- 1Division of Cardiology, Cape Heart Institute, University of Cape Town, Cape Town, South Africa
- 2SAMRC Extramural Unit on Intersection of Noncommunicable Diseases and Infectious Diseases, University of Cape Town, Cape Town, South Africa
- 3Department of Virology, School of Pathology, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa
- 4Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa
Hypertension (HTN) is a chronic medical condition characterized by systolic blood pressure of ≥140 mmHg and diastolic blood pressure >80 mmHg upon repeated measurements. It is one of the most common non-communicable diseases affecting 30% of the global population. Sub-Saharan Africa (SSA) has a high burden of infectious diseases, which contribute to the increased prevalence of hypertension. Furthermore, SSA has the highest number of people living with chronic infectious diseases, such as human immunodeficiency virus (HIV) and tuberculosis (TB). The pathogenesis of these conditions is associated with chronic, low-grade inflammation and immune activation that complicates various homeostatic functions, leading to increased risk of non-communicable diseases among this population. Furthermore, persistent immune activation leads to endothelial dysfunction, arterial stiffness, and altered vascular tone, which contribute to elevated and treatment-refractory blood pressure. However, immunological factors that contribute to the development and pathogenesis of hypertension remain poorly understood. Antiretroviral therapy and anti-TB medications further complicate this landscape by inducing metabolic disturbances and modulating drug metabolism, which affects the efficacy of anti-hypertensive medications. There is a paucity of data and studies reporting on immune dysregulation associated with HTN amongst people living with chronic infections such as HIV and TB. This review aims to highlight this gap in knowledge and the need for more translational research studies to improve health outcomes in hypertensive individuals living with HIV and TB in SSA. Understanding these intertwined immunological and pathophysiological mechanisms is crucial to developing targeted interventions for managing HTN, especially in this vulnerable population.
1 Introduction
Hypertension (HTN) is a chronic, cardiovascular condition in which the systolic blood pressure is ≥140 mmHg and/or diastolic blood pressure is >80 mmHg in the arteries upon repeated measurements (1). It is one of the major risk factors for morbidity and mortality, as it contributes to the development of cardiovascular conditions such as heart failure and stroke (2–5). It is one of the most common non-communicable diseases affecting 30% of the global population (6). In 2023, the World Health Organisation (WHO) reported that only 21% of adults with hypertension had their blood pressure controlled (7). The prevalence of hypertension in sub-Saharan Africa (SSA) increased significantly between the years 1999–2019 (8), reaching 34% in men and 48% in women (8, 9). Numerous factors which include increased burden of infectious disease, changes in diet, lifestyle and poor healthcare are contributing factors to this (8, 10–13). Key factors associated with the high prevalence of hypertension and its disparities across sub-Saharan Africa include dietary patterns (8, 14–16), abnormal body weight (both increased adiposity and underweight) (8, 17–19), population ageing (8, 18, 20–22), socioeconomic status (education and income), and psychosocial stressors (8, 14, 17, 20, 21). Infectious diseases such as coronavirus disease 2019 (COVID-19) (23, 24), human immunodeficiency virus (HIV) (25, 26) and tuberculosis (TB) (27) have also been implicated in the development of hypertension. Resistant hypertension (RH) is defined as BP that remain elevated despite the concurrent administration of three or more antihypertensive agents of different classes, including a diuretic (28). The exact aetiology of resistant hypertension is unknown; however, it is postulated to be associated with multiple factors such inappropriate treatment and limited access to healthcare, high-salt diet, volume overload and hyperactivation of the sympathetic nervous system (29, 30).
The highest number of people living with chronic infections such as HIV and TB is in SSA (31, 32). People living with HIV (PLWHIV) and TB are at an increased risk of developing hypertension (33, 34) (Table 1). The high burden of chronic infections in SSA poses a significant public health challenge as weakens the already under-resourced healthcare system and increases the risk of non-communicable disease in the population (34–37). Underlying conditions such as HTN pose a significant public health challenge around the world (33, 34, 38). Furthermore, the mechanisms affecting pathogenesis of infectious disease in people living with chronic infections remains poorly understood. Therefore, there is a paucity of data and research studies on immune mechanisms associated with development of hypertension amongst people living with chronic infections such as HIV and TB. This review aims to highlight this gap in knowledge and the need for more translational research studies to improve health outcomes in hypertensive individuals living with HIV and TB in SSA. Understanding these intertwined immunological and pathophysiological mechanisms is crucial to developing targeted interventions for managing HTN, especially among this vulnerable population.
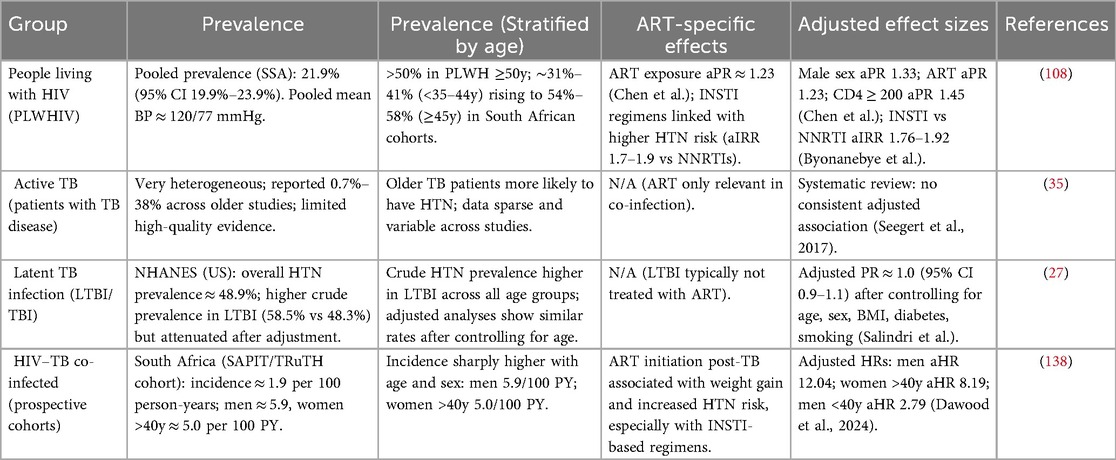
Table 1. Prevalence of HTN amongst different population groups living with chronic infections in SSA.
2 Inflammation and hypertension
Inflammation plays a crucial role in the pathogenesis of hypertension (39–42). It has been shown that T lymphocytes are required for the full development of angiotensin II– and DOCA-salt–induced hypertension and vascular dysfunction in mice; T cells (via AT1 receptor and NADPH oxidase–dependent ROS and cytokine production) infiltrate perivascular tissue and drive vascular oxidative stress, endothelial dysfunction and blood-pressure elevation (43). Immune activation in hypertension is kick-started by well-known pro-hypertensive stimuli such as, increased sympathetic outflow (44, 45), acute and chronic stress (46), excessive salt intake (47, 48), gut microbial dysbiosis (49, 50), local oxidative-stress (51) and vascular dysfunction (52). A key concept is that immune cells infiltrate organs critical to blood pressure regulation, such as the blood vessels (53), kidneys, heart (54), and brain, leading to chronic inflammation (43, 52, 55, 56). This chronic inflammatory state disrupts normal organ function and contributes to the onset and progression of hypertension (52). The inflammatory and immune mechanisms driving hypertension are regulated through a complex interaction between innate and adaptive immune cells (Figure 1) (52). The early development of hypertension involves activation of innate immune cells, such as macrophages, dendritic cells, and natural killer (NK) cells, which initiate inflammatory responses by releasing cytokines and chemokines (51, 52, 57). These signalling molecules facilitate the recruitment of additional immune cells, including T and B lymphocytes, to the site of inflammation (52). Once activated, T cells can differentiate into effector subsets; such as T helper 1 (Th1) and Th17 cells; which release cytokines that amplify the inflammatory response and contribute to vascular dysfunction (52, 58, 59).
