- 1Clinical Science Department, Ajman University, Ajman, United Arab Emirates
- 2Center of Medical and Bioallied Health Sciences Research, Ajman University, Ajman, United Arab Emirates
- 3Department of Restorative Dentistry & Endodontics, Faculty of Dentistry, SEGi University, Petaling Jaya, Selangor, Malaysia
Introduction: Teeth that have undergone endodontic treatment often suffer a considerable loss in structural strength, necessitating the insertion of a post into the root canal to maintain stability for both restoration and functionality. While NaOCl and EDTA are standard disinfectants, they come with drawbacks such as toxicity to surrounding tissues, potential allergic reactions, interference with resin sealer polymerization, and dentin erosion. Consequently, finding more effective alternatives is crucial.
Objectives: Effectiveness of various contemporary post space disinfectants, i.e., Er: YAG laser, Phthalocyanine (Pc) activated Photodynamic Therapy (PDT), Bioactive glass nanoparticles (BAGNPs) on the removal of the smear layer (SL) and the push-out bond strength (PBS) of quartz fiber post (QFP) to radicular dentin.
Methods: Forty-eight mature, single-rooted human mandibular premolars were selected for the study. After ensuring proper disinfection, standard root canal procedures were carried out on each tooth. Post space was prepared. The samples were randomly split into four groups based on the post-space disinfection protocols (n = 12). NaOCl + 17% EDTA, Er: YAG laser + 17% EDTA, Pc(PDT) 17% EDTA, and BAGNPs 17% EDTA. SL removing efficiency was evaluated using SEM on two samples from each group. Bonding of QFP was performed in the post space, followed by sectioning of the canal dentin (coronal, middle, apical). The PBS via universal testing machine and failure mode via stereomicroscopy were quantitatively evaluated. ANOVA was used to compare the results across the different groups, accompanied by the Tukey post hoc test p < 0.05.
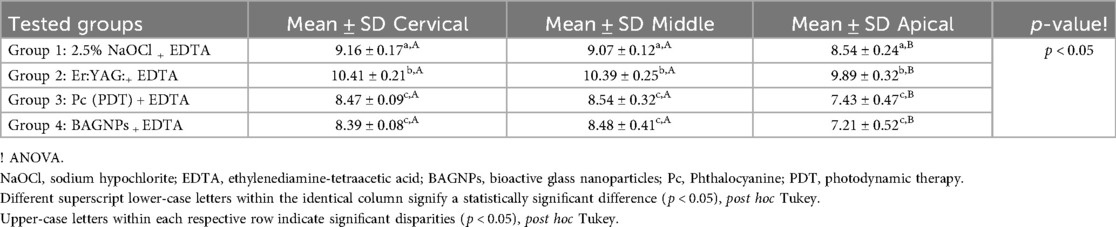
Results: Post space treated with Er: YAG+ EDTA presented the highest SL removal and maximum PBS. Whereas Group 4 (BAGNPs+EDTA) samples revealed the lowest SL removal efficacy and minimum PBS.
Conclusion: Er: YAG + EDTA used as a post-space disinfectant showed significantly better results in terms of SL removal and PBS across all three sections compared to the other groups. Er: YAG + EDTA could potentially serve as an alternative to 2.5% NaOCl + EDTA.
1 Introduction
Teeth that have been treated endodontically often experience a significant reduction in their structural integrity, making it necessary to place a post within the root canal to ensure stability for both restoration and function (1). In today's dental field, there is an increasing emphasis on aesthetic posts and cores, which has led to the innovation of metal-free post-and-core systems, particularly those utilizing zirconium dioxide and fiber-reinforced composite (FRC) materials (2). Quartz fiber posts (QFP) offer significant benefits in dental restoration, primarily because their elastic modulus is similar to that of dentin. This similarity enhances stress distribution and reduces the likelihood of root fractures when compared to metal posts (3, 4). Additionally, QFP offers superior aesthetics and enhanced light transmission, resulting in restorations that appear more natural. However, the post space must be meticulously cleaned and free from any smear layer (SL), as this factor significantly impacts the push-out bond strength (PBS) results (5).
In the field of endodontics, sodium hypochlorite (NaOCl) is commonly considered the preferred disinfectant for root canal cleaning due to its potent antimicrobial characteristics and its ability to effectively dissolve organic tissue. Nonetheless, NaOCl affects the organic elements of the SL (6). Therefore, to enhance decontamination, ethylenediaminetetraacetic acid (EDTA) is used after NaOCl application, as it efficiently removes the remaining inorganic debris and the SL that develops on tooth surfaces, thereby improving bond strength (6). Nonetheless, both NaOCl and EDTA are associated with limitations, including toxicity to the tissues around the root, possible allergic reactions, and disruption of resin sealer polymerization as well as dentin erosion (7). Therefore, the pursuit of more effective alternatives becomes essential.
Recent advancements in technology have facilitated the implementation of laser and photodynamic therapy (PDT) across various restorative and endodontic practices (8). Considering the wavelength of 2,940 nm, Er: YAG lasers are completely absorbed in the surface of target tissues, leading to minimal thermal propagation, which is advantageous for root canal therapy (9). Furthermore, work by AlSahaf has shown that Er: YAG laser, particularly in conjunction with EDTA, efficiently removes the SL and enhances the bonding of dental materials (10). Photodynamic therapy (PDT) in the field of dentistry represents a non-invasive approach that employs light-activated photosensitizers to eradicate bacteria, fungi, and certain cancer cells (11). Its application has expanded across various areas of dentistry. Numerous photosensitizers (PSs) with distinct compositions exist; however, phthalocyanine (Pc) has garnered considerable interest due to its remarkable antibacterial efficacy and color stability, as noted by Sahin and associates (12). Nevertheless, there remains a lack of literature evidence that has illuminated their effects on SL removal and the PBS of QFP, indicating a necessity for future research.
