- 1School of Medical, Jiangnan University, Wuxi, China
- 2Department of General Surgery, The Affiliated Wuxi No. 2 People’s Hospital of Jiangnan University, Wuxi, China
- 3Drug Clinical Trial Facility, Affiliated Hospital of Jiangnan University, WuXi, China
Background: Experimental drug management is an important part of clinical trial management and directly relates to the reliability of clinical trial data and the safety of participants. This study explores the implementation of centralized pharmacy management in clinical drug trials, addressing the limitations of decentralized approaches.
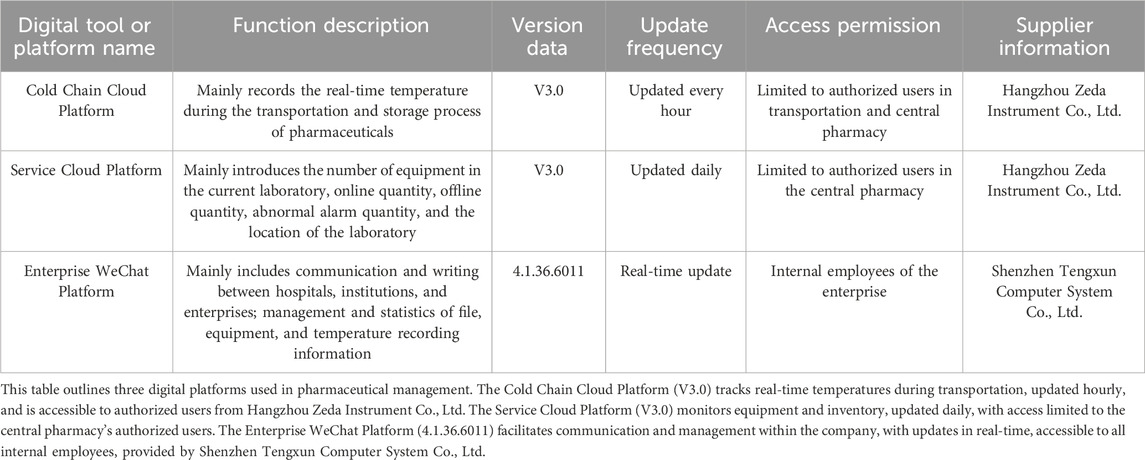
Methods: It involves the establishment of a centralized drug warehouse with unified processes and digital systems, primarily relying on the Cold Chain Cloud Platform, Service Cloud Platform, and Enterprise WeChat Platform, to enhance precision and efficiency.
Results: It indicated a significant reduction in error rates and improved adherence to clinical trial protocols. The centralized model also minimized protocol deviations and strengthened participant safety.
Conclusion: It highlights the benefits of centralized management in enhancing drug administration efficiency and maintaining high standards of trial conduct, suggesting future integration with Artificial Intelligence (AI) and Electronic Health Records (EHR) for more precise pharmaceutical services.
1 Introduction
In recent years, the decentralized approach to managing investigational drugs has demonstrated significant limitations (National Medical Products Administration, 2020a; National Medical Products Administration, 2020b), such as difficulties in coordination between departments, which reduces efficiency. In contrast, institutional centralized pharmacy management is characterized by its efficiency and standardization. Therefore, centralized pharmacy management is positioned as an emerging trend that is expected to enhance the safety of drug quality, storage, and utilization. Effective drug management throughout clinical trials is integral to the progression and precision of trial outcomes, influencing both data accuracy and reliability.
This significance is reflected in standards such as the current Good Practice for the Quality Management of Pharmaceutical Clinical Trials in China (No. 57 of 2020) and the ASHP Guidelines for the Management of Investigational Drug Products (Feng et al., 2020; Guo et al., 2019). As regulatory frameworks evolve with increasingly comprehensive drug management criteria, drug administrators must navigate these requirements to ensure accurate, complete, and practical application in daily operations while minimizing error risks.
Centralized drug warehouse management, employing unified processes, standardized operations, and digital information systems, enhances precision and efficiency across experimental drug storage, distribution, and retrieval, thereby raising overall management quality and productivity in drug logistics (Mohammad et al., 2021; Dagenais et al., 2022; Brodie et al., 2019). This centralized framework further minimizes error rates, safeguards participant welfare, and strengthens adherence to clinical trial protocols (Jones et al., 2020).
Effective management of investigational drugs is integral to ensuring the scientific integrity, accuracy, and impartiality of clinical trial outcomes (Sessler and Myles, 2020). With regulatory management on clinical trial drugs becoming increasingly rigorous and standardized, monitoring drug management practices has emerged as a core aspect of the on-site evaluation of trial institutions and project verifications (Spira et al., 2021; Thall et al., 2023). Currently, however, the regulatory framework lacks specific guidelines on investigational drug management, allowing institutions to establish tailored protocols aligned with hospital-specific needs (Magnuson et al., 2021). Irrespective of the chosen approach, adherence to Good Clinical Practice (GCP) standards remains paramount to affirming the scientific validity, authenticity, and reliability of clinical trial data.
Drug clinical trial institutions in China primarily implement three management models: centralized management, decentralized management, and a collaborative approach between institutions and specialized teams (hybrid management) (Gulden et al., 2019; Calabrese et al., 2023). There is a scarcity of international research specifically exploring the centralized management of drugs in clinical projects. Currently, centralized drug management is advantageous for the summarization of drug resources and the enhancement of management efficiency. In contrast, decentralized drug management can refine functions, including managing specific drugs at the department level, ensuring timely delivery to participants, and reducing data deviations in clinical projects caused by the drug transportation process (Thomas et al., 2020). This model may have advantages in flexibility and response speed, but it could face challenges in standardization and coordination. The hybrid model combines the strengths of both centralized and decentralized approaches, but it may increase management complexity.
At present, some institutions are plagued by chaotic personnel management, slow project progress, and inadequate drug monitoring (Fan et al., 2022). In contrast to other institutions, our hospital undertakes a larger number of projects and involves a broader range of departments. By leveraging a centralized pharmacy management platform to integrate project management, execution, and supervision, we have achieved smoother project progress and more standardized drug management. The application of this management platform effectively simplifies actual processes and maximizes the utilization of resources.
The centralized pharmacy of our hospital relies on a variety of digital platforms (Table 1) and adopts a modular design. Each module can be flexibly adjusted and expanded according to specific tasks. This design not only enhances the adaptability of the system, but also facilitates subsequent optimization and upgrades. In contrast, some existing models often have a fixed architecture, which makes it difficult to make effective adjustments for different tasks.
In November 2020, Our hospital utilizes a centralized management framework, establishing an independent GCP central pharmacy staffed with dedicated pharmacists to manage clinical trial drugs. This model satisfies specific regulatory demands for inventory, secured storage, controlled access, registration, and prescription management. Additionally, a dedicated dispensing window for clinical trial drugs is established. This minimizes issues such as substandard storage, irregular distribution and usage, and incomplete documentation (Liu et al., 2020; Lagreula et al., 2021; Cao et al., 2023). This study presents critical aspects of daily central warehouse management alongside a targeted implementation strategy.
