Abstract
Introduction:
Coronary artery ectasia (CAE)—diffuse dilatation ≥1.5× the adjacent segments is uncommon and lacks standardized management. Its coexistence with a hemodynamically significant myocardial bridge (MB) is unusual and may create competing disturbances in coronary flow that complicate diagnosis and treatment.
Case presentation:
An 80-year-old man with hypothyroidism, epilepsy, benign prostatic hyperplasia, and paroxysmal atrial fibrillation on rivaroxaban presented with acute precordial pain consistent with non–ST-segment elevation myocardial infarction (NSTEMI). He was hemodynamically stable; ECG showed inferior ST depression with T-wave inversion in V3–V4, and high-sensitivity troponin was elevated (Killip I, GRACE 177, CRUSADE 49). Early diagnostic angiography (<24 h) revealed diffuse three-vessel ectasia (Markis I) with slow TIMI-2 flow and a prominent mid-LAD MB (∼75% systolic “milking”); the intermediate branch had an ostial lesion with downstream aneurysmal dilatation and was not amenable to PCI. Echocardiography showed LVEF >65% with basal inferior/inferoseptal hypokinesia and severe left-atrial enlargement (57 ml/m2). A diagnosis of type 2 NSTEMI due to supply–demand mismatch in the setting of diffuse CAE and MB was established. He was treated with clopidogrel (single antiplatelet therapy) (INR 2.0–3.0), high-intensity statin, and beta-blocker, with symptomatic improvement and remained asymptomatic without recurrent ischemic events over a 4-month follow-up.
Conclusions:
Diffuse CAE with significant MB can precipitate NSTEMI without discrete obstructive lesions and challenges standard revascularization. In such anatomy, individualized conservative therapy—rate control and tailored antithrombotic management—may be preferable, while advanced imaging and diastolic physiology can refine diagnosis and selection for invasive strategies.
1 Introduction
Coronary artery ectasia (CAE) is defined as a diffuse dilatation of a coronary segment to at least 1.5 times the diameter of an adjacent normal segment; by contrast, “coronary artery aneurysm” (CAA) is typically used for focal dilatations, whereas CAE refers to more diffuse involvement (1). The reported prevalence of CAE varies by geography and population, ranging from ∼0.3% to 5% in angiographic series, with a male predominance; the right coronary artery (RCA) is most commonly affected (2, 3). Clinically, CAE presents heterogeneously—from stable angina to acute coronary syndromes (ACS), and even atypical anginal equivalents—reflecting a pathophysiologic substrate of sluggish flow, distal embolization, and thrombosis within ectatic segments (3). Although multiple mechanisms have been proposed (atherosclerosis, inflammatory and connective-tissue disorders), a standardized management algorithm is lacking (4). Prognosis is likewise variable, with observational data suggesting higher rates of adverse events, including no-reflow and stent thrombosis when percutaneous coronary intervention (PCI) is attempted in markedly ectatic or aneurysmal vessels (4).
Myocardial bridging (MB)—systolic compression of a tunneled coronary segment, most often the mid–left anterior descending (LAD) artery—is a relatively common anatomic variant. It is detected more frequently by coronary computed tomography angiography (CCTA) than by invasive angiography (approximately 19%–22% vs. 2%–6%) and can be associated with angina, ACS, arrhythmias, and, rarely, sudden death, particularly under tachycardic states that curtail diastolic perfusion (4, 5). The coexistence of diffuse CAE and a hemodynamically significant MB is unusual and may create competing perturbations of coronary flow—stasis and thrombus formation within ectatic segments, with dynamic systolic compression over the bridged segment—complicating both diagnosis and treatment strategies.
2 Case presentation
We report the case of an 80-year-old man with a medical history of hypothyroidism, epilepsy, benign prostatic hyperplasia, paroxysmal atrial fibrillation, and vertigo. He was independent in his daily activities and had good adherence to his prescribed medications. He reported no relevant family medical history. Chronic medications included lamotrigine, clonazepam, tamsulosin, dutasteride, and rivaroxaban 20 mg daily.
