Abstract
This study examines the ungulate and carnivore remains recovered from the Middle Palaeolithic site of Gruta da Figueira Brava, Portugal, to assess Neanderthal subsistence strategies in the region during late Marine Isotope Stage 5 (MIS-5). The site, now facing the Atlantic Ocean, was located up to 2 km inland at the time of occupation, providing access to both terrestrial and coastal environments. Despite extensive fragmentation and carbonate encrustation of the faunal assemblage, zooarchaeological and taphonomic analyses reveal a diversity of prey species, dominated by red deer (Cervus elaphus) and ibex (Capra pyrenaica), with lesser contributions from aurochs (Bos primigenius) and horses (Equus caballus). The skeletal element representation, along with cut marks, percussion marks and burning evidence suggest a complex and flexible approach to resource transport, processing and consumption. Neanderthals exploited both high-yield and marginal bone portions, maximising nutritional intake through cooking, defleshing and marrow extraction. The assemblage suggests that whole deer carcasses were occasionally transported to the site, while selective transport strategies were applied to larger taxa. The presence of carnivore remains, including bears (Ursus arctos), hyaenas (Crocuta crocuta), wolves (Canis lupus) and wild cats (Felis silvestris), with no evidence of human-carnivore interactions, suggests intermittent use of the cave by non-human predators during periods of human absence (e.g., for cat denning and bear hibernation or as a hyaena latrine).
1 Introduction
Neanderthals primarily exploited a variety of ungulate species. In the Iberian Middle Palaeolithic, red deer often predominates in faunal assemblages, while other Cervidae, Caprinae and Equidae are also regularly found; whereas Bovidae, Rhinocerotidae and Suidae remains are less abundant (e.g., Álvarez-Lao and García, 2011a,b; Álvarez-Lao and Méndez, 2016; Marín-Arroyo and Sanz-Royo, 2021; Moclán et al., 2021; Rosell et al., 2012; Sanz et al., 2019; Yravedra and Cobo-Sánchez, 2015). However, the relative abundance of different taxa largely depends on the geographical setting, local environmental conditions and species' seasonal availability (e.g., Salazar-García et al., 2013; Blasco and Fernández Peris, 2012; Blasco et al., 2013; Finlayson et al., 2012). Although less frequent, certain European Middle Palaeolithic contexts present faunal assemblages that indicate monospecific hunting practices (i.e., when the assemblage is dominated by a single taxon; e.g., Daujeard et al., 2017; Farizy et al., 1994; Gaudzinski and Roebroeks, 2000; Patou-Mathis, 2006). Regardless of whether driven by ecological factors or deliberate human choice, these animals were hunted through ambush and stalking techniques, with different age groups targeted during specific seasons, which demonstrates the adaptability and flexibility of Neanderthal hunting strategies (e.g., Patou-Mathis, 2000; Gaudzinski and Roebroeks, 2000). These cases suggest a well-coordinated predation strategy that involved pre-planned tactics, intensive group collaboration and a deep understanding of the landscape and prey behaviour necessary to effectively intercept herds at water streams or along their migratory routes, and then drive and trap them into natural features like cliffs, gorges, or swamps (e.g., Gaudzinski-Windheuser and Kindler, 2012; Rodríguez-Hidalgo et al., 2017; White et al., 2016).
Consequently, it is not unexpected that several studies analysing Neanderthal skeletal remains have characterised their diet as heavily meat-oriented, with enriched δ15N isotope values in comparison with other carnivores, such as lions and hyaenas (e.g., Bocherens et al., 2014; Jaouen et al., 2019). However, the consumption of various plant foods has also been demonstrated (e.g., Henry et al., 2014; Zilhão et al., 2020). Several authors have suggested that Neanderthal subsistence strategies varied as a function of the type of ecosystem they lived in—steppe-tundra in the north, variably wooded landscapes in the south, arid steppe in the east (e.g., Carrión et al., 2019; Stewart et al., 2019)—and it has also been recently shown that, when living by the sea, they developed accomplished coastal adaptations (e.g., Stringer et al., 2008; Zilhão et al., 2020). While higher diversity of prey does not necessarily equate to greater adaptability or flexibility per se—as it may be dependent on other factors, such as opportunistic catches, the development of new techniques, increased sedentism—, it contributes to the understanding of the complexity of the subsistence strategies used by these hominins. This maturing picture of Neanderthal subsistence patterns draws on several decades of research, which has progressively shifted the perception of Neanderthal behaviour from simplistic to increasingly complex.
However, Neanderthals were not the only predators consuming ungulates, inhabiting or visiting caves; other carnivores, such as bears, hyaenas, wolves, foxes, wild cats, among others, also occupied these spaces (e.g., Cobo-Sánchez et al., 2024; Dusseldorp, 2013; Romandini et al., 2018; Sanchis et al., 2019; Zilhão et al., 2010). As a result, faunal studies of the Palaeolithic period require comprehensive zooarchaeological and taphonomic analyses to distinguish human from non-human contributions to the assemblage. Once human involvement in a faunal accumulation is established, a detailed characterisation of subsistence strategies becomes possible. Given the rarity of direct evidence for hunting techniques in the Middle Palaeolithic, the quantitative and qualitative analysis of ungulate remains, combined with eco-ethological information on game species and their environments, plays a crucial role in reconstructing human subsistence behaviours. These analyses provide insights into acquisition and processing techniques, as well as consumption patterns, offering a better understanding of Neanderthal adaptations and their interaction with the biomes they exploited. The ability to select prey or to opportunistically catch it, to process carcasses efficiently and to respond to ecological variables demonstrates advanced cognitive skills, environmental awareness and strategic decision-making, which are all key indicators of behavioural complexity (e.g., Johansson, 2014; Langley et al., 2008; Rendu, 2022).
In this study, we employ zooarchaeological and taphonomic methods to examine the ungulate and carnivore remains recovered from the Middle Palaeolithic cave site of Gruta da Figueira Brava in central Portugal. Despite the small sample size of identifiable specimens and the presence of dense calcareous coatings on the bones' surfaces, we aim (1) to identify and reconstruct the palaeoecological areas of the surrounding landscape where ungulates were present and available for exploitation, (2) to characterise the agent(s) responsible for the bone accumulation in the cave, (3) to identify the hunting and carcass processing strategies associated with Neanderthal groups that inhabited the site during the later part of Marine Isotope Stage 5 (MIS-5).
2 The site
Gruta da Figueira Brava is located about 30 km (as the crow flies) south of Lisbon (Portugal), on the southern slope of the Arrábida mountain chain (Figure 1A). Currently, the cave features three main entrances but access to the interior is only possible through Entrance 1; the others are blocked by sediment and speleothems (Figures 1B, C). Area C, in the interior part of Entrance 2, was excavated in the 1980s (Antunes, 2000); the recent, 2010–2013 excavations took place in Entrance 3 and the interior area behind (Area F and the SEx trench; Figures 1B–D). Today, Mediterranean vegetation dominated by evergreen trees and shrubs with patches of more forested areas and others of more open terrain covers the limestone slopes rising behind the cave (Ribeiro, 1945). The palaeobotanical evidence indicates that, through the time of the cave's Middle Palaeolithic occupation, the surrounding landscape was much like at present (Zilhão et al., 2020).
Figure 1
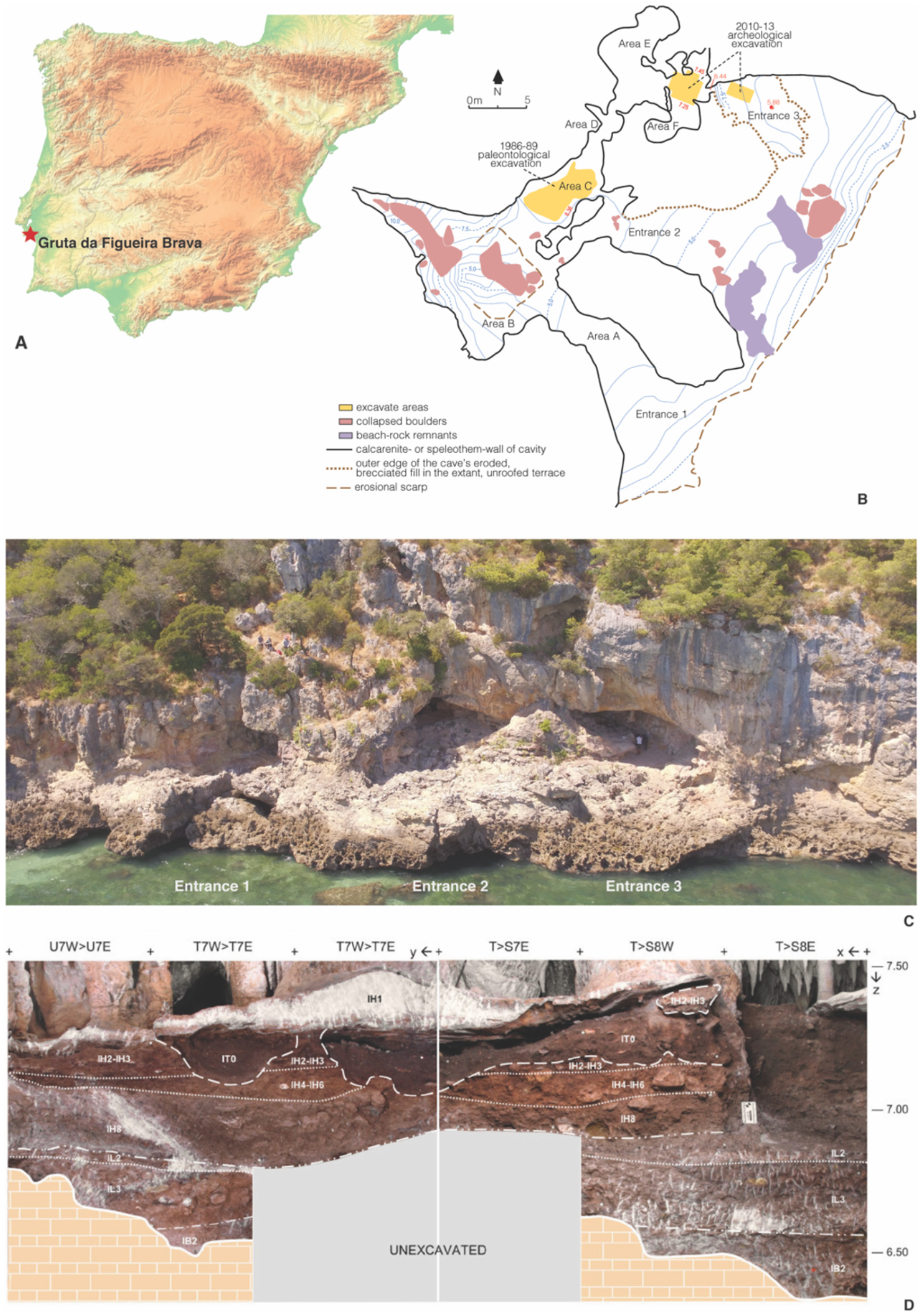
The Middle Palaeolithic site Gruta da Figueira Brava, Setúbal, Portugal. (A) Site location in the Iberian Peninsula. (B) Plan of the cavities—elevations are in metres above sea level. (C) The cave seen from the sea; the three different entrances are indicated. (D) Stratigraphic profile of Area F; units IL2 and IL3 belong in phase FB3, units IH2-IH3 to IH8 belong in phase FB4; IT0 denotes the reworked deposit. (A, C) are reproduced from Nabais et al. (2023a), published under a CC BY license, with permission from Frontiers, original copyright 2023. (B, D) are reproduced from Zilhão et al. (2020), published under a CC BY license, with permission from The American Association for the Advancement of Science, original copyright 2020.
The cave opens at the base of a biocalcarenite escarpment and onto an interglacial marine abrasion platform, 5 m above modern sea level. At the time of occupation, estimated distances to the coastline range between 750 m (during occupation phases FB1 and FB2, dated to MIS-5c) and 2,000 m (during occupation phase FB4, dated to MIS-5b) (Zilhão et al., 2020). The site therefore remained within easy reach of the seaside and associated coastal environments (e.g., estuaries, pools, lagoons). The ecotonal environment provided opportunities to exploit a wide range of ecological niches, as evidenced by the inhabitants' diverse diet. This included tortoises, crabs, birds, fish and shellfish (Nabais et al., 2023a,b; Nabais and Zilhão, 2019; Zilhão et al., 2020), as well as marine mammals and very large terrestrial mammals (e.g., rhinoceros), which were identified in the initial excavations conducted at the site during the 1980s (Antunes, 2000).
The coupled dating of sediments (by OSL) and speleothems (by U-series) places the occupation sequence in the ca. 86–106 ka interval. The oldest evidence comes from the SEx trench, where the MIS-5b levels were washed away due to Holocene sea level rise, but brecciated layers dated to MIS-5c could be archaeologically excavated and yielded charcoal, shells, bones and artefacts. The remains from the most recent occupation phase come entirely from Area F. Here, the Pleistocene deposit was sealed by flowstone (Figure 1D), but significant small mammal burrowing nonetheless resulted in a network of tunnels and chambers where intrusive Holocene items could be found among the deposit's reworked Pleistocene content. The Holocene intrusions are easy to recognise and, where mammals are concerned, mostly correspond to the bone remains of leporids, rodents and insectivores. The macro-mammal remains found in the reworked levels derive mostly, if not entirely, from sediments laid down during the FB4 occupation phase (Zilhão et al., 2020). However, through abundance of caution, we have counted them separately under the category “Reworked.” While these finds will be referenced when pertinent, all relative frequencies and other statistical measures mentioned in the text are calculated exclusively based on the MIS-5 in situ material.
3 Materials and methods
All materials recovered were studied and quantifications were done by spit and then agglomerated according to provenience: reworked; and MIS-5 occupation phases FB4, FB3 and FB2. Every bone and tooth fragment was examined, recorded and counted. Every bone fragment, whether it was triangulated on site or not, was assigned a unique database number. Abundance was assessed using the Number of Identified Specimens (NISP), the Minimum Number of Elements (MNE) and the Minimum Number of Individuals (MNI) (Grayson, 1984; Klein and Cruz-Uribe, 1984; Lyman, 1994, 2008; White, 1953). To assess body part representation of the main ungulates in the assemblage, the NISP was chosen over the Minimal Animal Units [MAU, as defined by Binford (1978, 1984)] in the production of Figure 4. This approach is justified by Lyman (2008, p. 248), who states that “NISP is not afflicted by problems of aggregation or definition, so evaluating skeletal completeness can be done with NISP to avoid the problems with the MNE and MAU.” Survival percentages of each body part were compared based on Brain's (1969) formula: %survivali = MNE × 100/number of elementi in the animal skeleton × MNI (Table 2). This formula is equivalent to Binford's %MAU (Lyman, 2008). The number of elements per animal skeleton followed Lyman (2008: 228).
Mammal identifications were carried out using the osteological reference collection of the Archaeosciences Laboratory (LARC, Lisbon, Portugal) of Património Cultural I.P., the Portuguese government's archaeological heritage management agency (Moreno-García et al., 2003). Mammal identifications were aided by several osteological atlases, like Hillson (2005), Pales and Lambert (1971, 1981), Pérez-Hidalgo and Cobo Rayán (1987), and Schmid (1972). The portion of the skeletal element present was recorded along with the anatomical part identified. Every element was sided (left, right, indeterminate), and ageing was recorded by analysing (1) tooth wear stages and (2) the state of fusion of long bone extremities. The different dentine patterns for Caprinae followed Payne's (1973, 1987) model. Grant's (1982) dentine patterns were used for Suidae and Bovinae. The teeth of Cervidae were described following Brown and Chapman's (1990, 1991) scheme. For age class estimation, long bones were recorded as unfused, fusing or fused (Reitz and Wing, 2008).
