- 1Department of Cardiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, Hunan, China
- 3Department of Cardiology, Songjiang Hospital and Songjiang Research Institute School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: Abdominal aortic aneurysm (AAA) is a disease with a relatively high mortality risk. Currently, diagnosis of AAA predominantly depends on imaging examinations, and there is an urgent need for simple and rapid screening biomarkers. The platelet to high-density lipoprotein cholesterol ratio (PHR) is an emerging biomarker reflects systemic inflammation and hypercoagulable states in cardiovascular diseases. The aim of this study is to elucidate the clinical significance of PHR in AAA.
Methods: A total of 156 patients with AAA and 113 healthy controls were enrolled. Among them, 80 patients with AAA had concomitant thrombosis. Serum samples were collected upon admission to the hospital and before treatment. The equation for PHR calculation is: PHR = [platelet (1,000 cells/µl)/HDL-C (mmol/L)]. The optimal PHR cut-off was determined via ROC analysis, and logistic regression identified AAA predictors.
Results: In this study, PHR level in patients with AAA was significantly higher than that of control group [127.00 (109.74, 163.42) vs. 192.35 (159.08, 231.66); <0.001]. Additionally, PHR value was even higher in AAA patients with thrombosis [161.80 (135.54, 193.41) vs. 220.61 (186.49, 262.27); <0.001]. ROC curve analysis revealed that PHR had a good predictive value for diagnosis of AAA, with an area under the curve (AUC) of 0.805. When it came to the diagnosis of AAA patients with thrombosis, the AUC of PHR was 0.814. Multivariate logistic regression analysis further demonstrated that PHR was an independent predictor in AAA (OR: 1.022, 95% CI: 1.012–1.031; P < 0.001) and AAA patients with thrombosis (OR: 1.016; 95% CI: 1.007–1.024; P < 0.001).
Conclusion: PHR has good diagnostic value for AAA and can serve as a rapid screening indicator for its clinical diagnosis.
1 Background
Abdominal aortic aneurysm (AAA) refers to a localized, progressive dilation of the abdominal aorta with a diameter ≥3 cm or exceeding 50% of the normal value (1). AAA typically manifests as a clinically occult condition, and patients often remain asymptomatic until the disease progresses (2). However, its rupture can lead to a mortality rate of more than 80%, representing a life-threatening aortic emergency (3, 4). Due to the high risk of rupture and associated mortality, timely diagnosis and personalized management of AAA are essential. Currently, the diagnosis of AAA mainly relies on imaging examination methods such as ultrasound, computed tomography angiography (CTA), or magnetic resonance angiography (MRA) (5, 6). In regions with limited resources or in urgent clinical situations, these examination methods may encounter difficulties such as high costs and poor accessibility. Over recent years, several serum biomarkers for AAA progression have been investigated, including D-dimer, matrix metalloproteinases (MMPs), C-reactive protein (CRP), inflammatory cytokines (e.g., IL-6, TNF-α) (1, 7–9). However, these biomarkers often exhibit limitations. For instance, they have relatively low sensitivity in diagnosing AAA, or are difficult to be widely applied in clinical practice. In recent years, there has been a notable increase in the number of biomarkers identified for evaluating the growth of AAAs. Among these, research has highlighted that elevated levels of circulating trimethylamine N-oxide (TMAO) are associated with a heightened risk of AAA development and can serve as a predictor for rapid AAA expansion (10). Despite these findings, TMAO has not yet gained widespread adoption in clinical practice. Therefore, it is an urgent priority to explore new biomarkers for the early screening of AAA, which can contribute to early intervention in AAA and thus prevent the occurrence of adverse events.
Abnormal lipid metabolism plays an important role in the occurrence and development of AAA (1, 11). High-density lipoprotein cholesterol (HDL-C), often termed the “good cholesterol”, inhibits the occurrence and development of AAA by facilitating reverse cholesterol transport to remove lipids from the arterial walls, inhibiting oxidative stress responses, and regulating immune responses (1, 11, 12). Clinical studies have shown that low HDL-C levels are independently associated with an increased risk of AAA (13). Moreover, platelets can also play a crucial role in the pathogenesis of AAA through multiple mechanisms, including participation in hemostasis and thrombosis, triggering of inflammatory responses, promotion of vascular remodeling, and degradation of the extracellular matrix, among others (14–16). Studies have demonstrated that treatment with low-dose aspirin is beneficial for patients with AAA, as it can reduce the volume of the aneurysms and decrease the risk of rupture of AAA (17, 18). A recent study demonstrated that the level of soluble glycoprotein VI (sGPVI) can predict the diagnosis of AAA and AAA growth rate, and may serve as a therapeutic target for AAA (19). Although sGPVI is not yet widely applied in clinical settings, its crucial role in platelet activation suggests that platelet-related indicators may have significant potential in predicting AAA.
The platelet to high-density lipoprotein cholesterol ratio (PHR), has garnered attention as a promising biomarker for AAA due to its integrative nature and practical clinical utility. The pathogenesis of AAA is multifaceted, encompassing both inflammatory and metabolic processes (20). Platelet activation and dyslipidemia are well-documented contributors to AAA development. Platelets play a crucial role in promoting inflammation and thrombosis, which are central to aneurysm formation and progression (21). In contrast, HDL-C exerts anti-inflammatory and anti-atherosclerotic effects. By amalgamating these two critical aspects, the PHR provides a composite measure that holistically reflects the interplay between inflammation and metabolism, both integral to AAA pathogenesis (22, 23).
Accumulating evidence underscores the association of PHR with various cardiovascular diseases, such as hypertension, heart failure, and stroke (20–23), and a retrospective multicenter study indicates that elevated PHR increases the long-term mortality risk in patients with CHD, serving as a high-risk marker to guide clinical intervention (24).Given the overlapping pathophysiological pathways between these conditions and AAA, it is plausible to hypothesize that PHR could serve as a valuable indicator for AAA. This hypothesis is further supported by the mechanistic linkages between platelet activation, lipid metabolism, and aneurysm development. Clinically, PHR stands out for its practicality and cost-effectiveness. It can be readily calculated from routine blood tests, making it easily accessible for widespread application across diverse healthcare settings (24). This accessibility is crucial for the routine monitoring and early detection of AAA, potentially enhancing clinical decision-making and patient outcomes.
