- Department of Restorative and Aesthetic Dentistry, College of Dentistry, University of Baghdad, Baghdad, Iraq
Neurogenic inflammation is pivotal in dental pulp repair, involving complex interactions between sensory nerves, immune cells, and dental pulp stem cells (DPSCs). This review aimed to identify the favorable pathways of neurogenic inflammation and neurogenic differentiation of DPSCs in the pulpal healing process. Also, to identify the techniques used to evaluate these inflammatory and differentiation processes. Both PubMed and Google Scholar databases were employed in the search strategy using keyword combinations based on MeSH terms. The search was performed for published articles in English from January 2014 to November 2024, including studies with histological and molecular findings. 29 articles only met the inclusion criteria. Neurogenic inflammation encompasses three main stages: initial (minutes to 24 h), intermediate (24 h to 3 days), and long-term response (3 days to several weeks). The immediate phase includes neuropeptide release, resulting in inflammation and recruitment of immune cells. The intermediate phase features the persistence of neuropeptides, nerve sprouting, and the initiation of repair processes. The long response phase involves resolving inflammation, angiogenesis, decreased neuropeptide levels, and neurogenesis mediated by DPSCs. Advanced methods such as IHC, RNA sequencing, electrophysiological studies, and micro-CT imaging have been employed to evaluate these mechanisms. However, limitations in real-time dynamic assessment highlight the necessity for more advanced and noninvasive procedures for direct evaluation of this complex process.
1 Introduction
The innervation of the dental pulp is exceedingly dense not only for perception but also for the maintenance of tooth vitality. The neurological components are mostly sensory trigeminal afferent axons with a few sympathetic efferent nerves. These sensory neurons have been shown to perform a wide range of actions in addition to their presumed sensory function, which is the reaction to the noxious stimuli affecting the tooth (1). Dental pulp reacts to injury or harmful stimuli through a complex interaction of neurogenic and immune responses, which occur over distinct temporal phases. In the immediate phase (minutes to 24 h), sensory nerves, especially C-fibers and A-δ fibers, release neuropeptides like substance P (SP) and calcitonin gene-related peptide (CGRP). This initiates vasodilation, attracts immune cells, and leads to neurogenic inflammation (2, 3). These actions are intensified by the activation of odontoblasts and the migration of dental pulp stem cells (DPSCs), which aid in early repair process (4). In the intermediate phase (24–72 h), the ongoing release of neuropeptides and nerve sprouting influence pain and tissue healing, while DPSCs further promote neurogenic differentiation (the process by which stem cells mature into neural lineages) and immune modulation. After injury, DPSCs can undergo neurogenic differentiation. This is critical for regenerating the damaged nerve fibers within the pulp, restoring sensory function (nociception), and contributing to the overall tissue repair response through the release of neurotrophic factors (5, 6). During the long-term phase (days to weeks), inflammation subsides, angiogenesis occurs, and tertiary dentin forms, all driven by neuropeptide signaling and DPSC-mediated regeneration (7, 8). To better understand the role of neurogenic inflammation and neurogenic differentiation in pulp repair, a range of advanced techniques have been employed over the last decades for evaluating both the neural and immune components of these processes (9). These methodologies enable precise detection of neuropeptides, cytokines, and immune cell markers involved in inflammation and the techniques used for stimulation and detection of neurogenic differentiation of DPSCs. Researchers have also been using a variety of lab models, from cell cultures to animal studies (10, 11), to see how neurons or their neuropeptides can modulate the pulp's inflammatory response. Animal models remain the gold standard model used by dental researchers for the majority of experimental dental studies in vivo (12–14). By carefully examining these techniques and models and acknowledging their limitations, proper guidance for future research can be obtained to improve our understanding of how the pulp heals and regenerates. Therefore, this review aimed to investigate the role of neurogenic inflammation and the mechanism of neurogenic differentiation of DPSCs in favorable pulp response. Also, to assess the methodology used to evaluate the neurogenic inflammatory process. This can assist in using this part of the overall pulp inflammatory process to obtain significant clinical outcomes.
2 Search strategy
PubMed and Google Scholar databases were used; the search strategy was based on MeSH terms in the following combinations: (“neurogenic” OR “CGRP” OR “substance P”) AND (“pulp” OR “dental” OR “dentistry”) AND (“healing” OR “repair” OR “regeneration” OR “regenerative”). This search was performed for articles published in English during January 2014 and November 2024. The studies included histological and molecular findings only. Case reports, narrative reviews, case series, clinical trials, social media sources, and studies with clinical and radiographic findings only were excluded. After initial research, 168 articles were identified, 52 were selected for title and abstract details, and only 29 met the inclusion criteria after full-text reading. This search strategy is well-suited for lab-based dental research that ensures systematic and efficient selection of relevant studies while promoting reproducibility and transparency of the research process. However, the reliance on MeSH terms and narrow keyword combinations may overlook studies that use different terminology.
3 Extracted data
The extracted data included studies, study sample, stimulative factors, testing procedure, and findings, listed in Table 1.
3.1 Temporal phases of neurogenic inflammation
3.1.1 Immediate response (minutes to 24 h)
The immediate phase begins minutes to hours after injury or exposure to harmful stimuli, such as bacterial invasion, mechanical trauma, orthodontic forces, or chemical irritants (such as LPS or hydrogen peroxide). Neuronal interaction has been observed early in the pulp reaction to stimuli. As shown in (Figure 1), it comprises the increased release of neuropeptides, including substance P (SP) and calcitonin gene-related peptide (CGRP), from their terminals (2, 15, 16). These neuropeptides are held in large, dense-core vesicles, mostly in C-fibers, and their release is induced by membrane depolarization and increases in intracellular calcium levels (3, 17, 18).
