- 1Department of Periodontics, Meenakshi Ammal Dental College and Hospital, Chennai, India
- 2Department of Periodontics, SRM Dental College, Ramapuram, Chennai, India
- 3Greatways Dental Clinic, Chennai, India
- 4Department of Periodontics, VYWS Dental College and Hospital, Amravati, India
- 5Department of Periodontology, SRM Institute of Science and Technology, Kattankulathur, India
Background: Dickkopf-1 is a glycoprotein that inhibits Wingless-related integration site signaling, impairing osteoblast and osteoclast functions, leading to bone loss and systemic inflammation linked to periodontitis and rheumatoid arthritis. Porphyromonas gingivalis exacerbates rheumatoid arthritis through citrullination and inflammation, highlighting their bidirectional relationship. To date no meta-analysis has examined the role of Dickkopf-1 in periodontitis, rheumatoid arthritis, and their comorbidity. Therefore, we conducted this meta-analysis to investigate the association and role of Dickkopf-1 in these comorbid conditions.
Methods: The present study was conducted in accordance with the guidelines of Transparent Reporting of Systematic Reviews and Meta-Analyses PRISMA statement (registered at PROSPERO under the number CRD42025643227). A total of 15 studies (14 case–control and 1 cross-sectional) were selected out of 386 using databases like PubMed and Google Scholar (by BM, JM, and DP). A random-effects model evaluated Dickkopf-1 levels in serum/gingival crevicular fluid in periodontitis and rheumatoid arthritis via standardized mean difference (SMD) and 95% confidence intervals (CI). Heterogeneity and publication bias were assessed using statistical metrics, forest plots, funnel plots, Begg's test, and Egger's regression.
Results: A total of 386 studies were retrieved and 15 were included in the meta-analysis, encompassing 4,438 participants (2,190 cases and 2,248 controls). The pooled SMD of 2.694 (p = 0.02; 95% CI: 1.170–6.203) indicated a significant association of Dickkopf-1 with periodontitis and/or rheumatoid arthritis compared to healthy controls. However, Egger's test revealed a t-value of 3.05 (p = 0.009), indicating significant publication bias.
Conclusion: Elevated Dickkopf-1 levels in rheumatoid arthritis and periodontitis patients suggest its critical role in the pathogenesis of both conditions. Hence, Dickkopf-1 holds therapeutic potential for managing interconnected inflammatory and bone disorders and may serve as a biomarker for diagnosing these diseases.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/search, PROSPERO CRD42025643227.
1 Introduction
Periodontitis is an inflammatory disease of the tooth-supporting structures, leading to periodontal breakdown, alveolar bone destruction, root exposure, and tooth mobility (1). It is a multifactorial condition influenced by pathogenic bacteria, plaque, calculus, genetics, environmental factors, health conditions, pregnancy, lifestyle, and immune responses (2, 3) Biomarkers, chemical mediators, and signaling molecules drive inflammation causing tissue damage, pocket formation, and further destruction (4–6). Periodontal pathogens and pro-inflammatory mediators can enter systemic circulation (7), contributing to bacteremia, toxemia, and systemic conditions like cardiovascular disease (8), diabetes (9), adverse pregnancy outcomes (10), respiratory diseases (11), chronic kidney disease (12), and rheumatoid arthritis (13), potentially triggering or exacerbating these conditions (14). Rheumatoid arthritis is a chronic autoimmune disease that mainly leads to joint inflammation and pain. This may further be influenced by genetic and environmental factors (15, 16). Studies have identified periodontal bacteria, such as Porphyromonas gingivalis, Prevotella intermedia, Prevotella melaninogenica, Tannerella forsythia, and Aggregatibacter actinomycetemcomitans in the synovium of patients with rheumatoid arthritis (17, 18) These findings, along with elevated serum antibody levels against these bacteria, suggest that periodontal bacteria or their DNA may translocate from periodontal tissues to the synovium, exacerbating joint inflammation (19).
Dickkopf proteins are glycoproteins that are secreted from tissues and cells and antagonize the Wingless-related integration site (Wnt) signaling pathway, which plays a crucial role in bone biology by regulating osteoblastic and osteoclastic activities and bone formation (20). The inactivation of Wnt signaling disrupts bone homeostasis, leading to bone resorption (21). Comprising four key members—Dickkopf-1, 2, 3, and 4—these proteins feature an N-terminal soggy domain and two conserved cysteine-rich domains (CRDs) encoded by the Dickkopf gene (22, 23). Detected in various tissues, including bone, skin, gingiva, and serum, Dickkopf proteins are associated with systemic diseases, such as autoimmune disorders, neurodegenerative diseases, cardiovascular diseases, diabetes, periodontitis, rheumatoid arthritis, and other bone conditions (24).
Dickkopf-1, first reported by Glinka et al. in 1998, plays a significant role in the shared pathogenesis of periodontitis and rheumatoid arthritis, two chronic inflammatory diseases linked by systemic interactions (25). Overexpressed in the periodontium of periodontitis patients, Dickkopf-1 blocks the Wnt signaling pathway, promoting bone loss by disrupting osteoblastic and osteoclastic activity (26). Elevated serum levels of Dickkopf-1 in periodontitis patients with early rheumatoid arthritis further link it to dysfunction in bone metabolism and pathological bone resorption (27). Both diseases are characterized by elevated pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, driving inflammation and tissue destruction (28–30). Periodontal pathogens such as P. gingivalis are implicated in rheumatoid arthritis pathogenesis through citrullination and systemic inflammation, highlighting a bidirectional relationship (17, 18). Neutralizing Dickkopf-1 has shown potential in enhancing bone regeneration; clinical evidence suggests that managing periodontitis can reduce systemic inflammation and improve rheumatoid arthritis outcomes. Addressing their comorbidity is crucial for mitigating disease progression and enhancing patient care (31).
To date, no systematic review has explored the relationship between Dickkopf-1 in periodontitis, rheumatoid arthritis, or their comorbidity. Although elevated Dickkopf-1 levels have been reported in various inflammatory diseases, its role in periodontitis and rheumatoid arthritis remains unclear. Therefore, the objective of this systematic review and meta-analysis was to investigate the association and contribution of Dickkopf-1 in periodontitis, rheumatoid arthritis, and their comorbidity. The clinical question (PICO question) guiding this review was whether Dickkopf-1 levels differ between patients with periodontitis and/or rheumatoid arthritis (participants) and healthy controls (comparators), based on measurements of Dickkopf-1 in serum or gingival crevicular fluid (GCF; outcome) using enzyme-linked immunosorbent assay (ELISA) (intervention).
