- 1Department of Oral Biology, Faculty of Dentistry, Universitas Indonesia, Jakarta, Indonesia
- 2Oral Science Research Center, Faculty of Dentistry, Universitas Indonesia, Jakarta, Indonesia
- 3Department of Oral Biology, Faculty of Dentistry, Universitas Hasanuddin, Makassar, Indonesia
- 4Department of Oral Biology, Faculty of Dentistry, Universitas Gadjah Mada, Yokyakarta, Indonesia
- 5Department of Oral Biology, Dental Medicine Faculty, Universitas Airlangga, Surabaya, Indonesia
Green leafy vegetables such as arugula are rich in nitrates that support oral health, yet their effects on oral microbial balance, especially in smokers, remain unclear. This study evaluated the survival and activity of nitrate-reducing bacteria (NRB; Veillonella spp. and Rothia spp.) in biofilm exposed to nitrate-containing arugula juice (3.25 or 6.25 μM). The proportions of NRB were compared with periodontopathogens (Porphyromonas gingivalis and Fusobacterium nucleatum). Using quantitative real-time PCR (qPCR), we assessed bacterial survival and transcription of nitrate reductase genes (narG and napA) in biofilm from smokers and non-smokers. The results revealed that nitrate-containing arugula juice increased NRB bacteria abundance while reducing periodontopathogen growth. A higher level of nitrate (6.25 μM) increased nitrate reductase expression. Prolonged exposure (9 h) sustained the growth-promoting effect on Rothia spp. These results suggest that non-smokers have more nitrate-reducing bacteria in their biofilm, which promotes oral microbial balance. Thus, smokers might be advised to consume nitrate-containing arugula juice to promote NRB, which may have health benefits.
1 Introduction
The human oral cavity hosts a complex microbial community that maintains health when in balance (symbiosis), but can lead to disease when disrupted (dysbiosis) (1). Oral dysbiosis is associated not only with oral issues such as caries and periodontal diseases but also systemic conditions, including diabetes, cancer, and cardiovascular disease (2, 3). Maintaining a healthy oral microbiota is therefore critical for overall health.
Cigarette smoking, a major public health concern, disrupts the oral microbiota by reducing diversity and promoting an anaerobic environment (4, 5). This disruption often occurs before clinical symptoms arise and is linked to an increased risk of systemic diseases (6). Although smoking is prevalent in Indonesia, little is known about its specific effect on the oral microbiota in this population, particularly on nitrate-reducing bacteria (NRB), which play a role in maintaining oral health. Understanding these effects could provide valuable insight into related oral dysbiosis in Indonesians.
Nitrate-rich vegetables, such as arugula (Eruca sativa), have gained attention for their potential to support oral health by promoting beneficial nitrate-reducing bacteria. These bacteria convert dietary nitrate into nitrite and then nitric oxide, an antimicrobial agent that helps prevent dysbiosis (1, 7). However, the role of arugula juice in mitigating the effects of smoking on oral health remains unclear. The aim of this study was to explore whether nitrate-containing arugula juice affects smokers’ salivary biofilm, specifically how it can promote nitrate-reducing bacteria and prevent dysbiosis. By exploring this, this study seeks to develop new strategies for managing smoking-related oral health challenges.
2 Materials and methods
2.1 Participants
The saliva donors included 12 smokers and 12 non-smokers aged 20–35 years, with a balanced male sex distribution (60%–75% in each group). The participants had good general health, absence of systemic disease, at least 20 teeth with no active caries (good cavity fillings were acceptable), no impacted teeth, no inflamed third molars, and no teeth with root canal treatment or periapical lesions.
To mitigate potential confounding factors that could influence the baseline oral microbiome composition and subsequently in vitro biofilm formation, we implemented strict inclusion and exclusion criteria. All participants, both smokers and non-smokers, were selected based on having good oral hygiene, as assessed by the simplified oral hygiene index (OHI-s) category (8), and plaque index (PI) < 1 (9, 10). Exclusion criteria included antibiotic or oral antiseptic use within the last month, oral protheses, orthodontic appliances, gingivitis, or chronic periodontal disease. Smokers were defined as those smoking at least one cigarette daily, while non-smokers had no history of tobacco use.
2.2 Saliva collection
It was suggested that the individuals adhere to their regular daily dietary routine. No particular dietary or drinking instructions were offered (11). Unstimulated saliva was collected in the morning after fasting for 1 h. After letting their saliva gather for approximately a minute, participants spat 2–3 ml of saliva into sterile tubes. Samples were stored on ice and frozen at −80°C until analysis. Ethical approval was granted by the Faculty of Dentistry, Universitas Indonesia (Protocol number: 010580724).
2.3 Nitrate estimation in saliva
Salivary nitrite was quantified using the Griess reaction (12). Test samples (100 µl) were mixed with Griess reagents (Promega Corporation, Madison, WI, USA), incubated for 10 min at room temperature, and absorbance was measured at 540 nm using an ELISA reader.
2.4 Preparation of arugula juice
Fresh arugula leaves were washed, fried, and stored at −4°C before use. A blend of 100 g leaves and 100 ml of cold phosphate buffer saline (PBS) was homogenized, centrifuged (12,000 rpm, 15 min, 4°C), filtered (0.22 µM), and stored at 4°C for up to 24 h (13).
2.5 Biofilm assay
Pooled saliva from smokers and non-smokers was centrifuged, and pellets were resuspended in PBS. Saliva (30 µl, containing bacteria/108 CFU ml) was mixed with arugula juice (30 µl, nitrate concentrations of 3.25 or 6.25 µM) and 40 µl of brain heart infusion (BHI) broth and then inoculated into 96-well plates. Biofilms grown without nitrate served as controls. Plates were incubated at 37°C aerobically and anaerobically (microaerophilic) using a gas mixture (H2 10%, CO2 10%, and N2 20%), incubated for 5 and 9 h, and biofilm bacteria were quantified using quantitative real-time PCR (qPCR).