He presented to the emergency department with acute precordial chest pain (8/10 on the visual analog scale) radiating to the left shoulder, without vasovagal symptoms. On admission, he was hemodynamically stable: heart rate 71 beats/min (regular rhythm), blood pressure 106/61 mmHg, and oxygen saturation 90% on room air. Physical examination revealed no signs of cardiopulmonary or hemodynamic instability. The initial electrocardiogram (ECG) showed ST-segment depression in the inferior leads and T-wave inversion in V3–V4, consistent with myocardial ischemia. High-sensitivity cardiac troponin was elevated, confirming non–ST-segment elevation myocardial injury compatible with an NSTEMI presentation. He was Killip class I, with a CRUSADE bleeding score of 49 and a GRACE score of 177 (high ischemic risk). Admission laboratory parameters—including hs-troponin-T 0.03 ng/ml, NT-proBNP 1,658 pg/ml, creatinine 0.96 mg/dl, LDL-C 88 mg/dl, HDL-C 38 mg/dl, triglycerides 192 mg/dl, and HbA1c 6.04%—are summarized in Table 1.
Table 1
| Test | Results | Reference range |
|---|---|---|
| WBC | 4.86 × 103/μl | 3.4–9.7 × 103/μl |
| Neutrophil | 2.48 × 103/μl | 2.2–4.8 × 103/μl |
| Lymphocyte | 1.62 × 103/μl | 1.1–3.2 × 103/μl |
| Hemoglobin | 15 g/dl | 14.0–18.0 g/dl |
| Platelet | 161,000/μl | 130,000–400,000/μl |
| Troponin T | 0.03 ng/ml | 0–0.01 ng/ml |
| Pro BNP | 1,658 pg/ml | <125 pg/ml |
| Creatinin | 0.96 mg/dl | 0.74–1.35 mg/dl |
| LDL | 88 mg/dl | <100 mg/dl |
| HDL | 38 mg/dl | 40–59 mg/dl |
| Triglycerides | 192 mg/dl | <150 mg/dl |
| HbA1c | 6.04% | 4–5, 6% |
Clinical–hematological profile of the patient.
Values are from admission laboratory testing. WBC, white blood cell count; NT-proBNP, N-terminal pro–B-type natriuretic peptide; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycated hemoglobin. Reference ranges according to the institutional laboratory.
