- 1Department of Translational Medicine and Laboratory for Technologies of Advanced Therapies (LTTA), University of Ferrara, Ferrara, Italy
- 2Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy
- 3Department of Biomedical Science, Lewyt College of Medicine, Long Island University, Brookville, NY, United States
- 4Faculty of Health Sciences, Curtin Medical School & Curtin Medical Research Institute (Curtin-MRI), Perth, WA, Australia
- 5Department of Environmental and Prevention Sciences, University of Ferrara, Ferrara, Italy
This article provides a narrative review of recent literature on the health impacts of climate change, synthesizing epidemiological findings, mechanistic insights, and policy implications across major exposure domains. Anthropogenic climate change is fundamentally altering global climate systems, with significant and multifaceted implications for human health. Epidemiological data indicate a strong correlation between ambient temperature fluctuations and cardiovascular mortality. Moreover, the frequency and severity of wildfires have intensified due to climate change, contributing to elevated levels of fine particulate matter (PM2.5) and resulting in substantial premature mortality. Climate change is modifying the geographic distribution and seasonality of vector-borne and zoonotic diseases, posing new challenges for infectious disease control. Air quality degradation alongside heightened UV radiation, contributes to a higher incidence of respiratory diseases, skin cancers, and ocular disorders. Climate-induced disruptions to agricultural systems are undermining food security, leading to increased malnutrition and related morbidity. Additionally, the psychological burden of climate-related events, including natural disasters and displacement, has been linked to rising rates of anxiety, post-traumatic stress disorder (PTSD), and affective disorders. These effects are more evident in vulnerable populations, including the elderly, individuals with pre-existing health conditions, those of lower socioeconomic status, and populations residing in low-income countries. Urgent mitigation strategies targeting greenhouse gas emissions are required to limit further climate-related health burdens. Concurrently, adaptive strategies must be implemented to bolster resilience across ecological, infrastructural, and health systems. Although public health systems are critical in addressing these challenges, a coordinated, multidisciplinary research agenda is imperative to elucidate the complex pathways linking climate change and health, and to develop evidence-based interventions aimed to reduce its negative impacts on human health.
Introduction
Climate change refers to the persistent modification of Earth’s climatic patterns, which encompasses more than just global warming. These alterations, driven by anthropogenic activities, include shifts in temperature, precipitation, and various climatological parameters. These changes have significant consequences for our planet, such as glacier melting, rising sea levels, increased occurrences of severe weather phenomena, and profound impacts on ecosystems, human activities, and health. Human health is affected by climate changes through increased occurrences of extreme weather events, degradation of air quality, threats to global food security, and alterations in the prevalence of communicable and non-transmissible disease frequencies on a global scale. Extreme weather events have led to increased mortality (Amirkhani et al., 2022) with a significant burden of mortality caused by heat exposure (Vicedo-Cabrera et al., 2021). The analysis of mortality in Europe during the summer of 2022, showed around 61,672 heat-related deaths, with 56% more heat-related deaths in women than men (Ballester et al., 2023). A different study conducted in 854 European cities projected that the rising temperature due to climate changes is expected to cause an increase in heat-related deaths that will exceed any decrease in cold-related deaths (Masselot et al., 2025). Ground-level ozone, tropical storms, hurricanes, and dust storms have also been linked to adverse cardiovascular outcomes (Aitken et al., 2022; Kazi et al., 2024). Regional disparities in temperature-related mortality risk in Europe have been documented (García-León et al., 2024) and strong evidence indicates that climate change affects more heavily specific population groups, such as individuals with pre-existing conditions, the elderly, pregnant women, outdoor workers, and population of lower socioeconomic classes or those living in underdeveloped countries (Ngcamu, 2023; Romanello et al., 2022). There is an urgent need for accelerated climate action to protect the health of present and future generations.
This article presents a narrative review of the health impact of climate change. We first discuss the multiple ways by which climate change affects human health, distinguishing between direct and indirect effects (Figure 1). McMichael (1993) was the first to categorize climate impacts on human health into direct and indirect mechanisms and this definition was later adopted by Watts et al. (2015). Direct effects refer to alterations in the frequency of extreme weather events, such as heatwaves, droughts, and heavy rainfall. Projections for the U.S. suggest that without mitigation, extreme temperatures could cause up to 26,574 annual deaths between 2036 and 2065, based on observed deaths from 2008 to 2019 (Khatana et al., 2024). Indirect effects include changes in the diffusion of vector-borne and waterborne diseases, air pollution, as well as occupational health impacts, food insecurity, undernutrition, displacement, psychological stress, and mental health outcomes (Tong et al., 2022). Among the indirect effects of climate change, food-and waterborne diseases represent significant health burdens globally, particularly in vulnerable populations. The World Health Organization estimates approximately 600 million cases of foodborne diseases annually, with children under five bearing 30% of the associated mortality burden (Estimating the Burden of Foodborne Diseases, n.d.). In Ecuador, waterborne diseases disproportionately affect indigenous communities, children, and the elderly, contributing to lower life expectancy in these populations (Ortiz-Prado et al., 2022). We then examine approaches to reduce the negative effects of climate change on human health (adaptation) and strategies to slow down or prevent further climate changes (mitigation).
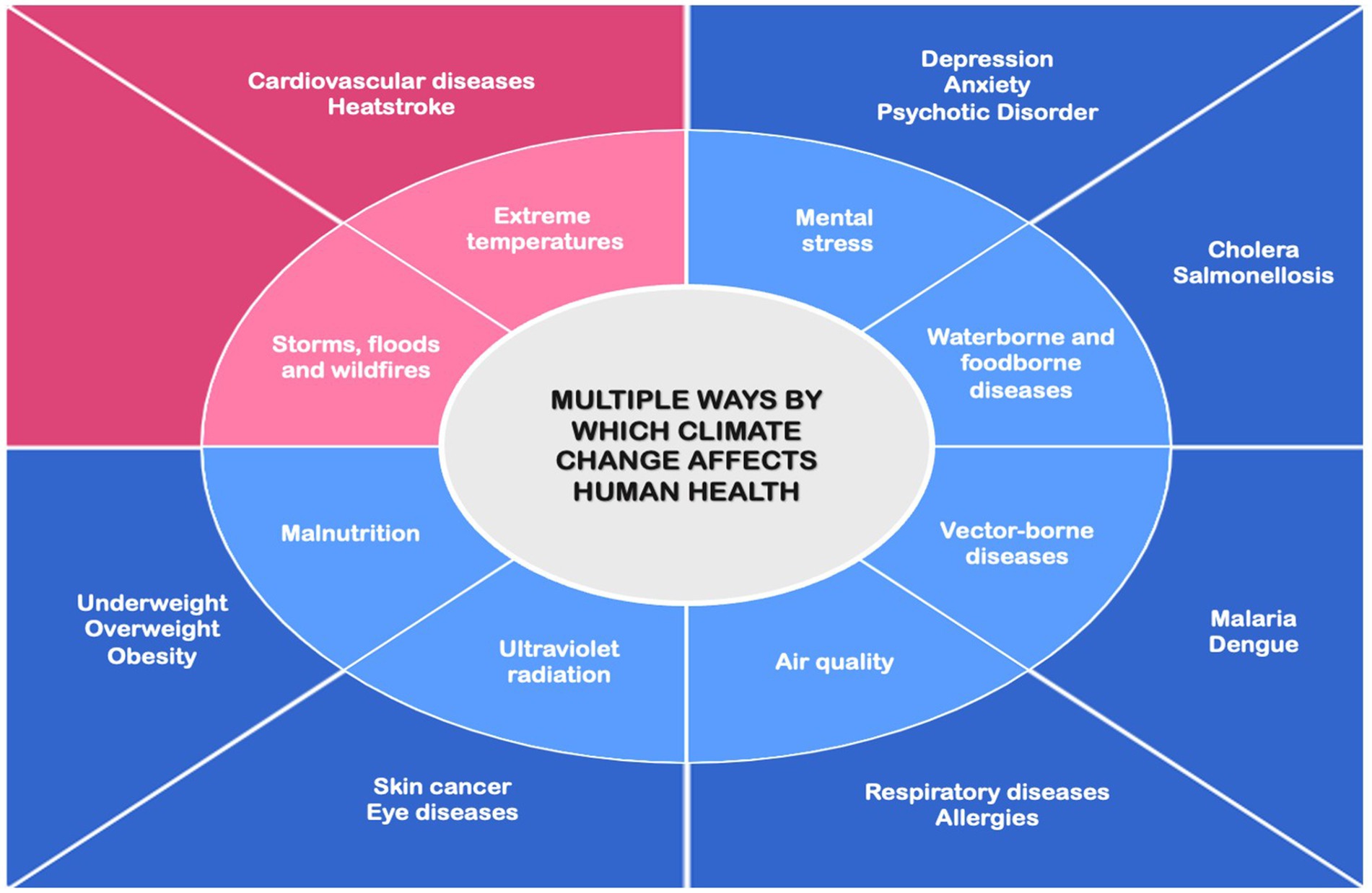
Figure 1. Schematic representation of the pathways through which climate change impacts human health. Light pink and light blue boxes represent direct and indirect causes, respectively. Dark pink and dark blue boxes represent the corresponding direct and indirect health effects. Direct effects are immediate impacts caused by environmental changes, while indirect effects are long-term consequences arising from ecological, environmental, or societal disruptions.
The review draws on peer-reviewed literature published primarily in the past decade, supplemented by landmark earlier studies. Relevant articles were identified through targeted searches in PubMed and expert knowledge of the field. Sources were selected to illustrate major exposure-outcome pathways and to integrate epidemiological (Supplementary Table 1), mechanistic and policy-relevant perspectives. As a narrative synthesis, this review does not apply formal systematic search methods or meta-analysis techniques, nor does it assess the strength of the evidence or systematically identify knowledge gaps.
Direct impacts of climate changes on human health
Extreme heat and cardiovascular diseases
Elevated temperatures associated with climate changes can profoundly affect cardiovascular health (Desai et al., 2023; Khraishah et al., 2022). Consistently, epidemiological and observational studies have demonstrated a correlation between extreme temperatures and increased mortality, particularly from cardiovascular diseases (Singh et al., 2024). A systematic review and meta-analysis of literature published between January 1, 1990, and March 10, 2022, found substantial heterogeneity in mortality and morbidity outcomes due to high temperatures and heatwaves. A 1 °C temperature rise was associated with a 2.1% increase in cardiovascular disease-related mortality, particularly from stroke and coronary heart disease. Morbidity due to arrhythmias, cardiac arrest, and coronary heart disease also increased. Heatwaves were also linked to a 17% increase in mortality risk, with greater intensity leading to higher risks (Liu et al., 2022). Vulnerable groups included women, individuals 65 and older, tropical climate residents, and those in lower-middle-income countries. A study conducted in Madrid (2015–2018) involving 6,514 patients revealed a 15.3% increase in the risk of acute cardiovascular events during periods of extreme heat, with males and non-Spanish populations being disproportionately affected (Salvador et al., 2023). Saucy et al., investigated the link between extreme temperature, pollution, aircraft noise, and cardiovascular mortality risk in Zurich, Switzerland. They found that heat poses a particularly stronger risk, especially for myocardial infarctions and hypertension-related deaths The study confirmed previous research showing that older women, especially those with lower socio-economic status and education, are the most vulnerable. Additionally, air pollution was shown to further increase heat-related mortality risk in heart failure cases, though not for all cardiovascular causes (Saucy et al., 2021). A study spanning 2000–2020 across 12 major Canadian cities used generalized additive models to assess daily mortality risks during extreme heat events. The findings revealed elevated mortality risks, particularly for non-accidental, cardiovascular, and respiratory causes. Older adults (aged 65 and above) were more vulnerable, with approximately 670 excess non-accidental deaths, including 115 cardiovascular deaths, attributed to extreme heat during the study period (Quick, 2024).
The pathophysiological mechanism underlying this increased risk for cardiovascular events is well described (Zhang et al., 2022). Briefly, during periods of high-temperature exposure or extreme heat events, the body initiates a thermoregulatory response primarily characterized by an increase in cutaneous blood flow to dissipate heat and maintain core body temperature (Chaseling et al., 2020) leading to sympathetic nervous system activation and elevation in heart rate and stroke volume (Criddle et al., 2024). Sweat evaporation is another major thermoregulatory effector to reduce core body temperature. Excessive sweating can lead to fluid loss, resulting in a reduction in blood volume, which in turn may lead to hypotension (Chaudhary et al., 2023) exacerbating conditions like heart failure and increase the risk of cardiovascular events. Additionally, the reduced plasma volume increases the concentration of red blood cells and platelets, as well as blood viscosity, which can lead to thromboembolism, thereby increasing the risk of ischemic stroke and myocardial infarction (Lim et al., 2013). High body temperature can trigger a systemic inflammatory response mediated by the overproduction of pro-inflammatory cytokines (Riggs et al., 2024). This inflammatory cascade, characterized by the release of inflammation mediators, such as tumor necrosis factor α (TNFα), interleukin 1β (IL-1β), interleukin 8 (IL-8) and interleukin 1 (IL-1), triggers a series of biochemical reactions within the blood vessels, leading to endothelial activation characterized by the adhesion of immune cells and platelets to the vascular endothelium, which facilitates complex interactions between coagulation factors. These events all contribute to a pro-thrombotic state, increasing the risk of intravascular clot formation and thromboembolic events (reviewed in Mol et al., 2024).
Heat stress leads also to a reduction in gut blood flow, which compromises the integrity of the intestinal epithelial barrier. As a result, increased permeability allows bacteria and endotoxins to translocate from the gut lumen into the systemic circulation, triggering the activation of both the innate and adaptive immune systems and contributing to the development of systemic inflammatory response syndrome (Pearce et al., 2013). Furthermore, following heat stress, the intestinal epithelium suffers from damage due to the disruption of the differentiation of intestinal stem cells into functional cells and, thus, to an imbalance of tissue homeostasis (Zan et al., 2023).
The elderly have been shown to be more susceptible to heat stress. This could be due to impaired thermoregulatory response, which is influenced not only by age but significantly by sex, as reported by a recent study showing that younger females exhibited the highest whole-body sweat loss, while older individuals, particularly older females, had reduced sweating (Atkins et al., 2024). The frequent use of medication could be another reason why the elderly are more susceptible to heat stress pathologies: heat exposure can affect drug absorption, distribution, and elimination potentially increasing the risk of cardiovascular mortality (Zhang et al., 2022).
Extreme heat and heatstroke
Extreme environmental heat can lead to heatstroke, a serious heat-related disorder characterized by a rapid elevation of core body temperature exceeding 40 °C. This condition results in damage to organs and tissues, caused not only by the cytotoxic effects of the heat but also from the release of inflammatory agents and by dysregulated coagulation (Bouchama and Knochel, 2002).
The elderly and individuals with cardiovascular disease or other chronic conditions that prevent efficient heat dissipation are at higher risk of heatstroke (Marchand and Gin, 2022). Heatstroke patients who develop disseminated intravascular coagulation (DIC) have a significantly higher risk of mortality, compared to those without DIC complications (Lin et al., 2024). The presence of cardiovascular disease also increases the risk of death associated to heatstroke (Lin et al., 2024).
Heatstroke is gaining increased prevalence throughout the world due to a steady rise in temperature, and significant mortality has been recorded among vulnerable populations. In Karachi, Pakistan, during an extreme heatwave on 24 June 2024, more than 1,500 people suffering of heatstroke were treated at various hospitals across the city (Khan and Mubeen, 2025).
Molecular and cellular mechanisms underlying heat-related pathologies
As climate change continues to drive the increasing frequency and intensity of heat waves, healthcare systems must implement proactive strategies to reduce risks of heat-related diseases, especially among vulnerable groups. Therefore, understanding the molecular mechanisms affected by heat exposure is essential for developing targeted prevention and treatment strategies against heatstroke and worsening of pre-existing pathologies caused by prolonged exposure to elevated environmental temperature.
In this paragraph we will review the most recent findings on the molecular and cellular processes affected by the heat (Figure 2). Studies involving human volunteers exposed to extreme heat in a sauna environment (75 °C) showed significant changes in gene expression even without a proportional increase in core body temperature. The genes with altered expression are involved in stress-related processes such as energy metabolism, cell survival, and immune function. Key changes also included mitochondrial dysfunction and altered protein synthesis (Bouchama et al., 2017).
