- 1Department of Cardiovascular Surgery, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, Guangzhou, Guangdong, China
- 2Department of Endocrinology, Harrison International Peace Hospital, Hengshui, Hebei, China
- 3Department of Cardiovascular Surgery, Harrison International Peace Hospital, Hengshui, Hebei, China
Objective: Thoracic endovascular aortic repair (TEVAR) is increasingly performed in patients with acute type B intramural hematoma (IMH). This study aimed to evaluate the effect of diabetes mellitus (DM) on postoperative clinical outcomes and aortic remodeling degree of acute type B IMH patients undergoing endovascular repair.
Methods: We retrospectively identified patients diagnosed with acute type B IMH at two medical centers between January 2017 and January 2024. Study subjects were divided into DM and none DM (nDM) groups. Baseline characteristics, procedure details, and postoperative outcomes were extracted for further analysis.
Results: Forty-four patients were included in the study and were divided into DM (n = 12) and nDM groups (n = 32). Preoperative demographic characteristics, laboratory biomarkers, and imaging data were similar between the two groups. Compared with nDM group, the DM group presented a slightly higher rate of patients without postoperative major adverse events (MAE) (75.0% vs 68.8%, p = 0.651). At the 1-year follow-up, the DM group exhibited a significantly higher degree of aortic remodeling, as assessed by the TAD/TLD ratio [total aortic diameter [TAD] divided by the true lumen diameter [TLD] at the maximal IMH thickness] (DM group: 1.09 ± 0.04; nDM group: 1.17 ± 0.10, p = 0.032). Cox multivariate regression revealed that a TAD/TLD ratio > 1.32 increased the incidence of postoperative MAE significantly.
Conclusions: DM is positively associated with the prognosis of patients with acute type B IMH undergoing TEVAR and promotes postoperative aortic remodeling. Moreover, a TAD/TLD ratio >1.32 independently predicts the incidence of postoperative MAE.
Introduction
Aortic intramural hematoma (IMH) is defined as the presence of blood within the aortic wall media, potentially resulting from the rupture of vasa vasorum within the aortic wall (1, 2). For acute Stanford type B IMH not involving the ascending aorta (1, 2), optimal medical treatment (OMT) was traditionally considered the best choice (3). However, with the high failure rates of OMT (4), thoracic endovascular repair (TEVAR) has been introduced and increasingly performed in patients with suitable indication, achieving better results (1, 2, 5, 6). Nevertheless, TEVAR outcomes are not always satisfactory and can be influenced by several factors such as the timing of endovascular repair and diabetes mellitus (DM) (7–12).
DM is a common characteristic among patients with IMH undergoing the TEVAR procedure, but its impact on prognostic outcomes remains controversial. Some studies have reported that DM may reduce the incidence of major adverse events (MAE) following the TEVAR procedure (9, 10), whereas other studies have shown conflicting results (11, 12).
In some previous studies, the degree of postoperative aortic remodeling, as assessed by imaging data, was positively associated with the prognosis following the TEVAR procedure (7, 13–15). In patients with aortic aneurysms, DM may reduce the incidence of MAE following the TEVAR procedure by protecting the aortic wall and prohibiting aortic enlargement (10, 16–18). However, it remains unclear how DM affects postoperative aortic remodeling of patients with IMH undergoing TEVAR. Thus, we conducted this study to evaluate the effect of DM on patients with acute type B IMH undergoing the TEVAR procedure and to assess the potential association of DM with postoperative aortic remodeling.
Methods
This study was authorized by the Guangdong Provincial People's Hospital's and Harrison International Peace Hospital's Ethical Committees. Informed consent was not required given the retrospective nature of this study. We utilized existing patient data, which posed minimal risk to the patients.
Study patients
We retrospectively evaluated individuals diagnosed with IMH at Harrison International Peace Hospital and Guangdong Provincial People's Hospital between January 2017 and January 2024. Finally, patients with acute Stanford type B IMH who had undergone the TEVAR procedure were included for further analysis. The exclusion criteria were as follows: (1) Stanford type A IMH; (2) chronic IMH; (3) trauma-induced entity; and (4) treatment other than TEVAR. Then, based on the presence of DM before operation, we divided the patients into DM and none DM (nDM) groups. In accordance with guidelines, all patients received standard management upon admission, including sedative therapy, pain relief, heart rate control, and antihypertensive medication (1–3). During hospitalization, all the patients underwent postoperative aortic computed tomography (CTA) once or twice.
