Abstract
Mast cell activation disease is a genetic disease entity characterized by a very pronounced clinical symptomatology with potential manifestations in virtually every organ and tissue. These arise from the inappropriate release of mast cell mediators and the accumulation of both morphologically normal and mutated mast cells. Owing to the high prevalence of the disease—estimated to be up to 17%—cardiologists and angiologists are frequently confronted with mast cell activation disease in their daily clinical practice, often without recognizing it. Therefore, every cardiologist and angiologist should possess basic knowledge about this disease and be aware of its cardiovascular challenges. This review summarizes the current state of knowledge on this highly complex disease, with a particular focus on the cardiovascular aspects.
1 Introduction to mast cell activation disease
Mast cell activation disease (MCAD; Table 1) is a genetic disease entity with a very pronounced clinical symptomatology. Clinical manifestations can be present in potentially every organ and tissue. The clinical picture of MCAD is characterized in the majority of cases by pathologically increased mast cell activity resulting in an inappropriate release of mast cell mediators, accompanied by the accumulation of both morphologically normal and mutated mast cells in one organ, organ segment, or multiple organs in the proven absence of other underlying diseases. Although a procedure for diagnosing MCAD has been established (see Section 1.2), it takes, on average, more than 5 years (1) for a definitive diagnosis. The prevalence of the MCAD variant mast cell activation syndrome (MCAS; Table 1) in the Northern Hemisphere is estimated at 4%–17% (2, 3). Consequently, people with mast cell disease are not only frequently seen in primary care but also in cardiology and angiology patient populations and in emergency rooms. Hence, cardiologists and angiologists are repeatedly, often unknowingly, confronted with patients with mast cell activation disease. Moreover, in patients with MCAD, many guideline-recommended cardiovascular therapies have limitations. Various drugs are (relatively) contraindicated, poorly tolerated, or their efficacy is poorly documented due to a lack of studies. This review, therefore, focuses on the dual challenge of MCAD and cardiovascular disease in everyday clinical practice. Its aim is to support the cardiovascular care of patients with MCAD, since it is not only stressful but also life-threatening for patients with MCAD when mast cell disease-specific characteristics are not taken into account (4). The presented cardiovascular findings and their recommended treatment are based on an extensive literature search, consensus recommendations from various international expert groups (5, 6), the WHO recommendations for the MCAD variant “systemic mastocytosis,” and our own extensive experience in the treatment of patients with MCAD.
Table 1
| Mast cell activation disease | |
|---|---|
| Systemic mastocytosis (SM) | Mast cell activation syndrome (MCAS) |
| Prevalence: 1–5/10,000 | Prevalence: up to 17% |
| Clinical variants | |
|
|
Current classification of systemic forms of primary mast cell activation disease (modified from (5)).
Primary mast cell disease must be distinguished from secondary (i.e., non-genetic) mast cell activation (often abbreviated to MCA), which occurs in a variety of clinical contexts and pathologies, including IgE-dependent allergic inflammation and other immunological and inflammatory reactions.
1.1 Pathogenesis of MCAD
MCAD, regardless of its various manifestations, represents a multifactorial, polygenic, and, thus, primary disease entity, i.e., the affected mast cell is mutationally altered (7), inducing pathologically increased activity. Due to the increased activity of affected mast cells, MCAD is characterized by an inappropriate exocytotic release of potentially more than 390 mast cell mediators stored in granules (8). These include pre-stored mediators, such as histamine, TNFα, and tryptase; numerous de novo-synthesized lipid mediators, including eicosanoids, chemokines, and cytokines; and many others (8). In addition to this exocytotic release, mediators can be released or transferred via several other mechanisms [reviewed in (8)]; under certain circumstances, almost all molecules formed by a mast cell can function as mediators. Due to the ubiquitous distribution of mast cells in the organism, symptoms can occur as a result of temporary dysfunctions in virtually all body systems, i.e., organs and tissues, including the cardiovascular system [for a comprehensive list of symptoms in the potential affected systems, see (8)]. In a few patients, all systems are affected, with the majority displaying subsets. The majority of symptoms in the affected systems are chronic and low-grade; some are persistent, but many are either episodic or waxing/waning (9). Depending on the type and quantitative extent of mast cell mediator exocytosis and the compensatory mechanisms inherent in all tissues to eliminate mast cell mediators and their functional effects, the severity of the clinical picture can range from trivial to disabling and even life-threatening (10). The multisystem combination of these symptoms is referred to as mast cell mediator release syndrome.
1.2 How to diagnose mast cell activation disease?
While in many diseases, a suspected diagnosis is suggested based on certain key symptoms, this is not the case in MCAD. Patients with MCAD usually describe the diverse clinical symptoms of mast cell mediator release syndrome. It is not uncommon to find a history of previous doctor hopping (recognizable by the bulging file of medical reports and findings that is usually brought to the consultation), with a multitude of individual diagnoses (especially functional bowel disorders, histamine intolerance, salicylate intolerance, multiple allergies, and multiple drug intolerances) that alone cannot explain the overall clinical picture and for which specific therapies have been unsuccessful. Such constellations should raise suspicion of MCAD. Using a standardized, validated medical history checklist (2, 11, 12), the suspicion of mast cell mediator release syndrome can be substantiated early on. A point score is used to analyze whether a patient's symptom constellation can be attributed with a probability of 95% to an unregulated increased release of mast cell mediators. The objection occasionally raised against the medical history checklist, i.e., that the symptoms and findings listed therein are largely non-specific and could be triggered by a variety of diseases, is insufficient. It must be countered that, of course, the relevant differential diagnoses must be excluded. Ultimately, however, the decisive factor in diagnosing mast cell mediator release syndrome is the fact that these symptoms and findings, which are non-specific in themselves, occur in a combination that affects multiple body systems simultaneously. In analogous checklists from other international working groups [e.g., (13)], the involvement of three body systems is considered sufficient to confirm the suspicion of mast cell mediator release syndrome. Our current version of the checklist is more restrictive. Only when five body systems are affected does the score suggest suspicion with a probability of 95% (12). Whether MCAD is the underlying cause of the mast cell mediator release syndrome must then be proven using diagnostic criteria [details in (10, 14, 15)].
1.3 Cursory overview of MCAD therapy
Due to its genetic causation, this disease is incurable in the current state of medical science. Once the disease has manifested clinically, the symptoms usually progress at varying rates if left untreated. Variables influencing and modifying the disease process may include other genetic predispositions (e.g., Ehlers–Danlos syndrome, IL-4R polymorphisms, and diamine oxidase and histamine N-methyltransferase polymorphisms), environmental factors such as diet and lifestyle, immunoglobulin levels and function, microbiome, the number and type of infections, or vitamin D metabolism. The totality of these variables influences the severity and progression of this disease. Therefore, a complex therapy is necessary after diagnosis [Table 2; for review (16)]. An essential prerequisite for drug therapy is that the affected patients adapt their lifestyle to their disease and minimize the frequency of triggering mast cell activation. If this does not happen, the therapy cannot be satisfactorily successful because mast cell activation repeatedly counteracts the mast cell-inhibiting effect of the medication. This also includes avoiding foods containing gluten, cow's milk protein, and baker's yeast for several weeks before or at the latest upon initiation of drug therapy, as these can weaken the mast cell-calming effect of the medication through non-specific histamine release (e.g., by wheat lectins and casomorphins) and/or stimulation of the immune system in the intestine (17). Similar effects can also be induced by alcohol or its metabolite, acetaldehyde, and by salicylates in medications and cosmetics (16). Due to the highly individualized genetic distortion of mast cells, only personalized therapy can be successful, which must be continually adapted to an individual’s needs in terms of composition and dosage over the course of the disease.
Table 2
| First-line long-term therapy (therapeutic aim: reduction of the unregulated, pathologically increased activity of mast cells) |
|---|
| Sustained-release ascorbic acid, H1-anthistamine, H2-anthistamine, cromoglicic acid, ketotifen (as an alternative to cromoglicic acid in cases of intolerance or to extend therapy), montelukast (a cysteinyl leukotriene receptor 1 antagonist), medicinal C. sativa buds, omalizumab (anti-IgE-antibody), and flavonoids (e.g., quercetin and luteolin) |
| More incisive additional drugs for long-term therapy (therapeutic aim: inhibition of mast cell proliferation and survival) |
| Interferon-α, imatinib, avrapitinib, and midostaurin |
| Optional symptom-oriented therapy |
Drug treatment for MCAD (16).
