heart Association; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; AF, atrial fibrillation; CVA, cerebrovascular disease; TIA, transient ischemic attack; ESRD, end-stage renal disease.
Echocardiographic and hemodynamic changes
The mean length of time between preoperative echocardiogram and TAVR was 92 ± 89 days (range 3–472 days), and the mean length of time between TAVR and postoperative echocardiogram was 22 ± 4 h (range 5–25 h). Echocardiographic and hemodynamic parameters before and after TAVR are summarized in Table 2. SBP remained unchanged at 135 mmHg, whereas diastolic blood pressure decreased significantly from 72 ± 8 to 58 ± 12 mmHg. Aortic valve area increased significantly, whereas the mean and peak AVG decreased significantly. Circumferential ESS decreased significantly from 122 ± 47 to 74 ± 32 kdyn/cm2, as well as valvuloarterial impedance from 5.25 ± 1.84 to 3.89 ± 0.87 mmHg/mL/m2. End-diastolic volume, end-systolic volume, and stroke volume index remained unchanged, whereas LVEF increased significantly from 55 ± 11% to 58 ± 9% (Figure 1). The percentage of patients with FSmw greater than two standard deviations below predicted increased significantly from 22% (8) to 78% (28) (Figure 2), whereas the average percent predicted FSmw decreased significantly from 89 ± 33% to 61 ± 15%. Similar decreases in average percent predicted FSmw were observed in patients with high (94 ± 34% to 63 ± 16%) vs. low (73 ± 24% to 55 ± 10%) mean AVG, normal (90 ± 36% to 58 ± 17%) vs. low (87 ± 29% to 66 ± 9%) stroke volume index, and preserved (88 ± 31% to 65 ± 14%) vs. reduced (90 ± 39% to 52 ± 12%) LVEF. There was not a strong association between preoperative cESS and the degree of postoperative decline in percent predicted FSmw (R2 = 0.58).
Table 2
| Variable | Pre-TAVR | Post-TAVR | P-value | % change |
|---|---|---|---|---|
| SBP (mmHg) | 135 ± 23 | 132 ± 19 | 0.40 | |
| DBP (mmHg) | 72 ± 8 | 58 ± 12 | <0.001 | −19 |
| HR (bpm) | 79 ± 15 | 78 ± 14 | 0.76 | |
| LVIDd (mm) | 45 ± 7 | 43 ± 7 | <0.01 | −4 |
| IVSTd (mm) | 12 ± 2 | 13 ± 2 | 0.14 | |
| PWTd (mm) | 12 ± 2 | 12 ± 2 | 0.93 | |
| LVIDs (mm) | 32 ± 8 | 30 ± 8 | 0.07 | |
| LVMI (g/m2) | 108 ± 33 | 105 ± 27 | 0.35 | |
| EDV (mL) | 127 ± 38 | 122 ± 27 | 0.27 | |
| ESV (mL) | 58 ± 29 | 52 ± 21 | 0.14 | |
| RWT | 0.55 ± 0.14 | 0.58 ± 0.12 | 0.07 | |
| AVA (cm2) | 0.71 ± 0.22 | 1.58 ± 0.52 | <0.001 | 124 |
| AVAi (cm2/m2) | 0.39 ± 0.13 | 0.88 ± 0.29 | <0.001 | 122 |
| Mean AVG (mmHg) | 48 ± 14 | 12 ± 6 | <0.001 | −74 |
| Peak AVG (mmHg) | 78 ± 20 | 20 ± 9 | <0.001 | −74 |
| cESS (kdyn/cm2) | 122 ± 47 | 74 ± 32 | <0.001 | −39 |
| Zva (mmHg/mL/m2) | 5.25 ± 1.84 | 3.89 ± 0.87 | <0.001 | −26 |
| SVi (mL/m2) | 38.10 ± 11.45 | 38.65 ± 8.99 | 0.72 | |
| LVEF (%) | 55 ± 11 | 58 ± 9 | <0.05 | 5 |
| FSmw (%) | 13 ± 3 | 13 ± 4 | 0.76 | |
| FSmw percent predicted (%) | 89 ± 33 | 61 ± 15 | <0.001 | −31 |
| High mean AVG | 94 ± 34 | 63 ± 16 | <0.001 | −33 |
| Low mean AVG | 73 ± 24 | 55 ± 10 | <0.05 | −25 |
| Normal baseline SVi | 90 ± 36 | 58 ± 17 | <0.001 | −36 |
| Low baseline SVi | 87 ± 29 | 66 ± 9 | <0.01 | −24 |
| Preserved baseline LVEF | 88 ± 31 | 65 ± 14 | <0.001 | −26 |
| Reduced baseline LVEF | 90 ± 39 | 52 ± 12 | <0.05 | −42 |
| FSmw ≥2 SD below predicted (%) | 22% (8) | 78% (28) | <0.001 | 250 |
Hemodynamic and echocardiographic variables before and after TAVR.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LVIDd, left ventricular internal diameter at end-diastole; IVSTd, interventricular septal thickness at end-diastole; PWTd, left ventricular posterior wall thickness at end-diastole; LVIDs, left ventricular internal diameter at end-systole; EDV, end-diastolic volume; ESV, end-systolic volume; RWT, relative wall thickness; AVA, aortic valve area; AVAi, aortic valve area indexed to body surface area; AVG, aortic valve pressure gradient; cESS, circumferential end-systolic wall stress; Zva, valvuloarterial impedance; Svi, stroke volume indexed to body surface area; LVEF, left ventricular ejection fraction; FSmw, midwall fractional shortening.
