- 1Division of Regenerative Dental Medicine and Periodontology, University Clinics of Dental Medicine, University of Geneva, Geneva, Switzerland
- 2Department of Periodontology, Faculty of Odontology, Malmo University, Malmo, Sweden
- 3Department of Periodontology, Blekinge Hospital, Karlskrona, Sweden
- 4Division of Conservative Dentistry and Periodontology, University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria
- 5Department of Periodontology, School of Dental Medicine, University of Bern, Bern, Switzerland
Introduction: Peri-implantitis is an inflammatory disease that compromises peri-implant tissues and supporting bone, potentially leading to implant loss. Although several surgical treatment strategies have been proposed, it remains unclear whether implant surface characteristics (smooth vs. rough) influence long-term treatment outcomes.
Methods: A systematic review was conducted to evaluate clinical studies with a minimum follow-up of 3 years that assessed the outcomes of surgical treatment of peri-implantitis in relation to implant surface type. Data extraction focused on recurrence of peri-implantitis, implant survival, clinical parameters, radiographic outcomes, and the type of surgical approach used (reconstructive vs. non-reconstructive).
Results: Seventeen clinical studies were included. Outcomes varied according to implant surface characteristics. Rough (modified) surfaces were generally associated with a higher risk of recurrence of peri-implantitis and implant loss compared with smooth (machined/turned) surfaces. Reconstructive surgical approaches, especially those involving bone grafts and membranes, demonstrated more favorable outcomes compared with non-reconstructive approaches.
Discussion: Despite observed trends, the certainty of the evidence remains low due to heterogeneity between studies, small sample sizes, and methodological limitations. Further well-designed long-term clinical trials are needed to clarify the role of implant surface characteristics in the long-term success of peri-implantitis surgical treatment.
Systematic Review Registration: PROSPERO (CRD420251129791).
1 Introduction
Dental implants have significantly advanced oral rehabilitation, providing highly predictable solutions for tooth replacement. For instance, a recent systematic review reported that long-term prospective studies on dental implants show high survival rates, typically exceeding 90% over 5–10 years and remaining around 78% after imputation at 20 years follow-up. In addition, five retrospective studies with ≥20 years of follow-up reported an implant survival rate of approximately 88%, including multifactorial causes (1).
However, despite the high survival rates, biological complications at implants are rather common. In particular, peri-implantitis, which is characterized by peri-implant mucosal inflammation and progressive bone loss, affects approximately 19.53% of patients and 12.53% of implants, highlighting its relevance in clinical practice (2). As the main etiological factor for peri-implantitis is the oral biofilm, microbial to implant surface interactions seem to play an important role in disease pathogenesis. Indeed, surface modifications (e.g., sandblasting, acid-etching, anodization, etc) aiming in enhancing implant surface bioactivity, substantially impact on microbial colonization and biofilm development (3–7). Indeed, although the incidence of peri-implantitis seems not to differ between modified and non-modified (i.e., turned) implants in the clinic, progression and severity of peri-implantitis appear linked to implant surface properties; specifically, pre-clinical in vivo studies indicate a faster disease progression at modified implants compared with turned implants, as well as differences in disease progression among various modified surfaces (5, 7). Moreover it seems that implant surface characteristics may impact on treatment outcomes both in the short-term but also on the long-term, with implants with a modified surface demonstrating less positive results and higher recurrence rates (3, 8, 9).
Despite technological advancements and improved treatment approaches, the impact of implant surface modifications on peri-implantitis outcomes remains unclear. Therefore, this systematic review aims to evaluate whether varying implant surface topographies influence clinical and radiographic outcomes following surgical peri-implantitis treatment in humans. The findings may offer critical insights guiding the selection of implant surface characteristics to enhance treatment efficacy.
2 Materials and methods
2.1 Study design
This review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered in PROSPERO (ID: CRD420251129791).
2.2 Search strategy
To identify relevant studies, we systematically searched PubMed, Embase, and the Cochrane Library. The search strategy was carried out in English language from database inception for articles published between 2000 and 2025. Two investigators (AZ and PG) independently reviewed the search results and screened the titles and abstracts. Full texts of all potentially eligible studies were obtained. In PubMed, the following search strategy was used: “(Periimplantitis OR peri-implantitis OR peri implantitis OR periimplant OR peri-implant OR peri implant) AND (treatment outcome OR therapy OR surgical treatment OR regenerative OR regeneration OR tissue regeneration OR reconstructive surgery OR bone graft OR bone substitute OR membranes OR surgical flap OR open flap debridement OR resective OR implantoplasty OR surface decontamination) AND (surface characteristics OR surface roughness OR material characteristics OR titanium surface OR implant types OR implant surfaces OR surface topography OR surface analysis) AND (implant survival OR bone loss OR recurrence OR retreatment OR radiographic stability OR long-term OR 3 years OR follow-up).” This search strategy was adapted to suit the other electronic sources. The reference lists of retrieved articles were also checked to identify additional studies of interest. Any inconsistencies were resolved by consensus with a third investigator (CG). The complete search strategies for all databases are provided in Appendix 2.
2.3 Criteria for considering studies for this review
2.3.1 Study design
Randomized controlled trials, prospective studies, retrospective studies, case-control studies, and case series were included. No specific cut-off criteria for sample size were applied, given the limited availability of data. Additionally, two case series with very small sample sizes were included due to their clinical relevance. Eligibility required that included studies explicitly reported the implant surface type(s) of the implants investigated.
2.3.2 Population
Human studies. Patients with osseointegrated dental implants diagnosed with peri-implantitis, treated surgically, with a follow-up period of at least 3 years (or an average ≥3 years).
2.3.3 Intervention
Surgical therapy for peri-implantitis.
2.3.4 Comparator
Different implant surface types, characterized by variations in macro-, micro-, and nano-scale surface roughness, topography, and material composition. Surfaces were categorized as non-modified (i.e turned, smooth, machihed), modified (rough), or mixed (hybrid), depending on their reported surface characteristics.
2.4 Outcomes
2.4.1 Primary outcome
• Percentage of implants with recurrence of peri-implantitis requiring re-treatment or explantation or simply defined as treatment failure by the authors.
2.4.2 Secondary outcomes
• Implant loss (due to any reason)
• Disease resolution defined by reduction of probing depth (PD) without bleeding on probing (BOP) or suppuration
• Radiographic bone loss or gain assessed by mean changes in bone levels or percentage of implants with stable bone levels post-treatment
• Mean probing depth (PD) post-treatment
Subgroup synthesis: The outcomes were further stratified based on implant surface types and surgical approach:
1. Turned (machined/non-modified surfaces)
• a. Non-reconstructive surgical approach
• b. Reconstructive surgical approach (regardless the technique or materials used)
2. Modified (rough surfaces)
• a. Non-reconstructive surgical approach
• b. Reconstructive surgical approach (regardless the technique or materials used)
3. Mixed or unspecified surfaces
• a. Non-reconstructive surgical approach
• b. Reconstructive surgical approach (regardless the technique or materials used)
2.5 Data collection
Two investigators independently extracted key data from the included articles. The inter-rater agreement for study selection was assessed using Cohen's kappa statistics. Inter-rater reliability was assessed using Cohen's kappa statistic on a subset of 20% of studies, yielding a kappa of 0.85, indicating a high level of agreement. Discrepancies were resolved through discussion or consultation with a third reviewer (CG). For each article, we extracted study features (i.e., study design, year of publication, number of enrolled patients), type of intervention, and outcome measures. Correct data extraction was controlled in a subset of randomly selected studies by the third investigator.
2.6 Assessment of risk of bias
Two investigators independently appraised the risk of bias of the included studies using the Cochrane Risk of Bias Tool 2.0 (RoB2) for RCTs. For non-RCTs the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used. Any inconsistencies were resolved by consensus with a third investigator (CG).
