- 1Nursing Department, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Cardiovascular Medicine, Cardiovascular Research Center, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Due to the aging population, the prevalence of aortic stenosis continues to rise, transcatheter aortic valve replacement (TAVR) has become an important method for treating severe symptomatic aortic stenosis. Although TAVR significantly improves the survival and symptoms of patients with aortic stenosis, this population is generally characterized by advanced age, frailty, and multiple comorbidities, posing challenges to postoperative functional recovery and quality of life improvement. Cardiac rehabilitation (CR) constitutes a cornerstone of secondary prevention for cardiovascular disease (CVD), and plays a pivotal role in optimizing outcomes for patients undergoing TAVR. This review aims to discuss the mechanistic, current practical evidence, existing challenges, and future directions of CR in TAVR patients.
1 Introduction
The prevalence of the most common valvular disease, aortic stenosis (AS), is still increasing, and its prevalence increases with age (1–3). In developed nations, the prevalence of severe aortic stenosis (AS) among individuals aged ≥75 years is 3.4% (4); a 2012–2015 sampling survey of valvular heart disease in China revealed a comparable AS prevalence of 3.4% in the same demographic (5). It is estimated that the number of patients with moderate to severe AS will nearly triple by 2060 (3, 6). TAVR is a safe and effective treatment for inoperable or high-risk patients with severe AS, and its indications have progressively expanded to include low-risk patients (7, 8). Nonetheless, the target population remains frail elderly patients (9). These patients have some postoperative problems, such as cardiac dysfunction, cognitive impairment, and poor recovery of quality of life (10–12). With the continuous growth and popularization of TAVR procedures, the demand for postoperative rehabilitation has surged, making it important to understand the CR outcomes of patients.
CR is a comprehensive cardiovascular disease rehabilitation intervention involving exercise, nutrition, psychology, medication, smoking cessation, and other aspects. Currently, the benefits of CR have been confirmed by numerous studies (13, 14), including reducing mortality, morbidity, and readmission rates, and improving quality of life and physical function, and enhancing metabolic, inflammatory, and molecular biomarkers of risk and resilience (15).
All AS patients may benefit from post-TAVR CR (16). Post-TAVR CR has been proven to be safe and beneficial, it can increase exercise capacity, improve quality of life, and reduce disability, and can reduce patient mortality, morbidity, and readmission rates (17–19). A study by Patel et al. confirmed that AS patients participating in CR had, compared to the control group, a 61% relative risk reduction in 1-year mortality, a 4.2% absolute risk reduction, and a 34% relative reduction in readmission risk (20). Furthermore, CR may also positively impact patients’ return to work and is cost-effective (21). Its implementation is recommended by clinical practice guidelines (22). The 2020 updated position statement from the European Association of Preventive Cardiology on secondary prevention and rehabilitation states that all patients after aortic valve replacement should receive a CR program (23). The 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease suggest that patients with asymptomatic mild AS can participate in all sports, while patients with symptomatic AS can consider light exercise that does not provoke symptoms for general health benefits (24). A Chinese expert consensus proposes that early exercise rehabilitation should be initiated as soon as possible after TAVR, provided patient safety is ensured (25).
However, in the real world, the referral rate for CR in TAVR patients is low (19, 26). A U.S. study found that only 39.8% (4027/10,124) of TAVR patients participated in CR (19). The CR referral rate in China is 12.4%, which is lower than the global average (34.4%) (27). The reasons for the low CR referral rate are multifaceted, including lack of or inefficient referral processes, limited health insurance coverage, scheduling conflicts, and inconvenience or long distances leading to excessive time and transportation costs for participating in CR (28, 29). A qualitative study further revealed the barriers and facilitators affecting CR acceptance in AS patients over 80 years old, primarily involving Perceptions and Understanding, Delivery and Accessibility, Perceived Impact of Exercise and Health and Life Changes, and Transportation (30). In conclusion, the risk of cardiovascular events remains high after TAVR, and CR can definitively improve patient prognosis and quality of life. However, the proportion of patients receiving CR in the real world is low. Future research could explore more accessible CR programs to address the multiple barriers TAVR patients face in participating in CR.
This review will discuss the pathophysiological mechanisms of CR in TAVR patients, comprehensive interventions covering the four core dimensions of exercise, nutrition, psychology, and risk factors, and then delve into the three major aspects of individualized clinical practice for complications, frailty, and digital health.
We conducted a systematic search of the literature to examine the existing evidence on CR after TAVR. Our search strategy combined keywords and Medical Subject Headings, including “transcatheter aortic valve replacement”, “TAVR”, “transcatheter aortic valve implantation”, “TAVI”, “cardiac rehabilitation”, “cardiovascular rehabilitation”, etc. These terms were combined using Boolean operators (AND, OR). Due to the limited number of studies identified in the initial search, we used a snowballing method to manually review the reference lists of relevant articles. The primary databases searched were PubMed, Web of Science, and Science Direct. The inclusion criteria included randomized controlled trials, cohort studies, retrospective studies, systematic reviews, and expert consensus publications; case reports were excluded.
2 The pathophysiological mechanisms of CR in TAVR patients
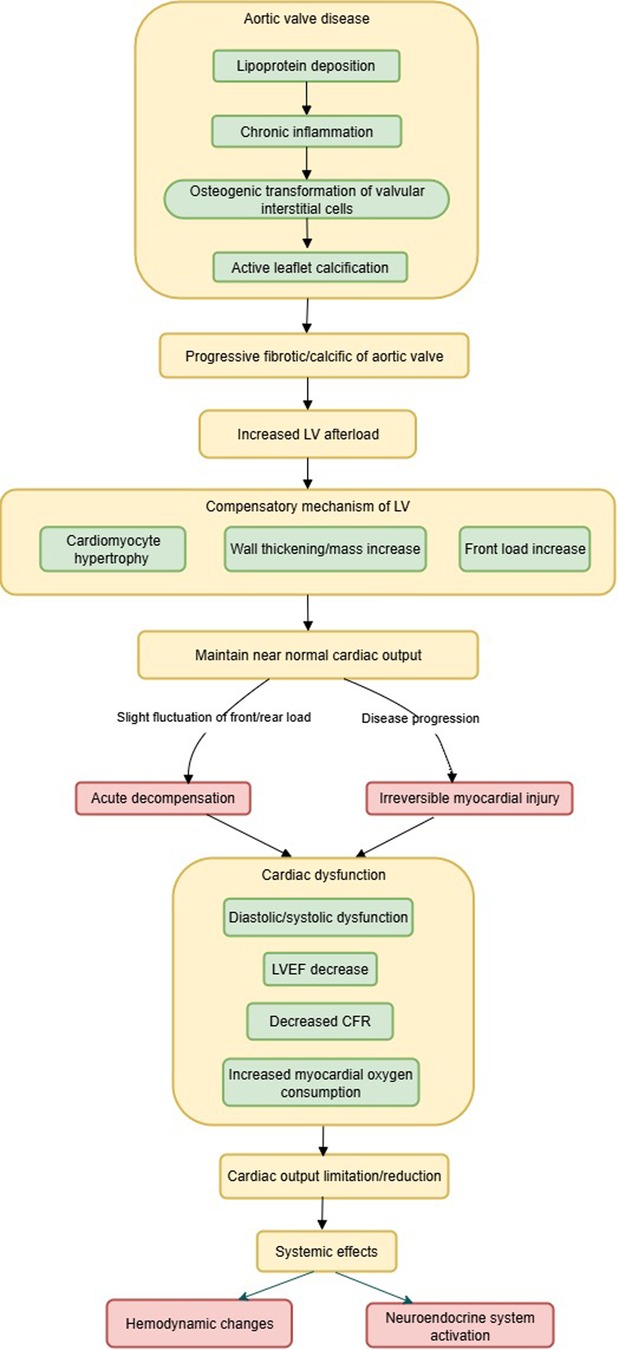
Clinical risk factors for the development of AS include advanced age, hypertension, hyperlipidemia, smoking, and diabetes (31), characterized by progressive fibrotic/calcific narrowing of the aortic valve, a process that is highly complex, involving lipoprotein deposition, chronic inflammation, osteogenic transformation of valvular interstitial cells, and active leaflet calcification (4). Progressive aortic valve stenosis leads to an increase in left ventricular (LV) afterload (32). The LV must continually overcome the resistance generated by the stenotic valve to maintain cardiac output, which over time leads to compensatory hypertrophy of LV cardiomyocytes, and increased LV wall thickness and mass (6, 33, 34). In the early stages of the disease, the body maintains a near-normal cardiac output through a delicate balance between left ventricular preload and afterload, with increased preload to provide adequate stroke volume, and increased afterload to maintain perfusion pressure. Subtle factors affecting preload or afterload may precipitate acute decompensation (35). Without intervention, disease progression leads to irreversible myocardial damage, diastolic/systolic dysfunction (6), decreased left ventricular ejection fraction (LVEF) (36), reduced coronary flow reserve (CFR) (37), and increased myocardial oxygen consumption (38), all leading to limited cardiac output.
