- 1Department of History, Faculty of Humanities, Chiang Mai University, Chiang Mai, Thailand
- 2Department of Anthropology, California Academy of Science, San Francisco, CA, United States
- 3Archaeological Research Facility, University of California, Berkeley, Berkeley, CA, United States
- 4Department of Anthropology, Boise State University, Boise, ID, United States
- 5Oak Ridge Institute for Science and Education, The U.S. Department of Agriculture (USDA), Agricultural Research Service (ARS), National Center for Agricultural Utilization Research (NCAUR), Peoria, IL, United States
- 6Unearthed Heritage Australia, Pty, Ltd., Melbourne, VIC, Australia
- 7The Second Regional Office of Fine Arts Department, Suphanburi, Thailand
- 8Laboratory for Cellular Metabolism and Engineering, The Institute of Biological Chemistry, Washington State University, Pullman, WA, United States
Introduction: The social and temporal dimensions of psychoactive plant use in prehistoric Southeast Asia remain elusive.
Method: Here, we employ novel biomolecular methods to provide direct evidence of betel nut chewing in ancient dental calculus from Nong Ratchawat, Central Thailand. Method validation confirmed reliable detection of key compounds through LC-MS analysis of experimental control samples.
Result: Subsequent analysis of 36 archaeological samples from six ~4,000-year-old burials revealed diagnostic arecoline derivatives in a single female burial, representing the earliest such evidence in Southeast Asia.
Discussion: This identification, despite the lack of traditional archaeological indicators, demonstrates the power of dental calculus analysis to illuminate prehistoric psychoactive substance use. This study establishes the antiquity of betel nut consumption in mid-Holocene Thailand and provides new methodological avenues for future research in Southeast Asia.
1 Introduction
Betel nut chewing remains deeply embedded in Southeast Asian social and cultural traditions, serving roles in religious ceremonies, recreational gatherings, and traditional medicine and curative practices (Zumbroich, 2008). The complex involves chewing a quid of “betel nut”—the seed of the areca palm (Areca catechu L.)—that is wrapped in betel leaf (Piper betle) with additives including limestone paste and other plants depending on local taste. Archaeobotanical betel nut plant remains from Spirit Cave and Ban Chiang Archaeological Sites in Northern and Northeastern Thailand first suggested the antiquity of this practice in the 1970s (Zumbroich, 2008), with subsequent research documenting betel nut remains across prehistoric sites throughout Mainland Southeast Asia dating from 14,000 BP to 1,500 BP (Higham, 2002, 2011; Higham and Thosarat, 1998; Liu, 2020; White and Hamilton, 2021).
While traditional betel nut practices persist in rural and ethnic minority communities, Thai governmental “modernization” policies implemented in the 1940s resulted in a sharp decline in betel nut chewing, especially in urban public spaces (Kampangsri et al., 2013). Modern policy approaches to psychoactive plants like betel nut (e.g., tobacco, cannabis, coca) typically emphasize health risks and disadvantages to health while disregarding sociocultural significance (Hernandez et al., 2017; Lin et al., 2008; Nalina and Rahim, 2007; Pfeiffer, 2013; Phumat et al., 2018).
Indirect archaeological evidence—including tooth staining and Arecaceae botanical fragments—suggests widespread betel nut use since the prehistoric period (c. 14,000 BP; Zumbroich, 2008). Few biomolecular studies have attempted to directly confirm past use of this important cultural psychoactive complex through the identification of associated alkaloids on stained teeth (Hocart and Frankhauser, 1996; Krais et al., 2017; Oxenham et al., 2002). To date, no studies have employed dental calculus analysis to detect betel nut biomarkers (Zumbroich, 2008).
Recent research demonstrates that archaeological dental calculus can illuminate ancient foodways, health and nutrition, diet and bacteria, milk consumption, among other topics (Charlier et al., 2010; Charlton et al., 2019; Delaney et al., 2023; Dudgeon and Tromp, 2014; Hardy et al., 2009; Juhola et al., 2019; Lertcharnrit and Niyomsap, 2020). Dental calculus analysis can also provide unprecedented insights into psychoactive plant use and direct evidence of individual use patterns (Eerkens et al., 2018; Preus et al., 2011). For example, our Ancient Metabolomics research team has conducted pioneering dental calculus studies examining tobacco use and gender/age dynamics among Indigenous North American peoples (Tushingham et al., 2018, 2020). We found that these mineralized plaque deposits preserve multiple microscopic and biomolecular indicators, with samples as small as 5 mg suitable for sequential testing. This initial research suggested the detection potential for other psychoactive plant compounds.
To explore the possibility of detecting betel nut compounds from archaeological contexts, our study implemented a two-phase approach: (1) Method validation using modern betel (P. betle) and Areca nut (A. catechu) control samples to test identification potential of key compounds including hydroxychavicol (associated with piper betle leaf), arecoline (a biomarker for betel nut), and nicotine (associated with tobacco, a modern betel nut additive) through liquid chromatography-mass spectrometry (LC-MS) analysis, and (2) Analysis of 36 archaeological dental calculus samples from six human burials from Nong Ratchawat, Central Thailand [4,000 BP (4,080–3,850 cal BP; 4,410–3,070 cal BP)]. No archaeological evidence typically associated with betel nut use (such as stained teeth, betel-related artifacts, or charred betel nut remains) has been found at the site, despite extensive investigations. Additionally, although periodontitis and tooth decay was observed in some individuals (Table 1), tooth wear and osteoarthritis of the temporomandibular joint (TMJ)—conditions associated with betel nut chewing in a study by Stone et al. (2020)—was not reported. Despite this, we knew it was possible that betel nut chewing took place, but was archaeologically “invisible” because of the absence of these markers. However, the absence of traditional archaeological markers does not preclude betel nut use at the site, as these practices may remain archaeologically “invisible” without biochemical detection methods.
This research addresses three key questions: (1) Can A. catechu and P. betle biomarkers be detected in Nong Ratchawat dental calculus samples?, (2) Do betel nut residues preserve uniformly throughout the mouth? (3) Was betel nut chewing a generalized behavior or limited to specific individuals at Nong Rachawat? We hypothesized that biomarkers would be detectable via LC-MS analysis, with stronger signals in premolar and molar samples than anterior teeth due to quid chewing mechanics. Based on ethnographic data, we expected betel nut chewing was practiced by all adult community members regardless of age, sex, and status.
Our results confirmed the detection potential for Areca compounds but not Piper compounds. Significantly, we identified arecoline and arecaidine derivatives in both control samples and three dental calculus samples from a single female burial at Nong Ratchawat, representing the earliest direct biomolecular evidence for arecoline in Southeast Asia. Below, we present our methods and findings, followed by a discussion of their implications for understanding prehistoric psychoactive plant use in the region.
2 Background: betel nut plants
Betel nut palm (Areca catechu L.) belongs to the palm family (Arecaceae) and is widespread throughout tropical South and Southeast Asia, the Pacific islands, and insular Oceania (Staples and Bevacqua, 2006). These trees reach heights of 30 m with stems 25–40 cm in diameter (Staples and Bevacqua, 2006). Their fruits (“betel nuts”) have smooth, brown to greenish-yellow-colored skins with a white pulp surrounding a brownish-orange core, ripening to yellow to orange or red (Staples and Bevacqua, 2006).
The nuts contain high concentrations of tannins along with gallic acid, fixed oil gum, volatile oil, lignin, iron peroxide, magnesium phosphate, and various salts (Amudhan et al., 2012). Their primary psychoactive compounds are arecaidine, guvacine, guvacoline, tetrahydropyridine alkaloids, more specifically arecoline–a nicotinic acid derivative that acts as a mild parasympathomimetic stimulant producing enhanced alertness, energy, euphoria, and relaxation. Indeed, betel nut chewing introduces a complex set of biological components into the bloodstream which results in various “physiological and psychosomatic responses” (Zumbroich, 2008, p. 90). Despite sharing the name “betel,” the leaf used to wrap pieces of the nut for chewing in traditional quids corresponds to a different species–Piper betle L., a vine in the Piperaceae family (Staples and Bevacqua, 2006). Similar to A. catechu, P. betle contains bioactive compounds, notably hydroxychavicol, a phenylpropanoid present in its leaves, a potent xanthine oxidase inhibitor in treating fungal infections and dental disorders (Ali et al., 2010).
2.1 Betel nut chewing in Southeast Asia
Contemporary betel nut chewing in Southeast Asia persists primarily among ethnic minorities and rural communities including Tai, Lao, Lahu, Lisu, Karen peoples (Gupta and Warnakulasuriya, 2002, p. 81; Krais et al., 2017; Zumbroich, 2008). While practices vary regionally, users typically combine dried or fresh nuts with P. betle leaf and limestone paste, which, in this region, is commonly pink in color due to the traditional preparation methods and turmeric additive in the mixture. Some preparations include Senegalia catechu bark filaments and tobacco (Nicotiana tabacum L.) as complimentary ingredients (Kozlakidis et al., 2022; Zumbroich, 2008; Ridge et al., 2001). Often, chewing produces copious amounts of red saliva (or juice) that requires frequent spitting or expectoration (Figure 1; Mae Wanna, June 2023 interview). While spitting is common in rural Thailand, in some parts of Southeast Asia and Micronesia the custom is for people to swallow the juice rather than spitting it out (Paulino et al., 2017, p. 5). Chewers report experiencing “a sense of wellbeing, heightened alertness, a warm body sensation, improved digestion and increased stamina” (Zumbroich, 2008, p. 90).

Figure 1. (A) Modern betel quid ingredients-Piper betle leaf, areca nut (Areca catechu L.), limestone paste, tobacco (Nicotiana tabacum L.), and Senegalia catechu bark filaments; (B) red liquid produced after chewing betel quid (Photos by Piyawit Moonkham).
