- 1Instituto de Geocronología y Geología Isotópica (CONICET-UBA), Consejo Nacional de Investigaciones Científicas y Técnicas, Ciudad de Buenos Aires, Argentina
- 2Instituto de Arqueología (FFyL, UBA), Consejo Nacional de Investigaciones Científicas y Técnicas, Ciudad de Buenos Aires, Argentina
- 3Department of Coevolution of Land Use and Urbanisation, Max Planck Institute of Geoanthropology, Jena, Germany
Pre-Hispanic pastoralist mobility has been a major topic in Andean archaeology and it is considered a key component of modern pastoral systems in the Andean highlands. Of particular interest here has been the exploration of changes in camelid breeding and herding between pre-Hispanic and modern pastoralist contexts. This paper examines variation in diet and territoriality of domestic camelids using intra-tooth enamel carbon and oxygen stable isotope measurements from modern and archaeological llama specimens from the Dry Puna of Argentina. We explore whether dietary and territoriality changes linked to seasonal mobility of modern llama herds are reflected in intra-tooth isotopic variation, and thus establish a modern frame of reference to study Late Holocene pastoralist mobility in the Puna highlands. Our preliminary results show the existence of moderate intra-tooth isotopic variation for enamel δ13CV − PDB and δ18OV − PDB values. Seasonal changes in plants and water consumed throughout the year dictated by the alternation of different pasture areas do not translate into significant isotopic variation for the modern camelid specimens analyzed. Moreover, intra-tooth series of archaeological camelid specimens show a similar patterning. This exposes the limitations of using carbon and oxygen stable isotope compositions measured in sequentially sampled camelid teeth to identify pastoralist mobility patterns in the tropical highlands of the Andes. Nonetheless, the consistency of our results shows continuities between pre-Hispanic and modern pastoralist practices in the Dry Puna of Argentina.
1 Introduction
South American camelids were domesticated in the Andes ca. 7,000 cal. years BP, a process that led to the appearance of two new domestic species: the llama (Lama glama) and the alpaca (Vicugna pacos) (Yacobaccio and Vilá, 2016). Indigenous herding practices—mostly aimed at obtaining meat and fiber—developed in different ecozones of the Andean region (Moore, 2016). Moreover, South American camelid domestication fostered the development of extended exchange networks among different Andean areas based on the use of llama caravans (Nielsen, 2006). The scale and characteristics of the mobility patterns of domestic South American camelids remain a pressing topic in archaeology, whether they comprise short-or-medium-distance mobility among vegetation patches as part of pastoralist practices or long-distance mobility within caravan exchange (Berenguer, 2004; Capriles, 2014; Mader et al., 2022; among others). Previous research carried out by archaeologists, ethnohistorians, and anthropologists mainly focused on studying the dynamics of llama caravan mobility and long-distance exchange in the Andes, overlooking the study of seasonal mobility and its relevance within the pastoral system, with only a few noteworthy exceptions in the South-Central Andes (Gil Montero, 1997; Göbel, 2001; Yacobaccio, 2007).
Stable isotope analyses of animal tissues can be used to investigate how herders managed domestic animals in the past and address issues such as mobility and foddering, which usually escape the scope and reach of traditional zooarchaeology (Balasse et al., 2002; Britton et al., 2008; Gillis et al., 2021; Lightfoot et al., 2020; Stevens et al., 2013, among others). Modern animal isotopic data can serve as a frame of reference to explore variation in isotopic values of archaeofaunal remains as well as provide important baseline isotope ecology information (Janzen et al., 2020; Makarewicz and Tuross, 2006; Thornton et al., 2011; among others). During the last two decades, stable isotope analyses have been increasingly applied to study the exploitation and management of South American camelids in the Andes (Eerkens et al., 2024; Finucane et al., 2006; Izeta et al., 2009; Knudson et al., 2012; López-Mendoza et al., 2022; Mengoni Goñalons, 2007; Mondini et al., 2019; Szpak et al., 2016; among many others). Nonetheless, most of these analyses have been performed on bulk samples —mostly bone collagen—, neglecting the information that could be obtained by performing sequential analyses on incremental tissues with fixed formation timelines, such as teeth or hair, with some exceptions (Alaica et al., 2022; Dufour et al., 2014; Santana-Sagredo et al., 2020; Szpak et al., 2014; Takigami et al., 2020). Isotopic analyses of sequentially sampled tissues have developed as a useful method to address the management of animal populations, especially when it comes to discussing seasonal mobility and dietary changes of domestic populations (Balasse et al., 2002; Makarewicz, 2017). Transhumant vertical mobility of domestic animals has been studied by tooth enamel sequential analyses of carbon and oxygen stable isotopes in several places around the globe, such as the Rift Valley (Balasse and Ambrose, 2005; Janzen et al., 2020), Anatolia (Henton et al., 2010; Makarewicz et al., 2017), the Lesser Caucasus (Chazin et al., 2019; Hirose et al., 2021; Tornero et al., 2016) and the eastern Iberian Peninsula (Alcàntara Fors et al., 2025; Messana et al., 2023; Valenzuela-Lamas et al., 2016).
Previous studies have addressed altitudinal mobility and grazing locations of South American camelids in the Andes exploring intra-tooth isotopic variation (Alaica et al., 2022; Dufour et al., 2014; Goepfert et al., 2013; Takigami et al., 2020). Nevertheless, such research is still quite rare and has thus far been mostly carried out in the Central Andes, with only one example for Argentina to date (Samec and Harrod, 2022). In this article, we present stable carbon and oxygen isotope measurements (δ13CV − PDB and δ18OV − PDB values) of llama tooth enamel to study dietary and territoriality changes linked to seasonal pastoralist mobility in the Dry Puna of Argentina. While intra-tooth isotopic variation in mammal teeth can track changes in dietary behavior and mobility patterns linked to human intervention on a seasonal scale (Lazzerini et al., 2021; Makarewicz et al., 2017; Tornero et al., 2018), the relationship between pastoralist mobility and seasonality in the Andean highlands, and their putative manifestation in intra-tooth isotopic variation in South American camelids, remains poorly understood. In many areas of the Andes, seasonal pastoralist mobility has been stressed as an important strategy that involves moving llama herds among different vegetation patches along an altitudinal gradient (Browman, 1974; Westreicher et al., 2007; Yacobaccio et al., 1998), potentially leading to seasonal changes in dietary and water resources used throughout the annual cycle (Dufour et al., 2014; Szpak et al., 2014).
Here, we analyse stable isotope compositions of modern and archaeological llama teeth following a sequential sampling procedure perpendicular to the growth axes of molars and premolars to understand how diet and territoriality of domestic South American camelids varied in a diachronic perspective. The information obtained through the analysis of teeth of modern llama specimens with known life-histories is used as a comparative dataset for the interpretation of the stable isotope compositions of llama tooth specimens recovered in Chayal Cave, a pastoralist archaeological site dated to the Late Holocene (Yacobaccio et al., 1997-1998). This site is an interesting case study due to its chronology, which places the human occupation of the cave at the cusp of colonial domination arriving in the region (Sica, 2016). We explore the temporal depth of current pastoralist practices when comparing a pre-Hispanic context with a modern one, the latter being largely affected by ongoing processes such as space delimitation by centralized authorities, household participation in a market economy, and young workers migration to large cities, just to name a few (Göbel, 2001; Tomasi, 2013). Previous investigations carried out in the Dry Puna of Argentina have studied domestic camelid management for the last 2,500 years using both zooarchaeology and bulk stable isotope analyses, leaving questions about the extent of the past pastoralist mobility ranges largely unanswered (Samec et al., 2020; Samec and Yacobaccio, 2021; Yacobaccio et al., 2011). This present study addresses these questions and discusses dietary and territoriality variations in llama herding for the past 1,000 years in the Dry Puna of Argentina, exploring continuities and discontinuities between pre-Hispanic and modern pastoralist systems and examining the plasticity of pastoralism when facing climate and social changes (Arzamendia et al., 2021; Göbel, 2001; Yacobaccio, 2014).
2 The study area
The Puna of Argentina is located between 22° and 27° S and between 3,000 and 5,000 masl. The region is a highland desert that contains several NE-SW oriented mountain ranges (Figure 1). It is characterized by high solar radiation and low atmospheric pressure due to altitude. The only sources of freshwater are a few rivers and springs scattered throughout the landscape. The climate in this area is complex, mainly driven by atmospheric circulation and topography. Precipitation is governed by the South American monsoon system and occurs mainly during the austral summer, i.e., between December and February (Zhou and Lau, 1998; Vuille and Keimig, 2004). Precipitation also exhibits a latitudinal gradient that delimits two sub-regions within the Puna of Argentina: the Dry Puna, located north of 24°S, with a mean annual precipitation of 300 mm/year; and the Salt Puna, located south of 24°S, with a mean annual precipitation below 100 mm/year (Bianchi et al., 2005).
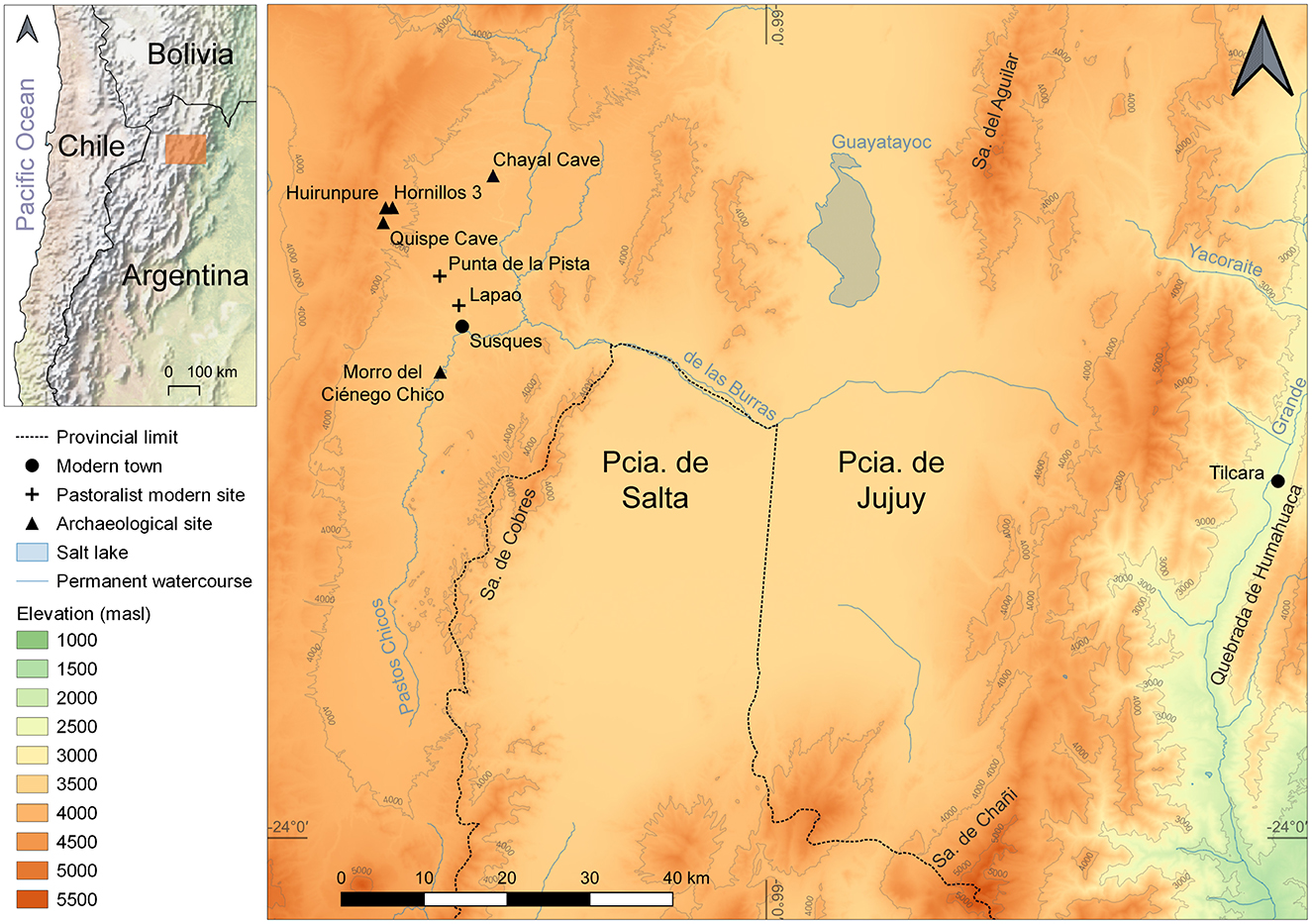
Figure 1. Map of the study area showing the location of the sites mentioned throughout the text. The image was created using QGIS and standardized sheets available online at: https://www.ign.gob.ar/NuestrasActividades/InformacionGeoespacial/CapasSIG.
The key plant communities identified in the dry Puna show an altitudinal arrangement (Braun Wilke et al., 1999; Cabrera, 1976; Ruthsatz and Movia, 1975). The shrub steppe is located between 3,500 and 3,900 masl. It is dominated by shrubs such as Parastrephia lepidophylla and Fabiana densa and exhibits a low relative proportion of herbs and grasses. It includes C3 shrubs and C3 grasses, as well as some C4 grasses. The mixed steppe is located between 3,900 and 4,100 masl. It represents an ecotonal landscape and is composed of both C3 grasses and C3 shrubs, with only a few C4 grass species. Finally, the grass steppe is located between 4,100 and 4,700 masl. It is dominated by Festuca spp. and other grasses, like Stipa spp. and Poa spp. which are mostly C3 species. Aside from this altitudinal arrangement, wetlands —locally called vegas— are scattered across the landscape between 3,500 and 4,700 masl. These are restricted patches with abundant plant cover throughout the year, composed mainly of hygrophilous C3 and C4 grasses such as Deyeuxia spp. and Muhlenbergia spp., and with a clear overall predominance of C3 species (Samec et al., 2017b).
