- 1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States
- 2Radiology Department, Harvard Medical School, Boston, MA, United States
- 3Center for Interdisciplinary Cardiovascular Sciences, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 4Center for Excellence in Vascular Biology, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 5Cardiovascular Research Center, Massachusetts General Hospital, Charlestown, MA, United States
Aim: Physiological bone mineralization and ectopic vascular calcification share similarities in the composition of calcium phosphate minerals. Evidence suggests a connection between the underlying biological mechanisms driving the deposition of bone mineral and cardiovascular calcification. Therefore, understanding the chemistry and composition of bone mineral and vascular calcification may be important for the development of effective treatments and diagnostic tools for cardiovascular diseases, as pharmacological interventions for the treatment of one process might affect the other. The goal of this study was to identify and compare compositional features of calcium phosphates in bone and calcified vascular tissues using phosphorus-31 (31P) solid state cross-polarization magic angle spinning (CPMAS) magnetic resonance (MR) spectroscopy, a specialized technique that provides compositional information unattainable through conventional chemical analysis.
Methods: Solid state MR spectra were acquired from biological specimens of human trabecular bone (n = 1), human vascular plaque (n = 1), human calcified aortic valves (n = 5), as well as calcified aortic tissues of apolipoprotein E-deficient mice (n = 1) fed a high cholesterol diet. Synthetic hydroxyapatite [Ca10(OH)2(PO4)6] and synthetic brushite (CaHPO4·2H2O) were used to model the solid state 31P MR spectra of the phosphate ion PO4–3 and hydrogen phosphate ion HPO4–2, respectively. Qualitative spectral features and quantitative metrics derived using Herzfeld-Berger analysis were assessed to characterize mineral composition and maturity.
Results: Solid state 31P MR spectra of all human specimens studied suggested a well-ordered crystal structure dominated by unprotonated phosphate (PO4–3), consistent with mature bone-like mineral. These specimens exhibited long CP time constants (700–900 µs) and modest chemical shift anisotropy. In contrast, the calcified mouse aorta spectrum showed pronounced sidebands, a short CP time constant (∼270 µs), and a more prominent HPO4–2 component—features indicative of immature, newly deposited mineral.
Conclusion: 31P solid state MR spectroscopy reveals differences in the phosphate and hydrogen phosphate ion content among the calcified tissues studied. This technique could potentially be an important complement to basic studies of pathological calcification in atherosclerosis and related calcific disorders.
1 Introduction
Physiological bone mineralization and pathological vascular calcification exhibit some similarities in their underlying mechanisms and calcium phosphate mineral composition (1, 2). Although the two processes are physiologically distinct, they share some cellular pathways and molecular mediators, including common proteins and regulatory factors (3–9). Inflammation and macrophage activities are key drivers of vascular calcification, and osteoclasts—the bone-resorbing cells—originate from the same hematopoietic lineage as macrophages (10, 11). Epidemiological studies have reported associations between osteoporosis and increased cardiovascular risk (12). Clinical observations further support this biological linkage: pharmacological agents used to treat cardiovascular diseases (CVDs) can influence bone metabolism, and conversely, treatments for osteoporosis may affect vascular calcification (13–15). Specifically, bisphosphonates (BPs), the most common anti-osteoporotic agents, inhibit bone resorption by binding to hydroxyapatite and suppressing osteoclast activity (16). BPs are non-hydrolyzable analogs of inorganic pyrophosphate, an endogenous inhibitor of vascular calcification (17, 18). BPs have been linked to altered lipid profiles and reduced aortic calcification (19). Moreover, statins have shown associations with increased bone mineral density (15). Estrogen deficiency, which accelerates bone loss, is also a known risk factor for atherosclerosis and vascular calcification (20, 21).
Vascular calcification is a key feature of atherosclerosis and a well-established predictor of cardiovascular events. While coronary artery calcium scores reflect total calcium burden, studies suggest that the chemical composition and structural organization of calcium deposits may more directly influence plaque stability and clinical outcomes (22–28). Beyond serving as a risk marker, mineral composition may reflect distinct stages of plaque development with differing biological implications, potentially clarifying mechanisms of disease and inform the design of targeted therapies aimed at specific phases of calcification.
In vertebrates, the final mineral product of biomineralization is a poorly crystalline, nonstoichiometric form of hydroxyapatite [Ca10(OH)2(PO4)6], often referred to as biological apatite. Solid state magnetic resonance (MR) spectroscopy evidence suggests that mature biological apatite may evolve from an initial precursor resembling dicalcium phosphate dihydrate (CaHPO4·2H2O, brushite), which is deposited in soft tissue during the earliest stages of mineralization (29). Alternative models of vertebrate mineralization propose other precursors—such as amorphous calcium phosphate [Ca3(PO4)2] or octacalcium phosphate [Ca8(HPO4)2(PO4)4·5H2O]—that may also transform into mature apatite over time. Hydroxyapatite, containing unprotonated phosphate (PO4−3), and brushite, containing protonated phosphate (HPO4−2), have been used as chemical models for the respective unprotonated and protonated phosphate ions in biological calcification. Therefore, one measure of the maturity of the biological mineral may be obtained by assessing the relative concentrations of HPO4−2 and PO4−2, given that mature bone mineral is dominated by PO4−3 and has a lower HPO4−2 concentration (29, 30).
Although several studies have characterized mineral phases in vascular calcification using techniques such as Fourier transform infrared spectroscopy (FTIR) and x-ray diffraction, these analyses have primarily provided qualitative identification of crystalline forms (e.g., hydroxyapatite, whitlockite) rather than quantitative differentiation of phosphate ion species. FTIR investigations by Rey et al. in bone (31, 32) and by Orzechowska et al. in calcified aortic valves (33) demonstrated valuable insight into phosphate protonation and carbonate substitution but required dehydrated, deproteinized specimens and did not resolve the relative abundance of HPO4−2 and PO4−3 in situ. In hydrated vascular tissues, conventional chemical analyses and vibrational spectroscopies remain limited by strong interference from residual water, proteins, and lipids (34). Clinical imaging modalities such as cardiac computed tomography (CT), intravascular ultrasound, and optical coherence tomography provide structural or qualitative compositional information but cannot assess mineral chemistry at the molecular level (35–41). In contrast, solid state MR spectroscopy offers a complementary approach capable of identifying and quantifying phosphate ion species in minimally processed mineralized tissues.
In this study, we employed two fundamental solid state MR spectroscopy techniques to analyze calcified tissue specimens: magic angle spinning (MAS) and cross-polarization (CP). The more common type of MR spectroscopy is performed on fluids, such as soft tissues in vivo or chemical solutions, where the signals from individual chemical groups (e.g., methyl groups, sodium ions, or water molecules) appear as resonance lines characterized primarily by their positions along the horizontal axis, known as the chemical shift, and measured in parts per million (ppm). Each chemical shift value uniquely corresponds to the identity of the respective chemical group. In contrast to fluids, in which rapid molecular tumbling and diffusion occur, molecules within solids are fixed in positions and orientations, making their chemical shifts orientation dependent. Consequently, MR spectra of solid substances, such as calcium phosphate mineral crystals present in calcified tissues, exhibit broad resonance patterns spanning a wide frequency range, complicating direct identification of chemical groups from single-valued chemical shifts. Such broad resonance profiles, known as powder patterns, reflect the geometric symmetry of the chemical group or ion. The central position of the powder pattern corresponds to the isotropic chemical shift that would be observed if the molecules were freely mobile, as in a fluid state.
MAS facilitates MR spectroscopy of solids by rapidly rotating the specimen around an axis inclined at approximately 54.7 degrees relative to the external magnetic field, the so-called “magic angle” (42). Rapid rotation partially averages orientation-dependent chemical shifts, analogous to random molecular tumbling in liquids, significantly reducing the breadth of powder patterns toward narrower resonance lines. To achieve a single-valued chemical shift, rotation rates must surpass the intrinsic width of the powder pattern, a condition that is technically challenging. Typically, lower rotation rates result in partial narrowing, producing a pattern characterized by sidebands spaced at intervals equal to the rotation frequency (e.g., Figure 1).