Betel nut use extends beyond physiological effects, serving multiple cultural reasons (Zumbroich, 2008). Recent ethnographic interviews conducted among Thai informants by the first author documented various traditional beliefs associated with betel nut chewing: black tooth staining is thought to prevent decay (Phor Beoi Boonda, August 2018 interview); chewing can cleanse the gums after a meal; and some communities credit ceremonial use with healing metal-related injuries (e.g., nail injuries) and wounds from animal attacks (Mae Wanna, July 2024 interview). The practice holds sacred status in numerous ritual contexts including harvest and raining season ceremonies such as Yok Kru (complimenting mentors), Thao Thnag Si (four guardian spirits), and Sueb Ja Ta (longevity ceremonies; Mea Kanya, March 2025 interview).
Both P. betle leaf and seeds serve medicinal and culinary purposes in many indigenous communities (Zumbroich, 2008). The resulting dental staining is also a common type of body ornamentation in Micronesia and Melanesia (Fitzpatrick et al., 2003; Krais et al., 2017; Zumbroich, 2008). Teeth staining is also performed as a part of individual rites of passages (e.g., coming of age and marriage; Zumbroich, 2008). In northern Thailand, betel nut and tobacco are standard offerings in various guardian spirit ceremonies (Mae Kampan, February 2025 interview). Despite increasingly negative perceptions of chewing culture, these traditional practices endure across the region (Mehrtash et al., 2017).
2.2 Archaeological background
Betel nut consumption and chewing culture is hypothesized to have been part of Southeast Asian and Micronesian culture for at least 4000 years (Fitzpatrick et al., 2003; Franke et al., 2015; Oxenham et al., 2002; Stone et al., 2020). While most archaeological evidence is indirect—consisting of archaeobotanical remains and stained teeth—several studies have attempted direct biomolecular identification of betel nut use (Hocart and Frankhauser, 1996; Krais et al., 2017; Oxenham et al., 2002; Stone et al., 2020).
2.2.1 Archaeobotanical evidence
The earliest betel nut plant remains in Thailand were recovered from ca. 9,000 BP burials at Spirit Cave in Mae Hong Son province, northwest Thailand (Figure 2; Oxenham et al., 2002). Additional macrobotanical evidence includes: betel leaf and nut remains in the deposits from the Sepik-Ramu basin (Glover, 1977; Zumbroich, 2008), as well as plant remains from Kuala Selingsing, Malaysian Peninsula (2,200–1,000 BP) and the Dongan Site, Papua New Guinea (5,500 BP; Swadling et al., 1991; Zumbroich, 2008; Figure 2).
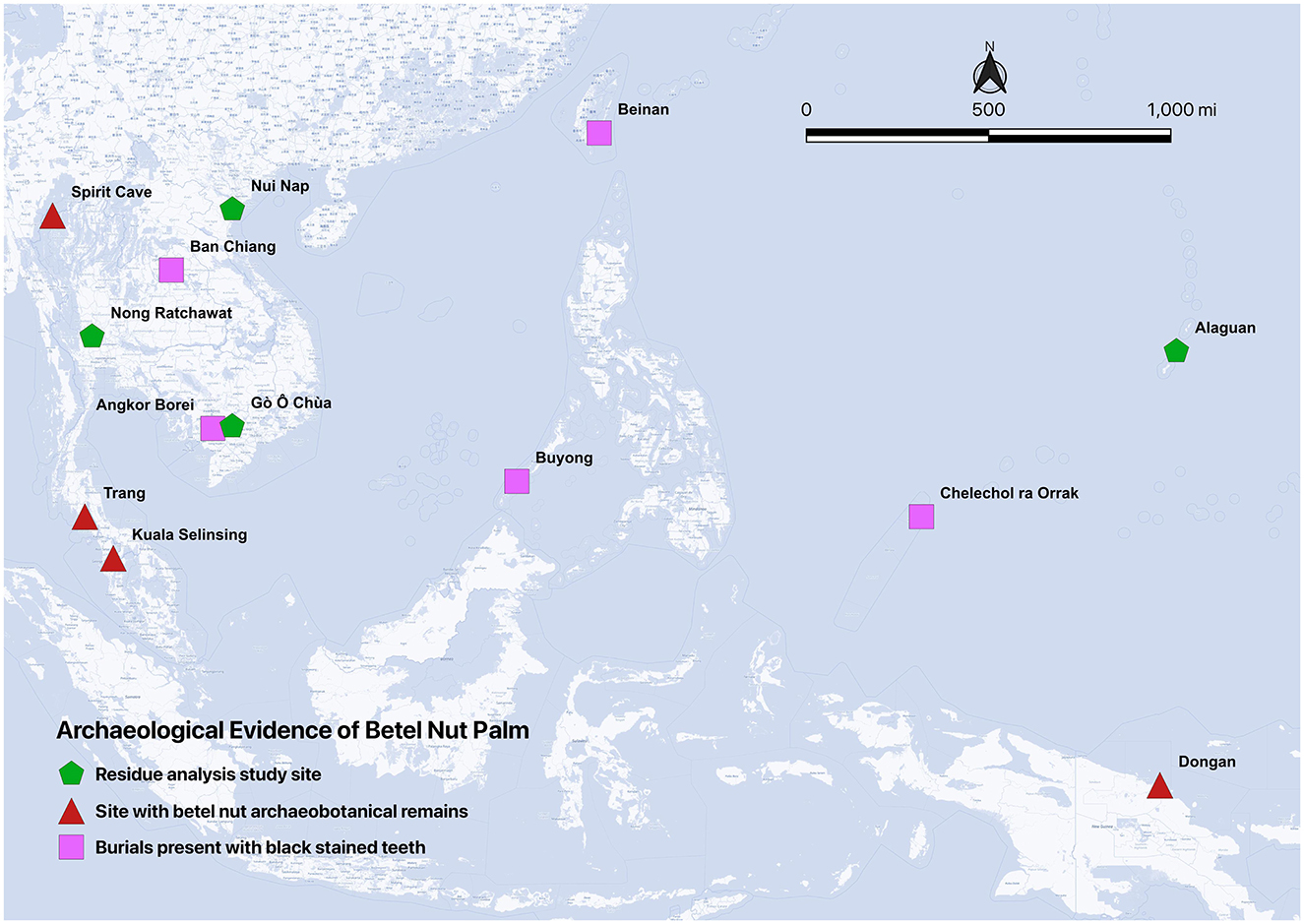
Figure 2. Archaeological sites with direct and indirect evidence of betel nut chewing practices in Southeast Asia, Micronesia and Melanesia. Thailand: Nong Ratchawat (this study), Spirit Cave (Oxenham et al., 2002), Trang (Zumbroich, 2008), Ban Chiang (Zumbroich, 2008), Taiwan: Beinan (Zumbroich, 2008), Vietnam: Go O Chua (Krais et al., 2017), Nui Nap (Oxenham et al., 2002), Malaysia: Kuala Selinsing (Swadling et al., 1991; Zumbroich, 2008), the Philippines: Buyong (Swadling et al., 1991), Papua New Guinea: Dongan (Swadling et al., 1991; Zumbroich, 2008). Palau: Chelechol ra Orrak (Stone et al., 2020), Mariana Island: Alaguan (Hocart and Frankhauser, 1996).
2.2.2 Dental, archival, and material culture evidence
Stained human teeth—hypothesized to be associated with betel nut chewing—have been identified at multiple sites. These include: Duyang, Philippines (5,000 BP), Peinan, eastern Taiwan (3,500–2,800 BP), Lean Buidane cave, Selababu Island, northeastern Indonesia (c. 2,000 BP), and Nui Nap (3,000–1,700 BP), Gò Ô Chùa (c. 2,550–2,150 BP), Vietnam (Krais et al., 2017; Oxenham et al., 2002), and Chelechol ra Orrak (c. 1000 BCE−200 CE), Palau (Stone et al., 2020). Chinese Han dynasty records (206 BCE−220 CE) mention black-stained teeth in ceremonial contexts in Annam (modern Vietnam; Oxenham et al., 2002). Historical artifacts including silver containers, bronze boxes, and ceramic figurines, associated with betel nut use have been found throughout Southeast Asia (Pindborg, 1973; Rooney, 1993).
2.2.3 Biomolecular studies
Hocart and Fankhauser pioneered the use of tetrahydropyridine alkaloids (arecoline, arecaidine, guvacine and guvacoline), as betel nut biomarkers in their study of a a historic Mariana Island population (Hocart and Frankhauser, 1996). The analysis of ground enamel samples from stained and unstained teeth from a female individual dated 1,100–1,700 CE led to the detection of arecaidine and guvacine as TMS-derivatives exclusively in the stained tooth sample (Hocart and Frankhauser, 1996). Oxenham et al. (2002) analyzed a black-stained tooth from a male burial at Bronze Age Nui Nap (450 BCE−250 CE) in northern Vietnam, detecting only non-diagnostic tannin derivatives that could not be specifically attributed to betel nut (Krais et al., 2017; Oxenham et al., 2002). Subsequently, Krais et al. (2017) examined a black-stained molar from a juvenile male burial from Iron Age Gò Ô Chùa (c. 600–400 BCE) in southern Vietnam (Krais et al., 2017). Their analysis identified arecoline and tentatively arecolidine in enamel samples but dentin samples yielded no positive results. To date, no studies have analyzed dental calculus for betel nut residues analysis in Southeast Asia.
2.3. Nong Ratchawat archaeological site
Nong Ratchawat spans three acres in the lower alluvial plain of the Tha Chin River Basin, Suphanburi province, central Thailand (Figure 3). The site, now largely consisting of wet rice paddy fields, has undergone significant agricultural disturbance (Wangthongchaicharoen, 2013). Accelerator Mass Spectrometry (AMS) dating of shell fragments from two main cultural layers by the Fine Arts Department of Thailand (hereafter FAD), places the site in the Bronze Age (c. 4,000–3,000 BP), supported by recovered pottery, animal bones, and stone tools characteristic of this period (Tanompolkrang, 2017; Wangthongchaicharoen, 2013).