3 Pastoralism in the Puna highlands from a diachronic perspective
South American camelids are the only large herd mammals that were domesticated in the Americas and have been an important asset for both ancient and modern Andean societies (Moore, 2016; Nielsen, 2024; Vilá and Arzamendia, 2022; among others). Investigations carried out in different areas located to the South of the Central Andes have provided substantial evidence that supports the occurrence of a local llama domestication process in the Puna of Atacama (Yacobaccio and Vilá, 2016). Archaeological sites located in the Puna highlands of Chile and Argentina show the appearance of llama-size individuals as early as 7,000 cal. years BP (Samec et al., 2014b; Yacobaccio et al., 2013), the presence of bone pathologies linked to captivity around 4,800–3,100 cal. years BP (Cartajena et al., 2007; Yacobaccio et al., 2018), and the existence of corrals and pens dated as early as 4,500 cal. years BP (Aschero and Yacobaccio, 1998-1999; Cartajena et al., 2007) (for details on the camelid domestication process see Yacobaccio, 2001, 2021). In the Dry Puna of Argentina, llama herding practices are attested as early as 2,700 cal. years BP by the large quantity of llama-size bones recovered at the sites of Huirunpure and Quispe Cave, as well as the presence of artifacts made from llama fiber within the burial of Morro del Ciénego Chico (Yacobaccio et al., 1997-1998, 2011). Around 1,000 cal. years BP, herding became the predominant subsistence strategy in the area, as inferred from the materials recovered at the archaeological sites of Chayal Cave and Hornillos 3 (Yacobaccio et al., 2011). From this point on, pastoralist practices enabled a diversified use of llama herds to obtain both primary and secondary products, as well as the development of long-distance exchange networks using large size llamas as pack-animals within the practice known as “caravaneo” (Capriles and Tripcevich, 2016).
Today, traditional llama breeding in the Dry Puna of Argentina is mostly oriented toward the production of fiber and meat (Yacobaccio, 2007). The herds are fed on natural pastures and mobility is practiced to avoid overgrazing the pasturelands managed by each household (Göbel, 2001). Within our study area, near the modern town of Susques, ethnoarchaeological studies show that the diet of the herds is determined by a complex and alternate use of different plant communities located at different altitudinal ranges within the territory of each household (Yacobaccio, 2007). Shrub steppes and vegas located below ~3,900 masl are used as summer pastures while mixed steppes and grass steppes located above ~3,900 masl are used as winter pastures (Yacobaccio et al., 1998). This use of the pasturelands during the annual cycle determines the access to shrub, grass, and herbaceous vegetation to be included in the diet of the herds. Given this mobility strategy, the settlement pattern is dispersed over the landscape, comprising sites that are used differentially throughout the year (Yacobaccio et al., 1998). Two types of settlements can be distinguished: residential bases and temporary sites. Residential bases are usually located near permanent water sources, such as rivers or vegas, and constitute complex structures with several rooms for different purposes (Yacobaccio et al., 1998). In the Susques area, the herders and their animals stay at the residential base for about eight months a year, making use of the pastures that grow in the vicinity of the settlement. In contrast, a part of the family group and their animals occupy temporary sites for two to three months during the winter, herding their llamas at the different plant communities located near these sites. These temporary sites are smaller, usually composed of a single room and a corral made of stone or shrub walls (Yacobaccio et al., 1998). Overall, the herd's seasonal mobility pattern is a vital component of the system, designed to avoid overgrazing productive patches and granting access to different resources for the llamas all year round.
4 Pastoralism in the Puna highlands from an isotopic perspective
Stable carbon isotope measurements of tooth enamel and other animal tissues can be employed to estimate the proportions of different types of plants in herbivore diets (Balasse, 2002; Cerling and Harris, 1999; Murphy et al., 2007). C3 plants (trees, shrubs, herbs, and temperate grasses), C4 plants (tropical grasses), and CAM plants (cacti and tropical epiphytes) exhibit different carbon fixation pathways leading to distinct δ13CV − PDB values (Ehleringer and Cerling, 2002; O'Leary, 1988; Vogel, 1993). Environmental conditions, such as temperature, water availability, irradiance, and atmospheric pressure, can influence the distribution of C3, C4, and CAM plants and their mean δ13CV − PDB values (Codron et al., 2005; Heaton, 1999; Tieszen, 1991). Research performed in the Andes has found that the distribution of C3 and C4 plants is influenced by altitudinal variation, according to its effects on temperature, precipitation, and irradiance (Cavagnaro, 1988; Panarello and Fernández, 2002; Szpak et al., 2013). Within our study area, C3 species are largely predominant, although a previous study revealed that C4 plants are present at lower elevations due to increased temperature and water stress and practically disappear above a certain threshold (~3,900–4,000 masl) where these conditions are no longer found (Samec et al., 2017b).
South American camelids are generalist herbivores with a strong selectivity toward grasses, although they may also feed on shrubs and other resources (Barri et al., 2014; Castellaro et al., 2004; Mosca Torres and Puig, 2010). This remains true for Lama glama, even though the diet of this domesticated species is influenced by human intervention through pastoralist practices. Previous studies showed that the variation in the local distribution of C3 and C4 plants shapes the variation identified in the δ13CV − PDB values measured on bulk bone collagen of modern domestic South American camelids from the Dry Puna, which show a negative correlation with altitude (Fernández and Panarello, 1999-2001; Samec et al., 2018; Yacobaccio et al., 2009). Current alternation of feeding areas within herding practices would be expected to constrain access to different plant communities located at different altitudes (Yacobaccio et al., 1998), although bulk bone collagen δ13CV − PDB values measured on modern llama herds have failed to capture this variability thus far (Samec et al., 2018; Yacobaccio et al., 2009). A previous study analyzed intra-tooth dentine δ13CV − PDB values on modern llamas from the Puna highlands and found relatively low ranges of variation, which could be related to the fact that dentine collagen only reflects the protein component of the diet (Samec and Harrod, 2022). In turn, δ13CV − PDB values measured on herbivore tooth enamel carbonates reflect the carbon isotopic composition of the whole diet during a fixed time-span and may capture seasonal dietary variation when sampled sequentially (Balasse, 2002; Makarewicz and Pederzani, 2017). Based on this information we expect tooth enamel δ13CV − PDB values measured from modern herded animals in the Dry Puna to show some variation linked to the alternation of vegetation patches located below 3,900 masl —with a certain amount of C4 grasses— and vegetation patches located above 3,900 masl —with a negligible presence of C4 grasses— used during the summer and winter months, respectively (Yacobaccio et al., 1998). If this isotopic pattern indeed exists, intra-tooth enamel δ13CV − PDB values would be a useful tool to identify diachronic variations in the proportion of C4 grasses in the diets of domestic South American camelids. This can also provide some clues to interpret sequentially sampled enamel δ13CV − PDB values measured on llama specimens recovered in pastoralist archaeological sites, such as Chayal Cave, to attest seasonal mobility among different grazing patches in the past —although the altitudinal arrangement of plant communities may have shifted due to climate change (Lupo et al., 2018).
Stable oxygen isotope measurements of herbivore tooth enamel reflect the isotopic composition of body water, which largely depends on the isotopic composition of water ingested directly by drinking and indirectly through feeding (Bryant and Froelich, 1995; Kohn et al., 1996; Sponheimer and Lee-Thorp, 1999). Ingested water δ18OV − SMOW values are usually linked to the δ18OV − SMOW values measured on local meteoric waters, which are expected to exhibit a negative correlation with altitude linked to temperature variations, as well as seasonal variation based on mean temperature and rainfall amount (Bowen and Wilkinson, 2002; Dansgaard, 1964; Rozanski et al., 1993). In the Andean tropics, seasonal rainfall variation is expected to cause lower δ18OV − SMOW values in meteoric water during summer as opposed to higher δ18OV − SMOW values during winter based on the amount effect (Gonfiantini et al., 2001; Rozanski and Araguás Araguás, 1995), although how this is registered in our study area remains to be established. Precipitation data obtained in previous studies carried out in nearby areas show lower δ18OV − SMOW values for rainfall occurring during summer (Jan-Feb-Mar) as opposed to the higher values registered in isolated rainfall events occurring during autumn or spring (Fernández and Panarello, 1989–1990; Rohrmann et al., 2014). In turn, Fernández and Panarello (1989–1990) measured rain samples and identified lower δ18OV − SMOW values in high-altitude areas in the North of the Province of Jujuy within the Dry Puna in Argentina. Rohrmann et al. (2014) found a negative correlation between elevation and δ18OV − SMOW values measured on stream water samples collected between 22° and 24° S in our study area. Nonetheless, the authors warn against simplistic models that link temperature-dependent isotope fractionation directly to surface elevation, especially in areas like the South-Central Andes (Rohrmann et al., 2014).
Our research has shown great variability in δ18OV − SMOW values measured in surface waters (vegas, creeks, and shallow pools) collected during just one season (autumn 2014) in the Dry Puna of Argentina (Samec et al., 2017a). In this sense, it is important to mention that δ18OV − SMOW values of surface waters in the Puna highlands do not depend exclusively on rainfall isotopic composition but are also affected by processes such as groundwater input, elevated evaporation, and the influence of climatic phenomena such as El Niño Southern Oscillation (ENSO), whose effects are not yet fully understood (Gat, 1996; Knudson, 2009; Squeo et al., 2006). Overall, the mean δ18OV − SMOW values of surface water bodies and plant leaves locally available for camelid consumption can be significantly impacted by the effects of evaporation and transpiration. In arid and semi-arid environments, still water bodies and plant leaves usually exhibit higher δ18OV − SMOW values than those measured on rainfall (Cernusak et al., 2016; Jasechko et al., 2013). As shown by previous studies, elevated evapotranspiration is closely linked to higher leaf water δ18OV − SMOW values, and C4 grasses usually show higher δ18OV − SMOW values when compared to C3 grasses while growing in low humidity conditions (Barbour, 2007; Helliker and Ehleringer, 2000). These are important aspects to consider when studying non-obligate drinking herbivores, such as llamas, since they obtain large amounts of water from plant leaves and usually exhibit higher δ18OV − PDB values than sympatric obligate drinking mammals (Levin et al., 2006; Sponheimer and Lee-Thorp, 1999).
Previous studies have identified a negative correlation between altitude and δ18OV − PDB values measured on tooth enamel and bone hydroxyapatite of South American camelids in the Central Andes (Dufour et al., 2014; Goepfert et al., 2013). For the Puna highlands of Argentina, Fernández and Panarello (1989–1990) found a correlation between δ18OV − SMOW values measured on meteoric waters and δ18OV − SMOW values measured on blood extracted from domestic and wild camelids. Based on this information, we expect considerable variation for intra-tooth enamel δ18OV − PDB values measured on modern Lama glama specimens. This variation should mainly respond to the seasonal alternation of vegetation patches and water resources located below 3,900 masl—with 18O enriched signals— and above 3,900 masl —with 18O depleted signals. Seasonal variation in rainfall amount will, in turn, affect mean δ18OV − SMOW values of the water sources available as well as mean plant leaf δ18OV − SMOW values in the study area, probably leading to higher δ18OV − SMOW values during the dry season and further complicating the interpretation of intra-tooth isotopic variation (Blumenthal et al., 2017). Overall, we expect tooth enamel δ18OV − PDB values measured in modern llamas to provide some clues on the effects of altitude alternation and seasonal rainfall regime in isotopic compositions. These results can provide a frame of reference to study pre-Hispanic pastoralist mobility through sequential stable isotope analyses of archaeological specimens recovered in pastoralist sites, such as Chayal Cave in the Dry Puna of Argentina. Nonetheless, it is important to consider that precipitation has been extremely variable during the last 3,000 years —especially during the last 1,000 years—, an aspect that has been linked to the increasing occurrence of ENSO (Kanner et al., 2013). Therefore, we must consider that periods of extremely low precipitation can affect the availability of grasses—both C3 and C4 species— used as pastures, as well as contribute to further alteration of mean δ18OV − SMOW values of water sources and plant leaves.
5 The archaeological site of Chayal Cave
Chayal Cave is located in the Chayal ravine at an altitude of 3,700 masl in the Department of Susques, Province of Jujuy, Argentina (Figure 1). The ravine is protected from the wind due to its sharp topography, primarily shaped by a small creek. The cave itself has a total surface area of 20 m2 of which 4.5 m2 have been excavated, revealing five natural layers bearing three occupational levels (layers 2, 3, 4). Two of these occupational levels were radiocarbon dated and their chronology spans from 1,180 to 320 cal. years BP (770–1630 cal. CE) (Yacobaccio et al., 1997-1998) (Table 1). This places the occupation of the cave on the verge of the arrival of colonial institutions linked to the Spanish Crown to the area (Figure 2). The excavations carried out at the site recovered well-preserved bone remains, lithic tools, and undecorated ceramic sherds—some linked to the Yavi style.
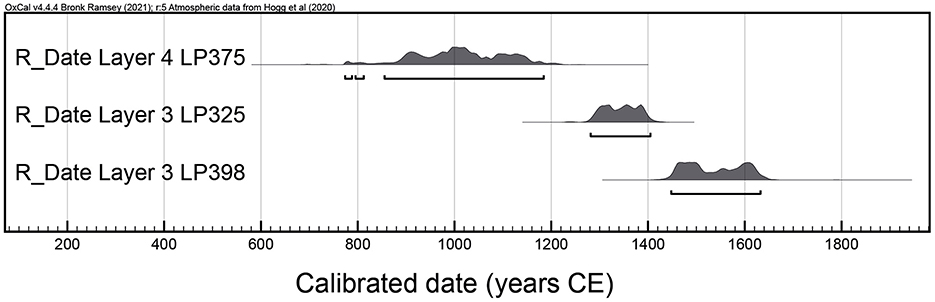
Figure 2. Plot of Chayal Cave's calibrated radiocarbon dates. Calibration was carried out with OxCal.v.4.4.4 (Bronk Ramsey, 2021) using the revised calibration curve for the Southern Hemisphere (Hogg et al., 2020).