In summary, the current first-line therapy consists of a long-term therapy consisting of an individually tailored combination of medications with sustained-release vitamin C, H1-antihistamine, H2-antihistamine, cromoglicic acid and/or ketotifen, montelukast, and if necessary, inhalation of medicinal Cannabis sativa buds [details in (16, 18)], aiming at reducing the extent of activation of mast cells and, depending on the active compound (antihistamines and montelukast), antagonizing their mediators at other mast cells and other immune cell populations (e.g., eosinophil granulocytes and dendritic cells), and preventing a secondarily induced pro-inflammatory state. Administered simultaneously with the mast cell activity-inhibiting therapy, symptom-oriented drug treatment should reduce the effects of the released mast cell mediators on effector cells by blocking the corresponding receptors on the effector cells, thus alleviating symptoms and preventing the establishment of a vicious circle. If this medication fails to achieve a tolerable disease intensity within a period of up to 3 months, it is indicated to escalate treatment to more profound therapeutics, such as immunosuppressive steroids (short-term use only), the anti-IgE antibody omalizumab, the anti-IL4/13 antibody dupilumab, kinase inhibitors (e.g., imatinib or avapritinib), or interferon-α. Their effects are based on the inhibition of mast cell proliferation and survival. A comprehensive, detailed description of treatment options is given in (16).
2 Cardiovascular symptoms of MCAD
The presence of mast cells has been documented in the human heart (19, 20, 21, 22). Mast cells reside in the interstitial space between the cardiomyocytes and are present in the endocardium, myocardium, and epicardium [for a review, see, e.g., (23, 24)]. Mast cells are also found around human coronary microvessels (23), in atherosclerotic plaques (25, 26), and often in close proximity to sensory nerves (22, 27, 94). Given the high density of mast cells in the cardiovascular system, up to 80% of patients with MCAD report cardiovascular symptoms (10, 28, 29, 30, 31, 32). However, there are currently no systematic studies on the occurrence of cardiovascular symptoms in MCAD; thus, in the presence of cardiovascular symptoms without a definite MCAD diagnosis, this diagnosis is rarely considered as the cause of the symptoms. The cardiovascular manifestations of mast cell activation linked to suspected causative mast cell mediators are summarized in Table 3.
Table 3
| Cardiovascular symptoms | Mediators |
|---|---|
| Cardiac symptoms | |
| Dysrhythmias (sinus tachycardia, ventricular tachycardia, bradycardia, and supraventricular and ventricular extrasystoles) | Histamine, PGD2, MMPs, endothelin 1 and 3, adenosine, triiodothyronine, 3-iodothyroacetic acid; 3-iodothyroanamine, and platelet-derived growth factor-A |
| Palpitations | Not yet investigated |
| Cardiac arrest | Histamine, PGD2, and leukotriens |
| Pericarditis | Not yet investigated |
| Heart failure (classic and atypical heart failure, e.g., takotsubo cardiomyopathy) | Histamine, tryptase, chymase, platelet activating factor, renin, IL-4 and -6, TNFα, fibroblast growth factor-2 and -7, and TGF-ß |
| Cardiac fibrosis | Tryptase, chymase, TGF-ß, and TNFα |
| Myocardial remodeling | TNFα, MMPs, platelet-derived growth factor α, ANGII, TGF-ß, IL-10 and -13, chymase, tryptase, histamine, CXCL10, and vascular endothelial growth factor α |
| Ischemia-reperfusion injury | TNFα, tryptase, ANGII, and histamine |
| Blood pressure anomalies | |
| Hypotension with presyncope or syncope | Histamine; tryptase; chymase; phospholipases; heparin; vasoactive intestinal polypeptide; PGD2; PGE2; platelet-activating factor; IL-6; nitric oxide; adrenomedullin, ACE 2; kallikrein 1; kallikrein-related peptidases 2, 8, and 9; kininogen 1; and natriuretic peptide A |
| Arterial hypertension | Histamine, chymase, carboxypeptidase A, renin, endothelin 1 and 3, PGD2 by metabolite, thromboxane A2, leukotriens, ANGII, angiotensinogen, and peptide YY |
| Vascular symptoms | |
| Arterial atherosclerosis | Tryptase; chymase; histamine; heparin; IL-4, -6, -8, and -1ß; IFNγ; CCL2; CCL3; CCL4; CCL-5; TNFα; vascular endothelial growth factor; fibroblast growth factor-2 and -7, biglycan; and apolipoprotein E |
| Restenosis | Chymase, tryptase, TGF-β, and histamine |
| Vein graft hyperplasia | Histamine and chymase |
| Aneurysm/artery dissection | Chymase; tryptase; cathepsin G, MMPs; IL-6, -8, and -1ß; IFNγ; CCL2; CCL-5; and adrenomedullin |
| Raynaud´s phenomenon | Histamine, serotonin, renin, ANGII, angiotensinogen, endothelin 1 and 3, PGD2 by metabolite, chymase, thromboxane A2, leukotriens, IL-6 and -8, and peptide YY |
| Vasculitis | Not yet investigated |
| Coronary syndromes with or without myocardial infarction (associated with rupture of an atherosclerotic plaque, partial or complete thrombosis, arterial embolism, and/or allergic coronary spasm) | ANGII, angiotensinogen, histamine, serotonin, IL-6, -8, CCL-5, endothelin 1 and 3, and leukotriens |
| Angioneogenesis | Angiogenin; CXCL12; CCL2; IL-8; platelet-derived growth factor; TNFα; IL-1β; IL-6; vascular endothelial growth factor; fibroblast growth factor-2; IFNγ; heparin; MMP-2, -9, -and 13; and angiopoetin-2 |
| Aberrant angiogenesis (hemangiomas, arteriovenous malformations, and telangiectasias) | Mediators involved in angioneogenesis are assumed but not proven to be involved |
| Livedo reticularis | Not yet investigated |
| Hemorrhoids | Not yet investigated |
| Varicosities | TGF-ß |
| Flush | Histamine and PGD2 |
| Hot flashes | Mediator-induced neural-mediated mechanisms may be involved |
| Clotting and bleeding abnormalities | |
| Tendency to bleed due to heparin release and hyperfibrinolysis | Heparin, vasoactive intestinal polypeptide, prostanoids, tryptase, chymase, tissue-type plasminogen activator, and urokinase |
| Thrombophilia | Histamine, factor VIII, and tryptase |
Cardiovascular symptoms of mast cell activation disease induced by mediators proven to be involved in the pathogenesis of the listed symptoms and dysfunctions (8, 47, 58, 76, 85, 89, 90, 91, 92, 93). The list is not exhaustive because there are probably more as yet unidentified mediators involved in the pathogenesis of the symptoms.
3 Selected cardiovascular symptoms, dysfunctions, and therapeutic challenges in MCAD
The following topics were selected for detailed discussion because they frequently occur in patients with MCAD and because at least some of these mediators responsible for the symptoms and dysfunctions have been identified. Moreover, the therapeutic options can be defined with a certain degree of specificity.
3.1 Cardiac fibrosis
Experimental and in vivo evidence suggests a critical role for an increase in myocardial mast cell density in the pathogenesis of cardiac fibrosis by inducing the expansion of activated myofibroblasts. Activated cardiac mast cells release a variety of potent pro-inflammatory and pro-fibrotic mediators (Table 3), which may contribute to cardiac fibrosis [(33, 34); Figure 1]. Mast cell-derived proteases participate in matrix metabolism through fibrogenic mediators and matricellular proteins or by exerting contact-dependent actions on fibroblast phenotype. Mast cell chymase converts angiotensin I to angiotensin II (ANGII) independently of angiotensin-converting enzyme (ACE). ANGII generation directly contributes to fibrosis by inducing the differentiation of fibroblasts to myofibroblasts. Mast cell tryptase can act directly on fibroblasts to induce proliferation and differentiation to the myofibroblast phenotype. Tryptase and chymase both act on transforming growth factor-ß (TGF-ß) to convert it to the active form, which also induces fibroblast differentiation to the myofibroblast phenotype and collagen deposition. Mast cell-derived tumor necrosis factor α (TNFα) and interleukin 1ß (IL-1ß) induce cardiomyocyte apoptosis, MMP-9 production, and inflammatory cell recruitment that enhances tissue remodeling. Additionally, mast cells release TGF-ß, further contributing to the activation and differentiation of fibroblasts. Cardiac fibrosis is associated with systolic and diastolic dysfunction, arrhythmogenesis, and adverse outcomes.
