- 1Department of Radiology, Mayo Clinic, Rochester, MN, United States
- 2Rush Alzheimer’s Disease Center, Department of Neurological Sciences, Rush University, Chicago, IL, United States
Background: Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) are two of the most common neurodegenerative diseases in older adults, and both show well-documented sex-specific differences in terms of clinical presentation, prevalence, and progression trajectories. However, the underlying neurobiological substrates that underpin these differences are poorly understood. In vivo biomarkers are well-suited to yield insights into how biological sex may shape disease pathophysiology in AD and DLB, and thus inform future research and precision medicine. The objective of this review is to synthesize recent evidence on sex differences related to biomarkers of AD and DLB.
Methods: We conducted a literature search of PubMed for studies published between January 2000 and May 2025 examining sex differences in neuroimaging and biofluid markers of mild cognitive impairment (MCI), AD, and/or DLB. Eligible studies were required to include sex-stratified or sex-interaction analyses in human participants with clinically defined MCI (due to AD or DLB), AD, or DLB.
Results: Of a total of 261 articles imported for screening, 63 met inclusion criteria, comprising 50 cross-sectional and 13 longitudinal investigations across biofluid markers (n = 18) studies, structural imaging (n = 18), functional imaging (n = 16), and molecular imaging (n = 11) studies. Women demonstrated initial cortical structural and metabolic advantages followed by accelerated decline. In MCI and AD, women were generally more susceptible to tau pathology and APOE ε4-related risk. In contrast, men with DLB showed greater metabolic and dopaminergic abnormalities, though women with DLB frequently exhibited mixed biomarker profiles. APOE ε4 conferred increased vulnerability in women for both conditions. Biofluid markers also revealed sex-specific expression patterns and associations with clinical outcomes.
Discussion: There is growing evidence that biological sex significantly influences the pathophysiology of AD and DLB as captured by in vivo biomarkers. These findings highlight the growing importance of analyses that consider sex differences in biomarker research and support the development of personalized diagnostic and therapeutic strategies in neurodegeneration. Future research should prioritize longitudinal studies to define optimal biomarker sequencing and therapeutic windows for each sex, while also investigating the genetic, hormonal, metabolic, pharmacological, and environmental mechanisms that underlie these sex differences, ultimately advancing precision medicine in neurodegenerative disease.
Introduction
Dementia affects over 55 million people worldwide, with Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB) representing two of the most common and clinically recognizable neurodegenerative causes, collectively accounting for a significant proportion of age-related dementia cases worldwide. AD affects approximately 60–70% of dementia patients and is characterized by progressive memory decline, amyloid-β (Aβ) and tau accumulation, and widespread cortical atrophy (Dubois et al., 2007; Scheltens et al., 2021). DLB represents 10–15% of dementia cases and presents with fluctuating cognition, visual hallucinations, parkinsonian motor symptoms, and is neuropathologically defined by α-synuclein pathology (McKeith et al., 2017; Vann Jones and O’Brien, 2013). Despite shared features of progressive cognitive decline, both AD and DLB exhibit distinct pathophysiological signatures that have become increasingly accessible to delineate through advances in the development of neuroimaging and biofluid markers.
The role of neuroimaging and biofluid markers has become central to early diagnosis, especially as the field has moved toward a biologic definition of disease rather than using a purely syndromic approach (Jack et al., 2018). Further, these biomarkers are also critical for disease monitoring and treatment development in both AD and DLB. Briefly, structural MRI reveals distinct patterns of cortical atrophy in AD, particularly in the medial temporal lobe, while DLB demonstrates more pronounced posterior cortical thinning and basal ganglia involvement (Mak et al., 2014). And, more recently positron emission tomography (PET) enables in vivo quantification of amyloid and tau deposition in both AD and DLB (Hall et al., 2017; Kantarci et al., 2016; Mak et al., 2021). Emerging neuroimaging techniques, including diffusion tensor imaging (DTI) and functional MRI (fMRI), along with plasma and cerebrospinal fluid (CSF) biomarkers, collectively provide additional insights into network connectivity, metabolic dysfunction, and pathological changes.
Yet, while these biomarkers have significantly improved diagnostic accuracy and differential diagnosis (Hansson et al., 2022; Kantarci et al., 2016), the extent to which their expression differs by biological sex remains a largely underexplored area of investigation. This knowledge gap is particularly striking, given the well-documented clinical and phenotypic differences between men and women in both AD and DLB presentation and progression, and that the biological mechanisms underlying these sex differences remain poorly understood (Carter et al., 2012). Women demonstrate a higher lifetime risk of developing AD, show faster rates of cognitive decline, and exhibit greater tau burden and cortical atrophy (Altmann et al., 2014; Buckley et al., 2019; Oveisgharan et al., 2018). The influence of the APOE ε4 allele, a major genetic risk factor for AD (Corder et al., 1993), also appears more pronounced in women, particularly regarding cognitive decline and associations with tau pathology (Sundermann et al., 2019). In contrast, DLB is diagnosed more frequently in men (Kane et al., 2018), a pattern consistently observed across prior American, European, and Japanese autopsy series that have found disproportionate numbers of men in pathologically-confirmed DLB cohorts (Barker et al., 2002; Fujishiro et al., 2008; Weiner et al., 1996). Men may present earlier with core features such as REM sleep behavior disorder and parkinsonism, while women with DLB tend to show more co-existing AD pathology (Diaz-Galvan et al., 2023) and are more likely to be underdiagnosed (Bayram et al., 2021). Together, these divergent clinical trajectories and presentations suggest that sex-specific biological pathways contribute to the pathophysiology of AD and DLB.
However, current biomarker thresholds and diagnostic frameworks do not account for sex-related variations, potentially limiting early detection and treatment optimization and therefore overlooking insights that could inform more precise diagnostic and therapeutic approaches (Nebel et al., 2018). For instance, a growing body of evidence suggests that sex differences have a bearing on treatment responses in disease-modifying trials, necessitating sex-stratified analyses to optimize therapeutic outcomes (Andrews et al., 2025). The cholinergic system—a primary target for AD and DLB treatments—exhibits marked sexual dimorphism too. Women showed higher frontal cortex cholinergic activity while men demonstrated greater hippocampal cholinergic function, with sex hormones exerting distinct trophic effects on cholinergic neurons through estrogen and androgen receptors (Giacobini and Pepeu, 2018). These neurobiological differences translate to clinically significant treatment disparities, with men showing stronger and more selective benefits from cholinesterase inhibitors in AD, potentially due to sex-specific differences in cholinergic system structure, function, pharmacokinetics, and aging-related changes (Giacobini and Pepeu, 2018).
Our review aims to synthesize current evidence on sex differences in in vivo biomarkers of AD and DLB and patients with mild cognitive impairment (MCI) due to AD or DLB. We included studies in MCI because it represents an intermediate stage between normal aging and dementia, marked by measurable cognitive decline with largely preserved daily functioning. Studying sex differences at this stage may reveal early biological mechanisms that precede dementia. By evaluating sex-specific findings from structural, functional, and molecular imaging, as well as biofluid markers modalities, we aim to identify consistent patterns and broad themes that may inform future research directions and support the advancement of personalized therapeutic strategies in AD and DLB.
Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. To identify relevant studies, we conducted a PubMed search limited to articles published in English between January 1, 2020, and May 27, 2025, with a focus on sex-related differences in imaging and biofluid markers of AD, DLB and MCI. The keywords were: ((“sex differences” OR “gender differences” OR “sex dimorphism” OR “sexual dimorphism” OR “sex-specific” OR “sex stratified” OR “sex dependent” OR “sex by” OR “sex X” OR “sex interaction” OR “gender interaction”) AND (“plasma biomarker*” OR “CSF biomarker*” OR “serum biomarker*” OR “blood biomarker*” OR neuroimaging OR MRI OR PET OR SPECT OR “magnetic resonance imaging” OR “positron emission tomography” OR “single-photon emission computed tomography” OR “brain imaging” OR “brain volume*” OR “brain atrophy”) AND (“Alzheimer’s disease” OR “Alzheimer disease” OR “AD” OR “Lewy bod*” OR “DLB” OR “mild cognitive impairment due to Alzheimer*” OR “MCI due to Alzheimer*” OR “Dementia with Lewy bodies”) NOT (animal* OR mice OR mouse OR rat OR rats OR rodent* OR “early onset” OR “early-onset” OR “familial” OR “autosomal dominant” OR “presenilin” OR “healthy control*” OR “healthy adult*” OR “cognitively normal”)) AND (“2020/01/01”[Date - Publication]: “2025/05/27”[Date - Publication]).”
Eligibility criteria
For inclusion, studies were required to: (1) report original data from human participants with MCI and/or AD and/or DLB; (2) investigate in vivo biomarkers using neuroimaging (such as structural MRI, functional MRI, PET) or fluid-based methods (CSF, plasma, or serum); (3) include sex-stratified results or analyses testing for sex-by-disease interactions within defined cognitive groups such as MCI, AD or DLB, and (4) be published in English in a peer-reviewed journal. Studies were excluded if they: focused exclusively on healthy or cognitively normal individuals, animal models, if they did not conduct sex-stratified or sex-interaction analyses within defined cognitive groups (i.e., MCI, AD, or DLB) or were review articles, editorials, or conference abstracts without peer-reviewed findings. All identified records were imported into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) for screening and data management. EM conducted the initial title and abstract screening within the Covidence platform, followed by full-text assessment of potentially eligible articles against the inclusion and exclusion criteria.
