Abstract
Graphical Abstract
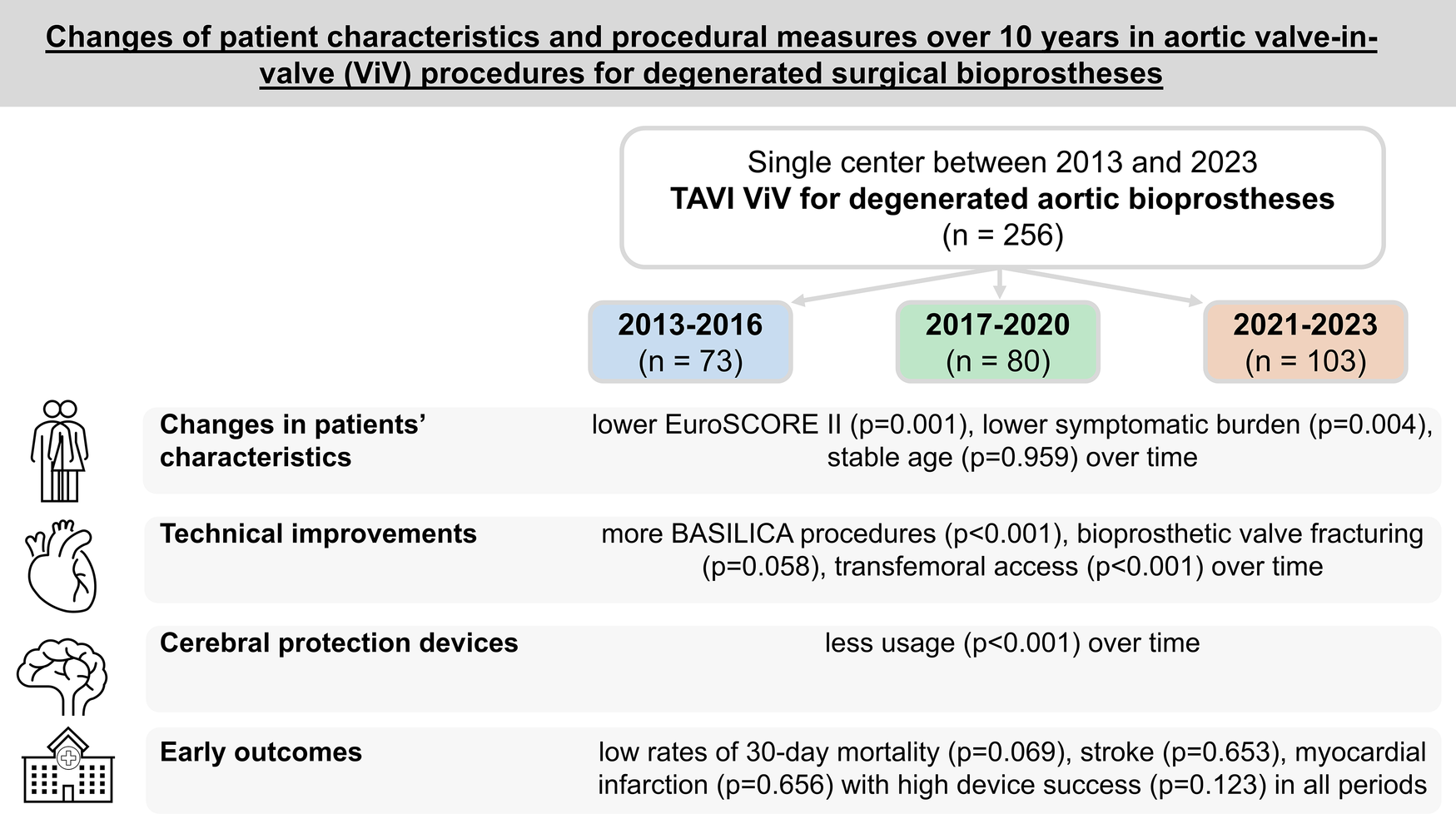
This single-center study investigates changes in patient characteristics and procedural measures over a period of 10 years in aortic valve-in-valve procedures for treatment of degenerated surgical bioprostheses. Baseline, procedural, early outcome, and echocardiographic parameters were retrospectively compared between three time periods (period 1: 2013–2016, period 2: 2017–2020, and period 3: 2021–2023). Main changes in characteristics, technical improvements, and early outcomes in patients are illustrated.
Objectives:
Aortic valve-in-valve procedures for treatment of degenerated surgical bioprostheses are an established therapy. In this study, we evaluated how the risk profiles, procedural approaches, and early outcomes for patients in these procedures changed over a period of 10 years.
Methods:
Baseline, procedural, early outcome, and echocardiographic parameters were retrospectively compared between three time periods (period 1: 2013–2016, period 2: 2017–2020, and period 3: 2021–2023).
Results:
Between 2013 and 2023, a total of 256 patients underwent valve-in-valve implantation in degenerated aortic bioprostheses at our center with a steady increase of patient numbers. The median age of the patients was 78.0 (interquartile range 72.2–82.4) years and remained unchanged over time. EuroSCORE II presented lower risk profiles in later periods (p = 0.001). Access proportions changed with transfemoral access in 100% of patients in period 3 (p < 0.001). Rates of BASILICA procedures (0% vs. 17.5% vs. 19.4%; p < 0.001) and valve fracturing steadily increased (0% vs. 6.3% vs. 7.8%; p = 0.058). Cerebral protection device use presented a distinct decline to 18.4% in period 3 (p < 0.001). Procedure time and length of intensive care unit stay decreased significantly over time. Early outcome parameters such as rates of permanent pacemaker implantation, bleeding, acute kidney injury, disabling stroke (0.0% vs. 1.3% vs. 1.0%; p = 0.653), and device success (91.8% vs. 92.5% vs. 98.1%; p = 0.123) showed no significant changes over time. The rate of 30-day mortality decreased to 0% in period 3 (p = 0.069).
Conclusion:
Advancements in technical approaches have expanded eligibility for patients previously considered unsuitable for aortic valve-in-valve procedures. In this study, it was found that early outcomes for patients were excellent, with improvement over time, highlighting the clinical efficacy and safety of the procedures.
Introduction
Aortic valve-in-valve (ViV) procedures for treatment of degenerated surgical bioprostheses are an established therapy. Because of technical improvements in transcatheter heart valves (THVs), increasing operator experience, and development of measures addressing unfavorable anatomies, ViV procedures have been increasingly performed over the last decade, even in patients previously considered not suitable for such procedures.
Unfavorable anatomies in aortic ViV procedures consist of low coronary ostia distance from the sewing ring of the index prosthesis with consecutive higher risk of coronary obstruction, especially in surgical bioprostheses with externally mounted leaflets, small bioprostheses (diameter ≤ 23 mm) (1) with anticipated detrimental hemodynamics after ViV in terms of elevated transvalvular pressure gradients or even patient–prosthesis mismatch, and hostile anatomies of iliac vessels in terms of severe calcification and/or tortuosity, a known risk factor for vascular complications and adverse outcomes following transcatheter aortic valve implantation (TAVI) for treatment of severe symptomatic native aortic valve stenosis.
To treat those patients at particularly high risk for periprocedural complications during the performance of ViV, several procedural refinements and changes in procedural measures have been implemented over the last decade: Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) was introduced in 2019 (2) to prevent coronary obstruction, which is associated with a mortality rate of up to 50% (3). Furthermore, bioprosthetic valve fracturing (BVF) and/or utilization of supraannular valves with a rather high implantation height was found to potentially avoid elevated postprocedural transvalvular pressure gradients/patient–prosthesis mismatch after ViV with consecutive early degeneration of implanted THV (4–6). Improvements in percutaneous vascular closure systems, as well as reduction of THV sheath sizes for transfemoral access, result in reduced vascular complication rates, which is of particular importance, because major vascular complications lead to prolonged hospital stay, higher mortality, as well as higher rates of bleeding complications, access site infections, and renal impairment (7–9).
To assess the influence of the described new techniques for aortic ViV procedures and illustrate the changing patient profiles and utilized periprocedural measures in a special subset of patients, we herein evaluate all aortic ViV procedures over a period of 10 years at our center.
Materials and methods
Patient cohort and study design
All patients who underwent TAVI in degenerated aortic bioprostheses as a ViV procedure at our center during the period between 2013 and 2023 were included in this analysis.