TEVAR procedure
To undergo the TEVAR procedure, the patients must meet one or more of the following indications: (1) maximum diameter of the aorta within the IMH ≥ 50 mm; (2) maximum IMH thickness ≥11 mm (1, 2). The assessment for the TEVAR procedure was performed by a multidisciplinary team consisting of cardiac surgeons, anesthesiologists, and radiologists. All patients underwent the TEVAR procedure in a hybrid operating room under general anesthesia. The criteria for procedural success were as follows: (1) successful delivery to the target lesion; (2) accurate deployment of the endograft; (3) absence of type I or III endoleaks, graft obstruction, and death during procedure (1, 2). The proximal landing zone of the aortic stent graft should extend at least 20 mm beyond the aortic segment unaffected by the IMH, combined with no more than 10% stent oversizing (1, 7).
Definitions
DM was diagnosed based on fasting plasma glucose levels ≥126 mg/dL, casual plasma glucose levels ≥200 mg/dL, hemoglobin A1c levels ≥6.5%, or treatment with anti-diabetic therapy (19, 20). We defined hypertension as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or treatment with antihypertensive therapy (21). Dyslipidemia was defined as follows: (1) low-density lipoprotein (LDL) cholesterol levels≥140 mg/dL; (2) high-density lipoprotein (HDL) cholesterol levels <40 mg/dL; (3) non-HDL cholesterol levels ≥170 mg/dL; (4) triglyceride levels ≥150 mg/dL; (5) or treated with antidyslipidemia therapy (22).
Based on the classification of IMH period (acute and subacute), the timing of TEVAR was determined using a cutoff of 7 days from symptom onset to intervention. Acute TEVAR (aTEVAR) was defined as intervention occurring within 1–7 days, while delayed TEVAR (dTEVAR) was defined as intervention occurring between 8 and 90 days (23).
On aortic CTA, the degree of aortic remodeling was evaluated using the ratio of the total aortic diameter (TAD) to the true lumen diameter (TLD) at the site of maximum IMH thickness (TAD/TLD ratio). TLD was defined as the distance between intimal surface, while the TAD was measured from adventitia to adventitia (Figure 1). A TAD/TLD ratio closest to 1.0 was considered indicative of better aortic remodeling (7).
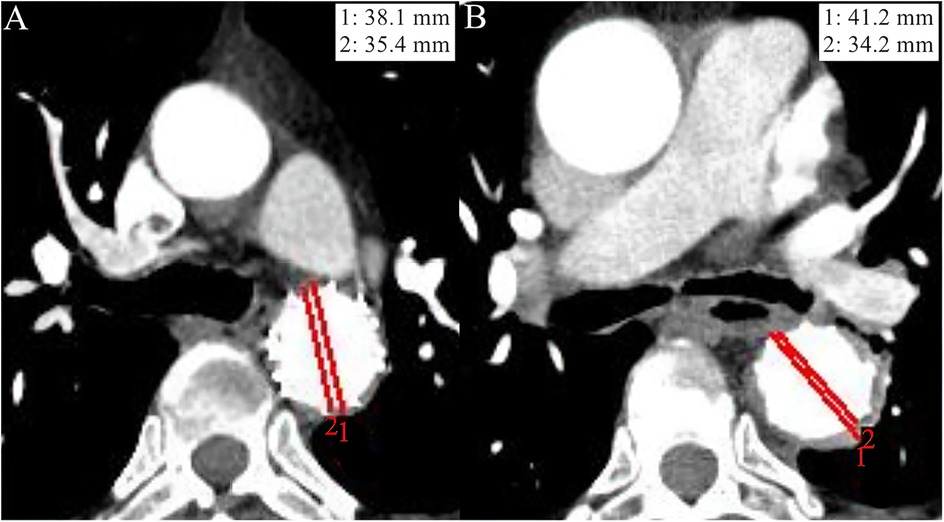
Figure 1. Imaging data from the aortic computed tomography angiography (CTA) of two study patients at the 1-year follow-up. The red line 1 indicates the total aortic diameter (TAD), while the red line 2 indicates the true lumen diameter (TLD). (A) An adult patient diagnosed with diabetes mellitus (DM) at admission had a TAD/TLD ratio of 1.08, indicating a high degree of aortic remodeling. (B) An adult patient without DM at admission showed relatively poor aortic remodeling degree as evaluated by a TAD/TLD ratio = 1.21, compared with patient A.