Coprolites were tentatively identified to species, or animal group size, based on their shape and size, and through comparison with those published by Sanz et al. (2016). These researchers established three distinct coprolite morphotypes. Morphotype 1, attributed to hyenids, is predominantly globular or spherical, occasionally pointed, and has flat or rounded extremities, a crumbly texture, sparse bone content, a length ranging from 19 to 51 mm and a diameter ranging from 18 to 50 mm. Morphotype 2 is linked to non-hyenid carnivores (such as lynx, fox or wolf), has a cylindrical or tube-like shape with the occasional sharp extremities, a spiral internal structure, several voids, abundant bone inclusions dominated by leporids, and ranges from 20 to 65 mm in length and from 18 to 50 mm in diameter. Finally, Morphotype 3, tentatively associated with larger carnivores (like bears), is larger and crumblier than all other morphotypes, displaying a length ranging from 21 to 87 mm and a diameter between 36 and 57 mm (Sanz et al., 2016).
The relatively large number of unidentified bone fragments could for the most part be assigned to size categories based on shape and thickness of the bone. Seven main animal size categories were created, adapted from the model used by Blasco and Fernández Peris (2012), Bunn (1986) and Díez et al. (1999): (1) Very Large Macrofauna: mammals larger than 1,000 kg (e.g. elephant, rhino); (2) Large Macrofauna: mammals from 300 to 1,000 kg (e.g. horse, aurochs, bear); (3) Medium Macrofauna: mammals from 100 to 300 kg (red deer and generic cervids); (4) Small Macrofauna: mammals from 20 to 100 kg (e.g. chamois, ibex, hyaena, wolf); (5) >Very Small Macrofauna: indeterminate mammal remains impossible to attribute to one of the animal groups but clearly larger than 20 kg; (6) Very Small Macrofauna: animals smaller than 20 kg (e.g. lynx, cat, fox); (7) Indeterminate Macrofauna: indeterminate remains that cannot be attributed to a specific category (mostly composed of heavily fragmented remains).
For a quick assessment of the assemblages' degree of fragmentation every bone (whether complete or fragmented) was assigned to a size interval in centimeters: 0–1, 1–2 cm and so on. Bone breakage was recorded following the criteria defined by Bunn (1983) and Villa and Mahieu (1991), adapted by Blasco and Fernández Peris (2012), and used in the analysis of several Palaeolithic faunal assemblages. Fracture outlines were recorded as transverse, curved/V-shaped, or longitudinal; fracture angles were noted as oblique, right or mixed; surface edges were recorded as jagged or smooth; and the time of fracture was described as old or new, i.e., before or after deposition.
Clear anthropogenic breakage can be assessed through several types of percussion marks, like percussion pits, percussion notches, impact flakes and adhering flakes (Blumenschine and Selvaggio, 1988; Capaldo and Blumenschine, 1994; Díez et al., 1999; Pickering and Egeland, 2006; White, 1992). Butchery marks, such as cuts, scrapes and chops were identified according to the criteria defined by Shipman and Rose (1983), Noe-Nygaard (1989) and Fisher (1995). Additional information was recorded, such as the number of striations (0 to n), striation distribution (isolated, clustered, crossed), striation orientation (oblique, longitudinal, transverse), striation delineation (straight or curved), striation location and side (posterior, anterior, medial, lateral) on the skeletal element. Trampling marks were distinguished from butchery marks following the protocol defined by Domínguez-Rodrigo et al. (2009a). Burning colour observations on bone were based on the schemes of Shipman et al. (1984) and Nicholson (1993), resulting in the creation of five different categories of analysis: (1) Not burnt, (2) Brown, (3) Black, (4) Grey and (5) White. If multiple colours were observed, they were recorded as a combination of categories, such as Brown-Black or Black-White, to accurately reflect the variation.
Carnivore marks were identified according to the categories defined by Binford (1981) and Fisher (1995): punctures, pits, scores, crenulations and digestion marks. The number of marks, their location on the anatomical element and their distribution (isolated, clustered, crossed) were also recorded. The largest width and the largest length of carnivore punctures were registered in millimetres (Andrés et al., 2012). Rodent gnawing was recorded as present or absent, and as to location on the anatomical element. Porcupine gnawing marks, as clearly illustrated by Binford (1984, p. 51), are considered amongst this rodent gnawing category. Other bone surface modifications, such as manganese stains, root etching or high concretion-coating, were also recorded.
4 Results
4.1 Taxonomic and body part frequencies
A total of 4,760 macro-mammal remains were analysed. The majority, representing 83.4% of the assemblage, originate from the occupation phases dated to MIS-5b and were recovered from Area F. Specifically, phase FB4 is the most significant contributor, accounting for 75% of the total remains. The remaining correspond to phases FB3 and FB2 (8.4%), and to the reworked assemblage (16.6%; Table 1).
Table 1
| Taxon | Phase FB4 | Phase FB3 | Phase FB2 | Rworked | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NISP | MNE | MNI | NISP | MNE | MNI | NISP | MNE | MNI | NISP | MNE | MNI | NISP | MNE | MNI | |
| Large macro-mammals | |||||||||||||||
| Equus caballus | 1 | 2 | 1 | – | – | – | – | – | – | – | – | – | 1 | 2 | 1 |
| Equus sp. | 14 | 11 | 4 | 2 | 2 | 2 | – | – | – | – | – | – | 16 | 13 | 6 |
| Bos sp. | 6 | 4 | 3 | – | – | – | – | – | – | 1 | 1 | 1 | 7 | 5 | 4 |
| Herbivore | 6 | 3 | – | 5 | 3 | – | 2 | 1 | – | 1 | 1 | – | 14 | 8 | – |
| Ursus arctos | 9 | 6 | 2 | 4 | 4 | 2 | – | – | – | – | – | – | 13 | 4 | 4 |
| Indeterminate | 36 | 8 | – | 19 | 5 | – | – | – | – | 1 | 1 | – | 56 | 14 | – |
| Sub-total | 72 | 34 | 10 | 30 | 14 | 4 | 2 | 1 | – | 3 | 3 | 1 | 107 | 46 | 15 |
| Medium macro-mammals | |||||||||||||||
| Cervus elaphus | 53 | 46 | 7 | 18 | 14 | 2 | – | – | – | 2 | 2 | 1 | 73 | 62 | 10 |
| Cervidae | 42 | 14 | 4 | 5 | 3 | 2 | 1 | 1 | 1 | 4 | 4 | 2 | 52 | 22 | 9 |
| Herbivore | 10 | 4 | – | – | – | – | – | – | – | 1 | 1 | – | 11 | 5 | – |
| Indeterminate | 81 | 14 | – | 8 | 1 | – | 4 | 2 | – | 2 | 2 | – | 95 | 19 | – |
| Sub-total | 186 | 78 | 11 | 31 | 18 | 4 | 5 | 3 | 1 | 9 | 9 | 3 | 231 | 108 | 19 |
| Small macro-mammals | |||||||||||||||
| Caprinae | 58 | 42 | 5 | 12 | 9 | 2 | – | – | – | 11 | 8 | 2 | 81 | 59 | 9 |
| Herbivore | 3 | 2 | – | – | – | – | – | – | – | 4 | 2 | – | 7 | 4 | – |
| Sus sp. | 4 | 4 | 2 | 1 | 1 | 1 | – | – | – | – | – | – | 5 | 5 | 3 |
| Hyaenidae | 3 | 3 | 1 | 1 | 1 | 1 | – | – | – | – | – | – | 4 | 4 | 2 |
| Canis lupus | 1 | 1 | 1 | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 |
| Martes sp. | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 | 1 | 1 | 1 |
| Carnivore | 33 | 4 | – | 3 | 2 | – | – | – | – | 1 | 1 | – | 37 | 7 | – |
| Indeterminate | 165 | 16 | – | 21 | 4 | – | 3 | 2 | – | 31 | 6 | – | 220 | 28 | – |
| Sub-total | 267 | 72 | 9 | 38 | 17 | 4 | 3 | 2 | – | 48 | 18 | 3 | 356 | 109 | 16 |
| >Very small macro-mammals | |||||||||||||||
| Herbivore | 24 | 5 | – | 4 | 3 | – | – | – | – | 3 | 1 | – | 31 | 9 | – |
| Indeterminate | 542 | 15 | – | 142 | 6 | – | 12 | 4 | – | 73 | 5 | – | 769 | 30 | – |
| Sub-total | 566 | 20 | – | 146 | 9 | – | 12 | 4 | – | 76 | 6 | – | 800 | 39 | – |
| Very small macro-mammals | |||||||||||||||
| Felis silvestris | 7 | 7 | 5 | 1 | 1 | 1 | – | – | – | 2 | 2 | 1 | 10 | 10 | 7 |
| Lynx pardinus | 1 | 1 | 1 | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 |
| Vulpes vulpes | 1 | 1 | 1 | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 |
| cf. Vulpes vulpes | – | – | – | – | – | – | – | – | – | 1 | 1 | 1 | 1 | 1 | 1 |
| Carnivore | – | – | – | – | – | – | – | – | – | 2 | 1 | – | 2 | 1 | – |
| Indeterminate | 480 | 29 | – | 28 | 3 | – | – | – | – | 344 | 17 | – | 852 | 49 | – |
| Sub-total | 489 | 38 | 7 | 29 | 4 | 1 | – | – | – | 349 | 21 | 2 | 867 | 63 | 10 |
| Indeterminate macro-mammals | |||||||||||||||
| Indeterminate | 1,989 | – | – | 105 | – | – | 1 | – | – | 304 | – | – | 2,399 | – | – |
| Sub-total | 1,989 | – | – | 105 | – | – | 1 | – | – | 304 | – | – | 2,399 | – | – |
| Total | 3,569 | 245 | 37 | 379 | 62 | 13 | 23 | 10 | 1 | 789 | 57 | 9 | 4,760 | 374 | 59 |
Number of Identified Specimens (NISP), Minimum Number of Elements (MNE) and Minimum Number of Individuals (MNI) of ungulate and carnivore remains by the different occupation phases in Gruta da Figueira Brava (2010–2013 excavations).
Approximately half of the assemblage (50.4% or n = 2,399) is indeterminate and cannot be assigned to any animal size categories. Within such categories, 41.9% (or n = 1,992) remain unidentifiable at family or species level. Consequently, excluding teeth, a considerable part of the faunal collection corresponds to non-identifiable bones (n = 4,008, or 84.2%). Only 7.8% (or NISP = 369) of the total analysed assemblage provides taxonomic information (Table 1), but the non-taxonomically identifiable portion of the assemblage offers valuable anatomical and taphonomical information, nonetheless.
Amongst the non-identifiable bone fragments, 75.5% (or n = 3,025) were recovered from phase FB4 levels (Figure 2A). More than half of the non-identifiable bones (n = 2,479, or 61.9%) cannot be categorised as either long or flat, they have been classified simply as indeterminate (Figure 2B). However, about 33% (or n = 1,331), most from phase FB4, correspond to long bone fragments, from which only 18 correspond to epiphyses. A total of 171 spongy bone fragments were identified within the indeterminate remains. Figures 2C–F presents a closer look at the non-identified bones recovered, in particular among animal size groups corresponding to the ungulates identified at the site, i.e. >Very Small Macrofauna, Small Macrofauna, Medium Macrofauna and Large Macrofauna. Therefore, even though the number of NISP is low, there is a clear presence of long bone remains of the appendicular skeleton, as well as (although in lower frequency) of flat bones of the axial skeleton (Figures 2B–F).
Figure 2
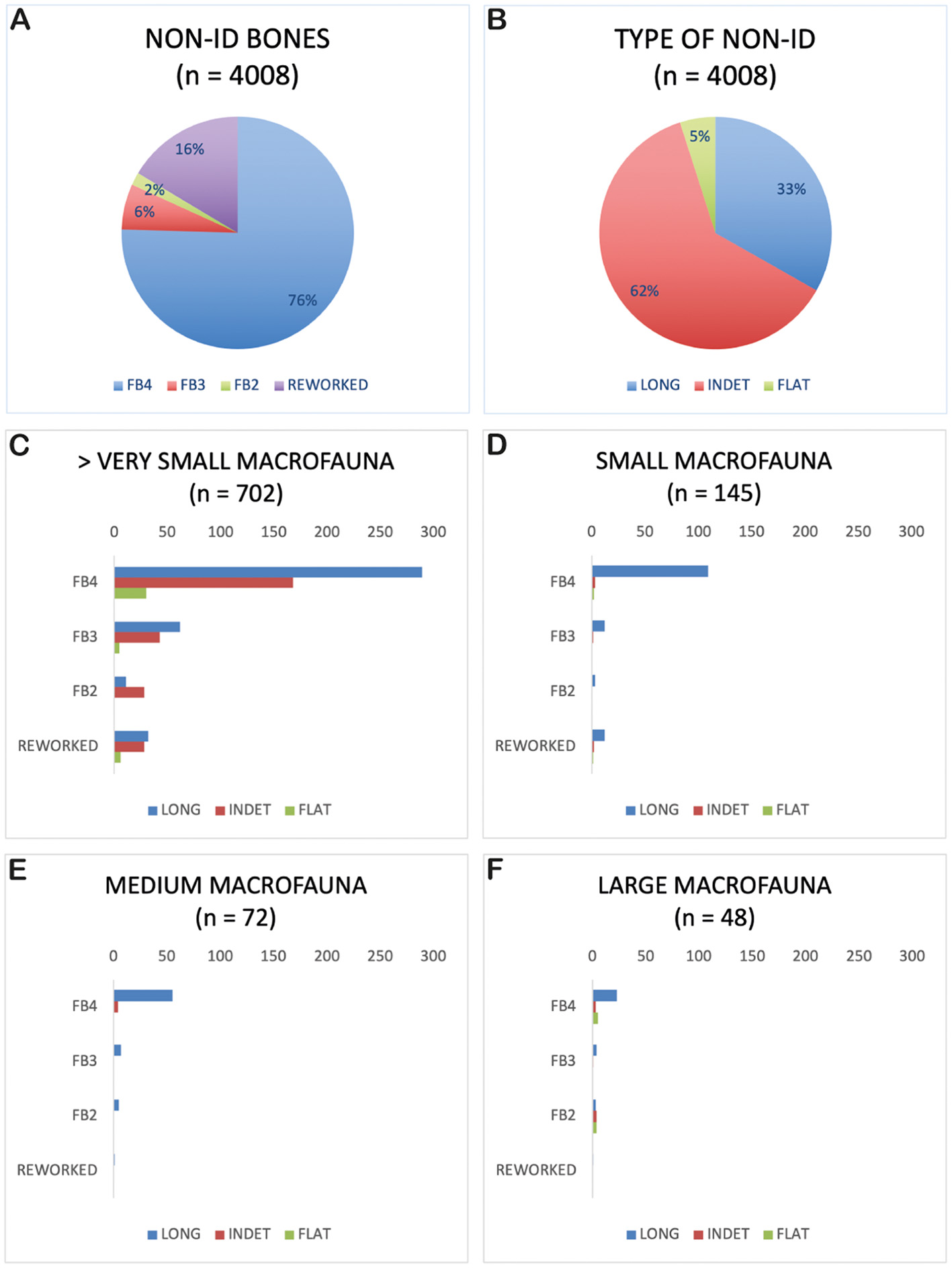
Pie charts illustrating the distribution (A) and type of fracture (B) of non-identified bone fragments across different occupation levels at Gruta da Figueira Brava. The histograms reflect relative abundances of skeletal elements from various ungulate size categories at Gruta da Figueira Brava: >Very Small Macrofauna (C), Small Macrofauna (D), Medium Macrofauna (E) and Large Macrofauna (F).