PHR not only integrates key pathophysiological components of AAA but also offers a practical and readily available marker for clinical use, making it a compelling candidate for further investigation in the diagnosis and management of AAA. Therefore, this study aims to investigate the clinical application value of PHR in AAA, with the expectation of providing a rapid and simple laboratory-based method to assist in the diagnosis of AAA.
2 Methods
2.1 Study population
In this study, patients with AAA who were treated in the Emergency Department and the Department of Vascular Surgery of our hospital from January 2018 to December 2018 were consecutively enrolled. Among them, patients with Marfan syndrome, acute myocardial infarction or stroke, malignant tumors, severe liver and kidney insufficiency, active inflammatory diseases, or other connective tissue diseases were excluded from the study. A total of 169 patients with AAA were included in this study. Meanwhile, we recruited 120 gender-matched healthy individuals who underwent comprehensive health examinations in the same hospital as the control group. AAA was diagnosed by chest radiograph, transesophageal echocardiography, computed tomography, or surgery, and it was determined whether there was concomitant thrombosis. AAA was defined as segmental abnormal dilation of the abdominal aorta, with a diameter increase of more than 50% compared to the diameter of the normal aorta. All healthy control individuals in this study underwent imaging examinations to confirm the absence of AAA.
Among the initial 289 patients, 20 were excluded due to the lack of complete data on the variables required for this study. Finally, 156 patients with AAA and 113 healthy control individuals were included in our analysis. This study was approved by the Ethics Committee, and signed informed consent was obtained from all patients before enrollment. The study protocol adhered to the Declaration of Helsinki, and all participants provided written confirmation of their willingness to participate.
2.2 Data collection and definitions
We collected a variety of data from the patients' medical records, covering cardiovascular risk factors, demographic characteristics, past medical history, laboratory test indicators and other data. Diabetes can be diagnosed if any of the following criteria is met: random blood glucose ≥11.1 mmol/L; fasting blood glucose ≥7.0 mmol/L; blood glucose 2 h after the oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L; or HbA1c ≥ 6.5%. The criteria for diagnosing hypertension are as follows: under resting conditions, systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, or the patient is currently receiving antihypertensive drug treatment. Coronary heart disease (CHD) is diagnosed according to relevant guidelines. In addition, the body mass index (BMI) is calculated by dividing the weight (in kg) by the square of the height (in m2).
2.3 Laboratory measurements
After the patients were admitted to the hospital, blood samples were collected from the anterior cubital veins before any medication was administered for the detection of multiple indicators. These indicators include blood routine tests, total cholesterol (TC); triglyceride (TG); low-density lipoprotein cholesterol (LDL-C); HDL-C, as well as serum creatinine, uric acid, blood urea nitrogen (BUN), etc. The calculation formula for the PHR is: PHR = [platelet (1,000 cells/µl)/HDL-C (mmol/L)]. All data were collected by professionals who were not involved in this study.
2.4 Statistical analysis
This study conducted statistical analyses using the Statistical Package for Social Sciences (SPSS) v.22. and GraphPad software 8.0.1. Continuous variables that conform to a normal distribution are represented by the mean ± standard deviation, and the t-test is applied for intergroup comparisons. Continuous variables that do not conform to a normal distribution are expressed as the median (interquartile range), and the Mann–Whitney U-test is chosen for intergroup comparisons. Categorical variables were presented in the form of counts and percentages (%), and the analysis of intergroup differences was completed through the Pearson's chi-squared (χ²) test or Fisher's exact test. The correlation between variables in patients with AAA was performed by Pearson or Spearman correlation analysis. The predictive value of the PHR for AAA and AAA with thrombosis was evaluated through the receiver operating characteristic (ROC) curve, and the optimal cut-off value of PHR was determined. Meanwhile, the sensitivity and specificity corresponding to each cut-off value were analyzed. Logistic regression analysis was used to screen the predictive factors for AAA and the predictive indicators for the presence of thrombosis in AAA patients. In the multivariate logistic regression model, variables with a P < 0.10 were included in the model. All statistical analysis was conducted two-sided, and the P-value < 0.05 was regarded as statistically significant.
3 Results
3.1 Baseline characteristics
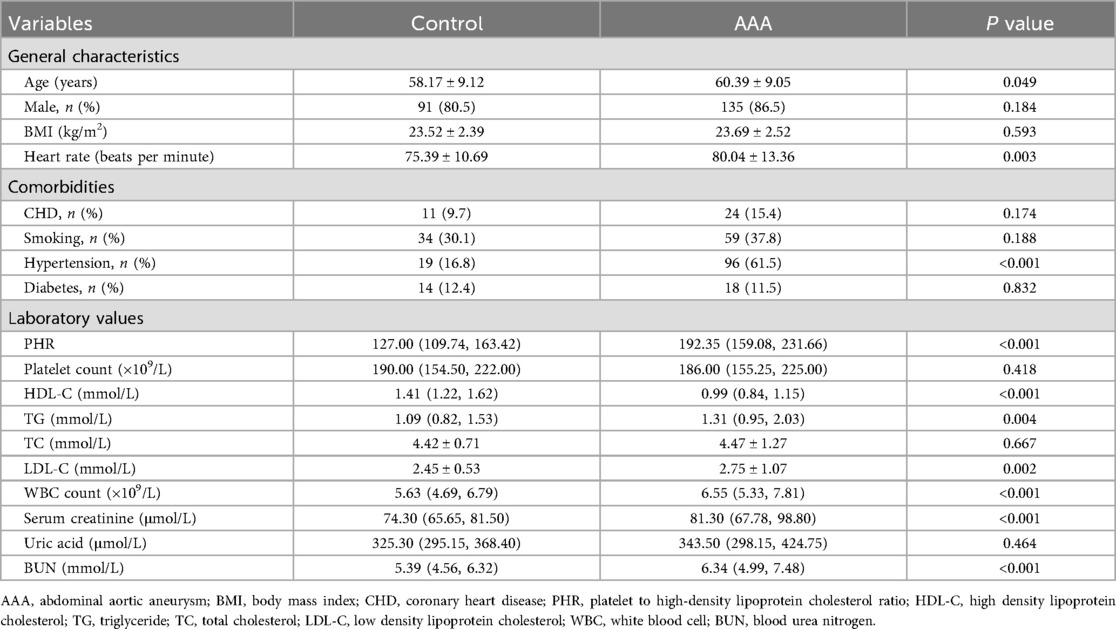
A total of 269 patients were included in this study, including 156 patients with AAA and 113 gender-matched healthy controls. The baseline characteristics of the study population are presented in Table 1.