2.6 PH and nitrate measurement in biofilms
Biofilm pH was assessed using a pH indicator strip. Our aim was to ascertain whether the pH was above or below 6 (14).
2.7 qPCR analysis
DNA and RNA were extracted from biofilm cells after removing extracellular DNA and non-viable cells (14). The Qubit assay kit with a Qubit fluorometer (Invitrogen) was used to measure the quantity and quality of the DNA and RNA. We measured the amount of bacteria's target DNA and mRNA transcription of nitrate-associated genes (narG and napA) in the biofilm using the SYBR green I binding dye and the particular primers listed in Table 1. The qPCR was carried out using the PCR procedure as previously reported (14) in LightCycler-96 (Roche). The abundance of each targeted bacteria was determined using the relative proportion to total bacteria (15, 16). Relative gene expression was calculated using the 2−ΔΔCt method, with nitrate-free biofilms acting as the control.
2.8 Statistical analysis
Data were analyzed using GraphPad PRISM 10. Differences between groups (e.g., nitrate levels, bacterial abundance, and gene expression) were assessed using Kruskal–Wallis ANOVA or Student's t-test, with significance set at p < 0.05.
3 Results
3.1 Salivary nitrate–nitrite levels (smokers vs. non-smokers)
The comparison of salivary nitrate–nitrite levels between the two groups (smoker and non-smoker) is summarized in Figure 1A. A Griess reaction standard curve (Figure 1B) validated the accuracy of the nitrate–nitrite measurement. This demonstrates that the assay was sensitive across a wide range of concentrations (0–150 µM), ensuring reliability in detecting the differences observed in smokers and non-smokers. We discovered that smoker participants had significantly lower nitrate–nitrite levels than non-smoking participants (p < 0.05).
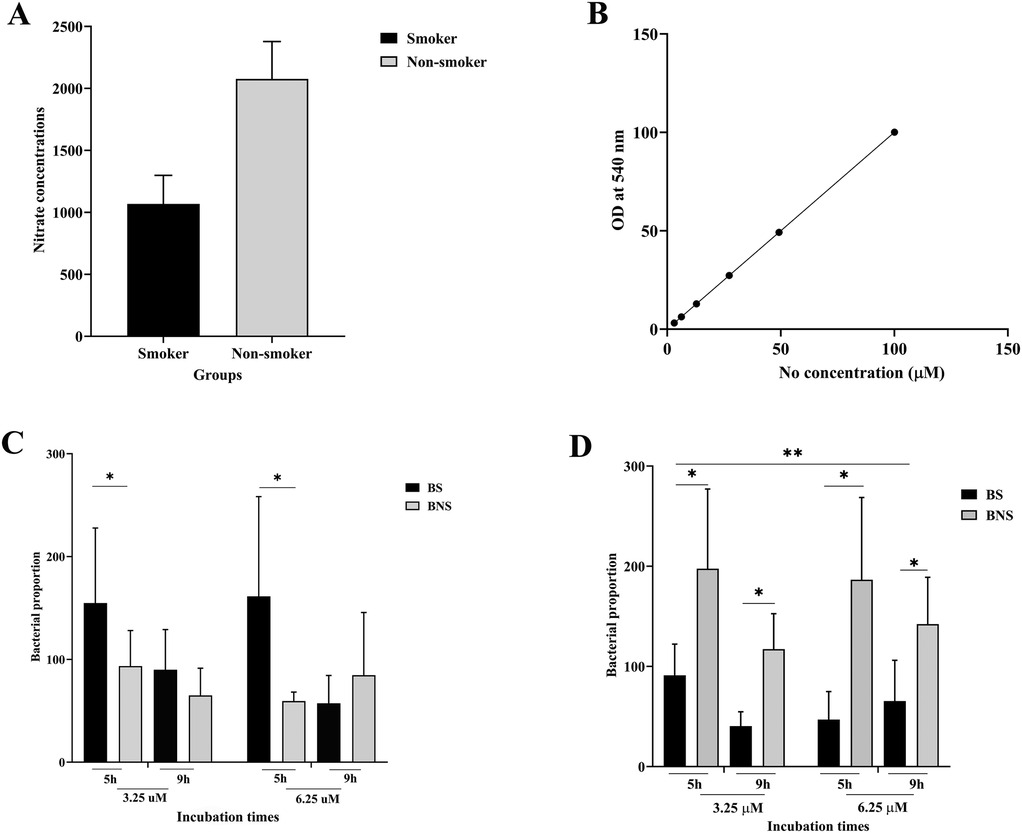
Figure 1. Comparison of nitrate-nitrite levels found in saliva and salivary bacterial counts from smokers (BS) and non-smokers (BNS) in biofilm. Salivary nitrate concentrations in non-smokers were substantially higher than in smokers (A), and nitrate concentrations were calculated using a standard curve (B). The bacterial proportions, which were evaluated in both aerobic (C) and anaerobic (D) conditions, were affected by varying arugula nitrate concentrations and incubation times. * indicates statistical significance (p < 0.005).
3.2 Impact of arugula nitrate on bacterial biofilms
As shown in Figures 1C,D, in aerobic conditions, bacteria from smokers (BS) showed higher abundance than those from non-smokers (BNS) at 5 h with both nitrate concentrations (3.25 and 6.25 µM). At 9 h, the growth of BS bacteria was no longer increasing but was still higher than BNS (with nitrate at 3.25 µM). The reverse was found in anaerobic conditions, as BNS consistently showed higher bacterial abundance under all nitrate concentrations. However, this increase diminished over time, as we observed at 9 h time period.
