- 1Department of Cardiology, University Hospital Marburg, Marburg, Germany
- 2County Hospital Loerrach, Department of Cardiology, Loerrach, Germany
Transcatheter edge-to-edge mitral and tricuspid valve repair (M-TEER, T-TEER) have emerged as meaningful treatment modalities among patients at high surgical risk suffering from valvular heart disease. While previous research has shown that optimal patient selection is crucial for treatment outcomes, recent studies have identified a multitude of factors that independently influence mortality. Although these findings can significantly support clinical decision-making, the large number of available studies renders an overview of this topic challenging. In this review, we provide a comprehensive overview of the currently identified factors associated with increased mortality after TEER. We also summarize the current evidence on published risk scores that stratify mortality risk after M-TEER and T-TEER. We aimed to provide clinical decision-making support for optimal patient selection and referral to TEER and to identify remaining gaps in evidence.
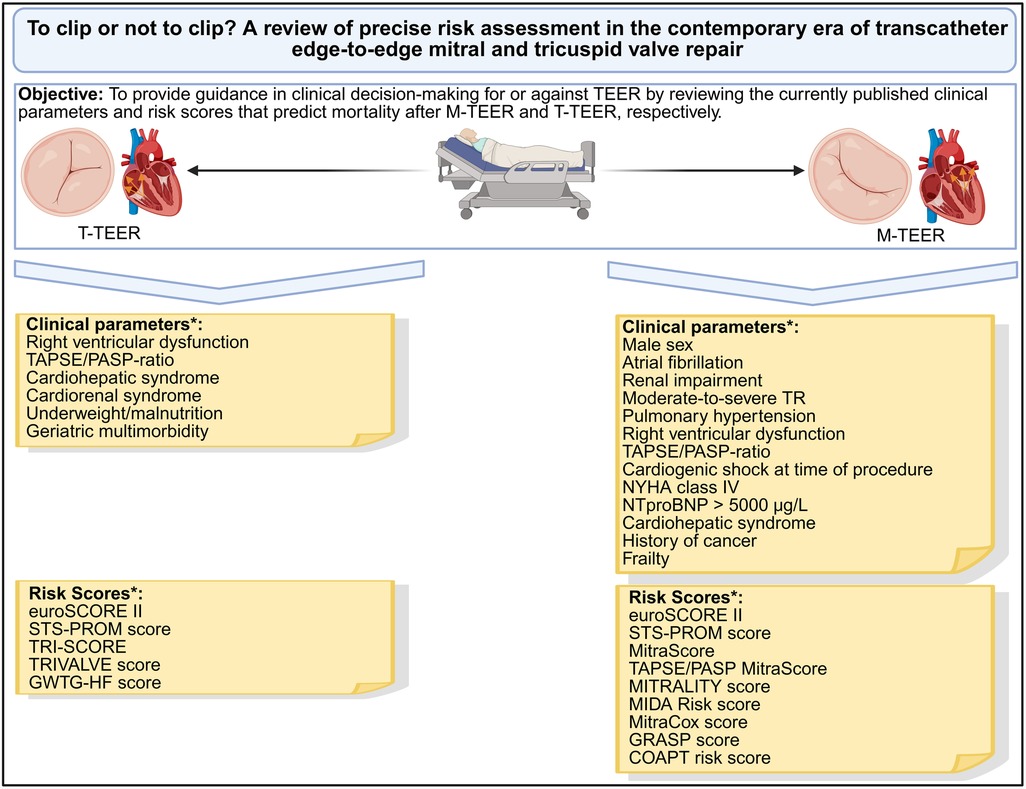
Graphical Abstract. *Clinical parameters or risk scores that were independently associated with mortality after M-TEER or T-TEER, respectively. M-TEER, transcatheter edge-to-edge mitral valve repair; NYHA, New-York-Heart-Association; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TEER, transcatheter edge-to-edge repair; TR, tricuspid valve regurgitation; T-TEER, transcatheter edge-to-edge tricuspid valve repair. Created in BioRender. Ausbüttel, F. (2025) https://BioRender.com/yu30ckm.
Introduction
In Western industrialized nations, mitral regurgitation (MR) is the most common form of valvular heart disease (VHD) in patients aged >75 years (1). Among patients suffering from congestive heart failure, severe tricuspid regurgitation (TR) serves as a surrogate parameter for advanced disease stage with a consecutive increase in mortality (2, 3). Since both VHDs are particularly common among elderly patients, whose increased age and higher rate of comorbidities lead to increased perioperative risk, these patients are frequently deemed ineligible candidates for surgical valve repair (4). Transcatheter edge-to-edge repair (TEER) was developed to ameliorate the overall therapeutic prognosis of this particular cohort of patients (5, 6). Both transcatheter edge-to-edge mitral valve repair (M-TEER) and transcatheter edge-to-edge tricuspid valve repair (T-TEER) have proven to be safe and efficacious treatment modalities for patients at high surgical risk who remain symptomatic despite optimal medical therapy (OMT) for VHD (7–12).
Owing to ongoing demographic changes, a further increase in procedure numbers are expected (4, 13). The lessons learned from the existing evidence on TEER revealed that optimal screening and referral of suitable candidates for TEER remain crucial to achieve favorable treatment outcomes (14, 15), although both M-TEER and T-TEER were proven successful in alleviating the symptom burden of VHD, quality of life and hospitalization rates in these distinct cohorts of patients (5, 6, 9, 12).
We therefore aimed to provide a comprehensive review of the existing evidence concerning the determinants of mortality in current “real-world“ patients who have undergone M-TEER or T-TEER. For this purpose, research was conducted into clinical parameters with a proven significant influence on mortality, as well as risk scores for predicting mortality, whose accuracy was presented using the area under the curve (AUC) value or c-statistics as reported in the original publication, respectively.