With rapid progress in nanotechnology, nanoparticles are showing great potential in the medical field. These tiny particles are characterized by at least one dimension ranging from 1–100 nm, which makes them highly effective for various applications (13). Obeid et al. explored the antimicrobial properties of Bioactive glass nanoparticles (BAGNPs) when used as an intracanal medicament against E. faecalis, resulting in the highest rate of bacterial elimination (14). However, there is a scarcity of studies that have clarified its role as a disinfectant for post spaces and its effect on SL removal and the bond strength of QFP.
The research was conducted under the hypothesis that the differences observed between groups would not be statistically significant in the SL removal capacity when utilizing contemporary post-space disinfectants in comparison to the conventional control. Additionally, it was anticipated that the strength of the bond between QFP and post space dentin with modern disinfectants would be analogous to that achieved with NaOCl and EDTA (control). Consequently, the objectives of this study were to evaluate the effectiveness of various contemporary post space disinfectants (NaOCl+17%EDTA, Er: YAG laser+17% EDTA, Phthalocyanine-Pc activated by PDT + 17% EDTA, and BAGNPs 17% EDTA) on the removal of the SL and PBS between QFP and the post space dentin.
2 Materials and methods
Preparation of BAGNPs Commercially available “standard bioactive glass 45S5 (Perioglass™, US Biomaterials Corp., Alachua, FL, USA)” was obtained from a certified supplier (Sigma Aldrich, Berlin, Germany). In addition, “bioactive glass nanopowder (BAG-np)” was synthesized using the “sol-gel” method by “Nano-Stream” (15). To formulate the oxide composition, silicon and phosphorus alkoxides were mixed with sodium and calcium sources, specifically sodium hydroxide and calcium hydroxide. The mixture was dissolved with the help of deionized water and ethyl alcohol, serving as solvents. The gel was created at 70°C and a pH of 2, then left to age for a week, followed by heat treatment at temperatures reaching 800°C (15). The resulting particles were examined using scanning electron microscopy (Quanta FEG 250, FEI, Hillsboro, OR, USA), operating at 120 kV to verify that a particle size of less than 100 nm was obtained.
Sample preparation: A total of 48 mandibular premolars, each with a single root and fully developed apices, which had been extracted due to periodontal issues, were used in this study. The study was approved by the Ajman University, UAE, Ethics Committee (Project Number: D-F-H-16-Jun-2025). The present study followed the checklist for reporting in vitro study (CRIS) guidelines and was conducted following the Declaration of Helsinki (16). The patient, whose teeth were utilized for experimental purposes in this study, provided written consent. Using the OpenEpi sample size calculator, the sample size was calculated (17, 18). Teeth that displayed curvature of 6-25° or greater on periapical radiograph, dilacerations, and fractured roots were excluded. The samples were subjected to disinfection with a thymol solution and were thoroughly cleaned using an ultrasonic scaler (Woodpecker Ultrasonic Scaler UDS-J, Guilin, Guangxi, China) to gently clean the specimens, removing any debris or remaining soft tissue. After cleaning, the samples were kept in a 0.9% saline solution at 37℃ to preserve their condition. The teeth were decoronated using a carborundum disc at the cementoenamel junction to ensure that each specimen maintained a consistent length of 15 mm (19).
Root canal treatment Each root canal was meticulously instrumented using ProTaper rotary system (Dentsply Sirona, Charlotte, North Carolina), progressing to the F3 file, and a standardized irrigation protocol was carried out, which included the application of 5 ml of 2.5% NaOCl (Supply Chain Management Co., Ltd, Jinan, Shandong, China) solution followed by 5 ml of 17% EDTA (Stardent Equipment Co., Limited, Guangdong, China) as a final disinfectant for 1 min. Canals were dried using paper points (Dentsply Maillefer). Resin-based Nonacrylic AH-26 sealer (Dentsply DeTrey, Beijing, China) was applied on the canal wall using a 20 K file rotated in a counterclockwise manner. A gutta-percha (GP) master cone (Dentsply Maillefer), coated with AH-26 sealer, was subsequently condensed into the canal using an endodontic condenser and plugger. A temporary filling was placed in the access cavity. The samples were then stored in 100% humidity at 37°C for 48 h. After this incubation period, each root was embedded securely into a cylindrical mold made from self-curing acrylic resin. The post-space preparation began 48 h after completing the root canal treatment (20).
Post space preparation Gutta-percha was carefully removed from the upper part of the canals using Peeso reamers sizes #2 and #3 (MANI Inc.). The post space was prepared to a depth of 9 mm below the cemental junction using a low-speed drill #3, as recommended by the manufacturer. The root canal treatment and post space preparation were performed by an experienced endodontist.
2.1 Group allocation
The samples were randomly assigned to four different groups, each following a specific disinfection protocol (n = 12).
2.1.1 Group 1 (NaOCl + EDTA)
The samples were rinsed using 5 ml of 2.5% NaOCl for 30 s and air dried. The samples were then treated with 17% EDTA for 30 s to aid in cleaning and SL removal from post space, using a 27-G irrigating tip (Endo-Eze; Ultradent, South Jordan, UT) (6).
2.1.2 Group 2 (Er: YAG laser + 17% EDTA)
Er: YAG laser (Lightwalker; Fotona, Ljubljana, Slovenia) was used to activate the irrigating solution at a wavelength of 2,940 nm. H14 handpiece and a conical PIPS fiber tip (600/9) was carefully positioned at the canal orifice. The Er: YAG laser was set on Super Short Pulse (SSP) with power 0.3W, frequency 15 Hz, and 20 MJ with no air and water spray. The fiber tip was kept perpendicular, steady, and at a constant distance to activate 2 ml of 3% sodium hypochlorite (NaOCl) refreshed continuously every 30 s. This process was repeated three times with 30 s pauses in between. The post-space was dried. The final step involved applying 17% EDTA for 30 s to complete the procedure (21).
