Abstract
This case report describes a young male patient admitted due to worsening of heart failure with recurrent embolic events. He had previously been diagnosed with hypertrophic cardiomyopathy (HCM) and left ventricular systolic dysfunction at other hospitals and was treated irregularly with medications including a beta-blocker and diuretics. On this admission, echocardiography and cardiac MR examination revealed left ventricular myocardial non-compaction combined with HCM. The coexistence of left ventricular hypertrophy and hypertrabeculation is rare, and the diagnosis has been updated according to the newly issued European Society of Cardiology guidelines. The present report describes the clinical features, therapy, and outcome of this disease.
1 Introduction
Previous cases demonstrated that both hypertrophic cardiomyopathy (HCM) and left ventricular non-compaction (LVNC) can rarely co-exist in the same patient (1–4). The mixed phenotype (HCM and LVNC) is associated with a more severe clinical course of the disease and worse cardiovascular complications (5). This case report describes a young male patient with HCM and hypertrabeculation.
2 Case presentation
A 39-year-old male patient was admitted to our hospital in April 2022 due to repeated chest tightness and shortness of breath for 5 years, aggravated for 20 days. He presented previous echocardiography examinations in other hospitals at admission, all indicated non-obstructive HCM, reduced left ventricular ejection fraction (the lowest was 12%), and enlarged left ventricle. He was repeatedly hospitalized and treated with irregular oral administration of a beta-blocker and diuretics before this admission. The previous electrocardiogram featured with sinus rhythm, frequent ventricular premature beats with short bursts of ventricular tachycardia, intraventricular conduction block, and occasional atrial premature beats. He was diagnosed with acute renal infarction in December 2020, acute hemorrhagic cerebral infarction in June 2021, and right middle cerebral artery occlusion and left middle cerebral artery inferior trunk occlusion in January 2022. He underwent emergency thrombectomy for an embolic event in 2022 and irregularly took oral warfarin after the first embolic event.
He was an ex-smoker with more than 10 years of smoking history, smoking approximately 10 cigarettes per day. He denied a history of hypertension, diabetes, and excessive alcohol exposure. There was no similar case in his family.
Physical examination at admission: a few moist rales were heard in the right lower lung, heart rate was 66 beats/min, a systolic murmur of grade 2/6 was heard in the apex of the heart, and there was no edema in either lower limb. Troponin I was 0.51 ng/mL, NT-pro BNP was 17,500 pg/mL, and D-dimer was 932 µg/L.
Electrocardiogram: sinus rhythm, high voltage in left ventricle, high voltage in right ventricle, premature ventricular beats, intraventricular conduction block, short P–R interval, abnormal ST segment and T wave changes. Chest CT scan showed the following: (1) interstitial pulmonary edema in both lungs, scattered cord foci in both lungs; (2) enlarged heart shadow, pericardial effusion, small amount of pleural effusion on both sides, and incomplete expansion of adjacent lung tissue, especially on the right side. Coronary angiography showed no obvious stenosis of the coronary artery.
Echocardiography: myocardial involvement [interventricular septum (IVS): 2.16 cm, left ventricular posterior wall (LVPW): 2.39 cm], left ventricular enlargement (LV: 6.6 cm), mild to moderate mitral regurgitation, mild tricuspid regurgitation, mild pulmonary hypertension, significantly reduced left ventricular function (EF: 20%), and a small amount of pericardial effusion. Significant trabeculae in the middle segment of the left ventricular free wall and the apical segment of the left ventricular wall were visualized, showing a “honeycomb-like” change. The ratio of the thickness of the non-compacted myocardium (∼16 mm) to the thickness of the compacted myocardium (∼8 mm) at the end of contraction was ∼2:1. Color Doppler ultrasound detected low-speed blood flow in the gaps between the myocardial recesses that communicated with the left heart cavity (Figure 1).