Given the high-risk ECG pattern and biomarker elevation, an early invasive strategy with diagnostic coronary angiography was undertaken within the first 24 h; percutaneous coronary intervention (PCI) was not performed due to the angiographic substrate. Before angiography, he received guideline-directed medical therapy, including dual antiplatelet therapy and high-intensity statin.
Coronary angiography demonstrated diffuse coronary ectasia involving all major epicardial vessels (Markis type I) with slow TIMI 2 flow. Maximal reference diameters exceeded 8.7 mm in the right coronary artery, 7.2 mm in the circumflex, and 7.1 mm in the left anterior descending (LAD) artery (Figure 1). The mid-LAD showed a prominent myocardial bridge (MB) with a characteristic “milking effect” (∼75% systolic compression), likely accentuated by the surrounding ectatic reference segment (Figure 2). The intermediate branch displayed a severe ostial lesion followed by aneurysmal dilatation (maximum diameter 6.5 mm) and was deemed unsuitable for PCI. Diffuse, non-obstructive atherosclerotic plaques were present throughout the coronary tree without significant fixed stenoseS.
Figure 1
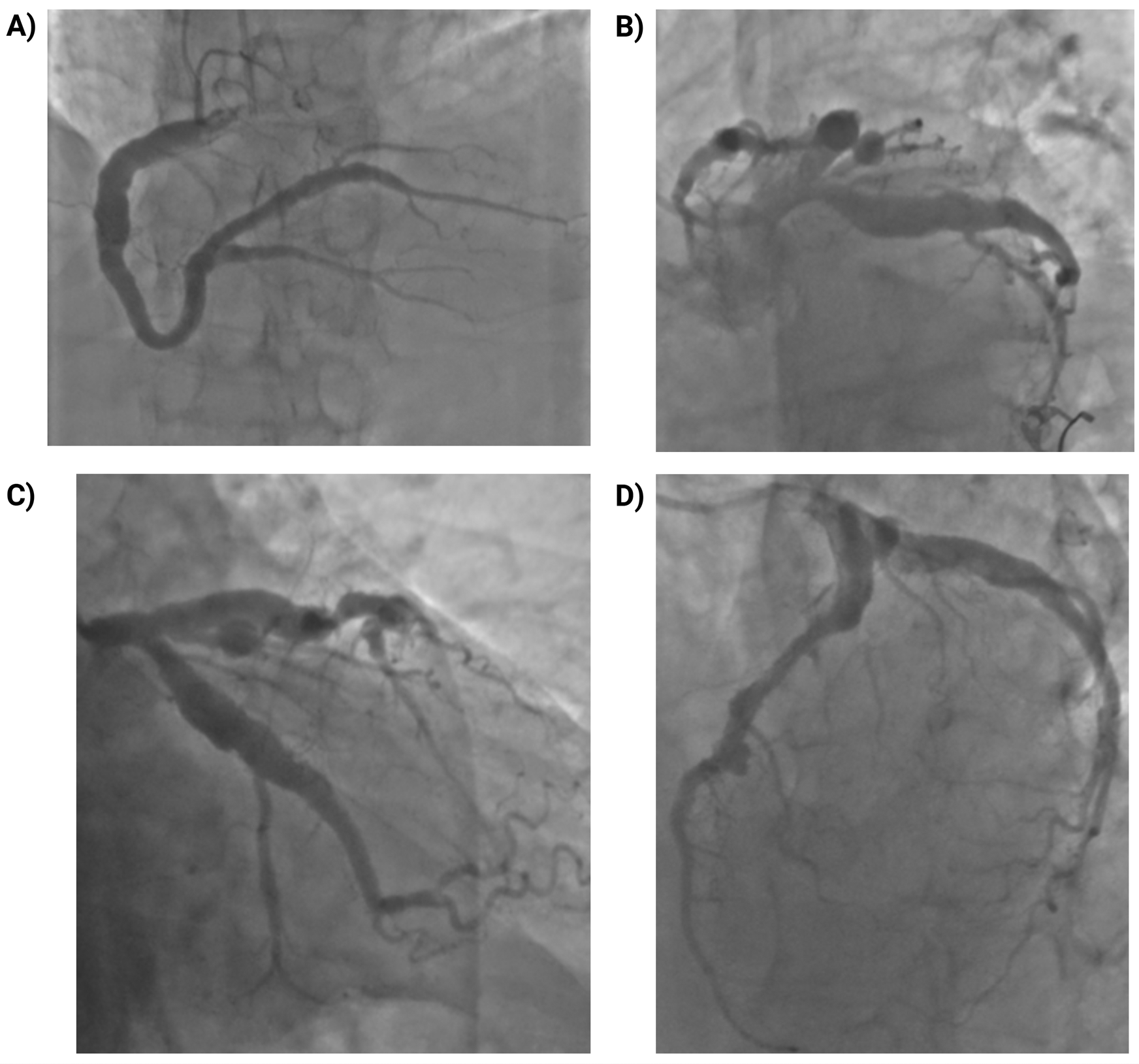
Coronary angiography showing diffuse multivessel coronary ectasia (markis type I). (A) Right coronary artery (RCA): marked ectasia with slow flow (TIMI 2); maximum reference diameter ∼8.7 mm; diffuse non-obstructive atherosclerosis. (B) Left main coronary artery (LMCA): ectatic with markedly enlarged ostium and trunk; no intraluminal lesions. (C) Left anterior descending artery (LAD): marked ectasia with diffuse atheroma; maximum reference diameter ∼7.1 mm. (D) Left circumflex artery (LCx): ectatic, large-caliber vessel with slow flow (TIMI 2); maximum reference diameter ∼7.2 mm; side branches with diffuse non-obstructive plaques.