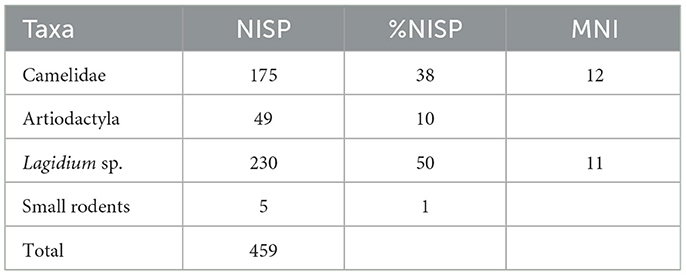
The bone assemblage recovered in layer 3 is very well-preserved and only 4% of the remains present weathering stages above 2 in the scale of Behrensmeyer (1978). Among the animal species present in this layer, viscachas (Lagidium sp.) predominate followed closely by South American camelids, which represent 38% of the total number of identified specimens (Table 2). There is no evidence of density-mediated attrition for the camelid remains since frequency and structural density of the anatomical parts recovered are not correlated (rs = 0.12 p < 0.05), according to the bone density global values published for South American camelids (Elkin, 1995). At the same time, the bones recovered in layer 3 show a low incidence of carnivore and rodent activity, given that only 7.6% of the remains have markings that can be attributed to carnivores and 4.5% to rodents. We estimated individual age-at-deaths for the specimens identified as camelids based on bone fusion stages as well as dental eruption and wear patterns (Yacobaccio et al., 1998). The age profile shows an overall abundance of immature specimens, since 45% of the camelid bones correspond to adult individuals, 33% to newborns, and 21% to juveniles. This age structure is somewhat different from the one registered in modern local pastoralist contexts, which point toward the importance of hunting—as well as herding— during the occupation of the site. Nevertheless, the osteometric analysis performed on the small number of complete and measurable camelid bones present at the site assigned them to the domestic species Lama glama (Yacobaccio et al., 1997-1998). Finally, the skeletal profile for South American camelids shows that the appendicular skeleton is well represented, especially limb parts with high contents of bone marrow (radio-ulnas, metatarsals, metacarpals, phalanges). This points toward the use of the site as a residential base (Yacobaccio et al., 1997-1998).
Previous studies measured bulk bone collagen δ13CV − PDB and δ15NAIR values and bulk bone hydroxyapatite δ13CV − PDB and δ18OV − PDB values on both domestic and wild South American camelids recovered in Chayal Cave (Samec et al., 2020; Samec and Yacobaccio, 2021). These studies concluded that camelid diets showed a high proportion of C3 plants for both wild and domestic species based on low bone collagen and hydroxyapatite δ13CV − PDB values, as well as highly variable bone collagen δ15NAIR values and bone hydroxyapatite δ18OV − PDB values. This investigation aims to explore camelid diets at the site in greater detail by addressing intra-annual variation in diet and territoriality of the domestic camelids herded by the inhabitants of the site using δ13CV − PDB and δ18OV − PDB values measured on sequentially sampled tooth enamel from llama specimens.
6 Materials and methods
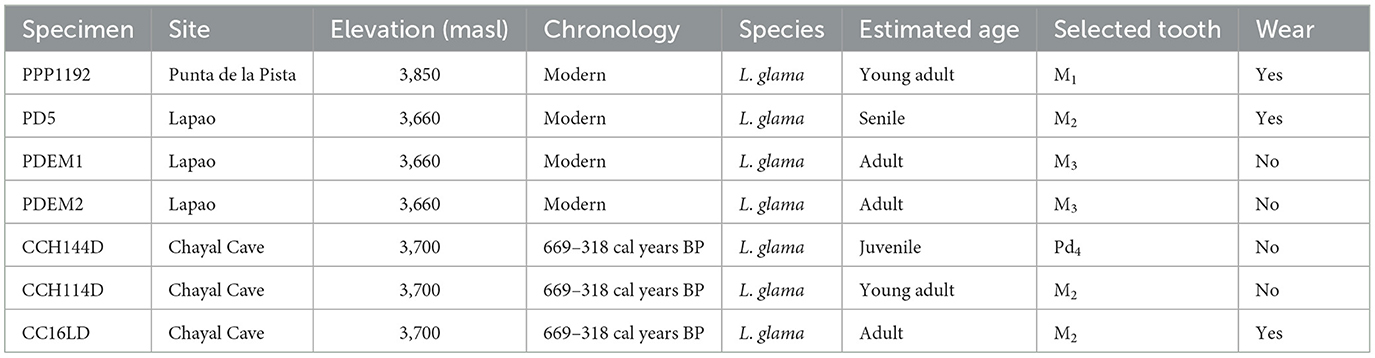
Our study includes two datasets: a modern one and an archaeological one. The modern dataset was collected as part of a previous ethnoarchaeological study (Yacobaccio et al., 1998). It is comprised of Lama glama mandible specimens that belonged to the same herd but were obtained from two different pastoralist sites with different functions: Lapao, a residential base located at 3,660 masl, and Punta de la Pista, a temporary site located at 3,850 masl (Samec et al., 2018). The archaeological dataset is comprised of Lama glama mandible specimens that were recovered from layer 3 within the stratigraphy of Chayal Cave, and their chronology is based on associated dates (Table 1) (Yacobaccio et al., 1997-1998). Overall, this study comprises four modern mandible specimens and three archaeological ones (Table 3) with a total number of 42 sequential intra-tooth enamel samples measured (Supplementary Table 1).
Mandible specimens were selected based on their overall availability and state of preservation and weathering—below or equal to 3 on the scale established by Behrensmeyer (1978). The tooth specimens analyzed here were still in place, strongly attached to the selected mandibles, posing some difficulties for sampling. Overall, the availability of suitable molars and premolars largely affected our sample size, also conditioned by the stage of eruption and wear in each case. The age-at-death of all specimens was established according to dental eruption and wear patterns for llamas and associated camelid species recognized in the literature (Fernández-Baca, 1962; Oporto et al., 1979; Wheeler, 1982). According to the categorization established by Yacobaccio et al. (1998), the specimens analyzed here belong to the following age classes: juvenile (12–24 months), young adult (24–36 months), adult (36–96 months), and senile (more than 96 months old) (Table 3). While the sequence of tooth eruption and wear is well-established for South American camelids, there is no available calendar for tooth formation. Figure 3 shows the timeline of eruption and wear for mandibular teeth of camelids based on previous studies (Fernández-Baca, 1962; Oporto et al., 1979; Wheeler, 1982). The time-spans represented here are considered as the minimum-maximum age range of eruption of each tooth crown, just before the wear process can commence. Tooth enamel is metabolically inert, which means that once formed, it is not remodeled and thus records the animal's life history over a particular time-span (Balasse, 2002). Therefore, the isotopic values presented here correspond to a time-span prior to the full eruption of the tooth in all cases. According to the literature, a molar crown can form over the course of a year depending on its length, and intra-tooth enamel samples are thought to represent intervals of weeks to months according to enamel maturation rates established for mammalian herbivore species (Green et al., 2018; Hoppe et al., 2004; Trayler and Kohn, 2017).
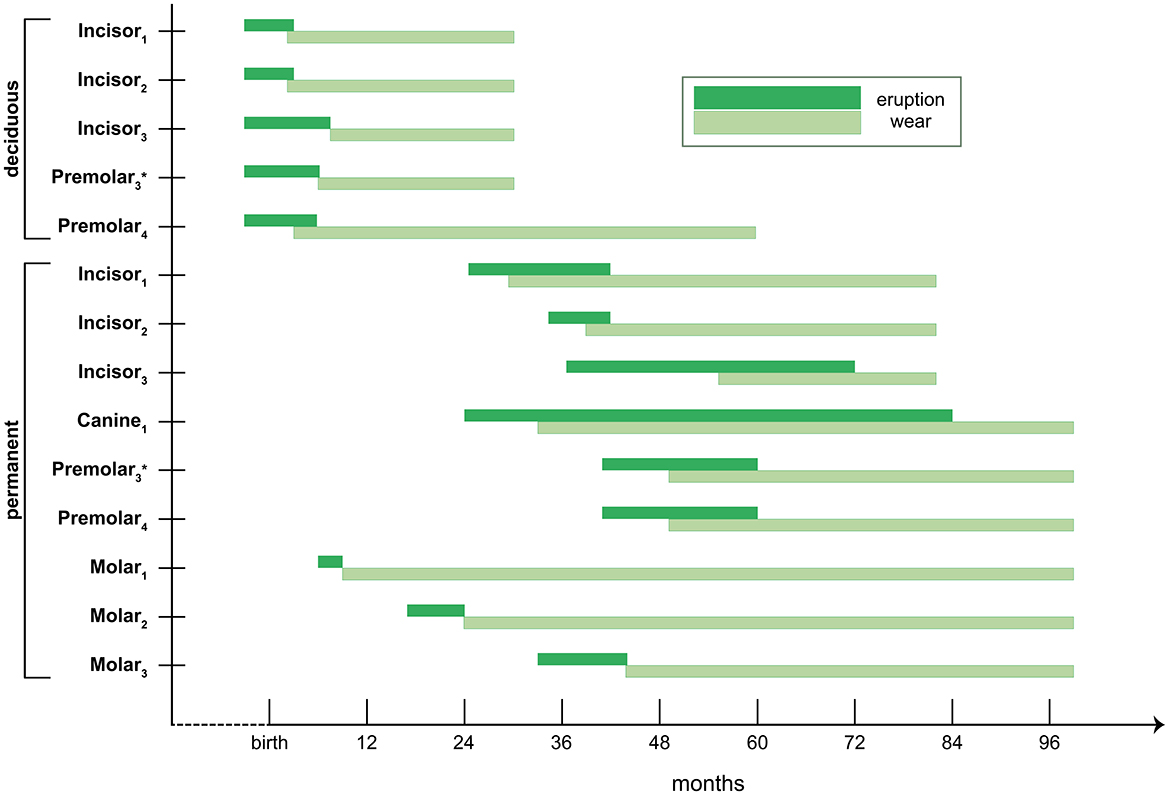
Figure 3. Unified timeline of mandibular teeth eruption and wear for South American camelids (modified from Samec et al., 2014a, based on the information published by Fernández-Baca, 1962; Oporto et al., 1979 and Wheeler, 1982). *According to Wheeler (1982), Pd3 is present in ~ 90% of mandible specimens and P3 in ~10%, whereas deciduous canines are only present in ~5% of male camelids and therefore were not included in the graph.
Ontogenetic processes will affect the isotopic signals recorded according to the time of tooth formation. This is particularly important for deciduous teeth—which mainly form before birth—and first molars—which will be affected by the effects of suckling (Balasse et al., 2001). According to ethnographic information, the weaning of newborn llamas occurs between six to eight months of age, largely determined by the availability of good pastures in that particular year. For this reason, we consider that the isotopic values measured on premolars and first molars might reflect some of these processes, whereas second molars will probably record diet after weaning almost completely, and third molars will have a purely post-weaning signal (Figure 3).
Intra-tooth sequential enamel samples were obtained by drilling horizontal grooves perpendicular to the tooth growth axis, maintaining ~2 mm between each groove and taking great care to avoid the underlying dentine (Balasse, 2002). The number of samples per tooth ranged between four and eight, according to the height of each crown (Table 3). The position of each sampling groove was measured in relation to the crown-root junction, with the closest sample to this point being the latest to form. Powdered enamel samples were pre-treated and measured at the laboratories of the Max Planck Institute of Geoanthropology (Jena, Germany). Each sample was treated with 1.5% sodium hypochlorite for 60 min and 0.1 M acetic acid for 10 min to remove any organics and secondary carbonates, respectively (Lee-Thorp et al., 2012). Measurements of δ13CV − PDB and δ18OV − PDB values for each sample were carried out on a Thermo Gas Bench 2 connected to a Thermo Delta V Advantage continuous-flow isotope ratio mass spectrometer using both internal (MERCK: δ13CV − PDB = −41.3 ‰, δ18OV − PDB = −14.4 ‰) and international (IAEA-603: δ13CV − PDB = 2.5 ‰, δ18OV − PDB = −2.4 ‰; IAEA-CO-8: δ13CV − PDB = −5.8 ‰, δ18OV − PDB = −22.7 ‰; USGS44: δ13CV − PDB = −42.2 ‰) standards calibrated against V-PDB reference values (Coplen et al., 1992; Craig, 1957). Replicates of internal standards showed analytical errors (SD) to be in the order of ± 0.2 ‰ for both δ13CV − PDB and δ18OV − PDB values.
We consider diagenesis to be a minor concern when conducting isotopic analyses on tooth enamel since this material is highly resistant to chemical alteration over time due to its crystallographic structure (Roche et al., 2010; Sponheimer and Lee-Thorp, 1999). This is particularly the case here, given that the archaeological samples studied come from a Late Holocene context. All modern δ13CV − PDB values were corrected by +1.5 ‰ (including those displayed in Table 4) to account for the fossil fuel effect (Marino and McElroy, 1991). Modern tooth δ13CV − PDB values shown in Figure 4 are uncorrected, whereas the rest of the δ13CV − PDB values mentioned throughout the text and shown in the rest of the tables and figures are corrected to account for this effect.
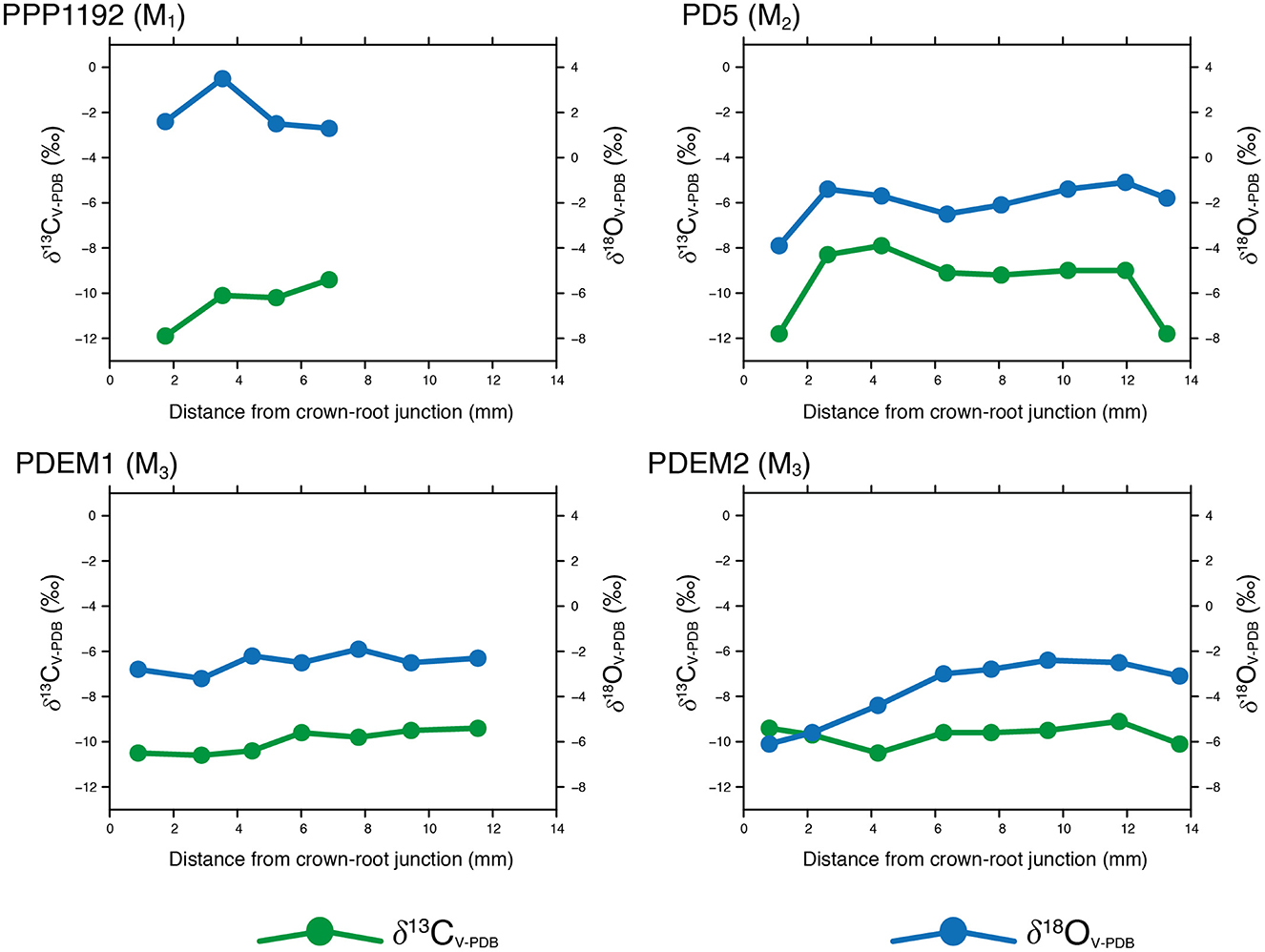
Figure 4. Intra-tooth enamel δ13CV − PDB (uncorrected) and δ18OV − PDB values measured on modern Lama glama specimens.