Figure 1
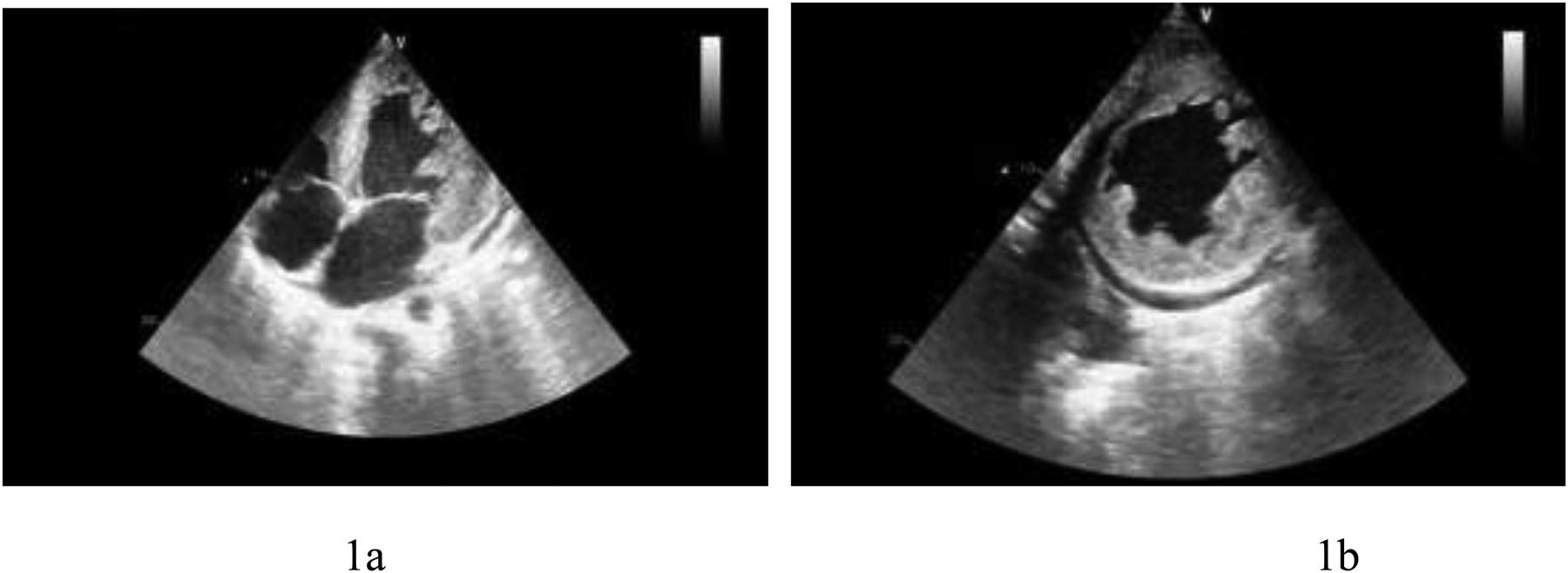
(a) Left ventricular apical four-chamber view showing left ventricular thickening and hypertrabeculation. (b) Left ventricular short-axis view presenting left ventricular thickening and hypertrabeculation.
Cardiac magnetic resonance (CMR) plain scan + enhanced scan showed the following: (1) left ventricular dilatation with decreased systolic function (EF 19%) thinning of the left ventricular free wall and apex, weakened movement, and increased and disordered trabeculae, considering LVNC; late enhancement of the left ventricular anterior wall, lateral wall, inferior wall, and apex, suggesting myocardial fibrosis or scar; (2) thickening of the left ventricular septum and right ventricular myocardium, with a high possibility of HCM; and (3) pericardial effusion (Figure 2).
Figure 2
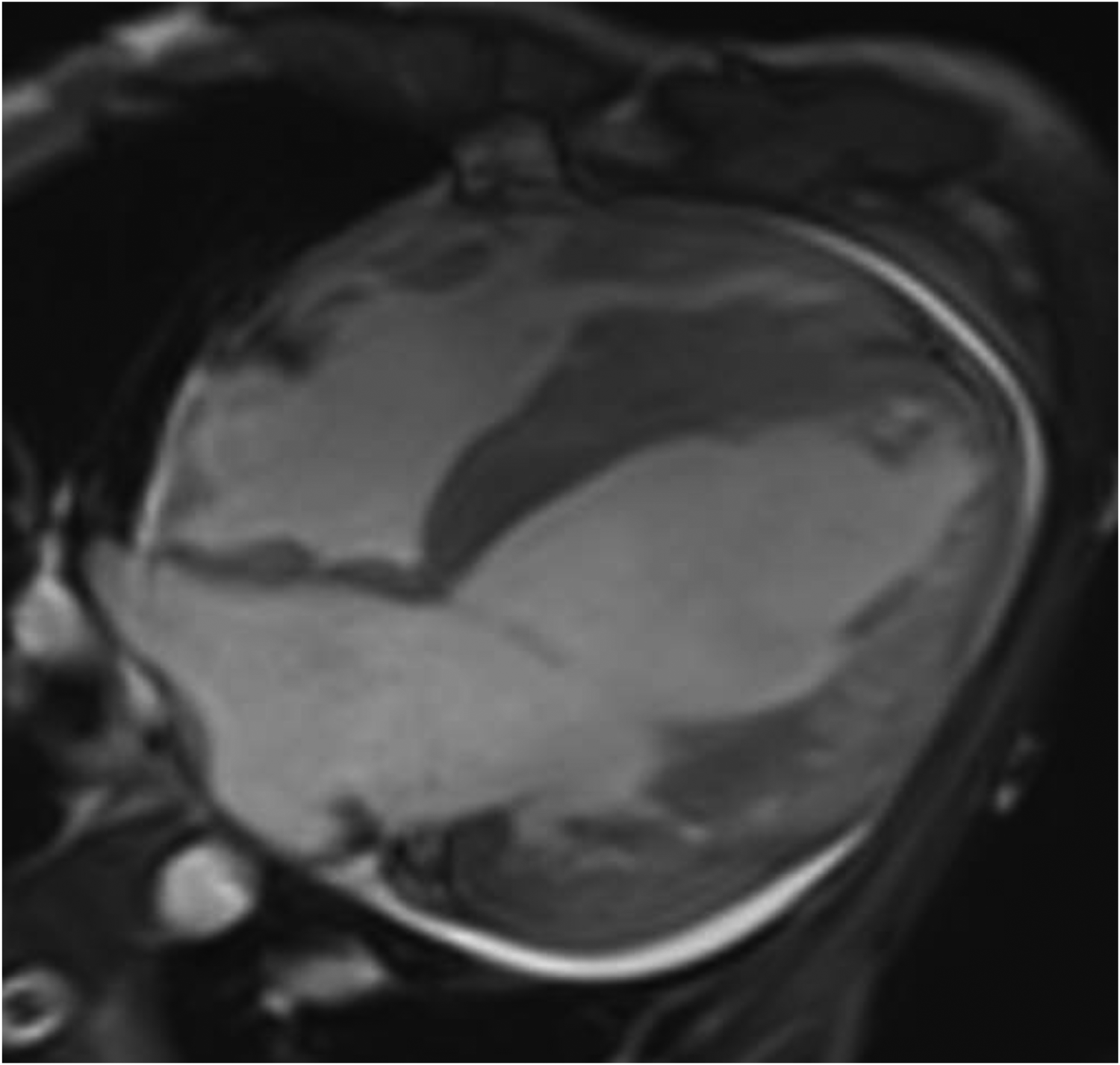
CMR four-chamber heart long axis: left ventricular dilatation, thinning of left ventricular free wall and apex, with increased and disordered trabeculae, presenting a spongy appearance.
The patient was diagnosed as non-obstructive HCM combined with LVNC and received recommended medication as recommended by the 2021 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure (6) and the 2014 ESC guidelines on diagnosis and management of HCM (7). The patient was discharged after symptom relief. The patient was at high risk of sudden cardiac death. Medication at discharge was as follows: metoprolol 47.5 mg qd, canagliflozin 100 mg qd, sacubitril/valsartan 50 mg bid, spironolactone 20 mg bid, furosemide 20 mg qd, and rivaroxaban 15 mg qd. Despite these medications, the patient died of sudden cardiac death 6 months later.
3 Discussion
LVNC and HCM are cardiomyopathies with distinct clinical presentations but may present common genetic mutations (4). Coexistence of HCM and LVNC is rare (8). Before the 2023 ESC guidelines for the management of cardiomyopathies, developed by the task force on the management of cardiomyopathies of the ESC (9), patient with this clinical phenotype was often diagnosed as HCM and LVNC. Now, this disease phenotype is named as HCM with hypertrabeculation (1).
