Abstract
Background:
Pancreatic neuroendocrine tumors (pNETs) are a group of uncommon tumors derived from peptide neurons and neuroendocrine cells, and account for roughly 2% to 4% of all pancreatic neoplasms. This study aimed to construct and validate a nomogram for predicting the prognosis of patients with pNETs. Our data came from the SEER database.
Methods:
A total of 5927 pNETs patients between 2004 and 2018 were included in this study. The nomogram was constructed base on eight prognostic factors and validated by C-index, ROC curve and calibration curves. A nomogram based on eight independent prognostic factors (patient age, sex, race, tumor grade, AJCC T, AJCC N, AJCC M, surgery, radiation, chemotherapy, tumor function and marital status) was developed for the prediction of CSS at 3 and 5 years.
Results:
The C-index and AUCs of the nomogram demonstrated that its superiority in discrimination over AJCC staging system. The calibration plots showed the good consistency between predictions and actual observations.
Conclusions:
In conclusion, our nomogram could better predict the prognosis of pNETs patients than AJCC staging system. The nomogram could be improved by integrating more important factors other than SEER database.
Introduction
Pancreatic neuroendocrine tumors (pNETs) are a group of uncommon tumors derived from peptide neurons and neuroendocrine cells, and account for roughly 2% to 4% of all pancreatic neoplasms (1, 2). The incidence of pNETs has increased from 1.07 to 5.25 per 100,000 in the United States over the past years (3). While few functioning pNETs secret hormones such as insulin, gastrin, glucagon, or other hormones and cause symptoms, most pNETs are nonfunctional (4). Due to the relative low incidence and non-functionality of pNETs, the prognosis of pNETs patients was rarely studied. Therefore, the prognosis of patients with pNETs is necessary to be further studied to help doctors and patients better understand this uncommon disease.
Currently, the American Joint Committee on Cancer (AJCC) TNM staging system is the most commonly used classification system for patients with pNETs (5). However, many significant prognostic factors such as gender, age, and grade were not included in AJCC system (6). Nomogram is an easy-to-use predictive system which is based on statistical model (7). It could be used to predict the prognosis of a disease by considering every selected factors, therefore this tool has been widely applied in clinical use (8–10).
This study aimed to construct and validate a nomogram for predicting the prognosis of patients with pNETs. The incorporated prognosis factors were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. And we further compared our nomogram with AJCC staging system to determine whether this model provides more accurate prediction.
Materials and methods
Ethics statement
Our data came from the SEER database and signed a data agreement (11187-Nov2021), so our study was exempt from ethical review. This article does not contain any studies with human participants performed by any of the authors.
Study population
We obtained patient data from the SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018) incidence database, using SEER*Stat version 8.3.9. A total of 10,773 pNETs patients were identified and 5,927 patients were finally included in this study. The flow chart for selecting research samples was shown in Figure 1. The following variables were used in the analysis: patient age, sex and race; Tumor grade, AJCC staging for the extent of tumor (T), extent of spread to lymph nodes (N), and presence of metastasis (M); Surgery (Y/N), radiation (Y/N) and chemotherapy (Y/N); Tumor function and marital status.
Figure 1

Flow chart of study patients’ enrollment.
The 3rd edition International Classification of Disease for Oncology (ICD-O-3) histology codes was used to select functioning and non-functioning pNETs. The functioning pNETs contained: Insulinoma (8151/3), Glucagonoma (8152/3), Gastrinoma (8153/3), Vipoma (8155/3), Somatostatinoma (8156/3), Enteroglucagonoma(8157/3) and ACTH-producing tumor (8158/3). NFpNETs contained: 8013/3, 8150/3, 8240/3-8246/3 and 8249/3.
Statistical analysis
Endpoint for the current analysis was cancer-specific death (CSS). For nomogram construction and validation, we randomly divided all the pNETs patients into training (n = 4,148) and validation (n = 1,779) cohorts, in a ratio of 7:3 (11, 12). Multivariate Cox proportional hazards regression analysis was performed to identify variables (P < 0.05) that significantly affected CSS in the training group. Using these identified prognostic factors, we constructed a nomogram for predicting 3- and 5-year CSS rates in pNETs patients.
The nomogram was validated internally in the training cohort and externally in the validation cohort. We also developed a risk stratification system based on the total points of each patient in the training cohort. The cutoff value of the total points was calculated by X-tile software (version 3.6.1). To evaluate the discriminative ability of the nomogram, we used the concordance index (C-index) and the receiver operating characteristic (ROC) curve and assessed the area under the curve (AUC) (13, 14). A C-index or AUC of 0.5 indicates a discrimination ability that is no better than chance, whereas that of 1.0 indicates a perfect discrimination ability (15). Calibration curves were constructed using a bootstrap approach, with 500 resamples, to compare the predicted CSS with the CSS observed in the study.