Patients with AAA were significantly older than healthy controls, with a faster heart rate and a higher prevalence of hypertension. However, there were no significant differences in gender, BMI, or smoking history between the two groups, and the prevalences of diabetes and CHD were similar. In terms of laboratory values, AAA patients also had higher levels of TG, LDL-C, WBC, serum creatinine, and BUN. However, their HDL-C level was significantly lower, and there were no significant differences in platelet count, TC, and uric acid levels. Interesting, the PHR level in AAA patients was significantly higher [192.35 (159.08, 231.66) vs. 127.00 (109.74, 163.42); P < 0.001] (Figure 1). To further assess the significance of PHR levels in AAA patients across different platelet count strata, we stratified all participants into two groups: one with platelet counts exceeding the group's mean (designated as the high platelet count group) and another with counts below this mean (low platelet count group). This stratification was based on the average platelet count determined for each participant group. Our analysis revealed a notable elevation in PHR across both AAA patient groups, as detailed in Additional File 1: Supplementary Figure S1.
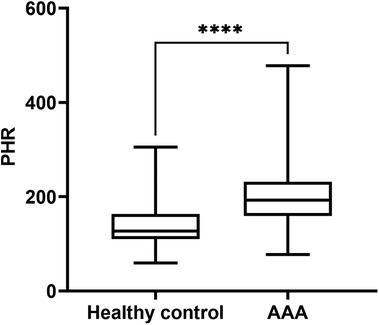
Figure 1. PHR levels in patients with AAA and healthy control group. Data were presented as median (min -max). AAA, abdominal aortic aneurysm; PHR, platelet to high-density lipoprotein cholesterol ratio; ****P < 0.0001.
3.2 The correlation between PHR and aneurysm diameter in patients with AAA
To further evaluate whether the PHR is associated with the disease progression in patients with AAA, we further examined the correlation between PHR and the diameter of AAA. The results showed that there was a significant positive correlation between the PHR level and the aneurysm diameter in AAA patients (r = 0.413, P < 0.001) (Figure 2A). This suggests that PHR has potential value in assessing the progression of AAA. Additionally, we conducted a detailed analysis of the correlation between HDL-C and platelet count in patients with AAA. Our results showed no significant correlation between these two variables (r = 0.076, P = 0.217) (Figure 2B), suggesting that HDL-C and platelets do not have a substitutable relationship. Further correlation analysis between HDL-C and aneurysm diameter in AAA patients revealed a significant negative correlation (r = −0.194, P = 0.015) (Figure 2C), which implies that lower HDL-C levels may be associated with aneurysm expansion. In contrast, a significant positive correlation was observed between platelet count and aneurysm diameter in AAA patients (r = 0.334, P < 0.001) (Figure 2D), indicating that higher platelet counts may be associated with aneurysm expansion.
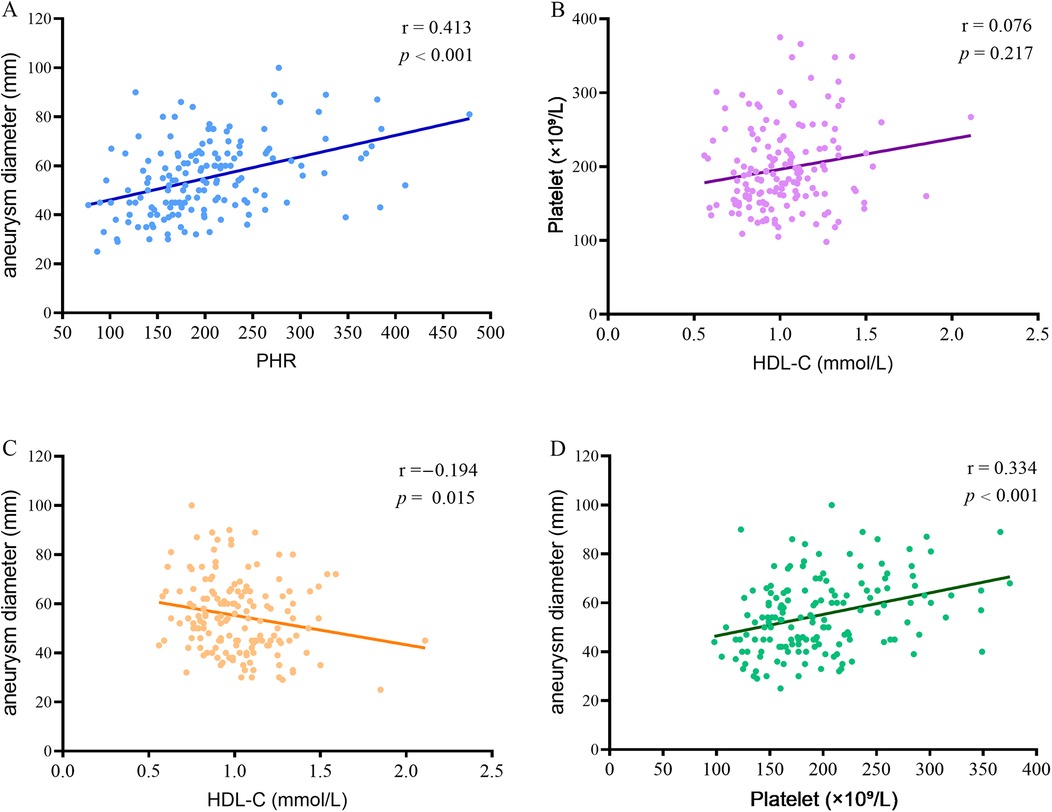
Figure 2. Correlation between PHR and aneurysm diameter in patients with AAA. (A) Correlation between PHR and aneurysm diameter in patients with AAA; (B) The correlation between HDL-C and platelet count in patients with AAA; (C) The correlation between HDL-C and aneurysm diameter in AAA patients; (D) The correlation between platelet count and aneurysm diameter in AAA patients. PHR, platelet to high-density lipoprotein cholesterol ratio; AAA, abdominal aortic aneurysm; HDL-C, high density lipoprotein cholesterol.