Results
Study selection and characteristics
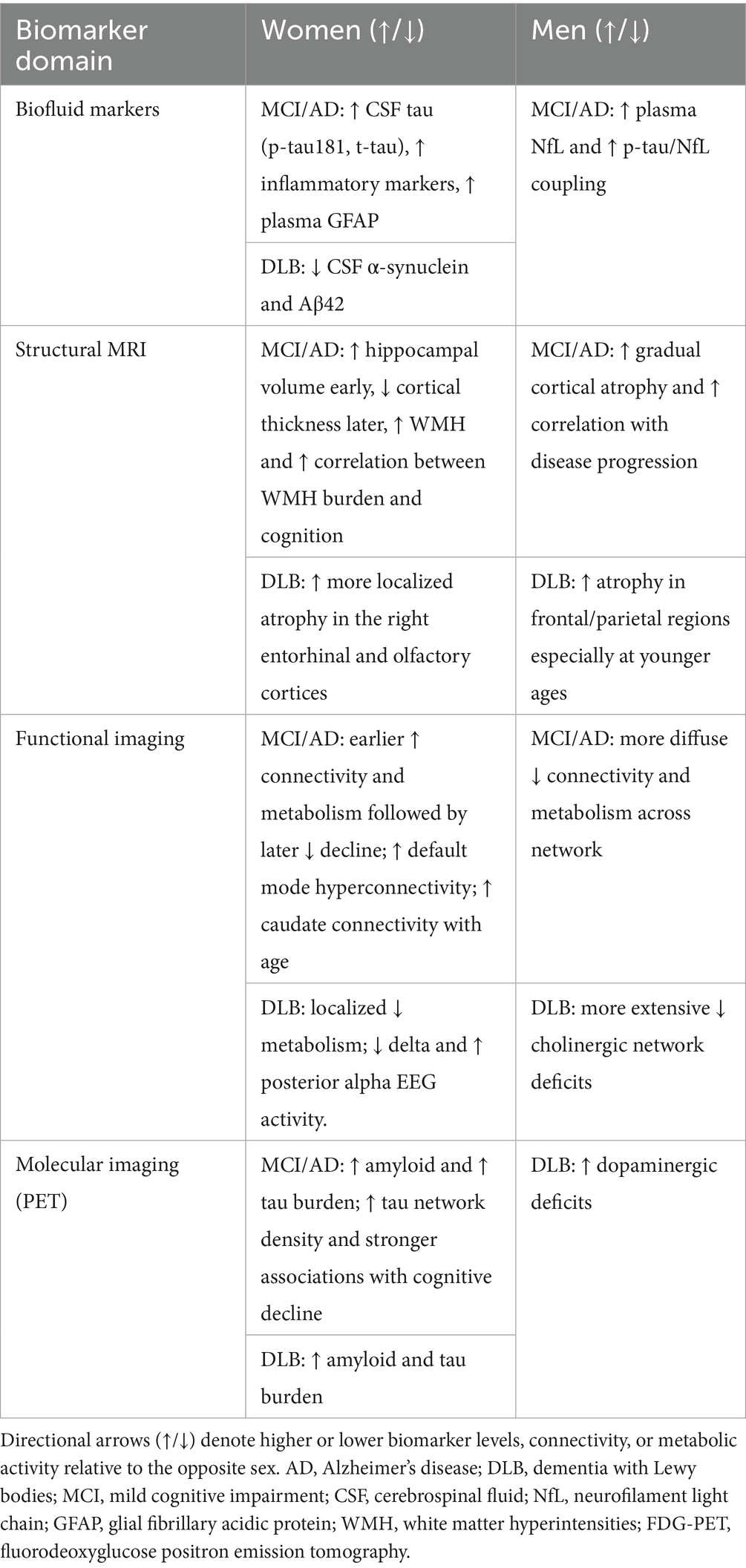
A total of 261 records were imported for screening (245 from PubMed, 16 through citation searching). After removing 3 duplicates, 258 studies remained for screening. Of these, 174 were excluded during title and abstract screening for not meeting eligibility criteria. The remaining 84 full-text articles underwent a detailed eligibility assessment, after which 21 studies were excluded resulting in a final inclusion of 63 studies. The complete study selection process is illustrated in the PRISMA flow diagram (Figure 1). Of the included studies, 50 were cross-sectional and 13 were longitudinal in design. Regarding patient populations, 22 studies examined combined AD dementia and MCI due to AD cohorts, followed by 16 focused on MCI due to AD, 13 on AD dementia, 11 on DLB, and 1 study included both AD dementia and DLB groups. Studies were categorized into four biomarker modalities: Biofluid markers (n = 18), structural imaging (n = 18), functional imaging (n = 16), molecular imaging (n = 11). Figure 2 presents an overview of the included studies, whereas Figure 3 depicts an alluvial plot visualizing the relationships between study designs, patient populations, and distribution of biomarker modalities across the literature. A summary of the principal sex-related biomarker patterns across modalities is presented in Table 1.
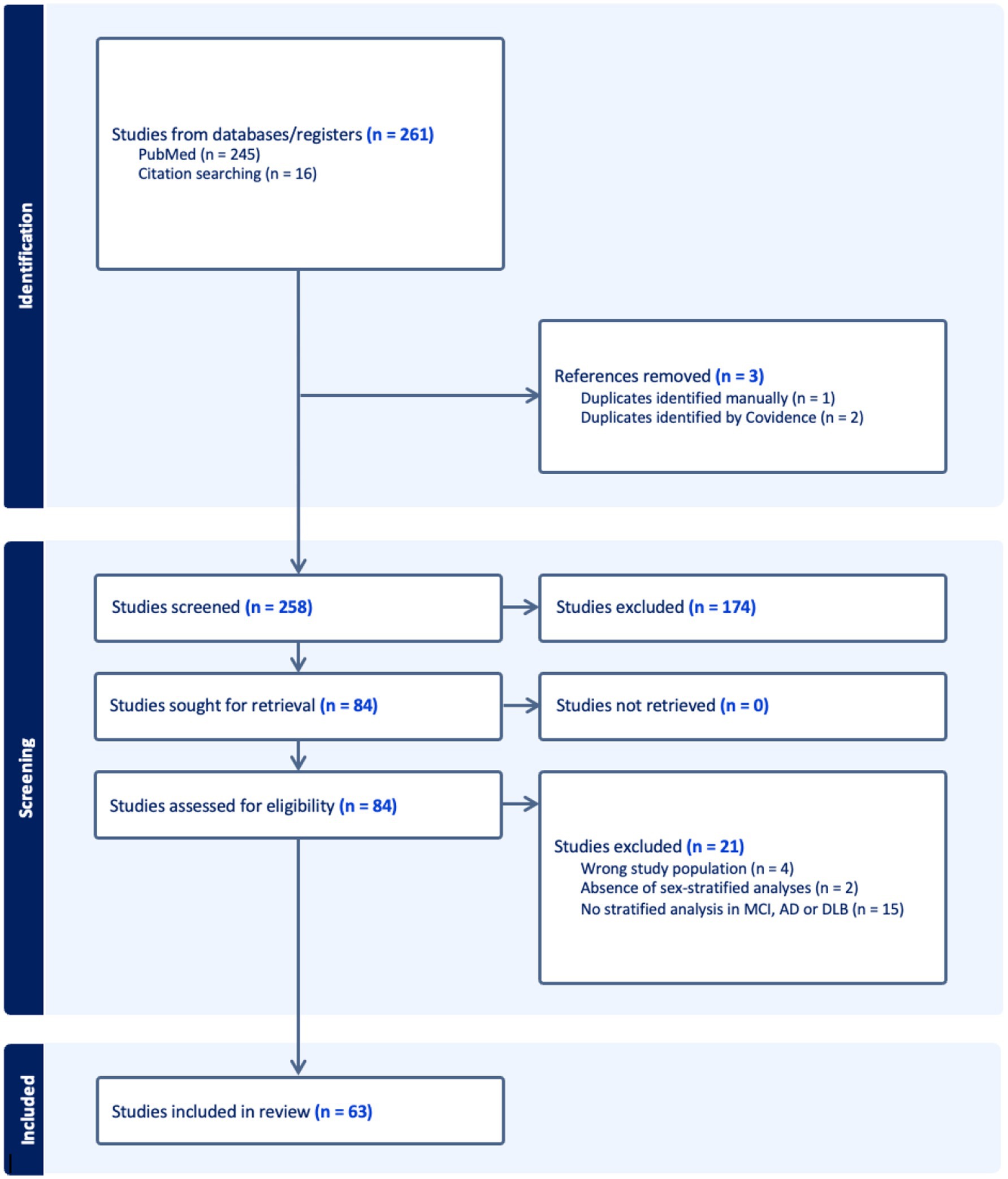
Figure 1. PRISMA flow diagram of study selection process. Flow diagram depicting the study selection process according to PRISMA guidelines. The final review included 63 studies that met all inclusion criteria. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MCI, Mild Cognitive Impairment; AD, Alzheimer’s Disease; DLB, Dementia with Lewy bodies.
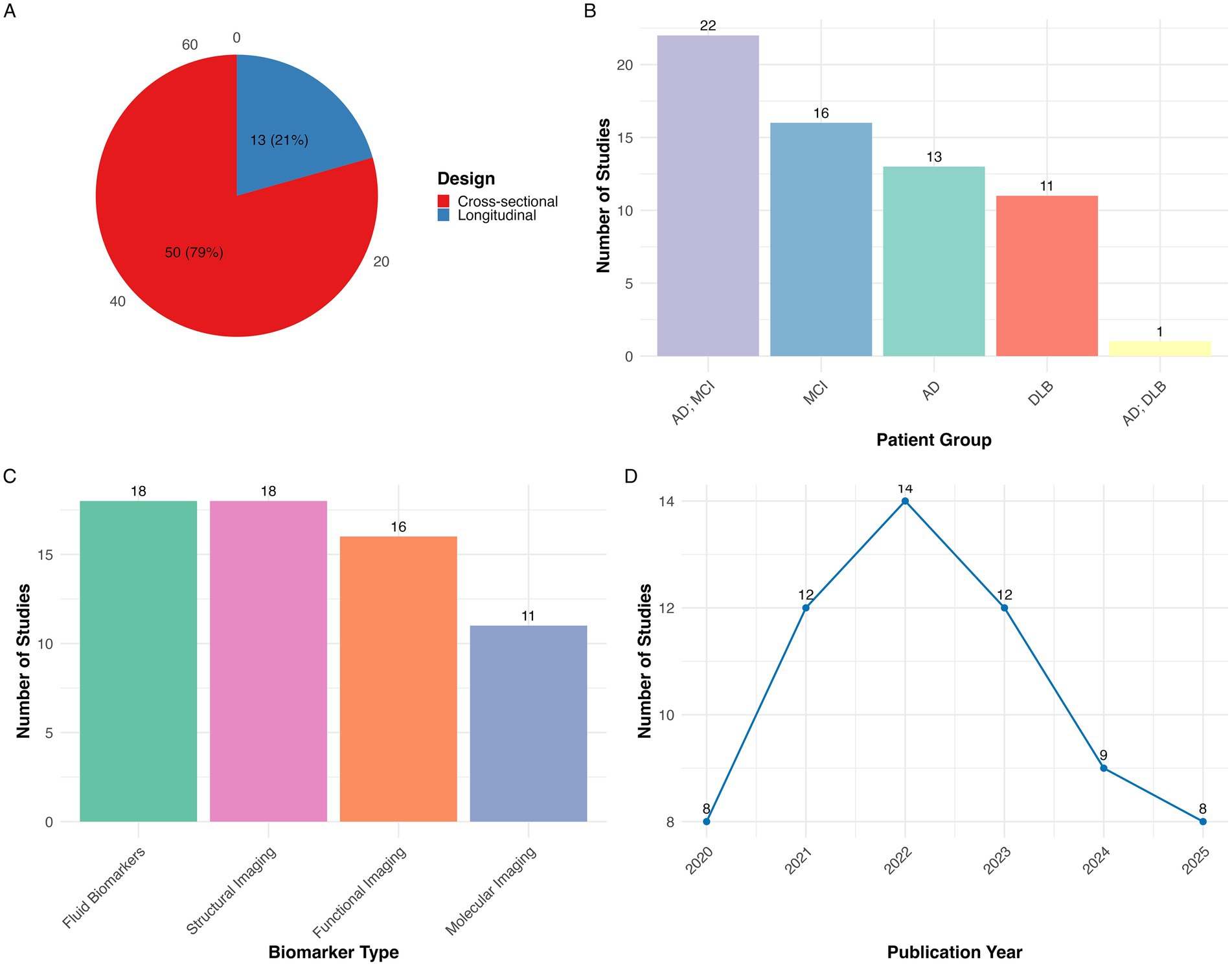
Figure 2. Summary of studies included in the systematic review of sex differences in dementia biomarkers (N = 63). (A) Distribution of studies by design showing that most studies were cross-sectional (n = 50, 78%) and the remainder were longitudinal (n = 13, 21%); (B) distribution of studies by patient group, indicating the number of studies conducted in AD+MCI, MCI, AD, DLB, AD+DLB; (C) distribution of studies by biomarker type, grouped into biofluid markers, structural imaging, functional imaging, and molecular imaging; (D) publication trend showing the annual number of studies from 2020 to 2025 with 2025 representing a partial year through May. AD, Alzheimer’s disease; MCI, Mild cognitive impairment; DLB, Dementia with Lewy bodies.