2 Methodology
This study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (32). The protocol was registered a priori in PROSPERO (CRD42025643227) and aimed to analyze the Dickkopf-1 level in serum and GCF in relation to periodontal disease and rheumatoid arthritis. The target search strategy was prepared based on the research question, the articles to be analyzed were selected in accordance with the inclusion and exclusion criteria of the research, and the data were extracted and analyzed after quality evaluation.
2.1 Search strategy
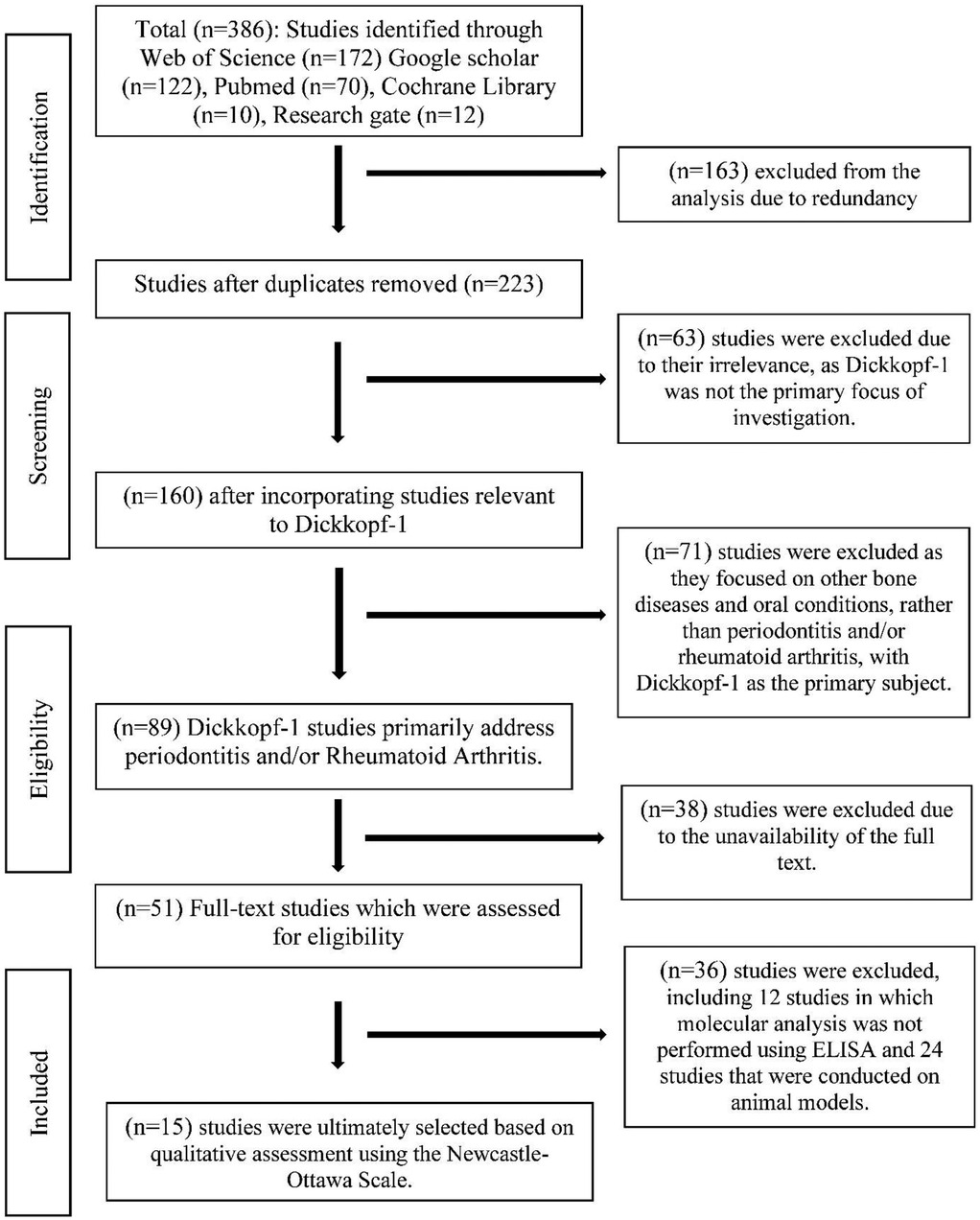
The structured electronic search was conducted by three authors (BM, JM, and DP) independently across multiple databases between 1 June and 31 December 2024. Relevant articles published between 2009 and 2022 were identified and included in the analysis. In addition to structured searches in bibliographic databases (PubMed, Web of Science, and Cochrane Library), gray literature was screened using Google Scholar and ResearchGate. These platforms were employed to identify potentially relevant unpublished, preprint, or non-indexed studies. However, given their lack of standardized indexing and formal peer-review mechanisms, findings from these sources were interpreted with caution. MeSH terms were used and the search strategy was detailed in Figure 1. In addition, pertinent studies were manually searched from the references of reviews and enrolled studies. The following MeSH terms were used for searching: Rheumatoid Arthritis, Arthritis, Chronic Periodontitis, Periodontitis, Periodontal disease, Dickkopf-1, and Dkk-1. Combining these terms with logical operators such as AND or OR, search phrases like Rheumatoid Arthritis“[MeSH]” AND [“Chronic Periodontitis”[MeSH] OR “Periodontitis”[MeSH] OR “Periodontal Diseases”[MeSH]] AND “Dickkopf-1 Protein”[MeSH] OR “DKK-1”[MeSH]) were used to identify relevant studies for the meta-analysis (Supplementary File S1).
2.2 Eligibility criteria
The electronic search, literature search, and study selection involved an initial screening of studies published between June and December 2024, using specified search terms and assessing full-text availability. Corresponding authors were contacted when necessary. The authors independently screened titles and abstracts, excluding studies based on two criteria: (1) molecular analysis not performed using ELISA and (2) studies involving animal models. The remaining studies were reviewed for full-text inclusion with disagreements resolved by discussion. The final set of relevant studies met the following inclusion criteria: (1) case–control or cross-sectional design, (2) case groups of periodontitis and/or rheumatoid arthritis patients, (3) serum or GCF Dickkopf-1 levels measured in both case and control groups, (4) ELISA-based molecular analysis, and (5) exclusion of non-English articles, case reports, mechanistic studies, animal studies, non-ELISA molecular analysis, and review articles.
