- 1Department of Genetic Medicine, Johns Hopkins School of Medicine, Baltimore, MD, United States
- 2Division of Geriatric Medicine and Gerontology, Johns Hopkins School of Medicine, Baltimore, MD, United States
- 3The Richman Family Precision Medicine Center of Excellence in Alzheimer’s Disease, Johns Hopkins School of Medicine, Baltimore, MD, United States
- 4Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine and Johns Hopkins Bayview Medical Center, Baltimore, MD, United States
Alzheimer’s disease (AD) is characterized by a long preclinical phase lasting more than a decade before the onset of its clinical phase of mild cognitive impairment (MCI) or dementia. Recent advances in psychedelic research underscore numerous neuroplastogenic and anti-inflammatory alterations induced by these compounds, making them promising therapeutic candidates for AD. In this mini review, we will briefly summarize the existing literature using human cerebral organoids to study the molecular and metabolic changes caused by various psychedelic compounds, focusing on their potential therapeutic applications for AD.
1 Introduction
Alzheimer’s disease (AD) is characterized by a long preclinical phase lasting more than a decade before the onset of its clinical phase of mild cognitive impairment (MCI) or dementia (Sperling et al., 2011; Bateman et al., 2012; Dubois et al., 2016). Its pathogenesis involves a cascade of interconnected biochemical and cellular changes, including the accumulation of beta-amyloid (Aβ) fibrils in extracellular plaques and the hyperphosphorylation of Tau in intracellular tangles. These alterations lead to neuroinflammation, synaptic dysfunction, neuronal degeneration, and ultimately to cognitive and functional decline (De Strooper and Karran, 2016). Although genetics plays a significant role (Sims et al., 2020), the precise causes and sequence of these pathological events are not yet completely understood, which can potentially explain the recurring therapeutic failures in AD research. Since treatments may be most effective in the early stages of the disease, it is crucial to identify AD in presymptomatic individuals and those experiencing cognitive decline before reaching the clinically defined MCI or dementia.
In the hippocampus, prefrontal cortex, and other areas of the brain that are susceptible to AD pathology, 5-HT2A receptors are highly present (Bryson et al., 2017). Psychedelic drugs, including d-lysergic acid diethylamide (LSD), psilocybin, 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), and DMT are potent serotonergic agonists with affinity to 5-hydroxytryptamine receptors (5-HTRs) (Nichols, 2016). Recent advances in psychedelic research underscore the numerous connections between these compounds and cognitive/affective alterations observed in older adults (Aday et al., 2020), making them appealing therapeutic candidates for AD.
Studies in rodents suggest that psychedelics support neurogenesis, neuroplasticity, and neuronal maturation by enhancing the development of neurites, dendritic spines, and synapses in neural progenitor cells, particularly where 5-HT2A receptors are highly expressed (Lima da Cruz et al., 2018; Morales-Garcia et al., 2020; Ly et al., 2018). These studies have revealed many cellular and molecular mechanisms of these drugs; however, expanding these findings to a human-relevant model is critical for evaluating the therapeutic potential of psychedelics.
Given the many differences between human and rodent brains (Xu et al., 2022), it has been challenging to model human brain physiology (particularly that of the hippocampus) in the laboratory. Laboratory-synthesized, human-derived 3D brain organoids represent an in vitro system that effectively models many aspects of the molecular architecture of the human brain (Chen et al., 2019; Porci’uncula et al., 2021). In this mini review we will briefly summarize the existing literature using cerebral organoids to study the molecular and metabolic alterations caused by various psychedelic compounds, focusing on their potential therapeutic applications for AD.
2 5-MeO-DMT effects
5-MeO-DMT is a short-acting psychedelic tryptamine that acts as a serotonin receptor agonist with affinity for other receptors, as well as serotonin and norepinephrine transporters (Ermakova et al., 2022).
5-MeO-DMT has been shown to induce anti-inflammatory pathways and other proteomic alterations in 45-day old human embryonic stem cell-derived cerebral organoids. Following exposure to 5-MeO-DMT for 24-h, shot-gun mass spectrometry (MS) revealed widespread changes in protein expression within toll-like receptor- and Gq-coupled receptor-mediated signaling cascades, ultimately leading to downregulation of transcriptional regulators of inflammatory cytokines, NFAT and NF-κβ (Dakic et al., 2017). Other downregulated proteins included srGAP, which is critical for the processes underpinning synaptic plasticity and higher cognitive functions (Dakic et al., 2017; Nguyen Thi Thanh et al., 2018), and mGluR5, which contributes to the rewarding effects of various drugs of abuse; thus, supporting the hypothesis that psychedelics carry a low risk of addiction (Johnson et al., 2018).
