- 1School of Politics and Public Administration, Yunnan Minzu University, Kunming, China
- 2School of Public Administration, North China University of Water Resources and Electric Power, Zhengzhou, China
- 3School of Government, Beijing Normal University, Beijing, China
- 4School of Management and Economics, Chuxiong Normal University, Chuxiong, China
- 5School of Government, Yunnan University, Kunming, China
Climate change has emerged as one of the most significant threats to global biodiversity, and climate adaptation has become a critical component of biodiversity conservation. This paper reviews adaptive management strategies for enhancing biodiversity resilience under climate change, based on a cross-scale framework. The findings reveal that: (1) Biodiversity conservation adaptation to climate change requires a cross-spatial scale framework, which highlights the vertical interaction and interdependencies between regional, landscape, and site-level strategies. (2) Adaptive management strategies vary across spatial scales. At the regional scale, dynamic planning based on assessment and monitoring is prioritized. Landscape-scale initiatives emphasize protected areas as the core, expanding their scope while restructuring networks through corridors, stepping stone, habitat matrix permeability, and climate refugia. At the site scale, efforts focus on in situ and ex situ conservation of keystone species, along with real-time monitoring of invasive species. (3) Future challenges in biodiversity conservation under climate change may include social inequity in adaptation efforts, delayed responses in dynamic landscape conservation planning, disruptions to species’s ecological networks, barriers to interdisciplinary collaboration, and insufficient attention to human-climate interactions. By highlighting the differential application of adaptation strategies across spatial scales and underscoring the critical importance of cross-scale collaboration, our findings provide important insights for advancing research and practice in biodiversity adaptation to climate change, offering a theoretical foundation and practical guidance for developing multi-level, operable climate-adaptive conservation policies.
1 Introduction
Global climate has changed more rapidly since 1950 than in any comparable period during the preceding million years (Stocker et al., 2013). Anthropogenic climate change now represents the most significant threat to biodiversity, significantly impacting species’ phenology, distribution and abundance, and further affecting ecosystem structure, function, stability and their feedback regulation to climate change (Urban, 2015). Climate change exerts profound and multidimensional pressures on biodiversity through interconnected pathways. Rising temperatures are triggering large-scale species redistribution, with many organisms shifting poleward and upward in elevation to track suitable climates (Pecl et al., 2017). Alarmingly, current extinction rates now exceed background rates by 100–1,000 times, with projected species losses of 5% at 2 °C warming and 16% at 4.3 °C (Bongaarts, 2019). Concurrently, climate-driven ecosystem degradation manifests through cascading effects: at 1 °C warming, mass coral bleaching becomes widespread (IPCC, 2002); a 2 °C increase severely disrupts most European ecosystems, drastically reducing Mediterranean plant diversity (Bakkenes et al., 2006); and beyond 3 °C, extensive forest loss is expected across Eurasia, eastern China, Canada, and the central U.S. (Scholze et al., 2006). The cumulative impacts are compounded by extreme events, as exemplified by Cyclone Idai, which reduced small herbivore populations in Mozambique by 28% within 20 months (Walker et al., 2023). Amid escalating extinction risks and ecosystem destabilization (Field et al., 2014), climate-resilient biodiversity conservation has become a global priority. The 2024 Convention on Biological Diversity (COP16) highlighted “climate change” and “biodiversity governance” as key agenda items (Climate-Diplomacy, 2024). Given that climate change exacerbates risks to both natural and human systems, advancing scientific understanding of its impacts on biodiversity and developing adaptive conservation strategies hold critical theoretical and practical significance for global biodiversity protection and international policy implementation.
Keeping track of research and practice on biodiversity adaptation to climate change will help us identify effective strategies. Over the past two decades, scientists have conducted a systematic reviewing of adaptation strategies proposed in existing research. Since Heller and Zavaleta (2009) comprehensively reviewed relevant research from 1975 to 2007 and categorized adaptation strategies (Heller and Zavaleta, 2009), McLaughlin et al. (2022) further traced research from 2007 to 2017 and found that, in comparison, climate change refugia, climate-adaptive assisted migration, and climate-adaptive genetics are three of the most latest and robust strategies for coping with climate change (McLaughlin et al., 2022). (iii) There are also reviews for a particular adaptation strategy, such as climate change adaptation planning for biodiversity conservation (Watson et al., 2012), land-use planning-based climate change adaptation (Schmitz et al., 2015), spatial planning for climate change adaptation (Reside et al., 2018), and habitat connectivity (Keeley et al., 2018). Nevertheless, biodiversity adaptation to climate change is a systematic process, reviewing existing research and practice based on an integrated framework is necessary. For example, Mawdsley et al. (2009) constructed an integrated framework for a taxonomy of natural resource management actions, and applied it to review existing research on biodiversity adaptation to climate change (Mawdsley et al., 2009).
The existing reviews have provided important inspiration for this paper, but it must also be realized that merely reviewing biodiversity adaptation strategies is not enough. An adaptation strategy may be applicable at the national or local government levels, but is too broad for protected areas (PAs), parks, watersheds, etc. In comparison, adaptation strategies that work for one particular species may be too granular for the landscape scale. Based on the above, current reviews of climate adaptation strategies remain overly generalized, and that it is essential to review and assess existing research and practice at different spatial scales. How can adaptive management strategies across multiple spatial scales effectively enhance the adaptive capacity of biodiversity to climate change? In contrast to approaches that classify conservation actions either by type (e.g., legal policies or direct species management) (Mawdsley et al., 2009) or by the nature of the strategy itself (e.g., modifying conservation plans) (Heller and Zavaleta, 2009), this paper establishes a multi-scale analytical framework for biodiversity adaptation to climate change based on landscape ecology, systematically reviewed the adaptive management strategies of biodiversity at different spatial scales of region-landscape-site, with a specific focus on synergistic interactions among three critical scales (regional, landscape, and site) to enhance ecological resilience. Furthermore, we systematically synthesize existing research and practical interventions in climate-adaptive biodiversity conservation across these scales, while identifying key challenges for future research.
2 Adaptation of biodiversity to climate change cross spatial scales
Based on the scale-dependence hypothesis (Chase et al., 2018), this study systematically identifies core adaptive management components across regional, landscape, and site scales under the guidance of landscape ecology and existing theoretical research. On this basis, a cross-scale biodiversity adaptation framework was constructed. The framework was preliminarily validated using the Delphi method and further applied in typical practical cases to examine its explanatory power and applicability (Figure 1).
2.1 Adaptation as a continuum of resistance, resilience and transformation
The systematic conceptualization of biological adaptation originates in Darwin’s theory of natural selection (1859), which emphasized organisms’ development of adaptive traits through genetic variation and environmental selection pressures (Darwin, 1859). Autonomous adaptation initially manifested through evolutionary responses to natural selection, exemplified by beak morphology changes in Galápagos finches (Grant and Grant, 2002). In the early 20th century, adaptation theory expanded to include niche differentiation and coevolution, such as the Red Queen hypothesis (Valen, 1973), though remaining confined to natural ecological processes. MacArthur and Wilson's (1967) theory of island biogeography significantly advanced understanding of species adaptation mechanisms (MacArthur and Wilson, 1967), laying foundations for conservation biology. The 1990s marked a pivotal transition period in which adaptation evolved into a cross-disciplinary policy instrument through the First Assessment Report by the Intergovernmental Panel on Climate Change (IPCC) and the United Nations Framework Convention on Climate Change (UNFCCC) (IPCC, 1990; UN, 1992), extending its application to disaster management, political ecology, rights protection, and food security (Smit and Wandel, 2006). While long considered as a component of ecological resilience, adaptation’s formal integration into mainstream biodiversity conservation frameworks occurred in the early 21st century. A critical turning point emerged with the 2010 Strategic Plan for Biodiversity (CBD, 2010), which for the first time explicitly incorporated adaptation into biodiversity conservation policies. Building upon this foundation, the IPCC Sixth Assessment Report (AR6, 2022) advanced the conceptual framework by proposing a “resistance-recovery-transformation” adaptation continuum (IPCC, 2022), systematically emphasizing the critical role of proactive human intervention in biodiversity conservation.
In contemporary conservation biology, the adaptation concept has evolved from its initial focus on innate species adaptability (MacArthur and Wilson, 1967) to policy-driven proactive strategies (CBD, 2010; IPCC, 2022) addressing anthropogenic climate impacts on biodiversity. This evolution marks a paradigm shift from studying natural ecological process to governing socio-ecological system (Sgrò et al., 2011). As biodiversity adapts to climate change, adaptation can be viewed as a continuum of resistance, recovery, and transformation, where resistance refers to the maintenance of the existing state from climate disturbances, while recovery is the process of returning to a state that was previously maintained after disturbance (Hodgson et al., 2015), and transformation means enabling or facilitating the transition to new conditions (Peterson St-Laurent et al., 2021). Policy interventions should account for ecosystem characteristics to enhance biodiversity’s capacity to recover from rapid climate change while maintaining ecological functions.
2.2 Biodiversity adaptation across spatial scale frameworks
Climate change impacts on biodiversity manifest through distinct scale-dependent processes (Ackerly et al., 2010). These impacts are simultaneously determined by macro-scale climate change patterns and mediated through species-ecosystem interactions (Wu and Li, 2006), necessitating an integrated cross-scale approach to biodiversity adaptation strategies (Phillips et al., 2025; Willis and Bhagwat, 2009). Cross-scale biodiversity adaptation refers to the multi-tiered conservation responses across spatial scales (regional, landscape, and site levels) in the context of climate change (Poiani et al., 2000), designed to address climate impacts operating at multiple scales. Landscape ecology offers the foundational theoretical framework for understanding these cross-scale interactions: (i) The spatial heterogeneity and diversity theory emphasizes the non-uniform distribution of landscape elements and their influence on ecological processes. Under climate change, biodiversity conservation targets similarly demonstrate marked spatial heterogeneity. (ii) The hierarchical patch dynamics paradigm conceptualizes ecosystems as dynamic mosaics of multi-level patches interconnected through ecological processes (Zhang et al., 2013). This hierarchical structure necessitates conservation strategies that establish cross-scale feedback mechanisms, where regional climate patterns influence landscape-scale habitat distribution, while site-specific microhabitat conditions reciprocally modulate local climatic features.
Figure 2 illustrates an operational (though imperfect) framework illustrating biodiversity adaptation across three spatial scales. Specifically: (i) The regional scale encompasses broader geographical areas containing multiple landscape types (Ekroos et al., 2016). (ii) The landscape represents habitat complexes with environmental gradients supporting multiple populations (Poiani et al., 2000). (iii) The site scale refers to homogeneous habitat patches supporting specific populations (Norris et al., 2020). In practice, regional biodiversity conservation planning needs to respond to global climate change and implement vulnerability assessments, conservation target setting, spatial project planning, and monitoring throughout implementation based on local resources and institutional capacity. The landscape scale emphasizes maximizing species and ecosystem diversity to enhance resilience. Specifically, this involves connecting PAs through corridors, stepping stones, and landscape matrix, supplemented by climate change refugia to aid species persistence and recovery, thereby enhancing the protected area network connectivity and improving landscape resilience. The site scale focuses on keystone species and invasive species, with conservation efforts prioritizing in-situ conservation while incorporating ex-situ measures; invasive species monitoring requires continuous assessment of their impacts on genetic diversity and ecosystem integrity.
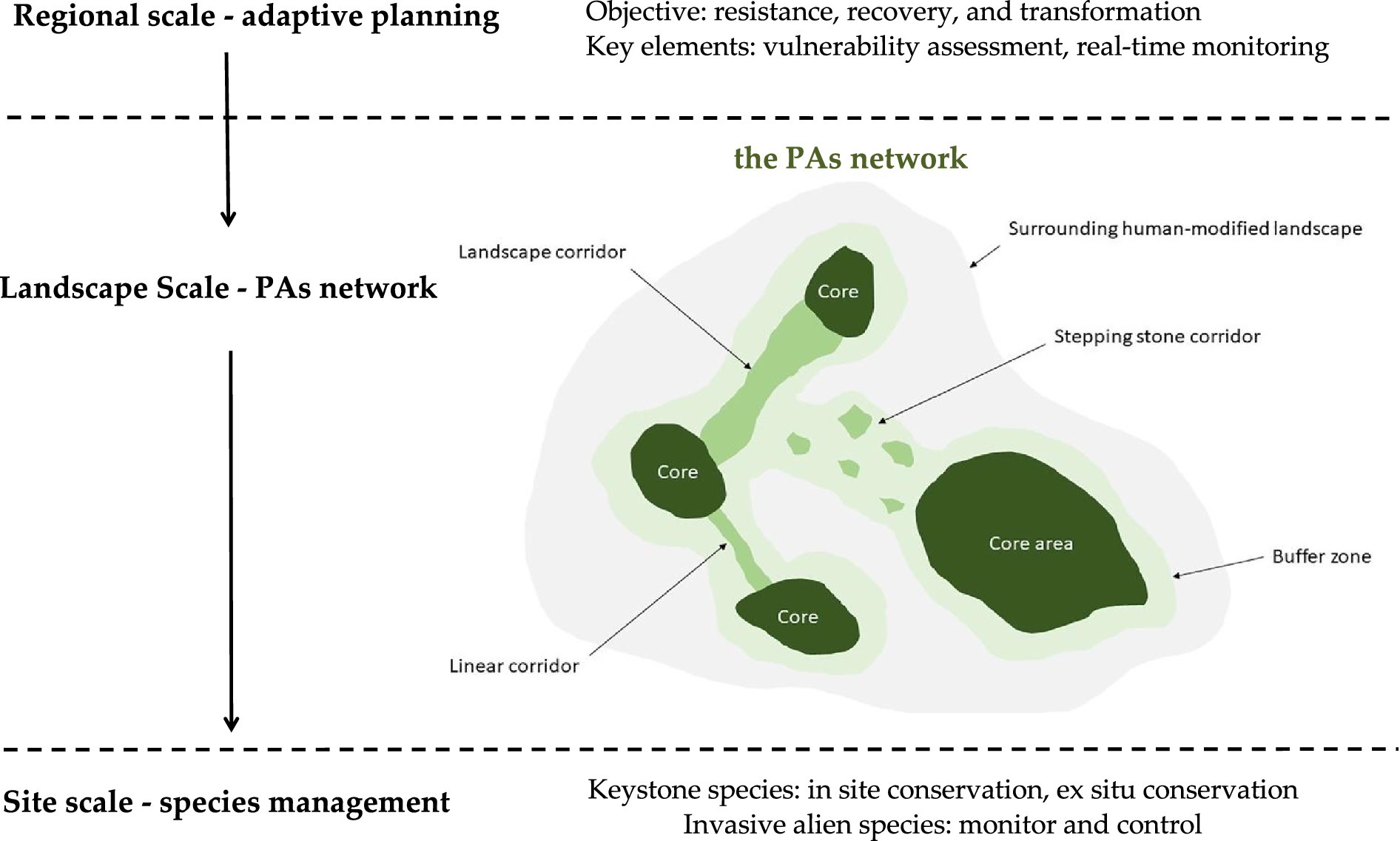
Figure 2. Biodiversity adaptation strategies across spatial scales. This figure was developed by the author based on existing research (Carver et al., 2021; Hole et al., 2011; Soule and Noss, 1998). The lines represent corridors, the blocks represent stepping stones and climate change refugia, and the rings surrounding the core areas, stepping stones, refugia, and corridors represent the matrix.
The conservation initiative in Yampa River Basin in Colorado, USA, exemplifies the application of this framework (Poiani et al., 2000). Initially, conservation efforts focused on protecting rare species. Since 1986, The Nature Conservancy (TNC) has implemented measures such as riparian land acquisition and vegetation restoration, primarily to protect the globally rare Acer negundo–Populus angustifolia/Cornus sericea riparian forest. In the late 1990s, with improved understanding of riparian ecosystem dynamics, TNC’s conservation focus shifted from a single forest type to conserving the entire riparian mosaic ecosystem, expanding conservation strategies from the site to the landscape level. After 2010, the Yampa River Basin was incorporated into the Upper Colorado River basin-wide conservation network, realizing the “Networks of Reserves” concept.