As clinical endpoints, we defined MAE, which included all-cause mortality, endoleak types I-IV, aortic rupture, new-onset aortic dissection (AD), re-intervention, spinal cord ischemia (SCI), stroke, and acute cardiac insufficiency (ACI).
Follow-up
We recommended that all patients undergo aortic CTA at 1, 3, 6, and 12 months after the intervention. Subsequently, we conducted a 1-year follow-up via telephone inquiry or outpatient service. Outcomes related to clinical endpoints and imaging data were recorded for analysis.
Statistical analyses
Continuous variables were presented in the form of mean ± standard deviation (M ± SD). Depending on the presence or absence of normality, the Student's t test or Mann–Whitney U-test was employed to compare the statistical difference. Dichotomous variables were expressed as frequency and percentage [n (%)]. Based on the sample size, we performed the Chi-square test or the Fisher's test for statistical analysis. We used the Kaplan–Meier (K-M) method to evaluate the 1-year outcomes of patients without postoperative MAE in each group. The optimal cutoff point for continuous data was determined based on previous studies. The log-rank test was used to compare statistical difference between K-M curves. We conducted Cox multivariate regression analysis to evaluate the association between variables (with a log-rank p value < 0.05 or traditionally recognized as risk factors) and postoperative MAE. The outcomes were presented as hazard ratio (HR) with corresponding 95% confidence intervals (CIs). Statistical analysis was performed using SPSS 26.0 software (IBM, White Plains, NY, USA), and a p value < 0.05 was considered to indicate a significant difference.
Results
Baseline characteristics
We identified 92 patients diagnosed with IMH at Harrison International Peace Hospital and Guangdong Provincial People's Hospital between January 2017 and January 2024. Forty-eight subjects were excluded because they met the exclusion criteria. Ultimately, 44 patients with acute type B IMH who had undergone TEVAR were included in this study. Twelve patients diagnosed with DM formed the DM group, while the remaining patients were assigned to the nDM group (n = 32).
Among the baseline characteristics, hypertension presented as the most frequent comorbidity, with no significant difference between the DM group and nDM group (91.7% for DM vs 90.6% for nDM, p = 0.915) (Table 1). There were no significant differences in the demographic characteristics, laboratory biomarkers, and imaging data between the two groups before TEVAR.
TEVAR details
TEVAR procedure was successfully performed in all patients (Table 1). The number and material of the endografts were similar between the two groups. In the case of proximal landing zone 2, we performed no coverage of left subclavian artery (LSA). The incidence of LSA reconstruction during the operation was higher in the nDM group, although there was no statistically significant difference between the two groups (DM group: 16.7%; nDM group: 31.3%, p = 0.333). Chimney technique, external fenestration, LSA-left carotid artery (LCA) bypass, or a Castor single-branched graft was employed to reconstruct LSA.
Outcomes at hospital and 1-year follow-up
Postoperative outcomes within 30-days are shown in Table 2. The measurements of postoperative TAD/TLD ratio before discharge were available for most patients, and there was no difference between the two groups (1.26 ± 0.16 for DM vs 1.25 ± 0.12 for nDM, p = 0.927). Overall, the incidence of postoperative MAE within 30-days was slightly higher in the nDM group, with no statistically significant difference was found (16.7% for DM vs 25.0% for nDM, p = 0.557). Only one death occurred in the nDM group, and there was no significant difference between the DM and nDM groups. Aortic rupture, one of the serious complications associated with the TEVAR procedure, was not observed in our study. On postoperative CTA before discharge, the incidence of endoleak type Ib and new-onset AD were also similar between the two groups, and no reintervention was needed (Figure 2). There were two cases of stroke and one case of SCI in the nDM group, while no cases of stroke or SCI were observed in the DM group. There were no significant inter-group differences, and all the three patients recovered before discharge. The rate of other postoperative complications, such as ACI, was also similar between the two groups.