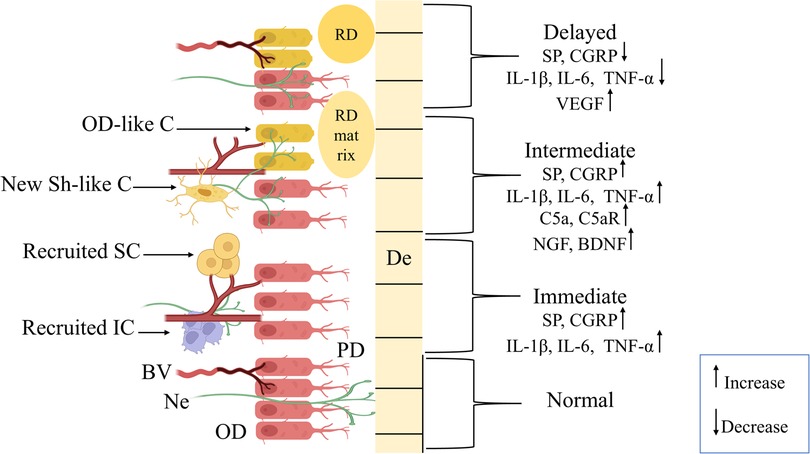
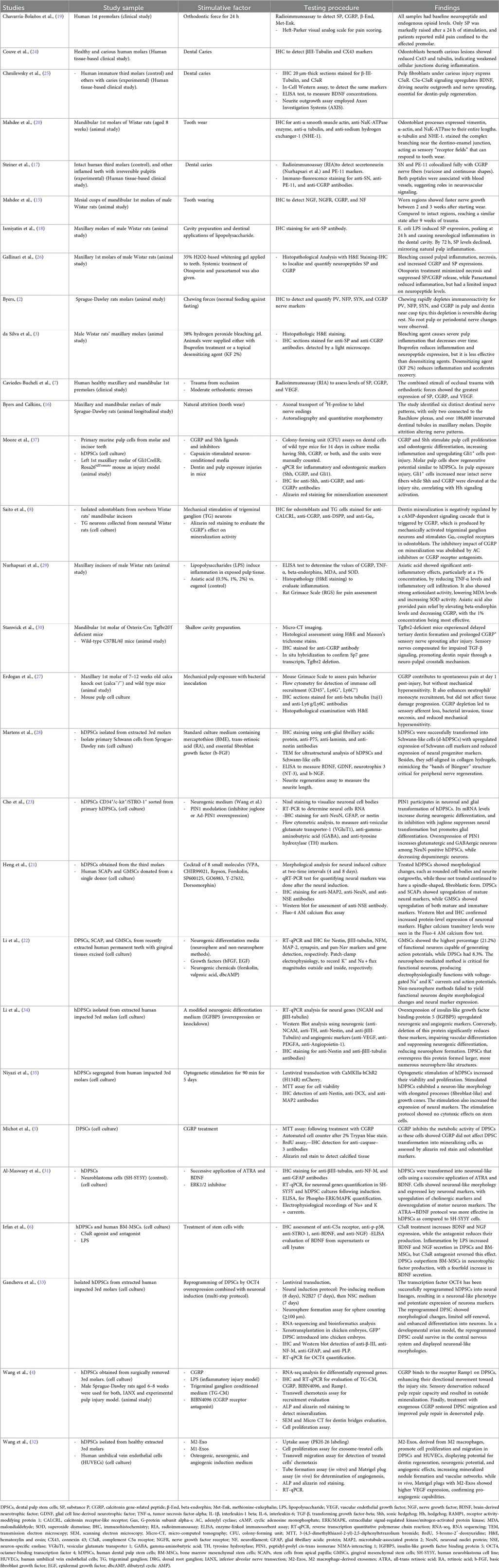
Figure 1. Graphic presentation showing the phases of the neurogenic inflammation process in dental pulp. Normal pulpal tissue exhibits odontoblasts (OD) aligned with intact cellular junctions supplied by normal blood vessels (BV), nerve fibers (Ne) extending between the OD layer through predentine (PD) up to dentin (De). The immediate phase (minutes to 24 h) represents instant pulpal tissue response to noxious stimuli through increased expression of calcitonin gene-related peptide (CGRP) and substance P (SP) neuropeptides, interleukins (IL)-1β, IL-6, and tumor-necrosis factor alpha (TNF-α). And, recruitment of immune cells (IC) and stem cells (SC) with dilation of BV and Ne retraction. In the intermediate phase (24 h to 3 days), a sustained increase in the levels of neuropeptides (CGRP, SP), IL-1β, IL-6, and TNF-α, with elevation in complement 5a and its receptor (C5a, C5aR), nerve growth factors, and brain-derived neurotrophic factor (BDNF). Also, this phase included neural sprouting with differentiation of new Schwann-like cells (New Sh-like C) and odontoblast-like cells (OD-like C), which started to produce tertiary dentin (RD) matrix to initiate the repair process. Finally, the long-term response phase (3 days to several weeks) is characterized by a marked decrease in CGRP, SP, IL-1β, IL-6, and TNF-α levels, an indication of inflammation resolution and neural sprout reduction. Besides, increased expression of vascular endothelial growth factor (VEGF) is associated with angiogenesis, with BV returning to a normal state, and RD formation.
Peptidergic sensory afferents, which account for approximately one-third of all dental pulp neurons, secrete calcitonin gene-related peptide upon stimulation. This neuropeptide binds to receptors on odontoblasts and other pulp cells containing receptor activity-modifying protein 1 (RAMP1) and calcitonin receptor-like receptor (CALCRL). In this way, inflammatory responses can be modulated through the stimulation of G-protein-coupled receptors in these cells. This results in an increase in the intracellular cyclic adenosine monophosphate and activation of adenylyl cyclase. Simultaneously, SP binds to the neurokinin-1 receptors that are found on the different cell types comprising the pulp, fibroblasts, odontoblasts, and endothelial cells. Furthermore, within 24 h of orthodontic force application, there was an obvious rise in SP concentration in the dental pulp. This illustrates the role of this neuropeptide in the initial inflammatory responses due to the application of mechanical orthodontic loading of the teeth (19). These neuropeptides, interacting with their respective receptors, can initiate an inflammatory response cascade such as vasodilation and increased vascular permeability, which result in plasma extravasation and subsequent edema. In conjunction with the mediators that immune cells, for instance, macrophages, neutrophils, and mast cells, are already present, will be recruited. These immune cells amplify the inflammatory processes with cytokines, especially the pro-inflammatory IL-1β, IL-6, and TNF-α. Additionally, SP promotes the degranulation of mast cells, which leads to a release of histamine and other inflammatory components, intensifying the inflammatory response (3). This reflexive relationship between sensory nerves and immune cells perpetuates inflammation of the pulp (2).
