- 1Research Unit of Prehistory and Anthropology, Department of Physical Science, Earth and Environment, University of Siena, Siena, Italy
- 2Centro Studi sul Quaternario ODV, Sansepolcro, Arezzo, Italy
- 3Paleogenetics Unit, Laboratory of Anthropology Molecular Anthropology, Department of Biology, University of Florence, Florence, Italy
During the Late Mousterian period Apulia (southeastern Italy) was characterized by frequent and prolonged aridity that could have caused the scarcity of vegetable foods and, consequently, a lack of important nutritional compounds. Zooarchaeological studies from several Mousterian contexts show that Apulian Neanderthals may have responded to this crisis by increasing the exploitation of ungulates. In particular, bone grease rendering was likely one of the dominant activities conducted on-site. Anthropologists and nutritionists have long recognized that the diets of modern-day hunter-gatherers may represent a reference standard for human nutrition in the past and a model for their adaptation to specific environmental conditions. In addition, evaluating of certain qualitative and quantitative aspects of the animal/plant nutrient intake and absorption may provide important information regarding the nutritional needs and the physiology of these human groups. In this analysis, we combine ethnographic data related to animal economic subsistence patterns of hunter-gatherers, zooarchaeological data from Late Mousterian assemblages located in Apulia, the physiology of medium-large ungulates, as well as new paleo genomic analyses of Neanderthals and modern humans. Analyzing and displaying multiple sources of information allowed us to quantify a low daily energy intake from carbohydrates for Late Mousterian populations in southern Italy, in contrast to a surplus of animal protein and fats, obtained from the specific treatment of carcasses inferred from the zooarchaeological data.
1 Introduction
Climate and environmental constraints have strongly influenced human behaviors and cultures forcing past communities to develop peculiar settlement techniques to overcome these limits (Agustí et al., 2009; Timmermann et al., 2022).
Several archaeological records provide evidence that Neanderthals were able to adapt to harsh environments through their hunting capabilities focused upon specific animal species (Gaudzinski, 1995, 1996; Jaubert et al., 1990; Farizy et al., 1994; Brugal et al., 1996; Airvaux, 2004), as well as exploiting wider spectrum of different resources (Blasco et al., 2022 and references therein, Hardy, 2019).
Banks et al. (2021) demonstrated that between MIS 5a and MIS 4, Western European Neanderthals developed highly adaptive cultural innovations to continue exploiting habitual environments affected by pronounced climate change. This coincided with the appearance of the Quina lithic production system during MIS 4 in some areas of Europe (Banks et al., 2021).
Analyzing occlusal molar microwear texture in Western Eurasian Paleolithic hominins, El Zaatari et al. (2016) demonstrated behavioral differences that distinguish modern humans from Neanderthals, in relation to fluctuations in climatic conditions of the Pleistocene. They evidenced that Neanderthals altered their diets in response to changing palaeoecological conditions, in contrast to Homo sapiens, who seem to have been less affected by slight changes in vegetation/climatic conditions, modifying their technological complexes accordingly.
Unquestionably, diet represents the functional link through which climatic and environmental factors can cause strong evolutionary pressures, leading to different patterns in resources use. In this regard, analysis of the nutritional qualities and energetic costs of acquiring and processing of foods can help identify the nutritional needs of the Paleolithic hunter-gatherers. Eaton et al. (1997) and Eaton and Konner (1985) reconstructed a Paleolithic diet and their model was revisited by Cordain et al. (2000, 2001). All these authors suggested lower consumption of carbohydrates and higher intakes of protein and polyunsaturated fatty acid (Eaton et al., 1998; Cordain et al., 2001).
When the environmental constraints are extended for a long time, even genomic mutations can occur (Ameur et al., 2012). Although an ever-increasing number of works in recent years have focused on understanding in greater depth the Neanderthal genome (Cerqueira Caio et al., 2012; Weasel, 2022; McArthur et al., 2021), little effort has been made to investigate the possible physiological characteristics that can be inferred from the expression of the identified genes in these human groups. Southern Italy offers us the opportunity to study the adaptations and responses of Neanderthals to climate changes and to different environmental conditions in depth, at a regional scale and over a long period, due to its richness in Middle Paleolithic sites with well dated long-term stratigraphies spanning from MIS 5 to MIS 3 (Boscato and Crezzini, 2012a,b; Boschin et al., 2021). In the last 20 years detailed zooarchaeological analyses on faunal assemblages from different sites in southern Italy referable to both Mousterian and Upper Paleolithic technocomplexes (MIS 4-3), give us the possibility of identifying differences in animal exploitation between Neanderthals and modern humans. It was possible to highlight a particular treatment of carcasses during the Mousterian, not recorded in Upper Paleolithic samples (Crezzini et al., 2023; Boscato et al., 2006; Boscato and Crezzini, 2012a,b).
In this work, considering the ethnographic data related to the economic subsistence patterns of hunter-gatherers, we merged zooarchaeological and palaeoecological data with the nutritional value of the anatomical parts in medium-large ungulate carcasses. Additionally, a paleo genome- analysis was carried out to investigate possible genetic bases of the differences in fat metabolism between Neanderthals and modern humans.
The aim of this work was to investigate the likely quality and quantity of the dietary macronutrients comprised in the Last Mousterian diet through peculiar zooarchaeological data from southern Italy.
2 Materials and methods
The zooarchaeological data considered in this study is related to Late Mousterian ungulate assemblage from Grotta di Santa Croce (SUs 525-535-539) dated to the end of MIS 4/beginning of MIS 3 (Boscato and Crezzini, 2006; Ciullo, 2016; Boscato, 2017; Crezzini et al., 2023) (Supplementary Figure 1B, see also text in Supplementary material). The faunal composition is dominated by only two species: the horse and the aurochs, while a third taxon, the red deer, is represented by a femur fragment (Supplementary Tables 1, 2).
The assemblage is characterized by strong fragmentation, and it mainly comprise 3–6 cm size class remains. This likely increased the high identification of isolated teeth compared to postcranial elements. Among the latter, carpal and tarsal bones, as well as phalanges and sesamoids are scarce or absent and long bones are mainly represented by diaphyseal fractions rather than epiphyseal portions (Supplementary Tables 2–4) (Crezzini et al., 2023). In addition, a high ratio of diaphysis/epiphysis fragments and a low quantity of spongy bone remains are recorded in the taxonomically unidentified sample (Boscato and Crezzini, 2012a,b).
The bone sample does not show alterations from carnivore activities or post-depositional processes. The relationship between aurochs and horse skeletal elements and bone density indicated that the BMD does not fully explain the peculiar skeletal frequencies of these taxa (Crezzini et al., 2023). Analysis of tooth eruption and wear points to the exploitation of adult individuals, rather than juveniles and sub-adults (Crezzini et al., 2023).
The ungulate assemblage composition from two other important Mousterian assemblages from Apulia, Grotta del Cavallo – SU F (Nardò – province of Lecce) and Riparo l'Oscurusciuto – SUs 2, 4, and 9 (Ginosa – province of Taranto) show strong similarities with that of Grotta di Santa Croce (Boscato et al., 2006, 2010; Boscato and Crezzini, 2006, 2012a,b; Boscato, 2017; Crezzini et al., 2023).
These samples (where the aurochs is the most common ungulate, followed by horse and/or red deer) are all dated to MIS 3 and the sites are close to each other (about 120 km as the crow flies, Supplementary Figure 1A) It is important to keep in mind that investigations carried out in these three sites are different depositional contexts: a rockshelter (Riparo l'Oscurusciuto), a cave (Grotta del Cavallo) and a talus (Grotta di Santa Croce). This reduces the possibility that similarities between samples are biased by similar post-depositional factors. No evidence of the systematic use of bones as fuel was recorded (Spagnolo et al., 2016): the lack or scarcity of spongy elements in Apulian assemblages, together with the evidence of fresh-bone breakage, it was interpreted as an intense exploitation of bone marrow and grease by Neanderthals (Crezzini et al., 2023).
An assessment of the presence of aurochs skeletal parts from Grotta di Santa Croce as a function of their economic significance, such as modified total products, skeletal fat utility, protein and white grease fat content was carried out by Crezzini et al. (2023), using the economic anatomy of nowadays bison examined by Emerson (1990, see data relate to the individual of bison indicate by Emerson with the name SAM). In our work, we enlarged this analysis by investigating the skeletal frequencies of Bos primigenius as a function of their caloric and energetic values, in order to investigate the possible energetic contribution provided by animal food in Neanderthal diet.
The calorie yield of the carcass products indicated by Emerson (1990) are reported in Tables 1, 2. In addition, we added in each of the tables two new columns.