2.3 Evaluation of methodological quality, data extraction, and review questions
All 15 studies were initially screened based on their titles and abstracts by authors. The methodological quality of the studies and the sources of bias were assessed using the Newcastle–Ottawa Scale for case–control studies (33). Information from all databases was exported to an Excel spreadsheet (Microsoft Office 2013). The review question of this study, based on the PICOS framework, focused on the comparison of Dickkopf-1 levels in serum or GCF in systemically and periodontally healthy individuals [controls/comparator, C), with the population comprising patients with periodontitis, rheumatoid arthritis, or both conditions (comorbidity, P). The intervention/exposure included the presence of inflammatory conditions such as periodontitis, RA, or both, as these are linked to elevated Dickkopf-1 levels (I). The outcome assessed was the mean concentration of Dickkopf-1 in serum or GCF (O). The study design included observational studies (case–control or cross-sectional) that utilized ELISA as the detection method for Dickkopf-1 (S).
2.4 Data items
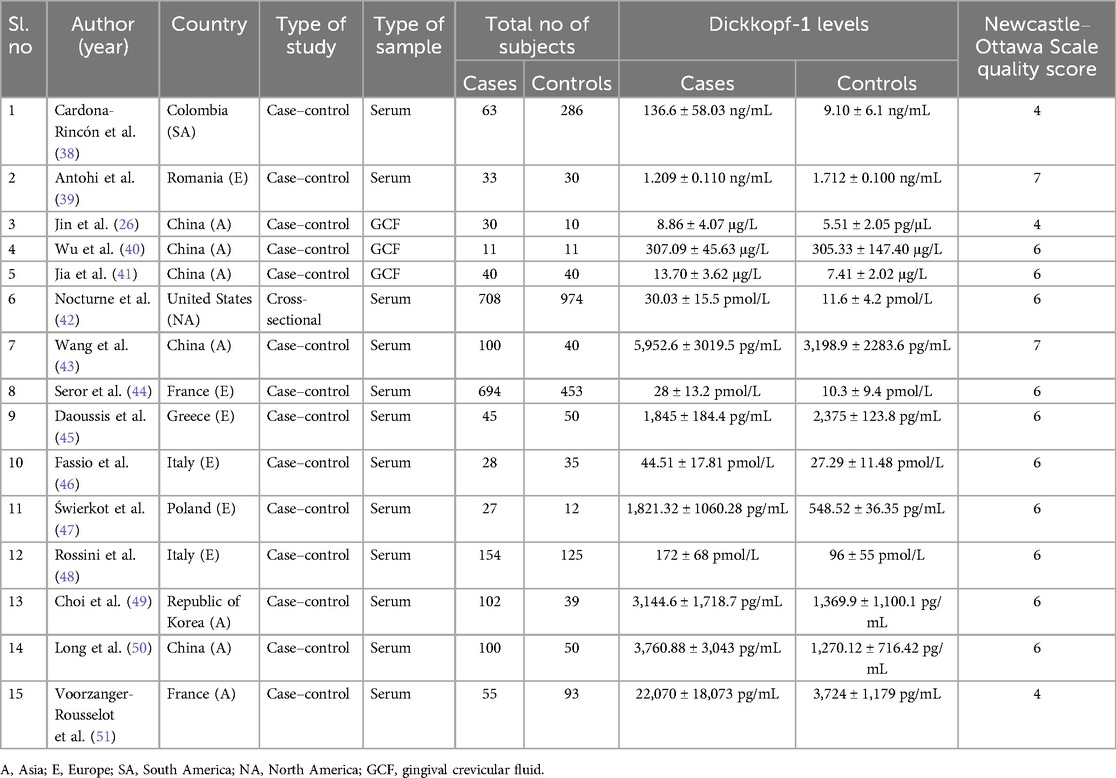
For subsequent analyses, the authors independently extracted the following data from the eligible studies: first author's name, year of publication, country, type of study, sample type (serum, GCF), molecular analysis method (ELISA), Dickkopf-1 levels, and the total number of participants, including controls and periodontitis and/or rheumatoid arthritis patients. Discrepancies in study selection, quality assessment, and data extraction were resolved through consensus discussions among the authors.
2.5 Statistical analysis
The analysis includes 15 studies, using the odds ratio (OR) as the effect size index. A random-effects model was employed, assuming that the selected studies represent a random sample from a broader pool of potential studies, with the results generalizable to this larger population (34). The relationship between serum or GCF Dickkopf-1 levels in patients with periodontitis and/or rheumatoid arthritis was assessed using the standardized mean difference (SMD) and 95% confidence interval (CI), incorporating the random-effects model to account for study heterogeneity (35). For all outcomes, the mean difference was used as the effect measure, with its corresponding confidence interval. A prediction interval was calculated to estimate the true effects. Statistical significance of the pooled SMD between patients and controls was evaluated using a Z-test. Heterogeneity across studies was assessed using the Q-statistic, I² statistic, Tau, Tau2, and forest plots (36). Publication bias was examined qualitatively and quantitatively using funnel plots, Begg's Mazumdar rank correlation test, and Egger's linear regression (37). All data analyses were conducted using Comprehensive Meta-Analysis version 4.
3 Results
3.1 Overview of studies included
3.1.1 Number of studies
A total of 386 studies were retrieved from Web of Science (n = 172), Google Scholar (n = 122), PubMed (n = 70), Cochrane Library (n = 10), and ResearchGate (n = 12) between 1 June and 31 December 2024 (Figure 1). Among the 386 studies, 15 studies were finally included in the meta-analysis, based on the inclusion and exclusion criteria, representing data from 4,438 participants (2,190 cases and 2,248 controls).
3.1.2 Study characteristics
The characteristics of the 15 included studies, consisting of 14 case–control studies and one cross-sectional study, are summarized in Table 1. These studies were conducted across Asia, Europe, and America and were published between 2009 and 2022. The included studies demonstrated moderate to high methodological quality based on the Newcastle–Ottawa Scale, with scores in the range of 4–7. Most studies adequately addressed participant selection and comparability, though a few showed limitations in outcome assessment. These quality differences were considered during interpretation, particularly regarding heterogeneity and the strength of evidence in studies with lower scores (Supplementary File S2). Among the studies, assessing the Dickkopf-1 levels in both serum and GCF, four compared Dickkopf-1 levels between periodontitis patients and healthy controls, one examined Dickkopf-1 levels in healthy controls and periodontitis associated with rheumatoid arthritis, and 10 focused on comparing Dickkopf-1 levels between rheumatoid arthritis patients and healthy controls. The total population data revealed that approximately 1,968 rheumatoid arthritis patients showed increased serum Dickkopf-1 levels compared to 1,821 healthy controls, and 45 rheumatoid arthritis patients showed decreased serum Dickkopf-1 levels compared to 50 healthy controls. Apart from this, 81 periodontitis patients had increased GCF Dickkopf-1 levels when compared to 61 healthy controls, and 33 periodontitis patients had decreased serum Dickkopf-1 levels when compared to 30 healthy controls. In addition, 63 patients with periodontitis along with rheumatoid arthritis showed increased serum Dickkopf-1 levels, compared to 286 healthy controls.