2.1.3 Group 3 (phthalocyanine-Pc 17% EDTA)
The post space was carefully filled with 1 ml of a 6 micromolar (µM) solution of Pc (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and left undisturbed for 5 min to allow for adequate interaction with the post space. Light-emitting diode (LED) Fotosan 630 (CMS Dental, Seoul, Korea) wavelength of 620–640 nm (85%) with a peak of 630 nm, and an intensity of 2–4 mW/cm2 to irradiate the post space for 60 s (22). Following the procedure, Pc was aspirated, and the post-space was dried. The final step involved applying 17% EDTA for 30 s to complete the procedure.
2.1.4 Group 4 (BAGNPs 17% EDTA)
The BAGNPs 1% solution was prepared by dissolving 1 g of BAGNPs into 100 ml of distilled water. The prepared 1% BAGNP solution (0.5 ml) was injected into the post space using an irrigating syringe and left for 1 min to take effect. The solution of BAGNPs was aspirated, and the post-space was dried. The final step involved applying 17% EDTA for 30 s to complete the procedure (23).
2.2 SL removal assessment
Once the intracanal treatments were completed, two teeth from each group were sectioned into two halves. This was done by carefully creating two longitudinal grooves on each tooth using a diamond-coated high-speed bur, taking special care not to breach the canal itself. The teeth were then split along the grooves using a mallet and chisel, with the help of a modified cementum spatula, producing mesial and distal halves of the root canal. Each half was then gold-plated with a 15–20 nm gold-palladium layer and observed using SEM (Quanta FEG 250, FEI, Hillsboro, OR, USA). During the experiment, the resolution achieved was approximately 40 nm. The evaluation of smear layer removal quality was conducted by two independent experts through the analysis of photographs acquired after scanning electron microscopy (SEM), utilizing a scoring system ranging from 1–5 as proposed by Hulsmann et al. (24). The criteria were established as follows:
Score 1: Complete removal of SL, with exposure of dentinal tubules (DT).
Score 2: Minimal SL present over the canal, with many DT observable.
Score 3: Presence of SL and debris obscuring the canal walls, with a limited number of DT visualized.
Score 4: Root canal surface entirely covered by SL DT, not visualized.
Score 5: Significant SL and debris obscuring the surface of the root canal.
Two independent endodontist specialists, blinded by the experimental groups, assessed the SL kappa score (0.78).
2.3 Bonding of quartz fiber post
QFP (RTD ILLUSION #3; St. Egreve, France) underwent a cleaning process that involved the application of 70% ethanol and then gently air-drying. Self-adhesive dual-cure resin cement (Maxcem Elite, BisCem, RelyX Unicem) was utilized to secure the posts in 10 teeth from each group. The methods for applying the cement were executed in line with the manufacturer's recommendations, a Lentulo spiral was used for the application process. Subsequently, the posts were gently placed to their full depth within the prepared cavities, applying light finger pressure for a few seconds. Excess luting material was carefully removed with a fine brush to maintain precision. To complete the process, the luting agent was cured by exposing it to light polymerization, with the LED light positioned directly at the coronal ends of the posts. The specimens, after preparation, were kept in distilled water to maintain hydration at a temperature of 37°C for 24 h before testing (25). Before the sectioning, samples were thermocycled (GeneBio System, Inc., Toronto, Canada), between temperatures of 5℃–55℃ for 15 s each bath for a total of 5,000 cycles.
2.4 Sectioning of the samples
The specimens were meticulously sectioned utilizing an Isomet butcher saw (Logix, Technova Noida, Gautam Buddha Nagar, Uttar Pradesh, India). The posts were positioned at a 90-degree angle to the root's longitudinal axis, ensuring proper alignment, while water cooling was utilized to mitigate thermal effects. From each specimen, three sections of 2 mm in thickness were obtained from each third of the root (coronal, middle, apical).
2.5 PBS testing
The PBS was quantitatively evaluated utilizing a universal testing machine (UTM) (EMIC DL 2,000; Sao Jose dos Pinhais, PR, Brazil) by exerting a force at a velocity of 0.5 mm/min. The posts were pushed in the apical-to-coronal direction to ensure they were moved toward the wider part of the root slice, helping to avoid any taper limitations during the procedure until the segment of the quartz post that had been relined was detached from the root slice. To articulate the bond strength in megapascals (MPa), the force at which failure occurred, measured in newtons, was divided by the surface area (mm2) of the post-dentin interface (26).
Fracture pattern assessment. The specimen failure mode was determined using a stereomicroscope (Leica, MZ125, Milton Keynes, UK) at x40 magnification. The fractured surfaces were classified as adhesive, cohesive, and admixed (27).
To assess failure types, cohesive, adhesive, or admixed, two independent endodontist specialists, blinded by the experimental groups, assessed the type of failure, using Kappa statistics (0.81).
Statistical analysis. The normality of the data distribution was evaluated through the application of the Kolmogorov–Smirnov test. Data analysis was conducted utilizing one-way ANOVA, supplemented by the Tukey post hoc test. All analytical procedures were performed utilizing SPSS software version 17 (SPSS Inc., Chicago, USA). The significance level of p < 0.05 was established.