To determine changes in the risk profiles, procedural data, and outcomes of patients over time, the cohort was divided into three subgroups based on the date of procedure (period 1: 2013–2016, period 2: 2017–2020, and period 3: 2021–2023).
Study procedure
Institutional standards for aortic ViV procedures have been described previously (10). In this study, access routes were planned and executed based on multislice computed tomography examination. Transfemoral access with local anesthesia was the first-line approach when possible. Patients in whom intentional leaflet laceration was performed, general anesthesia was used to enable transesophageal echocardiography guidance. Utilized vascular closure systems consisted of suture-based devices (ProGlide/ProStyle/ProStar; Abbott, Abbott Park, IL, USA) or a collagen plug-based device (MANTA; Teleflex, Wayne, PA, USA). In recent years, all those patients in whom intentional leaflet laceration was not performed were provided with single femoral puncture as interventional access, and non-interventional access was gained via the right-sided radial artery. The target height for THV valve deployment was alignment of both lower stent rims to create optimal postinterventional hemodynamics with full stent deployment of the THV. Intentional leaflet laceration was performed as previously described (11). Bioprosthetic valve fracture (BVF) was performed according to the discretion of the surgeons and in recent years after THV implantation. The application of cerebral embolic protection was left to operator discretion and consisted of the Sentinel device (Boston Scientific, Marlborough, MA, USA). In recent years, all patients were postoperatively transferred to a holding area until the first postoperative day and the stay until discharge was completed in the ward (10).
Statistical analysis
Outcome parameters were adjudicated in accordance with the updated standardized Valve Academic Research Consortium-3 (VARC-3) definitions (12).
Continuous variables were reported as medians with interquartile range (IQR; 25th percentile and 75th percentile) and compared using the Kruskal–Wallis test. Binary variables were shown as counts and frequencies and compared using the χ2 test.
Normality of continuous data deviation was tested by using the Shapiro–Wilk test.
Parameters predicting the VARC-3 composite endpoint device success were identified using logistic regression analysis. The parameters included in the model were the time period of the procedure, the femoral access route, the choice of THV type, cerebral protection, preballooning, postballooning, and the BASILICA procedure. Odds ratios, 95% confidence intervals (CI), and p-values were reported from this model.
For binary outcome parameters (including 30-day mortality, myocardial infarction, disabling stroke, acute kidney injury stage II or III, permanent pacemaker implantation, major vascular complications, type 3 or 4 bleeding, and device success), proportions were calculated for each study period. Exact 95% CIs were derived using binomial tests to provide reliable estimates of event rates independent of sample size distribution.
A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using the statistical software RStudio 2023.12.1 (R Foundation for Statistical Computing, Vienna, Austria). Graphs were created using GraphPad Prism, version 9.5.1 (GraphPad Software, San Diego, CA, USA).
Results
Study population
Between 2013 and 2023, a total of 256 patients underwent TAVI as a ViV procedure in degenerated bioprostheses at our center. Of these, n = 73 were assigned to the period 1 group (2013–2016), n = 80 to the period 2 group (2017–2020), and n = 103 to the period 3 group (2021–2023).
Baseline characteristics
The median age of the patients was 78.0 (IQR 72.2–82.4) years with a range of 48.3–96.2 years without any differences between the three groups. Risk stratification using EuroSCORE II presented a decrease of perioperative risk from 11.8% (IQR 6.0–15.5) in period 1 to 6.4% (IQR 2.8–16.0) in period 3 (p = 0.001). Patients in later periods presented with a lower symptomatic burden at the time of ViV as represented by lower percentages of New York Heart Association (NYHA) functional classes III or IV (89.0% vs. 77.5% vs. 67.3%; p = 0.004).
The durability of index surgical bioprostheses increased significantly over time [8.0 (IQR 6.0–11.0) vs. 11.0 (IQR 8.0–14.3) vs. 12.0 (IQR 9.0–14.0) years; p < 0.001]. Accordingly, a commonly considered early deteriorating surgical bioprosthesis with externally mounted leaflets presented the highest proportion as index valve in period 1 (34.2% vs. 23.8% vs. 7.8%; p < 0.001). An increase of deteriorated Perceval sutureless aortic valves (LivaNova, London, United Kingdom) treated by ViV was seen over time (0% vs. 1.3% vs. 8.7%; p = 0.004). Overall, 20.7% of patients had a surgical valve size of ≤21 mm, while in 36.7% of surgical valves, the true inner diameter measured ≤19 mm. The ViV procedure was performed in 49.6% of patients because of a structural valve deterioration of the surgically implanted aortic bioprostheses, which presented as a combined lesion with at least moderate stenosis and a concomitant moderate regurgitation component.
Detailed baseline characteristics are reported in Table 1.
Table 1
| Variable | All (n = 256) | 2013–2016 (n = 73) | 2017–2020 (n = 80) | 2021–2023 (n = 103) | p-Value |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 78.0 (72.2–82.4) | 78.5 (72.7–83.0) | 77.8 (73.5–82.4) | 77.6 (72.0–82.2) | 0.959 |
| Age (years), range | 48.3–96.2 | 51.1–89.8 | 50.7–90.1 | 48.3–96.2 | |
| Male sex, n (%) | 149 (58.4) | 35 (47.9) | 52 (65.0) | 62 (60.2) | 0.115 |
| BMI (kg/m2), median (IQR) | 26.5 (23.7–30.0) | 27.3 (23.7–30.1) | 26.5 (24.6–29.8) | 26.1 (23.3–29.7) | 0.656 |
| EuroSCORE II (%), median (IQR) | 8.1 (4.9–14.5) | 11.8 (6.0–15.5) | 6.3 (4.0–9.9) | 6.4 (2.8–16.0) | 0.001 |
| Severely reduced ejection fraction <30%, n (%) | 22 (8.6) | 6 (8.2) | 8 (10.0) | 7 (6.8) | 0.736 |
| Arterial hypertension, n (%) | 207 (80.9) | 59 (80.8) | 65 (81.3) | 83 (80.6) | 0.993 |
| Insulin-dependent diabetes mellitus, n (%) | 20 (7.8) | 6 (8.2) | 5 (6.3) | 9 (8.7) | 0.814 |
| Coronary artery disease, n (%) | 139 (54.3) | 48 (65.8) | 38 (47.5) | 53 (51.5) | 0.058 |
| Prior stroke, n (%) | 32 (12.5) | 14 (19.2) | 8 (10.0) | 10 (9.7) | 0.124 |
| Prior myocardial infarction, n (%) | 23 (9.0) | 7 (9.6) | 6 (7.5) | 10 (9.7) | 0.855 |
| Chronic lung disease, n (%) | 28 (10.9) | 13 (17.8) | 8 (10.0) | 7 (6.8) | 0.066 |
| Creatinine (mg/dL), median (IQR) | 1.17 (0.94–1.50) | 1.20 (0.93–1.47) | 1.30 (1.00–1.70) | 1.11 (0.92–1.39) | 0.116 |
| Dialysis, n (%) | 2 (0.8) | 1 (1.4) | 0 (0.0) | 1 (1.0) | 0.605 |
| NT-proBNP (ng/L), median (IQR) | 3,194.0 (1,387.3–7,615.3) | 3,853.0 (1,499.0–9,968.0) | 3,927.0 (1,559.0–7,778.0) | 2,977.5 (1,142.3–6,324.5) | 0.607 |
| NYHA stadium III or IV; n (%) | 195 (76.8) | 65 (89.0) | 62 (77.5) | 68 (67.3) | 0.004 |
| Any malignant disease, n (%) | 44 (17.2) | 17 (23.3) | 14 (17.5) | 13 (12.6) | 0.181 |
| Mean transvalvular gradient (mmHg), median (IQR) | 30.0 (18.0–39.0) | 33.0 (20.0–44.5) | 28.0 (18.0–36.0) | 29.0 (17.0–38.0) | 0.085 |
| Isolated at least moderate aortic valve stenosis, n (%) | 43 (16.8) | 12 (16.4) | 11 (13.8) | 20 (19.4) | 0.593 |
| Isolated at least moderate aortic valve regurgitation, n (%) | 77 (30.1) | 19 (26.0) | 25 (31.3) | 33 (32.0) | 0.667 |
| At least moderate aortic valve stenosis and regurgitation, n (%) | 127 (49.6) | 41 (56.2) | 40 (50.0) | 46 (44.7) | 0.322 |
| Time from index procedure (years), median (IQR) | 10.0 (7.0–14.0) | 8.0 (6.0–11.0) | 11.0 (8.0–14.3) | 12.0 (9.0–14.0) | <0.001 |
| Surgical valve type, n (%) | |||||
| Carpentier-Edwards Perimount, CE-SAV (Edwards Lifesciences, Irvine, CA, USA) |
62 (24.2) | 16 (21.9) | 17 (21.3) | 29 (28.2) | 0.481 |
| Medtronic Freestyle, Mosaic, Hancock (Medtronic, Minneapolis, MN, USA) |
87 (34.0) | 21 (28.8) | 29 (36.3) | 37 (35.9) | 0.538 |
| LivaNova Mitroflow (LivaNova, London, United Kingdom) |
52 (20.3) | 25 (34.2) | 19 (23.8) | 8 (7.8) | <0.001 |
| Sorin Pericarbon, Freedom Solo (Sorin Group, Milano, Italy) |
15 (5.9) | 7 (9.6) | 4 (5.0) | 4 (3.9) | 0.262 |
| St Jude Medical Trifecta (St. Jude Medical, St. Paul, MN, USA) |
10 (3.9) | 1 (1.4) | 3 (3.8) | 6 (5.8) | 0.322 |
| LivaNova Perceval (LivaNova, London, United Kingdom) | 10 (3.9) | 0 (0.0) | 1 (1.3) | 9 (8.7)) | 0.004 |
| Other/unknown | 20 (7.8) | 3 (4.1) | 7 (8.8) | 10 (9.7) | 0.368 |
| Stented surgical valve prosthesis, n (%) | 209 (81.6) | 64 (87.7) | 65 (81.3) | 80 (77.7) | 0.137 |
| Stentless surgical valve prosthesis, n (%) | 21 (8.2) | 8 (10.9) | 6 (7.5) | 7 (6.8) | 0.220 |
| Sutureless surgical valve prosthesis, n (%) | 10 (3.9) | 0 (0.0) | 1 (1.3) | 9 (8.7) | 0.004 |
| Surgical valve size ≤21 mm, n (%) | 53 (20.7) | 17 (23.3) | 19 (23.8) | 17 (16.5) | 0.487 |
| Surgical valve true ID ≤19 mm, n (%) | 94 (36.7) | 29 (39.7) | 30 (37.5) | 35 (34.0) | 0.298 |
Baseline characteristics.
