- 1Department of Intensive Care Unit, Madda Walabu University Goba Referral Hospital, Goba, Ethiopia
- 2Department of Emergency and Critical Care Nursing, School of Nursing, Faculty of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Medical Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4Department of Nursing, College of Health Sciences, Mattu University, Mattu, Ethiopia
- 5Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Sciences, Haramaya University, Harar, Ethiopia
Introduction: Septic shock is a global health issue causing high mortality rates in intensive care units, with limited evidence in Africa, including Ethiopia, regarding its incidence and predictors. The aim of this study was assess the incidence and predictors of mortality among patients with septic shock admitted to the ICU of Comprehensive Specialized Hospitals of the Northwest Amhara region.
Methods: A study involving 386 ICU patients with septic shock from 2019 to 2023 was conducted using a random sampling method and structured data extraction tool. Data was analyzed using EpiData and STATA, with variables selected for multivariate analysis.
Result: The overall incidence rate of septic shock was 10.4 per 100-person day of observation with a median survival time of 7, days and the proportion of deaths during the study period was 58.29%. In multivariate Cox proportional regression analysis, age 40–59 years (HR: 1.77, p = 0.005), age > 60 years (HR: 3.52, p < 0.001), delay ICU admission (HR: 1.93, p = 0.001), low MAP (HR: 2.56, p < 0.001), comorbidity (HR: 2.74, p < 0.001), complication (HR: 1.87, p = 0.012), ALF (HR: 1.84, p = 0.037), no pathogen identification (HR: 1.69, p = 0.035) were found significant predictors of mortality for patients with septic shock in the ICU.
Conclusion: The incidence of mortality in patients with septic shock admitted to the ICU was high and the main predictors were age> 60 years, low MAP, comorbidity, and delay ICU admission >6 h, Hence, Early recognition and appropriate treatment recommended by the International Sepsis Survival Campaign guideline should be implemented.
Background
Septic shock is defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality. It is characterized by hypotension, poor perfusion, lack of responsiveness to fluid resuscitation, and the requirement for vasopressors to maintain a normal range of blood pressure (BP) to keep mean arterial pressure (MAP) above 65 mmHg (1). On a continuum of severity from bad to worse, sepsis can range from the early phase that begins with infection and bacteremia to sepsis and septic shock, leading to multiple organ dysfunction syndrome and death (2).
Worldwide epidemiological data from systematic reviews showed a varying incidence of 13 to 300/100,000 people and 11/100,000 people annually for severe sepsis and septic shock, respectively (3). Every year, sepsis affects 31 million people worldwide (4), and some studies showed that sepsis has an incidence rate of 285 cases per 100,000 hospital admissions in Taiwan (5).
Different studies carried out in the USA, Germany and The Netherlands showed that the prevalence rates of sepsis among adult patients in the ICU ranged between 6.3 and 53% (6–8). According to a study, between 2010 and 2015, sepsis admissions increased by two and a half times, from 3.9% to 9.4%, in three acute care hospitals in the USA (9) and the study conducted in Brazil shows that the incidences of severe sepsis and septic shock in ICU were 5 and 11.5%, respectively (10).
World Health Organization reports showed that ~24 million new cases of septic shock occur each year, and the burden of septic shock is significantly greater in low- and middle-income nations (11). The rate of septic shock-related deaths is still high and appears to be increasing despite several therapeutic advances. Septic shock was the most common reason for mortality for ICU patients in ICU in the United States (9). One of the studies conducted in the United Kingdom revealed that septic shock, which has a death rate of 56%, affected 19.9% of patients referred to intensive care units (12).
Septic shock is highly aggressive and can cause progressive loss of function of several organs, but should be considered reversible, especially if identified and treated early in its course (13). In recent decades, the incidence and mortality of severe sepsis and septic shock have increased despite breakthroughs in our understanding of the pathophysiology, diagnosis, therapeutic therapy, and supportive care (14). Further consequences may result from septic shock. During their hospital stays, patients with sepsis were more likely to develop secondary illnesses such as pneumonia and bloodstream infections (15) and recurrent hospitalization (16).
The lengths of stay of patients in the ICU and in the hospital can both increase due to sepsis and septic shock. It was discovered that patients with sepsis had a hospital stay of 75% longer than patients without sepsis. Sepsis' potential to lead to organ failure was one potential contributing element to this problem (17). A German study showed high rates of acute and chronic death: 44.6% of septic shock patients passed away during their hospitalizations, and another 19.1% did so within the first year after diagnosis. The predictors of an increase in mortality were age, nosocomial origin of sepsis, diabetes, cerebrovascular disease, duration of stay in the intensive care unit, and renal replacement therapy (18).
Most of the studies on septic shock were conducted in developed countries. Sepsis, severe sepsis, and septic shock are more common in developing countries, yet there are few studies toward septic shock (19). A study conducted in two ICUs in Rwanda shows that the mortality rate was 82.1% for septic shock (20). According to a study carried out in Ethiopia, 26.5 out of 100 ICU admissions had sepsis or septic shock at the time of admission, which includes a 50.9% mortality rate (21).
There is limited information on the outcome of patients with septic shock admitted to the ICU in sub-Saharan African countries, according to the ability of the researchers to search for studies. Although septic shock has a high mortality rate in Africa, there is no survival study on the incidence and predictors of mortality among septic shock patients admitted to the ICU. Therefore, this study aims to evaluate the incidence and predictors of mortality among septic shock patients admitted to the ICU.
Methods
Study design and period
An institutional-based retrospective follow-up study was conducted among patients with septic shock admitted to the ICU from March 10, 2019, to March 9, 2023, and the data extraction period was from April 10, 2023, to May 25, 2023.
Study setting
The study was carried out in comprehensive specialized hospitals in northwest Amhara, Ethiopia. There are five comprehensive specialized hospitals in North Amhara, namely the University of Gondar, Debre Tabor, Tibebe Ghion, Felege Hiwot and Debre Markos comprehensive specialized hospitals. These hospitals are located at a distance of 727, 593, 490, 490, and 300 km, respectively, from Addis Ababa, the capital of Ethiopia. The first adult ICU started at Felege Hiwot Comprehensive Specialized Hospital in 2009 with three beds and two mechanical ventilators. Then the University of Gondar Comprehensive Specialized Teaching Hospital also started with four beds, two mechanical ventilators, one defibrillator, and four noninvasive monitors. Subsequently, it expanded into comprehensive specialized hospitals Tibebe Ghion, Debre Markos, and Debre Tabor in 2011, 2016, and 2020, respectively (22). Currently, a total of 51 functional adult ICU beds (22 in UoGCSH, 10 in FHCSH, nine in TGCSH, four in DMCSH and six in DTCSH) are available for critical patients. The ICUs offer a comparable standard of care and are equipped with mechanical ventilators, noninvasive patient monitoring machines, portable ultrasounds, electrocardiograms, defibrillators, and infusion pumps. The ICUs employ pulmonologists, anesthesiologists, neurologists, nurses, general practitioners, and residents.
Population
All septic shock patients aged over 18 years who were admitted to the ICU of comprehensive specialized hospitals in Northwest Amhara from March 10, 2019, to March 9, 2023, were included in the study. Those patients whose ICU follow-up time is < 24 h were excluded from the study due to incomplete records due to the infeasibility of time for the history of essential information, which is considered a variable of the study and another type of shock.
Sample size determination
The sample size for the primary objective was determined using simple population proportions with the following assumptions: 50.9% incidence of death among patients with septic shock patients in the ICU (21) with a 95% confidence level and a 5% error margin. Depending on this assumption, the sample size for the primary objective was calculated using the following formula:
By adding a non-response rate of 10%, the final sample size was 423.
Where n = sample size, Z = critical value of 95% CI = 1.96, p = proportion of septic shock death among ICU admitted patients (50.9%) = 0.509, and d = precision (marginal error) = 0.05.