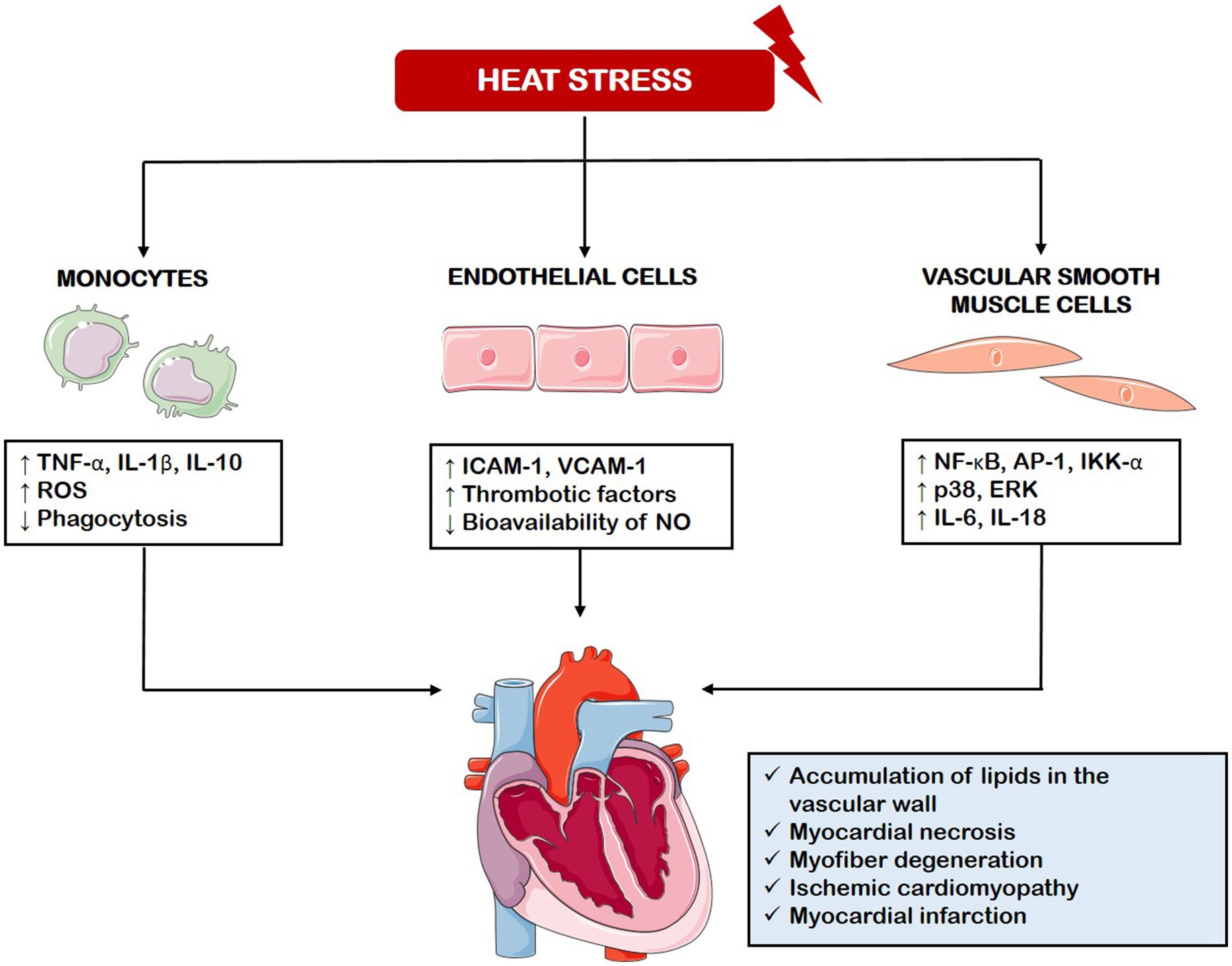
Figure 2. Cellular and molecular processes affected by heat stress. Heat stress disrupts molecular pathways in monocytes, endothelial cells, and vascular smooth muscle cells, leading to increased production of pro-inflammatory cytokines, reactive oxygen species, adhesion molecules, and thrombotic factors. These responses contribute to cardiac damage observed in animal models of heat stress and heatstroke. TNF-α, Tumor Necrosis Factor-alpha; IL-1β, Interleukin 1 beta; IL-10, Interleukin 10; ROS, Reactive Oxygen Species; ICAM-1, Intercellular Adhesion Molecule 1; VCAM-1, Vascular Adhesion Molecule 1; NO, Nitric Oxide; NF-kB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; AP-1, Activator Protein 1; IKK-α, IκB kinase alpha; ERK, Extracellular signal-Regulated Kinase; IL-6, Interleukin 6; IL-18, Interleukin 18.
The mechanisms underlying the inflammatory response associated with heat stress have been investigated across various cell types and tissues. In skeletal muscle, acute heat exposure (6 h at 37 °C) activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and activator protein 1 (AP-1) signaling pathways, indicated by elevated IkappaB kinase α (IKK-α) and nuclear AP-1 levels (Ganesan et al., 2017). In monocytes isolated from healthy volunteers, exposure to 43 °C further increases the lipopolysaccharide (LPS)-induced release of inflammatory mediators such as TNF-α, IL-1β, and interleukin 10 (IL-10), alongside with reactive oxygen species (ROS) production while suppressing their phagocytic activity. This altered response is accompanied by changes in immune-related surface molecules like the triggering receptor expressed on myeloid cells 1 (TREM-1), toll-like receptor 4 (TLR-4), and cluster of differentiation 86 (CD86) (Luo et al., 2019). In C2C12 myoblasts subjected to prolonged heat stress (15 h), phosphorylation of p38 and extracellular signal-regulated kinase (ERK) was enhanced, leading to the activation of inflammatory factors including the NLR family pyrin domain-containing 3 (NLRP3) inflammasome, interleukin 6 (IL-6), and interleukin 18 (IL-18), through a lysophosphatidic acid receptor-mediated mechanism and changes were suppressed by treatment with non-saponin component of ginseng (Chei et al., 2020). In fibroblasts, heat exposure at 43 °C resulted in increased expression of Z-DNA binding protein-1 (ZBP1) via heat shock factor 1 (HSF1), which then triggered receptor-interacting protein kinase 3 (RIPK3)-dependent necroptosis, a regulated form of cell death (Yuan et al., 2022). Lastly, pulmonary microvascular endothelial cells exposed to both heat stress and LPS showed significant endothelial glycocalyx (EGCX) degradation, along with elevated oxidative stress, pro-inflammatory cytokine production, and enhanced apoptosis (Chen et al., 2023). Developmental studies have shown that the Notch pathway, a major regulator of functions of cardiomyocyte, endothelial cells and macrophages (Fortini et al., 2017, 2025; Severi et al., 2024) is sensitive to changes in temperature (Moreno Acosta et al., 2023; Nomura et al., 2022).
Studies conducted on runners to investigate the effects of exertional heat have shown the induction of intestinal lesions, impaired toxin elimination, and increased release of inflammatory cytokines in plasma (Snipe et al., 2018). A recent study explored the protective effects of lonicerin, a flavonoid, against intestinal injury and systemic inflammation induced by heat stress. Using a rat model exposed to 42 °C, the research demonstrated that lonicerin reduced intestinal injury by activating the AMP-activated protein kinase/sirtuin 1 (AMPK/SIRT1) axis, promoting autophagy, and reducing the levels of inflammatory cytokines (Sun et al., 2025).
Animal models have significantly contributed to understanding of the effects of heat stress on the vascular system, helping to address the worsening of cardiovascular disease during heat waves. These models have confirmed in vitro findings of increased oxidative stress, inflammatory mediators, and thrombotic factors (Mol et al., 2024). This oxidative unbalance and release of inflammatory markers reduces nitric oxide (NO) bioavailability, increases lipid peroxidation, and promotes mitochondrial dysfunction and DNA damage, all of which contribute to endothelial dysfunction, a crucial event in the development of atherosclerosis and in the worsening other CVDs (Cimaglia et al., 2022; Fortini et al., 2021; Vieceli Dalla Sega et al., 2022, 2024). Consistently, in animals exposed to heat stress were observed histopathological changes, such as accumulation of fat cells and cholesterol deposition in the blood vessels, disruption of heart tissue, and, in severe heatstroke cases, myocardial necrosis and myofiber degeneration, signs associated with advanced ischemic cardiomyopathy and myocardial infarction. Various types of cardiomyopathies have also been observed (Figure 2) (Mol et al., 2024).
All these studies point to the use of anti-oxidant, anti-inflammatory, anti-apoptosis and anti-coagulant agents, including phytochemicals, to interfere with the heat-mediated damages to the cardiovascular system (Mol et al., 2024). Of interest, observation in patients and in vitro have shown that heat acclimation alleviates the heat stress-induced impairment of vascular endothelial cells (Wen et al., 2024).
Several studies have specifically focused on the molecular mechanism altered by heatstroke. Heatstroke alters gene expression in peripheral blood cells: transcriptome analysis of a cohort of subjects exposed to heat conditions who developed heatstroke versus those who did not revealed upregulation of heat shock proteins (HSPs), suppression of genes involved in ATP production, unfolded protein response, DNA repair, oxidative stress responses, and activation and impairment of genes related to innate or adaptive immunity, respectively (Bouchama et al., 2023). Exosome analysis from heatstroke patients identified significant up-or down-regulation of proteins such as serum amyloid A-1, von Willebrand factor, and histone H3, as well as genes associated with inflammation, platelet activation, and immune responses (Li Y. et al., 2023).
In rodent models of heatstroke, brain cell lesions were observed alongside a robust inflammatory response characterized by increased secretion of tumor necrosis factor-β1 (TNF-β1), cyclooxygenase-2 (COX-2), and intercellular adhesion molecule 1 (ICAM-1). Additionally, heatstroke upregulated metalloproteinases 2 (MMP2) and 9 (MMP9) and ROS, while downregulating anti-oxidative stress enzymes, such as superoxide dismutase. These effects were mitigated by inhibiting the JAK2/STAT3 (Janus kinase 2/signal transducers and activators of transcription 3) signaling pathway, suggesting its critical role in the heatstroke response (Tao et al., 2015). A different study in a rat model of heatstroke revealed that coagulation dysfunction associated with heat stroke is due ROS-mediated damage of the endothelial glycocalyx and that the antioxidant N-acetylcysteine protected endothelial glycocalyx integrity by attenuating ROS production (Peng et al., 2023).
Extreme cold
While global warming is the dominant trend, extreme cold events could occur due to the fact that the Arctic is warming faster than the rest of the globe (Rantanen et al., 2022), and these changes could influence the behavior of the polar vortex, leading to more extreme cold weather in lower latitudes (Yamanouchi and Takata, 2020).
Extreme cold also contributes substantially to mortality. A study across 50 U.S. cities found that extreme temperatures, both hot and cold, accounted for 5.74 and 1.59% of deaths due to myocardial infarction and cardiac arrest, respectively (Medina-Ramón and Schwartz, 2007). Similarly, a study in China analyzing 1.8 million deaths across 272 cities (2013–2015) found that 14% of deaths were attributed to non-optimal temperatures and extreme cold posed greater long-term risks, while extreme heat had immediate but short-lived effects. Elderly, women, individuals with low educational attainment, and residents of urban areas with inadequate heating were the most vulnerable groups (Chen et al., 2018).
Exposure to low temperatures can significantly exacerbate cardiovascular risks, as demonstrated by both acute and prolonged cold exposure. Cold environments increase cardiac workload, especially in individuals with pre-existing cardiovascular conditions such as coronary artery disease and heart failure, leading to greater strain on the heart and potentially causing ischemia, angina, and impaired exercise performance (Ikäheimo, 2018). The results of a meta-analysis showed that a decrease in temperature has been associated with a 1.6% increase in cardiovascular disease-related mortality and a 1.2% increase in morbidity for each 1 °C drop, with coronary heart disease mortality and aortic aneurysm morbidity being particularly impacted. Factors such as age, country income level, and climate zone influence the impact of cold weather on cardiovascular disease. Furthermore, cold spells significantly impact the outcome of cardiovascular diseases, raising mortality by 32.4% and morbidity by 13.8% (Fan et al., 2023). Of interest, a study conducted in Thessaloniki, Greece, where the population is acclimated to heat, highlighted higher cardiovascular mortality at moderately cold temperatures, with women more vulnerable to the effects of cold (Psistaki et al., 2023).
Storms, floods, and wildfires
Storms and floods affect human health by causing drowning, injuries caused by contact with objects in floodwater, hypothermia, and electrical injuries. Epidemiological studies have shown that climate change is increasing the risk of drowning (Sindall et al., 2022) and floods-related health risks, such as fatalities, injuries, and mental health challenges (Wu et al., 2024b).
Storms and floods have also an indirect effect on human health by facilitating the transmission of waterborne and vector-borne diseases (Brown and Murray, 2013; Okaka and Odhiambo, 2018). A comparative study conducted in Vietnam following heavy rainfall in late October 2008 revealed elevated occurrences of dengue fever, conjunctivitis, dermatitis, and psychological disorders in municipalities significantly impacted by flooding, in contrast to those not affected (Bich et al., 2011).
Floods have been linked to increased mortality risks, particularly in low-income regions and among older populations. A global study analyzing 47.6 million deaths across 761 communities in 35 countries found that flood-related mortality risks persisted for up to 60 days post-exposure (Yang et al., 2023). Additionally, a UK Biobank study revealed a 6.7% increase in all-cause mortality risk per unit increase in cumulative flood exposure, with varying risks depending on the cause and time after flooding (Wu et al., 2024a).
Wildfires are becoming more frequent and intense due to climate change. As reported by the United States Environmental Protection Agency, in the U.S. there has been an increase in wildfires since 1983, and the extension of the area burned has also grown, with peaks coinciding with many of the warmest years. Wildfires cause approximately 4,000 premature deaths annually in the U.S., with the West and metropolitan areas near fire sources being most affected by wildfire-induced particulate matter smaller than 2.5 micron in diameter (PM2.5) (Pan et al., 2023).
A recent study estimated that, in the U.S. from 2006 to 2020, climate change contributed to approximately 15,000 deaths associated with wildfire particulate matter (Law et al., 2025). Another study reported an increase in global population exposure to landscape-fire sourced air pollution during 2010–2019 compared to 2000–2009. This exposure was higher in low-income countries in comparison to high-income countries (Xu et al., 2023).
Indirect impact of climate changes on human health
Vector-borne disease
Vector-borne diseases are infections transmitted by the bites of blood-feeding arthropods, like ticks and mosquitoes. These diseases are among the most thoroughly researched in relation to climate change because of their prevalence and responsiveness to environmental conditions. Research has shown that rising temperatures driven by climate change can promote pathogen transmission by mosquitoes, thereby facilitating the spread of infectious diseases (Bellone et al., 2023; Thomson and Stanberry, 2022). Climate change has a substantial effect on the transmission of vector-borne diseases also by altering other factors, such as precipitation, and wind patterns. In the next paragraphs we will examine the most studied vector-borne disease in relation to climate changes: malaria and dengue fever.
Malaria, caused by a Plasmodium parasite transmitted by Anopheles mosquitoes has caused 409,000 deaths in the world in 2019 only (Al-Awadhi et al., 2021). An analysis conducted in Kenya revealed that the abundance, distribution and transmission of the malaria vectors carried by mosquitoes are influenced by environmental factors, such as precipitation and temperature (Kelly-Hope et al., 2009). In a study of the impact of temperature increase from 1970 to 2003 on malaria incidence in East Africa’s mountainous regions, a non-linear increase in malaria cases due to global warming was observed. However, the expected size of the epidemic was smaller than observed, indicating that other factors may amplify the effects of climate change on malaria transmission (Alonso et al., 2011). In agreement with this observation, it has been reported that the influence of climate changes on parasite transmission is not solely due to increased average temperature, but also to daily fluctuations. Indeed, these fluctuations make transmission possible at lower average temperatures than predicted and could potentially block transmission at higher average temperatures (Paaijmans et al., 2010). Furthermore, diurnal variation in temperature and humidity influences mosquitoes and parasites. A study conducted in India explored the incubation periods of Plasmodium falciparum and Plasmodium vivax across different microhabitats throughout the year. It was found that warm and stable conditions promote rapid parasite development and extend the lifespan of Plasmodium parasites, up to 15 days for Plasmodium falciparum and 24 days for Plasmodium vivax (Thomas et al., 2018). Increasing temperatures could also promote malaria transmission in Europe. Indeed, Anopheles mosquitoes are becoming more stable and widespread, increasing the risk of prolonged transmission periods in certain areas (Lovey and Schlagenhauf, 2023). As temperatures rise, it is expected a northward spread of Anopheles mosquitoes and an extended transmission season, potentially allowing malaria transmission for up to 6 months annually between 2051 and 2080 (Fischer et al., 2020).