4.1.1 Ungulates
No remains of Very Large Macrofauna were recovered in the 2010–2013 excavations (but note that elephant and rhino are listed among the 1980s Area C finds; Antunes, 2000). The largest species is the aurochs (Table 1), which was identified based on the presence of five tooth fragments (a permanent molar and incisors), a left naviculo-cuboid and a horn core fragment (Figure 4, Tables 2–4). Equids were mainly identified based on tooth remains (NISP = 13, of which five are deciduous; Figure 3F), but there is also one astragalus, two metatarsals and one metacarpal; these bones allow taxonomic attribution to Equus caballus (Figure 4; Tables 2–4).
Table 2
| Body part | Equidae | Bovidae | Cervidae | Caprinae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NISP | MNE | %S | NISP | MNE | %SI | NISP | MNE | %S | NISP | MNE | %S | |
| Cranial | ||||||||||||
| Antler/horncore | – | – | – | 1 | 1 | 12.50 | 9 | 4 | 10.53 | – | – | – |
| Skull | – | – | – | – | – | – | – | – | – | – | – | – |
| Mandible | – | – | – | – | – | – | 13 | 13 | 34.21 | 1 | 1 | 5.56 |
| Maxilla | – | – | – | – | – | – | 3 | 3 | 7.89 | 2 | 2 | 11.11 |
| Isolated teeth | 13 | 11 | 3.93 | 4 | 2 | 1.56 | 48 | 28 | 4.61 | 62 | 43 | 14.93 |
| Axial | ||||||||||||
| Vertebra | – | – | – | – | – | – | 2 | 2 | 1.50 | – | – | – |
| Ribs | – | – | – | – | – | – | – | – | – | – | – | – |
| Front limbs | ||||||||||||
| Scapula | – | – | – | – | – | – | – | – | – | 1 | 1 | 5.56 |
| Humerus | – | – | – | – | – | – | 1 | 1 | 2.63 | 1 | 1 | 5.56 |
| Radius | – | – | – | – | – | – | 1 | 1 | 2.63 | – | – | – |
| Ulna | – | – | – | – | – | – | – | – | – | – | – | – |
| Carpals | – | – | – | – | – | – | – | – | – | – | – | – |
| Metacarpal | 1 | 1 | 2.38 | – | – | – | 3 | 3 | 7.89 | 1 | 1 | 5.56 |
| Hind limbs | ||||||||||||
| Pelvis | – | – | – | – | – | – | 1 | 1 | 2.63 | – | – | – |
| Femur | – | – | – | – | – | – | – | – | – | – | – | – |
| Tibia | – | – | – | – | – | – | 2 | 2 | 5.26 | – | – | – |
| Tarsals | 1 | 1 | 1.19 | 1 | 1 | 2.50 | 3 | 3 | 1.58 | 1 | 1 | 1.11 |
| Metatarsal | 2 | 2 | 4.76 | – | – | – | 7 | 5 | 13.16 | – | – | – |
| Indet Limbs | ||||||||||||
| Metapodials | – | – | – | – | – | – | 18 | 6 | 7.89 | – | – | – |
| Phalanx 1 | – | – | – | – | – | – | 3 | 2 | 1.32 | 1 | 1 | 1.39 |
| Phalanx 2 | – | – | – | – | – | – | 2 | 2 | 1.32 | – | – | – |
| Phalanx 3 | – | – | – | – | – | – | 2 | 2 | 1.32 | – | – | – |
| MNI | 7 | 4 | 19 | 9 | ||||||||
Number of Identified Specimens (NISP), Minimum Number of Elements (MNE) and percentage of survival (%S) of the MIS-5 main ungulate body parts recovered in Gruta da Figueira Brava (2010–2013 excavations).
Table 3
| Taxa | Juvenile | Adult | Senile | |||
|---|---|---|---|---|---|---|
| NISP | MNI | NISP | MNI | NISP | MNI | |
| Ungulates | ||||||
| Equidae | 5 | 2 | 4 | 1 | – | – |
| Bovidae | – | – | 4 | 1 | – | – |
| Cervidae | 10 | 3 | 42 | 4 | 1 | 1 |
| Caprinae | 1 | 1 | 47 | 4 | – | – |
| Suidae | 1 | 1 | 2 | 1 | – | – |
| Carnivores | ||||||
| Ursus | – | – | 9 | 2 | – | – |
| Hyaenidae | – | – | – | – | – | – |
| Canis | – | – | – | – | – | – |
| Felis | – | – | 3 | 1 | – | – |
| Lynx | – | – | 1 | 1 | – | – |
| Vulpes | – | – | – | – | – | – |
Number of Identified Specimens (NISP) and Minimum Number of Individuals (MNI) of the MIS-5 teeth remains showing age information from ungulates and carnivores recovered in Gruta da Figueira Brava (2010–2013 excavations).
Table 4
| Taxa | Unfused | Fused | ||
|---|---|---|---|---|
| NISP | MNI | NISP | MNI | |
| Ungulates | ||||
| Equidae | – | – | 4 | 1 |
| Bovidae | – | – | 1 | 1 |
| Cervidae | 6 | 1 | 15 | 1 |
| Caprinae | 1 | 1 | 3 | 1 |
| Suidae | – | – | 1 | 1 |
| Carnivores | ||||
| Ursus | – | – | 3 | 1 |
| Hyaenidae | – | – | 4 | 2 |
| Canis | – | – | 1 | 1 |
| Felis | – | – | 5 | 2 |
| Lynx | – | – | – | – |
| Vulpes | – | – | 1 | 1 |
Number of Identified Specimens (NISP) and Minimum Number of Individuals (MNI) of the MIS-5 long bone remains showing states of epiphyseal fusion from ungulates and carnivores recovered in Gruta da Figueira Brava (2010–2013 excavations).
Figure 3
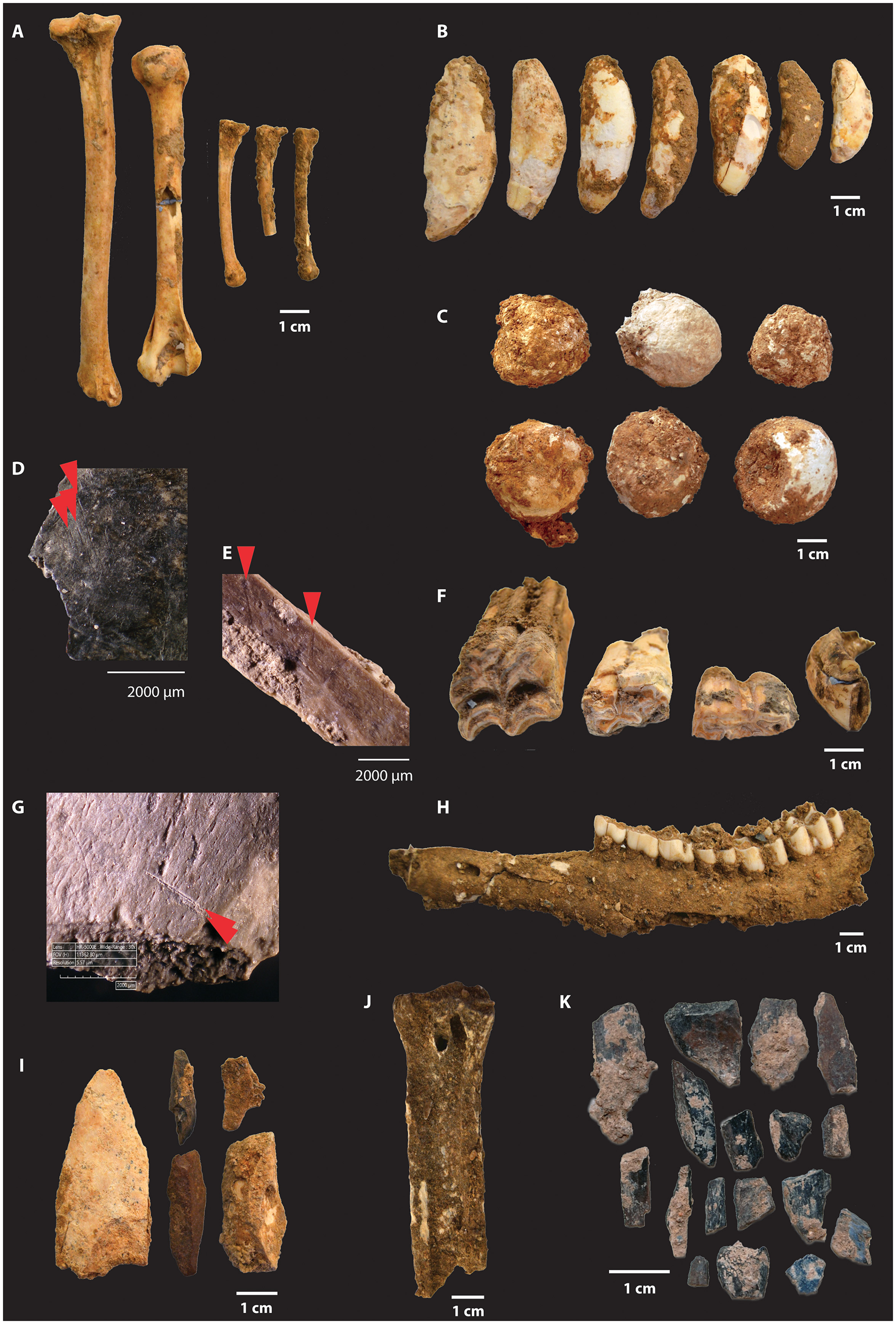
Middle Palaeolithic, MIS-5 ungulate and carnivore remains recovered in Area F of Gruta da Figueira Brava. (A)Felis silvestris (from left to right, tibia, humerus, two metatarsus 3 left and one metatarsus 2). (B)Ursus arctos canine teeth. (C) Hyaena coprolites. (D) Cut marks on long bone of >Very Small Macrofauna fragment. (E) Cut marks on long bone of >Very Small Macrofauna fragment. (F)Equus caballus (from left to right, two permanent premolars/molars, one deciduous premolar 4 and one permanent incisor 3). (G) Cut marks on long bone of >Very Small Macrofauna fragment. (H)Cervus elaphus mandible with permanent teeth P2, P3, P4, M1, M2, M3. (I) Impact flakes on indeterminate macro-mammal remains. (J)Cervus elaphus metatarsus with very straight longitudinal fracture. (K) Burnt bone fragments of indeterminate macro-mammals.
Figure 4
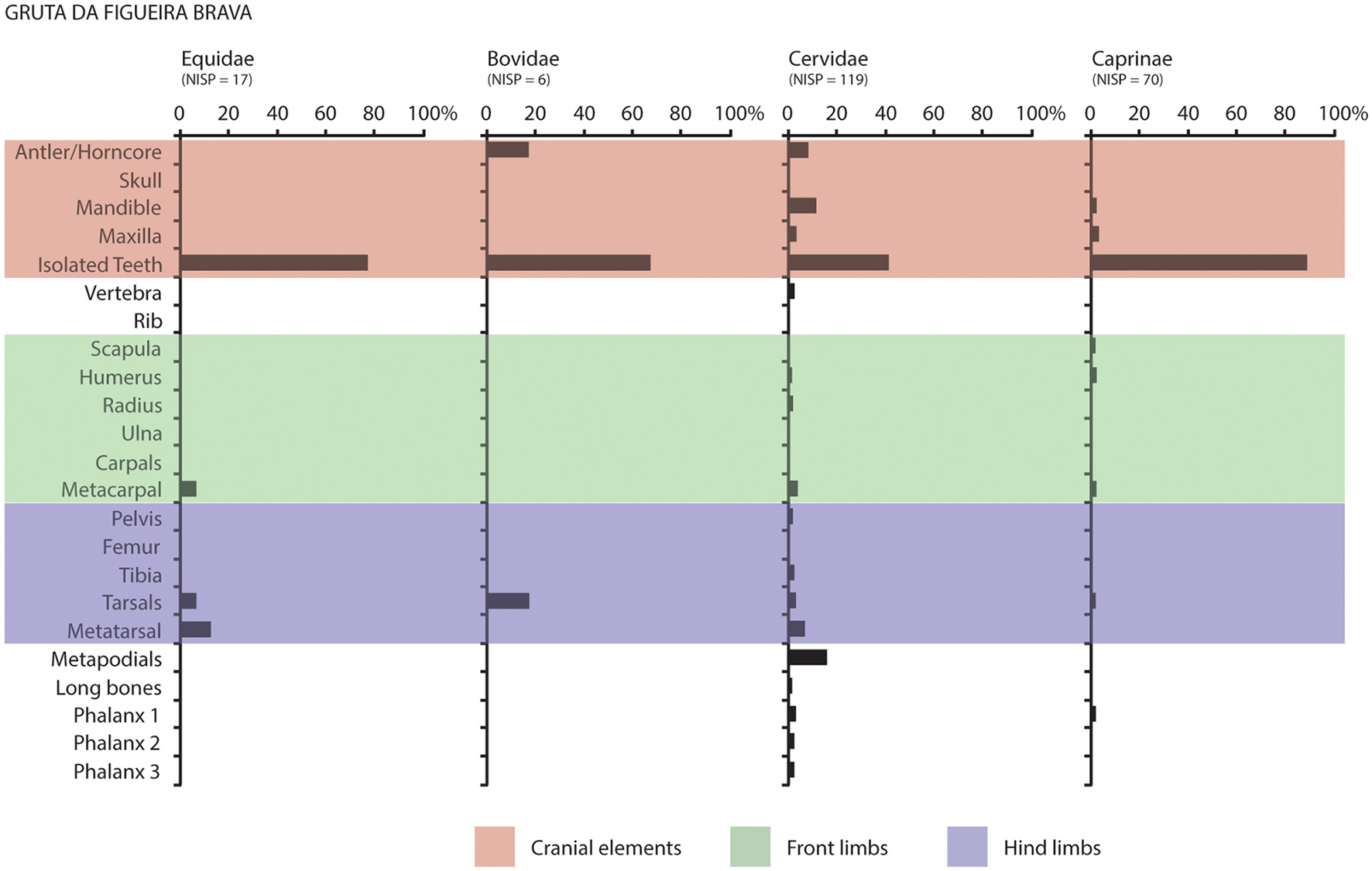
Body part representation of the most representative ungulate taxa in the Middle Palaeolithic, MIS-5 macro-mammal assemblage from Gruta da Figueira Brava.
Red deer is the best represented ungulate (Table 1). Identification as Cervus elaphus was possible for the majority of the remains, which suggests that most of the fragments assigned to Cervidae probably belong to the species too. Cranial elements—such as mandibles (NISP = 14; Figure 3H), maxillae (NISP = 4) and isolated teeth (NISP = 37)—predominate, but the appendicular skeleton is also represented by phalanges (NISP = 7) as much as hind limbs, tarsals (NISP = 8) and front limbs (NISP = 4; Figure 4; Table 2). The state of long bone fusion and the dental remains indicate that deer of all ages (from juvenile to senile) are represented in the assemblage (Tables 3, 4).
Caprines come close to deer in NISP counts (Table 1). They are mainly represented by isolated teeth (NISP = 69; of which three are deciduous), but there are two adult mandibles and two adult maxillae (Table 3). Front and hind limbs are evenly represented, by three elements each, as well as two first phalanges that could not be assigned to leg (Figure 4; Table 2). All bones are fused, except for a distal humerus (Table 4). Tooth morphology was explored in order to attempt species identification and, based on the LARC reference collection, Capra pyrenaica is the best candidate. Finally, boar is the less frequent ungulate; it is represented by two metapodials, two adult mandibular teeth and a juvenile mandible, all from Area F (Tables 1, 3, 4).
As explained before, more of the appendicular and axial skeletons of these taxa are represented amongst the long bone and flat bone fragments of the animal size categories >Very Small Macrofauna, Small Macrofauna, Medium Macrofauna and Large Macrofauna, as shown in Figure 2.