The sample size for the secondary objective was calculated using the double population proportion formula using Epi-info version 7.2.2 taking into account the significant predictor variables of related studies based on the following assumptions: two-sided confidence level = 95%, power = 80% and the ratio of exposed to non-exposed = 1:1 from a study conducted in Addis Ababa (21). As a result, the proportion of mortality from septic shock among males, comorbidity of cancer, and HIV among those who were exposed and not exposed was used for each group, and a 10% non-response rate was considered and the maximum sample size was 209.
When comparing the sample sizes calculated for both objectives, it was found that the sample size obtained from the first objective (423) was the highest. Therefore, to get a maximum sample size, the sample size calculated for the first objective was used for the study.
Sampling technique and procedures
The study was carried out in all five comprehensive specialized hospitals found in the Northwest Amhara district. A proportional allocation of study participants was applied for each hospital. A sampling frame was prepared by extracting the numbers of the chart of patients with septic shock admitted to the ICU of each hospital from March 10, 2019, to March 9, 2023, from the ICU log book. A total of 423 patient charts were selected using random numbers generated from the sample frame using STATA software version 17 (Figure 1).
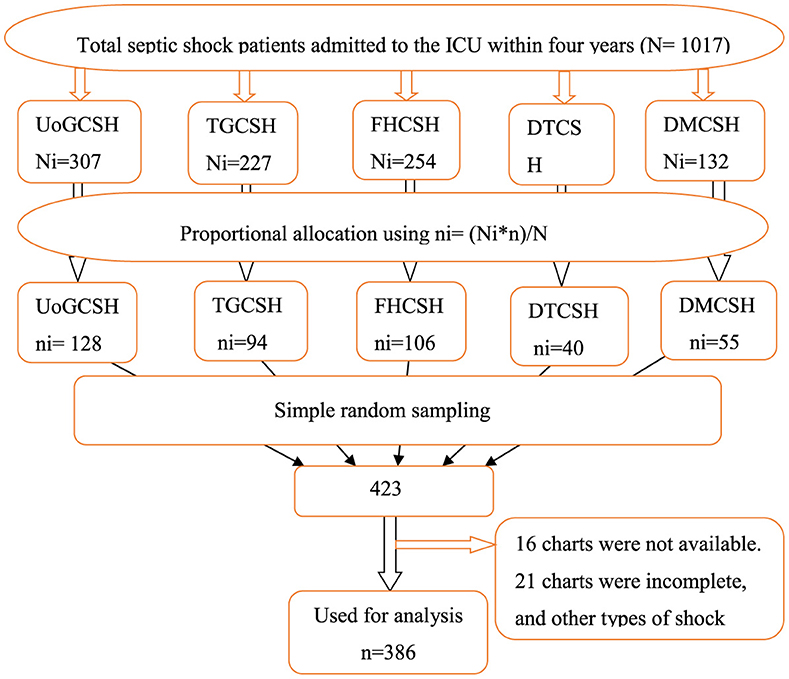
Figure 1. Schematic presentation of the sampling procedure used to select patients with septic shock admitted to the ICU of comprehensive specialized hospitals in the northwest of Amhara, Ethiopia, 2023.
Operational definitions
• Septic shock: those patients admitted to the ICU with the diagnosis of septic shock by physician decisions.
• Event: the occurrence of death during the follow-up period.
• Follow-up time: time from admission to the ICU to discharge outcome (censored or death).
• Censored: those ICU-admitted patients with septic shock, including survivors, who were discharged, transferred to wards, and those who left against medical advice, were censored.
• Comorbidity: the cooccurrence of one or more diseases or medical conditions in patients according to the Charlson Comorbidity Index (CCI) (23).
• Altered body temperature: temperature hyperthermia (>38°C) or hypothermia (< 36°C) (1).
• Altered white blood cell: white blood cell count >12,000/mm3 or < 4,000/mm3 (1).
• Low hemoglobin level: baseline hemoglobin < 9 g/dl.
• Hypoglycemia at admission: baseline blood sugar level < 70 md/dl.
• Organ dysfunction: organ failure related to septic shock documented/diagnosed by a physician during admission.
• Pathogen identification: if patients have evidence of microbiological culture tests by collecting samples from suspected parts of the body.
• Incomplete chart: is missing baseline records such as date of admission to the ICU and date of discharge for outcome variables (discharge condition).
• Duration of the illness: the time from the time the patient starts the illness to admission to the ICU.
• Delayed ICU admission: the time that elapsed or the waiting time of patients who required ICU admission after being diagnosed with septic shock until ICU admission in hours.
Data collection tools and procedures
A structured data abstraction tool was adapted from different studies (18, 21, 24–27) and the International Sepsis Survival Campaign guideline and used to obtain information from patient charts. The checklist contained sociodemographic factors, hospitalization-related factors, baseline clinical-related factors, and treatment-related factors. All relevant data were retrospectively collected from April 10, 2023, to May 25, 2023, from patient charts by trained nurses and supervised by a BSc nurse. The investigator was responsible for supervising the data collection and facilitating the process. The data collection process was examined every day.
Data quality control
A preliminary review of the chart was performed on 21 (5%) of the ICU patient charts at UoGCSH to ensure the availability of variables on the patient's chart. Based on the preliminary chart review findings, necessary corrections and modifications were made to the data extraction checklist prior to actual data collection. Before the data collection process started, a 1-day training was given to the data collectors and supervisor before the start of the data collection period on how to collect data, and the completeness and accuracy of the collected data was checked on the same day of collection. Before analyzing the data, any data errors were cleaned and the analysis of missing data was performed using STATA software to identify missing variables and determine the types of missing data.
Data processing and analysis
The data was coded and entered using EpiData version 4.6.6 statistical software, then exported to STATA version 17 for analysis. The descriptive statistics of the different variables were presented by frequency, cross-tabulation, pie chart, and bar chart. The outcome of each participant was dichotomized into censored and event. Incomplete data < 15% of the record were managed with the assumption of mean and mode imputation after determining that the missing data were completely random. During the entire follow-up period, the incidence rate of mortality was calculated. To describe the cumulative probability of death and the median survival time, the Kaplan–Meier curve (KM) was used. The Kaplan–Meier survival curve (KM) and the log-rank test method were implemented to test the occurrence of differences in the probability of death between groups. The proportional hazard assumption was checked by the Schoenfeld residual test and was satisfied (the p-value of each variable ranges from 0.1127 to 0.9736 and the global test result was 0.9702, which was insignificant). Multicollinearity was checked by variance inflation factors. Cox proportional-hazard regression was used to explore the association between each independent variable and the outcome variable. The fitness of the model was checked by the Cox-Snell residual test. Variables that had a p-value < 0.2 in the bivariate analysis were entered into the multivariate analysis and the adjusted hazard ratio (AHR) with a 95% confidence interval (CI) was calculated to assess the strength of the association. Variables with a p-value of 0.05 are considered statistically significant predictors of the incidence of septic shock.
Result
Sociodemographic characteristics of the study participants
A total of 423 charts were retrieved and 386 were found complete, giving a response rate of 91.3%. The mean age of the study participants during follow-up initiation was 45 ± 17 years. The most common age group affected by septic shock 159 (41.2%) were those aged 18 to 39 years. Approximately 199 (51.6%) were male and more than half of the 204 (53.1%) study participants were rural residence. Among the study participants, those patients over 60 years experienced more deaths 94 (89.5%) than other groups (Table 1).