Dengue is a viral disease transmitted by mosquito Aedes aegypti. A large body of evidence collected in Malaysia shows that climate change influences its transmission through rising temperatures, increased precipitation and humidity (Hii et al., 2016). A study conducted in Europe found that climatic conditions are becoming more favorable for the establishment of the Asian tiger mosquito (Aedes albopictus), another dengue vector, in northern regions, while southern areas are becoming less hospitable (Caminade et al., 2012). Similarly, in north-western China, raising temperatures and increased precipitation significantly impacted the distribution of Aedes albopictus (Wu et al., 2011), increasing the risk for future epidemic for dengue fever in this region. Consistently, in the region of Guangzhou, China, precipitation, humidity, and temperature have been found positively associated with dengue incidence, whereas wind speed was inversely related to the disease spread (Lu et al., 2009). Similar climate-related patterns have been observed in Singapore (Gui et al., 2021). Another meteorological event positively associated with the spread of dengue is typhoons because they contribute to extreme rainfall and elevated water accumulation, creating mosquito breeding sites (Kao et al., 2023). Similarly, increased rainfall in Bangladesh has led significant changes in river water levels, expanding areas suitable for mosquito breeding and leading to an increase in dengue hospitalizations (Hashizume et al., 2012). Another factor contributing to the spread of dengue is water storage practices. In Colombia, households store water during dry seasons which provide breeding sites for the mosquitoes responsible for the disease but not in cooler cities. This study shows that climate change impact A. aegypti production also through human behavioral adaptations. The multifaceted effects on mosquito breeding, distribution, and human behavior create a dynamic web of risk factors. Understanding these regional variations and their interactions is crucial for developing effective mitigation strategies and preventing future outbreaks (Padmanabha et al., 2010).
Waterborne and foodborne disease
Bacteria and protozoa naturally present in water, as well as zoonotic pathogens originating from animal waste, can enter our bodies through contaminated water, either by accidental ingestion, or direct contact with our eyes, ears, or wounds. Shellfish can accumulate pathogens from the aquatic environment, and the use of contaminated water for irrigation can spread them to crops. Climate change is amplifying our exposure to a range of waterborne and foodborne pathogens. Impacting the growth, survival, and virulence of pathogens, and indirectly disrupting ecosystems and animal habitats, potentially creating new pathways for zoonotic diseases to jump species (Dupke et al., 2023). In the next paragraphs we will discuss the most studied waterborne and foodborne pathogens in relation to climate changes: Vibrio and Salmonella.
Vibrio species, including V. cholerae, the causative agent of cholera, are waterborne pathogens transmitted through the consumption or exposure to contaminated water or seafood (Baker-Austin et al., 2018). Rising temperature, increased precipitation, and salinity fluctuations create favorable condition for the growth of these bacteria, influencing their geographical and seasonal distribution, ultimately affecting the risk of Vibrio infections (Velez et al., 2023). Consistently, regions endemic for cholera exhibit a strong correlation between water temperature and incidence of this disease (Reyburn et al., 2011). Heavy rainfall events, intensified by climate change, further promote pathogen transmission by altering water quality and microbial composition (Shih et al., 2021). Another environmental factor associated with the spread of Vibrio is rising ocean temperatures. A clear positive relationship between Vibrio abundance and sea surface temperature in the North Sea has been demonstrated through DNA analysis of formalin-fixed samples from the historical Continuous Plankton Recorder archive, collected over 44 years (Vezzulli et al., 2012).
Salmonella species are foodborne zoonotic agents whose growth is influenced by temperature, varying by the food type. For instance, its optimal growth temperature is around 37 °C in chicken (Juneja et al., 2007) and around 25 °C in cooked ham (Szczawińska et al., 2014). Climate change, particularly rising temperatures and extreme weather events, has been linked to increased Salmonella infections. Monthly data on Salmonella outbreaks in Mississippi, Tennessee, and Alabama were analysed over a nine-year period and correlated with meteorological data. The results showed a positive correlation between rising temperatures and Salmonella epidemics (Akil et al., 2014). A study conducted in Maryland showed a close link between extreme heat exposure, extreme precipitation, and Salmonella infection (Morgado et al., 2021). A positive correlation between high temperature and Salmonella cases has also been observed in Europe, where the incidence of salmonellosis rises during warmer periods (European Food Safety Authority and European Centre for Disease Prevention and Control, 2022). Climate change, particularly warmer temperatures and extreme weather events, appears to exacerbate Salmonella outbreaks, underscoring the importance of monitoring and mitigating the impacts of global warming on public health.
Air quality
Air quality and climate change are deeply interconnected through complex mechanisms. Both anthropogenic and natural sources of air pollution significantly contribute to climate change, while climate change, in turn, worsens air quality in polluted regions by altering ventilation patterns, precipitation models, and increasing the concentration of pollutants (Fiore et al., 2015; Wang et al., 2022).
A key air pollutant is tropospheric (or surface) ozone (O3), located in the lower atmosphere. The health effects of tropospheric ozone are severe and manifest both in the short and long term. Prolonged exposure to high levels of ozone has been associated with an increased risk of mortality from respiratory diseases, such as pneumonia and chronic obstructive pulmonary disease (Jerrett et al., 2009). Short-term effects include respiratory symptoms, including worsening of asthma, along with ocular irritations (Desqueyroux et al., 2002). Tropospheric ozone is influenced by the complex interaction between human activities and meteorological patterns and it is clearly affected by climate changes (Cooper, 2019). A U.S.-focused study evaluated how near-term climate change (by 2030) may impact ground-level ozone and public health. Using two climate models under different greenhouse gas scenarios, the researchers project temperature increases of 1–4 °C and ozone concentration rises of 1–5 ppb. These changes are expected to result in up to thousands of additional premature deaths and illnesses annually, along with increased healthcare utilization (Fann et al., 2015). Climate change can exacerbate the occurrence of allergic rhinitis and asthma by altering various aspects of the pollen season. Pollen grains can also interact with air pollutants and be affected by thunderstorms, further worsening respiratory symptoms (D’Amato et al., 2023). Lastly, climate change may affect fine particulate matter (PM10-2.5) (Bhattarai et al., 2024), which has adverse effects on vascular endothelial function (Li J. et al., 2023) and it has been linked to increased mortality related to heart failure (Saucy et al., 2021). Furthermore, PM10-2.5 together with nitrogen dioxide (NO2) and O3 has been linked to an increased risk of physical disabilities in older adults, underscoring the multiple ways in which air pollution impacts human health (Gao et al., 2024).
Ultraviolet radiation
Ozone in the stratosphere absorbs most of the ultraviolet radiation (UV) from the Sun. The Montreal Protocol, aimed to protect the stratospheric ozone layer by reducing the emission ozone-depleting substances produced by industrial activities, has led to gradual ozone recovery, with mid-latitudes expected to return to 1980 levels before mid-century. However, strong interactions between ozone depletion and meteorological condition affected by climate change such as clouds and aerosols. Limit our confidence in predicting the future levels of UV radiation (McKenzie et al., 2011). Increased UV exposure poses significant risks to human health, particularly to the eyes. UV-A and UV-B radiation contribute to cataracts and retinal damage, while short blue light (400–440 nm) increases the risk of age-related macular degeneration in older adults. Solar ultraviolet radiation (UVR) has well-established adverse effects on the skin, particularly in individuals with poorly melanized skin. These effects can be acute, such as erythema (Harrison and Young, 2002) or chronic, leading to skin cancers (Al-Sadek and Yusuf, 2024). Additionally, chronic UV exposure leads to photoaging, a gradual process marked by changes in skin metabolism and oxidative stress due to altered enzyme activity (Gromkowska-Kępka et al., 2021).
A literature review explored the association between climate change and the incidence of skin cancer It is reported that skin cancer rates have been rising globally, particularly since the mid-20th century, and that this increase is strongly linked to factors associated with climate change, such as the depletion of stratospheric ozone and consequent increased exposure to UV radiation (Parker, 2021). Climate change-related variables, including increased UV exposure, have been linked to a rising occurrence of cataracts, glaucoma, periocular tumors, and eye infections (Wong et al., 2024).
Malnutrition
Climate change is one of the main threats to food security by affecting the quantity, quality, accessibility of food from crops, fisheries, and livestock, with serious consequences for nutritional health (Heikonen et al., 2025; Myers et al., 2017). For example, global warming affects fishing, altering the chemical composition of aquatic environments such as salinity, oxygen concentration, and acidification, thus reducing the maximum body weight of fish species and limiting capture potential (Huang et al., 2021).
This uncertainty in food production exacerbates nutrient deficiencies and increases the vulnerability of populations already exposed to food insecurity (Swinburn et al., 2019). A study conducted in Kilifi County, Kenya, highlighted how changes in weather patterns negatively affect local nutritional quality and food production. In particular, irregular rainfall patterns for at least 4 years led to reduced milk production, lack of food diversity, increasing drought, and ultimately high levels of poverty, making the county one of the most affected by malnutrition in Kenya (Cheruiyot et al., 2022).
Several studies have also shown that the increase in temperature related to climate change is associated with an increase in hospital admissions for malnutrition. A study conducted in Brazil, analyzing data collected over a 15-year period in over 1,500 cities, found that every 1 °C increase in temperature was correlated with a 2.5% increase in malnutrition-related hospital admissions. The populations most vulnerable to these effects were those aged over 80, children aged 0–4 years, and adolescents aged 5–19 years. This study indicates that climate warming may increase malnutrition morbidity not only by threatening food security but also through reduced food intake, impaired digestion and absorption function, and fluid and electrolyte disturbances (Xu et al., 2019). There is strong evidence indicating that children’s health can be affected in a dual mode can be underweight (short, wasted, or deficient in essential micronutrients) and at the same time overweight or obese by these negative effects of climate changes to food systems (Agostoni et al., 2023). Predictive models on the availability of agricultural products in the context of climate change suggest that by 2050, global food availability per capita could decrease by 3.2%, with a 4% reduction in fruit and vegetable consumption and a 0.7% reduction in red meat consumption. This scenario is expected to cause approximately 529,000 climate-related deaths, making it urgent to adopt strategies to stabilize the climate and mitigate the negative impacts on agricultural systems and global health (Springmann et al., 2016).
The relation between climate changes, food production and human health is complex. Global warming impacts eating habits. For example, during heat periods, we tend to adopt a sedentary lifestyle, while rising prices of fruits and vegetables lead to greater consumption of processed foods and refreshing drinks, increasing the risk of developing obesity (An et al., 2018). Furthermore, food systems themselves contribute to climate change. Agriculture significantly contributes to climate change by emitting greenhouse gas (GHGs). Animal-based food production alone is responsible for 57% of food-related emissions, mainly due to enteric fermentation, manure, and feeding processes. Other contributors include synthetic fertilizers, fossil fuel use, and deforestation. Additionally, food processing, transportation, refrigeration, cooking, and food waste throughout the supply chain also emit GHGs and impact the climate (Filho et al., 2022).
Mental stress
Climate change has been linked to negative impacts on mental health. A review conducted in UK showed that people exposed to extreme weather events showed high prevalence of depression, anxiety, and post-traumatic stress disorder (PTSD) (Cruz et al., 2020). Individuals exposed to floods show a significant increase in levels of anxiety and depression, with key factors influencing poor mental health identified as water depth and the absence of flood alerts (Robin et al., 2020). The severity of flood-related disasters has also been linked to PTSD symptoms in tourists returning from affected areas, as demonstrated by a study on the 2004 Southeast Asian tsunami, which examined its repercussions on three Scandinavian tourist populations (Heir et al., 2011). A study using data from the 2017–2018 Houston Health Survey estimated the effects of Hurricane Harvey on a local community, showing that after the storm, Houston adults experienced an average increase of 1.12 days per month of poor physical health and an increase of 1.31 days per month of poor mental health. These effects were most pronounced in areas of the city where the storm caused more severe structural damage (Bozick, 2021). In fact, the likelihood of experiencing mental distress increases with the severity of disaster exposure, which causes not only physical injuries, loss of life, or significant family damage but also property damage, loss of social support, and socio-economic decline (Schwartz et al., 2017; Joseph et al., 2014; Zwiebach et al., 2010).
Heatwaves also have a direct impact on mental health, contributing to the onset of mood disorders, schizophrenia, and neurotic disorders. However, the associations between heat exposure (both high ambient temperatures and heatwaves) and mortality and morbidity related to mental health vary across studies and locations. An analysis showed that for every 1 °C increase in temperature there was a 2.2% increase in mental health-related mortality and a 0.9% increase in mental health-related morbidity with populations living in tropical climates, as well as individuals aged over 65, being particularly vulnerable (Liu et al., 2021). Similarly, at temperatures above 23 °C, each 1 °C increase has been associated with a greater than 7% increase in the risk of major depression, with men aged 65 and older being the most vulnerable to heat-related major depression (Chen et al., 2019).
Wildfires, attributed to climate change, have been found to have a significant impact on mental health, particularly when exposure levels are high (Silveira et al., 2021). Poor dietary habits, induced by climate change, can exacerbate mental health issues such as depression (Lassale et al., 2019). Food insecurity has been associated with psychotic disorders, depression, which can lead to domestic violence against women, and behavioral disorders in children, as demonstrated by studies conducted in England (Melchior et al., 2009).
Strategies to protect human health against climate changes
The strategies to be adopted to protect human health from climate change are based on two main pillars: mitigation and adaptation. Mitigation involves the reduction or prevention of GHG emissions to limit the extent of climate change, mainly through the development and deployment of clean energy technologies, sustainable practices, behavioral changes, policies and regulations, and international agreements. The European Environmental Agency aims to reach a 55% or greater reduction of GHG emission by 2030, in comparison to 1990, and a climate-neutrality objective by 2050. Adaptation, instead, focuses on building resilience and adapting to the changes that are already occurring (Mastrandrea et al., 2014).
In the next paragraphs, we will discuss examples of both approaches impacting the medical field. These include intervention measures to reduce the GHG emissions (carbon footprint) of hospitals, the promotion of urban green spaces and lifestyles aimed at reducing carbon emissions, the development of action plans to protect vulnerable populations during heat waves, including the enhancement of surveillance systems to detect and respond to climate-sensitive health issues. New public health strategies will be required to facilitate adaptation to existing climate changes.
Mitigation: reduction of greenhouse gas emissions by the healthcare system
Hospitals and healthcare systems contribute significantly to GHG emissions through energy consumption for heating, cooling, operating medical equipment, and performing medical procedures (Comes et al., 2024; Pichler et al., 2019; Tennison et al., 2021). Additionally, emissions arise from patients and staff transportation, as well as waste management. Consequently, it is imperative to develop novel strategies to reduce healthcare carbon footprint. One such strategy is improving prescribing practices. Optimizing medication prescribing can make a significant contribution to reducing the carbon footprint of healthcare facilities. A prime example is personalized medicine where treatment is tailored to a patient’s specific clinical, genetic, and environmental exposure profile (Evans et al., 2024; Swen et al., 2011). This approach not only enhances clinical outcomes but may also reduce resource use and hospital admissions. Some authors have nevertheless highlighted the environmental impact to generate, process, and store large amount of data necessary for a personalized approach to medical care (Samuel and Lucassen, 2023).
A case in point is the treatment of high cardiovascular risk type 2 diabetes with empagliflozin (EMPA) a sodium-glucose-transporter 2 inhibitor (SGLT2i) which reduces glucose levels by enhancing glucose excretion (Ndefo et al., 2015). In these patients, treatment with EMPA, as opposed to placebo, resulted in a reduced rate of the primary composite cardiovascular outcome and of death from any cause when the study drug was added to standard care (Zinman et al., 2015). Similarly, dapagliflozin (DAPA), a different inhibitor SGLT2, reduced the risk of both initial and overall non-elective hospitalisation for any cause in patients with T2D (Schechter et al., 2023). EMPA treatment was associated with a lower incidence of both total cardiovascular hospitalisations and heart failure-related admissions, when compared to sitagliptin, a dipeptidyl peptidase-4 inhibitor (DPP-4i) and GLP1-RA, an agonist of the glucagon-like peptide-1 receptor, both used to control glucose in older patients with T2D (Desai et al., 2022). Recent studies have shown that EMPA reduces the frequency of major cardiovascular events in patients with heart failure, regardless the presence of diabetes (McMurray et al., 2019; Packer et al., 2021; Voors et al., 2022). These findings suggest that EMPA, and gliflozins in general, by reducing the frequency of hospitalization for heart failure, could have positive effects on climate change by reducing the carbon footprint of healthcare facilities. Several studies have supported this hypothesis. A study compared data from 441 patients treated with EMPA to 13,122 patients treated with other antihyperglycemic agents and found that treatment with EMPA was associated with fewer visits to healthcare facilities compared with other branded antihyperglycemic agents (Raju et al., 2022). In a comparative study between EMPA and DPP-4i, that examined healthcare utilization and costs in diabetic patients, a significant reduction in hospitalisation frequency and healthcare costs was demonstrated in the group of patients treated with EMPA (Htoo et al., 2023).