3.2 Overall effect size analysis
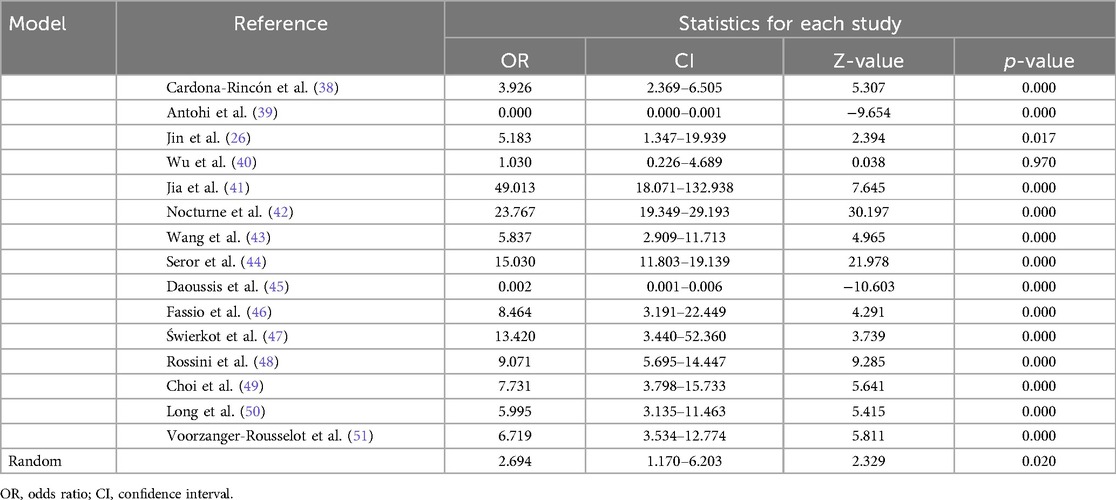
The pooled effect size was calculated as a standardized mean difference (SMD) of 2.694 [p-value = 0.02; 95% CI: 1.170–6.203] across the 15 included studies, indicating a significant association between periodontitis and/or rheumatoid arthritis in comparison to the control group. All 15 studies were analyzed to assess the strength of association between Dickkopf-1 levels in periodontitis and/or rheumatoid arthritis compared with controls. In total, 12 studies showed an OR >1 with a p-value <0.05, indicating statistical significance. However, the studies by Antohi et al. (39) and Daoussis et al. (45) had an OR <1. Wu et al. (40) had an OR of 1 and p-value >0.05, suggesting the result was not statistically significant (Table 2).
3.3 Heterogeneity
Based on the fixed random model analysis for heterogeneity among the 15 studies, it was observed that the Q-value = 473.9, p = 0.000 with a degree of freedom (d.f.) of 14, and I2 = 97%, indicating a substantial heterogeneity across the studies and suggesting that variability in study outcomes was higher than expected by chance alone. Further in the random model analysis, the Tau = 0.868 and Tau2 = 0.753 values indicated low variance between the studies. This suggests that the studies included in the meta-analysis were relatively heterogeneous among the included studies, indicating substantial variability in effect sizes across studies (Table 3).
3.3.1 Forest plot
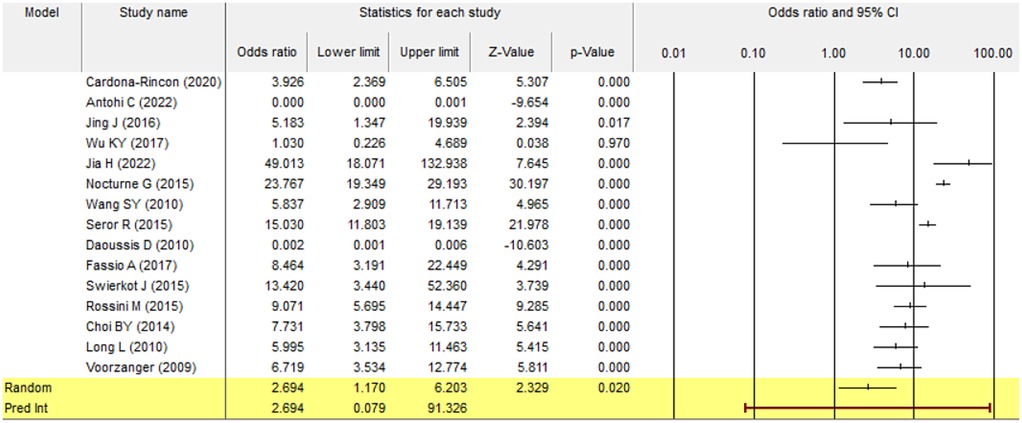
A forest plot represents the individual studies by horizontal lines and their respective confidence intervals. The pooled effect size, represented by the diamond shape at the bottom, indicates a statistically significant positive effect with a 95% confidence interval (1.170–6.203) and p-value of 0.020. Most individual study results were consistent with the pooled estimate, suggesting low heterogeneity; however, certain studies showed wide confidence intervals with a slight variance of results (Figure 2).
3.4 Publication bias
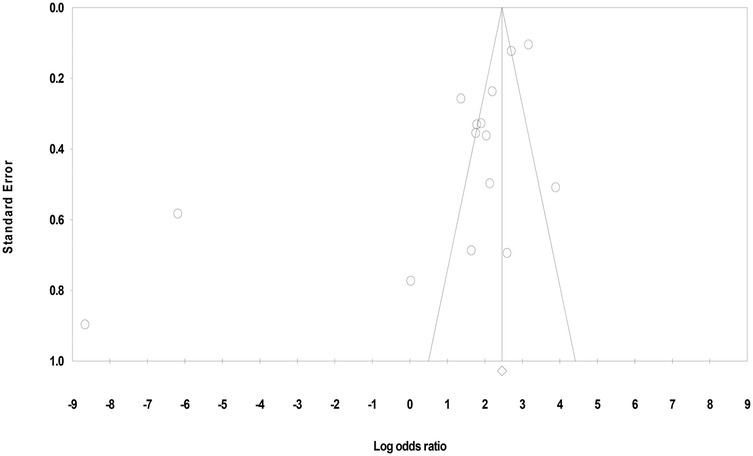
3.4.1 Funnel plot
The funnel plot appeared visually symmetrical; however, closer inspection revealed mild asymmetry, suggesting potential publication bias due to varying sample size between and within the studies and different types of sample analyzed. Under the fixed-effect model, the point estimate and 95% confidence interval for the combined studies was 11.67 and under the random-effects model the point estimate and 95% confidence interval for the combined studies was 2.69. Using Trim and Fill, these values remained unchanged (Figure 3).
