- 1Department of Geography, University of Exeter, Exeter, United Kingdom
- 2Plymouth Marine Laboratory, Plymouth, United Kingdom
- 3Department of Biosciences, University of Exeter, Exeter, United Kingdom
Electrochemical technology can be used to remove inorganic carbon from seawater and facilitate the removal of carbon dioxide (CO2) from the atmosphere. Electrochemical ‘Direct Ocean Carbon Capture and Storage’ (DOCCS) is a marine carbon dioxide removal (mCDR) method that removes atmospheric CO2 by releasing low-carbon seawater into the surface ocean, where it re-equilibrates with the atmosphere and stores atmospheric CO2. At the point of release, DOCCS discharge has low concentrations of dissolved inorganic carbon (DIC) and high pH, potentially causing unintended marine environmental impacts; however, its chemistry moves progressively towards that of ambient seawater as it dilutes and re-equilibrates with the atmosphere. To date, there are no published studies that investigate the impact of DOCCS discharge on marine ecosystems. Research from relevant analogues, where biological responses to low-DIC and/or high-pH seawater are investigated, provides some insight into potential DOCCS impacts. Despite this, significant evidence gaps remain. These evidence gaps are discussed alongside DOCCS-specific recommendations for future environmental impact research. Understanding the potential risks/benefits to marine ecosystems from discharge of low-DIC and high-pH seawater is critical to: (i) support licensing applications; (ii) develop any necessary mitigating actions; (iii) determine the net benefit of mCDR approaches; and (iv) stimulate informed public discourse about the acceptability of such approaches.
1 Introduction
1.1 Marine carbon dioxide removal
Analysis of emissions scenarios has shown that meeting the Paris Agreement goal to avoid global average temperature rise of more than 1.5°C above preindustrial levels will require not only the reduction of emissions but also the use of negative emissions technologies that can remove carbon dioxide (CO2) from the atmosphere (Bach et al., 2019; Masson-Delmotte et al., 2018). The ocean is the largest inorganic carbon reservoir in the Earth’s ocean–atmosphere-biosphere system (Ciais et al., 2014), estimated to hold approximately 38,000 Gt C, which is over 60 times the estimated carbon content of the atmosphere (Friedlingstein et al., 2023; Intergovernmental Panel on Climate Change (IPCC), 2023; Majumdar and Deutch, 2018). The ocean’s capacity to hold carbon draws attention to its potential use as a sink for atmospheric CO2 within climate mitigation strategies. Marine Carbon Dioxide Removal (mCDR) aims to accelerate the natural CO2 sequestration processes of the ocean through a range of different techniques (Bach et al., 2024). mCDR methods are typically less well understood (Smith et al., 2024) than terrestrial CDR methods at present, but mCDR has inherent advantages over land-based methods, including less competition for space (Johnson et al., 2024). All mCDR techniques manipulate the marine environment in some way and therefore have the potential to cause unintended environmental impacts.
Here, we discuss the potential marine environmental impacts of a mCDR approach that removes atmospheric CO2 by electrochemically removing CO2 from seawater. At the time of writing, the electrochemical removal of CO2 from seawater does not have an established name, and in the literature has been referred to as direct ocean capture (DOC) (Karunarathne et al., 2025; Al Yafiee et al., 2024), direct ocean removal (DOR) (Cross et al., 2023), electrochemical direct ocean capture (eDOC) (Aleta et al., 2023), and electrochemical ocean alkalinity cycling (OAC) (Eisaman, 2024). In this report, it will be referred to thereafter as Direct Ocean Carbon Capture and Storage (DOCCS) as this is used in the ‘State of CDR’ report (Smith et al., 2024). In this report, we will introduce and discuss some of the potential unintended environmental impacts that could be linked to DOCCS technology and highlight key knowledge gaps.
1.2 Direct Ocean Carbon Capture and Storage (DOCCS)
The vast majority of ocean carbon is stored in the form of dissolved inorganic carbon (DIC) (Equation 1) (Carroll et al., 2022). DOCCS achieves atmospheric CO2 removal by electrochemically, or otherwise, adjusting seawater to extract its DIC (Bach et al., 2024). DOCCS generates seawater depleted in DIC, which is released into the marine environment where it re-equilibrates with the atmosphere by ‘refilling’ with atmospheric CO2 (Halloran et al., 2025). DOCCS essentially uses the ocean’s natural carbon sequestration capability as a ‘pump’ or ‘pass through’ to draw CO2 out of the atmosphere (Aleta et al., 2023; Eisaman, 2024). Methods to remove DIC from seawater using DOCCS technology are broadly split into two: one which removes DIC in the form of a gas; and the other in the form of a solid (Aleta et al., 2023; Eisaman et al., 2018; National Academies of Sciences, Engineering, 2022). This report will focus its discussion on the former method, which due to significant energetic advantages and simplified output stream handling, is the approach being piloted today (Aleta et al., 2023).
Equation 1: Total seawater DIC (μmol kg-1) is the sum of three different inorganic carbon species, carbon dioxide (CO2 (aq)), bicarbonate (HCO3- (aq)), and carbonate (CO32- (aq)).
In electrochemical DOCCS, electrolysis of incoming seawater is used to physically divide it into two separate seawater streams enriched in hydrogen (H+) and hydroxide (OH-) ions, respectively, which can be used to manipulate the inorganic carbon chemistry of large volumes of seawater (Aleta et al., 2023; de Lannoy et al., 2018; Digdaya et al., 2020; Eisaman et al., 2012, 2018; Karunarathne et al., 2025; National Academies of Sciences, Engineering, 2022; Sharifian et al., 2021). This is because the balance of inorganic carbon species (CO2, HCO3-, and CO32-) influences the pH of seawater and vice versa (Cornwall et al., 2012; Millero, 2000) (Figure 1). The addition of acid (H+) to lower seawater pH to around 4, converts 100% of seawater DIC into CO2 (Aleta et al., 2023) (Figure 1), which can be liberated and vacuum stripped from seawater as a gas (de Lannoy et al., 2018). Removed CO2 is purified for long-term geological storage or utilised in other ways such as in synthetic fuel production (Eisaman et al., 2018), however it should be noted that any CO2 released from the use of DOCCS-derived products or services must ultimately be re-captured and stored to be considered CDR (Aleta et al., 2023). Acidic seawater that is depleted in DIC is then recombined with the electrochemically dissociated base (OH-) to restore seawater alkalinity to natural levels (Eisaman, 2024). The addition of base to low-DIC seawater increases the pH beyond the seawater’s original pH (Eisaman, 2024), due to depleted levels of inorganic carbon, and specifically carbonic acid, in that seawater. Treated water is then released into the marine environment where the atmosphere and ocean naturally equilibrate, drawing down atmospheric CO2 to replace the removed carbon (Aleta et al., 2023), typically over a period of months to years (Halloran et al., 2025).
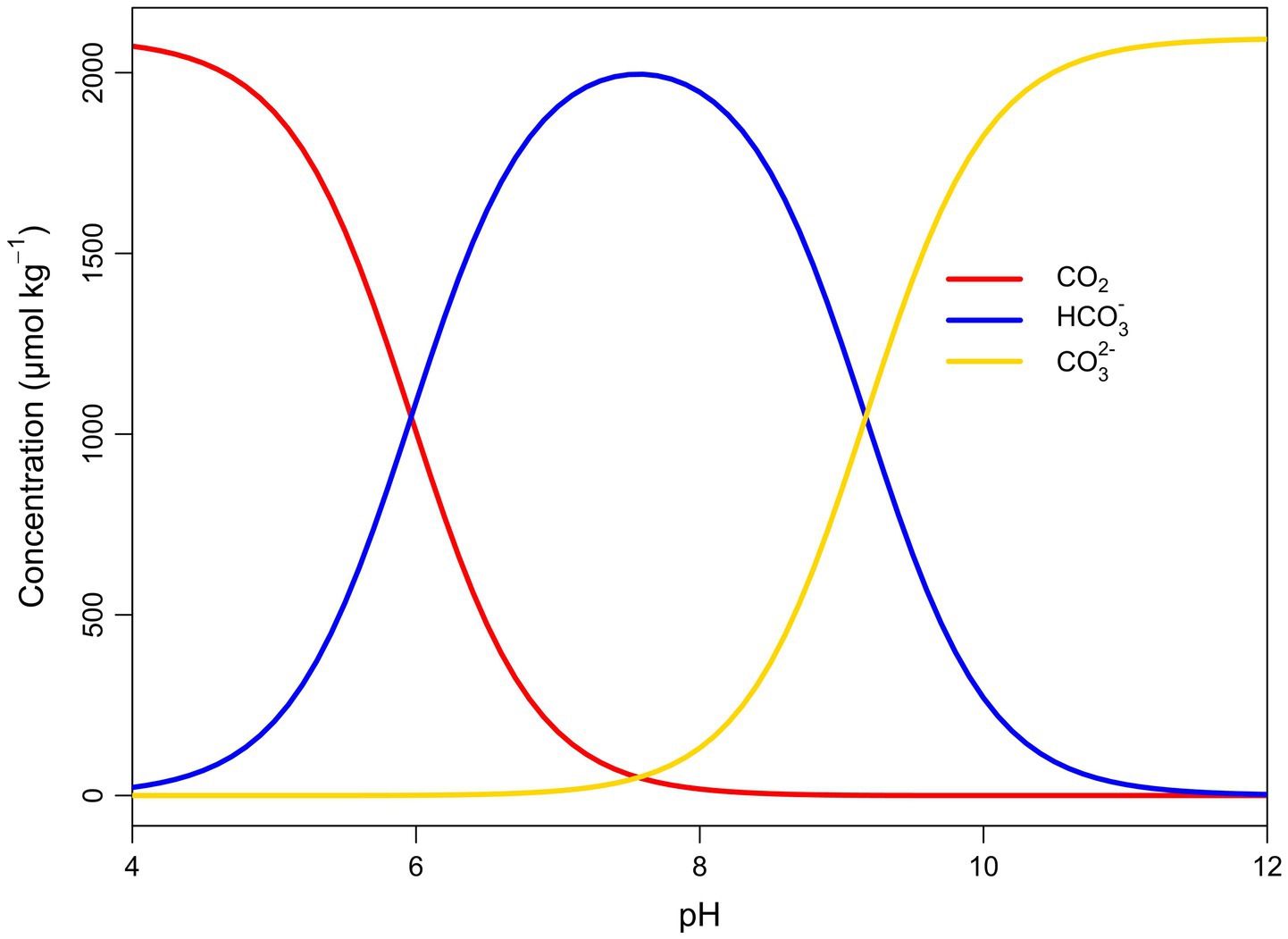
Figure 1. Bjerrum plot describing the relative concentrations (μmol kg-1) of different DIC species (Aqueous CO2, bicarbonate, and carbonate) in seawater at different pH levels. DIC (2095.78 μmol kg-1), salinity (35.03 psu), temperature (12.53°C), and hydrostatic pressure (0 bar) values from an annual mean in the Western English Channel (Kitidis et al., 2012). Plot produced with seacarb (Gattuso et al., 2024).