Figure 1
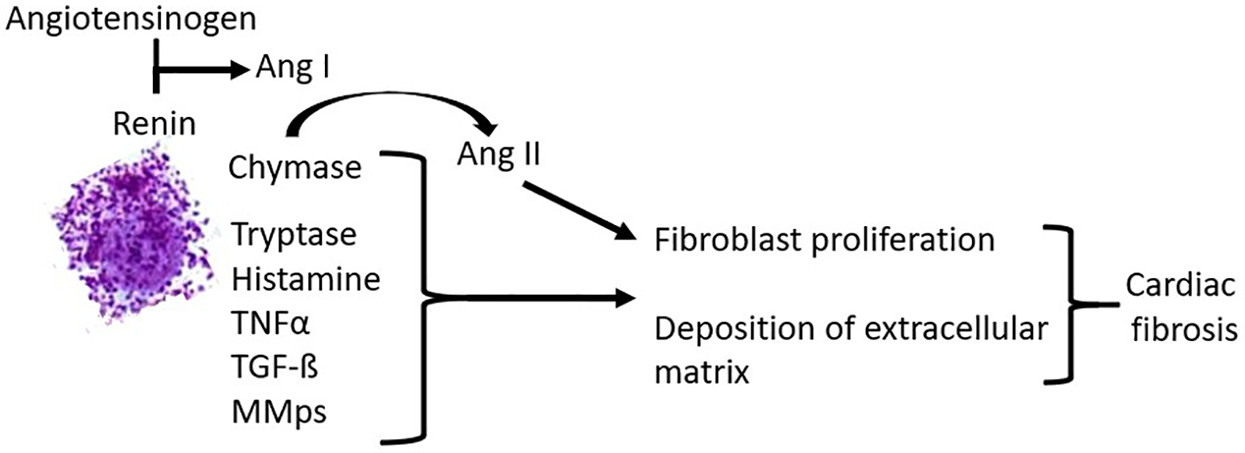
Pathogenetic effects of mast cells in cardiac fibrosis.
3.1.1 Therapeutic option
A blockade of mast cell activity using mast cell stabilizing drugs (Table 2) should be considered as a first-line therapy.
3.2 Dysrhythmias: sinus tachycardia, ventricular tachycardia, bradycardia, supraventricular and ventricular extrasystoles, and cardiac arrest
Dysrhythmias are frequently reported by patients with both MCAD variants (Table 1) (SM: 29% (35, 36); MCAS: ≥20% (29, 37, 38, 39, 40, 41)). In the worst case, MCAD can present as a cardiac emergency with cardiac arrest or ventricular fibrillation (42).
3.2.1 Sinus tachycardia
Mast cell activation can induce an increased release of norepinephrine (NE) from cardiac sympathetic nerves, leading to pathological amounts of NE resulting in arrhythmias and sudden cardiac death. Mast cells can increase NE release by targeting sympathetic nerves in two ways. First, ANGII can be formed in the heart locally by mast cell-derived renin [for review, see (22)] or independently of ACE by the proteases cathepsin-D (43) and chymase [e.g., (44)]. ANGII then binds to facilitatory presynaptic angiotensin AT1-receptors on the sympathetic nerve endings, leading to presynaptic release of NE (45). In addition, ANGII can also directly elicit cardiac arrhythmias without sympathetic involvement (46). Second, mast cell-derived histamine is known to act as a direct stimulator of histamine-H2-receptors on the cardiomyocytes in the sinoatrial node (47), inducing an increase in heart rate and contractility in animal models and in humans (48) to elicit sinus tachycardia [for review, see (49)]. Finally, prostaglandin D2, which is also a major mast cell mediator, has been shown to induce tachycardia in humans (50).
3.2.1.1 Therapeutic options
Mast cell mediator-induced supraventricular tachycardia should be treated when it causes extreme distress to the patient and when their heart rate persistently exceeds 100–120 beats per min. As a first step, the doses of drugs that reduce mast cell activity (Table 2), such as antihistamines (51), should be optimized. If this action is not sufficient to normalize the patient’s heart rate, further medical treatment should be considered. As a result of its pathophysiology, supraventricular tachycardia due to mast cell activation should be sensitive to treatment with direct renin inhibitors and AT1 receptor blockers. However, these drug classes can only be used in patients who do not have hypotension, since these drugs can induce a further decrease in blood pressure. In these patients, the funny current blocker ivabradine (52), which reduces heart rate in patients with sinus rhythm (but not in those with atrial fibrillation) without altering blood pressure (53), should be used next if available [as in Europe (14, 54)], since the calcium channel blocker verapamil is frequently ineffective (14). The use of ß-adrenoceptor antagonists should be avoided in patients with MCAD if possible. ß-Adrenoceptors play an important inhibitory role in the control of mast cell mediator release (55, 56). Hence, ß-adrenoceptor blockade disinhibits mast cell mediator release, usually leading to instantaneous aggravation of the symptoms of MCAD (57). If the use of ß-adrenoceptor antagonists is unavoidable, attempts must be made to block mast cell activity in patients with MCAD in parallel, e.g., by administering high doses of glucocorticoids. Finally, it should be noted that epinephrine, as an emergency medication to control anaphylactoid reactions, should be used in tachycardic patients with MCAD with particular caution because it has been reported to precipitate ventricular fibrillation (40). The antagonists of the two prostaglandin D2 receptors, prostaglandin D2 receptor 1 (DP1) and chemoattractant homologous receptor expressed on Th2 cells (CRTH2), may theoretically be a future approach to the treatment of tachycardia in MCAD. However, clinical approval of such drugs is delayed because of safety concerns.
3.2.2 Ventricular tachycardia
Ventricular tachycardia (VT) episodes in MCAD may only last a few seconds without causing harm, but episodes lasting more than a few seconds can be life-threatening. VT may sometimes result in ventricular fibrillation and turn into cardiac arrest (55). Pathophysiologically, the presumed causes of VT in association with MCAD include reentry circuits based on mast cell mediator-induced fibrosis and other myocardial tissue damage, as well as mast cell mediators, which are yet to be identified. Due to the above-discussed pathologically increased release of NE from cardiac sympathetic nerves, catecholaminergic polymorphic VT may occur.
3.2.2.1 Therapeutic options
Therapy may be directed either at terminating an episode of abnormal heart rhythm or at reducing the risk of VT episodes. Usually, MCAD-related VT is self-limiting. Long-term anti-arrhythmic therapy may be indicated to prevent recurrence of VT. In several patients with MCAD, catheter ablation has been performed as a potentially definitive treatment option for their recurrent VT. However, probably due to the complex pathophysiology of VT in these patients with MCAD, VT reappeared after a few weeks or months (personal observation).
3.2.3 Supraventricular and ventricular extrasystoles
Supraventricular (SVES) and ventricular extrasystoles (VES) are often found in patients with MCAD, in particular during flares of the disease but also after long-standing disease. The release of arrhythmogenic mast cell mediators is suspected to be the cause. The indication for drug therapy must be highly personalized.
3.2.4 Bradyarrhythmia
The most prominent effect of histamine on the atrioventricular (AV) node is a slowing of conduction [for review, see (49)] up to a third-degree atrioventricular block (48, 59). In addition to the effect of histamine on the AV node, fibrosis of the AV node region can occur during the course of MCAD progression, resulting in a progressive AV block beginning with a first-degree AV block, transitioning into a second-degree AV block according to Mobitz type I or II, and evolving into a third-degree AV block. The progression of the AV conduction disturbance may manifest clinically as standing vertigo, coordination difficulties, and a rapid decrease in resting heart rate after physical exertion to values below 50–40 beats per min and sometimes even slower. A rapid decrease in heart rate may cause a drop in systolic blood pressure to 100 mm Hg or less, necessitating a temporary shock position. This physical stress reaction induces additional activation of the MCAD with a release of cardiovascular mediators, which can lead to a further drop in blood pressure (rarely to hypertension) and/or SVES and/or VES.
3.2.4.1 Therapeutic option
Since the AV block in MCAD is not due to a temporary or reversible cause, the implantation of a dual-chamber pacemaker is indicated for any form of AV block of second degree or higher. After the implantation of the pacemaker, a temporarily rapid junctional escape rhythm and, in particular, SVES and VES may be observed due to released mast cell mediators. Since ß-adrenoceptor blockers should be avoided in MCAD (see Section 3.2.1), an individualized decision must be made as to whether and, if so, which antiarrhythmics should be used to suppress the dysrhythmias that are not eliminated by the pacemaker.
3.3 Heart failure
A role of mast cells in heart failure has been suggested [e.g., (60)]. In a Danish retrospective cohort study of 548 adults with systemic mastocytosis, 12 patients had congestive heart failure (61). In a prospective study with 18 patients with MCAS that was performed to investigate the suspected cardiac impact of increased systemic mast cell activation, diastolic left ventricular dysfunction was found using pulse wave- and/or tissue-Doppler imaging in 12 patients with MCAS, which represents the most sensitive sign of a myocardial structural alteration (62, 63). In five of these 12 patients, left ventricular hypertrophy was observed. Although there may be structural signs of heart failure in the majority of patients with MCAD, symptomatic chronic heart failure does not appear to be more prevalent than in the general population.
3.3.1 Role of mast cells in pathogenesis
Alterations in the pumping performance of the heart in patients with MCAD are probably due to fibrosis and to the remodeling effect of the prohypertrophic and profibrotic factors secreted by mast cells (Table 3, Figure 1).