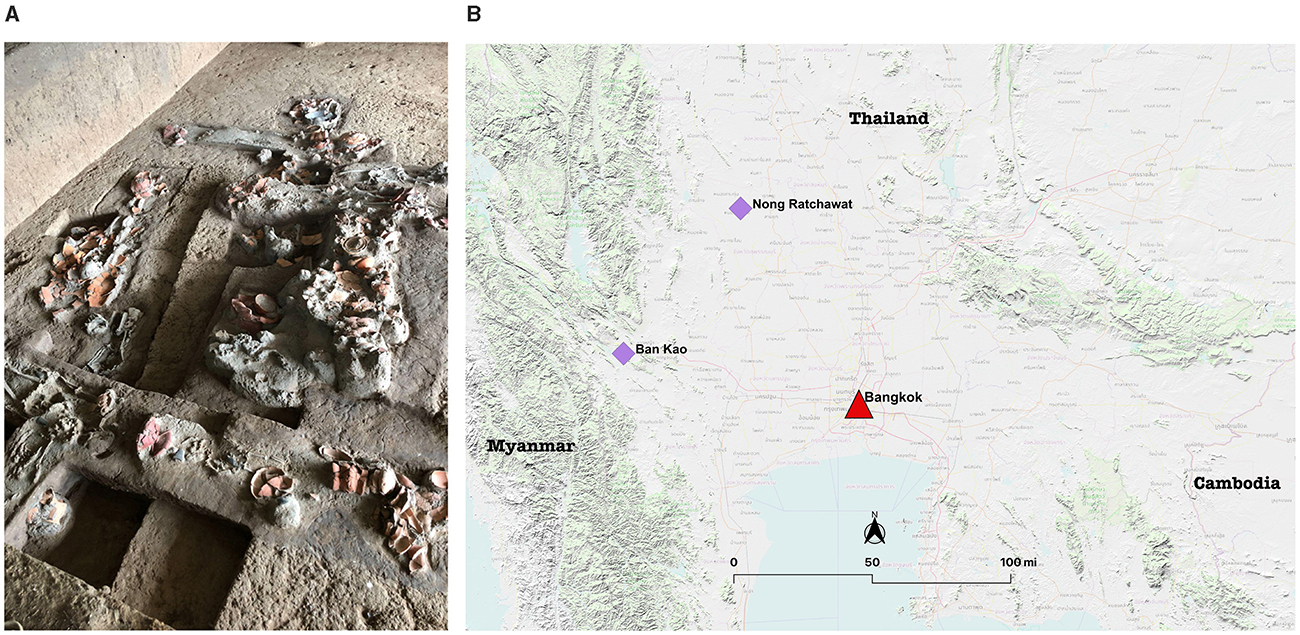
Figure 3. (A) Archaeological burials with associated artifacts and (B) Map of Nong Ratchawat archaeological site (Photos and created by Piyawit Moonkham).
Between 2003 and 2020, FAD excavations unearthed 156 human burials along with pottery [stylistically similar to ceramics from the Ban Kao site (Kanchanaburi Province, western Thailand)], beads, stone tools, and animal bones (Devanwaropakorn, 2018; Lertwinitnun, 2022, p. 28). The site contains four main layers with two prominent cultural layers (Lertwinitnun, 2022, p. 26; Tanompolkrang, 2017, p. 56–59). The lower layer contained polished stone axes and tools, bracelets, clay pottery, abundant animal bones (burnt or modified), and shell fragments. The human burials in this layer suggest spatial arrangement in two distinct locations, the central mound used for burials, and the edge of the mound used for residential purposes (Lertwinitnun, 2022, p. 41). AMS dating of shell fragments from this layer yielded dates of 4,080–3,850 cal BP (Duangsakul, 2013; Lertwinitnun, 2022, p. 41; Tanompolkrang, 2017, p. 59). The upper layer contained numerous burials, but fewer burial goods than the lower layer. Burials were also found within residential areas, indicating no clear distinction between burial and residential spaces. Shell fragments from this layer AMS dated to 4,410–3,070 cal BP (Tanompolkrang, 2017, p. 59). The abundance of stone bracelets in the upper layer suggests Nong Ratchawat may have been a manufacturing center at least 4000 years ago (Tanompolkrang, 2017). The community likely shared pottery production techniques and forms (e.g., clay tripod), traded, exchanged, and/or communicated with other Bronze Age sites around central, western, and northeastern Thailand, including Ban Kao, Ban Chiang, and Phromtin Tai. Evidence of rice chaff/husk temper in both cultural layers suggests that Nong Ratchawat was a farming village reliant on rice agriculture (Guedes et al., 2020; Higham and Thosarat, 1998; Lertwinitnun, 2022, p. 41). The site now serves as an archaeological museum and educational center overseen by FAD (Lertwinitnun, 2022; Wangthongchaicharoen, 2013; Figure 3).
Despite numerous skeletal discoveries at Nong Ratchawat, bioarchaeological studies remain limited, with most reports published in Thai. These studies examine burial ceremonies and practices, osteological indicators of health and disease, dental health, nutrition, and dental morphological variation (Devanwaropakorn, 2018; Duangsakul, 2013; Lertwinitnun, 2015, 2022; Sriharat, 2011; Tanompolkrang, 2017; Wangthongchaicharoen, 2013). Key findings include Sriharat's (2011) macroscopic and metric trait analysis of nine individuals that revealed weathered and spongy bone, periodontitis and benign tumors. Dental attrition and development indexes provided age-at-death estimates between 25 and 35 years (Sriharat, 2011). Wangthongchaicharoen (2013) examined 43 highly fragmentary human remains (80–90% incomplete), including four infants and one child, with adult deaths averaging 20–35 years of age. Approximately 20% of the study showed signs of tooth decay, alveolar process issues, and periodontitis (Wangthongchaicharoen, 2013). Lertwinitnun (2015) and Lertwinitnun (2022) documented periodontitis, tooth decay, abscesses, high dental calculus accumulation, and sex-differentiated linear enamel hypoplasia from malnutrition among 33 individuals in 2015 and 32 individuals in 2022.
Based on burial patterns and related evidence, Duangsakul (2009) described four burial groups at Nong Ratchawat. The first group had various burial goods with some teeth extracted as a part of a decorative practice common at the site (Lertwinitnun, 2022, p. 41). The second group showed similar decorative practices but with more elaborate burial goods such as polished stone axes and three-legged clay pots. These two groups dated to the early Neolithic period [4,200–3,500 BP (4,080–3,850 cal BP)] (Duangsakul, 2013; Lertwinitnun, 2022, p. 41). The third and fourth groups dated to the late Neolithic period [3,500–3,000 BP (4,410–3,070 cal BP)] (Lertwinitnun, 2022, p. 41; Tanompolkrang, 2017, p. 59) and were found with various grave goods including stone beads, polished stone axes, and round-based pottery (Lertwinitnun, 2022, p. 41). Based on dental morphology, the population is believed to display traits commonly found among populations in East and Southeast Asia (Lertwinitnun, 2022). Analysis of dental traits, burial patterns, and grave goods suggests the fourth group may have migrated from elsewhere during the late Neolithic period (3,500–3,000 cal BP; Lertwinitnun, 2022, p. 110–114). Tanompolkrang's (2017) stereomicroscopic analysis of 13 individuals revealed dental wear patterns consistent with both carnivorous and herbivorous diets.
3 Materials and methods
Residue extractions from both experimental control and archaeological samples were prepared in the Tushingham Laboratory at Washington State University (WSU). Initial LC-MS analyses of all samples were conducted at WSU's Gang Laboratory. Subsequent targeted analyses were performed on a subset of the samples under Brownstein's supervision at The U.S. Department of Agriculture (USDA) Agricultural Research Service (ARS) laboratory in Peoria.
3.1 Sample collection
3.1.1 Archaeological samples
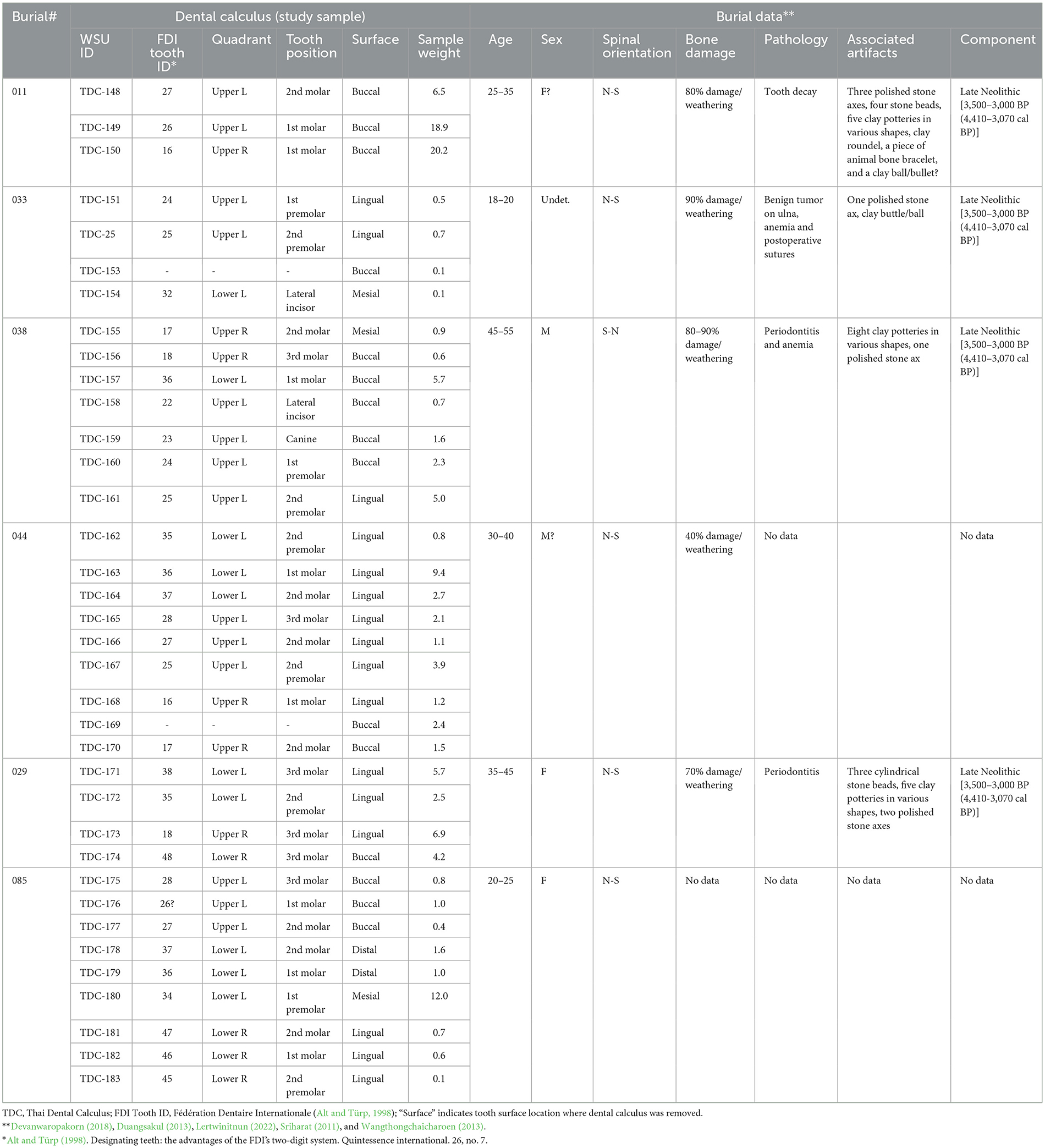
Thirty-six dental calculus samples were analyzed from six individuals (Burials no. 11, 29, 33, 38, 44, and 85) from Nong Ratchawat (TDC 148–183). Sample quantities per individual range from three to nine, depending on dental preservation and plaque deposit presence (Table 1). The collection is predominantly composed of posterior unstained teeth (31 molar and premolar samples), with three specimens from incisors and canines. Sample weights range from 0.1 to 20.2 mg. Bioarchaeological analysis identified three males, two females, and one individual of undetermined sex, aged 18 to 55 years old (Table 1; Lertwinitnun, 2022; Sriharat, 2011; Tanompolkrang, 2017).