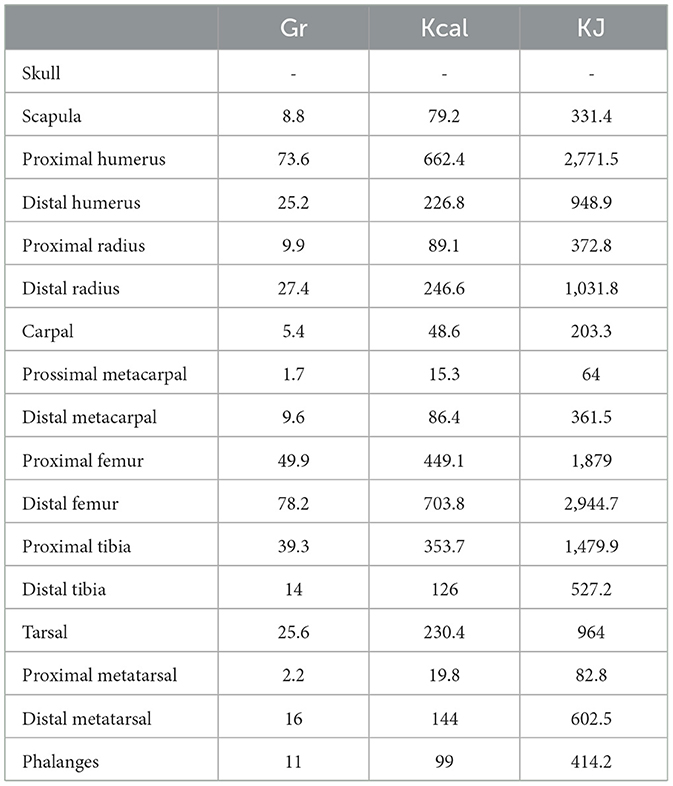
Table 1. Bone grease grams and energy yield of the bison carcass products (Emerson, 1990).
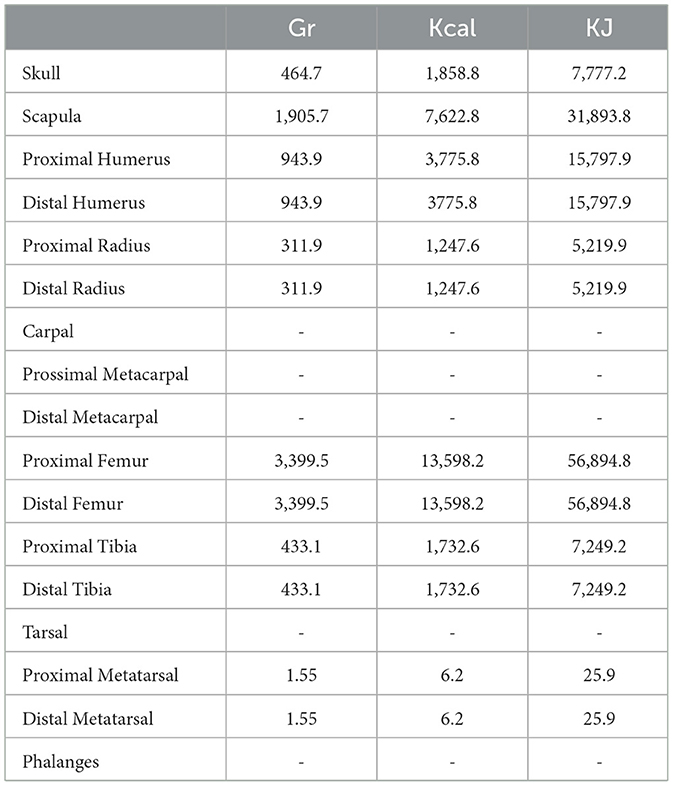
Table 2. Protein grams and energy yield of the bison carcass products (Emerson, 1990).
In Table 1 the new columns contain the bone grease grams and the relative energy yield (KJ) of carcass products, while in Table 2 the new columns contain protein grams and the relative energy yield (KJ) of carcass products. We calculated these values as follows:
The bone grease and the protein grams were calculated using the reverse process followed by Emerson to calculate the respective of calorie yield values (see Emerson, 1990, p. 888). To estimate the possible energetic contribution provided by plant food in Neanderthal diet, we considered the macronutrient composition and energy value of plants suggested by Eaton et al. (1998). Finally, we assumed a daily energy intake of 12,500 KJ, as suggested by Kuipers et al. (2010) for the Palaeolithic hunter-gatherers, and we used the equation suggested by Eaton and Konner (1985) for the determination of the projected macronutrient composition of pre-agricultural diets:
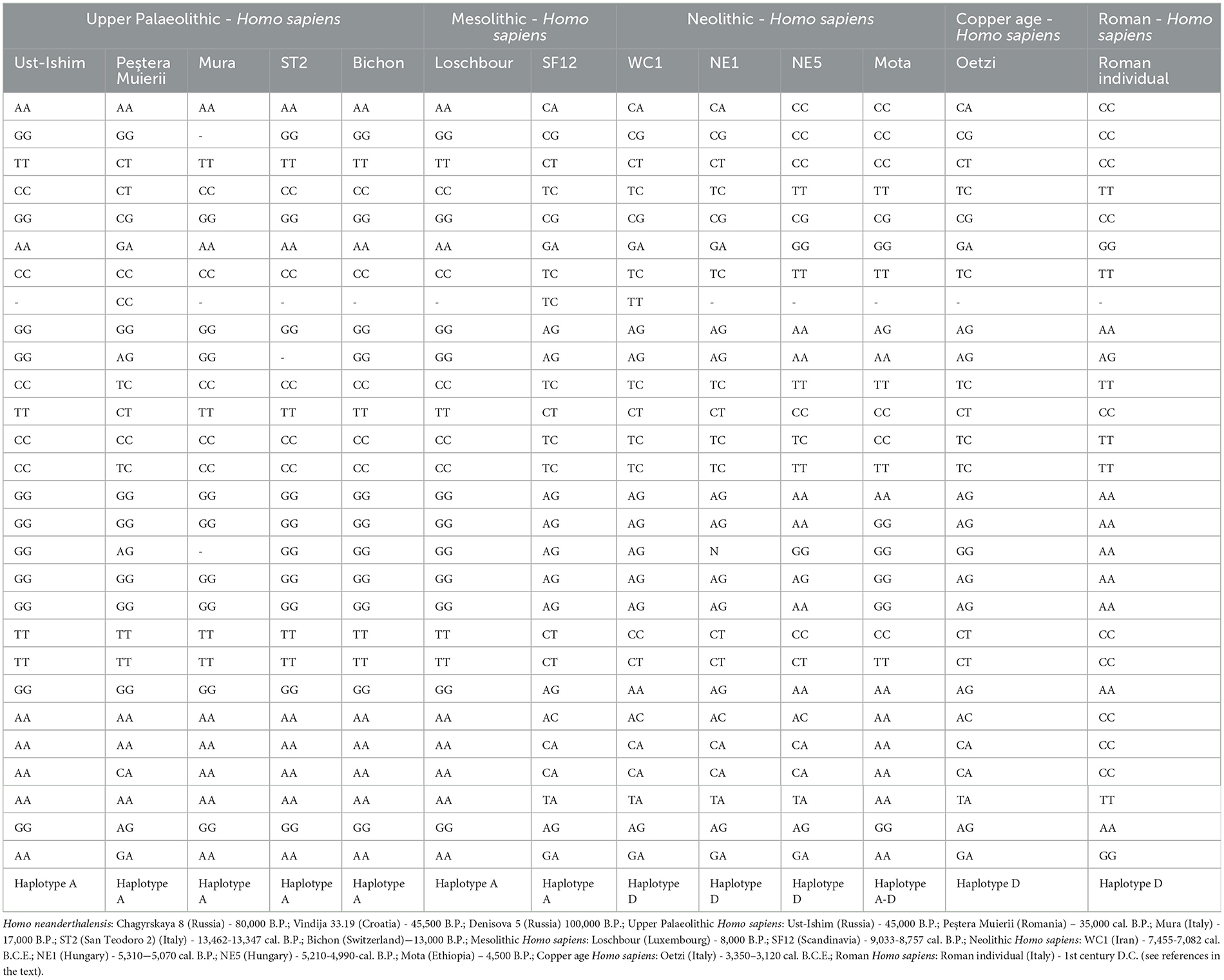
where Ca and Cp are the assumed proportions of animal and plant foods consumed, A is the mean energy content (KJ/gr) of animal foods and B is the mean energy content (KJ/gr) of plant foods. Considering a database of 21 wild animal foods and 44 plant foods, Eaton and Konner (1985) suggested the following values: A = 5.90 KJ/gr (or 1.41 Kcal/gr) and B = 5.40 KJ/gr (or 1.29 Kcal/gr). The variable x represents the total number of grams required to provide any given amount of food energy. For genomic investigations, among all the published human ancient genomes, we selected individuals present complete (or almost complete) genome with at least 2 × coverage and with available alignment files (in BAM format) mapped on Homo sapiens reference genome assembly GRCh37/hg19. Finally, 14 archaic human genomes were obtained (Table 3).