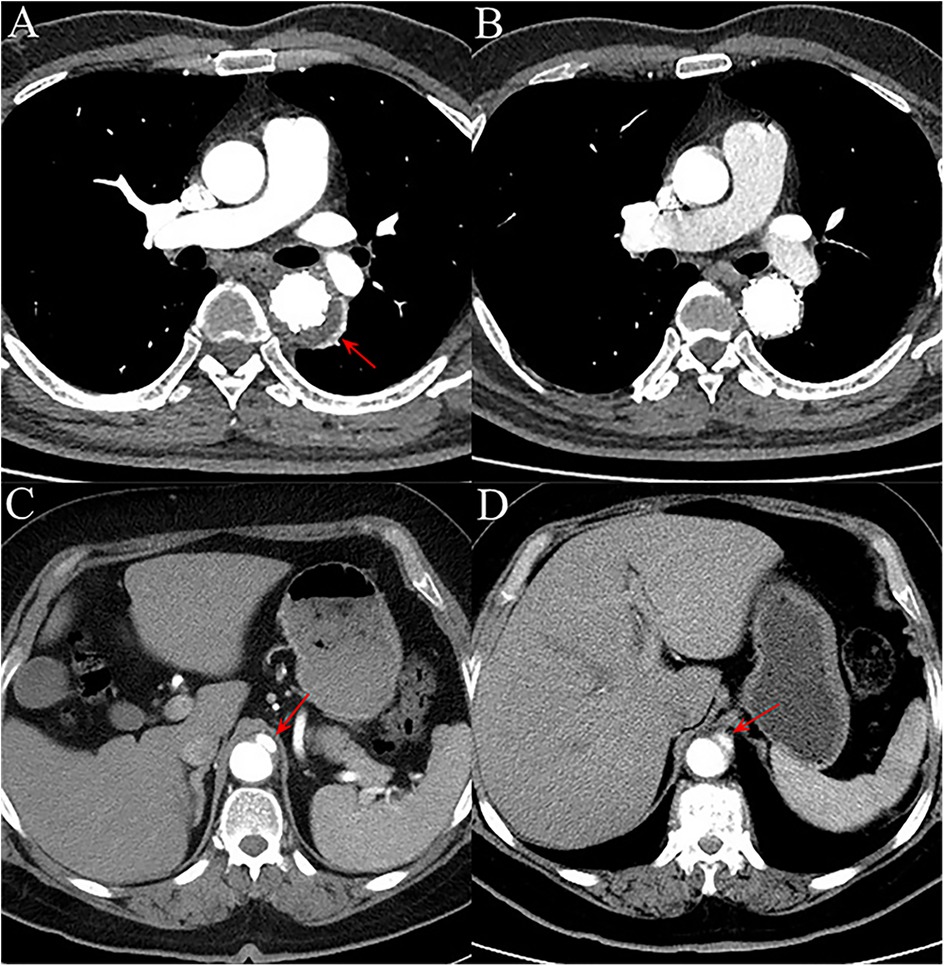
Figure 2. (A) on aortic computed tomography angiography (CTA) performed within 30 days after the procedure, a type Ib endoleak (red arrow) was observed in one patient, with contrast agent leaking into the aortic mid-layer from the distal landing zone of the endograft. (B) The endoleak had disappeared by the 1-year follow-up CTA. (C) In another patient, CTA within 30 days post-operation revealed a new-onset focal aortic dissection (red arrow) originating from the distal landing zone of the endograft, without involvement of major branch vessels. (D) At the 1-year follow-up, the aortic dissection (red arrow) remained stable in extent, with no significant progression.
Next, we performed 1-year follow-up for most patients (DM group: 100.0%; nDM group: 96.9%, p = 1.000) (Table 2). Overall, a similar incidence of MAE was investigated between the DM and nDM groups during 1-year follow-up. Endoleak type Ib occurred in one case in each group, and one new-onset AD occurred in the nDM group. However, none of the three patients required reintervention. On aortic CTA, the TAD/TLD ratio was significantly lower in the DM group (DM group: 1.09 ± 0.04; nDM: 1.17 ± 0.10, p = 0.032). Due to varying levels of patient compliance, a number of patients did not return to the hospital for 1-year CTA examination. To assess whether this introduced potential selection bias, we conducted a comparison between patients who completed 1-year CTA follow-up and those who did not. No significant differences were observed between the two groups (Table 3). In the K-M analysis, the DM group had a slightly higher proportion of patients without postoperative MAE. However, the log-rank test showed no significant difference between the DM and nDM groups (75.0% for DM vs 68.8% for nDM, p = 0.651) (Figure 3). In previous studies, aTEVAR has been associated with higher rates of postoperative MAE (7, 8). We also found that aTEVAR increased the incidence of MAE in our study; however, this increase was not statistically significant compared to dTEVAR (59.1% for aTEVAR vs 89.8% for dTEVAR, log-rank p = 0.134) (Figure 4A). Finally, we determined the optimal cutoff point of the TAD/TLD ratio as 1.32 in accordance with previous study (7), and a significantly lower proportion of patients without MAE was observed in the group with a TAD/TLD ratio >1.32 (50.0% vs 82.1%, p = 0.031) (Figure 4B). Then, we performed a multivariate Cox regression analysis, and found that the TAD/TLD ratio >1.32 was independently associated with a higher incidence of postoperative MAE (HR, 3.798; 95% CI, 1.038–13.901; p = 0.044) (Table 4).