2 Regulatory context
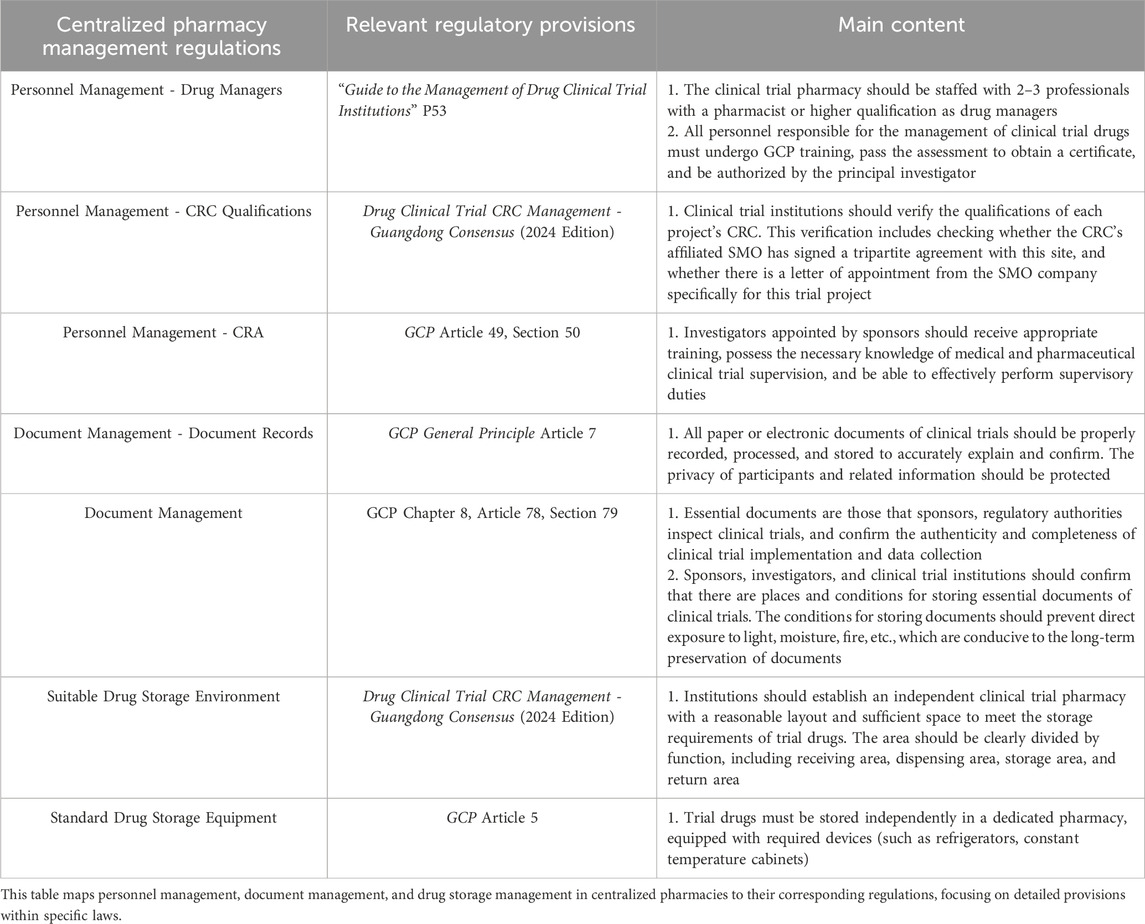
In China, the regulatory standards, including the Drug Clinical Trial Quality Management Standards and the General Rules, impose strict requirements on the management of investigational drugs and clinical trial data (Administration, 2004; Omidian and Omidi, 2022). They mandate detailed protocols for handling drugs and emphasize meticulous documentation practices. These regulations also outline essential qualifications for trial personnel and standards for pharmacy management and infrastructure (Cao et al., 2022; Lin et al., 2020; Tang et al., 2021). Furthermore, the Requirements for Drug Records and Data Management and the Key Points and Judgment Principles of Drug Registration Verification reinforce the need for stringent management practices and controlled documentation procedures (Wei et al., 2021; Zhou et al., 2022).
China’s GCP regulations are developed based on the Food and Drug Administration (FDA) and European Medicines Agency (EMA) regulations with localization modifications. In terms of the preparation of investigational medicinal products (IMPs), all three sets of regulations require that the preparation of IMPs should comply with the relevant requirements of Good Manufacturing Practice (GMP) for clinical trial medicinal products. The packaging and labeling should indicate that the products are for clinical trial use only and provide information on the clinical trial and the IMPs (Boesen et al., 2021).
Regarding the management of IMPs, the approval authorities differ among the three (Chiodin et al., 2019). China’s regulations require the sponsor to be responsible for providing IMPs to investigators and clinical trial institutions. IMPs shall not be provided before obtaining approval or filing from the ethics committee and the drug regulatory authority. The FDA requires the sponsor to provide IMPs and prohibits the provision of IMPs before obtaining approval from the IRB and the FDA. The EMA requires the sponsor to provide IMPs and prohibits the provision of IMPs before obtaining approval from the ethics committee and the EMA.
3 Drug management measures for clinical trials
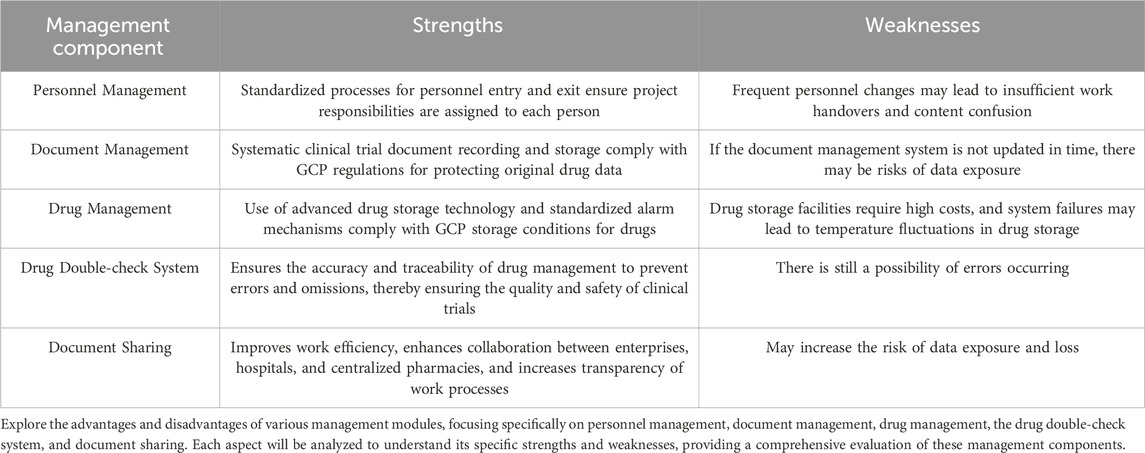
Centralized clinical drug management mainly consists of the following parts: personnel management, file management, appropriate drug storage conditions, complete temperature warning mechanism, systematic drug double-check system and sharing of medication files (Figure 1) (Table 2).