All statistical analyses were conducted using SPSS (version 24.0; SPSS, Chicago, IL, USA) and R software (version 4.1.2; http://www.r-project.org/). A P value of less than 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
A total of 5,927 pNETs patients from 2004 to 2018 were included in this study. The training and validation cohorts consisted of 4,148 and 1,779 cases, respectively, selected by the random split-sample method (split ratio: 7:3). In the total cohort of pNETs patients, the majority of patients were under 65 years old (59.0%), male (55.3%), and white (78.8%). Furthermore, most of the patients had well-differentiated grade (90.1%), T1 (34.1%), N0 (72.9%), and M0 (79.4%). Most of pNETs patients underwent surgery (75.4%), but only a small proportion of the patients received radiation therapy (3.36%) and chemotherapy (13.7%). 99.0% of pNETs were non-functional and a large number of pNETs patients were married (63.2%). The characteristics of pNETs patients in the training and validation cohorts were similar to those in the total cohort (Table 1).
Table 1
| Characteristics | Total cohort | Training cohort | Validation cohort |
|---|---|---|---|
| 5927 (100%) | 4148 (70%) | 1779 (30%) | |
| Age | |||
| <65 | 3496 (59.0%) | 2461 (59.3%) | 1035 (58.2%) |
| ≥65 | 2431 (41.0%) | 1687 (40.7%) | 744 (41.8%) |
| Sex | |||
| Male | 3280 (55.3%) | 2284 (55.1%) | 996 (56.0%) |
| Female | 2647 (44.7%) | 1864 (44.9%) | 783 (44.0%) |
| Race | |||
| W | 4668 (78.8%) | 3270 (78.8%) | 1398 (78.6%) |
| B | 673 (11.4%) | 469 (11.3%) | 204 (11.5%) |
| AI | 34 (0.57%) | 21 (0.51%) | 13 (0.73%) |
| API | 552 (9.31%) | 388 (9.35%) | 164 (9.22%) |
| Grade | |||
| low | 5341 (90.1%) | 3739 (90.1%) | 1602 (90.1%) |
| high | 586 (9.89%) | 409 (9.86%) | 177 (9.95%) |
| AJCC T | |||
| T1 | 2019 (34.1%) | 1405 (33.9%) | 614 (34.5%) |
| T2 | 1969 (33.2%) | 1371 (33.1%) | 598 (33.6%) |
| T3 | 1597 (26.9%) | 1126 (27.1%) | 471 (26.5%) |
| T4 | 342 (5.77%) | 246 (5.93%) | 96 (5.40%) |
| AJCC N | |||
| N0 | 4322 (72.9%) | 3018 (72.8%) | 1304 (73.3%) |
| N1 | 1605 (27.1%) | 1130 (27.2%) | 475 (26.7%) |
| AJCC M | |||
| M0 | 4706 (79.4%) | 3282 (79.1%) | 1424 (80.0%) |
| M1 | 1221 (20.6%) | 866 (20.9%) | 355 (20.0%) |
| Surgery | |||
| No | 1458 (24.6%) | 1019 (24.6%) | 439 (24.7%) |
| Yes | 4469 (75.4%) | 3129 (75.4%) | 1340 (75.3%) |
| Radiation | |||
| No | 5728 (96.6%) | 4004 (96.5%) | 1724 (96.9%) |
| Yes | 199 (3.36%) | 144 (3.47%) | 55 (3.09%) |
| Chemotherapy | |||
| No | 5117 (86.3%) | 3570 (86.1%) | 1547 (87.0%) |
| Yes | 810 (13.7%) | 578 (13.9%) | 232 (13.0%) |
| Tumor function | |||
| non-functioning | 5865 (99.0%) | 4104 (98.9%) | 1761 (99.0%) |
| functioning | 62 (1.05%) | 44 (1.06%) | 18 (1.01%) |
| Marital status | |||
| Married | 3745 (63.2%) | 2633 (63.5%) | 1112 (62.5%) |
| Single | 2182 (36.8%) | 1515 (36.5%) | 667 (37.5%) |
pNETs Patient characteristics in the study.
W, White; B, Black; AI, American Indian/Alaska Native; API, Asian or Pacific Islander; Low grade: grade I and II, high grade: grade III.
Screening for prognostic factors of CSS
Based on multivariate Cox proportional hazards regression analyses, we identified eight independent prognostic factors in the training cohort. Over 65 years old (hazard ratio [HR] = 1.48, P < 0.001), female (HR = 0.81, P < 0.01), poorly differentiated grade (HR = 4.53, P < 0.001), AJCC T2/T3/T4 (HR = 1.54/2.10/1.49, P < 0.01), AJCC N1 (HR = 1.61, P < 0.001), AJCC M1 (HR = 2.59, P < 0.001), surgery (HR = 0.26, P < 0.001) and single status (HR = 1.23, P < 0.05) were all significantly associated with CSS in pNETs patients (Figure 2 and Table 2).