A reduction in cardiac output leads to prioritized redistribution of blood flow to vital organs such as the brain, resulting in insufficient blood perfusion to skeletal muscles, increased local vascular resistance, and reduced shear stress and chronic inflammation, which inhibits nitric oxide (NO) release and consequently causes vascular endothelial dysfunction (39, 40).
Furthermore, AS activates multiple neuroendocrine systems, such as activation of the sympathetic nervous system (SNS) (41), the renin-angiotensin-aldosterone system (RAAS) (42), the natriuretic peptide system (BNP/NT-proBNP) (43), and inflammatory pathways (44). Research has confirmed that neurohormonal activity is reduced in patients after TAVR, resulting in significant clinical benefits (45). The pathophysiological mechanism of AS is shown in Figure 1.
Currently, no effective pharmacotherapy exists to alter the natural history of AS progression (32). TAVR can directly reverse AS, resulting in a significant reduction or even elimination of the aortic transvalvular pressure gradient, causing an abrupt decrease in LV afterload and a marked reduction in myocardial oxygen consumption. In AS patients with preserved systolic function, stroke volume and cardiac output usually increase rapidly. This improves systemic tissue perfusion, thereby alleviating patient symptoms such as dyspnea, fatigue, and angina.
CR cannot directly relieve valvular stenosis like TAVR does, but it plays an irreplaceable role in ensuring the smooth progress of TAVR and maintaining the surgical outcomes. The mechanisms of CR are multifaceted, interactive, and comprehensive, with its core function being to improve hemodynamics, reverse cardiac remodeling, and enhance overall functional capacity through regular exercise. Studies indicate that exercise training can improve ventilatory function, endothelial function, and enhance skeletal muscle metabolism, and reduce levels of inflammatory cytokines (e.g., tumor necrosis factor-α, and interleukin-6), exerting positive effects on peak VO2, autonomic nervous system function, central hemodynamic function, peripheral vascular and muscle function, and exercise capacity (15, 46–48). It must be emphasized that exercise training should be regular and incorporate aerobic exercise.
TAVR patients often experience anxiety, depression, and concerns about their prognosis. Depressive status is associated with poor nutritional choices, limited exercise, smoking, and reduced medication adherence, which may exacerbate heart disease (49). CR influences rehabilitation outcomes by providing psychological counseling, patient education, and behavioral interventions to help patients reduce psychological stress, improve nutrition, quit smoking, control blood pressure, blood glucose, and lipids, and enhance medication adherence.
3 Core components of integrated CR after TAVR: exercise, nutrition, psychological support, and risk factor management
CR is a multidimensional and personalized rehabilitation process, and the synergistic effect of all aspects is crucial for patient recovery and long-term health. In post-TAVR management, multimodal CR that integrates exercise, nutrition, psychological support, and risk factor control can provide comprehensive recovery support for patients. By improving patients’ psychological burden and nutritional status, it can also indirectly enhance their participation and completion rates of the rehabilitation program, thus forming a virtuous cycle.
A multicenter prospective cohort study implemented a three-week standardized multi-component inpatient CR for 136 elderly patients after TAVR, including patient education, personalized exercise training, dietary counseling, psychological support, and risk factor management. This study found that patients’ physical capacity, quality of life, anxiety, and frailty were significantly improved (50). A nurse-led multidimensional CR program (including exercise, medication management, nutritional guidance, psychological support, sleep management, health education, and smoking cessation assistance) also showed significant benefits for frailty status, 6MWT, walking speed, grip strength, etc. in patients with unstable angina (UA) undergoing percutaneous coronary intervention (PCI) (51). Takashi et al. found that comprehensive CR supervised by a multidisciplinary rehabilitation team can significantly reduce major adverse cardiovascular events in acute myocardial infarction (AMI) patients undergoing PCI (52). Moreover, the benefits of multimodal CR for heart failure and atrial fibrillation patients have also been confirmed (53, 54).
Currently, there are few large-scale randomized controlled trials related to CR after TAVR, Exercise-based CR is a key intervention for cardiovascular diseases (CVD), with most studies focusing on the exercise rehabilitation aspect. There is a need to increase exploration in areas such as nutrition and psychological support.
3.1 Exercise rehabilitation
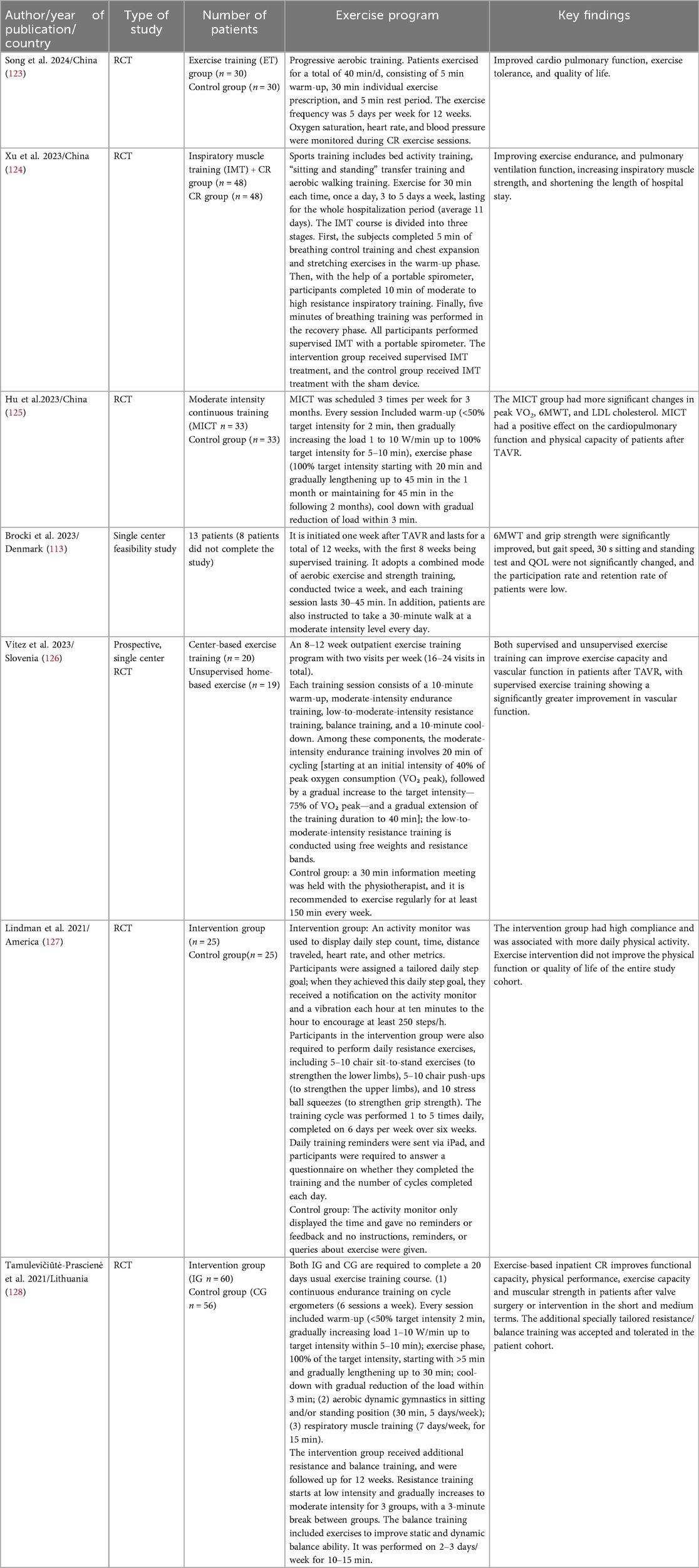
Studies show that 93.33% of TAVR patients lack regular physical activity and have low adherence (55). Sedentary behavior increases the risk of mortality and functional decline after TAVR (56), while moderate-to-vigorous physical activity and participation in CR can improve survival rates in patients after heart valve surgery [OR,1.52, (95% CI 1.03–2.24)] (57). Exercise-based CR programs after TAVR have been proven safe and can significantly improve physical capacity and health-related quality of life (9), but this may be independent of program duration (58). Currently, there is no unified protocol for post-TAVR exercise rehabilitation, with significant variations between study protocols, as shown in Table 1. Future efforts should further develop intervention strategies to enhance patients’ motivation and ability for physical activity and provide appropriate triggers.
3.2 Dietary/nutritional support
Dietary advice plays an important role in CR programs and is associated with improved metabolic and cardiovascular outcomes (59). t is reported that adherence to a Mediterranean diet can reduce cardiovascular events by 30% (59, 60). A study by Schwaab et al. found that CR can improve low-density lipoprotein cholesterol (LDL-C) levels in patients with myocardial infarction (MI), with the proportion of patients achieving an LDL-C concentration below 70 mg/dL or a reduction ≥50% increasing from only 2% at baseline to 42% after an average of 22 days of treatment (61). TAVR patients are often very frail (62); conducting nutritional risk assessments and providing personalized dietary advice are crucial for their early recovery (63). However, it is difficult for frail patients to follow dietary advice in actual practice.