Risk assessment prior to intervention
Previous studies have identified a variety of independent mortality predictors for both procedures, which have partly been summarized in scores for predicting mortality. These are presented separately for both procedures:
Transcatheter edge-to-edge mitral valve repair (M-TEER)
Clinical parameters with significant effects on mortality after M-TEER
One of the clinical predictors of mortality was male sex (16), which was associated with a higher rate of complicating comorbidities (17). Further comorbidities that were associated with significantly increased mortality included concomitant atrial fibrillation (AF) (18), renal impairment (19), moderate to severe tricuspid regurgitation (TR) (20, 21), pulmonary hypertension (22) and right ventricular dysfunction (RVD) (23, 24). For the measurement of RVD, the ratio of the echocardiographic values of the tricuspid annular plane systolic excursion (TAPSE) and the pulmonary arterial systolic pressure (PASP) was established, which defined the uncoupling between the right ventricle (RV) and the pulmonary artery (PA) as a marker of significantly worsened survival (25). With respect to left ventricular ejection fraction (LVEF) as a further central echocardiographic parameter, a wide range of studies revealed inconsistent results with no clear observable effect on mortality (26). However, the performance of M-TEER in patients with reduced LVEF due to cardiogenic shock was associated with an increased mortality rate (27). In the context of advanced heart failure, heart failure symptoms of New-York-Heart-Association (NYHA) class IV (28), elevated levels of NTproBNP > 5,000 µg/L (21) and secondary organ failure, e.g., occurrence of cardiohepatic syndrome (29), were also associated with reduced survival. Coronary artery disease (CAD), in contrast, was not found to be an independent predictor of mortality, but the presence of the disease is discussed as a surrogate parameter of increased morbidity, in which other diseases may be prevalent, which in turn negatively influence the all-cause mortality rate (30).
In addition to cardiac comorbidities, other complex diseases were also relevant in influencing mortality. A study by Tabata et al. demonstrated significantly higher inflammation parameters and worse survival among patients who underwent M-TEER and had a history of cancer (31). However, further studies are needed to clarify to what extent the negative influence can be attributed to the disease itself or possibly to the utilization of potentially cardiotoxic chemotherapy regimens. Frailty or a low body mass index (BMI) were also linked to increased procedural risk and, consequently, increased mortality (32). Last, previous valve surgery was found to be an operative factor associated with increased mortality in this interventional cohort (21, 28).
Risk scores for the stratification of mortality prior to M-TEER
Many of the aforementioned predictors were then incorporated with other parameters to develop corresponding risk scores. The European System for Cardiac Operative Risk Evaluation (euroSCORE) II and the Society of Thoracic Surgeons' Predicted Risk of Mortality (STS-PROM) score represent the most well-known and earliest risk scores for predicting mortality and were originally developed for patients undergoing cardiac surgery (33, 34). While the EuroSCORE II inquires a smaller number of parameters and estimates all-cause mortality (AUC-value 0.81 for patients undergoing cardiac surgery), the STS-PROM score offers additional risk parameters in addition to mortality, e.g., renal failure, prolonged intensive care stay, permanent stroke and the need for reoperation (c-statistics 0.799 for mortality and c-statistics 0.639 for reoperation in patients undergoing cardiac surgery, respectively). Their validity for predicting mortality was subsequently confirmed equally for patients who underwent M-TEER, enabling the utilization of both scores in clinical risk prediction (35). An analysis by Schneider et al. also revealed an inverse association between the STS-PROM score and the procedural success of M-TEER, as measured by the reduction success of MR to mild severity (36).
Nevertheless, both scores were supplemented by further scores during the ongoing M-TEER treatment period. The MitraScore predicts the mortality and hospitalization rate after M-TEER on the basis of eight clinical points (c-statistics 0.68), which was also validated by various analyses (37, 38). The score was later expanded by adding the TAPSE/PASP-ratio as the aforementioned marker of RV-PA uncoupling (39). The MITRALITY score was also developed on the basis of clinical parameters for risk stratification prior to M-TEER and demonstrated comparable precision to the previously established scores in subsequent analyses (37, 40) (AUC value 0.783). The Mitral Regurgitation International Database (MIDA) risk score (41) (no AUC value or c-statistics provided) and the Getting Reduction of mitrAl inSufficiency (GRASP) nomogram (42) were equally developed on the basis of clinical parameters (AUC value 0.78). The MitraCox score stratifies the risk of in-hospital mortality and includes an evaluation of the conducting cardiac center with respect to the annual number of performed procedures (43) (AUC value 0.82). In contrast, the Cardiovascular Outcomes Assessment of the Mitraclip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) risk score (44) focuses on the risk assessment of patients with functional MR who meet the inclusion criteria of the COAPT study (8) (AUC value 0.74). All in all, the risk scores available to date are primarily predictive of mortality among M-TEER patients. To our knowledge, the STS-PROM score is the only score that can predict the additional endpoints listed. The previously published scores for the prediction of mortality risk in M-TEER patients are listed in Table 1.