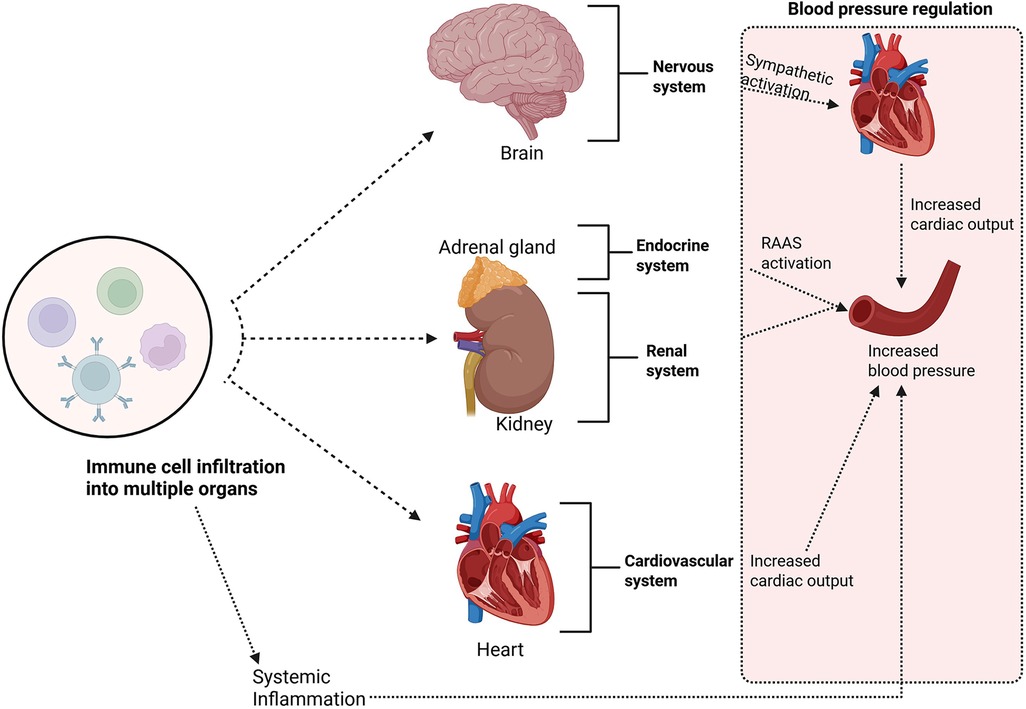
Figure 1. Immune cells infiltrate organs critical to blood pressure regulation, such as the blood vessels, kidneys, heart, and brain; leading to chronic inflammation. This chronic inflammatory state disrupts normal organ function and contributes to the onset and progression of hypertension. Created using Bio-render.
3 Immunity and hypertension
Hypertension has long been associated with activation of immune pathways (2), here we discuss the cells of the innate and adaptive immune system and their role in hypertension development. Dendritic cells (DCs) are specialised antigen-presenting cells (APCs) derived from hematopoietic progenitors in the bone marrow and are widely distributed across various tissues (60, 61). They possess a unique capacity to initiate T cell responses by capturing, processing, and presenting antigens to naïve T cells. Therefore, DCs serve as key orchestrators of the adaptive immune system (60, 61). DCs from hypertensive mice exhibit elevated surface expression of the B7 ligands CD80 and CD86, indicative of DC maturation and activation (51, 62). Inhibition of these costimulatory molecules has been shown to prevent the development of hypertension and the activation of T cells in models of both angiotensin II- and DOCA-salt induced hypertension (51, 62). In mice, oxidative stress in hypertension generates reactive γ-ketoaldehydes known as isoketals, which accumulate in and activate DCs to drive T-cell responses that contribute to elevated blood pressure (51). Plasma levels of F2-isoprostanes, oxidative stress markers produced alongside isoketals, were elevated in individuals receiving treatment for hypertension and significantly higher in those with resistant hypertension (51). Additionally, isoketal-modified proteins were notably increased in circulating monocytes and dendritic cells from hypertensive patients (51). These findings suggest a positive feed-forward loop between hypertension and DCs.
Over the years, monocytes and macrophages have been associated with hypertension development (55). This is evidenced by notable increases in their numbers and alterations in their phenotype observed within the vasculature, kidneys, heart, and brain across various models of hypertension, in comparison to normotensive controls (55, 63–68). In humans, macrophage subsets are grouped by the expression of CD14 and CD16. This breaks the macrophage populations into 3 subsets: CD14++/CD16−−(“classical monocytes or macrophages”), CD14+/CD16+ or CD14++/CD16+ (“intermediate monocytes or macrophages”), and CD14dim or +/CD16++ (“nonclassical monocytes or macrophages”) (69, 70). CD14+ and CD16+ macrophages are taken to be correlates of the M1 and M2 phenotypes (69). Directly implicating CD14+ human macrophages in the development of clinical hypertension is their robust expression of angiotensin-converting enzyme (69, 71), demonstrating their potential involvement in hypertension through participation in the renin-angiotensin-aldosterone system (RAAS), which may lead to feedforward activation of monocytes and “switching” between the M1 and M2 phenotypes (69). There are less studies on humans but the data that are available support a role for inflammation and macrophage polarization in essential hypertension, as well as cardiovascular disease (69). One study showed that the severity of hypertension was linked to the presence of CD68+ M1 inflammatory macrophages in the kidney, independent of race (69, 72). This indicates a possible role of macrophages in the development of hypertension.
Various subsets of T lymphocytes influence blood pressure regulation by modulating the local cytokine environment within cardiovascular regulatory organs (73). Hematopoietic stem cells in the bone marrow differentiate into naïve T cells which mature in the thymus before entering systemic circulation and migrating to distant tissues (73, 74). Single CD4+ T cells are classified as T helper cells (Th cells), CD8+ T cells are referred to as cytotoxic T cells, and CD1d+ T cells are known as natural killer T cells (73). Once an antigen is presented via the major histocompatibility complex (MHC) and binds to the T cell receptor (TCR) on the naïve CD4+ T cell, the T cell differentiates into distinct T helper (Th) lineages e.g., Th1, Th2, Th17, or T regulatory (Treg) in response to the local concentrations of specific cytokines. These T cell subsets provide helper functions by secreting specific cytokines that target other immune cells and modulate both vascular reactivity and renal sodium handling (73). Immune activation in hypertension is characterised by activation of T-cells via DC activation (75). T-cells then migrate to the vascular tree and the kidney causing inflammation and hypertension therefore, factors effecting T-cell activation and function are important mediators of essential hypertension (75). In an attempt to characterise T cells in newly diagnosed, treatment-naïve hypertensive individuals by assessing circulating levels of C-X-C chemokine receptor type 3 (CXCR3) chemokines, findings revealed that hypertensive patients had a higher proportion of immunosenescent, proinflammatory, and cytotoxic CD8+ T cells compared to healthy controls (76). Similarly in a different study, the frequency of both CD4+ and CD8+ CD45RO+ (memory) circulating T cells was higher in the hypertensive patients than in the normotensive controls (54). Hypertensive patients exhibited a higher frequency of CD4+ 1l-17A+ compared to normotensive controls. Interferon gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) were also increased in CD4+ T cells, and CD8+ T cells of hypertensive patients (54). These results demonstrate the role of T-cell driven inflammation in mediating hypertension.