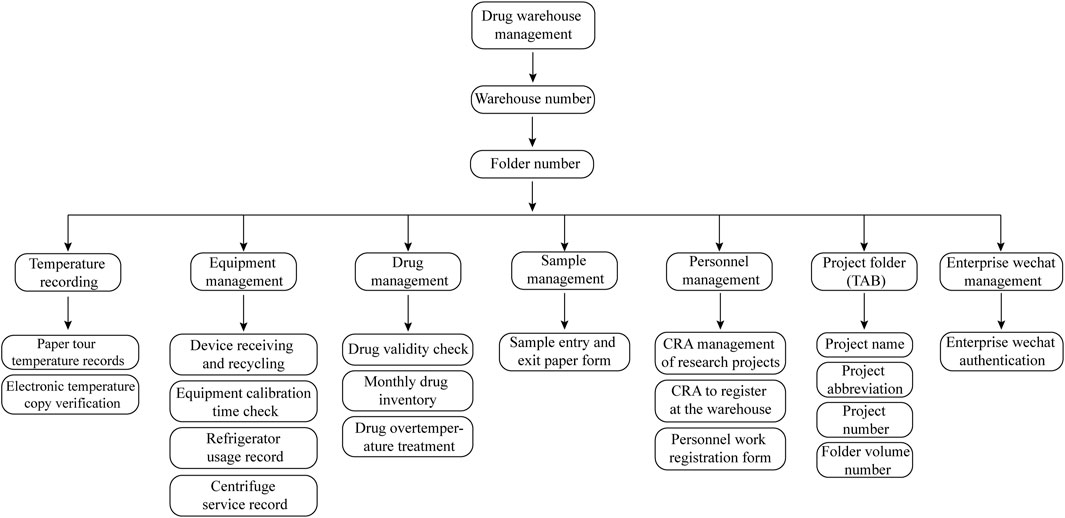
Figure 1. Classification and Coding Guidelines for Drug Folders. The drug warehouse document management flowchart is comprehensively structured, detailing six core areas: temperature monitoring, equipment management, drug inventory, sample tracking, personnel records, and project documentation. Temperature monitoring includes both paper-based and digital records. Equipment management encompasses documentation related to equipment acquisition, recycling, and utilization. Drug inventory management involves tracking stock levels and expiration dates. Sample tracking records each sample’s entry and exit from the warehouse. Personnel records document CRA and CRC registrations, while project documentation maintains an organized record of project names and identification numbers.
3.1 Personnel management
To comply with legal and regulatory standards, the central pharmacy requires the appointment of at least two drug administrators who are qualified in GCP. These administrators must have expertise in the clinical trial drug management system and be proficient in Standard Operation Procedures (SOPs) to ensure effective drug management (Zhang et al., 2020). Alternatively, these administrators must undergo annual GCP training and pass an accredited state-recognized assessment to maintain valid GCP certification (Miyauchi et al., 2022).
The study systematically examines the qualifications of clinical research coordinators (CRCs) across programs, focusing on whether affiliated Site Management Organization (SMO) companies have formalized tripartite agreements with sites and provided delegation letters specific to test projects. The audit further verifies the validity of CRCs’ IDs, the presence of updated GCP training certifications, institutional work numbers, and Principal Investigator (PI) authorization. Only CRCs meeting these approval standards may engage in authorized drug management activities, including drug verification, transport, temperature monitoring, and end-of-month inventory management (National Medical Products Administration, 2020b).
The study further assesses the credentials of clinical research associates (CRA), examining whether each CRA is affiliated with the sponsor or the appointed Contract Research Organization (CRO), holds an officially endorsed letter of appointment, and has been formally registered with the organization, including the acquisition of a CRA job number. Access to the drug supervision center’s storage facilities, as well as drug receipt and recycling areas, is restricted to CRAs meeting all qualification requirements.
3.2 File management
3.2.1 Controlled version and number of files
The Center establishes SOPs (JG-SOP-V2.0) governing the control of drug-related documents, designating the drug administrator as the sole recipient for these materials. Each clinical project’s CRA customizes the drug documents per the hospital’s template, followed by review and approval from the project team, PI, and Quality Assurance. After verification, the documents are printed, handed over to the drug administrator, and then uniformly filed into the project folder upon document retrieval and project completion. During trials, when updates to controlled documents are required or existing copies are exhausted, additional documents are issued. The CRA submits an application via the platform, secures approval for the updated or new documents, and delivers them to the drug administrator, completing the official record.
3.2.2 Record of files
Documentation must remain accurate, prompt, and comprehensive. Forms maintained by the center encompass drug inventory logs, participant drug issuance and retrieval records, transportation temperature logs, participant drug receipt forms, and storage temperature logs. Any irregularities during storage, such as temperature deviations, drug loss, or delivery discrepancies, must be promptly documented, with a monthly inventory check to confirm drug stocks. Additionally, GCP centralized pharmacy management requires three primary types of documentation: electronically derived temperature records indicating storage conditions (and their copies); logs for receiving, calibration, recovery, and maintenance of refrigerators, incubators, thermometers, and similar equipment; and registration records of CRA monitoring and site visits.
3.2.3 Folder management
Establishing standardized coding rules for folders optimizes file placement and retrieval processes. Folder storage must be systematically organized within designated cabinets, with storage spaces engineered for fire, theft, insect, and light protection (Myles, 2022). Additionally, folder locations should be dynamically realigned according to project progress. Implementing a rigorous approval process for folder access, borrowing, and return is essential to safeguard file security and confidentiality, restricting access solely to authorized personnel. Together, these measures promote uniform folder storage, expedite file retrieval, and ensure the authenticity and completeness of records, preserving both the integrity of documentation and the confidentiality of participants’ drug use data.
3.3 Appropriate drug storage conditions
3.3.1 Appropriate drug storage partitions
At the drug warehouse, areas for qualified, unqualified, and holding drugs are marked with colored bands. Expired and empty packages are stored in the nonconforming area, while overtemperature drugs are held pending review. Regular checks of expiration dates and temperatures are crucial for proper storage. Sections for expired drugs and packaging, along with isolation fridges and recycling bins, ensure timely disposal of unusable drugs.
3.3.2 Standard drug storage equipment
Effective GCP management mandates air conditioning, dehumidifiers, and humidifiers within drug storage areas to maintain requisite environmental conditions, with all equipment verified for proper function and supported by valid calibration certificates. A dynamic refrigerator information log should comprehensively record equipment details—serial numbers, storage durations, suppliers, calibration expiration dates, and sponsor retrieval dates. Regular monitoring of calibration status is essential, with timely notifications to ensure prompt professional recalibration. Annual calibration also necessitates temperature-monitoring tools, such as thermometers and temperature cards, accompanied by an electronic calibration certificate. Additionally, a dynamic log for temperature-monitoring equipment captures critical information, including serial numbers, storage timelines, providers, certificate validity, and sponsor retrieval schedules. Thermometers approaching calibration expiration must be promptly replaced, ensuring accuracy of exported temperature records.