3.3.2 Therapeutic options
The medical treatment options for patients with chronic heart failure [New York Heart Association (NYHA) class II–IV], based on the Guidelines of the European Society of Cardiology with a special focus on heart failure (64), are also valid for patients with MCAD and clinically manifest heart failure. The European Society of Cardiology Guidelines recommend, depending on the distortion of the ejection fraction, therapy with diuretics and SGLT-2 inhibitors, followed by the administration of angiotensin-AT1-receptor antagonists, ACE inhibitors, angiotensin receptor-neprilysin inhibitors, mineral corticoid receptor antagonists, and ß-adrenoceptor blockers. However, ACE inhibitors should be avoided in patients with MCAD if possible because they can significantly worsen MCAD: ACE is able to degrade bradykinin, which is an activator of mast cells (57). Therefore, the inhibition of ACE increases mast cell mediator releasability and, hence, autocrine mast cell activation with MCAD symptoms through the stabilization of bradykinin (57). ß-Adrenoceptor blockers should be used with great caution in patients with MCAD, as substantiated in Section 3.2.1. The use of SGLT-2 inhibitors should be considered in MCAD, as they could also possibly have a beneficial effect on MCAD itself. Since allergic reactions (e.g., urticaria) are possible, individual risk assessment and close monitoring in patients with MCAD are advisable, especially during the introduction phase of SGLT-2 inhibitors.
3.4 Hypotension with or without presyncope or syncope
Episodes of hypotension with lightheadedness or syncope as a manifestation of MCAD are reported by 22%–55% of the patients with MCAD (2, 29, 35, 36, 65), in contrast to a prevalence of 5% in the control group (2). The frequency of the episodes of syncope can vary from as frequently as daily to as rarely as once per year or never.
3.4.1 Role of mast cells in the pathogenesis
Mast cells can produce and release a number of mediators that induce vasodilatation (Table 3) and, thereby, induce hypotension up to vascular collapse.
3.4.2 Therapeutic options
In a scenario of acute hypotension, the simplest treatment is to lie back down immediately after feeling lightheaded upon standing. Symptoms will often disappear. In order to prevent mast cell mediator-induced hypotension, mast cell activity stabilizing drugs (Table 2), such as H1-antihistamines, should be the first-line therapy. Acetylsalicylic acid (80–325 mg orally once or twice daily in adults) may be beneficial in some patients who have high levels of vasodilating prostaglandins (36, 66, 67) if the patient's tolerance to use non-steroidal anti-inflammatory drugs (NSAIDs) without adverse effects is known. Alternatively, prostaglandin formation can be reduced through the selective inhibition of cycloxygenase-2, such as with etoricoxib in Europe or celecoxib in the US, though the potential cardiovascular risks of cycloxygenase-2-selective NSAIDs need to be acknowledged (68). Preliminary data from SM patients suggest the efficacy of the humanized monoclonal IgE-antibody omalizumab in the prevention of spontaneous episodes of systemic hypotension (69, 70, 71, 72, 73). In the case of recurrent anaphylactic syncope, acute treatment with epinephrine and corticosteroids should be considered. A future approach to the treatment of vascular symptoms in MCAD could be antagonists of the prostaglandin D2 receptors DP1 and CRTH2. However, no substances with these two targets have been approved thus far.
3.5 Systemic arterial hypertension
In up to 31% of patients with MCAD, marked recurrent or sustained elevation in arterial blood pressure due to mast cell activation has been observed (2, 29, 30, 74). Moreover, in patients with MCAD, alternating hypotensive and hypertensive episodes are often observed.
3.5.1 Role of mast cells in the pathogenesis
Mast cells can produce and release a number of mediators that induce vasoconstriction (Table 3) and, thereby, induce hypertension. In particular, ANGII is a potent vasoconstrictor. Another major mediator released in patients with MCAD, the vasodilator prostaglandin D2, can be metabolized by 11-ketoreductase to the biologically active metabolite 9α, 11ß-PGF2, which is a vasopressor (74). Thus, arterial hypertension in patients with MCAD may be linked to the metabolism of prostaglandin D2 in the individual patient. In addition, released endothelins could be important mediators in the induction of arterial hypertension.
3.5.2 Therapeutic options
As a result of its pathophysiology, therapy for hypertension in patients with MCAD must be personalized and is frequently developed through trial and error. Theoretically, MCAD-induced hypertension may be especially sensitive to treatment with direct renin inhibitors and AT1 receptor blockers. However, mast cell mediator-induced hypertension has often been shown to be refractory to these drugs. Calcium channel blockers such as amlodipine and, if these are insufficient, clonidine could be used. A blood pressure crisis due to an acute mast cell mediator release episode can be treated with nifedipine or clonidine, but in patients with MCAD, the blood pressure values may only decrease slowly over hours after administration. Nevertheless, the dosages of both drugs must be titrated very cautiously because the degradation of the causative hypertensive mast cell mediators can be shorter than the half-life of the drugs. If doses of nifedipine or clonidine are chosen that reduce the hypertension to normal blood pressure values, the drugs could induce dramatic hypotension within minutes when the hypertensive mediators are degraded. Therefore, in the interventional treatment for a hypertensive crisis in patients with MCAD, doses of both drugs should be chosen that will initially reduce the systolic blood pressure to an empirically found target value of 150–160 mmHg. As concentrations of hypertensive mast cell mediators in blood decrease, blood pressure will then continue to decline slowly over several hours, but will normally not reach extreme hypotensive levels. From the standard medication for the treatment of high blood pressure, ß-adrenoceptor antagonists and ACE inhibitors are problematic for the reasons mentioned above (Sections 3.2.1 and 3.3). The administration of endothelin receptor antagonists may be an approach to the treatment of arterial hypertension in patients with MCAD. In 2024, the US Federal Drug Administration approved the non-selective endothelin receptor antagonist aprocitentan for resistant hypertension. To date, there are no published studies on its use in patients with MCAD. In the future, if approved, antagonists of the prostaglandin D2 receptors DP1 and CRTH2 and peptide YY receptor antagonists, specifically targeting the Y1 and Y2 receptors, may become important drugs in the treatment of hypertension. In order to prevent mast cell mediator-induced hypertension, mast cell activity reducing therapy must be optimized (Table 2).
3.6 Arterial atherosclerosis
Atherosclerosis is a chronic inflammatory disease characterized by the formation of atherosclerotic plaques that consist of numerous cells, including smooth muscle cells, endothelial cells, immune cells, and foam cells. There is compelling evidence that mast cells play a pivotal role in the pathology of atherosclerosis [for a review, see, e.g., (75, 76)].
3.6.1 Role of mast cells in pathogenesis
Mast cells accumulate within atherosclerotic plaques during their progression and release a mélange of mediators, including histamine, tryptase, chymase, cathepsins, and cytokines, such as TNFα, IFNγ, CCL2, IL-6, and IL-8. These mediators promote vascular cell apoptosis, blood-borne inflammatory cell adhesion and recruitment, foam cell formation, lipid degradation, neovascularization of the plaque, plaque progression, instability, erosion, rupture, aggravation of the inflammation of the plaque environment, and thrombosis (76, 77).
3.6.2 Therapeutic options
The currently available therapeutics for atherosclerosis focus on alleviating hyperlipidemia and preventing thrombotic complications. The current first-line clinical drugs for lipid management are statins, which, in addition to their main effect of lowering cholesterol levels, have pleiotropic effects, e.g., reducing the activity of mast cells (16). Non-statin agents, including ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) therapies, complement statin therapy or are administered independently. In addition, therapies that disrupt atheroprogression through immunosuppressive mechanisms have been investigated in clinical trials [reviewed in (78)]. In this context, the drug-induced reduction in mast cell activity in patients with MCAD is an important preventive anti-atherogenic therapy, since mast cells actively contribute to atherosclerotic plaque progression and destabilization (Table 2). Cholesterol and triglycerides that are diet-independently increased in the blood in approximately 75% of MCAD patients (28) can be deposited in the artery walls, forming plaques that narrow the vessels and reduce blood flow.
3.7 Angina pectoris and coronary syndromes with or without myocardial infarction
3.7.1 Angina pectoris due to coronary heart disease
Angina pectoris can be the cardinal symptom of coronary artery disease, in which the underlying circulatory disorder is caused by stenosis of a coronary artery due to atherosclerosis. This form of angina pectoris responds to the administration of nitroglycerin spray, and the pain is aggravated by exertion in acute scenarios. The MCAD variant systemic mastocytosis has been proven to be associated with a higher incidence of acute coronary syndrome, even when plasma lipid levels are low (79).