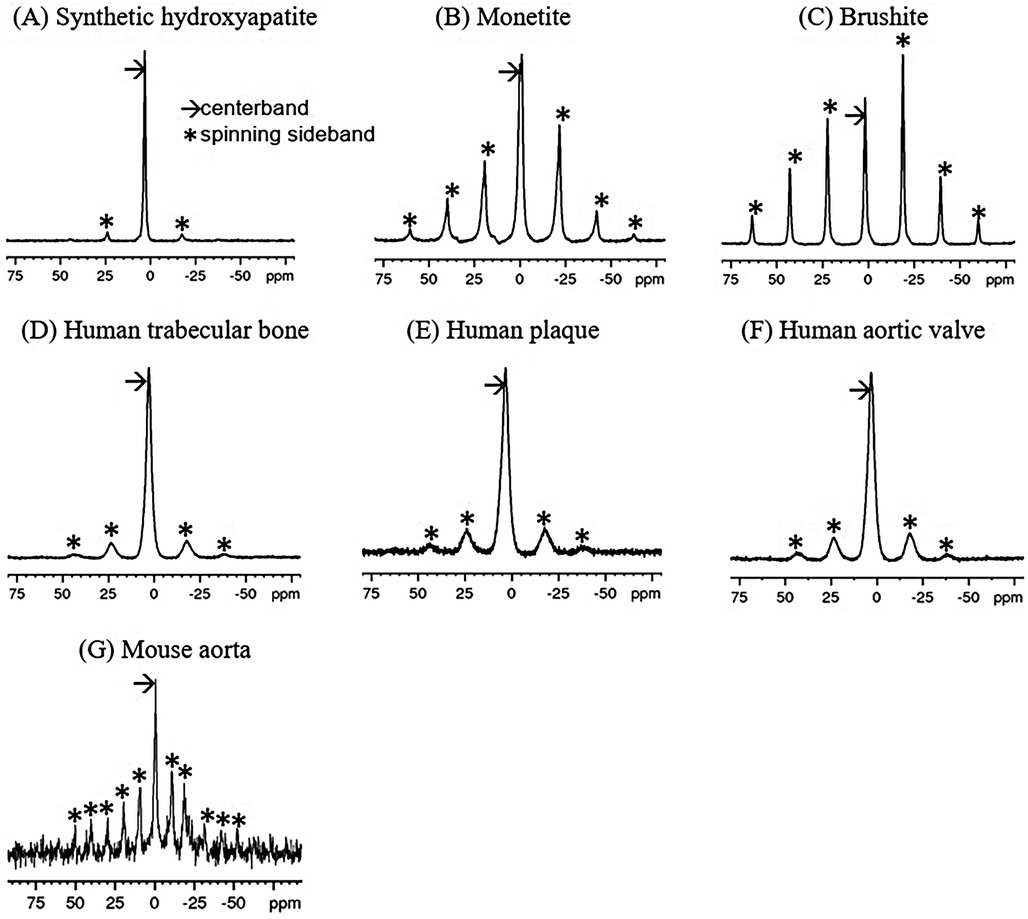
Figure 1. 31P single-pulse MAS spectra of synthetic calcium phosphate compounds (A–C) and 1H-31P CPMAS spectra of mineralized tissues (D–G). The spin rate for (A–F) was 5 kHz (∼21 ppm), while that for (G) was 2.5 kHz (∼10.5 ppm). Note that sidebands (*) are spaced at the spin rate. Chemical shifts are referenced to 85% H3PO4.
CP serves two main functions in solid state MR spectroscopy. First, CP can enhance signals from nuclear isotopes with inherently low signal-to-noise ratio (SNR), such as those with low MR frequencies, low isotopic or chemical abundances, or long spin-lattice relaxation times T1. This enhancement is achieved by transferring magnetization from isotopes with higher SNR (commonly protons, 1H) to the isotope of interest through simultaneous radiofrequency (RF) excitation. Second, CP is useful for spectral editing, differentiating chemical groups based on their proximity to the isotope source of magnetization. CP and MAS are frequently combined as cross-polarization magic angle spinning (CPMAS). It is important to note that CP magnetization transfer and the magnetization transfer effect in clinical MRI are fundamentally different. The former occurs via transverse magnetization exchange on microsecond-to-millisecond time scales, whereas the latter occurs on significantly longer (millisecond-to-second) time scales as a result of the diffusion of magnetization transported either by chemical diffusion of nuclear spins or by spin-spin coupling.
To date, aside from straightforward non-CP phosphorus-31 (31P) MAS measurements (43, 44), solid state MR techniques have not been utilized for examining vascular calcification. 1H-31P solid state CPMAS MR spectroscopy has proven effective in quantifying bone mineral and solid organic matrix ex vivo (45–51). In biological specimens, distinguishing phosphate ions such as PO4−3 and HPO4−2 based on chemical shifts (3.1 ppm and 1.4 ppm, respectively) may be challenging, as inherent crystallographic disorder leads to broad resonance linewidths even with MAS. Thus, measurement of CP transfer rates depend strongly on the proton-phosphorus distances, and may help discriminate between these ions. For instance, rapid CP occurs in HPO4−2 ions in which 1H and 31P nuclei are spaced by only a few tenths of a nanometer. Conversely, hydroxyapatite consists of spatially separated OH– ions (protons) and PO4−3 ions, leading to slower CP. Thus, the CP rate is reflective of the local crystal structure and can inform about the types of phosphate ions present, as established by prior studies in bone (47, 52). Indeed, 1H-31P CPMAS investigations of progressively mineralizing embryonic to mature chicken bone have demonstrated a clear reduction in HPO4−2 content from initial embryonic mineralization (approximately 8 days post-egg laying) to fully mature bone in 75-week-old chickens (30, 47). Given the biological parallels between bone mineralization and vascular calcification, we hypothesize that CPMAS could similarly reveal compositional variations within calcified vascular tissues, potentially providing insights into the mineral maturity of these tissues.
In this study, we applied CPMAS MR spectroscopy to investigate pathological calcifications from human plaque, human aortic valve, and the aorta of apolipoprotein E (ApoE)-deficient mice, along with human trabecular bone specimens. To characterize mineral maturity, we compared these spectra to reference spectra of calcium phosphates commonly used as mineral models of biological calcification. CP transfer rates were derived to assess differences in crystal structure and composition. Additionally, we applied Herzfeld-Berger (HB) analysis (53) to extract the principal values of the chemical shift tensor (CST), parameters mathematically modelling the orientation-dependent variation in chemical shifts, from the spectral data. Given adequate spectral quality, indicated by clear identification and assignment of spinning sidebands to phosphate ions (PO4−3 and HPO4−2), HB analysis can reliably determine both the relative abundance of these phosphate species and their respective chemical shift tensors, despite significant spectral overlap.
2 Materials and methods
2.1 Specimens
Commercial synthetic compounds included hydroxyapatite Ca10(OH)2(PO4)6, brushite (CaHPO4·2H2O), and monetite (CaHPO4) (Fisher Scientific, Hampton, NH, USA) were used without further purification or analysis. Human specimens included trabecular bone from autopsy (n = 1), calcified aortic plaque (n = 1) from autopsy, and calcified human aortic valves (n = 5) acquired at the time of surgical excision and placed in neutral buffered formalin. Calcified aortic tissue (aortic root and arch vessels) was harvested en bloc from 60-week-old ApoE-deficient mice (n = 1), that had been fed an atherogenic diet from 10 weeks of age; mouse specimens were preserved in formaldehyde buffer. All specimens were stored at −80°C until MR scanning.
Prior to MR spectroscopy, intravital fluorescence microscopy of the carotid artery in these mice was performed using a bisphosphonate-derived imaging agent (OsteoSense 750; dose 2 nmol/150 μL). µCT of the mice was also performed to detect calcification. The human aortic plaque specimen was obtained from autopsy and consisted of a macroscopically calcified aortic segment from a donor with advanced atherosclerosis. Morphological classification (e.g., sheet-like vs. nodular) was not performed, as only a small portion of the calcified tissue was retained for spectroscopy, and no additional imaging or histological characterization was available.
Animal studies were approved by the respective Institutional Animal Care and Use Committees. Use of discarded human specimens was approved by the Massachusetts General Hospital Institutional Review Board.