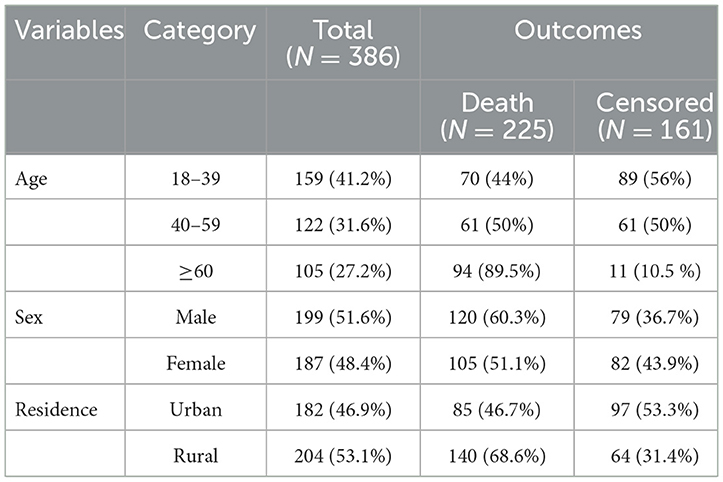
Table 1. Sociodemographic characteristics of patients with septic shock admitted to the ICU in Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Hospitalizations-related characteristics of the study participants
Among those patients with septic shock admitted to the ICU, 107 (27.2%) were admitted from a medical emergency and 70 (18.1%) from the medical ward. About 70 (18.7%) of the study participants had a history of more than seven days of illness before admission to the ICU. Regarding admission delay, more than half 204 (52.8%) of the study participants were delayed admission to the ICU for more than 6 h (Table 2).
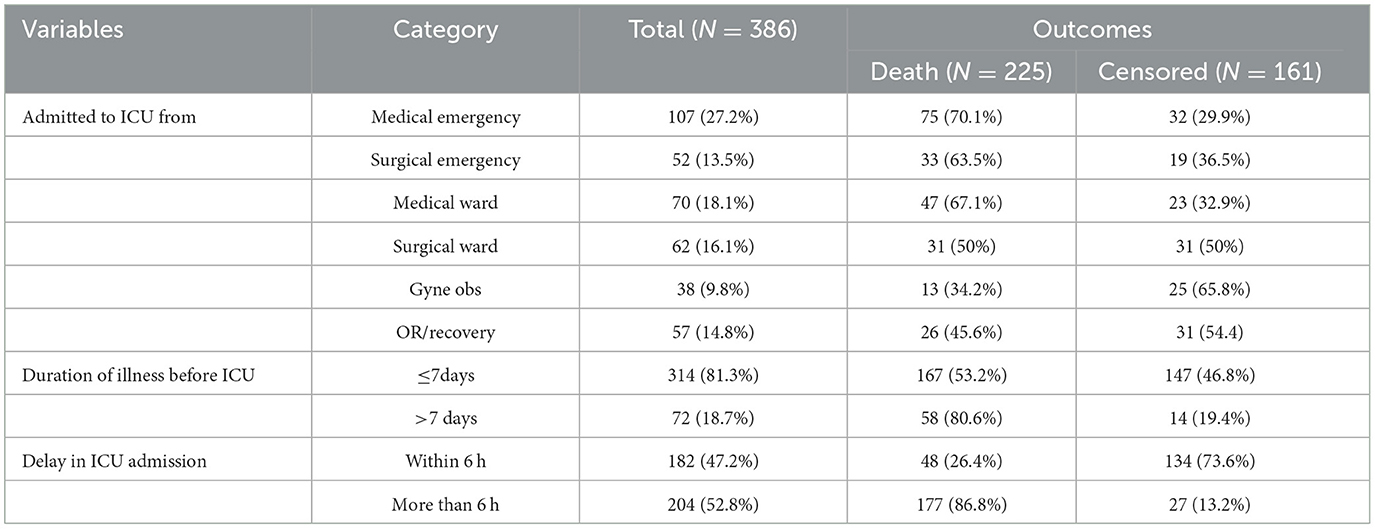
Table 2. Hospitalization-related factors of septic shock patients admitted to the ICU in Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Clinical-related characteristics of study participants
Baseline clinical characteristics of study participants
Approximately 228 (59.1%) patients with septic shock admitted to the ICU had low MAP (< 65 mmHg) at admission. One hundred seventy-nine (46.4%) of them had hypoxia, and 140 (36.3%) were admitted with a GCS level of < 9. More than half 217 (56.2%) of the study participants were tachycardic, and almost half of them had a low baseline hemoglobin level of 191 (49.5%) at admission (Table 3).

Table 3. Clinical characteristics related to septic shock patients admitted to the ICU of Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Baseline history and clinical characteristics of study participants
Approximately half 197 (51%) of the study participants had comorbidities, and 57 (28.9%) of them had multiple comorbidities. Hypertension was the largest of 58 (29.4%) comorbid diseases. During admission to the ICU, more than three-quarters 318 (82.4%) of the patients had acute organ failure related to septic shock, and respiratory and cardiovascular failure were the most frequent acute organ failures 142 (44.7%) and 131 (44.2%), respectively. During ICU follow-up, 252 (65.3%) of the study participants developed complications or new organ failure during their ICU stay, and 76 (30.2%) of them had multiple complications. ARDS 85 (33.7%) and AKI 82 (32.5%) were the most frequent types of complications, respectively, during stays in the ICU (Table 4).
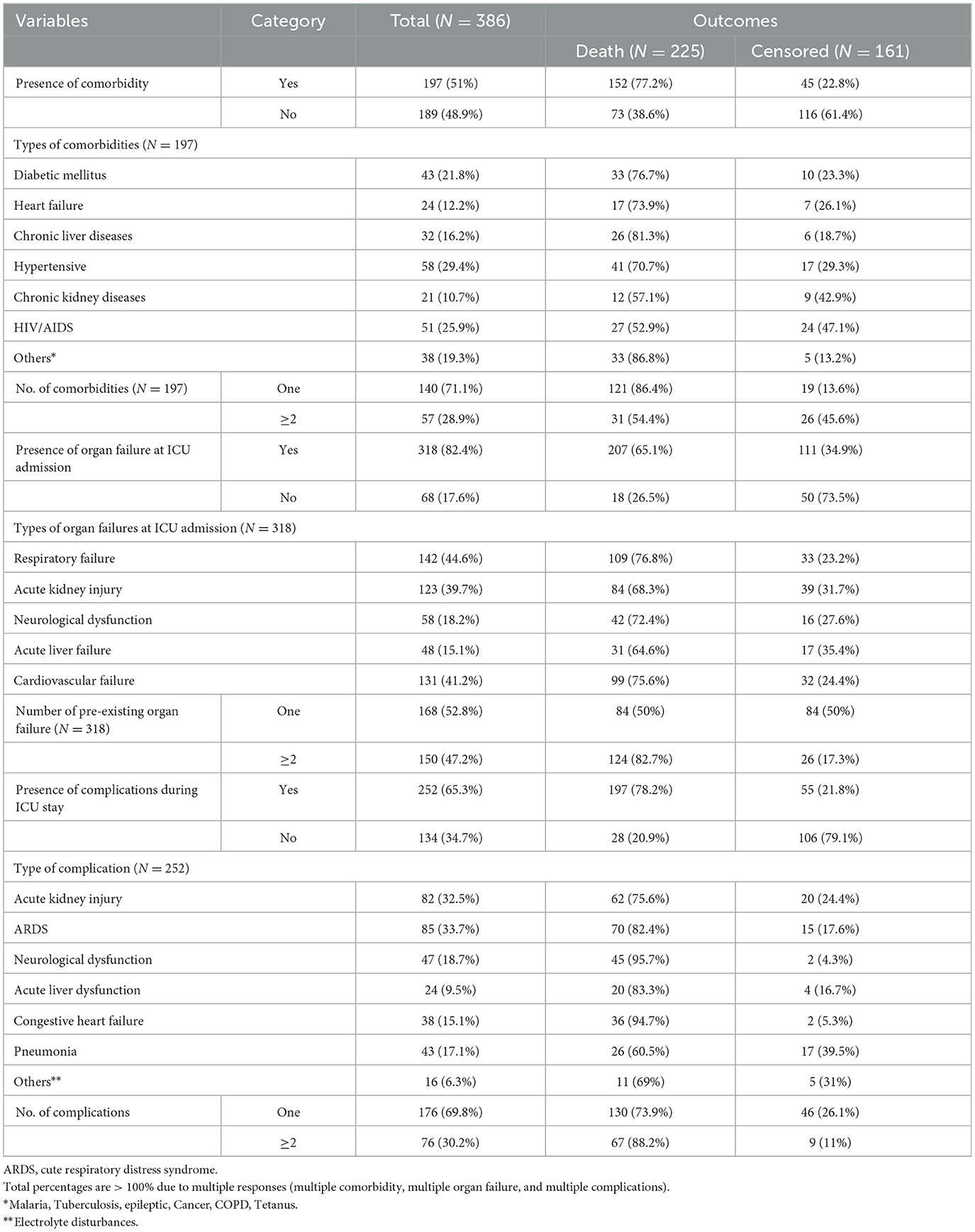
Table 4. Baseline history and ICU-related clinical characteristics of septic shock patients admitted to the ICU in Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Treatment-related characteristics of study participants
About 234 (60.2%) patients with septic shock started on vasopressors before admission to the ICU. Adrenaline and dopamine were the most frequent vasopressors during stays in the ICU, at 195 (85%) and 145 (41.7%), respectively. Of the patients, 337 (87.3%) were treated with a combination (more than two types) of antibiotic treatment and 71 (18.4%) of patients with septic shock had evidence of pathogen identification during the ICU follow-up time (Table 5).