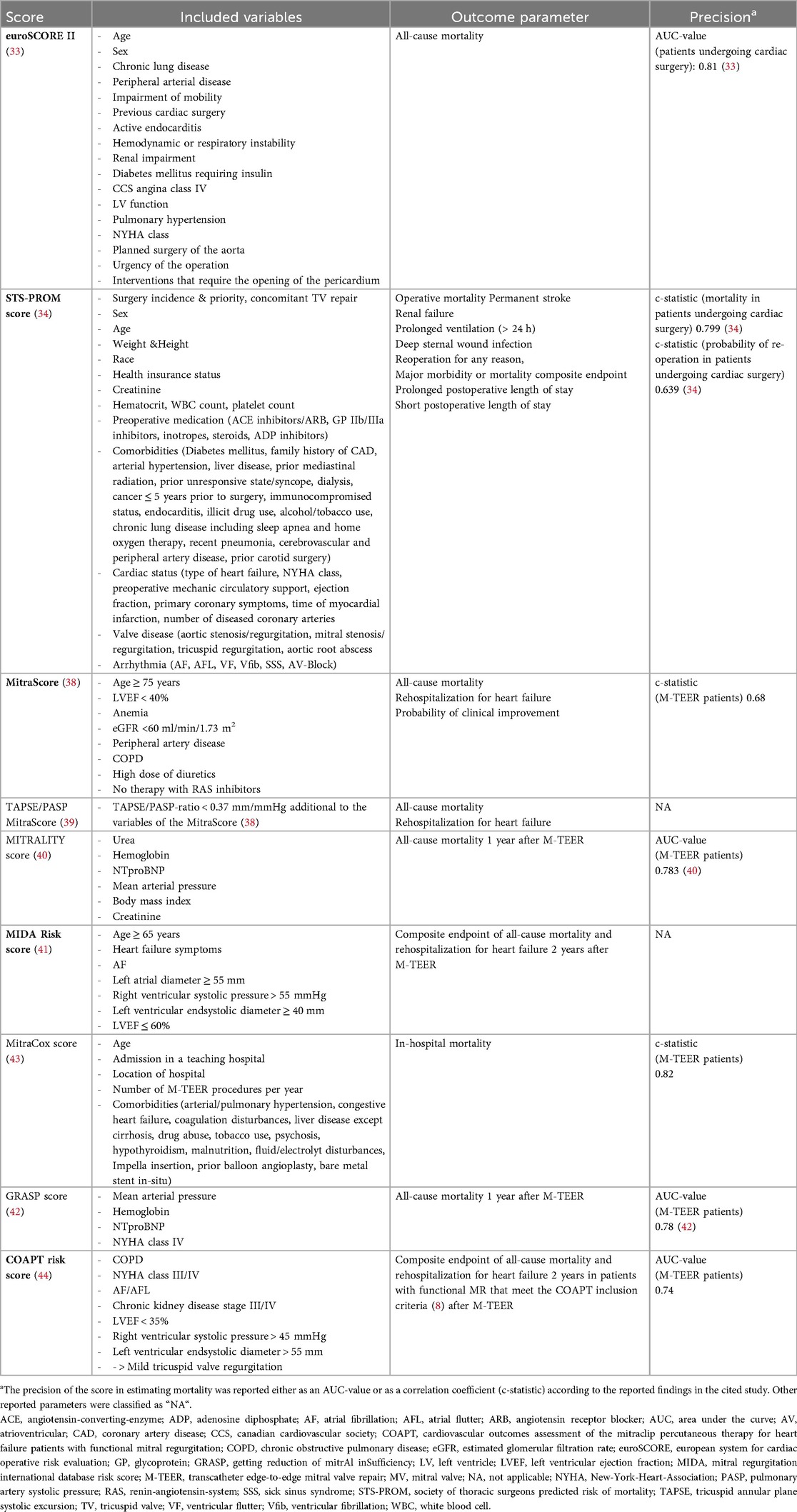
Table 1. Overview of currently published scores for predicting mortality in patients undergoing M-TEER.
Transcatheter edge-to-edge tricuspid valve repair (T-TEER)
Clinical parameters with significant effects on mortality after T-TEER
Since the first feasibility study on T-TEER was published ten years after the food and drug administration (FDA) approval of M-TEER and therefore represents a more recent form of treatment (6), the corresponding evidence with respect to mortality predictors is relatively limited. Similar to the M-TEER collective, concomitant pulmonary hypertension (45), RV-PA uncoupling (46, 47) and cardiohepatic and cardiorenal syndrome (48, 49) negatively impacted survival in patients who underwent T-TEER. The same applied to underweight (50) and malnutrition (51), which can also be regarded as markers of advanced cardiac and noncardiac morbidity. In this context, geriatric multimorbidity has emerged as a marker of reduced survival (52). Owing to the ongoing demographic changes, this factor occupies a relevant position in both TEER cohorts. In addition to the general risk assessment, the question arises as to whether geriatric multimorbidity can be optimized, which might have an impact on treatment outcomes. Thus, the conditions and effects of this parameter in both TEER collectives should be examined in more detail in future studies.
Risk scores for the stratification of mortality prior to T-TEER
With respect to the risk scores for the prediction of mortality in the T-TEER cohort, the EuroSCORE II (AUC-value 0.81 for patients undergoing cardiac surgery) and the STS-PROM score (c-statistics 0.799 for mortality and c-statistics 0.639 for reoperation in patients undergoing cardiac surgery, respectively) can also be applied, but no dedicated validation of the scores was performed in this specific cohort. However, they can still provide a risk assessment for isolated surgical tricuspid valve repair, although this procedure is rarely performed because of the elevated complication rate in addition to the increased average morbidity of the respective cohort (53). The additional predicted endpoints of the STS-PROM score could be applied equally in the T-TEER cohort, although differentiated validation studies of the score in these endpoints among this particular cohort are currently lacking. In contrast, the TRI-SCORE outperforms the EuroSCORE II and STS-PROM score in the prediction of 30-day, 1-year and 10-year mortality after T-TEER (54, 55) (e.g., AUC values for predictin 1-year mortality after T-TEER: 0.931 vs. 0.644 vs. 0.59, respectively). An additional score also composed of clinical and echocardiographic parameters is the TRIVALVE score, which predicts a combined endpoint of mortality and rehospitalization until one year after T-TEER (56) (AUC-value: 0.683). Furthermore, the Get With The Guidelines-Heart Failure (GWTG-HF) score was extrapolated to T-TEER patients after it was developed primarily to predict in-hospital mortality in patients hospitalized due to acute heart failure (57). In a published study by Kavsur et al., the GWTG-HF score proved to be equally predictive of the combined endpoint of mortality and rehospitalization until one year after T-TEER (57) (no AUC value or c-statistics reported). Finally, the GLIDE score was developed to predict procedural success prior to T-TEER, defined as the reduction in TR to a moderate or lower severity grade (58, 59). This score was not intended to directly predict mortality but could be considered metaphorically, as procedural T-TEER success has been shown to be a marker of reduced mortality and hospitalization rates (60). An overview of the currently available scores that predict mortality after T-TEER is outlined in Table 2.