IQR, interquartile range; BMI, body mass index; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; ID, inner diameter.
Procedural data
Procedure time declined over the study period [100.0 (83.0–123.0) vs. 102.5 (79.8–162.0) vs. 68.0 (50.0–105.0) minutes; p < 0.001], despite an increase of concomitant procedures (BASILICA, BVF).
Access proportions changed distinctly with an increase in the use of the transfemoral approach (75.3% in period 1, 98.8% in period 2, and 100% in period 3; p < 0.001). Correspondingly, the rate of transapical access declined from 17.8% (n = 13) in period 1 to 0% (n = 0) in later periods (p < 0.001).
While in periods 1 and 2, the most commonly used THV consisted of a supra-annular self-expanding (SE) THV, a shift to an intra-annular SE THV was seen in period 3 [5.5% (n = 4) vs. 1.3% (n = 1) vs. 12.6% (n = 13); p = 0.010]. The most frequently implanted THVs in the total cohort were Medtronic CoreValve/Evolut/Evolut Pro (Medtronic, Minneapolis, MN, USA) with a rate of 61.3% (n = 157), followed by Edwards Sapien/Sapien XT/Sapien 3/Sapien 3 Ultra (Edwards Lifesciences, Irvine, CA, USA) with a rate of 23.4% (n = 60), NVT Allegra (NVT, Vancouver, BC, Canada) with an implantation rate of 6.6% (n = 17), and Abbott Navitor (Abbott Park, IL, USA) with 5.1% (n = 13).
Over time, more BASILICA procedures for the prevention of coronary obstruction were performed (0% vs. 17.5% vs. 19.4%; p < 0.001), and rates of valve fracturing steadily increased (0% vs. 6.3% vs. 7.8%; p = 0.058).
The utilization of cerebral protection devices witnessed a distinct decline in period 3 (56.2% vs. 68.8% vs. 18.4%; p < 0.001).
Detailed procedural data are summarized in Table 2.
Table 2
| Variable | All (n = 256) | 2013–2016 (n = 73) | 2017–2020 (n = 80) | 2021–2023 (n = 103) | p-Value |
|---|---|---|---|---|---|
| Procedure time (min), median (IQR) | 90.0 (65.0–130.0) | 100.0 (83.0–123.0) | 102.5 (79.8–162.0) | 68.0 (50.0–105.0) | <0.001 |
| Access, n (%) | |||||
| Transfemoral | 237 (92.6) | 55 (75.3) | 79 (98.8) | 103 (100.0) | <0.001 |
| Transapical | 13 (5.1) | 13 (17.8) | 0 (0) | 0 (0) | <0.001 |
| Transaxillary | 4 (1.6) | 3 (4.1) | 1 (1.3) | 0 (0) | 0.092 |
| Transaortic | 2 (0.8) | 2 (2.7) | 0 (0) | 0 (0) | 0.080 |
| Implanted THV, n (%) | |||||
| Edwards Sapien/Sapien XT/Sapien 3/Sapien 3 Ultra (Edwards Lifesciences, Irvine, CA, USA) |
60 (23.4) | 11 (15.1) | 14 (17.5) | 35 (34.0) | 0.005 |
| Medtronic CoreValve/Evolut/Evolut Pro (Medtronic, Minneapolis, MN, USA) | 157 (61.3) | 54 (74.0) | 48 (60.0) | 55 (53.4) | 0.021 |
| JenaValve (JenaValve, Munich, Germany) |
3 (1.2) | 3 (4.1) | 0 (0) | 0 (0) | 0.022 |
| SJM Portico (St. Jude Medical, St. Paul, MN, USA) | 5 (2.0) | 4 (5.5) | 1 (1.3) | 0 (0) | 0.030 |
| Medtronic Engager (Medtronic, Minneapolis, MN, USA) | 1 (0.4) | 1 (1.4) | 0 (0) | 0 (0) | 0.284 |
| NVT Allegra (NVT, Vancouver, BC, Canada) | 17 (6.6) | 0 (0) | 17 (21.3) | 0 (0) | <0.001 |
| Abbott Navitor (Abbott Laboratories, Abbott Park, IL, USA) | 13 (5.1) | 0 (0) | 0 (0) | 13 (12.6) | <0.001 |
| SE THV intra-annular, n (%) | 18 (7.0) | 4 (5.5) | 1 (1.3) | 13 (12.6) | 0.010 |
| SE THV supra-annular, n (%) | 174 (68.0) | 54 (74.0) | 65 (81.3) | 55 (53.4) | <0.001 |
| BE THV intra-annular, n (%) | 60 (23.4) | 11 (15.1) | 14 (17.5) | 35 (34.0) | 0.005 |
| Preballooning, n (%) | 25 (9.8) | 5 (6.8) | 6 (7.5) | 14 (13.6) | 0.237 |
| Postballooning, n (%) | 129 (50.4) | 39 (53.4) | 45 (56.3) | 45 (43.7) | 0.200 |
| Contrast agent (mL), median (IQR) | 125.0 (74.5–188.3) | 127.0 (93.5–179.0) | 137.0 (99.0–199.5) | 95.0 (59.0–169.0) | 0.002 |
| Use of cerebral protection device, n (%) | 115 (44.9) | 41 (56.2) | 55 (68.8) | 19 (18.4) | <0.001 |
| Vascular closure system, n (%) | |||||
| MANTA (Teleflex, Wayne, PA, USA) | 118 (47.8) | 0 (0.0) | 37 (46.8) | 81 (82.7) | <0.001 |
| ProGlide (Abbott Laboratories, Abbott Park, IL, USA) | 59 (23.7) | 17 (23.3) | 41 (51.2) | 1 (1.0) | <0.001 |
| ProStyle (Abbott Laboratories, Abbott Park, IL, USA) | 13 (5.2) | 0 (0.0) | 0 (0.0) | 13 (13.2) | <0.001 |
| ProStar (Abbott Laboratories, Abbott Park, IL, USA) | 43 (17.3) | 41 (56.9) | 1 (1.3) | 1 (1.0) | <0.001 |
| BASILICA procedure, n (%) | 34 (13.3) | 0 (0.0) | 14 (17.5) | 20 (19.4) | <0.001 |
| BVF, n (%) | 13 (5.1) | 0 (0.0) | 5 (6.3) | 8 (7.8) | 0.058 |
Procedural data.