Table 3. Comparison of postoperative outcomes between patients with and without 1-year CTA follow-up.
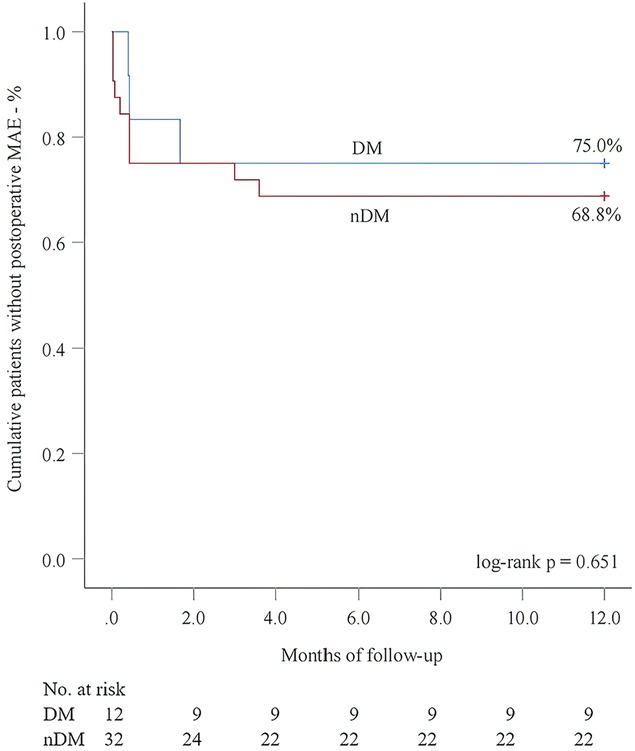
Figure 3. Kaplan–meier analysis for postoperative major adverse events (MAE) in relation to diabetes mellitus (DM).
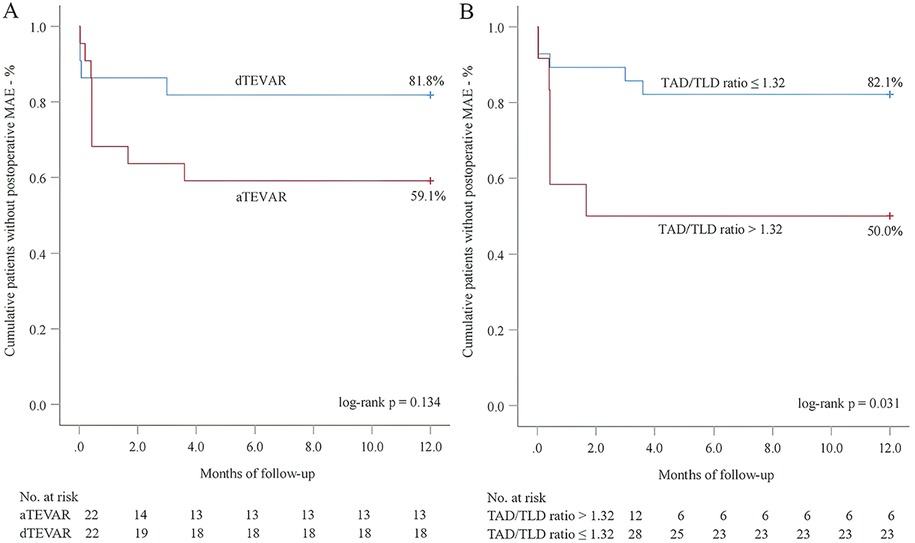
Figure 4. Kaplan–meier analysis for the association of major adverse events (MAE) with endovascular repair timing (A) and total aortic diameter (TAD)/true lumen diameter (TLD) ratio (B).