2.3 Cross-scale synergy in biodiversity adaptation
2.3.1 How to achieve synergy?
The core of the “regional-landscape-site” cross-scale framework lies in its multi-scale systemic integration, overcoming the limitations of traditional single-scale conservation approaches to develop comprehensive solutions for climate change complexities (Figure 3). This framework emphasizes vertical interactions and interdependencies, responding to climate change uncertainties through both spatial cascades and policy implementation.
a. In terms of spatial scales, vertical integration manifests through a species-landscape-region planning hierarchy. While site-scale species protection yields local benefits, it faces challenges in achieving broader ecosystem functional adaptation goals. Conversely, landscape-scale approaches achieve functional integration through ecological networks (corridors/refugia), yet face land-use conflicts (Mendonça et al., 2021), governance fragmentation (Dorst et al., 2022), and multi-stakeholder coordination challenges (Kauark-Fontes et al., 2023). Large-scale interventions require coordination at a broader regional scale. Scientific understanding of cross-scale adaptation challenges helps avoid maladaptive practices (Schuldt et al., 2023).
b. In terms of implementation, vertical interaction combines top-down resource allocation and policy dissemination with bottom-up feedback mechanisms across administrative levels (Kauark-Fontes et al., 2023; Puskás et al., 2021). The goals, geographical scope, practical measures, and implementation processes of biodiversity adaptation at the region-landscape-site scales are mutually matched (Table 1). Conceptualized as an implementation cycle, climate adaptation involves four iterative phases: planning, design, implementation, and engineering management and maintenance (Mirsafa et al., 2025). Specifically: (i) The planning stage focuses on the regional level, aiming to maintain the integrity and authenticity of the regional ecosystem. This stage involves planning across broad geographical areas spanning different landscapes, including the identification and diagnosis of macro-level issues, the setting of overall adaptive goals, and the specific layout of working units and sub-projects. (ii) The design stage focuses on the landscape level, aiming to maintain the integrity of the structure and function of ecosystems. In this stage, detailed designs of working units (e.g., protected area networks) within a complex of multiple ecosystems are required, along with the formulation of corresponding specific indicator systems and standards. (iii) The implementation stage takes place at the site level, achieving dynamic balance of the matrix ecosystem through species management. Specific project construction within species habitats requires the determination of adaptive measures based on species types and their implementation. (iv) Additionally, the management and maintenance stage covers monitoring and evaluation, adaptive management, and supervision and inspection throughout the entire process. Biodiversity conservation across different scales works in synergy with each other and is interconnected at each level, forming a cross - scale spatial three - dimensional network to achieve collaboration.
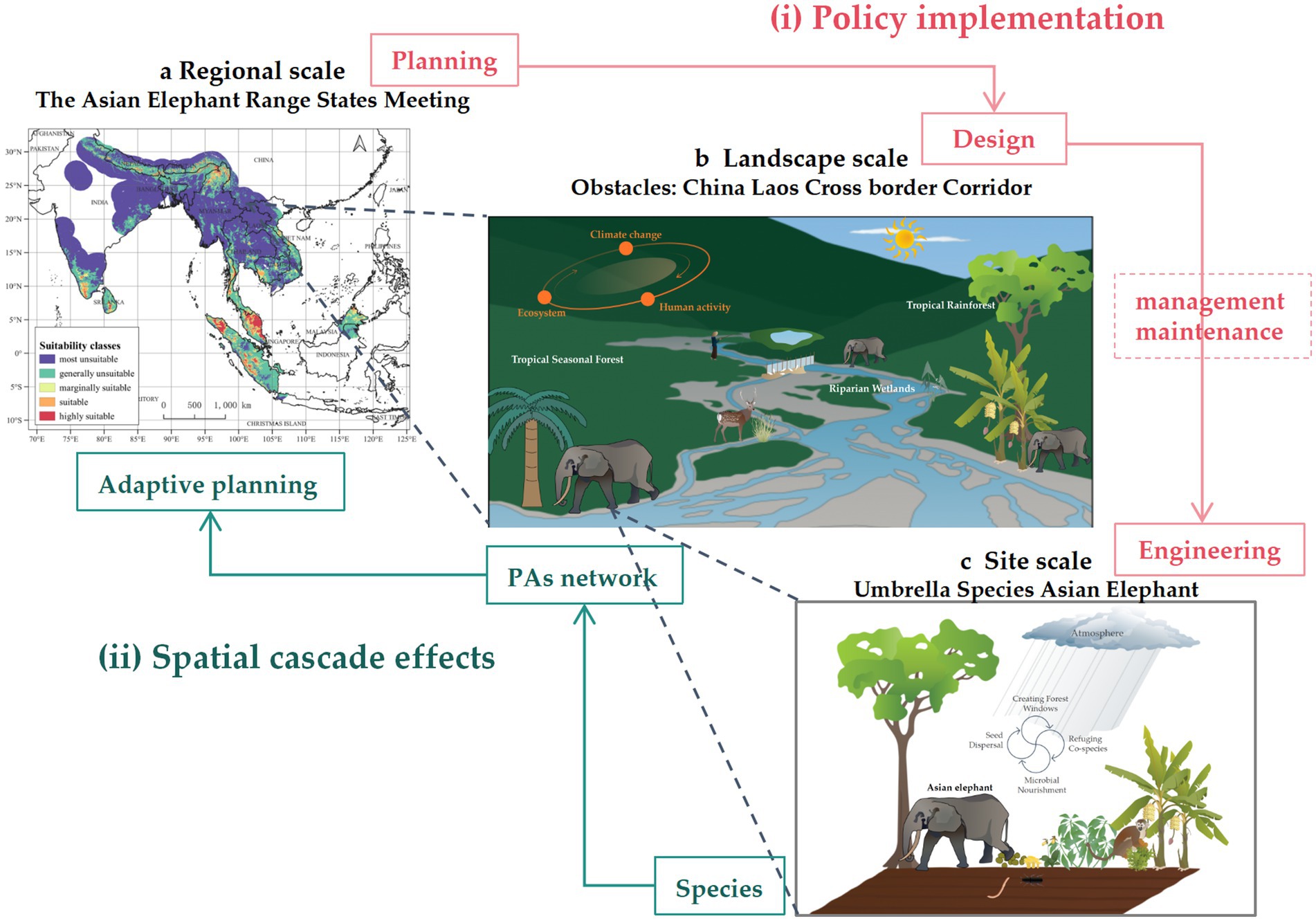
Figure 3. Cross-scale synergy in biodiversity adaptation strategies: Asian Elephants’ dry season migration. Note: Taking the dry-season migration of Asian elephants (Elephas maximus) as an example, cross-scale synergy unfolds across two dimensions: (i) Spatial cascade effects. At the site scale, during the dry season, Asian elephants enhance ecosystem drought resilience by creating forest gaps. However, at the landscape scale, the China-Laos Railway fragments traditional migration corridors. Meanwhile, at the regional scale, the Asian Elephant Range States Meeting promotes corridor connectivity, facilitating climate-adaptive movements toward wetlands. (ii) Policy implementation. When droughts prolong in border regions, the regional-scale Lancang-Mekong Cooperation Mechanism coordinates hydropower water releases. Guided by transnational agreements, the landscape-scale China-Laos Railway project adopts unified ecological standards, while site-scale mitigation measures—such as extended tunnels, wildlife bridges, isolation fences, and acoustic-optical barriers—“yield” to elephants, ensuring migration pathways. Data on Asian elephant distribution across 13 range countries were sourced from Xu et al. (2024). Other graphics elements were created using the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/imagelibrary/).
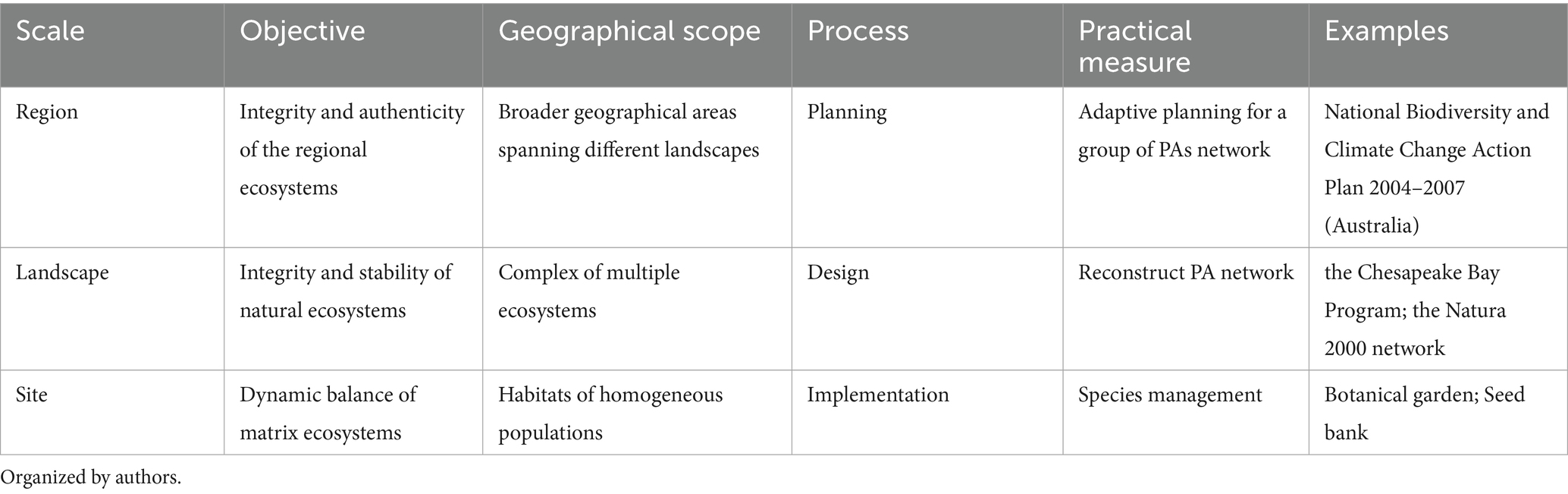
Table 1. Comparison of cross scale management strategies for biodiversity adaptation to climate change.
2.3.2 Why we need synergistic integration?
The primary advantages of vertical interactions and inter-dependencies are reflected in the ecological linkages across spatial scales and the hierarchical transmission and information feedback mechanisms in implementation.
a. Ecological interdependencies enable cross-scale conservation coordination, forming functional ecological networks that enhance the overall efficacy of biodiversity adaptation strategies. For instance, at the regional scale, climate models can identify refugia, providing a scientific basis for the design of corridors at the landscape scale. In turn, the construction of ecological networks at the landscape scale creates migration pathways for species conservation at the site scale.
b. In terms of governance mechanisms, hierarchical transmission and information feedback mechanisms optimize vertical governance structures and strengthen the effectiveness of policy implementation, ensuring the scientific and operational nature of cross-scale decision-making. In horizontal cooperation, environmental departments are often the leaders in formulating national biodiversity strategy policies, while other departments (i.e., transportation, energy, waste, drainage, and water) act as supporters. Planning among departments is mostly fragmented, and policy integration always faces conflicts of interest. This leads to isolated planning and mutual buck-passing among functional departments (Kauark-Fontes et al., 2023). For example, Bicentenario Park was envisioned as a transitional space connecting the old city park and the historic center of Bogotá, Colombia. However, stakeholder coordination failures have stalled construction progress (Fixsen, 2018). Even at global scales, protracted negotiations over Convention on Biological Diversity (CBD) funding mechanisms further exemplify these governance challenges. Conversely, the Chesapeake Bay Program in the United States and the Natura 2000 network in Europe demonstrate the potential for coordinated conservation across spatial scales. The Chesapeake Bay Program guides the restoration and protection of North America’s largest estuary through a regional partnership.1 Similarly, the Natura 2000 network integrates over 27,000 PAs across member states through standardized monitoring and management frameworks.2 These cases underscore that institutionalized cross-scale coordination is prerequisite for effective biodiversity governance.
2.3.3 Critical gaps in synergistic implementation
The critical gaps in biodiversity adaptation strategies across spatial scales are as follows:
a. Assessment of problem-governance scale alignment. Effective cross-scale management first requires a clear distinction between the spatio-temporal scale at which problems occur (problem scale) and the institutional scale at which governance is implemented (governance scale), and an assessment of the degree of alignment between the two (Padt et al., 2014). Many countries face horizontal mismatches between internal governance scales and the scale of climate change impacts. For example, there is inconsistency between the boundaries of river basins such as the Rhine, Meuse, or Scheldt (problem scale) and the administrative boundaries of member states (governance scale) in the European Union region (Dewulf et al., 2015).
b. Identification of ecological cascades across spatial scales. For instance, refuge planning at the regional scale needs to be coordinated with ecological corridors at the landscape scale and habitat restoration at the site scale (Phillips et al., 2025). However, there are still deficiencies in identifying and integrating ecological cascades across spatial scales (Le Provost et al., 2023; Li et al., 2025). In geographical modeling, the nonlinear characteristics of scale transformation and cross-scale interactions pose challenges to integrating multi-level ecological effects (Peters et al., 2007). For example, simple upscaling-downscaling based on traditional hierarchical theory cannot fully explain the complex interactions between different scales (Gonzalez et al., 2020; Koo, 2009). This makes it difficult to accurately predict ecosystem behavior and dynamics in cross-scale analyses, thereby limiting the synergy between conservation measures at different scales.
c. Policy implementation via vertical integration. To match policy practice with the scale of biodiversity conservation goals, it is necessary to (i) coordinate and integrate across levels under the same objective, (ii) share information and resources within appropriate scopes, and (iii) to facilitate the resolution of cross-boundary issues by connecting governance systems at different scales. Current policy implementation not only lacks coordination across different levels of jurisdiction but also faces temporal scale mismatches. That is, adaptation strategies that require long-term implementation cycles face challenges due to the tendency to pursue short-term economic benefits under the fixed political turnover cycles of governments (Kettunen and Ten Brink, 2012). The difficulty in effectively coordinating governance needs at different scales during policy implementation affects the achievement of biodiversity conservation goals.
d. Dynamic adaptive management. Dynamically adjusting management strategies based on multi-scale ecological monitoring data is key to biodiversity conservation. For example, the Dutch “Room for the River” program dynamically adjusts the setback distance of dikes based on annual flood simulations (Zevenbergen et al., 2015). However, there are still deficiencies in dynamic adaptive management, and the linkage mechanism between long-term dynamic monitoring and strategy adjustment has not yet been established (Zarzuelo Romero et al., 2025), which limits the flexibility and effectiveness of biodiversity adaptation strategies.
3 Regional-scale adaptation planning provides top-level design
3.1 Practices of adaptive planning
Adaptive planning provides a systematic framework for biodiversity adaptation through objective-driven resource allocation (Reside et al., 2018). Robust regional-scale adaptive planning should incorporate the following features: reversibility, preservation of future options, resistance to a variety of impacts, and permission for mid-course adjustments (Wilby and Vaughan, 2011). Effective planning must simultaneously address multiple interacting drivers of biodiversity loss, as climate change acts synergistically with habitat degradation, soil loss, nitrogen enrichment and acidification, single-focus efforts risk exacerbating the others. For example, the Kyoto Protocol addresses emission reduction plans, the Clean Development Mechanism, carbon sequestration, biodiversity conservation and human livelihoods (UNFCCC, 1997). For adaptive planning to remain flexible and robust, assessing species vulnerability and continuous real-time monitoring are key (Sutherland, 2006).
In 2002, the Strategic Plan of the CBD called for the integration of biodiversity concerns into relevant national sectoral and cross-sectoral plans, programs and policies. Developed countries and some large developing biodiversity powerhouses have placed a high priority on adaptive planning for biodiversity to climate change. Common categories of adaptive planning include the following: (i) Integrating climate adaptation into overall national development planning, which is the choice of most countries. For example, China has consistently included ecosystems as a key area of adaptation to climate change in A Review of China’s Climate Change Policies and Actions (2022), National Strategy for Climate Change Adaptation 2035 and National Plan for Climate Change (2014–2020). (ii) Embedding adaptation within existing sustainability frameworks, covering sectoral domains spanning disaster mitigation, water security, public health, environmental management, energy and national security (IPCC, 2022; UNEP, 2023). (iii) Developing dedicated adaptation plans. Australia was the first country in the world to issue a dedicated action plan for biodiversity conservation, and was the first to issue National Action Plan on Biodiversity and Climate Change, which integrated conservation and adaptation of biodiversity to climate change into key strategic planning (Booth, 2012), and developed adaptation strategy in Biodiversity and Climate Adaptation in Australia.
Globally, countries are issuing biodiversity-related national strategies or plans to respond to climate change. Developed economies concentrate planning efforts on climate change mitigation, deploying highly specified and operational initiatives, while developing countries prioritize adaptation objectives (UNEP, 2023). However, economic development priorities limit developing nations to framework-level plans that remain largely conceptual, failing to address current challenges. As the most climate-vulnerable nations, developing countries require: (i) implementation of the common but different responsibility (CBDR) principle to ensure climate justice, (ii) Global Climate Change Initiative (GCCI)-type mechanisms for equitable development (Persson et al., 2009), and (iii) institutional strengthening with adequate financing. In Australia, the Council of Ministers for Natural Resource Management leads adaptive planning, while the National Institute for Climate Change Adaptation develops policy guidance tools, and the Australian Biodiversity Fund provides financial security by establishing eco-banks that implement payments for ecosystem/environmental services (PES) (Salzman et al., 2018).
3.2 Crucial components of adaptive planning
Numerous implementation frameworks for adaptive planning have been proposed in existing research and practice: the Adaptation for Conservation Targets (ACT) (Cross et al., 2012), Climate-Smart Conservation (CSC) (Stein et al., 2014), and Portfolio Decision Analysis (PDA) (Convertino and Valverde, 2013) frameworks. However, from a process perspective, biodiversity adaptation planning generally follows a cyclical “Assess-Plan-Implement-Monitor” process (Watson et al., 2012). Specifically, during the assessment phase, risk and vulnerability analyses are conducted; the planning phase develops adaptive strategies; the implementation phase implements engineering, technological, and institutional measures; and the monitoring and adjustment phase utilizes monitoring data for subsequent dynamic optimization (Abrahms et al., 2017). To maintain flexibility and robustness in adaptive planning, various analytical frameworks and tools are employed, such as Robust Decision Making (Yousefpour and Hanewinkel, 2016), Iterative Risk Management (Döll and Romero-Lankao, 2017), and Scenario Planning (Star et al., 2016). Throughout the planning cycle, understanding and assessing species vulnerability and maintaining continuous real-time monitoring are crucial (Maris and Béchet, 2010; Sutherland, 2006). Here, monitoring serves as the linchpin connecting cyclical adaptive planning by: (i) providing baseline data for pre-implementation assessment and planning design; (ii) enabling post-implementation evaluation of management effectiveness; and (iii) informing future decision-making (Williams and Brown, 2012). The interdependent relationship between assessment and monitoring is particularly noteworthy, while assessment relies on monitoring data, monitoring ultimately serves assessment needs within this iterative framework.