IQR, interquartile range; SE THV, self-expanding transcatheter heart valve; BE THV, balloon-expanding transcatheter heart valve; BASILICA, bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVI; BVF, bioprosthetic valve fracturing.
Outcome
The length of intensive care unit (ICU) stay significantly decreased over time [1.0 (1.0–3.0) vs. 1.0 (1.0–2.0) vs. 1.0 (1.0–1.0) days; p < 0.001].
Outcome parameters according to VARC-3, such as myocardial infarction [2.7% (95% CI 0.3%–9.5%) vs. 2.5% (95% CI 0.3%–8.7%) vs. 1.0% (95% CI 0.0%–5.4%); p = 0.656], disabling stroke [0.0% (95% CI 0.0%–4.9%) vs. 1.3% (95% CI 0.0%–6.8%) vs. 1.0% (95% CI 0.0%–5.4%); p = 0.653], type 3 or 4 bleeding [8.2% (95% CI 3.1–17.0%) vs. 12.5% (95% CI 6.2–21.8%) vs. 4.9% (95% CI 1.6–11.1%); p = 0.180], major vascular complications [0.0% (95% CI 0.0%–4.9%) vs. 3.8% (95% CI 0.8–10.6%) vs. 1.9% (95% CI 0.2%–6.7%); p = 0.248], permanent pacemaker implantation [5.5% (95% CI 1.5–13.4%) vs. 1.3% (95% CI 0.0%–6.8%) vs. 3.9% (95% CI 1.1%–9.8%); p = 0.352], or acute kidney injury stage II or III [4.1% (95% CI 0.9–11.5%) vs. 1.3% (95% CI 0.0%–6.8%) vs. 1.0% (95% CI 0.0%–5.4%); p = 0.294], did not show significant changes over time. In one instance, conversion to sternotomy was performed because of a ventricular perforation in a patient undergoing VIV with BASILICA.
The rates of at least moderate paravalvular leakage (PVL) following ViV were stable over time (0 (0.0%) vs. 1 (1.3%) vs. 1 (1.0%); p = 0.655). The postprocedural transvalvular mean gradient decreased over time [17.3 (IQR 13.3–22.8) vs. 12.0 (IQR 9.0–18.0) vs. 11.0 (IQR 7.3–17.0) mmHg; p < 0.001].
The rates of VARC-3 endpoint device success numerically increased over time without significant differences, from 91.8% [n = 67, (95% CI 83.0–96.9%)] in period 1 to over 92.5% [n = 74, (95% CI 84.4–97.2%)] in period 2 and to 98.1% [n = 101, (95% CI 93.2–99.8%)] in period 3 (p = 0.123), because of a lower number of patients with postprocedural transvalvular mean gradients ≥ 20 mmHg. The rate of 30-day mortality declined from 5.5% (95% CI 1.5–13.6%) in period 1 to 0% (95% CI 0.0%–3.5%) in period 3 without any significance.
Detailed outcome parameters are given in Table 3. A comparison of outcome parameters for the three time periods of ViV procedures is shown in Figure 1.
Table 3
| Variable | All (n = 256) | 2013–2016 (n = 73) | 2017–2020 (n = 80) | 2021–2023 (n = 103) | p-Value |
|---|---|---|---|---|---|
| NT-proBNP [ng/L], median (IQR) | 2,392.5 (849.5–5,546) | 5,645.5 (2,158.0–7,105.5) | 2,271.0 (763.0–9,162.0) | 2,216.0 (849.0–4,456.0) | 0.356 |
| Valve malposition, n (%) | 6 (2.3) | 3 (4.1) | 1 (1.3) | 2 (1.9) | 0.476 |
| Pericardial tamponade, n (%) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0.474 |
| Hemodynamic shock, CPR, n (%) | 6 (2.3) | 0 (0.0) | 5 (6.3) | 1 (1.0) | 0.019 |
| Coronary ostia occlusion, n (%) | 2 (0.8) | 1 (1.4) | 1 (1.3) | 0 (0.0) | 0.506 |
| Aortic root rupture, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | / |
| Conversion to CPB, n (%) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0.474 |
| Length of ICU stay (days), median (IQR) | 1.0 (1.0–1.25) | 1.0 (1.0–3.0) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | <0.001 |
| Major vascular complication, n (%) | 5 (2.0) | 0 (0.0) | 3 (3.8) | 2 (1.9) | 0.248 |
| Type 3 or 4 bleeding, n (%) | 21 (8.2) | 6 (8.2) | 10 (12.5) | 5 (4.9) | 0.180 |
| Permanent pacemaker implantation, n (%) | 9 (3.6) | 4 (5.5) | 1 (1.3) | 4 (3.9) | 0.352 |
| Acute kidney injury stage II or III, n (%) | 5 (2.0) | 3 (4.1) | 1 (1.3) | 1 (1.0) | 0.294 |
| Myocardial infarction, n (%) | 5 (2.0) | 2 (2.7) | 2 (2.5) | 1 (1.0) | 0.656 |
| Disabling stroke, n (%) | 2 (0.8) | 0 (0.0) | 1 (1.3) | 1 (1.0) | 0.653 |
| Postprocedural mean gradient (mmHg), median (IQR) | 14.0 (9.0–19.0) | 17.3 (13.3–22.8) | 12.0 (9.0–18.0) | 11.0 (7.3–17.0) | <0.001 |
| PVL ≥ moderate, n (%) | 2 (0.8) | 0 (0.0) | 1 (1.3) | 1 (1.0) | 0.655 |
| VARC Device Success, n (%) | 242 (94.5) | 67 (91.8) | 74 (92.5) | 101 (98.1) | 0.123 |
| 30-day mortality, n (%) | 7 (2.7) | 4 (5.5) | 3 (3.8) | 0 (0.0) | 0.069 |
Outcome parameters at 30 days.
IQR, interquartile range; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; CPR, cardiopulmonary resuscitation; ICU, intensive care unit; PVL, paravalvular leakage; VARC, Valve Academic Research Consortium.
Figure 1
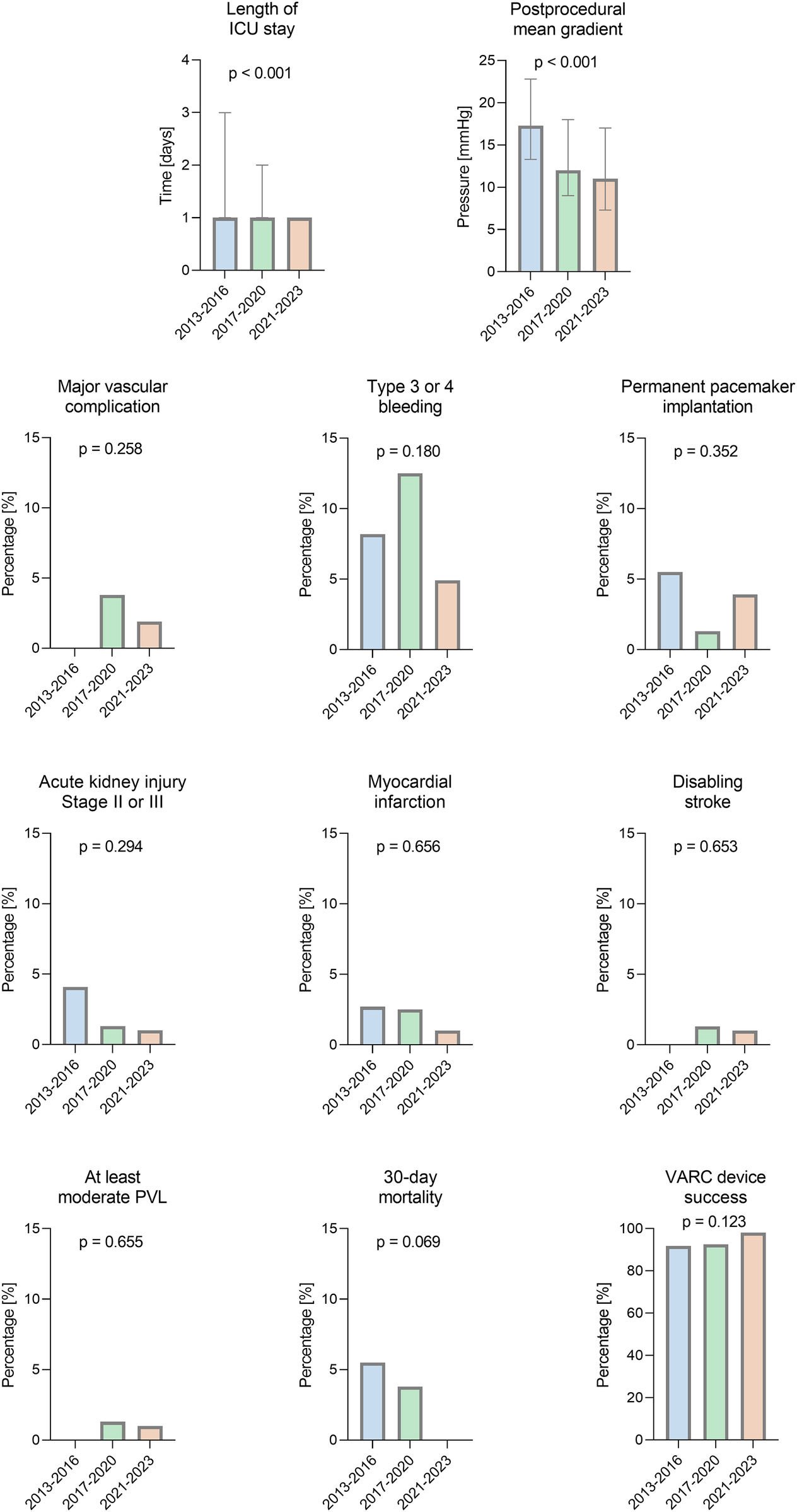
Comparison of outcomes for three time periods of valve-in-valve procedures. VARC-3 endpoints of ViV procedures for degenerated bioprostheses between 2013 and 2023 at our center. PVL, paravalvular leakage; ICU, intensive care unit.