3.4.2 Eggers' regression intercept
Egger's test (T = 3.05, p = 0.009) further supported the publication bias. Thus, despite the visual appearance in the funnel plot, statistical results suggested small-study effects or selective publication, warranting cautious interpretation of the findings.
3.4.3 Begg and Mazumdar’s rank correlation
Begg and Mazumdar's test yielded a Kendall's tau of −0.25 with a p-value of 0.18, indicating no significant rank correlation.
4 Discussion
Periodontitis is an inflammatory disease that causes periodontal tissue destruction, often linked to rheumatoid arthritis (5). Dickkopf-1 plays a central role in bone remodeling by inhibiting the Wnt signaling pathway, suppressing osteoblast function, and promoting osteoclast-mediated resorption. This disruption contributes to alveolar bone loss in periodontitis and joint erosion in rheumatoid arthritis (52, 53, 54). Pro-inflammatory cytokines, such as TNF-α and IL-1β, upregulate Dickkopf-1 expression, worsening tissue damage (55). Elevated Dickkopf-1 levels in serum and GCF serve as potential biomarkers for bone resorption and disease activity. These findings underscore Dickkopf-1 importance as both a diagnostic and therapeutic target (27).
This systematic review and meta-analysis assessed Dickkopf-1 as a biomarker for periodontitis, rheumatoid arthritis, and their comorbidity. Various studies have shown a significant impact of Dickkopf-1 in the progression of both periodontitis and rheumatoid arthritis. Diarra et al. showed that TNF-α stimulates Dickkopf-1 production linking inflammation to bone resorption (55). Vargas et al. found elevated serum Dickkopf-1 levels were a marker for joint damage in rheumatoid arthritis (56). De Pablo et al. suggested a bidirectional relationship between rheumatoid arthritis and periodontitis (57), while Kharlamova et al. highlighted P gingivalis in rheumatoid arthritis exacerbation (58). Ceccarelli et al. identified shared inflammatory mechanisms and genetic factors between both the diseases (59). Romero-Sánchez et al. linked elevated Dickkopf-1 in GCF to bone loss in periodontitis (27). Seror et al. found Dickkopf-1 predictive of rheumatoid arthritis structural progression, positioning it as a potential diagnostic and therapeutic target (44). Goes et al. identified Dickkopf-1 as a mediator of bone resorption in periodontitis through inhibition of osteoblast activity and promotion of osteoclastogenesis in mice model (60). Similarly, Buckland demonstrated the pivotal role of Dickkopf-1 in rheumatoid arthritis, particularly in joint destruction and inflammation (61).
Our analysis identified consistently elevated levels of Dickkopf-1 in patients with periodontitis and rheumatoid arthritis compared to healthy controls, as reported in 12 out of 15 studies, with an OR >1 and p-values <0.05, indicating a strong association of Dickkopf-1 with these conditions as a pro-inflammatory mediator. Another study carried out by Wu et al. observed Dickkopf-1 levels in periodontitis patients, which was non-significant, with an OR of 1.03 and a p-value >0.05, potentially attributable to the small sample size of 11 periodontitis patients in his study, limiting the strength of the correlation (40). On the other hand, two studies reported slightly reduced serum Dickkopf-1 levels in rheumatoid arthritis and periodontitis patients compared to healthy controls (39, 45). The dual role of Dickkopf-1 as an anti-oncogenic marker and a regulator of bone resorption may explain the decreased levels observed in periodontitis patients (39). Another study found reduced Dickkopf-1 levels in rheumatoid arthritis patients after anti-TNF α therapy (45).
Among the 15 studies reviewed, Jia et al. reported an odds ratio of 49 in periodontitis patients, indicating a strong significant association with Dickkopf-1 (41). Similarly, notable findings were observed in studies related to rheumatoid arthritis: Nocturne et al. demonstrated an odds ratio of 23, Seror et al. reported an odds ratio of 15, and Świerkot et al. documented an odds ratio of 13 (42, 44, 47). These findings highlight that Dickkopf-1 is highly associated with both periodontitis and rheumatoid arthritis. Since both conditions were characterized as bone-related diseases, the pivotal role of Dickkopf-1 in bone metabolism and pathology underscores its importance in the progression and regulation of these disorders. Our findings also indicate that Dickkopf-1 exhibits a significant association with periodontitis and rheumatoid arthritis, both as independent conditions and in their coexistence as comorbidities.
In the present meta-analysis on the role of Dickkopf-1 specifically in periodontitis alone, as well as its involvement in the context of comorbid periodontitis and rheumatoid arthritis, it was observed that elevated levels of Dickkopf-1 were consistently observed in studies by Jia et al., Jin et al., and Cardona-Rincón et al., suggesting its role as a pro-inflammatory marker that regulates bone remodeling by suppressing osteoblast activity and stimulating osteoclastogenesis (26, 38, 41). Its levels were elevated in periodontitis, linking inflammation to alveolar bone resorption, thereby positioning it as a potential diagnostic marker for periodontal disease. Dickkopf-1 acts by inhibiting the Wnt signaling pathway, enhances osteoclast activity, and accelerates bone degradation, a process observed in both periodontitis and rheumatoid arthritis. The increased expression of Dickkopf-1 contributes to the progression of alveolar bone loss and joint destruction, underscoring its role in comorbid conditions and highlighting its potential as a therapeutic target for mitigating bone loss.
In our study, the Q-value (473.9, p < 0.0001) and I2 (97%) indicate substantial heterogeneity among the included studies. Although Tau2 (0.753) reflects the between-study variance, Tau (0.868) gives the standard deviation of true effect sizes, suggesting moderate variability in effect sizes across studies. These values justify the use of a random-effects model and have been clearly explained to aid interpretation of the pooled effect size and enhance result transparency. Egger's test (T = 3.05, p = 0.009) revealed significant publication bias, suggesting both small-study effects and underreporting of studies with null results. This bias may inflate the pooled effect size and compromise the credibility and generalizability of Dickkopf-1 as a biomarker, highlighting the need for cautious interpretation, inclusion of gray literature, and further prospective research.
In the present study, the substantial heterogeneity observed in Dickkopf-1 levels across studies likely arises from methodological and population-related differences. Variations in disease definitions for periodontitis and rheumatoid arthritis, including the use of differing diagnostic criteria (e.g., CAL/PD vs. CDC/AAP for periodontitis; 1987 ACR vs. 2010 ACR/EULAR for rheumatoid arthritis), may have affected disease classification and Dickkopf-1 expression. Population diversity in terms of age, ethnicity, genetic background, and systemic health status further contributes to biomarker variability. Differences in sample types (serum vs. gingival crevicular fluid) introduce additional variability, reflecting systemic vs. localized inflammation. Geographic variation and environmental influences, along with differences in ELISA protocols (e.g., kit sensitivity, antibody specificity, and calibration methods), may also impact measured Dickkopf-1 levels. These factors collectively limit the comparability of findings and highlight the need for standardized diagnostic criteria and assay methodologies in future research.