Several factors make DOCCS an attractive approach for CDR. By volume, seawater contains approximately 100-150 times more inorganic carbon than air (Karunarathne et al., 2025), meaning that removal efficiency could be higher than in Direct Air Carbon Capture and Storage (DACCS). Further to this, rather than needing to pump air across a sorption surface, DOCCS carbon removal takes place over a large surface area downstream of the point of decarbonised water release (Al Yafiee et al., 2024). Carbon removed by DOCCS has the potential to be extremely durable, as the CO2 gas that is removed from seawater and then purified can be deposited in long-term geological storage (Smith et al., 2024). However, the efficacy and sustainability of this approach is still being researched (Al Yafiee et al., 2024). After re-equilibration with the atmosphere, seawater released by DOCCS is chemically indistinct from surrounding ambient seawater (Eisaman, 2024). This offers advantages over other mCDR techniques like Ocean Alkalinity Enhancement (OAE), which results in increased DIC, alkalinity and pH after equilibrium has been reached (Eisaman, 2024). Unlike DOCCS, OAE could also result in the addition of chemical impurities to seawater associated with mineral feedstocks (Bach et al., 2019; Ferderer et al., 2024; Flipkens et al., 2024; Geerts et al., 2025; Guo et al., 2025; Hutchins et al., 2023; Xin et al., 2024). That said, versions of OAE that use base electrochemically generated from seawater avoid this issue (Marín-Samper et al., 2024; Oschlies et al., 2025). Before re-equilibration with the atmosphere, seawater released into the surface ocean following DOCCS will have a changed inorganic carbon chemistry: characterised by low DIC concentrations and high pH (Eisaman, 2024), which could impact marine ecosystems.
1.3 Aims and objectives
The potential marine ecosystem impacts associated with releasing low-DIC, high-pH seawater into the marine environment currently represents one of the biggest unknowns and barriers towards large-scale deployment of DOCCS technology (Digdaya et al., 2020; Karunarathne et al., 2025; National Academies of Sciences, Engineering, 2022). First this report describes how DOCCS impacts the inorganic carbon chemistry of seawater, including how it changes downstream of the plant. Key perturbations of the inorganic carbon chemistry are then linked to potential marine ecosystem impact by referring to relevant research that may provide insight. Finally, the report highlights key knowledge gaps and provides recommendations for future research on DOCCS marine impacts.
2 Influence of DOCCS on seawater inorganic carbon chemistry
At the point of release into the marine environment, DOCCS discharge has low DIC concentrations and a high pH (Eisaman, 2024). Table 1 describes the inorganic carbon chemistry of seawater from the Western English Channel under ambient conditions (Kitidis et al., 2012) (inflow) and following 90% DIC removal from DOCCS (outflow). pH is high because of the addition of the basic (OH--enriched) seawater to the low-DIC seawater stream. Total alkalinity (TA) remains at ambient levels because the alkalinity change associated with the addition of base (OH-) during pH manipulation balances that from the acid (H+) added to remove the carbon. The net effect is that inorganic carbon speciation is perturbed in DOCCS discharge (relative to ambient seawater, see Table 1), with a higher relative proportion of DIC in the form of CO32- and a smaller relative proportion as HCO3- and CO2 (Figure 1). While the impacts of DOCCS on seawater inorganic carbon chemistry are generally consistent (low DIC and high pH) across different ocean regions, natural differences in salinity, temperature, DIC, and TA causes some variation of pH and DIC speciation for DOCCS discharge. In the supplementary materials, we reproduce DOCCS inorganic carbon chemistry calculations for Tropical and Polar waters.
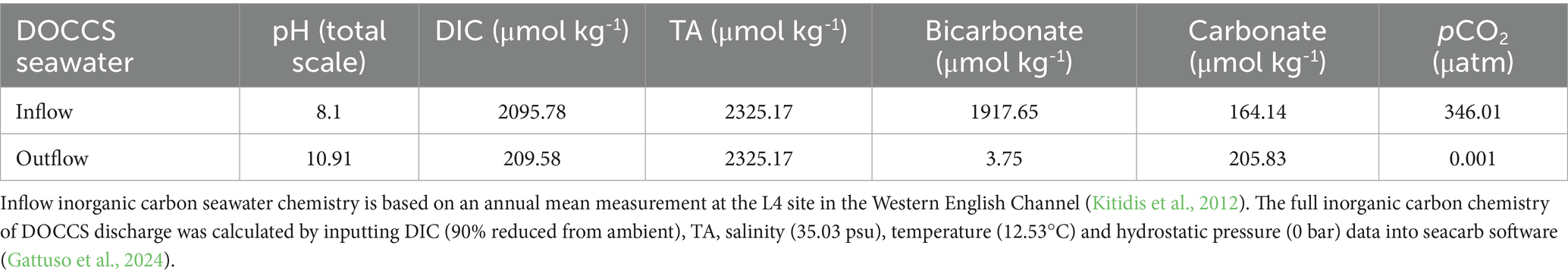
Table 1. Inflow and outflow inorganic carbon chemistry based on a 90% seawater DIC removal efficiency.
2.1 The impact of dilution and CO2 uptake on the inorganic carbon chemistry of DOCCS discharge
DOCCS discharge (low-DIC, high-pH seawater) released into the marine environment will become diluted by mixing with ambient seawater, such that the chemistry downstream of the discharge point eventually becomes indistinct from normal seawater (Eisaman, 2024) (Figure 2). In parallel to dilution, uptake of atmospheric CO2 by DOCCS discharge will return DIC (concentration and speciation) and pH to that of ambient seawater (Figure 3). The extent to which low-DIC, high-pH chemical perturbations persist in the marine environment will depend on the scale and frequency of DOCCS discharge, as well as the level of mixing and magnitude of air-sea CO2 flux.

Figure 2. Schematic demonstrating the dilution of DOCCS discharge in the marine environment. At the top of the image, pie charts are used to show the change in DIC concentration (size of the pie chart corresponds to the DIC concentration), and DIC speciation (CO2, HCO3-, CO32-). The left-hand pie chart represents DOCCS discharge when 90% of DIC has been removed. The right-hand pie chart represents ambient seawater inorganic carbon chemistry. Inorganic carbon chemistry values are based on data from the Western English Channel (Kitidis et al., 2012). Below the pie charts a scale bar shows how DOCCS discharge (which is low in DIC and high in pH) dilutes until its chemically indistinct from ambient seawater. The size of the arrows above the scale bar indicates the ‘CO2 uptake potential’ of DOCCS discharge as it is diluted with ambient seawater (greatest at the point of discharge and decreases until seawater is chemically indistinct from ambient seawater).

Figure 3. TA-DIC-pH contour plot illustrating the TA, DIC, and pH (total scale) transformations of DOCCS discharge as it goes through DOCCS processing. (1) The addition of acid (generated via seawater electrolysis) to seawater, lowers the pH, mobilising CO2; (2) the majority of seawater DIC (ca. 90%) is removed in the form of gas; (3) base addition (generated via seawater electrolysis) is added to seawater in the same molar quantities as the acid, increasing TA back to ambient levels; (4) re-equilibration with the atmosphere increases seawater DIC and lowers pH until the chemistry is indistinct from normal seawater. Red circle represents the inorganic carbon chemistry of ambient seawater and DOCCS discharge post-equilibration. Figure adapted from Eisaman (2024) and generated using seacarb (Gattuso et al., 2024), based on seawater inorganic carbon system and physical (temperature, salinity, pressure) data from the Western English Channel (Kitidis et al., 2012) (Table 1).
Figure 4 presents the inorganic carbon chemistry of DOCCS discharge as it dilutes with ambient seawater. As DOCCS discharge mixes with ambient seawater, DIC and TA both change at a predictable rate, because both behave linearly with respect to mixing (Dickson et al., 2007; Wolf-Gladrow et al., 2007). As DIC and TA concentrations for both DOCCS discharge and ambient seawater are known (Table 1), their resulting concentrations in any mixed sample can be calculated based on the dilution ratio (Table 2). TA is the same in both DOCCS discharge and ambient seawater (Table 1), so the concentration remains constant as the two mix (Figure 4 and Table 2). DIC is lower in DOCCS discharge compared to ambient seawater (Table 1) and so will increase as the two are mixed (Figure 4 and Table 2). While DIC and TA are considered conservative with respect to mixing (Wolf-Gladrow et al., 2007), pCO2, bicarbonate, carbonate, pH, and the calcium carbonate saturation state (Omega calcite and Omega aragonite) are not because of the non-linear nature of inorganic carbon system equilibria (Emerson and Hedges, 2008; Wolf-Gladrow et al., 2007). CO2 system solvers, such as ‘seacarb’ (used in this report) (Gattuso et al., 2024), can be used to predict the full inorganic carbon system when seawater DIC, TA, temperature, salinity, and pressure are known (Dickson et al., 2007). It is therefore possible to determine the full seawater inorganic carbon system at different dilution ratios of DOCCS discharge to ambient seawater (Figure 4). The dilution trends of the inorganic carbon system are comparable across different ocean regions, though there are subtle differences in pH and DIC speciation (see Supplementary materials for DOCCS inorganic carbon system calculations in polar and tropical seawater).

Figure 4. Inorganic carbon chemistry of DOCCS discharge as a function of dilution with ambient seawater. Modelling uses the ‘inflow’ and ‘outflow’ inorganic carbon chemistry and physical data shown in Table 1 to predict how the inorganic carbon chemistry of DOCCS discharge changes as it is diluted with ambient seawater. In the top plot TA, CO32-, DIC, HCO3-, and pCO2 trends are presented. In the bottom plot, pH (total scale) and the Calcite and Aragonite saturation states are presented. The full inorganic carbon chemistry of DOCCS discharge at different levels of dilution was generated in seacarb (Gattuso et al., 2024).

Table 2. DIC and TA calculations for DOCCS discharge at a range of dilution values when it is mixed with ambient seawater.
One phenomenon that calculations of seawater inorganic carbonate chemistry (Figure 4) do not account for is the formation of mineral precipitates that can occur in high-pH seawater. Aleta et al. (2023) has shown that a variety of solid carbonate and hydroxide precipitates are possible with dolomite (CaMg(CO3)2) precipitation dominating below pH 9.7, and artinite (Mg2CO3(OH)2) and brucite (Mg(OH)2) dominating above pH 9.7. The precipitation of carbonate and hydroxide minerals could result in the long-term removal of alkalinity (Bach, 2024; Gately et al., 2023; Halloran et al., 2025; Schulz et al., 2023), consequently reducing the pH and altering the speciation of inorganic carbon. It is important that DOCCS minimises precipitation in discharge because the removal of alkalinity would limit the capacity of DOCCS discharge to take up atmospheric CO2 (Halloran et al., 2025). Understanding how mineral precipitation could impact seawater chemistry in DOCCS discharge after it is released into the marine environment has not yet been thoroughly investigated.
To reduce the level of chemical perturbation in DOCCS discharge, DOCCS technology could hypothetically make use of specialist discharge systems that dilute DOCCS discharge and or encourage CO2 uptake before it is released into the marine environment. For instance, active sparging and passive cascades (National Academies of Sciences, Engineering, 2022), could be used to promote gas exchange in treated seawater, before DOCCS discharge is re-introduced into the marine environment. It should however be noted that pre-equilibration essentially turns DOCCS into DACCS and therefore no longer sidesteps DACCS’ challenges around movement of large volumes of air and the pressure drop associated with CO2 sorption (Erans et al., 2022).