2.7 Data synthesis
Preliminary analyses of available data revealed high heterogeneity, precluding meaningful meta-analysis. Therefore, a narrative synthesis was conducted. These limitations included significant heterogeneity in implant surface types, surgical techniques, and reported outcome measures across studies. To enhance clarity and readability, findings were systematically summarized in tables according to pre-defined outcomes and subgroup analyses.
Data extraction was performed separately for each treatment group within studies containing multiple groups, while data from studies with a single treatment group were extracted accordingly. Results were categorized based on implant surface types and surgical approaches as follows:
1. Turned (machined/non-modified surfaces) a. Non-reconstructive surgical approach b. Reconstructive surgical approach (regardless the technique or materials used)
2. Modified (rough surfaces) a. Non-reconstructive surgical approach b. Reconstructive surgical approach (regardless the technique or materials used)
3. Mixed or unspecified surfaces a. Non-reconstructive surgical approach b. Reconstructive surgical approach (regardless the technique or materials used)
Findings were systematically summarized in tables according to pre-defined outcomes and subgroup analyses to enhance clarity and readability.
Within each treatment group, data were systematically collected on key parameters, including sample size (number of participants and implants), criteria used to define peri-implantitis, type of bone substitute, membrane used (if applicable), follow-up periods, implant system, and implant surface characteristics.
3 Results
3.1 Study selection
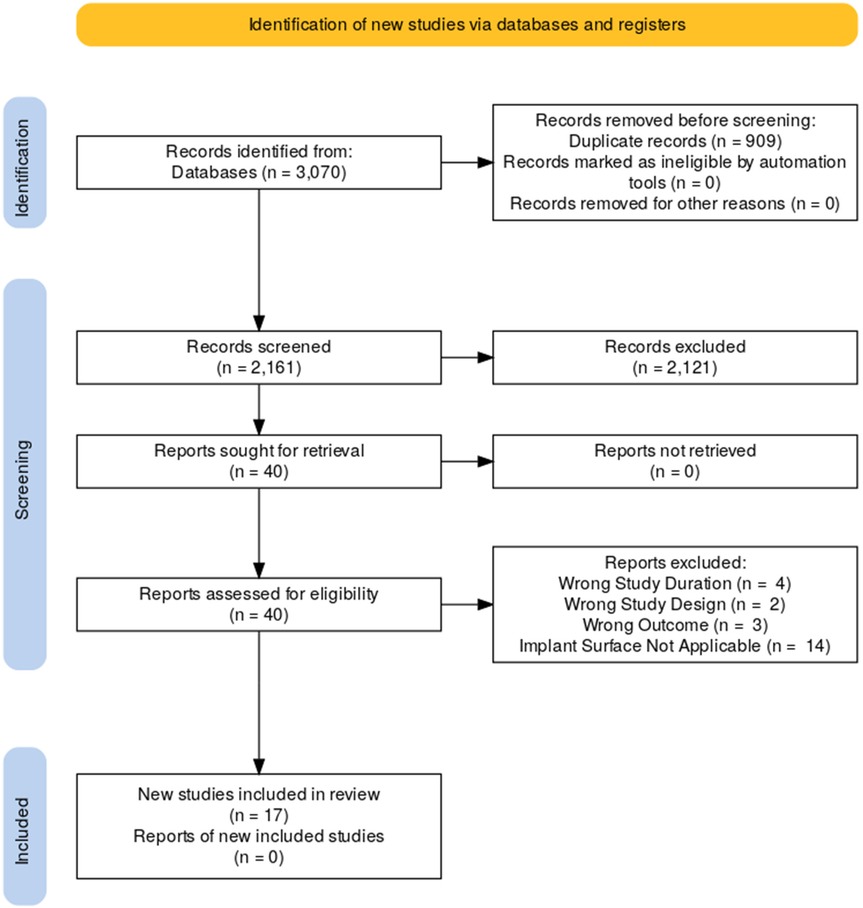
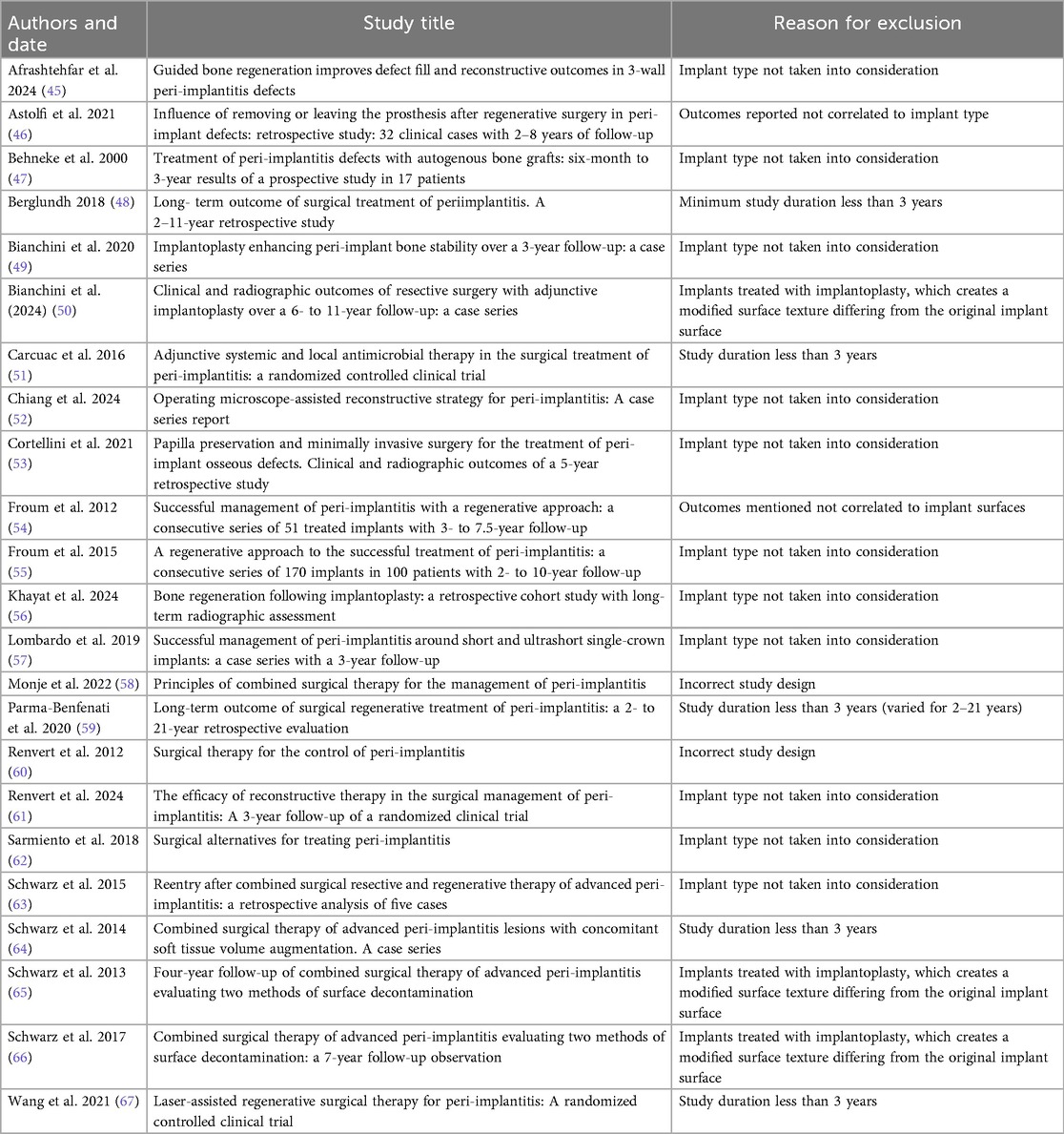
The literature search process is illustrated in the flowchart below (Figure 1). In total, there are 17 studies included in the analysis (8–24). Among them, 8 are prospective cohort studies, 3 are retrospective cohort studies, 1 are randomized controlled trials. The remaining studies include 1 each of the following types: prospective clinical study, retrospective observational study, and prospective case series. A detailed description of the study characteristics can be found in the results in Tables 1a,b.