5-MeO-DMT treatment upregulated the expression of NMDAR, CaMK2, and CREB—signaling molecules involved in long-term potentiation, learning, and memory (Dakic et al., 2017; Caya-Bissonnette and B’eique, 2024). 5-MeO-DMT significantly increased EFNB2, EPHB, intersectin, ELMO1, CDC42, RAC1, and integrins, which promote dendritic spine development. Finally, 5-MeO-DMT was shown to suppress cell death-related pathways upon activation of σ1-RS, which induces neuroprotection by modulating intracellular calcium levels and inhibiting the expression of pro-apoptotic genes (Figure 1) (Mueller et al., 2013).
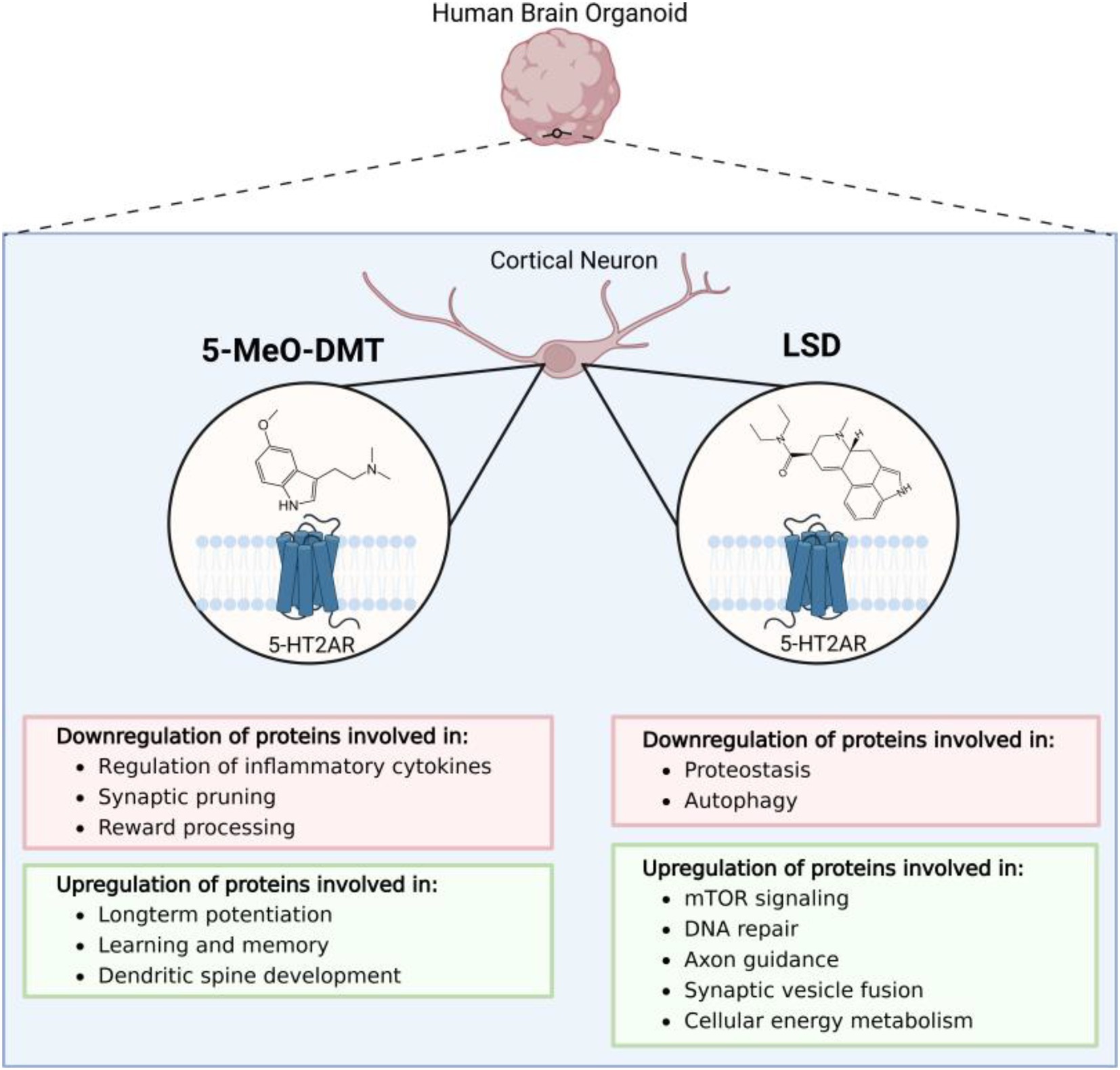
Figure 1. An overview of the state of the current literature evaluating the effects of psychedelic compounds in human cell-derived 3D brain organoids. Presently, only proteomics studies evaluating the effects of d-lysergic acid diethylamide (LSD) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) have been applied to cerebral organoid models.
3 LSD effects
LSD is another psychedelic that predominantly exerts its effects through 5-HT2A receptor antagonism, but also binds to dopaminergic, adrenergic, and other serotonergic receptors (De Gregorio et al., 2021). Furthermore, LSD allosterically binds tropomyosin receptor kinase B (TrkB), promoting its interaction with brain-derived neurotrophic factor (BDNF); a key molecule that mediates plastic changes underlying learning and memory (Figure 1) (Moliner et al., 2023).