3.2.1 Assessing species vulnerability
According to the IPCC (2007), the vulnerability of species to climate change encompasses three dimensions: exposure, susceptibility, and adaptive capacity (IPCC, 2007). (i) Exposure refers to the degree to which species are exposed to significant climate change, such as the proportion of species in regions experiencing rapid climate change. Generally, the faster the rate of climate change and the greater its intensity and frequency, the higher the exposure of species in that area. (ii) Susceptibility indicates the degree to which species are affected by climate hazards, determined by their intrinsic biological characteristics and emphasizing the outcomes of climate change impacts, such as species extinction or reduced abundance due to climate influences. (iii) Adaptive capacity denotes the ability of organisms to adjust to, exploit, and respond to potential damages, opportunities, or consequences (McCarthy et al., 2001). The intersection of high susceptibility, high exposure, and low adaptive capacity represents the greatest vulnerability (Hole et al., 2011).
Understanding species vulnerability under a range of potential future scenarios is important due to the uncertainty in climate change projections and in species, ecosystem and human responses. On one hand, direct threats to biodiversity from climate change may include: changes in phenology, changes in species distribution shifts, community composition alterations, ecosystem function changes and loss of living space. On the other hand, human responses to climate change also have an impact on biodiversity, mainly in the agriculture, water, health and energy sectors. For example, upslope shifts in cultivation due to climate (Warner et al., 2009). Glacial retreat due to climate change is significantly reducing dry season flows in glacial rivers, prompting the construction of upstream reservoirs to ensure adequate flows for hydroelectric power generation and downstream agricultural needs, which may reduce the diversity and abundance of organisms along the way (Vergara et al., 2007). In addition, using biofuels as an alternative resource is seen as a way to reduce greenhouse gas emissions, while crop-based biofuel production leads to the conversion of rainforests, savannas, grasslands and other natural ecosystems to agricultural land, which generates significant carbon debt and causes widespread degradation of natural ecosystems. These indirect threats can be as serious as, or even exceed in scale and scop the direct threats, while further affecting policy feasibility (Fargione et al., 2008).
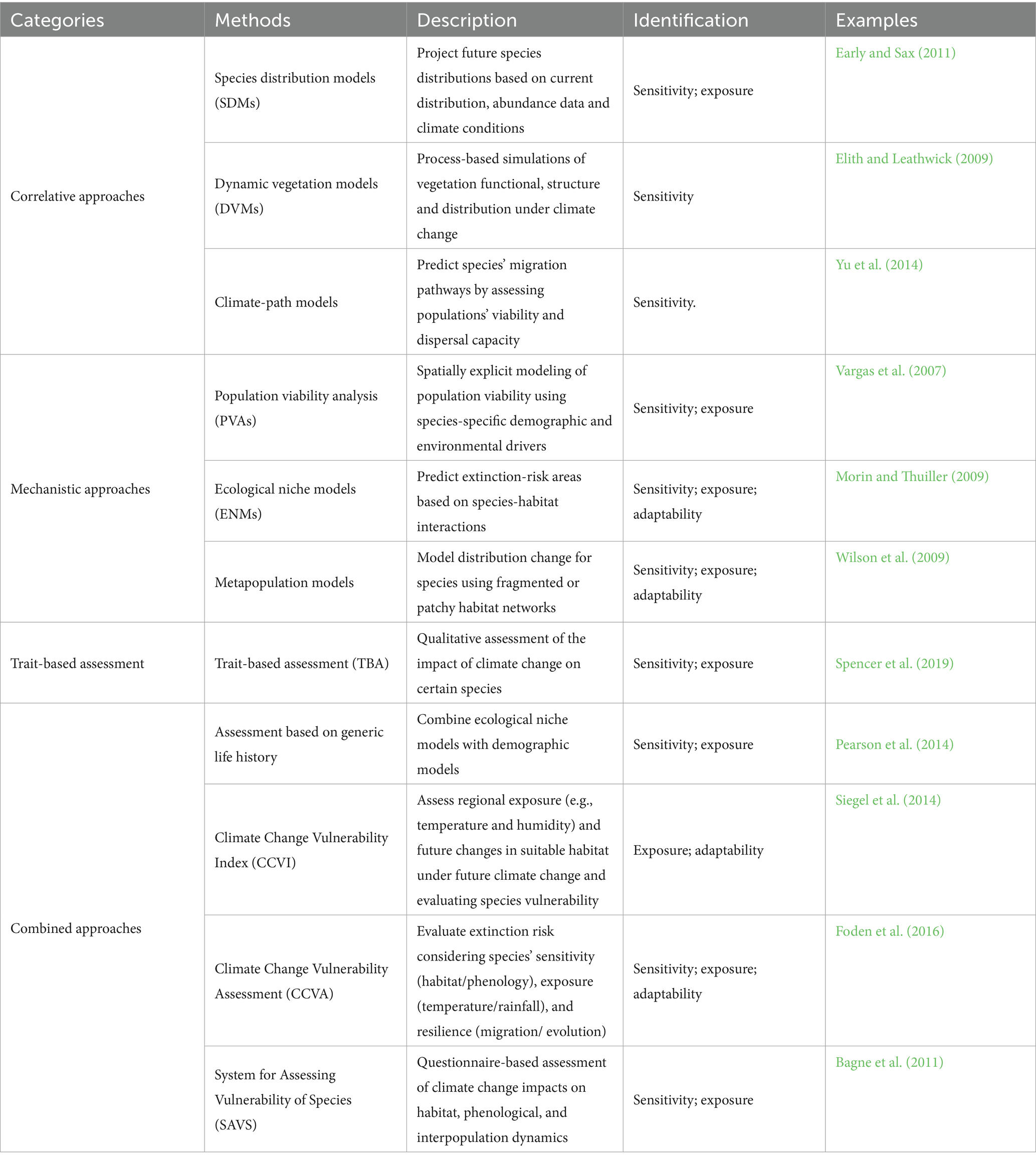
Assessing species vulnerability based on this understanding of vulnerability components is an important element of adaptation planning. For understanding and reconciling the expected interactions of the various elements, Williams et al. (2008) have integrated a working framework for species vulnerability assessments, which associates the interactions between vulnerability, exposure and adaptive capacity to guide biodiversity adaptation to climate change (Williams et al., 2008). Guided by the theoretical framework, identifying interactions between vulnerability, exposure and adaptive capacity and estimating vulnerability are the next important task (Pacifici et al., 2015). Based on the consideration of species distributional changes, population changes and extinction potential (Pacifici et al., 2015), methods for assessing species vulnerability are categorized into correlative, mechanistic, trait-based and combined approaches (Table 2).
(a)Correlative approaches are the most widely used, mainly based on observed species distributional changes in relation to climate change to predict the possible future suitable distribution areas of species. Species distribution models (SDMs), in particular, emphasize data-driven projections of potential distributional shifts (Jakubska-Busse et al., 2024). Employing an ensemble of SDMs under the RCP8.5 scenario, Dawe and Boutin (2016) projected that the climatically suitable habitat of the white-tailed deer (Odocoileus virginianus) would expand northward across North America by 2050 (Dawe and Boutin, 2016). Dynamic vegetation models (DVMs) simulate climate-driven vegetation succession (Heffernan et al., 2024). Yu et al. (2014) coupled the LPJ-GUESS model with outputs from 19 GCMs under RCP8.5 and demonstrated that evergreen broad-leaved forests are projected to replace deciduous forests in eastern China by 2100 (Yu et al., 2014). Climate-trajectory models highlight species’ capacity to persist and disperse by comparing future climatic analogues with current conditions. Ohlemüller et al. (2006) quantified the spatial extent of analogous and non-analogous climates across Europe to evaluate species’ adaptive capacity under climate change (Ohlemüller et al., 2006). While correlative approaches efficiently predict future habitat suitability, their reliability is contingent upon robust model selection and high-resolution climatic data (Elith and Leathwick, 2009) and may overlook biotic interactions influencing adaptive capacity (Meier et al., 2011).
(b)Mechanistic approaches focus on quantifying the probability of extinction under climate change by explicitly incorporating species-specific traits, physiological tolerances, and habitat interactions into viability assessments. Population viability analysis (PVA) exemplifies this strategy; it integrates physiological thresholds and the stochasticity of climatic events to estimate extinction risk, with particular emphasis on endangered taxa. Using PVA, Vargas et al. (2007) demonstrated that an increase in El Niño frequency elevates the probability of population collapse for the Galápagos penguin (Spheniscus mendiculus) to 78% within the next 100 years (Vargas et al., 2007). Although mechanistic approaches are powerful in integrating physiological and behavioral mechanisms underlying extinction risk, they demand extensive data and remain challenging to couple with non-climatic degradation (Foden et al., 2016).
(c)Trait-based assessment (TBA) refers to assessing the potential climate change impacts on a species by identifying the population, ecological niche and habitat characteristics of individual species through literature surveys, data compilation and expert consultation (Aguirre-Gutiérrez et al., 2025). A species is considered to have limited adaptive capacity if its habitat is within a restricted elevational range, if it has low genetic diversity or if it is dependent on only a few prey or host species. Applying this framework to the world’s avifauna, Foden et al. (2013) identified alpine endemics such as the Himalayan Snowcock (Tetraogallus himalayensis) as highly vulnerable because of their restricted altitudinal range and weak dispersal ability (Foden et al., 2013). Although TBA translates functional attributes into quantitative vulnerability scores, the weighting of individual traits requires expert calibration to minimise subjectivity.
(d)Combined approaches integrate correlative, mechanistic and TBA in a complementary manner to meet empirical needs. Pearson et al. (2014) coupled SDMs with PVA and demonstrated that dispersal barriers can trigger local extinctions of European amphibians even within climatically suitable habitats; such integration reduces predictive uncertainty, yet it demands extensive cross-disciplinary data support (Pearson et al., 2014).
3.2.2 Real-time monitoring throughout the process
Monitoring serves as a critical component of adaptive planning implementation, providing early warnings for emergent climate risks and establishing empirical bases for conservation effectiveness evaluation, with its outcomes requiring real-time integration into the planning revision process (Corelli et al., 2024). Specifically, monitoring objectives can be operationalized through three key dimensions: (i) Understanding how ecosystems, habitats, and species respond to climate change while identifying compounding stressors that may exacerbate these responses; (ii) Generating data for model development and validation to enhance predictive capacity for climate adaptation scenarios; (iii) Assessing the effectiveness of policy and management interventions (Bongaarts, 2019). As a representative case study, the European Biodiversity Observation Network (EU BON) project (2012–2017) established a continent-scale monitoring infrastructure through standardized permanent plots (accessible via https://monitoring.europabon.org), systematically recording species abundance, phenology, and microclimate data to analyze climate-land use interactions. The longitudinal datasets enabled refinement of species distribution models and evaluation of Natura 2000 PAs’ capacity to accommodate climate-induced species range shifts (EU BON, 2017).
Operationalizing sustained monitoring requires the iterative execution of three core tasks: (i) Standardization of indicator systems. Standardized monitoring based on the Essential Biodiversity Variables (EBVs) enables comparable metrics across genetic, species, and ecosystem levels, facilitating cross-project data integration and trend analysis (Geijzendorffer et al., 2016). For instance, the SoilBON network employs microbial diversity and soil organic carbon EBVs to assess policy impacts on subsurface biota, with springtail (Collembola) abundance serving as a rapid indicator of soil quality (Guerra et al., 2021). Likewise, the Global Coral Reef Monitoring Network adopts hard coral cover, macroalgal canopy cover, and fish diversity and abundance as three robust EBVs for reef health assessment (Obura et al., 2019). (ii) Multi-scale data integration and model validation. Species, ecosystem, climatic and remotely sensed data collected at nested scales are assimilated into analytical models to enhance the resolution of interaction effects (Jetz et al., 2019). Rogers et al. (2025) integrated multi-scale data via zero-inflated Bayesian regression to quantify the joint influence of climate and land use on freshwater fish assemblages in the northeastern United States (Rogers et al., 2025). (iii) Data sharing and attribution analysis. Scientifically rigorous designs facilitate seamless data exchange, enabling the timely detection of natural trends and extreme events while disentangling causal pathways. The Colorado Parks and Wildlife agency exemplifies this approach through the Colorado Beaver Activity Mapper, which fuses: GPS-mapped beaver dam, citizen science activity reports submitted via the Engage CPW platform, and ecological process data from interagency portals to identify the drivers of beaver–human conflict dynamics (CPW, 2025; Longwell, 2025).
Moreover, the protracted nature of climate change and the persistence of statistical noise necessitate long-term monitoring programs (Leung and Gonzalez, 2024). Most environmental time series require extended periods before underlying trends or variable relationships achieve statistical significance. Many ecological responses—including species migration and community succession—as well as cyclical climatic phenomena such as the El Niño, unfold over decades. For instance, the long-lived trees and limited seed dispersal mean that forest communities may require centuries to complete demographic turnover, whereas contemporary anthropogenic warming exceeds historical natural variability, resulting in marked lags between climatic forcing and forest response (Fastovich et al., 2025). Consequently, the informational value of biodiversity data increases exponentially with the length of time series (Robinson et al., 2005). Nevertheless, sustained biodiversity monitoring has been documented as a high-cost endeavor, with cumulative expenditures reaching the millions to billions of dollars—an issue repeatedly identified as a critical financing challenge in recent international assessments (CBD, 2024; NPWS, 2021; UNEP-MAP, 2022).
4 Reconfiguration of the network of PAs at landscape scale
4.1 Expanding the scope of PAs
The establishment of PAs continues to be the best strategy for biodiversity conservation at the global level (Bruner et al., 2001), enhancing species’ adaptive capacity. According to the IUCN (2018), the global PA network comprises 238,563 sites, covering 14.9% of terrestrial and 7.3% of marine areas (Elise et al., 2018). The advantages of PAs are that they effectively enrich baseline species diversity, achieve conservation targets, promote population connectivity, and maintain genetic adaptive potential. Expanding the scope of PAs and enhancing habitat quality will enhance the ability of species to adapt to climate change in their original habitat, especially amidst increasingly frequent extreme weather events (Abernathy et al., 2019). The CBD’s Aichi Target 11 explicitly incorporates PA coverage in Key Biodiversity Areas (KBAs) as a progress indicator (CBD, 2011). Climate-informed PA expansion requires five strategic considerations.
a. Focusing on potential changes in biodiversity distribution under climate change to fill gaps. Species currently outside PA networks, particularly rare and endemic species, should receive priority protection (Rodrigues et al., 2004). Concurrently, restoring ecosystem function requires focusing on gap species’ roles in reestablishing the “top carnivore-herbivore-primary producer” trophic network.
b. Planning PAs requires considering both replication and representation. Replication entails protecting multiple samples of the same type of ecosystem or population needs to be protected in different areas; when one area is climate-affected, surviving populations in other areas can serve as reintroduction sources (Mawdsley et al., 2009). Representation involves protecting a comprehensive portfolio of PAs, such as the protection of multiple genetically variable populations of a species, different communities of an ecosystem type or multiple habitats (Giraudo and Arzamendia, 2017). A key challenge is identifying representative PAs given climate-induced ecosystem transformations and novel species assemblages.
c. Prioritization should target areas with greater geographic and climatic diversity (S. Liu et al., 2025). Based on the principle of being the most species-rich and the most threatened, the 34 global biodiversity hotspots—covering merely 2.3% of land area but containing more than 75% of endangered mammals, birds and amphibians—demand urgent protection (Sgrò et al., 2011). Moreover, genetic diversity hotspots should also receive enhanced attention (Schmidt et al., 2024).
d. Fully utilizing the conservation value of keystone species, indicator species, pioneer species, umbrella species and flagship species. As Chinese Academy of Sciences Academician Li Zhensheng observed, a single gene can influence the rise and fall of a nation, a single species can shape the economic lifeline of a country, and healthy ecological community can improve regional environment (CAS, 2013). The presence and abundance of these species can have a major impact on ecosystems, and if lost, a ‘butterfly effect’ of change throughout the ecosystem can be triggered.
e. Selecting genotype-specific habitats for PA designation to promote in situ evolution of species (Dunlop and Brown, 2008), such as areas of steep ecological gradients, areas with recent significant geological or climatic changes (Cowling and Pressey, 2001), including island ecosystems (Cartwright, 2019).