7 Results
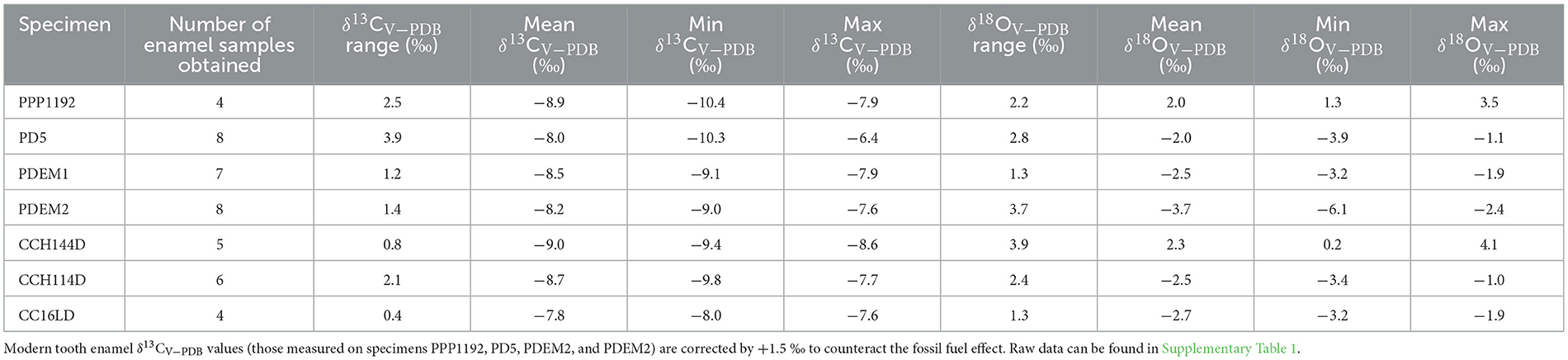
We present 42 pairs of δ13CV − PDB and δ18OV − PDB values measured on sequentially sampled tooth enamel of modern and archaeological Lama glama specimens (Supplementary Table 1). All δ13CV − PDB values range between −10.4 and −6.4 ‰, with a mean amplitude of intra-tooth variation of 1.8 ‰ ranging from 0.4 to 3.9 ‰. δ18OV − PDB values range between −6.1 and 4.1 ‰, with a mean amplitude of intra-tooth variation of 2.5 ‰ ranging from 1.3 to 3.9 ‰. Modern corrected δ13CV − PDB values range between −10.4 and −6.4 ‰, with a mean value of −8.3 ‰, and modern δ18OV − PDB values range between −6.1 and 3.5 ‰, with a mean value of −2.0 ‰. On the other hand, archaeological δ13CV − PDB values range between −9.8 and −7.6 ‰, with a mean value of −8.6 ‰, while archaeological δ18OV − PDB values range between −3.4 and 4.1 ‰, with a mean value of −1.0 ‰. Table 4 shows some basic univariate statistics for the δ13CV − PDB and δ18OV − PDB values series of both modern and archaeological specimens.
As shown in Figures 4, 5, intra-tooth δ13CV − PDB and δ18OV − PDB values series exhibit moderate variation and our results show consistent patterns when comparing modern and archaeological specimens. These results are in agreement with those obtained for domestic South American camelids in other areas of the Andes, which show similar ranges for intra-tooth enamel δ13CV − PDB and δ18OV − PDB values measured on molars of modern and archaeological camelids (Dufour et al., 2014; Goepfert et al., 2013). In addition, the intra-tooth enamel δ13CV − PDB range of variation resembles the intra-tooth dentine δ13CV − PDB variation identified in a previous study that comprised some of the same modern specimens analyzed here (Samec and Harrod, 2022). Overall, the relatively low δ13CV − PDB values presented here point toward the importance of C3 plants in the diets of both modern and archaeological specimens. Nonetheless, the δ13CV − PDB values series also show some variable contribution of C4 plants through time. Modern specimens show similar δ13CV − PDB values when the individual series are juxtaposed, which conforms to our expectations since they belong to the same herd. On the other hand, δ18OV − PDB values show greater variability, both on an intra-tooth and inter-tooth basis, for both the modern and the archaeological contexts. In addition, δ18OV − PDB values appear to be relatively high when compared to the enamel δ18OV − PDB values measured on domestic camelids from the highlands in the Central Andes (Dufour et al., 2014).
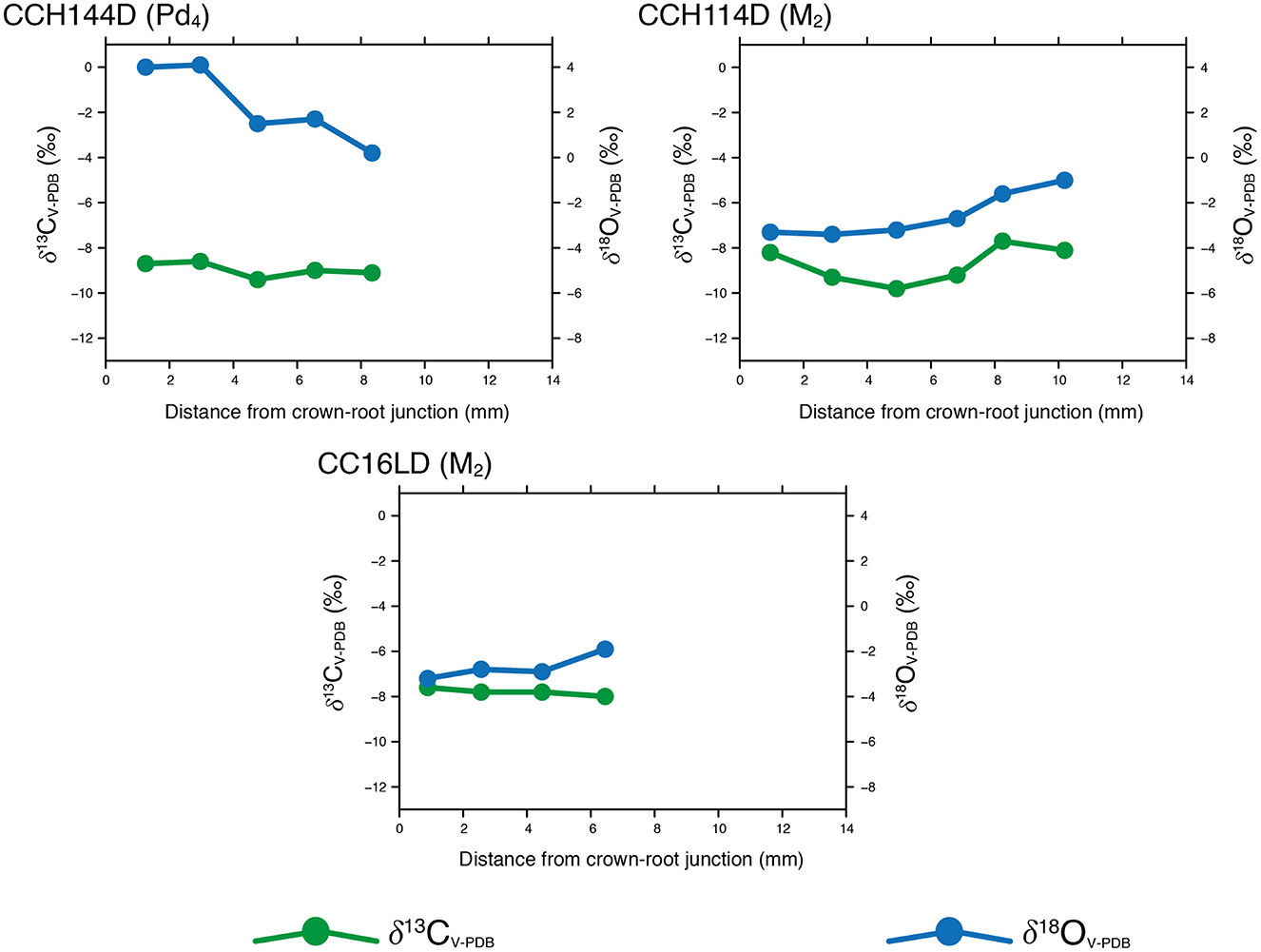
Figure 5. Intra-tooth enamel δ13CV − PDB and δ18OV − PDB values measured on archaeological Lama glama specimens.
Neither δ13CV − PDB nor δ18OV − PDB values series show a marked sinusoidal pattern for both modern and archaeological specimens, with δ13CV − PDB values exhibiting less intra-tooth variation than their δ18OV − PDB counterparts. The absence of a clear cyclical pattern challenges our expectations based on pastoralist mobility and seasonal alternation of pasturelands observed in ethnoarchaeological studies, at least for the modern series (Yacobaccio, 2007). This is particularly remarkable for the δ18OV − PDB values, as they do not reflect a clear variation linked to the prevailing summer rain regime identified in the study area. Some of the δ18OV − PDB values series show a certain degree of variation (PDEM2 or CCH114D), although it hardly approaches a clear sinusoidal pattern as seen in other mammal herbivorous species (Feranec et al., 2009; Makarewicz and Pederzani, 2017). Both PPP1192 (modern) and CCH144D (archaeological) specimens show higher δ18OV − PDB values and relatively high intra-tooth variation compared with the rest of the teeth analyzed here, something that could be explained by the fact that both teeth (M1 and Pd4) formed before weaning and represent different stages of development, thus likely capturing complex ontogenetic changes (Figures 4, 5).
8 Discussion
Tooth enamel δ13CV − PDB values measured on Lama glama specimens point toward the intra-annual importance of C3 plants in the diets of both modern and archaeological herds, as expected according to previous studies carried out in the Dry Puna of Argentina (Samec et al., 2018, 2020; Samec and Yacobaccio, 2021). The proportion of C3 and C4 (and CAM) plants consumed by the llamas analyzed here can be estimated using a two-end mixing model —as the one mentioned in Panarello et al. (2021)— considering the modern local plants δ13CV − PDB values already published (Samec et al., 2017b) (Table 5). To estimate dietary δ13CV − PDB values for the tooth enamel series and establish the proportions of the different photosynthetic types throughout the annual cycle, a discrimination factor of 12.9 ‰ can be used, following the information published by Cerling and Harris (1999) for Lama guanicoe —a wild South American camelid species closely related to the domestic Lama glama. The resulting estimated proportion of C3 plants in the diets of the llamas analyzed here ranges between 0.60 and 0.95 for both modern and archaeological specimens (Supplementary Table 1). These results are in line with the availability of C3 and C4 (and CAM) species in the plant communities identified in the study area. Plant communities found below 3,900 masl show an abundance of ~80% of C3 plants, whereas C3 species account for ~95% of the specimens surveyed in the communities located above that threshold (Samec et al., 2017b). We acknowledge that the availability of certain vegetation in the area does not equal herbivore consumption, but considering that South American camelids prefer palatable grasses (both C3 and C4 species) over other kinds of plants when available, we believe this to be a good approximation to their diets (Castellaro et al., 2004).
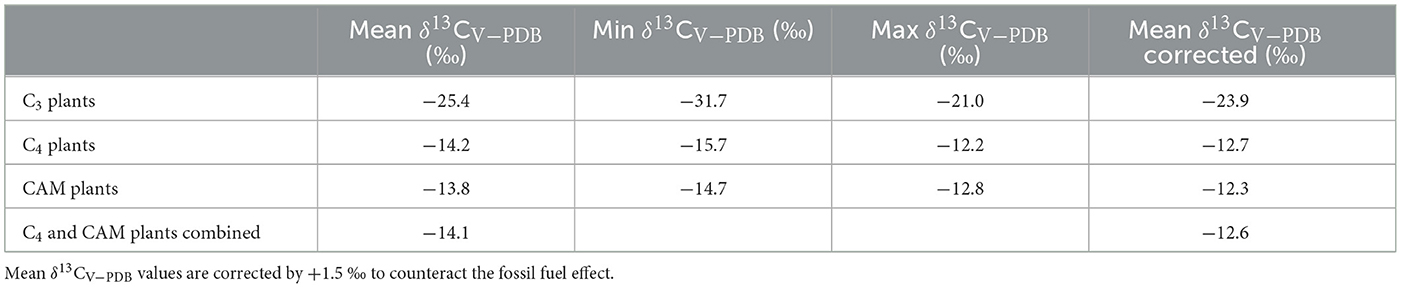
Table 5. Local baseline for stable carbon isotope values based on modern plant δ13CV − PDB values obtained in the study area (Samec et al., 2017b).