Figure 2
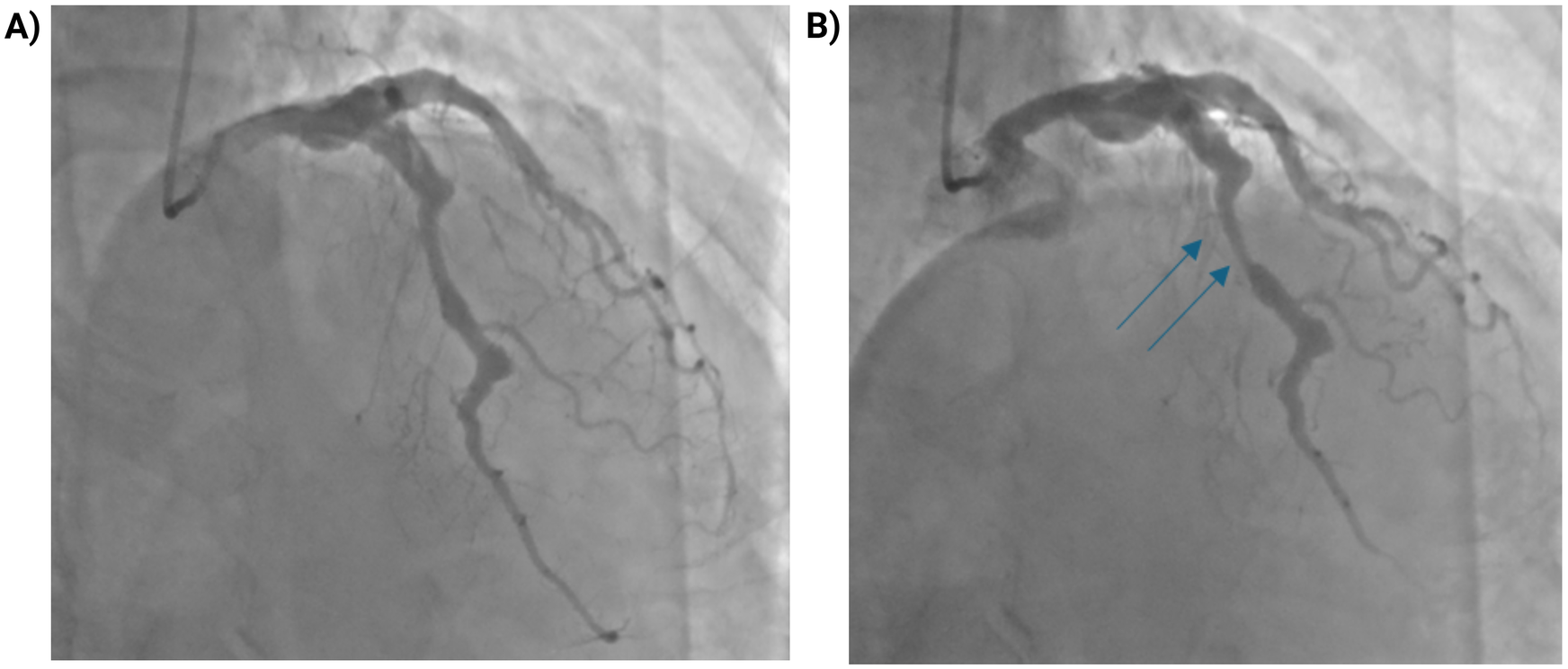
Myocardial bridging of the mid-LAD with dynamic systolic compression. (A) Coronary angiogram in diastole showing the mid-LAD segment. (B) During systole, dynamic compression of a myocardial bridge in the mid-LAD segment (blue arrows) produces a “milking effect” with approximately 75% systolic narrowing. The apparent degree of stenosis may be overestimated due to the surrounding coronary ectasia.
Based on the findings observed on coronary angiography, a Type 2 myocardial infarction secondary to supply–demand mismatch was established. The markedly slow and turbulent flow in the ectatic segments, combined with the hemodynamically significant myocardial bridge which further increased dynamic obstruction and impaired distal perfusion—supported this diagnosis.
Transthoracic echocardiography showed a preserved left ventricular ejection fraction (>65%). Regional wall-motion abnormalities were noted (hypokinesia of the basal inferior and basal inferoseptal segments). Diastolic function was grade I (mild). Right ventricular systolic function was normal [tricuspid annular plane systolic excursion (TAPSE) 20 mm]. The left atrium was severely enlarged (volume index 57 ml/m2). No significant valvular abnormalities were identified, and the pericardium was normal.
Given the high bleeding risk and the extensive ectatic anatomy without focal, flow-limiting stenosis, single antiplatelet therapy (SAPT) with clopidogrel was preferred, and anticoagulation was continued with warfarin (target INR 2.0–3.0). In line with contemporary recommendations for patients requiring long-term oral anticoagulation without recent stent implantation, we selected clopidogrel as the single antiplatelet agent and withheld aspirin to minimize major bleeding risk while maintaining adequate platelet inhibition. A high-intensity statin and a beta-blocker were also administered, and his pre-existing medications were maintained. He responded favorably to medical therapy with symptom improvement and was discharged in stable condition.
During follow-up, the patient was evaluated twice by the cardiology department: the first visit focused on optimizing medical therapy, and the second involved ordering an exercise stress test as part of the entry assessment for the cardiac rehabilitation program. Additional follow-up was conducted through scheduled phone calls and INR monitoring, both of which demonstrated good treatment adherence and no recurrent events. The first in-person visit took place one month after hospital discharge, and the second occurred three months later; the latter represented the final in-person contact of a four-month follow-up period. Throughout this interval, the patient remained asymptomatic, and no repeat coronary angiography or CCTA was deemed necessary given the absence of recurrent ischemic symptoms and stable laboratory parameters apart from routine INR monitoring (Figure 3).