4.2 Restoring landscape connectivity
4.2.1 Structural approaches to connectivity enhancement
In most cases, continuous and intact native habitats are the best solution for biodiversity conservation. However, in reality, a large number of economically or socially significant land-use types have separated PAs into ecological islands. Reconfiguring PA networks can help populations move along ecological corridors and increase population size, thus increasing adaptive resilience and improving resistance to climate change impacts. Corridors, stepping stones and matrices collectively transform scattered PAs into an interconnected network that retains a natural vegetation-like structure, forming PA networks. Linear corridors directly connect PAs through habitat patches of habitat, facilitating species dispersal (Stralberg et al., 2020b). For example, the tri-national Great Limpopo Transfrontier Conservation Area (GLTFCA) elephant-movement corridor network, completed in 2024 by South Africa, Mozambique, and Zimbabwe, provides approximately 15,000 African elephants and wildebeest with 3,500 km2 of continuous dry-season migration habitat (Bakari, 2025). Appropriately sized stepping stone patches shorten the spatial distance between suitable habitats and lower the energetic cost of cross-landscape movement (Schüßler et al., 2020). In the Atlantic Forest of Brazil, the Portal de Paranapanema restoration project established 90 agroforestry stepping stones (5–20 ha each) that reconnected forest fragments, restoring 1,800 ha of contiguous forest within a decade (Hilty et al., 2020). As the largest, most homogeneous, and most connected component of the landscape, the matrix plays a pivotal role in mitigating edge effects when its ecological quality is improved (Ruffell et al., 2017). Colombia’s Caribbean Ecological Connectivity Initiative converted 1.5 million ha of agricultural matrix (oil palm, pasture, and urban zones) into multifunctional sustainable-use zones through zonation, integrating them with core reserves and corridors to prevent protected-area isolation (FAO and UNEP, 2020).
Corridors and stepping stones constitute essential supplements to the matrix, offering structural guarantees for species movement and the continuity of key ecological processes. However, it must be noted that:
a. Corridor effectiveness is highly contingent upon the dispersal capacity of the focal taxa: volant birds may benefit, whereas amphibians and invertebrates often fail to use corridors that are too narrow or environmentally unsuitable. Lynch (2019) observed that contemporary urban greenway designs disproportionately cater to mammals and birds, neglecting the requirements of low-mobility species (Lynch, 2019). Moreover, Linear corridors are prone to induce edge effects, leading to abrupt changes in microenvironment parameters such as light and wind speed, and may become a diffusion pathway for invasive species (Bennett and Bennett, 2003). In experimental corridors at Savannah River Site, South Carolina, increased edge illumination significantly elevated densities of the invasive fire ant (Solenopsis invicta), resulting in a marked decline in native ant diversity (Resasco et al., 2023).
b. The successful implementation of stepping stones strategies hinges on critical thresholds of patch area and inter-patch distance, and static configurations may prove inadequate under climate-driven range shifts (Huntley et al., 2008). Saura et al. (2014) argue that stepping stones must attain sufficient area or quality to yield conservation benefits (Saura et al., 2014). Empirical work on the Tianshan Mountains further demonstrated that stepping stones design parameters (size, placement, and species composition) should be dynamically adjusted to match focal species’ dispersal distances (Han et al., 2022).
c. Matrix strategies confront multiple challenges: heterogeneity management complexity, socio-economic conflicts, and differential species responses. In Queensland, Australia, despite subsidies encouraging pastoralists to retain native vegetation, the economic appeal of high-return crops such as sugarcane has hindered effective heterogeneity management, and avian community structure has shown no significant improvement (Macinnis-Ng, 2014). Whether landscape connectivity restoration enhances biodiversity resilience to climate change depends on the coupled effects of climate velocity, habitat quantity and configuration, landscape fragmentation, the overall extent and elevational gradients of corridors–stepping stones–matrix, and species dispersal capacity. Consequently, dynamically adjusting the spatial extents and areas of corridors, stepping stones, and the matrix in accordance with climate-change scenarios and species dispersal traits has become the central challenge in contemporary landscape connectivity restoration.
4.2.2 Implementation frameworks and global applications
Research and practice in restoring landscape connectivity can be divided into two categories: focal species-based and network structure-based approaches.
a. Focal species-based connectivity, the more traditional approach, involves planning by predicting species’ future distribution based on their exposure, sensitivity, and resilience under climate change (Krosby et al., 2015). Key applications includ: (i) finding habitats or corridors that will remain valuable for certain priority species even under climate change (Fan et al., 2017); (ii) predicting species distributions based on climate change, and thus determining how to protected area or promote landscape connectivity (Choe et al., 2017). Published researches have also shown passionate concerns about ecological corridor planning for flagship species such as Asian elephants and giant pandas (Mandal and Das Chatterjee, 2023).
b. Network structure-based enhances landscape permeability by enriching the physical elements to facilitate species adaptation (Keeley et al., 2018). The specific methods can be summarized as follows: (i) Take advantage of the innate connectivity of waterways (Krosby et al., 2014); (ii) Mapping of environmental gradients based on macro-climatic gradients or land cover permeability (Rouget et al., 2006); (iii) Priority is given to the most natural areas with less human disturbance as corridors (Belote et al., 2016); (v) Design lattice-work corridor along latitudinal/longitudinal axes (Townsend and Masters, 2015); (iv) Maximize the continuity and diversity of the physical environment adjacent to the corridor (Beier and Brost, 2010).
Restoring landscape connectivity based on network structures has been incorporated into biodiversity conservation in multiple countries. Since 1992, to ensure ecological connectivity and habitat quality, The EU’s Natura 2000 network (established in 1992) covers nearly 28,000 sites (18% of land area), which is at the heart of the EU’s ecological conservation and climate change adaptation program (BISE, 2022). Mitigation banks in the U.A. adopt a range of strategies to conserve, manage and restore degraded habitats, connect fragmented habitats, create buffers and habitats for adaptive conservation of biodiversity (EPA, 2002). The Cape Floristic region of South Africa has a protected area plan that incorporates river corridors across mountains (Pressey et al., 2007). Australia has initiated large-scale landscape restoration and connectivity projects to combat climate change (Taylor and Figgis, 2007). In addition, countries have been developing green infrastructures to restore the connectivity between cities and nature. Green infrastructure is defined as an interconnected network of natural areas (e.g., rivers, wetlands, forests and wildlife habitats), and human-made environments (e.g., green spaces, parks, farmlands and pastures) (Canzonieri et al., 2007), the connectivity of which is essential for the survival of natural species, air and water quality, and human health and quality of life. In 2022, the EU launched biodiversity strategy for 2030, which designated “the improvement and restoration of ecosystems and their services through the development of green infrastructure” as one of its six headline targets (EU, 2022).
4.3 Identifying climate change refugia
4.3.1 Conceptual foundations and ecological significance
Escalating frequency, intensity, and duration of extreme climatic events impose substantial physiological and demographic stress on biota (Murali et al., 2023). Climate change refugia emerge as pivotal sanctuaries for species persistence and population recovery. Unlike landscape connectivity initiatives that design migration pathways based on species traits and dispersal capacity, refugia-oriented strategies focus on safeguarding residual populations to maintain genetic diversity (Ackerly et al., 2020). Refugia are defined as spatial habitats into which populations contract when confronted with climatic stress, providing critical buffering when necessary (Morelli et al., 2020). For taxa constrained within such refugia, migration or dispersal to more suitable habitats is often precluded by intrinsic or extrinsic barriers (Poulos et al., 2013). Consequently, facilitating population persistence within refugia is pivotal for both post-extinction recolonization and long-term adaptation under climate change, rendering the identification of refugia a priority for biodiversity conservation planning.
Refugia are species- and stressor-specific, shaped by the dynamic interplay between stressors and organisms (Greiser et al., 2020; Stewart, 2010).
a. Climate change as the dominant stressor. The velocity and magnitude of climatic shifts constitute the primary criteria for refugium identification (Szcodronski et al., 2024). If global warming is constrained to 2 °C, integrating climate-change refugia into an expanded protected-area network remains feasible (Saunders et al., 2023). For example, the U.S. National Park Service designated the meadow complex of Devils Postpile National Monument as a climate-change refugium and implemented invasive-tree removal to preserve its ecological function (Morelli et al., 2016). When warming exceeds 2 °C, however, most refugia will be restricted to high latitudes and elevations (Lawler et al., 2020).
b. Anthropogenic co-stressors. Intensive infrastructure development, habitat conversion and degradation, poaching, and pollution—are critical co-stressors. Contemporary refugia such as nearshore coral reefs of the Great Barrier Reef show reduced survival potential due to land-based runoff driven by human activities (van Woesik, 2025).
c. Stressor interactions. The compatibility between landscape attributes and the biophysical thresholds of refugial species is therefore essential (Keppel et al., 2024). Topography, soil type, ecosystem engineers (e.g., beavers), and microclimate modifiers (e.g., forest canopies) facilitate the formation and maintenance of climate-change refugia (Cartwright and Johnson, 2018; Frei et al., 2023; Kuntzemann et al., 2023; Stralberg et al., 2020a). Biophysical thresholds of refugial species serve as key indicators of whether a refugium effectively buffers ecosystems from climate change (Beaumont et al., 2019; Greiser et al., 2020). For instance, peat-forming bryophytes sustain hydrological feedback that maintains soil moisture, locally reducing fire and drought frequency and thereby promoting refugium formation; conversely, partial drainage or intensified drought reduces moisture retention; once critical biophysical thresholds are exceeded, the buffering capacity of climate-change refugia declines (Kuntzemann et al., 2023).
4.3.2 Technical challenges and uncertainties
Technically, climate change refuge identification depends on the accumulation of data across multiple species, such as relying on phylogenetic comparisons (Keppel et al., 2018), phylogenetic diversity metrics (Costion et al., 2015) and phylogenetic geographical analysis (Liu et al., 2025) to collect genomic data of certain species. Based on the collected species data, SDM is used for large-scale identification. Specifically, the habitat requirements, population dynamics and dispersal of a species are incorporated into bioclimatic models to predict the potential future distribution of a species at local, regional or larger spatial scales, and to identify refuge locations based on current distribution versus modelled future distribution projections (Briscoe et al., 2016). However, refugium identification confronts significant methodological, data and ecological challenges due to the complex interactions among diverse stressors, landscape contexts and refugial taxa.
a. Uncertainty in climate models. SDMs serve as a critical tool for identifying refugia (Georges et al., 2024), yet most models primarily correlate species distributions with macroclimatic variables, failing to comprehensively incorporate key ecological factors such as soil properties, vegetation structure, hydrology, land use, invasive species, and behavioral buffering. When projecting future ranges and identifying refugia, due to truncating all acceptable conditions for species, it may encounter niche truncation challenges (Anselmetto et al., 2025), which may either over or underestimate the true location of refugia. Furthermore, SDMs exhibit delayed responses to dynamic changes. Most current approaches rely on steady-state climate assumptions, rendering them inadequate for capturing the immediate impacts of extreme events (e.g., wildfires, droughts) on refugia. For instance, studies on post-glacial oak refugia demonstrate that species migration is strongly climate-driven (Hao et al., 2023), yet existing models struggle to integrate abrupt disturbances (e.g., flash droughts) that may disrupt refugial habitats.
b. Limitations in data acquisition across spatial and temporal scales. Data deficiencies create both taxonomic and spatial blind spots in refugia identification. Phylogenetic analyses and genetic diversity assessments rely heavily on existing genomic datasets, yet endangered species frequently lack such genetic information, compromising refugia prioritization. For instance, rare tropical rainforest plants often remain genomically uncharacterized, hindering accurate evaluation of their refugial potential (Costion et al., 2015). Similarly, the Refugia of endemic Pacific island birds have not been included in conservation plans due to genomic data gaps (Sherley, 2001).
Spatial blind spots emerge from reliance on high-resolution environmental data. Refugia identification proves particularly sensitive to spatial resolution and thermal buffering thresholds. Coarse-scale global climate datasets (e.g., 1-km resolution) systematically underestimate fine-scale temperature variations in topographically complex terrain, causing omission of critical microrefugia (e.g., ravines, caves) (Rosauer et al., 2013). Furthermore, the paucity of high-resolution environmental data in tropical, deep-sea, and polar regions biases identification efforts toward well-studied areas, leaving many potential refugia undetected (Morelli et al., 2020).
a. Stressor-species interaction gaps. Most studies focus on flagship species while neglecting community-level interaction dynamics, including competitive interactions among plant species, plant–animal relationships (herbivory, pollination, seed dispersal) (Ackerly et al., 2020); anthropogenic pressures (e.g., trawling impacts on marine refugia, deep-sea mining effects) (Zelli et al., 2025). Climate-human pressure interactions may drive species reassembly and loss, potentially altering ecosystem functioning and potentially unbalancing refugium network design (González-Trujillo et al., 2024).
b. Dynamic threshold uncertainty. Microclimatic stability thresholds differ among refugial species and even among life-history stages within the same species, yet these biological thresholds often defy clear identification (Hillebrand et al., 2020) and quantitative characterization (Groffman et al., 2006). Critical knowledge gaps persist regarding the biological threshold at which refugial processes lose their buffering capacity against climate change impacts (Costa et al., 2022).
5 Site scale emphasis on species
5.1 In situ and ex situ conservation for keystone species
In 2023, the IUCN released its latest Red List of Threatened Species, assessing 157,190 species, of which 44,016 are threatened with extinction (IUCN, 2023), highlighting the urgency. In situ conservation is one of the most effective methods for promoting the population recovery of keystone species, yet accelerating climate change has caused protected-area boundaries increasingly to misalign with shifting climatic envelopes, producing a pronounced spatio-temporal mismatch (IPCC, 2022). The first global assessment revealed that, among 11,633 terrestrial vertebrate species examined, 1,424 (12.2%) are unprotected gap species (Rodrigues et al., 2004), thus necessitating ex situ measures as a supplement. While ex situ measures can immediately avert acute threats for species unable to persist on site, they often do so at the expense of ecological context and evolutionary feedback (Minteer, 2014; Seddon et al., 2014). Integrating dynamic in situ management with ex-situ interventions to create a “parallel situ conservation” may mitigate mismatch risk while preserving ecological integrity (Feng et al., 2023).
5.1.1 In situ conservation for keystone species is the optimal choice
For keystone species, in situ conservation is one of the most effective ways to reduce the biodiversity loss on a global scale. The specific implementation is divided into three steps:
a. Using data on species richness, endemism, species threatened, taxonomic distinctiveness and habitat uniqueness to varying degrees to identify keystone species based on vulnerability and irreplaceability (Cottee-Jones and Whittaker, 2012).
b. Identifying KBAs on the basis of the previous step and four criteria: threatened species and ecosystem types, geographically restricted biodiversity, ecological integrity, and biological processes (Langhammer, 2007).
c. Ultimately, gradually implementing in situ conservation programs for different types of KBAs, such as biodiversity hotspots, plant diversity centers, endemic bird areas, and most valuable ecological areas (Bonn et al., 2002).
Thanks to international efforts, 14% of forests (Schmitt et al., 2009) and 88% of vertebrate species (Rodrigues et al., 2004) have been protected in 34 biodiversity hotspots around the world, and in situ conservation of species has been actively promoted. However, half of the world’s KBAs are still unprotected (Butchart et al., 2012). In addition, in situ conservation on the site scale has difficulty maintaining good ecological processes because of inadequate management effectiveness and the isolation of PAs from each other, and the loss of biodiversity at the population level is often prone to far-reaching ecological and evolutionary consequences, such as the loss of top predators. Under land use pressure, in situ conservation faces the enormous challenges of feeding 9 billion people by 2050 and biodiversity conservation.
5.1.2 Ex situ conservation aids retention of endangered species
Endangered species are more susceptible to climate change and there are climate thresholds beyond which the probability of extinction increases dramatically, thus making ex situ conservation an important initiative for the adaptive management of endangered species (Solomon et al., 2007). Ex situ conservation refers to the relocation of plants, animals and other organisms from areas that have become unsuitable to other areas that are suitable for survival in form of assisted dispersal (Liu et al., 2014), assisted migration (Guinan et al., 2025) and assisted colonization (Gallagher et al., 2015). Ex situ conservation requires three criteria to be met: (i) Seed to seed, requiring relocated plants and animals to be able to grow freely and survive through sexual reproduction; (ii) Representation requires that relocated species maintain genetic integrity and represent the genetic diversity of the population; (iii) Maintaining the population gene frequencies of genes after translocation to avoid outbreeding depression, genetic assimilation and intragression (Engelmann and Engels, 2002; Quinlan et al., 2025). Targets 12 and 13 of the Aichi Biodiversity Targets call for the conservation of biological genetic diversity through ex situ conservation projects (Geijzendorffer et al., 2017), and countries around the world are actively exploring ex situ conservation systems, which have been extended to cover wildlife, crops, domesticated animals and microbial strains.