In addition to prescribing practices, other clinical strategies can enhance environmental sustainability and reduce healthcare costs without compromising patient outcomes. To increase environmental sustainability and for health-care spending reduction, specialty drug dosing could be optimised. A retrospective analysis using a cohort of patients from the Veterans Health Administration showed that alternative pembrolizumab delivery strategies may offer environmental advantages over the current dosing without compromising clinical outcomes (Bryant et al., 2024). During the COVID-19 pandemic, the antidepressant fluvoxamine (selective serotonin reuptake inhibitor) was associated with a reduction in the risk of COVID-19 related hospitalization and mortality (Reis et al., 2022). Anaesthesia practices also present opportunities for environmental improvement. A French study assessed the environmental impact of two anaesthesia strategies over a two-year period. A strategy based exclusively on total intravenous anaesthesia (TIVA) and a mixed approach combining TIVA with volatile anaesthesia. The results showed a 20-fold reduction in the carbon footprint associated with hypnotic drugs in facilities that adopted the exclusive TIVA strategy (Bernat et al., 2024).
Preventive healthcare measures can greatly contribute to reduce the carbon footprint of healthcare. For example, colorectal cancer screenings, have been shown to reduce cancer care–related GHG emissions (Yusuf et al., 2024). Likewise, adopting diets associated with lower GHG emissions—such as those low in red meat—not only have a direct beneficial effect on the environment, but have been linked to reductions in metabolic syndrome and myocardial infarction which indirectly can lower hospitalization rates (Aljahdali et al., 2023; Álvarez-Álvarez et al., 2024; García et al., 2023). In another preventive strategy, identifying patients with multiple chronic conditions who live in urban heat islands and prescribing devices like air conditioner, heat pump, and/or air purifier could reduce heat-related hospitalization (DeVoe et al., 2023).
Technological advances also offer promising solutions for reducing the healthcare carbon footprint. Continuous glucose monitoring and personalized insulin delivery systems can improve disease management while potentially reducing the frequency of hospital visits (Pickup, 2015). Inhaler technology also plays a role. A recent study conducted in 12 European countries and the United States assessed the environmental impact of inhaler choices in the management of chronic obstructive pulmonary disease (COPD). Researchers evaluated the potential reduction in carbon footprint by switching patients from pressurized metered dose inhalers and dry powder inhalers to reusable soft-mist inhalers within the same therapeutic class. The results showed that this transition could significantly reduce CO₂ emissions, with reductions ranging from 9.5 to 92.6% (Janson et al., 2023). Similarly, a study evaluated the waste generated by eyedrop packaging used to treat dry eye disease in Germany and found that single-dose units produced substantially more waste than multi-dose units, emphasizing the need for more sustainable packaging solutions in ophthalmology to reduce the environmental footprint of dry eye therapy (Schilcher et al., 2024).
Telemedicine significantly contributes to reducing the carbon footprint of the healthcare sector by drastically lowering the emissions associated with patients transportation. A review of the existing studies has shown that each remote consultation avoids the emission of quantity of CO2 ranging between 372 and 0.7 kg. It has also been calculated that the emissions associated with telemedicine are negligible compared to the savings achieved (Purohit et al., 2021). Two Swedish rehabilitation centres reported that, replacing physical visits with teleconsultation appointments resulted in a significant reduction (40 to 70 times) in carbon emissions. These practices are particularly convenient for patients who can only attend appointments by car (Holmner et al., 2014). Telemedicine represents also an opportunity to reduce the environmental impact associated with the management and follow-up of cancer patients, who typically require numerous routine visits. A comparative study conducted by the National Cancer Institute in Florida looked at two groups of patients: those located within 1 h of the centre and those more than 1 h away. Telemedicine reduced carbon emissions by 424,471 kg for patients within 1 hour of the centre and by 2,744,248 kg for those more than 1 h away (Patel et al., 2023).
Socioeconomic status is a critical determinant of health outcomes and is exacerbated by the impact of climate change. Telemedicine is a promising strategy to mitigate these challenges by reducing healthcare costs. By decreasing the need for patient travel, which is a significant financial burden for disadvantaged populations, telemedicine can improve access to healthcare. Numerous studies have demonstrated that telemedicine lowers overall healthcare expenditures compared to in-person visits (Dullet et al., 2017; Miah et al., 2019; Robinson et al., 2017).
Mitigation: changing the urban settings
Over half of the world’s population now lives in urban areas, a trend that continues to increase due to economic development, cultural changes, and the pursuit of better living conditions. Projections suggest that by 2050, nearly 70% of the global population will reside in cities (Change, 2014). There is evidence showing that urbanization and climate changes are related. High temperature-related disruptive effects on agriculture productivity drives urbanization (Helbling and Meierrieks, 2023). Urbanization, in turn, has led to increased energy consumption, contributing to significant GHG emissions (accessed on 15 December 2022) (Luqman et al., 2023). Furthermore, increased settlement density alters local microclimates and exacerbates heat and pollution, creating the so called “urban heat island” (Cichowicz and Bochenek, 2024) which negatively affects human health (Antoniou et al., 2024). Modification of urban settings are therefore crucial mitigation and adaptation strategies to counteract the deleterious impacts of climate change.
A recent study has investigated, in a neighbourhood context, the effectiveness of the application of cool materials on urban surfaces in improving microclimatic parameters (Giorio and Paparella, 2023). A different approach for microclimate control to reinstate comfort and usability was tested in a case study employing a degraded and unused space [an amphitheatre in Isla de la Cartuja, Seville (Spain)] in which a system utilizing nocturnally cooled water was used to generate cold air during diurnal periods. The results showed that outdoor thermal comfort (below 28 °C) can be achieved even when surrounding temperatures exceed 40 °C (Medina et al., 2022). A crucial approach to achieve microclimate regulation and human thermal comfort in urban areas relies on green infrastructure, such as trees, green roofs, green walls and the less investigated blue infrastructures, such as ponds and fountains (Kumar et al., 2024). A study has examined the effects of different blue-green infrastructure strategies on the microclimate and human thermal comfort in Melbourne’s Central Business District. The use of simulation software, has revealed that tree-based blue-green infrastructures are the most effective in lowering air temperatures and enhancing thermal comfort (Balany et al., 2022).
Mitigation: lifestyle modification
Changing our lifestyle could significantly reduce our carbon footprint and, consequently, the causes of climate change. These changes include adopting sustainable transportation practices, improving household energy efficiency, and adopting other low-carbon consumption behaviors.
Switching from private car use to public transportation, cycling, or walking can significantly reduce greenhouse gas emissions in urban areas, as demonstrated by the implementation of travel demand management strategies in Seoul’s Green Transport Zone (Kwak et al., 2024). A study conducted across seven European cities calculated CO2 emissions using different transportation modes. The results show that daily CO2 emissions amount to 3.2 kg per person, with cars contributing 70% of these emissions, while bicycles account for only 1%. Furthermore, these emissions decrease by 14% for each trip made by bicycle and by 62% for each avoided car trip (Brand et al., 2021).
In addition to changing our daily travel habits, modifying our dietary habits can significantly reduce our carbon footprint. A study conducted in Iran to predict GHG emissions based on dietary choices showed that a diet high in vegetables and low in red and white meat, eggs, cereals, and sweets significantly reduce CO2 emissions (Noormohammadi et al., 2022). Plant-based dietary patterns significantly not only reduce GHG emissions but also mitigate land use and biodiversity loss, compared to conventional diets, even though the impact on water and energy use may vary depending on specific food choices (Carey et al., 2023).
Despite the documented environmental benefits of plant-based diets, strategies aimed at modifying consumer habits are difficult to implement. A study conducted in Switzerland showed that residents who were randomly given information on food labels specifying CO2 emissions were more motivated to change their eating habits. This suggests that providing information about CO2 emissions on food labels can strengthens individuals’ intentions to adopt more sustainable diets and supports policies promoting mandatory CO₂ labelling (Maier, 2024). To address the complexity of establishing a healthy nutrition system aimed at reducing the carbon footprint, a dietary model that minimizes GHG emissions while considering nutritional needs and food preferences has been proposed. Based on the analysis of the current dietary habits of Italian adults, two scenarios were considered: minimizing environmental impact and minimizing changes to current dietary habits. The combination of these two scenarios resulted in a dietary model of 2,100 kcal/day with 49% carbohydrates, 16% proteins, 31% fats, and 4% fibers, achieving a 49% reduction of GHG emission compared to the current average diet (Biasini et al., 2023).
Lifestyle modifications include actions aimed to the lowering of household carbon footprints such as the reduction of the need for water and space heating, in addition to promoting a circular economy (reducing, reusing, and recycling) (Andreou et al., 2022). Transforming waste matrices of apple, pear, and sugar beet crops in products with functional applications for the prevention of cardiovascular diseases represents a virtuous example of circular economy practices (Caliceti et al., 2022).
Adaptation: flood protection and early warning systems
Monitoring and managing extreme weather events can prevent deaths caused by climate changes. The United Nations recommends expanding existing Early Warning Systems for extreme weather globally as a key climate adaptation strategy. To support this goal, a study evaluated the effectiveness of short-term weather forecasts and showed that forecast accuracy varies by region. Tropical areas show poor forecast reliability, indicating a need for improved predictability, while extra-tropical regions have relatively reliable forecasts, highlighting the necessity of local interventions (Coughlan De Perez et al., 2022). Since 2004, Italy has implemented the Heat Health Warning Systems (HHWWS) that predicts extreme heat and its health impacts. This system supports risk-based prevention measures for vulnerable groups and raise public awareness. Italy’s HHWWS covers 27 major cities and uses city-specific models based on the relationship between temperature and mortality, issuing three-day graded warnings during summer (Michelozzi et al., 2010). Addressing flood-related challenges is another important strategy for adapting to climate changes. A study focused on pre-existing adaptation measures in the Central Veneto Area, a region where recent extreme climate events, especially floods and rising temperatures, are severely challenging the area’s resilience. The research identified adaptation measures, many of which were not explicitly labeled as such, underscoring the pivotal role of proactive local planning in fostering climate resilience (Litt et al., 2022). Yu et al. have investigated the flood risk in Ulsan, South Korea, by focusing on how different types of buildings—like homes, schools, and car-related facilities—are affected. It uses a system called PPS25 (Planning Policy Statement 25), which groups buildings by how vulnerable they are to floods. The results show that while some districts are fairly safe, others have many buildings in high-risk areas. The risk depends on things like the land’s shape, how the city has developed, and what the buildings are used for. This type of research can help city planners make smarter decisions to protect people and buildings from future floods (Yu et al., 2021).
Monitoring and early intervention are crucial for protecting health from other climate-related threats. Climate change has significantly affected pollen seasons, shifting them earlier and increasing their intensity. Automatic pollen monitoring systems (Plaza et al., 2022) could prevent the rise in allergic reactions associated with these shifts (D’Amato et al., 2020). Early intervention could also be crucial to protect health from vector-borne diseases. A study in Singapore demonstrated the value of incorporating meteorological data into disease prediction models. By analyzing a decade of dengue and weather data, researchers found that temperatures and rainfall-based models improved the accuracy of outbreak predictions, supporting their use in targeted public health intervention (Hii et al., 2012).
Biosensors can greatly help monitoring processes. Biosensors have been developed to detect Cryptosporidium in drinking water, a pathogen responsible for gastrointestinal infections, which is difficult to monitor due to its low concentrations (Siwak et al., 2023). Similarly, monitoring sea surface temperature, using readily available remote sensing data, could serve as an early warning signal for Vibrio parahaemolyticus (Vp) and to guide the implementation and cessation of preventive or control measures (Konrad et al., 2017). Emerging technologies, such as CRISPR-Cas-based biosensors, offer a promising tool for fast and accurate sensor system for the detection of these diseases (Zakiyyah et al., 2022). Beyond infectious disease control, biosensors could play other crucial role in human health. For example, heatstroke activates specific inflammatory pathways, including ROS-mediated NLRP3 inflammasome activation and release of cytokines like IL-1β and IL-18 (Du et al., 2024). Recent advances in inflammasome monitoring include caspase-1 biosensors that detect luciferase cleavage, enabling real-time tracking of inflammation dynamics in live animals. This method, which tracks the protease that activates IL-1β/IL-18, could be adapted for wearable heatstroke monitoring systems (Talley et al., 2019). Next-generation biosensors could contribute to multi-parameter systems that monitor both vital signs and biochemical markers of heat stress. These build upon existing solutions like the Heat Stroke Alert System, which uses wearable sensors to track core temperature and heart rate (Marambe et al., 2018) and skin-adhesive nano-sensor for real-time heatstroke detection (Mani et al., 2020). Biosensors have now extended their capabilities to monitor sweating patterns and thirst, which are critical for heatstroke prevention. For instance, a validated wearable system combines a capacitive humidity sensor (tracking real-time perspiration rates) with smartphone-based thirst alerts, utilizing vasopressin biomarker changes to indicate risk periods (Momose et al., 2023).
Adaptation: lifestyle changes
Lifestyle changes, such as increasing physical activity and shifting to a diet rich in plant-based foods while reducing meat intake, can serve as an effective strategy for both mitigate impact on climate and promote adaptation to climate change, by reducing its negative effects on human health. Research demonstrates that plant-based diets are associated with reduced inflammation and a lower risk of cardiometabolic diseases, which are increasingly exacerbated by the effects of climate change (Koelman et al., 2022). Of relevance, fruits and vegetables fermentation with Lactiplantibacillus plantarum, enhances their beneficial properties as regulators of the immune, digestive, and cardiovascular system (Marracino et al., 2022). A diet that puts an emphasis on fish foods, may provide health benefits. Fish is a good source of protein and often rich in healthy in polyunsaturated fatty acids (PUFA) and monounsaturated (MU)FAs with a significant contribution to the beneficial health effect of marine organisms’ intake given by the presence of the long-chain semi-essential omega-3 FAs, which are indispensable for many metabolic processes, protecting the circulatory system (Chiefa et al., 2024; Ferrara et al., 2024). Similarly, regular physical activity, which has been shown to significantly benefit cardiovascular health by modifying blood pressure, insulin sensitivity, and lipid profiles (Nystoriak and Bhatnagar, 2018) could build resilience against the health risks posed by rising temperatures (Deshayes and Périard, 2023).
Climate change is associated with increased cancer risk and it has been calculated 534,000 climate related deaths worldwide, including deaths from cancer, by 2050, as a result of changes in food supply due to climate changes (Hiatt and Beyeler, 2020; Springmann et al., 2016). In this context, nutritional chemoprevention could be a particularly promising approach especially for cancers that are associated with diet, such as colorectal, stomach, esophageal, liver, endometrial, breast, and prostate. Dietary phytochemicals have been shown to exert anti-inflammatory, antioxidant, and anticancer properties. For example, it has been shown that stilbene polyphenols, mostly accumulated in grapes and blueberries, prevent further progression of precancerous prostate lesions into full blown disease and can beneficially interfere with advanced disease and metastasis (Campanelli et al., 2023; Hemani et al., 2022; Li et al., 2013; Parupathi et al., 2022).
In reviewing the effectiveness of climate change measures on human health, a complex picture emerges. Studies by Boeckmann and Rohn (2014) and Bouzid et al. (2013) highlight the challenges in assessing the effectiveness of public health interventions and planned adaptation measures to protect against climate-related health risks, noting a general lack of rigorous study designs and inconclusive evidence. While some interventions show promise, a weak evidence base and methodological shortcomings, such as difficulties in attributing health outcomes to specific measures, hinder comprehensive evaluation. Similarly, Johar et al. (2025) found mixed evidence for the effectiveness of community-based heat adaptation interventions, despite some improvements in heat literacy. The research also reveals a narrow focus in existing studies. Dinh et al. (2024) and Luyten et al. (2023) found that research on the health impacts of climate change adaptation and mitigation often focuses on a limited range of health outcomes, primarily near-term benefits from reduced air pollution, while neglecting mental health, communicable diseases, and long-term climate-related health harms. On the other hand, several studies, including those by Markandya et al. (2018) and Shrestha et al. (2024), demonstrate the significant health co-benefits of climate change mitigation strategies. Markandya et al. (2018) found that the health co-benefits from reduced air pollution could substantially outweigh the costs of achieving Paris Agreement targets, particularly in countries like China and India. Shrestha et al. (2024) further supported this, showing that strategies like increasing renewable energy, active transport, and plant-based diets have a positive co-benefit of reducing cardiovascular disease. These findings underscore the importance of taking action on climate change not only for future climate benefits but also for immediate and tangible health gains.
Conclusive remarks
There is robust evidence that anthropogenic activities have significantly influenced climate change, resulting in complex and far-reaching impacts on human health. Climate change increases cardiovascular and respiratory mortality through temperature fluctuations, wildfire-related air pollution, and degraded air quality. It reshapes the spread of infectious diseases, undermines food security, and raises risks of malnutrition. Psychological impacts like anxiety, PTSD, and depression are worsening. Collectively, these adverse health effects are especially severe in vulnerable populations.