The impact of LSD on neural plasticity-related pathways has been evaluated using 45-day-old human-induced pluripotent stem cell (hiPSC)-derived cerebral organoids (Ornelas et al., 2022). After treatment with 10 nM LSD for 24-h, liquid-chromatography (LC)-MS revealed proteomic alterations involved in common cellular processes including DNA replication, mTOR signaling cascades, and dopamine neurotransmitter release cycle. Specifically, mTOR was significantly upregulated, which may promote psychedelic-induced structural plasticity in the prefrontal cortex (Ly et al., 2018). Other affected pathways reflect neuroplasticity and synaptic reorganization processes, particularly axon guidance, synaptic vesicle cycle, and long-term depression (Ornelas et al., 2022). In fact, there is evidence that neuronal plasticity is stimulated by LSD through both 5-HT2A and mTOR signaling (Ly et al., 2018).
In a subsequent study (Costa et al., 2024), hiPSC-derived 45-day old human cortical organoids were then exposed to 100 nM LSD for 24 h. LC/MS-MS-based shotgun proteomic analysis revealed a significant shift in the abundance of multiple proteins that modify processes involved in proteostasis, energy metabolism, and neuroplasticity. Most proteostasis proteins were downregulated, possibly prolonging the lifespan of synaptic proteins by slowing turnover rates, although it is unclear whether LSD regulates proteostasis directly or through indirect homeostatic effects. In addition, LSD altered the abundance of proteins associated with glycolysis, the TCA cycle and oxidative phosphorylation, and also increased lactate production, implying that psychedelics could trigger metabolic alterations to meet the high demands of neuroplasticity (Watts et al., 2018). LSD exposure led to upregulation of synaptic vesicle fusion proteins, suggesting an effect on neuroplasticity and neurotransmitter release.
4 Discussion
Preliminary research has highlighted the antidepressant, anxiolytic, and anti-addictive features of classic psychedelics (Watts et al., 2018). Furthermore, preclinical and neuroimaging studies point to a variety of biological mechanisms of action of psychedelics, including structural and functional enhancement of neuroplasticity (Lima da Cruz et al., 2018; Ly et al., 2018), anti-inflammatory properties (Flanagan and Nichols, 2018), shifts in critical signaling pathways (i.e., BDNF) (Ly et al., 2018; Hutten et al., 2020), and modifications of functional neural connectivity (Carhart-Harris et al., 2017; Barrett et al., 2020; Preller et al., 2020). The key pathophysiological processes of AD include decreased functional brain activity and connectivity (Dennis and Thompson, 2014), reduced serotonergic neurotransmission (Smith et al., 2017; Mecca, 2019) associated with neuropsychiatric symptoms (Butzlaff and Ponimaskin, 2016; Chakraborty et al., 2019), neuroinflammation, (Kinney et al., 2018) and alteration of key signaling pathways (i.e., BDNF) (Peng et al., 2005; Tanila, 2017). Therefore, for many patients with AD, classic psychedelics may offer therapeutic advantages that merit further investigation.
Although two-dimensional (2D) iPSC-derived neuronal cultures are valuable tools to simulate cellular responses, only one study has focused on the neuroprotective impact of the endogenous hallucinogen N,N-dimethyltryptamine (DMT) on human cortical neurons derived from iPSCs, monocyte-derived macrophages, and dendritic cells (Szabo et al., 2016). Studies investigating the effects of psychedelics in iPSC-derived neurons are exceedingly rare and, to our knowledge, have not yet been conducted in AD patient-derived models. Furthermore, we are not aware of any data that exist from post-mortem AD brain tissue studies. In light of these gaps, iPSC-derived organoids offer a valuable, sophisticated system that closely mimics the spatial architecture of the human brain and incorporates complex cell-to-cell interactions, which may influence drug responses (Salerno and Rehen, 2024).
Human brain organoids provide a unique in vitro imitation of physiologically relevant complex functions and processes of the human brain (Logan et al., 2019; Whiteley et al., 2022), and thus provide valuable data connecting preclinical and clinical studies. Importantly, organoids can be grown using patient-derived iPSCs to serve as tools for precision medicine approaches. Given the highly psychoactive properties of psychedelic substances and the heterogeneous neurobehavioral reactions they evoke (Moujaes et al., 2023), patient-derived organoids can be used to evaluate personalized treatment strategies that achieve optimal efficacy with minimal adverse effects (Park and Mook-Jung, 2022).
Considering that serotonergic degeneration is observed early in the course of AD (Smith et al., 2023) and that psychedelic compounds mediate their effects primarily through the 5-HT2A receptor, hindbrain serotonergic organoids could offer a unique platform to investigate how psychedelics influence serotonergic pathways in the context of AD pathology (Valiulahi et al., 2021; Zivko et al., 2024). The methodological framework developed for studying the response of serotonergic hindbrain organoids to escitalopram (Zivko et al., 2024), a selective serotonin reuptake inhibitor (SSRI), suggests that this platform can be used to explore the potential of psychedelics to treat AD.