While significant achievements have been made in ex situ conservation, it is crucial to recognize its distinctive characteristics. (i) Unlike climate change refugia that emphasize population relocation and long-term retention in natural systems, ex situ conservation focuses on species under human intervention. For example, wild plant conservation primarily utilizes botanical gardens and seed banks; food and agricultural plant preservation employs germplasm repositories and nurseries; wild animal conservation relies on zoos, safari parks, aquaria, and other captive breeding programs; while microbial conservation is accomplished through strain conservation (FAO, 2007). (ii) Ex situ conservation attempts may fail and even accelerate species extinction. A tragic example is the 1981 capture and captive breeding of Japan’s last five crested ibises (Nipponia nippon), which ultimately failed to prevent the species’ extinction in 2003 (Biodiversity Center of Japan, 2024). (iii) Under climate change scenarios, ecosystem transformations may become so profound that species reintroduction becomes unfeasible, potentially turning ex situ conserved populations into evolutionary relicts (Minteer, 2014). (iv) Technical constraints include: (a) climate model uncertainty and error may cause mis-translocation risk, as evidenced by the Poweshiek skipperling (Oarisma poweshiek) conservation program in the U. S., where climate model biases led to dramatic post-release survival declines (Runquist et al., 2025); (b) small population sampling reduces genetic diversity (Mclachlan et al., 2007). A global meta-analysis confirmed that inadequate wild population sampling resulted in significantly lower genetic diversity in restored populations compared to reference groups over 50 years, impairing long-term adaptive capacity (Wei et al., 2023); (c) microhabitat mismatches increase post-release mortality; and (d) translocated individuals can introduce or encounter novel pathogens. In Canada, the ex situ conservation of whitebark pine (Pinus albicaulis) faced dual threats: the absence of Clark’s nutcracker (Nucifraga columbiana), its natural seed disperser in native habitats, and the concurrent spread of white pine blister rust (Cronartium ribicola) to recipient sites (Sáenz-Romero et al., 2021).
5.2 Monitoring invasive alien species
Climate change is a pivotal factor in the acceleration of invasive species incursions (Liu et al., 2017). Throughout the sequential process of biological invasions, the repercussions of climate change can emerge at various stages. To begin with, the warming climate may expedite the developmental pace and amplify the reproductive cycles of invasive species. Following this, climate change has the potential to reshape the distribution and the spatial extent of an organism’s impact. Ultimately, the warming trend could augment the phenological flexibility, competitive prowess among species, and the growth-defense trade-offs of invasive flora, thereby disrupting the intricate relationships between hosts, pests, and predators (Gu et al., 2023). The encroachment of alien species, further perturbs biogeographical distributions, impinges on the diversity and genetic integrity of native species, and escalates the peril of extinction for indigenous taxa. It is estimated that invasive alien species and their management cost the global economy billions of dollars annually (IUCN, 2018). The Strategic Plan for Biodiversity (2011–2020) requires Parties to take urgent action to identify and prioritize invasive alien species pathways to prevent their introduction and rooting (CBD, 2015). To achieve these goals, the following aspects must be taken into account:
a. Border controls are the first barrier. Biosecurity mechanisms must be established at the national level to regulate intentional introductions, use technology to mitigate the effects of unintentional introductions, and encourage community participation in monitoring and managing invasive alien species. Almost all countries have introduced regulatory provisions related to invasive alien species based on risk assessment, establishing strict quarantine controls at borders to prohibit the import and trade of regulated species; or using a white-listing approach to prohibit the introduction of all nonnative species unless they are determined to be low risk (Genovesi et al., 2015).
b. Once invasive alien species have already invaded the region, eradication or impact mitigation must be pursued through conventional control, gene editing and other methods. Conventional control including physical control (Leary et al., 2013), chemical control (Simberloff et al., 2018) and biological control (Veitch et al., 2019). In the last decade, gene editing techniques have been progressively introduced, such as gene silencing, where specific genes of invasive species are not expressed or are not significantly expressed to reduce their spread (Martinez et al., 2020). Gene editing techniques are often used in combination with transgenesis and, while they can help to manage or eradicate of invasive alien species, their potential unintended consequences need to be addressed (Callaway, 2018).
c. The involvement of stakeholders from across society is crucial for controlling invasive alien species. Widespread community participation can collect more valuable data on invasive alien species. For example, people can readily identify and record invasive alien species through mobile phones and apps, which not only further increases their awareness of biosecurity and early monitoring capabilities, but also helps to record their location and spread pathways.
6 Conclusions and future prospects
6.1 Conclusion
Compared to other anthropogenic environmental threats, climate change will exert more gradual, more difficult-to-quantify, and largely irreversible impacts on biodiversity. Given biodiversity’s critical importance to human society and the inherent uncertainties of climate change, developing cross-scale adaptive management strategies is imperative. This paper examines adaptive management strategies to enhance biodiversity resilience under climate change, employing a cross-scale “region-landscape-site” framework. Key findings include:
a. Cross-spatial-scale synergy is key to biodiversity adaptation to climate change. This study proposes that multi-scale collaboration across “regional–landscape–site” levels can systematically enhance the adaptive capacity of biodiversity to climate change and reduce its vulnerability. This finding deepens existing adaptation frameworks: whereas Mawdsley et al. (2009) focused on a horizontal classification of action types and Heller and Zavaleta (2009) emphasized the functional aspects of strategies, this study highlights the spatial dimension of vertical integration across scales, thereby extending the theoretical framework. This perspective aligns with insights from case studies such as those in the Andes (Hole et al., 2011) and coastal wetlands (He et al., 2025), which also identify cross-scale coordination as central to effective adaptation. This study further formulates a universal theoretical model, suggesting that scale synergy can address uncertainties associated with climate change through both “ecological cascading” and “governance implementation” dimensions. The primary contribution of this research lies in constructing a systematic adaptation framework centered on “scale synergy,” moving beyond earlier research paradigms that categorized actions or strategies in isolation. It reveals the inherent connections and interactive mechanisms among multi-scale conservation strategies. This systemic perspective can help policymakers identify scale disconnects during implementation and offers significant theoretical value for the adaptive management of complex ecosystems. However, the practical application of this framework faces several challenges, which also represent limitations of this study. These include scale mismatches between problem identification and governance levels, difficulties in identifying ecological cascades across spatial scales, barriers to vertical policy integration, and delays in adaptive management feedback.
b. Biodiversity adaptation strategies at different scales have distinct emphases yet are mutually reinforcing. The regional scale focuses on top-down design and systematic assessment, providing strategic guidance for lower levels through macro-level planning and continuous monitoring. The landscape scale emphasizes spatial restructuring and network optimization—including protected area expansion, connectivity enhancement, and climate refuge identification—to facilitate species movement and persistence. The site scale prioritizes direct interventions targeting key species, such as in situ/ex situ conservation and invasive species control, representing the most immediate and concrete conservation actions. Although these strategies are widely recognized, the innovation of this study lies in integrating them into a coherent system with clearly defined hierarchical support relationships, elucidating the inter-dependencies among strategies across scales. For instance, species conservation at the site scale relies on landscape connectivity to support successful reintroduction and population recovery, while landscape optimization depends on regional-scale vulnerability assessments and planning prioritization. Together, these three scales form an organic whole: actions at lower levels facilitate the implementation of higher-level strategies, while upper-level planning provides the framework and basis for localized interventions. Nevertheless, integrating multi-scale strategies still faces resource- and knowledge-related barriers, such as: (i) competition for limited conservation funding among strategies operating at different scales, making optimal resource allocation challenging; and (ii) difficulties in effectively integrating site-level monitoring data into landscape and regional scales to support macro-decision-making, coupled with the generally insufficient resolution of regional climate models to provide precise guidance for site-specific management.
6.2 Future prospects
The adaptation of biodiversity to climate change is a long-term, social learning process that requires individuals and societies to increase their awareness of potential future changes and enhance their capacity to cope with them. Future efforts must address critical challenges, including social inequities, lags in dynamic landscape conservation planning, dynamic imbalances in species’ ecological networks, and barriers to interdisciplinary support.
a. At the regional scale, policies for biodiversity adaptation confront both domestic and international social inequities. Biodiversity conservation has significant positive externalities. However, biodiversity-rich regions are often less developed regions that face three constraints: development limitations imposed by conservation requirements. High dependence on climate-sensitive sectors (e.g., agriculture), and lack of technical and financial resources to withstand climate change (Marshall et al., 2016). Furthermore, climate change may exacerbate current regional inequalities (Feliciano et al., 2025). Therefore, future policy design and international cooperation must explicitly incorporate equity, fairness, and distributional considerations, evaluating how policies affect balanced regional development, stakeholders responses, and the burden placed on the poor, etc.
b. At the landscape scale, response lags in dynamic landscape-conservation planning pose a future challenge. Natural disturbances, succession and climate cycles drive spatial shifts in suitable habitats. Dynamic landscape conservation explicitly addresses biodiversity’s climate adaptation needs at this scale, incorporating both structural and functional connectivity responses to environmental change (Xu et al., 2025). By enhancing cross-scale, long-term monitoring, climate impact assessments, and risk analyses, this approach enables prediction of species redistribution and ecosystem transitions, quantification of landscape-level dynamics, and development of proactive conservation targets to guide adaptive management. Regrettably, a major constraint remains: the limited availability of large-scale, high-resolution, long-term ecological datasets essential for reliable implementation.
c. At the site scale, dynamic imbalances in species’ ecological networks hinder the maintenance of complex ecosystem stability, thereby impeding species adaptation. While flagship species such as the giant panda and Asian elephant attract policy attention and function as umbrella species, the neglect of non-charismatic or lower-trophic-level species can alter matrix ecosystem structure (Poiani et al., 2000), undermine network stability, and erode adaptive capacity. Ecological-network models require actions that explore the links between complexity and stability (Landi et al., 2018), and foster dynamic equilibrium within habitats, thereby promoting species adaptation and sustaining ecosystem services.
d. Interdisciplinary support across all three scales remains inadequate. Most existing proposals focus on ecology disciplines, underestimating the contribution of the social sciences. Effective biodiversity adaptation across spatial scales demands large geographical coverage, long time-frames, integration with species-conservation plans, natural resource management, and the livelihoods of local people, etc. (Frison, 2024). Capacity building must therefore be truly multidisciplinary, engaging atmospheric sciences, biology, ecology, geography, agronomy, sociology, economics and management.
e. It is imperative to heighten attention to the synergistic impacts of human activities and climate change on biodiversity. Climate change, human activities, and their interactions are the strongest drivers of biodiversity loss. Although the scientific community has directed significant concern towards climate change (Mazor et al., 2018), it is crucial to acknowledge that human actions, especially those involving excessive development, continue to pose the most significant threat to species at risk as noted in the IUCN Red List (Maxwell et al., 2016). Habitats conversion driven by agricultural expansion, deforestation, marine fishing, and urban sprawl—exacerbated by climate change—constitutes the primary cause of biodiversity decline. The cumulative amplification of multiple stressors can trigger cascading effects, potentially undermining conservation initiatives (Brook et al., 2008). Future research endeavors should concentrate on elucidating the intricate interplay between climate change and human activities in relation to biodiversity, and devising integrated planning and management strategies.
Author contributions
XM: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization, Visualization, Methodology. LJ: Investigation, Methodology, Writing – original draft. LS: Writing – original draft. ZQ: Project administration, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by the Yunnan Provincial Philosophy and Social Science Planning Key Project (ZX2025ZD06); the Yunnan Minzu University Talent Introduction Research Project (101520250000023).
Acknowledgments
We are grateful to the reviewers who provided extensive and helpful comments on an earlier version of this manuscript. We thank Professor Hongxin Wang for early input, and Professor David Ackerly, reviewer, for his thoughtful comments to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Abernathy, H. N., Crawford, D. A., Garrison, E. P., Chandler, R. B., Conner, M. L., Miller, K. V., et al. (2019). Deer movement and resource selection during hurricane Irma: implications for extreme climatic events and wildlife. Proc. R. Soc. B Biol. Sci. 286:20192230. doi: 10.1098/rspb.2019.2230
Abrahms, B., DiPietro, D., Graffis, A., and Hollander, A. (2017). Managing biodiversity under climate change: challenges, frameworks, and tools for adaptation. Biodivers. Conserv. 26, 2277–2293. doi: 10.1007/s10531-017-1362-4
Ackerly, D. D., Kling, M. M., Clark, M. L., Papper, P., Oldfather, M. F., Flint, A. L., et al. (2020). Topoclimates, refugia, and biotic responses to climate change. Front. Ecol. Environ. 18, 288–297. doi: 10.1002/fee.2204
Ackerly, D. D., Loarie, S. R., Cornwell, W. K., Weiss, S. B., Hamilton, H., Branciforte, R., et al. (2010). The geography of climate change: implications for conservation biogeography. Divers. Distrib. 16, 476–487. doi: 10.1111/j.1472-4642.2010.00654.x
Aguirre-Gutiérrez, J., Díaz, S., Rifai, S. W., Corral-Rivas, J. J., Nava-Miranda, M. G., González-M, R., et al. (2025). Tropical forests in the Americas are changing too slowly to track climate change. Science 387:eadl5414. doi: 10.1126/science.adl5414
Anselmetto, N., Morresi, D., Barbarino, S., Loglisci, N., Betts, M. G., and Garbarino, M. (2025). Species distribution models built with local species data perform better for current time, but suffer from niche truncation. Agric. For. Meteorol. 362:110361. doi: 10.1016/j.agrformet.2024.110361
Bagne, K. E., Friggens, M. M., and Finch, D. M. (2011). A System for Assessing Vulnerability of Species (SAVS) to Climate Change (No. RMRS-GTR-257; p. RMRS-GTR-257). Washington, DC: U.S. Department of Agriculture, Forest Service.
Bakari, M. (2025) Ecological Connectivity in the GLTFCA: 2024 Corridor Assessment Summary (International Online Workshop “Ecological Connectivity and Transboundary Conservation Practice: Tools and Insights”). IUCN Connectivity Conservation Specialist Group. Available online at: https://iucn.org/events/vital-sites-connectivity-2025 (Accessed August 11, 2025).
Bakkenes, M., Eickhout, B., and Alkemade, R. (2006). Impacts of different climate stabilisation scenarios on plant species in Europe. Glob. Environ. Chang. 16, 19–28. doi: 10.1016/j.gloenvcha.2005.11.001
Beaumont, L. J., Esperón-Rodríguez, M., Nipperess, D. A., Wauchope-Drumm, M., and Baumgartner, J. B. (2019). Incorporating future climate uncertainty into the identification of climate change refugia for threatened species. Biol. Conserv. 237, 230–237. doi: 10.1016/j.biocon.2019.07.013
Beier, P., and Brost, B. (2010). Use of land facets to plan for climate change: conserving the arenas, not the actors. Conserv. Biol. 24, 701–710. doi: 10.1111/j.1523-1739.2009.01422.x
Belote, R. T., Dietz, M. S., McRae, B. H., Theobald, D. M., McClure, M. L., Irwin, G. H., et al. (2016). Identifying corridors among large protected areas in the United States. PLoS One 11:e0154223. doi: 10.1371/journal.pone.0154223
Bennett, A. F., and Bennett, G. (2003). Linkages in the landscape: The role of corridors and connectivity in wildlife conservation. 2nd Edn. Gland, Switzerland; Cambridge, UK: IUCN.
Biodiversity Center of Japan (2024) Japanese Crested Ibis Available online at: https://www.biodic.go.jp/center/spec/toki_e.html (Accessed July 08, 2024).
BISE. (2022). Natura 2000 [non-profit website]. Available online at: https://biodiversity.europa.eu/natura2000/en/natura2000 (Accessed June 25, 2023).
Bongaarts, J. (2019). IPBES, 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the intergovernmental science-policy platform on biodiversity and ecosystem services. Popul. Dev. Rev. 45, 680–681. doi: 10.1111/padr.12283
Bonn, A., Rodrigues, A. S. L., and Gaston, K. J. (2002). Threatened and endemic species: are they good indicators of patterns of biodiversity on a national scale?: threat, endemism and biodiversity. Ecol. Lett. 5, 733–741. doi: 10.1046/j.1461-0248.2002.00376.x
Booth, T. H. (2012). Biodiversity and climate change adaptation in Australia: strategy and research developments. Adv. Clim. Chang. Res. 3, 12–21. doi: 10.3724/SP.J.1248.2012.00012
Briscoe, N. J., Kearney, M. R., Taylor, C. A., and Wintle, B. A. (2016). Unpacking the mechanisms captured by a correlative species distribution model to improve predictions of climate refugia. Glob. Chang. Biol. 22, 2425–2439. doi: 10.1111/gcb.13280
Brook, B. W., Sodhi, N. S., and Bradshaw, C. J. A. (2008). Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460. doi: 10.1016/j.tree.2008.03.011
Bruner, A. G., Gullison, R. E., Rice, R. E., and da Fonseca, G. A. B. (2001). Effectiveness of parks in protecting tropical biodiversity. Science 291, 125–128. doi: 10.1126/science.291.5501.125
Butchart, S. H. M., Scharlemann, J. P. W., Evans, M. I., Quader, S., Aricò, S., Arinaitwe, J., et al. (2012). Protecting important sites for biodiversity contributes to meeting global conservation targets. PLoS One 7:e32529. doi: 10.1371/journal.pone.0032529
Callaway, E. (2018). ‘Gene drive’ ban back on table—worrying scientists. Nature 563, 454–455. doi: 10.1038/d41586-018-07436-4
Canzonieri, C., Benedict, M. E., and Mcmahon, E. T. (2007). Green infrastructure: linking landscapes and communities. Landsc. Ecol. 22, 797–798. doi: 10.1007/s10980-006-9045-7
Cartwright, J. (2019). Ecological islands: conserving biodiversity hotspots in a changing climate. Front. Ecol. Environ. 17, 331–340. doi: 10.1002/fee.2058
Cartwright, J. M., and Johnson, H. M. (2018). Springs as hydrologic refugia in a changing climate? A remote sensing approach. Ecosphere 9, 1–22. doi: 10.1002/ecs2.2155
Carver, S., Convery, I., Hawkins, S., Beyers, R., Eagle, A., Kun, Z., et al. (2021). Guiding principles for rewilding. Conserv. Biol. 35, 1882–1893. doi: 10.1111/cobi.13730
CAS (2013). Focusing on plant species with extremely small populations [non-profit website] : Chinese Academy of Sciences (Accessed April 04, 2024).