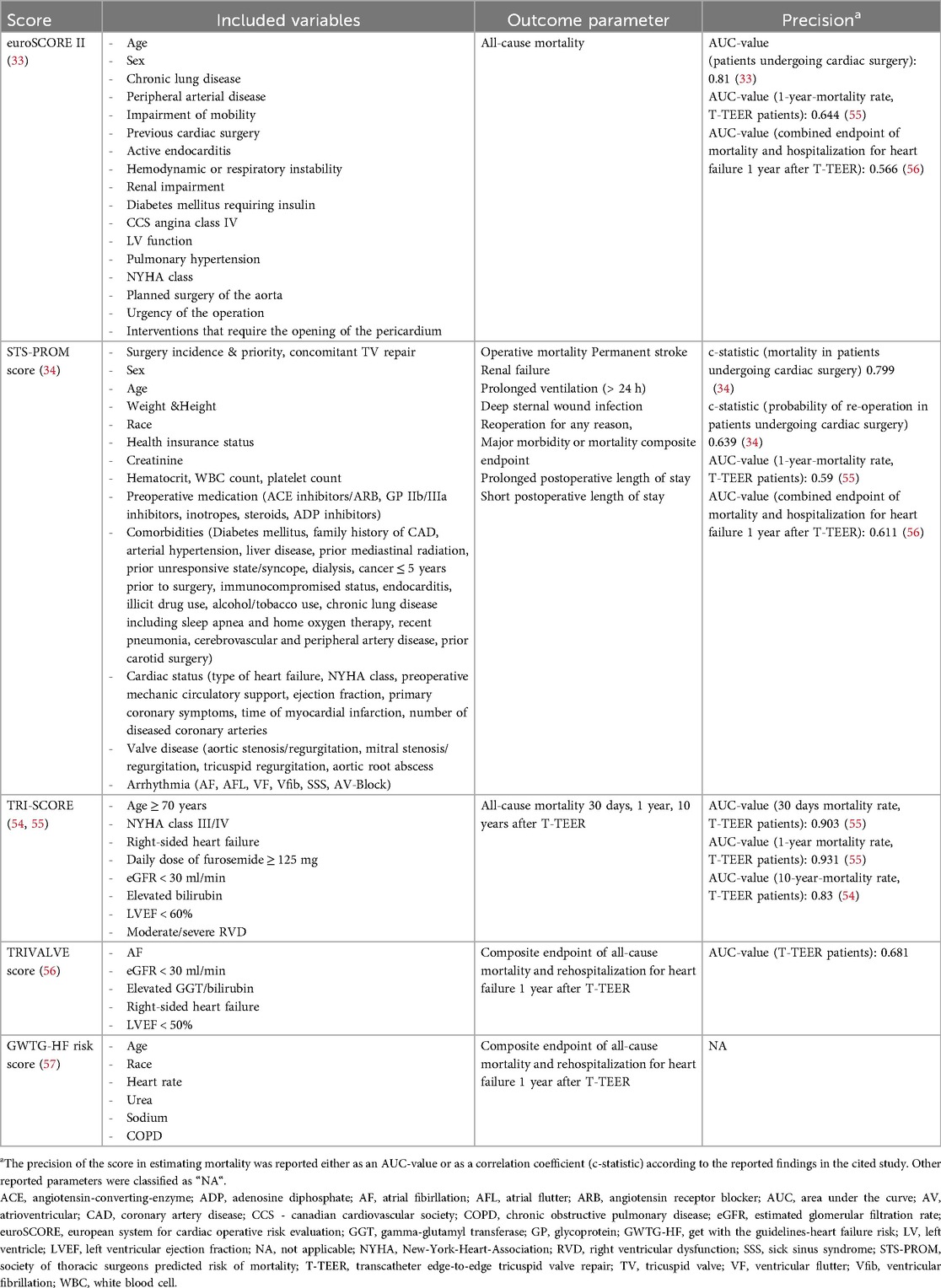
Table 2. Overview of currently published scores for predicting mortality in patients undergoing T-TEER.
In summary, previous research revealed a broad spectrum of cofactors that were independently associated with increased mortality following M-TEER and T-TEER. To the best of our knowledge, current evidence does not indicate a relevant influence of the choice of TEER system on mortality (61, 62). While favorable results have been reported for the newer M-TEER devices in terms of procedure-related characteristics and hospitalization rates, no results are yet available for mortality (63). However, some study findings showed that mortality can be significantly ameliorated by treatment of the underlying comorbidity or by TEER itself. For instance, the survival of patients who underwent M-TEER and had concomitant AF could be considerably improved by pulmonary vein isolation as a state-of-the-art therapy for AF to the extent that survival no longer differed from that of patients who underwent M-TEER without AF (64). Similarly, an improvement in RV-PA uncoupling after M-TEER was observed in the majority of patients, which was associated with improved survival after M-TEER (65). With respect to secondary organ failure due to advanced VHD, T-TEER led to an improvement in liver function, while renal function remained unaffected (66).
These recent data raise the question as to what extent the appropriate treatment of the underlying condition and the TEER procedure can contribute to improved survival, thereby justifying the referral of the patient for TEER. The available preliminary findings provide a significant contribution to existing evidence, which should, however, be further explored and augmented by future studies.
While the STS-PROM score represents the highest versatility in predicted endpoints among M-TEER patients (34), it can be supplemented by the MITRALITY score with the best AUC value to date (40), although comparability is hampered by inconsistencies in the reports. Among T-TEER patients, the TRI-SCORE has so far proven to be even better at predicting mortality than the well-known euroSCORE II and STS-PROM score models (54, 55). For future treatment, risk prediction should therefore be based on the identified comorbidities and a selection of the aforementioned scores. Nevertheless, depending on the results of upcoming studies, further scores should be developed that take into account any prognosis-improving therapies for comorbidities. In addition, other endpoints besides mortality, such as the occurrence of procedural complications and the reduction success of MR/TR, should also be investigated as further crucial endpoint parameters.
Limitations
Given that this review did not aim to serve as a systematic review, the effects of additional unknown or uncollected cofactors on mortality cannot be excluded. The respective limitations that were outlined in the particular studies should be considered when the presented findings are interpreted. Notably, individual confirmation of the feasibility of M-TEER by echocardiography constitutes the cornerstone of further risk assessment prior to the procedure. This review did not include a synthesis of the relevant echocardiographic feasibility criteria, therefore these should be considered separately in the relevant publications (67, 68). On the basis of the present analysis, no statement can be drawn regarding the risk prediction of patients undergoing transcatheter mitral and tricuspid valve replacement (TMVR, TTVR), who therefore must also be examined separately.