3.2.1 Effect of malnutrition on patients with TAVR
Malnutrition is a common yet often overlooked problem in TAVR patients, and its presence predicts a poor prognosis, particularly being significantly associated with increased mortality (64–66). A prospective multicenter cohort study of 1,158 patients undergoing aortic valve replacement (TAVR or surgical aortic valve replacement, SAVR) showed that compared to patients with normal nutritional status, older adults with signs and symptoms of malnutrition had a nearly threefold increase in one-year postoperative mortality (28% vs. 10%, P < 0.001) (67). This indicates that nutritional status is an important indicator for predicting one-year all-cause mortality in TAVR patients (68).
3.2.2 Assessment of nutritional risk in patients with TAVR
Nutritional status assessment is challenging, and traditional indicators such as body weight are often unreliable (65, 67). Multiple studies using different nutritional screening tools have revealed a high risk of malnutrition in this population. Mini Nutritional Assessment-Short Form (MNA-SF): Goldfarb et al. screened 1,158 patients (727 TAVR and 431 SAVR) for nutritional risk and found that 32.8% were at risk of malnutrition (67). Geriatric Nutritional Risk Index (GNRI): Data from a single-center prospective study of 433 TAVR patients showed that 61.4% of the patients were at nutritional risk before TAVR (mild 17.6%, moderate 40.6%, severe 3.2%) (69).The study also found that patients whose malnutrition improved after TAVR had better survival than those with persistent malnutrition (69). Another study involving 953 TAVR patients found that a low GNRI (<98, prevalence 35.2%) was associated with an increased risk of all-cause mortality (70).
Composite indices [the Controlling Nutritional Status score (CONUT), GNRI, Prognostic Nutritional Index (PNI)]: Ishizu et al. found in a study of 1,040 Japanese high-risk TAVR patients that the proportions of moderate/severe malnutrition were: CONUT 16.6%, GNRI 60.5%, PNI 13.8% (65). A study by Li et al. on 536 Chinese AS patients also reported similar discrepancies: CONUT 14.4%, NRI 44.4%, PNI 10.6% (71). Collectively, it is evident that NRI/GNRI detects a higher proportion of nutritional risk, while PNI shows the opposite trend.
MNA-SF, CONUT, NRI/GNRI, and PNI have been proven to predict the prognosis of TAVR patients (64, 66, 67, 72). Among them, MNA-SF involves patient self-reported nutritional status, CONUT assesses serum albumin levels, total cholesterol levels, and total lymphocyte count (64), NRI/GNRI assesses serum albumin and body weight/body mass index (66, 73), and PNI assesses serum albumin and total lymphocyte count (72). It is noteworthy that CONUT, NRI/GNRI, and PNI all measure the objective indicator of serum albumin, possibly suggesting that its level is associated with the prognosis of TAVR patients (74–76). Interestingly, two small studies comparing the value of GNRI, PNI, and CONUT in predicting one-year mortality after TAVR yielded conflicting results (one found CONUT/PNI superior, the other found GNRI superior) (77, 78).
Novel indicators are also being explored; the Triglycerides × Total Cholesterol × Body Mass Index (TCBI) is a simple nutritional scoring calculation method that has been validated in CVD patients (79, 80). Sudo et al. found that a low TCBI was associated with signs of right heart overload and an increased risk of 3-year mortality. Its addition to the EuroSCORE II enhanced the predictive value for all-cause mortality (81). A study utilizing the Malnutrition Universal Screening Tool (MUST) and 3-day food diaries administered by dietitians and nurses found that 8 patients (22%) were at medium risk and 3 patients (8%) were at high risk; 6 patients had a nutritional intake of <50% of their calculated requirements, and 3 of these patients required early nutritional support (62).
In summary, the prevalence of malnutrition in TAVR patients varies depending on the assessment tool used, but regardless of which nutritional indicator is used, the deterioration of malnutrition is associated with higher all-cause mortality (71). Future research is needed to clarify the clinical value of different nutritional assessment indicators in predicting adverse outcomes in TAVR patients.
3.2.3 Nutritional support for TAVR patients
Given that malnutrition can be reversed through nutritional intervention, early nutritional risk assessment before TAVR is crucial, as it helps clinicians identify patients who may benefit from nutritional support to improve the prognosis of TAVR patients. Therefore, routine screening of patients’ nutritional status should be performed before TAVR, and interventions should be provided when appropriate to improve outcomes (68). The PERFORM-TAVR trial, a multicenter randomized clinical trial with a parallel-group design exploring the effects of nutritional intervention, concluded on December 1, 2022. The trial aimed to assess whether protein supplementation combined with exercise could improve physical frailty in elderly TAVR patients (defined as an SPPB ≤8 or an SF36-PF ≤55). Patients in the intervention group received a protein-rich nutritional supplement twice daily from 4 weeks before TAVR until 12 weeks after discharge; the study results have not yet been published. A substantial body of research indicates that nutritional support is effective in improving the prognosis of patients with CVD (59, 82, 83). However, no other studies have specifically examined the independent effect of nutritional support on the rehabilitation of TAVR patients. Therefore, the results of the PERFORM-TAVR trial are highly anticipated, and it is hoped that more researchers will focus on improving the nutritional status of TAVR patients.
3.3 Psychological support
Compared to quality of life, the psychological health status of TAVR patients receives less attention, however, preoperative anxiety and depression are quite prevalent among TAVR patients (84). A systematic review found that the prevalence of depression and anxiety in TAVR patients was 15%–30% and 25%–30% (85). Depression is associated with mortality; the presence of depressive symptoms before TAVR is associated with a higher risk of short- and medium-term mortality (86). If depression persists postoperatively, the mortality risk is highest (86). Moreover, depression and cognitive impairment have an additive effect in predicting mortality risk after TAVR (Depression: HR,1.45, [95% CI, 1.13–1.86], P < 0.01; Cognitive Dysfunction(CD): HR,1.27, [95% CI, 1.02–1.59], P = 0.04; Depression and CD: HR,2.06, [95% CI, 1.44–2.96], P < 0.01) (87).
The effectiveness of psychological interventions for TAVR patients shows inconsistent results. A study found that CR significantly improved depressive symptoms in TAVR patients (PHQ-9 score decreased from 4.3 to 2.3, P < 0.01), but failed to improve anxiety symptoms (GAD-7 score decreased from 3.4 to 2.3, P = 0.25) (26). A RCT confirmed that CR after cardiac valve surgery improved physical capacity but did not improve mental health (88). A psychological education program specifically for cardiac valve surgery patients, implemented within CR, also failed to improve their physical and mental health status at 12 or 24 months (18). Another study reported no between-group differences in Hospital Anxiety and Depression Scale (HADS) scores at different time points after discharge (30 days, 6 months, 12 months), regardless of whether inpatient CR was arranged (16). Interestingly, one RCT observed spontaneous improvement in depression and anxiety scores in TAVR patients at 1 month postoperatively, and brief cognitive behavioral therapy (CBT) showed no additional advantage (84). However, this does not seem to be a universal phenomenon, as a large-sample study found that even after excluding patients with recorded anxiety or depression within 6 months before TAVR, the cumulative incidence of new-onset anxiety or depression 1 year after TAVR remained as high as 19% (89).
Anxiety and depressive symptoms may resolve spontaneously in some TAVR patients, and some studies have questioned the effectiveness of CR in improving the mental health of TAVR patients. However, mental health issues in TAVR patients are real and associated with poor prognosis. Future research aimed at optimizing post-TAVR mental health rehabilitation should more precisely focus on patient populations with elevated baseline psychological symptoms (90), and delve deeper into exploring more effective targeted interventions.
3.4 Risk factor management
TAVR significantly improves patients’ symptoms and long-term prognosis, but patients may still face various challenges postoperatively, such as complications, lack of rehabilitation knowledge, and long-term durability issues of bioprosthetic valves, etc. The effectiveness of CR is often measured by the reduction of CVD risk factors (91), CR manages risk factors through systematic patient education and behavioral interventions, including promoting smoking cessation, effectively controlling blood pressure, blood glucose, lipids, weight management, and improving medication adherence, thereby optimizing long-term prognosis, addressing patients’ knowledge gaps in rehabilitation, enhancing their postoperative self-management skills, and preparing them for long-term challenges such as valve durability.
Studies have found that cardiovascular risk factors such as smoking, hypercholesterolemia, and elevated serum creatinine and calcium levels accelerate disease progression in AS patients (92). The ACC/AHA guidelines for the management of patients with valvular heart disease clearly state that smoking is associated with adverse outcomes after TAVR (7). Smoking cessation is the most important modifiable cardiac risk factor and is recognized as a key secondary prevention strategy. Furthermore, conventional cardiovascular risk factors such as hypertension, diabetes, and dyslipidemia have a strong, independent, and graded association with the incidence of severe AS in the elderly, accounting for about one-third of its attributable risk (93). Studies have also found that smoking, diabetes, high cholesterol, and triglyceride levels are associated with accelerated bioprosthetic valve degeneration (94). In a multivariate regression model, gender, smoking, diabetes, and cholesterol levels were significant risk factors predicting the need for reoperation (94). Patients without these risk factors required reoperation after an average of 9.25 ± 0.88 years, which was significantly shortened to 4.05 ± 0.43 years in patients with 2 or 3 risk factors (P = .0002) (94). Therefore, for TAVR patients with these risk factors, the CR team must emphasize and guide strict control of these high-risk factors, which is crucial for delaying bioprosthetic valve degeneration and avoiding early reoperation.