In response, the global community has committed to concerted efforts to reduce GHG emissions through a variety of strategies. These include transforming healthcare delivery by emphasizing preventive care, adopting low-carbon technologies, and improving system-wide sustainability. At the individual level, lifestyle modifications—such as reduced reliance on carbon-intensive transportation, dietary shifts, and energy conservation—can contribute meaningfully to GHG reduction. Concurrently, adaptation strategies are essential for enhancing resilience. These include changes in urban planning, food systems, and health infrastructure to better withstand current and future climate stressors. A comprehensive analysis of existing knowledge gaps in the field—including the need for longitudinal investigations of cumulative exposures, rigorous assessments of adaptation and mitigation approaches effectiveness—lies beyond the scope of this article. Nonetheless, the prevailing consensus across peer-reviewed literature indicates that climate action has the potential to generate immediate and equitable health benefits. However, the authors collectively call for more comprehensive research with standardized methods to provide policymakers with better evidence for addressing health equity and promoting public health resilience in the face of a changing climate.
In this context, public health will play major role. Community health serves as the essential foundation for societal wellness, encompassing a wide array of proactive methods designed to protect and enhance the well-being of all individuals within the population. As the climate continues to evolve and present new challenges, this vital function assumes a greater significance, particularly in the critical area of public health adaptation. This forward-thinking strategy is not merely reactive; it equips communities with the necessary tools and resources to effectively confront health issues that arise as a direct consequence of climate change. It entails the development of comprehensive plans that are carefully crafted and executed with precision, followed by diligent monitoring to assess their success in safeguarding public health. Furthermore, it requires a willingness to make necessary adjustments to maintain community wellness in the face of ongoing environmental shifts. Public health leaders and professionals employ a diverse range of innovative tactics and strategies to tackle the myriad health threats posed by climate change, which can include everything from heat-related illnesses to the spread of infectious diseases.
Historically, the primary responsibility for protecting global health has rested heavily on the shoulders of local governments, who are tasked with implementing policies and programs that cater to the unique needs of their communities. However, a recent worldwide evaluation has revealed a concerning and notable disconnect between this crucial duty and the actions being undertaken, highlighting the urgent need for collaboration and commitment at all levels to ensure that public health remains a priority during an ever-changing environment. Astonishingly, a mere 10% of urban centers boasting populations exceeding one million have taken the initiative to launch health adaptation projects specifically designed to address the pressing challenges posed by climate change. Moreover, the few initiatives that have been implemented tend to focus primarily on mitigating the impacts of extreme weather occurrences, such as heatwaves and floods, while often neglecting the need for more substantial investments in crucial research and infrastructure that could provide long-term solutions. Alternative strategies, however, might encompass a wide array of innovative approaches, including the utilization of cutting-edge, data-driven disease monitoring technologies through advanced medical devices like biosensors, the implementation of effective medical treatments tailored to climate-related health issues, the employment of targeted vector control methods aimed at reducing the spread of diseases transmitted by insects, and the promotion of comprehensive public health programs designed to lower carbon emissions and enhance community resilience against the adverse effects of climate change.
Multidisciplinary research will be essential to create new tools, methods, and policies that can mitigate climate change and safeguard human health. To paraphrase an African proverb often cited in the context of child-rearing, we might conclude: “It will take a village to protect our health and the health of our planet.”
Author contributions
AO: Conceptualization, Investigation, Project administration, Writing – original draft, Writing – review & editing. AA: Conceptualization, Investigation, Project administration, Writing – original draft, Writing – review & editing. AS: Conceptualization, Investigation, Project administration, Writing – original draft, Writing – review & editing. PS: Conceptualization, Investigation, Writing – review & editing. AT: Writing – review & editing. FC: Writing – review & editing. FF: Conceptualization, Investigation, Supervision, Writing – review & editing. FV: Conceptualization, Investigation, Supervision, Writing – review & editing. AL: Conceptualization, Investigation, Supervision, Writing – review & editing. MV: Conceptualization, Investigation, Supervision, Writing – review & editing. LP: Conceptualization, Investigation, Supervision, Writing – review & editing. PR: Conceptualization, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work received support from National Recovery and Resilience Plan (NRRP), Mission 04 Component 2 Investment 1.5 – NextGenerationEU, Call for tender n. 3277 dated December 30, 2021; award number: 0001052 dated June 23, 2022, Fondazione Anna Maria Sechi per il Cuore (FASC) and 2023-FAR (Fondo Ateneo per la Ricerca Rizzo Paola, University of Ferrara).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fclim.2025.1647942/full#supplementary-material
References
Agostoni, C., Baglioni, M., La Vecchia, A., Molari, G., and Berti, C. (2023). Interlinkages between climate Change and food systems: the impact on child malnutrition-narrative review. Nutrients 15:416. doi: 10.3390/nu15020416
Aitken, W. W., Brown, S. C., and Comellas, A. P. (2022). Climate change and cardiovascular health. J. Am. Heart Assoc. 11:e027847. doi: 10.1161/JAHA.122.027847
Akil, L., Ahmad, H. A., and Reddy, R. S. (2014). Effects of climate change on Salmonella infections. Foodborne Pathog. Dis. 11, 974–980. doi: 10.1089/fpd.2014.1802
Al-Awadhi, M., Ahmad, S., and Iqbal, J. (2021). Current status and the epidemiology of malaria in the Middle East region and beyond. Microorganisms 9:338. doi: 10.3390/microorganisms9020338
Aljahdali, A. A., Campos, H., Granados, K., Jones, A. D., and Baylin, A. (2023). Diet-attributable greenhouse gas emissions and acute myocardial infarction in Costa Rica heart study. Nutrients 16:138. doi: 10.3390/nu16010138
Alonso, D., Bouma, M. J., and Pascual, M. (2011). Epidemic malaria and warmer temperatures in recent decades in an east African highland. Proc. Biol. Sci. 278, 1661–1669. doi: 10.1098/rspb.2010.2020
Al-Sadek, T., and Yusuf, N. (2024). Ultraviolet radiation biological and medical implications. Curr. Issues Mol. Biol. 46, 1924–1942. doi: 10.3390/cimb46030126
Álvarez-Álvarez, L., Vitelli-Storelli, F., Rubín-García, M., García, S., Bouzas, C., Ruíz-Canela, M., et al. (2024). Impact of Mediterranean diet promotion on environmental sustainability: a longitudinal analysis. Public Health 230, 12–20. doi: 10.1016/j.puhe.2024.02.010
Amirkhani, M., Ghaemimood, S., von Schreeb, J., El-Khatib, Z., and Yaya, S. (2022). Extreme weather events and death based on temperature and CO2 emission – a global retrospective study in 77 low-, middle-and high-income countries from 1999 to 2018. Prev. Med. Rep. 28:101846. doi: 10.1016/j.pmedr.2022.101846
An, R., Ji, M., and Zhang, S. (2018). Global warming and obesity: a systematic review. Obes. Rev. 19, 150–163. doi: 10.1111/obr.12624
Andreou, A., Fragkos, P., Fotiou, T., and Filippidou, F. (2022). Assessing lifestyle transformations and their systemic effects in energy-system and integrated assessment models: a review of current methods and data. Energies 15:4948. doi: 10.3390/en15144948
Antoniou, N., Montazeri, H., Blocken, B., and Neophytou, M. (2024). On the impact of climate change on urban microclimate, thermal comfort, and human health: multiscale numerical simulations. Build. Environ. 260:111690. doi: 10.1016/j.buildenv.2024.111690
Atkins, W. C., McKenna, Z. J., Jarrard, C. P., Foster, J., and Crandall, C. G. (2024). Thermoregulatory responses to very hot and dry heat exposure: interactions between age and sex. Physiology 39:726. doi: 10.1152/physiol.2024.39.S1.726
Baker-Austin, C., Oliver, J. D., Alam, M., Ali, A., Waldor, M. K., Qadri, F., et al. (2018). Vibrio spp. infections. Nat. Rev. Dis. Primers 4:8. doi: 10.1038/s41572-018-0005-8
Balany, F., Muttil, N., Muthukumaran, S., Wong, M. S., and Ng, A. W. M. (2022). Studying the effect of blue-green infrastructure on microclimate and human thermal comfort in Melbourne’s central business district. Sustainability 14:9057. doi: 10.3390/su14159057
Ballester, J., Quijal-Zamorano, M., Méndez Turrubiates, R. F., Pegenaute, F., Herrmann, F. R., Robine, J. M., et al. (2023). Heat-related mortality in Europe during the summer of 2022. Nat. Med. 29, 1857–1866. doi: 10.1038/s41591-023-02419-z
Bellone, R., Lechat, P., Mousson, L., Gilbart, V., Piorkowski, G., Bohers, C., et al. (2023). Climate change and vector-borne diseases: a multi-omics approach of temperature-induced changes in the mosquito. J. Travel Med. 30:taad062. doi: 10.1093/jtm/taad062
Bernat, M., Boyer, A., Roche, M., Richard, C., Bouvet, L., Remacle, A., et al. (2024). Reducing the carbon footprint of general anaesthesia: a comparison of total intravenous anaesthesia vs. a mixed anaesthetic strategy in 47,157 adult patients. Anaesthesia 79, 309–317. doi: 10.1111/anae.16221
Bhattarai, H., Tai, A. P. K., Val Martin, M., and Yung, D. H. Y. (2024). Responses of fine particulate matter (PM2.5) air quality to future climate, land use, and emission changes: insights from modeling across shared socioeconomic pathways. Sci. Total Environ. 948:174611. doi: 10.1016/j.scitotenv.2024.174611
Biasini, B., Donati, M., Rosi, A., Kalmpourtzidou, A., Bonaccio, M., Ruggiero, E., et al. (2023). An optimisation study to reduce greenhouse gas emissions from adults’ current diet in Italy. Eur. J. Pub. Health 33:ckad160.349. doi: 10.1093/eurpub/ckad160.349
Bich, T. H., Quang, L. N., Ha, L. T. T., Hanh, T. T. D., and Guha-Sapir, D. (2011). Impacts of flood on health: epidemiologic evidence from Hanoi, Vietnam. Glob. Health Action 4:6356. doi: 10.3402/gha.v4i0.6356
Boeckmann, M., and Rohn, I. (2014). Is planned adaptation to heat reducing heat-related mortality and illness? A systematic review. BMC Public Health 14:1112. doi: 10.1186/1471-2458-14-1112
Bouchama, A., Aziz, M. A., Mahri, S. A., Gabere, M. N., Dlamy, M. A., Mohammad, S., et al. (2017). A model of exposure to extreme environmental heat uncovers the human transcriptome to heat stress. Sci. Rep. 7:9429. doi: 10.1038/s41598-017-09819-5
Bouchama, A., and Knochel, J. P. (2002). Heat stroke. N. Engl. J. Med. 346, 1978–1988. doi: 10.1056/NEJMra011089
Bouchama, A., Rashid, M., Malik, S. S., Al Mahri, S., Yassin, Y., Abdullah, M., et al. (2023). Whole genome transcriptomic reveals heat stroke molecular signatures in humans. J. Physiol. 601, 2407–2423. doi: 10.1113/JP284031
Bouzid, M., Hooper, L., and Hunter, P. R. (2013). The effectiveness of public health interventions to reduce the health impact of climate change: a systematic review of systematic reviews. PLoS One 8:e62041. doi: 10.1371/journal.pone.0062041
Bozick, R. (2021). The effects of hurricane Harvey on the physical and mental health of adults in Houston. Health Place 72:102697. doi: 10.1016/j.healthplace.2021.102697
Brand, C., Dons, E., Anaya-Boig, E., Avila-Palencia, I., Clark, A., De Nazelle, A., et al. (2021). The climate change mitigation effects of daily active travel in cities. Transp. Res. Part D Transp. Environ. 93:102764. doi: 10.1016/j.trd.2021.102764
Brown, L., and Murray, V. (2013). Examining the relationship between infectious diseases and flooding in Europe: a systematic literature review and summary of possible public health interventions. Disaster Health. 1, 117–127. doi: 10.4161/dish.25216
Bryant, A. K., Lewy, J. R., Bressler, R. D., Chopra, Z., Gyori, D. J., Bazzell, B. G., et al. (2024). Projected environmental and public health benefits of extended-interval dosing: an analysis of pembrolizumab use in a US National Health System. Lancet Oncol. 25, 802–810. doi: 10.1016/S1470-2045(24)00200-6
Caliceti, C., Malaguti, M., Marracino, L., Barbalace, M. C., Rizzo, P., and Hrelia, S. (2022). Agri-food waste from apple, pear, and sugar beet as a source of protective bioactive molecules for endothelial dysfunction and its major complications. Antioxidants (Basel) 11:1786. doi: 10.3390/antiox11091786
Caminade, C., Medlock, J. M., Ducheyne, E., McIntyre, K. M., Leach, S., Baylis, M., et al. (2012). Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J. R. Soc. Interface 9, 2708–2717. doi: 10.1098/rsif.2012.0138
Campanelli, G., Deabel, R. A., Puaar, A., Devarakonda, L. S., Parupathi, P., Zhang, J., et al. (2023). Molecular efficacy of Gnetin C as dual-targeted therapy for castrate-resistant prostate cancer. Mol. Nutr. Food Res. 67:e2300479. doi: 10.1002/mnfr.202300479
Carey, C. N., Paquette, M., Sahye-Pudaruth, S., Dadvar, A., Dinh, D., Khodabandehlou, K., et al. (2023). The environmental sustainability of plant-based dietary patterns: a scoping review. J. Nutr. 153, 857–869. doi: 10.1016/j.tjnut.2023.02.001
Change, C. (2014) World urbanization prospects. On the other hand, the rural population is expected to remain 3.4 billion up to 2020, and it estimates it will decrease to approximately 3.2 billion by 2050. Almost 90% of the world’s rural population lived in Asia and 10.
Chaseling, G. K., Crandall, C. G., and Gagnon, D. (2020). Skin blood flow measurements during heat stress: technical and analytical considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R57–R69. doi: 10.1152/ajpregu.00177.2019
Chaudhary, P., Bharadwaj, A., Gupta, R. K., Kumar, S., and Sharma, S. (2023). “Heat stress and dehydration” in Adaptation under stressful environments through biological adjustments and interventions. eds. R. Tulsawani and D. Vohora (Singapore: Springer Nature Singapore), 369–376.