2.2 Are DOCCS discharge conditions novel?
Surface seawater has a naturally stable pH of about 8.1 due to its strong buffering capacity facilitated by high alkalinity (Hansen, 2002; Jiang et al., 2019; Middelburg et al., 2020). Despite seawater’s ability to withstand changes in pH, changes in pH and therefore inorganic carbon chemistry do occur. Ocean acidification, the term used to describe the gradually decreasing average ocean pH since the industrial revolution (Doney et al., 2009), and the consequent shifts in the inorganic carbon system (Raven et al., 2005), is occurring globally in response to rising atmospheric CO2 (Gattuso and Hansson, 2011). In addition, more localised but even greater magnitude acidification regularly occurs in coastal regions as a consequence of accelerated microbial respiration (and associated oxygen removal and CO2 production) driven by the aftermath of anthropogenic eutrophication when algal blooms crash (Baumann, 2019). DOCCS discharge, which is low-DIC and high-pH seawater, represents the opposite change in inorganic carbon chemistry to that observed during ocean acidification (see Table 3).

Table 3. Approximate changes in inorganic carbon chemistry after four different chemical perturbations.
As demonstrated by the DOCCS process, seawater pH can also be increased. OAE, another emerging mCDR technique, which achieves CDR by increasing seawater alkalinity, also generates high pH in seawater (Bach et al., 2019). Seawater manipulated by DOCCS and OAE experience the same inorganic carbon system shift due to high pH (Figure 1) (Halloran et al., 2025). However, seawater DIC remains high or increased in OAE, whereas seawater DIC is reduced in DOCCS discharge (Table 3) (Halloran et al., 2025).
High-pH seawater conditions can also occur naturally without any human intervention. In eutrophic marine environments where rates of primary production are high, DIC is consumed through photosynthesis and if the CO2 is not restored via air-sea exchange, for example, due to restrictions on mixing or convection of that water, lower pCO2 levels result, and pH is elevated (Hendriks et al., 2014; Hinga, 2002; Krause-Jensen et al., 2016; Legrand et al., 2018; Søgaard et al., 2011). Eutrophic marine environments may be a useful natural analogue for DOCCS, as high pH is achieved by DIC removal rather than by alkalinity adjustments. However, high rates of primary production also drive other changes in seawater chemistry such as nutrient removal (Kemp et al., 1997; Tilman et al., 1982) and oxygen addition (Cloern et al., 2014; Herfort et al., 2012). In natural analogues, low-DIC, high-pH seawater conditions will persist until DIC levels are replenished via a range of processes (e.g., CO2 production through respiration, air-sea CO2 exchange, or by mixing with water enriched in DIC). Previous work has discussed natural analogues with respect to various mCDR techniques, but not for DOCCS (Bach and Boyd, 2021).
3 How could DOCCS discharge impact marine organisms?
To date there have been no studies that have investigated the specific environmental impacts of low-DIC, high-pH seawater on marine organisms in the context of DOCCS. In the absence of technology-specific environmental impact studies, existing research, primarily from ocean acidification, OAE and eutrophic marine environments studies may provide insight into potential DOCCS marine impacts. Here we outline the key chemical perturbations associated with DOCCS discharge (based on the inorganic carbon chemical perturbations shown in Table 1 and Figure 4) and provide an initial perspective of the potential impacts to marine organisms based on studies that experimentally manipulate the inorganic carbon chemistry of seawater. The scope of the discussion is constrained to studies investigating biological impacts of chemical perturbations most relevant to the inorganic carbon chemistry regime of DOCCS discharge.
3.1 Low DIC
The reduction of DIC in DOCCS discharge is a significant perturbation to seawater chemistry that is required in order for DOCCS technology to achieve mCDR (Halloran et al., 2025). Low DIC in seawater could impact marine organisms that rely on DIC for important cellular processes such as photosynthesis, calcification, and acid–base regulation. Studies have shown that phytoplankton can become limited at DIC concentrations < 0.5 mM (Berge et al., 2010; Clark and Flynn, 2000; Hansen et al., 2007; Søderberg and Hansen, 2007). These experiments were able to manipulate DIC levels without changing the pH, thus removing pH as a confounding factor in their experiments.
This was an improvement on earlier studies such as those by Riebesell et al. (1993) and Chen and Durbin (1994), which investigated the impact of carbon limitation on phytoplankton by increasing pH. DIC limitation of growth and photosynthesis has also been observed in a range of macroalgal species (Borowitzka, 1981; Flores-Moya and Fernández, 1998; Frost-Christensen and Sand-Jensen, 1990; García-Sánchez et al., 1994, 2016; Lignell and Pedersen, 1989) with two studies showing significant reductions in macroalgal growth when seawater DIC was reduced to 150 μM (Andría et al., 2001; Jiang et al., 2016). Reduced calcification in macroalgae and marine bivalves has also been demonstrated under DIC limiting conditions (Borowitzka, 1981; Thomsen et al., 2015).
3.2 High pH
Internal pH plays a key role in many aspects of cell biology (Putnam, 2001). If an increase in seawater pH associated with DOCCS discharge influences internal pH in organisms, it could impact a wide range of biochemical processes (Putnam, 2001).
3.2.1 Impacts to internal physiology
Ocean acidification research has shown that decreasing pH, driven by an increasing concentration of carbonic acid in seawater, has the potential to impact marine organisms through a variety of physiological pathways, such as acid–base regulation, metabolism, and calcification (Fabry et al., 2008; Guinotte and Fabry, 2008; Pörtner, 2008; Pörtner et al., 2004).
It is worth stating that for many animals, in particular fish, the elevated environmental pCO2 (rather than the seawater pH itself) is thought to be the cause of most of the impacts observed (Brauner et al., 2012; Wilson, 2020). This is because all aquatic animals, by the necessity for respiratory gas exchange, are permeable to gases, hence as environmental CO2 rises, internal (blood and tissue) CO2 rises in parallel (Brauner et al., 2019; Wilson, 2020). In turn, this elevation of internal CO2 results in at least temporary internal acidification (reduced pH) termed “acidosis” (Esbaugh, 2017; Fabry et al., 2008). However, many marine animals including fish and crustaceans recover blood and intracellular pH rapidly (hours) through acid–base regulation processes that primarily involves excretion of excess H+ or uptake of additional HCO3- via the gills (Brauner et al., 2019; Fehsenfeld and Weihrauch, 2017; Wilson, 2020). Internal pH regulation is assumed to have an energetic cost that can therefore potentially divert energy away from other important physiological processes like growth, swimming, and reproduction, functions that are known to be impaired by elevated CO2 (Lefevre, 2019; Munday et al., 2019; Skov, 2019).
CO2-driven ocean acidification is also known to interfere with ecologically relevant sensory processes such as olfaction, which may be via a direct effect of seawater pH change on external-facing receptors in aquatic animals (Porteus et al., 2018, 2021).
Given the well-established impacts of relatively small reductions in seawater pH and elevations in CO2 on marine organisms, it is highly likely that the opposite scenario, high seawater pH and reduced CO2, will result in similarly important physiological impacts to those identified in ocean acidification research. While there is far less research on the impacts of high pH on marine organisms, research investigating the environmental impacts associated with OAE and eutrophic marine environments may provide insights. For example, published research suggests that marine primary producers such as phytoplankton (Berge et al., 2012; Chen and Durbin, 1994; Hansen, 2002; Oberlander et al., 2025; Pedersen and Hansen, 2003; Rai and Rajashekhar, 2014; Søderberg and Hansen, 2007; Søgaard et al., 2011; Taraldsvik et al., 2000), macroalgae (Borowitzka, 1981; Frost-Christensen and Sand-Jensen, 1990; Jiang et al., 2018; Lignell and Pedersen, 1989; Menéndez et al., 2001; Tee et al., 2015), and seagrass (Invers et al., 1997) have a growth/performance upper limit of ca. pH 9. Experiments investigating the impacts of high pH on non-phototrophic marine organisms are less common but also demonstrate impacts above pH 8 (Camatti et al., 2024; Comeau et al., 2017; Cripps et al., 2013; Hansen et al., 2017). Several of these studies highlighted acid–base regulation, membrane transport and metabolic processes as potential impact pathways at high pH (Cripps et al., 2013; Hansen, 2002; Pedersen and Hansen, 2003).
The only research to investigate the impact of elevated pH on higher trophic levels comes from studies focused on brackish and freshwater ecosystems, where high pH can develop due to underlying geochemistry (Brauner et al., 2012) or episodically as a result of eutrophication and high daytime photosynthesis rates (Scott et al., 2005). There is evidence to suggest that high pH could impact the internal physiology of marine fish. For example, excretion of nitrogenous waste, a critical function in aquatic organisms, becomes inhibited in freshwater fish at high pH, resulting in the toxic accumulation of ammonia in the blood (Thompson et al., 2015; Wilkie and Wood, 1991, 1996; Wood, 1993, 2022). However, marine fish may be more tolerant to these impacts, as research shows that toxicity at high pH decreases with higher freshwater calcium concentrations (Iwama et al., 1997; Wilkie and Wood, 1996; Yesaki and Iwama, 1992). Calcium concentration averages 10 mM in seawater compared to a global median of 0.65 mM for inland waters (Pinheiro et al., 2021).
Ion transport at the gills is also inhibited at high pH, in particular the uptake of sodium and chloride ions, which can result in the gradual reduction of these two essential ions in freshwater fish (Thompson et al., 2015; Wright and Wood, 1985). In marine fish, removal of sodium and chloride ions by active transport is required to mitigate their continuous inward diffusion from seawater (Wood, 2022). High seawater pH exposure in marine fish could therefore have the opposite effect, whereby sodium and chloride gradually accumulate, reaching potentially toxic levels. In addition, elevated environmental pH is associated with an internal alkalosis in freshwater fish (Wilkie and Wood, 1996) and reduced blood pCO2. This is the opposite outcome to that described above for fish exposed to ocean acidification but necessitates similar acid–base regulatory processes (but opposite direction) to restore internal pH, that will require energy with potential knock-on effects for other energy requiring processes.
3.2.2 Exacerbation of DIC limitation due to elevated pH
At high pH the relative concentrations of CO2 and HCO3- decrease while the relative concentration of CO32- increases (Figure 1). This means at high pH, CO2 and HCO3- comprise a smaller fraction of DIC, which is already reduced (due to DIC removal) in DOCCS discharge (Table 1 and Figure 4). The combined impact of a reduced proportion of CO2 and HCO3- in an already reduced pool of DIC, could exacerbate DIC limitation for some marine organisms, particularly for processes like photosynthesis, calcification, and acid–base regulation that use CO2 and HCO3- (Findlay et al., 2011; Raven, 1994; Wilson, 2020). As highlighted in Bach et al. (2019) and Faucher et al. (2025), some studies have shown that under high-pH conditions phytoplankton growth is impacted when pCO2 falls below ca. 100 μatm (Bach et al., 2011, 2015; Faucher et al., 2025; Sett et al., 2014). However DIC was greater than 1.3 mM in all these studies. Research investigating the performance of marine dinoflagellates in eutrophic marine environments, where pH is increased by the removal of DIC, have shown that the impacts of low DIC can be exacerbated when seawater is at high pH (Hansen et al., 2007; Søderberg and Hansen, 2007). Both studies showed that the ‘growth compensation point’ (the concentration of DIC required for maintenance of cells), increased with pH, indicating that DIC became limiting at a higher concentration as pH increased.