Moreover, we included 2 unpublished ancient individuals processed in the Laboratory of Anthropology (University of Florence) for other research projects. The haplotype analysis was based on the 28 SNP positions, previously found to distinguish haplotypes A and D (Ameur et al., 2012), spanning a 38.9 kb region that includes the promoter regions of FADS1 and FADS2. The variants were called using bcftools (RRID:SCR_005227, v1.16) (Li et al., 2009). Only the reads with a minimum mapping quality of 30 were used to call confident bases.
3 Results
3.1 Dietary macronutrient composition of the Apulian Neanderthals
During the Late Mousterian period Apulia was characterized by frequent and prolonged drought climate conditions.
Speleothem data (covariation of δ18O and δ13C) recorded on stalagmite PC from Pozzo Cucù Cave (Apulia, 40.90° N, 17.16° E), indicated two periods of extremely dry conditions: from 66.7+0.9/−1.2 to 65.6+1.1/−1.3 ka during MIS 4, and from 55.3+1.1/−2.5 to 54.9+1.1/−2.7 ka during MIS 3. The occurrence of these events fit with speleothem, lacustrine and marine records from the Mediterranean region. These periods are considered as the driest and probably coldest of the entire MIS 5–3-time span, at least in southern Italy, and the event at ~55 ka was certainly the driest of the entire record (Columbu et al., 2020 and references therein).
These climate conditions could have caused the scarcity of vegetable foods for Apulian Neanderthals (Boscato and Crezzini, 2012a; Boschin et al., 2021).
Consequently, in this analysis we use a P: A energy subsistence ratio of 15:85, considering mean subsistence economies by primary living environment in hunter-gatherer societies (Cordain et al., 2000, 2002; Speth, 2010). A significant value of plant contribution was assumed due to the archaeological evidence of plant use/collection from some Neanderthal sites (Henry et al., 2011; Hardy et al., 2012; Salazar-García et al., 2013; Power et al., 2018; Mariotti Lippi et al., 2023). Thus, through the equation suggested by Eaton and Konner (1985), the total number of grams required to provide the necessary amount of food energy in our model is:
Under these idealized and probably intermittent isocaloric conditions, the total daily food intake of 2,146 gr would have been provided by 1,824.1 gr of animal food (85%) and 321.9 gr of vegetable food (15%).
The daily energy intake derived from the consumption of these food quantities, respectively is (Supplementary Table 5):
3.1.1 The aurochs consumption
In the Grotta di Santa Croce assemblage, no remains of birds, fish and mollusk were recorded. Aurochs and horses (more rarely red deer) were hunted by Neanderthals (Supplementary Tables 1, 2) (Boscato et al., 2010; Crezzini et al., 2023). In the estimation of the macronutrients of these human groups we decided to consider only the exploitation of aurochs as animal food. Our decision was based on three different reasons: the need to simplify calculations in the construction of a food model for Neanderthals of Grotta di Santa Croce, the high presence of Bos primigenius in the Apulian Middle Palaeolithic zooarchaeological assemblages, indicating this species as one of the most widespread and exploited prey by humans in this area (Boscato et al., 2006, 2010; Boscato and Crezzini, 2006, 2012a,b; Boscato, 2017), and the availability of a detailed macronutrient quantitative and qualitative distributions in bovid carcass reported by Emerson in her study on the economic anatomy of nowadays adult bison (Emerson, 1990).
Emerson reported that the carcass weight of the examined individual was 640 Kg (Emerson, 1990, Table 2.4, p. 32). Levine et al. (1973) suggest that in ungulates in which the hooves, antlers, hide, and internal gastrointestinal contents were discarded, the remaining carcass represented 72% of the live weight. Therefore, we consider an edible carcass weighing 460.8 Kg. Pitts and Bullard (1968) demonstrated that the percentage body fat of a mammalian species can be estimated from its relative size via a formula:
Where FFM is the fat-free mass expressed in grams. For the bison examined by Emerson (1990) this value is 116.9 Kg (Emerson, 1990, note a, Table 6.7, p. 456, individual SAM), that give a percentage body fat of 19.5. This represents the x parameter included in the equation from Cordain et al. (2000 – Figure 3; p. 687):
useful to determine the relative contribution of fat energy from game food. Thus, an animal with 19.5% body fat would derive 71% of its energy as fat that, in our case, correspond to 7,641.02 KJ (10,762 KJ × 0.71) or to 1,826.24 Kcal (Supplementary Table 5). The relative contribution of protein energy from hunted animal food was also determined by using the equation proposed by Cordain et al. (2000 – Figure 3, p. 687):
We found that an animal with 19.5% body fat would derive 29% of its energy as protein, which corresponds to 3,120.98 KJ (10,762 KJ × 0.29) or to 745.93 Kcal (Supplementary Table 5).
3.1.2 The vegetal consumption
For the macronutrient composition and energy value of plant foods we considered the following distribution: 19 en% of fat, 13 en% of protein and 68 en% of carbohydrate (Eaton et al., 1998). This allowed us to determine, for an assumed P: A subsistence ratio of 15:85 and an energy intake of 12,500 KJ, the contribution of 243.75 KJ (0.13 × 0.15 × 12,500 KJ) or 58.25 Kcal from plant protein; the contribution of 356.25 KJ (0.19 × 0.15 × 12,500 KJ) or 85.14 Kcal, from plant fat; and a contribution of 1,275 KJ (0.68 × 0.15 × 12,500 KJ) or 304.73 Kcal from plant carbohydrates (Supplementary Table 5).
3.2 The intake and the metabolism of different macronutrients
In the previous paragraph we calculated the grams of animal and plant food that would have had to be consumed by Apulian Neanderthals on a P: A = 15%:85% diet and the energy contribution provided by different macronutrients.
Regarding animal food, we do not know which parts of the bovid carcass would have been preferably meet Neanderthal daily energy requirements. By comparing the macronutrients/energy data calculated in the previous paragraph and the economic values of bison parts reported by Emerson (1990) with the skeletal frequencies recorded at Grotta di Santa Croce (Supplementary Tables 2–4) (Crezzini et al., 2023), we aim to investigate some peculiar aspects of aurochs' exploitation by southern Italy Neanderthals, likely related to their nutritional needs.
In addition, the section on carbohydrates includes speculations on the possible contributions provided with plant food. Finally, we describe the results of our preliminary research on the fat metabolism related to characteristic mutations of FADS1 and FADS2 genes.
3.2.1 Lipids
Examining the energy intake (KJ) related to the different anatomical parts of bison published by Emerson (1990) it is noteworthy that the rare or absent aurochs skeletal elements at Grotta di Santa Croce are characterized by the highest values of bone grease (carpal and tarsal bones, proximal and distal femur, proximal humerus, distal radius, distal metapodials, phalanges) (Table 1; Supplementary Tables 2–4).
Exploitation of skeletal fat only from these bones could provide about 11,172 KJ (2,670 Kcal), a higher amount of energy than the total provided by animal food calculated in this paper (about 10,762 KJ or 2,572.2 Kcal, Supplementary Table 5), for an assumed P:A subsistence ratio of 15:85. Such amounts of energy from these skeletal elements can be obtained through only 296.7 gr of bone grease intake, a small amount in respect to the total grams of animal food that probably must have been included in the Neanderthal diet hypothesized in this study (1,824.1 gr). A low quantity of bone grease rendering (about 100 gr) from specific skeletal portions such as distal radius, carpals, distal tibia, tarsals, distal metapodial and phalanges (the rarest aurochs bones recorded in the Grotta di Santa Croce assemblage) provides about 4,104.5 KJ, more than half the energy from animal fat calculated in our model (7,641.02 KJ, Supplementary Table 5).
3.2.2 Protein
According to the hypothesized diet in this paper, Apulian Neanderthals should have obtained about 3,120.98 KJ (or 745.93 Kcal) from animal protein (Supplementary Table 5). At Grotta di Santa Croce the aurochs body parts are positively correlated with their protein quantity (p = 0.27, Crezzini et al., 2023, Figure 6, p. 8).
This correlation is not significant, but the highest MAU (Minimal Animal Units) values are related to the medial portions of humerus, tibia and femur (Supplementary Table 3). Excluding the scapula (which still present), these skeletal portions provide the largest amounts of energy from protein (Table 2). Archaeological records indicate that European Neanderthals and quasi-contemporary modern humans of the mid-Upper Paleolithic may have had to rely repeatedly on diets characterized by large (excessive) amounts of animal protein. This evidence comes from isotope studies of the fossil human remains: the collagen of these Late Pleistocene humans is more enriched in 15N than that of contemporary carnivores (Lee-Thorp and Sponheimer, 2006; Robbins et al., 2005). However, it is noted that the maximum amount of lean meat that a forager can safely consume is finite. This is due to the limit of the liver that can no longer effectively deaminate the amino acids to avoid a hazardous build-up of ammonia (hyperammonemia) and excess amino acids (hyperaminoacidemia) in the blood (Dimski, 1994; Husson et al., 2003; Powers-Lee and Meister, 1988).