Dickkopf-1 plays a pivotal role in bone metabolism by inhibiting the Wnt signaling pathway, thereby suppressing osteoblast activity and promoting osteoclast-mediated bone resorption. This mechanism underlies its involvement in both alveolar bone loss in periodontitis and joint destruction in rheumatoid arthritis. Pro-inflammatory cytokines, such as TNF-α and IL-1β, upregulate Dickkopf-1 expression, exacerbating tissue degradation. Several studies have identified elevated levels of Dickkopf-1 in serum and GCF as potential biomarkers of disease activity and bone resorption in both conditions. For example, Diarra et al. demonstrated that TNF-α induces Dickkopf-1 production, linking inflammatory signaling to bone loss (55). However, the meta-analysis revealed substantial heterogeneity (I2 = 97%, Q = 473.9, p < 0.0001), likely due to differences in study designs, sample sources, geographic variation, and diagnostic criteria, despite relatively low between-study variance (Tau2 = 0.753). Although these findings highlight the clinical relevance of Dickkopf-1 as a biomarker and possible therapeutic target, caution is warranted in interpretation due to methodological inconsistencies and potential publication bias.
This meta-analysis highlights several limitations that warrant consideration. The limited number of available studies examining the association between Dickkopf-1, periodontitis, and rheumatoid arthritis constrained the scope of analysis, particularly restricting the feasibility of subgroup analyses due to insufficient data on studies focused exclusively on periodontitis or its comorbidity with rheumatoid arthritis. Most included studies were cross-sectional or case–control in design, limiting causal inference, and none explored longitudinal outcomes. Significant heterogeneity (I2 = 97%, Q = 473.9, p < 0.0001) was observed across studies, likely attributable to differences in diagnostic criteria, population demographics, geographic origin, sample types (serum vs. gingival crevicular fluid), and variability in ELISA protocols, including assay sensitivity and manufacturer-specific differences. Furthermore, Dickkopf-1 assessment was limited to serum and GCF, with a lack of data on synovial fluid or saliva due to sparse literature, which may have offered broader insights into its biomarker potential. Although Egger's test indicated significant publication bias (p = 0.009), the exclusion of non-English studies and gray literature may further contribute to this bias. In addition, the geographic and ethnic diversity of study populations introduces variability that may impact generalizability. Collectively, these limitations underscore the need for well-designed, longitudinal, and mechanistic studies with standardized methodologies to clarify the clinical utility of Dickkopf-1 as a biomarker and therapeutic target in both periodontitis and rheumatoid arthritis.
In conclusion, the present meta-analysis highlights the potential involvement of Dickkopf-1 in the interplay between periodontitis and rheumatoid arthritis. Although elevated Dickkopf-1 levels in serum and GCF may indicate its relevance in these chronic inflammatory conditions, the findings should be interpreted with caution due to the observational design of the included studies and the presence of publication bias. At this stage, Dickkopf-1 can be considered a promising, but not yet established, biomarker or therapeutic target. Future research should focus on prospective cohort studies, investigation of Dickkopf-1 levels in additional biofluids such as synovial fluid and saliva, and clinical studies evaluating the impact of modulating Dickkopf-1 in therapeutic contexts to substantiate its clinical applicability.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
BM: Formal analysis, Writing – original draft, Data curation, Writing – review & editing. JM: Validation, Methodology, Data curation, Supervision, Project administration, Conceptualization, Writing – original draft, Investigation, Writing – review & editing. DP: Supervision, Writing – review & editing, Conceptualization, Writing – original draft, Investigation, Visualization, Validation, Data curation. VR: Validation, Writing – review & editing. PG: Writing – review & editing, Visualization. RR: Resources, Funding acquisition, Writing – review & editing. KT: Funding acquisition, Visualization, Writing – review & editing. GK: Writing – review & editing, Visualization, Validation, Investigation. GP: Funding acquisition, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely appreciate the support of our colleagues and the institution Meenakshi Ammal Dental College and Hospital, Chennai.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2025.1593218/full#supplementary-material
References
1. Könönen E, Gursoy M, Gursoy UK. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med. (2019) 8(8):1135. doi: 10.3390/jcm8081135
2. Abdulkareem AA, Al-Taweel FB, Al-Sharqi AJ, Gul SS, Sha A, Chapple IL. Current concepts in the pathogenesis of periodontitis: from symbiosis to dysbiosis. J Oral Microbiol. (2023) 15(1):2197779. doi: 10.1080/20002297.2023.2197779
3. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. (2015) 15(1):30–44. doi: 10.1038/nri3785
4. Łasica A, Golec P, Laskus A, Zalewska M, Gędaj M, Popowska M. Periodontitis: etiology, conventional treatments, and emerging bacteriophage and predatory bacteria therapies. Front Microbiol. (2024) 15:1469414. doi: 10.3389/fmicb.2024.1469414
5. Bhuyan R, Bhuyan SK, Mohanty JN, Das S, Juliana N, Abu IF. Periodontitis and its inflammatory changes linked to various systemic diseases: a review of its underlying mechanisms. Biomedicines. (2022) 10(10):2659. doi: 10.3390/biomedicines10102659
6. Ray RR. Periodontitis: an oral disease with severe consequences. Appl Biochem Biotechnol. (2023) 195(1):17–32. doi: 10.1007/s12010-022-04127-9
7. Martínez-García M, Hernández-Lemus E. Periodontal inflammation and systemic diseases: an overview. Front Physiol. (2021) 12:709438. doi: 10.3389/fphys.2021.709438
8. Nabila S, Choi J, Kim JE, Hahn S, Hwang IK, Kim TI, et al. Bidirectional associations between periodontal disease and systemic diseases: a nationwide population-based study in Korea. Sci Rep. (2023) 13(1):14078. doi: 10.1038/s41598-023-41009-4