There is growing evidence that late-life depressive symptoms are associated with an increased risk of incident dementia, a concept that has been codified into the construct of ‘mild behavioral impairment’ (Creese and Ismail, 2022). In the data set of the National Alzheimer’s Coordinating Center, the majority of participants who progressed from normal to impaired cognition presented behavioral symptoms prior to cognitive changes (Wise et al., 2019). Thus, the mechanisms underlying late-life depression may also be the basis for neurodegenerative disease. Many of the mechanisms identified by the aforementioned organoid studies are common to depression and AD, including decreased serotonergic innervation, neuroinflammation, and disruptions in crucial signaling pathways, including BDNF (Mendes-Silva et al., 2016; Linnemann and Lang, 2020; Bajaj and Mahesh, 2024). Clinical trials have shown that a single dose of a psychedelic can induce lasting physiological changes across multiple neural pathways without the need for sustained or repeated exposure to maintain these effects (Knudsen, 2023). This is analogous to the observation that a single dose of psilocybin was effective in treating major depression both in the short term (3 weeks) (Goodwin et al., 2022) and in a long-term open-label follow-up over 52 weeks (Goodwin et al., 2025), suggesting that a single psychedelic dose can induce long-term alterations in the human brain.
Psilocybin is another psychedelic that is being evaluated as a treatment for several neurological disorders (Carhart-Harris et al., 2021; Schindler et al., 2021; Daws et al., 2022). Although its application as a medical intervention for AD has been understudied, it has been recognized as a breakthrough therapy for major depressive disorder (Raison et al., 2023; Zheng et al., 2024). Considering the potential of psilocybin to induce neurogenesis, synaptogenesis, and synaptic plasticity (Jefsen et al., 2020; Shao et al., 2021; Raval et al., 2021), it may represent a strong candidate for trials in human brain organoids, with the aim of exploring its potential therapeutic benefit for AD. Furthermore, depression and anxiety are prominent symptoms of AD and can accelerate the progression of the disease (Ma, 2020; Agüera-Ortiz et al., 2021). Therefore, psilocybin may alleviate affective symptoms and even delay the course of the disease.
Despite a growing body of evidence supporting the therapeutic potential of psychedelics across various medical conditions, their use remains controversial due to lingering stigma around historical misuse. Notably, several perceived risks, such as addiction and neurotoxicity, have been refuted by recent research (Schlag et al., 2022). However, other potential risks, such as the exacerbation of delusions or hallucinations following high-dose psychedelic administration, remain a concern, particularly in individuals with advanced AD (Scarmeas et al., 2005). As a result, research into the therapeutic potential of psychedelics is increasingly focused on the earliest stages of the disease (Garcia-Romeu et al., 2022). In this context, a rational approach may involve eliminating the hallucinogenic effects of these compounds while preserving their therapeutic benefits, potentially minimizing adverse side effects (Yin and Gao, 2023). Moreover, to support this effort, advanced model systems such as organoids could offer the potential to understand the mechanisms underlying the heterogeneity of the clinical response, guiding the development of more targeted therapeutic strategies (Zhou et al., 2023; Giorgi et al., 2024; Smirnova and Hartung, 2024).
Altogether, strong evidence suggests that psychedelic drugs mediate plastogenic and anti-inflammatory processes in brain regions involved in AD pathology, which makes them promising cognitive enhancers and prospective therapeutic candidates. Given the high value of human cerebral organoids as tools for conducting preclinical research in a human-relevant environment, more studies in this direction are required to gain a comprehensive understanding of the mechanisms behind the neurorestorative impact of these compounds on the human brain.
Author contributions
XA: Writing – original draft, Writing – review & editing. RB: Visualization, Writing – review & editing. PR: Writing – review & editing. VM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. VM was supported from The Richman Family Precision Medicine Center of Excellence in Alzheimer’s disease at Johns Hopkins and the NIH RF1AG083801 grant. RB was supported by The National Institutes of Health Research (T32AG058527).
Acknowledgments
The authors would also like to acknowledge support by The Richman Family Precision Medicine Center of Excellence in Alzheimer’s disease at Johns Hopkins. Figures were created using BioRender.com.