2.2 MR spectroscopy
Spectra were acquired using a Bruker Biospin (Billerica, MA, USA) Avance III multinuclear MR spectrometer equipped with a 14 Tesla Magnex Scientific (Oxford, UK) wide bore magnet (f1H = 600 MHz and f31P = 243 MHz). Samples, either compounds or calcified portions of the biological specimens, were tightly packed into 4 mm zirconia MAS rotors with a volume of about 78 μL. Both single-pulse 31P MAS and 1H-31P CPMAS spectra were acquired. For CP acquisitions, the 90° RF pulse length was 4.5 µs (B1 field 55 kHz) on both 1H and 31P channels. Single-pulse 31P spectra used a 90° RF pulse of 4.1 µs. The MAS spin rate was 5 kHz for most specimens, with 32 averages per spectrum and 2 s recycle delay (TR), resulting in 64 s acquisition duration per spectrum. For mouse aortic samples, which had lower mineral content and showed spinning instability due to tissue heterogeneity, the MAS rate was reduced to 2.5 kHz. To compensate, 7,400 scans were acquired per spectrum, giving a total acquisition time of approximately 4.1 h to ensure adequate signal-to-noise ratio (SNR). CP spectra were also acquired with varying CP times to measure 1H-31P CP time constants and 1H rotating frame spin-lattice relaxation time constants for the buildup and loss of magnetization, respectively.
2.3 MR data analysis
Spectral data were processed offline using Bruker TopSpin 3.6.2 (Bruker, Billerica, MA, USA). Up to 2 ppm line broadening was applied to the mouse aorta spectra for reasonable SNR. For each series of CP spectra with varying CP times, the spectrum with the most intense centerband exhibited the highest SNR, and its phase correction was applied to all spectra in the series. Because it is typically not possible to use an internal chemical shift reference in solid state MR spectroscopy, a hydroxyapatite external reference set to 3.1 ppm (corresponding to 85% phosphoric acid defined as 0 ppm) was applied.
Herzfeld-Berger analysis was performed on the spectra used for phasing, employing the software tool ssNake (55) to extract the principle values of the CST. The principle values constitute a set of three numbers which can be mathematically expressed in multiple ways: as three principal values , , and where , or as the isotropic chemical shift (analogous to the chemical shift in the fluid state) , the total anisotropy , and the asymmetry parameter .
The biological specimens generally exhibit spectra with broader linewidths than those of the synthetic compounds, such that the small isotropic chemical shift difference between PO4−3 and HPO4−2 prevents these resonances from being separately resolvable. For these spectra, the ssNake program fit the sideband pattern to two CSTs, putatively PO4−3 and HPO4−2. The isotropic (liquid-state equivalent) chemical shift is given by Equation 1:
The total anisotropy , a measure of the overall width of the chemical shift powder pattern, can be calculated as Equation 2:
This quantity provides an estimate of the extent to which the point symmetry of the crystal lattice at the nucleus deviates from ideal tetrahedral geometry. When the total anisotropy is zero, the spectral line appears sharp. The asymmetry parameter , which quantifies the deviation of the chemical shift tensor from axial symmetry, is given by Equation 3:
Centerband peak areas were determined by integration to quantify signal intensity for extracting CP and rotating frame spin-lattice relaxation time constants as a function of CP contact time t. The signal was fit to Equation (4) using a nonlinear least squares approach:
The analysis models the signal as a composite of contributions from both PO4−3 and HPO4−2 ions, without attempting to resolve them separately. The rationale behind this model for the time dependence of the 31P signal is that the first exponential term describes the buildup of magnetization via rapid 1H to 31P polarization transfer, while the second exponential term accounts for signal decay due to relaxation in the proton rotating frame. Additional details are provided in the discussion section.
3 Results
3.1 31P MR spectra
31P MAS spectra of the synthetic calcium phosphates and 1H-31P CPMAS spectra biological specimens are shown in Figure 1. Because of the high point symmetry of the unprotonated phosphate in hydroxyapatite (Figure 1A), its chemical shift anisotropy is small, and the spinning sidebands are weak. Its centerband (isotropic chemical shift) occurs at 3.1 ppm. There is no indication of other types of phosphate ion in the spectrum. In contrast, monetite (Figure 1B) and brushite (Figure 1C) contain a single acidic phosphate HPO4−2 and no PO4−3. The lower symmetry of the HPO4−2 ion in these compounds results in a large chemical shift anisotropy and a prominent sideband pattern. Their isotropic shifts occur upfield from hydroxyapatite.
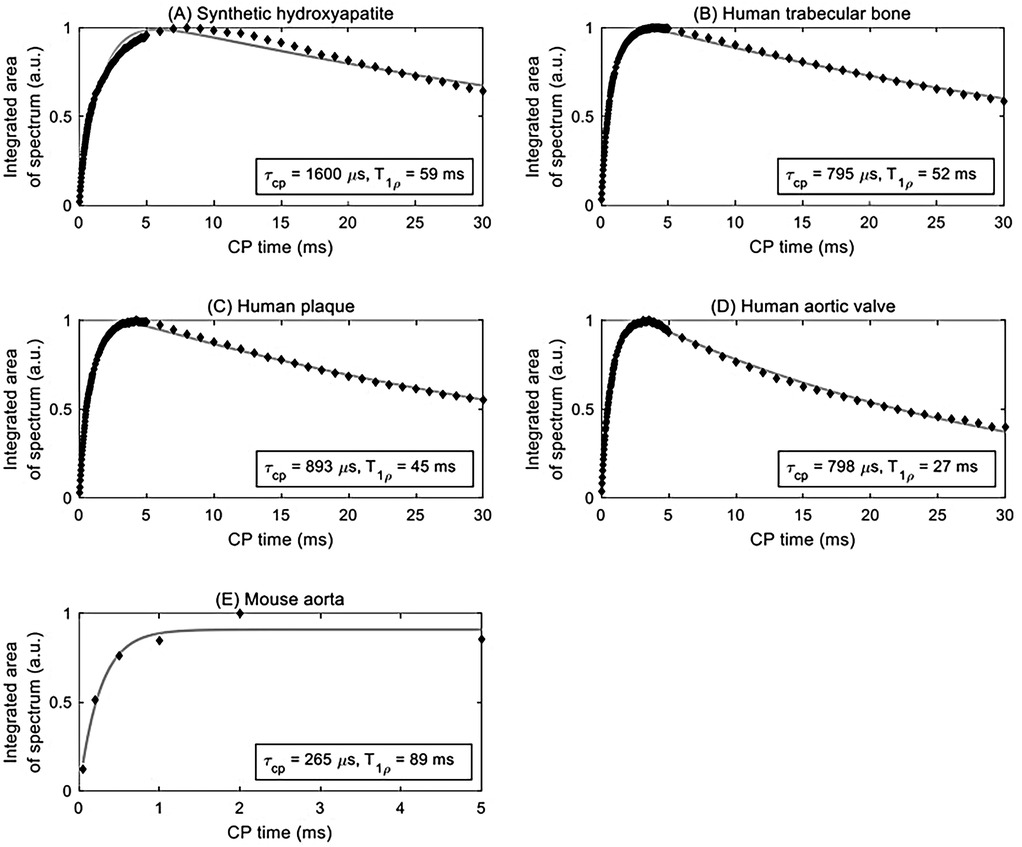
Figure 2. Curve fitting (solid grey line) of integrated area under the centerband (discrete points) provides an estimate of the CP time constant τCP and rotating frame spin-lattice relaxation time constant T1ρ for various samples: (A) synthetic hydroxyapatite, (B) human trabecular bone, (C) human plaque, (D) human aortic valve, and (E) mouse aorta.
The biological specimens exhibit broader spectral linewidths than the synthetic materials. The bone, plaque and calcified valve spectra (Figures 1D–F) resemble that of hydroxyapatite and have isotropic shifts of 3.1 ppm, but with somewhat more prominent sideband patterns similar to monetite and brushite. This suggests that the predominant phosphate ion is PO4−3, and that it is accompanied by a small amount of HPO4−2 which is contributing to the sidebands.
The mouse aorta spectrum is distinctly different from those of the other biological specimens in that the sidebands are more prominent, suggesting a higher level of HPO4−2 content (Figure 1G). The lower spin rate and SNR make visual comparison with the other spectra difficult. However, the analyses below reveal distinct differences from the other biological specimens.