HCM is often manifested as hypertrophy of the ventricular septum, while this case presented both hypertrophy and hypertrabeculation. Detraining and CMR can be used to differentiate cardiomyopathies from athlete's heart (10–12) (Figure 3). Despite standard medication at that time, and before the era of cardiac myosin inhibitors (13), the patient suffered sudden cardiac death within 6 months after discharge. Neither genetic studies nor an autopsy were performed on the patient. These missing data served as a significant limitation of the current report for the understanding of the underlying pathogenetic background of this patient.
Figure 3

Differentiating an athlete's heart from HCM.
It remains unknown if this patient with non-obstructive HCM might benefit from the cardiac myosin inhibitor therapy on top of the medication received. The cause of death might be related to malignant ventricular arrhythmias. It remained unknown if this patient might benefit from implantable cardioverter defibrillator (ICD) therapy or arrhythmia ablation. Nowadays, therapy options for HCM have been significantly expanded (14), especially for obstructive HCM. It is expected that more therapeutic options might also be available for patients with non-obstructive HCM and hypertrabeculation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Zhongda Hospital, Southeast University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YLi: Writing – original draft, Data curation, Conceptualization. YLiu: Writing – original draft, Data curation. XZ: Data curation, Writing – original draft. LC: Writing – review & editing, Supervision. QD: Supervision, Writing – review & editing. GM: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Gabra B Anavekar NS Haaf P . Hypertrophic cardiomyopathy and left ventricular non-compaction cardiomyopathy: two in one. Eur Heart J. (2023) 44(4):327. 10.1093/eurheartj/ehac718
2.