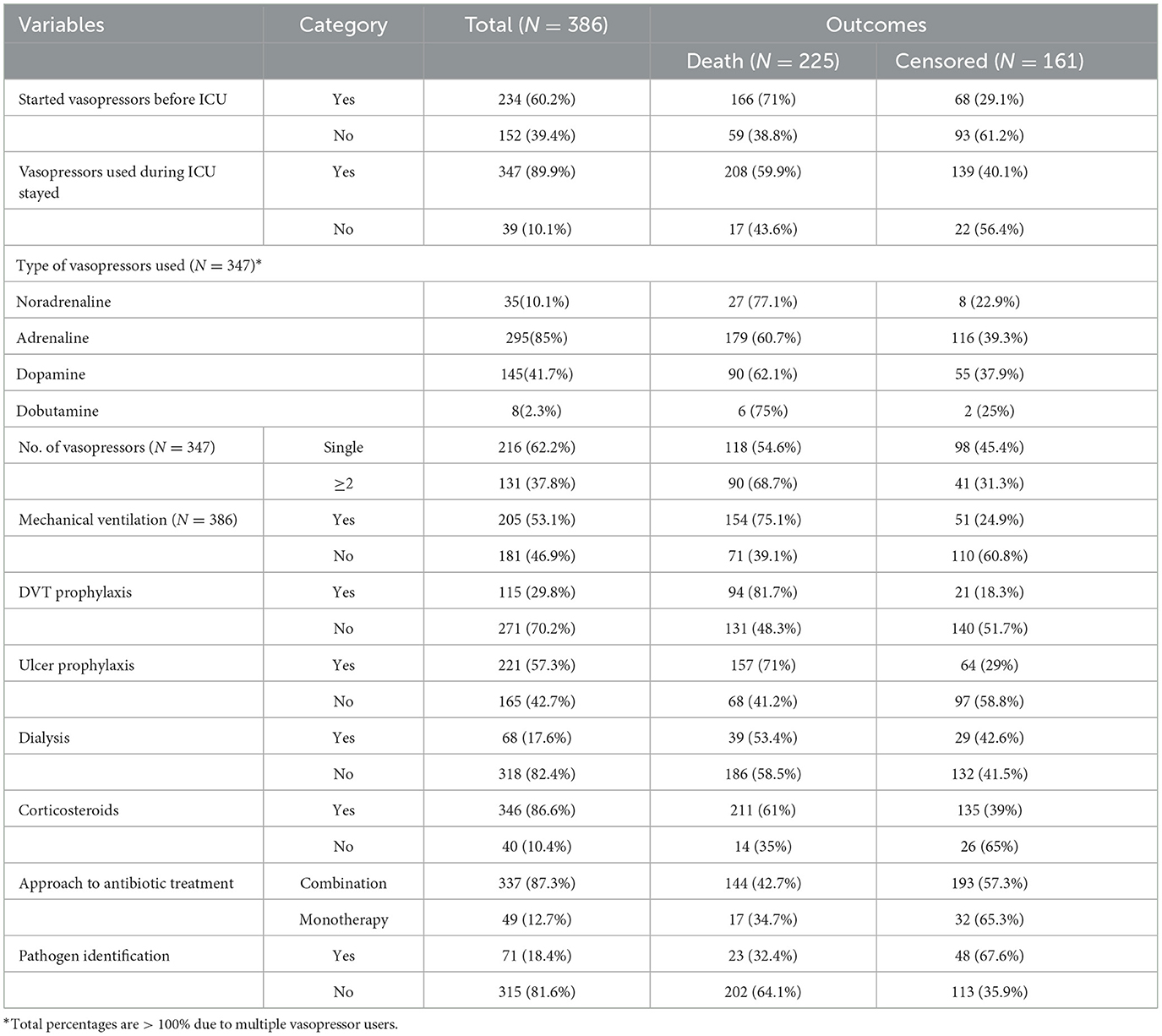
Table 5. Treatment-related characteristics of septic shock patients admitted to the ICU in Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Discharge conditions of septic shock patients admitted to the ICU
According to the current study, the proportion of death among patients with septic shock admitted to the ICU was 58.29% (Figure 2).
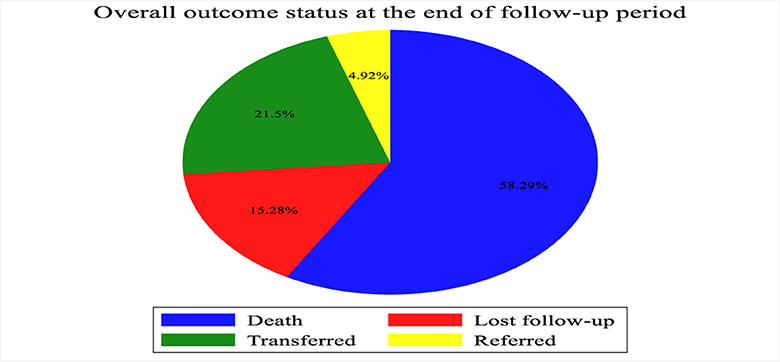
Figure 2. Outcome of patients with septic shock admitted to Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Mortality incidence among patients with septic shock patients
During the follow-up period, 225 patients with septic shock admitted to the ICU died, making the overall incidence density rate of death 10.4 per 100 person-day observation at 95% CI (9.10–11.8) and the median survival time was 7 days (IQR of 4–12 days). The total follow-up time for this cohort was 2,169 days, with a median follow-up time of 5 days (IQR of 3–7 days). Minimum and maximum follow-up times were 1 and 27 days, respectively. Among the deaths reported, nearly two-thirds of the 151 (61.1%) mortality was observed in the first weeks after admission to the ICU and 206 (85.6%) deaths occurred during the last 2 weeks of admission. The cumulative probability of death at the end of the first, fifth, 10th, 15th, and 20th days was 5%, 42.4%, 74%, 88.3%, and 95.3%, respectively. The proportion of death among septic shock patients admitted to the ICU during the follow-up period was 58.29% with 95% CI (53.3, 63.1).
Kaplan–Meier failure curve was used to describe the median survival time and the cumulative probability of death during the follow-up period (Figure 3).