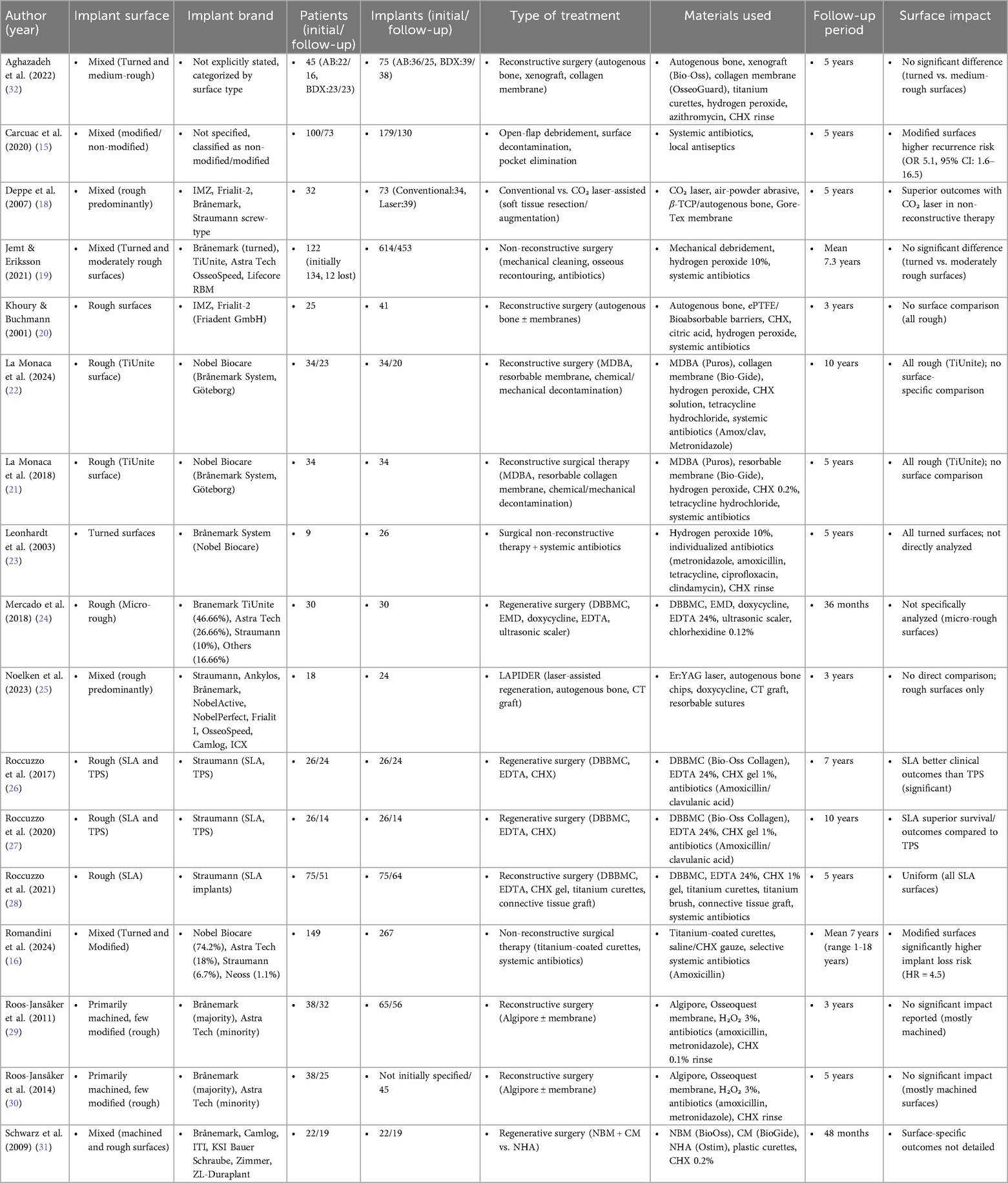
Table 1a. Summary of clinical studies evaluating treatments of peri-implantitis: surface types, materials, and outcomes.
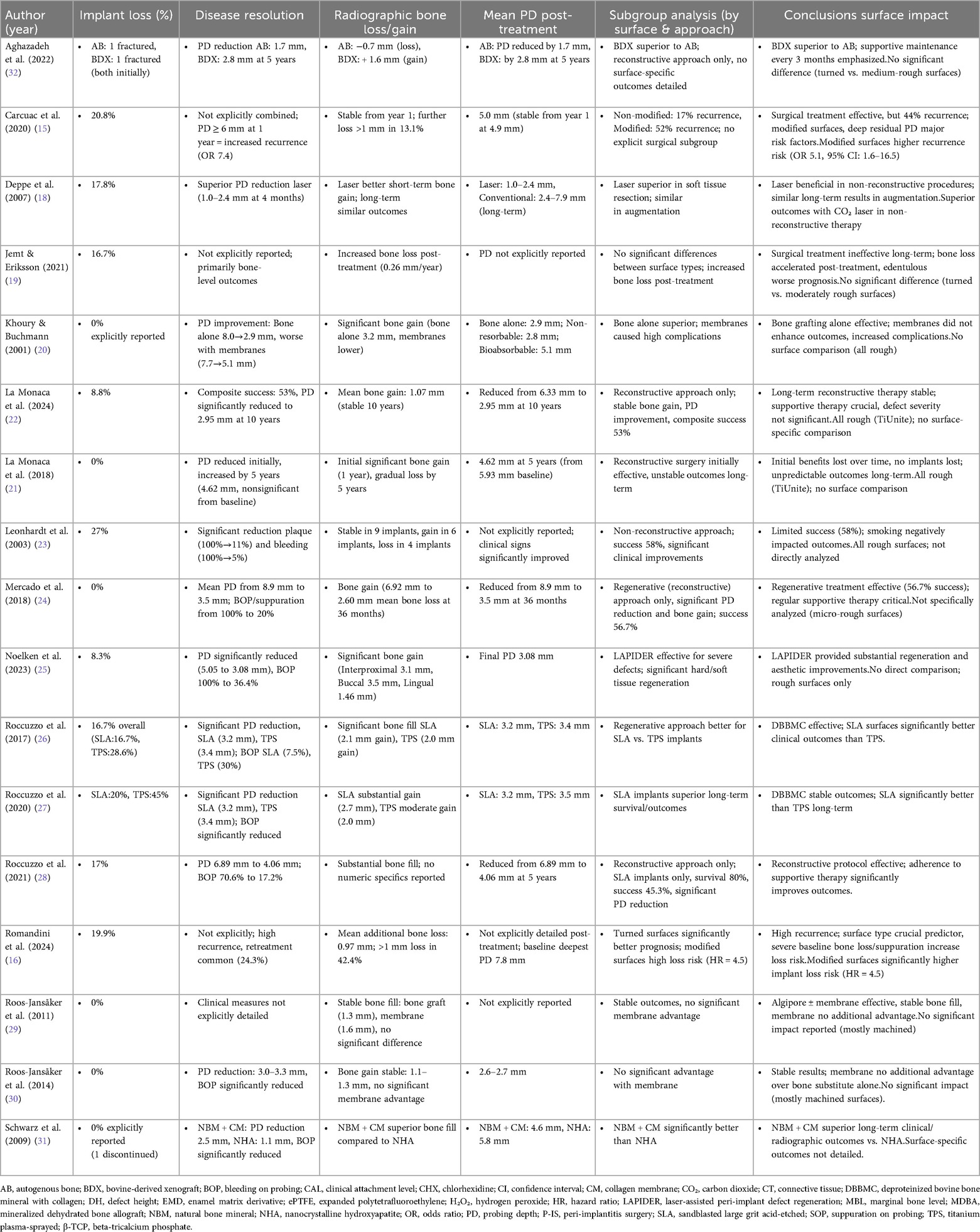
Table 1b. Comparative outcomes of clinical studies on peri-implantitis treatments: implant surfaces, secondary outcomes, and long-term surface impact.