Figure 3
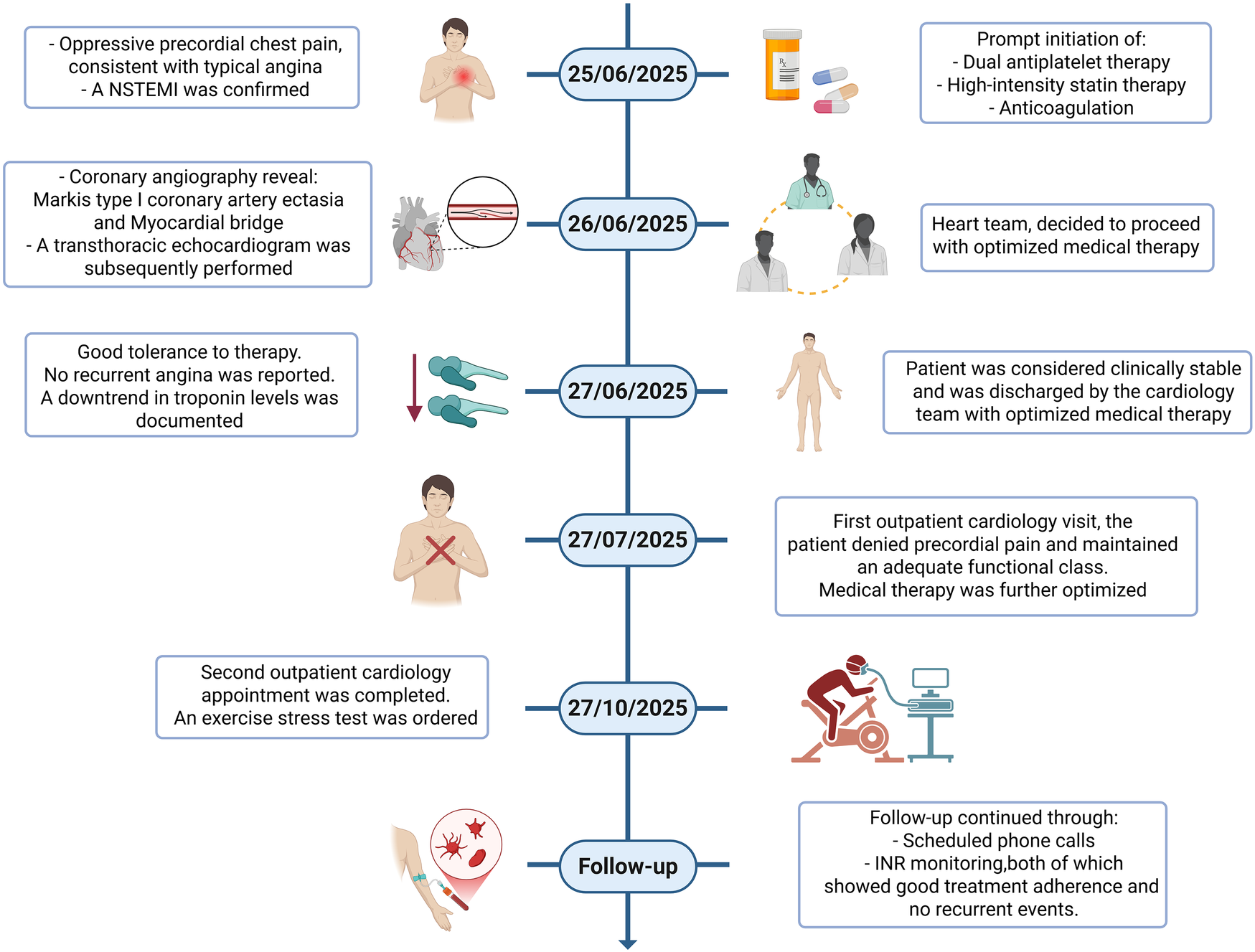
Chronological summary of the clinical course. Timeline illustrating symptom onset, emergency department presentation, ECG changes, biomarker trajectory, timing of coronary angiography, in-hospital management, discharge, and outpatient follow-up visits.