Figure 6 shows the δ13CV − PDB values series for estimated diets of modern and archaeological specimens as well as two theoretical thresholds for diets with 95% and 80% of C3 plants to evaluate the alternation of plant communities located below and above 3,900 masl. This figure shows a good fit between the estimated dietary δ13CV − PDB values for both archaeological and modern herds and the theoretical threshold for diets with 80% of C3 plants as expected for the plant communities located below 3900 masl, partly matching our expectations based on ethnoarchaeological studies performed in the area (Yacobaccio, 2007). In this sense, tooth enamel δ13CV − PDB values clearly reflect herding behavior and assign a fundamental role to those plant communities placed below the 3,900 masl threshold —where both C3 and C4 plant species can be found— and that are predominantly used during the wet season (Yacobaccio et al., 1998). Figure 6 also shows a slight variation in some of the specimens analyzed here —e.g. PD5 and CCH114D— that could be interpreted as dietary variations dictated by the use of different vegetational patches throughout the year, although most of the values accommodate nicely to the 80% C3 dietary threshold. Moreover, the modern δ13CV − PDB values individual series exhibit a good overlap for a fraction of its length, especially for specimens PD5, PDEM1, and PDEM2, an aspect that can be explained by the fact that these specimens belonged to the same herd and moved in tandem among vegetation patches. This pattern can be extended to the archaeological δ13CV − PDB values series, where CCH144D and CCH114D also show certain dietary overlap, although it is impossible to assess if these llamas belonged to the same herd even though they were both recovered from the same context (layer 3) at the archaeological site of Chayal Cave.
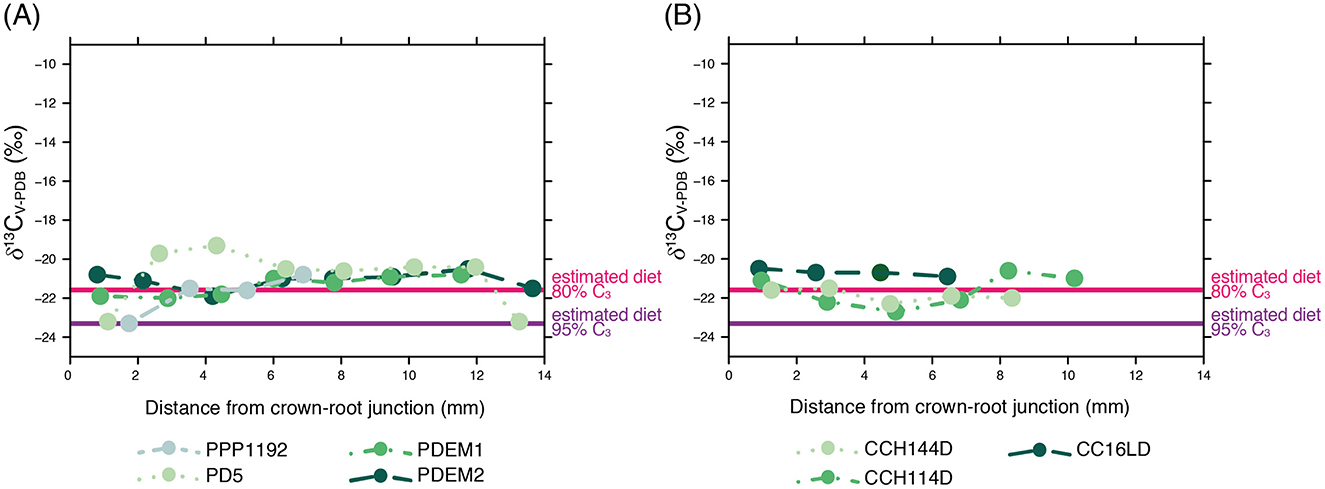
Figure 6. Estimated dietary δ13CV − PDB values for modern (A) and archaeological (B) Lama glama specimens and local theoretical thresholds for diets with 95% and 80% of C3 plants.
Intra-tooth variation in δ18OV − PDB values does not show a clear seasonal pattern, as opposed to the expectations based on pastoralist mobility among different altitudes and the prevailing summer rain regime registered in the study area. To explore this further, we used the equation published by Amiot et al. (2004) to estimate δ18OV − SMOW values of ingested water for the intra-tooth enamel δ18OV − PDB values series. This required us to first convert the values to the V-SMOW scale and calculate the equivalent phosphate δ18OV − SMOW values for our carbonate enamel values using the equations established in the literature (Coplen et al., 1983; Lécuyer et al., 2010). The results of these calculations for both modern and archaeological δ18OV − SMOW values series are presented in (Figure 7; Supplementary Table 1). In turn, this figure shows the mean annual δ18OV − SMOW value for local meteoric waters estimated using the OIPC (Online Isotopes in Precipitation Calculator) (Bowen, 2017), as well as the mean δ18OV − SMOW value for local stream water based on a recent survey (Rohrmann et al., 2014) and the mean value for local surface water bodies as established by our own survey (Samec et al., 2017a) (Table 6).
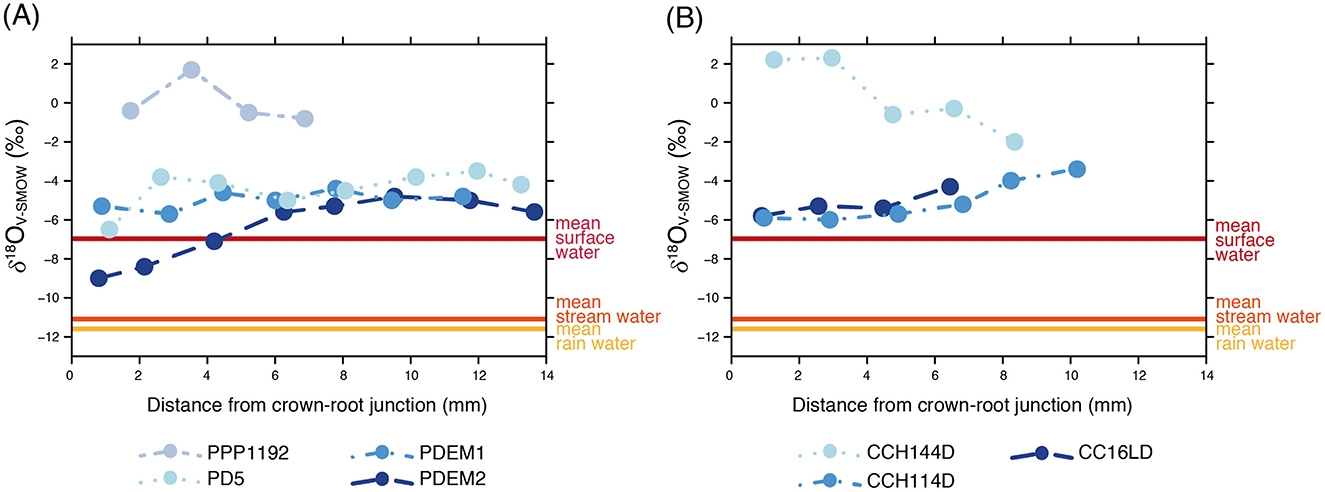
Figure 7. Estimated ingested water δ18OV − SMOW values for modern (A) and archaeological (B) Lama glama specimens and local mean δ18OV − SMOW values for surface, stream, and rain water.
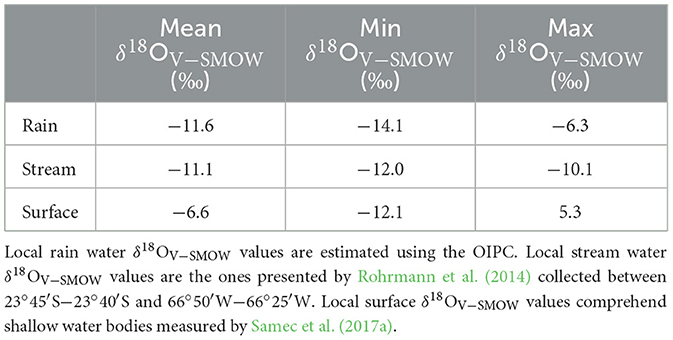
Table 6. Local baseline for stable oxygen isotope values based on modern water δ18OV − SMOW values obtained in the study area (Bowen, 2017; Rohrmann et al., 2014; Samec et al., 2017a).
Figure 7 shows that the estimated δ18OV − SMOW values for camelid ingested water are higher than the values of local precipitation and running streams. This suggests the use of highly evaporated water bodies as drinking sources and/or the consumption of plants with 18O enriched leaf water (Hoppe et al., 2004; Levin et al., 2006; Sealy et al., 2020). Surface waters —and particularly still water bodies such as natural shallow pools— show great variability in their stable isotope compositions, exhibiting relatively high δ18OV − SMOW values in dry environments (Lachniet and Patterson, 2006; Bowen et al., 2018). In turn, leaf water is highly sensitive to enrichment in 18O due to elevated evaporation, which can have major effects on herbivore enamel δ18OV − PDB values (Sponheimer and Lee-Thorp, 1999). These effects are more pronounced in animals that do not drink water every day, such as drought-tolerant mammals in water-limited habitats (Levin et al., 2006). This should be considered when studying tooth enamel δ18OV − PDB values measured on modern llamas in drought-prone areas such as the Dry Puna of Argentina. Moreover, if we consider the local paleoenvironmental information available for the time-span when Chayal Cave was occupied, this pattern can be extended to the last 1,000 years. Lupo et al. (2007) propose the occurrence of an arid pulse between 1,310 and 1,540 cal. years CE based on the analysis of different proxies from Lake Pululos —located North of our study area— which could have led to increased δ18OV − SMOW values for both surface and leaf waters due to drier environmental conditions.
Finally, our results suggest the existence of significant differences when comparing enamel δ18OV − PDB values of pre-weaned and post-weaned formed teeth (Figures 4, 5). This pattern could respond to the influence of ontogenetic changes in the oxygen isotopic composition of pre-weaned formed specimens, i.e., PPP1192 (M1) and CCH144D (Pd4), as they might affect the oxygen isotopic composition of body water. However, this pattern is not reflected in the δ13CV − PDB values measured on PPP1192 and CCH144D, which remain similar to the δ13CV − PDB values measured on post-weaned formed teeth. Unfortunately, there is not enough research on mammal enamel isotopic values of pre-weaned formed teeth to understand the causes of this patterning, which should be addressed in future studies that systematically compare isotopic values measured on different tooth tissues with different formation timelines (Samec et al., 2014a).
Overall, our results show moderate intra-tooth isotopic variability for both modern and archaeological camelids, reflecting dampened variations in diet and water intake through time. The absence of a clear seasonal pattern for both δ13CV − PDB and δ18OV − PDB values goes against what might be expected based on the seasonal alternation of pasture areas documented among modern herders (Yacobaccio, 2007). For carbon stable isotope values, the seasonal alternation among pasture patches located below and above 3900 masl identified by ethnoarchaeological studies evidently does not translate into considerable differences in terms of dietary proportions of C4 plants, matching the results found in intra-tooth dentine δ13CV − PDB values (Samec and Harrod, 2022). Nonetheless, some variation can be seen in certain individuals, probably corresponding to a decreased relative input of C4 plants during winter while herds are feeding at elevations above 3,900 masl. Likewise, the amplitude of most of the δ18OV − PDB values series does not reflect clear seasonal fluctuations and opposes the pattern expected based on seasonal pastoralist mobility and summer precipitation regime. In this case, the pattern that should result from the alternation of different plant communities located at different altitudes may be obscured by the combination of altitudinal mobility patterns coupled with seasonal changes in mean precipitation δ18OV − SMOW values resulting from the amount effect and the effects of changes in temperature and humidity on mean leaf water δ18OV − SMOW values, as well as the differential impacts of this on C3 and C4 plants (Janzen et al., 2020). Overall, the high δ18OV − PDB values identified in the camelid specimens analyzed here reveal their status as non-obligate drinkers heavily relying on pastures with 18O enriched leaf water due to evapotranspiration, thus capturing local environmental conditions (Levin et al., 2006). Moderate intra-tooth variation in both carbon and oxygen stable isotope compositions is also recorded on the archaeological llama specimens recovered in Chayal Cave, an aspect that complicates the use of sequentially sampled tooth enamel to identify seasonal dietary and mobility changes within the study area in the past. In this sense, we believe that some mobility among vegetational patches existed in the past as it does today, but intra-tooth enamel δ13CV − PDB and δ18OV − PDB values series can only capture it as long as this mobility implies switching between patches with significant and consistent baseline variability. A previous study analyzed bulk bone samples from Chayal Cave and suggested that the pastoralist inhabitants of the cave employed a small-scale mobility strategy (Samec and Yacobaccio, 2021), a finding that seems further sustained by the trends presented here. In this sense, modern domestic camelids provide the best analogy for reconstructing the diet and mobility of archaeological domestic camelids, and our results show consistencies among modern and archaeological datasets. Overall, the results discussed here show that llama herders and their animals can thrive in high-altitude environments by practicing a small-scale mobility strategy that allows them to counteract seasonal aridity impacts and avoid overgrazing preferred vegetational patches, fostering a sustainable pastoralist system in the long run.
9 Final remarks
Zooarchaeological evidence recovered from the pastoralist site of Chayal Cave, located in the Dry Puna of Argentina, highlights the importance of llama herding in this region for at least the last 1,000 years. Previous investigations carried out at the site discussed the characteristics of the pastoralist strategies employed by the inhabitants of the cave, although the extent of the mobility ranges remained in the realm of working hypothesis. This study tackled this question by using a novel technique previously unapplied in the study area, fostering the development of a frame of reference based on modern data.
We presented δ13CV − PDB and δ18OV − PDB values measured on sequentially sampled tooth enamel of modern and archaeological domestic South American camelids from the Dry Puna of Argentina. Overall, the δ13CV − PDB values discussed here show a clear intra-annual preponderance of C3 plants in the diets of both modern and archaeological herds and the signal exhibited by the tooth series is consistent with the current availability of C3 and C4 plants in the study area. Nonetheless, intra-tooth δ13CV − PDB variation does not exhibit a clear seasonal pattern as might be expected based on the use of different plant communities throughout the year, as attested by ethnoarchaeological studies performed in the area. δ18OV − PDB values also show the absence of a clear sinusoidal pattern for both modern and archaeological tooth series. In turn, estimated δ18OV − SMOW values for ingested water resulted higher than the mean δ18OV − SMOW values for local rainfall and stream waters, only partly matching the δ18OV − SMOW values measured on surface shallow water bodies. This suggests that the animals sampled here relied on evaporated water resources, probably indirectly consumed as pastures with high mean leaf δ18OV − SMOW values, given the non-obligate drinking character of the Lama glama species. At the same time, our study identified differences between pre-weaned and post-weaned formed teeth —particularly for oxygen stable isotope values—, highlighting the importance of considering ontogenetic processes when measuring isotopic compositions in animal tissues.