9. Păunică I, Giurgiu M, Dumitriu AS, Păunică S, Pantea Stoian AM, Martu MA, et al. The bidirectional relationship between periodontal disease and diabetes mellitus—a review. Diagnostics. (2023) 13(4):681. doi: 10.3390/diagnostics13040681
10. AlSharief M, Alabdurubalnabi E. Periodontal pathogens and adverse pregnancy outcomes: a narrative review. Life. (2023) 13(7):1559. doi: 10.3390/life13071559
11. Zhang Z, Wen S, Liu J, Ouyang Y, Su Z, Chen D, et al. Advances in the relationship between periodontopathogens and respiratory diseases. Mol Med Rep. (2024) 29(3):42. doi: 10.3892/mmr.2024.13166
12. Baciu SF, Mesaroș AȘ, Kacso IM. Chronic kidney disease and periodontitis interplay—a narrative review. Int J Environ Res Public Health. (2023) 20(2):1298. doi: 10.3390/ijerph20021298
13. de Molon RS, Rossa Jr C, Thurlings RM, Cirelli JA, Koenders MI. Linkage of periodontitis and rheumatoid arthritis: current evidence and potential biological interactions. Int J Mol Sci. (2019) 20(18):4541. doi: 10.3390/ijms20184541
14. Lohiya DV, Mehendale AM, Lohiya DV, Lahoti HS, Agrawal VN. Effects of periodontitis on major organ systems. Cureus. (2023) 15(9):e46299.37915876
15. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. (2018) 6(1):15. doi: 10.1038/s41413-018-0016-9
16. Jang S, Kwon EJ, Lee JJ. Rheumatoid arthritis: pathogenic roles of diverse immune cells. Int J Mol Sci. (2022) 23(2):905. doi: 10.3390/ijms23020905
17. Krutyhołowa A, Strzelec K, Dziedzic A, Bereta GP, Łazarz-Bartyzel K, Potempa J, et al. Host and bacterial factors linking periodontitis and rheumatoid arthritis. Front Immunol. (2022) 13:980805. doi: 10.3389/fimmu.2022.980805
18. Corrêa JD, Fernandes GR, Calderaro DC, Mendonça SM, Silva JM, Albiero ML, et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep. (2019) 9(1):8379. doi: 10.1038/s41598-019-44674-6
19. Martinez-Martinez RE, Abud-Mendoza C, Patiño-Marin N, Rizo-Rodríguez JC, Little JW, Loyola-Rodríguez JP. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. (2009) 36(12):1004–10. doi: 10.1111/j.1600-051X.2009.01496.x
20. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. (2006) 25(57):7469–81. doi: 10.1038/sj.onc.1210054
21. Heath DJ, Chantry AD, Buckle CH, Coulton L, Shaughnessy JD Jr, Evans HR, et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. (2009) 24(3):425–36. doi: 10.1359/jbmr.081104
22. Patel S, Barkell AM, Gupta D, Strong SL, Bruton S, Muskett FW, et al. Structural and functional analysis of Dickkopf 4 (Dkk4): new insights into Dkk evolution and regulation of Wnt signaling by Dkk and Kremen proteins. J Biol Chem. (2018) 293(31):12149–66. doi: 10.1074/jbc.RA118.002918
23. Baetta R, Banfi C. Dkk (Dickkopf) proteins: emerging new players in atherosclerosis. Arterioscler Thromb Vasc Biol. (2019) 39(7):1330–42. doi: 10.1161/ATVBAHA.119.312612
24. Huang Y, Liu L, Liu A. Dickkopf-1: current knowledge and related diseases. Life Sci. (2018) 209:249–54. doi: 10.1016/j.lfs.2018.08.019
25. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. (1998) 391(6665):357–62. doi: 10.1038/34848
26. Jin J, Wu KY, Xc CJ, Chi YT, Sun XJ, Yuan HB, et al. Levels of Dickkopf-l in gingival crevicular lluid and gingival tissue from chronic periodontitis. J Oral Sci Res. (2016) 32(4):379. doi: 10.13701/j.cnki.kqyxyj.2016.04.015
27. Romero-Sánchez C, Giraldo S, Heredia-P AM, De Avila J, Chila-Moreno L, Londoño J, et al. Association of serum and crevicular fluid Dickkopf-1 levels with disease activity and periodontitis in patients with early rheumatoid arthritis. Curr Rheumatol Rev. (2022) 18(2):124–35. doi: 10.2174/1573397117666211116105118
28. Cheng R, Wu Z, Li M, Shao M, Hu T. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int J Oral Sci. (2020) 12(1):2. doi: 10.1038/s41368-019-0068-8
29. Kondo N, Kuroda T, Kobayashi D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int J Mol Sci. (2021) 22(20):10922. doi: 10.3390/ijms222010922
30. Neurath N, Kesting M. Cytokines in gingivitis and periodontitis: from pathogenesis to therapeutic targets. Front Immunol. (2024) 15:1435054. doi: 10.3389/fimmu.2024.1435054
31. Negri S, Wang Y, Sono T, Qin Q, Hsu GC, Cherief M, et al. Systemic DKK1 neutralization enhances human adipose-derived stem cell mediated bone repair. Stem Cells Transl Med. (2021) 10(4):610–22. doi: 10.1002/sctm.20-0293
32. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
33. Ga W. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. In: 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000 July 3–5; Oxford, UK. Ottawa: Ottawa Hospital Research Institute (2000).
34. Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. (2009) 9:1–2. doi: 10.1186/1471-2288-9-86
35. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry. (2020) 81(5):11349. doi: 10.4088/JCP.20f13681
36. Ioannidis JP, Patsopoulos NA, Evangelou E. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Br Med J. (2007) 335(7626):914–6. doi: 10.1136/bmj.39343.408449.80
37. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74(3):785–94. doi: 10.1111/biom.12817
38. Cardona-Rincón AD, Acevedo-Godoy MA, Bello-Gualtero JM, Valle-Oñate R, Chalem-Choueka P, Perdomo SJ, et al. Association of Dickkopf-1 polymorphisms with radiological damage and periodontal disease in patients with early rheumatoid arthritis: a cross-sectional study. J Clin Rheumatol. (2020) 26(7S):S187–94. doi: 10.1097/RHU.0000000000001391
39. Antohi C, Salceanu M, Aminov L, Martu MA, Dascalu CG, Dodi G, et al. Assessment of systemic and maxillary bone loss in cancer patients with endo-periodontal lesions using Dkk-1 biomarker and dental radiological examinations. Appl Sci. (2022) 12(10):5235. doi: 10.3390/app12105235
40. Wu KY, Xu CJ, Chi YT, Sun XJ, Wang HF, Wang MM. Detection of Dickkopf-1 and alkaline phosphatase activity in gingival crevicular fluid from chronic periodontitis with Er: YAG laser as an adjunctive treatment. Shanghai J Stomatol. (2017) 26(3):285.
41. Jia H, Yang L, Wang P, Liu X, Kang K. Levels of DKK-1, MIP-1α and IL-17 in gingival crevicular fluid and serum of patients with chronic periodontitis and their clinical significance. J Chin Phys. (2022):1316–20.