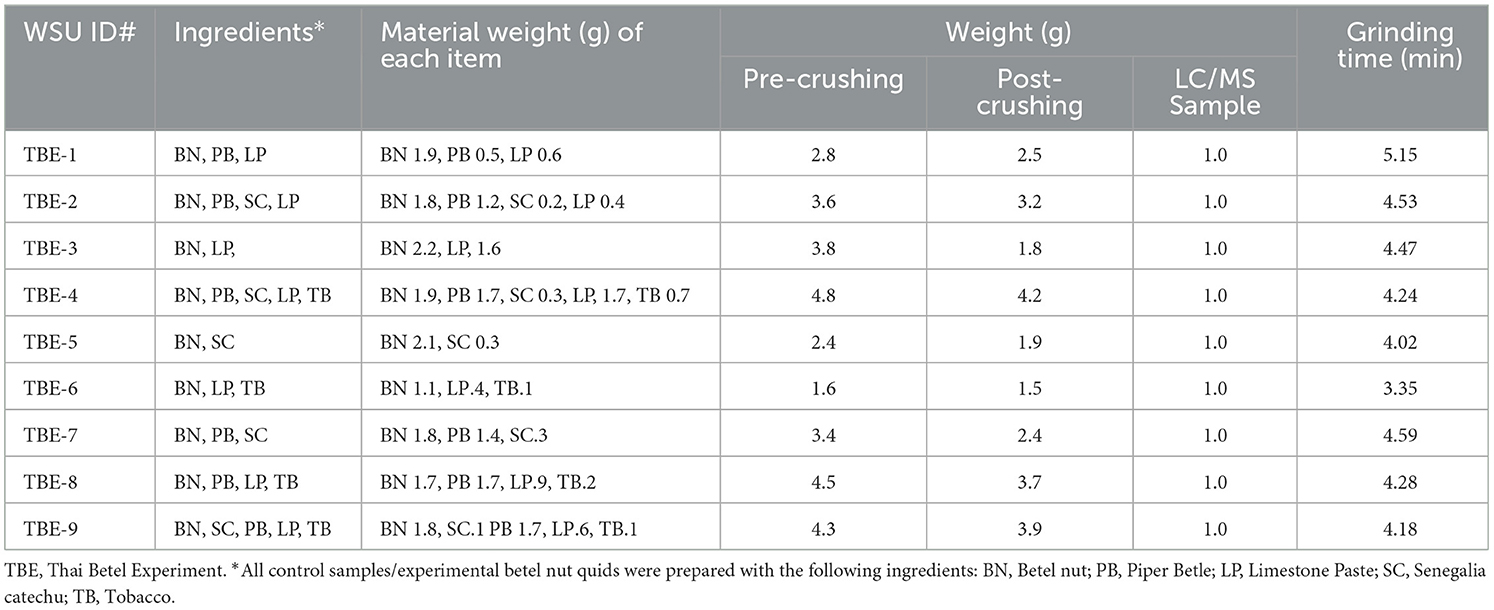
3.1.2 Control samples (experimental quids)
Control samples consisted of nine experimentally produced betel quid reference samples (TBE 1–9), created to establish realistic and reliable analytical controls for the systematic study of multi-ingredient compositions. This approach was designed to better understand chemical interactions between all components. Each experimental quid contained three core ingredients: dried betel nut fruit, pink/red limestone paste (made from cockleshells that are baked to a high temperature to produce unslaked lime, to which water is added; it is then pounded into an edible paste), and betel leaves (Piper betle). To reflect common modern chewing practices, some samples also incorporated Senegalia catechu bark filaments and small portions of loose tobacco. The approach and composition of various sourced materials was based on ethnographic fieldwork by the first author. Each sample was prepared in the mortar, ground and weighted. The grinding process involved adding saliva in each sample as well. The mortar and pestle was cleaned with fragrance-free soap and alcohol for each grinding process to prevent contamination. Human saliva was then added to each sample to replicate traditional practices and to produce realistic experimental controls. While biochemical reactions are complex, saliva helps to blend ingredients through the chewing process, and the addition of lime paste creates an alkaline oral environment that both releases betel nut's psychoactive compounds (arecoline and guvacoline) and converts them into arecaidine and guvacine, respectively; this conversion may further enhance stimulating effects. Piper betel leaf is thought to aid in alkaloid absorption, further contributing to these effects (Chu, 2001; Krais et al., 2017, p. 11–12).
Previous studies by Oxenham et al. (2002) and Krais et al. (2017) did not employ controls in their analysis, while Hocart and Frankhauser (1996) incorporated analysis of a whole betel nut fruit control in their study. Our methodology, however, is a significant advance as it involves analysis of complete quid compositions rather than isolated components, incorporating saliva to understand biochemical interactions, and creating standardized reference samples for comparative analysis. Detailed compositions for all experimental samples are provided in Table 2. This comprehensive approach enables more accurate identification and analysis of archaeological betel quid residues.
3.2 Sample processing (residue extractions)
For the 36 archaeological samples (TDC 148–183), processing began with careful mechanical grinding of plaque fragments with a stainless-steel tool while inside sample vials. Chemical residues then underwent sequential extraction by immersing samples for 10 min in 0.2 mL of 0.5% formic acid (FA) followed by 0.2 mL of an acetonitrile:2-propanol:water (APW) solution using a sonication bath. After extraction, samples were centrifuged, and 0.15 mL of the supernatant was transferred to secondary microvials. Both extracts and remaining calculus sediment in vials were then air-dried, with sediment preserved for future analysis. Dry extracts were resuspended in 0.1 mL of 0.1% formic acid/50% aqueous acetonitrile, vortexed, and centrifuged (21,000 × g for 10 min at 4°C). An 0.075 mL aliquot from each sample was transferred to amber vials for LC-MS analysis.
For control samples (TBE 1–9), 1.0 g of each experimental quid was placed in tubes for extraction and LC/MS analysis (Table 2). All samples underwent a similar sequential extraction process as archaeological samples, with an initial rinse of 3.0 mL of 0.5% FA and 3.0 mL of an APW. solution using a sonication bath. Samples were then placed in glass vials and rinsed with methyl tert-butyl ether (MTBE), submitted to 10 min of sonication, and liquid samples were collected and transferred to another tube to conduct LC-MS process.
3.3 Analytical methods
Chemical standards of nicotine, arecoline, and hydroxychavicol were purchased from Sigma-Aldrich (St. Louis, MO, USA) and included in the sample batch. Hydroxychavicol, associated with Piper betle leaves, could not be characterized under the established instrument settings and thus was excluded from analysis. Standards for arecaidine and guvacine were not available, but their putative presence was analyzed based on known molecular mass and associated signatures observed in the experimental control samples. This procedure allowed us to examine a broader metabolite signature associated with betel nut chewing. Lastly, a solvent blank (50% aqueous acetonitrile with 0.1% formic acid) was interspersed every five samples to control for cross-contamination.
Initial LC-MS analyses on all samples was performed at the WSU Gang Laboratory on a Waters Acquity ultra-performance liquid chromatography (UPLC) system (Waters Corporation, Milford, MA, USA) coupled to a Waters Synapt G2-S HDMS Q-TOF with lockspray ionization, which was operated using electrospray ionization (ESI) in the positive and resolution mode [23,000 FWHM (full width half maximum)] following the protocol developed by Brownstein et al. (2020).
Samples from Burial 11 (TDC-148, 149, 150) and TBE-5 were also run at USDA ARS Peoria on a Thermo Scientific (San Jose, CA, USA) Orbitrap ID-X mass spectrometer coupled to a Thermo Scientific Vanquish LC system all running under the Thermo Scientific Xcalibur v4.4.16.14 software. The MS was operated using a heated electrospray ionization (H-ESI) probe in the positive polarity mode. The ion transfer tube and vaporizer temperatures were set at 350°C and 400°C, respectively. The sheath, auxiliary, and sweep gas rates were set at 70, 20, and 10 arbitrary units, respectively. The mass resolution was 60,000 and the spray voltage was 3.450 kV for positive mode. The scan range was from 100 to 1,000 m/z with a scan time of 0.6 s and the RF lens was set at 60%. Targeted and untargeted ions detected at any given time interval were subjected to MS/MS fragmentation in the HCD using stepped collision energy (CE) mode at 15, 30, 45 CE units. The chromatographic conditions of the Vanquish LC system followed the conditions previously described in Brownstein et al. (2020).