CO2 is generally considered the main inorganic carbon substrate for terrestrial plant photosynthesis (Cooper and Filmer, 1969). However, CO2 is less readily available to marine plants than terrestrial plants because the diffusion of CO2 in seawater takes longer than in air (Reinfelder, 2011; Sun et al., 2023). To adapt to the challenge of CO2 limitation, marine primary producers have developed Carbon Concentrating Mechanisms (CCMs) that enhance inorganic carbon utilisation (Raven et al., 2011). CCMs commonly make use of the HCO3- pool, through carbonic anhydrase, an enzyme which converts HCO3- to CO2 (Beer et al., 2002; Reinfelder, 2011; Sun et al., 2023). In marine environments where CO2 becomes naturally depleted over time (e.g., rock pools), marine organisms with CCMs can continue functioning even when the concentration of CO2 is significantly reduced (Maberly, 1990; Murru and Sandgren, 2004). CCMs may help some marine producers tolerate DOCCS discharge conditions, as CO2 concentrations are lower than in ambient seawater (Table 1 and Figure 4). However, at very low dilution factors (ca. < 1), CCMs are unlikely to provide much benefit as the total pool of both CO2 and HCO3- is significantly reduced (Figure 4).
3.2.3 Increased calcium carbonate (CaCO3) saturation states
CaCO3 saturation state is a term used to describe how saturated seawater is with respect to CaCO3 minerals. The CaCO3 saturation state is ‘supersaturated’ when >1 and ‘undersaturated’ when <1 (Feely et al., 2004, 2008; Gangstø et al., 2008). When seawater is saturated, Ca2+ and CO32- ions are plentiful and assuming no complicating factors, mineral precipitation can occur inorganically. When seawater is undersaturated, CaCO3 dissolution is thermodynamically favoured. Calcifying marine organisms require additional carbon to build their CaCO3 structures, and saturated conditions provide a favourable environment for this to occur (Langdon et al., 2000). However, as saturation states decreases and become undersaturated, any exposed CaCO3 mineral structure can be subject to dissolution and the organism must use more energy to continue to calcify in these less favourable conditions (Findlay et al., 2011; Wood et al., 2008). In high-pH seawater a greater fraction of DIC is in the form of CO32- (Figure 1). At a dilution factor of ~ 0 - 2, the concentration of CO32- ions in DOCCS discharge temporarily increases above ambient levels (Figure 4), which increases the CaCO3 saturation state (Figure 4). Several studies have shown biological calcification increases in response to increasing TA and pH (Albright et al., 2016; Borowitzka, 1981; Gore et al., 2019). However, research by Bednaršek et al. (2025), which uses calcification response data from ocean acidification studies to try and predict how marine calcifiers may respond to high pH during OAE, suggests that the response of marine calcifiers to increasing pH is likely to be varied. The high CaCO3 saturation state observed at low dilutions (dilution factor ca. > 0 to 2) of DOCCS discharge could reduce dissolution of existing CaCO3 structures, potentially countering some of the projected impacts of ocean acidification on very local scales.
4 Key knowledge gaps and research suggestions
Based on what is currently known about the response of marine organisms to low DIC and/or high pH, its plausible that DOCCS discharge could impact marine organisms when the degree of seawater CO2 removal is high and any pre-dilution before discharge is low. However, major gaps in understanding regarding DOCCS environmental impacts remain. Addressing the knowledge gaps to support the responsible development and regulation of DOCCS requires a collaborative and coordinated response.
4.1 Lack of empirical environmental impact data for DOCCS
There is currently no published research that directly studies the environmental impacts of DOCCS on marine organisms/ecosystems. The combined impact of low DIC and high pH is central to understanding how marine organisms may be impacted by DOCCS discharge waters. Ocean acidification and OAE research highlights that marine organisms could be sensitive to inorganic carbon chemistry changes generated by DOCCS, however none of these experiments replicate the conditions experienced under DOCCS so should be used only to generate hypotheses.
The most relevant data on potential DOCCS impacts comes from studies investigating the performance of photosynthesising marine organisms in naturally occurring eutrophic environments. Various techniques were used to manipulate inorganic carbon chemistry in these studies, often holding either DIC or pH constant while adjusting the other (Andría et al., 2001; Borowitzka, 1981; Hansen et al., 2007; Søgaard et al., 2011). Holding one variable constant while changing another allows researchers to examine the independent effects of low DIC and high pH on marine organisms but does not address their combined impact. Many experiments that raise pH and hold DIC constant do so by adding alkalinity, which is similar to an OAE experiment (Rai and Rajashekhar, 2014). Other studies combined the effects of low DIC and high pH and assessed the impacts using “drift” experiments. Drift experiments allow DIC and pH to covary while photosynthetic organisms grow in a closed system (Berge et al., 2012). An alternative approach generates low-DIC and high-pH conditions by acidifying seawater, removing DIC as CO₂, and adding alkalinity (Hansen et al., 2007; Søderberg and Hansen, 2007). This technique, which allows for precise control of DIC and pH, and does not alter any other seawater chemistry, resembles DOCCS discharge conditions most closely. The combined approach of seawater DIC removal and alkalinity addition is likely to be the most appropriate technique for simulating DOCCS discharge conditions for environmental impact experiments as it requires minimal equipment and can be achieved in most laboratories.
While analogues of DOCCS discharge conditions are useful for directing research, they do not provide enough evidence to make strong conclusions about potential DOCCS environmental impacts. As DOCCS environmental impact experiments are currently in their infancy (CNN, 2025; BBC News, 2025) early research should investigate the short-term physiological response of key marine organism groups, both photosynthetic (phytoplankton, macroalgae) and non-photosynthetic (bivalves, fish), to short term DOCCS discharge exposure, at a range of dilution levels. Investigating direct and immediate impacts, could help early identification of important ecological sensitivities to DOCCS discharge, and shape future environmental impact research priorities.
The long-term marine ecosystem impacts of sustained exposure to low-DIC, high-pH conditions generated by DOCCS have not been studied. Short-term studies on individual marine organisms are in early development (CNN, 2025; BBC News, 2025) but there is no data on the ecosystem-wide, chronic changes that may unfold. Ocean acidification research has shown that stressors can cause long-term shifts in biodiversity, organism behaviour, and ecosystem services that are not picked up by acute or single-generation laboratory experiments (Dupont et al., 2008; Howald et al., 2022; Pedersen et al., 2014). Ultimately, long-term laboratory studies, similar to those conducted for ocean acidification research, will be needed to determine if chronic impacts arise due to sustained exposure to low-DIC, high-pH water.
Maintaining ‘non-equilibrated’ DOCCS conditions may be challenging in long-term laboratory studies as DOCCS discharge will have a natural tendency to draw in atmospheric CO2, changing the experimental conditions. Potential CO2 uptake by non-equilibrated seawater is also highlighted in ‘Guide to best practices in Ocean Alkalinity Enhancement Research’ (Iglesias-Rodríguez et al., 2023; Schulz et al., 2023). Simulating long-term DOCCS discharge conditions in a laboratory, may require either; a closed-system design, where the experiment is closed off from the atmosphere; or the continuous supply of freshly generated low-DIC, high-pH seawater into the experiment. Further, inorganic mineral precipitation will occur at high pH (discussed more in Section 4.2), which could cause the seawater chemistry to evolve through the experiment, again pointing to the need for mesocosms with a continuous supply of fresh decarbonised water (Halloran et al., 2025).
The positive or negative response of individual species to low-DIC, high-pH seawater must be considered within the context of complex and highly connected marine ecosystems as the net ecosystem response to DOCCS discharge may differ from the sum of individual species responses. Predicting secondary impacts (i.e., the impacts within an ecosystem that occur indirectly as a result of primary impact) due to low-DIC, high-pH seawater will be as challenging as it was for ocean acidification and OAE research. Net ecosystem impact studies will require a multifaceted approach that includes the collection of empirical data from laboratory- and field-based experiments, long-term and continuous environmental monitoring (including baseline monitoring), and ecological modelling. Prior to field experiments, single and multi-species mesocosm studies, as has been done for OAE (Bhaumik et al., 2025; Faucher et al., 2025; Sánchez et al., 2024), should be conducted to capture and explore the short to medium term response of natural ecosystems to DOCCS discharge and ensure that the risk of environmental impact in field experiments is low.
4.2 Chemical uncertainty for DOCCS discharge
Figure 4 demonstrates the expected theoretical inorganic carbon chemistry in DOCCS discharge at different mixing points downstream. However, as briefly mentioned in Section 2.1, the modelling used to determine the inorganic carbon chemistry does not consider inorganic precipitation that occurs in high-pH seawater. Inorganic precipitation could impact the marine environment through the removal of essential nutrients and/or by physical disturbance (Gately et al., 2023). The removal of dissolved ions via inorganic precipitation will have a marked effect on the inorganic carbon chemistry and ion concentration of DOCCS discharge (Gately et al., 2023; Halloran et al., 2025), changing its chemical profile.
For the former, inorganic precipitation can result in the removal of DIC and TA from the solution (Gately et al., 2023; Halloran et al., 2025; Ramírez et al., 2025), which will have subsequent impacts to the pH, and the relative proportions of the inorganic carbon species. Modelling of the inorganic carbon chemistry that does not consider inorganic precipitation may risk mis-characterising the inorganic carbon chemistry of DOCCS discharge when pH is high (ca. >9). To obtain accurate measurements of DIC and TA during DOCCS environmental impact experiments, seawater samples will need to be filtered to remove any precipitate, as has been suggested for OAE inorganic carbon chemistry measurements (Schulz et al., 2023).
pH can also influence the bioavailability of nutrients in seawater. Ocean acidification research has shown how low pH can change the bioavailability of micronutrients and lead to potential biological impacts in marine organisms (Cheriyan et al., 2024; Hoffmann et al., 2012; Millero et al., 2009; Stockdale et al., 2016). There is far less evidence for how high pH could impact the bioavailability of nutrients in seawater. At high pH, mineral precipitation can result in the reduction of dissolved nutrients such as Ca, Mg, Fe, Si, and P in seawater (Aleta et al., 2023; Gately et al., 2023; Moras et al., 2022), which could limit important physiological processes such as calcification (Dixon-Anderson, 2021; Ries, 2010). Experiments investigating the performance of phytoplankton in high-pH environments have briefly discussed the possibility of high pH directly impacting nutrient availability, though tend to attribute biological impacts to different mechanisms of the pH effect like intracellular impacts and CO2 limitation (Hansen, 2002; Hansen et al., 2007; Lundholm et al., 2004). Alternatively, phytoplankton research by Olsen et al. (2006), demonstrated that a photosynthesis-induced increase in seawater pH to 9, resulted in up to 20% of the phosphate in the system precipitating out of solution.
To develop a better understanding of how high pH influences inorganic carbon system dynamics and nutrient availability in DOCCS discharge, laboratory experiments and modelling should be combined to characterise the inorganic carbon chemistry and nutrient profiles of DOCCS discharge at a range of dilution levels. This is particularly important in scenarios where the degree of DOCCS carbon removal is high, and the level of dilution is low because high pH levels and abiotic mineral precipitation are more likely.
4.3 The impact of spatial scale
The extent and duration to which low-DIC, high-pH conditions persist in the marine environment following the release of DOCCS discharge will be determined by the rate of mixing with ambient seawater and by the rate of atmospheric CO2 uptake by DOCCS discharge (Bednaršek et al., 2025; Schulz et al., 2023). The uptake of CO2 by DOCCS discharge is expected to occur over the course of months to years (Halloran et al., 2025) and is influenced by physical, chemical, and biological factors (Halloran et al., 2025). Dilution of DOCCS discharge is likely to happen over much shorter timescales, though this is scale dependent and influenced by the outflow rate and the local hydrodynamic conditions.