Figure 1
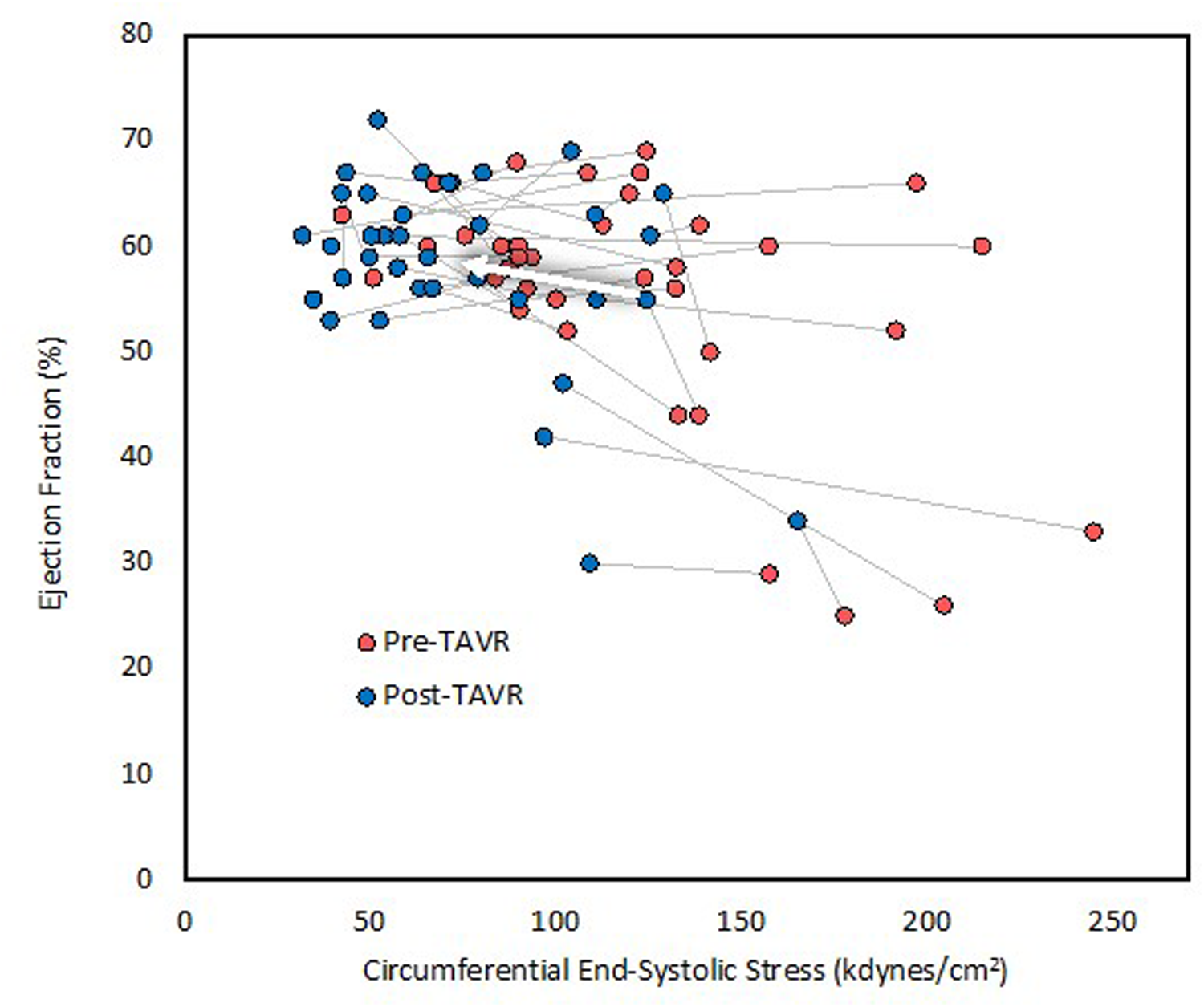
Left ventricular ejection fraction before and after TAVR. LVEF increased only marginally despite significant afterload reduction. The arrow indicates average change.