3.2 Time constants
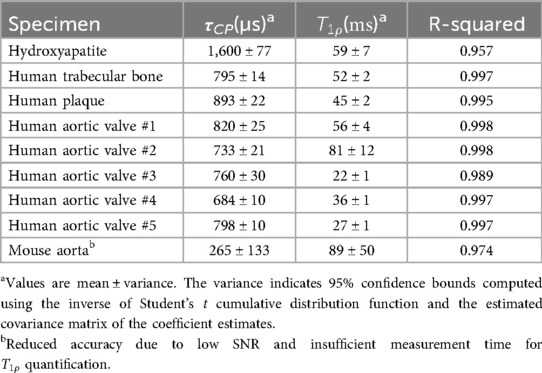
Signal curves as a function of CP time of selected specimens are shown in Figure 2, and the corresponding numerical results of the fits are summarized in Table 1. The time axis for the mouse aorta spectrum is limited to 5 ms because of challenges with spinning stability; this value therefore has higher uncertainty. All fits converged successfully.
Hydroxyapatite, containing only unprotonated phosphate, was found to have the longest CP time constant at 1,600 µs. For comparison, brushite, containing only protonated phosphate, was found in a previous study (47) to have a CP time constant of 140 µs. The biological specimens in this study fall in between these two extremes. The human specimens tend to be in the range of 700–900 µs, whereas the mouse aorta specimen has a much shorter CP time constant of about 270 µs, much closer to that of brushite.
3.3 Herzfeld-Berger chemical shift tensor
Chemical shift parameters based on Herzfeld-Berger analysis of the spinning sideband patterns are reported in Table 2. The isotropic chemical shifts are estimated to be accurate to ±0.2 ppm, and the chemical shift anisotropies are estimated to be accurate to ±2 ppm. The asymmetry parameters are estimated to be accurate to ±0.05. The sideband patterns of the synthetic compounds were fit to a single CST, whereas those of the biological specimens were fit to two CSTs, permitting the separation of unprotonated from protonated phosphates.
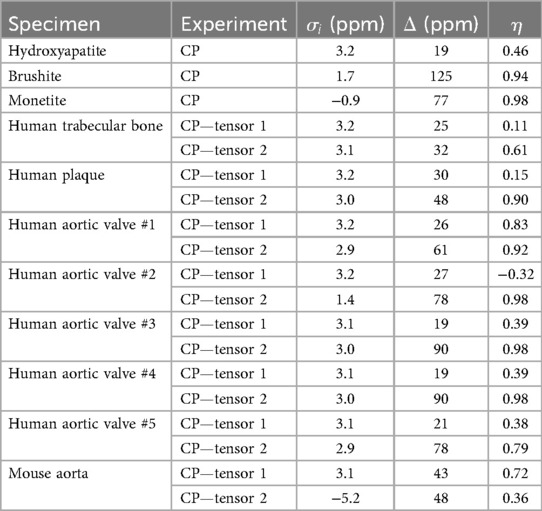
Table 2. 31P chemical shift tensor parameters of synthetic and biological calcium phosphate compounds.
The 31P chemical shift anisotropy of hydroxyapatite is small as expected because PO4−3 in hydroxyapatite is located at site of high tetrahedral symmetry. Brushite, which contains a single protonated phosphate ion HPO4−2, possesses a lower point symmetry, and therefore a larger chemical shift anisotropy than hydroxyapatite. The isotropic shift of brushite is shifted upfield (less positive) from hydroxyapatite, similar to the upfield shift with decreasing pH (increased protonation) found in aqueous orthophosphates (56, 57). The large value of from brushite indicates a nearly equidistant separation of the principal values of the tensor, and a highly symmetric powder pattern. The of monetite (CaHPO4, the anhydrous form of brushite), lies even further upfield.
Distinct from the “pure” environment of PO4−3 or HPO4−2 in the synthetic salts, biological calcium phosphates are mixed crystals containing both types of phosphate ions, as established by indirect wet chemical evidence (58, 59). Biological calcium phosphates also contain miscellaneous crystal lattice defects, vacancies and substitutions. The high surface area of biological calcium phosphate nanocrystals also substantially increases the microscopic heterogeneity of phosphate chemical environments. All of these factors increase the 31P spectral linewidths as readily apparent from the spectra in Figures 1D–G. Therefore, the analysis of the biological specimens parametrized the spectra as containing contributions from two chemical shift tensors representing PO4−3 and HPO4−2. Because HPO4−2 is the minor component, and because the PO4−3 and HPO4−2 spectra overlap almost perfectly, a lower accuracy is expected in the HPO4−2 parameters resulting from the fit. The isotropic chemical shift calculated from the first tensor of human trabecular bone, plaque and human aortic valves are all at 3.2 ppm (±0.2 ppm), which can be assigned to unprotonated phosphate based on its similarity to the hydroxyapatite isotropic shift. The chemical shift anisotropies calculated for the first tensor of the human specimens are somewhat larger than that of hydroxyapatite. The larger values are likely a reflection of the disordered nature of biological calcium phosphate crystals. The second tensor exhibits isotropic chemical shifts also at 3.2 ppm (±0.2 ppm), except for human aortic valve specimen #2 for which . The chemical shift anisotropy derived from the second tensor of the human specimens is increased as compared to those of the first tensor, suggestive of some level of “brushite-like” ions in the mineral crystals. The mouse aorta specimen exhibits a spectrum with considerable differences from the other biological specimens. One of the isotropic chemical shifts of mouse aorta lie substantially upfield , implying a higher HPO4−2 content of the crystals. This was further supported by the increased sideband intensities in Figure 1G. Furthermore, an earlier study reported HPO4−2 anisotropy and of a few bone specimens from chick embryo, adult chicken, calf embryo, adult calf, rabbit embryo and adult rabbit (47), which are consistently close to values of brushite.
The calculated chemical shift tensors can be used to generate simulated spinning patterns that closely resemble the original spinning patterns, as shown in Figure 3. For biological specimens characterized by two tensors, the composite pattern is the sum of two simulated patterns. The pattern with a stronger centerband and resonance closer to 3.1 ppm (green traces in Figures 3C–F) is associated with unprotonated phosphates, whereas the pattern with a weaker and broader centerband and resonance shifted upfield (less positive, blue traces in Figures 3C–F) is associated with protonated phosphates. Fitting the mouse spectrum to one tensor gives a reasonable result. The spectrum is too noisy to fit two tensors with reasonable confidence. However, the mouse spectrum implies the presence of freshly deposited mineral crystals as opposed to entirely mature hydroxyapatite crystals in the calcification of mouse aorta.