Pharmacological management is also a crucial component of CR, as the majority of patients post-TAVR require medication to prevent valve thrombosis and thromboembolic events. The European Society of Cardiology/European Association for Cardio-Thoracic Surgery and the ACC/AHA have issued guidelines for post-TAVR antithrombotic therapy (7, 95). However, due to a lack of high-quality evidence, specific antithrombotic regimens remain controversial (8). Pharmacologic regimens are individualized and adjusted by cardiologists based on the latest guidelines and patient-specific circumstances. Patients should strictly adhere to prescribed medications postoperatively and attend regular follow-up visits.
4 Clinical challenges and countermeasures in post-TAVR rehabilitation: complications, frailty, and individualized practice of digital health
4.1 Rehabilitation management of complications after TAVR
TAVR is a minimally invasive procedure, but patients have numerous and frequent postoperative complications (96). These complications undoubtedly increase the difficulty of CR management in TAVR patients and may even hinder the implementation of CR. Therefore,CR programs need to be individually adjusted based on the patient's personalized complication risks.
For patients with moderate/severe paravalvular aortic regurgitation (97), close monitoring for heart failure symptoms such as dyspnea and fatigue during rehabilitation is essential. The exercise rehabilitation should be conservative, avoiding resistance training that may cause drastic blood pressure fluctuations, and symptom-limited exercise can be performed. If severe vascular complications and bleeding occur (98), the timing of rehabilitation should be appropriately delayed. For patients via the femoral artery approach, exercise rehabilitation of the lower limb muscles can be initiated after the puncture site has healed. For patients with disabling stroke (99), the focus of CR should be comprehensive neuro-cardiac rehabilitation addressing cardiac, cerebral, and limb functions. The rehabilitation program should focus on training muscle strength, balance, coordination, and activities of daily living, and the multidisciplinary team should include neurologists. For patients with acute kidney injury (100), exercise intensity should be appropriately reduced. Rehabilitation mandates rigorous monitoring of fluid balance and electrolyte levels, and strenuous activities that may induce dehydration should be avoided prior to full renal function recovery. For patients with infective endocarditis (101), rehabilitation should commence gradually after the infection is controlled and safety is assessed by a multidisciplinary team, while simultaneously enhancing nutritional support for the patient. For patients who develop high-grade atrioventricular block (102), close monitoring of ECG evolution is required during rehabilitation before pacemaker implantation. During exercise rehabilitation, vigilance for bradycardia or even syncope is necessary.
It is reported that permanent pacemaker implantation (PPI) is the most common complication of TAVR, with a post-TAVR PPI incidence rate ranging between 2% and 51%. In the foreseeable future, a significant reduction in this type of complication after TAVR is unlikely (102). CR for this patient population faces unique challenges. Cardiac implantable electronic devices often impose a specific psychological burden on patients, such as concerns about limitations in daily life and device function (103), which may reduce their willingness to participate in rehabilitation and their quality of life. Rehabilitation personnel should provide timely activity guidance, proactively assess the patient's psychological state, and offer targeted psychological counseling. Infection after PPI and lead dysfunction are uncommon but represent a serious complication. Transvenous lead extraction is the gold standard for treating such complications (104). Rehabilitation personnel need to teach patients to promptly recognize signs of infection and inform them of the importance of postoperative antibiotic use.
4.2 Rehabilitation management of frailty after TAVR
TAVR patients are often elderly with numerous comorbidities, and the incidence of postoperative frailty varies depending on the assessment tool, the FRAILTY-AVR study reported an incidence ranging from 26% to 68% (105). Frailty is a significant risk factor for postoperative disability and mortality (95), making frailty in TAVR patients an important issue that cannot be ignored. Although CR has been proven to effectively improve frailty and health outcomes (106), particularly for frail patients with physical deconditioning, recent surgery, high psychological burden of disease, or cognitive limitations (15), the key to maximizing its benefits lies in integrating standardized frailty assessments into the CR protocol and formulating individualized rehabilitation strategies based on the results. The use of validated, standardized assessment tools is recommended in both clinical practice and research. In CR for TAVR patients, screening with standardized assessment tools before and during rehabilitation enables the objective quantification of frailty severity, providing a crucial basis for setting rehabilitation intensity. Currently, there is no consensus on the optimal method for measuring frailty. The Fried Frailty Phenotype is the most commonly used multidimensional measure (107), while the Clinical Frailty Scale is a simpler unidimensional measure. Both tools have been validated in TAVR patients for effectively predicting mortality (108, 109).The core of identifying frailty lies in using the assessment results to precisely guide CR practice. For patients with severe frailty, the goals are to maintain existing function, prevent disability, and reduce complications. The rehabilitation intensity in the initial phase needs to be extremely conservative, focusing on low-intensity, short-duration activities. The progression cycle should be longer than that for patients with mild/moderate frailty. CR for frail TAVR patients should not be limited to exercise rehabilitation alone but should adopt a multimodal, comprehensive CR approach. Post-TAVR frailty is closely associated with the occurrence of postoperative complications and poor recovery. When formulating a CR plan, it is essential to consider their frailty status alongside specific postoperative complication risks. Some scholars suggest that patients who participate in prehabilitation may exhibit higher adherence to postoperative CR (110), and it can also reduce the risk of postoperative complications, shorten hospital stays, promote recovery, and improve quality of life in patients undergoing major surgery (111). Therefore, rehabilitation can be initiated preoperatively for AS patients to enhance the physical and psychological readiness of frail patients, facilitating the smooth implementation of postoperative CR. It is worth mentioning that tele-rehabilitation offers distinct advantages for discharge CR in frail patients.
4.3 Application of digital CR in TAVR patients
Currently, digital health technologies are significantly enhancing the personalization, convenience, and monitoring level of CR programs. Research confirms that digital CR is as effective as, or even superior to, traditional CR in improving patient outcomes (28). Digital CR can better promote and sustain patients’ health behaviors, and is conducive to addressing the underutilization of CR to promote health equity (112). Several studies have utilized remote monitoring technologies such as the internet, wearable devices, and mobile applications to effectively address issues like geographical barriers to CR participation and low rehabilitation adherence in TAVR patients (113–116). Moreover, digital CR demonstrates effectiveness comparable to hospital-based CR programs in reducing the incidence of postoperative complications, readmission rates, and mortality (114, 117, 118).
Kohei et al. used remote monitoring applications and wireless ECG transmitters to conduct remote cardiac rehabilitation (RCR) management for TAVR patients and found that the patients’ peak VO2 and 6MWT values were significantly greater than before the intervention (114). A U.S. research team developed the multimodal Corrie Health digital platform, which utilizes smartphones, smartwatches, and wireless blood pressure monitors to assist 1,064 patients recovering from AMI in self-management. The intervention group using Corrie had a 52% lower risk of readmission within 30 days after discharge compared to the control group [HR, 0.48 (95% CI, 0.26–0.88), P = 0.018] (119). The research team is integrating the multimodal Corrie Health digital platform with CR to explore the efficacy and safety of digital CR for patients after AMI, coronary artery bypass graft surgery, PCI, cardiac valve surgery, or TAVR (120). Currently, the application of digital CR in TAVR patients is still insufficient, while its application in patients with coronary heart disease is relatively more extensive (51, 121, 122). Future exploration by more scholars in this field is anticipated.
5 Existing challenges and future directions
Although the benefits of CR for TAVR patients have been confirmed, its full potential has not been fully realized due to a series of challenges. Action must be taken to fully utilize CR to optimize the long-term outcomes of this growing and complex patient population.
Post-TAVR CR faces many challenges. Firstly, there is a lack of high-quality evidence and standardization. There are few large-scale randomized controlled trials focusing on CR after TAVR. This evidence gap leads to a lack of standardized, universally accepted CR protocols. In terms of exercise rehabilitation, current protocols show significant variations in intensity, frequency, and duration, hindering comparative effectiveness research and consistent clinical implementation. Secondly, CR participation rates and adherence are suboptimal, resulting from multiple barriers. Third, the high prevalence of frailty and multiple comorbidities complicates the implementation of CR. Additionally, patients often have concomitant malnutrition and psychological distress, which are frequently underemphasized in traditional exercise-centric CR models. Fourth, the management of postoperative complications. The high incidence and variety of post-TAVR complications necessitate careful, conservative adjustments to CR protocols, complicating the implementation of a “one-size-fits-all” approach and requiring an individualized strategy.
Future strategies could adopt personalized and multimodal CR, not just focusing on exercise rehabilitation. CR protocols should be comprehensive and personalized, integrating tailored exercise prescriptions with structured nutritional support and psychological interventions. The systematic use of validated assessments for frailty, nutrition, and mental health is crucial for designing these individualized protocols. Furthermore, robust clinical trials are needed to establish standardized protocols and confirm the efficacy of novel interventions like prehabilitation and digital CR for TAVR patients. The foundation for all these efforts must be strong multidisciplinary team support, ensuring seamless collaboration among cardiologists, rehabilitation specialists, nutritionists, psychologists, and nursing specialists to provide holistic care.