3.7.2 Vasospastic angina pectoris
Another cause of coronary artery narrowing in patients with MCAD is coronary artery vasospasm that can be detected acutely on an ECG by screening for signs of ischemia. However, the ECG changes are reversible and physical performance is good. Typically, these signs are no longer present on the ECG upon arrival at the emergency room, and troponin levels in the blood are not elevated. Furthermore, this form of angina pectoris responds to the administration of nitroglycerin spray, and the pain is aggravated by exertion in the acute scenario. Numerous case reports of patients with MCAD presenting with vasospastic angina pectoris have been published (31, 80, 81, 82, 83). We have made similar observations in our patient population (unpublished findings). Mast cells have been detected at the site of vasospasm in patients with variant angina, indicating a role in coronary artery spasm (19). Vasospastic angina pectoris in patients with MCAD seems to be due to the release of a plethora of vasoconstrictory mediators, such as serotonin, leukotriens, and endothelin, from mast cells (Table 3), which can trigger coronary artery vasospasm.
Therapeutic options for 3.7.1 and 3.7.2
In patients with MCAD, the treatment should initially dilate the coronary vessels in both variants. Vasodilator drugs, including nitrates, e.g., nitroglycerin and calcium channel blockers, should be considered as the first-line therapy. For the vasospastic variant, after initial relief of vasospasm with a vasodilator drug, blockade of mast cell activity with mast cell stabilizers (Table 2) should be attempted to prevent coronary artery vasospasm. Such optimized, long-term mast cell activity-inhibiting medication, always adapted to the specific trigger situation, should be used for the prophylaxis of mast cell-induced vasospastic angina pectoris.
3.7.3 Neuropathic non-cardiac angina pectoris
There are patients with MCAD whose angina pectoris does not respond to the application of vasodilators, is not triggered or worsened by exercise, and usually occurs spontaneously at rest. However, it can be alleviated by blocking mast cell activity. It is assumed that this angina pectoris reflects neuropathic pain resulting from the activation of mast cells. Mast cells are often in close proximity to sensory nerves (22, 27, 94), which may be relevant to the occurrence of non-cardiac angina pectoris. If C-nerve fibers in the thorax are activated by mast cell mediators, their activity is interpreted in the brain as angina pectoris-like. There are no systematic studies on the prevalence of neuropathic non-cardiac angina pectoris in patients with MCAD. However, non-cardiac angina pectoris is frequently observed in MCAD treatment centers. If the presence of MCAD is unknown in a patient, the frequency of rescue calls with admission of such a patient to the emergency room may be as high as once a week. In the absence of signs of ischemia on the ECG and pathologically altered specific blood values, as well as independence from exercise, emergency physicians should consider non-cardiac angina pectoris due to MCAD.
Therapeutic options for 3.7.3
Blockade of mast cell activity with mast cell activity-inhibiting drugs should be considered as the first-line therapy (Table 2).
3.7.4 Non-cardiac angina pectoris due to Roemheld symptom
Mast cell mediator-induced adhesions can lead to an accumulation of gas before the upper left intestinal flexure. In addition, extreme abdominal distension often occurs unpredictably and within minutes, independent of food intake. Focal swelling, segmental or local hypo-, hyper-, and dysmotility, with transport disturbances, induced by acute mast cell mediator release, appear to be responsible for this phenomenon. This flatulence results in pain from adjacent organs, such as cardiac pain. The resulting displacement of the cardiac axis in cases of pronounced flatulence in the upper small intestine and stomach can lead to the Roemheld symptom complex (84).
Therapeutic options for 3.7.4
In such cases, the use of liquid simethicone, heat (placing a heating pad on the abdomen or drinking 500 mL of warm water), and, if indicated due to the intensity of the symptoms, the administration of metamizole as a spasmolytic (check tolerance beforehand), as well as intravenous administration of antihistamines and fluid resuscitation with 5% glucose or an electrolyte solution, may be helpful in the acute scenario (84). Preventive treatment focuses on optimizing MCAD medication (Table 2) and dietary changes, e.g., avoiding gas-producing foods.
3.8 Clotting and bleeding abnormalities
Mast cell activation may affect hemostasis through vascular (e.g., endothelial cells) and cellular components (e.g., platelets, monocytes, and neutrophils) and by clotting and fibrinolytic factors released from activated mast cells [for a review, see (85)]. Since high concentrations of mast cell mediators related to secondary hemostasis can be achieved in circulation, clinically significant bleeding, thrombosis, or both simultaneously can occur in MCAD. The resulting platelet activation may additionally trigger mast cell activation, thereby stimulating mast cell mediator release and contributing to the distortion of hemostasis. Clinical signs of bleeding diathesis, such as hematoma formation, bruising, prolonged bleeding after biopsies, gingival bleeding, epistaxis, gastrointestinal hemorrhage, conjunctival hemorrhage, menorrhagia, or hemorrhagic ulcer disease, occur in approximately 50% of patients with MCAD; severe or fatal bleeding seems to be rare (85). Increased release of heparin from mast cells (the main source of heparin) can reach circulating concentrations as high as the heparin levels achieved during thromboprophylaxis through subcutaneously applied exogenous heparin and, thereby, could contribute to bleeding diathesis. However, the main cause of bleeding diathesis likely lies in a serious hyperfibrinolysis. Therefore, in all patients suspected of having MCAD, a mast cell disease-specific examination of the coagulation system should be carried out for diagnostic reasons and before surgeries to optimize the perioperative procedures.
3.8.1 Therapeutic options
To reduce thrombotic and bleeding tendencies in patients with MCAD in their everyday lives, mast cell activation should be reduced as much as possible by the class of medication found to be best for the individual patient (Table 2).
Hemostatic drug treatment for bleeding in patients with MCAD is only based on clinical evidence. Before and after invasive procedures, mast cell activity should be reduced as much as possible by administering medications and avoiding triggers such as temperature shock from a cold operating room or infusion of refrigerated fluids. Routine procedures to stop surgical bleeding are rarely effective when the bleeding is induced by mast cell activity. Unless contraindicated, tranexamic acid (TXA) 1 g should be administered intravenously 30 min before the first incision, and, depending on the intra- and postoperative bleeding situation, the TXA infusion should be continued for at least 12–24 h (total dosage 2–3 g/24 h). Depending on the clinical circumstances, TXA can be administered topically, orally, or intravenously. The risk of an arterial or venous thromboembolism when using TXA remains unclear but appears to be empirically low with the recommended dosages.
In cases in which bleeding is proven to be due to very high plasma heparin levels, heparin could be neutralized through protamine titration according to the patient's heparin levels. However, because of possible adverse reactions to protamine due to mast cell activation, protamine use should be limited to patients with MCAD with life-threatening bleeding, which is very likely the result of endogenous heparin.
In cases of severe thrombocytopenia (due to hypersplenism in an enlarged spleen, which is often present in MCAD, among other causes ), a transfusion of platelet concentrates should be considered.
Despite the potential risk of bleeding in patients with MCAD, thromboprophylaxis with low-molecular-weight heparin, unfractionated heparin, or fondaparinux should not be withheld when clinically indicated, including in the context of internal or surgical treatment.
Thrombophilia and a thromboembolism in patients with MCAD should be treated according to the respective guidelines.
3.9 Raynaud's phenomenon
Raynaud's phenomenon [RP; for review, see, e.g., (86)] is a condition characterized by episodic, excessive vasoconstriction triggered by cold or stress in the fingers and toes, albeit less pronounced in the latter. This leads to a distinctive sequence of color changes of the digits, which is usually livid in patients with MCAD. The majority of patients experience numbness and/or severe burning pain. Nailfold capillaroscopy may reveal reduced flow with marked sludge formation, episodes of stasis, and intermittent pendulum flow. Capillary density is often only slightly reduced without true avascular fields. Atypical capillaries, particularly torsions and vertex ectasias, may be present. Microbleeds may occasionally be observed. These changes may be accompanied by perivascular edema. Acral oscillography of the upper extremities shows reduced oscillations after cold exposure. Cold autoantibodies are not detectable in the blood. In the context of MCAD, the pathogenesis of RP presumably involves a complex interaction between the vascular wall, nerves, and mast cell mediators, disrupting the balance between vasoconstriction and vasodilation (Table 3). The prevalence of RP in MCAD has not been determined as yet. An estimation of up to 10% reflects our experiences.
3.9.1 Therapeutic options
Although RP is suggested to be induced by mast cell mediators in MCAD, its treatment remains elusive. After its manifestation in MCAD, RP initially responds a little to mast cell activity-inhibiting drugs, but as MCAD progresses, it no longer responds at all. Patients are advised to avoid cold exposure. Otherwise, the recommended medication is that for RP management in general: vasodilators (dihydropyridine calcium channel blockers, topical nitrates, pentoxifylline, and phosphodiesterase-5 inhibitors), vasoconstriction inhibitors (ANGII receptor antagonists and endothelin-1 receptor antagonists), and endothelial function modulators (limited evidence and no strong recommendation for the use of antiplatelet agents). Studies on whether these drugs have a beneficial effect in MCAD-related RP are missing.