3.4 Complete temperature warning mechanism
Initially, a dynamic drug inventory table is created, followed by the development of a dial based on this table to provide a clear visual representation of current drug inventory levels, as illustrated in Figure 2. This figure details the quantities of drugs nearing expiration or already expired, alongside their respective project names and precise expiration dates. By selecting a specific number within the item column, users can access a detailed breakdown of each drug in stock.
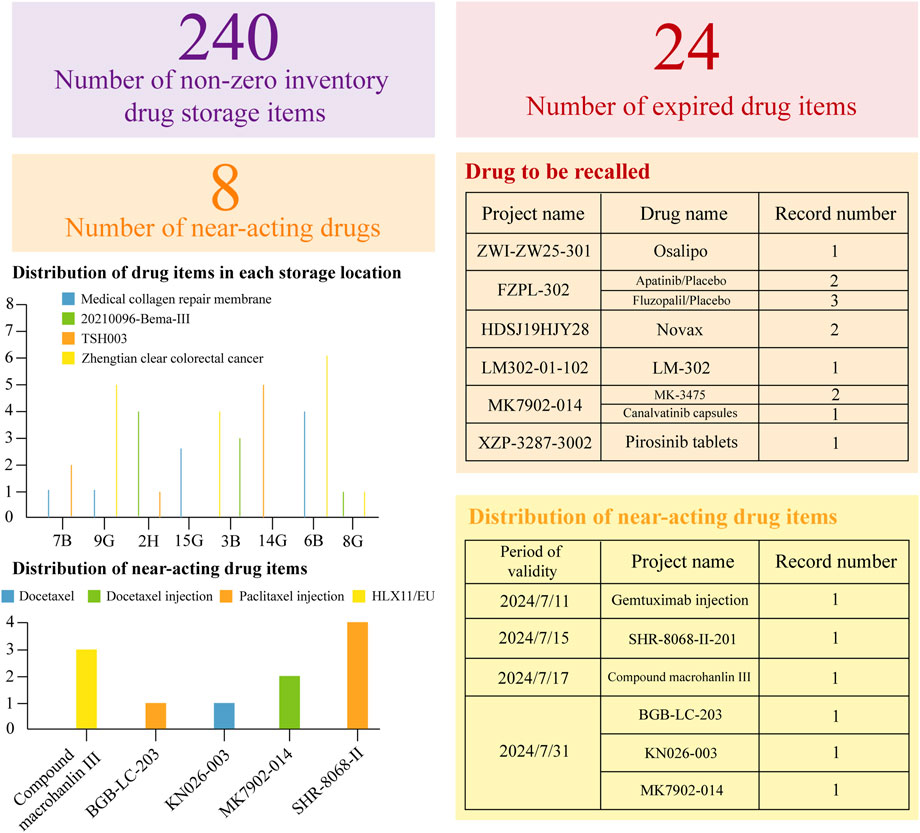
Figure 2. Dynamic Inventory Management Table for Clinical Trial Drugs. This statistical table is based on the WeChat Work platform. It has organized the current expiration dates of the drugs, providing a clear overview of their validity. Additionally, it has meticulously listed all the relevant drugs, ensuring a comprehensive inventory. Moreover, the table has accurately specified the storage locations for each drug, facilitating efficient management and easy access.
In the second place, the instrument panel incorporates dynamic thermometer tables and temperature cards to precisely represent the status of temperature monitoring equipment within the pharmacy. As illustrated in Figure 3, to assess the need for advance procurement or replacement with a newly calibrated thermometer supplied by the project team, the panel tracks metrics including the total thermometer inventory, active thermometers, replacement units, and thermometers due for calibration each month. Additionally, the dynamic table records both the quantity and provider of each thermometer, enhancing traceability and enabling prompt recovery and replacement in case of malfunction. Selecting specific color-coded indicators (e.g., “35 thermometers due for calibration this month”) reveals detailed equipment information and current status.
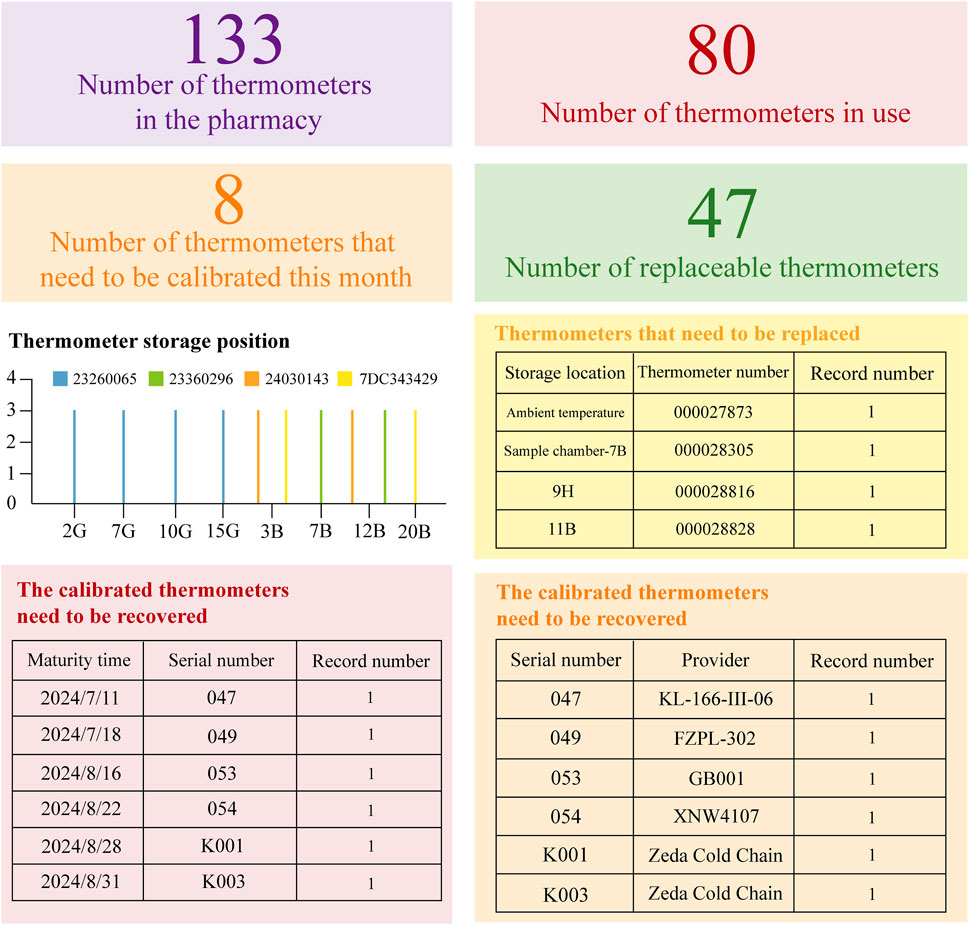
Figure 3. Dynamic Inventory Management Table for Temperature Recording Equipment. Based on the WeChat Work platform and Cold Chain Cloud Platform (www.zjueecloud.com), this dynamic table compiles extensive statistical data on the inventory of pharmacy thermometers. It includes information on their validity period, designated uses, and suppliers for each unit. Additionally, it provides a statistical analysis of the number and distribution of thermometers that are nearing their expiration dates.