3 Discussion
CAE exhibits a wide clinical spectrum. Some patients present with concomitant, functionally significant stenoses, whereas others—like ours—show diffuse ectasia without fixed critical obstruction. Presentations range from typical angina to ACS, including both STEMI and NSTEMI, driven by a combination of slow-flow/no-reflow, in situ thrombosis, and distal embolization (6). Most CAE in adults is acquired and associated with atherosclerosis; traditional risk factors include hypertension, smoking, and familial hypercholesterolemia, while less common etiologies comprise inflammatory and connective-tissue disorders (e.g., vasculitides, systemic lupus erythematosus, Ehlers–Danlos, and Kawasaki disease) (2).
Myocardial bridging (MB) is a relatively common anatomical variant whose true prevalence depends on the diagnostic modality—higher in autopsy series and CCTA, and lower by invasive angiography. The LAD—especially its proximal and mid segments—is most frequently involved; the LCx and RCA are less often affected. CCTA detects MB more often than conventional angiography (∼19%–22% vs. 2%–6%) (7). Physiologically, MB can precipitate supply–demand mismatch by accentuating systolic compression and abbreviating diastolic perfusion during tachycardia. Depth, length, and location of a MB all contribute to the severity of vessel compression; the duration of systolic narrowing is also relevant. In general, a MB is considered hemodynamically significant when there is a visually estimated ≥70% reduction in the minimal luminal diameter during systole, with ≥35% persistent narrowing extending into early diastole (7, 8). In our patient, angiography documented a prominent mid-LAD bridge with ∼75% systolic “milking effect”, superimposed on Markis type I three-vessel ectasia with TIMI 2 slow flow—two substrates that synergistically impair effective myocardial perfusion despite the absence of a discrete, focal culprit stenosis. Although the regional wall-motion abnormality was confined to the basal inferior and inferoseptal segments, which correspond predominantly to the right coronary artery territory, this finding is consistent with the markedly ectatic RCA and globally impaired TIMI 2 flow in all three epicardial vessels. We therefore interpret the event as the result of a combined mechanism—diffuse Markis type I ectasia with slow flow, particularly in the RCA, together with a hemodynamically significant mid-LAD bridge—rather than a single-vessel culprit lesion.
Revascularization in ectatic/aneurysmal culprit vessels is technically challenging and carries higher risks of no-reflow, distal embolization, and stent thrombosis; intermediate-term outcomes after PCI in this context include increased mortality, target vessel revascularization, and recurrent MI (1). Mechanistically, device sizing and apposition are problematic in markedly dilated segments; dense thrombus burden and sluggish flow favor acute/subacute thrombosis; and, when MB coexists, dynamic compression adds mechanical stress that may predispose to stent fracture or restenosis if a device is implanted across the bridged segment (9). Accordingly, in the absence of a focal, flow-limiting lesion, contemporary management often prioritizes optimized medical therapy with individualized antithrombotic regimens (4).
Evidence guiding antithrombotic strategy in CAE is limited. A small randomized trial in ACS suggested that DOAC plus SAPT reduces bleeding vs. DAPT while preserving ischemic protection (10). Notably, our patient experienced an ischemic event despite chronic rivaroxaban for atrial fibrillation. In this setting—diffuse Markis I ectasia, angiographic slow flow, and prior DOAC exposure at the time of presentation—the heart-team favored SAPT plus warfarin with INR monitoring to balance thrombotic and bleeding risks and to allow tight anticoagulation control (11). Case-based experience has likewise reported favorable outcomes with warfarin-based regimens in recurrent ischemia related to ectasia, though high-quality comparative data are lacking (11). Given the coexistence of MB, beta-blockade was instituted to lower heart rate and contractility, prolong diastole, and lessen systolic compression; nitrates were avoided due to the potential to exacerbate dynamic narrowing via reflex tachycardia and increased vessel compliance (5). High-intensity statin therapy was prescribed to target shared atherothrombotic risk factors.
Diagnostic interpretation in CAE with MB merits nuance. First, angiography can overestimate the apparent percent stenosis adjacent to ectatic reference segments, which can mislead revascularization decisions. Second, although invasive coronary angiography established the diagnosis in our case, CCTA offers superior anatomic delineation of MB and aneurysmal/ectatic morphology and may be preferred when the clinical context allows (7). Third, intravascular imaging (e.g., IVUS) improves diagnostic precision in aneurysmal/ectatic segments and can clarify plaque/thrombus composition; it was not available in our setting. Advanced intracoronary imaging (IVUS/OCT) and CCTA were not available in our institution at the time of presentation, which limited further anatomic and functional characterization and represents an important limitation of this report. Finally, when symptoms persist despite optimal therapy, functional assessment focused on diastolic physiology (e.g., diastolic FFR/iFR) may help quantify bridging significance and guide consideration of advanced therapies (9).