These results allowed us to explore the scope and limitations of using carbon and oxygen stable isotope measurements on sequentially sampled camelid teeth to identify seasonal mobility patterns within pastoralist strategies developed in the tropical highlands of the Andes. In this case, the results presented do not show a clear seasonal pattern that points toward significant changes in browse and water consumed throughout the year for the modern and archaeological camelid specimens analyzed. This may reflect the intrinsically small scale of the local herding strategies as well as the combination of environmental and cultural processes counteracting each other. Overall, modern pastoralist strategies evidence how the mobility of the herds —and herders— can offset commonly assumed altitudinal isotopic variations in vegetation and water resources and ultimately manage to avoid overexploiting productive patches. These results show how both pre-Hispanic and modern pastoralist practices seem perfectly catered to the challenges posed by the Andean highlands, stressing their importance as an enduring subsistence strategy in a changing environment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because animals were not alive when the study was performed. All animal tissues were part of vertebrate bone collections.
Author contributions
CS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. HY: Funding acquisition, Investigation, Writing – original draft. PR: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by CONICET (Argentina) PIP 0853, UBA (Argentina) UBACyT F-383, DAAD (Germany) Short-term Grant 91703285, ERC and European Union's Horizon 2020 (research and innovation programme) grant agreement number 850709 (PANTROPOCENE).
Acknowledgments
We thank Jana Ilgner, Sara Marzo, and Bianca Fiedler for their assistance during laboratory procedures. We are also grateful to all members of VICAM, and especially to Yanina Arzamendia and Jorge Baldo, for their collaboration. Our everlasting gratitude goes to the Susques community, especially to our friend Demetria, for welcoming us and allowing us to work in their lands. Finally, we acknowledge the reviewers for their insightful and encouraging comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fearc.2025.1557715/full#supplementary-material
Supplementary Table 1 | Raw carbon and oxygen stable isotope values and contextual information.
References
Alaica, A. K., Scaffidi, B. K., González La Rosa, L. M., Jennings, J., Knudson, K. J., and Tung, T. (2022). Flexible agropastoral strategies during the 1st millennium CE in southern Peru: Examining yunga Arequipa camelid husbandry practices during Wari expansion through stable isotope analysis (δ13C and δ15N) in the Majes and Sihuas Valleys. Quat. Int. 634, 48–64. doi: 10.1016/j.quaint.2022.06.015
Alcàntara Fors, R., Madgwick, R., Viñas-Caron, L. C., Nederbragt, A. J., and Saña Seguí, M. (2025). Cattle on the rocks: understanding cattle mobility, diet, and seasonality in the Iberian Peninsula. The Middle Neolithic site of Cova de les Pixarelles (Tavertet, Osona). PLoS ONE 20, e0317723. doi: 10.1371/journal.pone.0317723
Amiot, R., Lecuyer, C., Buffetaut, E., Fluteau, F., Legendre, S., and Martineau, F. (2004). Latitudinal temperature gradient during the cretaceous upper Campanian-Middle Maastrichtian: δ18O record of continental vertebrates. Earth Planet. Sci. Lett. 226, 255–272. doi: 10.1016/j.epsl.2004.07.015
Arzamendia, Y., Rojo, V., González, N. M., Baldo, J. L., Zamar, M. I., Lamas, H. E., et al. (2021). The Puna pastoralist system: a coproduced landscape in the Central Andes. Mt. Res. Dev. 41, R38–R49. doi: 10.1659/MRD-JOURNAL-D-21-00023.1
Aschero, C. A., and Yacobaccio, H. D. (1998-1999). 20 años después: Inca Cueva 7 reinterpretado. Cuadernos del INAPL 18, 7–18. Available online at: https://revistas.inapl.gob.ar/index.php/cuadernos/article/view/507
Balasse, M. (2002). Reconstructing dietary and environmental history from enamel isotopic analysis: time resolution of intra-tooth sequential sampling. Int. J. Osteoarchaeol. 12, 155–165. doi: 10.1002/oa.601
Balasse, M., and Ambrose, S. H. (2005). Mobilité altitudinale des pasteurs néolithiques dans la vallée du Rift (Kenya): premiers indices de l'analyse du δ13C de l'émail dentaire du cheptel domestique. Anthropozoologica 40, 147–166. Available online at: https://sciencepress.mnhn.fr/fr/periodiques/anthropozoologica/40/1/mobilite-altitudinale-des-pasteurs-neolithiques-dans-la-vallee-du-rift-kenya-premiers-indices-de-l-analyse-du-d13c-de-l-email-dentaire-du-cheptel-domestique
Balasse, M., Ambrose, S. H., Smith, A. B., and Price, T. D. (2002). The seasonal mobility model for prehistoric herders in the south-western Cape of South Africa assessed by isotopic analysis of sheep tooth enamel. J. Archaeol. Sci. 29, 917–932. doi: 10.1006/jasc.2001.0787
Balasse, M., Bocherens, H., Mariotti, A., and Ambrose, S. H. (2001). Detection of dietary changes by intra-tooth carbon and nitrogen isotopic analysis: an experimental study of dentine collagen of cattle (Bos taurus). J. Archaeol. Sci. 28, 235–245. doi: 10.1006/jasc.1999.0535
Barbour, M. M. (2007). Stable oxygen isotope composition of plant tissue: a review. Funct. Plant Biol. 34, 83–94. doi: 10.1071/FP06228
Barri, F. R., Falczuk, V., Cingolani, A. M., and Díaz, S. (2014). Dieta de la población de guanacos (Lama guanicoe) reintroducida en el Parque Nacional Quebrada del Condorito, Argentina. Ecol. Aust. 24, 203–211. doi: 10.25260/EA.14.24.2.0.23
Behrensmeyer, A. (1978). Taphonomic and ecologic information from bone weathering. Paleobiology 4, 150–162. doi: 10.1017/S0094837300005820
Berenguer, J. (2004). Caravanas, Interacción y Cambio en el Desierto de Atacama. Sirawi Ediciones, Santiago: Museo Chileno de Arte Precolombino.
Bianchi, A. R., Yañez, C. E., and Acuña, L. R. (2005). Bases de datos mensuales de las precipitaciones del Noroeste Argentino. Argentina: Informe del Proyecto Riesgo Agropecuario, INTA-SAGPYA.
Blumenthal, S. A., Levin, N. E., Brown, F. H., Brugal, J. P., Chritz, K. L., Harris, J. M., et al. (2017). Aridity and hominin environments. Proc. Natl. Acad. Sci. 114, 7331–7336. doi: 10.1073/pnas.1700597114
Bowen, G. J. (2017). The online isotopes in precipitation calculator, version 3.1. Available online at: http://www.waterisotopes.org (accessed November 15, 2024).
Bowen, G. J., Putman, A., Brooks, J. R., Bowling, D. R., Oerter, E. J., and Good, S. P. (2018). Inferring the source of evaporated waters using stable H and O isotopes. Oecologia 187, 1025–1039. doi: 10.1007/s00442-018-4192-5
Bowen, G. J., and Wilkinson, B. (2002). Spatial distribution of δ18O in meteoric precipitation. Geology 30, 315–318. doi: 10.1130/0091-7613(2002)030<0315:SDOOIM>2.0.CO;2
Braun Wilke, R. H., Picchetti, L. P. E., and Villafañe, B. S. (1999). Pasturas Montanas de Jujuy. Argentina: Facultad de Ciencias Agrarias, Universidad Nacional de Jujuy.
Britton, K., Müldner, G., and Bell, M. (2008). Stable isotope evidence for salt-marsh grazing in the Bronze Age Severn Estuary, UK: implications for palaeodietary analysis at coastal sites. J. Archaeol. Sci. 35, 2111–2118. doi: 10.1016/j.jas.2008.01.012
Bronk Ramsey, C. (2021). OxCal v.4.4.4 [software]. Available online at: https://c14.arch.ox.ac.uk/oxcal.html (accessed February 18, 2025).
Browman, D. L. (1974). Pastoral nomadism in the andes. Curr. Anthropol. 15, 188–196. doi: 10.1086/201455
Bryant, D. J., and Froelich, P. N. (1995). A model of oxygen isotope fractionation in body water of large mammals. Geochim. Cosmochim. Acta 59, 4523–4537. doi: 10.1016/0016-7037(95)00250-4
Cabrera, A. L. (1976). Regiones fitogeográficas argentinas. Enciclopedia Argentina de Agricultura y jardinería, 2da Edición, tomo II. Buenos Aires: Editorial Acme.
Capriles, J. (2014). Mobile communities and pastoralist landscapes during the formative period in the central altiplano of Bolivia. Latin Amer. Antiquity 25, 3–26. doi: 10.7183/1045-6635.25.1.3
Capriles, J. M., and Tripcevich, N. (editors) (2016). The Archaeology of Andean Pastoralism. Albuquerque, NM: University of New Mexico Press.
Cartajena, I., Núñez, L., and Grosjean, M. (2007). Camelid domestication in the western slope of the Puna de Atacama, Northern Chile. Anthropozoologica 42, 155–174. Available online at: https://sciencepress.mnhn.fr/fr/periodiques/anthropozoologica/42/2/la-domestication-des-camelides-sur-le-versant-occidental-de-la-puna-de-atacama-chili-du-nord
Castellaro, G., Ullrich, T., Wackwitz, B., and Raggi, A. (2004). Composición botánica de la dieta de alpacas (Lama pacos L.) y llamas (Lama glama L.) en dos estaciones del año, en praderas altiplánicas de un sector de la Provincia de Parinacota, Chile. Agric. Técn. 64, 353–364. doi: 10.4067/S0365-28072004000400004
Cavagnaro, J. B. (1988). Distribution of C3 and C4 grasses at different altitudes in a temperate arid region of Argentina. Oecologia 76, 273–277. doi: 10.1007/BF00379962
Cerling, T. E., and Harris, J. M. (1999). Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120, 347–363. doi: 10.1007/s004420050868
Cernusak, L. A., Barbour, M. M., Arndt, S. K., Cheesman, A. W., English, N. B., Feild, T. S., et al. (2016). Stable isotopes in leaf water of terrestrial plants. Plant Cell Environ. 39, 1087–1102. doi: 10.1111/pce.12703
Chazin, H., Gordon, G. W., and Knudson, K. J. (2019). Isotopic perspectives on pastoralist mobility in the Late Bronze Age South Caucasus. J. Anthropol. Archaeol. 54, 48–67. doi: 10.1016/j.jaa.2019.02.003
Codron, J., Codron, D., Lee-Thorp, J., Sponheimer, M., Bond, W., de Ruiter, D., et al. (2005). Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from an African savanna. J. Archaeol. Sci. 32, 1757–1772. doi: 10.1016/j.jas.2005.06.006
Coplen, T., Kendall, C., and Hopple, J. (1983). Comparison of stable isotope reference samples. Nature 302, 236–238. doi: 10.1038/302236a0
Coplen, T. B., Krouse, H. R., and Bohlke, J. K. (1992). Reporting of nitrogen-isotope abundances. Pure Appl. Chem. 64, 907–908. doi: 10.1351/pac199264060907
Craig, H. (1957). The natural distribution of radiocarbon and the exchange time of carbon dioxide between atmosphere and sea. Tellus 9, 1–17. doi: 10.3402/tellusa.v9i1.9078
Dansgaard, W. (1964). Stable isotopes in precipitation. Tellus 16, 436–467. doi: 10.1111/j.2153-3490.1964.tb00181.x
Dufour, E., Goepfert, N., Leon, B. G., Chauchat, C., Jordan, R. F., and Vásquez Sánchez, S. (2014). Pastoralism in Northern Peru during pre-Hispanic times: insights from the Mochica Period (100–800 AD) based on stable isotopic analysis of domestic camelids. PLoS ONE 9:e87559. doi: 10.1371/journal.pone.0087559
Eerkens, J. W., Vaughn, K. J., Linares-Grados, M., and Beckham, C. (2024). Long-term camelid husbandry and agricultural intensification in the Southern Nasca Region, Peru: Insight from faunal isotopes. Latin Amer. Antiquity 35, 694–711. doi: 10.1017/laq.2023.44
Ehleringer, J. R., and Cerling, T. E. (2002). “C3 and C4 photosynthesis,” in Encyclopedia of Global Environmental Change. The earth system: biological and ecological dimensions of global environmental change, ed. R.E. Munn (New York: Wiley), 186–190.
Elkin, D. C. (1995). Volume density of South American camelids skeletal parts. Inter. J. Osteoarchaeol. 5, 29–37. doi: 10.1002/oa.1390050104
Feranec, R. S., Hadley, E. A., and Paytan, A. (2009). Stable isotopes reveal seasonal competition for resources between late Pleistocene bison (Bison) and horse (Equus) from rancho La Brea, southern California. Palaeogeogr. Palaeoclimatol. Palaeoecol. 271, 153–160. doi: 10.1016/j.palaeo.2008.10.005
Fernández, J., and Panarello, H. O. (1989–1990). Isótopos en Arqueología. 1. Valores isotópicos del oxígeno en aguas meteóricas y su pasaje a la sangre humana y a la de camélidos de la Puna Jujeña a 4.000 m de altitud. Runa XIX, 47–58.
Fernández, J., and Panarello, H. O. (1999-2001). Isótopos del carbono en la dieta de herbívoros y carnívoros de los Andes Jujeños. Xama 12-14, 71–85.
Fernández-Baca, S. (1962). Algunos aspectos del desarrollo dentario en la alpaca (Lama pacos). Rev. Fac. Med. Vet. 16–17, 88–103.
Finucane, B. C., Maita Agurto, P., and Isbell, W. H. (2006). Human and animal diet at Conchopata, Peru: stable isotope evidence for maize agriculture and animal management practices during the Middle Horizon. J. Archaeol. Sci. 33, 1766–1776. doi: 10.1016/j.jas.2006.03.012
Gat, J. R. (1996). Oxygen and hydrogen isotopes in the hydrologic cycle. Ann. Rev. Earth Planet. Sci. 24, 225–262. doi: 10.1146/annurev.earth.24.1.225
Gil Montero, R. (1997). Unidades domésticas con residencias múltiples: Puna de Jujuy (Argentina), fines del siglo XVIII. Andes 8, 47–76.