If protein averages about 16% nitrogen, a widely used value to estimate total or “crude” protein that an 80 kg (176 lb) adult can consume is about 261 gr per day (range 221–305 g) (Cordain et al., 2000; Mann, 2000).
If we consider the meat nutritional value of the proximal humerus of bison reported by Emerson (1990) (Table 2), we can calculate the energy provided by the consumption of 200 gr of meat recovered from this anatomical part:
This suggests that Neanderthal (an 80 kg adult) could probably obtain the entire quantity of energy from animal protein (3,120.98 KJ) assumed in our model by consuming a “safe” amount of bovid protein.
3.2.3 Carbohydrates
In our hypothesized diet, the proportion of plants consumed is low. The amount that would have been consumed slightly exceeds 320 gr. The energy from fat and protein (356.25 and 243.75 KJ respectively, Supplementary Table 5) probably could be easily offset by the high availability of animal resources. On the other hand, plant carbohydrates would contribute the highest value: 1,275 KJ (Supplementary Table 5). This daily energy contribution from plant carbohydrates appears to be quite high if we consider the quality and quantity of plant resources recoverable in a prairie-steppe environment such as that hypothesized for the Late Mousterian in Apulia (Columbu et al., 2020; Boschin et al., 2021). Studies conducted to investigate biochemical characteristics of common steppe species indicate a low caloric content of their seeds (generally the plant structure with the higher nutritional value). Examining the seeds of Oryzopsis hymenoides (Poaceae), Kelrick and MacMahon (1985) determined a value of 4,058 calories per gram (Table 2, p. 67). This value corresponds to 16,9 KJ. The (soluble and structural) carbohydrates represent 49.1% of the total seed weight (Kelrick and MacMahon, 1985, Table 3, p. 68). Therefore, to reach the daily amount of carbohydrate energy from plants assumed in our model, Neanderthal would have had to consume daily about 75.4 gr of these seeds.
3.2.4 The paleogenome analysis
If the zooarchaeological data from the investigated Late Mousterian contexts of southern Italy indicated an intense exploitation of fats by Neanderthals, analogous analyses on faunal assemblages from different sites in southern Italy referable to Upper Palaeolithic, don't evidence this settlement pattern in modern humans, although the preys remained the same (Boscato and Crezzini, 2012b). To find a possible genetic basis for these resource needs by Neanderthal, we analyzed FADS1 and FADS2 genes for Neanderthals and modern human.
These genes, located in chromosome 11, encoding two key enzymes for the long-chain polyunsaturated fatty acids (LC-PUFAs) biosynthesis (Nakamura and Nara, 2004), the omega-3 docosahexaenoic (DHA) acid and the omega-6 arachidonic acid (AA). LC-PUFAs are essential to maintain the function of human brain and central nervous system and they can be supplied through dietary intake as DHA and AA directly or as their 18-carbon precursors. DHA is mainly found in fish, AA is also present in eggs, land-animal fats, and liver while the precursors are found in high quantities in some vegetable oils (Ratnayake and Galli, 2009). The conversion of the 18-carbon precursors to LC-PUFAs is done through a series of elongations and desaturations of the fatty-acid molecules and the efficiency of this process is strongly correlated with enzymes encoded by FADS1 and FADS2 (Schaeffer et al., 2006; Bokor et al., 2010). In extant human populations, two FAD haplotypes (A and D) were identified – defined by 28 closely linked SNPs – that differ in their ability for synthesizing LC-PUFAs (Ameur et al., 2012). Haplotype D, is characterized as an enzymatically efficient gene signature, showing significantly increased desaturase activity. It is nearly fixed in African populations, while in Europe and the Middle East shows a frequency of 75%. Haplotype A is associated with a reduction in the enzymatic efficiently and its frequency is close to 100% in American populations. Recent data suggests that the functionality of diverse fatty acid desaturase enzymes found in humans today have been shaped over the course of evolution by environmental, dietary, and nutritional selective pressures in combination with genetic events, such as duplications, losses, and mutations (Castro et al., 2012; Vicedomini et al., 2021). Moreover, Ameur et al. (2012) observed that primates and archaic humans, notably the Neanderthals and the Denisovans, have haplotypes that are very similar to haplotype A, suggesting that haplotype D likely appeared in modern human lineage after the split from the common ancestor with Neanderthals. According to this observation, we investigated the possible interaction between genetic variants of FAD genes and regional exclusive dietary in Neanderthals and ancient modern humans. With this aim, we analyzed genomic data of 16 ancient individuals from different regions and cultures, which include 3 high coverage genomes of Neanderthal (Prüfer et al., 2014, 2017; Mafessoni et al., 2020), 7 Upper Paleolithic and Mesolithic hunter-gatherers (Fu et al., 2014; Svensson et al., 2021; Higgins et al., 2024; Posth et al., 2023; Jones et al., 2015; Lazaridis et al., 2014; Günther et al., 2018), 5 Neolithic farmers (Lazaridis et al., 2014; Broushaki et al., 2016; Gamba et al., 2014; Gallego Llorente et al., 2015; Wang et al., 2023) and one individual from Roman age (Table 3), focusing on the 28 SNPs that distinguish haplotypes A and D.
Our analysis showed that all Neanderthals have nucleotide variants found on both human haplotypes but with high similarity with haplotype A, confirming previous results based on incomplete sequence of Neanderthal genome (Ameur et al., 2012). Interestingly, Chagyrskaya 8 and Vindija 33.19, who are more closely related to each other than to the Neanderthal from Denisova Cave (Mafessoni et al., 2020), share seven deletions absent in Denisova 5 (Table 3).
Regarding the Upper Paleolithic hunter-gatherers, all the specimens analyzed were homozygous for haplotype A, except for Peştera Muierii, an Early Upper Paleolithic woman from Romania (Svensson et al., 2021) which displays a higher genetic variability with 12 heterozygous variants. The ancestral haplotype A is also recovered in Mesolithic individuals from Luxembourg (Lazaridis et al., 2014) and from Scandinavia (Günther et al., 2018).
Starting from the Neolithic period, we observed the first evidence of the derived haplogroup D. Among the early farmers, Mota, a 4,500-year-old individual from Ethiopia (Gallego Llorente et al., 2015), carry mixed FADS haplotype consistent with a progressive reduction of haplotype A by recombination with haplotype D in African populations. Additionally, our results indicate that Neolithic individuals from Europe and the Near East were characterized by a high genetic variability in FAD genes as suggested by the observed heterozygosity which is not found in the Paleolithic specimens. In our ancient dataset, the first occurrence of homozygous haplotype D was detected in a historical individual from Roman period.
4 Discussion
4.1 Lipids
At Grotta Santa Croce aurochs body parts are positively and significantly correlated with Unsaturated Marrow Index (p = 0.006; Crezzini et al., 2023). Binford (1978) mentions that marrow with high oleic acid content being more desirable in Nunamiut people due to its taste. However, mere flavor can't represent the only reason when considering an adaptation for exploiting the marrow from specific ungulate body parts (Binford, 1978).
Besides a specific taste, differences in fat chemistry cause different consistency, appearances and melting points that may be useful both to obtain satiety, delay postprandial appetite sensations, increase energy intake, and for daily activities, like waterproofing clothes, heat production, mechanical buffering and lubrication (Hadley, 1985; Klaus, 2001).
In recent medical literature there is a lack of consensus regarding whether changes in the saturation profile of a high-fat meal can affect post-ingestion satiety and energy intake (Burton-Freeman, 2005; Kamphuis et al., 2001; Strik et al., 2010).
On the other hand, it is possible that the Palaeolithic hunter-gatherers were “aware” that the consumption of resources characterized by specific fat chemistry could have a long-term effect on their health (Outram, 1998).
Oleic acid, together with other18-C FA compounds, is an important precursor for the de novo biosynthesis of pivotal substances like LC-PUFA (DHA and AA) through elongations and desaturase activity by enzymes encoded by specific genes including FADS1 and FADS2.
The marrow and the bone grease of the distal limb bones, the most represented ungulate parts, together with the skull, in Palaeolithic contexts, are characterized by the higher percentage of oleic acid and other 18-carbon unsaturated fatty acids like ALA and LA (Morin, 2007).
The presence in both Neanderthal and modern humans of the ancestral FAD haplotypes (A), characterized as an enzymatically less efficient gene signatures could explain the significant exploitation of these parts of the ungulate carcasses to ensure the biosynthesis of a sufficient amount of LC-PUFA, achievable only through the intake of high quantity of their precursors.