Finally, the institution’s centralized sample processing method mandates unified management of equipment, including centrifuges and ultra-low temperature refrigerators. The dashboard, illustrated in Figure 4, is structured according to real-time equipment monitoring tables, detailing the total number of active centrifuges and refrigerators, units requiring calibration, and those marked as out of service. Additionally, the dynamic table delineates storage locations, assigned units, and precise calibration expiration dates. This configuration enables drug administrators to readily monitor equipment in the center’s drug warehouse, providing immediate access to usage, maintenance, and calibration details, thereby enhancing the robustness of the drug management system.
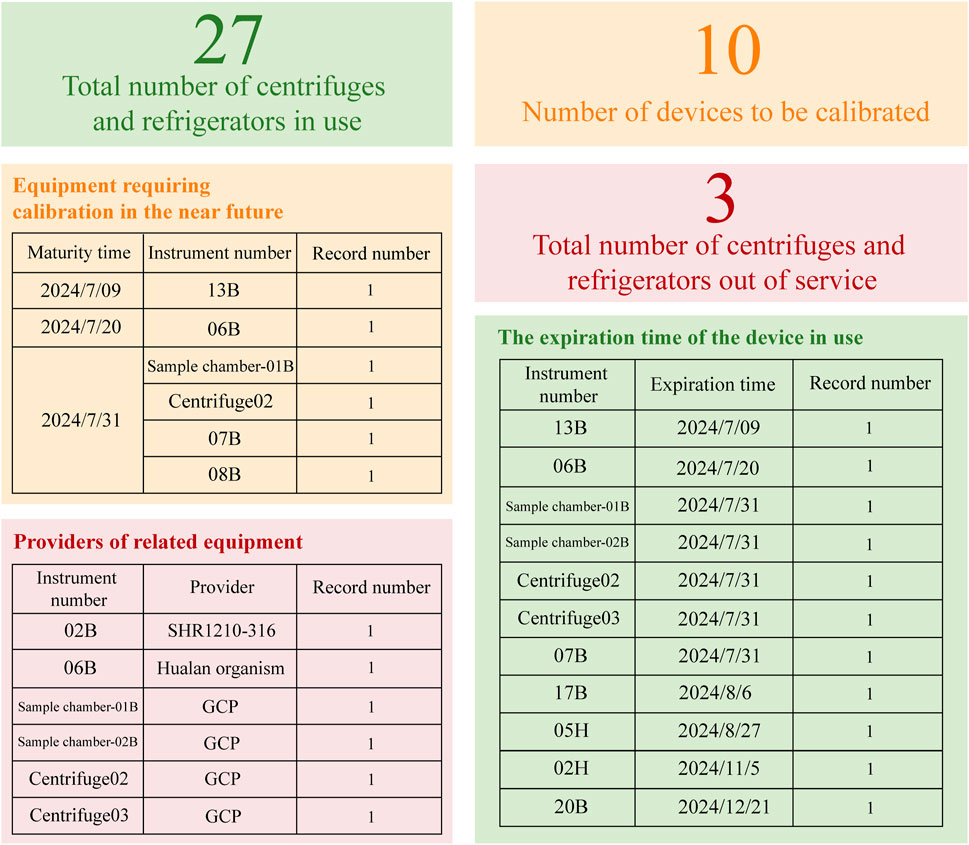
Figure 4. Dynamic Inventory Table for Refrigerators and Centrifuges. Based on the WeChat Work platform, this dynamic inventory table provides a clear overview of the equipment status, categorizing items into active, inactive, or those requiring calibration. It also details the calibration due dates for each item and includes supplier information related to specific equipment.
By developing and refining diverse dynamic charts and dashboards, enhanced management of drug and thermometer expiration dates, as well as calibration validity for related equipment, can be achieved. This approach ensures that clinical trial drugs are consistently maintained within appropriate storage conditions, thereby supporting rigorous drug management standards. These measures are designed to prevent the distribution of expired medications to participants, safeguarding their safety and wellbeing.
3.5 Systematic drug double-check system
The pharmacy at the Center has adopted a dual verification system for a closed-loop management process covering drug reception, storage, distribution, return, and recovery. Two administrators and a CRC or CRA verify all incoming drugs’ documentation, temperatures, calibration certificates, and quality reports, ensuring they match delivery and application data. Daily temperature checks and monthly inventory reconciliations are conducted to confirm inventory accuracy against both paper and digital records.
For drug distribution, two administrators verify prescriptions and, if necessary, randomization orders before dispensing medication, both signing to minimize errors. Some procedures require photographic evidence for comprehensive project team review. Unused drugs and packaging are returned by CRCs to administrators for inventory checks and recorded details (Nicholson, 2023).
Expired drugs, returned unused drugs, and used packaging are systematically recorded and verified by CRAs and administrators. The CRA also files a recovery application, uploading electronic documents and archiving originals securely. This dual-core system ensures a fully compliant, closed-loop process, minimizing error risks.
3.6 Sharing of medication files
The Center Pharmacy’s network platform facilitates secure, encrypted file exchanges with CRCs and CRAs, enhancing efficiency. It shares calibration certificates and monthly temperature records for warehouse equipment, alerts CRAs to expiring drugs via a dynamic table, and provides device-specific information for calibration and recycling. Electronic inventory ledgers grant CRCs access to monitor drug levels and flag expiring items. A mind map of the warehouse process is shared to ensure clear protocol understanding, reduce miscommunication, and boost workflow efficiency.
4 Outcomes
Centralized drug management has reduced the error rate in management. In the baseline phase, the most common type of error was incorrect dosing time, with an error rate of 17.6 per 100 doses. After the intervention, the error rate for incorrect dosing time significantly decreased from 17.6 to 9.8 (Table 3). Protocol deviations are mainly classified into five levels: Level 1 has no impact on data quality or subject safety; Level 2 has a minor impact on data quality; Level 3 has a minor impact on subject safety; Level 4 has a significant impact on data quality or subject safety; Level 5 results in subject death. Protocol deviations are common in clinical trials and need to be reduced through strict drug management and monitoring. The classification and handling measures of deviations are crucial for ensuring the scientific nature of the trial and the safety of subjects. According to the research, the severity of deviations is significant for guiding the handling and prevention of deviations (Table 4).