Figure 2
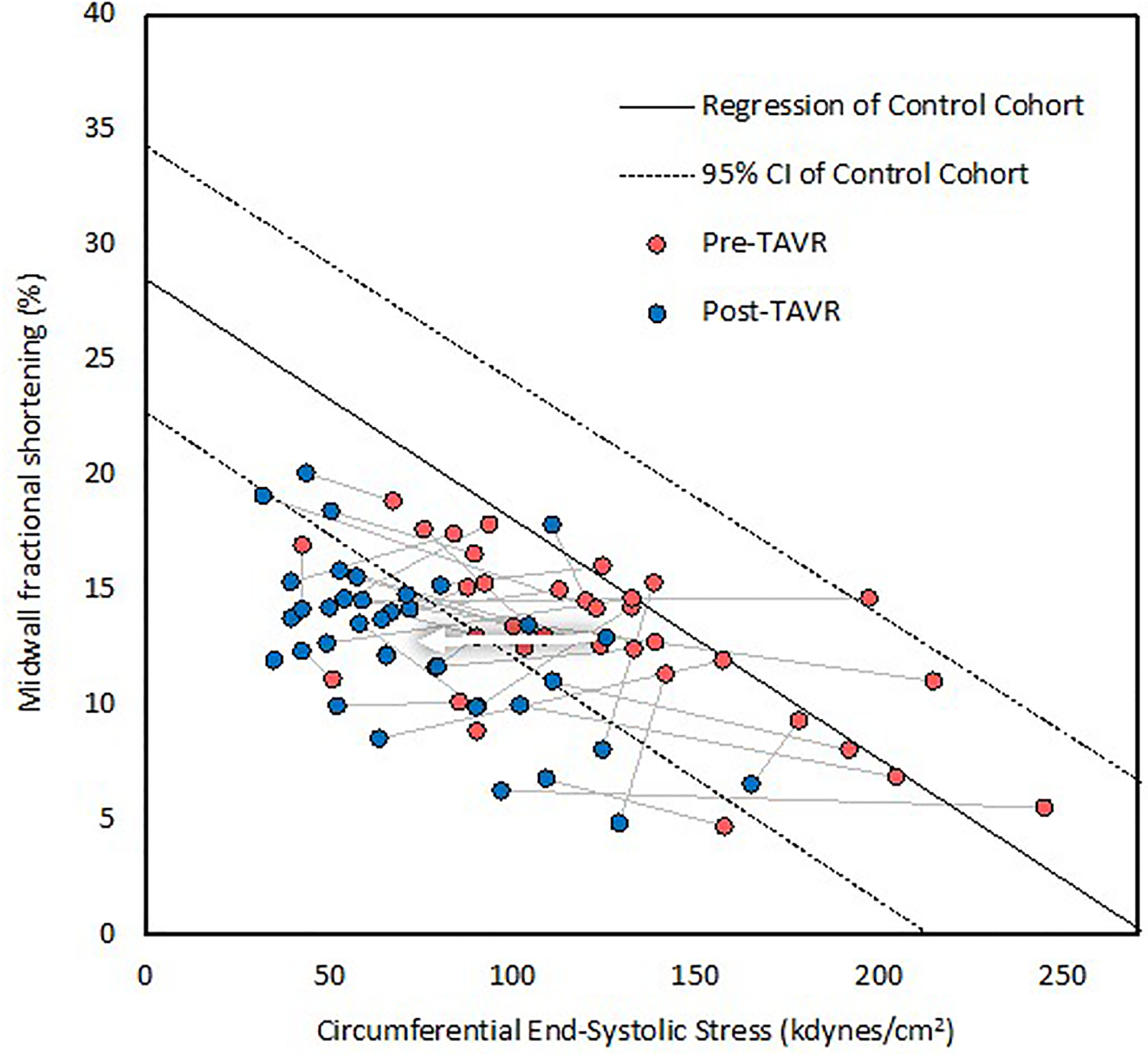
The stress-shortening relationship before and after TAVR. Each patient is represented by a line, with red dots indicating preoperative values and blue dots postoperative values. The dots falling within the 95% CI represent normal contractility. This graph demonstrates that contractility was impaired in many patients at baseline and, on average, declined further following TAVR, as evidenced by horizontal movement crossing the lower bound 95% confidence interval of normal. The arrow indicates average change. CI indicates confidence intervals.
Discussion
In this cohort of 36 medically complex patients with severe AS, LVEF was mildly decreased at baseline and increased only slightly following TAVR despite substantial afterload reduction, pointing to an immediate decline in LV contractility. FSmw corrected for cESS was markedly reduced at baseline and decreased further within 24 h of TAVR, in support of this conclusion. Thus, this contractility decline is evident in the immediate post-TAVR period, long before reverse remodeling can take place. It also occurred in the absence of new ischemic insults, pacemaker-related ventricular dyssynchrony, initiation of negative inotropic agents, and confounding effects of thoracotomy. Heart rate remained unchanged, ruling out the contribution from the Bowditch effect (tachycardia-mediated positive inotropy). Therefore, this contractility decline appears to be related to valvular unloading itself.
Accordingly, we hypothesize that systolic function in severe AS is supported by augmentation of contractility in response to high afterload. A similar phenomenon involving tandem increases in effective arterial elastance and ventricular elastance has been demonstrated in hypertensive heart disease and normal aging (34–36). Multiple mechanisms may mediate the interaction between afterload and contractility, including enhanced sympathetic tone and alterations in myocardial calcium handling. In decompensated severe AS, the classic triad of positional or exertional hypotension (37), endomyocardial ischemia (38, 39), and pulmonary venous congestion (40, 41) is a potent trigger of increased adrenergic tone. Remediation of these processes through aortic valve replacement, in turn, reduces adrenergic tone (42). Additionally, acute afterload elevation (e.g., through aortic cross-clamping) has been shown to induce homeometric autoregulation (i.e., the Anrep effect), wherein increased myocardial stretch leads to an increase in the calcium transient amplitude (43–46). Although in vitro studies investigating this phenomenon in chronic pressure overload states are lacking, the Anrep effect would provide an elegant connection between valvular stenosis and augmented contractility.