Chei, S., Song, J.-H., Oh, H.-J., Lee, K., Jin, H., Choi, S.-H., et al. (2020). Gintonin-enriched fraction suppresses heat stress-induced inflammation through LPA receptor. Molecules 25:1019. doi: 10.3390/molecules25051019
Chen, J., Ding, C., Cao, J., Tong, H., and Chen, Y. (2023). Heat stress combined with lipopolysaccharide induces pulmonary microvascular endothelial cell glycocalyx inflammatory damage in vitro. Immun Inflamm Dis 11:e1034. doi: 10.1002/iid3.1034
Chen, N.-T., Lin, P.-H., and Guo, Y.-L. L. (2019). Long-term exposure to high temperature associated with the incidence of major depressive disorder. Sci. Total Environ. 659, 1016–1020. doi: 10.1016/j.scitotenv.2018.12.434
Chen, R., Yin, P., Wang, L., Liu, C., Niu, Y., Wang, W., et al. (2018). Association between ambient temperature and mortality risk and burden: time series study in 272 main Chinese cities. BMJ 363:k4306. doi: 10.1136/bmj.k4306
Cheruiyot, S. J., Kimanthi, M., Shabani, J. S., Nyamu, N. F., Gathu, C., Agoi, F., et al. (2022). Climate change poses a threat to nutrition and food security in Kilifi County, Kenya. Afr J Prim Health Care Fam Med 14, e1–e4. doi: 10.4102/phcfm.v14i1.3718
Chiefa, F., Tedeschi, P., Cescon, M., Costa, V., Sarti, E., Salgado-Ramos, M., et al. (2024). Nutrients and quality aspects characterizing Ostrea edulis cultivated in Valli di Comacchio (northern Italy) across different seasons. Molecules 29:5546. doi: 10.3390/molecules29235546
Cichowicz, R., and Bochenek, A. D. (2024). Assessing the effects of urban heat islands and air pollution on human quality of life. Anthropocene 46:100433. doi: 10.1016/j.ancene.2024.100433
Cimaglia, P., Fortini, F., Vieceli Dalla Sega, F., Cardelli, L. S., Massafra, R. F., Morelli, C., et al. (2022). Relationship between PCSK9 and endothelial function in patients with acute myocardial infarction. Nutr. Metab. Cardiovasc. Dis. 32, 2105–2111. doi: 10.1016/j.numecd.2022.06.020
Comes, D. J., Bluiminck, S., Kooistra, E. J., de Nes, L., van Workum, F. T. W. E., Touw, H., et al. (2024). The carbon footprint of a laparoscopic cholecystectomy. Br. J. Surg. 111:znae225. doi: 10.1093/bjs/znae225
Cooper, O. R. (2019). Detecting the fingerprints of observed climate change on surface ozone variability. Sci Bull (Beijing) 64, 359–360. doi: 10.1016/j.scib.2019.02.013
Coughlan De Perez, E., Harrison, L., Berse, K., Easton-Calabria, E., Marunye, J., Marake, M., et al. (2022). Adapting to climate change through anticipatory action: the potential use of weather-based early warnings. Weather Climate Extremes 38:100508. doi: 10.1016/j.wace.2022.100508
Criddle, M. J., Haynes, M. A., Carter, M. H. H., Collis, M. J., Wigati, M. K., Dechichi, M. J. G. C., et al. (2024). Determinants of physiological response to exertional heat stress in humans. J. Clin. Exerc. Physiol. 13:408. doi: 10.31189/2165-7629-13-s2.408
Cruz, J., White, P. C. L., Bell, A., and Coventry, P. A. (2020). Effect of extreme weather events on mental health: a narrative synthesis and meta-analysis for the UK. Int. J. Environ. Res. Public Health 17:8581. doi: 10.3390/ijerph17228581
D’Amato, G., Annesi-Maesano, I., Biagioni, B., Lancia, A., Cecchi, L., D’Ovidio, M. C., et al. (2023). New developments in climate change, air pollution, pollen allergy, and interaction with SARS-CoV-2. Atmosphere 14:848. doi: 10.3390/atmos14050848
D’Amato, G., Chong-Neto, H. J., Monge Ortega, O. P., Vitale, C., Ansotegui, I., Rosario, N., et al. (2020). The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy 75, 2219–2228. doi: 10.1111/all.14476
Desai, R. J., Glynn, R. J., Everett, B. M., Schneeweiss, S., Wexler, D. J., Bessette, L. G., et al. (2022). Comparative effectiveness of Empagliflozin in reducing the burden of recurrent cardiovascular hospitalizations among older adults with diabetes in routine clinical care. Am. Heart J. 254, 203–215. doi: 10.1016/j.ahj.2022.09.008
Desai, Y., Khraishah, H., and Alahmad, B. (2023). Heat and the heart. Yale J. Biol. Med. 96, 197–203. doi: 10.59249/HGAL4894
Deshayes, T. A., and Périard, J. D. (2023). Regular physical activity across the lifespan to build resilience against rising global temperatures. EBioMedicine 96:104793. doi: 10.1016/j.ebiom.2023.104793
Desqueyroux, H., Pujet, J.-C., Prosper, M., Squinazi, F., and Momas, I. (2002). Short-term effects of low-level air pollution on respiratory health of adults suffering from moderate to severe asthma. Environ. Res. 89, 29–37. doi: 10.1006/enrs.2002.4357
DeVoe, J. E., Huguet, N., Likumahuwa-Ackman, S., Bazemore, A., Gold, R., and Werner, L. (2023). Precision ecologic medicine: tailoring care to mitigate impacts of climate change. J. Prim. Care Community Health 14:21501319231170585. doi: 10.1177/21501319231170585
Dinh, N. T. T., Tran, J., and Hensher, M. (2024). Measuring and valuing the health co-benefits of climate change mitigation: a scoping review. Lancet Planetary Health 8, e402–e409. doi: 10.1016/S2542-5196(24)00095-0
Du, G., Yang, Z., Wen, Y., Li, X., Zhong, W., Li, Z., et al. (2024). Heat stress induces IL-1β and IL-18 overproduction via ROS-activated NLRP3 inflammasome: implication in neuroinflammation in mice with heat stroke. Neuroreport 35, 558–567. doi: 10.1097/WNR.0000000000002042
Dullet, N. W., Geraghty, E. M., Kaufman, T., Kissee, J. L., King, J., Dharmar, M., et al. (2017). Impact of a university-based outpatient telemedicine program on time savings, travel costs, and environmental pollutants. Value Health 20, 542–546. doi: 10.1016/j.jval.2017.01.014
Dupke, S., Buchholz, U., Fastner, J., Förster, C., Frank, C., Lewin, A., et al. (2023). Impact of climate change on waterborne infections and intoxications. J. Health Monit. 8, 62–77. doi: 10.25646/11402
Estimating the Burden of Foodborne Diseases (n.d.). Available online at: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (Accessed May 29, 2025).
European Food Safety Authority and European Centre for Disease Prevention and Control (2022). The European Union one health 2021 zoonoses report. EFSA J. 20:e07666. doi: 10.2903/j.efsa.2022.7666
Evans, W., Meslin, E. M., Kai, J., and Qureshi, N. (2024). Precision medicine—are we there yet? A narrative review of precision medicine’s applicability in primary care. JPM 14:418. doi: 10.3390/jpm14040418
Fan, J.-F., Xiao, Y.-C., Feng, Y.-F., Niu, L.-Y., Tan, X., Sun, J.-C., et al. (2023). A systematic review and meta-analysis of cold exposure and cardiovascular disease outcomes. Front Cardiovasc Med 10:1084611. doi: 10.3389/fcvm.2023.1084611
Fann, N., Nolte, C. G., Dolwick, P., Spero, T. L., Brown, A. C., Phillips, S., et al. (2015). The geographic distribution and economic value of climate change-related ozone health impacts in the United States in 2030. J. Air Waste Manag. Assoc. 65, 570–580. doi: 10.1080/10962247.2014.996270
Ferrara, D., Cescon, M., Giacoppo, G., Costa, V., Purcaro, G., Spadafora, N. D., et al. (2024). Composition and nutritional values of fatty acids in marine organisms by one-step microwave-assisted extraction/derivatization and comprehensive two-dimensional gas chromatography-flame ionization detector. J. Chromatogr. B 1236:124074. doi: 10.1016/j.jchromb.2024.124074
Filho, W. L., Setti, A. F. F., Azeiteiro, U. M., Lokupitiya, E., Donkor, F. K., Etim, N. N., et al. (2022). An overview of the interactions between food production and climate change. Sci. Total Environ. 838:156438. doi: 10.1016/j.scitotenv.2022.156438
Fiore, A. M., Naik, V., and Leibensperger, E. M. (2015). Air quality and climate connections. J. Air Waste Manag. Assoc. 65, 645–685. doi: 10.1080/10962247.2015.1040526
Fischer, L., Gültekin, N., Kaelin, M. B., Fehr, J., and Schlagenhauf, P. (2020). Rising temperature and its impact on receptivity to malaria transmission in Europe: a systematic review. Travel Med. Infect. Dis. 36:101815. doi: 10.1016/j.tmaid.2020.101815
Fortini, F., Vieceli Dalla Sega, F., Caliceti, C., Aquila, G., Pannella, M., Pannuti, A., et al. (2017). Estrogen receptor β-dependent Notch1 activation protects vascular endothelium against tumor necrosis factor α (TNFα)-induced apoptosis. J. Biol. Chem. 292, 18178–18191. doi: 10.1074/jbc.M117.790121
Fortini, F., Vieceli Dalla Sega, F., Lazzarini, E., Aquila, G., Sysa-Shah, P., Bertero, E., et al. (2025). ErbB2-NOTCH1 axis controls autophagy in cardiac cells. Biofactors 51:e2091. doi: 10.1002/biof.2091
Fortini, F., Vieceli Dalla Sega, F., Marracino, L., Severi, P., Rapezzi, C., Rizzo, P., et al. (2021). Well-known and novel players in endothelial dysfunction: updates on a notch(ed) landscape. Biomedicine 9:997. doi: 10.3390/biomedicines9080997
Ganesan, S., Volodina, O., Pearce, S. C., Gabler, N. K., Baumgard, L. H., Rhoads, R. P., et al. (2017). Acute heat stress activated inflammatory signaling in porcine oxidative skeletal muscle. Physiol. Rep. 5:e13397. doi: 10.14814/phy2.13397
Gao, J., Mendes de Leon, C. F., Zhang, B., Weuve, J., Langa, K. M., D’Souza, J., et al. (2024). Long-term air pollution exposure and incident physical disability in older US adults: a cohort study. Lancet Healthy Longev 5:100629. doi: 10.1016/j.lanhl.2024.07.012
García, S., Pastor, R., Monserrat-Mesquida, M., Álvarez-Álvarez, L., Rubín-García, M., Martínez-González, M. Á., et al. (2023). Metabolic syndrome criteria and severity and carbon dioxide (CO2) emissions in an adult population. Glob. Health 19:50. doi: 10.1186/s12992-023-00948-3
García-León, D., Masselot, P., Mistry, M. N., Gasparrini, A., Motta, C., Feyen, L., et al. (2024). Temperature-related mortality burden and projected change in 1368 European regions: a modelling study. Lancet Public Health 9, e644–e653. doi: 10.1016/S2468-2667(24)00179-8
Giorio, M., and Paparella, R. (2023). Climate mitigation strategies: the use of cool pavements. Sustainability 15:7641. doi: 10.3390/su15097641
Gromkowska-Kępka, K. J., Puścion-Jakubik, A., Markiewicz-Żukowska, R., and Socha, K. (2021). The impact of ultraviolet radiation on skin photoaging – review of in vitro studies. J. Cosmet. Dermatol. 20, 3427–3431. doi: 10.1111/jocd.14033
Gui, H., Gwee, S., Koh, J., and Pang, J. (2021). Weather factors associated with reduced risk of dengue transmission in an urbanized tropical city. Int. J. Environ. Res. Public Health 19:339. doi: 10.3390/ijerph19010339
Harrison, G. I., and Young, A. R. (2002). Ultraviolet radiation-induced erythema in human skin. Methods 28, 14–19. doi: 10.1016/s1046-2023(02)00205-0
Hashizume, M., Dewan, A. M., Sunahara, T., Rahman, M. Z., and Yamamoto, T. (2012). Hydroclimatological variability and dengue transmission in Dhaka, Bangladesh: a time-series study. BMC Infect. Dis. 12:98. doi: 10.1186/1471-2334-12-98
Heikonen, S., Heino, M., Jalava, M., Siebert, S., Viviroli, D., and Kummu, M. (2025). Climate change threatens crop diversity at low latitudes. Nat Food 6, 331–342. doi: 10.1038/s43016-025-01135-w
Heir, T., Rosendal, S., Bergh-Johannesson, K., Michel, P.-O., Mortensen, E. L., Weisaeth, L., et al. (2011). Tsunami-affected Scandinavian tourists: disaster exposure and post-traumatic stress symptoms. Nord. J. Psychiatry 65, 9–15. doi: 10.3109/08039481003786394
Helbling, M., and Meierrieks, D. (2023). Global warming and urbanization. J. Popul. Econ. 36, 1187–1223. doi: 10.1007/s00148-022-00924-y
Hemani, R., Patel, I., Inamdar, N., Campanelli, G., Donovan, V., Kumar, A., et al. (2022). Dietary Pterostilbene for MTA1-targeted interception in high-risk premalignant prostate cancer. Cancer Prev. Res. (Phila.) 15, 87–100. doi: 10.1158/1940-6207.CAPR-21-0242
Hiatt, R. A., and Beyeler, N. (2020). Cancer and climate change. Lancet Oncol. 21, e519–e527. doi: 10.1016/S1470-2045(20)30448-4
Hii, Y. L., Zaki, R. A., Aghamohammadi, N., and Rocklöv, J. (2016). Research on climate and dengue in Malaysia: a systematic review. Curr. Environ. Health Rep. 3, 81–90. doi: 10.1007/s40572-016-0078-z
Hii, Y. L., Zhu, H., Ng, N., Ng, L. C., and Rocklöv, J. (2012). Forecast of dengue incidence using temperature and rainfall. PLoS Negl. Trop. Dis. 6:e1908. doi: 10.1371/journal.pntd.0001908
Holmner, A., Ebi, K. L., Lazuardi, L., and Nilsson, M. (2014). Carbon footprint of telemedicine solutions – unexplored opportunity for reducing carbon emissions in the health sector. PLoS One 9:e105040. doi: 10.1371/journal.pone.0105040
Htoo, P. T., Tesfaye, H., Schneeweiss, S., Wexler, D. J., Glynn, R., Schmedt, N., et al. (2023). 270-OR: health care utilization and cost associated with empagliflozin in older adults with type 2 diabetes—results from the EMPRISE study. Diabetes 72:270-OR. doi: 10.2337/db23-270-OR
Huang, M., Ding, L., Wang, J., Ding, C., and Tao, J. (2021). The impacts of climate change on fish growth: a summary of conducted studies and current knowledge. Ecol. Indic. 121:106976. doi: 10.1016/j.ecolind.2020.106976
Ikäheimo, T. M. (2018). Cardiovascular diseases, cold exposure and exercise. Temperature (Austin) 5, 123–146. doi: 10.1080/23328940.2017.1414014
Janson, C., Hernando Platz, J., Soulard, S., Langham, S., Nicholson, L., and Hartgers-Gubbels, E. S. (2023). Reducing carbon footprint by switching to reusable soft-mist inhalers. ERJ Open Res. 9, 00543–02022. doi: 10.1183/23120541.00543-2022
Jerrett, M., Burnett, R. T., Pope, C. A., Ito, K., Thurston, G., Krewski, D., et al. (2009). Long-term ozone exposure and mortality. N. Engl. J. Med. 360, 1085–1095. doi: 10.1056/NEJMoa0803894
Johar, H., Abdulsalam, F. I., Guo, Y., Baernighausen, T., Jahan, N. K., Watterson, J., et al. (2025). Community-based heat adaptation interventions for improving heat literacy, behaviours, and health outcomes: a systematic review. Lancet Planet. Health 9:101207. doi: 10.1016/S2542-5196(25)00007-5
Joseph, N. T., Matthews, K. A., and Myers, H. F. (2014). Conceptualizing health consequences of hurricane Katrina from the perspective of socioeconomic status decline. Health Psychol. 33, 139–146. doi: 10.1037/a0031661
Juneja, V. K., Valenzuela Melendres, M., Huang, L., Gumudavelli, V., Subbiah, J., and Thippareddi, H. (2007). Modeling the effect of temperature on growth of Salmonella in chicken. Food Microbiol. 24, 328–335. doi: 10.1016/j.fm.2006.08.004
Kao, B., Lin, C.-H., and Wen, T.-H. (2023). Measuring the effects of typhoon trajectories on dengue outbreaks in tropical regions of Taiwan: 1998-2019. Int. J. Biometeorol. 67, 1311–1322. doi: 10.1007/s00484-023-02498-0
Kazi, D. S., Katznelson, E., Liu, C.-L., Al-Roub, N. M., Chaudhary, R. S., Young, D. E., et al. (2024). Climate change and cardiovascular health: a systematic review. JAMA Cardiol. 9, 748–757. doi: 10.1001/jamacardio.2024.1321
Kelly-Hope, L. A., Hemingway, J., and McKenzie, F. E. (2009). Environmental factors associated with the malaria vectors Anopheles gambiae and Anopheles funestus in Kenya. Malar. J. 8:268. doi: 10.1186/1475-2875-8-268
Khan, A., and Mubeen, M. (2025). Heat stroke in the era of global warming: a call for urgent action. Ann. Glob. Health 91:1. doi: 10.5334/aogh.4519