3.3 The impact of arugula-derived nitrate on nitrate-reducing bacteria (Rothia spp. and Veillonella spp.)
As shown in Figures 2A,B, at 9 h and in aerobic conditions, there was a significant growth of Rothia and Veillonella spp. in the BNS samples, regardless of the nitrate concentrations (3.25 and .25 μM). After 5 h of incubation, a higher abundance of Rothia spp. was found in both conditions, irrespective of the sample's source (BS or BNS), and this remained so at 9 h for all arugula nitrate concentrations. We found that the increased proportion of Veillonela spp. was influenced by the duration of incubation, but their numbers were still lower than those of Rothia spp.
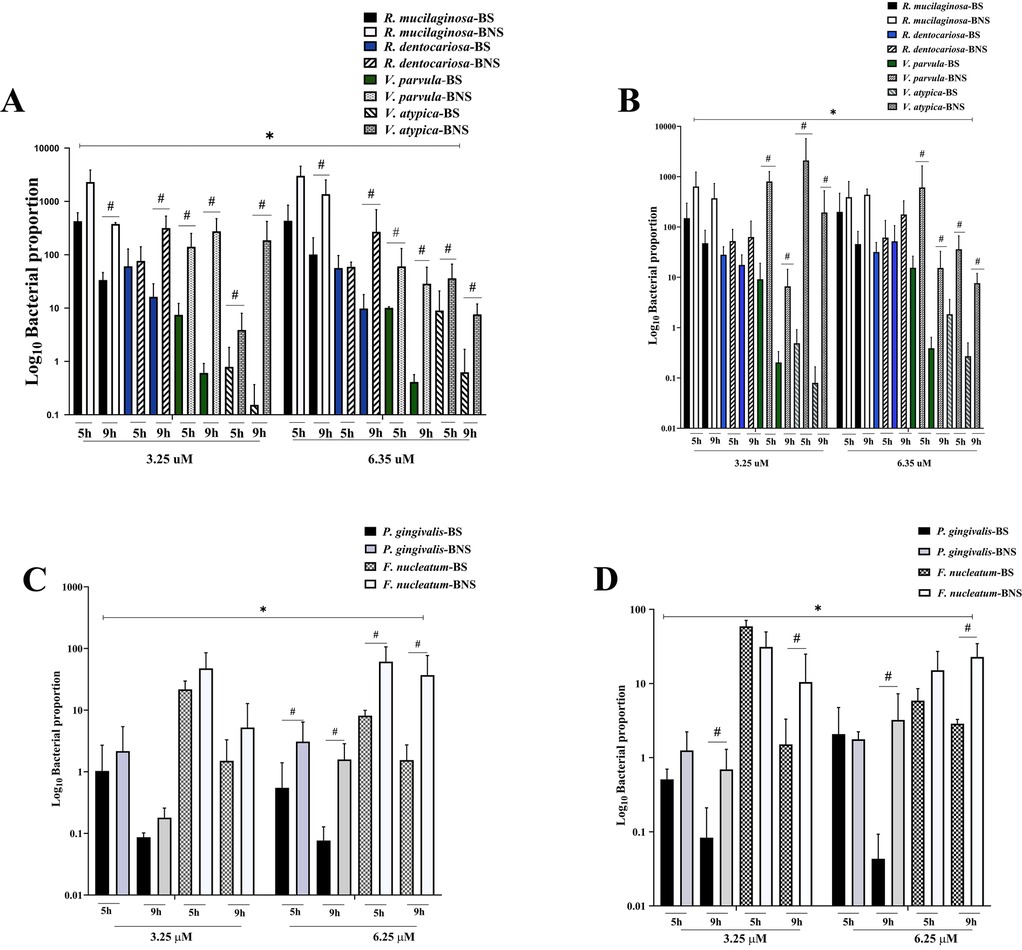
Figure 2. The assessment of nitrate-reducing bacteria and periodontopathogens in the biofilm assay. The bar graph shows that the numbers of nitrate-reducing bacteria (A,B) and periodontopathogens (C,D) varied significantly in both aerobic (A,C) and anaerobic (B,D) settings. The impact of environmental conditions on microbial communities was highlighted by the significant difference in nitrate-reducing bacteria proportions depending on arugula nitrate concentrations. However, the periodontopathogen counts showed how incubation time affected the growth of these bacteria, indicating that the biofilm environments have an impact. The results indicated the relationship between smoking status, specific experimental conditions, and different microbiological profiles. * indicates that nitrate concentration differences were statistically significant (p < 0.05). # denotes a statistically significant difference between BS and BNS (p < 0.05).
When comparing the NRB species, R. mucilaginosa showed robust growth, especially in biofilm derived from the BNS group, with significant variations. Furthermore, there were significant variations in V. parvula between the BS and BNS groups, with BNS samples exhibiting greater proportions under both nitrate concentrations. The amount of V. atypica in biofilm derived from BNS samples was significantly higher than that in biofilm derived from BS samples under all conditions (aerobic and anaerobic, and at 5 and 9 h).
3.4 Effect of arugula nitrate on periodontopathic bacteria (P. gingivalis and F. nucleatum)
We further investigated potential changes in periodontopathogens. As shown in Figure 2C, in aerobic conditions and at 5 h, the presence of arugula nitrate (3.25 μM) significantly suppressed the growth of both P. gingivalis and F. nucleatum in biofilms, irrespective of whether the bacteria originated from BS or BNS samples. Yet the growth inhibition occurred in a time-dependent manner. By 9 h, the growth suppression was more significant in the BS samples. Moreover, under anaerobic conditions, both nitrate concentrations (3.25 and 6.25 μM) were effective in reducing the growth of P. gingivalis and F. nucleatum, particularly after 9 h. The reduction was again more significant in the BS samples (Figure 2D).