Given that afterload is higher in AS patients with reduced LVEF (1, 27), our findings may be hypothesized to suggest positive inotropic autoregulatory mechanisms are more strongly activated in patients with reduced LVEF. Indeed, in our cohort, FSmw declined to a greater extent in patients with baseline reduced LVEF. Yet, prior studies have demonstrated an inverse relationship between baseline LVEF and the magnitude of LVEF improvement following TAVR (summarized in Table 3). Two explanations might resolve this seeming paradox. First, there was no strong correlation in our cohort between baseline wall stress and the magnitude of contractility decline; therefore, contractility augmentation in severe AS may not vary proportionately with afterload and instead occur as an “all or none” phenomenon at a certain afterload threshold. Second, the interaction between afterload and stroke volume is modified by the slope of the end-systolic pressure–volume relation (ESPVR). Patients with reduced LVEF have a higher prevalence and severity of contractile dysfunction and therefore typically experience marked improvement in LVEF with TAVR. Conversely, patients with preserved LVEF have less contractile dysfunction and steeper ESPVR slopes. Under these conditions, the impact of afterload reduction may be outweighed by an associated contractility decline, resulting in modest or no LVEF improvement. This phenomenon is illustrated in Figure 3.
Table 3
| Study | Subgroup | LVEF (% ± SD) | Time Interval | N | P-value | |
|---|---|---|---|---|---|---|
| Pre-TAVR | Post-TAVR | |||||
| Maes et al., 2019 (TOPAS-TAVI) (8) | LVEF <30% | 22 ± 5 | 34 ± 12 | 1 year | 128 | <0.001 |
| Pilgrim et al., 2011 (9) | LVEF ≤30% | 25 ± 4 | 34 ± 10 | Discharge | 37 | <0.001 |
| Fraccaro et al., 2012 (10) | LVEF <35% | 27.7 ± 6.0 | 35.4 ± 11.0 | Discharge | 46 | <0.0001 |
| O'Sullivan, et al., 2013 (11) | LVEF ≤40%, LFLG | 29.0 ± 6 | 38.3 ± 13.2 | 30 days | 20 | <0.0001 |
| O'Sullivan, et al., 2013 (11) | LVEF ≤40%, LFHG | 31.8 ± 7 | 50.6 ± 10.0 | 30 days | 31 | <0.0001 |
| Bauer et al., 2013 (12) | All | 32 ± 9 | 50 ± 17 | 30 days | 31 | <0.05 |
| Gotzmann et al., 2012 (13) | LFLG | 32 ± 6 | 46 ± 11 | 6 months | 15 | <0.01 |
| Van Linden et al., 2011 (14) | LVEF ≤40% | 32.5 ± 7.1 | 41.5 ± 10.1 | Discharge | 39 | <0.0001 |
| 53.9 ± 13 | 1 year | <0.0001 | ||||
| Kuneman et al., 2022 (15) | LVEF <40% | 33 ± 6 | 43 ± 10 | 1 year | 88 | <0.001 |
| Ewe et al., 2010 (16) | LVEF <50% | 37 ± 8 | 46 ± 11 | Discharge | 97 | <0.001 |
| Maes et al., 2019 (TOPAS-TAVI) (8) | LVEF 30−40% | 37 ± 7 | 41 ± 12 | 1 year | 165 | <0.001 |
| Elmariah et al., 2013 (PARTNER Cohort A Trial) (17) | LVEF <50% | 37.1 ± 9.2 | 48.6 ± 11.3 | 1 year | 108 | <0.0001 |
| Løgstrup et al., 2013 (18) | LVEF ≤50% | 39 ± 9.4 | 52 ± 12.5 | 1 year | 74 | <0.0001 |
| Deste et al., 2018 (19) | All | 39.3 ± 8.8 | 44.1 ± 10.1 | 30 days | 46 | <0.05 |
| Kuneman et al., 2022 (15) | LVEF 40–49% | 45 ± 3 | 52 ± 8 | 1 year | 122 | <0.001 |
| Harrington et al., 2021 (22) | LVEF <60% | 45.4 ± 10 | 46.7 ± 9.8 | 1 year | NRa | 0.15 |
| Sato et al., 2017 (20) | All | 50 ± 14 | 53 ± 13 | 10 days | 209 | <0.001 |
| Popma et al., 2014 (CoreValve Extreme) (21) | All | 54.5 ± 14.4 | 57.3 ± 11.6 | 1 year | 330 | NR |
| Pilgrim et al., 2011 (9) | LVEF >30% | 56 ± 10 | 57 ± 11 | Discharge | 219 | NR |
| Fraccaro et al., 2012 (10) | LVEF ≥35% | 56.5 ± 8.7 | 55.9 ± 9.2 | Discharge | 315 | 0.24 |
| Gotzmann et al., 2012 (13) | No LFLG | 57 ± 11 | 56 ± 8 | 6 months | 152 | >0.05 |
| Løgstrup et al., 2013 (18) | LVEF >50% | 57.9 ± 5.3 | 60 ± 7.7 | 1 year | 34 | >0.05 |
| Kuneman et al., 2022 (15) | LVEF ≥50% | 58 ± 5 | 59 ± 7 | 1 year | 350 | <0.05 |
| Ewe et al., 2010 (16) | LVEF ≥50% | 61 ± 7 | 59 ± 11 | Discharge | 50 | >0.05 |
| Harrington et al., 2021 (22) | LVEF ≥60% | 65.7 ± 5.5 | 53.5 ± 10.8 | 1 year | NRa | <0.01 |
Summary of studies investigating changes in LVEF following TAVR.