Procedural changes within the studied time period were analyzed for their impact on the VARC-3 endpoint “device success.” None of the examined variables (time period, BE THV, SE THV, transfemoral access, cerebral protection, preballooning, postballooning, BASILICA procedure) were identified as an independent factor predicting the endpoint device success.
A forest plot of the multivariate regression model used to identify the factors predicting the VARC-3 composite endpoint device success is shown in Figure 2.
Figure 2
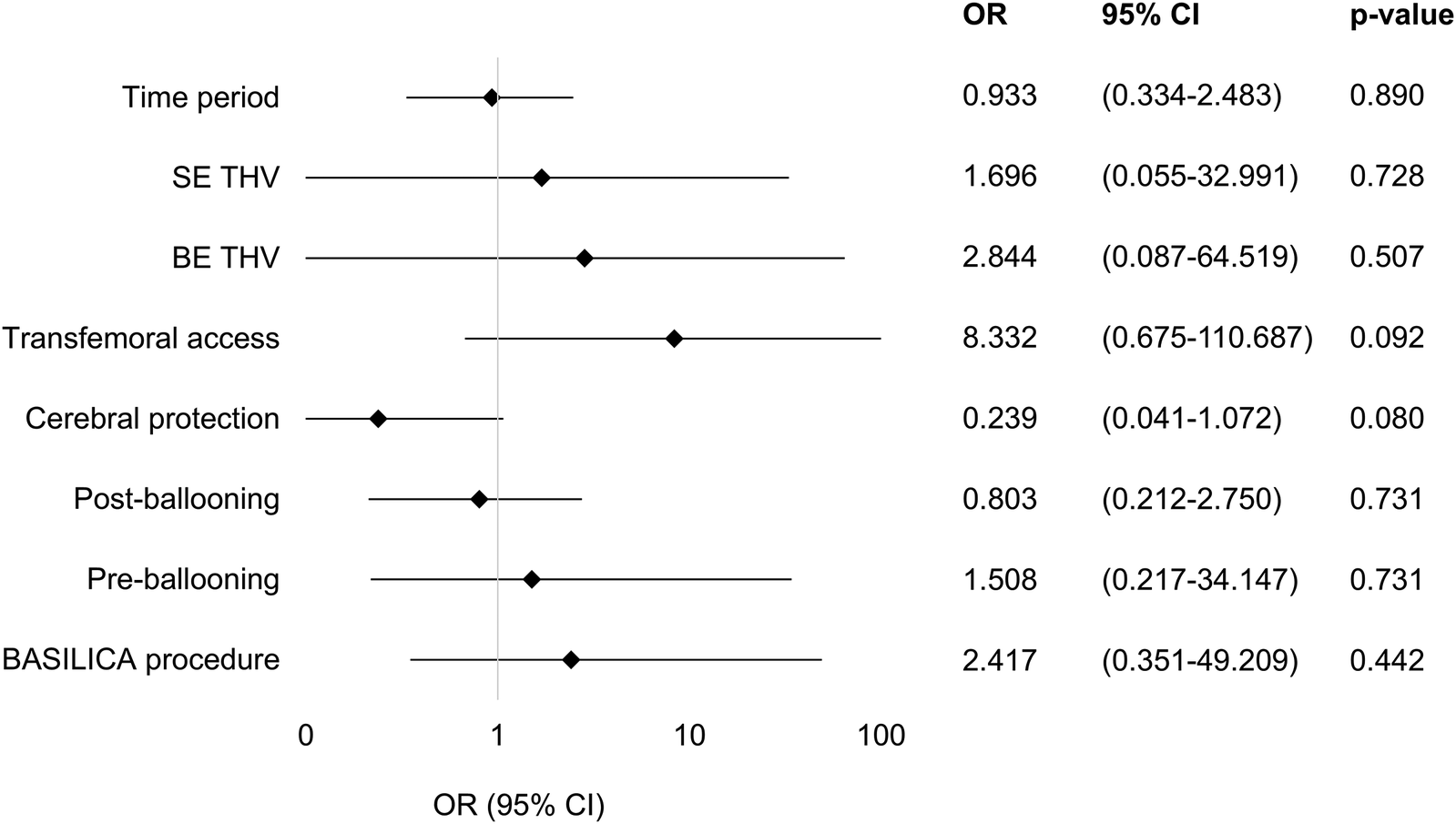
Factors predicting VARC-3 endpoint device success. A forest plot of the multivariate regression model used to identify the factors predicting the VARC-3 composite endpoint device success. Odds ratios and 95% confidence intervals of all tested parameters in the model are shown using a logarithmic x-axis. None of the variables included in the model were identified as independent predicting device success. OR, odds ratio; CI, confidence interval; SE THV, self-expanding transcatheter heart valve; BE THV, balloon-expanding transcatheter heart valve; BASILICA, bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVI.
Echocardiographic midterm results 12 months after implantation
An echocardiographic follow-up 12 months after ViV determined a mean transvalvular gradient of 12 (8–17) mmHg in the total cohort without significant differences between time periods. There was no transvalvular regurgitation in 96.8% of all patients, and none of the patients presented with a moderate or severe regurgitation.
Symptomatic burden represented by NYHA functional classes showed no significant differences between the patient groups (p = 0.366).
Detailed echocardiographic midterm results at 12 months after implantation are presented in Table 4.
Table 4
| Variable | All (n = 63) | 2013–2016 (n = 27) | 2017–2020 (n = 18) | 2021–2023 (n = 18) | p-Value |
|---|---|---|---|---|---|
| Mean transvalvular gradient (mmHg), median (IQR) | 12 (8–17) | 13 (9.5–21) | 11 (8.3–12) | 9 (6–16.8) | 0.124 |
| Left ventricular ejection fraction, n (%) | 0.007 | ||||
| 45–54% | 10 (15.9) | 1 (3.7) | 7 (38.9) | 2 (11.1) | |
| 30–44% | 8 (12.7) | 3 (11.1) | 2 (11.1) | 3 (16.7) | |
| <30% | 3 (4.8) | 0 (0.0) | 3 (16.7) | 0 (0.0) | |
| Transvalvular regurgitation, n (%) | 0.623 | ||||
| None | 61 (96.8) | 26 (96.3) | 17 (94.4) | 18 (100.0) | |
| Trace | 2 (3.2) | 1 (3.7) | 1 (5.6) | 0 (0.0) | |
| Mild | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Paravalvular regurgitation, n (%) | 0.369 | ||||
| None | 48 (76.2) | 21 (77.8) | 12 (66.7) | 15 (83.3) | |
| Trace | 7 (11.1) | 2 (7.4) | 3 (16.7) | 2 (11.1) | |
| Mild | 8 (12.7) | 4 (14.8) | 3 (16.7) | 1 (5.6) | |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NYHA, n (%) | 0.366 | ||||
| II | 19 (30.2) | 7 (25.9) | 5 (27.8) | 7 (38.9) | |
| II-III | 9 (14.3) | 7 (25.9) | 2 (11.1) | 0 (0.0) | |
| III | 3 (4.8) | 1 (3.7) | 1 (5.6) | 1 (5.6) | |
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Follow-up transthoracic echocardiography 12 months after implantation.