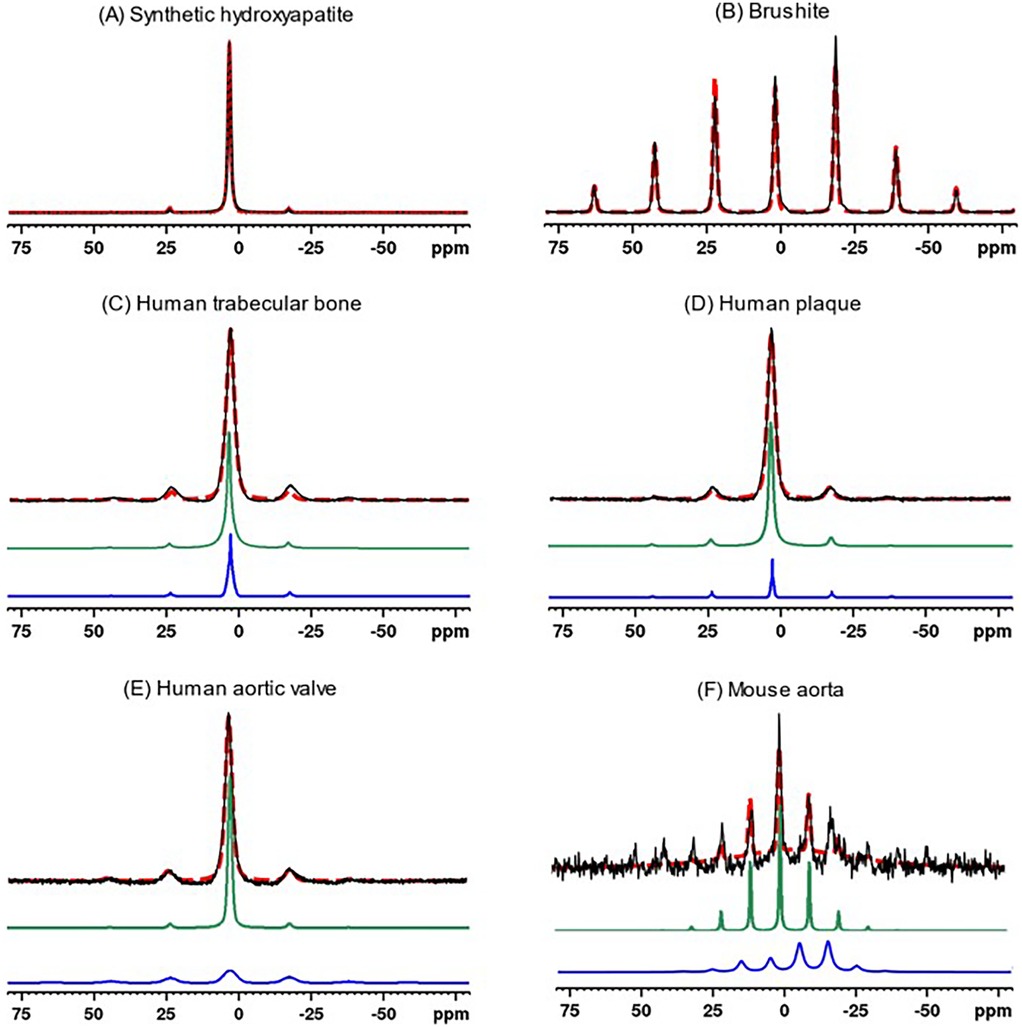
Figure 3. 31P MAS experimental spectra (solid black traces) of synthetic calcium phosphate compounds and biological specimens, and corresponding simulated spectra (red dashed traces) based on Herzfeld-Berger chemical shift tensor analysis. The synthetic compound spectra were fit to a single tensor, whereas biological specimens were fit to two tensors (individual simulated tensor spectra displayed as green and blue traces). The latter composite simulated spectra (red dashed traces) are the sums of the spectra from the two components. (A) Synthetic hydroxyapatite, (B) Brushite, (C) Human trabecular bone, (D) Human plaque, (E) Human aortic valve, (F) Mouse aorta.
4 Discussion
The CP rate depends strongly on the direct dipole-dipole coupling between 1H and 31P nuclei. This rate varies as the inverse sixth power of the internuclear distance and can therefore be used to discriminate between calcium phosphates with close vs. more distant 31P–1H spacing, as well as deduce general information reflective of the crystal geometry about the phosphate ion. also depends on the number of coupled spins. 31P in HPO4−2 transfers magnetization with 1H at a rapid rate because the participating nuclei are within the same ion and are in close proximity. At the other end of the scale, PO4−3 in hydroxyapatite transfers magnetization far more slowly because the closest protons are located in other, more distant, ions (e.g., OH− or HPO4−2) in the crystal lattice. In this case, 31P signals from nuclei separated from protons by more than a few tens of a nm, such as the distance between phosphate ions in calcium phosphate crystals and the protons in surrounding tissue and matrix, are fully suppressed.
T1ρ is the relaxation time constant for energy exchange between nuclear spins and the lattice, measured in the rotating frame of reference. It is analogous to the more familiar spin-lattice relaxation time constant T1. T1 is sensitive to molecular motions with correlation times comparable to the inverse of the Larmor frequency. For example, for a proton Larmor frequency of 600 MHz (B0 ∼ 14 T), the proton T1 is determined by molecular motion correlation times on the order of ns. A substantial degree of molecular motion (molecular tumbling or diffusion, chemical exchange, or even spin exchange) occurring at this rate will contribute to a shortening in the T1 value. Because T1ρ is measured in the rotating reference frame with respect to a spinlock RF field on the order of millitesla (ν1 ∼ kHz) rather than Tesla (ν0 ∼ tens to hundreds of MHz), it is sensitive to relatively slow molecular motions (e.g., motions of macromolecules, or slow spin exchange characteristic of substances with dilute, widely spaced, protons).
Spin-lattice relaxation of either type (T1ρ or T1) is mediated by the time-dependence (e.g., vibrations and motions of surrounding atoms and molecules) of the spin-spin coupling interaction (60). Since protons have a much stronger magnetic moment than phosphorus nuclei, they play the dominant role in spin-lattice relaxation. 31P nuclei therefore tend to undergo spin-lattice relaxation via spin-spin coupling to the proton “reservoir” (the collective assembly of protons in the crystal lattice, water molecules, bone matrix, etc.). Therefore, variations in T1ρ may be indicative of water content, presence of protons in the crystal lattice, and similar aspects of the chemical environment. However, there are no clear trends in the T1ρ data for the specimens studied here.
The spectra of all the human specimens resemble that of hydroxyapatite, except the human specimens exhibit more prominent sidebands, as does that of the mouse aorta. As found in earlier studies of animal bone, this is generally the case for mature bone mineral, which is primarily apatitic with a small content of HPO4−2 that contributes to the sidebands (45, 47). In contrast, calcification in the ApoE-deficient mouse resembled that of immature or embryonic bone. Moreover, calcified mouse aorta exhibits much shorter CP time constants, implying a higher content of HPO4−2 in the calcium phosphate crystals. Prior studies detected the presence of microcalcifications (1 μm–30 μm) in the aorta of ApoE-deficient mice fed a high cholesterol diet for as early as 12 weeks starting at 8–10 weeks of age (61, 62) using molecular imaging and microscopy. Despite the age of the ApoE-deficient mouse (60 weeks) in this study, its calcification did not resemble that observed in vascular calcification in humans. This result could be because calcification in humans may evolve over many years, far longer than the 60 weeks in the mice. Therefore, the use of mouse models for human calcified tissues should be viewed with the caveat that there may be fundamental differences between human and mouse calcification due to the very different time scales for the deposition and remodeling of the calcium phosphate crystals. This may in part explain the discrepancy in the response to statin therapy, which has been effective in ApoE-deficient mice (63–65) but exhibited controversial effects in human patients with atherosclerosis plaques (66–71). Calcification in the aged ApoE-deficient mouse thus likely models early preclinical calcification in human aortic valves (72), but not the mature pattern of calcification seen in patients with established aortic stenosis.
The small tissue size and relatively low mineral density of murine aortic calcifications constrain the signal-to-noise ratio attainable in solid state 1H-31P MR spectroscopy, limiting the feasibility of analyzing multiple age groups or lesion sizes within this dataset. Consequently, while the present findings clearly demonstrate compositional differences between human and murine calcifications, we cannot fully exclude the contribution of mineral age or lesion maturity to the observed variation. Future studies incorporating ApoE-deficient mice at different ages and comparing larger and smaller plaques within the same animal will be important to distinguish the relative influence of mineral age, lesion progression, and intrinsic compositional factors.
In addition to mineral age, crystal size and surface chemistry may also influence the apparent phosphate speciation (73). Biological apatite crystals are typically nanometer-scale platelets (e.g., 15–100 nm length, 0.5–4 nm in thickness) and are often coated by a hydrated, disordered surface layer (74) that contains labile ions and is prone to ionic exchange (75). Solid state MR spectroscopy studies have shown that HPO42− ions are disproportionately localized in these amorphous surface layers — for example, Von Euw et al. (76) estimated a ∼0.8 nm surface shell rich in protonated phosphate even on small particles. TEM imaging of synthetic apatite also reveals 1–2 nm amorphous surface layers (77). Because the surface-to-volume ratio is much higher in small crystals, a greater fraction of total phosphate may be in protonated form (HPO42−) relative to PO43−. Thus, the higher relative HPO42− content observed in mouse vs. human calcifications could partially reflect smaller, less-coalesced nanocrystals rather than purely younger mineral age.
Beyond nanoscale effects, variations in the extracellular environment can further modulate phosphate chemistry. During physiological bone mineralization, extracellular pH remains close to neutral, favoring PO4−3–rich apatite (78). In contrast, plaque microenvironments are often mildly acidic due to inflammation and hypoxia (79–81), which could shift the equilibrium toward protonated phosphate (HPO4−2). Variations in local pH, therefore, may contribute to the compositional differences observed between vascular and skeletal mineral.