NR, not reported; LFLG, low flow, low gradient; LFHG, low flow, high gradient.
A total of 397 patients; distribution between reduced and preserved LVEF subgroups not available.
Figure 3
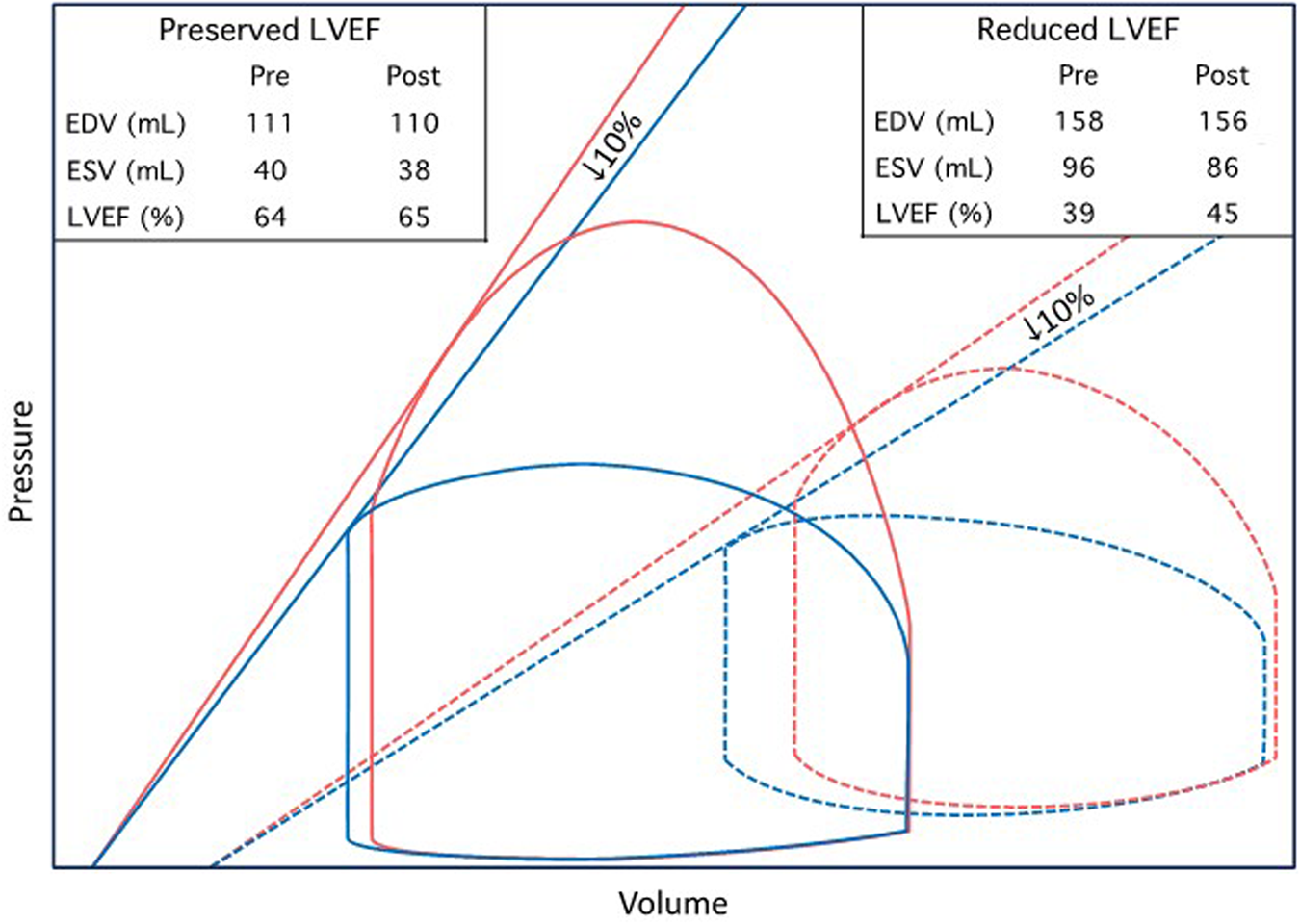
Theoretic pressure–volume diagrams depicting proposed changes in LVEF following TAVR. Two hypothetical patients with normal and abnormal LV systolic function, respectively, are illustrated. In the presence of impaired LV contractility (signified by decreased ESPVR slope), stroke volume is more sensitive to changes in afterload. Accordingly, patients with reduced LVEF experience a significant increase in LVEF following TAVR, driven by a reduction in valvuloarterial impedance, despite a 10% contractility decline. Conversely, patients with preserved baseline LVEF experience minimal or no increase despite substantial afterload reduction. Red and blue lines indicate before and after TAVR, respectively. The solid and dashed lines indicate preserved and reduced baseline LVEF, respectively.
The variability in LVEF response to TAVR reinforces the limitations of endocardial indices in the evaluation of systolic function in the presence of altered LV geometry and loading conditions. Importantly, regardless of the impact on LVEF, withdrawal of these positively inotropic mechanisms likely restores both ventricular efficiency (i.e., the ratio of stroke work to total LV energy expenditure) and contractile reserve (i.e., the ratio of resting to maximal contractility), thereby improving exercise capacity and resistance to acute stressors, such as infection, arrhythmia, and hypovolemia (47, 48). Therefore, unlike long-term postoperative contractility impairment, which reflects poor reverse modeling and predicts poor outcomes following TAVR (49), contractility decline in the immediate term may actually be an expected and even favorable biomarker.