IQR, interquartile range.
Discussion
This study highlights important temporal shifts in the practice and outcomes of ViV procedures. The main findings of this study are (I) aortic ViV procedures for degenerated bioprostheses showed significant changes in patient characteristics toward lower risk profiles and lower symptomatic burden with stable age over time, (II) advancements in technical approaches such as transfemoral access, BASILICA procedures, and valve fracturing have expanded eligibility for patients previously considered unsuitable for interventional treatment, leading to an increase of treated patients over time and improvement of hemodynamic outcome parameters, (III) the peak incidence of degenerated Mitroflow (LivaNova, London, United Kingdom) prostheses appears to have been reached, (IV) the impact of randomized controlled trials demonstrating the limited efficacy of cerebral protection devices has influenced clinical practice, (V) early outcomes such as device success, stroke, and 30-day mortality were found to be excellent in this study, with improvement over time, and 0% mortality was achieved in period 3, highlighting the clinical efficacy and safety of the ViV procedures.
Changes in patient characteristics
Patients presented with a significant decrease in risk profiles and a lower symptomatic burden in later time periods. A change in patient age could not be detected. These findings are in line with those of previous work from our group with reference to change in risk profiles and patient age in TAVI for symptomatic native aortic valve stenosis (13) and confirm the influence of randomized controlled trials comparing TAVI and SAVR in low-risk patients on clinical daily practice (NOTION, PARTNER 3, Evolut Low Risk trial) (14–16). Although redo SAVR is a safe therapy modality in contemporary practice (17), its inherently less invasive nature seems to play a crucial role in decision-making in the context of heart team discussions in this rather elderly patient collective.
The threshold for treating patients with ViV declined over time because of encouraging results and enhanced clinical safety over time. Therefore, patients in later time periods were treated earlier with less symptoms and lower-risk patients were treated with ViV because of the improved predictability of hemodynamic results.
Moreover, analyses have shown that the durability of the THV extends up to 10 years, which is similar to that of surgically implanted bioprostheses (18). Because the mean patient age of the investigated patient cohort is above the mean life expectancy of men and slightly below the mean life expectancy of women in Germany, it can be assumed that a majority of patients in the investigated patient cohort who underwent ViV will not outlive the implanted THV.
Within the context of aortic valve stenosis, the relevance of lifetime management remains particularly important for younger patients. Our results suggest that younger patients are still not increasingly undergoing TAVI as ViV procedures in degenerated aortic bioprostheses and rather receive redo SAVR at our center (19). This approach aligns with the current concepts of lifetime valve management, emphasizing individualized treatment strategies based on patient age and clinical risk. In younger patients with acceptable surgical risk, redo SAVR remains the preferred option to optimize long-term durability. With increasing risk profiles, ViV TAVI provides a less invasive alternative with favorable safety outcomes. However, in patients for whom a third intervention may be anticipated, the use of intra-annular transcatheter valves should be considered to facilitate a potential second ViV procedure in the future.
Periprocedural techniques
Over the last few years, reduced sheath sizes and improvements in delivery systems have made transfemoral access applicable to a higher number of patients. Consequently, the significance of alternative access routes, which are associated with increased perioperative mortality and morbidity, has declined (20). In this study, a shift toward transfemoral access was seen, with 100% transfemoral access in contemporary practice, accompanied by a shift to a second access via the radial artery. These modalities certainly contributed to the safety of ViV, with a decline, although not significant, in bleeding and vascular complications (21).
Over time, a significant increase of concomitant BASILICA and BVF procedures was seen in our ViV cohort. Most likely, these concomitant procedures contributed to the herein seen increase of patient numbers by expanding approachable anatomies for ViV. Specifically, patients with bioprostheses with externally mounted leaflets (MitroFlow, Trifecta), shallow sinuses of Valsalva, or a low valve to coronary distance were commonly considered unsuitable for ViV. However, with the implementation of BASILICA, ViV became applicable to those patients without a significant increase in perioperative risk (22). Furthermore, an increase in device success rates was seen accompanied by a steady decrease in postoperative transvalvular pressure gradients, which is most probably a consequence of higher rates of BVF in later time periods, sophisticated implant techniques using the cusp-overlap technique, and alignment of the proximal THV stent at the highest point of the bioprosthetic stent.
The cusp-overlap technique, initially developed for native TAVI with SE THV to optimize implantation height and reduce PVL and pacemaker rates, is of lesser relevance in ViV procedures. In ViV using SE THV, however, it may assist in accurate positioning by aligning the THV stent frame with the surgical bioprosthesis (“stent-on-stent”). For BE THV, cusp-overlap views are generally not necessary, as the radiopaque surgical stent frame provides sufficient fluoroscopic guidance during deployment.
Although not all bioprosthetic stents are fracturable, it has been shown that BVF leads to improved hemodynamics after ViV (23). Furthermore, the cusp-overlap technique used in TAVI for severe symptomatic aortic valve stenosis has been shown to improve the prediction of implantation height, leading to a subsequent reduction in PVL and permanent pacemaker implantation rates (24).
In several studies, implantation of SE THV in degenerated bioprostheses was associated with lower postprocedural transvalvular gradients compared with BE THV (25, 26). A subanalysis of the VIVID registry identified intra-annular THV design as an independent predictor for elevated postprocedural gradients (5). The rate of survival following SE or BE THV for ViV procedures was found to be similar (25). In this study, preferred implanted valves consisted of SE THV. However, the proportion of BE THV increased over time. Early mortality rates of patients did not change significantly over time. In the 12-month echocardiographic follow-up, no significant difference in the transvalvular gradients was observed across different time periods.
Although the proportion of small surgical bioprostheses remained unchanged over time, the persistently high device success rate observed in this cohort may reflect the early adoption of supra-annular SE THV in small surgical valves and a cautious patient selection strategy. These factors likely contributed to favorable postprocedural hemodynamics, even before BVF and other adjunctive techniques became routinely implemented.
Index bioprostheses
Bioprostheses with externally mounted leaflets were shown to have a higher incidence of early structural valve deterioration leading to higher reoperation rates when compared with bioprostheses with internally mounted leaflets. Furthermore, these valves demonstrated a higher all-cause mortality (27). In this study, we observed a peak incidence of degenerated bioprostheses with externally mounted leaflets in period 1. The decline of ViV for these specific valve types in later time periods may be explained by a reduced implantation rate as a direct consequence of these findings. Furthermore, an increase of sutureless valves treated by ViV was seen, a finding that is worth further observation.
Cerebral protection
Several randomized controlled trials have demonstrated the limited efficacy of cerebral protection devices in TAVI (28–31). In our study, a direct impact of these findings on clinical practice in later time periods was seen without compromising patient safety in terms of postprocedural stroke.
While the utilization of cerebral embolic protection devices has significantly decreased over time in our cohort, it remains higher than in recent randomized controlled trials investigating TAVI for severe symptomatic aortic valve stenosis in contemporary practice (DEDICATE trial) (32).
Clinical outcomes
In this cohort, excellent 30-day clinical outcomes with a device success rate of 98.1% and a mortality rate of 0% in time period 3 without significant changes over time were detected. Procedure duration presented with a significant decrease over time. ICU stay decreased significantly, mainly because of reduced ICU times in contemporary TAVI practice. At 12 months after ViV, similar transvalvular mean gradients and rates of PVL were described in echocardiographic follow-up.