3.3 The association between PHR and thrombosis in AAA patients
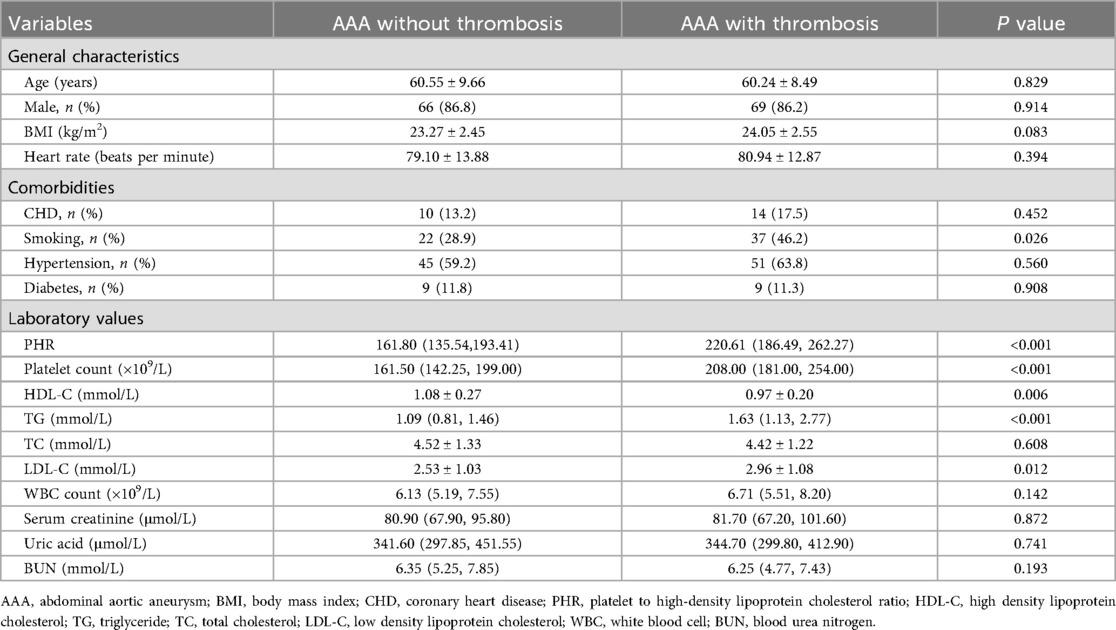
Among the 156 patients with AAA, a total of 80 patients had concomitant thrombosis. Subsequently, we further analyzed the differences in PHR between AAA patients with thrombosis and those without thrombosis. The results indicated that the PHR in AAA patients with thrombosis was significantly higher than that in AAA patients without thrombosis [220.61(186.49, 262.27) vs. 161.80(135.54, 193.41); P < 0.001] (Figure 3). Moreover, there were more smokers among AAA patients with thrombosis, and they had higher platelet counts, higher TG and LDL-C levels, and lower HDL-C levels (Table 2).
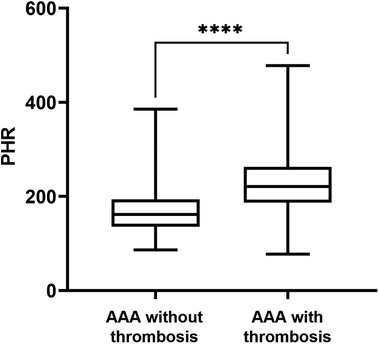
Figure 3. PHR levels in AAA patients divided by the thrombosis. Data were presented as median (min -max). AAA, abdominal aortic aneurysm; PHR, platelet to high-density lipoprotein cholesterol ratio; ****P < 0.0001.
3.4 Evaluation of the diagnostic value of PHR for AAA
To further evaluate the diagnostic performance of PHR in patients with AAA, we conducted a ROC curve analysis. The results showed that PHR had a good predictive value for the diagnosis of AAA. The area under the curve (AUC) was 0.805, and the optimal cut-off value was 170.7 (sensitivity: 84.96%, specificity: 66.03%) (Figure 4A). In addition, for the diagnosis of AAA complicated with thrombosis, the AUC of PHR was 0.814, and the optimal cut-off value was 204.6 (sensitivity: 86.84%, specificity: 66.25%) (Figure 4B). To assess whether the PHR offers superior diagnostic performance compared to platelet count and HDL-C individually, we conducted ROC curve analyses for both platelet count and HDL-C. Our findings revealed that HDL-C also holds considerable diagnostic value for AAA. However, when considering the overall diagnostic capabilities, PHR not only exhibited robust diagnostic efficacy for AAA but also significantly outperformed both platelet count and HDL-C in diagnosing AAA complicated with thrombosis (Additional File 1: Supplementary Figure S2). Furthermore, to determine if PHR surpasses traditional factors in diagnosing AAA, we compared the diagnostic performance of PHR alone, hypertension alone, and the combination of PHR and hypertension using ROC curves. The results indicated that PHR alone exhibits superior diagnostic performance compared to hypertension. Moreover, integrating PHR with hypertension further enhances diagnostic accuracy (Additional File 1: Supplementary Figure S3).
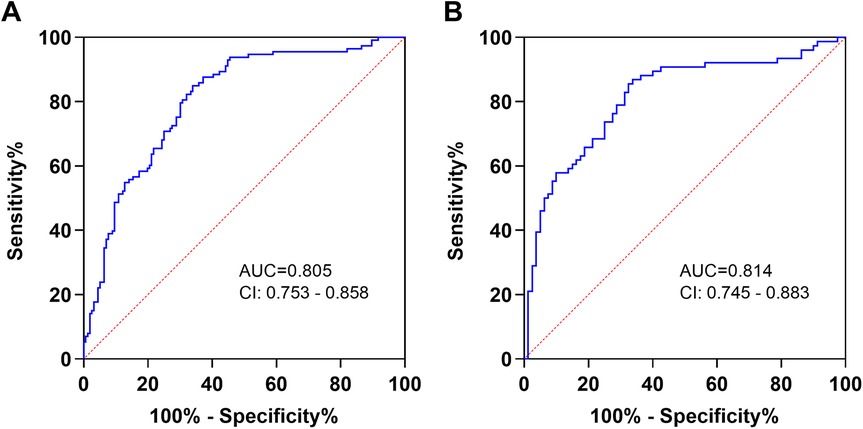
Figure 4. Diagnostic value of PHR in AAA patients. (A) The ROC curves of PHR to AAA patients and healthy controls. (B) The ROC curves of PHR to thrombosis in AAA patients. AUC, area under the curve; CI, confidence interval.