3.10 Cardiac catheterization and pacemaker implantation
The special precautions indicated for major surgical interventions in patients with MCAD (87) should also be applied to less invasive procedures, such as cardiac catheterization and pacemaker implantation. Both procedures should be performed under short-term anesthesia with propofol. Propofol is particularly suitable for this purpose because, in addition to its narcotic effect, it simultaneously inhibits mast cell activity. During these procedures, the tendency to bleed, which exists in almost all patients with MCAD, should be reduced through a preoperative administration of tranexamic acid (see Section 3.8).
Furthermore, it should be taken into account during pacemaker implantation that patients with mast cell disease have reduced ability to dissolve absorbable suture material (88). Therefore, absorbable suture material should not be used to prevent impaired postoperative wound healing. In addition, the implanted pacemaker should allow for MRI examinations with a magnetic flux density of 3 Tesla, as patients with MCAD must undergo repeated MRI scans due to their multisystem symptoms and comorbidities.
4 Limitations of this review
The significance of the above statements is limited because they are based only on small study groups, individual case reports, and the experience of MCAD experts. Although the majority of patients with MCAD complain of cardiovascular symptoms, no systematic studies have been conducted to date on the various cardiovascular symptoms. Nevertheless, there is no reason to doubt the causal involvement of mast cell mediators in the described symptoms in MCAD.
However, without results from systematic studies, no long-term prognostic data can be presented regarding the cardiovascular manifestations of MCAD, including mortality, and whether treatment interventions improve cardiovascular outcomes in MCAD.
Finally, most treatment recommendations are based on expert opinion, albeit obtained in the treatment of thousands of patients. Thus, there is a need for prospective randomized controlled trials.
5 Conclusion
Cardiologists and angiologists are often unknowingly confronted with mast cell activation disease in their daily clinical practice. Therefore, every cardiologist and angiologist should have a basic understanding of this disease and should be informed about its challenges in the cardiovascular system.
Statements
Author contributions
WT: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. GM: Conceptualization, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Publication of the article is financially supported by the Förderclub Mastzellforschung e.V. (Germany).
Conflict of interest
GJ Molderings is co-owner of the start-up company Mast Cell Sciences Ltd. WT is co-owner and president of Mast Cell Sciences Ltd.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACE, angiotensin-converting enzyme; ANGII, angiotensin II; CCL2, C-C motif chemokine ligand 2; CCL3, C-C motif chemokine ligand 3; CCL4, C-C motif chemokine ligand 4; CCL5, C-C motif chemokine ligand 5; CNS, central nervous system; CRTH2, chemoattractant homologous receptor expressed on TH2 cells; CXCL10, C-X-C motif chemokine 10; CXCL12, CXC motif chemokine 12; DP1, prostaglandin D2 receptor 1; ECG, electrocardiogram; IFNγ-interferon gamma; IL-1ß, interleukin-1ß; IL-4, interleukin-4; IL-6, interleukin-6; IL-8, interleukin-8; MCAD, mast cell activation disease; MCAS, mast cell activation syndrome; MMP, matrix metalloproteinase; NE, norepinephrine; NSAID, non-steroidal anti-inflammatory drugs; PGD2, prostaglandin D2; PGE2, prostaglandin E2; RP, Raynaud’s phenomenon; SGLT-2, sodium-glucose co-transporter 2; SM, systemic mastocytosis; SVES, supraventricular extrasystole; TGF-ß, transforming growth factor beta; TNFα, tumor necrosis factor α; TXA, tranexamic acid; VES, ventricular extrasystole; VT, ventricular tachycardia.
References
1.
Haenisch B Fröhlich H Herms S Molderings GJ . Evidence for contribution of epigenetic mechanisms in the pathogenesis of systemic mast cell activation disease. Immunogenetics. (2014) 66:287–97. 10.1007/s00251-014-0768-3
2.
Molderings GJ Haenisch B Bogdanow M Fimmers R Nöthen MM . Familial occurrence of systemic mast cell activation disease. PLoS One. (2013) 8:e76241. 10.1371/journal.pone.0076241
3.
Zaghmout T Maclachlan L Bedi N Gülen T . Low prevalence of idiopathic mast cell activation syndrome among 703 patients with suspected mast cell disorders. J Allergy Clin Immunol Pract. (2024) 12:753–61. 10.1016/j.jaip.2023.11.041
4.
Schwab D Raithel M Ell C Hahn EG . Severe shock under upper gastrointestinal endoscopy in a patient with systemic mastocytosis. Gastrointest Endoscop. (1999) 50:264–7. 10.1016/s0016-5107(99)70237-3
5.
Afrin LB Ackerley MB Bluestein LS Brewer JH Brook JB Buchanan AD et al Diagnosis of mast cell activation syndrome: a global “consensus-2”. Diagnosis. (2020) 8:137–52. 10.1515/dx-2020-0005
6.
Valent P Hartmann K Bonadonna P Gülen T Brockow K Alvarez-Twose I et al Global classification of mast cell activation disorders: an ICD-10-CM-adjusted proposal of the ECNM-AIM consortium. J Allergy Clin Immunol Pract. (2022) 10:1941–50. 10.1016/j.jaip.2022.05.007
7.
Molderings GJ . Systemic mast cell activation disease variants and certain genetically determined comorbidities may be consequences of a common underlying epigenetic disease. Med Hypotheses. (2022) 163:110862. 10.1016/j.mehy.2022.110862
8.
Molderings GJ Afrin LB . A survey of the currently known mast cell mediators with potential relevance for therapy of mast cell-induced symptoms. Naunyn Schmiedebergs Arch Pharmacol. (2023) 396:2881–91. 10.1007/s00210-023-02545-y
9.
Afrin LB Self S Menk J Lazarchick J . Characterization of mast cell activation syndrome. Am J Med Sci. (2017) 353:207–15. 10.1016/j.amjms.2016.12.013
10.
Afrin LB Butterfield JH Raithel M Molderings GJ . Often seen, rarely recognized: mast cell activation disease—a guide to diagnosis and therapeutic options. Ann Med. (2016) 48:190–201. 10.3109/07853890.2016.1161231
11.
Molderings GJ Kolck U Scheurlen C Brüss M Frieling T Raithel M et al Systemic mast cell disease with gastrointestinal symptoms—a diagnostic questionnaire. Dtsch Med Wochenschr. (2006) 131:2095–100. 10.1055/s-2006-951337
12.
Weinstock LB Pace LA Rezaie A Afrin LB Molderings GJ . Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci. (2021) 66:965–82. 10.1007/s10620-020-06264-9
13.
Hermine O Lortholary O Leventhal PS Catteau A Soppelsa F Baude C et al Case-control cohort study of patients’ perceptions of disability in mastocytosis. PLoS One. (2008) 3:e2266. 10.1371/journal.pone.0002266
14.
Molderings GJ Homann J Brettner S Raithel M Frieling T . Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. Dtsch Med Wochenschr. (2014) 139:1523–34. 10.1055/s-0034-1370055
15.
Zienkiewicz T Homann J Mücke M Seidel H Hertfelder HJ Weinstock LB et al Evaluation of a tryptase depletion index for better pathologic identification of mast cell activation syndrome. Z Gastroenterol. (2023) 61:268–74. 10.1055/a-1833-9226
16.
Molderings GJ Haenisch B Brettner S Homann J Menzen M Dumoulin FL et al Pharmacological treatment options for mast cell activation disease. Naunyn-Schmiedeberg’s Arch Pharmacol. (2016) 389:671–94. 10.1007/s00210-016-1247-1
17.
Bengtsen U Nilsson-Balknäs U Hanson LA Ahlstedt S . Double blind, placebo controlled food reactions do not correlate to IgE allergy in the diagnosis of staple food related gastrointestinal symptoms. Gut. (1996) 39:130–5. 10.1136/gut.39.1.130
18.
Molderings GJ . Die systemische Mastzellaktivierungserkrankung. Med Monatsschrift Pharm. (2024) 47:98–104.
19.
Forman MB Oates JA Robertson D Robertson RM Roberts LJ Virmani R . Increased adventitial mast cells in a patient with coronary spasm. N Engl J Med. (1985) 313:1138–41. 10.1056/NEJM198510313131807
20.
Dvorak AM . Mast-cell degranulation in human hearts. N Engl J Med. (1986) 315:969–70. 10.1056/nejm198610093151515
21.
Marone G De Crescenzo G Adt M Patella V Arbustini E Genovese A . Immunological characterization and functional importance of human heart mast cells. Immunopharmacology. (1995) 31:1–18. 10.1016/0162-3109(95)00037-3
22.
Reid AC Silver RB Levi R . Renin: at the heart of the mast cell. Immunol Rev. (2007) 217:123–40. 10.1111/j.1600-065X.2007.00514.x
23.