Compared with a meta-analysis of 5,500 patients treated with ViV procedures, rates of 30-day mortality, stroke, and permanent pacemaker implantation are lower in this analysis, highlighting the procedures’ safety (33). Data from the Valve-in-Valve International Data (VIVID) registry showed a 30-day mortality rate of 5.3% in 1,550 patients from 110 centers (34), and results from this analysis presented a 30-day mortality rate of 0% in latest time periods. As procedural experience increased and device technology improved, ViV implantation was offered to patients with lower baseline risk and fewer symptoms. This evolution probably contributed to the overall stability of adverse event rates, including stroke, pacemaker implantation, and mortality. Moreover, the lower complication rates may reflect both enhanced operator expertise and more refined patient selection in the later study period.
Compared with redo SAVR, ViV is associated with lower incidences of periprocedural complications (35), whereas the incidence of early mortality after ViV and redo SAVR is similar (35, 36). However, ViV is commonly considered to present significantly higher incidences of paravalvular leakage and increased mean transvalvular gradients compared with redo SAVR, and previously identified risk factors for adverse outcomes in ViV in terms of mortality are small bioprosthetic valves, age, and non-transfemoral access (36–38), emphasizing the need for a thorough heart team discussion of every individual patient with a deteriorated surgical bioprosthesis in order to determine the best treatment modality in the context of aortic valve lifetime management. Particularly, younger patients who most probably will outlive the implanted THV may benefit from redo SAVR with aortic annulus enlargement (39) to facilitate future ViV procedures.
Study limitations
The present study is limited by its retrospective, single-center design, which may affect the generalizability of the results. Patients were not randomized to a specific treatment strategy or time point, and potential selection bias with unmeasured confounders cannot be ruled out. In addition, the absence of a comparator group, such as patients undergoing redo surgical aortic valve replacement, precludes a direct comparison of outcomes between treatment modalities.
Conclusions
This 10-year study of aortic ViV procedures for degenerated bioprostheses showed significant changes in patient risk profile and procedural measures over time. Advancements in technical approaches such as transfemoral access, BASILICA procedures, and BVF have expanded eligibility for patients previously considered unsuitable for interventional treatment. In addition, the peak incidence of degenerated Mitroflow prostheses appears to have been reached. Moreover, the impact of randomized controlled trials demonstrating the limited efficacy of cerebral protection devices has influenced clinical practice. In this study, it was found that early outcomes such as device success, stroke, and 30-day mortality were excellent, with improvement over time, highlighting the clinical efficacy and safety of the ViV procedures.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by the Hamburg Ethics Committee (protocol code: PV4304, date of approval: January 31, 2013). This analysis was performed in accordance with the Declaration of Helsinki. Prospectively included patients consented to data collection as part of our prospective aortic valve registry (“HARbOR,” NCT NCT04227002). In retrospectively included patients, ethical approval and informed consent to participate were waived in compliance with the local legislation and institutional requirements (Hamburg Hospital Law, §12 HmbKHG). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. TD, OB, SL, and LW: Data curation, Project administration, Validation, Writing – review & editing. NSö, IH, LH, DG, LV-B, JS, and SP: Data curation, Validation, Writing – review & editing. EG: Investigation, Supervision, Validation, Writing – review & editing. SB: Investigation, Project administration, Supervision, Validation, Writing – review & editing. HR: Project administration, Supervision, Validation, Writing – review & editing, Investigation. NSc: Project administration, Supervision, Validation, Writing – review & editing. AS: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. TD was funded by the Clinician Scientist Programme of the German Center for Cardiovascular Research (Grant number DZHK; FKZ 81X3710109).
Conflict of interest
NS received travel compensation from Edwards Lifesciencesand St Jude Medical, and speaking and lecture fees and travelcompensation from Boston Scientific. AS received lecture fees/travel compensation from Abbott, Boston Scientific, andEdwards Lifesciences Inc. AS is a proctor for Abbott. MBreceived travel compensation from Abbott. SL received travelcompensation from Edwards Lifesciences Inc. HR received travelcompensation and lecture fees from Edwards Lifesciences Inc.and Medtronic Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Grammar and spelling were checked with ChatGPT (OpenAI); all substantive content remains the sole responsibility of the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors, wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BASILICA, bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during transcatheter aortic valve implantation; BE, balloon-expanding; BVF, bioprosthetic valve fracturing; ICU, intensive care unit; IQR, interquartile range; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PVL, paravalvular leakage; SAVR, surgical aortic valve replacement; SE, self-expanding; TAVI, transcatheter aortic valve implantation; THV, transcatheter heart valve; VARC-3, Valve Academic Research Consortium-3; ViV, valve-in-valve.
References
1.
Nuche J Abbas AE Serra V Vilalta V Nombela-Franco L Regueiro A et al Balloon- vs self-expanding transcatheter valves for failed small surgical aortic bioprostheses: 1-year results of the LYTEN trial. JACC Cardiovasc Interv. (2023) 16(24):2999–3012. 10.1016/j.jcin.2023.10.028
2.
Komatsu I Mackensen GB Aldea GS Reisman M Dvir D . Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. Part 2: how to perform BASILICA. EuroIntervention. (2019) 15(1):55–66. 10.4244/EIJ-D-19-00056
3.
Ribeiro HB Rodés-Cabau J Blanke P Leipsic J Kwan Park J Bapat V et al Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. (2018) 39(8):687–95. 10.1093/eurheartj/ehx455
4.
Pibarot P Simonato M Barbanti M Linke A Kornowski R Rudolph T et al Impact of pre-existing prosthesis-patient mismatch on survival following aortic valve-in-valve procedures. JACC Cardiovasc Interv. (2018) 11(2):133–41. 10.1016/j.jcin.2017.08.039
5.
Simonato M Webb J Kornowski R Vahanian A Frerker C Nissen H et al Transcatheter replacement of failed bioprosthetic valves: large multicenter assessment of the effect of implantation depth on hemodynamics after aortic valve-in-valve. Circ Cardiovasc Interv. (2016) 9(6):e003651. 10.1161/CIRCINTERVENTIONS.115.003651
6.
Allen KB Chhatriwalla AK Cohen DJ Saxon JT Aggarwal S Hart A et al Bioprosthetic valve fracture to facilitate transcatheter valve-in-valve implantation. Ann Thorac Surg. (2017) 104(5):1501–8. 10.1016/j.athoracsur.2017.04.007
7.
Hayashida K Lefèvre T Chevalier B Hovasse T Romano M Garot P et al Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. (2011) 4(8):851–8. 10.1016/j.jcin.2011.03.019
8.
Toggweiler S Gurvitch R Leipsic J Wood DA Willson AB Binder RK et al Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. (2012) 59(2):113–8. 10.1016/j.jacc.2011.08.069
9.
Mach M Okutucu S Kerbel T Arjomand A Fatihoglu SG Werner P et al Vascular complications in TAVR: incidence, clinical impact, and management. J Clin Med. (2021) 10(21):5046. 10.3390/jcm10215046
10.
Schaefer A Demal TJ Bhadra OD Grundmann D Voigtländer L Waldschmidt L et al Valve-in-valve procedures for degenerated surgical and transcatheter aortic valve bioprostheses using a latest-generation self-expanding intra-annular transcatheter heart valve. Front Cardiovasc Med. (2023) 10:1209184. Published 2023 September 1. 10.3389/fcvm.2023.1209184
11.
Westermann D Ludwig S Kalbacher D Spink C Linder M Bhadra OD et al Prevention of coronary obstruction in patients at risk undergoing transcatheter aortic valve implantation: the Hamburg BASILICA experience [published correction appears in Clin Res Cardiol. 2021 Oct;110(10):1693. doi: 10.1007/s00392-021-01904-0]. Clin Res Cardiol. (2021) 110(12):1900–11. 10.1007/s00392-021-01881-4
12.
VARC-3 WRITING COMMITTEE: GénéreuxPPiazzaNAluMCNazifTHahnRTPibarotPet alValve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. (2021) 77(21):2717–46. 10.1016/j.jacc.2021.02.038
13.
Demal TJ Weimann J Ojeda FM Bhadra OD Linder M Ludwig S et al Temporal changes of patient characteristics over 12 years in a single-center transcatheter aortic valve implantation cohort. Clin Res Cardiol. (2023) 112(5):691–701. 10.1007/s00392-023-02166-8
14.
Thyregod HGH Jørgensen TH Ihlemann N Steinbrüchel DA Nissen H Kjeldsen BJ et al Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur Heart J. (2024) 45(13):1116–24. 10.1093/eurheartj/ehae043
15.