4.1.2 Carnivores
Brown bear (Ursus arctos) is the best represented carnivore (Table 1; Figure 3B). The remains include adult teeth (two incisors and seven canines), a nearly complete atlas, one axis, a thoracic vertebra and a left humerus. The vertebral discs are all fused, but the calcareous concretions attached to the humerus do not allow proper observation of the state of long bone fusion (Table 4). Based on Andrews and Turner (1992), canines only erupt at 14 months, suggesting that the two bear individuals minimally represented in the assemblage were more than one year old.
Hyaenas were identified based on two metapodials, a left astragalus and a first phalanx. Most probably, they are of Crocuta crocuta, a species commonly found in Europe's Middle and Upper Pleistocene caves (Sanz et al., 2016). Amongst the 37 coprolites recovered, a total of 28 were complete and allowed measurements (Figures 3C, 5; Table 5); most (NISP = 22) cluster with Morphotype 1 and can be attributed to hyaenas, due to their spherical morphology, with flattened or concave ends (Horwitz and Goldberg, 1989; Larkin et al., 2000) and size, which compares well with available data for Crocuta crocuta. However, one very large coprolite is close to Morphotype 3 of Furninha and, therefore, is probably of bear. Apart from an outlier of very small size that does not relate to any of the species listed in Table 5 and in Figure 5, all the other non-hyaena coprolites (NISP = 4) cluster in Morphotype 2 and, thus, may belong to wolf (Canis lupus). Indeed, the wolf is represented at the site by a complete left calcaneum.
Figure 5
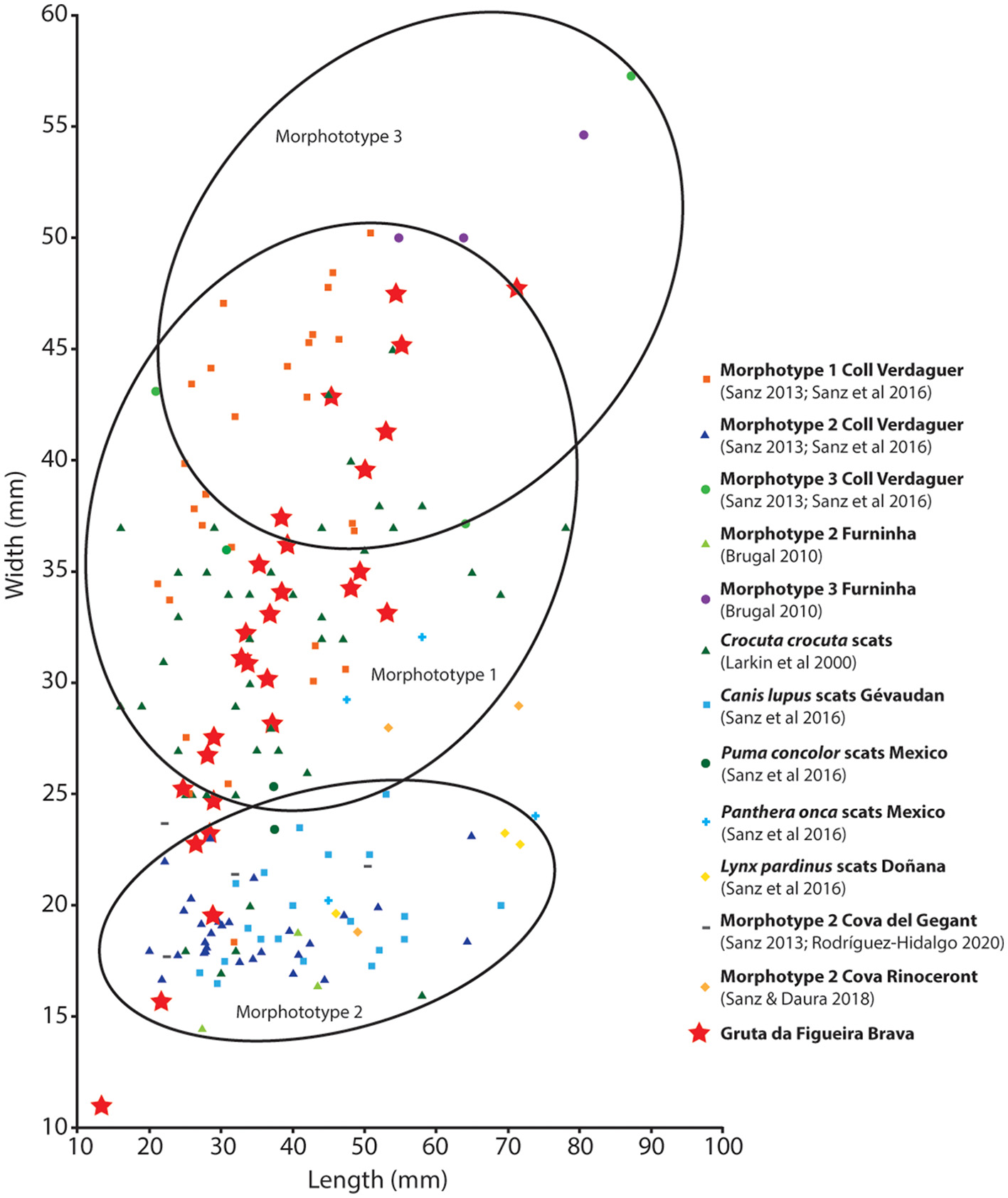
Comparative analysis of the coprolites from Gruta da Figueira Brava. The graph is adapted from Sanz et al. (2016). It includes updated information provided by Montserrat Sanz (pers. comm. in October 2020) and data from the 2010–2013 excavations as provided in Table 2.
Table 5
| ID | Unit | Phase | Taxon | Length (mm) | Width (mm) | Fragment size (cm) |
|---|---|---|---|---|---|---|
| 1 | Reworked | – | 53.02 | 33.16 | >5 | |
| 2 | Reworked | – | 39.24 | 36.22 | 3–4 | |
| 3 | IH2-IH3 | FB4 | – | 78.88 | – | >5 |
| 4 | IH2-IH3 | FB4 | Canis lupus | 26.47 | 22.86 | 2–3 |
| 5 | IH2-IH3 | FB4 | Crocuta crocuta | 36.47 | 30.21 | 3–4 |
| 6 | IH2-IH3 | FB4 | Crocuta crocuta | 52.88 | 41.37 | >5 |
| 7 | IH2-IH3 | FB4 | – | 43.09 | – | 4–5 |
| 8 | IH2-IH3 | FB4 | Crocuta crocuta | 38.31 | 37.45 | 3–4 |
| 9 | IH4 | FB4 | Crocuta crocuta | 35.40 | 35.31 | 3–4 |
| 10 | IH4 | FB4 | Crocuta crocuta | 28.12 | 26.85 | 2–3 |
| 11 | IH4 | FB4 | Crocuta crocuta | 36.82 | 33.16 | 3–4 |
| 12 | IH4 | FB4 | Ursus arctos | 71.18 | 47.73 | >5 |
| 13 | IH4 | FB4 | Canis lupus | 21.65 | 15.75 | 2–3 |
| 14 | IH4 | FB4 | Crocuta crocuta | 37.16 | 28.18 | 3–4 |
| 15 | IH6 | FB4 | Crocuta crocuta | 48.06 | 34.30 | 4–5 |
| 16 | IH6 | FB4 | Crocuta crocuta | 55.06 | 45.15 | >5 |
| 17 | IH6 | FB4 | Very small carnivore | 13.24 | 11.05 | 1–2 |
| 18 | IH6 | FB4 | – | 20.35 | – | 2–3 |
| 19 | IH6 | FB4 | Crocuta crocuta | 32.87 | 31.11 | 3–4 |
| 20 | IH6 | FB4 | – | – | 21.80 | 2–3 |
| 21 | IH6 | FB4 | Crocuta crocuta | 49.97 | 39.57 | 4–5 |
| 22 | IH6 | FB4 | Crocuta crocuta | 28.94 | 27.53 | 3–4 |
| 23 | IH6 | FB4 | Crocuta crocuta | 54.25 | 47.38 | >5 |
| 24 | IH6 | FB4 | Crocuta crocuta | 38.38 | 34.13 | 3–4 |
| 25 | IH6 | FB4 | – | 31.54 | – | 3–4 |
| 26 | IH8 | FB4 | – | 52.81 | – | >5 |
| 27 | IH8 | FB4 | Crocuta crocuta | 24.70 | 25.29 | 2–3 |
| 28 | IH8 | FB4 | Crocuta crocuta | 45.51 | 42.92 | 4–5 |
| 29 | IH8 | FB4 | Canis lupus | 28.90 | 19.58 | 2–3 |
| 30 | IH8 | FB4 | Canis lupus | 28.36 | 23.29 | 2–3 |
| 31 | IH8 | FB4 | Crocuta crocuta | 28.91 | 24.79 | 2–3 |
| 32 | IH8 | FB4 | – | 39.93 | – | 3–4 |
| 33 | IH8 | FB4 | Crocuta crocuta | 49.28 | 35.02 | 4–5 |
| 34 | IH8 | FB4 | – | 47.00 | – | 4–5 |
| 35 | IH8 | FB4 | – | 50.75 | – | >5 |
| 36 | IL3 | FB3 | Crocuta crocuta | 33.43 | 32.24 | 3–4 |
| 37 | IL3 | FB3 | Crocuta crocuta | 33.45 | 30.86 | 3–4 |
As for the smaller carnivores, the wildcat (Felis silvestris; Table 1 and Figure 3A) is represented by fused long bones—three metatarsals, a right humerus, a left tibia—and three maxillary fragments with permanent teeth (Tables 3, 4). A fragment of right maxilla with permanent premolars 2 and 3, and a fused left pelvis were also found in the reworked deposit. Lynx (Lynx pardinus) and fox (Vulpes vulpes; Tables 1, 3, 4) are represented by a left upper canine and a right fused fifth metacarpus, respectively.
4.2 Fragmentation and type of fracture
The macro-mammal assemblage is heavily fragmented: only 2.47% (or n = 98) of the in situ remains are complete, corresponding mainly to bones of limb extremities (i.e. metapodials, tarsals, carpals and phalanges) and coprolites; whereas 53.24% (or n = 2,114) of the fractured bones are smaller than 2 cm. Overall, fractures are preferentially curved/V-shaped (43.72%, or n = 1,736) and longitudinal (36.94%, or n = 1,467; Figure 3J); the former with most angles being oblique (56.31%, or n = 2,236), the latter with most being at right angles (35.81%, or n = 1,422) and occurring frequently on ungulate metapodials. Smooth edges are the most frequent (86.93%, or n = 3,452). Such fracture patterns denote bones that were broken when fresh for the curved/V-shaped types, whereas some degree of dry bone fracturing is evidenced by the right angles.
Out of a total of 18 mandibles, longitudinal fractures predominate (66.67%, or n = 12), followed by transverse fractures (33.33%, or n = 6). Of the 25 vertebrae found, only three are complete: most show fractures along their transversal axis (n = 18). Transversal fractures are also the most common among ribs (83.04%, or n = 93 from a total of 112 remains). Nearly half of the phalanges are complete (n = 7, from a total of 16), and six show transversal fractures. Most limb bones are broken (99.35%, or n = 1,078 from a total of 1,085), and longitudinal (54.29%, or n = 589) and curved/V-shaped (29.12%, or n = 316) fractures are predominant. These patterns remain valid if only deer is considered, as one would expect given that it is the most common taxa: out of a total of 33 deer limb bone fragments, 63.64% (or n = 21) are longitudinally broken and 24.24% (or n = 8) display curved/V-shaped breaks.
4.3 Bone surface modifications
The observation of bone surface modifications is hindered by the dense concretion coating covering the bones, which obscures potential marks and greatly reduces the likelihood of detecting additional surface alterations. Percussion marks and impact flakes are less affected by this condition and therefore represent the most frequently observed types of anthropogenic modification (Table 6; Figure 3I). Mostly, they concern unidentifiable fragments but, in a few cases—a distal humerus shaft impacted on the cranial side, two metapodials impacted on the shafts, a metatarsal and a metacarpal impacted on the cranial side of shaft ends—the bones could be determined to deer. Among percussion notches, only two are on identified remains—one on the dorsal side of a proximal shaft of a horse metacarpal, the other on the dorsal side of a proximal shaft of a deer first phalanx. No adhering flakes could be identified to taxon, although they are present on non-identifiable macrofaunal remains. Percussion pits are the least represented anthropogenic mark; in one case, the affected bone could be identified as a deer metatarsal. Cut marks were found on an ibex first phalanx from the reworked deposit; otherwise, all cut marks are on unidentified remains (Figures 3D, E, G). Carnivore marks are scarce and mostly represented by punctures on the root surface of mandibular teeth of caprines and cervids (Table 6).
Table 6
| Phase FB4 | Phase FB3 | Phase FB2 | Reworked | Total | |
|---|---|---|---|---|---|
| Burning | |||||
| Brown | 127 | 1 | – | 25 | 153 |
| Black | 450 | 7 | 3 | 44 | 504 |
| Grey | 16 | – | – | – | 16 |
| White | 7 | – | – | 1 | 8 |
| None | 2,969 | 309 | 82 | 719 | 4,079 |
| Total | 3,569 | 317 | 85 | 789 | 4,760 |
| % Burnt | 16.81 | 2.52 | 3.53 | 8.87 | 14.31 |
| Butchery | |||||
| Chops | 2 | – | – | 9 | 11 |
| Cuts | 10 | 2 | – | 9 | 21 |
| Scrapes | 1 | – | – | 1 | 2 |
| None | 3,556 | 315 | 85 | 770 | 4,726 |
| Total | 3,569 | 317 | 85 | 789 | 4,760 |
| % Butchery | 0.36 | 0.63 | 0 | 2.41 | 0.71 |
| Percussion | |||||
| Impact flake | 143 | 24 | 6 | 51 | 224 |
| Adhering flake | 12 | – | 1 | 1 | 14 |
| Percussion notch | 29 | 7 | 2 | 17 | 55 |
| Percussion pit | 6 | 2 | – | 4 | 12 |
| None | 3,379 | 284 | 76 | 716 | 4,455 |
| Total | 3,569 | 317 | 85 | 789 | 4,760 |
| % Percussion | 5.32 | 10.41 | 10.59 | 9.25 | 6.41 |
| Carnivore | |||||
| Carnivore pit | – | – | – | 1 | 1 |
| Carnivore puncture | 14 | – | – | 3 | 17 |
| Scoring | 1 | – | – | – | 1 |
| None | 3,554 | 317 | 85 | 785 | 4,741 |
| Total | 3,569 | 317 | 85 | 789 | 4,760 |
| % Carnivore | 0.38 | 0 | 0 | 0.51 | 0.40 |
Bone surface modifications observed in the macro-mammal assemblage from Gruta da Figueira Brava (2010–2013 excavations).
4.4 Burning
Thermo-alterations were observed in 611, i.e., 15.39% of the in situ remains analysed (Table 6; Figure 3K), which for the most part come from the FB4 deposit. Due to the very small size of the fragments (< 2 cm) and the lack of diagnostic features, only a few (n = 111, i.e., 18.17%) could be assigned to species or size category. Black burns predominate, followed by brown burns; grey and white thermo-alterations are rare (Table 6).
In most MIS-5 fragments, burning covers the surfaces of the remains entirely (i.e., is complete), but 41 (or 6.71%) fragments display thermo-alteration only on one side, mostly the exterior part of diaphyseal fragments. In contrast, burning only on the interior surface was observed in four shaft fragments. Shaft fragments are the most represented body part (n = 585), but there are also 24 burnt epiphyses and two black burnt tooth fragments. The latter are a maxillary canine fragment of bear from unit IH8 and one mandibular premolar of deer from unit IH4.