Furthermore, odontoblasts are also triggered by neurogenic inflammation. These cells evidenced receptors for neuropeptides, which can stimulate them to release inflammatory mediators and increase their intracellular cAMP levels to modulate cellular function. Although activation of CGRP receptors (CALCRL and RAMP) on odontoblasts by CGRP was reported to inhibit the mineralization process, potentially serving as a protective mechanism to prevent excessive dentin formation as a subsequent increase in pulpal pressure during inflammation (8). This stage also showed the retraction of CGRP fibers from the inflammatory region as indicated in (Figure 1) (15). Also, the odontoblastic processes, along with the sensory nerve fibrils, are evidenced to deeply extend into the dentin tubules. This can affect the pulpal warning system. A histo-immunological study indicated that under typical physiological circumstances, the odontoblast processes are terminated in a form of complex branching network at the dentin-enamel junction (20). This can serve as a “receptor field” to assess the integrity within this region. Therefore, when dentinal tubules were exposed by physiological wear, this can activate a series of actions, including odontoblast processes retraction, possible signaling to the afferent nerve fibers and the sub-odontoblast layer, through odontoblast cell bodies, initiating an inflammatory process (20).
Another role of the neurogenic mediators detected in the early stages of injury or inflammation is the stimulation of DPSCs recruitment and differentiation. It has been documented that CGRP is critical in stimulating DPSCs to polarize and move toward the injury site as a fundamental step in activating the repair process. Autonomous cell movement and collective interaction, termed leader-follower behavior, are crucial for the orderly healing of injured tissues (4, 5). Since DPSCs are of neural crest origin, they possess an intrinsic capacity for neurogenesis and can differentiate into neuron-like cells that harbor neuronal genes (21, 22). Such versatility is important for reconstituting certain parts of neural circuits located in the pulp, such as those involved in nociception and autonomic control, which enable the detection of damaging stimuli and blood flow control (22, 23).
3.1.2 Intermediate phase (24 h–72 h)
The inflammatory process continues during this stage through sustained neuropeptide release and initiation of the tissue repair mechanisms, including new odontoblast-like cells differentiation and production of hard tissue matrix (8). As illustrated in (Figure 1), the levels of SP and CGRP have been reported to be maintained during this stage, as SP expression may peak at 24 h before gradually declining at 72 h after stimulation (18). There can be two major functions of the elevated neuropeptide levels during this stage: First, it is composed of immune cell infiltration and nerve sprouting. Immune cells, such as the dendritic cells, the major antigen-presenting cells, are further evidenced during this stage in the reaction to caries as well as other injuries. They infiltrated the odontoblast layer and extended their processes into the reactionary dentin. Studies have shown that recruitment of dendritic cells occurs parallel to the sprouting of nerve fibers, and this can represent an organized neuroimmune response (24). The complement system, as part of innate immunity, is also involved in neurogenic inflammation. This activation results in the formation of the anaphylatoxins, including C5a, which affect inflammation and the regeneration of nerves. Besides, the expression of C5a receptor (C5aR) has been demonstrated on pulpal fibroblast cells that, upon interaction with C5a, promote the local production of brain-derived neurotrophic factor (BDNF). This may direct axon growth and sprouting toward the injury site (25).
The second role is the transmission of an exaggerated pain sensation as a result of neural sprouting. Nerve fibers are observed to sprout in reactionary dentin against which GAP-43, a marker for neuronal plasticity, is upregulated. This enhancement might be the reason for pain occurring during pulpitis upon caries, as a result of increased nerve fiber density in the affected areas (24). Subsequently, the administration of anti-inflammatory drugs, e.g., hydrocortisone and acetaminophen, by a material (e.g., gel or drug dispersion) was found to influence neuropeptides expression and reduce inflammation in dental pulp. For example, when hydrocortisone (Otosporin) was applied topically after bleaching, inflammation was attenuated. It suppressed the production of SP, CGRP, and facilitated tissue repair (26). Nerve sprouting also occurs as a result of orthodontic forces and is considered to be responsible for the perception of pain after mechanical force is applied (19). Furthermore, CGRP and SP can sensitize nociceptors, which results in mechanical hypersensitivity and spontaneous pain-like behaviors. As in the Calca knockout mice (where the CGRP gene is deleted), there is a significant decrease in spontaneous pain-like behavior but no significant change in mechanical hypersensitivity, which indicates that spontaneous pain sensation is essentially mediated by CGRP (27).
At the intermediate stage, DPSCs recruitment was also demonstrated after CGRP stimulation, and it was reported that DPSCs have a unique receptor named activity-modifying protein 1 (RAMP1) which directly interacts with CGRP, promoting collective attraction of DPSCs to the inflamed area, thus facilitating better repair of the pulp. Additionally, exogenous treatment of CGRP enhanced DPSC mobilization and led to favorable pulp repair quality. Thus, the sensory nerve could be a new target for stem cell-based pulp therapies (4). Besides, a previous study showed that DPSCs can be differentiated into Schwann-like cells using trans-retinoic acid (RA), and essential fibroblast growth factor (b-FGF) (28). This was in-vitro study, so it is unknown whether these cells could participate in the formation of new nerve fibers or act as sensory cells by themselves, which is not explained yet by recent studies, and can be a good source for new research. Moreover, DPSCs also exert their immunomodulatory function through the complement system, which was evident when C5aR blocking resulted in a reduction of BDNF levels in DPSCs. This anti-inflammatory activity serves to counterbalance excessive neuroinflammation and to influence neurotrophic secretion. As such, it reinforces the regenerative microenvironment and promotes axonal growth and synaptic connectivity (6).