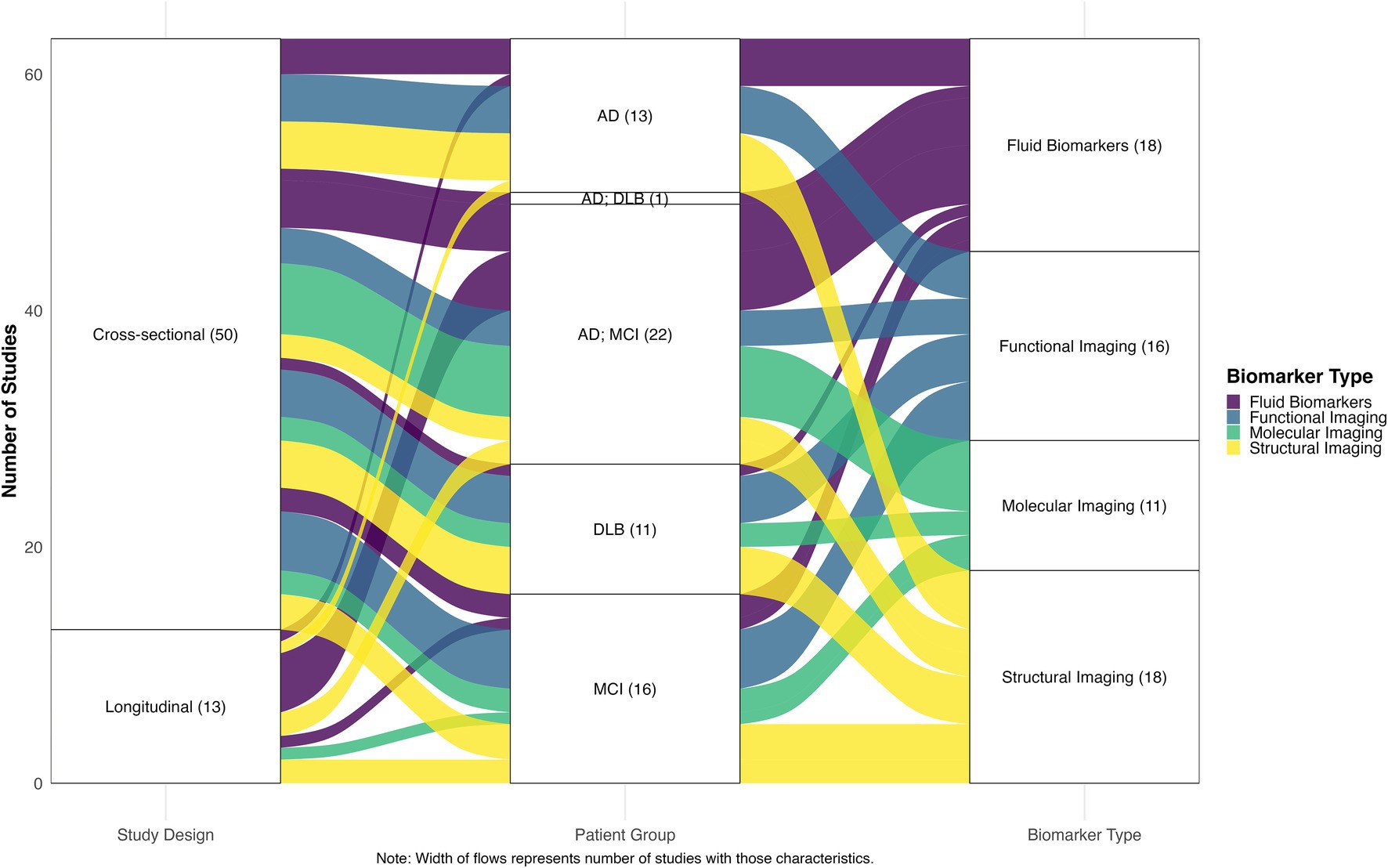
Figure 3. Alluvial plot of relationships between study designs, patient groups, and biomarker types. The width of the flows represents the number of studies with corresponding characteristics, illustrating the distribution and interconnections between methodological approaches across the included literature. AD, Alzheimer’s disease; MCI, mild cognitive impairment; DLB, Dementia with Lewy bodies.
Biofluid markers
Eighteen studies examined sex differences in biofluid markers across the AD and DLB spectrum, revealing distinct patterns in cerebrospinal fluid (CSF) and blood-based markers of neurodegeneration, tau pathology, amyloid deposition, neuroinflammation, and metabolic dysfunction. While CSF is not routinely collected in most persons with AD or DLB, several studies have examined sex differences in CSF biofluids. Among APOE ε4 non-carriers, women exhibited significantly higher CSF phosphorylated tau (p-tau) and total tau (t-tau) concentrations in both MCI and AD dementia stages (Babapour Mofrad et al., 2020). This finding was corroborated by Ren et al. (2024), who found significantly higher t-tau concentrations in CSF among women with MCI (Ren et al., 2024). In DLB, CSF protein patterns differed markedly from AD. Women with DLB had significantly lower CSF α-synuclein and amyloid-β42 levels compared to men, accompanied by shorter symptom duration and more frequent hallucinations at diagnosis (van de Beek et al., 2022). Additional studies focused specifically on CSF neuroinflammatory markers revealed pronounced sex differences in immune responses too. The neuroinflammatory marker sTNFR2 showed women-specific associations with cognitive decline across the AD spectrum, with this relationship mediated through CSF p-tau181 levels (Bernier et al., 2022). Elevated baseline CSF TNF-α, interleukin-9, and IL-12p40 levels were associated with higher rates of conversion to MCI/AD, with women showing significantly shorter times to conversion when TNF-α levels were elevated (Contreras et al., 2022). Matrix metalloproteinase-9 (MMP-9) demonstrated sex-specific associations with CSF biomarkers: in men, MMP-9 was associated with higher CSF Aβ42, while in women, it correlated with higher CSF t-tau (Tsiknia et al., 2022b).
Blood biomarkers are more practical compared to CSF biomarkers. Plasma biomarker studies demonstrated sex differences in tau and amyloid pathology, metabolism, neurodegeneration, and neuroinflammation that varied across disease stages and showed distinct predictive value by sex. Plasma p-tau181 levels showed stronger associations with amyloid deposition in women with MCI compared to men; however, this relationship reversed in dementia stages, where plasma p-tau181 correlated with amyloid burden in men but not women (Tsiknia et al., 2022a). The p-tau181/Aβ42 ratio emerged as a stronger predictor of progression to AD, specifically in men with MCI (Berezuk et al., 2023). Liu et al. (2022) demonstrated that lower plasma Aβ42 was associated with faster memory decline in women but not men and that higher plasma Aβ42 levels predicted lower future AD risk specifically in women (Liu et al., 2022). Conversely, men displayed steeper increases in plasma pTau181, pTau231, and neurofilament light chain (Nfl) compared to women, along with steeper declines in brain volume and cognitive function (Joynes et al., 2025). In contrast, Labonte et al. (2025) found that higher plasma NfL levels predicted worsening dementia status only in women (Labonte et al., 2025). Women demonstrated significantly higher plasma GFAP levels compared to men in both DLB and AD, indicating enhanced astroglial activation (Bolsewig et al., 2024).
CSF neuroinflammatory markers revealed pronounced sex differences in immune responses to neurodegeneration. The neuroinflammatory marker sTNFR2 showed women-specific associations with cognitive decline, with this relationship mediated through CSF p-tau181 levels (Bernier et al., 2022). Elevated baseline levels of CSF inflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin-9 (IL-9), and IL-12p40, were also associated with higher rates of progression from cognitively normal to MCI or AD dementia. Women with elevated CSF TNF-α levels showed significantly shorter times to conversion, indicating sex-specific vulnerability to neuroinflammation (Contreras et al., 2022).
Sex-stratified metabolomic studies have begun to uncover distinct biochemical signatures in AD, revealing that men and women may follow divergent metabolic trajectories as the disease progresses. Men exhibited the most prominent metabolic alterations, particularly in phosphatidylcholines (e.g., PC ae C44:4, PC ae C42:3, PC ae C44:6) and amino acids (e.g., glycine, proline, glutamine), which were consistently reduced in AD compared to controls. These metabolites were enriched in glycerophospholipid metabolism, aminoacyl-tRNA biosynthesis, and arginine and proline metabolism pathways. In contrast, women-specific alterations were detected primarily in medium-chain acylcarnitines, including C10:2, C12:1, and C9, with reductions observed in both MCI and AD stages (Zarzar et al., 2022). Arnold et al. (2020) confirmed widespread sex-specific metabolic disruptions, identifying 53 metabolites with consistent sex differences across the MCI and AD spectrum (Arnold et al., 2020). Specifically, phosphatidylcholines and sphingomyelins were negatively associated with AD biomarkers in men but positively associated in women, suggesting divergent lipid dynamics. Similarly, acylcarnitines correlated with tau burden and cognitive decline in women but not in men, while amino acids showed stronger inverse associations with AD pathology in men. Additional plasma biomarkers demonstrated sex-specific patterns across disease stages. van Kruining et al. (2023) demonstrated that elevated plasma ceramide species C18:0, C24:1, and combined ceramide chain lengths were associated with MCI diagnosis exclusively in men (van Kruining et al., 2023). The clinical potential of sex-specific metabolic signatures was demonstrated by Xu et al. (2021), who found that vanillylmandelic acid (VMA) and tryptophan significantly improved AD prediction accuracy in women when combined with CSF markers (AUC increased from 0.884 to 0.955). This improvement was not observed in men, highlighting a sex-specific predictive advantage (Xu et al., 2021). In alignment with these findings, Kawakami et al. (2022) confirmed significant sex differences in serum lipid biomarkers, though these differences did not enhance diagnostic accuracy when analyzed separately (Kawakami et al., 2022). Notably, Chang et al. (2023) reported that the presence of APOE ε4 may override or modify observed sex-based differences in serum metabolites of AD patients (Chang et al., 2023).