3.5 Univariate and multivariate logistic regression analyses
To investigate whether PHR could serve as a predictor for AAA, univariate and multivariate logistic regression analyses were performed, and the results are shown in Table 3. Univariate logistic regression analysis revealed that age, heart rate, hypertension, PHR, HDL-C, TG, LDL-C, WBC, serum creatinine, and BUN were significantly associated with AAA. Subsequently, multivariate regression analysis indicated that PHR (OR: 1.022; 95% CI: 1.012–1.031; P < 0.001) remained an independent predictor of AAA after adjusting for confounding factors. Additionally, hypertension, serum creatinine, and BUN were also associated with the presence of AAA.
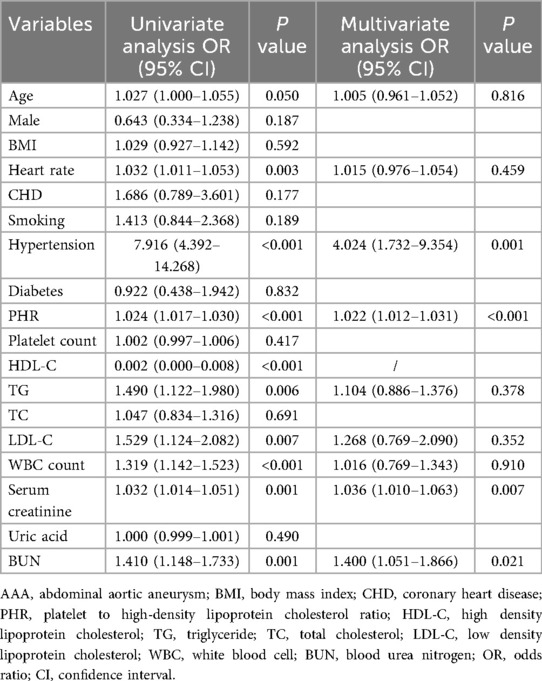
Table 3. Univariate and multivariate logistic regression analysis showing the independent predictors for AAA.
We further explored the predictive value of PHR for thrombosis in AAA patients, and the results are shown in Table 4. Univariate logistic regression analysis revealed that smoking, PHR, platelet count, HDL-C, TG, and LDL-C were closely associated with thrombosis. After adjusting for univariate predictors with P < 0.10, PHR (OR: 1.016; 95% CI: 1.007–1.024; P < 0.001) remained an independent factor for predicting thrombosis in AAA patients.
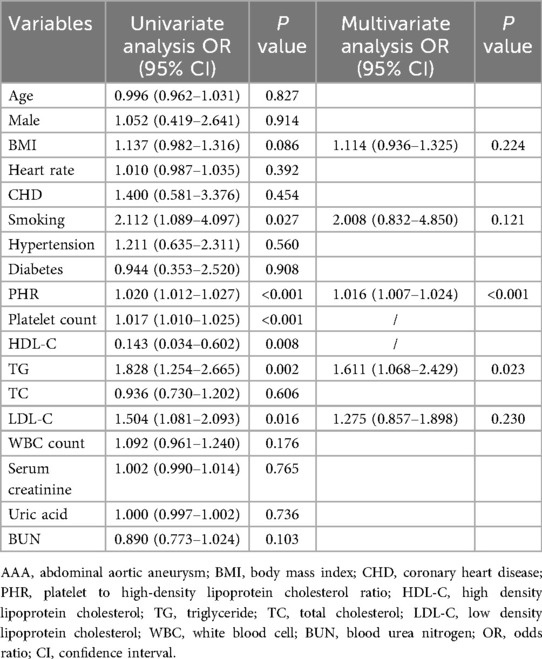
Table 4. Univariate and multivariate logistic regression analysis showing the independent predictors for thrombosis in AAA patients.
4 Discussions
In this study, we have demonstrated that the PHR is significantly elevated in patients with AAA, particularly in those with thrombosis, compared to controls. Importantly, we have identified a positive correlation between PHR and aneurysm diameter in AAA patients. Furthermore, our analysis indicates that PHR serves as an independent predictor of both AAA and AAA complicated with thrombosis. Specifically, at a cutoff value of 170.7, PHR achieves a sensitivity of 84.96% and a specificity of 66.03% for identifying AAA patients. At a higher cutoff value of 204.6, the sensitivity increases to 86.84%, with a specificity of 66.25% for detecting thrombosis in AAA patients. To our knowledge, this study represents the first to provide compelling evidence supporting the potential use of PHR as a novel diagnostic marker for AAA.
Studies have consistently shown that HDL-C levels are inversely related to the risk of AAA (13). For instance, a Danish registry study revealed that the average HDL-C concentration in AAA patients was significantly lower than that in patients with aorto-iliac occlusive diseases (25). Furthermore, there is a clear inverse correlation between HDL-C levels and AAA diameter, and therapies targeting HDL-C have been shown to effectively contribute to the prevention of AAA formation (26). HDL-C is primarily synthesized in the liver and is responsible for transporting cholesterol from extra-hepatic tissues to the liver for excretion (27). In AAA patients, however, the ability of HDL-C to promote cholesterol efflux from macrophages in the aortic wall is impaired (28). As a result, macrophages in the aortic wall of AAA patients become engorged with cholesterol, and the normal function of HDL-C in removing this cholesterol is disrupted, which may contribute to the development of AAA (28). Moreover, reduced levels of HDL-C can lead to a weakening of its anti-inflammatory, anti-oxidative, and anti-atherosclerotic effects, thereby promoting the occurrence and progression of AAA (27, 29).
In addition to abnormal lipid metabolism, hypertension—a traditional risk factor for AAA—also plays a significant role in the diagnosis of AAA. Our findings highlight that combining the PHR with hypertension significantly enhances diagnostic performance. This improvement can be explained by the following mechanisms: hypertension primarily damages the vascular wall structure through sustained blood pressure elevation, yet it fails to specifically identify other pathophysiological aspects of AAA. Conversely, PHR integrates platelet-driven inflammatory responses and HDL-C mediated anti-atherosclerotic effects, can precisely encapsulate the central pathological shifts in AAA's evolution and progression. This unique mechanistic profile empowers PHR to function not only as an independent AAA predictor but also to refine diagnostic precision when coupled with traditional risk factors like hypertension, thereby furnishing a more holistic foundation for the precise identification of AAA.