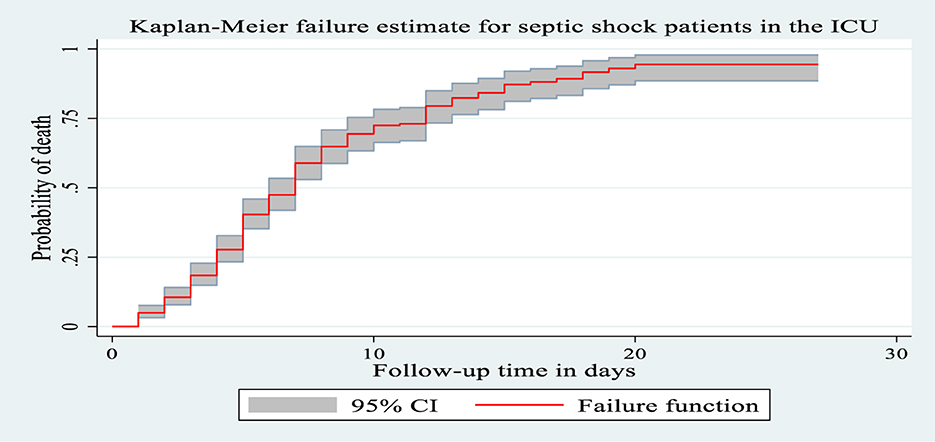
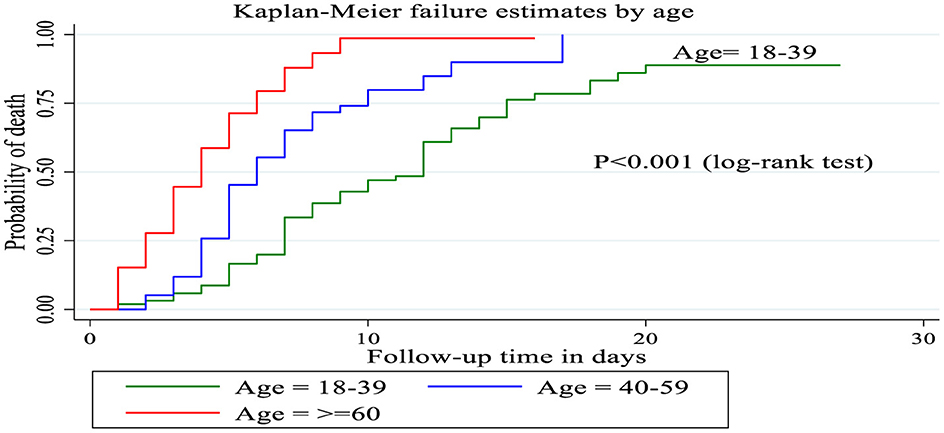
Figure 3. The Kaplan Meier failure curve for septic shock patients admitted to Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Kaplan–Meier curve with logarithmic rank test
Patients between the ages of 18 and 39 had a longer survival time (12 days), according to the Kaplan–Meier failure curve combined with the logarithmic rank test, compared to patients over the age of 60 (4 days) and those between 40 and 59 (6 days). The mortality incidence density rate among patients over 60 years of age, 40–59 years of age and 18–39 years of age was 22.2, 10.4, and 6.02 per 100 person days, respectively, without adjusting for other covariates (Figure 4).
Cox proportional hazard assumption test
The cox proportional hazard assumptions were tested for each predictor's variable by using the Schoenfeld residual test. The global test was carried out and it was found that it was insignificant (the p-value of each variable ranged between 0.1174 and 0.9736, and the overall global test was met (p-value = 0.9702; Table 6).

Table 6. Schoenfeld residual test for patients with septic shock admitted to the ICU of the Comprehensive Specialized Hospitals of Northwest Amhara, Amhara, Ethiopia, 2023.
The hazard function closely follows the 45-degree line, as demonstrated by the Cox-Snell residual plot, indicating that the model's goodness of fitness was met. The final model thus had good data fitting (Figure 5).
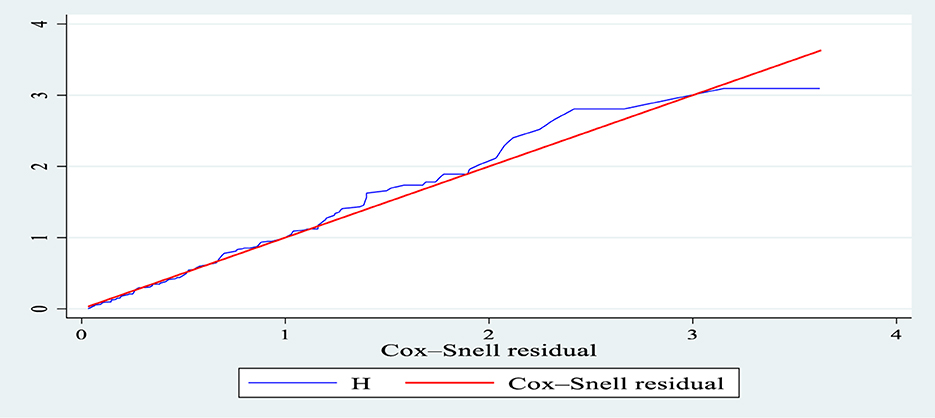
Figure 5. Nelson-Aalen cumulative hazard graph against Cox-Snell residual in patients with septic shock in the ICU of Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Predictors of death among septic shock patients admitted to the ICU
Before starting the Cox proportional hazard regression model, all variables were checked for multicollinearity, and those variables that had variance inflation factors >10 were excluded. Then all variables were entered into the bivariate Cox proportional hazard regression model. All variables that had (p-value < 0.2) on bivariable analysis were entered into the multivariate Cox proportional hazard regression model. Based on this, age, sex, residence, delay in ICU admission, duration of the illness, low MAP, tachycardia, altered bod T0, hypoxia, GCS level, hypoglycemia, thrombocytopenia, low hemoglobin, comorbidity, type of comorbidity (DM, HF, and HTN), presence of complication, type of complication (ARDS, ND, and ALF), vasopressors before the ICU and pathogen identification were candidates for multivariate analysis. However, only seven variables were found to be predictors of septic shock mortality. These variables were age, delay in admission to the ICU, low MAP, comorbidity, presence of complications, acute liver failure, and pathogen identification.
The risk of death among septic shock patients aged 40–59 and those over 60 years was 1.77 times (AHR = 1.77, 95% CI: 1.18, 2.66) and 3.5 times (AHR = 3.52, 95% CI: 2.31, 5.35) higher than among those aged between 18 and 39 years, respectively. Similarly, the death risk among patients with septic shock who had delayed admission to the ICU for more than 6 h was nearly 2 times (AHR = 1.93, 95% CI 1.33, 2.80) higher compared to those admitted in < 6 h. Patients with septic shock who had low MAP during admission to the ICU were 2.45 times (AHR = 2.45, 95% CI 1.53, 3.94) than their counterparts. Likewise, the risk of death among septic shock patients who had comorbidity was 2.7 times higher (AHR = 2.74, 95% CI: 1.81, 4.15) than among those who did not have comorbidity. Furthermore, the risk of death among patients who developed complications was 1.8 times (AHR = 1.867, 95% CI 1.146, 3.04) higher than among those who did not develop complications and having ALF complications increased the risk of death by 1.8 times (AHR = 1.84, 95% CI 1.04, 3.27) Furthermore, the risk of death among patients who did not have pathogen identification was nearly 1.69 times (AHR= 1.69, 95% CI 1.04, 2.76) higher than among those who have pathogen identification (Table 7).

Table 7. Bivariate and multivariate Cox proportional hazards regression analysis of mortality predictors among patients with septic shock admitted to the ICU of the Comprehensive Specialized Hospitals of Northwest Amhara, Ethiopia, 2023.