At scales comparable to other point source-pollution outflows, such as sewage outfalls or waste discharge from ships, DOCCS discharge is expected to dilute quickly, posing little risk to the marine environment (Hunt et al., 2010; Lewis and Riddle, 1989; Loehr et al., 2006; Tate et al., 2016; Water Research Centre Limited, 2024). At these scales, low-DIC, high-pH perturbations may only exist within a short distance (e.g., a few meters) of the point source. If DOCCS technology was scaled by several orders of magnitude, dilution would occur at a much slower rate (assuming DOCCS discharge was subject to the same mixing), and low-DIC, high-pH conditions could persist in the marine environment for longer and over greater spatial scales.
For prospective DOCCS sites, plume dilution modelling (Fennel et al., 2023; Hunt et al., 2010; Premathilake and Khangaonkar, 2019) can provide insight into how DOCCS discharge is expected to dilute and disperse within the marine environment. Dilution model outputs can then be coupled with inorganic carbon chemistry data (Figure 4) to predict the extent of chemical perturbation at different locations within the plume affected area. Biological response data from laboratory impact experiments, can also be integrated at this stage, enabling the assessment of environmental risk across the plume-affected area. Modelling dilution under different outflow scenarios enables early evaluation of the chemical perturbation and potential environmental impact as outflow rates are increased towards climate-target relevant scales.
4.4 Context dependent variability
The environmental impacts of DOCCS discharge will vary considerably based on the context in which they are deployed. In Section 4.3, the impact of scaling DOCCS technology on environmental risk was briefly discussed, however, there are many other context-dependent factors that will determine the extent to which DOCCS technology could impact the marine environment. For example, an offshore location may avoid water discharged by DOCCS interacting with particularly sensitive (e.g., benthic or coastal) environments. Some offshore marine organisms such as microbial, plankton, and higher-trophic mobile species could still be impacted, though some mobile species may be able to avoid DOCCS discharge conditions by moving into unaffected areas (Tierney, 2016). Conversely, proximity of DOCCS to productive coastal areas potentially risks exposing sensitive benthic marine ecosystems (e.g., seagrass and coral reef ecosystems), that are fixed in their position, as well as disrupting complex biogeochemical cycling. That said, there is an argument that coastal DOCCS deployment poses less environmental risk than offshore, as our scientific understanding of coastal zones far exceeds that of the open ocean (Bridges et al., 2023; Mishonov et al., 2024).
Also important to consider is the temporal context of DOCCS discharge release. The extent of environmental impact associated with DOCCS discharge may change over different timescales. For example, DOCCS discharge may have a larger impact on marine primary producers during the day, when photosynthesis is the dominant physiological process, compared to the night when rates of photosynthesis naturally decline (Harding et al., 1981). Seasonality could also change the course of environmental impact, by driving variability in physical, chemical, and biological conditions over the course of the year. On longer time scales, understanding how the potential environmental impacts of DOCCS discharge interact with aspects of climate change like ocean acidification and rising sea surface temperatures will also be important.
5 Conclusion
There has been very little study of the response of marine ecosystems to low-DIC and/or high-pH seawater. Understanding the potential environmental impacts is crucial for the overall development of DOCCS technology, but particularly for environmental safety and the social license to operate, especially at large scales and/or at high intensities. Existing research, discussed in Section 3, shows that marine organisms may be sensitive to low-DIC and/or high-pH seawater conditions. DOCCS discharge could therefore cause unintended environmental impacts at or near the point of discharge. Potential environmental impacts are likely to be more severe when the degree of CO2 removal is high (ca. >90%) and when DOCCS discharge is released at large scales. It would be irresponsible to deploy DOCCS technology at large scales, relative to the rates of dilution, until further work is conducted. Studies that assess the response of individual marine species to naturally generated low-DIC, high-pH conditions in eutrophic environments offer an interesting perspective on the potential impacts of DOCCS, but do not fulfil the necessary evidence requirements to build a comprehensive environmental risk assessment. An environmental impact evidence base from a range of marine organisms and ecosystems is urgently required to assess the impacts of DOCCS discharge. A key challenge is to generate an environmental impacts evidence base for DOCCS quickly enough to prevent harm to marine ecosystems from inappropriate operation, while enabling this technology to scale, if and where appropriate, to contribute to the mitigation of harm caused by climate change.
Author contributions
GH: Writing – original draft, Writing – review & editing, Visualisation. HF: Writing – review & editing. TB: Writing – review & editing. RW: Writing – review & editing. PH: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Department for Energy Security and Net Zero through Phase 2 of the Direct air capture and greenhouse gas removal programme, (SeaCURE) project, the UKRI EPSRC Impact Acceleration Account held by the University of Exeter (grant number EP/X525704/1), the UKRI NERC CO2 Removal Hub's Flexible Fund grant CREPE.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fclim.2025.1528951/full#supplementary-material
References
Al Yafiee, O., Mumtaz, F., Kumari, P., Karanikolos, G. N., Decarlis, A., and Dumée, L. F. (2024). Direct air capture (DAC) vs. Direct Ocean capture (DOC)–a perspective on scale-up demonstrations and environmental relevance to sustain decarbonization. Chem. Eng. J. 497:154421. doi: 10.1016/J.CEJ.2024.154421
Albright, R., Caldeira, L., Hosfelt, J., Kwiatkowski, L., Maclaren, J. K., Mason, B. M., et al. (2016). Reversal of ocean acidification enhances net coral reef calcification. Nature 531, 362–365. doi: 10.1038/nature17155
Aleta, P., Refaie, A., Afshari, M., Hassan, A., and Rahimi, M. (2023). Direct Ocean capture: the emergence of electrochemical processes for oceanic carbon removal. Energy Environ. Sci. 16, 4944–4967. doi: 10.1039/D3EE01471A
Andría, J. R., Brun, F. G., Pérez-Lloréns, J. L., and Vergara, J. J. (2001). Acclimation responses of Gracilaria sp. (Rhodophyta) and Enteromorpha intestinalis (Chlorophyta) to changes in the external inorganic carbon concentration. Bot. Mar. 44, 361–370. doi: 10.1515/BOT.2001.046/MACHINEREADABLECITATION/RIS
Bach, L. T. (2024). The additionality problem of ocean alkalinity enhancement. Biogeosciences 21, 261–277. doi: 10.5194/BG-21-261-2024
Bach, L. T., and Boyd, P. W. (2021). Seeking natural analogs to fast-forward the assessment of marine CO2 removal. Proc. Natl. Acad. Sci. USA 118:e2106147118. doi: 10.1073/PNAS.2106147118
Bach, L. T., Gill, S. J., Rickaby, R. E. M., Gore, S., and Renforth, P. (2019). CO2 removal with enhanced weathering and ocean alkalinity enhancement: potential risks and CO-benefits for marine pelagic ecosystems. Front. Climate 1:7. doi: 10.3389/FCLIM.2019.00007
Bach, L. T., Riebesell, U., Gutowska, M. A., Federwisch, L., and Schulz, K. G. (2015). A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework. Prog. Oceanogr. 135, 125–138. doi: 10.1016/J.POCEAN.2015.04.012
Bach, L. T., Riebesell, U., and Schulz, K. G. (2011). Distinguishing between the effects of ocean acidification and ocean carbonation in the coccolithophore Emiliania huxleyi. Limnol. Oceanogr. 56, 2040–2050. doi: 10.4319/LO.2011.56.6.2040
Bach, L. T., Vaughan, N. E., Law, C. S., and Williamson, P. (2024). Implementation of marine CO2 removal for climate mitigation: the challenges of additionality, predictability, and governability. Elementa 12:44. doi: 10.1525/ELEMENTA.2023.00034/200344
Baumann, H. (2019). Experimental assessments of marine species sensitivities to ocean acidification and co-stressors: how far have we come? Canad. J. Zool. 97, 399–408. doi: 10.1139/CJZ-2018-0198
BBC News. (2025). Could taking carbon out of the sea cool down the planet? - BBC News. Available at: https://www.bbc.co.uk/news/articles/cr788kljlklo (Accessed April 18, 2025).
Bednaršek, N., Van De Mortel, H., Pelletier, G., García-Reyes, M., Feely, R. A., and Dickson, A. G. (2025). Assessment framework to predict sensitivity of marine calcifiers to ocean alkalinity enhancement - identification of biological thresholds and importance of precautionary principle. Biogeosciences 22, 473–498. doi: 10.5194/BG-22-473-2025
Beer, S., Bjork, M., Hellblom, F., and Axelsson, L. (2002). Inorganic carbon utilization in marine angiosperms (seagrasses). Funct. Plant Biol. 29, 349–354. doi: 10.1071/PP01185
Berge, T., Daugbjerg, N., Andersen, B. B., and Hansen, P. J. (2010). Effect of lowered pH on marine phytoplankton growth rates. Mar. Ecol. Prog. Ser. 416, 79–91. doi: 10.3354/MEPS08780
Berge, T., Daugbjerg, N., and Hansen, P. J. (2012). Isolation and cultivation of microalgae select for low growth rate and tolerance to high pH. Harmful Algae 20, 101–110. doi: 10.1016/J.HAL.2012.08.006
Bhaumik, A., Faucher, G., Henning, M., Meunier, C. L., and Boersma, M. (2025). Prey dynamics as a buffer: enhancing copepod resilience to ocean alkalinity enhancement. Environ. Res. Lett. 20:9326. doi: 10.1088/1748-9326
Borowitzka, M. A. (1981). Photosynthesis and calcification in the articulated coralline red algae Amphiroa anceps and A. foliacea. Mar. Biol. 62, 17–23. doi: 10.1007/BF00396947
Brauner, C. J., Gonzalez, R. J., and Wilson, J. M. (2012). Extreme environments: hypersaline, alkaline, and ion-poor Waters. Fish Physiol. 32, 435–476. doi: 10.1016/B978-0-12-396951-4.00009-8
Brauner, C. J., Shartau, R. B., Damsgaard, C., Esbaugh, A. J., Wilson, R. W., and Grosell, M. (2019). Acid-base physiology and CO2 homeostasis: regulation and compensation in response to elevated environmental CO2. Fish Physiol. 37, 69–132. doi: 10.1016/BS.FP.2019.08.003
Brewer, P. G., and Goldman, J. C. (1976). Alkalinity changes generated by phytoplankton growth. Limnol. Oceanogr. 21, 108–117. doi: 10.4319/LO.1976.21.1.0108
Bridges, A. E. H., Barnes, D. K. A., Bell, J. B., Ross, R. E., Voges, L., and Howell, K. L. (2023). Filling the data gaps: transferring models from data-rich to data-poor deep-sea areas to support spatial management. J. Environ. Manag. 345:118325. doi: 10.1016/J.JENVMAN.2023.118325
Camatti, E., Valsecchi, S., Caserini, S., Barbaccia, E., Santinelli, C., Basso, D., et al. (2024). Short-term impact assessment of ocean liming: a copepod exposure test. Mar. Pollut. Bull. 198:115833. doi: 10.1016/j.marpolbul.2023.115833
Carroll, D., Menemenlis, D., Dutkiewicz, S., Lauderdale, J. M., Adkins, J. F., Bowman, K. W., et al. (2022). Attribution of space-time variability in Global-Ocean dissolved inorganic carbon. Glob. Biogeochem. Cycles 36:e2021GB007162. doi: 10.1029/2021GB007162
Chen, C. Y., and Durbin, E. G. (1994). Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar. Ecol. Prog. Ser. 109, 83–94.