Conclusion
Clinical decision-making in the current TEER era remains challenging but can be supported by a variety of cofactors that are linked to survival and by various risk scores that estimate all-cause mortality. Although none of these parameters can provide the sole criterion for deciding for or against TEER, the presented overview can significantly contribute to optimized patient selection with consecutive improvement in treatment outcomes. Further research is needed to investigate the effects of the treatment of comorbidities that significantly worsen mortality.
Author contributions
FA: Conceptualization, Methodology, Investigation, Resources, Writing – original draft, Formal analysis. C-FF: Formal analysis, Supervision, Methodology, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no generative AI was used in the creation of the manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet (London, England). (2006) 368:1005–11. doi: 10.1016/S0140-6736(06)69208-8
2. Wang N, Fulcher J, Abeysuriya N, McGrady M, Wilcox I, Celermajer D, et al. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: a systematic review and meta-analysis. Eur Heart J. (2019) 40:476–84. doi: 10.1093/eurheartj/ehy641
3. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. (2004) 43:405–9. doi: 10.1016/j.jacc.2003.09.036
4. Zhou S, Egorova N, Moskowitz G, Giustino G, Ailawadi G, Acker MA, et al. Trends in MitraClip, mitral valve repair, and mitral valve replacement from 2000 to 2016. J Thorac Cardiovasc Surg. (2021) 162:551–562.e4. doi: 10.1016/j.jtcvs.2019.12.097
5. Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (endovascular valve edge-to-edge REpair study) cohort. J Am Coll Cardiol. (2009) 54:686–94. doi: 10.1016/j.jacc.2009.03.077
6. Sorajja P, Whisenant B, Hamid N, Naik H, Makkar R, Tadros P, et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. (2023) 388:1833–42. doi: 10.1056/NEJMoa2300525
7. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. (2011) 364:1395–406. doi: 10.1056/NEJMoa1009355
8. Stone Gregg W, Lindenfeld J, Abraham WT, Saibal K, Scott LD, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. (2018) 379:2307–18. doi: 10.1056/NEJMoa1806640
9. Anker SD, Friede T, von Bardeleben R-S, Butler J, Khan M-S, Diek M, et al. Transcatheter valve repair in heart failure with moderate to severe mitral regurgitation. N Engl J Med. (2024) 391:1799–809. doi: 10.1056/NEJMoa2314328
10. Lurz P, Besler C, Schmitz T, Bekeredjian R, Nickenig G, Möllmann H, et al. Short-Term outcomes of tricuspid edge-to-edge repair in clinical practice. J Am Coll Cardiol. (2023) 82:281–91. doi: 10.1016/j.jacc.2023.05.008
11. Kodali SK, Hahn RT, Davidson CJ, Narang A, Greenbaum A, Gleason P, et al. 1-Year Outcomes of transcatheter tricuspid valve repair. J Am Coll Cardiol. (2023) 81:1766–76. doi: 10.1016/j.jacc.2023.02.049
12. von Bardeleben RS, Lurz P, Sorajja P, Ruf T, Hausleiter J, Sitges M, et al. Two-Year outcomes for tricuspid repair with a transcatheter edge-to-edge valve repair from the transatlantic TRILUMINATE trial. Circ Cardiovasc Interv. (2023) 16:e012888. doi: 10.1161/CIRCINTERVENTIONS.122.012888
13. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22:1342–56. doi: 10.1002/ejhf.1858
14. Nappi F, Nenna A, Sing SSA, Timofeeva I, Mihos C, Gentile F, et al. Mitral regurgitation: lessons learned from COAPT and MITRA-fr. J Thorac Dis. (2020) 12:2936–44. doi: 10.21037/jtd.2020.01.67
15. Schlotter F, Stolz L, Kresoja K-P, von Stein J, Fortmeier V, Koell B, et al. Tricuspid regurgitation disease stages and treatment outcomes after transcatheter tricuspid valve repair. JACC Cardiovasc Interv. (2025) 18:339–48. doi: 10.1016/j.jcin.2024.10.034
16. Kanitsoraphan C, Thangjui S, Techorueangwiwat C, Kewcharoen J, Rattanawong P, Nagamine T, et al. Gender difference in outcomes of patients undergoing MitraClip therapy: a systematic review and meta-analysis. Cardiovasc Revasc Med. (2022) 40:20–5. doi: 10.1016/j.carrev.2021.11.012
17. Ausbuettel F, Barth S, Chatzis G, Sassani K, Fischer D, Weyand S, et al. Sex-Specific disparities in outcomes of transcatheter edge-to-edge repair for mitral regurgitation: a multicenter “real-world”. Analysis. J Clin Med. (2023) 12:7231. doi: 10.3390/jcm12237231
18. Megaly M, Abraham B, Saad M, Omer M, Elbadawi A, Tawadros M, et al. Impact of atrial fibrillation on the outcomes after MitraClip®: a meta-analysis. Structural Heart. (2018) 2:531–7. doi: 10.1080/24748706.2018.1517952
19. Safiriyu I, Nagraj S, Otulana R, Saralidze T, Kokkinidis DG, Faillace R. Prognostic impact of Pre- and post-procedural renal dysfunction on late all-cause mortality outcome following transcatheter edge-to-edge repair of the mitral valve: a systematic review and meta-analysis. Cardiovasc Revasc Med. (2022) 42:6–14. doi: 10.1016/j.carrev.2022.03.023
20. Chitturi KR, Bhardwaj B, Murtaza G, Karuparthi PR, Faza NN, Goel SS, et al. Clinical impact of tricuspid regurgitation on transcatheter edge-to-edge mitral valve repair for mitral regurgitation. Cardiovasc Revasc Med. (2022) 41:1–9. doi: 10.1016/j.carrev.2022.01.027