3.2 Study populations
3.2.1 Peri-implantitis
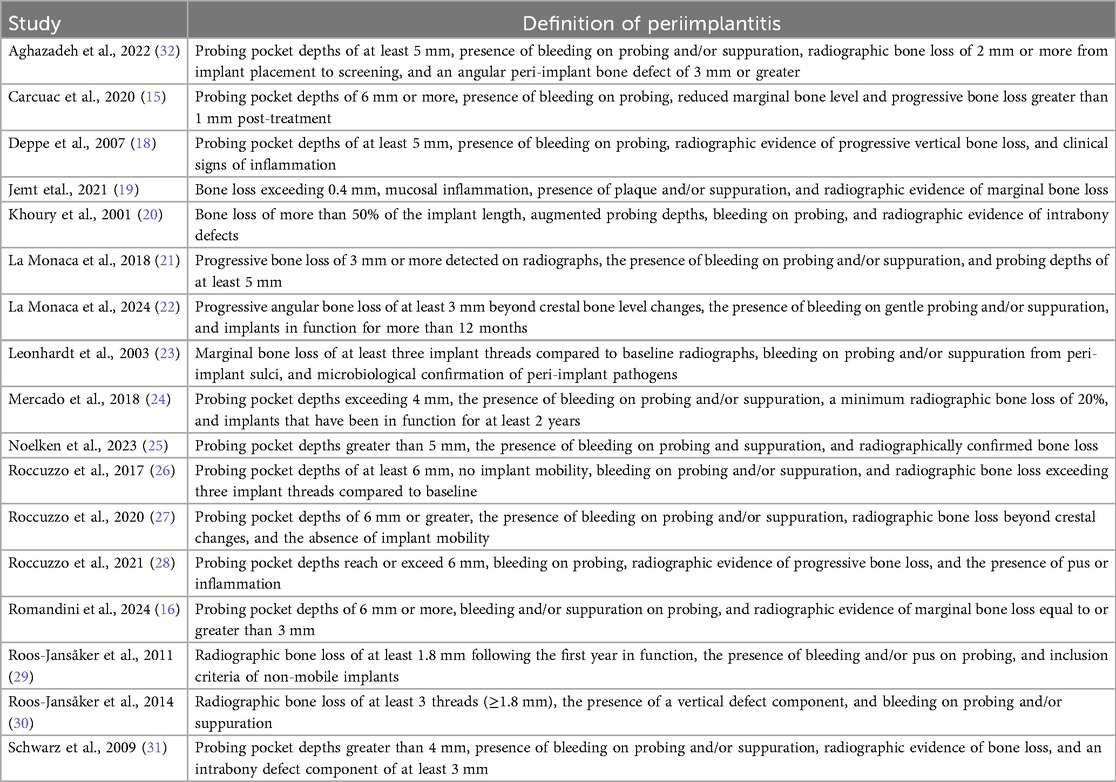
Across the 17 studies analyzed, various diagnostic criteria have been employed to identify peri-implantitis, reflecting differences in study designs and clinical considerations (8–24). The most commonly reported diagnostic parameters include probing depth (PD), bleeding on probing (BOP), suppuration, and radiographic evidence of bone loss.
3.2.2 Probing depth (PD)
A probing depth threshold of ≥6 mm is frequently used as a criterion to identify peri-implantitis, as observed in studies by Carcuac et al., Romandini et al., and Roccuzzo et al. (15, 16, 26–28). Other studies, such as Aghazadeh et al. and Noelken et al., set a threshold of ≥5 mm, which is similar to the >4 mm threshold considered indicative of disease by Mercado et al. and Schwarz et al. (24, 25, 31, 32). This variation highlights differences at diagnosis across studies.
3.2.3 Bleeding on probing (BOP) and suppuration
The presence of BOP and/or suppuration was consistently reported as a diagnostic marker in all studies. It serves as an indicator of ongoing inflammation and peri-implant tissue destruction. Studies such as La Monaca et al. and Khoury & Buchmann emphasize the importance of these clinical signs in combination with radiographic findings for accurate diagnosis (20–22).
3.2.4 Radiographic bone loss
Radiographic evaluation of bone loss is another widely accepted criterion for peri-implantitis diagnosis. The threshold for bone loss varies among studies, with the most commonly reported value being ≥3 mm, as seen in Romandini et al. (16). Other studies, including Carcuac et al. and Aghazadeh et al., defined progressive bone loss based on post-treatment changes or specific defect characteristics, such as angular defects of ≥3 mm (15, 32). A more conservative threshold of ≥1.8 mm was applied in studies such as Roos-Jansåker et al., reflecting the variability in bone loss progression (29, 30).
3.2.5 Variability in diagnostic criteria
Despite a general agreement on the primary diagnostic signs—probing depth, BOP/suppuration, and radiographic bone loss—variability exists in the specific thresholds and additional criteria applied across studies.
A detailed overview of the case definitions used to include patients with peri-implantitis in each study (treatment group) is provided in Table 2.
3.3 Primary outcome: recurrence and treatment failure
The included studies demonstrated that implant surface characteristics influenced recurrence rates following surgical peri-implantitis treatment. Modified (rough) surfaces consistently showed higher recurrence compared with turned (machined) surfaces. Carcuac et al. reported an overall recurrence of 44%, with a significantly increased risk for modified surfaces (OR 5.1) (15). Similarly, Romandini et al. found a retreatment rate of 24.3%. In contrast, studies involving turned surfaces, such as Leonhardt et al., reported more stable outcomes (23). These findings indicate that surface roughness is a key determinant of recurrence and long-term treatment stability. Studies by Schwarz et al., Mercado et al. and Noelken et al. documented relatively stable outcomes without explicitly reporting significant recurrence rates (24, 25, 31).
3.4 Secondary outcomes
3.4.1 Implant loss
Implant loss was more frequent among rough surface implants, especially TPS, with Roccuzzo et al. reporting loss in 45% of TPS implants (27), and Leonhardt et al. reporting 27% for turned surfaces (23). SLA surfaces demonstrated better survival than TPS, with 20% vs. 45% loss after 10 years (27). Modified surfaces were identified as a strong predictor of implant loss (HR 4.5) (16). Turned surfaces generally exhibited lower long-term loss risk, around 20%, compared to modified ones (16). Lower implant loss rates were generally associated with reconstructive surgical approaches, as observed by Noelken et al. 8.3% (25) and La Monaca et al. (22), 8.8%.
3.4.2 Disease resolution and probing depth (PD)
Reconstructive surgery generally improved PD irrespective of surface, but rough surfaces demonstrated greater variability. Mercado et al. (2018) reported PD reduction from 8.9 mm to 3.5 mm on micro-rough implants (24), while Noelken et al. achieved PD reduction from 5.05 mm to 3.08 mm in predominantly rough implants (25) identifying disease resolution. Roccuzzo et al. observed significant PD improvements for SLA implants compared with TPS (26, 27). Turned implants Leonhardt et al., also demonstrated significant PD reduction (23). Conversely, non-reconstructive surgical approaches such as that of Deppe et al. with predominantly rough surfaces showed initial short-term PD reductions with inconsistent long-term stability (18).