4 Results
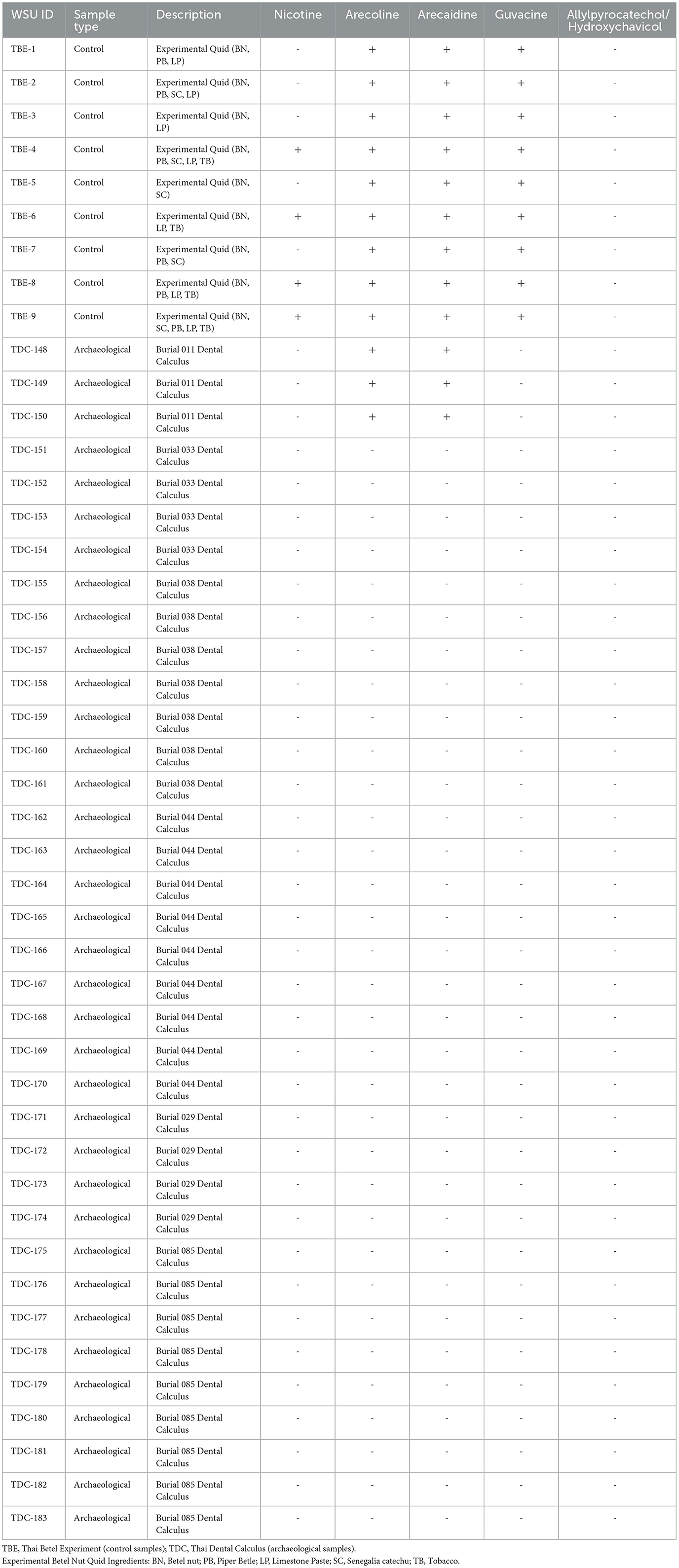
In total, nine control samples/experimental quids (TBE 1–9), and 36 archaeological dental calculus samples (TDC 148–183) were analyzed. Results are summarized in Table 3. The biomarker nicotine, associated with tobacco, a modern betel nut additive, was detected in tobacco-containing reference samples (TBE-4, TBE-6, TBE-8, TBE-9) but was absent in archaeological samples, as expected (Table 3). Signals for arecoline were present in all experimental controls (TBE-1–9) as well as in all three samples from Burial 11 (TDC-148, TDC-149, TDC-150; Figure 4). The remaining archaeological samples (n = 36) showed no matching signals (Table 3).
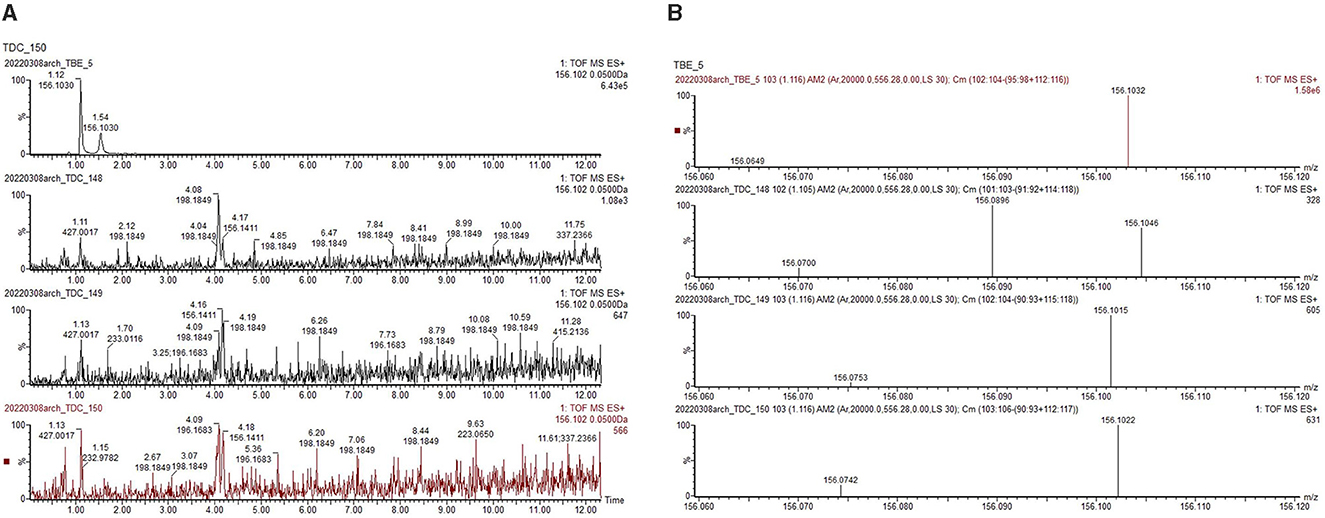
Figure 4. (A) Archaeological sample TDC 148–150 from Burial 11. (B) Chromatogram showing co-occurring peaks between reference materials (TBE) and archaeological samples. Mass spectra indicate weaker arecoline ion signals in archaeological samples (328, 695, 631) compared to reference sample (1.58e6 or 1,580,000).
The compound detected in Burial 11 archaeological samples matched arecoline's molecular weight and formula. However, its MSn fragmentation pattern differed from both reference samples and the arecoline standard (Figure 5). Targeted analysis at the USDA ARS laboratory employing an Orbitrap MS system confirmed the arecoline derivative peaks with the same molecular weight and formula, but with greater accuracy. However, the divergent MSn fragmentation pattern still persisted. Given Nong Ratchawat's antiquity (4,080–3,850 cal BP; 4,410–3,070 cal BP), this difference likely reflects structural transformation of the original compound, and this modification resulted in a different MSn fragmentation pattern. Residue analysis studies have only recently begun exploring degradation processes on the biomolecular level in detail (e.g., Huber et al., 2022). Future experiments exposing reference materials to various environmental conditions may help to elucidate these processes (Figure 5).
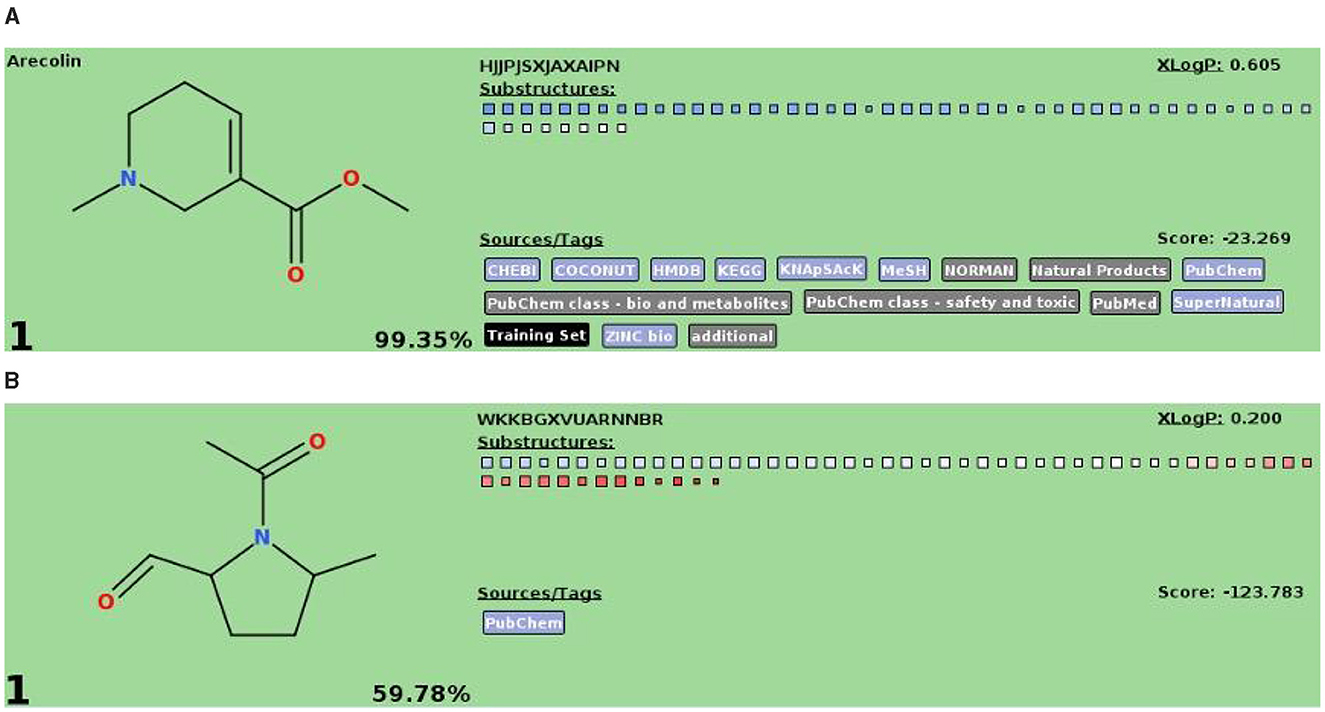
Figure 5. (A) Fragmentation pattern of arecoline compound. (B) Possible structural change in the fragmentation pattern of arecoline compound identified at Burial 11, Nong Ratchawat site.