By the end of this phase, a regeneration process is launched via upregulated NGF expression. These mediators act through p75 and TrkA receptors that odontoblasts and subodontoblasts express, regulating nerve growth, survival, and sensitivity in the response of the host tissues to damage. NGF can also mediate the expression of SP and CGRP in sensory nerves, and so it can affect the inflammatory reaction. In the inflamed pulp, the levels of NGF are upregulated, as well as the ability of DPSC to release BDNF, which might lead to higher susceptibility and more perceived pain during this phase (6, 15).
3.1.3 Long-term response (3 days to weeks or 56 days)
This phase includes events that lead to tissue healing under favorable conditions, primarily influenced by the severity of the injury and the intensity of the immune response. When the outcome of this process is beneficial and the scale tips toward tissue healing, numerous mechanisms work together to achieve this goal, marked by the resolution of inflammation and the initiation of tissue remodeling (16). One of these mechanisms is the upregulation of the vascular endothelial growth factor (VEGF) through the continuous release of CGRP and SP, as demonstrated in (Figure 1). VEGF is essential for the formation of new blood vessels, which secures oxygen supply and nutrients to the pulpal tissue, thereby promoting angiogenesis. This is considered to be a protective mechanism against hypoxia induced by marked inflammation or trauma, preventing tissue necrosis (7).
Yet another mechanism is provided by the reduction in neuropeptides as the SP and CGRP release diminishes in time with abating inflammation. The reduced number and density of nerve sprouts might result in decreasing chronic symptoms over time and help to understand the gradual relief of symptoms (15). Moreover, in response to pain, the body releases beta-endorphins, natural analgesic substances that suppress CGRP release and diminish pain (29). However, in the case of chronic inflammation or repetitive injury, neuropeptide levels can remain elevated, and chronic pain, hypersensitivity, poor wound healing, and possibly even tissue necrosis can result (3, 30). Furthermore, the signaling of CGRP and SP may influence odontoblast activity, eliciting the production of tertiary dentin following injury (7, 8). This role of CGRP in tertiary dentinogenesis was evident in a previous study, which found that the absence of transforming growth factor beta receptor 2 (Tgfbr2) in deficient mice can disrupt TGF-β signaling and impair tertiary dentin formation. at the same time, CGRP immunopositive (CGRP+) sensory afferents could compensate for this deficiency by increasing the duration of their axonal sprouting, favoring delayed formation of tertiary dentin, therefore highlighting the protective nature of CGRP during the repair process (30). In addition, sonic hedgehog (Shh) in combination with CGRP signaling, when promoting inflammation after injury, led to proliferation and transformation of DPSCs to odontoblast-like cells for dentin regeneration.
DPSCs have been demonstrated to express multiple neural markers, including glial markers (GFAP, p75, and laminin), and secrete many neurotrophic factors, including BDNF, GDNF, and NGF, through the action of M2 macrophage-derived exosomes (M2-Exos) (31, 32). These exosomes could promote neurogenic differentiation and angiogenesis, which suggests a sophisticated crosstalk between neural and vascular regeneration in the process of pulp repair (32). It has been reported that DPSCs can acquire neurogenic features such as Na+ and K+ currents and action potentials after neurogenic differentiation. Even though these cells do not entirely recapitulate the function of terminally differentiated neurons, they may help to partly compensate for neuronal signaling in the pulpal healing process (22). In addition, DPSCs might contribute to reducing inflammation and rebuilding the neural and vascular network at the end of this stage by releasing anti-inflammatory cytokines, ensuring the long-lasting function and vitality of the pulp (7).
In the context of the pulpal repair process, many experimental models have been designed to induce DPSCs' neurogenic differentiation. For instance, in an animal model, it has been shown that transplanted DPSCs can engraft to damaged pulp tissue and support the regeneration of neural and vascular structures (33). Accordingly, many in vitro techniques are applied to induce neurogenic differentiation in DPSCs that could be used for in-vivo in an attempt to produce neurological pulp tissue repair. For example, overexpressing octamer-binding transcription factor 4 (OCT4) could result in the reprogramming of DPSCs into neural lineages implanted in the chicken embryo model. This can induce cellular differentiation to neuronal-like morphology with elevation in the levels of neural markers justifying their role in neural repair (33). Pre-treatment of DPSCs with special molecules (VPA, CHIR99021, Repsox, Forskolin, SP600125, GO6983, Y-27632, Dorsomorphin) also produces transformations of DPSCs into more rounded cell bodies with neurite outgrowth (neural cell morphology). Along with increased expression of neural markers (MAP2, NeuN, and NSE) (21). Furthermore, human DPSCs were differentiated into neuron-like cells after 12 days in all-trans retinoic acid (ATRA) and BDNF treatment, and these cells acquired functional electrophysiological features and neuronal morphology that favor their incorporation into neural networks (31).
Proteins such as peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1); insulin-like growth factor binding protein 5 (IGFBP5) increase the neurogenic potential of DPSCs. For example, upregulated PIN1 expression promotes glutamatergic and GABAergic differentiation but suppresses glial differentiation, which may contribute to the recovery of neurons (23). Additionally, IGFBP5 elevates neurogenic-related markers, such as NCAM and nestin, and induces differentiation into neuron-like cells that are beneficial to pulp re-innervation (34). Furthermore, 470 nm, 15 Hz optical stimulation of DPSCs (optogenetic activation) for 5 days enhanced the expression of neural markers and induced neuron-like morphology of the cells. This indicates that optogenetic depolarization drives neurogenic differentiation without notable cytotoxic effects (35). Finally, the ERK/MAPK pathway has been found to be crucial for neurogenic differentiation of DPSCs. Knockdown of ERK1/2 completely inhibited the differentiation of neurons, which was supported by a decrease in the expression of mature neuronal markers (βIII-tubulin, NF-M, GFAP). This suggests that the ERK/MAPK pathway was involved in the sensory cholinergic neuronal transformation of DPSCs (31).
Approaches in the future could enhance the ability of DPSCs to aid in nerve-healing and function-restoration, providing opportunities for treating dental injuries and diseases. This may have implications for new forms of therapy that aid in recovery and stimulate regeneration in injured tissues as well. The application of DPSCs to custom treatments could advance dentistry and the overall well-being of patients. Having outlined the temporal phases, we now discuss the tools to evaluate these processes.