Figure 2
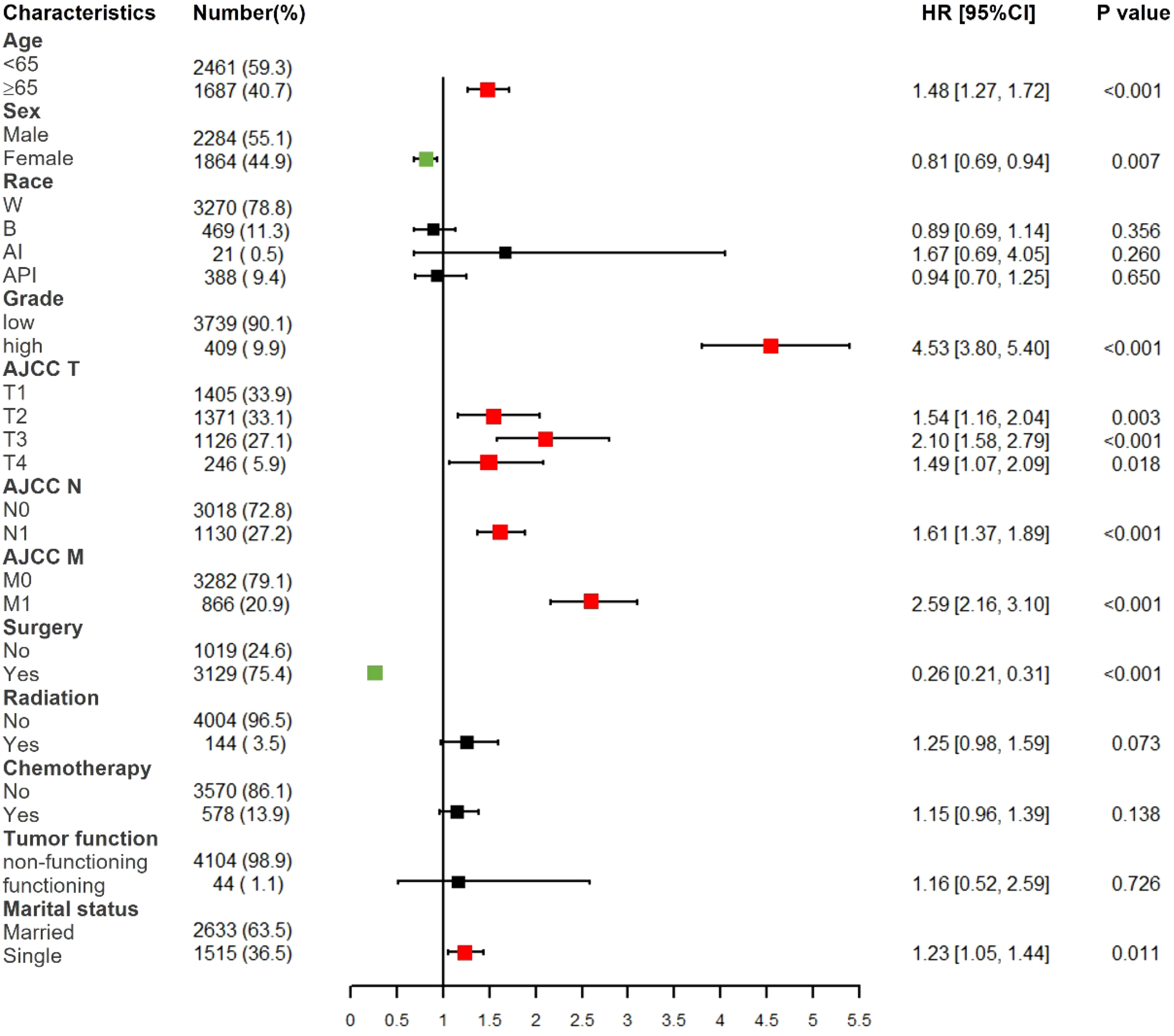
Forest plot of multivariate Cox regression analysis for CSS in pNETs patients. W, White; B, Black; AI, American Indian/Alaska Native; API, Asian or Pacific Islander; Low grade: grade I and II, high grade: grade III.
Table 2
| Characteristics | Multivariate analysis | |
|---|---|---|
| HR [95% CI] | P value | |
| Age | ||
| <65 | Reference | |
| ≥65 | 1.48 [1.27, 1.72] | <0.001*** |
| Sex | ||
| Male | Reference | |
| Female | 0.81 [0.69, 0.94] | 0.007** |
| Race | ||
| W | Reference | |
| B | 0.89 [0.69, 1.14] | 0.356 |
| AI | 1.67 [0.69, 4.05] | 0.260 |
| API | 0.94 [0.70, 1.25] | 0.650 |
| Grade | ||
| low | Reference | |
| high | 4.53 [3.80, 5.40] | <0.001*** |
| AJCC T | ||
| T1 | Reference | |
| T2 | 1.54 [1.16, 2.04] | 0.003** |
| T3 | 2.10 [1.58, 2.79] | <0.001*** |
| T4 | 1.49 [1.07, 2.09] | 0.018* |
| AJCC N | ||
| N0 | Reference | |
| N1 | 1.61 [1.37, 1.89] | <0.001*** |
| AJCC M | ||
| M0 | Reference | |
| M1 | 2.59 [2.16, 3.10] | <0.001*** |
| Surgery | ||
| No | Reference | |
| Yes | 0.26 [0.21, 0.31] | <0.001*** |
| Radiation | ||
| No | Reference | |
| Yes | 1.25 [0.98, 1.59] | 0.073 |
| Chemotherapy | ||
| No | Reference | |
| Yes | 1.15 [0.96, 1.39] | 0.138 |
| Tumor function | ||
| non-functioning | Reference | |
| functioning | 1.16 [0.52, 2.59] | 0.726 |
| Marital status | ||
| Married | Reference | |
| Single | 1.23 [1.05, 1.44] | 0.011* |
Multivariate Cox regression analysis based on all variables for pNETs patient cancer-specific survival (Training Cohort).