Our case complements the limited literature on the coexistence of CAE/CAA and MB, as summarized in Table 2. Czepe et al. reported CAE with MB diagnosed by CCTA but did not detail management, underscoring the variability in diagnostic pathways (9). Zhen Ye et al. described CAA with MB characterized by angiography and IVUS and managed medically with antiplatelet and anti-ischemic therapy, with favorable follow-up (12). In contrast, our patient presented with NSTEMI on a background of diffuse Markis I ectasia and a hemodynamically significant mid-LAD bridge; the combination of extensive ectasia, slow flow, and dynamic compression—without a discrete, stentable culprit—supported a conservative strategy of SAPT plus oral anticoagulation and rate-control therapy.
Table 2
| Reported cases | Sex/Origin | Age at presentation | Type of coronary ectasia | Diagnostic test/method | Treatment | Outcomes |
|---|---|---|---|---|---|---|
| Case 1. Ye Z. et al., 2021 (12) | Woman, Fujian Province, China | 54-year-old | Markis type III | Coronary Angiography (CAG) | Aspirin 100 mg/d, Rosuvastatin 10 mg/d, Metoprolol 47.5 mg/d, and Amlodipine 5 mg/d | The patient was in good condition at the 5-mo follow-up by phone. |
| Case 2. Czepe G. et al., 2024 (9) | Woman, Lublin, Poland | 78-year-old | Markis type III | Coronary computed tomography angiography (CCTA), | No specific description. Currently: clinical observation at a cardiology outpatient clinic, measures to modify risk factors and optimize treatment, including treatment of hypertension. |
There is no description of the follow-up. |
| Current report | Man, Quito, Ecuador | 80-year-old | Markis type I | Coronary angiography (CAG) | Clopidogrel 75 mg/d, warfarin (INR 2.0–3.0), high-intensity statin, beta-blocker, plus standard therapy for comorbidities. | Asymptomatic with no recurrent ischemic events during 4-month follow-up; therapeutic INR on serial monitoring. |
Summary of previous case reports of coronary ectasia plus myocardial bridging.
CAG, coronary angiography; CCTA, coronary computed tomography angiography; INR, international normalized ratio; d, day; mo, month.
Interventional or surgical therapies should be reserved for selected refractory cases—for example, deep or long bridges (>25 mm length or >5 mm depth), objective ischemia persisting despite optimized medical therapy, or when a discrete, flow-limiting stenosis is demonstrable (9). PCI across MB carries non-trivial risks, including in-stent restenosis and stent fracture; surgical options (myotomy or CABG) are considered when symptoms and ischemia remain refractory, but myotomy bears risks such as ventricular perforation, aneurysm formation, and bleeding (9). In our patient, interventional therapy was not pursued given the absence of a clear PCI target, the unfavorable ectatic substrate, and the favorable clinical response to medical therapy (4, 5, 11).
Finally, while CAE and CAA are distinct entities—diffuse vs. focal dilation (≥1.5× the reference diameter)—they share etiologies and pathophysiologic mechanisms and are sometimes conflated in clinical practice. To our knowledge, reports of myocardial infarction in the setting of Markis I CAE coexisting with MB remain scarce; our case underscores that this coexistence may be under-recognized in ACS and that outcomes can be acceptable with carefully individualized, conservative management. During follow-up, our patient remained asymptomatic, with therapeutic INR values and no recurrent ischemic events.
4 Conclusions
Diffuse coronary artery ectasia (Markis type I) coexisting with a hemodynamically significant mid-LAD myocardial bridge can precipitate NSTEMI without discrete obstructive lesions and challenges conventional revascularization strategies. In this anatomic context, PCI may be hazardous (thrombus burden, malapposition, no-reflow, device complications), making optimized medical therapy—rate control with beta-blockade, high-intensity statin, and individualized antithrombotic therapy—a reasonable first-line approach.