Discussion
According to the current study, the incidence density rate of mortality in patients with septic shock in the study was 10.4 per 100 person days of observation at 95% CI (9.10–11.8), and the median survival time was 7 days (IQR 4–12 days). The proportion of deaths among septic shock patients admitted to the ICU during the follow-up period was 58.29%, with a 95% CI of 53.3 to 63.1.
The result of this study is consistent with the study done in France (54.4%) (28). The reason behind this consistency could be because the study design they used and similar follow up time also increased the proportion of deaths, which may make it consistent with the current study. Most of their study participants had acute respiratory failure (83.6%) at admission compared to the current study (44.6%), and the patients were followed for 28 days even after being discharged from the ICU.
This finding is higher than the study conducted in Addis Ababa, which found that the overall ICU and the 28 days of mortality from sepsis and septic shock were 41.8 and 50.9%, respectively. The reason for this higher figure could be due to the severity of the case, as the study conducted in Addis Ababa included both sepsis (62%) and septic shock. In the current study, most of the participants had multiple organ dysfunction (47.2%) compared to the study conducted in Addis Ababa (21). Another reason for the difference could be that our study period was longer than theirs, as they mentioned in their limitations.
Furthermore, the findings of this study were higher than those of the study conducted in Germany (44.3%) (7), two studies in China (33.5%, 39%) (12, 29), Britain (44.2%) (30), a study conducted in five Asian countries (44.3%) (31), Pakistan (51.1%) (32), and a study conducted in North America (37.3%) (33). This higher mortality rate could be the result of delayed diagnosis or treatment, a lack of resources, a variation in the setting up of the ICU, poor service, or lack of early treatment such as vasopressors, as evidenced by the fact that 39.4% of the participants in our study did not take vasopressors before admission to the ICU. An inappropriate choice of drug may be one of the causes. International guidelines for the treatment of sepsis and septic shock (34) recommended norepinephrine as a first-line drug, but this study shows that more than three-quarters (76.2%) of patients took adrenaline as a first-line drug. It may be due to a lack of access to the drugs, which patients may not be able to afford, or it may be due to a registration problem that needs further study.
However, the result is lower than recent studies conducted in Croatia (63.4%) (35) and Rwanda (82.9%) (36). The reason behind this may be that the study conducted in Croatia included both septic shock patients admitted to the ICU and those patients who developed septic shock during their stay in the ICU, which may increase the result. Those patients who developed septic shock in the ICU (nosocomial infection) had more fatalities than those with community-acquired septic shock (37). The study conducted in Rwanda had fewer ICU beds (11 beds) than the current study, which may prolong the stay to admit patients to the ICU, and patients can deteriorate due to a lack of adequate care. Additionally, fewer people used vasopressors (32%) and mechanical ventilators (46.4%) during the study period than in the current study, suggesting a lack of resources relative to this study environment. HIV comorbidity was also more prevalent (19%) than in the current study, which can increase the mortality rate.
In another way, the finding of this study is lower than the studies in India (65.7%) (38) and Croatia (72.1%) (39), because the study conducted in India included only those patients who met the definition of septic shock of the criteria of sepsis 3 criteria (34); therefore, the severity of the diseases may not be the same as in the current study, and may be due to the nature of the prospective observational study designs they used that decrease the underestimation of the result. The study conducted in Croatia also followed a prospective study for 6 years and included those patients who developed septic shock in the ICU, which can increase the proportion of mortality. The presence of a high proportion of COPD can increase septic shock mortality, and those patients who develop septic shock after admission to the ICU are significant causes of mortality that were not included in the current study.
This study found that being younger had a preventive association with the mortality of septic shock in the ICU. Those patients older than 40 years have a higher mortality rate from septic shock in the ICU. Keeping other variables constant, those septic shock patients aged more than 60 years have three and a half times the hazard of death compared to those aged between 18 and 39 years, and those patients aged between 40 and 59 years also have a 1.77 times hazard of death compared to those aged between 18 and 39 years. This finding was supported by the studies conducted in India (38), France (28), and America (24). This may be due to comorbidities related to aging and the medical, social, and financial resources involved. According to a study in Morocco (40), being older increases septic shock due to multiple comorbidities and drugs they use, decreases endocrine dysfunction, increases the risk of colonization by gram-negative bacteria in the elderly, and decreases immunity as age increases.
The risk of death among patients who delayed admission to the ICU for more than 6 h after septic shock was diagnosed was nearly twice that of those who did not. The current study showed that half of the patients were delayed in being admitted to the ICU. This may be due to the scarcity of beds in the ICU or problems with early consultation with the ICU physician, or patients may not be willing to admit themselves to the ICU due to affordability, as evidenced by 15.3% of lost follow-up in this study, and some patients may not have a good attitude toward admission to the ICU (fear of admission to the ICU) that needs further study. This finding was consistent with a study conducted in the United Kingdom (41). Evidence showed that demand for ICU beds is increasing worldwide, delays in ICU admission are becoming a more frequent problem, and critically ill patients show further physiological deterioration and an increase in organ dysfunction while waiting for an ICU bed to become available. The treatment guidelines of the International Sepsis Survival Campaign recommend that patients with sepsis and septic shock are admitted to the ICU within 6 h of diagnosis (34). The study conducted in the USA supported this finding, as ~33.5% of septic shock patients admitted to the emergency department were referred to the ICU, with a median time delay of 24 (12 48) h. Training in health care can alleviate the problem of early diagnosis and decrease the time delay for patients with septic shock patients arriving at the ICU (13).
According to the findings of this study, nearly 60% of the patients had low MAP during admission to the ICU. Keeping other variables constant, the risk of death among patients with low MAP (< 65 mmHg) was 2.45 times higher than among those with high MAP (>65 mmHg). This is because low mean arterial pressure can cause decreased perfusion to vital organs, which can cause multiple organ failures. This was supported by the international definition of Sepsis 3 of sepsis and septic shock, as was the presence of low MAP and low serum lactate levels (< 2 mmol/L), which increased hospital mortality rates by >40%. Vasopressors were required to maintain a mean arterial pressure of 65 mm Hg or greater and a serum lactate level >2 mmol/L (>18 mg/dl) (1). The study conducted in Cameroon also supports the current study (37). Most of our setup used systolic blood pressure, but some studies suggested that it was better to use MAP for septic shock care (34). A proper mean arterial pressure titration in the early stages of treatment for patients with sepsis and septic shock corresponds to a successful outcome. Adequate mean arterial pressure is a crucial prerequisite for tissue and organ perfusion and is maintained throughout the treatment of sepsis patients (42).
The presence of comorbidities was also another independent predictor of mortality. The risk of death among patients with comorbidities was 2.7 times higher compared to their counterparts. This study was supported by studies conducted in Addis Ababa, Vietnam, Pakistan, and Germany that found chronic comorbid medical conditions present in 53%−64% of all patients with septic shock, which strongly affect critically ill patients with sepsis and septic shock patients (18, 21, 43, 44). The study showed that half of the patients had comorbidities such as diabetes mellitus, heart failure, hypertension, chronic liver diseases, chronic kidney disease, HIV/AIDS during admission to the ICU, among others.
The presence of complications or new organ failure during the follow-up of the ICU, such as ARDS, neurological dysfunction, acute liver failure, and congestive heart failure, increased the risk of death nearly two times compared to those without complications. Specifically, patients who had acute liver failure increased the risk of death by 1.84 times compared to those who did not have ALF. Because the liver acts as a lymphoid organ or mediated immune system in response to sepsis, liver dysfunction following sepsis and septic shock is an independent predictor of future multiorgan dysfunction and sepsis-related death, and the presence of pre-existing liver dysfunction aggravates the condition (45).