3 Results
3.1 SL removal assessment
SL removal from the post space after using different post space disinfection protocols is displayed in Table 1. The cervical third of Group 2 (Er: YAG:+ EDTA) treated teeth presented the highest SL removal (1.54 ± 0.06). Whereas, the apical third of Group 4 (BAGNPs+ EDTA) samples revealed the lowest SL removal (3.93 ± 0.72). Comparison among different tested groups exhibited that SL removal in Group 3 [Pc (PDT)+EDTA] (Cervical: 2.77 ± 0.23, Middle: 3.21 ± 0.28, and Apical: 3.84 ± 0.41) and Group 4 (Cervical: 2.69 ± 0.20, Middle: 3.12 ± 0.22, and Apical: 3.93 ± 0.72) presented comparable scores at all three-thirds of the canal (p > 0.05). However, Group 1 (2.5% NaOCl + EDTA) (Cervical: 2.11 ± 0.12, middle: 2.17 ± 0.04, apical: 2.84 ± 0.44) and Group 2 treated teeth (Cervical: 1.54 ± 0.06, middle: 1.58 ± 0.07, and apical: 2.56 ± 0.81) presented significant difference from each other and other tested groups (p < 0.05) (Figure 1).
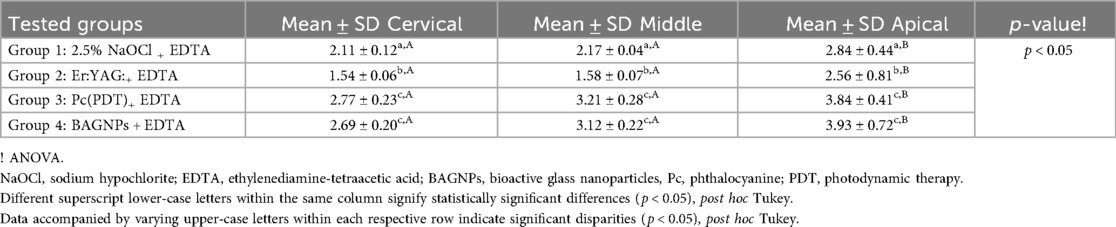
Table 1. SL removal efficacy from post space after the application of various disinfection protocols.
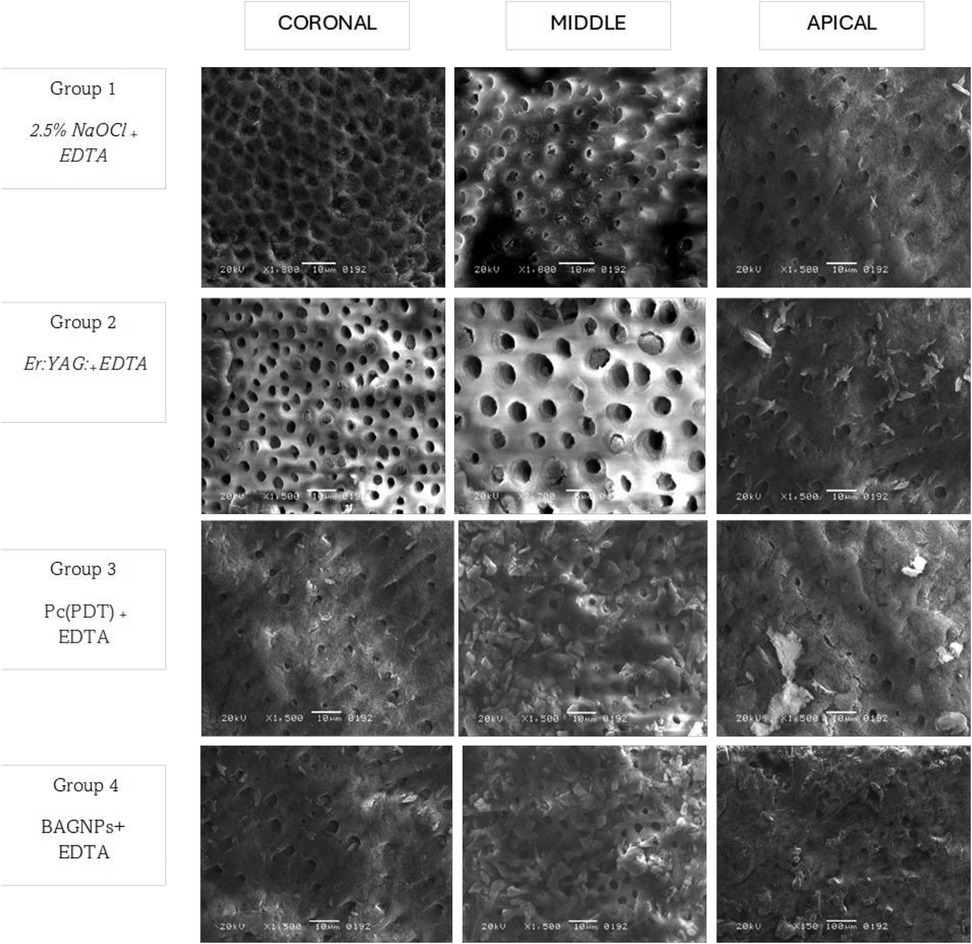
Figure 1. SEM micrograph of canal wall irrigated with 2.5% NaOCl + EDTA. The SEM image shows that dentinal tubules are open, and the SL has been partially removed in the coronal, middle, and apical thirds of the dentin. Areas of debris and smear layer are observable. Canal dentin irrigated with Er: YAG: +EDTA pronounced open dentinal tubules. Radicular canal irrigated with PC + EDTA and BAGNP + EDTA SEM images captured indicate the presence of SL and debris that covered the walls, revealing only a few dentinal tubules observed open in all three thirds (coronal, middle, and Apical).
Intra-group comparison reveals that smear removal efficacy was found to be comparable between the coronal and middle third in all groups (p > 0.05). However, the efficacy of SL was found to be significantly higher in the apical third in all groups (p < 0.05).