Cheriyan, E., Kumar, B. S. K., Gupta, G. V. M., and Rao, D. B. (2024). Implications of ocean acidification on micronutrient elements-iron, copper and zinc, and their primary biological impacts: a review. Mar. Pollut. Bull. 199:115991. doi: 10.1016/J.MARPOLBUL.2023.115991
Ciais, P., Sabine, C., Bala, G., Bopp, L., Brovkin, V., Canadell, J., et al. (2014). Carbon and other biogeochemical cycles. Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change,
Clark, D. R., and Flynn, K. J. (2000). The relationship between the dissolved inorganic carbon concentration and growth rate in marine phytoplankton. Proc. R. Soc. Lond. Ser. B Biol. Sci. 267, 953–959. doi: 10.1098/RSPB.2000.1096
Cloern, J. E., Foster, S. Q., and Kleckner, A. E. (2014). Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11, 2477–2501. doi: 10.5194/bg-11-2477-2014
CNN. (2025). UK project trials carbon capture at sea to help tackle climate change | CNN. Available at: https://edition.cnn.com/climate/carbon-capture-sea-seacure-spc/index.html (Accessed April 29, 2025).
Comeau, L. A., Sonier, R., Guyondet, T., Landry, T., Ramsay, A., and Davidson, J. (2017). Behavioural response of bivalve molluscs to calcium hydroxide. Aquaculture 466, 78–85. doi: 10.1016/J.AQUACULTURE.2016.09.045
Cooper, T. G., and Filmer, D. (1969). The active species of “CO2” utilized by ribulose diphosphate carboxylase. J. Biol. Chem. 244, 1081–1083. doi: 10.1016/S0021-9258(18)91899-5
Cornwall, C. E., Hepburn, C. D., Pritchard, D., Currie, K. I., Mcgraw, C. M., Hunter, K. A., et al. (2012). Carbon-use strategies in MACROALGAE: differential responses to lowered PH and implications for ocean ACIDIFICATION1. J. Phycol. 48, 137–144. doi: 10.1111/J.1529-8817.2011.01085.X
Cripps, G., Widdicombe, S., Spicer, J. I., and Findlay, H. S. (2013). Biological impacts of enhanced alkalinity in Carcinus maenas. Mar. Pollut. Bull. 71, 190–198. doi: 10.1016/J.MARPOLBUL.2013.03.015
Cross, J. N., Sweeny, C., Jewett, E. B., Feely, R. A., McElhany, P., Carter, B., et al. (2023). Strategy for NOAA carbon dioxide removal research: A white paper documenting a potential NOAA CDR science strategy as an element of NOAA’S climate interventions portfolio. Washington DC: NOAA.
de Lannoy, C. F., Eisaman, M. D., Jose, A., Karnitz, S. D., DeVaul, R. W., Hannun, K., et al. (2018). Indirect ocean capture of atmospheric CO2: part I. Prototype of a negative emissions technology. Int. J. Greenhouse Gas Control 70, 243–253. doi: 10.1016/J.IJGGC.2017.10.007
Dickson, A. G., Sabine, C. L., and Christian, J. R. (eds). (2007). Guide to best practices for ocean CO2 measurement. Sidney, British Columbia, North Pacific Marine Science Organization, 191pp. (PICES Special Publication 3; IOCCP Report 8). doi: 10.25607/OBP-1342
Digdaya, I. A., Sullivan, I., Lin, M., Han, L., Cheng, W. H., Atwater, H. A., et al. (2020). A direct coupled electrochemical system for capture and conversion of CO2 from oceanwater. Nat. Commun. 11, 1–10. doi: 10.1038/s41467-020-18232-y
Dixon-Anderson, I. S. (2021). Effect of magnesium concentration in seawater on marine invertebrate calcification. University of Otago. Available online at: https://ourarchive.otago.ac.nz/esploro/outputs/graduate/Effect-of-Magnesium-Concentration-in-Seawater/9926480168701891 (Accessed January 15, 2025).
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/ANNUREV.MARINE.010908.163834
Dupont, S., and Thorndyke, M. C.Commission Internationale pour l’Exploration Scientifique de la Mer Mediterranee (2008). Ocean acidification and its impact on the early life-history stages of marine animals. CIESM Workshop Monographs, 89–97.
Eisaman, M. D. (2024). Pathways for marine carbon dioxide removal using electrochemical acid-base generation. Front. Climate 6:1349604. doi: 10.3389/FCLIM.2024.1349604
Eisaman, M. D., Parajuly, K., Tuganov, A., Eldershaw, C., Chang, N., and Littau, K. A. (2012). CO2 extraction from seawater using bipolar membrane electrodialysis. Energy Environ. Sci. 5, 7346–7352. doi: 10.1039/C2EE03393C
Eisaman, M. D., Rivest, J. L. B., Karnitz, S. D., de Lannoy, C. F., Jose, A., DeVaul, R. W., et al. (2018). Indirect ocean capture of atmospheric CO2: part II. Understanding the cost of negative emissions. Int. J. Greenhouse Gas Control 70, 254–261. doi: 10.1016/J.IJGGC.2018.02.020
Emerson, S., and Hedges, J. (2008). Chemical oceanography and the marine carbon cycle. Cambridge: Cambridge University Press.
Erans, M., Sanz-Pérez, E. S., Hanak, D. P., Clulow, Z., Reiner, D. M., and Mutch, G. A. (2022). Direct air capture: process technology, techno-economic and socio-political challenges. Energy Environ. Sci. 15, 1360–1405. doi: 10.1039/D1EE03523A
Esbaugh, A. J. (2017). Physiological implications of ocean acidification for marine fish: emerging patterns and new insights. J. Comp. Physiol. 188, 1–13. doi: 10.1007/S00360-017-1105-6
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432. doi: 10.1093/ICESJMS/FSN048
Faucher, G., Haunost, M., Paul, A. J., Tietz, A. U. C., and Riebesell, U. (2025). Growth response of Emiliania huxleyi to ocean alkalinity enhancement. Biogeosciences 22, 405–415. doi: 10.5194/bg-22-405-2025
Feely, R. A., Sabine, C. L., Hernandez-Ayon, J. M., Ianson, D., and Hales, B. (2008). Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320, 1490–1492. doi: 10.1126/SCIENCE.1155676
Feely, R. A., Sabine, C. L., Lee, K., Berelson, W., Kleypas, J., Fabry, V. J., et al. (2004). Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366. doi: 10.1126/SCIENCE.1097329
Fehsenfeld, S., and Weihrauch, D. (2017). Acid–Base regulation in aquatic decapod crustaceans. Acid-Base Balance and Nitrogen Excretion in Invertebrates, 151–191.
Fennel, K., Long, M. C., Algar, C., Carter, B., Keller, D., Laurent, A., et al. (2023). Modelling considerations for research on ocean alkalinity enhancement (OAE). State the Planet 2, 1–29. doi: 10.5194/SP-2-OAE2023-9-2023
Ferderer, A., Schulz, K. G., Riebesell, U., Baker, K. G., Chase, Z., and Bach, L. T. (2024). Investigating the effect of silicate- and calcium-based ocean alkalinity enhancement on diatom silicification. Biogeosciences 21, 2777–2794. doi: 10.5194/BG-21-2777-2024
Findlay, H. S., Wood, H. L., Kendall, M. A., Widdicombe, S., Spicer, J. I., and Twitchett, R. J. (2011). Comparing the impact of high CO2 on calcium carbonate structures in different marine organisms 7, 565–575. doi: 10.1080/17451000.2010.547200
Flipkens, G., Dujardin, V., Salden, J., T’Jollyn, K., Town, R. M., and Blust, R. (2024). Olivine avoidance behaviour by marine gastropods (Littorina littorea L.) and amphipods (Gammarus locusta L.) within the context of ocean alkalinity enhancement. Ecotoxicol. Environ. Saf. 270:115840. doi: 10.1016/J.ECOENV.2023.115840
Flores-Moya, A., and Fernández, J. A. (1998). The role of external carbonic anhydrase in the photosynthetic use of inorganic carbon in the deep-water alga Phyllariopsis purpurascens (Laminariales, Phaeophyta). Planta 207, 115–119. doi: 10.1007/S004250050462/METRICS
Friedlingstein, P., O’Sullivan, M., Jones, M. W., Andrew, R. M., Bakker, D. C. E., Hauck, J., et al. (2023). Global Carbon Budget 2023. Earth Syst. Sci. Data 15, 5301–5369. doi: 10.5194/ESSD-15-5301-2023
Frost-Christensen, H., and Sand-Jensen, K. (1990). Growth rate and carbon affinity of Ulva lactuca under controlled levels of carbon, pH and oxygen. Mar. Biol. 104, 497–501. doi: 10.1007/BF01314356/METRICS
Gangstø, R., Gehlen, M., Schneider, B., Bopp, L., Aumont, O., and Joos, F. (2008). Modeling the marine aragonite cycle: changes under rising carbon dioxide and its role in shallow water CaCO3 dissolution. Biogeosciences 5, 1057–1072. doi: 10.5194/BG-5-1057-2008
García-Sánchez, M. J., Delgado-Huertas, A., Fernández, J. A., and Flores-Moya, A. (2016). Photosynthetic use of inorganic carbon in deep-water kelps from the strait of Gibraltar. Photosynth. Res. 127, 295–305. doi: 10.1007/S11120-015-0184-Z/TABLES/4
García-Sánchez, M. J., Fernández, J. A., and Niell, X. (1994). Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194, 55–61. doi: 10.1007/BF00201034
Gately, J. A., Kim, S. M., Jin, B., Brzezinski, M. A., and Iglesias-Rodriguez, M. D. (2023). Coccolithophores and diatoms resilient to ocean alkalinity enhancement: a glimpse of hope? Sci. Adv. 9:66. doi: 10.1126/SCIADV.ADG6066
Gattuso, J.-P., Epitalon, J.-M., Lavigne, H., and Orr, J. (2024). Seacarb: seawater carbonate chemistry. R package version 3.3.0. CRAN. Available online at: http://CRAN.R-project.org/package=seacarb
Geerts, L. J. J., Hylén, A., and Meysman, F. J. R. (2025). Review and syntheses: ocean alkalinity enhancement and carbon dioxide removal through marine enhanced rock weathering using olivine. Biogeosciences 22, 355–384. doi: 10.5194/BG-22-355-2025
Gore, S., Renforth, P., and Perkins, R. (2019). The potential environmental response to increasing ocean alkalinity for negative emissions. Mitig. Adapt. Strateg. Glob. Chang. 24, 1191–1211. doi: 10.1007/S11027-018-9830-Z/TABLES/6
Guinotte, J. M., and Fabry, V. J. (2008). Ocean acidification and its potential effects on marine ecosystems. Ann. N. Y. Acad. Sci. 1134, 320–342. doi: 10.1196/ANNALS.1439.013
Guo, J. A., Strzepek, R. F., Yuan, Z., Swadling, K. M., Townsend, A. T., Achterberg, E. P., et al. (2025). Effects of ocean alkalinity enhancement on plankton in the equatorial Pacific. Communic. Environ. 6, 1–8. doi: 10.1038/s43247-025-02248-7Lennart, &, & Bach, T
Halloran, P. R., Bell, T. G., Burt, W. J., Chu, S. N., Gill, S., Henderson, C., et al. (2025). Seawater carbonate chemistry based carbon dioxide removal: towards commonly agreed principles for carbon monitoring, reporting, and verification. Front. Climate 7:1487138. doi: 10.3389/FCLIM.2025.1487138
Hansen, P. J. (2002). Effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquat. Microb. Ecol. 28, 279–288. doi: 10.3354/AME028279
Hansen, B. W., Hansen, P. J., Nielsen, T. G., and Jepsen, P. M. (2017). Effects of elevated pH on marine copepods in mass cultivation systems: practical implications. J. Plankton Res. 39, 984–993. doi: 10.1093/PLANKT/FBX032
Hansen, P. J., Lundholm, N., and Rost, B. (2007). Growth limitation in marine red-tide dinoflagellates: effects of pH versus inorganic carbon availability. Mar. Ecol. Prog. Ser. 334, 63–71. doi: 10.3354/MEPS334063
Harding, L. W., Meeson, B. W., Prézelin, B. B., and Sweeney, B. M. (1981). Diel periodicity of photosynthesis in marine phytoplankton. Mar. Biol. 61, 95–105. doi: 10.1007/BF00386649/METRICS
Hendriks, I. E., Olsen, Y. S., Ramajo, L., Basso, L., Steckbauer, A., Moore, T. S., et al. (2014). Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 11, 333–346. doi: 10.5194/BG-11-333-2014
Herfort, L., Peterson, T. D., Prahl, F. G., McCue, L. A., Needoba, J. A., Crump, B. C., et al. (2012). Red waters of Myrionecta rubra are biogeochemical hotspots for the Columbia River estuary with impacts on primary/secondary productions and nutrient cycles. Estuar. Coasts 35, 878–891. doi: 10.1007/S12237-012-9485-Z/FIGURES/4
Hinga, K. R. (2002). Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 238, 281–300. doi: 10.3354/MEPS238281
Hoffmann, L. J., Breitbarth, E., Boyd, P. W., and Hunter, K. A. (2012). Influence of ocean warming and acidification on trace metal biogeochemistry. Mar. Ecol. Prog. Ser. 470, 191–205. doi: 10.3354/MEPS10082
Howald, S., Moyano, M., Crespel, A., Kuchenmüller, L. L., Cominassi, L., Claireaux, G., et al. (2022). Effects of ocean acidification over successive generations decrease resilience of larval European sea bass to ocean acidification and warming but juveniles could benefit from higher temperatures in the NE Atlantic. J. Exp. Biol. 225:35. doi: 10.1242/JEB.243802/275035
Hunt, C. D., Mansfield, A. D., Mickelson, M. J., Albro, C. S., Geyer, W. R., and Roberts, P. J. W. (2010). Plume tracking and dilution of effluent from the Boston sewage outfall. Mar. Environ. Res. 70, 150–161. doi: 10.1016/J.MARENVRES.2010.04.005
Hutchins, D. A., Fu, F.-X., Yang, S.-C., John, S. G., Romaniello, S. J., Andrews, M. G., et al. (2023). Responses of globally important phytoplankton groups to olivine dissolution products and implications for carbon dioxide removal via ocean alkalinity enhancement. Biorxiv 2023:536121. doi: 10.1101/2023.04.08.536121
Iglesias-Rodríguez, M. D., Rickaby, R. E. M., Singh, A., and Gately, J. A. (2023). Laboratory experiments in ocean alkalinity enhancement research. State of the Planet, 2-oae2023, 1–18. doi: 10.5194/SP-2-OAE2023-5-2023
Invers, O., Romero, J., and Pérez, M. (1997). Effects of pH on seagrass photosynthesis: a laboratory and field assessment. Aquat. Bot. 59, 185–194. doi: 10.1016/S0304-3770(97)00072-7
Intergovernmental Panel on Climate Change (IPCC). (2023). “Global carbon and other biogeochemical cycles and feedbacks” in Climate change 2021 – The physical science basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, (Cambridge University Press) 673–816.