Together, these studies highlight the multifaceted nature of sex differences in patterns of biofluid across the AD and DLB spectrum. Distinct patterns emerged across both CSF and plasma measures of tau, amyloid, neuroinflammation, neurodegeneration, and metabolism. Broadly, women tended to show greater vulnerability to tau pathology and neuroinflammatory processes, while men showed distinct amyloid dynamics and metabolic profiles. Notably, many of these associations were only detectable through sex-stratified or sex interaction analyses.
Structural MRI
Structural brain MRI analyses employed diverse methods including automated volumetry, cortical thickness mapping, visual rating scales (e.g., Fazekas, MTA), and white matter hyperintensities (WMH) quantification as a marker of small-vessel cerebrovascular disease. Several studies revealed sex-specific patterns of neurodegeneration across the AD spectrum. Cortical thickness analyses revealed distinct sex-specific trajectories across disease stages. Women maintained greater cortical thickness across widespread regions during normal aging and early disease stages but exhibited steeper rates of cortical thinning from MCI to AD relative to men (Cieri et al., 2022). Extending these findings, Inguanzo et al. (2025) employed longitudinal atrophy subtype modeling in amyloid-positive AD individuals to demonstrate that women exhibited earlier hippocampal atrophy and a greater burden of white matter abnormalities than men (Inguanzo et al., 2025). Men showed more gradual, consistent thinning patterns across all disease stages. And, sex-related structural differences appear to be more pronounced in later AD stages compared to the prodromal MCI phase (Sauty and Durrleman, 2023). Women with established AD demonstrated higher gray matter volumes compared to men at equivalent disease stages while showing more severe gray matter atrophy during the MCI phase (Fernández et al., 2024). There are also regionally specific sex differences. For example, hippocampal analyses revealed sex differences across disease stages. Among individuals with amnestic MCI, women—particularly Hispanic and White non-Hispanic women—exhibited significantly larger baseline hippocampal volumes than men, independent of age, education, and APOE ε4 status (Garcia et al., 2024).
Unsupervised clustering analysis of MCI patients identified sex-specific disease subtypes. Women were more frequently classified into poor-prognosis subtypes characterized by smaller hippocampal and amygdalar volumes and greater cognitive deficits, while men more often clustered into better-prognosis subtypes with larger regional volumes (Katabathula et al., 2023). Similarly, Massetti et al. (2022) found that MRI-based prediction of MCI-to-AD conversion was significantly more accurate in women and younger individuals (Massetti et al., 2022). Longitudinal multimodal analyses revealed that men showed greater sensitivity to structural neuroimaging and CSF biomarkers—particularly hippocampal volume and tau/Aβ ratios—relative to women during the MCI to AD progression (Sarica et al., 2024).
Brain-age delta, an index of structural aging derived from MRI, also showed sex-specific biomarker associations. In MCI, brain-age delta in women was positively associated with plasma NfL, while in men, it correlated with WMH burden, suggesting that different neurobiological processes underlie aging trajectories in men versus women (Cumplido-Mayoral et al., 2023). Supporting this, Morrison et al. (2024) found that WMH burden was more strongly associated with cognitive impairment in women, and that women experienced more cognitive decline than men for equivalent WMH loads (Morrison et al., 2024). Supporting the notion of sex-dependent vulnerability across multiple structural domains, Kim et al. (2023) reported that women with AD exhibited lower connectivity strength within rich-club (i.e., densely interconnected hubs) and feeder networks (i.e., networks linking these central hubs to more peripheral regions), particularly involving the thalamus. Interestingly, thalamic connectivity was associated with cognition in men during prodromal AD and in women during AD dementia, suggesting stage- and sex-specific relevance of this network (Kim et al., 2023).
Using similar structural MRI approaches, others have also investigated sex differences in DLB, revealing distinct patterns of regional atrophy and age-related vulnerability. In a multicenter study combining data from the European-DLB consortium and Mayo Clinic, men exhibited widespread gray matter volume loss across frontal, temporal, and parietal regions, while women showed more localized atrophy in the right entorhinal and olfactory cortices (Oltra et al., 2023). Sex differences in atrophy were age-dependent, with men showing more severe neurodegeneration at younger ages than women with DLB, before diminishing after 75 years of age. Importantly, this regional atrophy correlated with cognitive impairment and visual hallucinations only amongst DLB men (Oltra et al., 2023). These findings were broadly aligned with visual rating analyses, which demonstrated that frontal atrophy was preferentially associated with DLB in men (Abdelnour et al., 2020). Using network analyses, Habich et al. (2024) revealed that normative sex differences in gray matter network organization were diminished in DLB. This network-level convergence aligns with the observation that sex differences in regional atrophy tend to diminish with increasing age. Sex differences in WMH burden were not observed in DLB (Ferreira et al., 2021).
In summary, sex differences in brain structure are evident across the AD spectrum and in DLB, encompassing both global and region-specific alterations that also manifest in prognostic subtypes, biomarker relationships, and network organization.
Functional MRI
Data from a range of functional neuroimaging studies using functional MRI, FDG-PET, magnetoencephalography, and SPECT imaging have consistently revealed sex-dependent alterations in brain network organization, metabolic activity, and neurotransmitter pathways in both AD/MCI and DLB. One study found that women with MCI demonstrated significantly lower degree centrality (i.e., measure of how many direct network connections a brain region maintains), and global efficiency (i.e., the capacity of the brain network to efficiently integrate information across region) compared to men, with these sex differences diminishing during AD progression (Cieri et al., 2021). Age-related changes in caudate nodal strength, a graph-theoretical measure capturing how strongly a brain region is connected to the rest of the network, showed sex-specific patterns in MCI patients. Older age was associated with greater caudate connectivity strength in women but not in men (Yang et al., 2022). Complementing these findings, hippocampal connectivity to the precuneus cortex and brain stem was significantly stronger in men than women with MCI (Williamson et al., 2022). Additional network topology analyses revealed distinct sex-specific hyperactivity patterns: women showed default mode network-centered hyperactivity while men exhibited limbic system-centered hyperactivity across AD and MCI populations. Connectivity changes demonstrated stronger associations with network centrality in women (Wang et al., 2024). Resting-state fMRI studies in AD revealed complementary patterns, with men showing significantly lower connectivity strength within sensorimotor and attention networks, and network properties correlating with cognitive behavior specifically in men (Li et al., 2021).
Recent research is examining factors which may modulate the relation of sex with functional brain neuroimaging. One emerging area is the interaction between sex and genetic risk. Sex differences in connectivity has been found to interact with genetic risk factors. Indeed, Lin et al. (2020) demonstrated that among women MCI participants who were APOE ε4 carriers, fMRI showed reduced connectivity in multiple brain regions compared to non-carriers. Furthermore, a significant APOE-by-sex interaction was found, whereby cognitive scores were negatively associated with connectivity changes in men (Lin et al., 2020). Extending this line of work to later disease stages, Williamson et al. (2024) found that in AD dementia patients, intrahippocampal connectivity differences between APOE ε4 carriers and non-carriers only became apparent when analyses were stratified by sex (Williamson et al., 2024).
Metabolic brain changes evaluated using FDG-PET also showed sex-related trajectories. Longitudinal FDG-PET studies revealed sex-specific metabolic trajectories. In a South Korean AD cohort (n = 181), brain metabolic impairment at two-year follow-up was evident exclusively in women. Park et al. (2023) demonstrated in a longitudinal South Korean cohort (n = 181) of AD patients that brain metabolic impairment at two-year follow-up was evident only in women (Park et al., 2023). Similarly, Beheshti et al. (2021) found that while cognitively healthy women initially showed metabolically “younger” brains relative to men, this advantage was diminished in MCI AD (Beheshti et al., 2021). In early AD, higher serum VEGF levels were negatively associated with regional cerebral blood flow (rCBF) in the angular gyrus, with this effect driven primarily by men—suggesting a sex-specific link between angiogenesis and cerebral perfusion that may have therapeutic implications (Song et al., 2025).
Beyond metabolic and perfusion measures, electrophysiological and vascular imaging studies also revealed sex-dependent brain alterations. MCI patients showed sex-specific correlations between brain oscillations and CSF biomarkers. In men, lower CSF Aβ42 concentrations (reflecting greater amyloid burden) were associated with higher central–posterior theta power, a slower rhythm typically linked to memory encoding (Klimesch, 1999). However, in women, higher CSF t-tau levels were related to lower beta power, a faster oscillatory activity that has been associated with cognitive and emotional regulation (Chino-Vilca et al., 2022; Ray and Cole, 1985). Using dynamic contrast-enhanced MRI, blood–brain barrier permeability in the occipital cortex differed by sex in MCI, as permeability and education were predictive of cognitive scores exclusively in women (Moon et al., 2021).
While sex-related functional brain differences are well documented in MCI and AD, the extent to which they manifest in DLB has been less studied. Men with DLB exhibited more severe and diffuse disruptions in metabolic connectivity, particularly affecting cholinergic pathways including Ch4-perisylvian projections, while women with DLB showed more localized metabolic impairments (Caminiti et al., 2023). SPECT imaging revealed comparable striatal dopaminergic deficits between sexes. However, women demonstrated greater reductions in extrastriatal projections with more extensive long-distance disconnections between subcortical and cortical regions, while men exhibited more focal changes (Boccalini et al., 2023). The predominance of men was observed among cognitively unimpaired patients presenting with REM Sleep Behavior Disorder and reduced cardiac MIBG uptake (Fujishiro et al., 2021). Electrophysiological analyses further supported sex differences in DLB pathophysiology, as women with DLB demonstrated lower central-parietal delta activity and higher posterior alpha activity compared to men (Del Percio et al., 2025).
Collectively, these studies consistently demonstrated sex-dependent differences in network connectivity, metabolic activity as well as and neurophysiological signatures in AD and DLB. Women tended to showed earlier functional advantages followed by steeper decline, while men exhibited more gradual but widespread functional impairments. Moreover, APOE ε4 modified these patterns differentially by sex.