In conclusion, although post-TAVR CR still faces significant challenges, it also implies abundant opportunities. The future lies in embracing personalized, multimodal rehabilitation by leveraging the power of prehabilitation and digital technology, and promoting strong multidisciplinary collaboration. Through these synergistic efforts, CR can transform from an underutilized intervention into a rehabilitation modality that provides high-quality rehabilitation care for all TAVR patients.
Author contributions
HD: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. CZ: Data curation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. QZ: Data curation, Investigation, Validation, Writing – review & editing. DC: Data curation, Investigation, Supervision, Validation, Writing – review & editing. LX: Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. A part of the article processing charges were funded by Nursing Department, The First Affiliated Hospital of Chongqing Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eichler S, Völler H. Advances in cardiac rehabilitation: cardiac rehabilitation after transcatheter aortic valve implantation. Monaldi Arch Chest Dis. (2016) 86(1–2):758. doi: 10.4081/monaldi.2016.758
2. Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation. (2020) 141(21):1670–80. doi: 10.1161/CIRCULATIONAHA.119.043391
3. d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuttard J, Birks J, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE population cohort study. Eur Heart J. (2016) 37(47):3515–22. doi: 10.1093/eurheartj/ehw229
4. Lindman BR, Clavel M-A, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. (2016) 2:16006. doi: 10.1038/nrdp.2016.6
5. Yang Y, Wang Z, Chen Z, Wang X, Zhang L, Li S, et al. Current status and etiology of valvular heart disease in China: a population-based survey. BMC Cardiovasc Disord. (2021) 21(1):339. doi: 10.1186/s12872-021-02154-8
6. Kardos A. Invited editorial: professor Attila Kardos MD PhD FRCP FESC in search for a mechanism of poor outcome in patients with aortic valve sclerosis without aortic stenosis and left ventricular hypertrophy. Results of a populational observational study with normal LV geometry. Eur J Prev Cardiol. (2023) 30:451–3. doi: 10.1093/eurjpc/zwad042
7. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77(4):e72–227. doi: 10.1016/j.jacc.2020.11.018
8. Auffret V, Guedeney P, Leurent G, Didier R. Antithrombotic after TAVR: no treatment, No problem? JACC Cardiovasc Interv. (2023) 16(1):92–3. doi: 10.1016/j.jcin.2022.11.003
9. Sperlongano S, Renon F, Bigazzi MC, Sperlongano R, Cimmino G, D'Andrea A, et al. Transcatheter aortic valve implantation: the new challenges of cardiac rehabilitation. J Clin Med. (2021) 10(4):810. doi: 10.3390/jcm10040810
10. Cortés C, Amat-Santos IJ, Nombela-Franco L, Muñoz-Garcia AJ, Gutiérrez-Ibanes E, De La Torre Hernandez JM, et al. Mitral regurgitation after transcatheter aortic valve replacement: prognosis, imaging predictors, and potential management. JACC Cardiovasc Interv. (2016) 9(15):1603–14. doi: 10.1016/j.jcin.2016.05.025
11. Wu S, Liu Y, Ni T, Lv T, Yao Y, Yan M. Risk factors and prognosis of silent cerebral infarction after transcatheter aortic valve replacement. Sci Rep. (2025) 15(1):15006. doi: 10.1038/s41598-025-99173-8
12. Mwipatayi BP, Picardo A, Masilonyane-Jones TV, Larbalestier R, Thomas S, Turner J, et al. Incidence and prognosis of vascular complications after transcatheter aortic valve implantation. J Vasc Surg. (2013) 58(4):1028–36. doi: 10.1016/j.jvs.2013.03.046
13. Lavie CJ, Milani RV. Cardiac rehabilitation, exercise training, and psychosocial risk factors. J Am Coll Cardiol. (2006) 47(1):212. doi: 10.1016/j.jacc.2005.10.002
14. Angst F, Giger RD, Lehmann S, Sandor PS, Teuchmann P, Csordas A. Mental and psychosocial health and health related quality of life before and after cardiac rehabilitation: a prospective cohort study with comparison to specific population norms. Health Qual Life Outcomes. (2022) 20(1):91. doi: 10.1186/s12955-022-01994-y
15. Damluji AA, Tomczak CR, Hiser S, O'Neill DE, Goyal P, Pack QR, et al. Benefits of cardiac rehabilitation: mechanisms to restore function and clinical impact. Circ Res. (2025) 137(2):255–72. doi: 10.1161/CIRCRESAHA.125.325705
16. Kleczynski P, Trebacz J, Stapor M, Sobczynski R, Konstanty-Kalandyk J, Kapelak B, et al. Inpatient cardiac rehabilitation after transcatheter aortic valve replacement is associated with improved clinical performance and quality of life. J Clin Med. (2021) 10(10):2125. doi: 10.3390/jcm10102125
17. Tarro Genta F, Tidu M, Bouslenko Z, Bertolin F, Salvetti I, Comazzi F, et al. Cardiac rehabilitation after transcatheter aortic valve implantation compared to patients after valve replacement. J Cardiovasc Med (Hagerstown). (2017) 18(2):114–20. doi: 10.2459/JCM.0000000000000494
18. Sibilitz KL, Tang LH, Berg SK, Thygesen LC, Risom SS, Rasmussen TB, et al. Long-term effects of cardiac rehabilitation after heart valve surgery - results from the randomised CopenHeartVR trial. Scand Cardiovasc J. (2022) 56(1):247–55. doi: 10.1080/14017431.2022.2095432
19. Guduguntla V, Yaser JM, Keteyian SJ, Pagani FD, Likosky DS, Sukul D, et al. Variation in cardiac rehabilitation participation during aortic valve replacement episodes of care. Circ Cardiovasc Qual Outcomes. (2022) 15(7):e009175. doi: 10.1161/CIRCOUTCOMES.122.009175
20. Patel DK, Duncan MS, Shah AS, Lindman BR, Greevy RA, Savage PD, et al. Association of cardiac rehabilitation with decreased hospitalization and mortality risk after cardiac valve surgery. JAMA Cardiol. (2019) 4(12):1250–9. doi: 10.1001/jamacardio.2019.4032
21. Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the secondary prevention and rehabilitation section of the European association of preventive cardiology. Eur J Prev Cardiol. (2021) 28(5):460–95. doi: 10.1177/2047487320913379
22. Brown TM, Pack QR, Aberegg E, Brewer LC, Ford YR, Forman DE, et al. Core components of cardiac rehabilitation programs: 2024 update: a scientific statement from the American Heart Association and the American association of cardiovascular and pulmonary rehabilitation. Circulation. (2024) 150(18):e328–47. doi: 10.1161/CIR.0000000000001289
23. Abreu A, Frederix I, Dendale P, Janssen A, Doherty P, Piepoli MF, et al. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: the avenue towards EAPC accreditation programme: a position statement of the secondary prevention and rehabilitation section of the European association of preventive cardiology (EAPC). Eur J Prev Cardiol. (2021) 28(5):496–509. doi: 10.1177/2047487320924912
24. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. (2021) 42(1):17–96. doi: 10.1093/eurheartj/ehaa605
25. Ge J. Expert consensus on exercise rehabilitation after transcatheter aortic valve replacement. Chin J Interv Cardiol. (2020) 28(07):361–8. doi: 10.3969/j.issn.1004-8812.2020.07.001
26. Imran HM, Baig M, Mujib M, Beale C, Gaw A, Stabile L, et al. Comparison of phase 2 cardiac rehabilitation outcomes between patients after transcatheter versus surgical aortic valve replacement. Eur J Prev Cardiol. (2018) 25(15):1577–84. doi: 10.1177/2047487318792099
27. Kotseva K, De Bacquer D, Jennings C, McEvoy JW, Ryden L, Ray KK, et al. Cardiac rehabilitation in patients with coronary heart disease - provision, attendance, and outcomes: results from the INTERASPIRE survey from fourteen countries across six WHO regions. Glob Heart. (2025) 20(1):75. doi: 10.5334/gh.1458
28. Wongvibulsin S, Habeos EE, Huynh PP, Xun H, Shan R, Porosnicu Rodriguez KA, et al. Digital health interventions for cardiac rehabilitation: systematic literature review. J Med Internet Res. (2021) 23(2):e18773. doi: 10.2196/18773
29. Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, et al. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. (2020) 13(1):e005902. doi: 10.1161/CIRCOUTCOMES.119.005902
30. Nichol C, Das R, Barry G, Kelly M, Vogiatzis I, Adams N. A qualitative study of barriers and facilitators to the uptake of cardiac rehabilitation in octogenarians. Geriatrics (Basel, Switzerland). (2024) 9(6):161. doi: 10.3390/geriatrics9060161
31. Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. (2014) 56(6):565–71. doi: 10.1016/j.pcad.2014.02.006
32. Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. (2012) 60(19):1854–63. doi: 10.1016/j.jacc.2012.02.093
33. Dweck MR, Joshi S, Murigu T, Gulati A, Alpendurada F, Jabbour A, et al. Left ventricular remodeling and hypertrophy in patients with aortic stenosis: insights from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. (2012) 14(1):50. doi: 10.1186/1532-429X-14-50
34. Bartel T, Müller S. Preserved ejection fraction can accompany low gradient severe aortic stenosis: impact of pathophysiology on diagnostic imaging. Eur Heart J. (2013) 34(25):1862–3. doi: 10.1093/eurheartj/eht157
35. Avvedimento M, Angellotti D, Ilardi F, Leone A, Scalamogna M, Castiello DS, et al. Acute advanced aortic stenosis. Heart Fail Rev. (2023) 28(5):1101–11. doi: 10.1007/s10741-023-10312-7
36. Spilias N, Martyn T, Denby KJ, Harb SC, Popovic ZB, Kapadia SR. Left ventricular systolic dysfunction in aortic stenosis: pathophysiology, diagnosis, management, and future directions. Structural Heart. (2022) 6(5):100089. doi: 10.1016/j.shj.2022.100089
37. Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. (1982) 307(22):1362–6. doi: 10.1056/NEJM198211253072202
38. Smucker ML, Tedesco CL, Manning SB, Owen RM, Feldman MD. Demonstration of an imbalance between coronary perfusion and excessive load as a mechanism of ischemia during stress in patients with aortic stenosis. Circulation. (1988) 78(3):573–82. doi: 10.1161/01.CIR.78.3.573
39. Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. (1989) 80(4):769–81. doi: 10.1161/01.CIR.80.4.769
40. Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. (1998) 98(24):2709–15. doi: 10.1161/01.CIR.98.24.2709
41. Sobajima M, Ueno H, Onoda H, Kuwahara H, Tanaka S, Ushijima R, et al. Transcatheter aortic valve implantation improves cardiac sympathetic nerve activity on 123I-metaiodobenzylguanidine myocardial scintigraphy in severe aortic valve stenosis. Circ J. (2018) 82(2):579–85. doi: 10.1253/circj.CJ-17-0817
42. Walther T, Schubert A, Falk V, Binner C, Walther C, Doll N, et al. Left ventricular reverse remodeling after surgical therapy for aortic stenosis: correlation to renin-angiotensin system gene expression. Circulation. (2002) 106(12 Suppl 1):I23–6. doi: 10.1161/01.cir.0000032919.33237.4d
43. Bergler-Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, et al. B-type natriuretic peptide in low-flow, low-gradient aortic stenosis: relationship to hemodynamics and clinical outcome: results from the multicenter truly or Pseudo-severe aortic stenosis (TOPAS) study. Circulation. (2007) 115(22):2848–55. doi: 10.1161/CIRCULATIONAHA.106.654210
44. Dhayni K, Chabry Y, Hénaut L, Avondo C, Boudot C, Ouled-Haddou H, et al. Aortic valve calcification is promoted by interleukin-8 and restricted through antagonizing CXC motif chemokine receptor 2. Cardiovasc Res. (2023) 119(13):2355–67. doi: 10.1093/cvr/cvad117
45. Gotzmann M, Hehen T, Germing A, Lindstaedt M, Yazar A, Laczkovics A, et al. Short-term effects of transcatheter aortic valve implantation on neurohormonal activation, quality of life and 6-minute walk test in severe and symptomatic aortic stenosis. Heart (British Cardiac Society). (2010) 96(14):1102–6. doi: 10.1136/hrt.2009.180661
46. Adamopoulos S, Coats AJ, Brunotte F, Arnolda L, Meyer T, Thompson CH, et al. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am Coll Cardiol. (1993) 21(5):1101–6. doi: 10.1016/0735-1097(93)90231-O
47. Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. (2005) 100(1):93–9. doi: 10.1016/j.ijcard.2004.08.073
48. Downing J, Balady GJ. The role of exercise training in heart failure. J Am Coll Cardiol. (2011) 58(6):561–9. doi: 10.1016/j.jacc.2011.04.020
49. Vu T, Smith JA. The pathophysiology and management of depression in cardiac surgery patients. Front Psychiatry. (2023) 14:1195028. doi: 10.3389/fpsyt.2023.1195028
50. Eichler S, Salzwedel A, Reibis R, Nothroff J, Harnath A, Schikora M, et al. Multicomponent cardiac rehabilitation in patients after transcatheter aortic valve implantation: predictors of functional and psychocognitive recovery. Eur J Prev Cardiol. (2017) 24(3):257–64. doi: 10.1177/2047487316679527
51. Zhou T, Wang Y, Wang J, Liu J, Zhang N, Zhang X, et al. The effectiveness of nurse-led multidimensional digital cardiac rehabilitation in patients with unstable angina undergoing percutaneous coronary intervention: emulated target trial. J Med Internet Res. (2025) 27:e75325. doi: 10.2196/75325
52. Hiruma T, Nakayama A, Sakamoto J, Hori K, Nanasato M, Hosoda T, et al. Comprehensive cardiac rehabilitation following acute myocardial infarction improves clinical outcomes regardless of exercise capacity. Circ J. (2024) 88(6):982–92. doi: 10.1253/circj.CJ-23-0668
53. Yamazaki Y, Ikeda Y, Shima H, Mochizuki T, Sakamoto R, Sawano K. Impact of comprehensive cardiac rehabilitation in elderly patients with acute heart failure. J Cardiopulm Rehabil Prev. (2022) 42(4):286–7. doi: 10.1097/HCR.0000000000000703
54. Cersosimo A, Longo Elia R, Condello F, Colombo F, Pierucci N, Arabia G, et al. Cardiac rehabilitation in patients with atrial fibrillation. Minerva Cardiol Angiol. (2025) 73. doi: 10.23736/S2724-5683.25.06885-1
55. Shen Z, Qian X, Huang C, Zhou D, Xu X, Lv J, et al. Barriers and facilitators to physical activity after transcatheter aortic valve replacement: a mixed-methods study. J Rehabil Med. (2025) 57:jrm39974. doi: 10.2340/jrm.v57.39974
56. Sathananthan J, Lauck S, Piazza N, Martucci G, Kim DH, Popma JJ, et al. Habitual physical activity in older adults undergoing TAVR: insights from the FRAILTY-AVR study. JACC Cardiovasc Interv. (2019) 12(8):781–9. doi: 10.1016/j.jcin.2019.02.049
57. Lund K, Sibilitz KL, Berg SK, Thygesen LC, Taylor RS, Zwisler AD. Physical activity increases survival after heart valve surgery. Heart (British Cardiac Society). (2016) 102(17):1388–95. doi: 10.1136/heartjnl-2015-308827
58. Hosseinpour A, Azami P, Hosseinpour H, Attar A, Koushkie Jahromi M. Efficacy of exercise training-based cardiac rehabilitation programmes after transcatheter aortic valve implantation: a systematic review and meta-analysis. Int J Cardiol Cardiovasc Risk Prev. (2024) 20:200238. doi: 10.1016/j.ijcrp.2024.200238
59. Popiolek-Kalisz J, Mazur M, Perone F. The role of dietary education in cardiac rehabilitation. Nutrients. (2025) 17(6):1082. doi: 10.3390/nu17061082
60. Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M, Ros E. Benefits of the Mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis. (2015) 58(1):50–60. doi: 10.1016/j.pcad.2015.04.003
61. Schwaab B, Zeymer U, Jannowitz C, Pittrow D, Gitt A. Improvement of low-density lipoprotein cholesterol target achievement rates through cardiac rehabilitation for patients after ST elevation myocardial infarction or non-ST elevation myocardial infarction in Germany: results of the PATIENT CARE registry. Eur J Prev Cardiol. (2019) 26(3):249–58. doi: 10.1177/2047487318817082
62. Zanettini R, Gatto G, Mori I, Pozzoni MB, Pelenghi S, Martinelli L, et al. Cardiac rehabilitation and mid-term follow-up after transcatheter aortic valve implantation. J Geriatr Cardiolol (2014) 11(4):279–85. doi: 10.11909/j.issn.1671-5411.2014.04.001
63. Niebauer J, Bäck C, Bischoff-Ferrari HA, Dehbi H-M, Szekely A, Völler H, et al. Preinterventional frailty assessment in patients scheduled for cardiac surgery or transcatheter aortic valve implantation: a consensus statement of the European association for cardio-thoracic surgery (EACTS) and the European association of preventive cardiology (EAPC) of the European Society of Cardiology (ESC). Eur J Prev Cardiol. (2024) 31(2):146–81. doi: 10.1093/eurjpc/zwad304
64. Honda Y, Yamawaki M, Shigemitsu S, Kenji M, Tokuda T, Tsutumi M, et al. Prognostic value of objective nutritional status after transcatheter aortic valve replacement. J Cardiol. (2019) 73(5):401–7. doi: 10.1016/j.jjcc.2018.11.013
65. Ishizu K, Shirai S, Tashiro H, Kitano K, Tabata H, Nakamura M, et al. Prevalence and prognostic significance of malnutrition in older Japanese adults at high surgical risk undergoing transcatheter aortic valve implantation. J Am Heart Assoc. (2022) 11(19):e026294. doi: 10.1161/JAHA.122.026294