Iwama, G. K., McGeer, J. C., Wright, P. A., Wilkie, M. P., and Wood, C. M. (1997). Divalent cations enhance ammonia excretion in Lahontan cutthroat trout in highly alkaline water. J. Fish Biol. 50, 1061–1073. doi: 10.1111/J.1095-8649.1997.TB01630.X
Jiang, L. Q., Carter, B. R., Feely, R. A., Lauvset, S. K., and Olsen, A. (2019). Surface Ocean pH and buffer capacity: past, present and future. Sci. Rep. 9, 1–11. doi: 10.1038/s41598-019-55039-4
Jiang, H., Zou, D., and Chen, B. (2016). Effects of lowered carbon supplies on two farmed red seaweeds, Pyropia haitanensis (Bangiales) and Gracilaria lemaneiformis (Gracilariales), grown under different sunlight conditions. J. Appl. Phycol. 28, 3469–3477. doi: 10.1007/S10811-016-0882-8
Jiang, H., Zou, D., Lou, W., Deng, Y., and Zeng, X. (2018). Effects of seawater acidification and alkalization on the farmed seaweed, Pyropia haitanensis (Bangiales, Rhodophyta), grown under different irradiance conditions. Algal Res. 31, 413–420. doi: 10.1016/J.ALGAL.2018.02.033
Johnson, M., van Doorn, E., Hilmi, N., Marandino, C., McDonald, N., Thomas, H., et al. (2024). Can coastal and marine carbon dioxide removal help to close the emissions gap? Scientific, legal, economic, and governance considerations. Elementa 12:71. doi: 10.1525/ELEMENTA.2023.00071
Karunarathne, S., Andrenacci, S., Carranza-Abaid, A., Jayarathna, C., Maelum, M., Skagestad, R., et al. (2025). Review on CO2 removal from ocean with an emphasis on direct ocean capture (DOC) technologies. Sep. Purif. Technol. 353:128598. doi: 10.1016/J.SEPPUR.2024.128598
Kemp, W. M., Smith, E. M., Marvin-DiPasquale, M., and Boynton, W. R. (1997). Organic carbon balance and net ecosystem metabolism in Chesapeake Bay. Mar. Ecol. Prog. Ser. 150, 229–248.
Kitidis, V., Hardman-Mountford, N. J., Litt, E., Brown, I., Cummings, D., Hartman, S., et al. (2012). Seasonal dynamics of the carbonate system in the Western English Channel. Cont. Shelf Res. 42, 30–40. doi: 10.1016/J.CSR.2012.04.012
Krause-Jensen, D., Marbà, N., Sanz-Martin, M., Hendriks, I. E., Thyrring, J., Carstensen, J., et al. (2016). Long photoperiods sustain high pH in arctic kelp forests. Sci. Adv. 2:938. doi: 10.1126/SCIADV.1501938
Langdon, C., Takahashi, T., Sweeney, C., Chipman, D., Goddard, J., Marubini, F., et al. (2000). Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles 14, 639–654. doi: 10.1029/1999GB001195
Lefevre, S. (2019). Effects of high CO2 on oxygen consumption rates, aerobic scope and swimming performance. Fish Physiol. 37, 195–244. doi: 10.1016/BS.FP.2019.08.001
Legrand, E., Riera, P., Pouliquen, L., Bohner, O., Cariou, T., and Martin, S. (2018). Ecological characterization of intertidal rockpools: seasonal and diurnal monitoring of physico-chemical parameters. Reg. Stud. Mar. Sci. 17, 1–10. doi: 10.1016/j.rsma.2017.11.003
Lewis, R. E., and Riddle, A. M. (1989). Sea disposal: modelling studies of waste field dilution. Mar. Pollut. Bull. 20, 124–129. doi: 10.1016/0025-326X(88)90817-X
Lignell, A., and Pedersen, M. (1989). Effects of pH and inorganic carbon concentration on growth of Gracilaria secundata. Br. Phycol. J. 24, 83–89. doi: 10.1080/00071618900650071
Loehr, L. C., Beegle-Krause, C. J., George, K., McGee, C. D., Mearns, A. J., and Atkinson, M. J. (2006). The significance of dilution in evaluating possible impacts of wastewater discharges from large cruise ships. Mar. Pollut. Bull. 52, 681–688. doi: 10.1016/J.MARPOLBUL.2005.10.021
Lundholm, N., Hansen, P. J., and Kotaki, Y. (2004). Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera pseudo-nitzschia and Nitzschia. Mar. Ecol. Prog. Ser. 273, 1–15. doi: 10.3354/MEPS273001
Maberly, S. C. (1990). Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J. Phycol. 26, 439–449. doi: 10.1111/J.0022-3646.1990.00439.X
Majumdar, A., and Deutch, J. (2018). Research opportunities for CO2 utilization and negative emissions at the Gigatonne scale. Joule 2, 805–809. doi: 10.1016/J.JOULE.2018.04.018
Marín-Samper, L., Arístegui, J., Hernández-Hernández, N., Ortiz, J., Archer, S. D., Ludwig, A., et al. (2024). Assessing the impact of CO2-equilibrated ocean alkalinity enhancement on microbial metabolic rates in an oligotrophic system. Biogeosciences 21, 2859–2876. doi: 10.5194/BG-21-2859-2024
Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P. R., et al. (2018). IPCC, 2018: summary for policymakers. In: Global warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global.
Menéndez, M., Martínez, M., and Comín, F. A. (2001). A comparative study of the effect of pH and inorganic carbon resources on the photosynthesis of three floating macroalgae species of a Mediterranean coastal lagoon. J. Exp. Mar. Biol. Ecol. 256, 123–136. doi: 10.1016/S0022-0981(00)00313-0
Middelburg, J. J., Soetaert, K., and Hagens, M. (2020). Ocean alkalinity, buffering and biogeochemical processes. Rev. Geophys. 58:e2019RG000681. doi: 10.1029/2019RG000681
Millero, F. J. (2000). The carbonate system in marine environments. Chem. Process. Marine Environ. 9:41. doi: 10.1007/978-3-662-04207-6_2
Millero, F. J., Woosley, R., DiTrolio, B., and Waters, J. (2009). Effect of ocean acidification on the speciation of metals in seawater. Oceanography 22, 72–85. doi: 10.5670/OCEANOG.2009.98
Mishonov, A. V., Boyer, T. P., Baranova, O. K., Bouchard, C. N., Cross, S. L., Garcia, H. E., et al. (2024). World Ocean database 2023.