Conflict of interest
Rosenberg received research grants from the National Institutes of Aging, Alzheimer’s Clinical Trials Consortium, Richman Family Precision Medicine Center of Excellence on Alzheimer’s Disease, Eisai, Functional Neuromodulation, and Lilly; honoraria from Lilly, GLG, Leerink, Medalink, Novo Nordisk, Noble Insights, TwoLabs, Otsuka, MedaCorp, ExpertConnect, HMP Global, Worldwide Clinical Trials, Medscape, and Neurology Week.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aday, J. S., Bloesch, E. K., and Davoli, C. C. (2020). Can psychedelic drugs attenuate age-related changes in cognition and affect? J. Cogn. Enhanc. 4, 219–227. doi: 10.1007/s41465-019-00151-6
Agüera-Ortiz, R., Garc’ıa-Ramos, F. J., Grandas P’erez, J., L’opez-Álvarez, J. M., Montes Rodr’ıguez, F. J., Olazar’an Rodr’ıguez, J., et al. (2021). Depression in Alzheimer’s disease: a delphi consensus on etiology, risk factors, and clinical management. Front. Psych. 12:638651. doi: 10.3389/fpsyt.2021.638651
Bajaj, S., and Mahesh, R. (2024). Converged avenues: depression and Alzheimer’s disease–shared pathophysiology and novel therapeutics. Mol. Biol. Rep. 51:225. doi: 10.1007/s11033-023-09170-1
Barrett, F. S., Doss, M. K., Sepeda, N. D., Pekar, J. J., and Griffiths, R. R. (2020). Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci. Rep. 10:2214. doi: 10.1038/s41598-020-59282-y
Bateman, R. J., Xiong, C., Benzinger, T. L. S., Fagan, A. M., Goate, A., Fox, N. C., et al. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804. doi: 10.1056/NEJMoa1202753
Bryson, O., Carter, T., Norman, R., and Kanaan, R. (2017). 5-HT2A agonists: a novel therapy for functional neurological disorders? Int. J. Neuropsychopharmacol. 20, 422–427. doi: 10.1093/ijnp/pyx011
Butzlaff, M., and Ponimaskin, E. (2016). The role of serotonin receptors in Alzheimer’s disease. Oper. Med. Physiol. 2:18. doi: 10.20388/omp2016.001.0018
Carhart-Harris, R. L., Giribaldi, B., Watts, R., Baker-Jones, M., Murphy-Beiner, A., Murphy, R., et al. (2021). Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 384, 1402–1411. doi: 10.1056/NEJMoa2032994
Carhart-Harris, R. L., Roseman, L., Bolstridge, M., Demetriou, L., Pannekoek, J. N., Wall, M. B., et al. (2017). Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 7:13187. doi: 10.1038/s41598-017-13282-7
Caya-Bissonnette, L., and B’eique, J. C. (2024). Half a century legacy of long-term potentiation. Curr. Biol. 34, R640–R662. doi: 10.1016/j.cub.2024.05.008
Chakraborty, S., Lennon, J. C., Malkaram, S. A., Zeng, Y., Fisher, D. W., and Dong, H. (2019). Serotonergic system, cognition, and BPSD in Alzheimer’s disease. Neurosci. Lett. 704, 36–44. doi: 10.1016/j.neulet.2019.03.050
Chen, H. I., Song, H., and Ming, G.-l. (2019). Applications of human brain organoids to clinical problems. Dev. Dyn. 248, 53–64. doi: 10.1002/dvdy.24662
Costa, M. N., Goto-Silva, L., Nascimento, J. M., Domith, I., Karmirian, K., Feilding, A., et al. (2024). LSD modulates proteins involved in cell proteostasis, energy metabolism and neuroplasticity in human cerebral organoids. ACS Omega 9, 36553–36568. doi: 10.1021/acsomega.4c04712
Creese, B., and Ismail, Z. (2022). Mild behavioral impairment: measurement and clinical correlates of a novel marker of preclinical Alzheimer’s disease. Alzheimers Res. Ther. 14:2. doi: 10.1186/s13195-021-00949-7
Dakic, V., Nascimento, J. M., Sartore, R. C., Maciel, R. M., de Araujo, D. B., Ribeiro, S., et al. (2017). Short term changes in the proteome of human cerebral organoids induced by 5-MeO-DMT. Sci. Rep. 7:12863. doi: 10.1038/s41598-017-12779-5
Daws, R. E., Timmermann, C., Giribaldi, B., Sexton, J. D., Wall, M. B., Erritzoe, D., et al. (2022). Increased global integration in the brain after psilocybin therapy for depression. Nat. Med. 28, 844–851. doi: 10.1038/s41591-022-01744-z
De Gregorio, D., Aguilar-Valles, A., Preller, K. H., Heifets, B. D., Hibicke, M., Mitchell, J., et al. (2021). Hallucinogens in mental health: preclinical and clinical studies on LSD, psilocybin, MDMA, and ketamine. J. Neurosci. 41, 891–900. doi: 10.1523/JNEUROSCI.1659-20.2020