5 Discussion
5.1 Local palaeoenvironment
The macro-mammal assemblage from Gruta da Figueira Brava comes from a deposit encompassing the remains of three different human occupation phases (FB4, FB3 and FB2). Due to small sample size, particularly regarding the older occupation phases (refer to Table 1), the following discussion will consider the MIS-5 levels as a whole. This approach is necessary to ensure a comprehensive analysis, mitigating the limitations imposed by the smaller datasets for individual phases.
The ungulates exhibit diverse dietary behaviours, reflective of their ecological adaptability. Aurochs, for instance, likely thrived in dense forested environments, where their diet primarily would have consisted of grasses and graminoids. However, they also must have supplemented their feeding with a variety of forbs, as well as the leaves and branches of bushes and trees, reflecting a degree of dietary flexibility suitable for forested settings (van Vuure, 2005). In contrast, horses are more adapted to open, low-density forests and grassland ecosystems, where their grazing behaviour focuses on a diverse array of grasses (García García et al., 2009). This grazing specialisation is supported by isotopic and morphological evidence that indicates that their diet is dominated by C3 grasses typical of temperate environments (Schulz and Kaiser, 2012). Such ecological distinctions between these two large size taxa, suggest that the landscape surrounding Gruta da Figueira Brava featured denser, closed-canopy settings (where aurochs thrived), as well as more open, grass-dominated areas (where horses thrived).
Red deer display a mixed feeding strategy that shifts between browsing and grazing according to seasonal variations (e.g., Solounias and Semprebon, 2002). This dietary flexibility enables them to consume a wide variety of resources, including grasses, sedges, foliage of trees and shrubs (Azorit et al., 2012). Such adaptability not only facilitates survival in varied environments but also contributes to their widespread distribution across most of Iberia, where they prosper in heterogenous landscapes comprising forest, grassland and scrubland (Geist, 1998; Clutton-Brock, 1982). These ecological traits highlight the red deer's role as a generalist herbivore, well-suited to dynamic and patchy environments, which must have constituted most of Arrábida's territory, where the cave is located.
Ibex are highly specialised ungulates adapted to mountainous or rocky environments, where their robust limbs are adjusted to move on steep terrain. Despite this specialisation, their habitat can extend to other types of landscape depending on food availability, demonstrating a degree of ecological flexibility. Seasonally, ibex exhibit altitudinal migration patterns, occupying higher elevations during the summer and autumn months when food is abundant in those areas. In contrast, during the winter and spring, they descend to lower elevation open areas where vegetation remains accessible (Granados et al., 2001).
The presence of these taxa through the period of site occupation highlights the sustained presence of a diverse range of ecological niches in the terrestrial surrounding landscape. This mosaic of habitats, ranging from dense forests and open grasslands to mountainous land, provided the Gruta da Figueira Brava's inhabitants with access to a broad spectrum of macro-mammal resources. The variety of species exploited reflects the foraging strategies employed by the human groups, enabling them to capitalise on the seasonal and spatial distribution of fauna. This evidence supports the view that the cave served as a central hub for human activity over time, facilitating the exploitation of different habitats and explaining the resilience of the adaptive system.
5.2 Agents of bone accumulation
Given that no anthropogenic or carnivore-induced marks are to be seen on the bones of carnivores, the parsimonious explanation for their presence is natural demise while taking shelter at the site. The limited number of skeletal elements indicate that episodes of larger carnivore use of the cave were sporadic and primarily took place when humans were not there. This inference is consistent with the stratigraphic distribution of the remains. In the case of hyaenas, for example, they almost entirely (a) date to the three millennia-long hiatus between the end of phase FB3 and the beginning of phase FB4, or (b) post-date the near-complete sedimentary fill-up of Entrance 3 and Area F (Tables 1, 2; Zilhão et al., 2020), which made the site unsuitable for settlement and allowed for the interior to become a hyaena latrine, as revealed by the abundant coprolites and coprolite crumbs seen in the upper reaches of the infilling. Indeed, (a) the scarcity of carnivore skeletal remains, (b) the absence of juveniles, (c) the lack of digested bones, (d) the limited presence of jagged edges on bone fractures and (e) the low frequency of carnivore-induced marks (i.e., carnivore marks account for only 0.38% in phase FB4 and are entirely absent in phases FB3 and FB2, whereas percussion and cut marks show higher frequencies in all phases of occupation; Table 6), collectively exclude the possibility that the site was used as a den where large carnivores engaged in feeding behaviours. While some of these bone surface modifications may be obscured by carbonate concretions, this factor would equally affect evidence of both human and carnivore activity, as well as other potential agents of bone surface modification. Therefore, although the absence of such marks should not be interpreted as definitive evidence of nonexistence, the low carnivore-induce occurrence suggests that carnivores played a minimal role in bone accumulation and bone modification at the site.
Bears and wolves consume their prey at the kill site and are not significant accumulators of faunal remains (Domínguez-Rodrigo, 1993, 1994; Saladié et al., 2013; Sala and Arsuaga, 2013). Whenever wolves transport food back to the den, it tends to be as eaten meat parts that are regurgitated to feed their cubs (Domínguez-Rodrigo, 1994; Castel et al., 2010; Sauqué et al., 2018). However, neither digested bone remains, nor skeletal remains of young wolves were recovered. At archaeological sites, wolves can also be responsible for the modification of previously accumulated bones. The limited number of bite marks found on the root surface of mandibular teeth of deer and ibex can maybe relate to the scavenging of mandibles for marrow (or what was left of it) or as an attempt to extract the tooth pulp (Binford, 1981), either by wolf or one of the other carnivores represented in the cave's faunal assemblage. The involvement of the porcupine, a species represented at the site by a complete mandible (Cardoso et al., 2021), should not be excluded either. Even though, the possibility that these marks resulted from human activity cannot be ruled out, particularly when considering the marrow exploitation activities observed in other parts of the assemblage, as discussed below. If that were to be the case, then it would further support Neanderthal's primary access to these ungulates' carcasses.
The majority of cut marks are observed on non-identifiable diaphyseal remains. Given that most incisions are located on shaft portions, this pattern is indicative of meat removal and filleting, reflecting butchery practices. However, the proportion of faunal remains exhibiting cut marks within this assemblage is comparatively lower than that recorded at other Iberian sites of similar Middle Palaeolithic contexts, e.g., Level IV of Bolomor (7.1%; Blasco and Fernández Peris, 2012) and Level Jb of Abric Romaní (4%; Marín et al., 2017). These assemblages have generally been interpreted as representing occupation sites of varying durations. Nevertheless, it is important to consider once more that the faunal assemblage from Figueira Brava has not been fully released from its concretion coating, which obscures a significant portion of the bones' surface modifications.
Despite the low frequency of cut marks, other lines of evidence indicate hominin involvement in faunal processing. These include the presence of bone fractures and percussion marks consistent with carcass processing activities. A variety of percussion marks, which in this case are the most frequent and the more diagnostic of anthropogenic fracturing, are primarily observed on red deer remains—the most exploited ungulate at the site. The presence of curved/V-shaped fractures with oblique angles, suggests that bones were broken with hammerstones while still fresh, even though different bones from different species can show varying fracture patterns (e.g., Alcántara-García et al., 2006; Coil et al., 2017). Bone fractures and percussion marks are likely related with marrow extraction, and the presence of abundance longitudinal fractures on long bones supports this interpretation (e.g., Stavrova et al., 2019; Vettese et al., 2020).
The presence of right-angled fractures, however, is typically associated with dry bone breakage (e.g. Outram, 2001), suggesting an additional scenario. Since these fractures are predominantly found on long bones, it is plausible that these elements were processed for deferred marrow consumption (e.g., Binford, 1978) or repurposed as raw materials for bone tool production. Notably, ungulate long bones—especially metapodials—have long been known to be preferred for tool manufacture, with a chaîne opératoire involving longitudinal fracturing (e.g., Sadek-Kooros, 1972; Yesner and Bonnichsen, 1979). Whether used for consumption, tool production, or both, the observed modifications strongly indicate anthropogenic manipulation of the assemblage.
Moreover, the high incidence of bones exhibiting varying degrees of heat exposure, including those with black colouration associated with burning temperatures linked to cooking activities, aligns with patterns observed at coeval, humanly occupied sites. A notable parallel can be drawn with unit II of Ramandils Cave in France, where similar thermal modifications have been documented (Rusch et al., 2019). However, it is important to note that while some brown-burnt bones were detected at Gruta da Figueira Brava, they cannot be directly attributed to cooking activities. Instead, they may result from bones being buried and subsequently exposed to heat from fire production on the sediment layer above (Téllez et al., 2022). Although these brown burns do not necessarily reflect direct consumption of the affected bones, they provide evidence for repeated fire use within the cave, suggesting a certain intensity of occupation and site reutilisation.
The human consumption of ungulates at Figueira Brava represents a relatively minor component of the diverse array of resources exploited by Neanderthals inhabiting the cave. The presence of other faunal remains of smaller size—such as crabs, birds and tortoises (Nabais et al., 2023a,b; Nabais and Zilhão, 2019)—which have previously been identified as resulting from human accumulation, reinforces this interpretation. Furthermore, all macrofaunal remains were recovered from stratigraphic levels containing abundant lithic artefacts attributable to the Mousterian tradition (Zilhão et al., 2020). Collectively, and despite the limitations imposed on analysis by the bones' concretion-coated surface and the relatively small excavated area, these indicators suggest that the role of primary accumulator and primary modifiers of the site's faunal remains was played by humans.
Given the evidence for anthropogenic modification—including burning, intentional breakage and butchery, to be discussed in detail below—the ungulate bones at the site reflect human subsistence behaviour. This interpretation is further supported by the taxonomic composition of the assemblage (ibex, red deer, horse, aurochs), the age profiles of the animals (predominantly adults, with some juvenile remains) and the skeletal part representation (primarily long bones and heads). These characteristics suggest primary access to the carcasses, consistent with previous studies (e.g., Domínguez-Rodrigo, 1999; Gaudzinski and Roebroeks, 2000).
5.3 Ungulate hunting and processing
The transport of animal carcasses to archaeological sites is influenced by multiple factors, including the proximity to the kill site, the value placed on different parts of the carcass and the energetic cost associated with transport, but the prevailing view is that animals hunted near the site are more likely to be skeletally complete upon deposition, as shorter transport distances reduce the likelihood of carcass part attrition during transportation [see a good summary by Reitz and Wing (2008) and references therein; and also a recent example from Bertacchi et al. (2025)]. Skeletal part frequencies can therefore provide insight into hunting practices and the distance from the kill site to the habitation area. Less desirable portions of a carcass are often left at the kill site—normally body parts with lower nutritional yield—, while higher-value parts are transported to residential areas (e.g., Marín et al., 2017). However, these decisions are shaped by multiple factors including weather conditions, topography, the number of individuals participating in the hunt, the energy costs associated with carcass transportation and processing, prey body size and competition with other carnivores (e.g., Bunn et al., 1988; Monahan, 1998; Schoville and Otárola-Castillo, 2014). It should also be considered that the identification of “valuable” or “prestigious” elements is context-dependent, influenced by personal and cultural preferences, specific subsistence needs and environmental conditions (O'Connell et al., 1990). For example, cranial elements may be transported despite their weight and bulk, at a great energetic expense, driven by symbolic behaviour or the value of the brain and other soft tissues for nutritional or other purposes (e.g., Stiner, 1994; Baquedano et al., 2023).
Despite the limitations of the assemblage, Gruta da Figueira Brava's skeletal remains reveal distinct patterns of carcass transport, suggesting a range of exploitation strategies. Although utility indices—such as Binford's (1978) MUI, MI GUI and MGUI, or Metcalfe and Jones's (1988) FUI—are challenging to apply due to the limited sample size, some fundamental principles can still be considered in the analysis and interpretation of our assemblage. According to these researchers, the likelihood of a body part being transported increases with its nutritional value, while less valuable parts are more likely to be discarded. Consequently, if a specific body part with a given nutritional value is removed from the kill-site, body parts with lower nutritional value are also expected to be transported (Binford, 1978; Metcalfe and Jones, 1988).
Applying these principles to our assemblage, it is evident that high-meat-bearing elements, such as the humerus and scapula of ibex, were transported to the site, showing significant survival rates (Figure 4; Table 2). Similarly, high-utility elements such as the humerus, radius, pelvis and tibia of deer were also brought in (Figure 4; Table 2). Following the basic principles outlined earlier, this suggests that all other limb bones were transported as well. Given the well-documented practice of marrow extraction, which results in extensive bone breakage, most evidence of long bones is archaeologically represented by taxonomically indeterminate long bone fragments within the >Very Small, Small and Medium Macrofauna size categories. However, the relatively higher presence of deer and ibex distal limb bones (i.e. metapodials, carpals, tarsals and phalanges) indicates that entire limbs were transported to the site. This pattern aligns with meat-utility predictions, which suggest that if high-utility elements—such as the humerus or a tibia—were transported, then all associated lower-nutritional-value bones—like the metapodials, carpals/tarsals and phalanges—would also have been brought in.
The axial skeleton, primarily represented by ribs and vertebrae, is highly non-diagnostic. Additionally, ribs are prone to breakage, often resulting in their classification within the generic flat bone group. From the axial skeleton, only two deer vertebrae were identified; however, given the substantial number of flat bone fragments categorised within the >Very Small, Small and Medium Macrofauna size classes recovered from the site (Figures 2C–F), it is likely that additional axial elements are present but not easily identifiable. Conversely, cranial elements are well represented for both deer and ibex, exhibiting the highest skeletal survival rates (Figure 4; Table 2). This evidence is strongly indicative that complete carcasses of both deer and ibex were transported to the cave, where they were subsequently processed and consumed.
In contrast, the skeletal remains of aurochs and horses at Gruta da Figueira Brava primarily consist of cranial fragments and limb extremities. The traditional interpretation of this “head and foot pattern” suggests that the abundance of skulls, hands and feet, alongside the absence of meat-rich bones, is indicative of scavenging activities (Binford, 1984). However, the taxonomic and anatomical characterisation of the overall bone assemblage of Figueira Brava does not align with a scavenging scenario. Alternative explanations have been proposed for this distinctive skeletal representation.
Amongst the Hadza, hunters try to transport as much food as possible, while reducing the weight they are transporting back to the base camp. Thus, they discard bones that are easy to process, such as ribs and long bones (Monahan, 1998). At several archaeological sites, cranial elements are disproportionately represented in horse remains, suggesting a deliberate transport strategy. For example, in level Pa and Pb of Abric Romaní (in Spain), horses exhibit a higher presence of cranial elements (Marín et al., 2019). Similarly, at the Chinese Palaeolithic site of Lingjing, horse skulls and mandibles are the most frequently identified skeletal parts (Zhang et al., 2012). This pattern aligns with observations from modern hunter-gatherer societies, where equid meat and milk are considered valuable due to their higher essential fatty acid (EFA) content (Levine, 1998). Experimental studies further demonstrate that equid bones retain significant muscle attachments and nutritional content even after processing. As a result, humans could have prioritised transporting these nutrient-rich skeletal elements to base camps, where they had the necessary time and resources to extract all available nutrients—a strategy that remains relevant among contemporary hunter-gatherers (Lupo, 2006).