21. Boerlage-vanDijk K, Wiegerinck EMA, Araki M, Meregalli PG, Bindraban NR, Koch KT, et al. Predictors of outcome in patients undergoing MitraClip implantation: an aid to improve patient selection. Int J Cardiol. (2015) 189:238–43. doi: 10.1016/j.ijcard.2015.01.045
22. Wei Z, Shao X, An Z, Chang Y, Liu S, Luo Z, et al. The impact of pulmonary hypertension on prognosis in moderate-to-severe mitral regurgitation patients treated with transcatheter edge-to-edge mitral valve repair: a comprehensive meta-analysis. Front Cardiovasc Med. (2024) 11:1489674. doi: 10.3389/fcvm.2024.1489674
23. Doldi PM, Stolz L, Kalbacher D, Köll B, Geyer M, Ludwig S, et al. Right ventricular dysfunction predicts outcome after transcatheter mitral valve repair for primary mitral valve regurgitation. Eur J Heart Fail. (2022) 24:2162–71. doi: 10.1002/ejhf.2661
24. Karam N, Stolz L, Orban M, Deseive S, Praz F, Kalbacher D, et al. Impact of right ventricular dysfunction on outcomes after transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Imaging. (2021) 14:768–78. doi: 10.1016/j.jcmg.2020.12.015
25. Rmilah AA, Ghaly R, Pfeiffer C, Saeed MH, Khojah A, Jaber S, et al. Prognostic value of baseline RV dysfunction using TAPSE and TAPSE to PASP ratio in patients undergoing mitra-clip: a systematic review and meta-analysis. Int J Cardiovasc Imaging. (2025) 41:827–46. doi: 10.1007/s10554-025-03354-5
26. Scotti A, Massussi M, Latib A, Munafò A, Colombo A, Taramasso M, et al. Meta-Analysis of relation between left ventricular dysfunction and outcomes after transcatheter mitral edge-to-edge repair. Am J Cardiol. (2022) 175:88–96. doi: 10.1016/j.amjcard.2022.03.059
27. Saito T, Kuno T, Ueyama HA, Kampaktsis PN, Kolte D, Misumida N, et al. Transcatheter edge-to-edge mitral valve repair for mitral regurgitation in patients with cardiogenic shock: a systematic review and meta-analysis. Catheter Cardiovasc Interv. (2024) 103:340–7. doi: 10.1002/ccd.30944
28. Puls M, Lubos E, Boekstegers P, von Bardeleben RS, Ouarrak T, Butter C, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German transcatheter mitral valve interventions registry. Eur Heart J. (2016) 37:703–12. doi: 10.1093/eurheartj/ehv627
29. Stolz L, Orban M, Karam N, Lubos E, Wild M, Weckbach L, et al. Cardio-hepatic syndrome in patients undergoing mitral valve transcatheter edge-to-edge repair. Eur J Heart Fail. (2023) 25:872–84. doi: 10.1002/ejhf.2842
30. Giordano A, Pepe M, Biondi-Zoccai G, Corcione N, Finizio F, Ferraro P, et al. Impact of coronary artery disease on outcome after transcatheter edge-to-edge mitral valve repair with the MitraClip system. Panminerva Med. (2023) 65:443–53. doi: 10.23736/S0031-0808.23.04827-9
31. Tabata N, Weber M, Sugiura A, Öztürk C, Tsujita K, Nickenig G, et al. Impact of cancer history on clinical outcome in patients undergoing transcatheter edge-to-edge mitral repair. Clin Res Cardiol. (2021) 110:440–50. doi: 10.1007/s00392-020-01770-2
32. Carter-Storch R, Veien KT, Mogensen NSB, Banke A, Tofte-Hansen EU, Ali M, et al. Low body mass index and risk of mortality after mitral transcatheter edge-to-edge repair procedure: the “obesity paradox”. Catheter Cardiovasc Interv. (2024) 104:401–7. doi: 10.1002/ccd.31114
33. Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. (2012) 41:734–44. discussion 744-5. doi: 10.1093/ejcts/ezs043
34. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg. (2009) 88:S23–42. doi: 10.1016/j.athoracsur.2009.05.056
35. Adamo M, Capodanno D, Cannata S, Giannini C, Laudisa ML, Barbanti M, et al. Comparison of three contemporary surgical scores for predicting all-cause mortality of patients undergoing percutaneous mitral valve repair with the MitraClip system (from the multicenter GRASP-IT registry). Am J Cardiol. (2015) 115:107–12. doi: 10.1016/j.amjcard.2014.09.051
36. Schneider L, Markovic S, Mueller K, Felbel D, Gerçek M, Friedrichs K, et al. Mitral valve transcatheter edge-to-edge repair using MitraClip or PASCAL: a multicenter propensity score-matched comparison. JACC Cardiovasc Interv. (2022) 15:2554–67. doi: 10.1016/j.jcin.2022.10.028
37. de Sá Marchi MF, van den Dorpel M, Calomeni P, Chatterjee S, Adrichem R, Verhemel S, et al. Comparative analysis of different risk prediction tools after mitral transcatheter edge-to-edge repair. Int J Cardiol. (2024) 400:131768. doi: 10.1016/j.ijcard.2024.131768
38. Raposeiras-Roubin S, Adamo M, Freixa X, Arzamendi D, Benito-González T, Montefusco A, et al. A score to assess mortality after percutaneous mitral valve repair. J Am Coll Cardiol. (2022) 79:562–73. doi: 10.1016/j.jacc.2021.11.041
39. Shechter A, Vaturi M, Kaewkes D, Koren O, Koseki K, Solanki A, et al. Prognostic value of baseline tricuspid annular plane systolic excursion to pulmonary artery systolic pressure ratio in mitral transcatheter edge-to-edge repair. J Am Soc Echocardiogr. (2023) 36:391–401.e19. doi: 10.1016/j.echo.2022.12.026