Molecular imaging biomarkers of AD
Molecular neuroimaging is a rapidly evolving field. Recent studies using brain PET radiotracers for amyloid and tau demonstrated significant sex differences both with respect to the severity of pathological protein accumulation and distribution patterns. In the largest “real-world” amyloid PET datasets to date, Abu Raya et al. (2025) analyzed 10,361 patients with cognitive impairment—including both MCI and dementia—and found that women had significantly higher amyloid PET positivity rates and greater amyloid burden than men, independent of age, comorbidities, and other demographic and clinical covariates (Abu Raya et al., 2025). Women with MCI or AD exhibited significantly greater amyloid (PIB-PET) and tau (Flortaucipir-PET) accumulation in AD-relevant brain regions than men, despite being clinically comparable in terms of cognition (MMSE, CDR-Sum of Boxes, episodic memory) and cortical thickness (Edwards et al., 2021). Consistent with these findings, Buckley et al. (2020) found that MCI women exhibited higher tau-PET signal than men across widespread cortical regions—particularly among β-amyloid–positive women—and that elevated tau burden predicted faster cognitive decline in women compared to men, suggesting greater tau vulnerability and accelerated disease progression in women (Buckley et al., 2020).
Sex differences in tau burden were pronounced during the MCI phase, with tau deposition correlating more strongly with verbal memory performance in women (Banks et al., 2021). Men with prodromal AD showed significantly lower tau burden compared to women (Digma et al., 2020). Sex-by-APOE ε4 interactions were observed across multiple cohorts. Women who are APOE ε4 carriers showed higher tau burden in the medial temporal lobe regions compared to men carriers (Wang et al., 2021). This pattern was further substantiated by Yan et al. (2021), who found that male APOE ε4 homozygotes showed significantly higher tau deposition than male heterozygotes, whereas female APOE ε4 heterozygotes already showed greater tau deposition across multiple regions, suggesting a sex-dependent dose–response relationship between APOE ε4 and tau pathology (Yan et al., 2021).
Beyond regional analyses, network-level investigations revealed distinct sex-specific patterns of tau spread. Shokouhi et al. (2020) demonstrated that tau networks amongst women were characterized by significantly greater network density and more numerous direct regional connections compared to men, with these differences most evident at the MCI stage of AD (Shokouhi et al., 2020). The functional implications of these sex-specific tau patterns extend to neurochemical systems as well. Wang et al. (2025) demonstrated that hippocampal mGluR5 receptor availability was negatively associated with tau deposition and cognitive associations were only present in women with AD dementia, where tau deposition mediated the receptor-cognition relationship (Wang et al., 2025). In DLB, women were more likely to exhibit concurrent amyloid and tau pathology compared to men in a multicenter study of 417 patients (Ferreira et al., 2020). In summary, there are robust sex differences in both the burden and spatial distribution of amyloid and tau pathology, with women often showing higher pathological burden and stronger cognitive associations than men in both AD dementia and DLB.
Discussion
Our review demonstrates that biological sex has strong influences on biomarker expression across AD dementia and DLB, revealing three broad patterns with potential bearing for precision medicine and sex-informed therapeutic approaches. In the following section, we interpret the broad themes emerging from this review, outline several limitations of current research, and propose future research priorities for advancing our mechanistic understanding of sex differences in AD and DLB.
Women exhibit a distinctive pattern of early structural and metabolic advantages followed by steeper decline trajectories during disease progression. Structural neuroimaging studies demonstrate that women maintain larger hippocampal volumes and greater cortical thickness during normal aging and early disease stages including MCI (Cieri et al., 2022; Garcia et al., 2024), yet exhibit accelerated atrophy rates as pathological processes advance and dementia becomes manifest (Sauty and Durrleman, 2023). This structural pattern extends to metabolic function, where women initially demonstrate metabolically younger-appearing brains but experience more aggressive decline in MCI and AD dementia stages compared to a more gradual decline in FDG signal as observed in men (Beheshti et al., 2021; Park et al., 2023). Nonetheless, some studies report differing trajectories—for example, recent studies have shown that women with amyloid-positive AD have slower cognitive decline than men despite exhibiting earlier hippocampal atrophy and a greater burden of white matter abnormalities (Inguanzo et al., 2025) and men have demonstrated steeper increases in plasma pTau181, pTau231, and NFL (Joynes et al., 2025), underscoring the complexity and heterogeneity of sex effects across cohorts and in vivo biomarkers. It is likely that other factors, including some that researchers have yet to define and measure, play a role in the complex relation of sex to neurodegenerative disease expression.
The preservation of hippocampal volumes and cortical thickness during early disease stages may serve to relatively preserve episodic memory function while other cognitive domains become impaired, potentially partially explaining why women tend to present with impairments in verbal memory and semantic fluency rather than episodic memory deficits in early cognitive decline (Aggarwal and Mielke, 2023). An alternative or complementary explanation is that women may start from a higher baseline performance in these domains earlier in life, providing a cognitive reserve that temporarily offsets the impact of neuropathology. Such selective preservation may initially mask the clinical impact of accumulating tau pathology and could have important implications for detection and accurate early diagnosis, as they suggest that conventional biomarker thresholds may systematically underestimate disease burden in women during early stages while simultaneously overestimating progression risk in men. In addition, the stronger biomarker-clinical correlations that were documented in women across multiple biomarkers and modalities (Banks et al., 2021; Burke et al., 2019; Morrison et al., 2024; Tsiknia et al., 2022a; Yang et al., 2022) suggest differential trajectories, indicating that interpretation of biomarkers may require sex-specific frameworks to optimize diagnostic accuracy and therapeutic timing. Indeed, prediction models for MCI-to-AD conversion demonstrate significantly higher accuracy in women compared to men (Massetti et al., 2022) and hippocampal volume changes are more predictive of AD progression in women than men (Burke et al., 2019).
The literature also revealed sex-specific patterns of vulnerability that differ between AD and DLB, suggesting that disease-specific pathological processes (i.e., aggregation of amyloid and tau versus α-synuclein) may interact with biological sex. In AD, women demonstrate consistently higher tau burden across multiple brain regions (Buckley et al., 2019; Digma et al., 2020; Edwards et al., 2021), with the most pronounced sex differences appearing during the MCI stage, as tau deposition shows stronger associations with verbal memory performance in women (Banks et al., 2021). This extends beyond regional tau accumulation to broader network-level differences, with women showing greater tau network density and more extensive regional connections (Shokouhi et al., 2020). Women also demonstrate higher CSF phosphorylated and total tau levels during MCI and AD stages (Babapour Mofrad et al., 2020; Ren et al., 2024). In contrast, DLB demonstrates a vulnerability pattern in men characterized by widespread structural damage and severe metabolic connectivity disruptions, particularly affecting cholinergic pathways. Men with DLB exhibit more extensive gray matter atrophy across frontal, temporal, and parietal regions, with these structural changes appearing to correlate with cognitive impairment and visual hallucinations exclusively in men (Abdelnour et al., 2020; Oltra et al., 2023). Men also demonstrated more severe and diffuse disruptions in metabolic connectivity, particularly involving cholinergic pathways (Caminiti et al., 2023). However, women with DLB are more likely to exhibit concurrent amyloid and tau pathology (Diaz-Galvan et al., 2023; Ferreira et al., 2020) and show lower CSF α-synuclein and amyloid-β42 levels, shorter symptom duration, and more frequent hallucinations at diagnosis (van de Beek et al., 2022). This mixed pathology profile is corroborated by prior autopsy data demonstrating that despite having higher regional Lewy body, neurofibrillary tangle, and senile plaque burdens across multiple brain regions, women with pathologically-confirmed DLB remain significantly less likely to receive clinical diagnoses compared to men (Bayram et al., 2025; Nelson et al., 2010). This pattern suggests a more aggressive disease phenotype in women despite less extensive structural damage (Oltra et al., 2023), highlighting both the complexity of sex-disease interactions and potential diagnostic bias that may systematically underrecognize DLB in women.
The vulnerability to tau pathology in AD described previously among women may – at least in part – be further amplified by genetic risk factors. The APOE ε4 allele demonstrates sex-specific effects that exacerbate women’s susceptibility to tau accumulation and neurodegeneration. For instance, women who are APOE ε4 carriers exhibit accelerated tau accumulation in medial temporal regions, higher CSF tau levels, and stronger associations between plasma biomarkers and brain pathology compared to men (Liu et al., 2019; Wang et al., 2021). We speculate that the more severe tau pathology observed in women who are APOE ε4 carriers may mediate downstream functional consequences, as connectivity analyses revealed widespread network impairments, particularly affecting women who are carriers (Lin et al., 2020), though longitudinal studies with appropriate statistical models would be needed to define causal pathways. The dose–response relationship between APOE ε4 and pathological burden or neurodegenerative outcomes also appears to differ by sex. For instance, women who are heterozygotes demonstrate more pronounced tau effects than men who are heterozygotes, while men who are homozygotes show dose-dependent increases in tau deposition not observed in women who are homozygotes (Yan et al., 2021). This heightened vulnerability to APOE ε4 among women is supported by epidemiological evidence showing that women with at least one copy of APOE ε4 exhibit greater risk and faster cognitive decline relative to men (Neu et al., 2017). Furthermore, women with APOE ε4 show higher rates of conversion from MCI to AD compared to non-carrier women or men (Beydoun et al., 2012). The biological basis for this vulnerability in women may involve both developmental and adult hormonal influences on brain structure and function (R. Li and Singh, 2014). Indeed, endocrinological factors and particularly sex steroid hormones, have been associated with AD onset and progression, with age-related depletion of estrogens in women and androgens in men resulting in loss of neuroprotective effects (Rosario et al., 2011).
Several limitations in this review should be acknowledged. First, this review relied on a single database (PubMed) and screening was conducted by one reviewer, which may have introduced selection bias by potentially omitting eligible studies indexed in other databases or by limiting validation of study inclusion decisions. However, this risk was partly mitigated as we performed citation searching of reference lists from included articles to identify additional eligible studies where possible. There is still scarce data on how sex differences in biomarkers evolve longitudinally, particularly following the same sample through the transition from MCI to AD or prodromal α-synucleinopathies to DLB. Additionally, the underlying biological mechanisms driving these sex differences remain largely unexplored, including the roles of hormonal factors, hormone replacement therapy use, estrogen-mediated neuroprotective mechanisms, and their complex interactions with genetic risk factors such as APOE ε4 and others. Furthermore, the lack of large-scale, multi-center studies may limit the generalizability of findings across diverse populations and healthcare settings, a challenge that is compounded by the small sample sizes available for direct comparisons between women and men in many studies. This limitation is particularly more acute for biomarkers that are costly, technically demanding, or invasive— all of which factors that may limit scalability and further restrict the feasibility of adequately powered, sex-specific studies in AD and DLB. Such constraints not only diminish statistical power but also increase the risk of spurious findings, making it more challenging to detect subtle yet potentially meaningful sex-specific effects. They also restrict the generalizability of results across diverse populations and healthcare settings and limit our understanding of how sex differences manifest across different demographic and geographic contexts. To this end, ongoing initiatives that pool data across large consortiums such as the National Alzheimer’s Coordinating Center (NACC)1 and the European-DLB consortium2 offer promising opportunities to address these limitations and enable more robust analyses of sex differences in adequately powered, multi-site cohorts. In parallel, the growing awareness in the research community and development of data harmonization methods, such as ComBat and similar statistical approaches (Fortin et al., 2017), further facilitate these multi-center efforts by addressing site-specific technical variations and enabling more reliable cross-site comparisons.