Cirillo C Monda E Dellegrottaglie S Scatteia A Limongelli G . A case report of mixed left ventricular non-compaction/hypertrophic cardiomyopathy phenotype in a child. Eur Heart J Case Rep. (2025) 9(2):ytaf051. 10.1093/ehjcr/ytaf051
3.
Kochanska S Spalek M Wrobel G Korzeluch W Michalowska I Kuder T et al Coexistence of hypertrophic cardiomyopathy and left ventricular non-compaction cardiomyopathy—a description of two cases. Quant Imaging Med Surg. (2022) 12(7):4016–21. 10.21037/qims-21-730
4.
Hotta VT Monge NMS Fernandes F Arteaga-Fernandez E . Rare association of left ventricular non-compaction and hypertrophic cardiomyopathy. Eur Heart J Case Rep. (2020) 4(3):1–2. 10.1093/ehjcr/ytaa109
5.
Komissarova SM Rineiska NM Chakova NN Niyazova SS . Overlapping phenotype: left ventricular non-compaction and hypertrophic cardiomyopathy. Kardiologiia. (2020) 60(4):137–45. 10.18087/cardio.2020.4.n728
6.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Bohm M et al 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. 10.1093/eurheartj/ehab368
7.
Authors/Task Force members, ElliottPMAnastasakisABorgerMABorggrefeMCecchiFCharronPet al2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. (2014) 35(39):2733–79. 10.1093/eurheartj/ehu284
8.
Goncalves L Pires I Santos J Correia J Neto V Moreira D et al One genotype, two phenotype: hypertrophic cardiomyopathy with left ventricular non-compaction. Cardiol J. (2022) 29(2):366–7. 10.5603/Cj.2022.0020
9.
Arbelo E Protonotarios A Gimeno JR Arbustini E Barriales-Villa R Basso C et al 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J. (2023) 44(37):3503–626. 10.1093/eurheartj/ehad194
10.
Mascia G Olivotto I Brugada J Arbelo E Di Donna P Della Bona R et al Sport practice in hypertrophic cardiomyopathy: running to stand still? Int J Cardiol. (2021) 345:77–82. 10.1016/j.ijcard.2021.10.013
11.
Zujko-Kowalska K Kaminski KA Malek L . Detraining among athletes—is withdrawal of adaptive cardiovascular changes a hint for the differential diagnosis of physically active people?J Clin Med. (2024) 13(8:2343. 10.3390/jcm13082343
12.
Malek LA Milosz-Wieczorek B Marczak M . Diagnostic yield of cardiac magnetic resonance in athletes with and without features of the athlete’s heart and suspected structural heart disease. Int J Environ Res Public Health. (2022) 19(8):4829. 10.3390/ijerph19084829
13.
Lim J Kim HK . Cardiac myosin inhibitors in hypertrophic cardiomyopathy. J Cardiovasc Imaging. (2025) 33(1):7. 10.1186/s44348-025-00052-7
14.
Sikand N Stendahl J Sen S Lampert R Day S. Current management of hypertrophic cardiomyopathy. Br Med J (2025) 389:e077274. 10.1136/bmj-2023-077274
Summary
Keywords
left ventricular non-compaction/hypertrabeculation, hypertrophic cardiomyopathy, heart failure, cardiac MR, cardiomyopathy
Citation
Li Y, Liu Y, Zheng X, Chen L, Dai Q and Ma G (2025) Case Report: Hypertrophic cardiomyopathy meets left ventricular hypertrabeculation: from mixed cardiomyopathy to hypertrophic cardiomyopathy with hypertrabeculation. Front. Cardiovasc. Med. 12:1708374. doi: 10.3389/fcvm.2025.1708374
Received
18 September 2025
Revised
15 October 2025
Accepted
05 November 2025
Published
26 November 2025
Volume
12 - 2025
Edited by
Maryanne Caruana, University of Malta, Malta
Reviewed by
Giuseppe Mascia, University of Genoa, Italy
Tiziana Felice, Mater Dei Hospital, Malta
Updates
Copyright
© 2025 Li, Liu, Zheng, Chen, Dai and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Qiming Dai 13815879581@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.