Patella V Marinò I Lampärter B Arbustini E Adt M Marone G . Human heart mast cells. Isolation, purification, ultrastructure, and immunologic characterization. J Immunol. (1995) 154:2855–65. 10.4049/jimmunol.154.6.2855
24.
Smorodinova N Bláha M Melenovský V Rozsívalová K Přidal J Ďurišová M et al Analysis of immune cell populations in atrial myocardium of patients with atrial fibrillation or sinus rhythm. PLoS One. (2017) 12:e0172691. 10.1371/journal.pone.0172691
25.
Bot I de Jager SC Bot M van Heiningen SH de Groot P Veldhuizen RW et al The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circ Res. (2010) 106:89–92. 10.1161/CIRCRESAHA.109.204875
26.
Kovanen PT . Mast cells as potential accelerators of human atherosclerosis-from early to late lesions. Int J Mol Sci. (2019) 20:4479. 10.3390/ijms20184479
27.
Morrey C Brazin J Seyedi N Corti F Silver RB Levi R . Interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunction. J Pharmacol Exp Ther. (2010) 335:76–84. 10.1124/jpet.110.172262
28.
Alfter K von Kügelgen I Haenisch B Frieling T Hülsdonk A Haars U et al New aspects of liver abnormalities as part of the systemic mast cell activation syndrome. Liver Int. (2009) 29:181–6. 10.1111/j.1478-3231.2008.01839.x
29.
Alvarez-Twose I González de Olano D Sánchez-Muñoz L Matito A Esteban-López MI Vega A et al Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J Allergy Clin Immunol. (2010) 125:1269–78. 10.1016/j.jaci.2010.02.019
30.
Jennings S Russell N Jennings B Slee V Sterling L Castells M et al The mastocytosis society survey on mast cell disorders: patient experiences and perceptions. J Allergy Clin Immunol Pract. (2014) 2:70–6. 10.1016/j.jaip.2013.09.004
31.
Paratz ED Khav N Burns AT . Systemic mastocytosis, Kounis syndrome and coronary intervention: case report and systematic review. Heart Lung Circ. (2017) 26:772–8. 10.1016/j.hlc.2016.12.009
32.
Greiner G Sprinzl B Górska A Ratzinger F Gurbisz M Witzeneder N et al Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood. (2021) 137:238–47. 10.1182/blood.2020006157
33.
Kong P Christia P Frangogiannis NG . The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. (2014) 71:549–74. 10.1007/s00018-013-1349-6
34.
Levick SP Widiapradja A . Mast cells: key contributors to cardiac fibrosis. Int J Mol Sci. (2018) 19:231. 10.3390/ijms19010231
35.
Horan RF Austen KF . Systemic mastocytosis: retrospective review of a decade’s clinical experience at the Brigham and women’s hospital. J Invest Dermatol. (1991) 96(3 Suppl):S5–14. 10.1111/1523-1747.ep12468899
36.
Valabhji J Robinson S Johnston D Bellamy M Davies W Bain BJ . Unexplained loss of consciousness: systemic mastocytosis. J R Soc Med. (2000) 93:141–2. 10.1177/014107680009300309
37.
Crawhall JC Wilkinson RD . Systemic mastocytosis: management of an unusual case with histamine (H1 and H2) antagonists and cyclooxygenase inhibition. Clin Invest Med. (1987) 10:1–4.
38.
Bazan-Socha S Rudzki Z Maciejewicz J Witkoś T Szczeklik A . Clinical variability in two cases of systemic mastocytosis. Pol Arch Med Wewn. (2001) 105:311–5.
39.
Ricciardi L Saitta S Isola S Bonanno D Quattrocchi P Giannetto L et al Systemic mastocytosis associated with recurrent paroxysmal atrial fibrillation. Allergy. (2005) 60:542–3. 10.1111/j.1398-9995.2005.00592.x
40.
Rohr SM Rich MW Silver KH . Shortness of breath, syncope, and cardiac arrest caused by systemic mastocytosis. Ann Emerg Med. (2005) 45:592–4. 10.1016/j.annemergmed.2005.02.002
41.
Molderings GJ Brettner S Homann J Afrin LB . Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. (2011) 4:10. 10.1186/1756-8722-4-10
42.
Ridolo E Triggiani M Montagni M Olivieri E Ticinesi A Nouvenne A et al Mastocytosis presenting as cardiac emergency. Intern Emerg Med. (2013) 8:749–52. 10.1007/s11739-013-1012-0
43.
Hackenthal E Hackenthal R Hilgenfeldt U . Isorenin, pseudorenin, cathepsin D and renin. A comparative enzymatic study of angiotensin-forming enzymes. Biochim Biophys Acta, Enzymol. (1978) 522:574–88. 10.1016/0005-2744(78)90089-x
44.
Moyazaki M Takai S Jin D Muramatsu M . Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. (2006) 112:668–76. 10.1016/j.pharmthera.2006.05.008
45.
Molderings GJ Likungu J Hentrich F Göthert M . Facilitatory presynaptic angiotensin receptors on the sympathetic nerves of the human saphenous vein and pulmonary artery. Potential involvement in ß-adrenoceptor-mediated facilitation of noradrenaline release. Naunyn-Schmiedeberg’s Arch Pharmacol. (1988) 338:228–33. 10.1007/BF00173392
46.
Iravanian S Dudley SC Jr . The renin–angiotensin–aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm. (2008) 5:S12–7. 10.1016/j.hrthm.2008.02.025
47.
Gergs U Weisgut J Griethe K Mißlinger N Kirchhefer U Neumann J . Human histamine H2 receptors can initiate cardiac arrhythmias in a transgenic mouse. Naunyn-Schmiedeberg’s Arch Pharmacol. (2021) 394:1963–73. 10.1007/s00210-021-02098-y
48.
Vigorito C Russo P Picotti GB Chiariello M Poto S Marone G . Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol. (1983) 5:531–7. 10.1097/00005344-198307000-00004
49.
Wolff AA Levi R . Histamine and cardiac arrhythmias. Circ Res. (1986) 58:1–16. 10.1161/01.res.58.1.1
50.
Heavey DJ Lumley P Barrow SE Murphy MB Humphrey PPA Dollery CT . Effects of intravenous infusions of prostaglandin D2 in man. Prostaglandins. (1984) 28:755–67. 10.1016/0090-6980(84)90033-9
51.
Salvucci F Codella R Coppola A Zacchei I Grassi G Anti ML et al Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation. Front Cardiovasc Med. (2023) 10:1202696. 10.3389/fcvm.2023.1202696
52.
Bucchi A Baruscotti M DiFrancesco D . Current-dependent block of rabbit sino-atrial node I(f) channels by ivabradine. J Gen Physiol. (2002) 120:1–13. 10.1085/jgp.20028593
53.
Joannides R Moore N Iacob M Compagnon P Lerebours G Menard JF et al Comparative effects of ivabradine, a selective heart rate-lowering agent, and propranolol on systemic and cardiac haemodynamics at rest and during exercise. Br J Clin Pharmacol. (2006) 61:127–37. 10.1111/j.1365-2125.2005.02544.x
54.
Zellerhoff S Hinterseer M Felix Krull B Schulze-Bahr E Fabritz L Breithardt G et al Ivabradine in patients with inappropriate sinus tachycardia. Naunyn-Schmiedeberg’s Arch Pharmacol. (2010) 382:483–6. 10.1007/s00210-010-0565-y
55.
Peachell P . Regulation of mast cells by β-agonists. Clin Rev Allergy Immunol. (2006) 31:131–41. 10.1385/CRIAI:31:2:131
56.
Wang XS Lau HYA . β-adrenoceptor-mediated inhibition of mediator release from human peripheral blood-derived mast cells. Clin Exp Pharmacol Physiol. (2006) 33:746–50. 10.1111/j.1440-1681.2006.04435.x
57.
Nassiri M Babina M Dölle S Edenharter G Ruëff F Worm M . Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. (2015) 135:491–9. 10.1016/j.jaci.2014.09.004
58.
Butterfield JH Weiler CR . Presentation of untreated systemic mastocytosis as recurrent, pulseless-electrical-activity cardiac arrests resistant to cardiac pacemaker. Int Arch Allergy Immunol. (2014) 163:130–4. 10.1159/000356487
59.
Thomas D Dragodanne C Frank R Prier A Chomette G Grosgogeat Y . Systemic mastocytosis with myo-pericardial localization and atrioventricular block. Arch Mal Coeur Vaiss. (1981) 74:215–21.
60.
Batlle M Pérez-Villa F Lázaro A Garcia-Pras E Ramirez J Ortiz J et al Correlation between mast cell density and myocardial fibrosis in congestive heart failure patients. Transplant Proc. (2007) 39:2347–9. 10.1016/j.transproceed.2007.06.047
61.