Our review highlights the need for prospective, sex-stratified studies to better understand how biological sex shapes biomarker expression and disease progression. Firstly, longitudinal studies are well-suited to define the optimal timing and sequencing of different biomarker modalities for women and men, with particular attention to identifying the narrow therapeutic windows that appear to precede accelerated decline in women (Beheshti et al., 2021; Park et al., 2023). More sophisticated neuroimaging techniques, such as Neurite Orientation Dispersion and Density Imaging (NODDI), may yield deeper insights into microstructural sex-related differences (Mak et al., 2025b; Zhang et al., 2012), while emerging α-synuclein biomarkers, including CSF seed amplification assays and other synuclein-based fluid biomarkers may reveal sex-specific patterns in Lewy body pathology (Mak et al., 2025a). Building on the promising findings from single-modality studies, future multi-modal investigations could elucidate how metabolic differences interact with genetic risk factors, neuroimaging profiles, and longitudinal clinical trajectories to influence sex-specific disease mechanisms too. Importantly, the sex-related in vivo biomarker patterns identified in this review would need validation through larger, well-characterized autopsy series and to determine whether imaging vs. pathological correlations differ between sexes. Additionally, research examining sex differences in response to emerging disease-modifying therapies, including anti-amyloid, anti-tau, and anti-α-synuclein treatments, will be essential for optimizing personalized treatment strategies. In the long term, we recommend that clinical studies and trials should be designed from the outset with adequate power to detect sex-specific treatment effects, as distinct pathological vulnerabilities in men and women may translate into differential therapeutic responses. As described, sex-stratified analytical approaches have already shown promise for improving diagnostic accuracy and informing personalized interventions (Massetti et al., 2022; Tsiknia et al., 2022a; Wang et al., 2021).
Conclusion
Our review demonstrates that biological sex shapes biomarker expression in AD dementia and DLB, and poses challenges to current diagnostic and therapeutic paradigms that are largely agnostic to sex differences. Understanding these sex-specific mechanisms offers novel opportunities to improve early detection, refine therapeutic timing, and optimize personalized interventions for men and women. To these ends, the growing emphasis on sex-stratified analyses by major funding agencies such as the NIH and Alzheimer’s Association is anticipated to help address knowledge gaps in both AD and DLB research and advance our understanding of sex-specific mechanisms across neurodegenerative conditions. Ultimately, embracing biological sex as a fundamental determinant of neurodegenerative disease may transform our approach to preventing, diagnosing, and treating these conditions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EM: Writing – original draft, Writing – review & editing. KK: Supervision, Writing – review & editing. ZA: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. E.M. was supported by ADRC Developmental Pilot Grant (P30 AG062677) and the Women Health Pilot Grant research, Mayo Clinic, USA. K.K was supported by grants from the NIH (R01 AG093767, K12 AR 084222). ZA was supported by the National Institute on Aging grant R01AG074549.
Conflict of interest
KK consults for Biogen, Eisai, and BioArctic, received research support from Avid Radiopharmaceuticals and Eli Lilly, and receives funding from NIH and Alzheimer’s Drug Discovery Foundation, and the Katherine B. Andersen Endowed Professorship. ZA receives research funds from the National Institutes of Health, the Illinois Department of Public Health, and industry for the conduct of clinical trials and/or consulting (including Amylyx, Eisai, Novo Nordisk, Summus, California Institute of Regenerative Medicine) and also occasionally serves as an expert legal witness and is on the Editorial Board of the journal Frontiers in Dementia.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Abdelnour, C., Ferreira, D., Oppedal, K., Cavallin, L., Bousiges, O., Wahlund, L. O., et al. (2020). The combined effect of amyloid-β and tau biomarkers on brain atrophy in dementia with Lewy bodies. Neuroimage Clin 27:102333. doi: 10.1016/j.nicl.2020.102333
Abu Raya, M., Zeltzer, E., Blazhenets, G., Schonhaut, D. R., Allen, I. E., Carrillo, M. C., et al. (2025). Sex differences in amyloid PET in a large, real-world sample from the imaging dementia-evidence for amyloid scanning (IDEAS) study. Alzheimers Dement. 21:e70304. doi: 10.1002/alz.70304
Aggarwal, N. T., and Mielke, M. M. (2023). Sex differences in Alzheimer’s Disease. Neurol. Clin. 41, 343–358. doi: 10.1016/j.ncl.2023.01.001
Altmann, A., Tian, L., Henderson, V. W., and Greicius, M. D.Alzheimer’s Disease Neuroimaging Initiative Investigators (2014). Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573. doi: 10.1002/ana.24135
Andrews, D., Ducharme, S., Chertkow, H., Sormani, M. P., and Collins, D. L.Alzheimer’s Disease Neuroimaging Initiative (2025). The higher benefit of lecanemab in males compared to females in CLARITY AD is probably due to a real sex effect. Alzheimers Dement. 21:e14467. doi: 10.1002/alz.14467
Arnold, M., Nho, K., Kueider-Paisley, A., Massaro, T., Huynh, K., Brauner, B., et al. (2020). Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun. 11:1148. doi: 10.1038/s41467-020-14959-w
Babapour Mofrad, R., Tijms, B. M., Scheltens, P., Barkhof, F., van der Flier, W. M., Sikkes, S. A. M., et al. (2020). Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 95, e2378–e2388. doi: 10.1212/WNL.0000000000010629
Banks, S. J., Andrews, M. J., Digma, L., Madsen, J., Reas, E. T., Caldwell, J. Z. K., et al. (2021). Sex differences in Alzheimer’s disease: do differences in tau explain the verbal memory gap? Neurobiol. Aging 107, 70–77. doi: 10.1016/j.neurobiolaging.2021.05.013
Barker, W. W., Luis, C. A., Kashuba, A., Luis, M., Harwood, D. G., Loewenstein, D., et al. (2002). Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the state of Florida brain Bank. Alzheimer Dis. Assoc. Disord. 16, 203–212. doi: 10.1097/00002093-200210000-00001
Bayram, E., Coughlin, D. G., Banks, S. J., and Litvan, I. (2021). Sex differences for phenotype in pathologically defined dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry 92, 745–750. doi: 10.1136/jnnp-2020-325668
Bayram, E., Coughlin, D. G., Koga, S., Ross, O. A., Litvan, I., and Dickson, D. W. (2025). Sex differences for regional pathology in people with a high likelihood of Lewy body dementia phenotype based on underlying pathology. Alzheimer’s Dem. Diagnosis Assessment Dis. Monitoring 17:e70083. doi: 10.1002/dad2.70083
Beheshti, I., Nugent, S., Potvin, O., and Duchesne, S. (2021). Disappearing metabolic youthfulness in the cognitively impaired female brain. Neurobiol. Aging 101, 224–229. doi: 10.1016/j.neurobiolaging.2021.01.026
Berezuk, C., Khan, M., Callahan, B. L., Ramirez, J., Black, S. E., Zakzanis, K. K., et al. (2023). Sex differences in risk factors that predict progression from mild cognitive impairment to Alzheimer’s dementia. J. Int. Neuropsychol. Soc. 29, 360–368. doi: 10.1017/S1355617722000297
Bernier, R. A., Banks, S. J., Panizzon, M. S., Andrews, M. J., Jacobs, E. G., Galasko, D. R., et al. (2022). The neuroinflammatory marker sTNFR2 relates to worse cognition and tau in women across the Alzheimer’s disease spectrum. Alzheimer's Dement. Diagn. Assess. Dis. Monit. 14:e12284. doi: 10.1002/dad2.12284
Beydoun, M. A., Boueiz, A., Abougergi, M. S., Kitner-Triolo, M. H., Beydoun, H. A., Resnick, S. M., et al. (2012). Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol. Aging 33, 720–731.e4. doi: 10.1016/j.neurobiolaging.2010.05.017
Boccalini, C., Nicastro, N., Peretti, D. E., Caminiti, S. P., Perani, D., and Garibotto, V. (2023). Sex differences in dementia with Lewy bodies: An imaging study of neurotransmission pathways. Eur. J. Nucl. Med. Mol. Imaging 50, 2036–2046. doi: 10.1007/s00259-023-06132-4
Bolsewig, K., van Unnik, A. A. J. M., Blujdea, E. R., Gonzalez, M. C., Ashton, N. J., Aarsland, D., et al. (2024). Association of plasma amyloid, P-tau, GFAP, and NfL with CSF, clinical, and cognitive features in patients with dementia with Lewy bodies. Neurology 102:e209418. doi: 10.1212/WNL.0000000000209418
Buckley, R. F., Mormino, E. C., Rabin, J. S., Hohman, T. J., Landau, S., Hanseeuw, B. J., et al. (2019). Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 76, 542–551. doi: 10.1001/jamaneurol.2018.4693
Buckley, R. F., Scott, M. R., Jacobs, H. I. L., Schultz, A. P., Properzi, M. J., Amariglio, R. E., et al. (2020). Sex mediates relationships between regional tau pathology and cognitive decline. Ann. Neurol. 88, 921–932. doi: 10.1002/ana.25878
Burke, S. L., Hu, T., Fava, N. M., Li, T., Rodriguez, M. J., Schuldiner, K. L., et al. (2019). Sex differences in the development of mild cognitive impairment and probable Alzheimer’s disease as predicted by hippocampal volume or white matter hyperintensities. J. Women Aging 31, 140–164. doi: 10.1080/08952841.2018.1419476
Caminiti, S. P., Boccalini, C., Nicastro, N., Garibotto, V., and Perani, D. (2023). Sex differences in brain metabolic connectivity architecture in probable dementia with Lewy bodies. Neurobiol. Aging 126, 14–24. doi: 10.1016/j.neurobiolaging.2023.02.004