3.4.3 Radiographic bone changes
Bone regeneration outcomes were surface-dependent. Reconstructive procedures around rough implants, particularly SLA, showed consistent bone gain (Roccuzzo et al. + 2.1 mm; + 2.7 mm) (26, 27). TPS implants demonstrated less favorable long-term stability, even with grafting (27). Smooth (turned) surfaces were rarely evaluated in regenerative contexts, limiting conclusions. Khoury & Buchmann reported substantial bone gain (3.2 mm) on rough implants with autografts (20), while Roos-Jansåker et al. found stable bone gain (1.1–1.6 mm) in predominantly machined implants (29, 30).
3.5 Subgroup analyses
3.5.1 Turned surfaces
Showed moderate long-term stability, but implant loss remained high when treated non-reconstructively (23). Reconstructive data were limited but suggested stable outcomes (29, 30).
3.5.2 Modified (rough) surfaces
Non-reconstructive approaches resulted in high recurrence and implant loss (15, 16). Reconstructive approaches improved outcomes, with SLA surfaces outperforming TPS (26, 27).
3.5.3 Mixed surfaces
Outcomes were heterogeneous. Laser-assisted non-reconstructive therapy demonstrated short-term benefits Deppe et al. (18), but Jemt & Eriksson reported long-term bone loss regardless of surface type (19). Reconstructive treatments showed better results with natural bone mineral combined with a collagen membrane (NBM + CM) compared to nanocrystalline hydroxyapatite (NHA) (31), but surface-specific differences remained underreported. Aghazadeh et al. reported improved outcomes with xenograft (BDX) usage (32).
The detailed study characteristics and outcomes are presented in Tables 1a,b.
3.6 Risk of bias
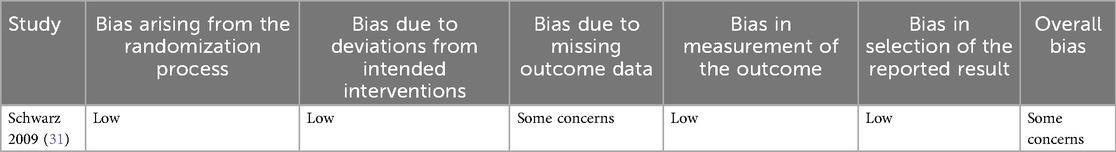
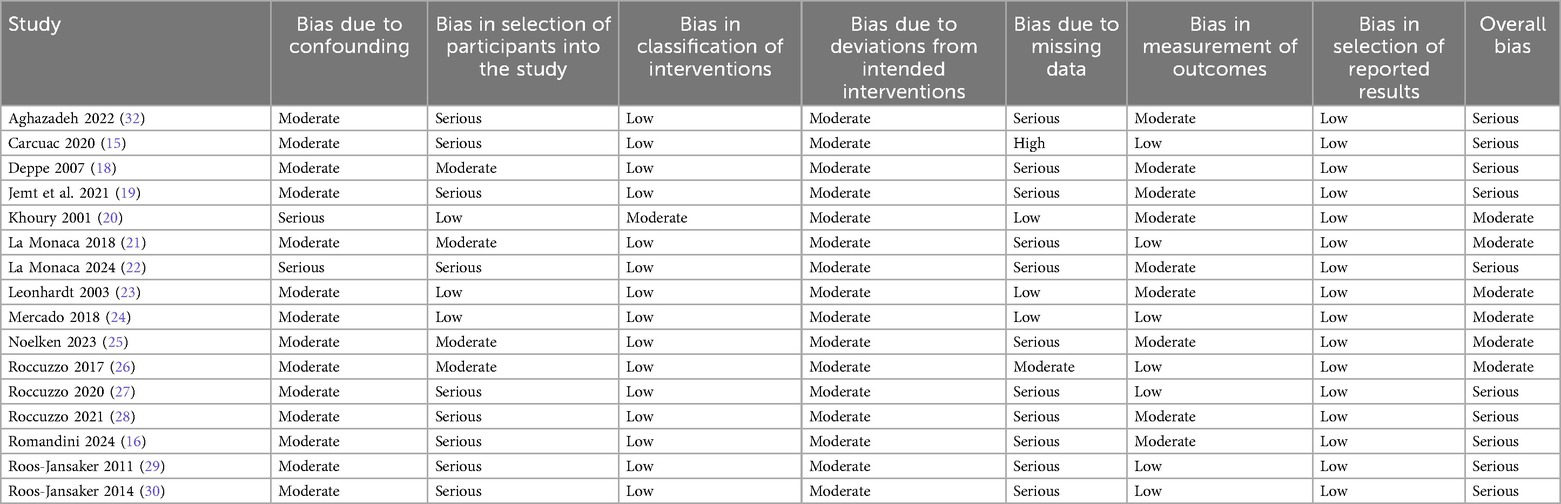
The risk of bias assessment for the 17 included studies highlights several concerns across different domains (Tables 3a and b).
The RCT (Schwarz 2009) had some concerns (31).
Among the non-randomized studies, serious risk of bias was frequently noted in selection of participants e.g., Aghazadeh, Carcuac, and La Monaca (15, 17, 22) and missing data e.g., Roccuzzo, Romandini, Roos-Jansaker (16, 27, 30). However, intervention classification and reporting of results were generally at low risk across studies. Confounding and deviations from interventions were rated as moderate risk in most cases.
Ten studies were judged to have a serious overall bias, primarily due to participant selection and missing data. The remaining studies had a moderate overall bias, with issues mainly related to confounding and missing data.
Certainty of evidence for the main outcomes was assessed using the GRADE approach and is presented in Supplementary Table S1. Overall, the certainty was judged to be very low to low across all outcomes, primarily due to serious risk of bias, high heterogeneity of diagnostic criteria and outcome definitions, small sample sizes, and imprecision of effect estimates.
4 Discussion
This systematic review aimed to evaluate the impact of implant surface characteristics on the long-term outcomes of surgical treatment of peri-implantitis. The main findings indicate that modified (rough) surfaces are consistently associated with higher recurrence and implant loss compared with turned (machined) surfaces. Within rough surfaces, SLA implants achieved more favorable outcomes than TPS, particularly in reconstructive contexts (15, 16, 26–28). In contrast, smooth implants demonstrated comparatively lower recurrence (23). These results underscore that implant surface topography can be a determinant of surgical treatment prognosis.
The effectiveness of peri-implantitis surgery is influenced by both treatment modality and implant surface. For non-regenerative procedures, modified surfaces were repeatedly associated with worse outcomes: Carcuac et al. reported a 44% recurrence rate with rough implants (15), while Romandini et al. identified modified surfaces such as TiUnite and SLA as predictors of implant loss (16). In contrast, turned surfaces showed more stable disease suppression (23).
For regenerative approaches, implant surface also played a key role. SLA implants demonstrated favorable long-term bone gain and PD reduction (26–28), whereas TPS implants performed poorly even when grafting was applied (27). Although smooth implants were less frequently studied in regenerative contexts, available evidence suggests they may perform adequately when combined with supportive therapy (29, 30).
Bone regeneration outcomes differed substantially according to surface characteristics. Greater bone fill was generally reported around rough surfaces when grafting materials were used. Khoury and Buchmann observed a 2.4 mm gain at 12 months using autogenous grafts on rough implants (20). Roccuzzo et al. reported significant defect reduction with xenografts, particularly in SLA implants, while TPS implants showed limited stability (26, 27). Comparable results with alloplastic materials were also noted (33, 34). However, bone regeneration around smooth surfaces was less favorable: Roos-Jansåker et al. reported limited improvement with alloplastic grafts (30). Thus, while rough implants may predispose to recurrence, they also appear to support more pronounced bone regeneration after reconstructive procedures.