4 Immunity and HIV/TB
4.1 Immune dysregulation and the human immunodeficiency virus
Human immunodeficiency virus (HIV)) is an infection that targets and damages the immune system, specifically the CD4+ white blood cells that play a crucial role in immune defence (77). For successful entry, the virus must engage both the CD4 receptor and one of two co-receptors on the host cell surface—the C-C chemokine receptor type 5 (CCR5) or the C-X-C chemokine receptor type 4 (CXCR4) (78–80). Beyond CD4+ cells, HIV infects macrophages and dendritic cells, disrupting antigen presentation and cytokine networks (78). By destroying these cells, HIV progressively weakens the body's ability to fight off opportunistic infections such as tuberculosis (77). The combination of persistent viral replication, mucosal barrier breakdown, and innate immune sensing (via Toll-like receptors and inflammasomes) sustains a state of chronic inflammation and immune activation, which paradoxically accelerates immune exhaustion and CD4+ decline despite antiviral responses (81).
Despite effective antiretroviral therapy (ART), immune dysregulation continues as a result of persistent latent viral reservoirs and sustained low-level immune activation (82). T-cell exhaustion, characterized by increased expression of inhibitory receptors such as PD-1 (83, 84), TIM-3, and LAG-3 (84), diminishes HIV-specific immune responses (85), while dysregulated cytokine production drives inflammation and immune activation (86, 87). Chronic inflammation accelerates immunosenescence and promotes the development of non-communicable diseases, including cardiovascular disease and certain cancers. Therefore, immune dysregulation in HIV extends beyond CD4+ T-cell depletion, representing a complex disorder characterized by sustained immune activation, impaired regulatory mechanisms, and incomplete immune recovery, even in the context of effective ART (88, 89).
4.2 Immune activation in Tuberculosis
Tuberculosis (TB) is a chronic infectious disease caused by the bacterium Mycobacterium tuberculosis (M.tb) (90). It primarily affects the lungs (pulmonary TB) but can also involve other organs (extrapulmonary TB) (90). TB is transmitted through airborne particles when an infected person coughs, sneezes, or speaks. Once inhaled, the bacteria can remain dormant (latent TB) or progress to active disease, especially in individuals with weakened immune systems (90). The innate immune system serves as the initial defense against M.tb infection (91). This largely depends on initial interactions with host innate immune cells, such as macrophages, dendritic cells, neutrophils, and natural killer cells (92). Multiple innate-like leukocytes also contribute to the host defense against M.tb, including non-conventional T-cell subsets such as mucosal-associated invariant T (MAIT) cells, CD1-restricted T lymphocytes, and natural killer T (NKT) cells (92). The initiation of innate immune responses to M.tb infection begins with pathogen recognition. During phagocytosis, conserved pathogen-associated molecular patterns (PAMPs) on the M.tb surface are detected by pattern recognition receptors (PRRs) expressed on host immune cells (92). In addition to phagocytosis, autophagy, apoptosis and the inflammasome activation, innate immune cells also trigger inflammatory cytokine and chemokine production to eliminate invading pathogens (93). M.tb manipulates host immune and metabolic pathways to evade clearance and establish persistent infection. Early studies in murine models, subsequently corroborated in humans (94), identified IFN-γ, TNF, and IL-1β as key cytokines required for effective immune control of M.tb (95–98). Signalling through the IL-1 receptor is modulated by the IL-1 receptor antagonist (IL-1ra), which competitively inhibits IL-1 binding (99). Elevated circulating concentrations of IL-1ra have been reported in individuals with active tuberculosis and proposed as a potential biomarker for disease activity and therapeutic response (100). Nonetheless, the molecular mechanisms underlying increased IL-1ra expression during active TB remain poorly understood.
5 Discussion
5.1 Intersection between infectious disease and non-communicable diseases
An estimated 40.8 million people were living with HIV globally at the end of 2024, with only 77% receiving antiretroviral therapy (ART) (36). The SSA region carries the greatest number of PLWHIV, in 2019, approximately 26 million individuals in SSA were living with HIV (32). Moreover, SSA accounted for 670,000 of the 1.5 million new HIV infections and 280,000 of the 650,000 AIDS-related deaths reported globally in 2021 (101). Hypertension is an increasingly common concern among adults living with HIV, particularly those receiving ART (102). While ART has significantly improved survival outcomes for PLWHIV, it has also been associated with an increasing burden of cardiovascular disease (103–106) (Figure 2). The pooled hypertension prevalence in PLWHIV in SSA was 21.9% alongside mean systolic blood pressure/diastolic blood pressure levels of 120/77 mmHg, withs significantly higher hypertension prevalence among males, ART users, and individuals with CD4 counts ≥200 cells/mm3 (107). These results underscore the need for cardiovascular risk integration into HIV care, especially as ART access and life expectancy continue to rise. The underlying mechanisms driving hypertension and cardiovascular disease in PLWHIV remain incompletely understood (103). In one study, the prevalence of hypertension among PLWHIV in SSA varied widely, ranging from 2.0%–50.2%, with most cases observed in individuals receiving ART (103). A retrospective Zambian cohort of PLWHIV initiating ART found that males developed hypertension earlier (2 years) compared to females (6 years) after ART initiation. In multivariable analysis, higher baseline SBP/MAP and use of certain ART (protease inhibitors) predicted incident hypertension in males but not in females (108).
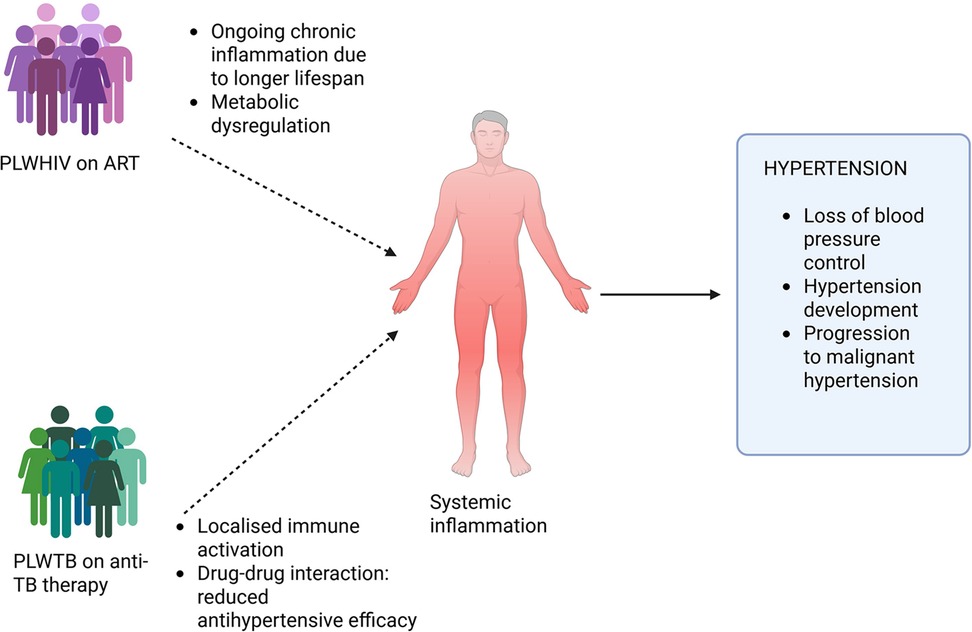
Figure 2. Chronic immune activation, inflammation, endothelial dysfunction, and metabolic derangements are induced by HIV infection, TB, and their respective treatments. ART is associated with metabolic complications which increase the risk of hypertension. Similarly, anti-tuberculosis medications, such as rifampicin, can interfere with the pharmacokinetics of commonly used antihypertensive drugs leading to subtherapeutic drug levels and poor blood pressure control. Created using Bio-render.