3.2 Techniques for assessment of neurogenic inflammation and neurogenic differentiation of dental pulp stem cells
Assessing neurogenic inflammation and differentiation of dental pulp stem cells employs various methodologies ranging from conventional to more sophisticated technologies. Beginning with histological methods, by using non-specific staining, such as hematoxylin and eosin, to assess general histology, inflammation, and necrosis (26). Another more specific staining, including Masson's trichrome staining for collagen and connective tissue analysis (30) and alizarin red staining for calcium deposits and mineralization detection (5). Also, Nissl staining to visualize neuronal cell bodies (23) and trypan blue staining to discriminate between live and dead cells in cell culture, dead cells take the blue stain due to a compromised cell membrane, while live cells repel the dye because of having an intact membrane (5). These stains are assessed mainly under a light microscope. However, subjectivity in scoring means that inflammatory grading (e.g., mild/moderate/severe) or target tissue visualization relies on the examiner's judgment (3). Furthermore, static photos cannot mimic the dynamic processes and real-time progression of inflammation (20).
Immunohistochemistry (IHC), on the other hand, is an antigen-antibody-based reaction in which the primary antibody binds to a special tissue antigen (coupled to an enzyme or fluorophore) to be detected by chromogenic or fluorescent labeling (26). This technique has been extensively applied in dental pulp research for staining tissue sections or isolated culture cells due to its reliability and reproducibility in investigating neurogenic inflammation across various study models (3, 24, 26). It can be utilized to detect cellular receptors (C5aR and NGFR) (25), cytoskeletal proteins (vimentin, α-tubulin, α-actin, and nestin), homeostatic ion transmitters (NaK-ATPase and NHE-1), growth factors (Heng et al.), nerves (CGRP and NF, and glial markers (NaK-ATPase and NHE-1) (20). Advanced live-cell imaging techniques using molecules loaded with calcium and cAMP-sensitive fluorescent dyes. This technique allows simultaneous monitoring of intracellular calcium levels (Ca²+ flux) in neurons and cAMP levels in odontoblasts, in real-time signaling fashion. This approach demonstrated that CGRP released from neurons acts as a key mediator in the axon reflex, a neural mechanism that amplifies inflammatory responses in the pulp (8). Nonetheless, IHC is semi-quantitative, lacking precise measurement, which can render comparisons between samples subjective (3, 24). Issues with antibody specificity can result in false positives or negatives due to cross-reactivity or inadequate antibody performance (18). Also, autofluorescence can pose a challenge, as dental pulp tissue may naturally fluoresce, obscuring accurate signals (2). Moreover, photobleaching occurs when fluorophores degrade with prolonged light exposure, diminishing signal intensity along with limited examination time (25).
Another class of immunostaining and molecular detection techniques includes radioimmunoassay (RIA), which is a highly sensitive technique used to quantify neuropeptides such as SP, CGRP, β-Endorphin (β-End), and Methionine-Enkephalin (Met-Enk) in pulp tissue cellular cultures (7, 17). Byers et al. (16) used axonal transport labeling with 3H-proline to trace sensory nerve endings in rat molar dentine. In which autoradiography was employed to detect radio-labeled dentinal tubules, providing insights into the distribution and response of trigeminal nerve endings to tooth wear (attrition). This technique allowed for the quantification of innervated dentinal tubules and the assessment of changes in nerve patterns due to attrition (16). However, radioactive materials necessitate careful handling, disposal, and adherence to safety protocols (17). Despite the high costs and technical demands, its multiplexing capability is limited, typically measuring one analyte at a single time (7). Other types of immunological and molecular techniques, such as in-cell western assays and enzyme-linked immunosorbent essay (ELISA), provide quantitative data on protein secretion, such as BDNF levels, confirming the protein expression of neural markers like nestin and βIII-tubulin (29). However, cross-reactivity of antibodies may result in binding to structurally similar proteins, skewing results (29). In addition to dynamic range, constraints indicate that very high or low analyte concentrations may fall outside the assay's detection limits (28). Another potent laser-based immunological technique for examining physical and chemical characteristics of cells or particles in a fluid solution is flow cytometry. It can be used in the profiling of immune cells during pulp inflammation, such as detection of CD45+ (a pan-leukocyte marker), Ly6G+ (neutrophils), and Ly6C+ (monocytes) (27). Besides, it can be employed in the characterization of stem cells' neural differentiation, e.g., measuring of NeuN, GFAP, Nestin, neural markers of differentiated DPSCs (23).
Other techniques used gene expression to indicate neurogenic differentiation. This can be done by spatial localization of gene expression (in situ hybridization), quantifying specific genes [quantitative real-time polymerase chain reaction (RT-qPCR)], or by analyzing the entire transcriptomes (RNA-sequencing) under neural induction conditions. in situ hybridization, the labeled probe is applied to tissue sections and allowed to bind (hybridize) to its target sequence to visualize gene activity directly in tissues. Making it invaluable for studies linking genetic changes (e.g., Tgfbr2 deletion) to cellular phenotypes (e.g., defective odontoblasts) (30). In comparison, RNA sequencing is used to uncover how the transcription factor OCT4 reprograms DPSCs into neural lineage cells. By comparing gene expression profiles between OCT4-overexpressing DPSC and controls (33). In addition to gene expression analysis via reverse transcription quantitative polymerase chain reaction (RT- qPCR) to quantify mRNA levels of neurogenic markers such as nestin, GDNF, and SOX 1 (22, 31). However, post-transcriptional discrepancies are present when mRNA levels do not always correspond to protein expression (34) and the fluctuation of reference genes under experimental conditions may result in housekeeping gene variability (4). Another type of gene-based technique is the use of knockout or transgenic methods, which based on the inactivation of a specific gene to identify the missed process within the genetically modified animal. In a study used a knockout mouse model (specifically, Calca−/− mice) to assess the role of CGRP in neurogenic inflammation during dental pulp injury. Interestingly, CGRP knockout did not affect mechanical hypersensitivity, suggesting that CGRP is more involved in spontaneous pain rather than evoked mechanically induced pain (27). Although the knockout methodology can specify the exact role of the inactivated gene, it may also affect the overall life of the genetically modified animal, resulting in a series of unexpected consequences (36). Therefore, careful evaluation of the role of these genes should be performed to prevent unsuccessful procedures.