W, White; B, Black; AI, American Indian/Alaska Native; API, Asian or Pacific Islander; Low grade: grade I and II, high grade: grade III. *:P<0.05;**:P<0.01;***:P<0.001.
Nomogram construction
We constructed a nomogram for predicting the 3- and 5- years CSS of pNETs patients based on the independent prognostic factors from the training cohort (Figure 3). The nomogram indicated that tumor grade contributed the most to prognosis, followed by surgery, AJCC M, AJCC T, AJCC N, age, sex and marital status. When applying the nomogram for a single pNETs patient, the total score of the patient could be calculated by adding each score of the selected variables. Then the prediction of 3- and 5- years survival rates for the patient could be read on the nomogram.
Figure 3
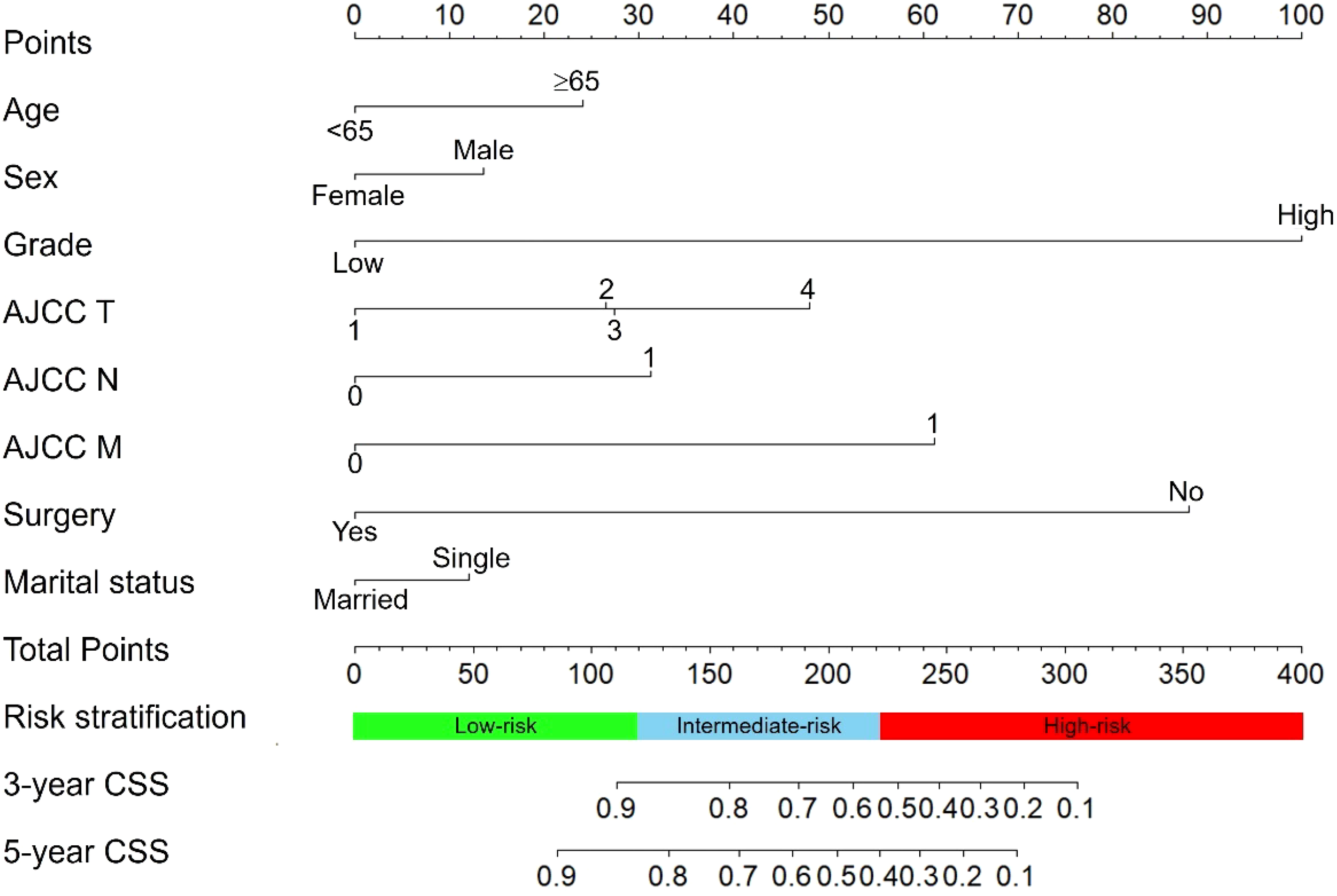
The nomogram predicting CSS in pNETs patients. Each factor was given a point on the basis of the nomogram. The total points were obtained by adding the given points of all factors. The estimated 3- and 5-year probabilities of CSS of the individual patient can be easily obtained from the nomogram based on the total points.