Mack MJ Leon MB Thourani VH Pibarot P Hahn RT Genereux P et al Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. (2023) 389(21):1949–60. 10.1056/NEJMoa2307447
16.
Forrest JK Deeb GM Yakubov SJ Gada H Mumtaz MA Ramlawi B et al 4-Year Outcomes of patients with aortic stenosis in the evolut low risk trial. J Am Coll Cardiol. (2023) 82(22):2163–5. 10.1016/j.jacc.2023.09.813
17.
Raschpichler M Kiefer P Otto W Noack T Gerber M De Waha S et al Redo surgical aortic valve replacement for bioprosthetic structural valve deterioration. Eur J Cardiothorac Surg. (2024) 66(4):ezae353. 10.1093/ejcts/ezae353
18.
Ternacle J Hecht S Eltchaninoff H Salaun E Clavel MA Côté N et al Durability of transcatheter aortic valve implantation. EuroIntervention. (2024) 20(14):e845–64. 10.4244/EIJ-D-23-01050
19.
Windecker S Okuno T Unbehaun A Mack M Kapadia S Falk V . Which patients with aortic stenosis should be referred to surgery rather than transcatheter aortic valve implantation?Eur Heart J. (2022) 43(29):2729–50. 10.1093/eurheartj/ehac105
20.
Carroll JD Mack MJ Vemulapalli S Herrmann HC Gleason TG Hanzel G et al STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. (2020) 76(21):2492–516. 10.1016/j.jacc.2020.09.595
21.
Grundmann D Kim W Kellner C Adam M Braun D Tamm AR et al Femoral or radial secondary access in TAVR: a subanalysis from the multicenter PULSE registry. JACC Cardiovasc Interv. (2024) 17(24):2923–32. 10.1016/j.jcin.2024.09.020
22.
AlBadri A Joseph J Patel V Patel D Koren O Cheng W et al Hemodynamic and mid-term outcomes for transcatheter aortic valve replacement in degenerated internally stented valves. JACC Cardiovasc Interv. (2023) 16(5):542–54. 10.1016/j.jcin.2023.01.381
23.
Hallak O Fischer K Ailawadi S Valencia D Yatsynovich Y Nazir R et al Effects of bioprosthetic valve fracturing on valve-in-valve transcatheter aortic valve implantation transvalvular gradients. Tex Heart Inst J. (2024) 51(2):e238304. 10.14503/THIJ-23-8304
24.
Wienemann H Maier O Beyer M Portratz M Tanaka T Mauri V et al Cusp overlap versus standard three-cusp technique for self-expanding evolut transcatheter aortic valves. EuroIntervention. (2023) 19(2):e176–87. 10.4244/EIJ-D-22-01030
25.
Ahmad D Sá MP Yousef S Brown JA Doshi N Kliner DE et al Supra-annular self-expanding versus balloon-expandable valves for valve-in-valve transcatheter aortic valve replacement. Am J Cardiol. (2024) 231:55–61. 10.1016/j.amjcard.2024.08.032
26.
Ochiai T Yoon SH Sharma R Miyasaka M Nomura T Rami T et al Outcomes of self-expanding vs. balloon-expandable transcatheter heart valves for the treatment of degenerated aortic surgical bioprostheses—a propensity score-matched comparison. Circ J. (2018) 82(10):2655–62. 10.1253/circj.CJ-18-0157
27.
Yokoyama Y Sakurai Y Kuno T Takagi H Fukuhara S . Externally mounted versus internally mounted leaflet aortic bovine pericardial bioprosthesis: meta-analysis. Gen Thorac Cardiovasc Surg. (2023) 71(4):207–15. 10.1007/s11748-022-01904-5
28.
Kapadia SR Kodali S Makkar R Mehran R Lazar RM Zivadinov R et al Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. (2017) 69(4):367–77. 10.1016/j.jacc.2016.10.023
29.
Levi A Linder M Seiffert M Witberg G Pilgrim T Tomii D et al The impact of cerebral embolic protection devices on characteristics and outcomes of stroke complicating TAVR. JACC Cardiovasc Interv. (2024) 17(5):666–77. 10.1016/j.jcin.2023.12.033
30.
Harmouch W Karnkowska B Thakker R Rasmussen P Shalaby M Khalife W et al Cerebral embolic protection in transcatheter aortic valve implantation using the sentinel cerebral protection system: a systematic review and meta-analysis. Cardiol Ther. (2024) 13(2):299–314. 10.1007/s40119-024-00359-4
31.
Kharbanda RK Kennedy J Jamal Z Dodd M Evans R Bal KK et al Routine cerebral embolic protection during transcatheter aortic-valve implantation. N Engl J Med. (2025) 392(24):2403–12. 10.1056/NEJMoa2415120
32.
Blankenberg S Seiffert M Vonthein R Baumgartner H Bleiziffer S Borger MA et al Transcatheter or surgical treatment of aortic-valve stenosis. N Engl J Med. (2024) 390(17):1572–83. 10.1056/NEJMoa2400685
33.
Mahmoud AN Gad MM Elgendy IY Mahmoud AA Taha Y Elgendy AY et al Systematic review and meta-analysis of valve-in-valve transcatheter aortic valve replacement in patients with failed bioprosthetic aortic valves. EuroIntervention. (2020) 16(7):539–48. 10.4244/EIJ-D-19-00928
34.
Aziz M Simonato M Webb JG Abdel-Wahab M McElhinney D Duncan A et al Mortality prediction after transcatheter treatment of failed bioprosthetic aortic valves utilizing various international scoring systems: Insights from the Valve-in-Valve International Data (VIVID). Catheter Cardiovasc Interv. (2018) 92(6):1163–70. 10.1002/ccd.27714
35.
Tran JH Itagaki S Zeng Q Leon MB O'Gara PT Mack MJ et al Transcatheter or surgical replacement for failed bioprosthetic aortic valves. JAMA Cardiol. (2024) 9(7):631–9. 10.1001/jamacardio.2024.1049
36.
Nasir MM Ikram A Usman M Sarwar J Ahmed J Hamza M et al Valve-in-Valve transcatheter aortic valve replacement versus redo-surgical aortic valve replacement in patients with aortic stenosis: a systematic review and meta-analysis. Am J Cardiol. (2024) 225:151–9. 10.1016/j.amjcard.2024.04.057
37.
Bleiziffer S Simonato M Webb JG Rodés-Cabau J Pibarot P Kornowski R et al Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur Heart J. (2020) 41(29):2731–42. 10.1093/eurheartj/ehaa544
38.
Wilbring M Kappert U Haussig S Winata J Matschke K Mangner N et al Hemodynamic follow-up after valve-in-valve TAVR for failed aortic bioprosthesis. J Card Surg. (2022) 37(12):4654–61. 10.1111/jocs.17048
39.
Yang B . Aortic annular enlargement with Y-incision/rectangular patch. Ann Cardiothorac Surg. (2024) 13(3):294–302. 10.21037/acs-2023-aae-0151
Summary
Keywords
TAVI, TAVR, valve-in-valve, aortic valve, bioprosthesis, lifetime management
Citation
Knochenhauer T, Demal TJ, Bhadra OD, Ludwig S, Sörensen NA, von der Heide I, Hannen L, Grundmann D, Voigtländer-Buschmann L, Waldschmidt L, Schirmer J, Pecha S, Girdauskas E, Blankenberg S, Reichenspurner H, Schofer N and Schaefer A (2025) Changes in patient characteristics and procedural measures over a 10-year period in aortic valve-in-valve procedures for degenerated surgical bioprostheses. Front. Cardiovasc. Med. 12:1680733. doi: 10.3389/fcvm.2025.1680733
Received
06 August 2025
Accepted
20 October 2025
Published
10 November 2025
Volume
12 - 2025
Edited by
Andreas Kalogeropoulos, Mitera Hospital, Greece
Reviewed by
Birgid Gonska, Ulm University Medical Center, Germany
Ioannis Lianos, Guy’s and St Thomas' NHS Foundation Trust, United Kingdom
Updates
Copyright
© 2025 Knochenhauer, Demal, Bhadra, Ludwig, Sörensen, von der Heide, Hannen, Grundmann, Voigtländer-Buschmann, Waldschmidt, Schirmer, Pecha, Girdauskas, Blankenberg, Reichenspurner, Schofer and Schaefer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Tim Knochenhauer t.knochenhauer@uke.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.