Discussion
In this study, the proportion of patients with DM was 27.3%, which is similar to the values reported in previous studies (9–12). As one of the most important prognostic indicators, all-cause mortality has been reported to be higher in patients with DM after TEVAR (12, 24, 25), while a similar result was also observed in some other studies (11, 25, 26). Another study reported that dietary therapy for DM was associated with significantly higher mortality during hospitalization and follow-up (11). In our study, 10 patients received oral medications alone, while two patients received insulin therapy in combination with oral medications in the DM group. During the 30-day postoperative period, one postoperative death was observed in patients without DM, while no deaths occurred in patients with DM. There was no significant inter-group difference. Such a result might be attributed to the absence of dietary management in the DM group.
In addition to mortality, SCI and stroke are considered serious complications following the TEVAR procedure. In our study, there were two cases of stroke and one case of SCI in the nDM group, while no such cases were observed in patients with DM. Although no significantly statistical inter-group differences were observed and the three patients recovered before discharge, DM appeared to decrease the incidence of nervous system complications following the TEVAR procedure. In some previous studies, DM acted as an independent risk factor of SCI and stroke after TEVAR (25, 27). However, the incidence of postoperative stroke was also recognized to be associated with extensive manipulation in the aortic arch region, which might occur during procedures such as LSA revascularization or coverage of LSA (27). In our study, no LCSA coverage was performed during operation. Moreover, there was a lower rate of LSA reconstruction in the DM group, decreased operations at the aortic arch, and resulted in the relatively lower incidence of nervous system complications.
Overall, compared with patients with DM, a higher incidence of postoperative MAE was investigated in patients without DM, but it did not reach statistical significance. This result has also been reported in some studies (10, 11). Traditionally, the aortic remodeling degree was considered to be positively associated with the prognostic outcomes of TEVAR procedure (7, 13, 15), which was confirmed by our study. We determined poor aortic remodeling degree as a TAD/TLD ratio >1.32 after intervention. Through multivariate regression analysis, we found that this ratio was significantly associated with a higher incidence of postoperative MAE. aTEVAR is negatively associated with the aortic remodeling degree, and it increases the rate of postoperative MAE (7, 8). Nevertheless, in our study, comparable patients underwent aTEVAR between both groups, and aTEVAR did not increase the rate of MAE in the K-M analysis.
Although postoperative TAD/TLD ratio was comparable between patients with and without DM within 30-days after TEVAR, a significantly lower data was investigated in the DM group at 1-year follow-up. In other words, patients with DM might achieve a better long-term aortic remodeling, resulting in a relatively lower incidence of postoperative MAE. This may be explained by the protective effects of DM on the aortic wall. Hyperglycemia promotes the formation of extracellular matrix and reduces its degradation in the aorta. Additionally, patients with DM typically have a greater intima-media thickness in the aorta, which helps to mitigate aortic wall stress (12, 28, 29). The putative protective effect of hyperglycemia on the aortic wall may also prohibit the development and progression of aortic aneurysm and aortic dissection, subsequently decreasing some serious complications such as mortality and aortic rupture (10, 16–18).
First, the relatively small sample size, particularly within subgroup analyses, may have limited the statistical power to detect significant differences in certain outcome measures. Second, this was a retrospective study conducted at two tertiary centers, which may introduce selection bias and limit the generalizability of the findings. Additionally, mid- to long-term follow-up data were not available for all patients, which may affect the assessment of durability outcomes. Future prospective, multicenter studies with larger cohorts and longer follow-up periods are warranted to validate these findings.
Conclusion
In patients with acute type B IMH undergoing the TEVAR procedure, DM slightly decreases the incidence of postoperative MAE. This effect may be attributed to the significantly higher degree of aortic remodeling observed after intervention. Moreover, a TAD/TLD ratio >1.32 independently predicts the incidence of postoperative MAE.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Harrison International Peace Hospital and Guangdong Provincial People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because, given the retrospective nature of the study and the minimal risk to participants.
Author contributions
FY: Formal analysis, Writing – review & editing, Data curation, Writing – original draft, Methodology, Visualization, Conceptualization, Software. MM: Writing – review & editing, Conceptualization, Software, Writing – original draft, Visualization. BW: Funding acquisition, Formal analysis, Writing – review & editing, Data curation, Writing – original draft, Methodology. HX: Formal analysis, Methodology, Writing – review & editing, Data curation, Visualization, Software.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Medical Science Research Project of Hebei [grant number 20232168].