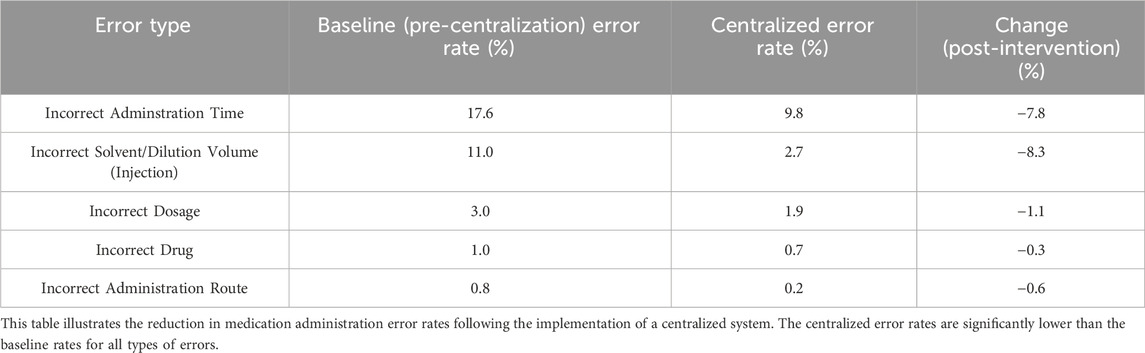
Table 3. Comparison of Error Rates Before and After the Implementation of Centralized Clinical Drug Management.
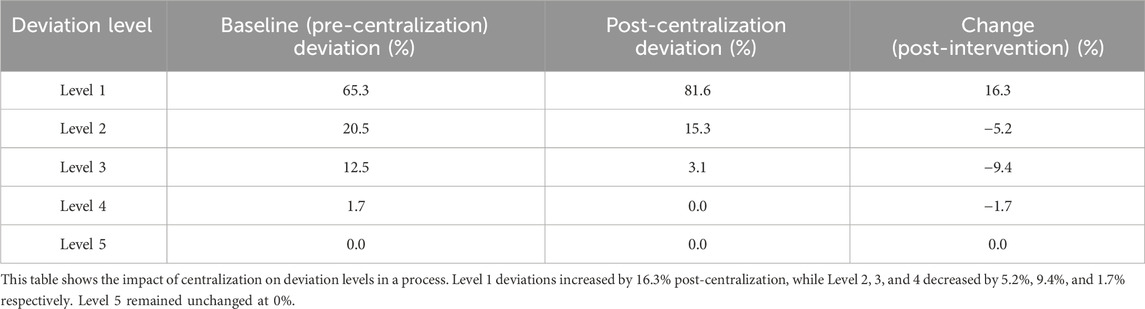
Table 4. Comparison of Protocol Deviations Before and After the Implementation of Centralized Clinical Drug Management.
5 Limitations
The centralized drug management system exhibits certain limitations, given the late establishment of the dedicated team and ongoing adaptations to the standards set forth by the National Bureau of Drug Clinical Trial Institutions Supervision and Inspection Points and Judgment Principles (Trial). Firstly, some members may lack sufficient experience in clinical trials. This could lead to errors in trial design, execution, and management. To mitigate this potential risk, our institution regularly organizes standardized clinical trial training sessions. After the training, participants are assessed, and we also arrange for team members to visit and exchange experiences with more established teams.
Secondly, with limited clinical project resources, our newly established team may face constraints in terms of funding, equipment, and human resources, which could affect the quality and speed of trials. In this regard, our hospital actively recruits advanced medical teams from home and abroad to provide technical support for clinical project recruitment and implementation. In terms of publicity, we collaborate with higher-level management departments and lower-level community hospitals to popularize clinical trial projects among the public. This helps them gain a basic understanding of these projects and provides a pool of potential participants for future clinical trials.
Thirdly, in terms of regulatory compliance, new teams may face the challenge of quickly adapting to and complying with the constantly changing clinical trial regulations and guidelines. This requires team members to keep up with the times, continuously track the latest international information on clinical trial projects, and integrate it with domestic regulations and guidelines to form a theoretical system suitable for our hospital’s institution.
Lastly, regarding technological adaptability, new institutions may need time to adapt to and adopt the latest clinical trial technologies and methods. This demands that we stay at the forefront of international technology. For example, by employing artificial intelligence and machine learning, AI technology can be utilized in clinical trial data management for data collection and integration, data cleaning and standardization, data monitoring and quality control, as well as data analysis and prediction. I believe that these limitations and the lessons learned from them can make our drug clinical projects more standardized and efficient.
6 Discussion and conclusion
Since November 2020, the center has managed 224 clinical trial projects (Figure 5), with 96 currently in active research stages requiring ongoing liaison with drug regulatory authorities. Among these, 49 projects constitute the primary workload. Specialized trials, including oncology and pediatric endocrinology, are characterized by extended timelines, complex treatment protocols, and elevated incidences of adverse and serious adverse events. The center’s drug storage facility maintains a substantial inventory, necessitating regular calibration and upkeep of numerous devices, alongside extensive management of both handwritten and electronic records. Beyond documentation duties, the drug administrator oversees meticulous logging and maintenance of equipment—such as thermometers and refrigerators—including details on acquisition, servicing, calibration, and eventual decommissioning, while coordinating with professionals for on-site calibration as needed. Daily temperature monitoring is recorded on controlled temperature sheets, with all data exported, securely encrypted, and uploaded to the platform monthly for secure sharing. The two drug administrators further manage daily operations encompassing drug receipt, maintenance, inventory control, distribution, and recycling.