Most studies report the prevalence of hypertension in PLWHIV, while data on the detailed mechanisms of how HIV and TB dysregulate the immune activation in hypertension remain scarce. Nonetheless, a mechanistic approach to how HIV dysregulates immune activation in the setting of hypertension can be summarised in 4 steps. (1) Chronic immune activation and pro-inflammatory cytokines: In addition to traditional risk factors and the impact of ART on blood pressure, inflammatory markers such as IL-17A, IFN-γ, and CD4+ T cells have been linked to hypertension in PLWHIV undergoing ART (103). The same authors propose that HIV viral proteins and ART interact with the immune system to synergistically contribute to kidney injury, vascular dysfunction, and alterations in sympathetic nervous activity, ultimately promoting the development of hypertension (103). Similarly, in another study assessment of inflammatory biomarkers in plasma demonstrated an association between HIV-induced inflammation and increased blood pressure levels (109). In an attempt to investigate how T-cell activation/exhaustion and monocyte subsets correlate with arterial stiffness, the authors noted that individuals with HIV who displayed elevated levels of PD-1+ exhausted CD4+ and CD8+ T cells showed evidence of stiffer arteries early in ART treatment (110). (2) Microbial translocation, monocyte activation and endothelial injury: HIV damages gut mucosa early on, allowing lipopolysaccharides (LPS) into the circulation (111). LPS/sCD14-mediated monocyte activation sustains systemic inflammation and directly impairs endothelial function (112), linking microbial translocation to vascular dysfunction and hypertension risk. (3) Viral proteins and immune cell-vascular cross talk: HIV proteins and viral-protein-expressing immune cells can act on endothelial and smooth muscle cells to increase oxidative stress, inflammasome activation, and local cytokine production, promoting vasoconstriction and remodelling (103). Recent work using the Tg26 mouse model of HIV shows CD4+ T cells expressing viral proteins can drive hypertension through IL-1α and NOX1 pathways (113). (4) Monocyte/macrophage and T-cell phenotypes that favour hypertension: HIV shifts innate and adaptive cell populations toward activated, inflammatory phenotypes (pro-inflammatory monocytes, senescent/activated CD8+ T cells, dysfunctional Tregs) (103). These cells infiltrate kidneys and vessels, producing cytokines and ROS that raise systemic vascular resistance and alter renal sodium handling (103, 113).
There is limited evidence regarding whether HIV infection contributes to an increased risk of resistant hypertension (RH) (114). A case–control study in Malawi (114) has been designed to evaluate these associations, though findings are not yet available. Current research on HIV–associated hypertension predominantly centres on cardiovascular disease mechanisms in PLWHIV; therefore, the precise contributors to hypertension remain poorly characterized. Comprehensive studies are required to assess whether the same biological pathways underlying HIV-related CVD are responsible for increased blood pressure. In addition, large multinational longitudinal cohorts are needed to define the mechanisms and predictors of hypertension in PLWHIV relative to people living without HIV (PWoH). The mechanisms linking immune activation or inflammation to hypertension and RH in the context of HIV remain poorly understood and require further investigation.
Although the global burden of TB is declining, it remains a significant public health challenge in many low- and middle-income countries (LMICs) (34). In 2021, 10.6 million individuals developed TB globally and an estimated 1.6 million people died from the disease (115, 116). LMICs accounted for 80% of all TB cases and deaths, with the WHO African region contributing 23% of new infections (115, 116). TB ranks as the second deadliest infectious disease and the 13th leading cause of death worldwide (115, 116). TB has been implicated in the pathogenesis of hypertension through diverse immunological and pathophysiological mechanisms (34). While there is no specific data on immune mechanisms in TB and hypertension in SSA, or globally, many believe that activation of immune responses in TB may impair endothelial function (Figure 2), thereby increasing the risk of cardiovascular disease and potentially contributing to the development of hypertension (34, 117, 118). TB may cause pulmonary hypertension by damaging the pulmonary vessels (34, 119), and may cause systemic hypertension via TB infection in the kidney, thereby causing damage to the renal tissue, decreased renal function, and impaired blood pressure regulation (34, 120, 121).
There are plausible, partly well-worked mechanisms by which active or latent M.tb infection can dysregulate immunity in ways that increase blood-pressure and cardiovascular risk. The evidence is a mix of epidemiology, clinical case reports, immunology, and animal/biomarker studies; causal chains are biologically plausible but not yet proven in randomized trials. M.tb elicits strong cellular immunity (IFN-γ, TNF-α, IL-1 family, IL-6) (118). Persistent or recurrent antigen exposure (active disease, poorly controlled latent infection, or post-treatment immune remodelling) raises circulating pro-inflammatory cytokines (122) that are known drivers of vascular inflammation, endothelial dysfunction, arterial stiffness and BP elevation.
Inflammation and immune activation in TB further complicate the management of hypertension. TB, as a chronic inflammatory condition, may trigger a complex sequence of immune responses that contribute to the development of atherosclerotic plaque formation (117). This process involves infection-induced antibodies cross-reacting with self-antigens, including heat-shock proteins (HSPs), which are a family of stress-responsive proteins expressed by cells under various physiological stress conditions (123). Human HSP60 exhibits approximately 40%–50% identical resemblance with heat-shock proteins found in Mycobacterium species (118). A similar mechanism may underlie the development of hypertension in individuals with TB (118). It has been shown that overexpression of HSPs can provoke autoimmune responses, resulting in the infiltration of macrophages and T-lymphocytes into renal tissue; an effect linked to hypertension in experimental models (118). Additionally, patients with essential hypertension have been found to exhibit elevated levels of anti-HSP70 and anti-HSP65 antibodies (118).
Immune activation in TB may exacerbate the inflammatory milieu that underlies hypertension by contributing to vascular dysfunction and elevated blood pressure (Figure 2). Epidemiological data further suggest a heightened risk of hypertension among individuals with latent TB, linking infectious disease status with chronic cardiovascular risk. At present, there is no data to suggest TB contributes to the development of RH, however it is not rare to come across cases where TB presents itself as malignant or uncontrolled hypertension (124).
5.2 Impact of ART and anti-TB medication on pathogenesis of hypertension
HIV treatment relies on combination ART to improve therapeutic outcomes (77). The widespread success of ART has been accompanied by a rising prevalence of NCDs among PLWHIV (125). As individuals with HIV experience longer lifespans due to effective ART, chronic comorbidities such as hypertension, have become prominent contributors to morbidity and mortality in this population (126–128). PLWHIV who are receiving combination ART have a higher risk of developing hypertension compared to those without HIV infection (129, 130). Prolonged use of highly active antiretroviral therapy (HAART) (beyond two years) is associated with a significantly increased risk of systolic hypertension, even after controlling for age, body mass index, race, and smoking (131). Other cardiovascular risk factors mediated by ART include hypertriglyceridemia, hypercholesterolemia, and atherosclerosis (132), well known risk factors for hypertension (133, 134).
The WHO endorses the use of fixed-dose combination regimens for anti-tuberculosis therapy, comprising isoniazid, rifampicin, pyrazinamide, and ethambutol as the standard first-line treatment (135). Rifampicin may reduce the efficacy of various antihypertensive medications by inducing cytochrome P450 enzymes, thereby accelerating their metabolism (136). The effects of rifampicin on blood pressure control and antihypertensive drug levels in 24 hypertensive patients with end-stage chronic kidney disease undergoing maintenance haemodialysis was studied. All participants had stable blood pressure (≤140/90 mmHg) on consistent antihypertensive regimens before starting rifampicin-based treatment for TB (137). However, after rifampicin initiation, there was a significant decrease in plasma concentrations of commonly used anti hypertensives. This decrease in levels correlated well with worsening of hypertension (137).
6 Conclusion
The growing intersection between infectious diseases and non-communicable conditions represents one of the most pressing challenges for health systems in SSA. Among PLWHIV and TB, the emergence and persistence of hypertension and resistant hypertension have significant implications for long-term morbidity and mortality. This complex clinical overlap is driven by a convergence of factors, including chronic immune activation, inflammation, endothelial dysfunction, and metabolic derangements induced by HIV infection, TB, and their respective treatments. ART, while lifesaving, is associated with metabolic complications which increase the risk of hypertension. Similarly, anti-tuberculosis medications, most notably rifampicin, can interfere with the pharmacokinetics of commonly used antihypertensive drugs, leading to subtherapeutic drug levels and poor blood pressure control. Moreover, HIV and TB themselves exert profound immunological effects that may contribute to vascular inflammation and endothelial injury, further complicating the pathophysiology of hypertension in co-infected individuals.