Burial 11's distinctive chemical profile extends beyond the presence of arecoline. While guvacine is definitely absent from all archaeological samples, there is a candidate signal for arecaidine, which matches expected m/z values yet not retention time. This signal accounts for significant peaks in all experimental controls as well as the three Burial 11 samples. Moreover, untargeted principal components analysis of weight-standardized peak area values, examining a filtered set of 126 chemical features unaffected by contamination or instrument noise, revealed that the Burial 11 samples separated from the remaining collection along Component 1. The statistical weight of PC1 indicates that this woman consumed a unique combination of substances that were not ingested by the other individuals examined in this study (Figure 6).
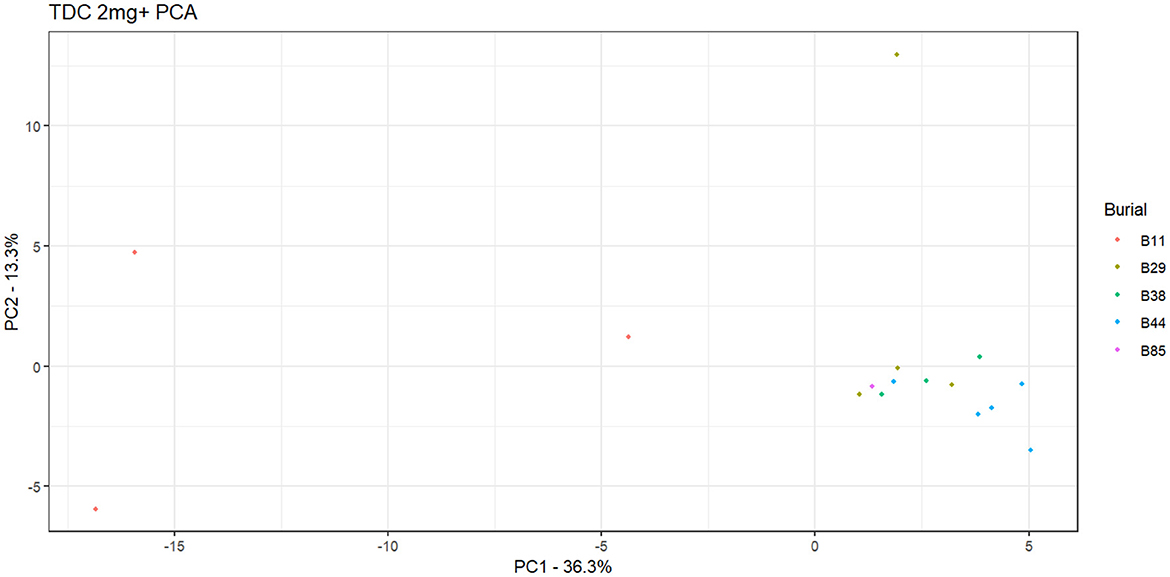
Figure 6. Principal Component Analysis (PCA) scatterplot showing burial associations for calculus samples exceeding 2 mg in weight.
5 Discussion
The presence of arecoline and arecaidine identified in Burial 11's dental calculus suggests that betel nut chewing was practiced in Thailand by about 4000 years ago. The isolation of positive results is noteworthy, indicating both protocol accuracy and limited betel nut consumption in Bronze Age Central Thailand. Hence, we further solidify a growing body of chemical residue data tracing the antiquity of betel nut chewing in Southeast Asia, while demonstrating successful arecoline and arecaidine extraction from human dental calculus. Compared to prior studies of enamel samples which involve grinding of tooth surfaces, our approach is less invasive and thus may encourage additional studies in the future.
Although only six burials were analyzed, drawing firm conclusions about why betel nut signatures were only detected in Burial 11 is challenging. Burial 11 is notable for the ritual breaking of pottery prior to interment and the inclusion of distinctive clay and stone objects—specifically, one clay roundel, five clay vessels in various shapes, and three stone beads (Lertwinitnun, 2022, p. 197; Sriharat, 2011, p. 125). While the deliberate breaking of pottery and placement of round objects is a practice found in many or most other burials at the site, three stone beads and the sophisticated form of five clay vessels are reported as relatively rare.
At present, there is no clear evidence from the available reports to suggest that Burial 11 represents special treatment, elevated social status, or unique ritual significance compared to most other individuals interred at the site (Lertwinitnun, 2022, p. 197; Sriharat, 2011, p. 125). However, the presence of stone beads as grave goods may point toward some degree of individual variation, possibly relating to gender, age, or personal identity.
Previous studies reveal interesting gender patterns in betel nut consumption. Oxenham et al. (2002) and Krais et al. (2017) examined male specimens exclusively, noting higher frequencies of reddish brown/black-stained teeth among men. Hocart and Frankhauser (1996) analyzed stained and unstained teeth of one female. All three studies suggest tooth blackening served as a rite of passage for both sexes (Table 4). While these studies provide valuable insight into staining patterns associated with betel nut use, differentiating these dynamics in archaeological contexts remains a challenge (e.g., staining from habitual vs. occasional chewers, as well as purposeful staining practices, such as those documented in communities like the Mariana Islanders). Thus, future research should incorporate comparative analyses of staining patterns for modern users in order to develop more refined diagnostic criteria.
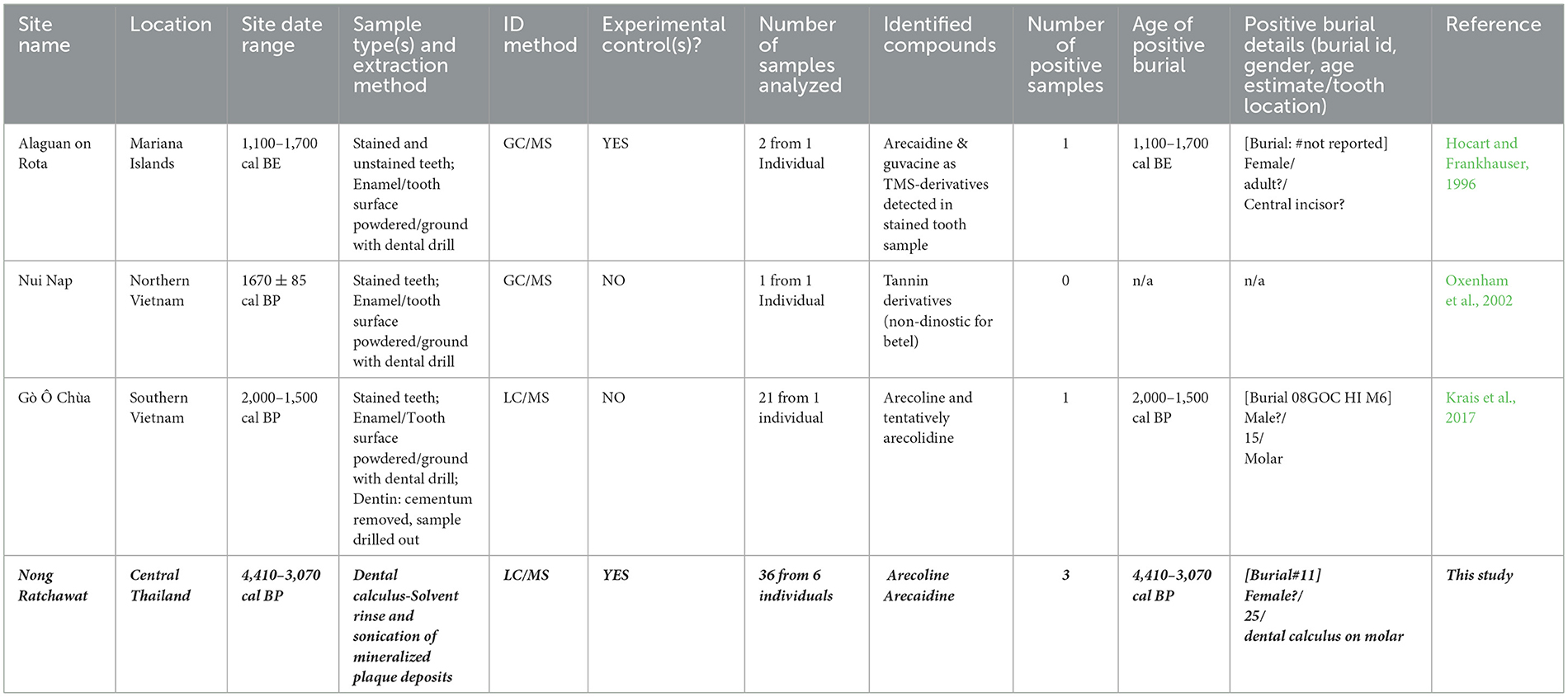
Table 4. Previous and current biomolecular studies of betel nut Chewing in Southeast Asian archaeological sites.
Recent ethnographic interviews conducted by the first author also indicate a generational shift: compared to two generations ago, women now predominantly chew betel nut while men favor tobacco, though men still participate in betel nut consumption during specific religious ceremonies and injury healing rituals (Mae Wanna, May 2023 interview; Mae Kampan, February 2025 interview). Given tobacco's 16th-century introduction to Southeast Asia (Hammond, 2009), betel nut use may have been more gender-neutral in the past (Mae Kanya, February 2025 interview). Ongoing research explores these changing gender dynamics.