Another class of assessment techniques is cell culture and functional assays, including electrophysiological recordings (patch-clamp), calcium flux assays, and neurite outgrowth in stem cell cultures. These procedures were performed to assess voltage-gated ion channel activity, action potential capacity of differentiated neurons, and length of neurites extended by dorsal root ganglion (DRG) neurons co-cultured with DPSCs, respectively (21, 28). The colony-forming unit (CFU) assay is used to measure the number of colonies produced from single cells under given culture conditions to evaluate the clonogenicity and proliferative capacity of cells, especially stem cells. This was employed in evaluating the effects of CGRP and Shh on DPSCs' proliferation and differentiation (37). While, transwell migration assay is used to assess DPSCs' capacity for migration in response to various chemotactic circumstances. It was used to evaluate how CGRP and M2-Exos affect DPSC migration toward damage sites (4, 32). However, these functional tests have technical difficulties that require skilled operators and stable cell preparations, considered time-consuming and cost-effective for large-scale studies (31). On the other hand, neurosphere formation assays to assess DPSCs neurogenic differentiation through evaluating functional properties (self-renewal) of neural progenitor-like cells by measuring sphere formation in suspension culture, and the expression of neural stem cell markers (e.g., Nestin, SOX1) (23). Since neurospheres are 3D clusters of cells grown in suspension, they are not pure neural stem cells and contain mixed cell populations. This cellular heterogeneity may complicate data interpretation (33). Another functional assay is through the evaluation of mitochondrial activity in live cells by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric test. Metabolically active cells decrease the yellow MTT tetrazolium salt to purple formazan crystals, which are then dissolved, and the absorbance is measured. Such a test is used to quantify metabolically active DPSCs after CGRP treatment (5) and to assess the viability of optogenetically stimulated hDPSCs when no cytotoxicity is observed (35).
For microscopy and imaging techniques, several instruments are used to serve this purpose. Confocal microscopy, which provides high-resolution images, is mainly used for visualizing fluorescently labeled specimens. Transmission electron microscopy (TEM) and scanning electron microscopy (Niyazi et al.) both provide ultra-high-resolution imaging that goes beyond what is possible with light microscopy. TEM is mainly used for ultrastructural analysis of the interior aspect of specimens, as in the observation of myelin-like structures and neurofilaments, and to validate the Schwann cell-like development of DPSCs (28). While SEM is primarily applied to study the surface topography of samples. For instance, SEM was utilized to examine the dentin pulp interface and the deposition of new mineralized dentin matrix following nerve injury (4). Finally, Micro-CT Imaging is a high-resolution, non-destructive 3D imaging approach that allows for the visualization and micrometer-scale analysis of the interior microstructure of mineralized dental tissues. Such as to determine and compare tertiary dentin volume and density after dentinal injury in wild-type mice against Tgfbr2-deficient mice (30).
The limitation in available knowledge is partly due to flaws in the assessment procedure. As noted in the included studies, the most common assessment technique is IHC. This technique is considered invasive and provides only static images of dynamic tissue changes. To date, no technique offers direct visualization of real-time, dynamic living tissue reactions. For this reason, more advanced and noninvasive procedures are needed for direct evaluation of the living sample to gain a better understanding of the complexity of this process. On the other hand, animal models and in vitro studies have significantly advanced our understanding of neurogenic inflammation in dental pulp repair, they present critical limitations that necessitate validation through human in vivo studies. Among these limitations regarding animal models are the physiological differences (rodent dental pulp anatomy, immune responses, and healing mechanisms differ from humans) (16). And pain assessment challenges [rodent pain behaviors (e.g., grimace scales) are indirect substitutions for human subjective pain experiences, limiting translational relevance] (29). While in vitro studies' main limitation is the lack of microenvironment complexity (22).
There are another cutting-edge technique that represent the future of research in this field which have not been shown among the current review electronic search. One of these techniques is spatial transcriptomics, enables researchers to quantify gene expression and identify the specific locations of that expression within a tissue sample (38). Another methodology is the in vivo imaging that can provide real-time viewing of the biological processes within a living organism, encompassing a range of technologies such as functional neural imaging for calcium detection to assess neuronal activity using advanced microscopy techniques (39). Organoid models are also an advanced methodology creating tiny, simplified, three-dimensional replicas of organs cultivated from stem cells, replicating essential characteristics of the actual organ's design, cellular variety, and functionality. Among these the neural organoids which have effectively supplemented or replaced animal models to address the distinctive characteristics of human nervous system development (40). Finally, CRISPR screening is a powerful functional genomics tool that uses the CRISPR-Cas9 “gene-editing scissors” to systematically deactivate (or modify) any gene in the genome to underpin its impact on a specific biological process. These advanced technologies offer substantial promise for pinpointing the etiologies and therapeutic targets of neurological disorders (41).
4 Conclusions
This narrative review classified the role of neurogenic inflammation within the stage of inflammation into initial, intermediate, and delayed, and each phase is characterized by specific cellular and molecular events. During the immediate phase (minutes to 24 h), neuropeptides are released from sensory nerves, which directly lead to vasodilatation, local inflammation, immune cell recruitment, and early inflammatory signals. The subsequent intermediate phase (24–72 h) is characterized by the persistence of the neuropeptides, nerve sprouting, and the onset of repair processes. Lastly, the long response phase (3 days to weeks) is linked to inflammatory resolution, marked by angiogenesis, reduced neuropeptide levels, and neurogenesis mediated by DPSCs differentiation, and the formation of tertiary dentin. Advanced methods, including IHC, RNA sequencing, electrophysiological studies, and micro-CT imaging, have played a crucial role in the evaluation of these mechanisms. However, limitations in real-time dynamic assessment require the need for non-invasive high-resolution technology to explore the process of pulpal healing. Future studies should focus on the modulation of neurogenic pathways and DPSC-based therapies for enhancing pulp regeneration.