3.2 PBS testing
The PBS of QFP after using different post-space disinfection protocols is displayed in Table 2. The cervical third of Group 2 (Er: YAG + EDTA) treated teeth presented the highest PBS (10.41 ± 0.21 MPa). Whereas, the apical third of Group 4 (BAGNPs + EDTA) samples revealed the lowest PBS (7.21 ± 0.52 MPa). Comparison among different tested groups exhibited that Group 3 [Pc (PDT) + EDTA] (Cervical: 8.47 ± 0.09 MPa, Middle: 8.54 ± 0.32 MPa, and Apical: 7.43 ± 0.47 MPa) and Group 4 (Cervical: 8.39 ± 0.08 MPa, Middle: 8.48 ± 0.41 MPa, and Apical: 7.21 ± 0.52 MPa) disinfected canals presented comparable PBS at all three thirds (p > 0.05). However, Group 1 (2.5% NaOCl + EDTA) (Cervical: 9.16 ± 0.17 MPa, middle: 9.07 ± 0.12 MPa, apical: 8.54 ± 0.24 MPa) and Group 2 treated teeth (Cervical: 10.41 ± 0.21 MPa, middle: 10.39 ± 0. MPa, and apical: 9.89 ± 0.32 MPa) presented significant differences from each other and other tested groups (p < 0.05).
An intra-group comparison reveals that PBS was found to be comparable between the coronal and middle third in all groups (p > 0.05). However, the PBS was found to be significantly lower in the apical third in all groups (p < 0.05).
3.3 Failure mode assessment
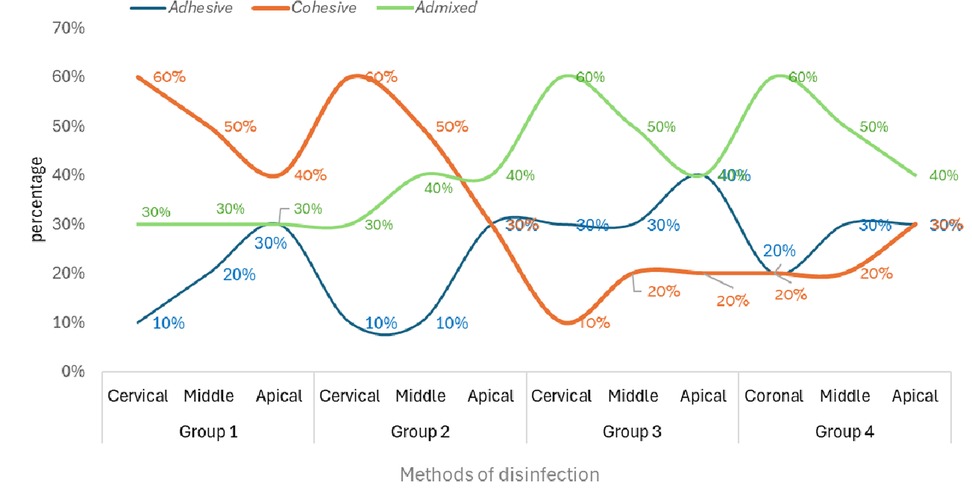
Figure 2 displays the percentage of failure modes for each investigated group. Groups 1 and 2 presented cohesive failures the most. Whereas Groups 3 and 4 exhibited admixed failures predominantly.
4 Discussion
The present investigation was predicated on the assertion that there would be no statistically significant difference in the SL removal efficacy when utilizing contemporary post-space disinfectants, Er: YAG laser, PC (PDT), BAGNP, in combination with EDTA as a final disinfectant, as opposed to the conventional method (2.5% NaOCl + EDTA). Furthermore, it was also hypothesized that the bond strength of QFP, when affixed to post-space following contemporary post-space disinfection, would be comparable to that obtained with NaOCl and EDTA. Considering the results of the present investigation, it was observed that the proposed hypothesis was entirely rejected, as the experimental group Er: YAG + EDTA showed significantly higher bond integrity and SL removal efficacy, while Pc(PDT) and BAGNP exhibited significantly lower and comparable bond strength values and SL removal efficacy compared to the control.
Based on the existing indexed academic literature, it can be asserted that the SL, which constitutes a delicate film of debris adhering to the walls of the root canal post-instrumentation, typically diminishes the bond strength of both posts and sealing materials (28, 29). Er: YAG laser pretreated root canal presented the highest scores of SL removal and PBS of QFP with the root canal dentin. Er: YAG laser is particularly effective on hard tissues because its 2,940 nm wavelength is readily absorbed by both hydroxyapatite and water (30). This enables it to effectively cut and remove dental hard tissues like dentin and enamel, often eliminating the need for local anesthetics (31). Additionally, combining the Er: YAG laser with EDTA has been shown to enhance the removal of the SL, creating better conditions for bonding restorative materials (32). The Er: YAG laser operates on the principle that its energy is absorbed by water molecules in the SL, leading to rapid vaporization and tissue removal (33). This process effectively removes the SL from the canal wall. Lab-based analysis by Celiksoz and coworkers has provided evidence that Er: YAG laser treatment, particularly in conjunction with EDTA, is effective in the removal of SL and significantly boosts the adhesion properties of dental materials, which justifies the outcomes of the existing study (34). However, some researchers have reported contradictory outcomes and revealed that the Er: YAG laser produces melted and sealed dentinal tubules, which eventually decreases the bond strength. This is following the findings of ex vivo analysis conducted by Alsahhaf and coworkers (11). Different laser parameters (frequency, power, power density), structure (human/bovine dentin and enamel), and material type may have contributed to differences in outcomes.