There are examples from Qesem and Tabun caves, in Israel (Blasco et al., 2024; Kuhn and Stiner, 2019) showing habitation sites where some carcasses were initially processed at kill sites and then transported to the cave for further butchering. This suggests a selective transport strategy, where only the most nutritionally valuable portions of the skeleton were brought back to the residential site, consistent with the observations of Lupo (2006). In France, at Pech de l'Azé IV, Niven (2013) documented a high representation of reindeer skulls, which she linked to the presence of fire within the site. This association suggests that controlled use of fire facilitated the efficient processing of cranial remains, allowing for their regular transport. This explanation could also account for the strong presence of large ungulate skulls at other sites. Experimental work on cow heads processing further supports this hypothesis, as percussion-based removal of cranial parts has been shown to result in the detachment of multiple teeth, thus increasing the number of cranial elements identified in an assemblage (Baquedano et al., 2023). A recent case study comes from the Epigravettian horse-killing site at Stránská skála IV in the Czech Republic, where animals were extensively dismembered and processed on-site, while the heads were likely transported to the base camp (Svoboda et al., 2020). However, no alternative explanations have been proposed beyond the hypothesis that these heads were used primarily for their fat content. These finds collectively suggest that the transport and processing of large ungulates followed a structured strategy influenced by nutritional optimisation, technological capabilities and environmental factors rather than simple scavenging behaviours.
This ungulate skeletal representation and transport strategy based on size-weight found in Gruta da Figueira Brava—where deer and ibex were transported whole while only selected parts of aurochs and horse were brought back to the base camp—is similar to one found in other Middle Palaeolithic sites, such as Abric Romaní (see Marín et al., 2017 and references therein). However, it should be highlighted that this variation in different-size-ungulate transport strategies appears to be influenced not only by species-specific factors but also by a combination of ecological, logistical and social variables. As well summarised by Faith et al. (2009) the proximity of the kill site to the habitation area likely played a role, as short distances would have facilitated the transport of entire carcasses, whereas longer distances may have necessitated selective transport of body parts. Additionally, the physical condition of the prey at the time of capture, such as its age, health and weight, could have influenced decisions about which parts to transport. The size and composition of the hunting group may have impacted transport strategies, as larger groups would have had greater capacity for carrying whole carcasses, while smaller groups might have prioritised the most nutritionally valuable portions. The presence of potential threats from competing predators could have further shaped these decisions, with hunters needing to balance efficiency with risk minimisation (Faith et al., 2009).
Carcass transport decisions may also reflect the potential for grease and marrow extraction. Assemblages indicative of bone grease exploitation are typically characterised by a marked underrepresentation of epiphyses, basipodials and acropodials (e.g., carpals, tarsals, phalanges), and spongy bone fragments, coupled with an overrepresentation of long bone diaphysis (e.g., Blasco et al., 2024; Crezzini et al., 2023). These patterns suggest deliberate selection and intensive processing of cancellous-rich skeletal parts for the purpose of fat extraction. High levels of fragmentation, the prevalence of small-sized shaft fragments, the presence of percussion marks are also consistent with grease rendering practices (Outram, 2001; Morin, 2007). The highly fragmented faunal assemblage from Gruta da Figueira Brava exhibits a notably low representation of indeterminate epiphyses, a pattern that could maybe reflect intensive processing activities possibly related to grease exploitation.
Even though utility indices are challenging to apply due to the limited size of the sample, the abundance of long bone fragments suggests the introduction of elements rich in medullary cavities filled with marrow (Binford, 1978; Thomas and Mayer, 1983). Among ungulates, such as red deer, exploitation for marrow is demonstrated by breakage patterns, particularly limb bones and metapodials. Marrow extraction is a vital dietary strategy, providing a rich source of animal fat with higher caloric value than carbohydrates and protein (Mead et al., 1986; Outram, 2001). Bone marrow, notably richer in essential fatty acids, represents a high-quality resource (Brink, 1997). Marrow extraction is a low-cost activity, requiring minimal time and effort, particularly for defleshed bones like mandibles and metapodials (e.g. Blasco et al., 2019; Marean and Cleghorn, 2003; Outram, 2001). At Gruta da Figueira Brava, percussion marks are frequently observed on shaft fragments of ungulate long bones, with the most compelling evidence found on red deer remains. Additionally, a percussion notch has been identified on a horse metacarpal. While similar patterns could not be confirmed for other ungulates, primarily due to the extensive fragmentation of long bones, that same high degree of fragmentation itself suggests intensive percussion activity, likely aimed at marrow extraction.
Before marrow extraction, bones must first be skinned, separated from the carcass and defleshed. It was not possible to determine whether incisions were present on articular areas of long bones—features typically associated with dismemberment—, which is likely due to the highly fragmentary state of the assemblage and the presence of calcareous concretions that complicate cut mark identification. However, the presence of incisions on an ibex phalanx at Gruta da Figueira Brava likely reflects skinning, an initial stage in carcass processing. Skinning typically involves making precise cuts around the extremities, particularly on the phalanges, to facilitate the removal of the hide. This practice is crucial not only for obtaining the skin, which may have been used for clothing or other functional purposes, but also as a preparatory step for butchery and further processing of the carcass. The location of the cut marks on the phalanx aligns with previously documented patterns of skinning in archaeological assemblages, where incisions on extremities are commonly associated with this activity (Binford, 1981; Campana and Crabtree, 2019; Faith et al., 2009; Shipman and Rose, 1983; Soulier and Costamagno, 2017).
Among the observable cut marks in Gruta da Figueira Brava, the majority are concentrated on long bone shafts. This distribution of cut marks and their longitudinal orientation indicates a strong focus on the processing of long bone diaphyses, which are normally associated with meat-bearing elements. Such patterns have been generally interpreted as evidence of subsistence strategies associated with filleting (e.g., Domínguez-Rodrigo et al., 2009a,b; Galán and Domínguez-Rodrigo, 2013; Livraghi et al., 2020; Soulier and Costamagno, 2017), which reflect primary access to carcasses, wherein early-stage butchery activities were conducted to remove muscle tissue (e.g., Noe-Nygaard, 1989; Marean, 1998). The location of these marks thus suggests that Neanderthals at the site were engaging in butchering techniques, emphasising meat extraction rather than secondary scavenging. Additionally, the anatomical placement of the cut marks provides insight into the butchery techniques employed. The concentration on the midshaft region of long bones suggests the use of cutting motions to detach muscle fibres efficiently (Galán and Domínguez-Rodrigo, 2013). Taken together, this evidence points to an organised and deliberate approach to ungulate carcass processing, demonstrating the economic use of these faunal resources within Neanderthal subsistence behaviours.
The burning patterns observed at the site further elucidate on subsistence practices. Although burnt bones are relatively low in number, the predominance of black burns suggests exposure to moderate temperatures, typically reaching up to 400°C (Nicholson, 1993). This temperature range is consistent with controlled fire use, such as roasting, rather than accidental burning from open flames or natural wildfires. The presence of partially burnt elements further supports this interpretation, as these are often associated with cooking activities where bones are exposed to heat for a limited duration rather than being completely incinerated (Gifford-Gonzalez, 1993; Pearce and Luff, 1994; Montón-Subías, 2002).
Additionally, certain burnt remains, such as bear canines and other elements not typically associated with human consumption, indicate secondary burning events rather than intentional roasting or food processing. These elements were likely affected by hearth fires ignited above previously deposited skeletal remains, suggesting repeated occupation phases where discarded bones were later exposed to combustion (e.g. Téllez et al., 2022). This pattern aligns with finds from other Palaeolithic sites, where fire use not only served subsistence purposes but also played a role in site maintenance and waste disposal (e.g., Pietraszek et al., 2022; Starkovich et al., 2020). These burning patterns reinforce the evidence for deliberate fire use and structured food processing strategies.
In sum, Gruta da Figueira Brava's faunal assemblage reveals how, during late MIS-5 times, local Neanderthal populations exploited the ungulate resources made available by the surrounding environment. Such resources, though, constituted but one component of a broader and diverse dietary spectrum that also included small prey and aquatic resources, as previously described. The transport and processing of entire deer and ibex carcasses, along with the selective transport of high-value body parts of aurochs and horse, demonstrate a strategic approach to resource exploitation and energy efficiency. Additionally, practices such as marrow extraction, defleshing and cooking highlight the cave inhabitants' capacity to maximise the nutritional potential of various food sources, contributing to a diversified and well-rounded diet.
6 Conclusion
The macro-mammal assemblage from Gruta da Figueira Brava offers compelling insights into the complex and diverse subsistence strategies employed by Neanderthal populations during MIS-5. The taxonomic composition and skeletal part representation indicate a well-structured approach to hunting, carcass transport and resource processing. The transport of complete ibex and red deer carcasses, alongside the selective transport of high-value elements from larger ungulates, such as aurochs and horses, suggests an adaptive strategy influenced by ecological conditions, prey size and transport logistics.
Further evidence of butchery activities, including cut marks on long bone shafts reflecting defleshing activities, incisions on phalanges suggesting skinning, percussion marks indicative of marrow extraction and the burning of skeletal elements, highlights the comprehensive use of ungulate resources. The presence of structured burning patterns, particularly black burns associated with controlled fire use, suggests that cooking played an integral role in Neanderthal dietary practices. Moreover, the identification of bones affected by secondary burning events implies long-term site occupation and repeated use of hearths.
While carnivore activity was present, the absence of digested bone and the lack of carnivore juvenile remains, is inconsistent with denning. The hyaena coprolites, nonetheless, indicate their intermittent use of the interior of the cave as a latrine when humans were absent. However, the low frequency of carnivore-induced modifications coupled with the stratigraphic context of their remains, indicates that human activity was the dominant factor in bone accumulation. The presence of anthropogenic modifications further reinforces this interpretation. The site's faunal assemblage also aligns with broader Middle Palaeolithic trends observed at other Neanderthal occupation sites, where ungulate exploitation following intentional carcass processing and selective transport depending on weight-size were central components of subsistence behaviour.
The diversity of ungulate prey reflects the exploitation of a range of ecological niches within the surrounding landscape, including patches of forest, grassland and shrubland. This further highlights the adaptability and efficiency of the region's Neanderthal subsistence strategies. Ultimately, the integration of ungulate hunting with the exploitation of small prey and aquatic resources reflects a diversified subsistence system that maximised food availability across different ecological zones and different times of the year, which accentuates the versatility of Neanderthal diets and behaviour in coastal Atlantic occupations. This adaptive flexibility underscores Neanderthals' capacity to respond to environmental variability and resource distribution, reinforcing their role as skilled hunters and efficient resource managers within the Middle Palaeolithic landscape.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because these are archaeological materials, no animals were harmed at any point of this research.
Author contributions
MN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. JZ: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was part of the project n° 526079 “Neanderthal Subsistence in Portugal: small and large prey consumption during the Marine Isotope Stage 5” funded by the London Arts and Humanities Partnership (LAHP). Other financial support has been provided by Portuguese funds through FCT - Fundação para a Ciência e a Tecnologia in the framework of the project UID/00698: Centre for Archaeology, University of Lisbon; and by the project “Archaeology and Evolution of Early Humans in the Western façade of Iberia” (PTDC/HAR-ARQ/30413/2017). Additional financial support has been provided by the project NEANDIVERSITY2 (PID2022-138590NB-C41) funded by Spanish MICIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”; and by MN's current postdoc contract part of the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 101034349, and from the State Research Agency of the Spanish Ministry of Science and Innovation through the Program Maria de Maeztu Unit of Excellence (CEX2019-000945-M). We acknowledge the use of the Vertebrate Reference Collection (Osteoteca) of the Archaeosciences Laboratory of the Património Cultural I.P., in Lisbon, Portugal. We also extend our gratitude to the PRISC infrastructure (Portuguese Research Infrastructure of Scientific Collections) for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PS declared a shared affiliation with the author MN at the time of review.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alcántara-García V. Barba Egido R. Barral del Pino J. M. Crespo Ruiz A. B. Eiriz Vidal A. I. Falquina Aparicio Á. et al . (2006). Determinación de procesos de fractura sobre huesos frescos: un sistema de análisis de los ángulos de los planos de fracturación como discriminador de agentes bióticos. Trabajos Prehist.61, 25–38. 10.3989/tp.2006.v63.i1.3
2
Álvarez-Lao D. Méndez M. (2016). Latitudinal gradientes and indicator species in ungulate paleoassemblages during the MIS 3 in W Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol.449, 455–462. 10.1016/j.palaeo.2016.02.050
3
Álvarez-Lao D. J. García N. (2011a). Geographical distribution of Pleistocene cold adapted large mammal faunas in the Iberian Peninsula. Quat. Int.233, 159–170. 10.1016/j.quaint.2010.04.017
4
Álvarez-Lao D. J. García N. (2011b). Southern dispersal and palaeoecological implications of woolly rhinoceros (Coelodonta antiquitatis): review of the Iberian occurrences. Quat. Sci. Rev.30, 2002–2017. 10.1016/j.quascirev.2011.05.005
5
Andrés M. Gidna A. Yravedra J. Domínguez-Rodrigo M. (2012). A study of dimensional differences of tooth marks (pits and scores) on bones modified by small and large carnivores. Archaeol. Antropol. Sci.4, 209–219. 10.1007/s12520-012-0093-4
6
Andrews P. Turner A. (1992). Life and death of the Westbury bears. Ann. Zool. Fennici28, 139–149.
7
Antunes M. (2000). Last Neanderthals in Portugal. Odontologic and Other Evidence. Classe das Ciências. Tomo XXXVIII.Lisboa: Academia das Ciências de Lisboa.
8
Azorit C. Tellado S. Oya A. Moro J. (2012). Seasonal and specific diet variations in sympatric red and fallow deer of southern Spain: a preliminary approach to feeding behaviour. Anim. Prod. Sci.52:720. 10.1071/AN12016
9
Baquedano E. Arsuaga J. L. Pérez-González A. Laplana C. Márquez B. Huguet R. et al . (2023). A symbolic Neanderthal accumulation of large herbivore crania. Nat. Hum. Behav.7, 342–352. 10.1038/s41562-022-01503-7
10
Bertacchi A. Kaliba P. Thompson J. (2025). Short-distance hunting strategies of Late Quaternary foragers in the miombo woodlands of Malawi. J. Anthropol. Archaeol.77:101656. 10.1016/j.jaa.2025.101656
11
Binford L. (1978). Nunamiut Ethnoarchaeology. New York, NY: Academic Press.
12
Binford L. (1981). Bones: Ancient Men and Modern Myths. New York, NY: Academic Press.
13
Binford L. (1984). Faunal Remains from Klasies River Mouth.New York, NY: Academic Press.
14
Blasco R. Fernández Peris J. (2012). Small and large game: human use of diverse faunal resources at Level IV of Bolomor Cave (Valencia, Spain). Comptes Rendus Palevol11, 265–282. 10.1016/j.crpv.2012.01.003
15
Blasco R. Rosell J. Arilla M. Margalida A. Villalba D. Gopher A. et al . (2019). Bone marrow storage and delayed consumption at Middle Pleistocene Qesem Cave, Israel (420 to 200 ka). Sci. Adv. 5:eaav9822. 10.1126/sciadv.aav9822
16
Blasco R. Rosell J. Assaf E. Barkai R. Gopher A. (2024). Exploring the lack of articular ends at the Middle Pleistocene site of Qesem Cave, Israel. J. Hum. Evol.189:103509. 10.1016/j.jhevol.2024.103509
17
Blasco R. Rosell J. Fernández-Peris J. Arsuaga J. L. Bermúdez Castro J. M. Carbonell E. et al . (2013). Environmental availability, behavioural diversity and diet: a zooarchaeological approach from the TD10-1 sublevel of Gran Dolina (Sierra de Atapuerca, Burgos, Spain) and Bolomor Cave (Valencia, Spain). Quat. Sci. Rev.70, 124–144. 10.1016/j.quascirev.2013.03.008
18
Blumenschine R. Selvaggio M. (1988). Percussion marks on bone surfaces as a new diagnostic of hominid behaviour. Nature333, 763–765. 10.1038/333763a0
19
Bocherens H. Drucker D. G. Madelaine S. (2014). Evidence for a 15N positive excursion in terrestrial foodwebs at the Middle to Upper Palaeolithic transition in south-western France: implications for early modern human palaeodiet and palaeoenvironment. J. Hum. Evol.69, 31–43. 10.1016/j.jhevol.2013.12.015
20
Brain C. K. (1969). The contribution of Namib desert Desert Hottentots to the understanding of Australopithecine bone accumulations. Sci. Papers Namib Desert Res. Station39, 13–22.