40. Zweck E, Spieker M, Horn P, Iliadis C, Metze C, Kavsur R, et al. Machine learning identifies clinical parameters to predict mortality in patients undergoing transcatheter mitral valve repair. JACC Cardiovasc Interv. (2021) 14:2027–36. doi: 10.1016/j.jcin.2021.06.039
41. Kavsur R, Spieker M, Iliadis C, Metze C, Transier M, Tiyerili V, et al. Mitral regurgitation international database (MIDA) score predicts outcome in patients with heart failure undergoing transcatheter edge-to-edge mitral valve repair. J Am Heart Assoc. (2021) 10:e019548. doi: 10.1161/JAHA.120.019548
42. Buccheri S, Capodanno D, Barbanti M, Popolo Rubbio A, Di Salvo ME, Scandura S, et al. A risk model for prediction of 1-year mortality in patients undergoing MitraClip implantation. Am J Cardiol. (2017) 119:1443–9. doi: 10.1016/j.amjcard.2017.01.024
43. Kathavarayan Ramu S, Agrawal A, Shekhar S, Bansal A, Isogai T, Yun J, et al. Mitracox score predicts in-hospital mortality in patients admitted for transcatheter edge-to-edge mitral valve repair. Am J Cardiol. (2023) 207:39–47. doi: 10.1016/j.amjcard.2023.08.160
44. Shah N, Madhavan MV, Gray WA, Brener SJ, Ahmad Y, Lindenfeld J, et al. Prediction of death or HF hospitalization in patients with severe FMR: the COAPT risk score. JACC Cardiovasc Interv. (2022) 15:1893–905. doi: 10.1016/j.jcin.2022.08.005
45. Stolz L, Kresoja K-P, von Stein J, Fortmeier V, Koell B, Rottbauer W, et al. Impact of pulmonary hypertension on outcomes after transcatheter tricuspid valve edge-to-edge repair. JACC Cardiovasc Interv. (2025) 18:325–36. doi: 10.1016/j.jcin.2024.10.023
46. Brunner S, Stolz L, Kresoja K-P, von Stein J, Fortmeier V, Koell B, et al. The relevance of right ventricular function and dimension in patients undergoing transcatheter tricuspid edge-to-edge repair. JACC Cardiovasc Interv. (2025) 18:1737–45. doi: 10.1016/j.jcin.2025.05.037
47. Koschutnik M, Donà C, Nitsche C, Kammerlander AA, Dannenberg V, Brunner C, et al. Impact of right ventricular-to-pulmonary artery coupling on remodeling and outcome in patients undergoing transcatheter edge-to-edge mitral valve repair. Clin Res Cardiol. (2025) 114:156–67. doi: 10.1007/s00392-023-02318-w
48. Jorde UP, Benza R, McCarthy PM, Ailawadi G, Whisenant B, Makkar R, et al. Impact of renal and liver function on clinical outcomes following tricuspid valve transcatheter edge-to-edge repair. J Am Coll Cardiol. (2024) 84:2446–56. doi: 10.1016/j.jacc.2024.08.044
49. Stolz L, Orban M, Besler C, Kresoja K-P, Braun D, Doldi P, et al. Cardiohepatic syndrome is associated with poor prognosis in patients undergoing tricuspid transcatheter edge-to-edge valve repair. JACC Cardiovasc Interv. (2022) 15:179–89. doi: 10.1016/j.jcin.2021.10.033
50. Vogelhuber J, Tenaka T, Sudo M, Sugiura A, Öztürk C, Kavsur R, et al. Impact of body mass index in patients with tricuspid regurgitation after transcatheter edge-to-edge repair. Clin Res Cardiol. (2024) 113:156–67. doi: 10.1007/s00392-023-02312-2
51. Pagnesi M, Adamo M, Stolz L, Pancaldi E, Kresoja K-P, von Stein J, et al. Malnutrition and outcomes in patients with tricuspid regurgitation undergoing transcatheter tricuspid valve repair. Eur J Heart Fail. (2025) 27(7):1304–14. doi: 10.1002/ejhf.3623
52. Schäfer M, Körber MI, Vimalathasan R, Mauri V, Iliadis C, Metze C, et al. Risk stratification of patients undergoing percutaneous repair of mitral and tricuspid valves using a multidimensional geriatric assessment. Circ Cardiovasc Qual Outcomes. (2021) 14:e007624. doi: 10.1161/CIRCOUTCOMES.120.007624
53. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/eurheartj/ehab395
54. Sala A, Carino D, Lorusso R, Zancanaro E, Bargagna M, Del Forno B, et al. TRI-SCORE: a single-centre validation study. Interdiscip Cardiovasc Thorac Surg. (2023):36(6):ivad085. doi: 10.1093/icvts/ivad085
55. Gröger M, Friedl S, Ouerghemmi D, Tadic M, Bruß E, Felbel D, et al. TRI-SCORE is superior to EuroSCORE II and STS-score in mortality prediction following transcatheter edge-to-edge tricuspid valve repair. Clin Res Cardiol. (2023) 112:1436–45. doi: 10.1007/s00392-023-02246-9
56. Russo G, Pedicino D, Pires Marafon D, Adamo M, Alessandrini H, Andreas M, et al. TRIVALVE Score: a risk score for mortality/hospitalization prediction in patients undergoing transcatheter tricuspid valve intervention. JACC Cardiovasc Interv. (2024) 17:2170–9. doi: 10.1016/j.jcin.2024.08.009
57. Kavsur R, Hupp-Herschel HE, Sugiura A, Tanaka T, Öztürk C, Weber M, et al. Prognostic significance of the get with the guidelines-heart failure (GWTG-HF) risk score in patients undergoing trans-catheter tricuspid valve repair (TTVR). Heart Vessels. (2021) 36:1903–10. doi: 10.1007/s00380-021-01874-3
58. Rudolph F, Narang A, Körber MI, Friedrichs KP, Kirchner J, Ivannikova M, et al. Assessment of the GLIDE score for prediction of mild tricuspid regurgitation following tricuspid transcatheter edge-to-edge repair. JACC Adv. (2025) 4:101523. doi: 10.1016/j.jacadv.2024.101523
59. Gerçek M, Narang A, Körber MI, Friedrichs KP, Puthumana JJ, Ivannikova M, et al. GLIDE Score: scoring system for prediction of procedural success in tricuspid valve transcatheter edge-to-edge repair. JACC Cardiovasc Imaging. (2024) 17:729–42. doi: 10.1016/j.jcmg.2024.04.008