The satisfactory performance of NaOCL and EDTA is in agreement with the outcomes of lab-based analysis conducted by Aljamhan and colleagues (35) as well as Alkhudhairy et al (36). The oxidative characteristics of NaOCl solution lead to the degradation of dentinal collagen and interfere with the polymerization process of bonding cement (37). Conversely, EDTA eliminates the oxidative layer, enhancing the surface's capacity to adhere to resin cement, which in turn boosts bond strength (38). In this study, a combination of 2.5% NaOCl and EDTA was employed to achieve a synergistic effect. This effect is further corroborated by SEM images, which reveal that the majority of dentinal tubules are open and SL has been eliminated in the coronal and middle sections of the dentin.
BAGNPs and PC-PDT-treated canals exhibited the least and comparable results in terms of SL removal and bond integrity compared to other comparative groups. This marks the first study to acknowledge the role of BAGNPs as a root canal irrigant in the removal of SL and PBS of QFP. In an earlier study, conducted by Sadooq and colleagues, it was found that BAGNPs remarkably display antibacterial properties (39). Nonetheless, in this study, BAGNPs demonstrate limited effectiveness in eliminating SL. According to the author, the absence of proteolytic activity, lack of chelating abilities, and issues related to particle size might impede its effectiveness in SL removal. In the present study, PC activated by PDT was utilized as a photosensitizer because of its excellent absorption in the phototherapeutic range, remarkable light stability, significant phototoxicity, and minimal dark toxicity, along with its efficiency in generating singlet oxygen (22). However, PC-PDT in the present study demonstrated low SL removal efficacy, and PBS. This outcome can be attributed to the lower hydrophilicity of PC-PDT, which negatively hindered PBS and poor SL removal efficacy (22). Both types of post-space disinfection (PC-PDT, BAGNPs), the outcome can be confirmed by the SEM image showing the presence of SL and debris that covered the walls and orifice opening of DT, revealing only a few dentinal tubules observed to be open.
The intragroup comparison revealed that across all the examined groups, the apical segment exhibited markedly lower SL removal and PBS of QFP. This phenomenon can be attributed to the fact that the apical area of the canal contains complexities such as variations in curvature, canal dimensions, taper, diameter, ramifications, deltas, isthmuses, and the permeability of dentin (40). Concerning the failure mode, it was noted that samples in groups 1 and 2 demonstrated cohesive failure type in abundance. Cohesive failures are attributed to adhesive system issues, C-factor stresses, sclerotic dentin, and air bubble incorporation (41). Whereas, an adhesive failure pattern is observed due to incomplete smear layer removal, poor adhesive penetration, and incomplete polymerization (42).
The current investigation reveals several inherent constraints. This study is defined by an ex vivo methodology; therefore, it is crucial to conduct extensive longitudinal clinical research to accurately ascertain the desirable characteristics of the newest post-space irrigants that are being utilized. In addition, the use of laser and photodynamic therapy (PDT) was performed with singular parameters; hence, alternative parameters must be assessed too. The absence of advanced surface analysis techniques like Raman spectroscopy and x-ray photoelectron spectroscopy (XPS) could restrict a more detailed understanding of the groups that were tested. It is essential to evaluate the antibacterial effectiveness of these modern canal disinfectants against E. faecalis, as this bacterium is the leading cause of root canal infections and treatment failures.
5 Conclusion
In the study, the application of Er: YAG + EDTA laser for post-space disinfection achieved superior results in Smear layer removal and push-out bond strength compared to all other groups examined. These results indicate that Er: YAG + EDTA can be effectively used as an alternative to conventional canal irrigation techniques when bonding a quartz post to canal dentin.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Deanship of Graduate Studies and Research. Research Ethic Committee Ajman University Project Number: D-F-H-16-Jun. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
OM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Investigation, Writing – review & editing. EZ: Software, Visualization, Validation, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript. The authors declared that they did not use any of the artificial intelligence–assisted technologies [such as Large Language Models (LLMs), chatbots, or image creators] in the production of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saraiva L, Aguiar T, Costa L, Correr-Sobrinho L, Muniz L, Mathias P. Effect of different adhesion strategies on fiber post cementation: push-out test and scanning electron microscopy analysis. Contemp Clin Dent. (2013) 4(4):443–7. doi: 10.4103/0976-237X.123022
2. Parisi C, Valandro LF, Ciocca L, Gatto MRA, Baldissara P. Clinical outcomes and success rates of quartz fiber post restorations: a retrospective study. J Prosthet Dent. (2015) 114(3):367–72. doi: 10.1016/j.prosdent.2015.03.011
3. Asmussen E, Peutzfeldt A, Heitmann T. Stiffness, elastic limit, and strength of newer types of endodontic posts. J Dent. (1999) 27(4):275–8. doi: 10.1016/S0300-5712(98)00066-9
4. Mao H, Chen Y, Yip KHK, Smales RJ. Effect of three radicular dentine treatments and two luting cements on the regional bond strength of quartz fibre posts. Clin Oral Investig. (2011) 15(6):869–78. doi: 10.1007/s00784-010-0453-3
5. Alkahtany MF. Extrusion bond strength of glass fiber post to radicular dentin after final irrigation using MTAD, EDTA, pineapple peel extract, and riboflavin. Photodiagnosis Photodyn Ther. (2022) 39:102982. doi: 10.1016/j.pdpdt.2022.102982
6. Bhargava KY, Aggarwal S, Kumar T, Bhargava S. Comparative evaluation of the efficacy of three anti-oxidants vs NAOCL and EDTA: used for root canal irrigation in smear layer removal–Sem study. Int J Pharm Pharm Sci. (2015) 7(6):366–71.
7. Alici O, Hubbezoglu I. The efficacy of four cavity disinfectant solutions and two different types of laser on the micro-shear bond strength of dentin adhesives. Cumhur Dent J. (2018) 21(1):9–17. doi: 10.7126/cumudj.389990
8. Zain E, Chew HP. Update on clinical detection methods for noncavitated fissure caries. World J Dent. (2012) 11(1):81–8.