Risk stratification system
After calculation by X-tile software (Figure S1), all patients were grouped into the low-risk (score: 0–121.2), intermediate-risk (score: 121.2–222.7), and high-risk groups (score: 222.7–378.1). Compared to low-risk group, HR of intermediate-risk group and high-risk group were 6.57 (P < 0.001) and 29.68 (P < 0.001). The C-index of the risk stratification system was 0.825. The Kaplan-Meier survival curves also showed the good discrimination power of the risk stratification system (Figure 4).
Figure 4
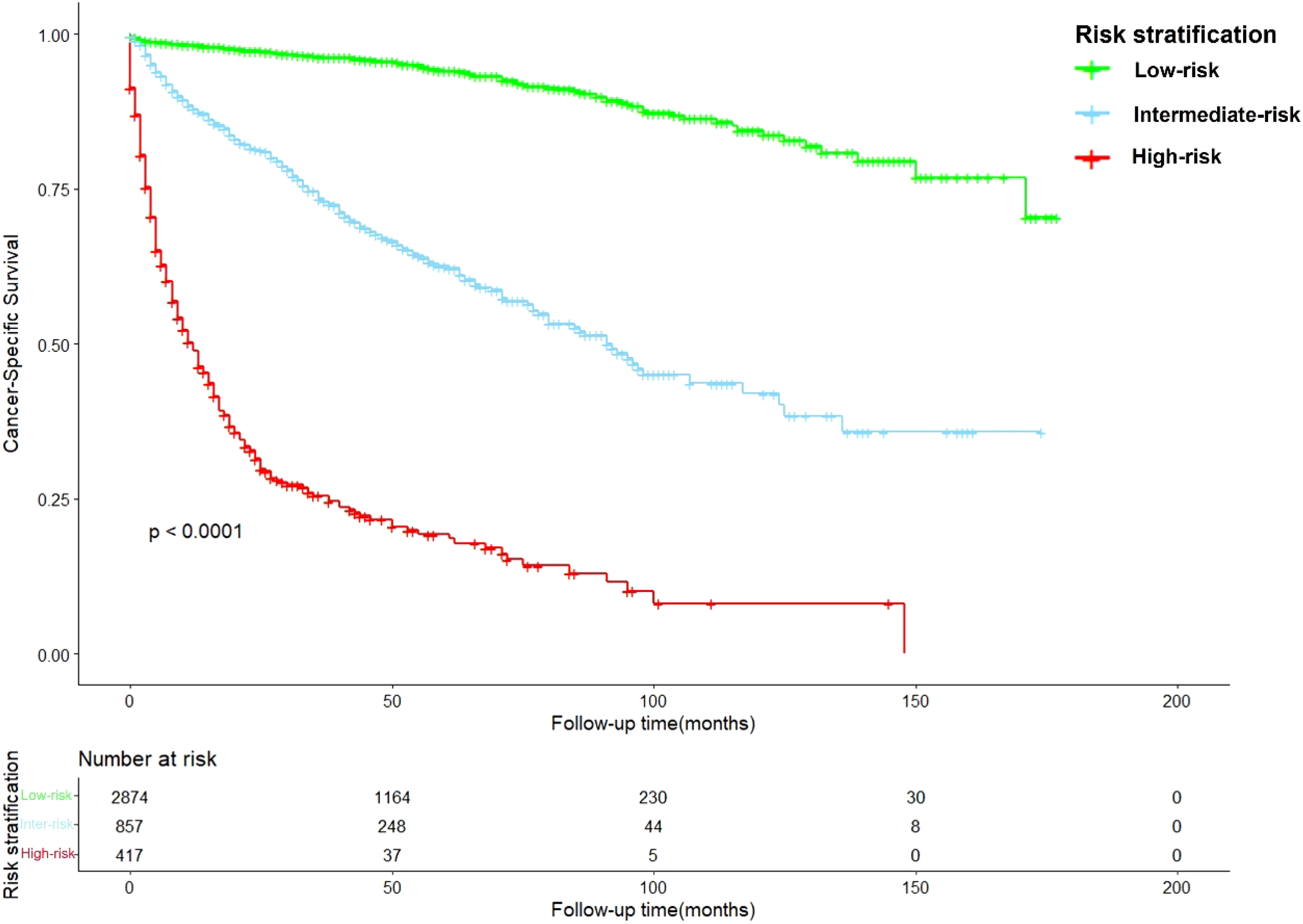
The Kaplan-Meier survival curves based on the risk stratification system.
Nomogram validation
The C-index of the nomogram was higher than which based on the AJCC staging system in both training cohort (0.856 vs. 0.781) and validation cohort (0.84 vs. 0.772). Also, the AUCs of the nomogram were higher than AJCC staging system in both training (3-year AUC: 0.829 vs. 0.818, 5-year AUC: 0.819 vs. 0.808, Figures 5A, B) and validation (3-year AUC: 0.831 vs. 0.809, 5-year AUC: 0.816 vs. 0.802, Figures 5C, D) cohorts for 3- and 5- years. The C-index and AUCs of the nomogram demonstrated that its superiority in discrimination over AJCC staging system. Furthermore, Figure 6 showed the good consistency between predictions and actual observations when using the nomogram for pNETs patients.