This case also highlights interpretive pitfalls of angiography (overestimation of stenosis adjacent to ectatic segments) and the potential value of CCTA/IVUS and diastolic physiologic assessment when available. With careful risk–benefit appraisal, avoidance of nitrates in MB, and structured follow-up (including INR monitoring), favorable outcomes are achievable, while registries and comparative studies are needed to refine antithrombotic selection and indications for invasive or surgical treatment in CAE with concomitant MB.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MR-C: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft. FR-A: Data curation, Investigation, Methodology, Resources, Software, Validation, Writing – original draft. CA: Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft. SD: Investigation, Methodology, Resources, Software, Validation, Writing – original draft. JG: Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft. JI-C: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Kawsara A Núñez Gil IJ Alqahtani F Moreland J Rihal CS Alkhouli M . Management of coronary artery aneurysms. JACC Cardiovasc Interv. (2018) 11:1211–23. 10.1016/j.jcin.2018.02.041
2.
Yuan M Li R Wu Q Chen Q . Prevalence and angiographic characteristics of coronary artery ectasia among patients with coronary artery disease: a retrospective analysis between 2014 and 2022. Tohoku J Exp Med. (2023) 261:165–71. 10.1620/tjem.2023.J065
3.
Richards GHC Hong KL Henein MY Hanratty C Boles U . Coronary artery ectasia: review of the non-atherosclerotic molecular and pathophysiologic concepts. Int J Mol Sci. (2022) 23:5195. 10.3390/ijms23095195
4.
Esposito L Di Maio M Silverio A Cancro FP Bellino M Attisano T et al Treatment and outcome of patients with coronary artery ectasia: current evidence and novel opportunities for an old dilemma. Front Cardiovasc Med. (2022) 8:805727. 10.3389/fcvm.2021.805727
5.
Yuan S-M . Myocardial bridging. Braz J Cardiovasc Surg. (2015) 31:60–2. 10.5935/1678-9741.20150082
6.
Zhou L Gong X Dong T Cui H Chen H Li H . Wellens’ syndrome: incidence, characteristics, and long-term clinical outcomes. BMC Cardiovasc Disord. (2022) 22:176. 10.1186/s12872-022-02560-6
7.
Sternheim D Power DA Samtani R Kini A Fuster V Sharma S . Myocardial bridging: diagnosis, functional assessment, and management. J Am Coll Cardiol. (2021) 78:2196–212. 10.1016/j.jacc.2021.09.859
8.
Danek BA Kearney K Steinberg ZL . Clinically significant myocardial bridging. Heart. (2024) 110:81–6. 10.1136/heartjnl-2022-321586
9.
Czepe G Przybylski P Czekajska-Chehab E . Novel case of coronary artery ectasia and myocardial bridging in one segment detected by coronary computed tomography angiography. J Clin Imaging Sci. (2025) 15:1. 10.25259/JCIS_149_2024
10.
Araiza-Garaygordobil D Gopar-Nieto R Sierra-Lara Martínez JD Mullasari AS Belderrain-Morales N Nájera-Rojas NA et al A randomized trial of antithrombotic therapy in patients with acute coronary syndrome and coronary ectasia. Am Heart J. (2025) 281:103–11. 10.1016/j.ahj.2024.11.012
11.
Bhuiyan M Badar F Ashraf A Chryssos ED Iftikhar A . Recurrent ST-elevation myocardial infarction (STEMI) in coronary artery aneurysm secondary to atherosclerosis. Cureus. (2022) 14(9):e28757. 10.7759/cureus.28757
12.
Ye Z Dong X-F Yan Y-M Luo Y-K . Coronary artery aneurysm combined with myocardial bridge: a case report. World J Clin Cases. (2021) 9:3996–4000. 10.12998/wjcc.v9.i16.3996
Summary
Keywords
coronary artery ectasia, myocardial bridging, NSTEMI, antithrombotic therapy, conservative management
Citation
Rojas-Cadena M, Rodríguez-Arcentales F, Arteaga C, Davila S, Gaibor JC and Izquierdo-Condoy JS (2025) Uncommon association of coronary artery ectasia and myocardial bridge presenting as non–ST-segment elevation myocardial infarction: a case report. Front. Cardiovasc. Med. 12:1727448. doi: 10.3389/fcvm.2025.1727448
Received
17 October 2025
Revised
24 November 2025
Accepted
26 November 2025
Published
09 December 2025
Volume
12 - 2025
Edited by
Tommaso Gori, Johannes Gutenberg University Mainz, Germany
Reviewed by
Robert-Mihai Enache, Fundeni Clinical Institute, Romania
Vegim Zhaku, State University of Tetova, North Macedonia
Updates
Copyright
© 2025 Rojas-Cadena, Rodríguez-Arcentales, Arteaga, Davila, Gaibor and Izquierdo-Condoy.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Juan S. Izquierdo-Condoy juan1izquierdo11@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.