CBD (2010) Strategic Plan for Biodiversity 2011–2020 Secretariat of the Convention on Biological Diversity. Available online at: https://www.cbd.int/sp (Accessed April 15, 2023).
CBD. (2011). Aichi biodiversity targets. Available online at: https://www.cbd.int/sp/targets/ (Accessed April 15, 2023).
CBD (2015) Global Biodiversity Outlook 4—A mid-term assessment of progress towards the implementation of the Strategic Plan for Biodiversity 2011–2020 Secretariat of the Convention on Biological Diversity. Available online at: https://www.cbd.int/gbo4/ (Accessed April 15, 2023).
CBD. (2024). Monitoring framework for the Kunming-Montreal Global Biodiversity Framework (COP16). Available online at: https://www.cbd.int/conferences/2024/cop-16/documents (Accessed July 11, 2025).
Chase, J. M., McGill, B. J., McGlinn, D. J., May, F., Blowes, S. A., Xiao, X., et al. (2018). Embracing scale-dependence to achieve a deeper understanding of biodiversity and its change across communities. Ecol. Lett. 21, 1587–1754. doi: 10.1111/ele.13151
Choe, H., Thorne, J. H., Hijmans, R., Kim, J., Kwon, H., and Seo, C. (2017). Meta-corridor solutions for climate-vulnerable plant species groups in South Korea. J. Appl. Ecol. 54, 1742–1754. doi: 10.1111/1365-2664.12865
Climate-Diplomacy (2024) UN Biodiversity Conference 2024 (CBD COP16) Available online at: https://climate-diplomacy.org/events/un-biodiversity-conference-2024-cbd-cop16 (Accessed April 08, 2025).
Convertino, M., and Valverde, L. J. (2013). Portfolio decision analysis framework for value-focused ecosystem management. PLoS One 8:e65056. doi: 10.1371/journal.pone.0065056
Corelli, V., Boerder, K., Hunter, K. L., Lavoie, I., and Tittensor, D. P. (2024). The biodiversity adaptation gap: management actions for marine protected areas in the face of climate change. Conserv. Lett. 17:e13003. doi: 10.1111/conl.13003
Costa, F. R. C., Schietti, J., Stark, S. C., and Smith, M. N. (2022). The other side of tropical forest drought: do shallow water table regions of Amazonia act as large-scale hydrological refugia from drought? New Phytol. 237, 714–733. doi: 10.1111/nph.17914
Costion, C. M., Edwards, W., Ford, A. J., Metcalfe, D. J., Cross, H. B., Harrington, M. G., et al. (2015). Using phylogenetic diversity to identify ancient rain forest refugia and diversification zones in a biodiversity hotspot. Divers. Distrib. 21, 279–289. doi: 10.1111/ddi.12266
Cottee-Jones, H. E. W., and Whittaker, R. J. (2012). Perspective: the keystone species concept: a critical appraisal. Front. Biogeogr. 4:533. doi: 10.21425/F5FBG12533
Cowling, R. M., and Pressey, R. L. (2001). Rapid plant diversification: planning for an evolutionary future. Proc. Natl. Acad. Sci. 98, 5452–5457. doi: 10.1073/pnas.101093498
CPW (2025). Beaver Conservation and Management Strategy. Engage CPW. Available online at: https://engagecpw.org/beaver-conservation-and-management-strategy (Accessed August 08, 2025).
Cross, M. S., Zavaleta, E. S., Bachelet, D., Brooks, M. L., Enquist, C. A. F., Fleishman, E., et al. (2012). The adaptation for conservation targets (ACT) framework: a tool for incorporating climate change into natural resource management. Environ. Manag. 50, 341–351. doi: 10.1007/s00267-012-9893-7
Darwin, C. R. (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. 1st Edn. London, UK: John Murray.
Davis, E. B., Koo, M. S., Conroy, C., Patton, J. L., and Moritz, C. (2008). The California hotspots project: identifying regions of rapid diversification of mammals. Mol. Ecol. 17, 120–138. doi: 10.1111/j.1365-294X.2007.03469.x
Dawe, K. L., and Boutin, S. (2016). Climate change is the primary driver of white-tailed deer (Odocoileus virginianus) range expansion at the northern extent of its range; land use is secondary. Ecol. Evol. 6, 6435–6451. doi: 10.1002/ece3.2316
Dewulf, A., Meijerink, S., and Runhaar, H. (2015). Editorial: the governance of adaptation to climate change as a multi-level, multi-sector and multi-actor challenge: a European comparative perspective. J. Water Clim. Chang. 6, 1–8. doi: 10.2166/wcc.2014.000
Döll, P., and Romero-Lankao, P. (2017). How to embrace uncertainty in PARTICIPATORY climate change RISK MANAGEMENT-a roadmap: PARTICIPATORY RISK MANAGEMENT. Earths Future 5, 18–36. doi: 10.1002/2016EF000411
Dorst, H., van der Jagt, A., Toxopeus, H., Tozer, L., Raven, R., and Runhaar, H. (2022). What’s behind the barriers? Uncovering structural conditions working against urban nature-based solutions. Landsc. Urban Plann. 220:104335. doi: 10.1016/j.landurbplan.2021.104335
Dunlop, M., and Brown, P. R. (2008). Implications of climate change for Australia’s National Reserve System: a preliminary assessment (Report to the Department of Climate Change). Canberra, Australia: Department of Climate Change.
Early, R., and Sax, D. F. (2011). Analysis of climate paths reveals potential limitations on species range shifts: climate paths. Ecol. Lett. 14, 1125–1133. doi: 10.1111/j.1461-0248.2011.01681.x
Ekroos, J., Ödman, A. M., Andersson, G. K. S., Birkhofer, K., Herbertsson, L., Klatt, B. K., et al. (2016). Sparing land for biodiversity at multiple spatial scales. Front. Ecol. Evol. 3:145. doi: 10.3389/fevo.2015.00145
Elise, B., Naomi, K., Neil, B., Trevor, S., Natasha, A., and Kathy, M. (2018) Protected Planet Report 2018 UNEP World Conservation Monitoring Centre. Available online at: https://livereport.protectedplanet.net/pdf/Protected_Planet_Report_2018.pdf (Accessed August 08, 2021).
Elith, J., and Leathwick, J. R. (2009). Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697. doi: 10.1146/annurev.ecolsys.110308.120159
Engelmann, F., and Engels, J. M. M. (2002). “Technologies and strategies for ex situ conservation” in Managing plant genetic diversity. eds. J. M. M. Engels, V. R. Rao, A. H. D. Brown, and M. T. Jackson. 1st ed (Wallingford, UK: CABI Publishing), 89–103.
EPA (2002) National Wetlands Mitigation Action Plan. Washington, DC, USA: Environmental Protection Agency
EU (2022) EU biodiversity strategy for 2030. European Commission. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM (Accessed May 05, 2023).
EU BON (2017) Building the European Biodiversity Observation Network (No. Grant Agreement No. 308454). Available online at: https://cordis.europa.eu/project/id/308454/reporting (Accessed May 05, 2023).
Fan, D., Sun, Z., Li, B., Kou, Y., Hodel, R. G. J., Jin, Z., et al. (2017). Dispersal corridors for plant species in the Poyang Lake Basin of Southeast China identified by integration of phylogeographic and geospatial data. Ecol. Evol. 7, 5140–5148. doi: 10.1002/ece3.2999
FAO (2007). Global Plan of Action for Animal Genetic Resources and the Interlaken Declaration. 1st Edn. Rome, Italy: FAO.
FAO and UNEP (2020). The state of the world’s forests 2020: Forests, biodiversity and people. Rome, Italy: FAO.
Fargione, J., Hill, J., Tilman, D., Polasky, S., and Hawthorne, P. (2008). Land clearing and the biofuel carbon debt. Science 319, 1235–1238. doi: 10.1126/science.1152747
Fastovich, D., Meyers, S. R., Saupe, E. E., Williams, J. W., Dornelas, M., Dowding, E. M., et al. (2025). Coupled, decoupled, and abrupt responses of vegetation to climate across timescales. Science 389, 64–68. doi: 10.1126/science.adr6700
Feliciano, D., Smith, P., and Mabhaudhi, T. (2025). Climate change exacerbates inequalities between small-scale and large-scale farmers in South Africa’s fruit export market. Reg. Environ. Chang. 25:28. doi: 10.1007/s10113-024-02354-w
Feng, C., Zhang, J., and Huang, H. (2023). Parallel situ conservation: a new plant conservation strategy to integrate in situ and ex situ conservation of plants. Biodivers. Sci. 31:23184. doi: 10.17520/biods.2023184
Field, C. B., Barros, V. R., Mastrandrea, M. D., Mach, K. J., Barnett, J., Burkett, V. R., et al. (2014). Climate change 2014: Impacts, adaptation, and vulnerability. Cambridge, UK and New York, USA: IPCC.
Fixsen, A. (2018). The Pride and Prejudice of Bogota’s Bicentenario Park : ArchDaily. Available online at: https://www.archdaily.com/904371/the-pride-and-prejudice-of-bogotas-bicentenario-park (Accessed May 10, 2021).
Foden, W. B., Butchart, S. H. M., Stuart, S. N., Vié, J.-C., Akçakaya, H. R., Angulo, A., et al. (2013). Identifying the world’s most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS One 8:e65427. doi: 10.1371/journal.pone.0065427
Foden, W. B., Young, B., Baker, D. J., and Bickford, D. (2016). IUCN SS C Guidelines for Assessing Species’ Vulnerability to Climate Change. Cambridge, UK: IUCN Species Survival Commission.
Frei, K., Vojtkó, A., Farkas, T., Erdős, L., Barta, K., E-Vojtkó, A., et al. (2023). Topographic depressions can provide climate and resource microrefugia for biodiversity. IScience 26:108202. doi: 10.1016/j.isci.2023.108202
Frison, E. (2024). We cannot afford another lost year for food and climate action. Bull. At. Sci. 80, 158–161. doi: 10.1080/00963402.2024.2339071
Gallagher, R. V., Makinson, R. O., Hogbin, P. M., and Hancock, N. (2015). Assisted colonization as a climate change adaptation tool: assisted colonization and climate change. Austral Ecol. 40, 12–20. doi: 10.1111/aec.12163
Geijzendorffer, I. R., Cohen-Shacham, E., Cord, A. F., Cramer, W., Guerra, C., and Martín-López, B. (2017). Ecosystem services in global sustainability policies. Environ. Sci. Pol. 74, 40–48. doi: 10.1016/j.envsci.2017.04.017
Geijzendorffer, I. R., Regan, E. C., Pereira, H. M., Brotons, L., Brummitt, N., Gavish, Y., et al. (2016). Bridging the gap between biodiversity data and policy reporting needs: an essential biodiversity variables perspective. J. Appl. Ecol. 53, 1341–1350. doi: 10.1111/1365-2664.12417
Genovesi, P., Carboneras, C., Vilà, M., and Walton, P. (2015). EU adopts innovative legislation on invasive species: a step towards a global response to biological invasions? Biol. Invasions 17, 1307–1311. doi: 10.1007/s10530-014-0817-8
Georges, V., Vaz, S., Carbonara, P., Fabri, M.-C., Fanelli, E., Follesa, M. C., et al. (2024). Mapping the habitat refugia of Isidella elongata under climate change and trawling impacts to preserve vulnerable marine ecosystems in the Mediterranean. Sci. Rep. 14:6246. doi: 10.1038/s41598-024-56338-1
Giraudo, A. R., and Arzamendia, V. (2017). Descriptive bioregionalisation and conservation biogeography: what is the true bioregional representativeness of protected areas? Aust. Syst. Bot. 30:403. doi: 10.1071/SB16056
Gonzalez, A., Germain, R. M., Srivastava, D. S., Filotas, E., Dee, L. E., Gravel, D., et al. (2020). Scaling-up biodiversity-ecosystem functioning research. Ecol. Lett. 23, 757–776. doi: 10.1111/ele.13456
González-Trujillo, J. D., Naimi, B., Assis, J., and Araújo, M. B. (2024). Reshuffling of Azorean coastal marine biodiversity amid climate change. J. Biogeogr. 51, 2546–2555. doi: 10.1111/jbi.15008
Grant, P. R., and Grant, B. R. (2002). Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296, 707–711. doi: 10.1126/science.1070315
Greiser, C., Ehrlén, J., Meineri, E., and Hylander, K. (2020). Hiding from the climate: characterizing microrefugia for boreal forest understory species. Glob. Chang. Biol. 26, 471–483. doi: 10.1111/gcb.14874
Groffman, P. M., Baron, J. S., Blett, T., Gold, A. J., Goodman, I., Gunderson, L. H., et al. (2006). Ecological thresholds: the key to successful environmental management or an important concept with no practical application? Ecosystems 9, 1–13. doi: 10.1007/s10021-003-0142-z
Gu, S., Qi, T., Rohr, J. R., and Liu, X. (2023). Meta-analysis reveals less sensitivity of non-native animals than natives to extreme weather worldwide. Nat. Ecol. Evol. 7, 2004–2027. doi: 10.1038/s41559-023-02235-1
Guerra, C. A., Bardgett, R. D., Caon, L., Crowther, T. W., Delgado-Baquerizo, M., Montanarella, L., et al. (2021). Tracking, targeting, and conserving soil biodiversity. Science 371, 239–241. doi: 10.1126/science.abd7926
Guinan, A., Evans, A. E., Plotkin, A. B., and Bradley, B. A. (2025). Identifying candidate plants for climate-informed restoration. Restor. Ecol. 33:e70030. doi: 10.1111/rec.70030
Han, L., Wang, Z., Wei, M., Wang, M., Shi, H., Ruckstuhl, K., et al. (2022). Small patches play a critical role in the connectivity of the Western Tianshan landscape, Xinjiang, China. Ecol. Indic. 144:109542. doi: 10.1016/j.ecolind.2022.109542
Hao, Q., Liu, H., Cheng, Y., and Song, Z. (2023). The LGM refugia of deciduous oak and distribution development since the LGM in China. Sci. China Earth Sci. 66, 80–91. doi: 10.1007/s11430-021-9981-9
He, Q., Li, Z., Daleo, P., Lefcheck, J. S., Thomsen, M. S., Adams, J. B., et al. (2025). Coastal wetland resilience through local, regional and global conservation. Nat. Rev. Biodivers. 1, 50–67. doi: 10.1038/s44358-024-00004-x
Heffernan, E., Epstein, H., Declan McQuinn, T., Rogers, B. M., Virkkala, A.-M., Lutz, D., et al. (2024). Comparing assumptions and applications of dynamic vegetation models used in the Arctic-boreal zone of Alaska and Canada. Environ. Res. Lett. 19:093003. doi: 10.1088/1748-9326/ad6619
Heller, N. E., and Zavaleta, E. S. (2009). Biodiversity management in the face of climate change: a review of 22 years of recommendations. Biol. Conserv. 142, 14–32. doi: 10.1016/j.biocon.2008.10.006
Hillebrand, H., Donohue, I., Harpole, W. S., Hodapp, D., Kucera, M., Lewandowska, A. M., et al. (2020). Thresholds for ecological responses to global change do not emerge from empirical data. Nat. Ecol. Evol. 4, 1502–1509. doi: 10.1038/s41559-020-1256-9
Hilty, J., Worboys, G. L., Keeley, A., Woodley, S., Lausche, B. J., Locke, H., et al. (2020). Guidelines for conserving connectivity through ecological networks and corridors. Glad, Switzerland: IUCN, International Union for Conservation of Nature.
Hodgson, D., McDonald, J. L., and Hosken, D. J. (2015). What do you mean, ‘resilient’? Trends Ecol. Evol. 30, 503–506. doi: 10.1016/j.tree.2015.06.010
Hole, D. G., Young, K. R., Seimon, A., Gomez, C., Hoffmann, D., Paez, K. S., et al. (2011). “Adaptive management for biodiversity conservation under climate change—A tropical Andean perspective” in Climate change and biodiversity in the tropical Andes. ed. C. Gonda. [São Paulo, Brazil: Inter-American Institute for Global Change Research (IAI)].
Huntley, B., Collingham, Y. C., Willis, S. G., and Green, R. E. (2008). Potential impacts of climatic change on European breeding birds. PLoS One 3:e1439. doi: 10.1371/journal.pone.0001439
IPCC (1990) FAR Climate Change: Scientific Assessment of Climate Change. Available online at: https://www.ipcc.ch/report/ar1/wg1/ (Accessed May 05, 2021).
IPCC (2002) Climate Change and Biodiversity. Available online at: https://www.ipcc.ch/publication/climate-change-and-biodiversity-2/ (Accessed May 05, 2021).
IPCC (2007) AR4 Climate Change 2007: Impacts, Adaptation, and Vulnerability Available online at: https://www.ipcc.ch/report/ar4/wg2/ (Accessed May 05, 2021).