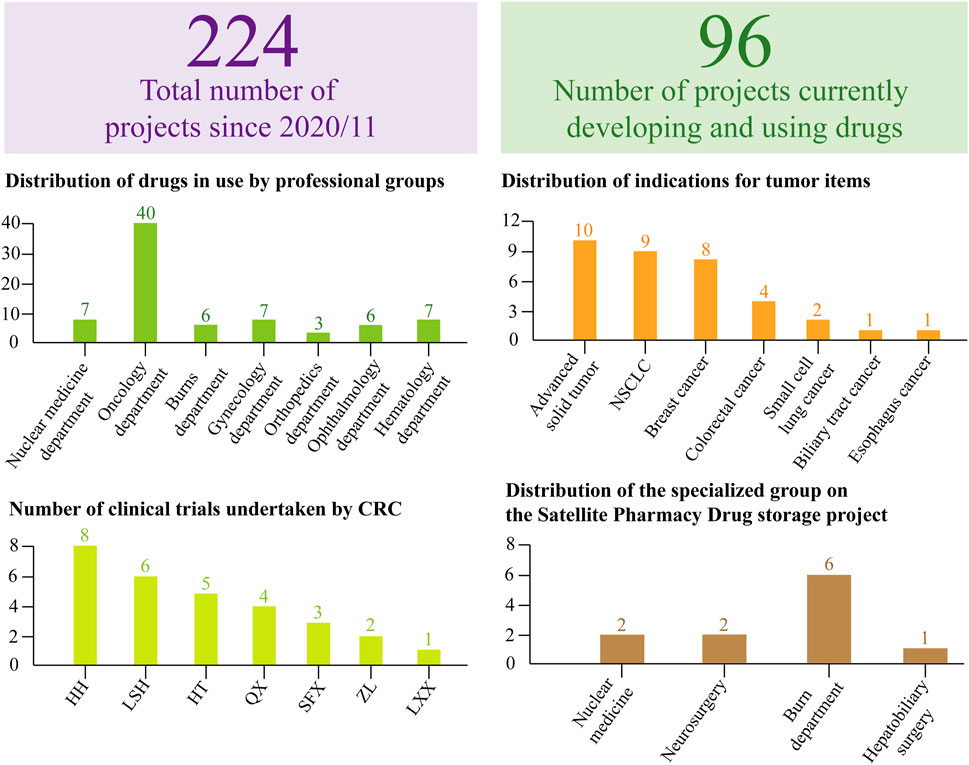
Figure 5. Dynamic Inventory Table for Clinical Trial Projects. Based on the WeChat Work platform and Service Cloud Platform, the dynamic project table provides a structured overview of total projects and those actively in development. For projects currently under investigation, statistical analysis includes the distribution across hospital professional groups, specific indications per project, and assigned personnel managing both the projects and corresponding sample processing. This streamlined approach to data and process management supports the efficient completion of tasks, ensuring both quality and quantity.
In terms of the costs associated with the construction of a centralized pharmacy, initially, new employees require training on standard operating procedures and other protocols. This involves expenses for training materials, venue rentals, and instructor fees. To attract and retain high-quality talent, a competitive compensation system must be in place to ensure team stability and professionalism. Additionally, there are costs for purchasing hardware such as servers, network equipment, and security devices. Factors such as brand, performance, and capacity of the equipment need to be considered. During the use of these devices, regular maintenance and potential replacement due to technological advancements are necessary, incurring additional costs. Furthermore, the purchase of commercial software, including operating systems and database software, entails software licensing fees and customization costs.
Regarding the benefits of a centralized pharmacy, professional operation and maintenance by personnel, along with robust equipment protection, help prevent security incidents such as data breaches, tampering, and loss. This reduces the risk of system crashes due to vulnerabilities or equipment malfunctions, thereby maintaining the normal operational order of the pharmacy. Workflow automation through software tools enhances work efficiency. Moreover, coordinated management of personnel, equipment, and software leads to efficient resource utilization (Table 5).
Since November 2020, the clinical trial center has been subjected to five verifications by the National Bureau, with no corrective actions reported for drug or sample management. This outcome directly reflects the National Bureau’s acknowledgment of the Center’s standards in these areas. In practice, the centralized management model has significantly improved drug administration efficiency, minimized protocol deviations, and strengthened participant drug safety.
While centralized drug management provides considerable benefits, satellite pharmacies remain integral within the four specialized groups due to unique protocol demands, including requirements for intraoperative or emergency drug administration for specific projects (Song and Kim, 2020). However, management of thermometers, refrigerators, drug expiration management, as well as drug receipt and retrieval processes in these satellite pharmacies, is collaboratively maintained by the Professional Group and the central pharmacy’s drug manager.
Looking to the future, the development direction of clinical centralized pharmacies will shift towards the integration of AI and EHR (Kabella et al., 2025; Shermock et al., 2023). By integrating the centralized pharmacy system with EHR, comprehensive sharing and integration of patient medical information can be achieved. This allows for a more thorough understanding of patients’ conditions and medication use, enabling the provision of more precise pharmaceutical services. Based on the integrated EHR data, AI can offer pharmacists more accurate decision support for medication use. For instance, by analyzing patients’ medical history, genetic information, and current conditions, AI can predict their reactions to certain medications, assisting pharmacists in selecting the most appropriate drugs and dosages to improve treatment outcomes.
Author contributions
YT: Writing – original draft, Writing – review and editing. XF: Funding acquisition, Writing – review and editing, Formal Analysis, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We express our gratitude to the Affiliated Hospital of Jiangnan University for their financial support.
Acknowledgments
We express our gratitude to Bullet Edits Limited for their linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Administration, SFAD (2004). Good quality management for drug clinical trials. Chin. J. New Med. Clin. Med. 23 (1), S1–S5.
Boesen, K., Gøtzsche, P. C., and Ioannidis, J. P. A. (2021). EMA and FDA psychiatric drug trial guidelines: assessment of guideline development and trial design recommendations. Epidemiol. Psychiatr. Sci. 30, e35. doi:10.1017/S2045796021000147
Brodie, F., Nguyen, T., and Eydelman, M. (2019). US Food and drug administration regulatory programs for innovative technologies. JAMA Ophthalmol. 137 (12), 1349–1350. doi:10.1001/jamaophthalmol.2019.3708
Calabrese, E. J., Pressman, P., Hayes, A. W., Dhawan, G., Kapoor, R., Calabrese, V., et al. (2023). Hormesis, biological plasticity, and implications for clinical trial research. Ageing Res. Rev. 90 (Sep), 102028. doi:10.1016/j.arr.2023.102028
Cao, N. Q., Marcus, A., Altarawneh, L., and Kwon, S. (2023). Priority-based replenishment policy for robotic dispensing in central fill pharmacy systems: a simulation-based study. Health Care Manag. Sci. 26 (2), 344–362. doi:10.1007/s10729-023-09630-x
Cao, Y., Liao, L., Liu, X., Zheng, Q., Xu, Z., and Niu, H. (2022). Trend of drug clinical trials in mainland China from 2009 to 2020. Curr. Med. Res. Opin. 38 (9), 1499–1507. doi:10.1080/03007995.2022.2103960
Chiodin, D., Cox, E. M., Edmund, A. V., Kratz, E., and Lockwood, S. H. (2019). Regulatory affairs 101: introduction to investigational new drug applications and clinical trial applications. Clin. Transl. Sci. 12 (4), 334–342. Epub 2019 Apr 12. doi:10.1111/cts.12635
Dagenais, S., Russo, L., Madsen, A., Webster, J., and Becnel, L. (2022). Use of real-world evidence to drive drug development strategy and inform clinical trial design. Clin. Pharmacol. Ther. 111 (1), 77–89. doi:10.1002/cpt.2480
Fan, J., Liu, X., Li, Y., Xia, H. S., Yang, R., Li, J., et al. (2022). Quality problems of clinical trials in China: evidence from quality related studies. Trials 23 (1), 343. doi:10.1186/s13063-022-06281-1
Feng, H. P., Wang, Z. R., and Zheng, X. M. (2020). Analysis of problems and countermeasures in the management of drugs used in clinical trials. China Med. Guide 18 (34), 33–35. doi:10.12677/ajmp.2022.113049
Gulden, C., Kirchner, M., Schuttler, C., Hinderer, M., Kampf, M., Prokosch, H. U., et al. (2019). Extractive summarization of clinical trial descriptions. Int. J. Med. Inf. 129 (Sep), 114–121. doi:10.1016/j.ijmedinf.2019.05.019
Guo, W., Xie, L. L., and Cao, L. Y. (2019). The influence and thinking of joining ICH on the work of China's drug clinical trial institutions. Chin. Pharm. 30 (11), 1445–1448.