De Strooper, B., and Karran, E. (2016). The cellular phase of Alzheimer’s disease. Cell 164, 603–615. doi: 10.1016/j.cell.2015.12.056
Dennis, E. L., and Thompson, P. M. (2014). Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol. Rev. 24, 49–62. doi: 10.1007/s11065-014-9249-6
Dubois, B., Hampel, H., Feldman, H. H., Scheltens, P., Aisen, P., Andrieu, S., et al. (2016). Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323. doi: 10.1016/j.jalz.2016.02.002
Ermakova, A. O., Dunbar, F., Rucker, J., and Johnson, M. W. (2022). A narrative synthesis of research with 5-MeO-DMT. J. Psychopharmacol. 36, 273–294. doi: 10.1177/02698811211050543
Flanagan, T. W., and Nichols, C. D. (2018). Psychedelics as anti-inflammatory agents. Int. Rev. Psychiatry 30, 363–375. doi: 10.1080/09540261.2018.1481827
Garcia-Romeu, S., Darcy, S., Jackson, H., White, T., and Rosenberg, P. (2022). Psychedelics as novel therapeutics in Alzheimer’s disease: rationale and potential mechanisms. Curr. Top. Behav. Neurosci. 56, 287–317. doi: 10.1007/78542021267
Giorgi, C., Lombardozzi, G., Ammannito, F., Scenna, M. S., Maceroni, E., Quintiliani, M., et al. (2024). Brain organoids: a game-changer for drug testing. Pharmaceutics 16:443. doi: 10.3390/pharmaceutics16040443
Goodwin, G. M., Aaronson, S. T., Alvarez, O., Arden, P. C., Baker, A., Bennett, J. C., et al. (2022). Single-dose psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med. 387, 1637–1648. doi: 10.1056/NEJMoa2206443
Goodwin, G. M., Aaronson, S. T., Alvarez, O., Carhart-Harris, R., Chai-Rees, J., Croal, M., et al. (2025). The role of the psychedelic experience in psilocybin treatment for treatment-resistant depression. J. Affect. Disord. 372, 523–532. doi: 10.1016/j.jad.2024.12.061
Hutten, N. R. P. W., Mason, N. L., Dolder, P. C., Theunissen, E. L., Holze, F., Liechti, M. E., et al. (2020). Low doses of LSD acutely increase BDNF blood plasma levels in healthy volunteers. ACS Pharmacol. Transl. Sci. 4, 461–466. doi: 10.1021/acsptsci.0c00099
Jefsen, O. H., Elfving, B., Wegener, G., and Müller, H. K. (2020). Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J. Psychopharmacol. 35, 483–493. doi: 10.1177/0269881120959614
Johnson, M. W., Griffiths, R. R., Hendricks, P. S., and Henningfield, J. E. (2018). The abuse potential of medical psilocybin according to the 8 factors of the controlled substances act. Neuropharmacology 142, 143–166. doi: 10.1016/j.neuropharm.2018.05.012
Kinney, J. W., Bemiller, S. M., Murtishaw, A. S., Leisgang, A. M., Salazar, A. M., and Lamb, B. T. (2018). Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 4, 575–590. doi: 10.1016/j.trci.2018.06.014
Knudsen, G. M. (2023). Sustained effects of single doses of classical psychedelics in humans. Neuropsychopharmacology 48, 145–150. doi: 10.1038/s41386-022-01361-x
Lima da Cruz, R. V., Moulin, T. C., Petiz, L. L., and Leão, R. N. (2018). A single dose of 5-MeO-DMT stimulates cell proliferation, neuronal survivability, morphological and functional changes in adult mice ventral dentate gyrus. Front. Mol. Neurosci. 11:312. doi: 10.3389/fnmol.2018.00312
Linnemann, C., and Lang, U. E. (2020). Pathways connecting late-life depression and dementia. Front. Pharmacol. 11:279. doi: 10.3389/fphar.2020.00279
Logan, S., Arzua, T., Canfield, S. G., Seminary, E. R., Sison, S. L., Ebert, A. D., et al. (2019). Studying human neurological disorders using induced pluripotent stem cells: from 2D monolayer to 3D organoid and blood brain barrier models. Compr. Physiol. 9, 565–611. doi: 10.1002/cphy.c180025
Ly, C., Greb, A. C., Cameron, L. P., Wong, J. M., Barragan, E. V., Wilson, P. C., et al. (2018). Psychedelics promote structural and functional neural plasticity. Cell Rep. 23, 3170–3182. doi: 10.1016/j.celrep.2018.05.022
Ma, L. (2020). Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Front. Aging Neurosci. 12:9. doi: 10.3389/fnagi.2020.00009
Mecca, A. P. (2019). AD molecular: molecular imaging of Alzheimer’s disease: PET imaging of neurotransmitter systems. Prog Mol Biol Transl Sci 165, 139–165. doi: 10.1016/bs.pmbts.2019.04.003