IPCC (2022) Sixth Assessment Report. Available online at: https://www.ipcc.ch/assessment-report/ar6/ (Accessed June 18, 2023).
IUCN (2018). Invasive alien species and sustainable development [Non-profit website]. Cambridge, UK; New York, USA: IUCN.
IUCN (2023). The IUCN red list of threatened species [non-profit website]. Gland, Switzerland: IUCN.
Jakubska-Busse, A., Wysocki, A., Domagała, P. J., Brudzińska-Kosior, A., Sporek, M., and Kosior, G. (2024). Expanding the boundaries in the face of global warming: A lesson from genetic and ecological niche studies of Centaurium erythraea in Europe. Sci. Total Environ. 953:176134. doi: 10.1016/j.scitotenv.2024.176134
Jetz, W., McGeoch, M. A., Guralnick, R., Ferrier, S., Beck, J., Costello, M. J., et al. (2019). Essential biodiversity variables for mapping and monitoring species populations. Nature Ecol. Evol. 3, 539–551. doi: 10.1038/s41559-019-0826-1
Kauark-Fontes, B., Marchetti, L., and Salbitano, F. (2023). Integration of nature-based solutions (NBS) in local policy and planning toward transformative change. Evidence from Barcelona, Lisbon, and Turin. Ecol. Soc. 28:225. doi: 10.5751/ES-14182-280225
Keeley, A. T. H., Ackerly, D. D., Cameron, D. R., Heller, N. E., Huber, P. R., Schloss, C. A., et al. (2018). New concepts, models, and assessments of climate-wise connectivity. Environ. Res. Lett. 13:073002. doi: 10.1088/1748-9326/aacb85
Keppel, G., Ottaviani, G., Harrison, S., Wardell-Johnson, G. W., Marcantonio, M., and Mucina, L. (2018). Towards an eco-evolutionary understanding of endemism hotspots and refugia. Ann. Bot. 122, 927–934. doi: 10.1093/aob/mcy173
Keppel, G., Stralberg, D., Morelli, T. L., and Bátori, Z. (2024). Managing climate-change refugia to prevent extinctions. Trends Ecol. Evol. 39, 800–808. doi: 10.1016/j.tree.2024.05.002
Kettunen, M., and Ten Brink, P. (2012). Nature, green economy and sustainable development: the outcomes of UN Rio+20 conference on sustainable development. Nat. Conserv. 2, 1–6. doi: 10.3897/natureconservation.2.3704
Koo, K.-A. (2009) Distribution of Picea rubens and global warming: A systems approach [University of Georgia]. Available online at: https://openscholar.uga.edu/record/18638 (Accessed May 05, 2021).
Krosby, M., Breckheimer, I., Pierce, D., Singleton, P. H., Hall, S. A., Halupka, K. C., et al. (2015). Focal species and landscape “naturalness” corridor models offer complementary approaches for connectivity conservation planning. Landsc. Ecol. 30, 2121–2132. doi: 10.1007/s10980-015-0235-z
Krosby, M., Norheim, R., Theobald, D., and McRae, B. (2014) Riparian climate-corridors: Identifying priority areas for conservation in a changing climate (Mapping Pacific Northwest Riparian Areas: Measuring Current Condition And Prioritizing For Climate Change Adaptation) Available online at: https://www.sciencebase.gov/catalog/item/53c938c6e4b092c1b256558f (Accessed May 05, 2021).
Kuntzemann, C. E., Whitman, E., Stralberg, D., Parisien, M.-A., Thompson, D. K., and Nielsen, S. E. (2023). Peatlands promote fire refugia in boreal forests of northern Alberta, Canada. Ecosphere 14:e4510. doi: 10.1002/ecs2.4510
Landi, P., Minoarivelo, H. O., Brännström, Å., Hui, C., and Dieckmann, U. (2018). Complexity and stability of ecological networks: a review of the theory. Popul. Ecol. 60, 319–345. doi: 10.1007/s10144-018-0628-3
Langhammer, P. F. (2007). Identification and gap analysis of key biodiversity areas: Targets for comprehensive protected area systems. Gland, Switzerland: IUCN.
Lawler, J. J., Rinnan, D. S., Michalak, J. L., Withey, J. C., Randels, C. R., and Possingham, H. P. (2020). Planning for climate change through additions to a national protected area network: implications for cost and configuration. Philos. Trans. Royal Soc. B Biol. Sci. 375:20190117. doi: 10.1098/rstb.2019.0117
Le Provost, G., Schenk, N. V., Penone, C., Thiele, J., Westphal, C., Allan, E., et al. (2023). The supply of multiple ecosystem services requires biodiversity across spatial scales. Nat. Ecol. Evol. 7, 236–249. doi: 10.1038/s41559-022-01918-5
Leary, J. J. K., Gooding, J., Chapman, J., Radford, A., Mahnken, B., and Cox, L. J. (2013). Calibration of an herbicide ballistic technology (HBT) helicopter platform targeting Miconia calvescens in Hawaii. Invasive Plant Sci. Manag. 6, 292–303. doi: 10.1614/IPSM-D-12-00026.1
Leung, B., and Gonzalez, A. (2024). Global monitoring for biodiversity: uncertainty, risk, and power analyses to support trend change detection. Sci. Adv. 10:eadj1448. doi: 10.1126/sciadv.adj1448
Li, S., Pu, J., Deng, X., Dong, B., and Su, Y. (2025). Improving ecological barrier area sustainability integrating ecosystem service interaction and social–ecological system coupling. Land Degrad. Dev. 36, 2420–2437. doi: 10.1002/ldr.5506
Liu, S., Gao, J., Xiao, B., Guo, W., Yu, Q., Wang, A., et al. (2025). Genetic differentiation and historical dynamics of the endemic species Rheum pumilum on the Qinghai-Tibetan plateau inferred from phylogeography implications. BMC Plant Biol. 25, 162–117. doi: 10.1186/s12870-025-06164-y
Liu, X., Li, X., Liu, Z., Tingley, R., Kraus, F., Guo, Z., et al. (2014). Congener diversity, topographic heterogeneity and human-assisted dispersal predict spread rates of alien herpetofauna at a global scale. Ecol. Lett. 17, 821–829. doi: 10.1111/ele.12286
Liu, Y., Oduor, A. M. O., Zhang, Z., Manea, A., Tooth, I. M., Leishman, M. R., et al. (2017). Do invasive alien plants benefit more from global environmental change than native plants? Glob. Chang. Biol. 23, 3363–3370. doi: 10.1111/gcb.13579
Longwell, A. (2025). Colorado is crafting a plan to manage and protect beavers—And it wants your input. Available online at: https://www.aspentimes.com/news/colorado-protect-beavers-environmentalists-wildlife-biologists/ (Accessed August 05, 2025).
Lynch, A. J. (2019). Creating effective urban greenways and stepping-stones: four critical gaps in habitat connectivity planning research. J. Plann. Lit. 34, 131–155. doi: 10.1177/0885412218798334
MacArthur, R. H., and Wilson, E. O. (1967). The theory of island biogeography. Princeton, New Jersey, USA: Princeton University Press.
Macinnis-Ng, C. (2014). Biodiversity and environmental change: Monitoring, challenges and direction. Melbourne, Australia: CSIRO Publishing.
Mandal, M., and Das Chatterjee, N. (2023) Species specific corridor demarcation: case of Asian elephant. M. Mandal and N. Das Chatterjee (M. Mandal and N. Das Chatterjee Geo-spatial analysis of Forest landscape for wildlife management (85–101). Cham: Springer International Publishing.
Maris, V., and Béchet, A. (2010). From adaptive management to adjustive management: a pragmatic account of biodiversity values: pragmatic account of biodiversity values. Conserv. Biol. 24, 966–973. doi: 10.1111/j.1523-1739.2009.01437.x
Marshall, N. A., Crimp, S., Curnock, M., Greenhill, M., Kuehne, G., Leviston, Z., et al. (2016). Some primary producers are more likely to transform their agricultural practices in response to climate change than others. Agric. Ecosyst. Environ. 222, 38–47. doi: 10.1016/j.agee.2016.02.004
Martinez, B., Reaser, J. K., Dehgan, A., Zamft, B., Baisch, D., McCormick, C., et al. (2020). Technology innovation: advancing capacities for the early detection of and rapid response to invasive species. Biol. Invasions 22, 75–100. doi: 10.1007/s10530-019-02146-y
Mawdsley, J. R., O’Malley, R., and Ojima, D. S. (2009). A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv. Biol. 23, 1080–1089. doi: 10.1111/j.1523-1739.2009.01264.x
Maxwell, S. L., Fuller, R. A., Brooks, T. M., and Watson, J. E. M. (2016). Biodiversity: the ravages of guns, nets and bulldozers. Nature 536, 143–145. doi: 10.1038/536143a
Mazor, T., Doropoulos, C., Schwarzmueller, F., Gladish, D. W., Kumaran, N., Merkel, K., et al. (2018). Global mismatch of policy and research on drivers of biodiversity loss. Nature Ecol. Evol. 2, 1071–1074. doi: 10.1038/s41559-018-0563-x
McCarthy, J. J., Canziani, O. F., Leary, N. A., Dokken, D. J., and White, K. S. (2001). Climate change 2001: Impacts, adaptation, and vulnerability: Contribution of working group II to the third assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press.
Mclachlan, J. S., Hellmann, J. J., and Schwartz, M. W. (2007). A framework for debate of assisted migration in an era of climate change. Conserv. Biol. 21, 297–302. doi: 10.1111/j.1523-1739.2007.00676.x
McLaughlin, B. C., Skikne, S. A., Beller, E., Blakey, R. V., Olliff-Yang, R. L., Morueta-Holme, N., et al. (2022). Conservation strategies for the climate crisis: an update on three decades of biodiversity management recommendations from science. Biol. Conserv. 268:109497. doi: 10.1016/j.biocon.2022.109497
Meier, E. S., Edwards, T. C., Kienast, F., Dobbertin, M., and Zimmermann, N. E. (2011). Co-occurrence patterns of trees along macro-climatic gradients and their potential influence on the present and future distribution of Fagus sylvatica. J. Biogeogr. 38, 371–382. doi: 10.1111/j.1365-2699.2010.02405.x
Mendonça, R., Roebeling, P., Fidélis, T., and Saraiva, M. (2021). Socio-economic models to assess and policy instruments to steer the impact of nature-based solutions: A review. The Sustainable City. Southampton, UK: WIT Press.
Mirsafa, M., Castaldo, A. G., and Lemes de Oliveira, F. (2025). Enabling nature-based solutions for climate adaptation in cities of the global south: planning dimensions and cross-cutting pathways for implementation. Environ. Dev. Sustain. doi: 10.1007/s10668-025-06256-7
Morelli, T. L., Barrows, C. W., Ramirez, A. R., Cartwright, J. M., Ackerly, D. D., Eaves, T. D., et al. (2020). Climate-change refugia: biodiversity in the slow lane. Front. Ecol. Environ. 18, 228–234. doi: 10.1002/fee.2189
Morelli, T. L., Daly, C., Dobrowski, S. Z., Dulen, D. M., Ebersole, J. L., Jackson, S. T., et al. (2016). Managing climate change Refugia for climate adaptation. PLoS One 11:e0159909. doi: 10.1371/journal.pone.0159909
Morin, X., and Thuiller, W. (2009). Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90, 1301–1313. doi: 10.1890/08-0134.1
Murali, G., Iwamura, T., Meiri, S., and Roll, U. (2023). Future temperature extremes threaten land vertebrates. Nature 615, 461–467. doi: 10.1038/s41586-022-05606-z
Norris, K., Terry, A., Hansford, J. P., and Turvey, S. T. (2020). Biodiversity conservation and the earth system: mind the gap. Trends Ecol. Evol. 35, 919–926. doi: 10.1016/j.tree.2020.06.010
NPWS (2021) Prioritised Action Framework for Nature 2000 in Ireland Available online at: https://docslib.org/doc/11623786/prioritised-action-framework-paf-for-natura-2000-in-ireland (Accessed August 18, 2023).
Obura, D. O., Aeby, G., Amornthammarong, N., Appeltans, W., Bax, N., Bishop, J., et al. (2019). Coral reef monitoring, reef assessment technologies, and ecosystem-based management. Front. Mar. Sci. 6:580. doi: 10.3389/fmars.2019.00580
Ohlemüller, R., Gritti, E. S., Sykes, M. T., and Thomas, C. D. (2006). Towards European climate risk surfaces: the extent and distribution of analogous and non-analogous climates 1931-2100: European climate risk surfaces. Glob. Ecol. Biogeogr. 15, 395–405. doi: 10.1111/j.1466-822X.2006.00245.x
Pacifici, M., Foden, W. B., Visconti, P., Watson, J. E. M., Butchart, S. H. M., Kovacs, K. M., et al. (2015). Assessing species vulnerability to climate change. Nat. Clim. Chang. 5, 215–224. doi: 10.1038/nclimate2448
Padt, F., Opdam, P., Polman, N., and Termeer, C. (Eds.) (2014). Scale-sensitive governance of the environment. In Scale-sensitivity as a governance capability: observing, acting and enabling. (Chichester, West Sussex; Hoboken, NJ: John Wiley & Sons, Ltd).
Pearson, R. G., Stanton, J. C., Shoemaker, K. T., Aiello-Lammens, M. E., Ersts, P. J., Horning, N., et al. (2014). Life history and spatial traits predict extinction risk due to climate change. Nat. Clim. Chang. 4, 217–221. doi: 10.1038/nclimate2113
Pecl, G. T., Araújo, M. B., Bell, J. D., Blanchard, J., Bonebrake, T. C., Chen, I.-C., et al. (2017). Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355:eaai9214. doi: 10.1126/science.aai9214
Persson, Å., Klein, R. J. T., Siebert, C. K., Atteridge, A., Müller, B., Hoffmaister, J., et al. (2009). Adaptation finance under a Copenhagen agreed outcome. Stockholm, Sweden: Stockholm Environment Institute.
Peters, D. P. C., Bestelmeyer, B. T., and Turner, M. G. (2007). Cross–scale interactions and changing pattern–process relationships: consequences for system dynamics. Ecosystems 10, 790–796. doi: 10.1007/s10021-007-9055-6
Peterson St-Laurent, G., Oakes, L. E., Cross, M., and Hagerman, S. (2021). R–R–T (resistance–resilience–transformation) typology reveals differential conservation approaches across ecosystems and time. Commun. Biol. 4:39. doi: 10.1038/s42003-020-01556-2
Phillips, K. B. B., Lineman, B., Murray, G. L. D., Nelson, S. J., Sperduto, D. D., and Tourville, J. C. (2025). Refugia or at risk? Alpine snowbank communities in the face of climate change. Conserv. Sci. Pract. :e70079. doi: 10.1111/csp2.70079
Phillips, S. J., Williams, P., Midgley, G., and Archer, A. (2008). Optimizing dispersal corridors for the cape Proteaceae using network flow. Ecol. Appl. 18, 1200–1211. doi: 10.1890/07-0507.1
Poiani, K. A., Richter, B. D., Anderson, M. G., and Richter, H. E. (2000). Biodiversity conservation at multiple scales: functional sites, landscapes, and networks. Bio Science 50, 133–146. doi: 10.1641/0006-3568(2000)050[0133:BCAMSF]2.3.CO;2
Poulos, D. E., Harasti, D., Gallen, C., and Booth, D. J. (2013). Biodiversity value of a geographically restricted soft coral species within a temperate estuary: biodiversity value of a soft coral habitat. Aquat. Conserv. Mar. Freshw. Ecosyst. 23, 838–849. doi: 10.1002/aqc.2362
Pressey, R. L., Cabeza, M., Watts, M. E., Cowling, R. M., and Wilson, K. A. (2007). Conservation planning in a changing world. Trends Ecol. Evol. 22, 583–592. doi: 10.1016/j.tree.2007.10.001
Puskás, N., Abunnasr, Y., and Naalbandian, S. (2021). Assessing deeper levels of participation in nature-based solutions in urban landscapes – a literature review of real-world cases. Landsc. Urban Plan. 210:104065. doi: 10.1016/j.landurbplan.2021.104065
Quinlan, E. J., Layman, C. A., and Silman, M. R. (2025). Climate-mediated hybridisation and the future of Andean forests. J. Biogeogr., 1–16. doi: 10.1111/jbi.15113
Resasco, J., Burt, M. A., Orrock, J. L., Haddad, N. M., Shoemaker, D., and Levey, D. J. (2023). Transient effects of corridors on polygyne fire ants over a decade. Ecol. Entomol. 48, 263–268. doi: 10.1111/een.13214
Reside, A. E., Butt, N., and Adams, V. M. (2018). Adapting systematic conservation planning for climate change. Biodivers. Conserv. 27, 1–29. doi: 10.1007/s10531-017-1442-5
Robinson, R. A., Learmonth, J. A., Hutson, A. M., Macleod, C. D., Sparks, T. H., Leech, D. I., et al. (2005) Climate Change and Migratory Species (BTO Research Report 414) Thetford, UK: British Trust for Ornithology.