3.5 The impact of arugula-derived nitrate on transcription of narG and napA
In comparison to the mRNA expression of the nitrate reductase-associated genes (narG and napA), the results demonstrated that under aerobic conditions (Figure 3A), transcription levels of both genes were consistently higher than in anaerobic conditions (Figure 3B). In both aerobic and anaerobic conditions, BNS frequently showed higher gene expression than BNS. In almost all conditions, narG exhibited greater expression levels than napA. Additionally, gene expression was higher at higher nitrate levels (6.25 µM) than at 3.25 µM, especially for narG. For all conditions, transcription levels typically decreased with time (5–9 h), especially for napA.
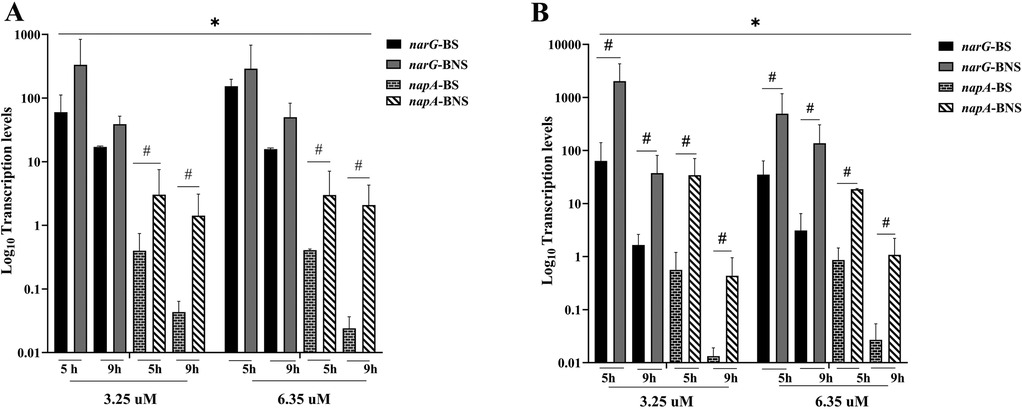
Figure 3. Nitrate-associated genes (narG and napA) transcription levels in biofilm from non-smokers (BNS) and smokers (BS). (A) Gene expression variations between groups under aerobic conditions for 5 and 9 h at different nitrate concentrations (3.25 and 6.25 µM). (B) shows the influence of environmental variables on gene transcription levels under anaerobic conditions with similar nitrate concentrations and incubation times. The findings show a significant difference in narG and napA expression, indicating unique metabolic capabilities according to experimental conditions and smoking status. * indicates that nitrate concentration differences were statistically significant (p < 0.05). # indicates a significant difference between BS and BNS (p < 0.05).
4 Discussion
The results of this study highlight the complex interplay between bacterial origin (smokers vs. non-smokers), environmental conditions (nitrate, oxygen), and the expression of nitrate-associated genes. We found that smoking had a significant impact on salivary nitrate-nitrite levels. This is in line with a previous study that found that smokers who practice adequate oral hygiene may have a less efficient nitrate reduction pathway than non-smokers (17, 18). Earlier studies reported that smoking mostly affects the oral environment by disturbing its balance, which results in dysbiotic oral communities and elevated oxidative stress (19–21). Vegetables high in dietary nitrate could be incorporated into the biofilm environment in order to restore the imbalance (22).
The present investigation demonstrated that the biofilms from smokers and non-smokers respond differently to the availability of oxygen and arugula nitrate, while the pH of the biofilms’ spent media remained constant (in the range of 5.5–7) in both anaerobic and aerobic conditions, regardless of nitrate concentrations (not shown). When smokers’ and non-smokers’ bacteria were compared, we observed that under aerobic conditions, BS biofilms initially showed greater abundance under both nitrate concentrations (3.25 and 6.35 μM). This suggests that smokers’ bacteria initially had a higher potential for aerobic nitrate use. This could be because the smokers’ oral microbiome had changed to support nitrate metabolism, possibly as a result of stresses associated with smoking (5, 23). However, this advantage diminished over time, as we observed at the 9 h time period. This could be due to competitive interactions, resource depletion, or shifts in community dynamics (24).
In contrast, in an anaerobic environment, BNS displayed higher bacterial abundance at both nitrate concentrations during the early time points (5 h). This result suggests that under anaerobic conditions, BNS may maintain nitrate-reducing capacity more effectively, possibly reflecting adaptations to low-oxygen niches typical of a healthy oral environment, which smoking disrupts through oxidative and metabolic shifts (25). This finding aligns with existing research suggesting nitrate metabolism can stimulate eubiosis in individuals without periodontitis (26), as we found in our smoker participants with good OHI. Further investigation is needed to understand the underlying mechanisms and potential implications for smokers’ oral health.