This paradox may be explained by surface-related biology. Rough surfaces are harder to decontaminate and accumulate more plaque (35, 36), yet they may stabilize the coagulum and promote defect fill (37). Accordingly, radiographic bone gain does not necessarily correspond to re-osseointegration, as several animal studies identified connective tissue interposition rather than true reattachment (38–40).
The role of membranes in guided bone regeneration (GBR) has also been linked to implant surfaces. Khoury et al. showed greater bone gain with non-resorbable membranes around rough implants (20), while Deppe et al. observed comparable results with resorbable membranes (18). These data suggest that both membrane type and surface roughness influence regenerative outcomes. Furthermore, clinical studies and experimental models in dogs indicate that rough surfaces generally achieve greater defect fill than smooth surfaces under GBR conditions (37).
Surface characteristics may also impact soft tissue attachment. Excessively smooth surfaces can impair mucosal adhesion, as Quirynen et al. observed attachment loss on polished abutments compared with stable CAL around commercially available surfaces (41). Other studies support that maintaining a certain degree of roughness enhances soft tissue sealing (42). These findings provide a biological explanation for the improved clinical outcomes of rough implants after GBR, despite their higher susceptibility to recurrence.
Interpretation of the evidence is complicated by considerable heterogeneity. Defect morphology influences outcomes, with narrower defects showing better results (17, 43), yet most studies failed to provide detailed descriptions, limiting cross-study comparisons. Moreover, peri-implantitis definitions varied widely: Roccuzzo et al. required ≥6 mm PD and bone loss exceeding three implant threads (26), while Mercado et al. used ≥4 mm PD and ≥20% radiographic bone loss (24). Measurement variability further complicates interpretation (44). Such inconsistencies directly affect assessment of surface-related outcomes and hinder robust comparisons across studies.
This review is limited by the substantial heterogeneity among the included studies, particularly in peri-implantitis diagnostic criteria, defect morphology, surgical techniques, and outcome measures. Most studies were small in size, lacked standardized definitions, and many were judged to have a serious overall risk of bias, especially in participant selection and missing data. Confounding variables were insufficiently controlled, further reducing certainty. Furthermore, the restriction to English-language studies may have introduced language bias, potentially leading to omission of relevant non-English publications. Applying the GRADE framework, the certainty of the available evidence was rated as very low to low for all main outcomes, reflecting methodological shortcomings and heterogeneity among the included studies. These limitations restrict the generalizability of the findings and reinforce the need for well-designed, adequately powered randomized controlled trials with standardized definitions and longer follow-up.
5 Conclusion
The effectiveness of peri-implantitis surgery is influenced by implant surface characteristics and treatment modality. Modified surfaces are generally more prone to recurrence and implant loss, with SLA implants performing better than TPS, while turned surfaces appear less susceptible but remain insufficiently studied in regenerative contexts. Reconstructive approaches combined with supportive care consistently provide the most favorable outcomes. Given the very low to low certainty of the evidence with heterogenous results, current findings should be interpreted with caution, and well-designed long-term randomized trials with standardized definitions and consistent surface classifications are urgently needed. Future trials should adopt standardized outcome definitions (e.g., PD thresholds, BOP, radiographic bone loss criteria) to allow comparability across studies. Research should focus on RCTs directly comparing surface types, long-term follow-up, and adjustment for confounding factors such as defect morphology and maintenance compliance. Addressing these gaps will clarify the role of implant surface modifications.
5.1 Clinical implications
When planning peri-implantitis surgery, implant surface characteristics should be taken into account, but they must not be considered in isolation. Evidence indicates that reconstructive approaches yield more reliable outcomes than non-reconstructive ones, particularly for rough implants, with SLA surfaces performing more favorably than TPS. Turned (machined) surfaces appear less prone to recurrence, although data on regenerative protocols remain scarce. These observations suggest that implant surface may influence prognosis, yet it represents only one part of a complex clinical picture.
Patient-related risk factors (such as smoking, systemic conditions, low compliance and/or adherence to supportive care) exert a profound effect on long-term success and may outweigh surface-related differences. Surgical decision-making should therefore be individualized, integrating implant surface type, defect morphology, patient risk profile, and anticipated compliance. The use of biomaterials and barrier membranes may enhance regenerative outcomes around rough implants, but clinicians should be cautious, as radiographic bone gain does not necessarily reflect true re-osseointegration, and complete defect resolution is rarely achievable.
Nevertheless, these clinical implications must be interpreted with caution. The available evidence is heterogeneous, often based on small studies with differing peri-implantitis definitions, inconsistent outcome measures, and a serious overall risk of bias. The evidence was rated as very low to low for all main outcomes. This means that while current data can guide clinical choices, they cannot provide definitive recommendations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
PG: Conceptualization, Formal analysis, Methodology, Data curation, Writing – original draft, Writing – review & editing, Investigation. CG: Formal analysis, Methodology, Data curation, Writing – review & editing. AS: Conceptualization, Formal analysis, Methodology, Data curation, Investigation, Writing – review & editing, Project administration. AZ: Supervision, Conceptualization, Methodology, Formal analysis, Data curation, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdmed.2025.1661369/full#supplementary-material
References
1. Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res. (2019) 21(Suppl 1):4–7. doi: 10.1111/cid.12742
2. Rupp F, Liang L, Geis-Gerstorfer J, Scheideler L, Hüttig F. Surface characteristics of dental implants: a review. Dent Mater. (2018) 34(1):40–57. doi: 10.1016/j.dental.2017.09.007
3. Shayeb MA, Elfadil S, Abutayyem H, Shqaidef A, Marrapodi MM, Cicciù M, et al. Bioactive surface modifications on dental implants: a systematic review and meta-analysis of osseointegration and longevity. Clin Oral Investig. (2024) 28(11):592. doi: 10.1007/s00784-024-05958-y
4. Souza JCM, Sordi MB, Kanazawa M, Ravindran S, Henriques B, Silva FS, et al. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. (2019) 94:112–31. doi: 10.1016/j.actbio.2019.05.045
5. Matos GRM. Surface roughness of dental implant and osseointegration. J Maxillofac Oral Surg. (2021) 20(1):1–4. doi: 10.1007/s12663-020-01437-5