In SSA, these challenges are compounded by systemic issues such as limited access to diagnostics, poor integration of care for infectious and chronic diseases, and fragmented health service delivery. The traditional disease-specific focus of public health programs has left a critical gap in managing comorbid non-communicable diseases in populations with high burdens of HIV and TB. As a result, hypertension often remains undiagnosed or poorly controlled, increasing the risk of cardiovascular events, renal disease, and premature death. To mitigate this growing burden, there is an urgent need for integrated models of care that combine infectious disease management with routine screening and treatment of non-communicable diseases, including hypertension. Future research should prioritize understanding the immunopathogenic mechanisms linking HIV, TB, and hypertension, and explore context-appropriate strategies to overcome drug–drug interactions and improve therapeutic outcomes. Strengthening health systems to provide holistic, continuous, and coordinated care will be essential in addressing this emerging syndemic and improving long-term outcomes for affected populations across sub-Saharan Africa.
Author contributions
PL: Writing – original draft, Writing – review & editing, Conceptualization. MZ: Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. MZ is supported by the South African Medical Research Council (SAMRC) Early Investigator Programme award through its Division of Research Capacity Development under the Research Capacity Development Initiative from funding received from the South African National Treasury. The content and findings reported/illustrated are the sole deduction, view and responsibility of the researcher and do not reflect the official position and sentiments of the SAMRC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Fact Sheets: Hypertension (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/hypertension (Accessed August 4, 2025).
2. Muralitharan R, Marques FZ, O'Donnell JA. Recent advancements in targeting the immune system to treat hypertension. Eur J Pharmacol. (2024) 983:177008. doi: 10.1016/j.ejphar.2024.177008
3. Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. (2006) 24(6):1201–8. doi: 10.1097/01.hjh.0000226212.34055.86
4. McManus M, Liebeskind DS. Blood pressure in acute ischemic stroke. J Clin Neurol. (2016) 12(2):137–46. doi: 10.3988/jcn.2016.12.2.137
5. Suzanne Oparil MAZ, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. (2003) 139(9):761–76. doi: 10.7326/0003-4819-139-9-200311040-00011
6. Poulter NR, Borghi C, Damasceno A, Jafar TH, Khan NA, Kokubo Y, et al. May Measurement Month: results of 12 national blood pressure screening programmes between 2017 and 2019. Eur Heart J Suppl. (2022) 24:F1–5. doi: 10.1093/eurheartjsupp/suac045
8. Gafane-Matemane LF, Craig A, Kruger R, Alaofin OS, Ware LJ, Jones ES, et al. Hypertension in sub-Saharan Africa: the current profile, recent advances, gaps, and priorities. J Hum Hypertens. (2024) 39:95–110. doi: 10.1038/s41371-024-00913-6
9. (NCD-RisC), N.R.F.C. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398(10304):957–80. doi: 10.1016/S0140-6736(21)01330-1
10. Ngianga-Bakwin Kandala CCN, Dukhi N, Sewpaul R, Davids A, Reddy SP. Mapping the burden of hypertension in South Africa: a comparative analysis of the national 2012 SANHANES and the 2016 demographic and health survey. Int J Environ Res Public Health. (2021) 18(10):5445. doi: 10.3390/ijerph18105445
11. Reddy SP, Mbewu AD, Williams DR, Harriman NW, Sewpaul R, Morgan JW, et al. Race, geographical location and other risk factors for hypertension: South African national health and nutrition examination survey 2011/12. SSM Popul Health. (2021) 16:100986. doi: 10.1016/j.ssmph.2021.100986
12. Ndong AK, van der Linden EL, Beune EJ, Meeks KA, Danquah I, Bahendeka S, et al. Serum potassium concentration and its association with hypertension among Ghanaian migrants and non-migrants: the RODAM study. Atherosclerosis. (2022) 342:36–43. doi: 10.1016/j.atherosclerosis.2021.12.006
13. Aliyu AA, Amadu L. Urbanization, cities, and health: the challenges to Nigeria—a review. Ann Afr Med. (2017) 16(4):149–58. doi: 10.4103/aam.aam_1_17
14. Ntiyani N, Letamo G, Keetile M. Prevalence of and factors associated with hypertension, diabetes, stroke and heart attack multimorbidity in Botswana: evidence from STEPS 2014 survey. PLoS One. (2022) 17(3):e0265722. doi: 10.1371/journal.pone.0265722
15. Tozivepi SN, Takawira S, Chikaka E, Mundagowa P, Chadambuka EM, Mukora-Mutseyekwa F. The nexus between adherence to recommended lifestyle behaviors and blood pressure control in hypertensive patients at mutare provincial hospital, Zimbabwe: a cross-sectional study. Patient Prefer Adherence. (2021) 15:1027–37. doi: 10.2147/PPA.S306885
16. Ware LJ, Chidumwa G, Charlton K, Schutte AE, Kowal P. Predictors of hypertension awareness, treatment and control in South Africa: results from the WHO-SAGE population survey (wave 2). J Hum Hypertens. (2019) 33(2):157–66. doi: 10.1038/s41371-018-0125-3
17. Craig LS, Gage AJ, Thomas AM. Prevalence and predictors of hypertension in Namibia: a national-level cross-sectional study. PLoS One. (2018) 13(9):e0204344. doi: 10.1371/journal.pone.0204344
18. Omar SM, Musa IR, Osman OE, Adam I. Prevalence and associated factors of hypertension among adults in Gadarif in Eastern Sudan: a community-based study. BMC Public Health. (2020) 20(1):291. doi: 10.1186/s12889-020-8386-5
19. Abdelbagi O, Musa IR, Musa SM, ALtigani SA, Adam I. Prevalence and associated factors of hypertension among adults with diabetes mellitus in Northern Sudan: a cross-sectional study. BMC Cardiovasc Disord. (2021) 21(1):168. doi: 10.1186/s12872-021-01983-x
20. Appiah F, Ameyaw EK, Oduro JK, Baatiema L, Sambah F, Seidu A-A, et al. Rural-urban variation in hypertension among women in Ghana: insights from a national survey. BMC Public Health. (2021) 21(1):2150. doi: 10.1186/s12889-021-12204-7
21. Okello S, Muhihi A, Mohamed SF, Ameh S, Ochimana C, Oluwasanu AO, et al. Hypertension prevalence, awareness, treatment, and control and predicted 10-year CVD risk: a cross-sectional study of seven communities in East and West Africa (SevenCEWA). BMC Public Health. (2020) 20(1):1706. doi: 10.1186/s12889-020-09829-5
22. Ezeala-Adikaibe BA, Mbadiwe CN, Okafor UH, Nwobodo UM, Okwara CC, Okoli CP, et al. Prevalence of hypertension in a rural community in southeastern Nigeria; an opportunity for early intervention. J Hum Hypertens. (2023) 37(8):694–700. doi: 10.1038/s41371-023-00833-x
23. Zhang V, Fisher M, Hou W, Zhang L, Duong TQ. Incidence of new-onset hypertension post–COVID-19: comparison with influenza. Hypertension. (2023) 80(10):2135–48. doi: 10.1161/HYPERTENSIONAHA.123.21174
24. Trimarco V, Izzo R, Pacella D, Trama U, Manzi MV, Lombardi A, et al. Incidence of new-onset hypertension before, during, and after the COVID-19 pandemic: a 7-year longitudinal cohort study in a large population. BMC Med. (2024) 22(1):127. doi: 10.1186/s12916-024-03328-9
25. Rodríguez-Arbolí E, Mwamelo K, Kalinjuma AV, Furrer H, Hatz C, Tanner M, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania—a prospective cohort study. PLoS One. (2017) 12(3):e0172089. doi: 10.1371/journal.pone.0172089
26. Selebalo MS, Bryden BJ, Thompson DM, Sanders JE. Prevalence of hypertension and associated factors in people living with HIV at senkatana clinic Maseru. Afr J Prim Health Care Fam Med. (2025) 17(1):4813. doi: 10.4102/phcfm.v17i1.4813
27. Salindri AD, Auld SC, Gujral UP, Urbina EM, Andrews JR, Huaman MA, et al. Tuberculosis infection and hypertension: prevalence estimates from the US national health and nutrition examination survey. medRxiv. (2024) 14(3):e075176. doi: 10.1136/bmjopen-2023-075176
28. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. (2018) 71(6):1269–324. doi: 10.1161/HYP.0000000000000066