Acknowledgments
We thank LetPub (https://www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Isselbacher EM, Preventza O, Hamilton Black J 3rd, Augoustides JG, Beck AW, Bolen MA, et al. 2022 Acc/AHA guideline for the diagnosis and management of aortic disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation. (2022) 146(24):e334–482. doi: 10.1161/CIR.0000000000001106
2. Bossone E, LaBounty TM, Eagle KA. Acute aortic syndromes: diagnosis and management, an update. Eur Heart J. (2018) 39(9):739–749d. doi: 10.1093/eurheartj/ehx319
3. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 Esc guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European society of cardiology (ESC). Eur Heart J. (2014) 35(41):2873–2926. doi: 10.1093/eurheartj/ehu281
4. Mesar T, Lin MJ, Kabir I, Dexter DJ, Rathore A, Panneton JM. Medical therapy in type B aortic intramural hematoma is associated with a high failure rate. J Vasc Surg. (2020) 71(4):1088–1096. doi: 10.1016/j.jvs.2019.07.084
5. Ye K, Qin J, Yin M, Jiang M, Li W, Lu X. Acute intramural hematoma of the descending aorta treated with stent graft repair is associated with a better prognosis. J Vasc Interv Radiol. (2017) 28(10):1446–53. doi: 10.1016/j.jvir.2017.06.034
6. Schoenhoff FS, Zanchin C, Czerny M, Makaloski V, Gahl B, Carrel T, et al. Aorta related and all-cause mortality in patients with aortic intramural haematoma. Eur J Vasc Endovasc Surg. (2017) 54(4):447–53. doi: 10.1016/j.ejvs.2017.07.001
7. Mesar T, Alie-Cusson FS, Lin MJ, Dexter DJ, Rathore A, Stokes GK, et al. Impact of thoracic endovascular aortic repair timing on aortic remodeling in acute type B aortic intramural hematoma. J Vasc Surg. (2022) 75(2):464–72. doi: 10.1016/j.jvs.2021.08.059
8. Xiang D, Wu F, Chen L, Liang H, Xiong B, Liang B, et al. Timing of endovascular repair impacts long-term outcomes of uncomplicated acute type B aortic dissection. J Vasc Surg. (2022) 75(3):851–60. doi: 10.1016/j.jvs.2021.09.017
9. Liu H, Shi L, Zeng T, Ji Q, Shi Y, Huang Y, et al. Type 2 diabetes mellitus reduces clinical complications and mortality in Stanford type B aortic dissection after thoracic endovascular aortic repair: a 3-year follow-up study. Life Sci. (2019) 230:104–10. doi: 10.1016/j.lfs.2019.05.055
10. Taimour S, Franzén S, Zarrouk M, Acosta S, Nilsson P, Miftaraj M, et al. Nationwide comparison of long-term survival and cardiovascular morbidity after acute aortic aneurysm repair in patients with and without type 2 diabetes. J Vasc Surg. (2020) 71(1):30–8.e33. doi: 10.1016/j.jvs.2019.01.063
11. Summers SP, Rastogi V, Yadavalli SD, Wang SX, Schaller MS, Jones DW, et al. The association between diabetes mellitus and its management with outcomes following endovascular repair for descending thoracic aortic aneurysm. J Vasc Surg. (2023) 78(2):313–23. doi: 10.1016/j.jvs.2023.02.024
12. Takahara M, Iida O, Tazaki J, Nishikawa R, Nanto K, Chiba Y, et al. Clinical features and prognosis of patients with and without diabetes mellitus undergoing endovascular aortic aneurysm repair. BMC Endocr Disord. (2022) 22(1):92. doi: 10.1186/s12902-022-01008-4
13. Sailer AM, Nelemans PJ, Hastie TJ, Chin AS, Huininga M, Chiu P, et al. Prognostic significance of early aortic remodeling in acute uncomplicated type B aortic dissection and intramural hematoma. J Thorac Cardiovasc Surg. (2017) 154(4):1192–200. doi: 10.1016/j.jtcvs.2017.04.064
14. Tian C, Shu C, Luo M, Fang K, Zhang Y. Survival and aortic remodeling outcomes in patients with type B aortic intramural hematoma in the endovascular era: an observational cohort study. J Vasc Surg. (2022) 76(1):70–8. doi: 10.1016/j.jvs.2022.01.143
15. Dohle DS, Laverne T, Bavaria J, Savino D, Vallabhajosyula P, Szeto WY, et al. Aortic remodelling after thoracic endovascular aortic repair in acute and chronic type B aortic dissections. Eur J Cardiothorac Surg. (2020) 58(4):730–7. doi: 10.1093/ejcts/ezaa118