Author contributions
MA: Writing – original draft, Writing – review & editing. AM: Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to express their gratitude to the scientific committee of the Department of Esthetic and Restorative Dentistry at the University of Baghdad College of Dentistry, and Dr. Mohammad H. Nekoofar for his review and valuable comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Byers M, Henry M, Närhi M. Dental innervations and its responses to tooth injury. In: Hargreaves KM, Goodis HE, Tay FR, editors. Seltzer and Bender’s Dental Pulp. 2nd ed. Chicago, IL: Quintessence Publishing Co (2012). p. 133–58.
2. Byers MR. Chewing causes rapid changes in immunoreactive nerve patterns in rat molar teeth: implications for dental proprioception and pain. Arch Oral Biol. (2019) 107:104511. doi: 10.1016/j.archoralbio.2019.104511
3. Da Silva LMAV, Cintra LTA, Gallinari MDO, Benetti F, Rahal V, Ervolino E, et al. Influence of pain-relieving therapies on inflammation and the expression of proinflammatory neuropeptides after dental bleaching treatment. Restor Dent Endod. (2020) 45:e20–0. doi: 10.5395/rde.2020.45.e20
4. Wang C, Liu X, Zhou J, Zhang X, Zhou Z, Zhang Q. Sensory nerves drive migration of dental pulp stem cells via the CGRP-Ramp1 axis in pulp repair. Cell Mol Life Sci. (2024a) 81:1–20. doi: 10.1007/s00018-024-05400-2
5. Michot B, Casey SM, Gibbs JL. Effects of calcitonin gene-related peptide on dental pulp stem cell viability, proliferation, and differentiation. J Endod. (2020) 46:950–6. doi: 10.1016/j.joen.2020.03.010
6. Irfan M, Kim JH, Druzinsky RE, Ravindran S, Chung S. Complement C5aR/LPS-induced BDNF and NGF modulation in human dental pulp stem cells. Sci Rep. (2022) 12:2042. doi: 10.1038/s41598-022-06110-0
7. Caviedes-Bucheli J, Lopez-Moncayo LF, Muñoz-Alvear HD, Gomez-Sosa JF, Diaz-Barrera LE, Curtidor H, et al. Expression of substance P, calcitonin gene-related peptide and vascular endothelial growth factor in human dental pulp under different clinical stimuli. BMC Oral Health. (2021) 21:1–8. doi: 10.1186/s12903-021-01519-x
8. Saito N, Kimura M, Ouchi T, Ichinohe T, Shibukawa Y. Gαs-coupled CGRP receptor signaling axis from the trigeminal ganglion neuron to odontoblast negatively regulates dentin mineralization. Biomolecules. (2022) 12:1747. doi: 10.3390/biom12121747
9. Bhat R, Shetty S, Rai P, Kumar BK, Shetty P. Revolutionizing the diagnosis of irreversible pulpitis–current strategies and future directions. J Oral Biosci. (2024) 66:272–80. doi: 10.1016/j.job.2024.03.006
10. Baban DA. Effect of sulcular green tea extract irrigation on experimental rabbit’ s gingivitis (A histopathological study). J Baghdad Coll Dent. (2020) 32:19–27. doi: 10.26477/jbcd.v32i1.2754
11. Ahmed MS, Mahdee AF, Mohammed S. Cavity preparation model in rat maxillary first molars: a pilot study. J Baghdad Coll Dent. (2023) 35:1–9. doi: 10.26477/jbcd.v35i4.3504
12. Abed SS, Athra'a Y. Expression of syndecan 1 on periodontium treated with topical application of aloe-vera. J Baghdad Coll Dent. (2016) 28:82–6. doi: 10.12816/0031129
13. Natiq N, Athra'a Y. The effect of thymosin beta 4 on developing dental tissue (experimental study on rats). J Baghdad Coll Dent. (2016) 28:69–74. doi: 10.12816/0031111
14. Mahdee AF, Ali AH, Gillespie JI. Structural and functional relations between the connective tissue and epithelium of enamel organ and their role during enamel maturation. J Mol Histol. (2021) 52:975–89. doi: 10.1007/s10735-021-09992-y
15. Mahdee A, Eastham J, Whitworth J, Gillespie J. Evidence for changing nerve growth factor signalling mechanisms during development, maturation and ageing in the rat molar pulp. Int Endod J. (2019) 52:211–22. doi: 10.1111/iej.12997
16. Byers MR, Calkins DF. Trigeminal sensory nerve patterns in dentine and their responses to attrition in rat molars. Arch Oral Biol. (2021) 129:105197. doi: 10.1016/j.archoralbio.2021.105197
17. Steiner R, Fischer-Colbrie R, Bletsa A, Laimer J, Troger J. Secretoneurin and PE-11 immunoreactivity in the human dental pulp. Arch Oral Biol. (2018) 86:13–7. doi: 10.1016/j.archoralbio.2017.11.005
18. Ismiyatin K, Wahluyo S, Soetojo A, Rahayu RP, Utomo H, Anindya C. The expression of pulpal substance P after dentinal application of escherichia coli lipopolysaccharide. Saudi Endod J. (2019) 9:169–73. doi: 10.4103/sej.sej_92_18
19. Chavarría-Bolaños D, Martinez-Zumaran A, Lombana N, Flores-Reyes H, Pozos-Guillen A. Expression of substance P, calcitonin gene-related peptide, β-endorphin and methionine-enkephalin in human dental pulp tissue after orthodontic intrusion: a pilot study. Angle Orthod. (2014) 84:521–6. doi: 10.2319/060313-423.1
20. Mahdee A, Alhelal A, Eastham J, Whitworth J, Gillespie J. Complex cellular responses to tooth wear in rodent molar. Arch Oral Biol. (2016) 61:106–14. doi: 10.1016/j.archoralbio.2015.10.004