Cohen SS Skovbo S Vestergaard H Kristensen T Møller M Bindslev-Jensen C et al Epidemiology of systemic mastocytosis in Denmark. Br J Haematol. (2014) 166:521–8. 10.1111/bjh.12916
62.
Kolck UW Alfter K Homann J von Kügelgen I Molderings GJ . Cardiac mast cells: implications for heart failure. J Am Coll Cardiol. (2007) 49:1107. 10.1016/j.jacc.2006.12.018
63.
Nagueh SF Appleton CP Gillebert TC Marino PN Oh JK Smiseth OA et al Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. (2009) 10:165–93. 10.1093/ejechocard/jep007
64.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M . ESC Scientific Document Group. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2024) 26:5–17. 10.1002/ejhf.3024
65.
Travis WD Li CY Bergstralh EJ Yam LT Swee RG . Systemic mast cell disease. Analysis of 58 cases and literature review. Medicine (Baltimore). (1988) 67:345–68.
66.
Butterfield JH . Survey of aspirin administration in systemic mastocytosis. Prostaglandins Other Lipid Mediators. (2009) 88:122–4. 10.1016/j.prostaglandins.2009.01.001
67.
Ono E Taniguchi M Mita H Fukutomi Y Higashi N Miyazaki E et al Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin Exp Allergy. (2009) 39:72–80. 10.1111/j.1365-2222.2008.03104.x
68.
De Vecchis R Baldi C Di Biase G Ariano C Cioppa C Giasi A et al Cardiovascular risk associated with celecoxib or etoricoxib: a meta-analysis of randomized controlled trials which adopted comparison with placebo or naproxen. Minerva Cardioangiol. (2014) 62:437–48.
69.
Carter MC Robyn JA Bressler PB Walker JC Shapiro GG Metcalfe DD . Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. (2007) 119:1550–1. 10.1016/j.jaci.2007.03.032
70.
Kontou-Fili K Filis CI Voulgari C Panayiotidis PG . Omalizumab monotherapy for bee sting and unprovoked “anaphylaxis” in a patient with systemic mastocytosis and undetectable specific IgE. Ann Allergy Asthma Immunol. (2010) 104:537–9. 10.1016/j.anai.2010.04.011
71.
Bell MC Jackson DJ . Prevention of anaphylaxis related to mast cell activation syndrome with omalizumab. Ann Allergy Asthma Immunol. (2012) 108:383–4. 10.1016/j.anai.2012.02.021
72.
Kibsgaard L Skjold T Deleuran M Vestergaard C . Omalizumab induced remission of idiopathic anaphylaxis in a patient suffering from indolent systemic mastocytosis. Acta Derm Venereol. (2014) 94:363–4. 10.2340/00015555-1687
73.
Jagdis A Vadas P . Omalizumab effectively prevents recurrent refractory anaphylaxis in a patient with monoclonal mast cell activation syndrome. Ann Allergy Asthma Immunol. (2014) 113:115–6. 10.1016/j.anai.2014.05.001
74.
Roberts LJ Oates JA . Biochemical diagnosis of systemic mast cell disorders. J Invest Dermatol. (1991) 96(3 Suppl):S19–24. 10.1111/1523-1747.ep12468945
75.
Lindstedt KA Mäyränpää MI Kovanen PT . Mast cells in vulnerable atherosclerotic plaques—a view to a kill. J Cell Mol Med. (2007) 11:739–58. 10.1111/j.1582-4934.2007.00052.x
76.
Elieh-Ali-Komi D Bot I Rodríguez-González M Maurer M . Cellular and molecular mechanisms of mast cells in atherosclerotic plaque progression and destabilization. Clin Rev Allergy Immunol. (2024) 66:30–49. 10.1007/s12016-024-08981-9
77.
Lagraauw HM Wezel A van der Velden D Kuiper J Bot I . Stress-induced mast cell activation contributes to atherosclerotic plaque destabilization. Sci Rep. (2019) 9:2134. 10.1038/s41598-019-38679-4
78.
Wu Y Xu Y Xu L . Pharmacological therapy targeting the immune response in atherosclerosis. Int Immunopharmacol. (2024) 141:112974. 10.1016/j.intimp.2024.112974
79.
Indhirajanti S van Daele PLA Bos S Mulder MT Bot I Roeters van Lennep JE . Systemic mastocytosis associates with cardiovascular events despite lower plasma lipid levels. Atherosclerosis. (2018) 268:152–6. 10.1016/j.atherosclerosis.2017.11.030
80.
Ward BR Schwartz LB . Systemic mastocytosis presenting as Prinzmetal (variant) angina. J Allergy Clin Immunol. (2011) 127(Suppl 2):AB188.
81.
Fassio F Almerigogna F . Kounis syndrome (allergic acute coronary syndrome): different views in allergologic and cardiologic literature. Intern Emerg Med. (2012) 7:489–95. 10.1007/s11739-012-0754-4
82.
González-de-Olano D Matito A Sánchez-López P Sánchez-Muñoz L Morgado JM Teodósio C et al Mast cell-related disorders presenting with Kounis syndrome. Int J Cardiol. (2012) 161:56–8. 10.1016/j.ijcard.2012.06.041
83.
Kounis NG Koniari I Velissaris D Tzanis G Hahalis G . Kounis syndrome—not a single-organ arterial disorder but a multisystem and multidisciplinary disease. Balkan Med J. (2019) 36:212–21. 10.4274/balkanmedj.galenos.2019.2019.5.62
84.
Raithel M Homann J Rieker RJ Molderings GJ . Gastrointestinal manifestations of systemic mast cell activation disease—a practice-oriented guide to clinical picture, diagnostics and therapy. Z Gastroenterol. (2025) 63:155–68. 10.1055/a-2468-5553
85.
Seidel H Hertfelder HJ Oldenburg J Kruppenbacher JP Afrin LB Molderings GJ . Effects of primary mast cell disease on hemostasis and erythropoiesis. Int J Mol Sci. (2021) 22:8960. 10.3390/ijms22168960
86.
Devgire V Hughes M . Raynaud’s phenomenon. Br J Hosp Med. (2019) 80:658–64. 10.12968/hmed.2019.80.11.658
87.
Sido B Homann J Hertfelder HJ Zienkiewicz T Christians KP Schablin P et al Surgical interventions in patients with systemic mast cell activation disease: recommendations for perioperative management. Chirurg. (2019) 90:548–56. 10.1007/s00104-019-0935-z
88.
Knüpfer HE Keppler V Zienkiewicz T Molderings GJ . Cystic diseases in urology: recommendations for patients with systemic mast cell disease. Urologie. (2022) 61:1115–21. 10.1007/s00120-022-01841-4
89.
Kolck UW Haenisch B Molderings GJ . Cardiovascular symptoms in patients with systemic mast cell activation disease. Transl Res. (2016) 174:23–32. 10.1016/j.trsl.2015.12.012
90.
Kritikou E Kuiper J Kovanen PT Bot I . The impact of mast cells on cardiovascular diseases. Eur J Pharmacol. (2016) 778:103–15. 10.1016/j.ejphar.2015.04.050
91.
Kennedy S Wu J Wadsworth RM Lawrence CE Maffia P . Mast cells and vascular diseases. Pharmacol Ther. (2013) 138:53–65. 10.1016/j.pharmthera.2013.01.001
92.
Yang MQ Ma YY Ding J Li JY . The role of mast cells in ischemia and reperfusion injury. Inflammation Res. (2014) 63:899–905. 10.1007/s00011-014-0763-z
93.
Sun K Li YY Jin J . A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduction Targeted Ther. (2021) 6:79. 10.1038/s41392-020-00455-6
94.
Silver RB Reid AC Mackins CJ Askwith T Schaefer U Herzlinger D et al Mast cells: a unique source of renin. Proc Natl Acad Sci U S A. (2004) 101:13607–12. 10.1073/pnas.0403208101
Summary
Keywords
mast cell activation disease, systemic mastocytosis, mast cell activation syndrome, mast cell mediator release syndrome, mast cell mediators, cardiovascular symptoms
Citation
Taumann W and Molderings GJ (2025) Cardiovascular manifestations in mast cell activation disease: key insights for cardiologists and angiologists. Front. Cardiovasc. Med. 12:1705201. doi: 10.3389/fcvm.2025.1705201
Received
14 September 2025
Accepted
14 October 2025
Published
10 November 2025
Volume
12 - 2025
Edited by
Michiaki Nagai, Hiroshima Asa Medical Association Hospital, Japan
Reviewed by
Fabrizio Salvucci, Istituto di Medicina Biologica (ImBio), Italy
Pengxin Xie, Peking University Third Hospital, China
Rodica Radu, Grigore T. Popa University of Medicine and Pharmacy, Romania
Beliz Karataş, Erzincan Universitesi Tip Fakultesi, Türkiye
Updates
Copyright
© 2025 Taumann and Molderings.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Gerhard J. Molderings molderings@uni-bonn.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.