Our findings complement indirect archaeological evidence including reddish brown/black-stained teeth, Areca fruit fragments, and clay figurines from contemporary and older sites around Southeast Asia (Zumbroich, 2008). While modern studies emphasized betel nut's negative impacts on oral health (Cox et al., 2004) leading to Thailand's 1941 public consumption ban during “modernization” (Kampangsri et al., 2013, p. 4337), this perspective neglects its profound social and cultural significance and separates it from traditional belief systems (Hernandez et al., 2017; Lin et al., 2008; Nalina and Rahim, 2007; Phumat et al., 2018). Like traditional tobacco use in indigenous North American communities (Tushingham et al., 2018), coca chewing in Andean South America (Pfeiffer, 2013), betel nut chewing transcends physiological effects to serve important social and ceremonial functions integral to local traditions throughout Southeast Asia (Zumbroich, 2008). Archaeological and ethnographic evidence demonstrates betel nut's deep cultural roots in Thai and Southeast Asian culture, featuring in social gatherings facilitating cultural transmission, religious activities, spirit shrine offerings, and guardian spirit rituals (e.g., use as the offerings for various ceremonies for spirit shrines and guardian spirits; Mae Kampan, February 2025 interview). Despite government modernization policies reducing urban consumption (Kampangsri et al., 2013; Phumat et al., 2018), betel nut chewing persists in rural Thai communities, highlighting its enduring cultural significance.
6 Summary
Our study identified arecoline and arecaidine signals in three dental calculus samples from a likely female burial interred ~4,000 BP (4,080–3,850 cal BP; 4,410–3,070 cal BP) at the Nong Ratchawat site (Lertwinitnun, 2022, p. 194; Tanompolkrang, 2017, p. 59). Despite differing fragmentation patterns from the arecoline, and time retention from arecaidine standards, matching molecular weights and formula indicate betel nut consumption. This study represents the first dental calculus analysis identifying betel nut residues and provides the earliest biomolecular evidence of betel nut use in Southeast Asia—predating studies by Hocart and Frankhauser (1996), Krais et al. (2017), and Oxenham et al. (2002) by at least 1000 years.
The absence of traditional archaeological indicators (black-stained teeth, betel nut fragments) demonstrates the value of biomolecular studies in revealing otherwise invisible behaviors (Tushingham et al., 2018, 2020). The lack of tooth staining in Burial 11 despite molecular evidence of betel nut use raises interesting questions. Multiple factors could explain this discrepancy: differences in consumption methods (such as drinking rather than chewing), variations in frequency or duration of use, post-consumption tooth cleaning practices, or taphonomic processes affecting stain preservation. Future experimental and comparative research may help elucidate the relationship between consumption patterns and visible archaeological markers.
The established protocol for biomolecular identification of dental calculus provides a platform for analyzing human remains across Southeast Asian sites. This research advances our understanding of psychoactive plant use patterns, communal practices, enhances comprehension of sociocultural worldviews, and offers a fresh approach to studying gender-based consumption patterns and disparities, and human-environment interactions.
As of 2020, 156 burials have been excavated at Nong Ratchawat (Lertwinitnun, 2022, p. 28). Having analyzed only six of these, significant potential exists for additional biomolecular analysis of dental calculus from the remaining individuals. Future ethnoarchaeological research will examine sociocultural aspects of psychoactive plant utilization, gender and age consumption patterns, and the evolving social and cultural roles of these practices among various Thai and Southeast Asian communities.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
PM: Conceptualization, Investigation, Writing – review & editing, Methodology, Supervision, Writing – original draft, Data curation, Formal analysis, Resources. ST: Methodology, Resources, Project administration, Data curation, Funding acquisition, Visualization, Conceptualization, Supervision, Writing – original draft, Investigation, Formal analysis, Writing – review & editing. MZ: Formal analysis, Methodology, Data curation, Investigation, Validation, Conceptualization, Writing – review & editing. KB: Formal analysis, Validation, Writing – review & editing. CD: Resources, Writing – review & editing. SD: Resources, Writing – review & editing. DG: Formal analysis, Project administration, Data curation, Methodology, Funding acquisition, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Science Foundation under Grant #1918966 and #1419506, the Lakeside Foundation for visiting scholars at the California Academy of Sciences, and UC Berkeley. Funding for open access publishing of this article was provided by the University of California Libraries.
Acknowledgments
We appreciate Dr. Anna Berim for her assistance in the laboratory. The authors would like to thank Sureeratana Bubpha, the Fine Arts Department of Thailand and their staff for their hospitality and assistance during the data collection in Thailand.
Conflict of interest
CD was employed by Unearthed Heritage Australia, Pty, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, I., Khan, F. G., Suri, K. A., Gupta, B. D., Satti, N. K., Dutt, P., et al. (2010). In vitro antifungal activity of hydroxychavicol isolated from Piper betle L. Ann. Clin. Microbiol. Antimicrob. 9, 1–9. doi: 10.1186/1476-0711-9-7
Alt, K. W., and Türp, J. C. (1998). “Roll call: thirty-two white horses on a red field. The advantages of the FDI two-digit system of designating teeth,” in Dental Anthropology: Fundamentals, Limits and Prospects (Vienna: Springer), 4155.
Amudhan, M. S., Begum, V. H., and Hebbar, K. (2012). A review on phytochemical and pharmacological potential of Areca catechu L. seed. Int. J. Pharm. Sci. Res. 3, 4151–4157. doi: 10.13040/IJPSR.0975-8232.3(11).4151-57
Brownstein, K. J., Tushingham, S., Damitio, W. J., Nguyen, T., and Gang, D. R. (2020). An ancient residue metabolomics-based method to distinguish use of closely related plant species in ancient pipes. Front. Mol. Biosci. 7, 133–133. doi: 10.3389/fmolb.2020.00133
Charlier, P., Huynh-Charlier, I., Munoz, O., Billard, M., Brun, L., and de La Grandmaison, G. L. (2010). The microscopic (optical and SEM) examination of dental calculus deposits (DCD). Potential interest in forensic anthropology of a bio-archaeological method. Leg. Med. 12, 163–171. doi: 10.1016/j.legalmed.2010.03.003
Charlton, S., Ramsøe, A., Craig, O. E., Alexander, M., Speller, C. F., Collins, M., et al. (2019). New insights into neolithic milk consumption through proteomic analysis of dental calculus. Archaeol. Anthropol. Sci. 11, 6183–6196. doi: 10.1007/s12520-019-00911-7
Chu, N. S. (2001). Effects of Betel chewing on the central and autonomic nervous systems. J. Biomed. Sci. 8, 229–36. doi: 10.1007/BF02256596
Cox, S., Piatkov, I., Vickers, E. R., and Ma, G. (2004). High-performance liquid chromatographic determination of arecoline in human saliva. J. Chromatogr. A 1032, 93–95. doi: 10.1016/j.chroma.2003.11.076
Delaney, S., Alexander, M., and Radini, A. (2023). More than what we eat: investigating an alternative pathway for intact starch granules in dental calculus using experimental archaeology. Quat. Int. 653, 19–32. doi: 10.1016/j.quaint.2022.03.004
Devanwaropakorn, K. (2018). A Comparative Study of Dental Variations of Prehistoric Communities at Ban Kao (Kanchanaburi) and Nong Ratchawat (Suphanburi), Thailand [BA Thesis]. Bangkok, Thailand: Silpakorn University.
Duangsakul, S. (2009). Recent excavation of nong ratchwat archaeological site at Nongyasai District, Suphanburi. Damrong J. Fac. Archaeol. Silpakorn Univ. 8, 1–18.
Duangsakul, S, . (ed.). (2013). “Nong Ratchawat Archaeological Site, Nong Ratchawat Subdistrict, Nong Yasai District, Suphanburi Province,” in Proceedings of Archaeology of Agricultural Societies: From Ban Kao to Nong Ratchawat, Bangkok: Thailand, 197–224.
Dudgeon, J. V., and Tromp, M. (2014). Diet, geography and drinking water in Polynesia: microfossil research from archaeological human dental calculus, Rapa Nui (Easter Island). Int. J. Osteoarchaeol. 24, 634–648. doi: 10.1002/oa.2249
Eerkens, J. W., Tushingham, S., Brownstein, K. J., Garibay, R., Perez, K., Murga, E., et al. (2018). Dental calculus as a source of ancient alkaloids: detection of nicotine by LC-MS in calculus samples from the Americas. J. Archaeol. Sci. Rep. 18, 509–515. doi: 10.1016/j.jasrep.2018.02.004
Fitzpatrick, S. M., Nelson, G. C., and Reeves, R. (2003). The prehistoric chewing of betel nut (Areca catechu) in western Micronesia. People Cult. Oceania 19, 55–65. doi: 10.11501/11225790
Franke, A. A., Mendez, A. J., Lai, J. F., Arat-Cabading, C., Li, X., and Custer, L. J. (2015). Composition of betel specific chemicals in saliva during betel chewing for the identification of biomarkers. Food Chem. Toxicol. 80, 241–246. doi: 10.1016/j.fct.2015.03.012
Glover, I. C. (1977). Prehistoric plant remains from Southeast Asia, with special reference to rice. South Asian Archaeol. 7, 7–37.
Guedes, J. D., Hanson, S., Lertcharnrit, T., Weiss, A. D., Pigott, V. C., Higham, C. F., et al. (2020). Three thousand years of farming strategies in central Thailand. Antiquity 94, 966–982. doi: 10.15184/aqy.2020.8
Gupta, P. C., and Warnakulasuriya, S. (2002). Global epidemiology of areca nut usage. Addict. Biol. 7, 77–83. doi: 10.1080/13556210020091437
Hammond, S. K. (2009). Global patterns of nicotine and tobacco consumption. Nicotine Tob. Res. 2009, 3–28. doi: 10.1007/978-3-540-69248-5_1
Hardy, K., Blakeney, T., Copeland, L., Kirkham, J., Wrangham, R., and Collins, M. (2009). Starch granules, dental calculus and new perspectives on ancient diet. J. Archaeol. Sci. 36, 248–255. doi: 10.1016/j.jas.2008.09.015
Hernandez, B. Y., Zhu, X., Goodman, M. T., Gatewood, R., Mendiola, P., Quinata, K., et al. (2017). Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS ONE 12:e0172196. doi: 10.1371/journal.pone.0172196
Higham, C. (2011). The bronze age of Southeast Asia: new insight on social change from Ban Non Wat. Camb. Archaeol. J. 21, 365–389. doi: 10.1017/S0959774311000424
Higham, C., and Thosarat, R. (1998). Prehistoric Thailand: From Early Settlement to Sukhothai. Bangkok: River Books.