Carter, C. L., Resnick, E. M., Mallampalli, M., and Kalbarczyk, A. (2012). Sex and gender differences in Alzheimer’s disease: recommendations for future research. J Womens Health (Larchmt) 21, 1018–1023. doi: 10.1089/jwh.2012.3789
Chang, R., Trushina, E., Zhu, K., Zaidi, S. S. A., Lau, B. M., Kueider-Paisley, A., et al. (2023). Predictive metabolic networks reveal sex- and APOE genotype-specific metabolic signatures and drivers for precision medicine in Alzheimer’s disease. Alzheimers Dement. 19, 518–531. doi: 10.1002/alz.12675
Chino-Vilca, B., Rodríguez-Rojo, I. C., Torres-Simón, L., Cuesta, P., Vendrell, A. C., Piñol-Ripoll, G., et al. (2022). Sex specific EEG signatures associated with cerebrospinal fluid biomarkers in mild cognitive impairment. Clin. Neurophysiol. 142, 190–198. doi: 10.1016/j.clinph.2022.08.007
Cieri, F., Yang, Z., Cordes, D., and Caldwell, J. Z. K. (2021). Sex differences of brain functional topography revealed in normal aging and Alzheimer’s disease cohort. J Alzheimer's Dis 80, 979–984. doi: 10.3233/JAD-201596
Cieri, F., Zhuang, X., Cordes, D., Kaplan, N., Cummings, J., and Caldwell, J. (2022). Relationship of sex differences in cortical thickness and memory among cognitively healthy subjects and individuals with mild cognitive impairment and Alzheimer disease. Alzheimer's Res Ther 14:36. doi: 10.1186/s13195-022-00973-1
Contreras, J. A., Aslanyan, V., Albrecht, D. S., Mack, W. J., and Pa, J. (2022). Higher baseline levels of CSF inflammation increase risk of incident mild cognitive impairment and Alzheimer’s disease dementia. Alzheimers Dement. 14:e12346. doi: 10.1002/dad2.12346
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science (New York, N.Y.) 261, 921–923. doi: 10.1126/science.8346443
Cumplido-Mayoral, I., García-Prat, M., Operto, G., Falcon, C., Shekari, M., Cacciaglia, R., et al. (2023). Biological brain age prediction using machine learning on structural neuroimaging data: multi-cohort validation against biomarkers of Alzheimer’s disease and neurodegeneration stratified by sex. eLife 12:81067. doi: 10.7554/eLife.81067
Del Percio, C., Lizio, R., Lopez, S., Noce, G., Jakhar, D., Carpi, M., et al. (2025). Resting-state electroencephalographic rhythms depend on sex in patients with dementia due to Parkinson’s and Lewy body diseases: an exploratory study. Neurobiol. Dis. 206:106807. doi: 10.1016/j.nbd.2025.106807
Diaz-Galvan, P., Przybelski, S. A., Lesnick, T. G., Schwarz, C. G., Senjem, M. L., Gunter, J. L., et al. (2023). Β-amyloid load on PET along the continuum of dementia with Lewy bodies. Neurology 101, e178–e188. doi: 10.1212/WNL.0000000000207393
Digma, L. A., Madsen, J. R., Rissman, R. A., Jacobs, D. M., Brewer, J. B., and Banks, S. J. (2020). Women can bear a bigger burden: ante- and post-mortem evidence for reserve in the face of tau. Brain Commun. 2:fcaa025. doi: 10.1093/braincomms/fcaa025
Dubois, B., Feldman, H. H., Jacova, C., Dekosky, S. T., Barberger-Gateau, P., Cummings, J., et al. (2007). Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746. doi: 10.1016/S1474-4422(07)70178-3
Edwards, L., La Joie, R., Iaccarino, L., Strom, A., Baker, S. L., Casaletto, K. B., et al. (2021). Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer’s continuum: greater tau-PET retention in females. Neurobiol. Aging 105, 86–98. doi: 10.1016/j.neurobiolaging.2021.04.003
Fernández, A., Cuesta, P., Marcos, A., Montenegro-Peña, M., Yus, M., Rodríguez-Rojo, I. C., et al. (2024). Sex differences in the progression to Alzheimer’s disease: a combination of functional and structural markers. GeroScience 46, 2619–2640. doi: 10.1007/s11357-023-01020-z
Ferreira, D., Nedelska, Z., Graff-Radford, J., Przybelski, S. A., Lesnick, T. G., Schwarz, C. G., et al. (2021). Cerebrovascular disease, neurodegeneration, and clinical phenotype in dementia with Lewy bodies. Neurobiol. Aging 105, 252–261. doi: 10.1016/j.neurobiolaging.2021.04.029
Ferreira, D., Przybelski, S. A., Lesnick, T. G., Lemstra, A. W., Londos, E., Blanc, F., et al. (2020). β-Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology 95, e3257–e3268. doi: 10.1212/WNL.0000000000010943
Fortin, J.-P., Parker, D., Tunç, B., Watanabe, T., Elliott, M. A., Ruparel, K., et al. (2017). Harmonization of multi-site diffusion tensor imaging data. NeuroImage 161, 149–170. doi: 10.1016/j.neuroimage.2017.08.047
Fujishiro, H., Ferman, T. J., Boeve, B. F., Smith, G. E., Graff-Radford, N. R., Uitti, R. J., et al. (2008). Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J. Neuropathol. Exp. Neurol. 67, 649–656. doi: 10.1097/NEN.0b013e31817d7a1d
Fujishiro, H., Ota, K., Yamagata, M., Ito, T., Hieda, S., Suga, H., et al. (2021). Early diagnosis of prodromal dementia with Lewy bodies using clinical history of probable REM sleep behaviour disorder and cardiac (123) I-MIBG scintigraphy in memory clinics. Psychogeriatr. Off. J. Jap. Psychogeriatric Soc. 21, 288–295. doi: 10.1111/psyg.12662
Garcia, P., Mendoza, L., Padron, D., Duarte, A., Duara, R., Loewenstein, D., et al. (2024). Sex significantly predicts medial temporal volume when controlling for the influence of ApoE4 biomarker and demographic variables: a cross-ethnic comparison. JINS 30, 128–137. doi: 10.1017/S1355617723000358
Giacobini, E., and Pepeu, G. (2018). Sex and gender differences in the brain cholinergic system and in the response to therapy of Alzheimer disease with cholinesterase inhibitors. Curr. Alzheimer Res. 15, 1077–1084. doi: 10.2174/1567205015666180613111504
Habich, A., Oltra, J., Schwarz, C. G., Przybelski, S. A., Oppedal, K., Inguanzo, A., et al. (2024). Grey matter networks in women and men with dementia with Lewy bodies. NPJ Parkinsons Dis 10:84. doi: 10.1038/s41531-024-00702-5
Hall, B., Hall, B. A., Mak, E., Cervenka, S., Aigbirhio, F. I., Franklin, I. A., et al. (2017). In vivo tau PET imaging in dementia: pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Res. Rev. 36, 50–63. doi: 10.1016/j.arr.2017.03.002
Hansson, O., Edelmayer, R. M., Boxer, A. L., Carrillo, M. C., Mielke, M. M., Rabinovici, G. D., et al. (2022). The Alzheimer’s association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 18, 2669–2686. doi: 10.1002/alz.12756
Inguanzo, A., Poulakis, K., Oltra, J., Maioli, S., Marseglia, A., Ferreira, D., et al. (2025). Atrophy trajectories in Alzheimer’s disease: how sex matters. Alzheimer's Res Ther 17:79. doi: 10.1186/s13195-025-01713-x
Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562. doi: 10.1016/j.jalz.2018.02.018
Joynes, C., Bilgel, M., An, Y., Moghekar, A. R., Ashton, N. J., Kac, P. R., et al. (2025). Sex differences in the trajectories of plasma biomarkers, brain atrophy, and cognitive decline relative to amyloid onset. Alzheimers Dement. 21:e14405. doi: 10.1002/alz.14405
Kane, J. P. M., Surendranathan, A., Bentley, A., Barker, S. A. H., Taylor, J.-P., Thomas, A. J., et al. (2018). Clinical prevalence of Lewy body dementia. Alzheimer's Res Ther 10:19. doi: 10.1186/s13195-018-0350-6
Kantarci, K., Lowe, V. J., Boeve, B. F., Senjem, M. L., Tosakulwong, N., Lesnick, T. G., et al. (2016). AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann. Neurol. 81, 58–67. doi: 10.1002/ana.24825
Katabathula, S., Davis, P. B., and Xu, R. (2023). Sex-specific heterogeneity of mild cognitive impairment identified based on multi-modal data analysis. J Alzheimer's Dis 91, 233–243. doi: 10.3233/JAD-220600
Kawakami, J., Piccolo, S. R., Kauwe, J. K., and Graves, S. W. (2022). Gender differences contribute to variability of serum lipid biomarkers for Alzheimer’s disease. Biomark. Med 16, 1089–1100. doi: 10.2217/bmm-2022-0462
Kim, S.-J., Bae, Y. J., Park, Y. H., Jang, H., Kim, J. P., Seo, S. W., et al. (2023). Sex differences in the structural rich-club connectivity in patients with Alzheimer’s disease. Front. Aging Neurosci. 15:1209027. doi: 10.3389/fnagi.2023.1209027
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29, 169–195. doi: 10.1016/S0165-0173(98)00056-3
Labonte, J., Sugarman, M. A., Pettway, E., Zetterberg, H., Blennow, K., Ashton, N. J., et al. (2025). Sex differences on tau, astrocytic, and neurodegenerative plasma biomarkers. J Alzheimer's Dis 105, 443–452. doi: 10.1177/13872877251329468
Li, R., and Singh, M. (2014). Sex differences in cognitive impairment and Alzheimer’s Disease. Front. Neuroendocrinol. 35, 385–403. doi: 10.1016/j.yfrne.2014.01.002
Li, X., Zhou, S., Zhu, W., Li, X., Gao, Z., Li, M., et al. (2021). Sex difference in network topology and education correlated with sex difference in cognition during the Disease process of Alzheimer. Front. Aging Neurosci. 13:639529. doi: 10.3389/fnagi.2021.639529
Lin, H., Sun, Y., Li, M., Zhan, Y., Lin, L., Ding, Z., et al. (2020). Sex modulates the apolipoprotein E ε4 effect on white matter and cortical functional connectivity in individuals with amnestic mild cognitive impairment. Eur. J. Neurol. 27, 1415–1421. doi: 10.1111/ene.14226
Liu, C., Li, Y., Nwosu, A., Ang, T. F. A., Liu, Y., Devine, S., et al. (2022). Sex-specific biomarkers in Alzheimer’s disease progression: Framingham heart study. Alzheimer Dem. 14:e12369. doi: 10.1002/dad2.12369
Liu, M., Paranjpe, M. D., Zhou, X., Duy, P. Q., Goyal, M. S., Benzinger, T. L., et al. (2019). Sex modulates the ApoE ε4 effect on brain tau deposition measured by 18F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics 9, 4959–4970. doi: 10.7150/thno.35366
Mak, E., Nicastro, N., Malpetti, M., Savulich, G., Surendranathan, A., Holland, N., et al. (2021). Imaging tau burden in dementia with Lewy bodies using [18F]-AV1451 positron emission tomography. Neurobiol. Aging 101, 172–180. doi: 10.1016/j.neurobiolaging.2020.11.006