Focusing on our Mousterian faunal assemblage, these dietary habits could have been particularly crucial for Apulian Neanderthal due to specific environment constraints. During the frequent and prolonged drought climate conditions that characterized the Late Mousterian period in this region (Columbu et al., 2020), the availability of 18-C FA compounds, contained in high quantities in vegetable food could have been very scarce.
Examining the percentages of oleic acid in caribou anatomical parts published by Binford (1978), it's possible to note that the most exploited skeletal elements at Grotta di Santa Croce contain higher amounts of oleic acid in skeletal grease than in marrow (Supplementary Table 3).
The specific exploitation of bone grease by Apulian Neanderthal could be related to the necessity of increasing the intake/absorption of 18-carbon precursors to LC-PUFAs from animal resources, through intense bone fragmentation/destruction of the limb bone epiphyses, the carpals, the tarsals, and the phalanges.
4.2 Proteins
Despite the large number of studies, the amount of protein that one can safely consume on a long-term basis is a topic in need of much more research, and different amounts are suggested according to different human groups (past and present) (Speth, 2010). The human body can adapt to changes in consumption by regulating the enzymes involved in protein metabolism and urea synthesis. This process of adaptation may take a few days to complete, a period longer than the duration of many of the nutritional studies designed to explore the effects of high-protein intakes (Institute of Medicine, 2005).
It is noted that arctic people present genome mutation that allows them avoid toxicity with ingestion of high amounts of meat (Clemente et al., 2014), through a diet with a P:A ratio very unbalanced toward animal resources (Binford, 1978; Sinclair, 1953).
If as calculated in our work, already low amounts of protein could meet the animal protein energy requirements of Apulian Mousterians, what advantage could a probable excess of protein intake (probably above the safe limit of 261 g for an adult weighing 80 kg) have? This issue is addressed in the next paragraph.
4.3 Carbohydrates
The gathering pattern of plant food in Palaeolithic can be predicted by the “optimal foraging theory” (Hawkes et al., 1982; Simms, 1987). Studies of hunter-gatherers have shown that the plants were collected with a general prioritization that maximizes the rate of energy capture relative to energy expenditure. More than 100 plant species can be used, but only a small fraction of these were regularly consumed and providing the greatest ratio of energy capture to energy expenditure (Simms, 1987; O'Connell and Hawkes, 1981).
This optimal foraging has been complex to implement in a prairie-steppe environment like that probably present in Apulia during the Late Mousterian. Zimov et al. (2012) indicate that typical steppe which developed during the Late Pleistocene in Europe is understood to have comprised primarily plant taxa as Cyperaceae, Artemisia, and Gramineae. Many species of Cyperaceae store carbohydrates in their underground systems, but not all are edible, and some require processing to access the nutrients (Zhang et al., 2024). Artemisia is a genus of bitter-tasting aromatic herbs and shrubs with strong chemical constituents that can be poisonous (Bora and Sharma, 2011). Gramineae (Poaceae) is a family of grasses widely spread in different vegetal ecosystems. Despite this, it includes taxa that store their energy as carbohydrates largely in its rhizomes and so it must be dug out (Hardy et al., 2022).
Other Gramineae species produce small edible seeds are characterized by low rates of energy. This is the case of Oryzopsis hymenoides (Eriocoma hymenoides) described in the previous paragraph: in respect to the amount admitted in our Neanderthal model, a low return of carbohydrate energy from this type of plant could be obtained, with high energy expenditure due to the collection of large quantity of their small seeds. The carbohydrate intake in these human groups could thus have been low.
As suggested by Hardy et al. (2022), although individual and short-term survival is possible on a relatively low-carbohydrate diet, Neanderthals had large, energy-expensive brains and led physically active lifestyles, suggesting that for optimal health they would have required a high intake of carbohydrates, in particular glucose. The European Food Information Council (EUFIC) indicates that a modern normal weight adult requires 200 gr of glucose per day, two-thirds of which (about 130 grams) is specifically needed by the brain to cover its glucose needs (Howarth et al., 2012). Despite the brain accounts for ~2% of the body weight, it consumes ~20% of glucose-derived energy (Institute of Medicine, 2005; Sweeting et al., 2021).
One hypothesis is that to meet this need, high-performance physiological processes capable of regulating blood glucose might have been present in Neanderthals. Among these processes, gluconeogenesis that needs to be given the most consideration (Hardy et al., 2022).
Gluconeogenesis (i.e., de novo synthesis of glucose) occurs in the liver and kidneys, it is stimulated by the diabetogenic hormones (glucagon, growth hormone, epinephrine, and cortisol). It is a metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates like glycerol (derived from fat breakdown), lactate (derived from muscles), propionate and certain specific amino acids obtained from the breakdown of proteins (for example, alanine). These amino acids are converted into urea through the urea cycle, whereas their carbon skeletons are transformed into other intermediates, mostly glucose.
Gluconeogenesis becomes vital during periods of metabolic stress, such as starvation (Acheson et al., 1984). It is interesting to note that Veldhorst et al. (2009) investigated gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet and they demonstrated that forty-two percent of the increase in energy expenditure after this diet was related to an increase in gluconeogenesis. The cost of gluconeogenesis was 33% of the energy content of the produced glucose (Veldhorst et al., 2009).
Our hypothesis is that the Neanderthal very-high-protein diet could have stimulated the process of gluconeogenesis. A probable excess of protein intake could have furnished a high quantity of amino-acids. The bio synthesis of glucose from these compounds may have compensated for the carbohydrate requirement deficit in Neanderthal due to low plant consumption.
5 Conclusions
The estimation of the dietary macronutrient ratio performed in this paper has permitted to better understand of the specific treatments of ungulate carcasses by Neanderthals, evidenced through the zooarchaeological studies carried out in southern Italy.
The scarcity/lack of epiphyses and spongy bones of ungulates recorded in assemblages can be explained by the intensive exploitation of these parts, characterized by a high fat energy yield. Paleogenomic data from the analysis of FADS1 and FADS2 genes underlined the importance for Apulian Neanderthals of 18-carbon unsaturated fatty acid intake from animal resources, to address the low availability of these compounds from plant resources. A high consumption of animal protein for the human groups investigated in this study was also determined. This could have meant a surplus of these substances in respect to the daily protein energy needs, providing high quantities of amino acids, important non-carbohydrate precursors for the gluconeogenesis. The gluconeogenesis products could have compensated for the low carbohydrate intake from plant consumption.
Our results stimulate investigating the possible presence of enzymatically related gene signatures for high-performance gluconeogenesis in Neanderthals. Obtaining data on this biochemical process in the context of Neanderthal physiology could be important to highlight new differences between these human groups compared with modern humans.
Like all metabolic processes, gluconeogenesis is characterized by different phases and dynamics that may characterize the metabolic structure of the species and express its specific nutritional requirements and chronic diseases.
Probably the most important aspect of this process for the topics discussed in this paper, is the hepatic insulin signaling that regulates gluconeogenetic activity (Onyango, 2022). In modern populations, if insulin target cells of the liver become less responsive to insulin, the hepatic insulin resistance (HIR) occurs, which, promoting excessive gluconeogenesis, contributes to the development of (pre)diabetes (Basu et al., 2013).
Paradoxically, HIR also promotes de novo lipid synthesis (Smith et al., 2020), leading to excessive deposition of fat in the liver (hepatic steatosis) and secretion of very low-density lipoproteins (VLDL) into the blood (Meshkani and Khosrow, 2009; Cook et al., 2015). These metabolic dysregulations cause dyslipidemia, cardiovascular disease and atherosclerosis (Meshkani and Khosrow, 2009; Berndt et al., 2021), which can progress to cirrhosis and hepatocellular carcinoma (Lu et al., 2021).
Specific metabolic mechanisms in the absorption and production of fats may have preserved Neanderthals from these diseases are related to gluconeogenic overactivity.
Finally, it's important to note that the breakdown of the proteins and the turn them into amino acids, pivotal substrates for gluconeogenesis, can also be obtained by chemical reactions that occur in specific food processing. This is the case of the external fermentation. Besides amino acids, the fermentation of lipids also produces long chain fatty acids (LCFAs) and glycerol, other important substrates for gluconeogenesis.
The external fermentation is hypothesized as an activity conducted by the Mousterian hunter gatherers at Grotta di Santa Croce (Crezzini et al., 2023).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
JC: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AM: Conceptualization, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. CC: Conceptualization, Methodology, Software, Writing – review & editing. DC: Funding acquisition, Methodology, Resources, Validation, Writing – review & editing. AR: Writing – review & editing. PB: Writing – review & editing. AM: Writing – review & editing. FB: Formal analysis, Funding acquisition, Resources, Supervision, Visualization, Writing – review & editing, Writing – original draft.