Concerning nitrate-reducing bacteria (Rothia spp. and Veillonella spp.), our results are aligned with earlier studies demonstrating that the NRB is necessary for the oral nitrate–nitrite–nitric oxide (NO) pathway (27, 28). We found that the NRB in BNS samples showed significant growth at 9 h in aerobic biofilm conditions, regardless of nitrate concentrations (3.25 or 6.35 µM). By comparing their growth patterns, we found that the NRB increased over time in all biofilm conditions. Rothia spp., particularly R. mucilaginosa, showed robust growth, especially in biofilm from non-smokers. Veillonella spp. also increased, but to a lesser extent. These findings highlight the potential of arugula nitrate to promote the growth of beneficial nitrate-reducing bacteria, which may contribute to a healthier oral environment (29). This suggests that in our in vitro setting, aerobic environments combined with nitrate availability favored the metabolic activity and growth of these bacteria. In anaerobic conditions, Rothia spp. showed a consistently higher abundance after 5 h of incubation and sustained growth at 9 h, independent of the sample origin (BS or BNS). This indicates that when the biofilm matures, the microenvironment is conducive to nitrate respiration by aerobic or facultative anaerobic bacteria (30), such as Rothia spp. Thus, our results indicated that adding nitrate from arugula juice provides Rothia spp. with an exogenous source of a crucial substrate for anaerobic respiration. The finding may be explained by the fact that the bacteria may thrive in anaerobic environments because nitrate acts as an alternative electron acceptor, allowing these organisms to efficiently carry out anaerobic respiration (31).
Conversely, our data revealed that Veillonella spp. grew in a way that was significantly impacted by the period of incubation, but their numbers were consistently lower than those of Rothia spp. Therefore, while responsive to nitrate, Veillonella spp. appears to grow more slowly or be less adapted compared to Rothia spp., particularly under anaerobic conditions. This suggests that Veillonella spp. may require longer adaptation or exhibit slower growth under these conditions. This finding highlights the species-specific kinetics of nitrate metabolism (32). Nevertheless, a dose-dependent response was demonstrated by the fact that the proportions of Rothia and Veillonella species were frequently larger in the 6.25 µM nitrate concentration compared to the 3.25 µM concentration. This suggests that high nitrate levels in arugula juice promote the growth or activity of NRB, which are environmentally adapted to both aerobic and anaerobic settings (28). Thus, when nitrate is exposed, Rothia spp. and Veillonella spp. seem to have a selection advantage. Rothia spp. operates efficiently under aerobic and anaerobic conditions, while Veillonella spp. prefers anaerobic conditions for optimal activity. The results highlight the metabolic versatility of Rothia spp. and how artificial manipulation of nitrate levels and oxygen availability in controlled experiments can influence microbial growth patterns. This aligns with broader studies on biofilm ecology and bacterial adaptability to nutrient and oxygen gradients (33). Taken together, our study suggests that the significant growth of nitrate-reducing bacteria in response to nitrate exposure (from arugula juice) highlights their ecological adaptability.
Considering that smoking may increase the risk of developing periodontitis (34), it is important to evaluate if, in addition to health-associated oral bacteria (NRB), the addition of nitrate exogen affects the dysbiosis-associated periodontal pathogens (P. gingivalis and F. nucleatum). This study revealed that arugula nitrate may reduce the accumulation of periodontopathogen-associated dysbiotic bacteria, which was not observed previously when nitrate was added to a healthy community (35). Our in vitro study revealed an important finding: periodontopathic bacteria from non-smokers appeared to be larger in number than those from smokers in all biofilm conditions. This suggests that smoking-related dysbiosis may suppress these species in environments enriched with nitrate. In addition, anaerobic conditions tend to have greater bacterial proportions than aerobic ones, which highlights that periodontopathic bacteria grow in oxygen-limited situations (36).
Interestingly, F. nucleatum seems to outcompete P. gingivalis in aerobic conditions, particularly over 9 h and with higher nitrate concentrations. In contrast, under anaerobic conditions, especially after 9 h, both nitrate concentrations (3.25 and 6.25 µM) were effective in inhibiting the development of P. gingivalis. Again, the decrease was more noticeable in BS samples, which may be an indication of differences in converting nitrate to nitrite, leading to the susceptibility of smokers’ periodontopathic bacteria to environments containing NO. These findings support recent studies suggesting that some anaerobes related to periodontitis are vulnerable to oxidative stress, which renders them exposed to the antibacterial effects of NO (37–39). Since F. nucleatum plays a role in converting nitrate to nitrite and linking the aerobic and anaerobic niches (22, 40), the bacterium benefits from nitrite formation while maintaining strict anaerobic bacteria such as P. gingivalis (41). However, under aerobic conditions, the drastic nitric depletion in BS at 9 h may indicate increased nitrite utilization by P. gingivalis or other anaerobes as they adapt to oxygen stress. This behavior aligns with studies showing that P. gingivalis can metabolize nitrite under microaerophilic stress (42, 43). However, the mechanisms behind P. gingivalis's higher sensitivity to nitric oxide than F. nucleatum and how these interactions could be used clinically to treat periodontal disease require further research, especially in light of the growth inhibition patterns observed, particularly in BS samples and after longer incubation periods.
According to our data, non-smokers’ oral bacteria may prefer anaerobic metabolism because of the changed oxygen tension in smokers’ mouths (44). This could lead to a decrease in the prevalence or metabolic activity of nitrate-reducing bacteria, as evidenced by the aforementioned decrease in salivary nitrate–nitric concentration. By referring to the results of the transcription levels of nitrate-associated genes, we found that in the presence of arugula nitrate (3.25 or 6.25 µM) and both anaerobic and aerobic environments, we found that non-smoking-associated bacteria may upregulate these genes more efficiently than smokers’ bacteria, leading to the nitrate-reducing bacteria being more metabolically active. This could indicate an in vivo event in which smoking promotes an alteration in the composition of oral bacteria, which are dominated by dysbiotic bacteria (45). Oxygen levels affect the pattern of narG and napA expression. While narG is often implicated in anaerobic reduction, napA is active in both anaerobic and aerobic environments and may act as an alternative mechanism when oxygen levels are low (45). Both genes’ higher transcription at 9 h, particularly in BNS, suggests that the microbiota in BNS is better able to adjust to nitrate availability, which improves nitrate metabolism in aerobic environments.