Previous studies have demonstrated that platelet count is closely associated with poor postoperative prognosis in patients with ruptured AAA (30). Specifically, patients with decreased platelet counts are at a significantly increased risk of bleeding and multiple organ failure, while those with elevated platelet levels are more prone to developing deep venous thrombosis and pulmonary embolism (30). In our current study, although no significant difference in platelet count was observed between AAA patients and healthy controls, the PHR was significantly elevated in AAA patients, irrespective of their platelet count levels. Moreover, a positive correlation was established between platelet count and aneurysm diameter, underscoring the close relationship between platelet count and the severity of AAA. This finding contrasts with previous studies that reported a significant decrease in platelet count among patients with ruptured AAA (30). The discrepancy can be attributed to the differing study populations. Our study predominantly included patients without acute rupture, resulting in relatively mild platelet consumption. Conversely, the prior study centered on patients undergoing surgical repair for ruptured AAA, a context where platelet activation and consumption are more pronounced, thereby increasing the likelihood of a reduced platelet count. This distinction in study populations accounts for the divergent findings regarding platelet count.
In addition, our study also found that the PHR level was higher in AAA patients with thrombosis. Moreover, the ROC curve indicated that PHR had a relatively high diagnostic value for thrombosis in AAA patients. Multivariate regression analysis showed that PHR was an independent predictor of thrombosis in AAA patients. Thrombosis is very common in AAA patients (31). The role of thrombosis in the progression of AAA is complex, but overall, its harmful effects outweigh its protective effects (32). Studies have shown that intraluminal thrombosis is closely associated with the rapid growth of AAA (33). Therefore, early screening for thrombosis in AAA patients is crucial for patient risk stratification and treatment.
Platelets and HDL-C are closely related to thrombosis in AAA. Platelets are of great significance in the process of formation and the growth of the thrombosis in AAA. Platelets play a crucial role in the formation and growth of thrombosis in AAA. Thrombosis occurs when platelets adhere to the complex formed by collagen and von Willebrand factor (vWF) (32). Subsequently, the binding of fibrinogen to activated integrin αIIbβ3 on platelets leads to platelet aggregation (32). Moreover, platelets within the thrombus, which have secretory functions, release soluble CD40 ligand (sCD40l), soluble P-selectin, and other substances into the bloodstream (34). These released substances can enhance the activation and accumulation of inflammatory cells (35), thereby accelerating the development of both thrombus and AAA. In addition to platelets, HDL-C also plays an important regulatory role in thrombosis. Studies suggest that HDL-C exerts an antithrombotic effect by preventing the self-association of vWF and the subsequent adhesion of platelets (36). Our study demonstrates that the PHR, a composite index composed of platelets and HDL-C, has a relatively high diagnostic value for thrombosis in AAA patients. However, due to the small sample size in this study, further verification by large-sample clinical studies is still needed.
The positive correlation between PHR and AAA diameter observed in our study is consistent with prior research linking low HDL-C levels to the formation and progression of AAA (13, 37). HDL-C deficiency promotes macrophage infiltration and foam cell formation in the aortic wall, while platelet hyperactivity accelerates intraluminal thrombosis formation (28, 32, 33). The PHR metric integrates these dual pathways: low HDL-C fosters a microenvironment conducive to inflammation, while elevated platelet counts exacerbate thrombus-mediated injury. This synergy likely amplifies extracellular matrix degradation and aneurysmal dilation. Clinically, the correlation between PHR and aneurysm diameter suggests that systemic metabolic-inflammatory derangements, rather than localized pathology alone, drive AAA growth. The predictive utility of the PHR may outperform traditional cardiovascular risk factors due to its composite reflection of the pro-thrombotic and anti-inflammatory imbalance (38, 39).
Although the diagnostical performance of PHR in AAA is encouraging, there are several limitations that need to be mentioned. Firstly, the retrospective, single-center design with a small sample size limits causal inference and accuracy, necessitating validation in larger, prospective cohorts. Secondly, the present study primarily focuses on the diagnostic value of the PHR in patients with AAA. It did not encompass long-term follow-up of AAA patients, nor did it evaluate the impact of PHR on the prognosis of AAA patients. Future research endeavors will involve extended follow-up of AAA patients to explore the correlation between PHR and acute AAA rupture, as well as to monitor the dynamic changes in PHR levels following treatment. Thirdly, non-invasive imaging at diagnosis can directly visualizes the presence of intraluminal thrombus along with aneurysm diameter in AAA patients. However, frequent follow-up imaging is often impractical, making it challenging to dynamically monitor the formation of intraluminal thrombus in AAA patients and to assess the dynamic process of thrombus development. In contrast, the PHR can be readily calculated from routine blood test results, offering the advantages of ease of use and low cost. This biomarker not only facilitates follow-up but also enables dynamic monitoring and assessment of thrombus formation in AAA patients, thus serving as a practical alternative or supplementary tool to imaging examinations.
5 Conclusion
Our findings reveal that the PHR possesses substantial diagnostic value for patients with AAA. Notably, PHR maintains robust diagnostic performance even in AAA patients with thrombus formation. These results suggest that PHR has the potential to serve as a practical and scalable clinical tool for the diagnosis of AAA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Ethics Committee at the Second Xiangya Hospital of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XD: Data curation, Writing – review & editing, Visualization, Writing – original draft, Formal analysis. JX: Data curation, Investigation, Writing – review & editing, Visualization, Formal analysis. SZ: Supervision, Writing – original draft, Writing – review & editing, Methodology, Project administration, Conceptualization. LL: Writing – original draft, Funding acquisition, Conceptualization, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the project of Hunan Administration of Traditional Chinese Medicine (No. B2024116), and Hunan Provincial Health High-Level Talent Scientific Research Project (No. R2023077).
Acknowledgments
We are indebted to all members who contributed to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1687265/full#supplementary-material
Abbreviations
AAA, abdominal aortic aneurysm; PHR, platelet to high-density lipoprotein cholesterol ratio; HDL-C, high density lipoprotein cholesterol; CTA, computed tomography angiography; MRA, magnetic resonance angiography; MMPs, matrix metalloproteinases; OGTT, oral glucose tolerance test; SBP, systolic blood pressure; DBP, diastolic blood pressure; CHD, coronary heart disease; TG, triglycerides; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; WBC, white blood cell; BUN, blood urea nitrogen; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; CRP, C-reactive protein; vWF, von Willebrand factor; AUC, area under the curve; OR, odds ratio; CI, confidence interval.