60. Besler C, Orban M, Rommel K-P, Braun D, Patel M, Hagl C, et al. Predictors of procedural and clinical outcomes in patients with symptomatic tricuspid regurgitation undergoing transcatheter edge-to-edge repair. JACC Cardiovasc Interv. (2018) 11:1119–28. doi: 10.1016/j.jcin.2018.05.002
61. Sugiura A, Vogelhuber J, Öztürk C, Schwaibold Z, Reckers D, Goto T, et al. PASCAL Versus MitraClip-XTR edge-to-edge device for the treatment of tricuspid regurgitation: a propensity-matched analysis. Clin Res Cardiol. (2021) 110:451–9. doi: 10.1007/s00392-020-01784-w
62. Oliveri F, Al Amri I, Montero-Cabezas J, Bingen B, van der Kley F, Arslan F, et al. Mitral valve transcatheter edge-to-edge repair with PASCAL vs MitraClip: a systematic review and meta-analysis. J Invasive Cardiol. (2023) 35(11). doi: 10.25270/jic/23.00218
63. Okuno T, Izumo M, Kuwata S, Akashi YJ, Yamamoto M, Kubo S, et al. Early- versus newer-generation transcatheter mitral valve edge-to-edge repair systems: insights from the OCEAN-mitral registry. JACC. Asia. (2025) 5:1110–20. doi: 10.1016/j.jacasi.2025.05.013
64. Ausbuettel F, Barth S, Chatzis G, Fischer D, Kerber S, Mueller J, et al. Catheter ablation of concomitant atrial fibrillation improves survival of patients undergoing transcatheter edge-to-edge mitral valve repair. Front Cardiovasc Med. (2023) 10:1229651. doi: 10.3389/fcvm.2023.1229651
65. Adamo M, Inciardi RM, Tomasoni D, Dallapellegrina L, Estévez-Loureiro R, Stolfo D, et al. Changes in right ventricular-to-pulmonary artery coupling after transcatheter edge-to-edge repair in secondary mitral regurgitation. JACC Cardiovasc Imaging. (2022) 15:2038–47. doi: 10.1016/j.jcmg.2022.08.012
66. Karam N, Braun D, Mehr M, Orban M, Stocker TJ, Deseive S, et al. Impact of transcatheter tricuspid valve repair for severe tricuspid regurgitation on kidney and liver function. JACC Cardiovasc Interv. (2019) 12:1413–20. doi: 10.1016/j.jcin.2019.04.018
67. Tanaka T, Vogelhuber J, Öztürk C, Silaschi M, Bakhtiary F, Zimmer S, et al. Eligibility for transcatheter tricuspid valve interventions in patients with tricuspid regurgitation. JACC Cardiovasc Interv. (2024) 17:2732–44. doi: 10.1016/j.jcin.2024.09.019
68. Hausleiter J, Stocker TJ, Adamo M, Karam N, Swaans MJ, Praz F. Mitral valve transcatheter edge-to-edge repair. EuroIntervention. (2023) 18:957–76. doi: 10.4244/EIJ-D-22-00725
Glossary
ACE angiotensin-converting-enzyme
ADP adenosine diphosphate
AF atrial fibrillation
AFL atrial flutter
ARB angiotensin receptor blocker
AUC area under the curve
AV atrioventricular
BMI body mass index
CAD coronary artery disease
CCS canadian cardiovascular society
COAPT risk score cardiovascular outcomes assessment of the mitraclip percutaneous therapy for heart failure patients with functional mitral regurgitation risk score
COPD chronic obstructive pulmonary disease
eGFR estimated glomerular filtration rate
euroSCORE II european system for cardiac operative risk evaluation II
FDA food and drug administration
GGT gamma-glutamyl transferase
GP glycoprotein
GRASP normogram getting reduction of mitrAl inSufficiency normogram
GWTG-HF score get with the guidelines-heart failure risk score
LV left ventricle
LVEF left ventricular ejection fraction
MIDA risk score mitral regurgitation international database risk score
MR mitral valve regurgitation
M-TEER transcatheter edge-to-edge mitral valve repair
MV mitral valve
NA not applicable
NYHA New-York-Heart-Association
OMT optimal medical therapy
PA pulmonary artery
PASP pulmonary arterial systolic pressure
RAS renin-angiotensin-system
RV right ventricle
RVD right ventricular dysfunction
SSS sick sinus syndrome
STS-PROM score society of thoracic surgeons predicted risk of mortality score
TAPSE tricuspid annular plane systolic excursion
TMVR transcatheter mitral valve replacement
TR tricuspid valve regurgitation
T-TEER transcatheter edge-to-edge tricuspid valve repair
TTVR transcatheter tricuspid valve replacement
TV tricuspid valve
VF ventricular flutter
Vfib ventricular fibrillation
VHD valvular heart disease
WBC white blood cell
Keywords: M-TEER, T-TEER, mortality prediction, transcatheter edge-to-edge repair, mitraclip, triclip, PASCAL
Citation: Ausbuettel F and Fichera C-F (2025) To clip or not to clip? A review of precise risk assessment in the contemporary era of transcatheter edge-to-edge mitral and tricuspid valve repair. Front. Cardiovasc. Med. 12:1693291. doi: 10.3389/fcvm.2025.1693291
Received: 26 August 2025; Accepted: 31 October 2025;
Published: 14 November 2025.
Edited by:
Elena Aikawa, Brigham and Women's Hospital and Harvard Medical School, United StatesReviewed by:
Basel Alqeeq, Islamic University of Gaza, PalestineCopyright: © 2025 Ausbuettel and Fichera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felix Ausbuettel, ZmVsaXhAYXVzYnVldHRlbC5pbmZv
 Felix Ausbuettel
Felix Ausbuettel Carlo-Federico Fichera2
Carlo-Federico Fichera2