Taken together, our in vitro experiment suggests that more nitrate and anaerobic conditions encourage the growth of periodontopathic bacteria, especially F. nucleatum. These conditions are probably made possible by nitrate-reducing bacteria, which alter the biofilm environment to encourage anaerobiosis and resource availability. Because smokers’ biofilms have lower bacterial proportions, smoking-related dysbiosis appears to influence this interaction.
5 Limitations of this study
This study has some limitations. First, it was designed as a pilot in vitro investigation, thus, it might not fully capture the complexity of the oral environment in vivo. Given the pilot nature of this in vitro study and its focus on assessing the feasibility and preliminary effect of arugula juice on biofilm, a formal power analysis was not conducted. The sample size was deemed sufficient to establish the in vitro model and provide initial indications of potential effects. However, we acknowledge that this study was not designed to provide definitive conclusions about differences in the in vivo oral microbiome between smokers and non-smokers. Future studies with in vivo sampling and analysis would require a power analysis to determine the appropriate sample size for such a comparison. Additionally, the study focused solely on smokers and non-smokers, and the analysis was limited to a small number of distinct bacterial species. Finally, larger-scale in vivo studies are needed to confirm these findings and assess the clinical significance of arugula juice for smokers’ oral health. A more thorough picture would be obtained by analyzing the entire microbial community.
6 Conclusion
This in vitro study provides preliminary evidence that nitrate-rich arugula juice may benefit smokers’ oral health by supporting the growth of nitrate-reducing bacteria and possibly inhibiting periodontopathogens. However, further research is necessary to fully understand the complex relationships among nitrate, oxygen levels, bacterial species, and smoking-related dysbiosis. To confirm these results and ascertain the clinical significance and proper application of arugula juice as a potential therapy or preventive approach for strengthening smokers’ oral health, larger-scale in vivo research is essential.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Faculty of Dentistry Universitas Indonesia. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
EB: Conceptualization, Resources, Writing – review & editing. BB: Conceptualization, Formal analysis, Funding acquisition, Writing – original draft. TF: Data curation, Investigation, Validation, Writing – review & editing. CT: Investigation, Supervision, Project administration, Writing – review & editing. HS: Conceptualization, Resources, Writing – review & editing. RI: Validation, Writing – review & editing. IR: Validation, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was supported by the Ministry of Higher Education, Science and Technology, the Republic of Indonesia (No. 051/E5/PG.02.00.PL/2024).
Acknowledgments
We wish to thank Aliya, Safira, Namira, and Annet for their contribution in collecting oral samples. We thank Anissa, Vivi, and Asti for their assistance in the laboratory. The authors would like to acknowledge all the study participants and the clinical clearance committee for providing permission and ethical clearance to conduct this study.
Conflict of interest
The authors declare that there were no commercial or financial relationships that might be construed as a potential conflict of interest throughout the research.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rosier BT, Marsh PD, Mira A. Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J Dent Res. (2018) 97(4):371–80. doi: 10.1177/0022034517742139
2. Chopra A, Franco-Duarte R, Rajagopal A, Choowong P, Soares P, Rito T, et al. Exploring the presence of oral bacteria in non-oral sites of patients with cardiovascular diseases using whole metagenomic data. Sci Rep. (2024) 14(1):1476. doi: 10.1038/s41598-023-50891-x
3. Li Y, Qian F, Cheng X, Wang D, Wang Y, Pan Y, et al. Dysbiosis of oral microbiota and metabolite profiles associated with type 2 diabetes mellitus. Microbiol Spectr. (2023) 11(1):e0379622. doi: 10.1128/spectrum.03796-22
4. Al Kawas S, Al-Marzooq F, Rahman B, Shearston JA, Saad H, Benzina D, et al. The impact of smoking different tobacco types on the subgingival microbiome and periodontal health: a pilot study. Sci Rep. (2021) 11(1):1113. doi: 10.1038/s41598-020-80937-3
5. Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. (2016) 10(10):2435–46. doi: 10.1038/ismej.2016.37
6. Roberts FA, Darveau RP. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: symbiosis and dysbiosis. Periodontol 2000. (2015) 69(1):18–27. doi: 10.1111/prd.12087
7. Lundberg JO, Carlstrom M, Weitzberg E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. (2018) 28(1):9–22. doi: 10.1016/j.cmet.2018.06.007
8. Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. (1964) 68:7–13. doi: 10.14219/jada.archive.1964.0034
9. Loe H, Silness J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand. (1963) 21:533–51. doi: 10.3109/00016356309011240