66. González Ferreiro R, Muñoz-García AJ, López Otero D, Avanzas P, Pascual I, Alonso-Briales JH, et al. Nutritional risk index predicts survival in patients undergoing transcatheter aortic valve replacement. Int J Cardiol. (2019) 276:66–71. doi: 10.1016/j.ijcard.2018.11.097
67. Goldfarb M, Lauck S, Webb JG, Asgar AW, Perrault LP, Piazza N, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. (2018) 138(20):2202–11. doi: 10.1161/CIRCULATIONAHA.118.033887
68. Eichler S, Salzwedel A, Harnath A, Butter C, Wegscheider K, Chiorean M, et al. Nutrition and mobility predict all-cause mortality in patients 12 months after transcatheter aortic valve implantation. Clin Res Cardiol. (2018) 107(4):304–11. doi: 10.1007/s00392-017-1183-1
69. González Ferreiro R, López Otero D, Álvarez Rodríguez L, Otero García Ó, Pérez Poza M, Antúnez Muiños PJ, et al. Prognostic impact of change in nutritional risk on mortality and heart failure after transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2021) 14(2):e009342. doi: 10.1161/CIRCINTERVENTIONS.120.009342
70. Seoudy H, Al-Kassou B, Shamekhi J, Sugiura A, Frank J, Saad M, et al. Frailty in patients undergoing transcatheter aortic valve replacement: prognostic value of the geriatric nutritional risk Index. J Cachexia Sarcopenia Muscle. (2021) 12(3):577–85. doi: 10.1002/jcsm.12689
71. Li B, Zhang S, Xu C, Huang M, Xiong Z, Hui Z, et al. Association between the malnutrition Status and all-cause mortality in patients with moderate and severe aortic stenosis: a prospective cohort study. J Am Heart Assoc. (2025) 14(3):e037086. doi: 10.1161/JAHA.124.037086
72. Mas-Peiro S, Hoffmann J, Seppelt PC, De Rosa R, Murray M-I, Walther T, et al. Value of prognostic nutritional index for survival prediction in trans-catheter aortic valve replacement compared to other common nutritional indexes. Acta Cardiol. (2021) 76(6):615–22. doi: 10.1080/00015385.2020.1757854
73. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent J-P, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82(4):777–83. doi: 10.1093/ajcn/82.4.777
74. Hsieh WC, Aboud A, Henry BM, Omara M, Lindner J, Pirk J. Serum albumin in patients undergoing transcatheter aortic valve replacement: a meta-analysis. Rev Cardiovasc Med. (2019) 20(3):161–9. doi: 10.31083/j.rcm.2019.03.524
75. Liu G, Hu X, Long M, Du Z-M, Li Y, Hu C-H. Meta-analysis of the impact of pre-procedural Serum albumin on mortality in patients undergoing transcatheter aortic valve replacement. Int Heart J. (2020) 61(1):67–76. doi: 10.1536/ihj.19-395
76. Seoudy H, Shamekhi J, Voigtländer L, Ludwig S, Frank J, Kujat T, et al. C-reactive protein to albumin ratio in patients undergoing transcatheter aortic valve replacement. Mayo Clin Proc. (2022) 97(5):931–40. doi: 10.1016/j.mayocp.2021.11.022
77. Okuno T, Koseki K, Nakanishi T, Sato K, Ninomiya K, Tomii D, et al. Evaluation of objective nutritional indexes as predictors of one-year outcomes after transcatheter aortic valve implantation. J Cardiol. (2019) 74(1):34–9. doi: 10.1016/j.jjcc.2019.02.017
78. Kucukosmanoglu M, Kilic S, Urgun OD, Sahin S, Yildirim A, Sen O, et al. Impact of objective nutritional indexes on 1-year mortality after transcatheter aortic valve implantation: a prospective observational cohort study. Acta Cardiol. (2021) 76(4):402–9. doi: 10.1080/00015385.2020.1747177
79. Maruyama S, Ebisawa S, Miura T, Yui H, Kashiwagi D, Nagae A, et al. Impact of nutritional index on long-term outcomes of elderly patients with coronary artery disease: sub-analysis of the SHINANO 5 year registry. Heart Vessels. (2021) 36(1):7–13. doi: 10.1007/s00380-020-01659-0
80. Ishiwata S, Yatsu S, Kasai T, Sato A, Matsumoto H, Shitara J, et al. Prognostic effect of a novel simply calculated nutritional index in acute decompensated heart failure. Nutrients. (2020) 12(11):3311. doi: 10.3390/nu12113311
81. Sudo M, Shamekhi J, Aksoy A, Al-Kassou B, Tanaka T, Silaschi M, et al. A simply calculated nutritional index provides clinical implications in patients undergoing transcatheter aortic valve replacement. Clin Res Cardiol. (2024) 113(1):58–67. doi: 10.1007/s00392-023-02220-5
82. Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the women’s health initiative randomized controlled dietary modification trial. JAMA. (2006) 295(6):655–66. doi: 10.1001/jama.295.6.655
83. Jimenez-Torres J, Alcalá-Diaz JF, Torres-Peña JD, Gutierrez-Mariscal FM, Leon-Acuña A, Gómez-Luna P, et al. Mediterranean Diet reduces atherosclerosis progression in coronary heart disease: an analysis of the CORDIOPREV randomized controlled trial. Stroke. (2021) 52(11):3440–9. doi: 10.1161/STROKEAHA.120.033214
84. Edwards KS, Chow EKH, Dao C, Hossepian D, Johnson AG, Desai M, et al. Impact of cognitive behavioral therapy on depression symptoms after transcatheter aortic valve replacement: a randomized controlled trial. Int J Cardiol. (2020) 321:61–8. doi: 10.1016/j.ijcard.2020.08.007
85. Suen W-L, Bhasin S, Betti V, Bruckel JT, Oldham MA. Mental health and transcatheter aortic valve replacement: a scoping systematic review. Gen Hosp Psychiatry. (2024) 86:10–23. doi: 10.1016/j.genhosppsych.2023.11.009
86. Patel A, Leon MB. Is depression an important new mortality risk factor after aortic valve replacement or simply a component of the geriatric disease spectrum? JAMA Cardiology. (2018) 3(3):198–9. doi: 10.1001/jamacardio.2017.5065
87. El-Sabawi B, Cloud H, Patel JN, Bell SP, Elmariah S, Fearon WF, et al. Association of depression and cognitive dysfunction with patient-centered outcomes after transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2023) 16(8):e012875. doi: 10.1161/CIRCINTERVENTIONS.123.012875
88. Sibilitz KL, Berg SK, Rasmussen TB, Risom SS, Thygesen LC, Tang L, et al. Cardiac rehabilitation increases physical capacity but not mental health after heart valve surgery: a randomised clinical trial. Heart (British Cardiac Society). (2016) 102(24):1995–2003. doi: 10.1136/heartjnl-2016-309414
89. Wegermann ZK, Mack MJ, Arnold SV, Thompson CA, Ryan M, Gunnarsson C, et al. Anxiety and depression following aortic valve replacement. J Am Heart Assoc. (2022) 11(9):e024377. doi: 10.1161/JAHA.121.024377
90. Hansen D. Exercise intervention after transcatheter aortic valve implantation: current evidence and issues to be resolved. Eur J Prev Cardiol. (2018) 25(8):791–3. doi: 10.1177/2047487318765258
91. Weinberger AH, Mazure CM, McKee SA, Caulin-Glaser T. The association of tobacco use and gender to cardiac rehabilitation outcomes: a preliminary investigation. J Subst Use. (2014) 19(1-2):171–5. doi: 10.3109/14659891.2013.765515
92. Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. (2000) 101(21):2497–502. doi: 10.1161/01.CIR.101.21.2497
93. Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, et al. Association between cardiovascular risk factors and aortic stenosis: the CANHEART aortic stenosis study. J Am Coll Cardiol. (2017) 69(12):1523–32. doi: 10.1016/j.jacc.2017.01.025
94. Nollert G, Miksch J, Kreuzer E, Reichart B. Risk factors for atherosclerosis and the degeneration of pericardial valves after aortic valve replacement. J Thorac Cardiovasc Surg. (2003) 126(4):965–8. doi: 10.1016/S0022-5223(02)73619-2
95. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
96. Han X-S, Wang G, Miao Y, Tian Z-Y, Zhang Y-C, Li Z-Y, et al. Optimal evaluation and treatment of cardiac rehabilitation for patients following TAVR: a personalized program based on the five prescriptions. Aging Dis. (2025) 15. doi: 10.14336/AD.2025.0716
97. Sinning J-M, Vasa-Nicotera M, Chin D, Hammerstingl C, Ghanem A, Bence J, et al. Evaluation and management of paravalvular aortic regurgitation after transcatheter aortic valve replacement. J Am Coll Cardiol. (2013) 62(1):11–20. doi: 10.1016/j.jacc.2013.02.088
98. Généreux P, Cohen DJ, Mack M, Rodes-Cabau J, Yadav M, Xu K, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol. (2014) 64(24):2605–15. doi: 10.1016/j.jacc.2014.08.052
99. Kapadia SR, Huded CP, Kodali SK, Svensson LG, Tuzcu EM, Baron SJ, et al. Stroke after surgical versus transfemoral transcatheter aortic valve replacement in the PARTNER trial. J Am Coll Cardiol. (2018) 72(20):2415–26. doi: 10.1016/j.jacc.2018.08.2172