Khatana, S. A. M., Szeto, J. J., Eberly, L. A., Nathan, A. S., Puvvula, J., and Chen, A. (2024). Projections of extreme temperature-related deaths in the US. JAMA Netw. Open 7:e2434942. doi: 10.1001/jamanetworkopen.2024.34942
Khraishah, H., Alahmad, B., Ostergard, R. L., AlAshqar, A., Albaghdadi, M., Vellanki, N., et al. (2022). Climate change and cardiovascular disease: implications for global health. Nat. Rev. Cardiol. 19, 798–812. doi: 10.1038/s41569-022-00720-x
Koelman, L., Egea Rodrigues, C., and Aleksandrova, K. (2022). Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 13, 101–115. doi: 10.1093/advances/nmab086
Konrad, S., Paduraru, P., Romero-Barrios, P., Henderson, S. B., and Galanis, E. (2017). Remote sensing measurements of sea surface temperature as an indicator of Vibrio parahaemolyticus in oyster meat and human illnesses. Environ. Health 16:92. doi: 10.1186/s12940-017-0301-x
Kumar, P., Debele, S. E., Khalili, S., Halios, C. H., Sahani, J., Aghamohammadi, N., et al. (2024). Urban heat mitigation by green and blue infrastructure: drivers, effectiveness, and future needs. Innovation (Camb) 5:100588. doi: 10.1016/j.xinn.2024.100588
Kwak, J., Ku, D., Jo, J., Kim, D., Bencekri, M., Choi, M., et al. (2024). Travel demand management strategies to mitigate climate change. Proc. Inst. Civ. Eng. 177, 64–75. doi: 10.1680/jmuen.23.00026
Lassale, C., Batty, G. D., Baghdadli, A., Jacka, F., Sánchez-Villegas, A., Kivimäki, M., et al. (2019). Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol. Psychiatry 24, 965–986. doi: 10.1038/s41380-018-0237-8
Law, B. E., Abatzoglou, J. T., Schwalm, C. R., Byrne, D., Fann, N., and Nassikas, N. J. (2025). Anthropogenic climate change contributes to wildfire particulate matter and related mortality in the United States. Commun. Earth Environ. 6:336. doi: 10.1038/s43247-025-02314-0
Li, K., Dias, S. J., Rimando, A. M., Dhar, S., Mizuno, C. S., Penman, A. D., et al. (2013). Pterostilbene acts through metastasis-associated protein 1 to inhibit tumor growth, progression and metastasis in prostate cancer. PLoS One 8:e57542. doi: 10.1371/journal.pone.0057542
Li, Y., Li, H., Ma, W., Maegele, M., Tang, Y., and Gu, Z. (2023). Proteomic profiling of serum exosomes reveals acute phase response and promotion of inflammatory and platelet activation pathways in patients with heat stroke. PeerJ 11:e16590. doi: 10.7717/peerj.16590
Li, J., Liu, F., Liang, F., Yang, Y., Lu, X., and Gu, D. (2023). Air pollution exposure and vascular endothelial function: a systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 30, 28525–28549. doi: 10.1007/s11356-023-25156-9
Lim, Y.-H., Kim, H., and Hong, Y.-C. (2013). Variation in mortality of ischemic and hemorrhagic strokes in relation to high temperature. Int. J. Biometeorol. 57, 145–153. doi: 10.1007/s00484-012-0542-x
Lin, Q.-W., Zhong, L.-C., He, L.-P., Zeng, Q.-B., Zhang, W., Song, Q., et al. (2024). A newly proposed heatstroke-induced coagulopathy score in patients with heat illness: a multicenter retrospective study in China. Chin. J. Traumatol. 27, 83–90. doi: 10.1016/j.cjtee.2023.08.001
Litt, G., Bertin, M., Negretto, V., and Musco, F. (2022). Reinterpreting spatial planning cultures to define local adaptation cultures: a methodology from the Central Veneto region case. Sustainability 14:7344. doi: 10.3390/su14127344
Liu, J., Varghese, B. M., Hansen, A., Xiang, J., Zhang, Y., Dear, K., et al. (2021). Is there an association between hot weather and poor mental health outcomes? A systematic review and meta-analysis. Environ. Int. 153:106533. doi: 10.1016/j.envint.2021.106533
Liu, J., Varghese, B. M., Hansen, A., Zhang, Y., Driscoll, T., Morgan, G., et al. (2022). Heat exposure and cardiovascular health outcomes: a systematic review and meta-analysis. Lancet Planet. Health 6, e484–e495. doi: 10.1016/S2542-5196(22)00117-6
Lovey, T., and Schlagenhauf, P. (2023). Rising temperatures and the threat of malaria in Europe: an unwelcome return? Rev. Med. Suisse 19, 849–852. doi: 10.53738/REVMED.2023.19.825.849
Lu, L., Lin, H., Tian, L., Yang, W., Sun, J., and Liu, Q. (2009). Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 9:395. doi: 10.1186/1471-2458-9-395
Luo, J., Chen, Y., Ding, C., Qiu, J., Chen, Y., Lin, Y., et al. (2019). Heat stress combined with lipopolysaccharide alter the activity and superficial molecules of peripheral monocytes. Int. J. Immunopathol. Pharmacol. 33:2058738419828891. doi: 10.1177/2058738419828891
Luqman, M., Rayner, P. J., and Gurney, K. R. (2023). On the impact of urbanisation on CO2 emissions. NPJ Urban Sustain. 3:6. doi: 10.1038/s42949-023-00084-2
Luyten, A., Winkler, M. S., Ammann, P., and Dietler, D. (2023). Health impact studies of climate change adaptation and mitigation measures – a scoping review. J. Clim. Change Health 9:100186. doi: 10.1016/j.joclim.2022.100186
Maier, M. (2024). Increasing the uptake of plant-based diets: an analysis of the impact of a CO2 food label. J. Environ. Psychol. 93:102216. doi: 10.1016/j.jenvp.2023.102216
Mani, G. K., Nimura, Y., and Tsuchiya, K. (2020). Advanced artificial electronic skin based pH sensing system for heatstroke detection. ACS Sens. 5, 911–916. doi: 10.1021/acssensors.0c00207
Marambe, Y. H. B., Niroshani, D. T., Rathnayake, P. D. C. T. K., Dayananda, S. H. C. K., and De Silva, D. H. (2018). Heat stroke alert system. Presented at the 2018 IEEE 6th region 10 humanitarian technology conference (R10-HTC), IEEE, Malambe, Sri Lanka, pp. 1–6. doi: 10.1109/R10-HTC.2018.8629819
Marchand, M., and Gin, K. (2022). The cardiovascular system in heat stroke. CJC Open 4, 158–163. doi: 10.1016/j.cjco.2021.10.002
Markandya, A., Sampedro, J., Smith, S. J., Van Dingenen, R., Pizarro-Irizar, C., Arto, I., et al. (2018). Health co-benefits from air pollution and mitigation costs of the Paris agreement: a modelling study. Lancet Planet. Health 2, e126–e133. doi: 10.1016/S2542-5196(18)30029-9
Marracino, L., Punzo, A., Severi, P., Nganwouo Tchoutang, R., Vargas-De-la-Cruz, C., Fortini, F., et al. (2022). Fermentation of Vaccinium floribundum berries with Lactiplantibacillus plantarum reduces oxidative stress in endothelial cells and modulates macrophages function. Nutrients 14:1560. doi: 10.3390/nu14081560
Masselot, P., Mistry, M. N., Rao, S., Huber, V., Monteiro, A., Samoli, E., et al. (2025). Estimating future heat-related and cold-related mortality under climate change, demographic and adaptation scenarios in 854 European cities. Nat. Med. 31, 1294–1302. doi: 10.1038/s41591-024-03452-2
Mastrandrea, M., Abdrabo, M., Adger, W., Federation, Y., Anisimov, O., Arent, D., et al. (2014). Climate Change 2014: Impacts, adaptation, and vulnerability—IPCC WGII AR5 summary for policymakers. Geneva, Switzerland: IPCC.
McKenzie, R. L., Aucamp, P. J., Bais, A. F., Björn, L. O., Ilyas, M., and Madronich, S. (2011). Ozone depletion and climate change: impacts on UV radiation. Photochem. Photobiol. Sci. 10, 182–198. doi: 10.1039/c0pp90034f
McMichael, A. J. (1993). Global environmental change and human population health: a conceptual and scientific challenge for epidemiology. Int. J. Epidemiol. 22, 1–8. doi: 10.1093/ije/22.1.1
McMurray, J. J. V., Solomon, S. D., Inzucchi, S. E., Køber, L., Kosiborod, M. N., Martinez, F. A., et al. (2019). Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381, 1995–2008. doi: 10.1056/NEJMoa1911303
Medina, D. C., Delgado, M. G., Amores, T. R. P., Toulou, A., Ramos, J. S., and Domínguez, S. Á. (2022). Climatic control of urban spaces using natural cooling techniques to achieve outdoor thermal comfort. Sustainability 14:14173. doi: 10.3390/su142114173
Medina-Ramón, M., and Schwartz, J. (2007). Temperature, temperature extremes, and mortality: a study of acclimatisation and effect modification in 50 US cities. Occup. Environ. Med. 64, 827–833. doi: 10.1136/oem.2007.033175
Melchior, M., Caspi, A., Howard, L. M., Ambler, A. P., Bolton, H., Mountain, N., et al. (2009). Mental health context of food insecurity: a representative cohort of families with Young children. Pediatrics 124, e564–e572. doi: 10.1542/peds.2009-0583
Miah, S., Dunford, C., Edison, M., Eldred-Evans, D., Gan, C., Shah, T. T., et al. (2019). A prospective clinical, cost and environmental analysis of a clinician-led virtual urology clinic. Ann. R. Coll. Surg. Engl. 101, 30–34. doi: 10.1308/rcsann.2018.0151
Michelozzi, P., de’ Donato, F. K., Bargagli, A. M., D’Ippoliti, D., De Sario, M., Marino, C., et al. (2010). Surveillance of summer mortality and preparedness to reduce the health impact of heat waves in Italy. Int. J. Environ. Res. Public Health 7, 2256–2273. doi: 10.3390/ijerph7052256
Mol, N., Priya, A., Singh, A. K., Mago, P., Shalimar,, and Ray, A. K. (2024). Unravelling the impacts of climatic heat events on cardiovascular health in animal models. Environ. Res. 248:118315. doi: 10.1016/j.envres.2024.118315
Momose, H., Takasaka, M., Watanabe-Asaka, T., Hayashi, M., Maejima, D., Kawai, Y., et al. (2023). Heatstroke risk informing system using wearable perspiration ratemeter on users undergoing physical exercise. Sci. Rep. 13:416. doi: 10.1038/s41598-023-27492-9
Moreno Acosta, O. D., Boan, A. F., Hattori, R. S., and Fernandino, J. I. (2023). Notch pathway is required for protection against heat stress in spermatogonial stem cells in medaka. Fish Physiol. Biochem. 49, 487–500. doi: 10.1007/s10695-023-01200-w
Morgado, M. E., Jiang, C., Zambrana, J., Upperman, C. R., Mitchell, C., Boyle, M., et al. (2021). Climate change, extreme events, and increased risk of salmonellosis: foodborne diseases active surveillance network (FoodNet), 2004-2014. Environ. Health 20:105. doi: 10.1186/s12940-021-00787-y
Myers, S. S., Smith, M. R., Guth, S., Golden, C. D., Vaitla, B., Mueller, N. D., et al. (2017). Climate change and global food systems: potential impacts on food security and undernutrition. Annu. Rev. Public Health 38, 259–277. doi: 10.1146/annurev-publhealth-031816-044356
Ndefo, U. A., Anidiobi, N. O., Basheer, E., and Eaton, A. T. (2015). Empagliflozin (Jardiance): a novel SGLT2 inhibitor for the treatment of type-2 diabetes. P T 40, 364–368.
Ngcamu, B. S. (2023). Climate change effects on vulnerable populations in the global south: a systematic review. Nat. Hazards 118, 977–991. doi: 10.1007/s11069-023-06070-2
Nomura, T., Nagao, K., Shirai, R., Gotoh, H., Umeda, M., and Ono, K. (2022). Temperature sensitivity of notch signaling underlies species-specific developmental plasticity and robustness in amniote brains. Nat. Commun. 13:96. doi: 10.1038/s41467-021-27707-5
Noormohammadi, M., Eini-Zinab, H., Rezazadeh, A., Omidvar, N., and Sobhani, S. R. (2022). A step toward a sustainable diet by reducing carbon footprint: a case study in Iran. J. Environ. Health Sustain. Dev. 7, 1583–1593. doi: 10.18502/jehsd.v7i1.8963
Nystoriak, M. A., and Bhatnagar, A. (2018). Cardiovascular effects and benefits of exercise. Front Cardiovasc. Med. 5:135. doi: 10.3389/fcvm.2018.00135
Okaka, F. O., and Odhiambo, B. D. O. (2018). Relationship between flooding and out break of infectious diseases in Kenya: a review of the literature. J. Environ. Public Health 2018, 1–8. doi: 10.1155/2018/5452938
Ortiz-Prado, E., Simbaña-Rivera, K., Cevallos, G., Gómez-Barreno, L., Cevallos, D., Lister, A., et al. (2022). Waterborne diseases and ethnic-related disparities: a 10 years nationwide mortality and burden of disease analysis from Ecuador. Front. Public Health 10:1029375. doi: 10.3389/fpubh.2022.1029375
Paaijmans, K. P., Blanford, S., Bell, A. S., Blanford, J. I., Read, A. F., and Thomas, M. B. (2010). Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA 107, 15135–15139. doi: 10.1073/pnas.1006422107
Packer, M., Anker, S. D., Butler, J., Filippatos, G., Ferreira, J. P., Pocock, S. J., et al. (2021). Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-reduced trial. J. Am. Coll. Cardiol. 77, 1381–1392. doi: 10.1016/j.jacc.2021.01.033
Padmanabha, H., Soto, E., Mosquera, M., Lord, C. C., and Lounibos, L. P. (2010). Ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. EcoHealth 7, 78–90. doi: 10.1007/s10393-010-0301-6
Pan, S., Gan, L., Jung, J., Yu, W., Roy, A., Diao, L., et al. (2023). Quantifying the premature mortality and economic loss from wildfire-induced PM2.5 in the contiguous U.S. Sci. Total Environ. 875:162614. doi: 10.1016/j.scitotenv.2023.162614
Parker, E. R. (2021). The influence of climate change on skin cancer incidence – a review of the evidence. Int J Womens Dermatol 7, 17–27. doi: 10.1016/j.ijwd.2020.07.003
Parupathi, P., Campanelli, G., Deabel, R. A., Puaar, A., Devarakonda, L. S., Kumar, A., et al. (2022). Gnetin C intercepts MTA1-associated neoplastic progression in prostate cancer. Cancers (Basel) 14:6038. doi: 10.3390/cancers14246038
Patel, K. B., Gonzalez, B. D., Turner, K., Alishahi Tabriz, A., Rollison, D. E., Robinson, E., et al. (2023). Estimated carbon emissions savings with shifts from in-person visits to telemedicine for patients with cancer. JAMA Netw. Open 6:e2253788. doi: 10.1001/jamanetworkopen.2022.53788
Pearce, S. C., Mani, V., Boddicker, R. L., Johnson, J. S., Weber, T. E., Ross, J. W., et al. (2013). Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 8:e70215. doi: 10.1371/journal.pone.0070215
Peng, N., Geng, Y., Ouyang, J., Liu, S., Yuan, F., Wan, Y., et al. (2023). Endothelial glycocalyx injury is involved in heatstroke-associated coagulopathy and protected by N-acetylcysteine. Front. Immunol. 14:1159195. doi: 10.3389/fimmu.2023.1159195
Pichler, P.-P., Jaccard, I. S., Weisz, U., and Weisz, H. (2019). International comparison of health care carbon footprints. Environ. Res. Lett. 14:064004. doi: 10.1088/1748-9326/ab19e1
Pickup, J. C. (2015). Banting memorial lecture 2014* technology and diabetes care: appropriate and personalized. Diabet. Med. 32, 3–13. doi: 10.1111/dme.12613
Plaza, M. P., Kolek, F., Leier-Wirtz, V., Brunner, J. O., Traidl-Hoffmann, C., and Damialis, A. (2022). Detecting airborne pollen using an automatic, real-time monitoring system: evidence from two sites. Int. J. Environ. Res. Public Health 19:2471. doi: 10.3390/ijerph19042471
Psistaki, K., Dokas, I. M., and Paschalidou, A. K. (2023). Analysis of the heat-and cold-related cardiovascular mortality in an urban Mediterranean environment through various thermal indices. Environ. Res. 216:114831. doi: 10.1016/j.envres.2022.114831
Purohit, A., Smith, J., and Hibble, A. (2021). Does telemedicine reduce the carbon footprint of healthcare? A systematic review. Future Healthc. J. 8, e85–e91. doi: 10.7861/fhj.2020-0080
Quick, M. (2024). The impacts of extreme heat events on non-accidental, cardiovascular, and respiratory mortality: an analysis of 12 Canadian cities from 2000 to 2020. Health Rep. 35, 3–15. doi: 10.25318/82-003-x202400600001-eng