10. Dhaifullah E, Al-Maweri SA, Koppolu P, Elkhtat E, Mostafa D, Mahgoub M. Body mass index and periodontal health status among young Saudi adults: a cross-sectional study. Ann Saudi Med. (2019) 39(6):433–40. doi: 10.5144/0256-4947.2019.433
11. Aurer A, Aleksic J, Ivic-Kardum M, Aurer J, Culo F. Nitric oxide synthesis is decreased in periodontitis. J Clin Periodontol. (2001) 28(6):565–8. doi: 10.1034/j.1600-051x.2001.028006565.x
12. Wadhwa D, Bey A, Hasija M, Moin S, Kumar A, Aman S, et al. Determination of levels of nitric oxide in smoker and nonsmoker patients with chronic periodontitis. J Periodontal Implant Sci. (2013) 43(5):215–20. doi: 10.5051/jpis.2013.43.5.215
13. Khaksar G, Assatarakul K, Sirikantaramas S. Effect of cold-pressed and normal centrifugal juicing on quality attributes of fresh juices: do cold-pressed juices harbor a superior nutritional quality and antioxidant capacity? Heliyon. (2019) 5(6):e01917. doi: 10.1016/j.heliyon.2019.e01917
14. Bachtiar EW, Bachtiar BM. Effect of cell-free spent media prepared from Aggregatibacter actinomycetemcomitans on the growth of Candida albicans and Streptococcus mutans in co-species biofilms. Eur J Oral Sci. (2020) 128(5):395–404. doi: 10.1111/eos.12725
15. Bachtiar BM, Bachtiar EW, Kusumaningrum A, Sunarto H, Soeroso Y, Sulijaya B, et al. Porphyromonas gingivalis association with inflammatory markers and exosomal miRNA-155 in saliva of periodontitis patients with and without diabetes diagnosed with COVID-19. Saudi Dent J. (2023) 35(1):61–9. doi: 10.1016/j.sdentj.2022.12.002
16. Navidshad B, Liang JB, Jahromi MF. Correlation coefficients between different methods of expressing bacterial quantification using real time PCR. Int J Mol Sci. (2012) 13(2):2119–32. doi: 10.3390/ijms13022119
17. Antonello G, Blostein F, Bhaumik D, Davis E, Gogele M, Melotti R, et al. Smoking and salivary microbiota: a cross-sectional analysis of an Italian alpine population. Sci Rep. (2023) 13(1):18904. doi: 10.1038/s41598-023-42474-7
18. Bachtiar EW, Putri AC, Bachtiar BM. Salivary nitric oxide, simplified oral hygiene Index, and salivary flow rate in smokers and non-smokers: a cross-sectional study. F1000Res. (2019) 8:1744. doi: 10.12688/f1000research.20099.1
19. Caliri AW, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res. (2021) 787:108365. doi: 10.1016/j.mrrev.2021.108365
20. Grine G, Royer A, Terrer E, Diallo OO, Drancourt M, Aboudharam G. Tobacco smoking affects the salivary gram-positive bacterial population. Front Public Health. (2019) 7:196. doi: 10.3389/fpubh.2019.00196
21. Chattopadhyay S, Malayil L, Chopyk J, Smyth E, Kulkarni P, Raspanti G, et al. Oral microbiome dysbiosis among cigarette smokers and smokeless tobacco users compared to non-users. Sci Rep. (2024) 14(1):10394. doi: 10.1038/s41598-024-60730-2
22. Vanhatalo A, Blackwell JR, L'Heureux JE, Williams DW, Smith A, van der Giezen M, et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. (2018) 124:21–30. doi: 10.1016/j.freeradbiomed.2018.05.078
23. Huang Q, Wu X, Zhou X, Sun Z, Shen J, Kong M, et al. Association of cigarette smoking with oral bacterial microbiota and cardiometabolic health in Chinese adults. BMC Microbiol. (2023) 23(1):346. doi: 10.1186/s12866-023-03061-y
24. Luo A, Wang F, Sun D, Liu X, Xin B. Formation, development, and cross-species interactions in biofilms. Front Microbiol. (2021) 12:757327. doi: 10.3389/fmicb.2021.757327
25. Senaratne NLM, Yung On C, Shetty NY, Gopinath D. Effect of different forms of tobacco on the oral microbiome in healthy adults: a systematic review. Front Oral Health. (2024) 5:1310334. doi: 10.3389/froh.2024.1310334
26. Rosier BT, Takahashi N, Zaura E, Krom BP, MartInez-Espinosa RM, van Breda SGJ, et al. The importance of nitrate reduction for oral health. J Dent Res. (2022) 101(8):887–97. doi: 10.1177/00220345221080982
27. Liddle L, Burleigh MC, Monaghan C, Muggeridge DJ, Sculthorpe N, Pedlar CR, et al. Variability in nitrate-reducing oral bacteria and nitric oxide metabolites in biological fluids following dietary nitrate administration: an assessment of the critical difference. Nitric Oxide. (2019) 83:1–10. doi: 10.1016/j.niox.2018.12.003
28. Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. (2005) 113(1):14–9. doi: 10.1111/j.1600-0722.2004.00184.x
29. Zhang H, Qin L. Positive feedback loop between dietary nitrate intake and oral health. Nutr Res. (2023) 115:1–12. doi: 10.1016/j.nutres.2023.04.008
30. Zhou P, Manoil D, Belibasakis GN, Kotsakis GA. Veillonellae: beyond bridging species in oral biofilm ecology. Front Oral Health. (2021) 2:774115. doi: 10.3389/froh.2021.774115
31. Martin-Rodriguez AJ, Rhen M, Melican K, Richter-Dahlfors A. Nitrate metabolism modulates biosynthesis of biofilm components in uropathogenic Escherichia coli and acts as a fitness factor during experimental urinary tract infection. Front Microbiol. (2020) 11:26. doi: 10.3389/fmicb.2020.00026
32. Hung JH, Zhang SM, Huang SL. Nitrate promotes the growth and the production of short-chain fatty acids and tryptophan from commensal anaerobe Veillonella dispar in the lactate-deficient environment by facilitating the catabolism of glutamate and aspartate. Appl Environ Microbiol. (2024) 90(8):e0114824. doi: 10.1128/aem.01148-24