Several limitations of our study must be acknowledged. Our cohort was small, nonrandom, and derived from a single academic medical center, predisposing to sampling error and selection bias. While we excluded patients with clear perioperative confounders of systolic function, it is difficult to definitively rule out a preoperative contractility decline due to progression of AS-associated cardiomyopathy, particularly in patients with long time intervals between preoperative echocardiogram and TAVR. Similarly, given its procedural necessity, we were unable to exclude rapid pacing as a potential confounder of contractility. However, myocardial stunning secondary to extreme tachycardia is typically a transient and subclinical phenomenon, lasting on the order of minutes in the majority of patients (50). Accordingly, rapid pacing alone is unlikely to have caused such a profound decrease in contractility in this cohort. Lastly, Zva decreased to a lesser degree (26%) than did cESS (39%) and mean AVG (74%). Although blood pressure did not increase, this discrepancy may reflect increased arterial load, as has previously been demonstrated following TAVR (51). Therefore, afterload may be slightly underestimated by cESS following TAVR, predisposing to error during the calculation of predicted FSmw.
In summary, systolic function in severe AS seems to be supported by positive inotropic autoregulatory mechanisms. These may include increased adrenergic tone and the Anrep effect. Restoration of contractile reserve through downregulation of these mechanisms represents one of many favorable hemodynamic effects of aortic valve replacement.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the UMass Chan Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. MG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. MP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. GA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TAVR, transcatheter aortic valve replacement; LVEF, left ventricular ejection fraction; FSmw, midwall fractional shortening; cESS, circumferential end-systolic wall stress; Zva, valvuloarterial impedance; AVG, transaortic valvular gradient; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; LVIDd, left ventricular internal diameter at end-diastole; LVIDs, left ventricular internal diameter at end-systole; PWTd, left ventricular posterior wall thickness at end-diastole; IVSTd, interventricular septal thickness at end-diastole; RWT, relative wall thickness; ESPVR, end-systolic pressure–volume relation; AF, atrial fibrillation; MI, myocardial infarction; CVA, cerebrovascular accident; TIA, transient ischemic attack; ESRD, end-stage renal disease; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; BSA, body surface area; BMI, body mass index.
References
1.
Ito S Pislaru C Miranda W Nkomo V Connolly H Pislaru S et al Left ventricular contractility and wall stress in patients with aortic stenosis with preserved or reduced ejection fraction. JACC Cardiovasc Imaging. (2020) 13:357–69. 10.1016/j.jcmg.2019.01.009
2.
Aldrugh S Valle J Parker M Harrington C Aurigemma G . Prevalence of left ventricular hypertrophy caused by systemic hypertension preceding the development of severe aortic stenosis. Am J Cardiol. (2021) 150:89–94. 10.1016/j.amjcard.2021.03.036
3.
Basso C Michaud K d'Amati G Banner J Lucena J Cunningham K et al Cardiac hypertrophy at autopsy. Virchows Arch. (2021) 479:79–94. 10.1007/s00428-021-03038-0
4.
Ross J . Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J Am Coll Cardiol. (1985) 5:811–26. 10.1016/s0735-1097(85)80418-6
5.
Ballo P Mondillo S Motto A Faraguti S . Left ventricular midwall mechanics in subjects with aortic stenosis and normal systolic chamber function. J Heart Valve Dis. (2006) 15:639–50.
6.
Bernardo B Weeks K Pretorius L McMullen J . Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. (2010) 128:191–227. 10.1016/j.pharmthera.2010.04.005
7.
Rajappan K Rimoldi O Dutka D Ariff B Pennell D Sheridan D et al Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. (2002) 105:470–6. 10.1161/hc0402.102931
8.
Maes F Lerakis S Barbosa Ribeiro H Gilard M Cavalcante J Makkar R et al Outcomes from transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis and left ventricular ejection fraction less than 30%: a substudy from the TOPAS-TAVI registry. JAMA Cardiol. (2019) 4:64–70. 10.1001/jamacardio.2018.4320
9.
Pilgrim T Wenaweser P Meuli F Huber C Stortecky S Seiler C et al Clinical outcome of high-risk patients with severe aortic stenosis and reduced left ventricular ejection fraction undergoing medical treatment or TAVI. PLoS One. (2011) 6:e27556. 10.1371/journal.pone.0027556
10.
Fraccaro C Al-Lamee R Tarantini G Maisano F Napodano M Montorfano M et al Transcatheter aortic valve implantation in patients with severe left ventricular dysfunction: immediate and mid-term results, a multicenter study. Circ Cardiovasc Interv. (2012) 5:253–60. 10.1161/CIRCINTERVENTIONS.111.964213
11.
O'Sullivan C Stortecky S Heg D Pilgrim T Hosek N Buellesfeld L et al Clinical outcomes of patients with low-flow, low-gradient, severe aortic stenosis and either preserved or reduced ejection fraction undergoing transcatheter aortic valve implantation. Eur Heart J. (2013) 34:3437–50. 10.1093/eurheartj/eht408
12.