29. Edward J Filippone GVN, Foy AJ. Controversies in hypertension V: resistant and refractory hypertension. Am J Med. (2024) 137(1):12–22. doi: 10.1016/j.amjmed.2023.09.015
30. Nagarajan N, Jalal D. Resistant hypertension: diagnosis and management. Adv Chronic Kidney Dis. (2019) 26(2):99–109. doi: 10.1053/j.ackd.2019.03.002
31. Wondmeneh TG, Wondmeneh RG. Risky sexual behaviour among HIV-infected adults in Sub-Saharan Africa: a systematic review and meta-analysis. Biomed Res Int. (2023) 2023:6698384. doi: 10.1155/2023/6698384
32. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
33. Godongwana M, De Wet-Billings N. Time to hypertension development among people living with HIV in South Africa: a longitudinal analysis of the national income dynamics survey (NIDS). Heliyon. (2021) 7(8):e07750. doi: 10.1016/j.heliyon.2021.e07750
34. Seegert AB, Rudolf F, Wejse C, Neupane D. Tuberculosis and hypertension—a systematic review of the literature. Int J Infect Dis. (2017) 56:54–61. doi: 10.1016/j.ijid.2016.12.016
35. Olubayo LAI, Mathema T, Kabudula C, Micklesfield LK, Mohamed SF, Kisiangani I, et al. The prevalence, incidence, and sociodemographic risk factors of HIV among older adults in sub-Saharan Africa (AWI-Gen): a multicentre, longitudinal cohort study. Lancet Healthy Longev. (2025) 6(3):100690. doi: 10.1016/j.lanhl.2025.100690
37. Organization, W.H. and W.H.O. Staff, Global Tuberculosis Report 2013. Geneva: World Health Organization (WHO) (2013).
38. Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. JAIDS J Acquir Immune Defic Syndr. (2012) 61(5):600–5. doi: 10.1097/QAI.0b013e31827303d5
39. Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. (2020) 36(5):635–47. doi: 10.1016/j.cjca.2020.01.013
40. Rodriguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The immunological basis of hypertension. Am J Hypertens. (2014) 27(11):1327–37. doi: 10.1093/ajh/hpu142
41. Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. (2006) 15(2):152–8. doi: 10.1097/01.mnh.0000203189.57513.76
42. Huang Z, Chen C, Li S, Kong F, Shan P, Huang W. Serum markers of endothelial dysfunction and inflammation increase in hypertension with prediabetes Mellitus. Genet Test Mol Biomarkers. (2016) 20(6):322–7. doi: 10.1089/gtmb.2015.0255
43. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. (2007) 204(10):2449–60. doi: 10.1084/jem.20070657
44. Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE. Cross talk between AT1 receptors and toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. (2016) 310(3):H404–15. doi: 10.1152/ajpheart.00247.2015
45. Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. (2010) 107(2):263–70. doi: 10.1161/CIRCRESAHA.110.217299
46. Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, et al. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. (2012) 71(9):774–82. doi: 10.1016/j.biopsych.2012.01.017
47. Ruggeri Barbaro N, Van Beusecum J, Xiao L, do Carmo L, Pitzer A, Loperena R, et al. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc Res. (2021) 117(5):1358–71. doi: 10.1093/cvr/cvaa207
48. Norlander AE, Saleh MA, Pandey AK, Itani HA, Wu J, Xiao L, et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight. (2017) 2(13):e92801. doi: 10.1172/jci.insight.92801
49. Liu L, Zhou Q, Xu T, Deng Q, Sun Y, Fu J, et al. Non-differential gut microbes contribute to hypertension and its severity through co-abundances: a multi-regional prospective cohort study. iMeta. (2025) 4(1):e268. doi: 10.1002/imt2.268
50. Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature. (2017) 551(7682):585–9. doi: 10.1038/nature24628
51. Kirabo A, Fontana V, de Faria APC, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. (2014) 124(10):4642–56. doi: 10.1172/JCI74084
52. Guzik TJ, Nosalski R, Maffia P, Drummond GR. Immune and inflammatory mechanisms in hypertension. Nat Rev Cardiol. (2024) 21(6):396–416. doi: 10.1038/s41569-023-00964-1
53. Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. (2018) 114(11):1547–63. doi: 10.1093/cvr/cvy112
54. Itani HA, McMaster WG, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. (2016) 68(1):123–32. doi: 10.1161/HYPERTENSIONAHA.116.07237
55. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. (2019) 19(8):517–32. doi: 10.1038/s41577-019-0160-5
56. Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. (2018) 215(1):21–33. doi: 10.1084/jem.20171773
57. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. (2015) 116(6):1022–33. doi: 10.1161/CIRCRESAHA.116.303697
58. Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. (2014) 63(4):797–803. doi: 10.1161/HYPERTENSIONAHA.113.02883
59. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. (2010) 55(2):500–7. doi: 10.1161/HYPERTENSIONAHA.109.145094
60. Christ A, Temmerman L, Legein B, Daemen MJAP, Biessen EAL. Dendritic cells in cardiovascular diseases. Circulation. (2013) 128(24):2603–13. doi: 10.1161/CIRCULATIONAHA.113.003364
61. Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. (2007) 7(1):19–30. doi: 10.1038/nri1996
62. Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, et al. Inhibition and genetic ablation of the B7/CD28T-cell costimulation axis prevents experimental hypertension. Circulation. (2010) 122(24):2529–37. doi: 10.1161/CIRCULATIONAHA.109.930446
63. Abumiya T, Masuda J, Kawai J, Suzuki T, Ogata J. Heterogeneity in the appearance and distribution of macrophage subsets and their possible involvement in hypertensive vascular lesions in rats. Lab Invest. (1996) 75(2):125–36. Available online at: https://pubmed.ncbi.nlm.nih.gov/8765313/8765313
64. Mervaala EMA, Müller DN, Park J-K, Schmidt F, Löhn M, Breu V, et al. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. (1999) 33(1 Pt 2):389–95. doi: 10.1161/01.HYP.33.1.389
65. Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, et al. CC Chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. (2000) 36(3):360–3. doi: 10.1161/01.HYP.36.3.360
66. Haller H, Behrend M, Park JK, Schaberg T, Luft FC, Distler A. Monocyte infiltration and c-fms expression in hearts of spontaneously hypertensive rats. Hypertension. (1995) 25(1):132–8. doi: 10.1161/01.HYP.25.1.132
67. Eng E, Veniant M, Floege J, Fingerle J, Alpers CE, Menard J, et al. Renal proliferative and phenotypic changes in rats with two-kidney, one-clip Goldblatt hypertension. Am J Hypertens. (1994) 7(2):177–85. doi: 10.1093/ajh/7.2.177
68. Liu Y, Jacobowitz DM, Barone F, McCarron R, Spatz M, Feuerstein G, et al. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J Cereb Blood Flow Metab. (1994) 14(2):348–52. doi: 10.1038/jcbfm.1994.43
69. Harwani SC. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res. (2018) 191:45–63. doi: 10.1016/j.trsl.2017.10.011
70. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. (2010) 116(16):e74–80. doi: 10.1182/blood-2010-02-258558
71. Ulrich C, Heine GH, Garcia P, Reichart B, Georg T, Krause M, et al. Increased expression of monocytic angiotensin-converting enzyme in dialysis patients with cardiovascular disease. Nephrol Dial Transplant. (2006) 21(6):1596–602. doi: 10.1093/ndt/gfl008
72. Hughson MD, Gobe GC, Hoy WE, Manning RD, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. (2008) 52(1):18–28. doi: 10.1053/j.ajkd.2008.03.023
73. Zhang J, Crowley SD. Role of T lymphocytes in hypertension. Curr Opin Pharmacol. (2015) 21:14–9. doi: 10.1016/j.coph.2014.12.003
74. Zhu J, Paul WE. CD4T Cells: fates, functions, and faults. Blood. (2008) 112(5):1557–69. doi: 10.1182/blood-2008-05-078154
75. Patrick DM, Van Beusecum JP, Kirabo A. The role of inflammation in hypertension: novel concepts. Curr Opin Physiol. (2021) 19:92–8. doi: 10.1016/j.cophys.2020.09.016
76. Youn J-C, Yu HT, Lim BJ, Koh MJ, Lee J, Chang D-Y, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. (2013) 62(1):126–33. doi: 10.1161/HYPERTENSIONAHA.113.00689
77. WHO. Health Topics: HIV (2025) Available online at: https://www.who.int/health-topics/hiv-aids#tab=tab_1 (Accessed August 5, 2025).