References
1. Golledge J, Thanigaimani S, Powell JT, Tsao PS. Pathogenesis and management of abdominal aortic aneurysm. Eur Heart J. (2023) 44(29):2682–97. doi: 10.1093/eurheartj/ehad386
2. van Leeuwen GL, Kooijman MA, Schuurmann RCL, van Leeuwen BL, van Munster BC, van der Wal-Huisman H, et al. Health literacy and disease knowledge of patients with peripheral arterial disease or abdominal aortic aneurysm: a scoping review. Eur J Vasc Endovasc Surg. (2024) 67(6):935–47. doi: 10.1016/j.ejvs.2024.03.040
3. Musto L, Smith A, Pepper C, Bujkiewicz S, Bown M. Risk factor-targeted abdominal aortic aneurysm screening: systematic review of risk prediction for abdominal aortic aneurysm. Br J Surg. (2024) 111(9):znae239. doi: 10.1093/bjs/znae239
4. Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. (2021) 18(5):331–48. doi: 10.1038/s41569-020-00472-6
5. Seike Y, Azuma N, Ohki T, Morikage N, Kodama A, Sumi M, et al. Current Status of diagnostic process in asymptomatic abdominal aortic aneurysm in Japan. Ann Vasc Dis. (2025) 18(1):25–00025. doi: 10.3400/avd.oa.25-00025
6. Ho VT, Cabot JH, George EL, Garcia-Toca M, Chen JH, Asch SM, et al. Expansion of abdominal aortic aneurysm screening and ultrasound utilization and diagnosis. JAMA Surg. (2023) 158(12):1349–51. doi: 10.1001/jamasurg.2023.4662
7. Cai H, Pan B, Xu J, Liu S, Wang L, Wu K, et al. D-Dimer is a diagnostic biomarker of abdominal aortic aneurysm in patients with peripheral artery disease. Front Cardiovasc Med. (2022) 9:890228. doi: 10.3389/fcvm.2022.890228
8. Badger SA, Soong CV, O'Donnell ME, Mercer C, Young IS, Hughes AE. C-reactive protein (CRP) elevation in patients with abdominal aortic aneurysm is independent of the most important CRP genetic polymorphism. J Vasc Surg. (2009) 49(1):178–84. doi: 10.1016/j.jvs.2008.07.081
9. Jabłońska A, Zagrapan B, Neumayer C, Eilenberg W, Scheuba A, Brostjan C, et al. Polymorphisms in the IL-6 and TNF-α gene are associated with an increased risk of abdominal aortic aneurysm. Int J Cardiol. (2021) 329:192–7. doi: 10.1016/j.ijcard.2020.12.051
10. Cameron SJ, Li XS, Benson TW, Conrad KA, Wang Z, Fleifil S, et al. Circulating trimethylamine N-oxide and growth rate of abdominal aortic aneurysms and surgical risk. JAMA Cardiol. (2025) 10(10):1000–1009. doi: 10.1001/jamacardio.2025.2698
11. Harrison SC, Holmes MV, Burgess S, Asselbergs FW, Jones GT, Baas AF, et al. Genetic association of lipids and lipid drug targets with abdominal aortic aneurysm: a meta-analysis. JAMA Cardiol. (2018) 3(1):26–33. doi: 10.1001/jamacardio.2017.4293
12. Perswani P, Ismail SM, Mumtaz H, Uddin N, Asfand M, Khalil ABB, et al. Rethinking HDL-C: an in-depth narrative review of its role in cardiovascular health. Curr Probl Cardiol. (2024) 49(2):102152. doi: 10.1016/j.cpcardiol.2023.102152
13. Peng Z, Qiu P, Guo H, Zhu C, Zheng J, Pu H, et al. Association between high-density lipoprotein cholesterol and risk of abdominal aortic aneurysm among males and females aged 60 years and over. J Vasc Surg. (2025) 81(4):894–904.e6. doi: 10.1016/j.jvs.2024.12.004
14. Sun W, Zheng J, Gao Y. Targeting platelet activation in abdominal aortic aneurysm: current knowledge and perspectives. Biomolecules. (2022) 12(2):206. doi: 10.3390/biom12020206
15. Feige T, Bosbach A, Krott KJ, Mulorz J, Chatterjee M, Ortscheid J, et al. GP VI-mediated platelet activation and procoagulant activity aggravate inflammation and aortic wall remodeling in abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. (2024) 44(11):2294–317. doi: 10.1161/atvbaha.123.320615
16. Wagenhäuser MU, Mulorz J, Krott KJ, Bosbach A, Feige T, Rhee YH, et al. Crosstalk of platelets with macrophages and fibroblasts aggravates inflammation, aortic wall stiffening, and osteopontin release in abdominal aortic aneurysm. Cardiovasc Res. (2024) 120(4):417–32. doi: 10.1093/cvr/cvad168
17. Wemmelund H, Jørgensen TM, Høgh A, Behr-Rasmussen C, Johnsen SP, Lindholt JS. Low-dose aspirin and rupture of abdominal aortic aneurysm. J Vasc Surg. (2017) 65(3):616–25.e4. doi: 10.1016/j.jvs.2016.04.061
18. Hariri E, Matta M, Layoun H, Badwan O, Braghieri L, Owens AP 3rd, et al. Antiplatelet therapy, abdominal aortic aneurysm progression, and clinical outcomes. JAMA Netw Open. (2023) 6(12):e2347296. doi: 10.1001/jamanetworkopen.2023.47296
19. Benson TW, Pike MM, Spuzzillo A, Hicks SM, Ali S, Pham M, et al. Soluble glycoprotein VI predicts abdominal aortic aneurysm growth rate and is a novel therapeutic target. Blood. (2024) 144(16):1663–78. doi: 10.1182/blood.2023021655
20. Hou N, Zhou H, Li J, Xiong X, Deng H, Xiong S. Macrophage polarization and metabolic reprogramming in abdominal aortic aneurysm. Immun Inflamm Dis. (2024) 12(11):e1268. doi: 10.1002/iid3.1268
21. Li N. Platelets as an inter-player between hyperlipidaemia and atherosclerosis. J Intern Med. (2024) 296(1):39–52. doi: 10.1111/joim.13794