Mendes-Silva, P., Pereira, K. S., Tolentino-Araujo, G. T., Nicolau, E. d. S., Silva-Ferreira, C. M., Teixeira, A. L.’u., et al. (2016). Shared biologic pathways between Alzheimer disease and major depression: a systematic review of MicroRNA expression studies. Am. J. Geriatr. Psychiatry 24, 903–912. doi: 10.1016/j.jagp.2016.07.017
Moliner, R., Girych, M., Brunello, C. A., Kovaleva, V., Biojone, C., Enkavi, G., et al. (2023). Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 26, 1032–1041. doi: 10.1038/s41593-023-01316-5
Morales-Garcia, J. A., Calleja-Conde, J., Lopez-Moreno, J. A., Alonso-Gil, S., Sanz-SanCristobal, M., Riba, J., et al. (2020). N,n-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo. Transl. Psychiatry 10:331. doi: 10.1038/s41398-020-01011-0
Moujaes, F., Preller, K. H., Ji, J. L., Murray, J. D., Berkovitch, L., Vollenweider, F. X., et al. (2023). Toward mapping neurobehavioral heterogeneity of psychedelic neurobiology in humans. Biol. Psychiatry 93, 1061–1070. doi: 10.1016/j.biopsych.2022.10.021
Mueller, B. H., Park, Y., Daudt, D. R., Ma, H.-Y., Akopova, I., Stankowska, D. L., et al. (2013). Sigma-1 receptor stimulation attenuates calcium influx through activated L-type voltage gated calcium channels in purified retinal ganglion cells. Exp. Eye Res. 107, 21–31. doi: 10.1016/j.exer.2012.11.002
Nguyen Thi Thanh, H., Kutzner, A., and Heese, K. (2018). Brain plasticity, cognitive functions and neural stem cells: a pivotal role for the brain-specific neural master gene SRGAP2–FAM72. Biol. Chem. 399, 55–61. doi: 10.1515/hsz-2017-0190
Ornelas, I. M., Cini, F. A., Wießner, I., Marcos, E., Ara’ujo, D.’a. B., Goto-Silva, L., et al. (2022). Nootropic effects of LSD: behavioral, molecular and computational evidence. Exp. Neurol. 356:114148. doi: 10.1016/j.expneurol.2022.114148
Park, J.-C., and Mook-Jung, I. (2022). Toward brain organoid-based precision medicine in neurodegenerative diseases. Organ 2:e21. doi: 10.51335/organoid.2022.2.e21
Peng, S., Wuu, J., Mufson, E. J., and Fahnestock, M. (2005). Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 93, 1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x
Porci’uncula, L. O., Goto-Silva, L., Ledur, F. F., and Rehen, S. K. (2021). The age of brain organoids: tailoring cell identity and functionality for normal brain development and disease modeling. Front. Neurosci. 15:674563. doi: 10.3389/fnins.2021.674563
Preller, K. H., Duerler, P., Burt, J. B., Ji, J. L., Adkinson, B., Stämpfli, P., et al. (2020). Psilocybin induces time-dependent changes in global functional connectivity. Biol. Psychiatry 88, 197–207. doi: 10.1016/j.biopsych.2019.12.027
Raison, C. L., Sanacora, G., Woolley, J., Heinzerling, K., Dunlop, B. W., Brown, R. T., et al. (2023). Single-dose psilocybin treatment for major depressive disorder: a randomized clinical trial. JAMA 330, 843–853. doi: 10.1001/jama.2023.14530
Raval, N. R., Johansen, A., Donovan, L. L., Ros, N. F., Ozenne, B., Hansen, H. D., et al. (2021). A single dose of psilocybin increases synaptic density and decreases 5-HT2A receptor density in the pig brain. Int. J. Mol. Sci. 22:835. doi: 10.3390/ijms22020835
Salerno, J. A., and Rehen, S. (2024). Human pluripotent stem cells as a translational toolkit in psychedelic research in vitro. iScience 27:109631. doi: 10.1016/j.isci.2024.109631
Scarmeas, N., Brandt, J., Albert, M., Hadjigeorgiou, G., Papadimitriou, A., Dubois, B., et al. (2005). Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch. Neurol. 62, 1601–1608. doi: 10.1001/archneur.62.10.1601
Schindler, E. A. D., Sewell, R. A., Gottschalk, C. H., Luddy, C., Flynn, L. T., Lindsey, H., et al. (2021). Exploratory controlled study of the migraine-suppressing effects of psilocybin. Neurotherapeutics 18, 534–543. doi: 10.1007/s13311-020-00962-y
Schlag, A. K., Aday, J., Salam, I., Neill, J. C., and Nutt, D. J. (2022). Adverse effects of psychedelics: from anecdotes and misinformation to systematic science. J. Psychopharmacol. 36, 258–272. doi: 10.1177/02698811211069100
Shao, L.-X., Liao, C., Gregg, I., Davoudian, P. A., Savalia, N. K., Delagarza, K., et al. (2021). Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544.e4. doi: 10.1016/j.neuron.2021.06.008