16. Taimour S, Avdic T, Franzén S, Zarrouk M, Acosta S, Nilsson P, et al. Survival, cardiovascular morbidity, and reinterventions after elective endovascular aortic aneurysm repair in patients with and without diabetes: a nationwide propensity-adjusted analysis. Vasc Med. (2019) 24(6):539–46. doi: 10.1177/1358863X19870243
17. De Rango P, Cao P, Cieri E, Parlani G, Lenti M, Simonte G, et al. Effects of diabetes on small aortic aneurysms under surveillance according to a subgroup analysis from a randomized trial. J Vasc Surg. (2012) 56(6):1555–63. doi: 10.1016/j.jvs.2012.05.078
18. Arun D, Munir W, Schmitt LV, Vyas R, Ravindran JI, Bashir M, et al. Exploring the correlation and protective role of diabetes Mellitus in aortic aneurysm disease. Front Cardiovasc Med. (2021) 8:769343. doi: 10.3389/fcvm.2021.769343
19. Zhong J. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43(Suppl 1):S14–31. doi: 10.2337/dc20-S002
20. Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese Clinical practice guideline for diabetes 2019. J Diabetes Investig. (2020) 11(4):1020–76. doi: 10.1111/jdi.13306
21. Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 Acc/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. (2018) 71(6):1269–324. doi: 10.1161/HYP.0000000000000066
22. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 Esc/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058.27567407
23. Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. (2013) 126(8):730.e19. doi: 10.1016/j.amjmed.2013.01.020
24. Tarbunou YA, Smith JB, Kruse RL, Vogel TR. Outcomes associated with hyperglycemia after abdominal aortic aneurysm repair. J Vasc Surg. (2019) 69(3):763–73. doi: 10.1016/j.jvs.2018.05.240
25. Gambardella I, Worku B, Lau C, Tranbaugh RF, Tabaie S, Ivascu N, et al. Diabetes Mellitus is an independent predictor of spinal cord injury after descending thoracic and thoracoabdominal aneurysm repair: maximum likelihood conditional regression in a propensity-score matched cohort. Ann Surg. (2023) 278(2):e382–88. doi: 10.1097/SLA.0000000000005572
26. Png CYM, Tadros RO, Kang M, Beckerman WE, Tardiff ML, Vouyouka AG, et al. The protective effects of diabetes Mellitus on post-EVAR AAA growth and reinterventions. Ann Vasc Surg. (2017) 43:65–72. doi: 10.1016/j.avsg.2016.10.059
27. Zha Z, Pan Y, Zheng Z, Wei X. Prognosis and risk factors of stroke after thoracic endovascular aortic repair for Stanford type B aortic dissection. Front Cardiovasc Med. (2021) 8:787038. doi: 10.3389/fcvm.2021.787038
28. Norman PE, Davis TM, Le MT, Golledge J. Matrix biology of abdominal aortic aneurysms in diabetes: mechanisms underlying the negative association. Connect Tissue Res. (2007) 48(3):125–31. doi: 10.1080/03008200701331524
Keywords: intramural hematoma, Stanford type B, Diabetes Mellitus, TEVAR, Aortic remodeling, Major adverse events
Citation: Yu F, Miao M, Wang B and Xian H (2025) Prognostic value of diabetes mellitus for acute type B intramural hematoma patients undergoing endovascular repair. Front. Cardiovasc. Med. 12:1683361. doi: 10.3389/fcvm.2025.1683361
Received: 11 August 2025; Revised: 8 November 2025;
Accepted: 14 November 2025;
Published: 27 November 2025.
Edited by:
Eugenio Martelli, University of Rome Tor Vergata, ItalyReviewed by:
Takayuki Okada, Kansai Medical University Medical Center, JapanMauro Fresilli, University of Rome Tor Vergata, Italy
Copyright: © 2025 Yu, Miao, Wang and Xian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Wang, aGlwaHdiQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship.
‡ORCID:
Bin Wang
orcid.org/0000-0003-0569-2862
 Fumin Yu
Fumin Yu Miao Miao2,†
Miao Miao2,† Bin Wang
Bin Wang