21. Heng BC, Jiang S, Yi B, Gong T, Lim LW, Zhang C. Small molecules enhance neurogenic differentiation of dental-derived adult stem cells. Arch Oral Biol. (2019) 102:26–38. doi: 10.1016/j.archoralbio.2019.03.024
22. Li D, Zou X-Y, El-Ayachi I, Romero LO, Yu Z, Iglesias-Linares A, et al. Human dental pulp stem cells and gingival mesenchymal stem cells display action potential capacity in vitro after neuronogenic differentiation. Stem Cell Rev. (2019) 15:67–81. doi: 10.1007/s12015-018-9854-5
23. Cho Y-A, Kim D-S, Song M, Bae W-J, Lee S, Kim E-C. Protein interacting with never in mitosis A-1 induces glutamatergic and GABAergic neuronal differentiation in human dental pulp stem cells. J Endod. (2016) 42:1055–61. doi: 10.1016/j.joen.2016.04.004
24. Couve E, Osorio R, Schmachtenberg O. Reactionary dentinogenesis and neuroimmune response in dental caries. J Dent Res. (2014) 93:788–93. doi: 10.1177/0022034514539507
25. Chmilewsky F, About I, Chung SH. Pulp fibroblasts control nerve regeneration through complement activation. J Dent Res. (2016) 95:913–22. doi: 10.1177/0022034516643065
26. Gallinari MDO, Cintra LTÂ, Benetti F, Rahal V, Ervolino E, Briso ALF. Pulp response of rats submitted to bleaching and the use of different anti-inflammatory drugs. PLoS One. (2019) 14:e0210338. doi: 10.1371/journal.pone.0210338
27. Erdogan O, Michot B, Xia J, Alabdulaaly L, Yesares Rubi P, Ha V, et al. Neuronal–immune axis alters pain and sensory afferent damage during dental pulp injury. Pain. (2024) 165:392–403. doi: 10.1097/j.pain.0000000000003029
28. Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, et al. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. (2014) 28:1634. doi: 10.1096/fj.13-243980
29. Nurhapsari A, Cilmiaty R, Prayitno A, Purwanto B, Soetrisno S. The role of asiatic acid in preventing dental pulp inflammation: an in vivo study. Clin Cosmet Investig Dent. (2023) 15:109–19. doi: 10.2147/CCIDE.S408158
30. Stanwick M, Fenesha F, Hamid A, Kang K, Kanniard D, Kim I, et al. Impaired tertiary dentin secretion after shallow injury in Tgfbr2-deficient dental pulp cells is rescued by extended CGRP signaling. Int J Mol Sci. (2024) 25:6847. doi: 10.3390/ijms25136847
31. Al-maswary AA, O’Reilly M, Holmes AP, Walmsley AD, Cooper PR, Scheven BA. Exploring the neurogenic differentiation of human dental pulp stem cells. PLoS One. (2022) 17:e0277134. doi: 10.1371/journal.pone.0277134
32. Wang Y, Mao J, Wang Y, Jiang N, Shi X. Multifunctional exosomes derived from M2 macrophages with enhanced odontogenesis, neurogenesis and angiogenesis for regenerative endodontic therapy: an in vitro and in vivo investigation. Biomedicines. (2024b) 12:441. doi: 10.3390/biomedicines12020441
33. Gancheva MR, Kremer K, Breen J, Arthur A, Hamilton-Bruce A, Thomas P, et al. Effect of octamer-binding transcription factor 4 overexpression on the neural induction of human dental pulp stem cells. Stem Cell Rev. (2024) 20:797–815. doi: 10.1007/s12015-024-10678-7
34. Li J, Diao S, Yang H, Cao Y, Du J, Yang D. IGFBP5 promotes angiogenic and neurogenic differentiation potential of dental pulp stem cells. Dev Growth Differ. (2019) 61:457–65. doi: 10.1111/dgd.12632
35. Niyazi M, Zibaii MI, Chavoshinezhad S, Hamidabadi HG, Dargahi L, Bojnordi MN, et al. Neurogenic differentiation of human dental pulp stem cells by optogenetics stimulation. J Chem Neuroanat. (2020) 109:101821. doi: 10.1016/j.jchemneu.2020.101821
36. Bailey J. Genetic modification of animals: scientific and ethical issues. In: Kenneth Shapiro Animals & Society Institute, USA. Animal Experimentation: Working Towards a Paradigm Change. Boston: Brill (2019). p. 443–79.
37. Moore E, Michot B, Erdogan O, Ba A, Gibbs J, Yang Y. CGRP and shh mediate the dental pulp cell response to neuron stimulation. J Dent Res. (2022) 101:1119–26. doi: 10.1177/00220345221086858
38. Williams CG, Lee HJ, Asatsuma T, Vento-Tormo R, Haque A. An introduction to spatial transcriptomics for biomedical research. Genome Med. (2022) 14:68. doi: 10.1186/s13073-022-01075-1
39. Yang W, Yuste R. In vivo imaging of neural activity. Nat Methods. (2017) 14:349–59. doi: 10.1038/nmeth.4230
40. Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. (2020) 21:571–84. doi: 10.1038/s41580-020-0259-3
Keywords: neuropeptides, pulp innervation, regeneration, pulp response, CGRP
Citation: Ahmed MS and Mahdee AF (2025) The role of neurogenic inflammation in pulp repair and the techniques used for its assessment (narrative review). Front. Dent. Med. 6:1686734. doi: 10.3389/fdmed.2025.1686734
Received: 15 August 2025; Accepted: 25 September 2025;
Published: 9 October 2025.
Edited by:
Wenpeng Song, Peking University Hospital of Stomatology, ChinaReviewed by:
Gestter Willian Lattari Tessarin, University Center in the North of São Paulo (UNORTE), BrazilKeGui Hou, The Hospital of Shunyi District, China
Copyright: © 2025 Ahmed and Mahdee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anas F. Mahdee, YS5mLm1haGRlQGNvZGVudGFsLnVvYmFnaGRhZC5lZHUuaXE=
 Muna Sh. Ahmed
Muna Sh. Ahmed Anas F. Mahdee
Anas F. Mahdee