Gillis, R. E., Bulatović, J., Penezić, K, Spasić, M, Tasić, N. N, and Makarewicz, C.A. (2021). Of herds and societies-Seasonal aspects of Vinča culture herding and land use practices revealed using sequential stable isotope analysis of animal teeth. PLoS ONE 16:e0258230. doi: 10.1371/journal.pone.0258230
Göbel, B. (2001). “El ciclo anual de la producción pastoril en Huancar (Jujuy, Argentina),” in El uso de los camélidos a través del tiempo, eds. G. L. Mengoni Goñalons, D. E. Olivera, H. D. Yacobaccio. 91–115.
Goepfert, N., Dufour, E., Gutiérrez, B., and Chauchat, C. (2013). Origen geográfico de camélidos en el periodo mochica (100–800 AD) y análisis isotópico secuencial del esmalte dentario: enfoque metodológico y aportes preliminares. Bulletin l'Institut Français d'Etudes Andines 42, 25–48. doi: 10.4000/bifea.869
Gonfiantini, R., Roche, M-. A., Olivry, J-. C., Fontes, J-. C., and Zuppi, G. M. (2001). The altitude effect on the isotopic composition of tropical rains. Chem. Geol. 181, 147–167. doi: 10.1016/S0009-2541(01)00279-0
Green, D. R., Olack, G., and Colman, A. S. (2018). Determinants of blood water δ18O variation in a population of experimental sheep: implications for paleoclimate reconstruction. Chem. Geol. 485, 32–43. doi: 10.1016/j.chemgeo.2018.03.034
Heaton, T. H. E. (1999). Spatial, species, and temporal variations in the 13C/12C ratios of C3 plants: implications for palaeodiet studies. J. Archaeol. Sci. 26, 637–649. doi: 10.1006/jasc.1998.0381
Helliker, B. R., and Ehleringer, J. R. (2000). Establishing a grassland signature in veins: 18O in the leaf water of C3 and C4 grasses. Proc. Natl. Acad. Sci. 97, 7894–7898. doi: 10.1073/pnas.97.14.7894
Henton, E., Meier-Augenstein, W., and Kemp, H. (2010). The use of oxygen isotopes in sheep molars to investigate past herding practices at the Neolithic settlement of Çatalhöyük, central Anatolia. Archaeometry 52, 429–449. doi: 10.1111/j.1475-4754.2009.00492.x
Hirose, M., Naito, Y. I., Kadowaki, S., Arai, S., Guliyev, F., and Nishiaki, Y. (2021). Investigating early husbandry strategies in the southern Caucasus: intra-tooth sequential carbon and oxygen isotope analysis of Neolithic goats, sheep, and cattle from Göytepe and Haci Elamxanli Tepe. J. Archaeol. Sci. Rep. 36, 102869. doi: 10.1016/j.jasrep.2021.102869
Hogg, A., Heaton, T., Hua, Q., Palmer, J., Turney, C., Southon, J., et al. (2020). SHCal20 Southern hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 62, 759–778. doi: 10.1017/RDC.2020.59
Hoppe, K. A., Stover, S. M., Pascoe, J. R., and Amundson, R. (2004). Tooth enamel biomineralization in extant horses: implications for isotopic microsampling. Palaeogeogr. Palaeoclimatol. Palaeoecol. 206, 355–365. doi: 10.1016/j.palaeo.2004.01.012
Izeta, A. D., Laguens, A. G., Marconetto, M. B., and Scattolin, M. C. (2009). Camelid handling in the meridional Andes during the first millennium AD: A preliminar approach using stable isotopes. Int. J. Osteoarchaeology 19, 204–214. doi: 10.1002/oa.1066
Janzen, A., Balasse, M., and Ambrose, S. H. (2020). Early pastoral mobility and seasonality in Kenya assessed through stable isotope analysis. J. Archaeol. Sci. 117, 105099. doi: 10.1016/j.jas.2020.105099
Jasechko, S., Sharp, Z., Gibson, J., Birks, S. J., Yi, Y., and Fawcett, P. (2013). Terrestrial water fluxes dominated by transpiration. Nature 496, 347–350. doi: 10.1038/nature11983
Kanner, L. C., Burns, S. J., Cheng, H., Edwards, R. L., and Vuille, M. (2013). High-resolution variability of the South American summer monsoon over the last seven millennia: insights from a speleothem record from the central Peruvian Andes. Quat. Sci. Rev. 75, 1–10. doi: 10.1016/j.quascirev.2013.05.008
Knudson, K. J. (2009). Oxygen isotope analysis in a land of environmental extremes: the complexities of isotopic work in the Andes. Int. J. Osteoarchaeol. 19, 171–191. doi: 10.1002/oa.1042
Knudson, K. J., Gardella, K. R., and Yaeger, J. (2012). Provisioning Inka feasts at Tiwanaku, Bolivia: the geographic origins of camelids in the Pumapunku complex. J. Archaeol. Sci. 39, 479–491. doi: 10.1016/j.jas.2011.10.003
Kohn, M. J., Schoeninger, M. J., and Valley, J. W. (1996). Herbivore tooth oxygen isotope compositions: effects of diet and physiology. Geochim. Cosmochim. Acta 60, 3889–3896. doi: 10.1016/0016-7037(96)00248-7
Lachniet, M. S., and Patterson, W. P. (2006). Use of correlation and multiple stepwise regression to evaluate the climatic controls on the stable isotope values of Panamanian surface waters. J. Hydrol. 324, 115–140. doi: 10.1016/j.jhydrol.2005.09.018
Lazzerini, N., Coulon, A., Simon, L., Marchina, C., Fiorillo, D., Turbat, T., et al. (2021). The isotope record (δ13C, δ18O) of vertical mobility in incremental tissues (tooth enamel, hair) of modern livestock: a reference set from the Mongolian Altai. Quat. Int. 595, 128–144. doi: 10.1016/j.quaint.2021.04.008
Lécuyer, C., Balter, V., Martineau, F., Fourel, F., Bernard, A., Amiot, R., et al. (2010). Oxygen isotope fractionation between apatite-bound carbonate and water determined from controlled experiments with synthetic apatites precipitated at 10-37°C. Geochim. Cosmochim. Acta 74, 2072–2081. doi: 10.1016/j.gca.2009.12.024
Lee-Thorp, J. A., Likius, A., Mackaye, H. T., Vignaud, P., Sponheimer, M., and Brunet, M. (2012). Isotopic evidence for an early shift to C4 resources by Pliocene hominins in Chad. Proc. Natl. Acad. Sci.109, 20369–20372. doi: 10.1073/pnas.1204209109
Levin, N. E., Cerling, T. E., Passey, B. H., Harris, J. M., and Ehleringer, J. R. (2006). A stable isotope aridity index for terrestrial environments. Proc. Natl. Acad Sci. 103, 11201–11205. doi: 10.1073/pnas.0604719103
Lightfoot, E., Jones, P. J., Joglekar, P. P., Tames-Demauras, M., Smith, E., Muschinski, J., et al. (2020). Feeding the herds: Stable isotope analysis of animal diet and its implication for understanding social organisation in the Indus Civilisation, Northwest India. Archaeol. Research Asia 24:100212. doi: 10.1016/j.ara.2020.100212
López-Mendoza, P., Samec, C., Núñez, L., Carrasco, C., Loyola, R., and Cartajena, I. (2022). El manejo territorial de los camélidos en la circumpuna de Atacama desde el Arcaico al Formativo (10.000-2400 aP): Una aproximación isotópica y taxonómica. Latin Amer. Antiquity 33, 575–595. doi: 10.1017/laq.2021.66
Lupo, L., Kulemeyer, J., Torres, G., Oxman, B., and Schittek, K. (2018). “Paleoecología del Cuaternario tardío de la Puna del Noroeste argentino,” in La Puna argentina: Naturaleza y Cultura, eds. H. R. Grau, M. D. P. Babot, A. E. Izquierdo, A. Grau (Serie de Conservación de la Naturaleza) (Tucumán: Fundación Miguel Lillo), 54–72.
Lupo, L., Morales, M. R., Yacobaccio, H. D., Maldonado, A., and Grosjean, M. (2007). “Cambios ambientales en la Puna Jujeña durante los últimos 1200 años: explorando su impacto en la economía pastoril,” in Pacarina, XVI Congreso Nacional de Arqueología Argentina, Tomo III (San Salvador de Jujuy: Facultad de Humanidades y Ciencias Sociales, Universidad Nacional de Jujuy), 151–156.
Mader, C., Reindel, M., and Isla, J. (2022). “Camelids as cargo animals by the Paracas culture (800-200 BC) in the Palpa valleys of southern Peru,” in Caravans in Socio-Cultural Perspective: Past and Present, eds. P. B. Clarkson, C. M. Santoro (London: Routledge), 174–192. doi: 10.4324/9781003179276-11
Makarewicz, C., and Tuross, N. (2006). Foddering by Mongolian pastoralists is recorded in the stable carbon (δ13C) and nitrogen (δ15N) isotopes of caprine dental collagen. J. Archaeol. Sci. 33, 862–870. doi: 10.1016/j.jas.2005.10.016
Makarewicz, C. A. (2017). Sequential δ13C and δ18O analyses of early Holocene bovid tooth enamel: resolving vertical transhumance in Neolithic domesticated sheep and goats. Palaeogeogr. Palaeoclimatol. Palaeoecol. 485, 16–29. doi: 10.1016/j.palaeo.2017.01.028
Makarewicz, C. A., Arbuckle, B. S., and Öztan, A. (2017). Vertical transhumance of sheep and goats identified by intra-tooth sequential carbon (δ13C) and oxygen (δ18O) isotopic analyses: evidence from Chalcolithic Köçk Höyük, central Turkey. J. Archaeol. Sci. 86, 68–80. doi: 10.1016/j.jas.2017.01.003
Makarewicz, C. A., and Pederzani, S. (2017). Oxygen (δ18O) and carbon (δ13C) isotopic distinction in sequentially sampled tooth enamel of co-localized wild and domestic caprines: complications to establishing seasonality and mobility in herbivores. Palaeogeogr. Palaeoclimatol. Palaeoecol. 485, 1–15. doi: 10.1016/j.palaeo.2017.01.010
Marino, B. D., and McElroy, M. B. (1991). Glacial-to-interglacial variations in the carbon isotopic composition of atmospheric CO2. Nature 349, 127–131. doi: 10.1038/349127a0
Mengoni Goñalons, G. L. (2007). Camelid management during Inca times in N. W. Argentina: models and archaeozoological indicators. Anthropozoologica 42, 129–141. Available online at: https://sciencepress.mnhn.fr/fr/periodiques/anthropozoologica/42/2/gestion-des-camelides-pendant-la-periode-inca-au-nord-ouest-de-l-argentine-modeles-et-indicateurs-archeozoologiques
Messana, C., Tornero, C., Madgwick, R., Lamb, A. L., Evans, J., and Colominas, L. (2023). Between valleys, plateaus, and mountains: unveiling livestock altitudinal mobility in the Iron Age Iberian Peninsula (3rd c. BC) through a multi-isotope approach. Front. Environ. Archaeol. 2:1245725. doi: 10.3389/fearc.2023.1245725
Mondini, M., Grant, J., Panarello, H. O., Samec, C., López, P., Núñez, M. L., et al. (2019). Composición isotópica del carbono y el nitrógeno en camélidos arqueológicos de la Puna de Atacama: una comparación entre ambas vertientes de los Andes. Cuad. del INAPL Series Especiales 7, 172–181. Available online at: https://revistas.inapl.gob.ar/index.php/series_especiales/article/view/1330
Moore, K. (2016). “Early domesticated camelids in the andes,” in The Archaeology of Andean Pastoralism, eds. J. Capriles, N. Tripcevich (Albuquerque, NM: University of New Mexico Press), 17–38.
Mosca Torres, M. E., and Puig, S. (2010). Seasonal diet of vicuñas in the Los Andes protected area (Salta, Argentina): are they optimal foragers? J. Arid Environ. 74, 450–457. doi: 10.1016/j.jaridenv.2009.10.002
Murphy, B. P., Bowman, D. M. J. S., and Gagan, M. K. (2007). Sources of carbon isotope variation in kangaroo bone collagen and tooth enamel. Geochim. Cosmochim. Acta 71, 3847–3858. doi: 10.1016/j.gca.2007.05.012
Nielsen, A. E. (2006). “Estudios internodales e interacción interregional en los Andes Circumpuneños: Teoría, método y ejemplos de aplicación,” in Esferas de Interacción Prehistóricas y Fronteras Nacionales Modernas: Los Andes Sur Centrales, ed. H. Lechtman (Lima: IEP), 29–62.
Nielsen, A. E. (2024). “Herders and llamas, companion species in the Southern Andes during the last three millenia,” in Nature(s) in Construction, eds. M. L. Pochettino, et al. (Cham: The Latin American Studies Book Series, Springer), 501–517. doi: 10.1007/978-3-031-60552-9_30
O'Leary, M. H. (1988). Carbon isotopes in photosynthesis. Bioscience 38, 325–336. doi: 10.2307/1310735
Oporto, N., Bigatti, R., and Larrieu, E. (1979). Determinación de edades en guanaco (Lama guanicoe) en base a su dentición. Rev. Arg. Produc. Anim. 4, 965–983.