6. Romero-Serrano M, Romero-Ruiz MM, Herrero-Climent M, Rios-Carrasco B, Gil-Mur J. Correlation between implant surface roughness and implant stability: a systematic review. Dent J (Basel). (2024) 12(9):276. doi: 10.3390/dj12090276
7. Kupka JR, König J, Al-Nawas B, Sagheb K, Schiegnitz E. How far can we go? A 20-year meta-analysis of dental implant survival rates. Clin Oral Investig. (2024) 28(10):541. doi: 10.1007/s00784-024-05929-3
8. Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol. (2018) 89(Suppl 1):S304–s12. doi: 10.1002/JPER.17-0588
9. Diaz P, Gonzalo E, Villagra LJG, Miegimolle B, Suarez MJ. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health. (2022) 22(1):449. doi: 10.1186/s12903-022-02493-8
10. Hussein A, Shah M, Atieh MA, Alhimairi S, Amir-Rad F, Elbishari H. Influence of implant surfaces on peri-implant diseases – A systematic review and meta-analysis. Int Dent J. (2025) 75(1):75–85. doi: 10.1016/j.identj.2024.10.007
11. Stavropoulos A, Bertl K, Winning L, Polyzois I. What is the influence of implant surface characteristics and/or implant material on the incidence and progression of peri-implantitis? A systematic literature review. Clin Oral Implants Res. (2021) 32(Suppl 21):203–29. doi: 10.1111/clr.13859
12. Garaicoa-Pazmino C, Lin GH, Alkandery A, Parra-Carrasquer C, Suárez-López Del Amo F. Influence of implant surface characteristics on the initiation, progression and treatment outcomes of peri-implantitis: a systematic review and meta-analysis based on animal model studies. Int J Oral Implantol (Berl. (2021) 14(4):367–82.34726847
13. Vernon JJ, Raïf EM, Aw J, Attenborough E, Jha A, Do T. Dental implant surfaces and their interaction with the oral microbiome. Dentistry Review. (2022) 2(4):100060. doi: 10.1016/j.dentre.2022.100060
14. Wassmann T, Kreis S, Behr M, Buergers R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int J Implant Dent. (2017) 3(1):32. doi: 10.1186/s40729-017-0093-3
15. Carcuac O, Derks J, Abrahamsson I, Wennström JL, Berglundh T. Risk for recurrence of disease following surgical therapy of peri-implantitis-A prospective longitudinal study. Clin Oral Implants Res. (2020) 31(11):1072–7. doi: 10.1111/clr.13653
16. Romandini M, Bougas K, Alibegovic L, Hosseini S, Carcuac O, Berglundh T, et al. Long-term outcomes and prognostic factors of surgical treatment of peri-implantitis - A retrospective study. Clin Oral Implants Res. (2024) 35(3):321–9. doi: 10.1111/clr.14228
17. Aghazadeh A, Persson GR, Renvert S. Impact of bone defect morphology on the outcome of reconstructive treatment of peri-implantitis. Int J Implant Dent. (2020) 6. doi: 10.1186/s40729-020-00219-5
18. Deppe H, Horch HH, Neff A. Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int J Oral Maxillofac Implants. (2007) 22(1):79–86.17340900
19. Jemt T, Eriksson J. Bone loss before and after peri-implantitis surgery: a 7-year retrospective observational study. Int J Oral Maxillofac Implants. (2021) 36(6):1199–210. doi: 10.11607/jomi.9175
20. Khoury F, Buchmann R. Surgical therapy of peri-implant disease: a 3-year follow-up study of cases treated with 3 different techniques of bone regeneration. J Periodontol. (2001) 72(11):1498–508. doi: 10.1902/jop.2001.72.11.1498
21. La Monaca G, Pranno N, Annibali S, Cristalli MP, Polimeni A. Clinical and radiographic outcomes of a surgical reconstructive approach in the treatment of peri-implantitis lesions: a 5-year prospective case series. Clin Oral Implants Res. (2018) 29(10):1025–37. doi: 10.1111/clr.13369
22. La Monaca G, Pranno N, Annibali S, Polimeni A, Cristalli MP. A 10-year follow-up of reconstructive treatment of peri-implantitis using mineralized dehydrated allograft and resorbable membrane: a retrospective case series. Clin Oral Implants Res. (2024) 36:325–38. doi: 10.1111/clr.14385
23. Leonhardt A, Dahlén G, Renvert S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol. (2003) 74(10):1415–22. doi: 10.1902/jop.2003.74.10.1415
24. Mercado F, Hamlet S, Ivanovski S. Regenerative surgical therapy for peri-implantitis using deproteinized bovine bone mineral with 10% collagen, enamel matrix derivative and doxycycline-A prospective 3-year cohort study. Clin Oral Implants Res. (2018) 29(6):583–91. doi: 10.1111/clr.13256
25. Noelken R, Westphal L, Schiegnitz E, Al-Nawas B. Hard and soft tissue regeneration of severe peri-implantitis defects with the laser-assisted peri-implant defect regeneration technique: 3-year results. Int J Implant Dent. (2023) 9(1):3. doi: 10.1186/s40729-023-00467-1
26. Roccuzzo M, Pittoni D, Roccuzzo A, Charrier L, Dalmasso P. Surgical treatment of peri-implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7-year-results. Clin Oral Implants Res. (2017) 28(12):1577–83. doi: 10.1111/clr.13028
27. Roccuzzo M, Fierravanti L, Pittoni D, Dalmasso P, Roccuzzo A. Implant survival after surgical treatment of peri-implantitis lesions by means of deproteinized bovine bone mineral with 10% collagen: 10-year results from a prospective study. Clin Oral Implants Res. (2020) 31(8):768–76. doi: 10.1111/clr.13628
28. Roccuzzo M, Mirra D, Pittoni D, Ramieri G, Roccuzzo A. Reconstructive treatment of peri-implantitis infrabony defects of various configurations: 5-year survival and success. Clin Oral Implants Res. (2021) 32(10):1209–17. doi: 10.1111/clr.13818
29. Roos-Jansåker AM, Lindahl C, Persson GR, Renvert S. Long-term stability of surgical bone regenerative procedures of peri-implantitis lesions in a prospective case-control study over 3 years. J Clin Periodontol. (2011) 38(6):590–7. doi: 10.1111/j.1600-051X.2011.01729.x
30. Roos-Jansåker AM, Persson GR, Lindahl C, Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a 5-year follow-up. J Clin Periodontol. (2014) 41(11):1108–14. doi: 10.1111/jcpe.12308
31. Schwarz F, Sahm N, Bieling K, Becker J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four-year clinical follow-up report. J Clin Periodontol. (2009) 36(9):807–14. doi: 10.1111/j.1600-051X.2009.01443.x
32. Aghazadeh A, Persson GR, Stavropoulos A, Renvert S. Reconstructive treatment of peri-implant defects-results after three and five years. Clin Oral Implants Res. (2022) 33(11):1114–24. doi: 10.1111/clr.13994
33. Wohlfahrt JC, Lyngstadaas SP, Rønold HJ, Saxegaard E, Ellingsen JE, Karlsson S, et al. Porous titanium granules in the surgical treatment of peri-implant osseous defects: a randomized clinical trial. Int J Oral Maxillofac Implants. (2012) 27(2):401–10.22442781
34. Roccuzzo M, Bonino F, Bonino L, Dalmasso P. Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: comparative results of a prospective study on two different implant surfaces. J Clin Periodontol. (2011) 38(8):738–45. doi: 10.1111/j.1600-051X.2011.01742.x
35. Subramani K, Jung RE, Molenberg A, Hammerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. (2009) 24(4):616–26.19885401
36. Saulacic N, Schaller B. Prevalence of peri-implantitis in implants with turned and rough surfaces: a systematic review. J Oral Maxillofac Res. (2019) 10(1):e1. doi: 10.5037/jomr.2019.10101
37. Persson LG, Berglundh T, Lindhe J, Sennerby L. Re-osseointegration after treatment of peri-implantitis at different implant surfaces. An experimental study in the dog. Clin Oral Implants Res. (2001) 12(6):595–603. doi: 10.1034/j.1600-0501.2001.120607.x