9. Al Ahdal K, Maawadh AM, Al Deeb L, Alshamrani AS, Almohareb T, Alrahlah A. Effect of malachite green, ocimum sanctum, and Er, Cr: YSGG laser on antimicrobial activity against S.mutans and CAD disinfection bonded to resin restoration. Photodiagnosis Photodyn Ther. (2023) 42:103571. doi: 10.1016/j.pdpdt.2023.103571
10. Alsahhaf A. Efficacy of canal disinfectants temoporfin, carbon nanoparticles, and Er: YAG laser on martens hardness, smear layer removal, and bond strength of glass fiber posts to canal dentin. Photodiagnosis Photodyn Ther. (2025) 52:104490–104490. doi: 10.1016/j.pdpdt.2025.104490
11. Banci HA, Strazzi-Sahyon HB, Duarte MAH, Cintra LTA, Gomes-Filho JE, Chalub LO, et al. Influence of photodynamic therapy on bond strength and adhesive interface morphology of MTA based root canal sealer to different thirds of intraradicular dentin. Photodiagnosis Photodyn Ther. (2020) 32:102031. doi: 10.1016/j.pdpdt.2020.102031
12. Şahin ÖH, Korucu H, Aydin ZU. Evaluation of the effects of different photosensitizers used in antimicrobial photodynamic therapy on tooth discoloration: spectrophotometric analysis. Lasers Med Sci. (2024) 39(1):1–9. doi: 10.1007/s10103-024-04085-0
13. Duvvuri SNR, Arafath MM, Alla RK, Bhaskar V, Kumaran K. Evaluation of the shear bond strength of the orthodontic composites modified with various concentrations of TiO2 nanoparticles: an in vitro study. Uttar Pradesh J Zool. (2023) 44(1):88–94. doi: 10.56557/upjoz/2023/v44i13395
14. Obeid MF, El-Batouty KM, Aslam M. The effect of using nanoparticles in bioactive glass on its antimicrobial properties. Restor Dent Endod. (2021) 46(4):e58. doi: 10.5395/rde.2021.46.e58
15. Bokov D, Turki Jalil A, Chupradit S, Suksatan W, Javed Ansari M, Shewael IH, et al. Nanomaterial by Sol-Gel method: synthesis and application. Adv Materi Sci Eng. (2021) 2021:5102014.
16. Goodyear MDE, Krleza-Jeric K, Lemmens T. The FDA and the declaration of Helsinki: a new rule seems to be more about imperialism than harmonisation. Br Med J. (2007) 338:624–5.
17. Alhamdan MM. Canal disinfectants: potassium titanyl phosphate laser, magnesium oxide nanoparticles, and aloe-emodin PDT on smear layer removal and bond strength of glass fiber post to root dentin. Photodiagnosis Photodyn Ther. (2025) 53:104615.40318758
18. Alkhudhairy F. Neodymium-doped yttrium vanadate laser and phthalocyanine photosensitizer doped chitosan nanoparticle activated via photodynamic therapy on smear layer and push-out bond strength of fiber post: an in vitro SEM, EDX assessment. J Photochem Photobiol B Biol. (2025) 272:113263.
19. Alturaiki SA, Bamanie AA, Albulowey MA, Al Daafas AA, Almalki A, Alqerban A. Disinfection of radicular dentin using riboflavin, rose Bengal, curcumin, and porfimer sodium on extrusion bond strength of fiber post to radicular dentin. Photodiagnosis Photodyn Ther. (2022) 37:102625. doi: 10.1016/j.pdpdt.2021.102625
20. Al Ahdal K, Al Deeb L, Al-Hamdan RS, Bin-Shuwaish MS, Al Deeb M, Maawadh AM, et al. Influence of different photosensitizers on push-out bond strength of fiber post to radicular dentin. Photodiagnosis Photodyn Ther. (2020) 31:101805. doi: 10.1016/j.pdpdt.2020.101805
21. DiVito E, Peters OA, Olivi G. Effectiveness of the erbium: YAG laser and new design radial and stripped tips in removing the smear layer after root canal instrumentation. Lasers Med Sci. (2012) 27(2):273–80. doi: 10.1007/s10103-010-0858-x
22. Gök T, Er Karaoglu G, Korucu H. Effect of phthalocyanine, methylene blue and toluidine blue photosensitizers on the adhesive interface of fiber posts: a confocal laser microscopy study. Lasers Med Sci. (2025) 40(1):1–9. doi: 10.1007/s10103-025-04369-z
23. Mierzejewska ŻA, Rusztyn B, Łukaszuk K, Borys J, Borowska M, Antonowicz B. The latest advances in the use of nanoparticles in endodontics. Appl Sci. (2024) 14(17):7912. doi: 10.3390/app14177912
24. Hülsmann M, Rümmelin C, Schäfers F. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endod. (1997) 23(5):301–6. doi: 10.1016/S0099-2399(97)80410-4
25. Vohra F, Bukhari IA, Sheikh SA, Naseem M, Hussain M. Photodynamic activation of irrigation (using different laser prototypes) on push out bond strength of fiber posts. Photodiagnosis Photodyn Ther. (2020) 30:101716. doi: 10.1016/j.pdpdt.2020.101716
26. Altaf A, Santhosh L, Srirekha A, Panchajanya S, Jaykumar T. Effect of riboflavin on push-out bond strength between fiber post and root dentin using adhesive cement—an in vitro study. J Clin Res Dent. (2019) 2(1):1–5. doi: 10.33309/2639-8281.020105