A previous study (47) examining the spectrum of Ca(H2PO4)2·H2O has ruled out the possibility of the sideband patterns observed in biological calcium phosphates being associated with the protonated phosphate ion H2PO4−. The chemical shift anisotropy of Ca(H2PO4)2·H2O is much smaller than those of the biological specimens. Additionally, it is unlikely that there is a significant H2PO4− content in biological calcium phosphates because an unusually acidic cellular environment would be required to maintain a substantial amount of H2PO4− in vivo. Therefore, it was assumed that only two phosphate ions (PO4−3 and HPO4−2) contribute to the spectra. In principle, each sideband pattern is affected by not only the 31P chemical shift anisotropy, but also 1H-31P and 31P-31P dipolar interactions. However, these secondary effects are expected to be relatively minor under conditions of high magnetic field strength and 5 kHz magic angle spinning.
The goal of this study was to provide fundamental insights into the structure of calcified cardiovascular tissues but translational parallels that merit exploration may exist. Cardiovascular MRI in humans is being increasingly explored on 0.5 Tesla commercial systems (82, 83) and the relaxation frequency governing T1 exchange at this field is not that different from T1ρ at 14T (∼36× with the 55 kHz B1 field used here). Moreover, portable MRI systems (Hyperfine Inc) operating in the low milli-Tesla range have been FDA-approved for point-of-care neuroimaging and T1 of carotid plaque on these systems would involve a relaxation frequency very similar to T1ρ at 14T. These low field systems are based on proton MRI, and the lower polarization of 31P would undoubtedly be challenging. However, hyperpolarization of 31P in aqueous solution has been described (84) and may be able to mitigate the low SNR of organic phosphates in atherosclerotic plaque. While spatial resolution and SNR are indeed limited in low-field MRI, some valuable insights may be able to be obtained by comparing in vivo T1 measurements on low field systems with T1ρ measurements ex vivo at 14T.
In addition to broader methodological extensions, future studies could perform solid state 1H-31P MR spectroscopy on calcified tissues dissected from different regions within the same plaque or from plaques at distinct anatomical locations. Such site-specific spectroscopy would allow for comparison of mineral phase composition and maturity across microenvironments that vary in cellular activity, lipid accumulation, or inflammatory state (85). In the present study, detailed morphological characterization of the calcified plaque specimen (e.g., nodular vs. sheet-like) was not available due to limited tissue availability and absence of complementary imaging. When combined with histological and microscopic analyses in future work, this spectroscopy-based approach could offer a spatially informed understanding of mineral heterogeneity within human plaques and clarify how phosphate speciation relates to local biological processes such as inflammation, lipid deposition, and necrosis.
5 Conclusions
Solid state MR spectroscopy adds another dimension to the study of pathologic calcification. It provides a means to identify the presence of various phosphate ions in the calcium phosphate mineral. Human trabecular bone, calcified atherosclerotic plaque, and calcified aortic valve specimens were found to have similar CPMAS spectra, whereas the spectra of experimentally induced calcified mouse aortic calcification are somewhat different and suggestive of immature, recently deposited bone. Although solid state MR spectroscopy has been used to study bone in several contexts, it has rarely been applied to pathological ectopic calcification. While this methodology is not directly applicable to clinical use, it could be an important complement to basic studies of pathological calcification in atherosclerosis and related calcific disorders.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Massachusetts General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Brigham and Women's Hospital Institutional Review Board. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JY: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CF: Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. EA: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. DS: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. BM: Data curation, Methodology, Resources, Writing – original draft, Writing – review & editing. AK: Formal analysis, Software, Validation, Writing – original draft, Writing – review & editing. JA: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. EA: NIH R01HL174066, the Leducq Foundation PRIMA network, and a research grant from Pfizer. BM: NIH T32HL007208 (DES). Martinos Center 14T MR spectrometer facility: NIH S10RR013026, S10OD023406, and S10OD038220. JY and JA: NIH R01AR075077 (grant R01EB029818).
Acknowledgments
This has been released as a preprint at medRxiv (54).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bostrom K. Insights into the mechanism of vascular calcification. Am J Cardiol. (2001) 88(2A):20E–2E. doi: 10.1016/s0002-9149(01)01718-0
2. Shioi A, Mori K, Jono S, Wakikawa T, Hiura Y, Koyama H, et al. Mechanism of atherosclerotic calcification. Z Kardiol. (2000) 89(Suppl 2):75–9. doi: 10.1007/s003920070103
3. Epple M, Lanzer P. How much interdisciplinarity is required to understand vascular calcifications? Formulation of four basic principles of vascular calcification. Z Kardiol. (2001) 90(Suppl 3):2–5. doi: 10.1007/s003920170036
4. LeGeros RZ. Formation and transformation of calcium phosphates: relevance to vascular calcification. Z Kardiol. (2001) 90(Suppl 3):116–24. doi: 10.1007/s003920170032
5. Tomazic BB. Physiochemical principles of cardiovascular calcification. Z Kardiol. (2001) 90(Suppl 3):68–80. doi: 10.1007/s003920170046
6. Peters F, Epple M. Simulating arterial wall calcification in vitro: biomimetic crystallization of calcium phosphates under controlled conditions. Z Kardiol. (2001) 90(Suppl 3):81–5. doi: 10.1007/pl00022850
7. Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. (1999) 84(2):166–78. doi: 10.1161/01.res.84.2.166
8. Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. (2000) 87(7):E10–7. doi: 10.1161/01.res.87.7.e10
9. Fitzpatrick LA, Turner RT, Ritman ER. Endochondral bone formation in the heart: a possible mechanism of coronary calcification. Endocrinology. (2003) 144(6):2214–9. doi: 10.1210/en.2002-0170
10. Goldring SR. Inflammatory mediators as essential elements in bone remodeling. Calcif Tissue Int. (2003) 73(2):97–100. doi: 10.1007/s00223-002-1049-y
11. Doherty TM, Uzui H, Fitzpatrick LA, Tripathi PV, Dunstan CR, Asotra K, et al. Rationale for the role of osteoclast-like cells in arterial calcification. FASEB J. (2002) 16(6):577–82. doi: 10.1096/fj.01-0898hyp
12. Lampropoulos CE, Papaioannou I, Cruz DP. Osteoporosis–a risk factor for cardiovascular disease? Nat Rev Rheumatol. (2012) 8(10):587–98. doi: 10.1038/nrrheum.2012.120
13. Caffarelli C, Montagnani A, Nuti R, Gonnelli S. Bisphosphonates, atherosclerosis and vascular calcification: update and systematic review of clinical studies. Clin Interv Aging. (2017) 12:1819–28. doi: 10.2147/CIA.S138002
14. Santos LL, Cavalcanti TB, Bandeira FA. Vascular effects of bisphosphonates-a systematic review. Clin Med Insights Endocrinol Diabetes. (2012) 5:47–54. doi: 10.4137/CMED.S10007
15. Ylitalo R. Bisphosphonates and atherosclerosis. Gen Pharmacol. (2000) 35(6):287–96. doi: 10.1016/s0306-3623(01)00121-5
16. Recker RR, Lewiecki EM, Miller PD, Reiffel J. Safety of bisphosphonates in the treatment of osteoporosis. Am J Med. (2009) 122(2 Suppl):S22–32. doi: 10.1016/j.amjmed.2008.12.004
17. Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. (1996) 97(12):2692–6. doi: 10.1172/jci118722