22. Huang Y, Hou X, Lv F, Gong Z. Association of the platelets to high density lipoprotein cholesterol ratio and risk of heart disease events in middle-aged and elderly Chinese population: a retrospective cohort study utilizing the CHARLS database. Rev Cardiovasc Med. (2025) 26(2):26403. doi: 10.31083/rcm26403
23. Chen J, Wang B, Liu C, Li C, Meng T, Wang J, et al. Association between platelet to high-density lipoprotein cholesterol ratio (PHR) and hypertension: evidence from NHANES 2005–2018. Lipids Health Dis. (2024) 23(1):346. doi: 10.1186/s12944-024-02342-3
24. Wang B, Wang J, Liu C, Hu X. The potential of platelet to high-density lipoprotein cholesterol ratio (PHR) as a novel biomarker for heart failure. Sci Rep. (2024) 14(1):23283. doi: 10.1038/s41598-024-75453-7
25. Burillo E, Lindholt JS, Molina-Sánchez P, Jorge I, Martinez-Pinna R, Blanco-Colio LM, et al. ApoA-I/HDL-C levels are inversely associated with abdominal aortic aneurysm progression. Thromb Haemost. (2015) 113(6):1335–46. doi: 10.1160/th14-10-0874
26. Hellenthal FA, Pulinx B, Welten RJ, Teijink JA, van Dieijen-Visser MP, Wodzig WK, et al. Circulating biomarkers and abdominal aortic aneurysm size. J Surg Res. (2012) 176(2):672–8. doi: 10.1016/j.jss.2011.09.040
27. Lu D, Si K, Huo G. Association between uric acid to high-density lipoprotein cholesterol ratio and abdominal aortic aneurysm: a single-center retrospective study. J Inflamm Res. (2025) 18:3217–26. doi: 10.2147/jir.S508355
28. Martínez-López D, Cedó L, Metso J, Burillo E, García-León A, Canyelles M, et al. Impaired HDL (high-density lipoprotein)-mediated macrophage cholesterol efflux in patients with abdominal aortic aneurysm-brief report. Arterioscler Thromb Vasc Biol. (2018) 38(11):2750–4. doi: 10.1161/atvbaha.118.311704
29. Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. (2015) 102(2):276–94. doi: 10.3945/ajcn.114.100305
30. Bradbury AW, Bachoo P, Milne AA, Duncan JL. Platelet count and the outcome of operation for ruptured abdominal aortic aneurysm. J Vasc Surg. (1995) 21(3):484–91. doi: 10.1016/s0741-5214(95)70291-1
31. Bontekoe J, Matsumura J, Liu B. Thrombosis in the pathogenesis of abdominal aortic aneurysm. JVS Vasc Sci. (2023) 4:100106. doi: 10.1016/j.jvssci.2023.100106
32. Ma X, Xia S, Liu G, Song C. The detrimental role of intraluminal thrombus outweighs protective advantage in abdominal aortic aneurysm pathogenesis: the implications for the anti-platelet therapy. Biomolecules. (2022) 12(7):942. doi: 10.3390/biom12070942
33. Zhu C, Leach JR, Wang Y, Gasper W, Saloner D, Hope MD. Intraluminal thrombus predicts rapid growth of abdominal aortic aneurysms. Radiology. (2020) 294(3):707–13. doi: 10.1148/radiol.2020191723
34. Dib PRB, Quirino-Teixeira AC, Merij LB, Pinheiro MBM, Rozini SV, Andrade FB, et al. Innate immune receptors in platelets and platelet-leukocyte interactions. J Leukoc Biol. (2020) 108(4):1157–82. doi: 10.1002/jlb.4mr0620-701r
35. Yokoyama S, Ikeda H, Haramaki N, Yasukawa H, Murohara T, Imaizumi T. Platelet P-selectin plays an important role in arterial thrombogenesis by forming large stable platelet-leukocyte aggregates. J Am Coll Cardiol. (2005) 45(8):1280–6. doi: 10.1016/j.jacc.2004.12.071
36. Chung DW, Chen J, Ling M, Fu X, Blevins T, Parsons S, et al. High-density lipoprotein modulates thrombosis by preventing von Willebrand factor self-association and subsequent platelet adhesion. Blood. (2016) 127(5):637–45. doi: 10.1182/blood-2014-09-599530
37. Cagli K, Tok D, Turak O, Gunertem E, Yayla C, Lafci G, et al. Monocyte count-to-high-density lipoprotein-cholesterol ratio is associated with abdominal aortic aneurysm size. Biomark Med. (2016) 10(10):1039–47. doi: 10.2217/bmm-2016-0157
38. Wanhainen A, Mani K, Golledge J. Surrogate markers of abdominal aortic aneurysm progression. Arterioscler Thromb Vasc Biol. (2016) 36(2):236–44. doi: 10.1161/atvbaha.115.306538
39. Krishna SM, Seto SW, Jose RJ, Biros E, Moran CS, Wang Y, et al. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler Thromb Vasc Biol. (2015) 35(2):389–98. doi: 10.1161/atvbaha.114.304732
Keywords: abdominal aortic aneurysm, PHR, diagnostic marker, thrombosis, platelet, high-density lipoprotein cholesterol
Citation: Du X, Xu J, Zhang S and Liu L (2025) Diagnostic value of platelet to high-density lipoprotein cholesterol ratio in abdominal aortic aneurysms. Front. Cardiovasc. Med. 12:1687265. doi: 10.3389/fcvm.2025.1687265
Received: 17 August 2025; Accepted: 14 October 2025;
Published: 30 October 2025.
Edited by:
Norbert Gerdes, Heinrich Heine University Düsseldorf, GermanyReviewed by:
Hong Jin, Karolinska Institutet (KI), SwedenTyler Benson, University of Cincinnati, United States
Copyright: © 2025 Du, Xu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shilan Zhang, Y2FyZGlvX3NoaWxhbjUyMUAxNjMuY29t; Ling Liu, ZmVsaXVsaW5nQGNzdS5lZHUuY24=
 Xiao Du1
Xiao Du1 Jin Xu
Jin Xu Shilan Zhang
Shilan Zhang Ling Liu
Ling Liu