Figure 5
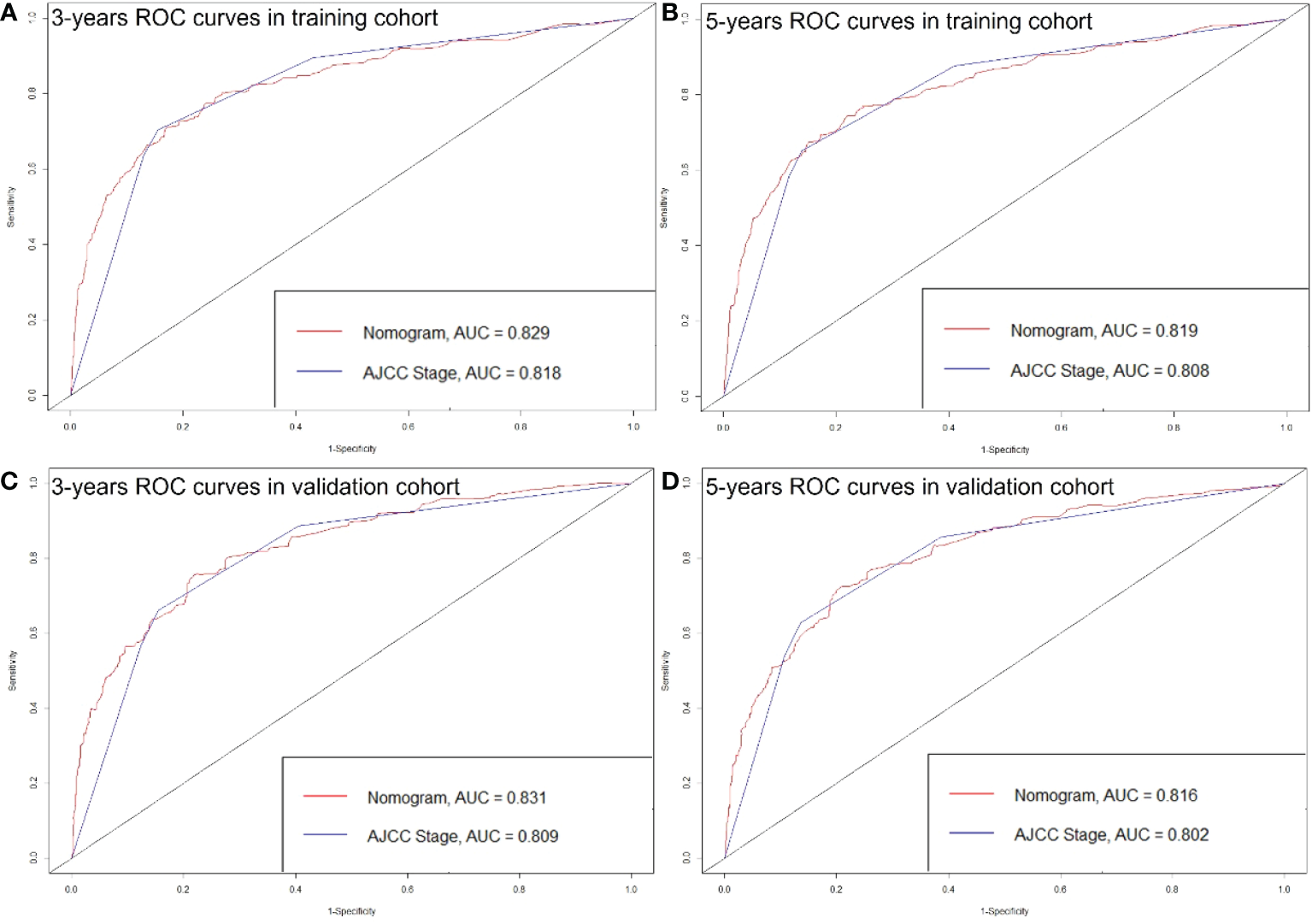
ROC curves of the Nomogram and AJCC stage in prediction of prognosis at 3- (A) and 5-year (B) point in the training cohort. ROC curves of the Nomogram and AJCC stage in prediction of prognosis at 3- (C) and 5-year (D) point in the validation cohort.