Jones, E. F., Buatti, J. M., Shu, H. K., Wahl, R. L., Kurland, B. F., Linden, H. M., et al. (2020). Clinical trial design and development work group within the quantitative imaging network. Tomography 6 (2), 60–64. doi:10.18383/j.tom.2019.00022
Kabella, D., Apollonio, D., Young, H., and Knight, K. R. (2025). The rise of clinical decision support algorithms in pain management 2009-2024. J. Gen. Intern Med. doi:10.1007/s11606-025-09600-9
Lagreula, J., Maes, F., Wouters, D., Quennery, S., and Dalleur, O. (2021). Optimizing pharmacists' detection of prescribing errors: Comparison of on-ward and central pharmacy services. J. Clin. Pharm. Ther. 46 (3), 738–743. doi:10.1111/jcpt.13339
Lin, L. F., Chen, Y. R., Yan, L., Liu, Y., Ni, J., Yang, H., et al. (2020). Analysis of clinical trials of new drugs in China as of 2019. Drug Discov. Today 25 (12), 2080–2088. doi:10.1016/j.drudis.2020.09.030
Liu, S., Luo, P., Tang, M. M., Hu, Q., Polidoro, J. P., Sun, S., et al. (2020). Providing pharmacy services during the coronavirus pandemic. Int. J. Clin. Pharm. 42 (2), 299–304. doi:10.1007/s11096-020-01017-0
Magnuson, A., Bruinooge, S. S., Singh, H., Wilner, K. D., Jalal, S., Lichtman, S. M., et al. (2021). Modernizing clinical trial eligibility criteria: recommendations of the ASCO-friends of cancer research performance status work group. Clin. Cancer Res. 27 (9), 2424–2429. doi:10.1158/1078-0432.CCR-20-3868
Miyauchi, E., Morita, S., Nakamura, A., Hosomi, Y., Watanabe, K., Ikeda, S., et al. (2022). Updated analysis of NEJ009: gefitinib-alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated EGFR. J. Clin. Oncol. 40 (31), 3587–3592. doi:10.1200/JCO.21.02911
Mohammad, A. K., Kunwar, S. V., Sarebjeet, K. S., Singh, S., Wilhite, A., Dasgupta, S., et al. (2021). Platinum-resistant ovarian cancer: from drug resistance mechanisms to liquid biopsy-based biomarkers for disease management. Semin. Cancer Biol. 77 (Dec), 99–109. doi:10.1016/j.semcancer.2021.08.005
Myles, P. S. (2022). Future of clinical trial methodology. Anesth. Analg. 134 (4), 668–673. doi:10.1213/ANE.0000000000005818
National Medical Products Administration (2020a). Drug administration law of the people's Republic of China (order of the president No. 31). China Democratic Legal Publishing House.
National Medical Products Administration (2020b). Good practice for the quality management of drug clinical trials (No. 57 of 2020). Natl. Health Comm.
Nicholson, W. (2023). Clinical trial reporting and representation-an opportunity to advance Health equity. JAMA Surg. 158 (2), 190–191. doi:10.1001/jamasurg.2022.6601
Omidian, H., and Omidi, Y. (2022). Blockchain in pharmaceutical life cycle management. Drug Discov. Today 27 (4), 935–938. doi:10.1016/j.drudis.2022.01.018
Sessler, D. I., and Myles, P. S. (2020). Novel clinical trial designs to improve the efficiency of research. Anesthesiology 132 (1), 69–81. doi:10.1097/ALN.0000000000002989
Shermock, S. B., Shermock, K. M., and Schepel, L. L. (2023). Closed-loop medication management with an electronic Health record system in U.S. And Finnish hospitals. Int. J. Environ. Res. Public Health 20 (17), 6680. doi:10.3390/ijerph20176680
Song, S. Y., and Kim, E. Y. (2020). The clinical trial transparency in oncology significantly increased over the recent years. J. Clin. Epidemiol. 119, 100–108. doi:10.1016/j.jclinepi.2019.11.018
Spira, A., Stewart, M. D., Jones, S., Chang, E., Fielding, A., Richie, N., et al. (2021). Modernizing clinical trial eligibility criteria: recommendations of the ASCO-friends of cancer research laboratory reference ranges and testing intervals work group. Clin. Cancer Res. 27 (9), 2416–2423. doi:10.1158/1078-0432.CCR-20-3853
Tang, W. F., Huang, Y., Zhou, D. S., Chen, Y., Ren, S., Li, Y., et al. (2021). Evolving drug regulatory landscape in China: a clinical pharmacology perspective. Clin. Transl. Sci. 14 (4), 1222–1230. doi:10.1111/cts.12987
Thall, P. F., Zang, Y., Chapple, A. G., Yuan, Y., Lin, R., Marin, D., et al. (2023). Novel clinical trial designs with dose optimization to improve long-term outcomes. Clin. Cancer Res. 29 (22), 4549–4554. doi:10.1158/1078-0432.CCR-23-2222
Thomas, D., Zachariah, S., and Baker, D. (2020). Decentralize-change-recentralize model of drug information networks in Health centers: decentralized drug information services. Innov. Pharm. 11 (1), 19. doi:10.24926/iip.v11i1.3032
Wei, J. S., Guo, Y. L., Wang, Y., Wu, Z., Bo, J., Zhang, B., et al. (2021). Clinical development of CAR T cell therapy in China: 2020 update. Cell Mol. Immunol. 18 (4), 792–804. doi:10.1038/s41423-020-00555-x
Zhang, L., Huang, Y. M., Huang, X. X., Liu, K., Yu, Y., Sun, S., et al. (2020). Ambulatory care pharmacy practice in China: status and future efforts. Int. J. Clin. Pharm. 42 (2), 321–325. doi:10.1007/s11096-020-00998-2
Keywords: clinical trials, drug management, implementation plan, standardization, centralized management mode
Citation: Tang Y and Fang X (2025) Centralized daily drug management in clinical trial institutions and specific implementation plans. Front. Drug Saf. Regul. 5:1620314. doi: 10.3389/fdsfr.2025.1620314
Received: 29 April 2025; Accepted: 09 June 2025;
Published: 20 June 2025.
Edited by:
Jeff Guo, University of Cincinnati, United StatesReviewed by:
Wenmin Du, Nanjing Medical University, ChinaLusi Agus Setiani, Pakuan University, Indonesia
Copyright © 2025 Tang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolong Fang, ODEzNDM5NzNAcXEuY29t
 Yu Tang
Yu Tang Xiaolong Fang3*
Xiaolong Fang3*