42. Nocturne G, Pavy S, Boudaoud S, Seror R, Goupille P, Chanson P, et al. Increase in Dickkopf-1 serum level in recent spondyloarthritis. Data from the DESIR cohort. PLoS One. (2015) 10(8):e0134974. doi: 10.1371/journal.pone.0134974
43. Wang SY, Liu YY, Ye H, Guo JP, Li RU, Liu X, et al. Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol. (2011) 38(5):821–7. doi: 10.3899/jrheum.100089
44. Seror R, Boudaoud S, Pavy S, Nocturne G, Schaeverbeke T, Saraux A, et al. Increased Dickkopf-1 in recent-onset rheumatoid arthritis is a new biomarker of structural severity. Data from the ESPOIR cohort. Sci Rep. (2016) 6(1):18421. doi: 10.1038/srep18421
45. Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. (2010) 62(1):150–8. doi: 10.1002/art.27231
46. Fassio A, Idolazzi L, Viapiana O, Benini C, Vantaggiato E, Bertoldo F, et al. In psoriatic arthritis Dkk-1 and PTH are lower than in rheumatoid arthritis and healthy controls. Clin Rheumatol. (2017) 36(10):2377–81. doi: 10.1007/s10067-017-3734-2
47. Świerkot J, Gruszecka K, Matuszewska A, Wiland P. Assessment of the effect of methotrexate therapy on bone metabolism in patients with rheumatoid arthritis. Arch Immunol Ther Exp. (2015) 63:397–404. doi: 10.1007/s00005-015-0338-x
48. Rossini M, Viapiana O, Adami S, Fracassi E, Idolazzi L, Dartizio C, et al. In patients with rheumatoid arthritis, Dickkopf-1 serum levels are correlated with parathyroid hormone, bone erosions and bone mineral density. Clin Exp Rheumatol. (2015) 33(1):77–83.25438096
49. Choi BY, Chang SH, Cho HJ, Kang EH, Shin K, Song YW, et al. The association of radiographic progression with serum R-spondin 1 (RSPO1) levels or Dickkopf-1 (DKK1)/RSPO1 ratios in rheumatoid arthritis patients: clinical evidence for reciprocal inhibition between DKK1 and RSPO1. Scand J Rheumatol. (2014) 43(6):453–61. doi: 10.3109/03009742.2014.905629
50. Long L, Liu Y, Wang S, Zhao Y, Guo J, Yu P, et al. Dickkopf-1 as potential biomarker to evaluate bone erosion in systemic lupus erythematosus. J Clin Immunol. (2010) 30:669–75. doi: 10.1007/s10875-010-9436-z
51. Voorzanger-Rousselot N, Ben-Tabassi NC, Garnero P. Opposite relationships between circulating Dkk-1 and cartilage breakdown in patients with rheumatoid arthritis and knee osteoarthritis. Ann Rheum Dis. (2009) 68(9):1513–4. doi: 10.1136/ard.2008.102350
52. Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. (2009) 113(3):517–25. doi: 10.1182/blood-2008-03-145169
53. Daoussis D, Andonopoulos AP. The emerging role of Dickkopf-1 in bone biology: is it the main switch controlling bone and joint remodeling? Semin Arthritis Rheum. (2011) 41(2):170–7. doi: 10.1016/j.semarthrit.2011.01.006
54. Li S, Yin Y, Yao L, Lin Z, Sun S, Zhang J, et al. TNF-a treatment increases DKK1 protein levels in primary osteoblasts via upregulation of DKK1 mRNA levels and downregulation of miR-335-5p. Mol Med Rep. (2020) 22(2):1017–25.32468044
55. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. (2007) 13(2):156–63. doi: 10.1038/nm1538
56. Vargas RR, Madrid EM, Rodríguez CG, Sarabia FN, Quesada CD, Ariza RA, et al. Association between serum Dickkopf-1 levels and disease duration in axial spondyloarthritis. Reumatol Clín. (2017) 13(4):197–200. doi: 10.1016/j.reumae.2016.04.016
57. De Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. (2008) 35(1):70–6.18050377
58. Kharlamova N, Jiang X, Sherina N, Potempa B, Israelsson L, Quirke AM, et al. Antibodies to Porphyromonas gingivalis indicate interaction between oral infection, smoking, and risk genes in rheumatoid arthritis etiology. Arthritis Rheumatol. (2016) 68(3):604–13. doi: 10.1002/art.39491
59. Ceccarelli F, Saccucci M, Di Carlo G, Lucchetti R, Pilloni A, Pranno N, et al. Periodontitis and rheumatoid arthritis: the same inflammatory mediators? Mediat Inflamm. (2019) 2019(1):6034546. doi: 10.1155/2019/6034546
60. Goes P, Dutra C, Lösser L, Hofbauer LC, Rauner M, Thiele S. Loss of Dkk-1 in osteocytes mitigates alveolar bone loss in mice with periodontitis. Front Immunol. (2019) 10:2924. doi: 10.3389/fimmu.2019.02924
Keywords: periodontitis, rheumatoid arthritis, pro-inflammatory biomarker, Dickkopf-1 (DKK-1), Wnt signaling pathway, bone remodeling
Citation: Maharavi B, Mahendra J, Ponnaiyan D, Rajaram V, Gyanchand P, Rughwani RR, Thakare KS, Kumar G and Patil G (2025) Evaluating Dickkopf-1 as a biomarker: insights into periodontitis, rheumatoid arthritis, and their comorbidity—a systematic review and meta-analysis. Front. Dent. Med. 6:1593218. doi: 10.3389/fdmed.2025.1593218
Received: 13 March 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Deepti Shrivastava, Al Jouf University, Saudi ArabiaReviewed by:
Franz Tito Coronel Zubiate, Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas, PeruNazurah Nik Eezammuddeen, Universiti Teknologi MARA, Malaysia
Copyright: © 2025 Maharavi, Mahendra, Ponnaiyan, Rajaram, Gyanchand, Rughwani, Thakare, Kumar and Patil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaideep Mahendra, ZHJqYWlkZWVwLnBlcmlvQG1hZGNoLmVkdS5pbg==; Deepa Ponnaiyan, ZGVlcGFfcG9ubmFpeWFuQHlhaG9vLmNvLmlu
 Bawatharani Maharavi
Bawatharani Maharavi Jaideep Mahendra
Jaideep Mahendra Deepa Ponnaiyan
Deepa Ponnaiyan Vijayalakshmi Rajaram
Vijayalakshmi Rajaram Pragya Gyanchand
Pragya Gyanchand Roshan R. Rughwani
Roshan R. Rughwani Kaustubh Suresh Thakare
Kaustubh Suresh Thakare Gayathri Kumar
Gayathri Kumar Gauri Patil
Gauri Patil