38. Persson LG, Araújo MG, Berglundh T, Gröndahl K, Lindhe J. Resolution of peri-implantitis following treatment. An experimental study in the dog. Clin Oral Implants Res. (1999) 10(3):195–203. doi: 10.1034/j.1600-0501.1999.100302.x
39. Schwarz F, Jepsen S, Herten M, Sager M, Rothamel D, Becker J. Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. J Clin Periodontol. (2006) 33(8):584–95. doi: 10.1111/j.1600-051X.2006.00956.x
40. You TM, Choi BH, Zhu SJ, Jung JH, Lee SH, Huh JY, et al. Treatment of experimental peri-implantitis using autogenous bone grafts and platelet-enriched fibrin glue in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2007) 103(1):34–7. doi: 10.1016/j.tripleo.2006.01.005
41. Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. (1996) 11(2):169–78.8666447
42. Dorkhan M, Yücel-Lindberg T, Hall J, Svensäter G, Davies JR. Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral Health. (2014) 14(75). doi: 10.1186/1472-6831-14-75
43. Polyzois I, Renvert S, Bosshardt DD, Lang NP, Claffey N. Effect of bio-Oss on osseointegration of dental implants surrounded by circumferential bone defects of different dimensions: an experimental study in the dog. Clin Oral Implants Res. (2007) 18(3):304–10. doi: 10.1111/j.1600-0501.2007.01207.x
44. Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. (2010) 81(2):231–8. doi: 10.1902/jop.2009.090269
45. Afrashtehfar KI, Alfallaj HA, Fernandez E, Hussaini S. Guided bone regeneration improves defect fill and reconstructive outcomes in 3-wall peri-implantitis defects. Evid Based Dent. (2024) 26:29–31. doi: 10.1038/s41432-024-01073-9
46. Astolfi V, Gómez-Menchero A, Ríos-Santos JV, Bullón P, Galeote F, Ríos-Carrasco B, et al. Influence of removing or leaving the prosthesis after regenerative surgery in peri-implant defects: retrospective study: 32 clinical cases with 2 to 8 years of follow-up. Int J Environ Res Public Health. (2021) 18(2):645. doi: 10.3390/ijerph18020645
47. Behneke A, Behneke N, d'Hoedt B. Treatment of peri-implantitis defects with autogenous bone grafts: six-month to 3-year results of a prospective study in 17 patients. Int J Oral Maxillofac Implants. (2000) 15(1):125–38.10697947
48. Berglundh T, Wennström JL, Lindhe J. Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clin Oral Implants Res. (2018) 29(4):404–10. doi: 10.1111/clr.13138
49. Bianchini MA, Galarraga-Vinueza ME, Bedoya KA, Correa BB, de Souza Magini R, Schwarz F. Implantoplasty enhancing peri-implant bone stability over a 3-year follow-up: a case series. Int J Periodontics Restorative Dent. (2020) 40(1):e1–8. doi: 10.11607/prd.3849
50. Bianchini MA, Kuhlkamp LF, Schwarz F, Galarraga-Vinueza ME. Clinical and radiographic outcomes of resective surgery with adjunctive implantoplasty over a 6- to 11-year follow-up: a case series. Int J Periodontics Restorative Dent. (2024) 44(4):466–76. doi: 10.11607/prd.6756
51. Carcuac O, Derks J, Charalampakis G, Abrahamsson I, Wennström J, Berglundh T. Adjunctive systemic and local antimicrobial therapy in the surgical treatment of peri-implantitis: a randomized controlled clinical trial. J Dent Res. (2016) 95(1):50–7. doi: 10.1177/0022034515601961
52. Chiang YC, Sirinirund B, Rodriguez A, Velasquez D, Chan HL. Operating microscope-assisted reconstructive strategy for peri-implantitis: a case series report. Clin Adv Periodontics. (2024) 14(3):149–56. doi: 10.1002/cap.10265
53. Cortellini P, Cortellini S, Bonaccini D, Tonetti MS. Papilla preservation and minimally invasive surgery for the treatment of peri-implant osseous defects. Clinical and radiographic outcomes of a 5-year retrospective study. Clin Oral Implants Res. (2021) 32(11):1384–96. doi: 10.1111/clr.13826
54. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. (2012) 32(1):11–20.22254219
55. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. (2015) 35(6):857–63. doi: 10.11607/prd.2571
56. Khayat P, Aidan D, Calatrava J, Wang HL. Bone regeneration following implantoplasty: a retrospective cohort study with long-term radiographic assessment. Int J Periodontics Restorative Dent. (2024) 0(0):1–17. doi: 10.11607/prd.7422
57. Lombardo G, Marincola M, Cicconetti A, Simancas-Pallares MA, Pighi J, Lehrberg J, et al. Successful management of peri-implantitis around short and ultrashort single-crown implants: a case series with a 3-year follow-up. Int J Dent. (2019) 2019:5302752. doi: 10.1155/2019/5302752
58. Monje A, Schwarz F. Principles of combined surgical therapy for the management of peri-implantitis. Clin Adv Periodontics. (2022) 12(1):57–63. doi: 10.1002/cap.10186
59. Parma-Benfenati S, Tinti C, Romano F, Roncati M, Aimetti M. Long-Term outcome of surgical regenerative treatment of peri-implantitis: a 2- to 21-year retrospective evaluation. Int J Periodontics Restorative Dent. (2020) 40(4):487–96. doi: 10.11607/prd.4647
60. Renvert S, Polyzois I, Claffey N. Surgical therapy for the control of peri-implantitis. Clin Oral Implants Res. (2012) 23(Suppl 6):84–94. doi: 10.1111/j.1600-0501.2012.02554.x
61. Renvert S, Giovannoli J-L, Rinke S. The efficacy of reconstructive therapy in the surgical management of peri-implantitis: a 3-year follow-up of a randomized clinical trial. J Clin Periodontol. (2024) 51(10):1267–76. doi: 10.1111/jcpe.14049
62. Sarmiento HL, Norton M, Korostoff J, Ko KI, Fiorellini JP. Surgical alternatives for treating peri-implantitis. Int J Periodontics Restorative Dent. (2018) 38(5):665–71. doi: 10.11607/prd.3639
63. Schwarz F, John G, Becker J. Reentry after combined surgical resective and regenerative therapy of advanced peri-implantitis: a retrospective analysis of five cases. Int J Periodontics Restorative Dent. (2015) 35:647–53. doi: 10.11607/prd.2320
64. Schwarz F, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin Oral Implants Res. (2014) 25(1):132–6. doi: 10.1111/clr.12103
65. Schwarz F, Hegewald A, John G, Sahm N, Becker J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J Clin Periodontol. (2013) 40(10):962–7. doi: 10.1111/jcpe.12143
66. Schwarz F, John G, Schmucker A, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: a 7-year follow-up observation. J Clin Periodontol. (2017) 44(3):337–42. doi: 10.1111/jcpe.12648
67. Wang CW, Ashnagar S, Gianfilippo RD, Arnett M, Kinney J, Wang HL. Laser-assisted regenerative surgical therapy for peri-implantitis: a randomized controlled clinical trial. J Periodontol. (2021) 92(3):378–88. doi: 10.1002/JPER.20-0040
Appendix
Keywords: peri-Implantitis, surgical peri-implantitis treatment, treatment outcome, explantation, implant surface, long-term outcomes, bone loss, implant survival
Citation: Gardelis P, Giannopoulou C, Stavropoulos A and Zekeridou A (2025) Impact of implant surface modifications on long-term outcome of surgical peri-implantitis treatment: a systematic review. Front. Dent. Med. 6:1661369. doi: 10.3389/fdmed.2025.1661369
Received: 7 July 2025; Accepted: 5 September 2025;
Published: 24 September 2025.
Edited by:
Giuseppe Troiano, University of Foggia, ItalyReviewed by:
Rok Gašperšič, University of Ljubljana, SloveniaPatricia Miguez, University of North Carolina at Chapel Hill, United States
Matteo Serroni, G. d'Annunzio University of Chieti and Pescara, Italy
Copyright: © 2025 Gardelis, Giannopoulou, Stavropoulos and Zekeridou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alkisti Zekeridou, YWxraXN0aS56ZWtlcmlkb3VAdW5pZ2UuY2g=
 Panagiotis Gardelis
Panagiotis Gardelis Catherine Giannopoulou
Catherine Giannopoulou Andreas Stavropoulos
Andreas Stavropoulos Alkisti Zekeridou
Alkisti Zekeridou