Rodrigues, A. S. L., Andelman, S. J., Bakarr, M. I., Boitani, L., Brooks, T. M., Cowling, R. M., et al. (2004). Effectiveness of the global protected area network in representing species diversity. Nature 428, 640–643. doi: 10.1038/nature02422
Rogers, J. B., DiRenzo, G. V., Quiñones, R. M., Richards, T., and Roy, A. H. (2025). Climate and land use drivers of freshwater fish biodiversity in the northeastern United States. Biol. Conserv. 310:111337. doi: 10.1016/j.biocon.2025.111337
Rosauer, D. F., Anderson, B. J., Welbergen, J. A., Moritz, C., Ferrier, S., Harwood, T. D., et al. (2013). Climate change refugia for terrestrial biodiversity: Defining areas that promote species persistence and ecosystem resilience in the face of global climate change. Gold Coast, Australia: National Climate Change Adaptation Research Facility (NCCARF).
Rouget, M., Cowling, R. M., Lombard, A. T., Knight, A. T., and Kerley, G. I. H. (2006). Designing large-scale conservation corridors for pattern and process. Conserv. Biol. 20, 549–561. doi: 10.1111/j.1523-1739.2006.00297.x
Ruffell, J., Clout, M. N., and Didham, R. K. (2017). The matrix matters, but how should we manage it? Estimating the amount of high-quality matrix required to maintain biodiversity in fragmented landscapes. Ecography 40, 171–178. doi: 10.1111/ecog.02097
Runquist, E., Miller, P., Stapleton, S., Smith, T., Cuthrell, D., and Nordmeyer, C. (2025). Using Population Viability Analysis (PVA) to inform and adapt ex situ conservation activities benefitting a Critically Endangered butterfly (Version 5, p. 1995123 bytes) [Dataset]. Raleigh, NC, USA: Dryad.
Sáenz-Romero, C., O’Neill, G., Aitken, S. N., and Lindig-Cisneros, R. (2021). Assisted migration field tests in Canada and Mexico: lessons, limitations, and challenges. Forests 12:Article 1. doi: 10.3390/f12010009
Salzman, J., Bennett, G., Carroll, N., Goldstein, A., and Jenkins, M. (2018). The global status and trends of payments for ecosystem services. Nat. Sustain. 1, 136–144. doi: 10.1038/s41893-018-0033-0
Saunders, S. P., Grand, J., Bateman, B. L., Meek, M., Wilsey, C. B., Forstenhaeusler, N., et al. (2023). Integrating climate-change refugia into 30 by 30 conservation planning in North America. Front. Ecol. Environ. 21, 77–84. doi: 10.1002/fee.2592
Saura, S., Bodin, Ö., and Fortin, M.-J. (2014). Editor’s choice: stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J. Appl. Ecol. 51, 171–182. doi: 10.1111/1365-2664.12179
Schmidt, C., Karachaliou, E., Vandergast, A., Crandall, E., Falgout, J., Hunter, M., et al. (2024). The global protected area network does not harbor genetically diverse populations. Available online at: https://ecoevorxiv.org/repository/view/6966/ (Accessed May 18, 2025).
Schmitt, C. B., Burgess, N. D., Coad, L., Belokurov, A., Besançon, C., Boisrobert, L., et al. (2009). Global analysis of the protection status of the world’s forests. Biol. Conserv. 142, 2122–2130. doi: 10.1016/j.biocon.2009.04.012
Schmitz, O. J., Lawler, J. J., Beier, P., Groves, C., Knight, G., Boyce, D. A., et al. (2015). Conserving biodiversity: practical guidance about climate change adaptation approaches in support of land-use planning. Nat. Areas J. 35, 190–203. doi: 10.3375/043.035.0120
Scholze, M., Knorr, W., Arnell, N. W., and Prentice, I. C. (2006). A climate-change risk analysis for world ecosystems. Proc. Natl. Acad. Sci. 103, 13116–13120. doi: 10.1073/pnas.0601816103
Schuldt, A., Liu, X., Buscot, F., Bruelheide, H., Erfmeier, A., He, J.-S., et al. (2023). Carbon–biodiversity relationships in a highly diverse subtropical forest. Glob. Chang. Biol. 29, 5321–5333. doi: 10.1111/gcb.16697
Schüßler, D., Mantilla-Contreras, J., Stadtmann, R., Ratsimbazafy, J. H., and Radespiel, U. (2020). Identification of crucial stepping stone habitats for biodiversity conservation in northeastern Madagascar using remote sensing and comparative predictive modeling. Biodivers. Conserv. 29, 2161–2184. doi: 10.1007/s10531-020-01965-z
Seddon, P. J., Griffiths, C. J., Soorae, P. S., and Armstrong, D. P. (2014). Reversing defaunation: restoring species in a changing world. Science 345, 406–412. doi: 10.1126/science.1251818
Sgrò, C. M., Lowe, A. J., and Hoffmann, A. A. (2011). Building evolutionary resilience for conserving biodiversity under climate change: conserving biodiversity under climate change. Evol. Appl. 4, 326–337. doi: 10.1111/j.1752-4571.2010.00157.x
Sherley, G. (2001) Bird Conservation Priorities and a Draft Conservation Strategy for the Pacific Islands region Secretariat of the Pacific Regional Environment Programme (SPREP). Available online at: https://library.sprep.org/content/bird-conservation-priorities-and-draft-avifauna-conservation-strategy-pacific-islands (Accessed June 08, 2022).
Siegel, R. B., Pyle, P., Thorne, J. H., Holguin, A. J., Howell, C. A., Stock, S., et al. (2014). Vulnerability of birds to climate change in California’s Sierra Nevada. Avian Conserv. Ecol. 9:art7. doi: 10.5751/ACE-00658-090107
Simberloff, D., Keitt, B., Will, D., Holmes, N., Pickett, E., and Genovesi, P. (2018). Yes we can! Exciting progress and prospects for controlling invasives on islands and beyond. West. N. Am. Nat. 78:942. doi: 10.3398/064.078.0431
Smit, B., and Wandel, J. (2006). Adaptation, adaptive capacity and vulnerability. Glob. Environ. Change 16, 282–292. doi: 10.1016/j.gloenvcha.2006.03.008
Solarz, W., Najberek, K., Tokarska-Guzik, B., and Pietrzyk-Kaszyńska, A. (2023). Climate change as a factor enhancing the invasiveness of alien species. Environ. Socio Econ. Stud. 11, 36–48. doi: 10.2478/environ-2023-0022
Solomon, S., Canziani, O. F., Palutikof, J., Linden, P.van der, and Hanson, C. (2007). Climate change 2007: The physical science basis: Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press.
Soule, M., and Noss, R. (1998). Rewilding and biodiversity: complementary goals for continental conservation. Wild Earth, Fall, 1–11. Available online at: https://www.mendeley.com/catalogue/62f394f2-5677-3245-93f2-c5d9ed031715/ (Accessed June 08, 2022).
Spencer, P. D., Hollowed, A. B., Sigler, M. F., Hermann, A. J., and Nelson, M. W. (2019). Trait-based climate vulnerability assessments in data-rich systems: an application to eastern Bering Sea fish and invertebrate stocks. Glob. Chang. Biol. 25, 3954–3971. doi: 10.1111/gcb.14763
Star, J., Rowland, E. L., Black, M. E., Enquist, C. A. F., Garfin, G., Hoffman, C. H., et al. (2016). Supporting adaptation decisions through scenario planning: enabling the effective use of multiple methods. Clim. Risk Manag. 13, 88–94. doi: 10.1016/j.crm.2016.08.001
Stein, B. A., Glick, P., Edelson, N., and Staudt, A. (2014) Climate-smart conservation: Putting adaption principles into practice. National Wildlife Federation. Available online at: https://pubs.usgs.gov/publication/70093621 (Accessed June 08, 2022).
Stewart, J. R. (2010). Refugia revisited: individualistic responses of species in space and time. Proc. R. Soc. Lond. B Biol. Sci. 277, 661–671. doi: 10.1098/RSPB.2009.1272
Stocker, T. F., Qin, D., and Plattner, G.-K. (2013). 2013: technical summary. In Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (33–115). Cambridge University Press.
Stralberg, D., Arseneault, D., Baltzer, J. L., Barber, Q. E., Bayne, E. M., Boulanger, Y., et al. (2020a). Climate-change refugia in boreal North America: what, where, and for how long? Front. Ecol. Environ. 18, 261–270. doi: 10.1002/fee.2188
Stralberg, D., Carroll, C., and Nielsen, S. E. (2020b). Toward a climate-informed north American protected areas network: incorporating climate-change refugia and corridors in conservation planning. Conserv. Lett. 13:e12712. doi: 10.1111/conl.12712
Sutherland, W. J. (2006). Predicting the ecological consequences of environmental change: a review of the methods*. J. Appl. Ecol. 43, 599–616. doi: 10.1111/j.1365-2664.2006.01182.x *Ecological predictions
Szcodronski, K. E., Wade, A. A., Burton, S. E., and Hossack, B. R. (2024). Incorporating projected climate conditions to map future riparian refugia. Conserv. Sci. Pract. 6:e13183. doi: 10.1111/csp2.13183
Taylor, M., and Figgis, P. (Eds.). Protected areas buffering nature against climate change. (2007). In Proceedings of a WWF and IUCN world commission on protected areas symposium.
Townsend, P. A., and Masters, K. L. (2015). Lattice-work corridors for climate change: a conceptual framework for biodiversity conservation and social-ecological resilience in a tropical elevational gradient. Ecol. Soc. 20:art1. doi: 10.5751/ES-07324-200201
UN. (1992) United Nations Framework Convention on Climate Change. United Nations. Available online at: https://www.un.org/zh/documents/treaty/A-AC.237-18(PARTII)-ADD.1 (Accessed June 08, 2022).
UNEP (2023) Adaptation Gap Report 2023: Underfinanced. Underprepared. Inadequate investment and planning on climate adaptation leaves world exposed. Nairobi. Available online at: https://primarysources.brillonline.com/browse/climate-change-and-law-collection/underfinanced-underprepared-inadequate-investment-and-planning-on-climate-adaptation-leaves-world-exposed;cccc025220231156 (Accessed May 18, 2025).
UNEP-MAP (2022) Status of Implementation of the Ecosystem Approach (EcAp) Roadmap–Comparative Analysis of the Second Phase (2019–2021) of the Integrated Monitoring and Assessment Programme (IMAP) for Biodiversity and Non-indigenous Species (No. UNEP(DEPI)/MED IG.26/Inf.7) Athens, Greece: United Nations Environment Programme Mediterranean Action Plan (UNEP-MAP).
UNFCCC (1997) Kyoto Protocol to the United Nations Framework Convention on Climate Change. Kyoto, Japan: United Nations Framework Convention on Climate Change (UNFCCC).
Urban, M. C. (2015). Accelerating extinction risk from climate change. Science 348, 571–573. doi: 10.1126/science.aaa4984
van Woesik, R. (2025). Paleo reefs provide clues for contemporary climate-change refugia. Cell Rep Sustain 2:100289. doi: 10.1016/j.crsus.2024.100289
Vargas, F. H., Lacy, R. C., Johnson, P. J., Steinfurth, A., Crawford, R. J. M., Boersma, P. D., et al. (2007). Modelling the effect of El Niño on the persistence of small populations: the Galápagos penguin as a case study. Biol. Conserv. 137, 138–148. doi: 10.1016/j.biocon.2007.02.005
Veitch, C. R., Clout, M. N., Martin, A. R., Russell, J. C., and West, C. J. (Eds.). (2019). Island invasives: scaling up to meet the challenge. Proceedings of the international conference on island invasives 2017. IUCN, International Union for Conservation of Nature.
Vergara, W., Deeb, A., Valencia, A., Bradley, R., Francou, B., Zarzar, A., et al. (2007). Economic impacts of rapid glacier retreat in the Andes. EOS Trans. Am. Geophys. Union 88, 261–264. doi: 10.1029/2007EO250001
Walker, R. H., Hutchinson, M. C., Becker, J. A., Daskin, J. H., Gaynor, K. M., Palmer, M. S., et al. (2023). Trait-based sensitivity of large mammals to a catastrophic tropical cyclone. Nature 623, 757–764. doi: 10.1038/s41586-023-06722-0
Wang, F., McShea, W. J., Li, S., and Wang, D. (2018). Does one size fit all? A multispecies approach to regional landscape corridor planning. Divers. Distrib. 24, 415–425. doi: 10.1111/ddi.12692
Warner, K., Charles, E., Alex de, S., and Susana, B. (2009). Search of shelter: Mapping the effects of climate change on human migration and displacement. The 6th session of the ad hoc working group on Long-term cooperative action under the convention (AWG-LCA 6). Bonn: United Nations University.
Watson, J. E. M., Rao, M., Ai-Li, K., and Yan, X. (2012). Climate change adaptation planning for biodiversity conservation: a review. Adv. Clim. Chang. Res. 3, 1–11. doi: 10.3724/SP.J.1248.2012.00001
Wei, X., Xu, Y., Lyu, L., Xiao, Z., Wang, S., Yang, T., et al. (2023). Impacts of ecological restoration on the genetic diversity of plant species: a global meta-analysis. J. Appl. Ecol. 60, 1149–1160. doi: 10.1111/1365-2664.14390
Wilby, R. L., and Vaughan, K. (2011). Hallmarks of organisations that are adapting to climate change: hallmarks of adapting organisations. Water Environ. J. 25, 271–281. doi: 10.1111/j.1747-6593.2010.00220.x
Williams, B. K., and Brown, E. D. (2012). Adaptive management: The U.S. Department of the Interior applications Guid. Washington, DC, US: Adaptive Management Working Group, U.S. Department of the Interior.
Williams, S. E., Shoo, L. P., Isaac, J. L., Hoffmann, A. A., and Langham, G. (2008). Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 6:e325. doi: 10.1371/journal.pbio.0060325
Willis, K. J., and Bhagwat, S. A. (2009). Biodiversity and climate change. Science 326, 806–807. doi: 10.1126/science.1178838
Wilson, R. J., Davies, Z. G., and Thomas, C. D. (2009). Modelling the effect of habitat fragmentation on range expansion in a butterfly. Proc. R. Soc. B Biol. Sci. 276, 1421–1427. doi: 10.1098/rspb.2008.0724
Wu, J., and Hobbs, R. (2007). Key topics in landscape ecology. Cambridge, UK: Cambridge University Press.
Wu, J., and Li, H. (2006). “Perspectives and methods of scaling” in Scaling and uncertainty analysis in ecology. eds. J. Wu, K. B. Jones, H. Li, and O. L. Loucks (Dordrecht, Netherlands: Springer Netherlands), 17–44.
Xu, D., Peng, J., Liu, M., Jiang, H., Tang, H., Dong, J., et al. (2025). Bridging climate refuges for climate change adaptation: a spatio-temporal connectivity network approach. Geogr. Sustain. 6:100235. doi: 10.1016/j.geosus.2024.08.012
Xu, H., Jiang, L., and Liu, Y. (2024). Mapping the potential distribution of Asian elephants: Implications for conservation and human–elephant conflict mitigation in South and Southeast Asia. Ecological Informatic, 80, 102518. doi: 10.1016/j.ecoinf.2024.102518
Yousefpour, R., and Hanewinkel, M. (2016). Climate change and decision-making under uncertainty. Curr. Forestry Rep. 2, 143–149. doi: 10.1007/s40725-016-0035-y
Yu, M., Wang, G., Parr, D., and Ahmed, K. F. (2014). Future changes of the terrestrial ecosystem based on a dynamic vegetation model driven with RCP8.5 climate projections from 19 GCMs. Clim. Chang. 127, 257–271. doi: 10.1007/s10584-014-1249-2
Zarzuelo Romero, C., López-Ruiz, A., Bermúdez, M., Ortega-Sánchez, M., and Caballero, I. (2025) Monitoring intertidal ecosystems: assessing spatio–temporal variability with Sentinel-2 and Landsat 8. Int. J. Appl. Earth. Obs.1422:104676, doi: 10.1016/j.jag.2025.104676
Zelli, E., Ellis, J., Pilditch, C., Rowden, A. A., Anderson, O. F., Geange, S. W., et al. (2025). Identifying climate refugia for vulnerable marine ecosystem indicator taxa under future climate change scenarios. J. Environ. Manag. 373:122635. doi: 10.1016/j.jenvman.2024.122635
Zevenbergen, C., Rijke, J., van Herk, S., and Bloemen, P. J. T. M. (2015). Room for the river: a stepping stone in adaptive delta management. Int. J. Water Gov. 3, 121–140. Available online at: https://journals.open.tudelft.nl/ijwg/article/view/5881
Keywords: climate change, biodiversity, adaptation, region-landscape-site scale, cross-spatial scale
Citation: Mengzhi X, Jixia L, Shixin L and Qianming Z (2025) How biodiversity conservation adapts to climate change: from a cross-spatial scale framework. Front. Clim. 7:1646318. doi: 10.3389/fclim.2025.1646318
Edited by:
Xixi Wang, Old Dominion University, United StatesReviewed by:
Qiyao Han, Nanjing Agricultural University, ChinaEdith Joana Singini, University of Stellenbosch, South Africa
Copyright © 2025 Mengzhi, Jixia, Shixin and Qianming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Qianming, enFtMTk4NkBjeHRjLmVkdS5jbg==
 Xu Mengzhi
Xu Mengzhi Li Jixia2
Li Jixia2 Zhang Qianming
Zhang Qianming