Raju, A., Pimple, P., Stafkey-Mailey, D., Farrelly, E., and Shetty, S. (2022). Healthcare costs and resource utilization associated with the use of Empagliflozin versus other Antihyperglycemic agents among patients with type 2 diabetes mellitus and cardiovascular disease: a real-world retrospective cohort analysis. Diabetes Ther 13, 25–42. doi: 10.1007/s13300-021-01173-0
Rantanen, M., Karpechko, A. Y., Lipponen, A., Nordling, K., Hyvärinen, O., Ruosteenoja, K., et al. (2022). The arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 3:168. doi: 10.1038/s43247-022-00498-3
Reis, G., Dos Santos Moreira-Silva, E. A., Silva, D. C. M., Thabane, L., Milagres, A. C., Ferreira, T. S., et al. (2022). Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob. Health 10, e42–e51. doi: 10.1016/S2214-109X(21)00448-4
Reyburn, R., Kim, D. R., Emch, M., Khatib, A., von Seidlein, L., and Ali, M. (2011). Climate variability and the outbreaks of cholera in Zanzibar, East Africa: a time series analysis. Am. J. Trop. Med. Hyg. 84, 862–869. doi: 10.4269/ajtmh.2011.10-0277
Riggs, D. W., Cobbold, S., Sears, C. G., Malovichko, M., Sithu, I., Sansbury, B. E., et al. (2024). Associations between short-term outdoor heat measures and markers of immune response and inflammation. Circulation 149. doi: 10.1161/circ.149.suppl_1.P297
Robin, C., Beck, C., Armstrong, B., Waite, T. D., and Rubin, G. J.English National Study of Flooding and Health Study Group, et al. (2020). Impact of flooding on health-related quality of life in England: results from the National Study of flooding and health. Eur. J. Pub. Health 30, 942–948. doi: 10.1093/eurpub/ckaa049
Robinson, J. D., Prochaska, J. D., and Yngve, D. A. (2017). Pre-surgery evaluations by telephone decrease travel and cost for families of children with cerebral palsy. SAGE Open Med. 5:2050312117720046. doi: 10.1177/2050312117720046
Romanello, M., Di Napoli, C., Drummond, P., Green, C., Kennard, H., Lampard, P., et al. (2022). The 2022 report of the lancet countdown on health and climate change: health at the mercy of fossil fuels. Lancet 400, 1619–1654. doi: 10.1016/S0140-6736(22)01540-9
Salvador, C., Gullón, P., Franco, M., and Vicedo-Cabrera, A. M. (2023). Heat-related first cardiovascular event incidence in the city of Madrid (Spain): vulnerability assessment by demographic, socioeconomic, and health indicators. Environ. Res. 226:115698. doi: 10.1016/j.envres.2023.115698
Samuel, G., and Lucassen, A. M. (2023). The environmental impact of data-driven precision medicine initiatives. Camb. Prisms Precis. Med. 1:e1. doi: 10.1017/pcm.2022.1
Saucy, A., Ragettli, M. S., Vienneau, D., de Hoogh, K., Tangermann, L., Schäffer, B., et al. (2021). The role of extreme temperature in cause-specific acute cardiovascular mortality in Switzerland: a case-crossover study. Sci. Total Environ. 790:147958. doi: 10.1016/j.scitotenv.2021.147958
Schechter, M., Wiviott, S. D., Raz, I., Goodrich, E. L., Rozenberg, A., Yanuv, I., et al. (2023). Effects of dapagliflozin on hospitalisations in people with type 2 diabetes: post-hoc analyses of the DECLARE-TIMI 58 trial. Lancet Diabetes Endocrinol. 11, 233–241. doi: 10.1016/S2213-8587(23)00009-8
Schilcher, A. V., Roth, M., Steindor, F. A., Helweh, R., and Geerling, G. (2024). “Dirty dry eye” – a waste volume analysis from topical therapy in keratoconjunctivitis sicca. Graefes Arch. Clin. Exp. Ophthalmol. 262, 2917–2924. doi: 10.1007/s00417-024-06431-y
Schwartz, R. M., Gillezeau, C. N., Liu, B., Lieberman-Cribbin, W., and Taioli, E. (2017). Longitudinal Impact of Hurricane Sandy Exposure on Mental Health Symptoms. Int J Environ Res Public Health. 14, 957. doi: 10.3390/ijerph14090957
Severi, P., Ascierto, A., Marracino, L., Ouambo Talla, A. W., Aquila, G., Martino, V., et al. (2024). 17β-estradiol inhibits Notch1 activation in murine macrophage cell line RAW 264.7. Mol. Biol. Rep. 51:1134. doi: 10.1007/s11033-024-10058-x
Shih, Y.-J., Chen, J.-S., Chen, Y.-J., Yang, P.-Y., Kuo, Y.-J., Chen, T.-H., et al. (2021). Impact of heavy precipitation events on pathogen occurrence in estuarine areas of the Puzi River in Taiwan. PLoS One 16:e0256266. doi: 10.1371/journal.pone.0256266
Shrestha, P., Nukala, S. K., Islam, F., Badgery-Parker, T., and Foo, F. (2024). The co-benefits of climate change mitigation strategies on cardiovascular health: a systematic review. Lancet Regional Health Western Pacific 48:101098. doi: 10.1016/j.lanwpc.2024.101098
Silveira, S., Kornbluh, M., Withers, M. C., Grennan, G., Ramanathan, V., and Mishra, J. (2021). Chronic mental health sequelae of climate Change extremes: a case study of the deadliest Californian wildfire. Int. J. Environ. Res. Public Health 18:1487. doi: 10.3390/ijerph18041487
Sindall, R., Mecrow, T., Queiroga, A. C., Boyer, C., Koon, W., and Peden, A. E. (2022). Drowning risk and climate change: a state-of-the-art review. Inj. Prev. 28, 185–191. doi: 10.1136/injuryprev-2021-044486
Singh, N., Areal, A. T., Breitner, S., Zhang, S., Agewall, S., Schikowski, T., et al. (2024). Heat and cardiovascular mortality: An epidemiological perspective. Circ. Res. 134, 1098–1112. doi: 10.1161/CIRCRESAHA.123.323615
Siwak, A. M., Baker, P. G., and Dube, A. (2023). Biosensors as early warning detection systems for waterborne Cryptosporidium. Water Sci. Technol. 88, 615–630. doi: 10.2166/wst.2023.229
Snipe, R. M. J., Khoo, A., Kitic, C. M., Gibson, P. R., and Costa, R. J. S. (2018). The impact of exertional-heat stress on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profile. Eur. J. Appl. Physiol. 118, 389–400. doi: 10.1007/s00421-017-3781-z
Springmann, M., Mason-D’Croz, D., Robinson, S., Garnett, T., Godfray, H. C. J., Gollin, D., et al. (2016). Global and regional health effects of future food production under climate change: a modelling study. Lancet 387, 1937–1946. doi: 10.1016/S0140-6736(15)01156-3
Sun, H., Zhang, L.-H., Wang, J.-H., Chen, R., Liu, Y., Zhang, P.-C., et al. (2025). Lonicerin regulates AMPK/SIRT1/autophagy pathway to attenuate heat stress intestinal injury and inhibit inflammation. Int. Immunopharmacol. 154:114549. doi: 10.1016/j.intimp.2025.114549
Swen, J. J., Nijenhuis, M., de Boer, A., Grandia, L., Maitland-van der Zee, A. H., Mulder, H., et al. (2011). Pharmacogenetics: from bench to byte--an update of guidelines. Clin. Pharmacol. Ther. 89, 662–673. doi: 10.1038/clpt.2011.34
Swinburn, B. A., Kraak, V. I., Allender, S., Atkins, V. J., Baker, P. I., Bogard, J. R., et al. (2019). The global Syndemic of obesity, undernutrition, and climate Change: the lancet commission report. Lancet 393, 791–846. doi: 10.1016/S0140-6736(18)32822-8
Szczawińska, M. E., Szczawiński, J., and Łobacz, A. (2014). Effect of temperature on the growth kinetics of Salmonella Enteritidis in cooked ham. Bull. Vet. Inst. Pulawy 58, 47–56. doi: 10.2478/bvip-2014-0008
Talley, S., Kalinina, O., Winek, M., Paik, W., Cannon, A. R., Alonzo, F., et al. (2019). A caspase-1 biosensor to monitor the progression of inflammation in vivo. J. Immunol. 203, 2497–2507. doi: 10.4049/jimmunol.1900619
Tao, Z., Cheng, M., Wang, S.-C., Lv, W., Hu, H.-Q., Li, C.-F., et al. (2015). JAK2/STAT3 pathway mediating inflammatory responses in heatstroke-induced rats. Int. J. Clin. Exp. Pathol. 8, 6732–6739.
Tennison, I., Roschnik, S., Ashby, B., Boyd, R., Hamilton, I., Oreszczyn, T., et al. (2021). Health care’s response to climate change: a carbon footprint assessment of the NHS in England. Lancet Planet Health 5, e84–e92. doi: 10.1016/S2542-5196(20)30271-0
Thomas, S., Ravishankaran, S., Justin, N. A. J. A., Asokan, A., Kalsingh, T. M. J., Mathai, M. T., et al. (2018). Microclimate variables of the ambient environment deliver the actual estimates of the extrinsic incubation period of plasmodium vivax and plasmodium falciparum: a study from a malaria-endemic urban setting, Chennai in India. Malar. J. 17:201. doi: 10.1186/s12936-018-2342-1
Thomson, M. C., and Stanberry, L. R. (2022). Climate change and vectorborne diseases. N. Engl. J. Med. 387, 1969–1978. doi: 10.1056/NEJMra2200092
Tong, S., Bambrick, H., Beggs, P. J., Chen, L., Hu, Y., Ma, W., et al. (2022). Current and future threats to human health in the Anthropocene. Environ. Int. 158:106892. doi: 10.1016/j.envint.2021.106892
Velez, K. E. C., Leighton, R. E., Decho, A. W., Pinckney, J. L., and Norman, R. S. (2023). Modeling pH and temperature effects as climatic hazards in Vibrio Vulnificus and Vibrio Parahaemolyticus planktonic growth and biofilm formation. Geohealth 7:e2022GH000769. doi: 10.1029/2022GH000769
Vezzulli, L., Brettar, I., Pezzati, E., Reid, P. C., Colwell, R. R., Höfle, M. G., et al. (2012). Long-term effects of ocean warming on the prokaryotic community: evidence from the vibrios. ISME J. 6, 21–30. doi: 10.1038/ismej.2011.89
Vicedo-Cabrera, A. M., Scovronick, N., Sera, F., Royé, D., Schneider, R., Tobias, A., et al. (2021). The burden of heat-related mortality attributable to recent human-induced climate change. Nat. Clim. Chang. 11, 492–500. doi: 10.1038/s41558-021-01058-x
Vieceli Dalla Sega, F., Cimaglia, P., Manfrini, M., Fortini, F., Marracino, L., Bernucci, D., et al. (2022). Circulating biomarkers of endothelial dysfunction and inflammation in predicting clinical outcomes in diabetic patients with critical limb ischemia. Int. J. Mol. Sci. 23:10641. doi: 10.3390/ijms231810641
Vieceli Dalla Sega, F., Fortini, F., Licastro, D., Monego, S. D., Degasperi, M., Ascierto, A., et al. (2024). Serum from COVID-19 patients promotes endothelial cell dysfunction through protease-activated receptor 2. Inflamm. Res. 73, 117–130. doi: 10.1007/s00011-023-01823-y
Voors, A. A., Angermann, C. E., Teerlink, J. R., Collins, S. P., Kosiborod, M., Biegus, J., et al. (2022). The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat. Med. 28, 568–574. doi: 10.1038/s41591-021-01659-1
Wang, R., Yang, Y., Xing, X., Wang, L., Chen, J., Tang, X., et al. (2022). Stringent emission controls are needed to reach clean air targets for cities in China under a warming climate. Environ. Sci. Technol. 56, 11199–11211. doi: 10.1021/acs.est.1c08403
Watts, N., Adger, W. N., Agnolucci, P., Blackstock, J., Byass, P., Cai, W., et al. (2015). Health and climate change: policy responses to protect public health. Lancet 386, 1861–1914. doi: 10.1016/S0140-6736(15)60854-6
Wen, J., Lin, Z., Cheng, J., Li, C., Wang, L., Zou, Y., et al. (2024). Heat acclimation alleviates the heat stress-induced impairment of vascular endothelial cells. Tissue Cell 90:102520. doi: 10.1016/j.tice.2024.102520
Wong, Y. L., Wong, S. W., Ting, D. S. J., Muralidhar, A., Sen, S., Schaff, O., et al. (2024). Impacts of climate change on ocular health: a scoping review. J. Climate Change Health 15:100296. doi: 10.1016/j.joclim.2023.100296
Wu, Y., Gasevic, D., Wen, B., Yang, Z., Yu, P., Zhou, G., et al. (2024a). Floods and cause-specific mortality in the UK: a nested case-control study. BMC Med. 22:188. doi: 10.1186/s12916-024-03412-0
Wu, F., Liu, Q., Lu, L., Wang, J., Song, X., and Ren, D. (2011). Distribution of Aedes albopictus (Diptera: Culicidae) in northwestern China. Vector Borne Zoonotic Dis. 11, 1181–1186. doi: 10.1089/vbz.2010.0032
Wu, Y., Wen, B., Gasevic, D., Patz, J. A., Haines, A., Ebi, K. L., et al. (2024b). Climate Change, floods, and human health. N. Engl. J. Med. 391, 1949–1958. doi: 10.1056/NEJMsr2402457
Xu, R., Ye, T., Yue, X., Yang, Z., Yu, W., Zhang, Y., et al. (2023). Global population exposure to landscape fire air pollution from 2000 to 2019. Nature 621, 521–529. doi: 10.1038/s41586-023-06398-6
Xu, R., Zhao, Q., Coelho, M. S. Z. S., Saldiva, P. H. N., Abramson, M. J., Li, S., et al. (2019). The association between heat exposure and hospitalization for undernutrition in Brazil during 2000-2015: a nationwide case-crossover study. PLoS Med. 16:e1002950. doi: 10.1371/journal.pmed.1002950
Yamanouchi, T., and Takata, K. (2020). Rapid change of the Arctic climate system and its global influences – overview of GRENE Arctic climate change research project (2011–2016). Pol. Sci. 25:100548. doi: 10.1016/j.polar.2020.100548
Yang, Z., Huang, W., McKenzie, J. E., Xu, R., Yu, P., Ye, T., et al. (2023). Mortality risks associated with floods in 761 communities worldwide: time series study. BMJ 383:e075081. doi: 10.1136/bmj-2023-075081
Yu, I., Park, K., and Lee, E. H. (2021). Flood risk analysis by building use in urban planning for disaster risk reduction and climate change adaptation. Sustainability 13:13006. doi: 10.3390/su132313006
Yuan, F., Cai, J., Wu, J., Tang, Y., Zhao, K., Liang, F., et al. (2022). Z-DNA binding protein 1 promotes heatstroke-induced cell death. Science 376, 609–615. doi: 10.1126/science.abg5251
Yusuf, H., Gupta, V., Osaghae, I., and Kumar, A. (2024). Longitudinal impact of screening colonoscopy on greenhouse gas emissions. PLoS One 19:e0307133. doi: 10.1371/journal.pone.0307133
Zakiyyah, S. N., Ibrahim, A. U., Babiker, M. S., Gaffar, S., Ozsoz, M., Zein, M. I. H. L., et al. (2022). Detection of tropical diseases caused by mosquitoes using CRISPR-based biosensors. Trop. Med. Infect Dis. 7:309. doi: 10.3390/tropicalmed7100309
Zan, G.-X., Qin, Y.-C., Xie, W.-W., Gao, C.-Q., Yan, H.-C., Wang, X.-Q., et al. (2023). Heat stress disrupts intestinal stem cell migration and differentiation along the crypt-villus axis through FAK signaling. Biochim. Biophys. Acta, Mol. Cell Res. 1870:119431. doi: 10.1016/j.bbamcr.2023.119431
Zhang, S., Rai, M., Matthies-Wiesler, F., Breitner-Busch, S., Stafoggia, M., de’Donato, F., et al. (2022). Climate change and cardiovascular disease–the impact of heat and heat-health action plans. Eur. Soc. Cardiol. 22:18.
Zinman, B., Wanner, C., Lachin, J. M., Fitchett, D., Bluhmki, E., Hantel, S., et al. (2015). Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128. doi: 10.1056/NEJMoa1504720
Keywords: climate change, cardiovascular disease, adaptation, mitigation, human health
Citation: Ouambo Talla AW, Ascierto A, Sanvido A, Severi P, Trabuio A, Ceccolini F, Fortini F, Vieceli Dalla Sega F, Levenson AS, Vaccarezza M, Pasti L and Rizzo P (2025) The heat, the heart and beyond: a narrative review of the many ways climate change impacts human health. Front. Clim. 7:1647942. doi: 10.3389/fclim.2025.1647942
Edited by:
Adugna Woyessa, Ethiopian Public Health Institute, EthiopiaReviewed by:
Andrea Mirkovic, University of Kragujevac, SerbiaAshtyn Tracey Areal, Institute for Environmental Medicine, Karolinska Institute Solna, Sweden
Copyright © 2025 Ouambo Talla, Ascierto, Sanvido, Severi, Trabuio, Ceccolini, Fortini, Vieceli Dalla Sega, Levenson, Vaccarezza, Pasti and Rizzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Rizzo, cnp6cGxhQHVuaWZlLml0
†These authors have contributed equally to this work and share first authorship
 Achille Wilfred Ouambo Talla1†
Achille Wilfred Ouambo Talla1† Alessia Ascierto
Alessia Ascierto Paolo Severi
Paolo Severi Francesco Vieceli Dalla Sega
Francesco Vieceli Dalla Sega Anait S. Levenson
Anait S. Levenson Mauro Vaccarezza
Mauro Vaccarezza Paola Rizzo
Paola Rizzo