Figure 6
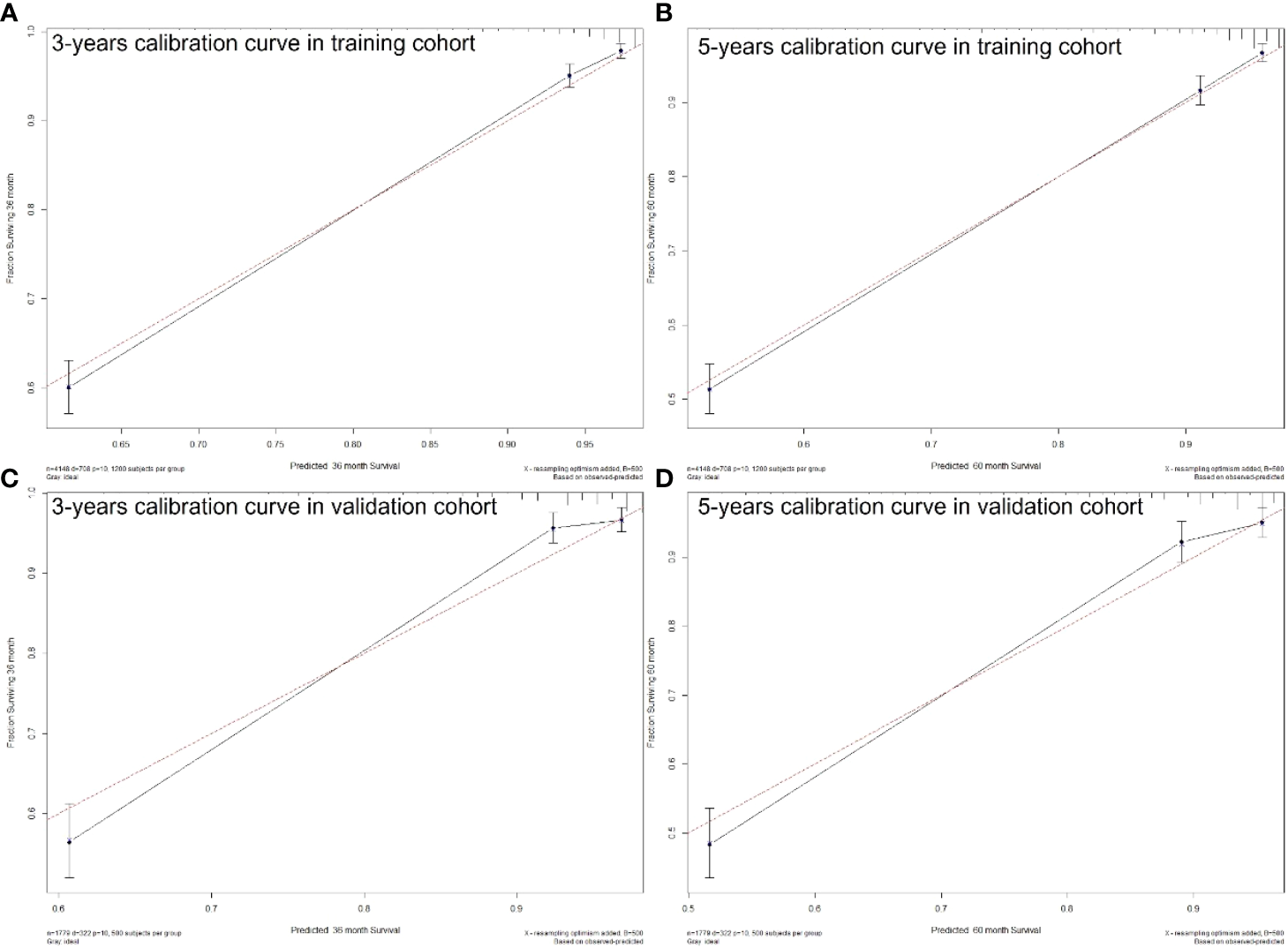
3- (A) and 5-years (B) calibration curves for probability of pNETs patients CSS nomogram construction in training cohort. 3- (C) and 5-years (D) calibration curves for probability of pNETs patients CSS nomogram construction in validation cohort. (Bootstrap = 500 repetitions).
Discussion
Although the AJCC staging system is widely used for predicting prognosis in pNETs patients, it has inherent defects because it neglects many additional risk factors other than the TNM factors. Our study obtained 12 factors of pNETs patients from SEER database, and found 8 independent prognosis factors including: patient age, sex, race, tumor grade, AJCC T, AJCC N, AJCC M, surgery, radiation, chemotherapy, tumor function and marital status. Therefore, the C-index and AUCs of our nomogram were higher than which of AJCC staging system (C-index: 0.856 vs. 0.781; 3-year AUCs: 0.829 vs. 0.818 in training cohort).
A previous study demonstrated that younger than 60 years old and female were two protective factors for non-functional pNETs patients (16), though the results were not validated. In our study, over 65 years old (HR = 1.48, P < 0.001) and female (HR = 0.81, P < 0.01) were two independent prognosis factors for pNETs patients. Because 99.0% of pNETs in our study were non-functional, therefore the results in our study were consistent with previous one. The reason why female patients have better prognosis may be attributed to the protective effect of estrogen digestive tract tumors (17, 18).
The treatment for patients with pNETs was still controversial (19). Tumor resection was recommended for early-stage pNETs patients (20, 21), and our study verified that pNETs patients after surgery had a better prognosis than patients without surgery (HR = 0.26, P < 0.001). Radiation and chemotherapy could also be optional used for patients with unresectable pNETs, though the curative effect was not distinct enough (22). In our study, no significant protective effect was observed in pNETs patients who underwent radiation therapy (HR = 1.25, P = 0.07) or chemotherapy (HR = 1.15, P = 0.14).
Marital status has been found to have effects on the prognosis of many cancers such as bladder cancer (23) and renal cell carcinoma (24). It could be attributed to that single patients lack the emotional support and social interaction from partners and they are prone to have unhealthy lifestyles such as alcoholism, tobacco consumption and drug abuse. Our study also proved that single patients with pNETs had worse prognosis than patients in marriage (HR = 1.23, P < 0.05).