78. Masenga SK, Mweene BC, Luwaya E, Muchaili L, Chona M, Kirabo A. HIV–host cell interactions. Cells. (2023) 12(10):1351. doi: 10.3390/cells12101351
79. Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV-1 target cells in the CNS. J Neurovirol. (2015) 21(3):276–89. doi: 10.1007/s13365-014-0287-x
80. Woodham AW, Skeate JG, Sanna AM, Taylor JR, Da Silva DM, Cannon PM, et al. Human immunodeficiency virus immune cell receptors, coreceptors, and cofactors: implications for prevention and treatment. AIDS Patient Care STDS. (2016) 30(7):291–306. doi: 10.1089/apc.2016.0100
81. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. (2006) 12(12):1365–71. doi: 10.1038/nm1511
82. Kulpa DA, Paiardini M, Silvestri G. Immune-mediated strategies to solving the HIV reservoir problem. Nat Rev Immunol. (2025) 25(7):542–53. doi: 10.1038/s41577-025-01136-7
83. Wondmagegn T, Birhan W, Derb E, Lemma M, Yohannes H, Anteneh DE, et al. Serum sPD-1 as a marker of T cell exhaustion in ART-naïve, ART-experienced, and intestinal parasite co-infected HIV-positive adults at the university of Gondar comprehensive specialized hospital, Northwest Ethiopia, 2024. BMC Infect Dis. (2025) 25(1):765. doi: 10.1186/s12879-025-11158-0
84. Fenwick C, Joo V, Jacquier P, Noto A, Banga R, Perreau M, et al. T-cell exhaustion in HIV infection. Immunol Rev. (2019) 292(1):149–63. doi: 10.1111/imr.12823
85. Muñoz-Muela E, Trujillo-Rodríguez M, Serna-Gallego A, Saborido-Alconchel A, Ruiz-Mateos E, López-Cortés LF, et al. HIV-1-specific T-cell responses and exhaustion profiles in people with HIV after switching to dual therapy vs. Maintaining triple therapy based on integrase inhibitors. Biomed Pharmacother. (2023) 168:115750. doi: 10.1016/j.biopha.2023.115750
86. Obeagu EI. Influence of cytokines on the recovery trajectory of HIV patients on antiretroviral therapy: a review. Medicine (Baltimore). (2025) 104(1):e41222. doi: 10.1097/MD.0000000000041222
87. Dufour C, Gantner P, Fromentin R, Chomont N. The multifaceted nature of HIV latency. J Clin Invest. (2020) 130(7):3381–90. doi: 10.1172/JCI136227
88. Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. (2012) 10(9):655–66. doi: 10.1038/nrmicro2848
89. Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. (2013) 9(2):e1003174. doi: 10.1371/journal.ppat.1003174
90. Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. (2016) 2(1):16076. doi: 10.1038/nrdp.2016.76
91. Rahman F. Characterizing the immune response to mycobacterium tuberculosis: a comprehensive narrative review and implications in disease relapse. Front Immunol. (2024) 15:1437901. doi: 10.3389/fimmu.2024.1437901
92. Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. (2017) 14(12):963–75. doi: 10.1038/cmi.2017.88
93. Khan N, Vidyarthi A, Javed S, Agrewala JN. Innate immunity holding the flanks until reinforced by adaptive immunity against mycobacterium tuberculosis infection. Front Microbiol. (2016) 7:328. doi: 10.3389/fmicb.2016.00328
94. Moreira-Teixeira L, Mayer-Barber K, Sher A, O’Garra A. Type I interferons in tuberculosis: foe and occasionally friend. J Exp Med. (2018) 215(5):1273–85. doi: 10.1084/jem.20180325
95. Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. (1993) 178(6):2243–7. doi: 10.1084/jem.178.6.2243
96. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to mycobacterium tuberculosis infection. J Exp Med. (1993) 178(6):2249–54. doi: 10.1084/jem.178.6.2249
97. Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against mycobacterium tuberculosis in mice. Immunity. (1995) 2(6):561–72. doi: 10.1016/1074-7613(95)90001-2
98. Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, et al. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during mycobacterium tuberculosis infection. Immunity. (2011) 35(6):1023–34. doi: 10.1016/j.immuni.2011.12.002
99. Arend WP, Joslin FG, Thompson RC, Hannum CH. An IL-1 inhibitor from human monocytes. Production and characterization of biologic properties. J Immunol. (1989) 143(6):1851–8. doi: 10.4049/jimmunol.143.6.1851
100. Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with mycobacterium tuberculosis. Eur Respir J. (2014) 43(5):1472–86. doi: 10.1183/09031936.00151413
101. Moyo E, Moyo P, Murewanhema G, Mhango M, Chitungo I, Dzinamarira T. Key populations and Sub-Saharan Africa’s HIV response. Front Public Health. (2023) 11:1079990. doi: 10.3389/fpubh.2023.1079990
102. Caiazzo E, Sharma M, Rezig AOM, Morsy MI, Czesnikiewicz-Guzik M, Ialenti A, et al. Circulating cytokines and risk of developing hypertension: a systematic review and meta-analysis. Pharmacol Res. (2024) 200:107050. doi: 10.1016/j.phrs.2023.107050
103. Masenga SK, Hamooya BM, Nzala S, Kwenda G, Heimburger DC, Mutale W, et al. Patho-immune mechanisms of hypertension in HIV: a systematic and thematic review. Curr Hypertens Rep. (2019) 21(7):56. doi: 10.1007/s11906-019-0956-5
104. Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. (2003) 33(4):506–12. doi: 10.1097/00126334-200308010-00012
105. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. (2007) 92(7):2506–12. doi: 10.1210/jc.2006-2190
106. Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. (2012) 18(4):264–76. doi: 10.1007/s13365-012-0092-3
107. Chen A, Chan Y-K, Mocumbi AO, Ojji DB, Waite L, Beilby J, et al. Hypertension among people living with human immunodeficiency virus in sub-saharan Africa: a systematic review and meta-analysis. Sci Rep. (2024) 14(1):16858. doi: 10.1038/s41598-024-67703-5
108. Masenga SK, Povia JP, Mutengo KH, Hamooya BM, Nzala S, Heimburger DC, et al. Sex differences in hypertension among people living with HIV after initiation of antiretroviral therapy. Front Cardiovasc Med. (2022) 9:1006789. doi: 10.3389/fcvm.2022.1006789
109. Batavia AS, Severe P, Lee MH, Apollon A, Zhu YS, Dupnik KM, et al. Blood pressure and mortality in a prospective cohort of HIV-infected adults in Port-au-Prince, Haiti. J Hypertens. (2018) 36(7):1533–9. doi: 10.1097/HJH.0000000000001723
110. Kelly C, Mwandumba HC, Heyderman RS, Jambo K, Kamng’ona R, Chammudzi M, et al. HIV-related arterial stiffness in Malawian adults is associated with the proportion of PD-1-expressing CD8+ T cells and reverses with antiretroviral therapy. J Infect Dis. (2019) 219(12):1948–58. doi: 10.1093/infdis/jiz015