21
Brink J. W. (1997). Fat content in leg bones of Bison bison, and applications to archaeology. J. Archaeol. Res.24, 259–274. 10.1006/jasc.1996.0109
22
Brown W. Chapman G. (1990). The dentition of fallow deer (Dama dama): a scoring scheme to assess age from wear of the permanent molariform teeth. J. Zool.221, 659–682. 10.1111/j.1469-7998.1990.tb04023.x
23
Brown W. Chapman G. (1991). The dentition of red deer (Cervus elaphus): a scoring scheme to assess age from wear of the permanent molariform teeth. J. Zool.224, 519–536. 10.1111/j.1469-7998.1991.tb03783.x
24
Bunn H. T. (1983). “Evidence on the diet and subsistence patterns of Plio-Pleistocene hominids at Koobi Fora, Kenya, and Olduvai Gorge, Tanzania,” in Animals and Archaeology. International Series 163, eds. J. Clutton-Brock, and C. Grigson (Oxford: British Archaeological Reports), 21–30.
25
Bunn H. T. (1986). Patterns of skeletal representation and hominid subsistence activities at Olduvai Gorge, Tanzania, and Koobi Fora, Kenya. J. Hum. Evol.15, 673–690. 10.1016/S0047-2484(86)80004-5
26
Bunn H. T. Bartram L. E. Kroll E. M. (1988). Variability in bone assemblage formation from Hadza hunting, scavenging, and carcass processing. J. Antrhopol. Archaeol.7, 412–457. 10.1016/0278-4165(88)90004-9
27
Campana D. V. Crabtree P. (2019). Evidence for skinning and craft activities from the Middle Palaeolithic Shanidar Cave, Iraq. J. Archaeol. Sci. Rep.25, 7–14. 10.1016/j.jasrep.2019.03.024
28
Capaldo S. Blumenschine R. (1994). A quantitative diagnosis of notches made by hammerstone percussion and carnivore gnawing on bovid long bones. Am. Antiq.59, 724–748. 10.2307/282345
29
Cardoso J. L. Detry C. Zilhão J. (2021). The first appearance of Hystrix (rodentia, mammalia) in Portugal, Last Interglacial, Gruta da Figueira Brava (Setúbal). Quaternaire32, 173–182. 10.4000/quaternaire.18541
30
Carrión J. S. Lalueza C. Stewart J. (2019). Neanderthals: ecology and evolution. Quat. Sci. Rev.217, 1–6. 10.1016/j.quascirev.2019.05.008
31
Castel J. C. Coumont M. P. Boudadi-Maligne M. Prucca A. (2010). Rôle et origine des grans carnivores dans les accumulations naturelles. Les cas des loups (Canis lupus) de l'Igue du Gral (Sauliac-sur-Célé, Lot, France). Rev. Paléobiol.29, 411–425.
32
Clutton-Brock T. H. (1982). Red Deer: Behavior and Ecology of the Two Sexes.Chicago, IL: University of Chicago Press.
33
Cobo-Sánchez L. Rufà A. Cascalheira J. (2024). Alternating carnivore and Neanderthal activities at Escoural Cave: insights from the taphonomic and machine learning analysis of leporid remains. Front. Environ. Archaeol.3:1473266. 10.3389/fearc.2024.1473266
34
Coil R. Tappen M. Yezzi-Woodley K. (2017). New analytical methods for comparing bone fracture angles: a controlled study of hammerstone and hyena (Crocuta crocuta) long bone breakage. Archaeometry59, 900–917. 10.1111/arcm.12285
35
Crezzini J. Boscato P. Ronchitelli A. Boschin F. (2023). A peculiar exploitation of ungulates at Grotta di Santa Croce: bone grease rendering and nutritional patterns among Neanderthals in southern Italy. Hist. Biol. 36, 2133–2145. 10.1080/08912963.2023.2242630
36
Daujeard C. Vettese D. Britton K. Béarez P. Boulbes N. Crégut-Bonnoure E. et al . (2017). Neanderthal selective hunting of reindeer? The case study of Abri du Maras (south-eastern France). Archaeol. Anthropol. Sci.11, 985–1011. 10.1007/s12520-017-0580-8
37
Díez J. Fernández-Jalvo Y. Rosell J. Cáceres I. (1999). Zooarchaeology and taphonomy of Aurora Stratum (Gran Dolina, Sierra de Atapuerca, Spain). J. Hum. Evol.37, 623–652. 10.1006/jhev.1999.0346
38
Domínguez-Rodrigo M. (1993). La formación de las acumulaciones óseas de macrofauna: revisión de los criterios de discernimiento de los agentes biológicos no antrópicos desde un enfoque ecológico. Zephyrus46, 103–122.
39
Domínguez-Rodrigo M. (1994). Las razones adaptativas del comportamiento subsistencial de los animales carnívoros y sus estrategias iniciales de consumo de presas: relevancia en el proceder tafonómico. Cuad. Prehist. Arqueol. Castellon16, 9–17.
40
Domínguez-Rodrigo M. (1999). Distinguishing between apple and oranges: the application of modern cut mark studies to the Plio-Pleistocene (a reply to Monahan). J. Hum. Evol.33, 793–800. 10.1006/jhev.1999.0358
41
Domínguez-Rodrigo M. de Juana S. Galán A. B. Rodríguez M. (2009a). A new protocol to differentiate trampling marks from butchery cut marks. J. Archaeol. Sci.36, 2643–2654. 10.1016/j.jas.2009.07.017
42
Domínguez-Rodrigo M. Mabulla A. Bunn H. T. Barba R. Diez-Martín F. Egeland C. P. et al . (2009b). Unraveling hominin behavior at another anthropogenic site from Olduvai Gorge (Tanzania): new archaeological and taphonomic research at BK, Upper Bed II. J. Hum. Evol.57, 260–283. 10.1016/j.jhevol.2009.04.006
43
Dusseldorp G. L. (2013). “Neanderthals and cave hyenas: co-existence, competition or conflict?” in Zooarchaeology and Modern Human Origins. Vertebrate Paleobiology and Paleoanthropology, eds. J. Clark, and J. Speth (Dordrecht: Springer), 191–208. 10.1007/978-94-007-6766-9_12
44
Faith J. T. Domínguez-Rodrigo M. Gordon A. D. (2009). Long-distance carcass transport at Olduvai Gorge? A quantitative examination of Bed I skeletal element abundances. J. Hum. Evol.56, 247–256. 10.1016/j.jhevol.2008.12.008
45
Farizy D. David F. Jaubert J. (1994). Hommes et Bisons du Paléolithique Moyen à Mauran (Haute-Garonne). Gallia Préhistoire, XXXe Suppléments. Paris: CNRS éditions.
46
Finlayson C. Brown K. Blasco R. Rosell J. Negro J. J. Bortolotti G. R. et al . (2012). Birds of a feather: Neanderthal exploitation of raptors and corvids. PLoS ONE7:e45927. 10.1371/annotation/5160ffc6-ec2d-49e6-a05b-25b41391c3d1
47
Fisher J. (1995). Bone surface modifications in zooarchaeology. J. Archaeol. Method Theory, 2, 7–68. 10.1007/BF02228434
48
Galán A. B. Domínguez-Rodrigo M. (2013). An experimental study of the anatomical distribution of cut marks created by filleting and disarticulation on long bone ends. Archaeometry55, 1132–1149. 10.1111/j.1475-4754.2012.00730.x
49
García García N. Feranec R. S. Arsuaga J. L. Bermúdez de Castro J. M. Carbonell E. (2009). Isotopic analysis of the ecology of herbivores and carnivores from the Middle Pleitocene deposits of Sierra de Atapuerca, northern Spain. J. Archaeol. Sci.36, 1142–1151. 10.1016/j.jas.2008.12.018
50
Gaudzinski S. Roebroeks W. (2000). Adults only. Reindeer hunting at the Middle Palaeolithic site Salzgitter Lebenstedt, Northern Germany. J. Hum. Evol.38, 497–521. 10.1006/jhev.1999.0359
51
Gaudzinski-Windheuser S. Kindler L. (2012). Research perspectives for the study of Neanderthal subsistence strategies based on the analysis of archaeozoological assemblages. Quat. Int.247, 59–68. 10.1016/j.quaint.2010.11.029
52
Geist V. (1998). Deer of the World. Their Evolution, Behavior, and Ecology. Pennsylvania, PA: Stackpole Books, Mechanicsburg.
53
Gifford-Gonzalez D. (1993). “Gaps in zooarchaeological analyses of butchery: is gender an issue?” in From Bones to Behaviour. Ethnoarchaeological and Experimental Contributions to the Interpretation of Faunal Remains, ed. J. Hudson (Carbondale: Center for Archaeological Investigations, Southern Illinois University), 181–199.
54
Granados J. E. Pérez J. M. Márquez F. J. Serrano E. Soriguer R. C. Fandos P. et al . (2001). La cabra montés (Capra pyrenaica, SCHINZ 1838). Galemys13, 3–37.
55
Grant A. (1982). “The use of tooth wear as a guide to the age of domestic ungulates,” in Ageing and Sexing Animal Bones from Archaeological Sites. BAR British Series 109, eds. B. Wilson, C. Grigson, and S. Payne (Oxford: Archaeopress), 91–108.
56
Grayson D. K. (1984). Quantitative Zooarchaeology: Topics in the Analysis of Archaeological Faunas. Orlando: Academic Press.
57
Henry A. Brooks A. Piperno D. (2014). Plant foods and the dietary ecology of Neanderthals and early modern humans. J. Hum. Evol.69, 44–54. 10.1016/j.jhevol.2013.12.014
58
Hillson S. (2005). Teeth, 2nd Edn. Cambridge: Cambridge University Press.
59
Horwitz L. K. Goldberg P. (1989). A study of Pleistocene and Holocene hyaena coprolites. J. Archaeol. Sci.16, 71–94. 10.1016/0305-4403(89)90057-5
60
Jaouen K. Richards M. P. Le Cabec A. Welker F. Rendu W. Hublin J. J. et al . (2019). Exceptionally high δ15N values in collagen single amino acids confirm Neandertals as high-trophic level carnivores. Proc. Natl Acad. Sci.116, 4928–4933. 10.1073/pnas.1814087116
61
Johansson S. (2014). The thinking Neanderthals: what do we know about Neanderthal cognition?Wiley Interdiscip. Rev. Cogn. Sci.5, 613–620. 10.1002/wcs.1317
62
Klein R. Cruz-Uribe G. (1984). The Analysis of Animal Bones from Archaeological Sites.Chicago, IL: University of Chicago Press.
63
Kuhn S. Stiner M. (2019). Hearth and home in the Middle Pleistocene. J.. Anthropol. Res. 75. 10.1086/704145
64
Langley M. Clarkson C. Ulm S. (2008). Behavioural complexity in Eurasian Neanderthal populations: a chronological examination of the archaeological evidence. Cambridge Archaeol. J.18, 289–307. 10.1017/S0959774308000371
65
Larkin N. R. Alexander J. Lewis M. D. (2000). Using experimental studies of recent faecal material to examine hyaena coprolites from the West Runton Freshwater Bed, Norfolk, UK. J. Archaeol. Sci.27, 19–31. 10.1006/jasc.1999.0437
66
Levine M. A. (1998). Eating horses: the evolutionary significance of Hippophagy. Antiquity72, 90–100. 10.1017/S0003598X00086300
67
Livraghi A. Fanfarillo G. Dal Colle M. Romandini M. Peresani M. (2020). Neanderthal ecology and the exploitation of cervids and bovids at the onset of MIS4: a study on De Nadale cave, Italy. Quat. Int. 586, 24–41. 10.1016/j.quaint.2019.11.024
68
Lupo K. D. (2006). What explains the carcass field processing and transport decisions of contemporary hunter-gatherers? Measures of economic anatomy and zooarchaeological skeletal part representation. J. Archaeol. Method Theory13, 9–66. 10.1007/s10816-006-9000-6
69
Lyman R. L. (1994a). Quantitative units and terminology in zooarchaeology. Am. Antiq.59, 36–71. 10.2307/3085500
70
Lyman R. L. (2008). Quatitative Paleozoology. Cambridge: Cambridge University Press. 10.1017/CBO9780511813863
71
Marean C. (1998). A critique of the evidence for scavenging by Neandertals and early modern humans: new data from Kobeh Cave (Zagros Mountains, Iran) and Die Kelders Cave 1 Layer 10 (South Africa). J. Hum. Evol.35, 111–136. 10.1006/jhev.1998.0224
72
Marean C. W. Cleghorn N. (2003). Large mammal skeletal element transport: applying foraging theory in a complex taphonomy system. J. Taphonomy1, 15–42.
73
Marín J. Rodríguez-Hidalgo A. Vallverdú J. Gómez de Soler B. Rivals F. Rabuñal J. R. et al . (2019). Neanderthal logistic mobility during MIS3: zooarchaeological perspective of Abric Romaní level P (Spain). Quat. Sci. Rev.225:106033. 10.1016/j.quascirev.2019.106033
74
Marín J. Saladié P. Rodríguez-Hidalgo A. Carbonell E. (2017). Ungulate carcass transport strategies at the Middle Palaeolithic site of Abric Romaní (Capellades, Spain). Comptes Rendus Palevol16, 103–121. 10.1016/j.crpv.2015.11.006
75
Marín-Arroyo A. B. Sanz-Royo A. (2021). What Neanderthals and AMH ate: reassessment of the subsistence across the Middle-Upper Palaeolithic transition in the Vasco-Cantabrian region of SW Europe. J. Quat. Sci.37, 320–334. 10.1002/jqs.3291
76
Mead J. F. Alfin-Slater R. B. Howton D. R. Popják G. (1986). Lipids: Chemistry, Biochemistry, and Nutrition. New York, NY: Plenum Press. 10.1007/978-1-4613-2107-1
77
Metcalfe D. Jones K. T. (1988). A reconsideration of animal body-part utility indices. Am. Antiq.53, 486–504. 10.2307/281213
78
Moclán A. Huguet R. Márquez B. Laplana C. Galindo-Pellicena M. García N. et al . (2021). A Neanderthal hunting camp in the central system of the Iberian Peninsula: a zooarchaeological and taphonomic analysis of the Navalmaíllo Rock Shelter (Pinilla del Valle, Spain). Quat. Sci. Rev.269:107142. 10.1016/j.quascirev.2021.107142
79
Monahan C. M. (1998). The Hadza Carcass transport debate revisited and its Archaeological Implications. J. Archaeol. Sci.25, 405–424. 10.1006/jasc.1997.0241
80
Montón-Subías S. (2002). “Cooking in zooarchaeology: is this issue still raw?” in Consuming Passions and Patterns of Consumption, eds. P. Miracle, and N. Milner (Cambridge: McDonald Institute of Archaeological Research), 7–15.
81
Moreno-García M. Pimenta C. Davis S. Gabriel S. (2003). “A osteoteca: uma ferramenta de trabalho,” in Paleoecologia Humana e Arqueociências. Um programa Multidisciplinar Para a Arqueologia sob a Tutela da Cultura. Trabalhos de Arqueologia 29, eds. M. Moreno-García, and J. Mateus(Lisboa: Instituto Português de Arqueologia), 235–262.