Funding
The authors declare that financial support was received for the research and/or publication of this article. The authors thank the University of Siena for the financial support provided for the publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fearc.2025.1558698/full#supplementary-material
References
Acheson, K. J., Schutz, Y., Bessard, T., Ravussin, E., Jequier, E., Flatt, J. P., et al. (1984). Nutritional influences on lipogenesis and thermogenesis after a carbohydrate meal. Am. J. Physiol. 246, 62–70. doi: 10.1152/ajpendo.1984.246.1.E62
Agustí, J., Blain, H. A., Cuenca-Bescós, G., and Bailon, S. (2009). Climate forcing of first hominid dispersal in Western Europe. J. Hum. Evol. 57, 815–821. doi: 10.1016/j.jhevol.2009.06.005
Airvaux, J. (2004). “Le site paléolithique de chez-Pinaud à Jonzac, Charente-Maritime,” in Prehistoire du Sud-Ouest, Vol. 8, 108.
Ameur, A., Enroth, S., Johansson, A., Zaboli, G., Igl, W., Johansson, A. C., et al. (2012). Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 90, 809–820. doi: 10.1016/j.ajhg.2012.03.014
Arrighi, S., Freguglia, M., Ranaldo, F., and Ronchitelli, A. (2009). Production and use in the lithic industry of the Mousterian in Santa Croce (Bisceglie, Italy). Humna E24, 91–106.
Banks, W. E., Moncel, M. H., Raynal, J. P., Cobos, M. E., Romero-Alvarez, D., Woillez, M. N., et al. (2021). An ecological niche shift for Neanderthal populations in Western Europe 70,000 years ago. Sci. Rep. 11:5346. doi: 10.1038/s41598-021-84805-6
Basu, R., Barosa, C., Jones, J., Dube, S., Carter, R., Basu, A., et al. (2013). Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J Clin Endocrinol Metab. 98, E409–E417. doi: 10.1210/jc.2012-3056
Berndt, N., Kolbe, E., Gajowski, R., Eckstein, J., Ott, F., Meierhofer, D., Matz-Soja, M., et al. (2021). Functional consequences of metabolic zonation in murine livers: insights for an old story. Hepatology 73, 795–810. doi: 10.1002/hep.31274
Binford, L. R. (1978). Dimensional analysis of behavior and site structure: learning from an Eskimo hunting stand. Am. Antiq. 43, 330–361. doi: 10.2307/279390
Blasco, R., Cochard, D., Colonese, A. C., Laroulandie, V., Meier, J., Morin, E., et al. (2022). “Small animal use by Neanderthals,” in Updating Neanderthals, eds. F. Romagnoli, F. Rivals, and S. Benazzi (London: Academic Press), 123–143. doi: 10.1016/B978-0-12-821428-2.00010-X
Bokor, S., Dumont, J., Spinneker, A., Gonzalez-Gross, M., Nova, E., Widhalm, K., et al. (2010). HELENA Study Group Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J. Lipid Res. 51, 2325–2333. doi: 10.1194/jlr.M006205
Bora, K. S., and Sharma, A. (2011). The genus Artemisia: a comprehensive review. Pharm. Biol. 49, 101–109. doi: 10.3109/13880209.2010.497815
Boscato, P. (2017). Ambienti e economia nel Paleolitico medio della Puglia: lo studio delle faune. Studi di Preistoria e Protostoria. 4, 119–124.
Boscato, P., and Crezzini, J. (2006). The exploitation of ungulate bones in Homo neanderthalensis and Homo sapiens. Hum. Evol. E21, 311–320. doi: 10.1007/s11598-006-9031-8
Boscato, P., and Crezzini, J. (2012a). “Il deposito musteriano del Riparo l'Oscurusciuto (Ginosa - TA): la fauna a grandi mammiferi delle UUSS 1÷9,” in Proceedings of the 6°Convegno Nazionale di Archeozoologia, eds. J. De Grossi Mazzorin, D. Saccà, and C. Tozzi May 21–24, 2009; Orecchiella (IT). 25–32.
Boscato, P., and Crezzini, J. (2012b). Middle-upper palaeolithic transition in Southern Italy: Uluzzian macromammals from Grotta del Cavallo (Apulia). Quat. Int. 252, 90–98. doi: 10.1016/j.quaint.2011.03.028
Boscato, P., Crezzini, J., Freguglia, M., Gambassini, P., Ranaldo, F., and Ronchitelli, A. (2010). “Activités de subsistance et exploitation des ressources de l'environnement à S. Croce (Bisceglie - Bari - Italie du Sud): les unités stratigraphiques 546 et 535 du Paléolithique moyen”, in Settlement dynamics of the middle paleolithic and middle stone age, eds. N. J. Conard and A. Delagnes (Vol. III. Kerns Verlag: Tübingen), 265–282.
Boscato, P., Crezzini, J., and Pellegrini, A. (2006). “Le parti mancanti: faune del Paleolitico medio nel deposito esterno della U,” in Archaeozoological studies in honour of Alfredo Riedel, ed. B. Sala (Bolzano: Ufficio Beni Archeologici), 39–50.
Boschin, F., Columbu, A., Spagnolo, V., Crezzini, J., Bahain, J. J., Falguères, C., et al. (2021). Human occupation continuity in southern Italy towards the end of the Middle Palaeolithic: a palaeoenvironmental perspective from Apulia. J. Q. Sci. 37, 204–216. doi: 10.1002/jqs.3319
Broushaki, F., Thomas, M. G., Link, V., López, S., van Dorp, L., Kirsanow, K., et al. (2016). Early neolithic genomes from the eastern fertile crescent. Science, 353, 499–503. doi: 10.1126/science.aaf7943
Brugal, J-. P., Costamagno, S., Jaubert, J., and Mourre, V. (1996). “Les gisements paléolithiques de Coudoulous (Tour de Faure, Lot, France),” in Proceeding of the XIII International Congress of Prehistoric and Protohistoric Sciences; September 8–14; Forlì (Italy).
Burton-Freeman, B. (2005). Sex and cognitive dietary restraint influence cholecystokinin release and satiety in response to preloads varying in fatty acid composition and content. J. Nutr. 135, 1407–1414. doi: 10.1093/jn/135.6.1407
Cardini, L. (1954). Scoperte e scavi paletnologici in Italia 1954: Bisceglie, Bari. Rivista Scienze Preistoriche IX:128.
Castro, L. F., Monroig, Ó., Leaver, M. J., Wilson, J., Cunha, I., Tocher, D. R., et al. (2012). Functional desaturase Fads1 (Δ5) and Fads2 (Δ6) orthologues evolved before the origin of jawed vertebrates. PLoS ONE. 7:31950. doi: 10.1371/journal.pone.0031950
Cerqueira Caio, C. S., Paixão-Côrtes, V. R., Zambra, F. M. B., Salzano, F. M., Hünemeier, T., Bortolini, M. C., et al. (2012). Predicting Homo pigmentation phenotype through genomic data: from Neanderthal to James Watson. Am. J. Hum. Biol. 24, 705–709. doi: 10.1002/ajhb.22263
Ciullo, S. (2016). Archeozoologia nella Grotta di Santa Croce (Bisceglie - BT). Le unità stratigrafiche (bachelor thesis). University of Siena, Siena, Italy. 538–549.
Clemente, F. J., Cardona, A., Inchley, C. E., Peter, B. M., Jacobs, G., Pagani, L., et al. (2014). A selective sweep on a deleterious mutation in CPT1A in Arctic populations. Am. J. Hum. Genet. 95, 584–589. doi: 10.1016/j.ajhg.2014.09.016
Columbu, A., Chiarini, V., Spötl, C., Benazzi, S., Hellstrom, J., Cheng, H., et al. (2020). Speleothem record attests to stable environmental conditions during Neanderthal-modern human turnover in southern Italy. Nat. Ecol. Evol. 4, 1188–1195. doi: 10.1038/s41559-020-1243-1
Cook, J. R., Langlet, F., Kido, Y., and Accili, D. (2015). Pathogenesis of selective insulin resistance in isolated hepatocytes. J. Biol. Chem. 290, 13972–13980. doi: 10.1074/jbc.M115.638197
Cordain, L., Eaton, S. B., Miller, J. B., Mann, N., and Hill, K. (2002). The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur. J. Clin. Nutr. 56, S42–S52. doi: 10.1038/sj.ejcn.1601353
Cordain, L., Miller, J. B., Eaton, S. B., Mann, N., Holt, S. H., Speth, J. D., et al. (2000). Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 71, 682–692. doi: 10.1093/ajcn/71.3.682
Cordain, L., Watkins, B. A., and Mann, N. J. (2001). Fatty acid composition and energy density of foods available to African hominids. Evolutionary implications for human brain development. World Rev. Nutr. Diet 90, 144–161. doi: 10.1159/000059813
Crezzini, J., Boscato, P., Ronchitelli, A., and Boschin, F. (2023). A peculiar exploitation of ungulates at Grotta di Santa Croce: bone grease rendering and nutritional patterns among Neanderthals in southern Italy. Hist. Biol. 36, 2133–2145. doi: 10.1080/08912963.2023.2242630
Dimski, D. S. (1994). Ammonia metabolism and the urea cycle: function and clinical implications. J. Vet. Intern. Med. 8, 73–78. doi: 10.1111/j.1939-1676.1994.tb03201.x
Eaton, S. B., Eaton, S. B., and Konner, M. J. III (1997). Paleolithic nutrition revisited: a twelve-year retrospective on its nature and implications. Eur. J. Clin. Nutr. 51, 207–216. doi: 10.1038/sj.ejcn.1600389
Eaton, S. B., Eaton, S. B., Sinclair, A. J. III, Cordain, L., and Mann, N. J. (1998). Dietary intake of long-chain polyunsaturated fatty acids during the paleolithic. World Rev. Nutr. Diet 83, 12–23. doi: 10.1159/000059672
Eaton, S. B., and Konner, M. (1985). Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med. 312, 283–289. doi: 10.1056/NEJM198501313120505
El Zaatari, S., Grine, F. E., Ungar, P. S., and Hublin, J-. J. (2016). Neandertal versus modern human dietary responses to climatic fluctuations. PLoS ONE 11:e0153277. doi: 10.1371/journal.pone.0153277
Emerson, A. M. (1990). Archaeological Implications of Variability in the Economic Anatomy of Bison (dissertation). Washington State University, United States.