27. Strazzi-Sahyon HB, da Silva PP, Nakao JM, da Silva PZ, Nunes LP, Seron MA, et al. Influence of two photodynamic therapy sessions and different photosensitizers on the bond strength of glass-fiber posts in different regions of intraradicular dentin. Photodiagnosis Photodyn Ther. (2021) 33:102193. doi: 10.1016/j.pdpdt.2021.102193
28. Violich DR, Chandler NP. The smear layer in endodontics–a review. Int Endod J. (2010) 43:2–15. doi: 10.1111/j.1365-2591.2009.01627.x
29. Bolhari B, Ehsani S, Etemadi A, Shafaq M, Nosrat A. Efficacy of Er,Cr:YSGG laser in removing smear layer and debris with two different output powers. Photomed Laser Surg. (2014) 32(10):527–32. doi: 10.1089/pho.2014.3766
30. Yildirim AZ, Unver S, Mese A, Bayram C, Denkbas EB, Cevik P. Effect of argon plasma and Er:YAG laser on tensile bond strength between denture liner and acrylic resin. J Prosthet Dent. (2020) 124(6):799.e1–e5. doi: 10.1016/j.prosdent.2020.08.009
31. Uzun I, Keskin C, Özsu D, Güler B, Aydemir H. Push-out bond strength of oval versus circular fiber posts irradiated by erbium-doped yttrium aluminum garnet laser. J Prosthet Dent. (2016) 116(3):425–30. doi: 10.1016/j.prosdent.2016.01.023
32. Alonaizan FA, Alofi RS, Alfawaz YF, Alsahhaf A, Al-Aali KA, Vohra F, et al. Effect of photodynamic therapy, Er,Cr:YSGG, and Nd:YAG Laser on the push-out bond strength of fiber post to root dentin. Photobiomodulation, photomedicine. Laser Surg. (2020) 38(1):24–9. doi: 10.1089/photob.2019.4687
33. Razumova S, Brago A, Kryuchkova A, Troitskiy V, Bragunova R, Barakat H. Evaluation of the efficiency of smear layer removal during endodontic treatment using scanning electron microscopy: an in vitro study. BMC Oral Health. (2025) 25(1):151. doi: 10.1186/s12903-025-05510-8
34. Ozge C, Yilmaz NA, Balin E. Effect of er: yag laser on repair bond strength of a nano hybrid composite. J Stomatol. (2022) 75(2):122–9. doi: 10.5114/jos.2022.117408
35. Aljamhan AS, Alrefeai MH, Alhabdan A, Alzehiri MH, Naseem M, Vohra F, et al. Interaction of zirconium oxide nanoparticle infiltrated resin adhesive with dentin conditioned by phosphoric acid and Er, Cr: YSGG laser. J Appl Biomater Funct Mater. (2022) 20:22808000221087349. doi: 10.1177/22808000221087349
36. Almutairi B, Alkhudhairy F. Laser-activated irrigation via photon-induced photoacoustic streaming and shock wave enhanced emission on smear layer removal efficacy, pushout bond strength, and sealer adaptation: a SEM assessment. Microsc Res Tech. (2025) 88(6):1806–15. doi: 10.1002/JEMT.24821
37. Ari H, Yaşar E, Bellí S. Effects of NaOCl on bond strengths of resin cements to root canal dentin. J Endod. (2003) 29(4):248–51. doi: 10.1097/00004770-200304000-00004
38. Kuruvilla A, Jaganath BM, Krishnegowda SC, Ramachandra PKM, Johns DA, Abraham A. A comparative evaluation of smear layer removal by using edta, etidronic acid, and maleic acid as root canal irrigants: an in vitro scanning electron microscopic study. J Conserv Dent. (2015) 18(3):247–51. doi: 10.4103/0972-0707.157266
39. Sadoq BE, Britel MR, Bouajaj A, Maâlej R, Abid M, Douiri H, et al. A review on antibacterial activity of nanoparticles. Biointerface Res Appl Chem. (2023) 13:1–19. doi: 10.33263/BRIAC135.405
40. da Silva PNF, Martinelli-Lobo CM, Bottino MA, de Melo RM, Valandr LF. Bond strength between a polymer-infiltrated ceramic network and a composite for repair: effect of several ceramic surface treatments. Braz Oral Res. (2018) 32:1–9. doi: 10.1590/1807-3107bor-2018.vol32.0028
41. Aljamhan AS, Alrefeai MH, Alhabdan A, Alkhudhairy F, Abrar E, Alhusseini SA. Push out bond strength of glass fiber post to radicular dentin irrigated with Nisin and MTAD compared to methylene blue photodynamic therapy. Photodiagnosis Photodyn Ther. (2021) 34:102304. doi: 10.1016/j.pdpdt.2021.102304
Keywords: phthalocyanine, bioactive glass nanoparticles, smear layer, quartz fiber post, scanning electron microscope, sustainable development goals (SDG) 4 & 9
Citation: Mahmoud O and Zain E (2025) Effect of Er: YAG laser, phthalocyanine activated photodynamic therapy, and bioactive glass nanoparticles on smear layer removal and push out bond strength of quartz fiber posts to canal dentin: a SEM assessment. Front. Dent. Med. 6:1665937. doi: 10.3389/fdmed.2025.1665937
Received: 21 July 2025; Accepted: 29 September 2025;
Published: 21 October 2025.
Edited by:
Vasudev Ballal, Manipal Academy of Higher Education, IndiaReviewed by:
Vini Mehta, D.Y. Patil Vidyapeeth, IndiaPaul Sharpe, University of Exeter, United Kingdom
Copyright: © 2025 Mahmoud and Zain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Okba Mahmoud, by5tYWhtb3VkQGFqbWFuLmFjLmFl
†ORCID:
Okba Mahmoud
orcid.org/0000-0002-3355-1429
Erum Zain
orcid.org/0000-0002-2301-630X
 Okba Mahmoud
Okba Mahmoud Erum Zain3,†
Erum Zain3,†