33. Davey ME, O'Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. (2000) 64(4):847–67. doi: 10.1128/MMBR.64.4.847-867.2000
34. Tymkiw KD, Thunell DH, Johnson GK, Joly S, Burnell KK, Cavanaugh JE, et al. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol. (2011) 38(3):219–28. doi: 10.1111/j.1600-051X.2010.01684.x
35. Rosier BT, Buetas E, Moya-Gonzalvez EM, Artacho A, Mira A. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep. (2020) 10(1):12895. doi: 10.1038/s41598-020-69931-x
36. de Andrade KQ, Almeida-da-Silva CLC, Coutinho-Silva R. Immunological pathways triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: therapeutic possibilities? Mediators Inflamm. (2019) 2019:7241312. doi: 10.1155/2019/7241312
37. Allaker RP, Silva Mendez LS, Hardie JM, Benjamin N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol Immunol. (2001) 16(4):253–6. doi: 10.1034/j.1399-302X.2001.160410.x
38. Backlund CJ, Worley BV, Sergesketter AR, Schoenfisch MH. Kinetic-dependent killing of oral pathogens with nitric oxide. J Dent Res. (2015) 94(8):1092–8. doi: 10.1177/0022034515589314
39. Backlund CJ, Sergesketter AR, Offenbacher S, Schoenfisch MH. Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J Dent Res. (2014) 93(11):1089–94. doi: 10.1177/0022034514529974
40. Thurnheer T, Karygianni L, Flury M, Belibasakis GN. Fusobacterium species and subspecies differentially affect the composition and architecture of supra- and subgingival biofilms models. Front Microbiol. (2019) 10:1716. doi: 10.3389/fmicb.2019.01716
41. Diaz PI, Zilm PS, Rogers AH. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology (Reading). (2002) 148(Pt 2):467–72. doi: 10.1099/00221287-148-2-467
42. Boutrin MC, Wang C, Aruni W, Li X, Fletcher HM. Nitric oxide stress resistance in Porphyromonas gingivalis is mediated by a putative hydroxylamine reductase. J Bacteriol. (2012) 194(6):1582–92. doi: 10.1128/JB.06457-11
43. Reher VG, Zenobio EG, Costa FO, Reher P, Soares RV. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J Oral Sci. (2007) 49(4):271–6. doi: 10.2334/josnusd.49.271
44. Hanioka T, Tanaka M, Takaya K, Matsumori Y, Shizukuishi S. Pocket oxygen tension in smokers and non-smokers with periodontal disease. J Periodontol. (2000) 71(4):550–4. doi: 10.1902/jop.2000.71.4.550
45. Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, et al. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. (2014) 9(3):e88645. doi: 10.1371/journal.pone.0088645
46. Nogales B, Timmis KN, Nedwell DB, Osborn AM. Detection and diversity of expressed denitrification genes in estuarine sediments after reverse transcription-PCR amplification from mRNA. Appl Environ Microbiol. (2002) 68(10):5017–25. doi: 10.1128/AEM.68.10.5017-5025.2002
47. Tomas I, Regueira-Iglesias A, Lopez M, Arias-Bujanda N, Novoa L, Balsa-Castro C, et al. Quantification by qPCR of pathobionts in chronic periodontitis: development of predictive models of disease severity at site-specific level. Front Microbiol. (2017) 8:1443. doi: 10.3389/fmicb.2017.01443
48. Miranda TS, Feres M, Retamal-Valdés B, Perez-Chaparro PJ, Maciel SS, Duarte PM. Influence of glycemic control on the levels of subgingival periodontal pathogens in patients with generalized chronic periodontitis and type 2 diabetes. J Appl Oral Sci. (2017) 25(1):82–9. doi: 10.1590/1678-77572016-0302
49. Uchibori S, Tsudukibashi O, Goto H, Kobayashi T, Aida M. A novel selective medium for the isolation and distribution of Rothia dentocariosa in oral cavities. J Microbiol Methods. (2012) 91(1):205–7. doi: 10.1016/j.mimet.2012.07.004
50. Kanady JA, Aruni AW, Ninnis JR, Hopper AO, Blood JD, Byrd BL, et al. Nitrate reductase activity of bacteria in saliva of term and preterm infants. Nitric Oxide. (2012) 27(4):193–200. doi: 10.1016/j.niox.2012.07.004
51. Altemani F, Barrett HL, Callaway LK, McIntyre HD, Dekker Nitert M. Reduced abundance of nitrate-reducing bacteria in the oral microbiota of women with future preeclampsia. Nutrients. (2022) 14(6):1–13. doi: 10.3390/nu14061139
Keywords: arugula, biofilm, nitrate-reducing bacteria, periodontopathogens, nitrate-associated genes
Citation: Bachtiar BM, Rieuwpassa IE, Susilowati H, Indrawati R, Theodorea CF, Fath T and Bachtiar EW (2025) Influence of nitrate-containing arugula juice on nitrate-reducing oral bacteria and periodontopathogens in smokers’ biofilm. Front. Dent. Med. 6:1545479. doi: 10.3389/fdmed.2025.1545479
Received: 15 December 2024; Accepted: 14 April 2025;
Published: 9 May 2025.
Edited by:
Wen Zhou, Case Western Reserve University, United StatesReviewed by:
Xiangsong Bai, Peking University, ChinaHao Shih, China Medical University Hospital, Taiwan
Copyright: © 2025 Bachtiar, Rieuwpassa, Susilowati, Indrawati, Theodorea, Fath and Bachtiar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Endang W. Bachtiar, ZW5kYW5nMDRAdWkuYWMuaWQ=
 Boy M. Bachtiar
Boy M. Bachtiar Irene E. Rieuwpassa3
Irene E. Rieuwpassa3 Heni Susilowati
Heni Susilowati Citra F. Theodorea
Citra F. Theodorea Turmidzi Fath
Turmidzi Fath Endang W. Bachtiar
Endang W. Bachtiar