Farizy, C., David, F., and Jaubert, J. (1994). Hommes et Bisons du Palèolithique moyen a Mauran (Haute Garonne). Paris: ed. CNRS.
Fu, Q., Li, H., Moorjani, P., Jay, F., Slepchenko, S. M., Bondarev, A. A., et al. (2014). Genome sequence of a 45,000-year-old modern human from western Siberia. Nature 514, 445–449. doi: 10.1038/nature13810
Gallego Llorente, M., Jones, E. R., Eriksson, A., Siska, V., Arthur, K. W., Arthur, J. W., et al. (2015). Ancient Ethiopian genome reveals extensive Eurasian admixture throughout the African continent. Science 350, 820–822. doi: 10.1126/science.aad2879
Gamba, C., Jones, E. R., Teasdale, M. D., McLaughlin, R. L., Gonzalez-Fortes, G., Mattiangeli, V., et al. (2014). Genome flux and stasis in a five millennium transect of European prehistory. Nat. Commun. 5:5257. doi: 10.1038/ncomms6257
Gaudzinski, S. (1995). Wallertheim revisited: a re-analysis of the fauna from the Middle Palaeolithic site of Wallertheim (Rheinhessen/Germany). J Archaeol Sci. 22, 51–66. doi: 10.1016/S0305-4403(95)80162-6
Gaudzinski, S. (1996). On bovid assemblages and their consequences for the knowledge of subsistence patterns in the Middle Palaeolithic. Proceedings of the Prehistoric Society, 62, 19–39. doi: 10.1017/S0079497X00002723
Günther, T., Malmström, H., Svensson, E. M., Omrak, A., Sánchez-Quinto, F., Kilinç, G. M., et al. (2018). Population genomics of Mesolithic Scandinavia: investigating early postglacial migration routes and high-latitude adaptation. PLoS Biol. 16:e2003703. doi: 10.1371/journal.pbio.2003703
Hadley, N. F. (1985). The Adaptive Role of Lipids in Biological Systems. Hoboken: John Wiley and Sons.
Hardy, K. (2019). Paleomedicine and the use of plant secondary compounds in the Paleolithic and Early Neolithic. Evol Anthropol. 28, 60–71. doi: 10.1002/evan.21763
Hardy, K., Bocherens, H., Miller, J. B., and Copeland, L. (2022). Reconstructing Neanderthal diet: the case for carbohydrates. J. Hum. E162:103105. doi: 10.1016/j.jhevol.2021.103105
Hardy, K., Buckley, S., Collins, M. J., Estalrrich, A., Brothwell, D., Copeland, L., et al. (2012). Neanderthal medics? Evidence for food, cooking and medicinal plants entrapped in dental calculus. Naturwissenschaften 99, 617–626. doi: 10.1007/s00114-012-0942-0
Hawkes, K., Hill, K., and O'Connell, J. F. (1982). Why hunters gather: optimal foraging and the Ache of eastern Paraguay. Am. Ethnol. 9, 379–398. doi: 10.1525/ae.1982.9.2.02a00100
Henry, A. G., Brooks, A. S., and Piperno, D. R. (2011). Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium). Proc. Natl. Acad. Sci. U. S. A. 108:486e491. doi: 10.1073/pnas.1016868108
Higgins, O. A., Modi, A., Cannariato, C., Diroma, M. A., Lugli, F., Ricci, S., et al. (2024). Life history and ancestry of the late Upper Palaeolithic infant from Grotta delle Mura, Italy. Nat. Commun. 15:8248. doi: 10.1038/s41467-024-51150-x
Howarth, C., Gleeson, P., and Attwell, D. (2012). Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow. Metab. 32, 1222–1232. doi: 10.1038/jcbfm.2012.35
Husson, A., Brasse-Lagnel, C., Fairand, A., Renouf, S., and Lavoinne, A. (2003). Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem. 270, 1887–1899. doi: 10.1046/j.1432-1033.2003.03559.x
Institute of Medicine. (2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Institute of Medicine, Panel on Macronutrients, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: National Academies Press.
Jaubert, J., Lorblanchet, M., Laville, H., Slot-Moller, R., Turq, A., Brugal, J-. P., et al. (1990). Les Chasseurs d'Auroch de La Borde. Paris, ed. de la Maison des Sciences del'Homme. doi: 10.4000/books.editionsmsh.36013
Jones, E., Gonzalez-Fortes, G., Connell, S., Siska, V., Eriksson, A., Martiniano, R., et al. (2015). Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Commun. 6:8912. doi: 10.1038/ncomms9912
Kamphuis, M. M., Westerterp-Plantenga, M. S., and Saris, W. H. (2001). Fat-specific satiety in humans for fat high in linoleic acid vs fat high in oleic acid. Eur. J. ClinNutr. 55, 499–508. doi: 10.1038/sj.ejcn.1601222
Kelrick, M. I., and MacMahon, J. A. (1985). Nutritional and physical attributes of seeds of some common sagebrush-steppe plants: Some implications for ecological theory and management. J. Range Manage. 38, 65–69. doi: 10.2307/3899336
Klaus, S. (2001). Adipose Tissues. Georgetown: Eurekah.com/Landes Bioscience. doi: 10.1201/9781498712644
Kuipers, R. S., Luxwolda, M. F., Janneke Dijck-Brouwer, D. A., Eaton, S. B., Crawford, M. A., Cordain, L., et al. (2010). Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Brit. J. Nutr. 104, 1666–1687. doi: 10.1017/S0007114510002679
Lazaridis, I., Patterson, N., Mittnik, A., Renaud, G., Mallick, S., et al. (2014). Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413. doi: 10.1038/nature13673
Lee-Thorp, J., and Sponheimer, M. (2006). Contributions of biogeochemistry to understanding hominin dietary ecology. Yearb. Phys. Anthropol. 49, 131–148. doi: 10.1002/ajpa.20519
Levine, E., Collins, V., Varner, S., Williams, G., and Hardenbrook, H. J. (1973). Dietary protein for man and animal. Ill Vet 16, 10–14.
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinforma. Oxf. Engl. 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Lu, Q., Tian, X., Wu, H., Huang, J., Li, M., Mei, Z., et al. (2021). Metabolic changes of hepatocytes in NAFLD. Front. Physiol. 12:710420. doi: 10.3389/fphys.2021.710420
Mafessoni, F., Grote, S., de Filippo, C., Slon, V., Kolobova, K. A., Viola, B., et al. (2020). A high-coverage neandertal genome from Chagyrskaya Cave. PNAS 117, 15132–15136. doi: 10.1073/pnas.2004944117
Mallegni, F., Piperno, M., and Segre, A. (1987). Human remains of Homo sapiens neanderthalensis from the pleistocene deposit of Sants Croce Cave, Bisceglie(Apulia), Italy. Am. J. Phys. Anthropol. 72, 421–429. doi: 10.1002/ajpa.1330720402
Mann, N. (2000). Dietary lean red meat and human evolution. Eur. J. Nutr. 39, 71–79. doi: 10.1007/s003940050005
Mariotti Lippi, M., Aranguren, B., Arrighi, S., Attolini, D., Benazzi, S., Boschin, F., et al. (2023). New evidence of plant food processing in Italy before 40ka. Quat. Sci. Rev. 312:108161. doi: 10.1016/j.quascirev.2023.108161
McArthur, E., Rinker, D. C., and Capra, J. A. (2021). Quantifying the contribution of Neanderthal introgression to the heritability of complex traits. Nat. Commun. 12:4481. doi: 10.1038/s41467-021-24582-y
Meshkani, R., and Khosrow, A. (2009). Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin. Biochem. 42, 1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018
Morin, E. (2007). Fat composition and Nunamiut decision-making: a new look at the marrow and bone grease indices. J. Archaeol Sci. 34, 69–82. doi: 10.1016/j.jas.2006.03.015
Nakamura, M. T., and Nara, T. Y. (2004). Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 24, 345–376. doi: 10.1146/annurev.nutr.24.121803.063211
O'Connell, J. F., and Hawkes, K. (1981). “Alyawara plant use and optimal foraging theory,” in Hunter-Gatherer Foraging Strategies, eds. B. Winterhalder and E. A. Smith (Chicago: University of Chicago), 99–125.