This study found that 252 (65.2%) patients developed complications during their stay in the ICU. This finding was supported by studies conducted in Addis Ababa (21), Egypt (46), Beirut (25), China (29, 47), and Pakistan (48). An international definition of Sepsis 3 referred to the fact that septic patients were more likely to develop organ failure related to low perfusion toward vital organs and could easily develop other complications (1).
The current study also found that the absence of pathogen identification during an ICU stay increased the risk of death by 1.7 times compared to those who had pathogen identification. According to this study, 315 (81.6%) had no pathogen identification during hospital stays. Similarly, the study conducted in Addis Ababa also shows that microbiological culture samples were collected from 32.4% of patients with septic shock admitted to the ICU, and only 10.5% of the patients developed microbiologic diagnoses related to sepsis or septic shock-related microbiologic diagnoses (21). This may be due to a lack of lab accessibility, patients may not be able to afford it, and the problem of early decision making. International guidelines for sepsis management recommend microbiological identification for all patients with suspected sepsis and septic shock patients (34). The study conducted in Croatia supports this finding, as only empirical treatment without pathogen identification increased septic shock mortality (35), and another study conducted in France also referred to the fact that septic shock treatment without pathogen identification increased mortality (49). The inability to have a specific antibiogram is one of the greatest therapeutic challenges in the treatment of septic shock in the ICU (37). Therefore, pathogen identification plays a crucial role in improving septic shock outcomes in the ICU.
Limitation of the study
The findings of this study could be affected by the fact that it is a retrospective study and rely on records; locating data for all variables is challenging, and those with insufficient information are omitted from the analysis. Variables related to physiological and laboratory investigations used to predict the severity of the disease, such as modified SOFA score, time to start antibiotics and fluid balance during the ICU stay, were not included. Furthermore, this study did not include patients who developed septic shock during stays in the ICU.
Conclusions
Overall mortality in patients with septic shock admitted to the ICU was found to be high compared to previous studies conducted in Ethiopia and some of the studies conducted globally. Being older, especially older than 60 years, having delayed admission to the ICU, low MAP (< 65 mmHg) at admission, comorbidities, complications during ICU stay such as ALF, and not having pathogen identification during the ICU stay were independent predictors of mortality, which could be minimized by providing appropriate ICU care services. For more aggressive ICU therapy aimed at reducing death, variables related to mortality from septic shock should be evaluated during septic shock treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Gondar College of Medicine and Health Science, Ethical Review Committee of the School of Nursing, with ethical approval number S/N/189/2015. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
TA: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. BL: Project administration, Supervision, Writing – review & editing. AZ: Conceptualization, Methodology, Writing – original draft. EE: Conceptualization, Formal analysis, Methodology, Project administration, Writing – review & editing. TD: Formal analysis, Methodology, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
First and foremost, we thank the Department of Emergency and Critical Care Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, for facilitating this research work. Next, we also extend our deepest gratitude and sincere appreciation to the referral hospitals in the Northwest part of the Amhara regional state for their invaluable and unreserved help throughout this research project. Last but not least, we would like to thank our data collectors and dearest colleagues for their cooperation and sharing of ideas.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHR, adjusted hazard ratio; AKI, acute kidney injury; ALF, acute liver failure; ARDS, acute respiratory distress syndrome; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; CLD, chronic liver disease; COPD, chronic obstructive pulmonary diseases; DM, diabetes mellitus; DVT, deep vein thrombosis; GCS, Glasgow Coma Scale; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; KM, Kaplan–Meier; MAP, mean arterial pressure; ND, neurological dysfunction; SICU, surgical intensive care unit; SOFA, sequential organ failure assessment; UoGCSH, University of Gondar Comprehensive Specialized Hospital; WBC, white blood cells; WHO, World Health Organization.
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Neviere R, Parsons PE, Finlay G. Sepsis syndromes in adults: Epidemiology, definitions, clinical presentation, diagnosis, and prognosis. Monografía en [Internet] Wolters Kluwer: UpToDate (2017).
3. Jawad I, Lukšić I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health. (2012) 2:010404. doi: 10.7189/jogh.01.010404
4. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. (2016) 193:259–72. doi: 10.1164/rccm.201504-0781OC
5. Shen H-N, Lu C-L, Yang H-H. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest. (2010) 138:298–304. doi: 10.1378/chest.09-2205
6. Angus DC, Pires Pereira CA, Silva E. Epidemiology of severe sepsis around the world. Endocr Metab Immune Disord Drug Targets. (2006) 6:207–12. doi: 10.2174/187153006777442332
7. SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicentre INSEP study. Intensive Care Med. (2016) 42:1980-9. doi: 10.1007/s00134-016-4504-3
8. van Gestel A, Bakker J, Veraart CP, van Hout BA. Prevalence and incidence of severe sepsis in Dutch intensive care units. Crit Care. (2004) 8:1–10. doi: 10.1186/cc2858
9. Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. (2014) 5:4–11. doi: 10.4161/viru.27372
10. Elias ACGP, Matsuo T, Grion CMC, Cardoso LTQ, Verri PH. Incidence and risk factors for sepsis in surgical patients: a cohort study. J Crit Care. (2012) 27:159–66. doi: 10.1016/j.jcrc.2011.08.001
12. Shankar-Hari M, Harrison D, Rubenfeld G, Rowan KJBB. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth. (2017) 119:626–36. doi: 10.1093/bja/aex234
13. Rezende E, Junior JMS, Isola AM, Campos EV, Amendola CP, Almeida SL. Epidemiology of severe sepsis in the emergency department and difficulties in the initial assistance. Clinics. (2008) 63:457–64. doi: 10.1590/S1807-59322008000400008
14. Park DW, Chun BC, Kim JM, Sohn JW, Peck KR, Kim YS, et al. Epidemiological and clinical characteristics of community-acquired severe sepsis and septic shock: a prospective observational study in 12 university hospitals in Korea. J Korean Med Sci. (2012) 27:1308–14. doi: 10.3346/jkms.2012.27.11.1308
15. van Vught LA, Klouwenberg PMCK, Spitoni C, Scicluna BP, Wiewel MA, Horn J, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. (2016) 315:1469–79. doi: 10.1001/jama.2016.2691
16. Ogura H, Gando S, Saitoh D, Takeyama N, Kushimoto S, Fujishima S, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. (2014) 20:157–62. doi: 10.1016/j.jiac.2013.07.006
17. Opal SM, Van Der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. (2015) 277:277–93. doi: 10.1111/joim.12331
18. Schmidt K, Gensichen J, Fleischmann-Struzek C, Bahr V, Pausch C, Sakr Y, et al. Long-term survival following sepsis: results of a single-center registry study with 4-year follow-up. Dtsch Arztebl Int. (2020) 117:775. doi: 10.3238/arztebl.2020.0775
19. Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. (2010) 376:1339–46. doi: 10.1016/S0140-6736(10)60446-1
20. Riviello ED, Kiviri W, Fowler RA, Mueller A, Novack V, Banner-Goodspeed VM, et al. Predicting mortality in low-income country ICUs: the Rwanda Mortality Probability Model (R-MPM). PLoS ONE. (2016) 11:e0155858. doi: 10.1371/journal.pone.0155858
21. Mulatu HA, Bayisa T, Worku Y, Lazarus JJ, Woldeyes E, Bacha D, et al. Prevalence and outcome of sepsis and septic shock in intensive care units in Addis Ababa, Ethiopia: a prospective observational study. Afr J Emerg Med. (2021) 11:188–95. doi: 10.1016/j.afjem.2020.10.001
22. Messelu MA, Tilahun AD, Beko ZW, Endris H, Belayneh AG, Tesema GA. Incidence and predictors of mortality among adult trauma patients admitted to the intensive care units of comprehensive specialized hospitals in Northwest Ethiopia. Eur J Med Res. (2023) 28:1–12. doi: 10.1186/s40001-023-01056-z
23. Fabbian F, De Giorgi A, Ferro S, Lacavalla D, Andreotti D, Ascanelli S, et al., editors. Post-operative All-cause Mortality in Elderly Patients Undergoing Abdominal Emergency Surgery: Role of Charlson Comorbidity Index. MDPI (2021). doi: 10.3390/healthcare9070805
24. Black LP, Hopson C, DeVos E, Fernandez R, Guirgis F, Garvan C. 4332 Septic shock epidemiology and sociodemographic predictors of mortality: results from One Florida Data Trust Cohort. J Clin Transl Sci. (2020) 4:36–7. doi: 10.1017/cts.2020.145
25. Kattouf N, Assaf M, Haidar S, Bachir R, El Sayed M, BouChebl R. The risk factors for mortality among septic trauma patients: a retrospective cohort study using the National Trauma Data Bank. Emerg Med Int. (2022) 2022:6386078. doi: 10.1155/2022/6386078
26. Shibru H, Muhie OA, Yesuf T, Greffie ES, Mitiku T, Admasu Z, et al. Outcome of septic shock and associated factors at the University of Gondar Hospital: a retrospective cohort study. J Intensive Crit Care. (2020) 6:18. doi: 10.36648/2471-8505.6.5.18
27. Vélez JW, Aragon DC, Donadi EA, Carlotti AP. Risk factors for mortality from sepsis in an intensive care unit in Ecuador: a prospective study. Medicine. (2022) 101:e29096. doi: 10.1097/MD.0000000000029096
28. Boussekey N, Cantrel J, Dorchin Debrabant L, Langlois J, Devos P, Meybeck A, et al. Epidemiology, prognosis, and evolution of management of septic shock in a French intensive care unit: a five years survey. Critical Care Res Pract. (2010) 2010:436427. doi: 10.1155/2010/436427
29. Wang M, Jiang L, Zhu B, Li W, Du B, Kang Y, et al. The prevalence, risk factors, and outcomes of sepsis in critically ill patients in China: a multicenter prospective cohort study. Front Med. (2020) 7:593808. doi: 10.3389/fmed.2020.593808
30. Khwannimit B, Bhurayanontachai R. The epidemiology of, and risk factors for, mortality from severe sepsis and septic shock in a tertiary-care university hospital setting. Epidemiol Infect. (2009) 137:1333–41. doi: 10.1017/S0950268809002027
31. Phua J, Koh Y, Du B, Tang Y-Q, Divatia JV, Tan CC, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ. (2011) 342:d3245. doi: 10.1136/bmj.d3245
32. Asghar A, Hashmi M, Rashid S, Khan FH. Incidence, outcome and risk factors for sepsis-a two year retrospective study at surgical intensive care unit of a teaching hospital in Pakistan. J Ayub Med Coll Abbottabad. (2016) 28:79.
33. Vincent J-L, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. (2019) 23:1–11. doi: 10.1186/s13054-019-2478-6
34. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247.
35. Vucelić V, Klobučar I, Duras Cuculić B, Gverić Grginić A, Prohaska Potočnik C, Jajić I, et al. Sepsis and septic shock–an observational study of the incidence, management, and mortality predictors in a medical intensive care unit. Croat Med J. (2020) 61:429–39. doi: 10.3325/cmj.2020.61.429
36. Hopkinson DA, Mvukiyehe JP, Jayaraman SP, Syed AA, Dworkin MS, Mucyo W, et al. Sepsis in two hospitals in Rwanda: a retrospective cohort study of presentation, management, outcomes, and predictors of mortality. PLoS ONE. (2021) 16:e0251321. doi: 10.1371/journal.pone.0251321
37. Metogo Mbengono JA, Tochie JN, Ndom Ntock F, Nzoaungo YB, Kona S, Ngono Ateba G, et al. The epidemiology, therapeutic patterns, outcome, and challenges in managing septic shock in a Sub-Saharan African Intensive Care Unit: a Cross-Sectional Study. Hosp Pract Res. (2019) 4:117–21. doi: 10.15171/hpr.2019.24
38. Mohan A, Shrestha P, Guleria R, Pandey RM, Wig N. Development of a mortality prediction formula due to sepsis/severe sepsis in a medical intensive care unit. Lung India. (2015) 32:313. doi: 10.4103/0970-2113.159533
39. Degoricija V, Sharma M, Legac A, Gradišer M, Šefer S, Vučičević Ž. Survival analysis of 314 episodes of sepsis in medical intensive care unit in university hospital: impact of intensive care unit performance and antimicrobial therapy. Croat Med J. (2006) 47:385–97.
40. Nasa P, Juneja D, Singh O. Severe sepsis and septic shock in the elderly: an overview. World J Crit Care Med. (2012) 1:23. doi: 10.5492/wjccm.v1.i1.23
41. Harris S, Singer M, Sanderson C, Grieve R, Harrison D, Rowan K. Impact on mortality of prompt admission to critical care for deteriorating ward patients: an instrumental variable analysis using critical care bed strain. Intensive Care Med. (2018) 44:606–15. doi: 10.1007/s00134-018-5148-2
42. Zhong X, Li H, Chen Q, Hao P, Chen T, Mai H, et al. Association between different MAP levels and 30-day mortality in sepsis patients: a propensity-score-matched, retrospective cohort study. BMC Anesthesiol. (2023) 23:116. doi: 10.1186/s12871-023-02047-7
43. Jamil B, Qureshi KA, Khan M, Ujan VA. Assessment of four mortality prediction models in intensive care unit patients with sepsis. Renal Fail. (2004) 5:33.
44. Trong TN, Thao DT, Minh VP, Truyen NV. Septic shock outcome and factors associated with mortality in the intensive care unit in Vietnam. J Med Assoc Thailand. (2021) 104:1249–54. doi: 10.35755/jmedassocthai.2021.08.11596
45. Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. (2014) 33:498–510. doi: 10.3109/08830185.2014.889129
46. Madkour AM, ELMaraghy AA, Elsayed MM. Prevalence and outcome of sepsis in respiratory intensive care unit. Egypt J Bronchol. (2022) 16:29. doi: 10.1186/s43168-022-00135-9
47. Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PLoS ONE. (2014) 9:e107181. doi: 10.1371/journal.pone.0107181
48. Ullah AR, Hussain A, Ali I, Samad A, Shah STA, Yousef M, et al. A prospective observational study assessing the outcome of Sepsis in intensive care unit of a tertiary care hospital, Peshawar. Pak J Med Sci. (2016) 32:688. doi: 10.12669/pjms.323.9978
Keywords: Ethiopia, ICU, incidence, mortality, predictors, septic shock
Citation: Ayenew Mekuria T, Liyew Wudu B, Zegeye AF, Eshete Tadesse E and Demis Nimani T (2024) Incidence of mortality and its predictors among septic shock patients admitted to the intensive care unit of comprehensive specialized hospitals in the northwest of Amhara, Ethiopia. Front. Disaster Emerg. Med. 2:1405753. doi: 10.3389/femer.2024.1405753
Received: 23 March 2024; Accepted: 10 June 2024;
Published: 26 June 2024.
Edited by:
Theodore Chan, University of California, San Diego, United StatesReviewed by:
Amanda Frantz, University of Florida, United StatesPeter A. Ward, University of Michigan, United States
Copyright © 2024 Ayenew Mekuria, Liyew Wudu, Zegeye, Eshete Tadesse and Demis Nimani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teshome Demis Nimani, dGVzaG9tZWRlbWlzMTEyQGdtYWlsLmNvbQ==
†ORCID: Teshome Demis Nimani orcid.org/0000-0002-8982-9362
 Tesfaye Ayenew Mekuria1
Tesfaye Ayenew Mekuria1 Teshome Demis Nimani
Teshome Demis Nimani