Bauer F Coutant V Bernard M Stepowski D Tron C Cribier A et al Patients with severe aortic stenosis and reduced ejection fraction: earlier recovery of left ventricular systolic function after transcatheter aortic valve implantation compared with surgical valve replacement. Echocardiography. (2013) 30:865–70. 10.1111/echo.12171
13.
Gotzmann M Lindstaedt M Bojara W Ewers A Mügge A . Clinical outcome of transcatheter aortic valve implantation in patients with low-flow, low gradient aortic stenosis. Catheter Cardiovasc Interv. (2012) 79:693–701. 10.1002/ccd.23240
14.
Van Linden A Kempfert J Blumenstein J Holzhey D Rastan A Mohr F et al Transapical aortic valve implantation off-pump in patients with impaired left ventricular function. Ann Thorac Surg. (2011) 92:18–23. 10.1016/j.athoracsur.2011.03.041
15.
Kuneman J Butcher S Singh G Wang X Hirasawa K van der Kley F et al Prognostic implications of change in left ventricular ejection fraction after transcatheter aortic valve implantation. Am J Cardiol. (2022) 177:90–9. 10.1016/j.amjcard.2022.04.060
16.
Ewe S Ajmone Marsan N Pepi M Delgado V Tamborini G Muratori M et al Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. (2010) 160:1113–20. 10.1016/j.ahj.2010.09.003
17.
Elmariah S Palacios I McAndrew T Hueter I Inglessis I Baker J et al Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the placement of aortic transcatheter valves (PARTNER) trial (cohort A). Circ Cardiovasc Interv. (2013) 6:604–14. 10.1161/CIRCINTERVENTIONS.113.000650
18.
Løgstrup B Andersen H Thuesen L Christiansen E Terp K Klaaborg K et al Left ventricular global systolic longitudinal deformation and prognosis 1 year after femoral and apical transcatheter aortic valve implantation. J Am Soc Echocardiogr. (2013) 26:246–54. 10.1016/j.echo.2012.12.006
19.
Deste W Gulino S Zappulla P Iacono F Sicuso R Indelicato A et al Early recovery of left ventricular systolic function after transcatheter aortic valve implantation. J Cardiovasc Echogr. (2018) 28:166–70. 10.4103/jcecho.jcecho_13_18
20.
Sato K Kumar A Jones B Mick S Krishnaswamy A Grimm R et al Reversibility of cardiac function predicts outcome after transcatheter aortic valve replacement in patients with severe aortic stenosis. J Am Heart Assoc. (2017) 6:e005798. 10.1161/JAHA.117.005798
21.
Popma J Adams D Reardon M Yakubov S Kleiman N Heimansohn D et al Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. (2014) 63:1972–81. 10.1016/j.jacc.2014.02.556
22.
Harrington C Sorour N Gottbrecht M Nagy A Kovell L Truong V et al Effect of transaortic valve intervention for aortic stenosis on myocardial mechanics. Am J Cardiol. (2021) 146:56–61. 10.1016/j.amjcard.2021.01.021
23.
Mangano D Van Dyke D Ellis R . The effect of increasing preload on ventricular output and ejection in man. Limitations of the Frank-Starling mechanism. Circulation. (1980) 62:535–41. 10.1161/01.cir.62.3.535
24.
Krayenbuehl H Hess O Monrad E Schneider J Mall G Turina M . Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. (1989) 79:744–55. 10.1161/01.cir.79.4.744
25.
Everett R Tastet L Clavel M Chin C Capoulade R Vassiliou V et al Progression of hypertrophy and myocardial fibrosis in aortic stenosis: a multicenter cardiac magnetic resonance study. Circ Cardiovasc Imaging. (2018) 11:e007451. 10.1161/CIRCIMAGING.117.007451
26.
Laursen K Carter-Storch R Pellikka P Ali M Mogensen N Øvrehus K et al Changes in afterload and contractility in patients with severe aortic stenosis after transcatheter aortic valve replacement. Eur Heart J Imaging Methods Pract. (2025) 3:qyaf063. 10.1093/ehjimp/qyaf063
27.
Aurigemma G Harrington C . Left ventricular systolic function and outcome in aortic stenosis: the long- and short-axis of it. JACC Cardiovasc Imaging. (2020) 13:370–3. 10.1016/j.jcmg.2019.03.010
28.
Baumgartner H Hung J Bermejo J Chambers J Edvardsen T Goldstein S et al Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. (2017) 30:372–92. 10.1016/j.echo.2017.02.009
29.
Shimizu G Zile M Blaustein A Gaasch W . Left ventricular chamber filling and midwall fiber lengthening in patients with left ventricular hypertrophy: overestimation of fiber velocities by conventional midwall measurements. Circulation. (1985) 71:266–72. 10.1161/01.cir.71.2.266
30.
de Simone G Devereux R Roman M Ganau A Saba P Alderman M et al Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol. (1994) 23:1444–51. 10.1016/0735-1097(94)90390-5
31.
Devereux R Alonso D Lutas E Gottlieb G Campo E Sachs I et al Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. (1986) 57:450–8. 10.1016/0002-9149(86)90771-x
32.
Lang R Badano L Mor-Avi V Afilalo J Armstrong A Ernande L et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39. 10.1016/j.echo.2014.10.003
33.
Aurigemma G Silver K McLaughlin M Mauser J Gaasch W . Impact of chamber geometry and gender on left ventricular systolic function in patients > 60 years of age with aortic stenosis. Am J Cardiol. (1994) 74:794–8. 10.1016/0002-9149(94)90437-5
34.