82
Morin E. (2007). Fat composition and Nunamiut decision-making: a new look at the marrow and bone grease indices. J. Archaeol. Sci.34, 69–82. 10.1016/j.jas.2006.03.015
83
Nabais M. Dupont C. Zilhão J. (2023a). The exploitation of crabs by Last Interglacial Iberian Neanderthals: the evidence from Gruta da Figueira Brava (Portugal). Front. Environ. Archaeol.2:1097815. 10.3389/fearc.2023.1097815
84
Nabais M. Pimenta C. Zilhão J. (2023b). Human-bird interaction in Last Interglacial Iberia: a combined approach using skeletal part analysis, bone surface modification, bird ethology and ethnography. J. Archaeol. Sci. Rep.49:104023. 10.1016/j.jasrep.2023.104023
85
Nabais M. Zilhão J. (2019). The consumption of tortoise among Last Interglacial Iberian Neanderthals. Quat. Sci. Rev.217, 225–246. 10.1016/j.quascirev.2019.03.024
86
Nicholson R. (1993). A morphological investigation of burnt animal bone and an evaluation of its utility in archaeology. J. Archaeol. Sci.20, 411–428. 10.1006/jasc.1993.1025
87
Niven L. (2013). “A diachronic evaluation of neanderthal cervid exploitation and site use at Pech de l'Aze IV, France,” in Zooarchaeology and Modern Human Origins: Human Hunting Behavior during the Later Pleistocene, eds. J. L. Clark, and J. D. Speth (Dordrecht: Springer Netherlands), 151–161. 10.1007/978-94-007-6766-9_9
88
Noe-Nygaard N. (1989). Man-made trace fossils on bones. Hum. Evol.4, 461–491. 10.1007/BF02436295
89
O'Connell J. Hawkes K. Blurton-Jones N. (1990). Reanalysis of large mammal body part transport among the Hadza. J. Archaeol. Sci.17, 301–316. 10.1016/0305-4403(90)90025-Z
90
Outram A. K. (2001). A new approach to identifying bone marrow and grease exploitation: why the “Indeterminate” fragments should not be ignored. J. Archaeol. Sci.28, 401–410. 10.1006/jasc.2000.0619
91
Pales L. Lambert C. (1971). Atlas Ostéologique pour server à l'identification des mammifères du Quaternaire. I. Les membres. Paris: Éditions du Centre National de la Recherche Scientifique.
92
Pales L. Lambert C. (1981). Atlas Ostéologique pour server à l'identification des mammifères du Quaternaire. II. Tête – Rachis Ceintures scapulaire et pelvienne. Paris: Éditions du Centre National de la Recherche Scientifique.
93
Patou-Mathis M. (2000). Neanderthal subsistence behaviours in Europe. Int. J. Osteoarchaeol.10, 379–395. 10.1002/1099-1212(200009/10)10:5<379::AID-OA558>3.0.CO;2-4
94
Patou-Mathis M. (2006). “Comportements de subsistence des Néandertaliens d'Europe,” in Neanderthals in Europe. Proceedings of the International Conference held in the Gallo-Roman Museum in Tougereu (September 17-19th 2004), vol. 117, eds. B. Demansin, and M. Otte (Liège: Eraul), 67–76.
95
Payne S. (1973). Kill-off patterns in sheep and goats: the mandibles from Asuan Kale. Anatol. Stud.23, 281–303. 10.2307/3642547
96
Payne S. (1987). Reference codes for wear states in the mandibular cheek teeth of sheep and goats. J. Archaeol. Sci.14, 609–614. 10.1016/0305-4403(87)90079-3
97
Pearce J. Luff R. (1994). “The taphonomy of cooked bone,” in Whither Environmental Archaeology? eds. R. Luff, and P. Rowley-Conwy (Oxford: Oxbow Books), 51–56.
98
Pérez-Hidalgo T. Cobo Rayán R. (1987). Atlas Anatomico de Ursidos del Pleistoceno Holoceno de la Peninsula Iberica. Madrid: Federación Madrileña de Espeleología.
99
Pickering T. Egeland C. (2006). Experimental patterns of hammerstone percussion damage on bones: implications for inferences of carcass processing by humans. J. Archaeol. Sci.33, 459–469. 10.1016/j.jas.2005.09.001
100
Pietraszek A. Zaidner Y. Shahack-Gross R. (2022). The distribution and treatment of fire remains across Unit V of the Middle Paleolithic open-air site of Nesher Ramla, Israel. Quat. Int. 624, 107–116. 10.1016/j.quaint.2021.03.027
101
Reitz E. Wing E. (2008). Zooarchaeology. Cambridge: Cambridge University Press. 10.1017/CBO9780511841354
102
Rendu W. (2022). “Selection versus opportunism: a view from Neanderthal subsistence strategies,” in Updating Neanderthals: Understanding Behavioural Complexity in the Late Middle Palaeolithic, eds. F. Romagnoli, F. Rivals, and S. Benazzi (London: Academic Press), 109–122. 10.1016/B978-0-12-821428-2.00013-5
103
Ribeiro O. (1945). Portugal. O Mediterrâneo e o Atlântico.Lisboa: Livraria Sá da Costa Editora.
104
Rodríguez-Hidalgo A. Saladié P. Ollé A. Arsuaga J. L. Bermúdez de Castro J. M. Carbonell E. (2017). Human predatory behavior and the social implications of comunal hunting based on evidence from the TD10.2 bison boné bed at TGran Dolina (Atapuerca, Spain). J. Hum. Evol.105, 89–122. 10.1016/j.jhevol.2017.01.007
105
Romandini M. Terlato G. Nannini N. Tagliacozzo A. Benazzi S. Peresani M. et al . (2018). Bears and humans, a Neanderthal tale. Reconstructing uncommon behaviors from zooarchaeological evidence in southern Europe. J. Archaeol. Sci.90, 71–91. 10.1016/j.jas.2017.12.004
106
Rosell J. Cáceres I. Blasco R. Bennàsar M. Bravo P. Campeny G. et al . (2012). A zooarchaeological contribution to establish occupational patterns at level J of Abric Romaní (Barcelona, Spain). Quat. Int.247, 69–84. 10.1016/j.quaint.2011.01.020
107
Rusch L. Gregoire S. Pois V. Moigne A. M. (2019). Neanderthal and carnivore occupations in unit II form the Upper Pleistocene site of Ramandils Cave (Port-la-Nouvelle, Aude, France). J. Archaeol. Sci. Rep.28:102038. 10.1016/j.jasrep.2019.102038
108
Sadek-Kooros H. (1972). Primitive bone fracturing: a method of research. Am. Antiq.37, 369–382. 10.2307/278436
109
Sala N. Arsuaga J. L. (2013). Taphonomic studies with wild brown bears (Ursus arctos) in the mountains of northern Spain. J. Archaeol. Sci.40, 1389–1396. 10.1016/j.jas.2012.10.018
110
Saladié P. Huguet R. Díez J. C. Rodríguez-Hidalgo A. Carbonell E. (2013). Taphonomic modifications produced by modern brown bears (Ursus arctos). Int. J. Osteoarchaeol.23, 13–33. 10.1002/oa.1237
111
Salazar-García D. Power R. Sanchis Serra A. Villaverde V. Walker M. Henry A. et al . (2013). Neanderthal diets in central and southeastern Mediterranean Iberia. Quat. Int.318, 3–18. 10.1016/j.quaint.2013.06.007
112
Sanchis A. Real C. Sauqué V. Núñez-Lahuerta C. Égüez N. Tormo C. et al . (2019). Neanderthal and carnivore activities at Llonin Cave, Asturias, northern Iberian Peninsula: Faunal study of Mousterian levels (MIS 3). Comptes Rendus Palevol18, 113–141. 10.1016/j.crpv.2018.06.001
113
Sanz M. Daura J. Éguez N. Brugal J. P. (2016). Not only hyenids: a multi-scale analysis of Upper Pleistocene carnivore coprolites in Cova del Coll Verdaguer (NE Iberian Peninsula). Palaeogeogr. Palaeoclimatol. Palaeoecol.443, 249–262. 10.1016/j.palaeo.2015.11.047
114
Sanz M. Rivals F. García D. Zilhão J. (2019). Hunting strategy and seasonality in the last interglacial occupation of Cueva Antón (Murcia, Spain). Archaeol. Anthropol. Sci.11, 3577–3594. 10.1007/s12520-018-0768-6
115
Sauqué V. Sanchis A. Madurell-Malapeira J. (2018). Late Pleistocene leopards as a bone accumulator: taphonomic results from S'Espasa cave and other Iberian key sites. Hist. Biol.30, 821–834. 10.1080/08912963.2017.1343313
116
Schmid E. (1972). Atlas of Animal Bones. Amsterdam: Elsevier.
117
Schoville B. J. Otárola-Castillo E. (2014). A model of hunter-gatherer skeletal element transport: the effect of prey body size, carrier, and distance. J. Hum. Evol.73, 1–14. 10.1016/j.jhevol.2014.06.004
118
Schulz E. Kaiser T. M. (2012). Historical distribution, habitat requirements and feeding ecology of the genus Equus (Perissodactyla). Mamm. Rev.43, 111–123. 10.1111/j.1365-2907.2012.00210.x
119
Shipman P. Foster G. Schoeninger M. (1984). Burnt bones and teeth: an experimental study of color, morphology, crystal structure and shrinkage. J. Archaeol. Sci.11, 307–325. 10.1016/0305-4403(84)90013-X
120
Shipman P. Rose J. (1983). Early hominid hunting, butchering and carcass processing behaviors: approaches to the fossil record. J. Anthropol. Archaeol.2, 57–98. 10.1016/0278-4165(83)90008-9
121
Solounias N. Semprebon G. M. (2002). Advances in the reconstruction of ungulate ecomorphology with application to early fossil equids. Am. Mus. Novit.3366, 1–49. 10.1206/0003-0082(2002)366<0001:AITROU>2.0.CO;2
122
Soulier M. C. Costamagno S. (2017). Let the cutmarks speak! Experimental butchery to reconstruct carcass processing. J. Archaeol. Sci. Rep.11, 782–802. 10.1016/j.jasrep.2016.12.033
123
Starkovich B. Elefanti P. Karkanas P. Panagopoulou E. (2020). Site use and maintenance in the middle Palaeolithic at Lakonis I (Peloponnese, Greece). J. Paleolithic Archaeol.3, 157–186. 10.1007/s41982-018-0006-x
124
Stavrova T. Borel A. Daujeard C. Vettese D. (2019). A GIS based approach to long bone breakage patterns derived from marrow extraction. PLoS ONE14:e0216733. 10.1371/journal.pone.0216733
125
Stewart J. R. García-Rodríguez O. Knul M. V. Sewell L. Montgomery H. Thomas M. G. et al . (2019). Palaeoecological and genetic evidence for Neanderthal power locomotion as an adaptation to a Woodland environment. Quat. Sci. Rev.217, 310–315. 10.1016/j.quascirev.2018.12.023
126
Stiner M. (1994). Honor Among Thieves: A zooarchaeological study of Neanderthal ecology.Princeton, NJ: Princeton University Press.
127
Stringer C. B. Finlayson J. C. Barton R. N. E. Fernández-Jalvo Y. Cáceres I. Sabin R. C. et al . (2008). Neanderthal exploitation of marine mammals in Gibraltar. Proc. Natl. Acad. Sci. USA105, 14319–14324. 10.1073/pnas.0805474105
128
Svoboda J. Borinová S. Lengyel G. Pokorny P. Prichystal A. Sázelová S. et al . (2020). Last Glacial Maximum landscape and Epigravettian horse hunting stretgy in Central Europe: the case of Stránská skála IV. Prehled Výzkumu61, 59–70. 10.47382/pv0611-06
129
Téllez E. Saladié P. Pineda A. Marín J. Vallverdú J. Chacón M. G. et al . (2022). Incidental burning on bones by Neanderthals: the role of fire in the Qa level of Abric Romaní rock-shelter (Spain). Archaeol. Anthropol. Sci.14:119. 10.1007/s12520-022-01577-4
130
Thomas D. H. Mayer D. (1983). “Behavioral faunal analysis of selected horizons,” in The Archaeology of Monitor Valley, Vol. 2: Gatecliff Shelter, ed. D. H. Thomas (New York, NY: Anthropological Papers of the American Museum of Natural History), 353–391.
131
van Vuure C. T. (2005). Retracing the Aurochs: History, Morphology and Ecology of an Extinct Wild Ox. Sofia, Moscow: Pensoft Publishers.
132
Vettese D. Blasco R. Cáceres I. Gaudzinski-Windheuser S. Moncel M. H. Hohenstein U. et al . (2020). Towards an understanding of hominin marrow extraction strategies: a proposal for a percussion mark terminology. Archaeol. Anthropol. Sci.12:48. 10.1007/s12520-019-00972-8
133
Villa P. Mahieu E. (1991). Breakage patterns of human long bones. J. Hum. Evol.21, 27–48. 10.1016/0047-2484(91)90034-S
134
White M. Pettitt P. Schreve D. (2016). Shoot first, ask questions later: Interpretative narratives of Neanderthal hunting. Quat. Sci. Rev.140, 1–20. 10.1016/j.quascirev.2016.03.004
135
White T. (1953). A method of calculating the dietary percentage of various food animals utilized by aboriginal peoples. Am. Antiq.18, 396–398. 10.2307/277116
136
White T. (1992). Prehistoric Cannibalism at Mancos 5MTURM-2346. Princeton, NJ: Princeton University Press. 10.1515/9781400852925
137
Yesner D. Bonnichsen R. (1979). Caribou metapodial shaft splinter technology. J. Archaeol. Sci.6, 303–308. 10.1016/0305-4403(79)90015-3
138
Yravedra J. Cobo-Sánchez L. (2015). Neanderthal exploitation of ibex and chamois in southwestern Europe. J. Hum. Evol.78, 12–32. 10.1016/j.jhevol.2014.10.002
139
Zhang S. Li Z. Zhang Y. Gao X. (2012). Skeletal element distributions of the large herbivores from the Lingjing site, Henan Province, China. Sci. China Earth Sci.55, 246–253. 10.1007/s11430-011-4279-x
140
Zilhão J. Angelucci D. Araújo M. Arnold L. Badal E. Callapez P. et al . (2020). Last interglacial Iberian Neandertals as Fisher-Hunter-Gatherers. Science367:eaaz7943. 10.1126/science.aaz7943
141
Zilhão J. Angelucci D. E. Argant J. Brugal J. P. Carrión J. S. Carvalho R. et al . (2010). “Humans and hyenas in the Middle Palaeolithic of Gruta da Oliveira (Almonda karstic system, Torres Novas, Portugal),” in Actas de la 1ª Reunion de Científicos sobre cubiles de hiena (y otros grandes carnívoros) en los yacimientos de la Península Ibérica, eds. E. Baquedano, and J. Rosell (Madrid: Museo Arqueológico Regional), 299–308.
Summary
Keywords
palaeodiet, subsistence strategies, taphonomy, Middle Palaeolithic, Pleistocene
Citation
Nabais M and Zilhão J (2025) Ungulates and carnivores from the late MIS-5 Neanderthal occupation of Gruta da Figueira Brava (Portugal). Front. Environ. Archaeol. 4:1564495. doi: 10.3389/fearc.2025.1564495
Received
21 January 2025
Accepted
07 April 2025
Published
20 May 2025
Volume
4 - 2025
Edited by
Antonio Rodríguez-Hidalgo, Spanish National Research Council (CSIC), Spain
Reviewed by
Juan Marín, National University of Distance Education (UNED), Spain
Palmira Saladie, Institut Català de Paleoecologia Humana i Evolució Social (IPHES), Spain
Francesco Boschin, University of Siena, Italy
Updates
Copyright
© 2025 Nabais and Zilhão.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariana Nabais mariananabais@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.