100. Sinning J-M, Ghanem A, Steinhäuser H, Adenauer V, Hammerstingl C, Nickenig G, et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. (2010) 3(11):1141–9. doi: 10.1016/j.jcin.2010.09.009
101. Del Val D, Panagides V, Mestres CA, Miró JM, Rodés-Cabau J. Infective endocarditis after transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 81(4):394–412. doi: 10.1016/j.jacc.2022.11.028
102. Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: current Status and future perspectives. Circulation. (2017) 136(11):1049–69. doi: 10.1161/CIRCULATIONAHA.117.028352
103. Fumagalli S, Pieragnoli P, Haugaa KH, Potpara TS, Rasero L, Ramacciati N, et al. The influence of age on the psychological profile of patients with cardiac implantable electronic devices: results from the Italian population in a multicenter study conducted by the European heart rhythm association. Aging Clin Exp Res. (2018) 31(9):1219–26. doi: 10.1007/s40520-018-1088-5
104. Barca L, Mascia G, Di Donna P, Sartori P, Bianco D, Della Bona R, et al. Long-Term outcomes of transvenous lead extraction: a comparison in patients with or without infection from the Italian region with the oldest population. J Clin Med. (2023) 12(13):4543. doi: 10.3390/jcm12134543
105. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. (2017) 70(6):689–700. doi: 10.1016/j.jacc.2017.06.024
106. MacEachern E, Quach J, Giacomantonio N, Theou O, Hillier T, Abel-Adegbite I, et al. Cardiac rehabilitation and frailty: a systematic review and meta-analysis. Eur J Prev Cardiol. (2024) 31(16):1960–76. doi: 10.1093/eurjpc/zwae239
107. Li Z, Dawson E, Moodie J, Martin J, Bagur R, Cheng D, et al. Measurement and prognosis of frail patients undergoing transcatheter aortic valve implantation: a systematic review and meta-analysis. BMJ Open. (2021) 11(3):e040459. doi: 10.1136/bmjopen-2020-040459
108. Strom JB, Xu J, Orkaby AR, Shen C, Song Y, Charest BR, et al. Role of frailty in identifying benefit from transcatheter versus surgical aortic valve replacement. Circ Cardiovasc Qual Outcomes. (2021) 14(12):e008566. doi: 10.1161/CIRCOUTCOMES.121.008566
109. Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, et al. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. (2017) 135(21):2013–24. doi: 10.1161/CIRCULATIONAHA.116.025630
110. McCann M, Stamp N, Ngui A, Litton E. Cardiac prehabilitation. J Cardiothorac Vasc Anesth. (2019) 33(8):2255–65. doi: 10.1053/j.jvca.2019.01.023
111. McIsaac DI, Kidd G, Gillis C, Branje K, Al-Bayati M, Baxi A, et al. Relative efficacy of prehabilitation interventions and their components: systematic review with network and component network meta-analyses of randomised controlled trials. BMJ (Clinical Research ed.). (2025) 388:e081164. doi: 10.1136/bmj-2024-081164
112. Golbus JR, Lopez-Jimenez F, Barac A, Cornwell WK, Dunn P, Forman DE, et al. Digital technologies in cardiac rehabilitation: a science advisory from the American Heart Association. Circulation. (2023) 148(1):95–107. doi: 10.1161/CIR.0000000000001150
113. Brocki BC, Andreasen JJ, Aarøe J, Andreasen J, Thorup CB. Exercise-based real-time telerehabilitation for older patients recently discharged after transcatheter aortic valve implantation: an extended feasibility study. J Geriatr Cardiol. (2023) 20(11):767–78. doi: 10.26599/1671-5411.2023.11.003
114. Ashikaga K, Doi S, Yoneyama K, Suzuki N, Kuwata S, Koga M, et al. Efficacy and safety of home-based cardiac telemonitoring rehabilitation in patients after transcatheter aortic valve implantation: single-center usability and feasibility study. JMIR Rehabilitation and Assistive Technologies. (2023) 10:e45247. doi: 10.2196/45247
115. Lorenzoni G, Azzolina D, Fraccaro C, Di Liberti A, D'Onofrio A, Cavalli C, et al. Using wearable devices to monitor physical activity in patients undergoing aortic valve replacement: protocol for a prospective observational study. JMIR Res Protoc. (2020) 9(11):e20072. doi: 10.2196/20072
116. Straiton N, Hollings M, Gullick J, Gallagher R. Wearable activity trackers objectively measure incidental physical activity in older adults undergoing aortic valve replacement. Sensors (Basel, Switzerland). (2023) 23(6):3347. doi: 10.3390/s23063347
117. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart (British Cardiac Society). (2016) 102(15):1183–92. doi: 10.1136/heartjnl-2015-308966
118. Tobia JM, Heinein S, Soliman F, La Placa T, Lee L, Sethi A, et al. Outcomes of telemedicine TAVR preoperative evaluations. JACC. Advances. (2025) 4(10 Pt 2):102087. doi: 10.1016/j.jacadv.2025.102087
119. Marvel FA, Spaulding EM, Lee MA, Yang WE, Demo R, Ding J, et al. Digital health intervention in acute myocardial infarction. Circ Cardiovasc Qual Outcomes. (2021) 14(7):e007741. doi: 10.1161/CIRCOUTCOMES.121.007741
120. Isakadze N, Kim CH, Marvel FA, Ding J, MacFarlane Z, Gao Y, et al. Rationale and design of the mTECH-rehab randomized controlled trial: impact of a Mobile technology enabled corrie cardiac rehabilitation program on functional Status and cardiovascular health. J Am Heart Assoc. (2024) 13(2):e030654. doi: 10.1161/JAHA.123.030654
121. Zhang S, Wang Y, Wu J, Ma C, Meng X. Effectiveness of smartwatch device on adherence to home-based cardiac rehabilitation in patients with coronary heart disease: randomized controlled trial. JMIR Mhealth Uhealth. (2025) 13:e70848. doi: 10.2196/70848
122. Nishio R, Dohi T, Yokoyama M, Nakade T, Takahashi N, Chikata Y, et al. Wearable devices in remote cardiac rehabilitation with and without weekly online coaching for patients with coronary artery disease: randomized controlled trial. JMIR Mhealth Uhealth. (2025) 13:e63797. doi: 10.2196/63797
123. Song J, Chen X, Wang B, Cheng Y, Wang Y. Effect of exercise-based cardiac rehabilitation on patients with chronic heart failure after transcatheter aortic valve replacement: a randomized controlled trial. J Cardiopulm Rehabil Prev. (2025) 45(1):51–6. doi: 10.1097/HCR.0000000000000912
124. Xu L, Wei J, Liu J, Feng Y, Wang L, Wang S, et al. Inspiratory muscle training improves cardiopulmonary function in patients after transcatheter aortic valve replacement: a randomized clinical trial. Eur J Prev Cardiol. (2023) 30(2):191–202. doi: 10.1093/eurjpc/zwac269
125. Hu Q, Li Y-S, Ren Q, Liang Y-C, Zhang J, Wang Y-X, et al. Efficacy and safety of moderate-intensity continuous training on the improvement of cardiopulmonary function in patients after transcatheter aortic valve replacement (ENERGY): a randomized controlled trial. J Am Med Dir Assoc. (2023) 24(11):1783–90. doi: 10.1016/j.jamda.2023.04.025
126. Vitez L, Bunc M, Jug B. The effects of exercise training on exercise capacity and vascular function after transcatheter aortic valve implantation-A pilot study. J Cardiovasc Dev Dis. (2023) 10(8):343. doi: 10.3390/jcdd10080343
127. Lindman BR, Gillam LD, Coylewright M, Welt FGP, Elmariah S, Smith SA, et al. Effect of a pragmatic home-based mobile health exercise intervention after transcatheter aortic valve replacement: a randomized pilot trial. European Heart Journal. Digital Health. (2021) 2(1):90–103. doi: 10.1093/ehjdh/ztab007
128. Tamulevičiūtė-Prascienė E, Beigienė A, Thompson MJ, Balnė K, Kubilius R, Bjarnason-Wehrens B. The impact of additional resistance and balance training in exercise-based cardiac rehabilitation in older patients after valve surgery or intervention: randomized control trial. BMC Geriatr. (2021) 21(1):23. doi: 10.1186/s12877-020-01964-3
Keywords: aortic stenosis, transcatheter aortic valve replacement, cardiac rehabilitation, multimodal intervention, frailty
Citation: Duan H, Zhang C, Zhang Q, Chen D and Xue L (2025) Cardiac rehabilitation for TAVR patients: mechanisms, current status, and future directions. Front. Cardiovasc. Med. 12:1701764. doi: 10.3389/fcvm.2025.1701764
Received: 10 September 2025; Accepted: 5 November 2025;
Published: 25 November 2025.
Edited by:
Guo-wei Tu, Fudan University, ChinaReviewed by:
Giuseppe Mascia, University of Genoa, ItalyNicola Pierucci, Sapienza University of Rome, Italy
Copyright: © 2025 Duan, Zhang, Zhang, Chen and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Xue, MzY0MDIyMDkxQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Huan Duan
Huan Duan Chuan Zhang
Chuan Zhang Qi Zhang1,2
Qi Zhang1,2