Sims, R., Hill, M., and Williams, J. (2020). The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 23, 311–322. doi: 10.1038/s41593-020-0599-5
Smirnova, L., and Hartung, T. (2024). The promise and potential of brain organoids. Adv. Healthc. Mater. 13:e2302745. doi: 10.1002/adhm.202302745
Smith, G. S., Barrett, F. S., Joo, J. H., Nassery, N., Savonenko, A., Sodums, D. J., et al. (2017). Molecular imaging of serotonin degeneration in mild cognitive impairment. Neurobiol. Dis. 105, 33–41. doi: 10.1016/j.nbd.2017.05.007
Smith, G. S., Protas, H., Kuwabara, H., Savonenko, A., Nassery, N., Gould, N. F., et al. (2023). Molecular imaging of the association between serotonin degeneration and beta-amyloid deposition in mild cognitive impairment. NeuroImage Clin. 37:103322. doi: 10.1016/j.nicl.2023.103322
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292. doi: 10.1016/j.jalz.2011.03.003
Szabo, A., Kovacs, A., Riba, J., Djurovic, S., Rajnavolgyi, E., and Frecska, E. (2016). The endogenous hallucinogen and trace amine N,N-dimethyltryptamine (DMT) displays potent protective effects against hypoxia via sigma-1 receptor activation in human primary iPSC-derived cortical neurons and microglia-like immune cells. Front. Neurosci. 10:423. doi: 10.3389/fnins.2016.00423
Tanila, H. (2017). The role of BDNF in Alzheimer’s disease. Neurobiol. Dis. 97, 114–118. doi: 10.1016/j.nbd.2016.05.008
Valiulahi, P., Vidyawan, V., Puspita, L., Oh, Y., Juwono, V. B., Sittipo, P., et al. (2021). Generation of caudal-type serotonin neurons and hindbrain-fate organoids from hPSCs. Stem Cell Rep. 16, 1938–1952. doi: 10.1016/j.stemcr.2021.06.006
Watts, M. E., Pocock, R., and Claudianos, C. (2018). Brain energy and oxygen metabolism: emerging role in normal function and disease. Front. Mol. Neurosci. 11:216. doi: 10.3389/fnmol.2018.00216
Whiteley, J. T., Fernandes, S., Sharma, A., Mendes, A. P. D., Racha, V., Benassi, S. K., et al. (2022). Reaching into the toolbox: stem cell models to study neuropsychiatric disorders. Stem Cell Rep. 17, 187–210. doi: 10.1016/j.stemcr.2021.12.015
Wise, E. A., Rosenberg, P. B., Lyketsos, C. G., and Leoutsakos, J.-M. (2019). Time course of neuropsychiatric symptoms and cognitive diagnosis in National Alzheimer’s coordinating centers volunteers. Alzheimers Dement (N Y). 11, 333–339. doi: 10.1016/j.dadm.2019.02.006
Xu, N., LaGrow, T. J., Anumba, N., Lee, A., Zhang, X., Yousefi, B., et al. (2022). Functional connectivity of the brain across rodents and humans. Front. Neurosci. 16:816331. doi: 10.3389/fnins.2022.816331
Yin, Y. N., and Gao, T. M. (2023). Non-hallucinogenic psychedelic analog design: a promising direction for depression treatment. Neurosci. Bull. 39, 170–172. doi: 10.1007/s12264-022-00933-7
Zheng, S., Ma, R., Yang, Y., and Li, G. (2024). Psilocybin for the treatment of Alzheimer’s disease. Front. Neurosci. 18:1420601. doi: 10.3389/fnins.2024.1420601
Zhou, J.-Q., Zeng, L.-H., Li, C.-T., He, D.-H., Zhao, H.-D., Xu, Y.-N., et al. (2023). Brain organoids are new tool for drug screening of neurological diseases. Neural Regen. Res. 18, 1884–1889. doi: 10.4103/1673-5374.367983
Zivko, C., Sagar, R., Xydia, A., Lopez-Montes, A., Mintzer, J., Rosenberg, P. B., et al. (2024). iPSC-derived hindbrain organoids to evaluate escitalopram oxalate treatment responses targeting neuropsychiatric symptoms in Alzheimer’s disease. Mol. Psychiatry 29, 3644–3652. doi: 10.1038/s41380-024-02629-y
Keywords: Alzheimer’s disease, brain organoids, psychedelics, stem cells, neuroplasticity
Citation: Androni X, Boyd RJ, Rosenberg PB and Mahairaki V (2025) Psychedelics meet human brain organoids: insights into proteomics and potential for Alzheimer’s disease treatment. Front. Dement. 4:1605051. doi: 10.3389/frdem.2025.1605051
Edited by:
Charbel Moussa, Georgetown University, United StatesReviewed by:
Ioannis N. Charalampopoulos, University of Crete, GreeceCopyright © 2025 Androni, Boyd, Rosenberg and Mahairaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vasiliki Mahairaki, dm1hY2hhaTFAamhtaS5lZHU=
 Xenia Androni
Xenia Androni Rachel J. Boyd
Rachel J. Boyd Paul B. Rosenberg
Paul B. Rosenberg Vasiliki Mahairaki
Vasiliki Mahairaki