Lam C Roger V Rodeheffer R Bursi F Borlaug B Ommen S et al Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. (2007) 115:1982–90. 10.1161/CIRCULATIONAHA.106.659763
35.
Saba P Ganau A Devereux R Pini R Pickering T Roman M . Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens. (1999) 17:1007–15. 10.1097/00004872-199917070-00018
36.
Chantler P Lakatta E . Arterial-ventricular coupling with aging and disease. Front Physiol. (2012) 3:90. 10.3389/fphys.2012.00090
37.
Dumonteil N Vaccaro A Despas F Labrunee M Marcheix B Lambert E et al Transcatheter aortic valve implantation reduces sympathetic activity and normalizes arterial spontaneous baroreflex in patients with aortic stenosis. JACC Cardiovasc Interv. (2013) 6:1195–202. 10.1016/j.jcin.2013.06.012
38.
Nitta K Fukuda Y Takahari K Takeda A Higashihara T Morita Y et al Factors influencing cardiac sympathetic nervous function in patients with severe aortic stenosis: assessment by 123I-metaiodobenzylguanidine myocardial scintigraphy. Heart Lung Circ. (2022) 31:671–7. 10.1016/j.hlc.2021.09.022
39.
Armour J . Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res. (1999) 41:41–54. 10.1016/s0008-6363(98)00252-1
40.
Floras J . Arterial baroreceptor and cardiopulmonary reflex control of sympathetic outflow in human heart failure. Ann N Y Acad Sci. (2001) 940:500–13. 10.1111/j.1749-6632.2001.tb03701.x
41.
Borovac J D'Amario D Bozic J Glavas D . Sympathetic nervous system activation and heart failure: current state of evidence and the pathophysiology in the light of novel biomarkers. World J Cardiol. (2020) 12:373–408. 10.4330/wjc.v12.i8.373
42.
Kadoya Y Zen K Tamaki N Nakamura S Fujimoto T Yashige M et al Serial changes in cardiac sympathetic nervous function after transcatheter aortic valve replacement: a prospective observational study using 123I-meta-iodobenzylguanidine imaging. J Nucl Cardiol. (2022) 29:2652–63. 10.1007/s12350-021-02799-0
43.
von Anrep G . On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol. (1912) 45:307–17. 10.1113/jphysiol.1912.sp001553
44.
Allen D Kurihara S . The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. (1982) 327:79–94. 10.1113/jphysiol.1982.sp014221
45.
Alvarez B Pérez N Ennis I Camilión de Hurtado M Cingolani H . Mechanisms underlying the increase in force and Ca(2+) transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res. (1999) 85:716–22. 10.1161/01.res.85.8.716
46.
Cingolani H Pérez N Cingolani O Ennis I . The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol. (2013) 304:175–82. 10.1152/ajpheart.00508.2012
47.
van Zalen J Badiani S Hart L Marshall A Beale L Brickley G et al The importance of contractile reserve in predicting exercise tolerance in asymptomatic patients with severe aortic stenosis. Echo Res Pract. (2019) 6:43–52. 10.1530/ERP-19-0005
48.
Monge García M Santos A . Understanding ventriculo-arterial coupling. Ann Transl Med. (2020) 8:795. 10.21037/atm.2020.04.10
49.
Pedersen A Povlsen J Rasmussen V Frederiksen C Christiansen E Terkelsen C et al Prognostic implications of residual left ventricular hypertrophy and systolic dysfunction in aortic stenosis following transcatheter aortic valve replacement. Int J Cardiovasc Imaging. (2023) 39:13–22. 10.1007/s10554-022-02688-8
50.
Axell R Giblett J White P Klein A Hampton-Til J O'Sullivan M et al Stunning and right ventricular dysfunction is induced by coronary balloon occlusion and rapid pacing in humans: insights from right ventricular conductance catheter studies. J Am Heart Assoc. (2017) 6:e005820. 10.1161/JAHA.117.005820
51.
Yotti R Bermejo J Gutiérrez-Ibañes E Pérez del Villar C Mombiela T Elízag J et al Systemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacement. J Am Coll Cardiol. (2015) 65:423–33. 10.1016/j.jacc.2014.10.067
Summary
Keywords
aortic stenosis, homeometric autoregulation, ventricular adaptation, transcatheter aortic valve replacement, echocardiography
Citation
Doerr AJ, Gottbrecht M, Kakouros N, Parker MW, Harrington CM and Aurigemma GP (2026) Homeometric autoregulation in severe aortic stenosis: insights from transcatheter aortic valve replacement. Front. Cardiovasc. Med. 12:1677372. doi: 10.3389/fcvm.2025.1677372
Received
31 July 2025
Revised
19 November 2025
Accepted
24 November 2025
Published
12 January 2026
Volume
12 - 2025
Edited by
Wei-Hsian Yin, Cheng Hsin General Hospital, Taiwan
Reviewed by
Audrey Adji, Victor Chang Cardiac Research Institute, Australia
Tien Ping Tsao, Cheng Hsin General Hospital, Taiwan
Updates
Copyright
© 2026 Doerr, Gottbrecht, Kakouros, Parker, Harrington and Aurigemma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Matthew Gottbrecht matthew.gottbrecht@umassmemorial.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.