Moras, C. A., Bach, L. T., Cyronak, T., Joannes-Boyau, R., and Schulz, K. G. (2022). Ocean alkalinity enhancement—avoiding runaway CaCO3 precipitation during quick and hydrated lime dissolution. Biogeosciences 19, 3537–3557. doi: 10.5194/bg-19-3537-2022
Munday, P. L., Jarrold, M. D., and Nagelkerken, I. (2019). Ecological effects of elevated CO2 on marine and freshwater fishes: from individual to community effects. Fish Physiol. 37, 323–368. doi: 10.1016/BS.FP.2019.07.005
Murru, M., and Sandgren, C. D. (2004). Habitat matters for inorganic carbon acquisition in 38 species of red MACROALGAE (RHODOPHYTA) from PUGET sound, Washington, USA1. J. Phycol. 40, 837–845. doi: 10.1111/J.1529-8817.2004.03182.X
National Academies of Sciences, Engineering. (2022). A research strategy for ocean-based carbon dioxide removal and sequestration. Washington, DC: The National Academies Press. doi: 10.17226/26278
Oberlander, J. L., Burke, M. E., London, C. A., and MacIntyre, H. L. (2025). Assessing the impacts of simulated ocean alkalinity enhancement on viability and growth of nearshore species of phytoplankton. Biogeosciences 22, 499–512. doi: 10.5194/BG-22-499-2025
Olsen, L. M., Öztürk, M., Sakshaug, E., and Johnsen, G. (2006). Photosynthesis-induced phosphate precipitation in seawater: ecological implications for phytoplankton. Mar. Ecol. Prog. Ser. 319, 103–110. doi: 10.3354/MEPS319103
Oschlies, A., Bach, L. T., Fennel, K., Gattuso, J.-P., and Mengis, N. (2025). Perspectives and challenges of marine carbon dioxide removal. Front. Climate 6:1506181. doi: 10.3389/FCLIM.2024.1506181
Pedersen, S. A., Håkedal, O. J., Salaberria, I., Tagliati, A., Gustavson, L. M., Jenssen, B. M., et al. (2014). Multigenerational exposure to ocean acidification during food limitation reveals consequences for copepod scope for growth and vital rates. Environ. Sci. Technol. 48, 12275–12284. doi: 10.1021/ES501581J
Pedersen, M. F., and Hansen, P. J. (2003). Effects of high pH on the growth and survival of six marine heterotrophic protists. Mar. Ecol. Prog. Ser. 260, 33–41. doi: 10.3354/MEPS260033
Pinheiro, J. P. S., Windsor, F. M., Wilson, R. W., and Tyler, C. R. (2021). Global variation in freshwater physico-chemistry and its influence on chemical toxicity in aquatic wildlife. Biol. Rev. 96, 1528–1546. doi: 10.1111/BRV.12711
Porteus, C. S., Hubbard, P. C., Uren Webster, T. M., van Aerle, R., Canário, A. V. M., Santos, E. M., et al. (2018). Near-future CO2 levels impair the olfactory system of a marine fish. Nat. Climate Change 8, 737–743. doi: 10.1038/s41558-018-0224-8
Porteus, C. S., Roggatz, C. C., Velez, Z., Hardege, J. D., and Hubbard, P. C. (2021). Acidification can directly affect olfaction in marine organisms. J. Exp. Biol. 224:86. doi: 10.1242/JEB.237941/270986
Pörtner, H. O. (2008). Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar. Ecol. Prog. Ser. 373, 203–217. doi: 10.3354/MEPS07768
Pörtner, H. O., Langenbuch, M., and Reipschläger, A. (2004). Biological impact of Elevated Ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718. doi: 10.1007/S10872-004-5763-0
Premathilake, L., and Khangaonkar, T. (2019). FVCOM-plume – a three-dimensional Lagrangian outfall plume dilution and transport model for dynamic tidal environments: model development. Mar. Pollut. Bull. 149:110554. doi: 10.1016/J.MARPOLBUL.2019.110554
Rai, S. V., and Rajashekhar, M. (2014). Effect of pH, salinity and temperature on the growth of six species of marine phytoplankton. J. Algal Biomass Utln 2014, 55–59.
Ramírez, L., Pozzo-Pirotta, L. J., Trebec, A., Manzanares-Vázquez, V., Díez, J. L., Arístegui, J., et al. (2025). Ocean alkalinity enhancement (OAE) does not cause cellular stress in a phytoplankton community of the subtropical Atlantic Ocean. Biogeosciences 22, 1865–1886. doi: 10.5194/BG-22-1865-2025
Raven, J. A. (1994). Carbon fixation and carbon availability in marine phytoplankton. Photosynth. Res. 39, 259–273. doi: 10.1007/BF00014587/METRICS
Raven, J., Caldeira, K., Elderfield, H., Hoegh-Guldberg, O., Liss, P., Riebesell, U., et al. (2005). Ocean acidification due to increasing atmospheric carbon dioxide : The Royal Society. Available online at: https://royalsociety.org/news-resources/publications/2005/ocean-acidification/
Raven, J. A., Giordano, M., Beardall, J., and Maberly, S. C. (2011). Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth. Res. 109, 281–296. doi: 10.1007/S11120-011-9632-6/TABLES/1
Reinfelder, J. R. (2011). Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu. Rev. Mar. Sci. 3, 291–315. doi: 10.1146/ANNUREV-MARINE-120709-142720/
Riebesell, U., Wolf-Gladrow, D. A., and Smetacek, V. (1993). Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361, 249–251. doi: 10.1038/361249a0
Ries, J. B. (2010). Review: geological and experimental evidence for secular variation in seawater mg/ca (calcite-aragonite seas) and its effects on marine biological calcification. Biogeosciences 7, 2795–2849. doi: 10.5194/BG-7-2795-2010
Sánchez, N., Goldenberg, S. U., Brüggemann, D., Jaspers, C., Taucher, J., and Riebesell, U. (2024). Plankton food web structure and productivity under ocean alkalinity enhancement. Sci. Adv. 10:eado0264. doi: 10.1126/SCIADV.ADO0264
Schulz, K. G., Bach, L. T., and Dickson, A. G. (2023). Seawater carbonate chemistry considerations for ocean alkalinity enhancement research: theory, measurements, and calculations. State Planet 2, 1–14. doi: 10.5194/SP-2-OAE2023-2-2023
Scott, D. M., Lucas, M. C., and Wilson, R. W. (2005). The effect of high pH on ion balance, nitrogen excretion and behaviour in freshwater fish from an eutrophic lake: a laboratory and field study. Aquat. Toxicol. 73, 31–43. doi: 10.1016/J.AQUATOX.2004.12.013
Sett, S., Bach, L. T., Schulz, K. G., Koch-Klavsen, S., Lebrato, M., and Riebesell, U. (2014). Temperature modulates Coccolithophorid sensitivity of growth, photosynthesis and calcification to increasing seawater pCO2. PLoS One 9:e88308. doi: 10.1371/JOURNAL.PONE.0088308
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C., and Vermaas, D. A. (2021). Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814. doi: 10.1039/D0EE03382K
Smith, S., Geden, O., Gidden, M., Lamb, W., Nemet, G., Minx, J., et al. (2024). The state of carbon dioxide removal. 2nd Ed. doi: 10.17605/OSF.IO/F85QJ
Søderberg, L. M., and Hansen, P. J. (2007). Growth limitation due to high pH and low inorganic carbon concentrations in temperate species of the dinoflagellate genus Ceratium. Marine Ecology Progress Series, 351, 103–112. doi: 10.3354/MEPS07146
Søgaard, D. H., Hansen, P. J., Rysgaard, S., and Glud, R. N. (2011). Growth limitation of three Arctic Sea ice algal species: effects of salinity, pH, and inorganic carbon availability. Polar Biol. 34, 1157–1165. doi: 10.1007/S00300-011-0976-3
Stockdale, A., Tipping, E., Lofts, S., and Mortimer, R. J. G. (2016). Effect of ocean acidification on organic and inorganic speciation of trace metals. Environ. Sci. Technol. 50, 1906–1913. doi: 10.1021/ACS.EST.5B05624
Sun, J., Zhao, C., Zhao, S., Dai, W., Liu, J., Zhang, J., et al. (2023). Diversity of CO2 concentrating mechanisms in macroalgae photosynthesis: a case study of Ulva sp. J Mar Sci Eng 11:1911. doi: 10.3390/JMSE11101911
Taraldsvik, M., Myklestad, S., and Myklestad, S. M. (2000). European journal of phycology the effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum the effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum. Eur. J. Phycol. 35, 189–194. doi: 10.1080/09670260010001735781
Tate, P. M., Scaturro, S., and Cathers, B. (2016). Marine Outfalls. Springer Handbook of Ocean Engineering, 711–740. doi: 10.1007/978-3-319-16649-0_32
Tee, M. Z., Yong, Y. S., Rodrigues, K. F., and Yong, W. T. L. (2015). Growth rate analysis and protein identification of Kappaphycus alvarezii (Rhodophyta, Gigartinales) under pH induced stress culture. Aquacul. Rep. 2, 112–116. doi: 10.1016/J.AQREP.2015.09.001
Thompson, W. A., Rodela, T. M., and Richards, J. G. (2015). The effects of strain and ploidy on the physiological responses of rainbow trout (Oncorhynchus mykiss) to pH 9.5 exposure. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 183, 22–29. doi: 10.1016/J.CBPB.2014.12.005
Thomsen, J., Haynert, K., Wegner, K. M., and Melzner, F. (2015). Impact of seawater carbonate chemistry on the calcification of marine bivalves. Biogeosciences 12, 4209–4220. doi: 10.5194/BG-12-4209-2015
Tierney, K. B. (2016). Chemical avoidance responses of fishes. Aquat. Toxicol. 174, 228–241. doi: 10.1016/J.AQUATOX.2016.02.021
Tilman, D., Kilham, S. S., and Kilham, P. (1982). Phytoplankton community ecology: the role of limiting nutrients. Annu. Rev. Ecol. Syst. 13, 349–372.
Water Research Centre Limited. (2024). Pre-trial audit of the planetary and South West Water Ocean alkalinity enhancement pilot Available online at: www.wrcgroup.com (Accessed December 12, 2024).
Wilkie, M. P., and Wood, C. M. (1991). Nitrogenous waste excretion, Acid-Base regulation, and lonoregulation in rainbow trout (Oncorhynchus mykiss). Exposed Extremely Alkaline Water 64, 1069–1086. doi: 10.1086/PHYSZOOL.64.4.30157957
Wilkie, M. P., and Wood, C. M. (1996). The adaptations of fish to extremely alkaline environments. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 113, 665–673. doi: 10.1016/0305-0491(95)02092-6
Wilson, R. W. (2020). The effects of CO2 and related water chemistry on fish: climate change vs. aquaculture. Bulletin of the European Association of Fish Pathologists 40, 49–54.
Wolf-Gladrow, D. A., Zeebe, R. E., Klaas, C., Körtzinger, A., and Dickson, A. G. (2007). Total alkalinity: the explicit conservative expression and its application to biogeochemical processes. Mar. Chem. 106, 287–300. doi: 10.1016/J.MARCHEM.2007.01.006
Wood, C. M. (1993). “Ammonia and urea metabolism and excretion” in Physiology of fishes. ed. M. E. Brown (Boca Raton: CRC Press), 379–425.
Wood, C. M. (2022). Conservation aspects of osmotic, acid-base, and nitrogen homeostasis in fish. Fish Physiol. 39, 321–388. doi: 10.1016/BS.FP.2022.04.007
Wood, H. L., Spicer, J. I., and Widdicombe, S. (2008). Ocean acidification may increase calcification rates, but at a cost. Proc. Biol. Sci. 275, 1767–1773. doi: 10.1098/rspb.2008.0343
Wright, P. A., and Wood, C. M. (1985). An analysis of branchial Ammonia excretion in the freshwater rainbow trout: effects of environmental Ph change and sodium uptake blockade. J. Exp. Biol. 114, 329–353. doi: 10.1242/JEB.114.1.329
Xin, X., Faucher, G., and Riebesell, U. (2024). Phytoplankton response to increased nickel in the context of ocean alkalinity enhancement. Biogeosciences 21, 761–772. doi: 10.5194/BG-21-761-2024
Keywords: marine environmental impacts, carbon dioxide removal, Direct Ocean Carbon Capture and Storage (DOCCS), mCDR, marine carbon dioxide removal, high pH
Citation: Hooper G, Findlay HS, Bell TG, Wilson RW and Halloran PR (2025) Removal of dissolved inorganic carbon from seawater for climate mitigation: potential marine ecosystem impacts. Front. Clim. 7:1528951. doi: 10.3389/fclim.2025.1528951
Edited by:
Maribel I. García-Ibáñez, Spanish Institute of Oceanography (IEO), SpainReviewed by:
Martin Johnson, Ecodiversity Ltd., IrelandNicolas Metzl, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2025 Hooper, Findlay, Bell, Wilson and Halloran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guy Hooper, Z2ZkaDIwMUBleGV0ZXIuYWMudWs=
 Guy Hooper
Guy Hooper Helen S. Findlay
Helen S. Findlay Thomas G. Bell
Thomas G. Bell Rod W. Wilson
Rod W. Wilson Paul R. Halloran
Paul R. Halloran