Onyango, A. N. (2022). Excessive gluconeogenesis causes the hepatic insulin resistance paradox and its sequelae. Heliyon 8, 8–12. doi: 10.1016/j.heliyon.2022.e12294
Outram, A. K. (1998). The identification and palaeoeconomic context of prehistoric bone marrow and grease exploitation (dissertation). University of Dhuram, Durham, United Kingdom.
Pitts, G. C., and Bullard, T. R. (1968). “Some interspecific aspects of body composition in mammals,” in Body Composition in Animals and Man. Washington, DC: National Academy of Sciences, 45–70.
Posth, C., Yu, H., Ghalichi, A., Rougier, H., Crevecoeur, I., Huang, Y., et al. (2023). Palaeogenomics of upper palaeolithic to neolithic European hunter-gatherers. Nature 615, 117–126. doi: 10.1038/s41586-023-05726-0
Power, R. C., Salazar-García, D. C., Rubini, M., Darlas, A., Harvati, K., Walker, M., et al. (2018). Dental calculus indicates widespread plant use within the stable Neanderthal dietary niche. J. Hum. E119, 27e−41. doi: 10.1016/j.jhevol.2018.02.009
Powers-Lee, S. G., and Meister, A. (1988). “Urea synthesis and ammonia metabolism,” in The Liver: Biology and Pathobiology2 2nd Edn, eds. I. M. Arias, W. B. Jakoby, H. Popper, D. Schachter, D. A. Shafritz (New York, NY: Raven Press), 317–329.
Prüfer, K., de Filippo, C., Grote, S., Mafessoni, F., Korlević, P., Hajdinjak, M., et al. (2017). A high-coverage Neandertal genome from Vindija Cave in Croatia. Science 358, 655–658. doi: 10.1126/science.aao1887
Prüfer, K., Racimo, F., Patterson, N., Jay, F., Sankararaman, S., Sawyer, S., et al. (2014). The complete genome sequence of a Neanderthal from the Altai Mountains. Nature 505, 43–49. doi: 10.1038/nature12886
Ranaldo, F., Arrighi, S., Freguglia, M., Boscato, P., and Ronchitelli, A. (2017). La variabilitàtecnica del Musteriano della Grotta di Santa Croce (Bisceglie - BT). Studi diPreistoria e Protostoria 4, 153–158.
Ratnayake, W. M., and Galli, C. (2009). Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: a background review paper. Ann. Nutr. Metab. 55, 8–43. doi: 10.1159/000228994
Robbins, C. T., Felicetti, L. A., and Sponheimer, M. (2005). The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia 144, 534–540. doi: 10.1007/s00442-005-0021-8
Salazar-García, D. C., Power, R. C., Serra, A. S., Villaverde, V., Walker, M. J., Henry, A. G., et al. (2013). Neanderthal diets in central and southeastern Mediterranean Iberia. Quat. Int. 318, 3–18. doi: 10.1016/j.quaint.2013.06.007
Schaeffer, L., Gohlke, H., Müller, M., Heid, I. M., Palmer, L. J., Kompauer, I., et al. (2006). Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 15, 1745–1756. doi: 10.1093/hmg/ddl117
Segre, A. G., and Cassoli, P. F. (1987). “Giacimento preistorico del Pleistocene medio e superiore della grotta di S. Croce, Bisceglie (Bari),” Proceedings ofthe XXV Riunione Scientifica I.I.P.P. Monopoli, 111–118.
Simms, S. R. (1987). Behavioral Ecology and Hunter-Gatherer Foraging. Oxford, United Kingdom: BAR International Series. 381, 1–157. doi: 10.30861/9780860544937
Sinclair, H. M. (1953). The diet of Canadian Indians and Eskimos. Proc. Nutr. Soc. 12, 69–82. doi: 10.1079/PNS19530016
Smith, G., Shankaran, I., Yoshino, M., Schweitzer, M., Chondronikola, G. G., Beals, M., et al. (2020). Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 130, 1453–1460. doi: 10.1172/JCI134165
Spagnolo, V., Marciani, G., Aureli, D., Berna, F., Boscato, P., Ranaldo, F., et al. (2016). Between hearths and volcanic ash: the SU 13 palimpsest of theOscurusciuto rock shelter (Ginosa - Southern Italy): analytical and inter-pretative questions. Quat. Int. 417, 105–121. doi: 10.1016/j.quaint.2015.11.046
Speth, J. D. (2010). The Paleoanthropology and Archaeology of Big-Game Hunting. New York: Springer. doi: 10.1007/978-1-4419-6733-6
Strik, C. M., Lithander, F. E., McGill, A. T., MacGibbon, A. K., McArdle, B. H., Poppitt, S. D., et al. (2010). No evidence of differential effects of SFA, MUFA or PUFA on post-ingestive satiety and energy intake: a randomised trial of fatty acid saturation. Nutr. J. 9:24. doi: 10.1186/1475-2891-9-24
Svensson, E., Günther, T., Hoischen, A., Hervella, M., Munters, A. R., et al. (2021). Genome of Peştera Muierii skull shows high diversity and low mutational load in pre-glacial Europe. Curr. Biol. 31, 2973–2983.e9. doi: 10.1016/j.cub.2021.04.045
Sweeting, A., Mijatovic, J., Brinkworth, G. D., Markovic, T. P., Ross, G. P., Brand-Miller, J., et al. (2021). The carbohydrate threshold in pregnancy and gestational diabetes: how low can we go? Nutrients 13:2599. doi: 10.3390/nu13082599
Timmermann, A., Yun, K-. S., Raia, P., Ruan, J., Mondanaro, A., Zeller, E., et al. (2022). Climate effects on archaic human habitats and species successions. Nature 604, 495–501. doi: 10.1038/s41586-022-04600-9
Veldhorst, M. A., Westerterp-Plantenga, M. S., and Westerterp, K. R. (2009). Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am. J. Clin. Nutr. 90, 519–526. doi: 10.3945/ajcn.2009.27834
Vicedomini, R., Righetti, N., Polit, L., Condemi, S., Longo, L., Carbone, A., et al. (2021). Genetic dietary adaptation in Neandertal, Denisovan and Sapiens revealed by gene copy number variation. bioRxiv [Preprint]. doi: 10.1101/2021.10.30.466563
Wang, K., Prüfer, K., Krause-Kyora, B., Childebayeva, A., Schuenemann, V. J., Coia, V., et al. (2023). High-coverage genome of the Tyrolean Iceman reveals unusually high Anatolian farmer ancestry. Cell Genom. 3:100377. doi: 10.1016/j.xgen.2023.100377
Weasel, L. (2022). How Neanderthals became white: the introgression of race into contemporary human evolutionary genetics. Am. Nat. 200, 129–139. doi: 10.1086/720130
Zhang, H., Zhu, Z., Di, Y., Luo, J., Su, X., Shen, Y., et al. (2024). Understanding the triacylglycerol-based carbon anabolic differentiation in Cyperus esculentus and Cyperus rotundus developing tubers via transcriptomic and metabolomic approaches. BMC Plant Biol. 24:1269. doi: 10.1186/s12870-024-05999-1
Keywords: Neanderthal diet, late Mousterian, southern Italy, genome analysis, zooarchaeology, macronutrient compositions
Citation: Crezzini J, Modi A, Cannariato C, Caramelli D, Ronchitelli A, Boscato P, Moroni A and Boschin F (2025) Neanderthal had a “crush” on fats. Macronutrient estimation in Middle Paleolithic (Late Mousterian) hunter-gatherers of southern Italy. Front. Environ. Archaeol. 4:1558698. doi: 10.3389/fearc.2025.1558698
Received: 10 January 2025; Accepted: 21 May 2025;
Published: 18 June 2025.
Edited by:
Mariana Nabais, Institut Català de Paleoecologia Humana i Evolució Social (IPHES), SpainReviewed by:
Juan Marín, National University of Distance Education (UNED), SpainJose A. Solano, University of Granada, Spain
Copyright © 2025 Crezzini, Modi, Cannariato, Caramelli, Ronchitelli, Boscato, Moroni and Boschin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacopo Crezzini, amFjb3BvLmNyZXp6aW5pQHVuaXNpLml0
 Jacopo Crezzini
Jacopo Crezzini Alessandra Modi3
Alessandra Modi3 David Caramelli
David Caramelli