Panarello, H. O., and Fernández, J. (2002). Stable carbon isotope measurements on hair from wild animals from altiplanic environments of Jujuy. Radiocarbon 44, 709–716. doi: 10.1017/S0033822200032161
Panarello, H. O., Tessone, A., Killian Galván, V. A., Samec, C. T., Kochi, S., Pirola, M., et al. (2021). 35 años de análisis de isótopos estables en la arqueología argentina: conceptos, fundamentos, metodología y aplicaciones. Cuad. del INAPL 30, 1–41. Available online at: https://revistas.inapl.gob.ar/index.php/cuadernos/article/view/1136
Roche, D., Ségalen, L., Balan, E., and Delattre, S. (2010). Preservation assessment of Miocene-Pliocene tooth enamel from Tugen Hills (Kenyan Rift Valley) through FTIR, chemical and stable-isotope analyses. J. Archaeol. Sci. 37, 1690–1699. doi: 10.1016/j.jas.2010.01.029
Rohrmann, A., Strecker, M. R., Bookhagen, B., Mulch, A., Sachse, D., Pingel, H., et al. (2014). Can stable isotopes ride out the storms? The role of convection for water isotopes in models, records, and paleoaltimetry studies in the central Andes. Earth Planet. Sci. Lett. 407, 187–195. doi: 10.1016/j.epsl.2014.09.021
Rozanski, K., and Araguás Araguás, L. (1995). Spatial and temporal variability of stable isotope composition of precipitation over the South American continent. Bulletin de l'Institut Français d'Études Andines 24, 379–390. doi: 10.3406/bifea.1995.1189
Rozanski, K., Araguás-Araguás, L., and Gonfiantini, R. (1993). “Isotopic patterns in modern global precipitation,” in Climate Change in Continental Isotopic Records, Geophys. Monogr. Ser. 78, eds. P. K. Swart, et al. (Washington, DC: AGU), 1–36. doi: 10.1029/GM078p0001
Ruthsatz, B., and Movia, C. (1975). Relevamiento de las estepas andinas del noreste de la provincia de Jujuy. Argentina: FECYT.
Samec, C., Arzamendia, Y., Baldo, J., Panarello, H., and Yacobaccio, H. (2014a). “Intra-individual variation in carbon and nitrogen isotope composition from bone and dentin collagen from a modern wild camelid in the Dry Puna of Argentina,” in Libro de Resúmenes de la 12th International Conference of Archaeozoology (Córdoba: Universidad Nacional de Córdoba), 138.
Samec, C., and Harrod, C. (2022). Muestreo secuencial de piezas dentales de camélidos domésticos y silvestres de las tierras altas de Atacama: primeros resultados de las composiciones isotópicas del carbono y el nitrógeno. Intersec. Antropol. 23, 51–66. doi: 10.37176/iea.23.1.2022.657
Samec, C., Morales, M., Pirola, M., Panarello, H., and Yacobaccio, H. (2017a). “Composición isotópica del oxígeno e hidrógeno en aguas meteóricas y superficiales de la Provincia de Jujuy, Argentina,” in Abstract retrieved from Segundo Taller Arqueología e Isótopos Estables en el Sur de Sudamérica (Argentina: San Rafael).
Samec, C., and Yacobaccio, H. (2021). Zooarqueología y análisis de isótopos estables en un contexto pastoril: el caso de Cueva Chayal (Jujuy, Argentina). Est. Atacameños 67:e4240. doi: 10.22199/issn.0718-1043-2021-0017
Samec, C. T., Morales, M. R., and Yacobaccio, H. D. (2014b). Exploring human subsistence strategies and environmental change through stable isotopes in the Dry Puna Argentina. Inter. J. Osteoarchaeol. 24, 134–148. doi: 10.1002/oa.2332
Samec, C. T., Pirola, M., Yacobaccio, H. D., and Panarello, H. O. (2020). Assessing prehispanic herding strategies through stable isotope analysis: A case study from the Dry Puna of Argentina. Environ. Archaeol. 25, 353–364. doi: 10.1080/14614103.2018.1549348
Samec, C. T., Yacobaccio, H. D., and Panarello, H. O. (2017b). Carbon and nitrogen isotope composition of natural pastures in the Dry Puna of Argentina: A baseline for the study of prehistoric herd management strategies. Archaeol. Anthropol. Sci. 9, 153–163. doi: 10.1007/s12520-015-0263-2
Samec, C. T., Yacobaccio, H. D., and Panarello, H. O. (2018). Stable isotope compositions of South American camelids in the Dry Puna of Argentina: A frame of reference for the study of prehistoric herding and hunting strategies. J. Archaeol. Sci. Rep. 18, 628–636. doi: 10.1016/j.jasrep.2017.10.042
Santana-Sagredo, F., Dufour, E., Goepfert, N., Zazzo, A., Franco-Jordán, R., and Vásquez-Sánchez, S. (2020). New bioarchaeological evidence and radiocarbon dates from the Lambayeque/Sicán culture camelids from the El Brujo complex (Northern Coast of Peru): implications for funerary and herd management practices. Environ. Archaeol. 25, 333–352. doi: 10.1080/14614103.2018.1556960
Sealy, J., Naidoo, N., Hare, V. J., Brunton, S., and Faith, J. T. (2020). Climate and ecology of the palaeo-Agulhas plain from stable carbon and oxygen isotopes in bovid tooth enamel from Nelson Bay Cave, South Africa. Quat. Sci. Rev. 235:105974. doi: 10.1016/j.quascirev.2019.105974
Sica, G. (2016). Procesos comunes y trayectorias diferentes en torno a las tierras de los Pueblos de Indios de Jujuy. Siglo XVI al XIX. Rev. Museo Antropol. 9, 171–186. doi: 10.31048/1852.4826.v9.n2.15891
Sponheimer, M., and Lee-Thorp, J. A. (1999). Oxygen isotopes in enamel carbonate and their ecological significance. J. Archaeol. Sci. 26, 723–728. doi: 10.1006/jasc.1998.0388
Squeo, F. A., Aravena, R., Aguirre, E., Pollastri, A., Jorquera, C. B., and Ehleringer, J. R. (2006). Groundwater dynamics in a coastal aquifer in north-central Chile: implications for groundwater recharge in an arid ecosystem. J. Arid Environ. 67, 240–254. doi: 10.1016/j.jaridenv.2006.02.012
Stevens, R. E., Lightfoot, E., Hamilton, J., Cunliffe, B. W., and Hedges, R. E. M. (2013). One for the master and one for the dame: stable isotope investigations of Iron Age animal husbandry in the Danebury Environs. Archaeol. Anthropol. Sci. 5, 95–109. doi: 10.1007/s12520-012-0114-3
Szpak, P., Chicoine, D., Millaire, J. F., White, C. D., Parry, R., and Longstaffe, F. J. (2016). Early horizon camelid management practices in the Nepeña Valley, North-Central Coast of Peru. Environ. Archaeol. 21, 230–245. doi: 10.1179/1749631415Y.0000000002
Szpak, P., Millaire, J. F., White, C. D., and Longstaffe, F. J. (2014). Small scale camelid husbandry on the north coast of Perú (Virú Valley): insight from stable isotope analysis. J. Anthropol. Archaeol. 36, 110–129. doi: 10.1016/j.jaa.2014.08.005
Szpak, P., White, C. D., Longstaffe, F. J., Millaire, J. F., and Vásquez Sánchez, V. (2013). Carbon and nitrogen isotopic survey of northern Peruvian plants: baselines for paleodietary and paleoecological studies. PLOS ONE 8:e53763. doi: 10.1371/journal.pone.0053763
Takigami, M., Uzawa, K., Seki, Y., Chocano, D. M., and Yoneda, M. (2020). Isotopic evidence for camelid husbandry during the formative period at the Pacopampa site, Peru. Environ. Archaeol. 25, 262–278. doi: 10.1080/14614103.2019.1586091
Thornton, E. K., DeFrance, S. D., Krigbaum, J., and Williams, P. R. (2011). Isotopic evidence for Middle Horizon to 16th Century camelid herding in the Osmore Valley, Peru. Inter. J. Osteoarchaeol. 21, 544–567. doi: 10.1002/oa.1157
Tieszen, L. L. (1991). Natural variations in the carbon isotope values of plants: implications for archaeology, ecology, and paleoecology. J. Archaeol. Sci. 18, 227–248. doi: 10.1016/0305-4403(91)90063-U
Tomasi, J. (2013). Espacialidades pastoriles en las tierras altoandinas. Asentamientos y movilidades en Susques, puna de Atacama (Jujuy, Argentina). Rev. Geogr. Norte Grande 55, 67–87. doi: 10.4067/S0718-34022013000200006
Tornero, C., Aguilera, M., Ferrio, J. P., Arcusa, H., Moreno-García, M., Garcia-Reig, S., et al. (2018). Vertical sheep mobility along the altitudinal gradient through stable isotope analyses in tooth molar bioapatite, meteoric water and pastures: a reference from the Ebro valley to the Central Pyrenees. Quat. Int. 484, 94–106. doi: 10.1016/j.quaint.2016.11.042
Tornero, C., Balasse, M., Balaçescu, A., Chataigner, C., Gasparyan, B., and Montoya, C. (2016). The altitudinal mobility of wild sheep at the Epigravettian site of Kalavan 1 (Lesser Caucasus, Armenia): evidence from a sequential isotopic analysis in tooth enamel. J. Hum. Evol. 97, 27–36. doi: 10.1016/j.jhevol.2016.05.001
Trayler, R. B., and Kohn, M. J. (2017). Tooth enamel maturation reequilibrates oxygen isotope compositions and supports simple sampling methods. Geochim. Cosmochim. Acta 198, 32–47. doi: 10.1016/j.gca.2016.10.023
Valenzuela-Lamas, S., Jiménez-Manchón, S., Evans, J., López, D., Jornet, R., and Albarella, U. (2016). Analysis of seasonal mobility of sheep in Iron Age Catalonia (north-eastern Spain) based on strontium and oxygen isotope analysis from tooth enamel: First results. J. Archaeol. Sci. Rep. 6, 828–836. doi: 10.1016/j.jasrep.2015.08.042
Vilá, B., and Arzamendia, Y. (2022). South American camelids: their values and contributions to people. Sustain. Sci. 17, 707–724. doi: 10.1007/s11625-020-00874-y
Vogel, J. C. (1993). “Variability of carbon isotope fractionation during photosynthesis,” in Stable Isotopes and Plant Carbon-Water Relations, eds. J. R. Ehleringer, A. E. Hall, G. D. Farquhar (San Diego: Academic Press), 29–38. doi: 10.1016/B978-0-08-091801-3.50010-6
Vuille, M., and Keimig, F. (2004). Interannual variability of summertime convective cloudiness and precipitation in the central Andes derived from ISCCP-B3 data. J Clim. 17, 3334–3348. doi: 10.1175/1520-0442(2004)017<3334:IVOSCC>2.0.CO;2
Westreicher, C. A., Mérega, J. L., and Palmili, G. (2007). The economics of pastoralism: study on current practices in South America. Nomadic Peoples 11, 87–105. doi: 10.3167/np.2007.110205
Yacobaccio, H. (2014). Pastoreo, movilidad y sequías. Cuad. del INAPL Series Especiales 2, 113–121. Available online at: https://revistas.inapl.gob.ar/index.php/series_especiales/article/view/1680
Yacobaccio, H. D. (2001). “La domesticación de camélidos en el Noroeste Argentino,” in Historia Argentina Prehispánica 1, eds. E. Berberián, A. Nielsen (Córdoba: Brujas), 7–40.
Yacobaccio, H. D. (2007). Andean camelid herding in the South Andes: ethoarchaeological models for archaeozoological research. Anthropozoologica 42, 143–154. Available online at: https://sciencepress.mnhn.fr/fr/periodiques/anthropozoologica/42/2/l-elevage-des-camelides-dans-les-andes-du-sud-modeles-ethnoarcheologiques-appliques-l-archeozoologie
Yacobaccio, H. D. (2021). The domestication of South American camelids: a review. Anim. Front. 11:65. doi: 10.1093/af/vfaa065
Yacobaccio, H. D., Madero, C. M., and Malmierca, M. P. (1998). Etnoarqueología de Pastores Surandinos. Buenos Aires: Grupo Zooarqueología de Camélidos.
Yacobaccio, H. D., Madero, C. M., Malmierca, M. P., and Reigadas, M. C. (1997-1998). Caza, domesticación y pastoreo de camélidos en la Puna Argentina. Rel. Soc. Arg. Antrop. XXII-XXIII, 389-418.
Yacobaccio, H. D., Morales, M. R., and Samec, C. T. (2009). Towards an isotopic ecology of herbivory in the Puna ecosystem: new results and patterns on Lama glama. Int. J. Osteoarchaeol. 19, 144–155. doi: 10.1002/oa.1050
Yacobaccio, H. D., Morales, M. R., Solá, P., Samec, C. T., Hoguin, R, and Oxman, B. I. (2013). Mid-Holocene occupation of the Dry Puna in NW Argentina: evidence from the Hornillos 2 rockshelter. Quat. Int. 307, 38–49. doi: 10.1016/j.quaint.2012.09.028
Yacobaccio, H. D., Samec, C., Marcoppido, G., and Vilá, B. (2018). Domesticación de camélidos y patologías óseas: un caso de estudio de la puna argentina. Cuad. del INAPL Series Especiales 6, 71–79. Available online at: https://revistas.inapl.gob.ar/index.php/series_especiales/article/view/1355
Yacobaccio, H. D., and Vilá, B. L. (2016). A model for llama (Lama glama Linnaeus, 1758) domestication in the southern Andes. Anthropozoologica 51, 5–13. doi: 10.5252/az2016n1a1
Yacobaccio, H. D., Catá, M. P., Morales, M. R., Solá, P., Alonso, M. S., Rosenbusch, M., et al. (2011). “El uso de cuevas por pastores andinos: el caso de Cueva Quispe (Susques, Puna de Jujuy),” in Arqueología en las Tierras Altas de Argentina. Evolución y Cambio Cultural, eds. H. Muscio, G. López (Oxford: BAR, Editorial John Hedges), 33–47.
Keywords: stable carbon isotope analysis, stable oxygen isotope analysis, sequential sampling, llama herding, seasonal mobility
Citation: Samec CT, Yacobaccio HD and Roberts P (2025) Late Holocene camelid pastoralism in the Puna highlands of Argentina: an integrative approach using stable isotope analysis of tooth enamel. Front. Environ. Archaeol. 4:1557715. doi: 10.3389/fearc.2025.1557715
Received: 09 January 2025; Accepted: 07 March 2025;
Published: 15 April 2025.
Edited by:
Richard Madgwick, Cardiff University, United KingdomReviewed by:
Claudia Costa, University of Algarve, PortugalNelson J. Almeida, University of Evora, Portugal
Copyright © 2025 Samec, Yacobaccio and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Celeste T. Samec, Y2VsZXN0ZXNhbWVjQGdtYWlsLmNvbQ==; Patrick Roberts, cm9iZXJ0c0BnZWEubXBnLmRl
 Celeste T. Samec
Celeste T. Samec Hugo D. Yacobaccio
Hugo D. Yacobaccio Patrick Roberts
Patrick Roberts