111. Blodget E, Shen C, Aldrovandi G, Rollie A, Gupta SK, Stein JH, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. (2012) 7(8):e42624. doi: 10.1371/journal.pone.0042624
112. Lepper PM, Kleber ME, Grammer TB, Hoffmann K, Dietz S, Winkelmann BR, et al. Lipopolysaccharide-binding protein (LBP) is associated with total and cardiovascular mortality in individuals with or without stable coronary artery disease–results from the Ludwigshafen Risk and Cardiovascular Health Study (LURIC). Atherosclerosis. (2011) 219(1):291–7. doi: 10.1016/j.atherosclerosis.2011.06.001
113. Kress TC, Barris CT, Kovacs L, Khakina BN, Jordan CR, Bruder-Nascimento T, et al. CD4+ T cells expressing viral proteins induce HIV-associated endothelial dysfunction and hypertension through interleukin 1α-mediated increases in endothelial NADPH oxidase 1. Circulation. (2025) 151(16):1187–203. doi: 10.1161/CIRCULATIONAHA.124.070538
114. Gondwe J, Ndovie M, Khuluza F, Banda CG. Association between HIV and treatment-resistant hypertension in Malawian adults: a protocol for a case-control study. BMJ Open. (2023) 13(8):e069280. doi: 10.1136/bmjopen-2022-069280
115. Sisay Asgedom Y, Ambaw Kassie G, Melaku Kebede T. Prevalence of tuberculosis among prisoners in sub-Saharan Africa: a systematic review and meta-analysis. Front Public Health. (2023) 11:1235180. doi: 10.3389/fpubh.2023.1235180
117. Huaman MA, Henson D, Ticona E, Sterling TR, Garvy BA. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines. (2015) 1(1):10. doi: 10.1186/s40794-015-0014-5
118. Rodríguez-Iturbe B, Pons H, Quiroz Y, Lanaspa MA, Johnson RJ. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol. (2014) 10(1):56–62. doi: 10.1038/nrneph.2013.248
119. Marjani M, Baghaei P, Malekmohammad M, Tabarsi P, Sharif-Kashani B, Behzadnia N, et al. Effect of pulmonary hypertension on outcome of pulmonary tuberculosis. Braz J Infect Dis. (2014) 18(5):487–90. doi: 10.1016/j.bjid.2014.02.006
120. Shen T-C, Huang K-Y, Chao C-H, Wang Y-C, Muo C-H, Wei C-C, et al. The risk of chronic kidney disease in tuberculosis: a population-based cohort study. QJM: Int J Med. (2015) 108(5):397–403. doi: 10.1093/qjmed/hcu220
121. Studer UE, Weidmann P. Pathogenesis and treatment of hypertension in renal Tuberculosis. Eur Urol. (1984) 10(3):164–9. doi: 10.1159/000463780
122. Wang Y, Sun Q, Zhang Y, Li X, Liang Q, Guo R, et al. Systemic immune dysregulation in severe tuberculosis patients revealed by a single-cell transcriptome atlas. J Infect. (2023) 86(5):421–38. doi: 10.1016/j.jinf.2023.03.020
123. Epstein SE, Zhu J, Najafi AH, Burnett MS. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. (2009) 119(24):3133–41. doi: 10.1161/CIRCULATIONAHA.109.849455
124. Mehta AA, Ashok A, Praveen VP, Kiran Kumar B. Tuberculosis presenting as uncontrolled hypertension. Respir Med Case Rep. (2024) 50:102063. doi: 10.1016/j.rmcr.2024.102063
125. Singh U, Olivier S, Cuadros D, Castle A, Moosa Y, Zulu T, et al. The met and unmet health needs for HIV, hypertension, and diabetes in rural KwaZulu-natal, South Africa: analysis of a cross-sectional multimorbidity survey. Lancet Glob Health. (2023) 11(9):e1372–82. doi: 10.1016/S2214-109X(23)00239-5
126. Fiseha T, Belete AG, Dereje H, Dires A. Hypertension in HIV-infected patients receiving antiretroviral therapy in northeast Ethiopia. Int J Hypertens. (2019) 2019(1):4103604. doi: 10.1155/2019/4103604
127. Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. (2011) 53(11):1120–6. doi: 10.1093/cid/cir627
128. Chhoun P, Ngin C, Tuot S, Pal K, Steel M, Dionisio J, et al. Non-communicable diseases and related risk behaviors among men and women living with HIV in Cambodia: findings from a cross-sectional study. Int J Equity Health. (2017) 16(1):125. doi: 10.1186/s12939-017-0622-y
129. Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-infected adults: novel pathophysiologic mechanisms. Hypertension. (2018) 72(1):44–55. doi: 10.1161/HYPERTENSIONAHA.118.10893
130. Gazzaruso C, Bruno R, Garzaniti A, Giordanetti S, Fratino P, Sacchi P, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. (2003) 21(7):1377–82. doi: 10.1097/00004872-200307000-00028
131. Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. (2005) 19(9):953–60. doi: 10.1097/01.aids.0000171410.76607.f8
132. Dressman J, Kincer J, Matveev SV, Guo L, Greenberg RN, Guerin T, et al. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest. (2003) 111(3):389–97. doi: 10.1172/JCI200316261
133. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. (2018) 34(5):575–84. doi: 10.1016/j.cjca.2017.12.005
134. Naidu S, Ponnampalvanar S, Kamaruzzaman SB, Kamarulzaman A. Prevalence of metabolic syndrome among people living with HIV in developing countries: a systematic review. AIDS Patient Care STDS. (2017) 31(1):1–13. doi: 10.1089/apc.2016.0140
135. WHO. Tuberculosis (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (Accessed August 9, 2025).
136. Yogesh S, Nallathambi N, Seshadri H, Gautham R, Naidu SP, Navvin S, et al. Assessing the effect of the anti-tuberculosis drug rifampicin on known hypertensive patients with tuberculosis in a tertiary care center. Cureus. (2023) 15(11):e49701. doi: 10.7759/cureus.49701
137. Agrawal A, Agarwal SK, Kaleekal T, Gupta YK. Rifampicin and anti-hypertensive drugs in chronic kidney disease: pharmacokinetic interactions and their clinical impact. Indian J Nephrol. (2016) 26(5):322–8. doi: 10.4103/0971-4065.176145
Keywords: hypertension, immune dysregulation, human immunodeficiency virus (HIV), tuberculosis, chronic infections
Citation: Letuka P and Zulu MZ (2025) Intersecting epidemics: immune dysregulation associated with HIV and tuberculosis syndemic contribute to increased risk of hypertensive disease in Sub-Saharan Africa. Front. Cardiovasc. Med. 12:1717609. doi: 10.3389/fcvm.2025.1717609
Received: 2 October 2025; Accepted: 31 October 2025;
Published: 17 November 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Prem Prakash, Meharry Medical College, United StatesCopyright: © 2025 Letuka and Zulu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Z. Zulu, bWljaGFlbC56dWx1QHdpdHMuYWMuemE=
 Pheletso Letuka
Pheletso Letuka Michael Z. Zulu
Michael Z. Zulu