Hocart, C. H., and Frankhauser, B. (1996). Betel nut residues in archaeological samples of human teeth from the Mariana Islands. Experientia 52, 281–285. doi: 10.1007/BF01920724
Huber, B., Vassão, D. G., Roberts, P., Wang, Y.V., and Larsen, T. (2022). Chemical modification of biomarkers through accelerated degradation: implications for ancient plant identification in archaeo-organic residues. Molecules 27:3331. doi: 10.3390/molecules27103331
Juhola, T., Henry, A. G., Kirkinen, T., Laakkonen, J., and Väliranta, M. (2019). Phytoliths, parasites, fibers, and feathers from dental calculus and sediment from Iron Age Luistari cemetery, Finland. Quat. Sci. Rev. 222:105888. doi: 10.1016/j.quascirev.2019.105888
Kampangsri, W., Vatanasapt, P., Kamsa-Ard, S., Suwanrungruang, K., and Promthet, S. (2013). Betel quid chewing and upper aerodigestive tract cancers: a prospective cohort study in Khon Kaen, Thailand. Asian Pac. J. Cancer Prev. 14, 4335–4338. doi: 10.7314/APJCP.2013.14.7.4335
Kozlakidis, Z., Cheong, I. H., and Wang, H. (2022). Betel nut and arecoline: past, present, and future trends. Innov. Digit. Health Diagn. Biomark. 2, 64–72. doi: 10.36401/IDDB-22-05
Krais, S., Klima, M., Huppertz, L. M., Auwärter, V., Altenburger, M. J., and Neukamm, M. A. (2017). Betel nut chewing in Iron Age Vietnam? Detection of areca catechu alkaloids in dental enamel. J. Psychoact. Drugs 49, 11–17. doi: 10.1080/02791072.2016.1264647
Lertcharnrit, T., and Niyomsap, N. (2020). Heritage management, education, and community involvement in Thailand: a central Thai community case. J. Community Archaeol. Herit. 7, 187–197 doi: 10.1080/20518196.2020.1767378
Lertwinitnun, S. (2015). A Study of Oral Health of Prehistoric Human Remains at Nong Ratchawat Archaeological Site, Nong Yasai District, Suphanburi Province [BA Thesis]. Bangkok, Thailand: Silpakorn University.
Lertwinitnun, S. (2022). Non-Metric Dental Traits in the Prehistoric Population from Nong Ratchawat Archaeological Site, Nong Ya Sai District, Suphan Buri Province [MA Thesis]. Bangkok, Thailand: Silpakorn University,
Lin, W. Y., Chiu, T.Y., Lee, L. T., Lin, C. C., Huang, C. Y., and Huang, K. C. (2008). Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. Am. J. Clin. Nutr. 87, 1204–1211. doi: 10.1093/ajcn/87.5.1204
Liu, C. H. (2020). Bioarchaeology of prehistoric central Thailand: a heterarchical view. Am. Anthropol. 122, 908–911. doi: 10.1111/aman.13489
Mehrtash, H., Duncan, K., Parascandola, M., David, A., Gritz, E. R., Gupta, P. C., et al. (2017). Defining a global research and policy agenda for betel quid and areca nut. Lancet Oncol. 18, e767–e775. doi: 10.1016/S1470-2045(17)30460-6
Nalina, T., and Rahim, Z. H. (2007). The crude aqueous extract of Piper betle L. and its antibacterial effect towards Streptococcus mutans. Am. J. Biotechnol. Biochem. 3, 10–15. doi: 10.3844/ajbbsp.2007.10.15
Oxenham, M. F., Locher, C., Cuong, N. L., and Thuy, N. K. (2002). Identification of areca catechu (betel nut) residues on the dentitions of bronze age inhabitants of Nui Nap, Northern Vietnam. J. Archaeol. Sci. 29, 909–915. doi: 10.1006/jasc.2001.0767
Paulino, Y. C., Hurwitz, E. L., Ogo, J. C., Paulino, T. C., Yamanaka, A. B., Novotny, R., et al. (2017). Epidemiology of areca (betel) nut use in the Mariana Islands: findings from the University of Guam/University of Hawai‘i cancer center partnership program. Cancer Epidemiol. 50, 241–246. doi: 10.1016/j.canep.2017.08.006
Pfeiffer, S. (2013). Rights of indigenous peoples and the international drug control regime: the case of traditional coca leaf chewing. Goettingen J. Int. Law 5, 287–324. doi: 10.3249/1868-158-l-5-l-pfeiffer
Phumat, P., Khongkhunthian, S., Wanachantararak, P., and Okonogi, S. (2018). Effects of Piper betle fractionated extracts on inhibition of Streptococcus mutans and Streptococcus intermedius. Drug Discov. Ther. 12, 133–141. doi: 10.5582/ddt.2018.01021
Pindborg, J. J. (1973). Illustration of betel (areca) nut chewing in sawankalok celadon figurines from Thailand. J. Hist. Med. Allied Sci. 28, 46–47. doi: 10.1093/jhmas/XXVIII.1.46
Preus, H. R., Marvik, O. J., Selvig, K. A., and Bennike, P. (2011). Ancient bacterial DNA (aDNA) in dental calculus from archaeological human remains. J. Archaeol. Sci. 38, 1827–1831. doi: 10.1016/j.jas.2011.03.020
Ridge, C., Akanle, O., and Spyrou, N. M. (2001). Elemental composition of betel nut and associated chewing materials. J. Radioanal. Nucl. Chem. 249, 67–70. doi: 10.1023/A:1013273421535
Rooney, D. (1993). Betel Chewing Traditions in South-East Asia. Kuala Lumphur: Oxford University Press.
Sriharat, J. (2011). A Study of Human Remains from Nong Ratchawat Archaeological Site, Nong Ratchawat Subdistrict, Nong Yasai District, Suphanburi Province [BA Thesis]. Bangkok, Thailand: Silpakorn University.
Staples, G. W., and Bevacqua, R. F. (2006). Areca catechu (betel nut palm). Species Profiles Pac. Isl. Agrofor. 1, 1–9.
Stone, J. H., Nelson, G. C., and Fitzpatrick, S. M. (2020). Temporomandibular joint osteoarthritis at Chelechol ra Orrak, Palau. Int. J. Paleopathol. 28, 20–31. doi: 10.1016/j.ijpp.2019.12.001
Swadling, P., Aroho, N., and Ivuyo, B. (1991). Settlements associated with the inland Sepik-Ramu Sea. Bull. Indo-Pac. Prehist. Assoc. 11, 92–112. doi: 10.7152/bippa.v11i0.11376
Tanompolkrang, W. (2017). Pattern of Toothwear and Dietary Behavior at Nong Rachawat, Suphan Buri [Master's Thesis]. Bangkok, Thailand: Silpakorn University.
Tushingham, S., Eerkens, J.W., Berim, A., Brownstein, K.J., and Gang, D.R. (2020). Age and gender dynamics of tobacco use residue analysis of dental calculus and archaeological pipe. in protohistoric village organization and territorial maintenance: the archaeology of Síi Túupentak (CA-ALA-565/H) in the San Francisco Bay Area. Center for Archaeological Research at Davis, UC-Davis Publication Number 20, 345–358.
Tushingham, S., Snyder, C. M., Brownstein, K. J., Damitio, W. J., and Gang, D. R. (2018). Biomolecular archaeology reveals ancient origins of indigenous tobacco smoking in North American Plateau. Proc. Nat. Acad. Sci. 115, 11742–11747. doi: 10.1073/pnas.1813796115
Wangthongchaicharoen, N. (2013). Analytical Report of Human Remains at Nong Ratchawat Archaeological Site, Nong Yasai District, Suphanburi Province, Thailand. Suphanburi: The 2nd Regional Office of the Fine Arts Department of Thailand (FAD), Suphanburi, (unpublished report, in Thai).
White, J. C., and Hamilton, E. G. (2021). The metal age of Thailand and Ricardo's Law of comparative advantage. Archaeol. Res. Asia 27:100305. doi: 10.1016/j.ara.2021.100305
Keywords: betel (areca) nuts, psychoactive plants, ancient residue, Nong Ratchawat, Thailand, archaeobotany, LC-MS, dental calculus
Citation: Moonkham P, Tushingham S, Zimmermann M, Brownstein KJ, Devanwaropakorn C, Duangsakul S and Gang DR (2025) Earliest direct evidence of bronze age betel nut use: biomolecular analysis of dental calculus from Nong Ratchawat, Thailand. Front. Environ. Archaeol. 4:1622935. doi: 10.3389/fearc.2025.1622935
Received: 05 May 2025; Accepted: 11 June 2025;
Published: 31 July 2025.
Edited by:
Barbara Huber, Max Planck Institute of Geoanthropology, GermanyReviewed by:
Scott M. Fitzpatrick, University of Oregon, United StatesLaura Juliana Prieto Pabon, Academy of Sciences of the Czech Republic (ASCR), Czechia
Copyright © 2025 Moonkham, Tushingham, Zimmermann, Brownstein, Devanwaropakorn, Duangsakul and Gang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piyawit Moonkham, cGl5YXdpdC5tb29ua2hhbUBjbXUuYWMudGg=; Shannon Tushingham, c3R1c2hpbmdoYW1AY2FsYWNhZGVteS5vcmc=
 Piyawit Moonkham
Piyawit Moonkham Shannon Tushingham
Shannon Tushingham Mario Zimmermann4
Mario Zimmermann4 David R. Gang
David R. Gang