18. Bakhshian Nik A, Hutcheson JD, Aikawa E. Extracellular vesicles as mediators of cardiovascular calcification. Front Cardiovasc Med. (2017) 4:78. doi: 10.3389/fcvm.2017.00078
19. Burnett JR, Vasikaran SD. Cardiovascular disease and osteoporosis: is there a link between lipids and bone? Ann Clin Biochem. (2002) 39(Pt 3):203–10. doi: 10.1258/0004563021902134
20. Christian RC, Harrington S, Edwards WD, Oberg AL, Fitzpatrick LA. Estrogen status correlates with the calcium content of coronary atherosclerotic plaques in women. J Clin Endocrinol Metab. (2002) 87(3):1062–7. doi: 10.1210/jcem.87.3.8354
21. Kiel DP, Kauppila LI, Cupples LA, Hannan MT, Donnell O, Wilson CJ, et al. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the framingham heart study. Calcif Tissue Int. (2001) 68(5):271–6. doi: 10.1007/BF02390833
22. Vliegenthart R, Oudkerk M, Hofman A, Oei H-HS, van Dijck W, van Rooij FJA, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. (2005) 112:572–7. doi: 10.1161/CIRCULATIONAHA.104.488916
23. Martin SS, Blaha MJ, Blankstein R, Agatston A, Rivera JJ, Virani SS, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation. (2014) 129(1):77–86. doi: 10.1161/circulationaha.113.003625
24. Ferencik M, Schlett CL, Ghoshhajra BB, Kriegel MF, Joshi SB, Maurovich-Horvat P, et al. A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol. (2012) 110(2):183–9. doi: 10.1016/j.amjcard.2012.02.066
25. Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. (2004) 110(22):3424–9. doi: 10.1161/01.Cir.0000148131.41425.E9
26. Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. (2014) 311(3):271–8. doi: 10.1001/jama.2013.282535
27. Neves PO, Andrade J, Monção H. Coronary artery calcium score: current status. Radiol Bras. (2017) 50(3):182–9. doi: 10.1590/0100-3984.2015.0235
28. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. (2018) 72(4):434–47. doi: 10.1016/j.jacc.2018.05.027
29. Villa-Bellosta R. Vascular calcification: key roles of phosphate and pyrophosphate. Int J Mol Sci. (2021) 22(24):13536. doi: 10.3390/ijms222413536
30. Wu Y, Ackerman JL, Strawich ES, Rey C, Kim HM, Glimcher MJ. Phosphate ions in bone: identification of a calcium-organic phosphate complex by 31p solid-state NMR spectroscopy at early stages of mineralization. Calcif Tissue Int. (2003) 72(5):610–26. doi: 10.1007/s00223-002-1068-8
31. Rey C, Collins B, Goehl T, Dickson IR, Glimcher MJ. The carbonate environment in bone mineral: a resolution-enhanced Fourier transform infrared spectroscopy study. Calcif Tissue Int. (1989) 45(3):157–64. doi: 10.1007/bf02556059
32. Rey C, Combes C, Drouet C, Glimcher MJ. Bone mineral: update on chemical composition and structure. Osteoporos Int. (2009) 20(6):1013–21. doi: 10.1007/s00198-009-0860-y
33. Orzechowska S, Wrobel A, Goncerz G, Podolec P, Rokita E. Physicochemical and micro-tomographic characterization of inorganic deposits associated with aortic stenosis. J Heart Valve Dis. (2014) 23:40–7. PMID: 24779327
34. Drouet C. Apatite formation: why it may not work as planned, and how to conclusively identify apatite compounds. Biomed Res Int. (2013) 2013:490946. doi: 10.1155/2013/490946
35. Larose E, Yeghiazarians Y, Libby P, Yucel EK, Aikawa M, Kacher DF, et al. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation. (2005) 112(15):2324–31. doi:doi: 10.1161/CIRCULATIONAHA.105.538942
36. Wang G, Zhang Y, Hegde SS, Bottomley PA. High-resolution and accelerated multi-parametric mapping with automated characterization of vessel disease using intravascular mri. J Cardiovasc Magn Reson. (2017) 19(1):89. doi: 10.1186/s12968-017-0399-6
37. Correia LCL, Atalar E, Kelemen MD, Ocali O, Hutchins GM, Fleg JL, et al. Intravascular magnetic resonance imaging of aortic atherosclerotic plaque composition. Arterioscler, Thromb, Vasc Biol. (1997) 17(12):3626–32. doi:doi: 10.1161/01.ATV.17.12.3626
38. Larose E, Kinlay S, Selwyn AP, Yeghiazarians Y, Yucel EK, Kacher DF, et al. Improved characterization of atherosclerotic plaques by gadolinium contrast during intravascular magnetic resonance imaging of human arteries. Atherosclerosis. (2008) 196(2):919–25. doi: 10.1016/j.atherosclerosis.2007.02.020
39. O’Malley PG, Taylor AJ, Jackson JL, Doherty TM, Detrano RC. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am J Cardiol. (2000) 85(8):945–8. doi: 10.1016/s0002-9149(99)00906-6
40. van Soest G, Goderie TP, Gonzalo N, Koljenović S, van Leenders GL, Regar E, et al. Imaging atherosclerotic plaque composition with intracoronary optical coherence tomography. Neth Heart J. (2009) 17(11):448–50. doi: 10.1007/bf03086301
41. Hitchner E, Zayed MA, Lee G, Morrison D, Lane B, Zhou W. Intravascular ultrasound as a clinical adjunct for carotid plaque characterization. J Vasc Surg. (2014) 59(3):774–80. doi: 10.1016/j.jvs.2013.09.028
42. Ackerman JL. Chapter 15 solid state NMR spectroscopy. In: Macomber RS, editor. A Complete introduction to Modern NMR Spectroscopy. I. New York, NY: John Wiley & Sons Inc. (1997). p. 283–304.
43. Guo W, Morrisett JD, DeBakey ME, Lawrie GM, Hamilton JA. Quantification in situ of crystalline cholesterol and calcium phosphate hydroxyapatite in human atherosclerotic plaques by solid-state magic angle spinning nmr. Arterioscler, Thromb, Vasc Biol. (2000) 20(6):1630–6. doi: 10.1161/01.ATV.20.6.1630
44. Guo W, Morrisett JD, Lawrie GM, DeBakey ME, Hamilton JA. Identification of different lipid phases and calcium phosphate deposits in human carotid artery plaques by mas NMR spectroscopy. Magn Reson Med. (1998) 39(2):184–9. doi: 10.1002/mrm.1910390204
45. Ramanathan C, Ackerman JL. ADRF differential cross polarization spectroscopy of synthetic calcium phosphates and bone mineral. J Magn Reson. (1997) 127(1):26–35. doi: 10.1006/jmre.1997.1173
46. Ramanathan C, Wu Y, Pfleiderer B, Lizak MJ, Garrido L, Ackerman JL. Adrf-cp surface-coil spectroscopy of synthetic calcium phosphates and bone mineral. J Magn Reson. (1996) 121(2):127–38. doi: 10.1006/jmra.1996.0152
47. Wu Y, Glimcher MJ, Rey C, Ackerman JL. A unique protonated phosphate group in bone mineral not present in synthetic calcium phosphates. Identification by phosphorus-31 solid state NMR spectroscopy. J Mol Biol. (1994) 244(4):423–35. doi: 10.1006/jmbi.1994.1740
48. Wu Y, Chesler DA, Glimcher MJ, Garrido L, Wang J, Jiang HJ, et al. Multinuclear solid-state three-dimensional mri of bone and synthetic calcium phosphates. Proc Natl Acad Sci U S A. (1999) 96(4):1574–8. doi: 10.1073/pnas.96.4.1574
49. Weiger M, Stampanoni M, Pruessmann KP. Direct depiction of bone microstructure using mri with zero echo time. Bone. (2013) 54(1):44–7. doi: 10.1016/j.bone.2013.01.027
50. Wu Y, Ackerman JL, Chesler DA, Graham L, Wang Y, Glimcher MJ. Density of organic matrix of native mineralized bone measured by water- and fat-suppressed proton projection MRI. Magn Reson Med. (2003) 50(1):59–68. doi: 10.1002/mrm.10512
51. Wu Y, Dai G, Ackerman JL, Hrovat MI, Glimcher MJ, Snyder BD, et al. Water- and fat-suppressed proton projection MRI (WASPI) of rat femur bone. Magn Reson Med. (2007) 57(3):554–67. doi: 10.1002/mrm.21174
52. Kuhn LT, Wu Y, Rey C, Gerstenfeld LC, Grynpas MD, Ackerman JL, et al. Structure, composition, and maturation of newly deposited calcium-phosphate crystals in chicken osteoblast cell cultures. J Bone Miner Res. (2000) 15(7):1301–9. doi: 10.1359/jbmr.2000.15.7.1301
53. Herzfeld J, Berger AE. Sideband intensities in NMR spectra of samples spinning at the magic angle. J Chem Phys. (1980) 73(12):6021–30. doi: 10.1063/1.440136
54. Yao J, Farrar CT, Aikawa E, Sosnovik DE, Moon BF, Kulkarni A, et al. Ex vivo phosphorus-31 solid-state magnetic resonance spectroscopy identifies compositional differences between bone mineral and calcified vascular tissues. medRxiv [Preprint]. medRxiv:2025.09.27.25336805 (2025). Available online at: https://doi.org/10.1101/2025.09.27.25336805 (Accessed November 13, 2025).