Mak, E., Przybelski, S. A., Wiste, H. J., Fought, A. J., Schwarz, C. G., Senjem, M. L., et al. (2025a). Influence of alpha-synuclein on glucose metabolism in Alzheimer’s disease continuum: analyses of α-synuclein seed amplification assay and FDG-PET. Alzheimers Dement. 21:e14571. doi: 10.1002/alz.14571
Mak, E., Reid, R. I., Przybelski, S. A., Fought, A. M., Lesnick, T. G., Schwarz, C. G., et al. (2025b). Cortical microstructural abnormalities in dementia with Lewy bodies and their associations with Alzheimer’s disease copathologies. NPJ Parkinsons Dis. 11:124. doi: 10.1038/s41531-025-00944-x
Mak, E., Su, L., Williams, G. B., and O’Brien, J. T. (2014). Neuroimaging characteristics of dementia with Lewy bodies. Alzheimer's Res Ther 6:18. doi: 10.1186/alzrt248
Massetti, N., Russo, M., Franciotti, R., Nardini, D., Mandolini, G. M., Granzotto, A., et al. (2022). A machine learning-based holistic approach to predict the clinical course of patients within the Alzheimer’s disease spectrum. J Alzheimer's Dis 85, 1639–1655. doi: 10.3233/JAD-210573
McKeith, I. G., Boeve, B. F., Dickson, D. W., Halliday, G., Taylor, J.-P., Weintraub, D., et al. (2017). Diagnosis and management of dementia with Lewy bodies. Neurology 89, 88–100. doi: 10.1212/wnl.0000000000004058
Moon, Y., Lim, C., Kim, Y., and Moon, W.-J. (2021). Sex-related differences in regional blood-brain barrier integrity in non-demented elderly subjects. Int. J. Mol. Sci. 22:2860. doi: 10.3390/ijms22062860
Morrison, C., Dadar, M., and Collins, D. L. (2024). Sex differences in risk factors, burden, and outcomes of cerebrovascular disease in Alzheimer’s disease populations. Alzheimers Dement. 20, 34–46. doi: 10.1002/alz.13452
Nebel, R. A., Aggarwal, N. T., Barnes, L. L., Gallagher, A., Goldstein, J. M., Kantarci, K., et al. (2018). Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 14, 1171–1183. doi: 10.1016/j.jalz.2018.04.008
Nelson, P. T., Schmitt, F. A., Jicha, G. A., Kryscio, R. J., Abner, E. L., Smith, C. D., et al. (2010). Association between male gender and cortical Lewy body pathology in large autopsy series. J. Neurol. 257, 1875–1881. doi: 10.1007/s00415-010-5630-4
Neu, S. C., Pa, J., Kukull, W., Beekly, D., Kuzma, A., Gangadharan, P., et al. (2017). Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 74, 1178–1189. doi: 10.1001/jamaneurol.2017.2188
Oltra, J., Habich, A., Schwarz, C. G., Nedelska, Z., Przybelski, S. A., Inguanzo, A., et al. (2023). Sex differences in brain atrophy in dementia with Lewy bodies. Alzheimers Dement. 20, 1815–1826. doi: 10.1002/alz.13571
Oveisgharan, S., Arvanitakis, Z., Yu, L., Farfel, J., Schneider, J. A., and Bennett, D. A. (2018). Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 136, 887–900. doi: 10.1007/s00401-018-1920-1
Park, J.-C., Lim, H., Byun, M. S., Yi, D., Byeon, G., Jung, G., et al. (2023). Sex differences in the progression of glucose metabolism dysfunction in Alzheimer’s disease. Exp. Mol. Med. 55, 1023–1032. doi: 10.1038/s12276-023-00993-3
Ray, W. J., and Cole, H. W. (1985). EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science 228, 750–752. doi: 10.1126/science.3992243
Ren, C., Wang, W. Q., Li, H. H., Li, B. Y., Shi, K. N., Yang, L. N., et al. (2024). Valuing the importance of sex differences in prodromal Alzheimer’s disease based on structural magnetic resonance imaging. J Alzheimer's Dis 102, 347–358. doi: 10.1177/13872877241289790
Rosario, E. R., Chang, L., Head, E. H., Stanczyk, F. Z., and Pike, C. J. (2011). Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol. Aging 32, 604–613. doi: 10.1016/j.neurobiolaging.2009.04.008
Sarica, A., Pelagi, A., Aracri, F., Arcuri, F., Quattrone, A., and Quattrone, A. (2024). Sex differences in conversion risk from mild cognitive impairment to Alzheimer’s disease: an explainable machine learning study with random survival forests and SHAP. Brain Sci. 14:201. doi: 10.3390/brainsci14030201
Sauty, B., and Durrleman, S. (2023). Impact of sex and APOE-ε4 genotype on patterns of regional brain atrophy in Alzheimer’s disease and healthy aging. Front. Neurol. 14:1161527. doi: 10.3389/fneur.2023.1161527
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer’s disease. Lancet 397, 1577–1590. doi: 10.1016/S0140-6736(20)32205-4
Shokouhi, S., Taylor, W. D., Albert, K., Kang, H., and Newhouse, P. A. (2020). In vivo network models identify sex differences in the spread of tau pathology across the brain. Alzheim. Dem. 12:e12016. doi: 10.1002/dad2.12016
Song, B. X., Jiang, G., Wong, M., Gallagher, D., MacIntosh, B. J., Andreazza, A. C., et al. (2025). Neuroimaging meets neurophysiology: vascular endothelial growth factor and regional cerebral blood flow in early Alzheimer’s disease. J. Neurophysiol. 133, 924–929. doi: 10.1152/jn.00604.2024
Sundermann, E. E., Maki, P., Biegon, A., Lipton, R. B., Mielke, M. M., Machulda, M., et al. (2019). Sex-specific norms for verbal memory tests may improve diagnostic accuracy of amnestic MCI. Neurology 93, e1881–e1889. doi: 10.1212/WNL.0000000000008467
Tsiknia, A. A., Edland, S. D., Sundermann, E. E., Reas, E. T., Brewer, J. B., Galasko, D., et al. (2022a). Sex differences in plasma p-tau181 associations with Alzheimer’s disease biomarkers, cognitive decline, and clinical progression. Mol. Psychiatry 27, 4314–4322. doi: 10.1038/s41380-022-01675-8
Tsiknia, A. A., Sundermann, E. E., Reas, E. T., Edland, S. D., Brewer, J. B., Galasko, D., et al. (2022b). Sex differences in Alzheimer’s disease: plasma MMP-9 and markers of disease severity. Alzheimer's Res Ther 14:160. doi: 10.1186/s13195-022-01106-4
van de Beek, M., Ooms, F. A. H., Ebenau, J. L., Barkhof, F., Scheltens, P., Teunissen, C. E., et al. (2022). Association of the ATN research framework with clinical profile, cognitive decline, and mortality in patients with dementia with Lewy bodies. Neurology 98, e1262–e1272. doi: 10.1212/WNL.0000000000200048
van Kruining, D., Losen, M., Crivelli, S. M., de Jong, J. J. A., Jansen, J. F. A., Backes, W. H., et al. (2023). Plasma ceramides relate to mild cognitive impairment in middle-aged men: the Maastricht study. Alzheimers Dement. 15:e12459. doi: 10.1002/dad2.12459
Vann Jones, S. A., and O’Brien, J. T. (2013). The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol. Med. 44, 673–683. doi: 10.1017/s0033291713000494
Wang, Y.-T. T., Pascoal, T. A., Therriault, J., Kang, M. S., Benedet, A. L., Savard, M., et al. (2021). Interactive rather than independent effect of APOE and sex potentiates tau deposition in women. Brain Commun. 3:fcab126. doi: 10.1093/braincomms/fcab126
Wang, Y., Wang, J., Chen, X., Lin, Z., You, Z., He, K., et al. (2025). Tau pathology is associated with postsynaptic metabotropic glutamate receptor 5 (mGluR5) in early Alzheimer’s disease in a sex-specific manner. Alzheimers Dement. 21:e70004. doi: 10.1002/alz.70004
Wang, S., Wang, Y., Xu, F. H., Tian, X., Fredericks, C. A., Shen, L., et al. (2024). Sex-specific topological structure associated with dementia via latent space estimation. Alzheimers Dement. 20, 8387–8401. doi: 10.1002/alz.14266
Weiner, M. F., Risser, R. C., Cullum, C. M., Honig, L., White, C., Speciale, S., et al. (1996). Alzheimer’s disease and its Lewy body variant: a clinical analysis of postmortem verified cases. Am. J. Psychiatry 153, 1269–1273. doi: 10.1176/ajp.153.10.1269
Williamson, J., James, S. A., Mukli, P., Yabluchanskiy, A., Wu, D. H., Sonntag, W., et al. (2024). Sex difference in brain functional connectivity of hippocampus in Alzheimer’s disease. GeroScience 46, 563–572. doi: 10.1007/s11357-023-00943-x
Williamson, J., Yabluchanskiy, A., Mukli, P., Wu, D. H., Sonntag, W., Ciro, C., et al. (2022). Sex differences in brain functional connectivity of hippocampus in mild cognitive impairment. Front. Aging Neurosci. 14:959394. doi: 10.3389/fnagi.2022.959394
Xu, J., Green, R., Kim, M., Lord, J., Ebshiana, A., Westwood, S., et al. (2021). Sex-specific metabolic pathways were associated with Alzheimer’s disease (AD) endophenotypes in the European medical information framework for AD multimodal biomarker discovery cohort. Biomedicine 9:1610. doi: 10.3390/biomedicines9111610
Yan, S., Zheng, C., Paranjpe, M. D., Li, Y., Li, W., Wang, X., et al. (2021). Sex modifies APOE ε4 dose effect on brain tau deposition in cognitively impaired individuals. Brain J. Neurol. 144, 3201–3211. doi: 10.1093/brain/awab160
Yang, Z., Caldwell, J. Z. K., Cummings, J. L., Ritter, A., Kinney, J. W., Cordes, D., et al. (2022). Sex modulates the pathological aging effect on caudate functional connectivity in mild cognitive impairment. Front. Psych. 13:804168. doi: 10.3389/fpsyt.2022.804168
Zarzar, T. G., Lee, B., Coughlin, R., Kim, D., Shen, L., and Hall, M. A. (2022). Sex differences in the metabolome of Alzheimer’s disease progression. Front. Radiol. 2:782864. doi: 10.3389/fradi.2022.782864
Keywords: dementia, Lewy bodies (LBD), neuroimaging, biomarker, sex differences, Alzheimer
Citation: Mak E, Kantarci K and Arvanitakis Z (2025) Sex differences in in vivo biomarkers of neurodegenerative dementia. Front. Dement. 4:1706177. doi: 10.3389/frdem.2025.1706177
Edited by:
Dorina Cadar, Brighton and Sussex Medical School, United KingdomReviewed by:
Velandai Srikanth, Monash University, AustraliaMaricedes Acosta-Martinez, Stony Brook University, United States
Copyright © 2025 Mak, Kantarci and Arvanitakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elijah Mak, bWFrLmVsaWphaEBtYXlvLmVkdQ==
 Elijah Mak
Elijah Mak Kejal Kantarci
Kejal Kantarci Zoe Arvanitakis2
Zoe Arvanitakis2