55. van Meerten SGJ, Franssen WMJ, Kentgens APM. Ssnake: a cross-platform open-source NMR data processing and fitting application. J Magn Reson. (2019) 301:56–66. doi: 10.1016/j.jmr.2019.02.006
56. Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. (1984) 1(3):307–15. doi: 10.1002/mrm.1910010303
57. Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. (1973) 248(20):7276–8. doi: 10.1016/S0021-9258(19)43389-9
58. Biltz RM, Pellegrino ED. The hydroxyl content of calcified tissue mineral. Calcif Tissue Res. (1971) 7(3):259–63. doi: 10.1007/bf02062614
59. Biltz RM, Pellegrino ED. The nature of bone carbonate. Clin Orthop Relat Res. (1977) 129:279–92. doi: 10.1097/00003086-197711000-00040
60. Wu Y, Ackerman JL, Kim HM, Rey C, Barroug A, Glimcher MJ. Nuclear magnetic resonance spin-spin relaxation of the crystals of bone, dental enamel, and synthetic hydroxyapatites. J Bone Miner Res. (2002) 17(3):472–80. doi: 10.1359/jbmr.2002.17.3.472
61. Aikawa E, Nahrendorf M, Figueiredo J-L, Swirski FK, Shtatland T, Kohler RH, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. (2007) 116(24):2841–50. doi:doi: 10.1161/CIRCULATIONAHA.107.732867
62. Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. (2016) 15(3):335–43. doi: 10.1038/nmat4519
63. Xian JZ, Lu M, Fong F, Qiao R, Patel NR, Abeydeera D, et al. Statin effects on vascular calcification. Arterioscler, Thromb, Vasc Biol. (2021) 41(4):e185–e92. doi: 10.1161/ATVBAHA.120.315737
64. Lei Y, Cui Q, Yang G, Piao L, Inoue A, Wu H, et al. Statins mitigate stress-related vascular aging and atherosclerosis in apoe-deficient mice fed high fat-diet: the role of glucagon-like peptide-1/adiponectin axis. Front Cell Dev Biol. (2021) 9:687868. doi: 10.3389/fcell.2021.687868
65. Nakamura K, Sasaki T, Cheng XW, Iguchi A, Sato K, Kuzuya M. Statin prevents plaque disruption in apoe-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis. (2009) 206(2):355–61. doi: 10.1016/j.atherosclerosis.2009.02.014
66. Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebo-controlled clinical trials on 2344 patients. Can J Cardiol. (2011) 27(6):800–8. doi: 10.1016/j.cjca.2011.03.012
67. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38(36):2739–91. doi: 10.1093/eurheartj/ehx391
68. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. (2014) 129(23):2440–92. doi: 10.1161/cir.0000000000000029
69. Arsenault BJ, Boekholdt SM, Mora S, DeMicco DA, Bao W, Tardif JC, et al. Impact of high-dose atorvastatin therapy and clinical risk factors on incident aortic valve stenosis in patients with cardiovascular disease (from TNT, IDEAL, and SPARCL). Am J Cardiol. (2014) 113(8):1378–82. doi: 10.1016/j.amjcard.2014.01.414
70. Parolari A, Tremoli E, Cavallotti L, Trezzi M, Kassem S, Loardi C, et al. Do statins improve outcomes and delay the progression of non-rheumatic calcific aortic stenosis? Heart. (2011) 97(7):523–9. doi: 10.1136/hrt.2010.215046
71. Healy A, Berus JM, Christensen JL, Lee C, Mantsounga C, Dong W, et al. Statins disrupt macrophage rac1 regulation leading to increased atherosclerotic plaque calcification. Arterioscler, Thromb, Vasc Biol. (2020) 40(3):714–32. doi: 10.1161/ATVBAHA.119.313832
72. Tanaka K, Sata M, Fukuda D, Suematsu Y, Motomura N, Takamoto S, et al. Age-associated aortic stenosis in apolipoprotein e-deficient mice. JACC. (2005) 46(1):134–41. doi: 10.1016/j.jacc.2005.03.058
73. Farlay D, Panczer G, Rey C, Delmas PD, Boivin G. Mineral maturity and crystallinity index are distinct characteristics of bone mineral. J Bone Miner Metab. (2010) 28(4):433–45. doi: 10.1007/s00774-009-0146-7
74. Wang B, Zhang Z, Pan H. Bone apatite nanocrystal: crystalline structure, chemical composition, and architecture. Biomimetics (Basel). (2023) 8(1):90. doi: 10.3390/biomimetics8010090
75. Iafisco M, Delgado López JM, Drouet C. Nanocrystalline apatites: synthesis, physical-chemical and thermodynamic characterization. In: Iafisco M, Delgado-Lopez JM, editors. Apatites: Synthesis, Structural Characterization and Biomedical Applications. New York: Nova Science Publishers (2014). Chapter 2, eBook, ISBN 978-1-63321-500-9
76. Von Euw S, Wang Y, Laurent G, Drouet C, Babonneau F, Nassif N, et al. Bone mineral: new insights into its chemical composition. Sci Rep. (2019) 9(1):8456. doi: 10.1038/s41598-019-44620-6
77. Bertolotti F, Carmona FJ, Dal Sasso G, Ramírez-Rodríguez GB, Delgado-López JM, Pedersen JS, et al. On the amorphous layer in bone mineral and biomimetic apatite: a combined small- and wide-angle x-ray scattering analysis. Acta Biomater. (2021) 120:167–80. doi: 10.1016/j.actbio.2020.04.026
78. Arnett T. Extracellular ph regulates bone cell function. J Nutr. (2008) 138:415S–8. doi: 10.1093/jn/138.2.415S
79. Liu CL, Zhang X, Liu J, Wang Y, Sukhova GK, Wojtkiewicz GR, et al. Na(+)-h(+) exchanger 1 determines atherosclerotic lesion acidification and promotes atherogenesis. Nat Commun. (2019) 10(1):3978. doi: 10.1038/s41467-019-11983-3
80. Ma J, Zhao X, Xu X, Lixin A, Liu Q, Qu P. A bibliometric analysis of strategies for atherosclerosis treatment with organic nanoparticles. Pharmaceutics. (2025) 17(9):1131. doi: 10.3390/pharmaceutics17091131
81. Naghavi M, John R, Naguib S, Siadaty M, Grasu R, Kurian K, et al. pH heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis. (2002) 164:27–35. doi: 10.1016/S0021-9150(02)00018-7
82. Campbell-Washburn AE, Varghese J, Nayak KS, Ramasawmy R, Simonetti OP. Cardiac mri at low field strengths. J Magn Reson Imaging. (2024) 59(2):412–30. doi: 10.1002/jmri.28890
83. Campbell-Washburn AE, Ramasawmy R, Restivo MC, Bhattacharya I, Basar B, Herzka DA, et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology. (2019) 293(2):384–93. doi: 10.1148/radiol.2019190452
84. Nardi-Schreiber A, Gamliel A, Harris T, Sapir G, Sosna J, Gomori JM, et al. Biochemical phosphates observed using hyperpolarized 31p in physiological aqueous solutions. Nat Commun. (2017) 8(1):341. doi: 10.1038/s41467-017-00364-3
Keywords: calcium phosphate, hydroxyapatite, magnetic resonance spectroscopy, plaque, vascular calcification
Citation: Yao J, Farrar CT, Aikawa E, Sosnovik DE, Moon BF, Kulkarni A and Ackerman JL (2025) Ex vivo phosphorus-31 solid state magnetic resonance spectroscopy identifies compositional differences between bone mineral and calcified vascular tissues. Front. Cardiovasc. Med. 12:1689116. doi: 10.3389/fcvm.2025.1689116
Received: 20 August 2025; Accepted: 5 November 2025;
Published: 26 November 2025.
Edited by:
Shihua Shi, Friedrich Miescher Institute for Biomedical Research (FMI), SwitzerlandCopyright: © 2025 Yao, Farrar, Aikawa, Sosnovik, Moon, Kulkarni and Ackerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingting Yao, ankxMDE1QG1naC5oYXJ2YXJkLmVkdQ==
 Jingting Yao
Jingting Yao Christian T. Farrar1,2
Christian T. Farrar1,2 Elena Aikawa
Elena Aikawa David E. Sosnovik
David E. Sosnovik Aditi Kulkarni
Aditi Kulkarni Jerome L. Ackerman
Jerome L. Ackerman