It was believed that patients with functional pNETs had a longer survival than those with nonfunctional pNETs (25–27). Due to the lack of specific symptoms, most of patients with nonfunctional pNETs could be diagnosed at a relatively advanced stage and had a worse prognosis. However, in our study, no significant prognosis difference was observed between functional pNETs patients and nonfunctional pNETs patients (P = 0.726). The reason could be the relatively small sample size of functional pNETs patients in our study (n = 62).
It should be noted that our study also has some limitations. First, this large-sample study was based on the SEER database, which may have some inherent biases. And our nomogram was internally validated, and it would be better to be validated externally using other populations.
Conclusions
In conclusion, we constructed and validated a nomogram for predicting the 3- and 5-year CSS in pNETs patients. The proposed nomogram considered eight independent prognosis factors: patient age, sex, race, tumor grade, AJCC T, AJCC N, AJCC M, surgery, radiation, chemotherapy, tumor function and marital status. We have confirmed the precise calibration and excellent discrimination power of our nomogram. The predictive power of this nomogram may be improved by considering other potential important factors that we could not be obtained from the SEER database, and also by external validation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Our data came from the SEER database and signed a data agreement (11187-Nov2021), so our study was exempt from ethical review. This article does not contain any studies with human participants performed by any of the authors.
Author contributions
KL: conceptualization. GH and WS: data curation. YZ and BR: formal analysis. YL: supervision. KL: writing—original draft. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Key R&D Project of Shaanxi Province (No. 2020GXLH-Z-001), the Scientific Development Funding of the First Affiliated Hospital of Xi’an Jiaotong University (No. 2022QN-17) and Shaanxi Natural Science Basic Research Program(No.2023-JC-QN-0879).
Acknowledgments
We thank the staff at the SEER database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2022.1088133/full#supplementary-material
References
1
Milan SA Yeo CJ . Neuroendocrine tumors of the pancreas. Curr Opin Oncol (2012) 24(1):46–55. doi: 10.1097/CCO.0b013e32834c554d
2
Yao JC Eisner MP Leary C Dagohoy C Phan A Rashid A et al . Population-based study of islet cell carcinoma. Ann Surg Oncol (2007) 14(12):3492–500. doi: 10.1245/s10434-007-9566-6
3
Yao JC Hassan M Phan A Dagohoy C Leary C Mares JE et al . One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the united states. J Clin Oncol (2008) 26(18):3063–72. doi: 10.1200/JCO.2007.15.4377
4
Zerbi A Falconi M Rindi G Delle Fave G Tomassetti P Pasquali C et al . Clinicopathological features of pancreatic endocrine tumors: a prospective multicenter study in Italy of 297 sporadic cases. Am J Gastroenterol (2010) 105(6):1421–9. doi: 10.1038/ajg.2009.747
5
Scarpa A Mantovani W Capelli P Beghelli S Boninsegna L Bettini R et al . Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol (2010) 23(6):824–33. doi: 10.1038/modpathol.2010.58
6
Liu K Huang G Chang P Zhang W Li T Dai Z et al . Construction and validation of a nomogram for predicting cancer-specific survival in hepatocellular carcinoma patients. Sci Rep (2020) 10(1):21376. doi: 10.1038/s41598-020-78545-2
7
Iasonos A Schrag D Raj GV Panageas KS . How to build and interpret a nomogram for cancer prognosis. J Clin Oncol (2008) 26(8):1364–70. doi: 10.1200/JCO.2007.12.9791
8
Li W Xiao Y Xu X Zhang Y . A novel nomogram and risk classification system predicting the cancer-specific mortality of patients with initially diagnosed metastatic cutaneous melanoma. Ann Surg Oncol (2021) 28(7):3490–500. doi: 10.1245/s10434-020-09341-5
9
Zhang H Li X Zhang Y Huang C Wang Y Yang P et al . Diagnostic nomogram based on intralesional and perilesional radiomics features and clinical factors of clinically significant prostate cancer. J Magn Reson Imaging. (2021) 53(5):1550–8. doi: 10.1002/jmri.27486
10
Zuo Z Zhang G Song P Yang J Li S Zhong Z et al . Survival nomogram for stage IB non-Small-Cell lung cancer patients, based on the SEER database and an external validation cohort. Ann Surg Oncol (2021) 28(7):3941–50. doi: 10.1245/s10434-020-09362-0
11
Collins GS Reitsma JB Altman DG Moons KGM . Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Eur Urology. (2015) 67(6):1142–51. doi: 10.1016/j.eururo.2014.11.025
12
Lei Z Li J Wu D Xia Y Wang Q Si A et al . Nomogram for preoperative estimation of microvascular invasion risk in hepatitis b virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg (2016) 151(4):356–63. doi: 10.1001/jamasurg.2015.4257
13
Hanley JA McNeil BJ . The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. (1982) 143(1):29–36. doi: 10.1148/radiology.143.1.7063747
14
Wolbers M Koller MT Witteman JC Steyerberg EW . Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. (2009) 20(4):555–61. doi: 10.1097/EDE.0b013e3181a39056
15
Balachandran VP Gonen M Smith JJ DeMatteo RP . Nomograms in oncology: More than meets the eye. Lancet Oncol (2015) 16(4):e173–80. doi: 10.1016/S1470-2045(14)71116-7
16
Ge LN Li HJ Dong L Shang GM Wang WY Li Y et al . Nomogram for predicting survival of patients with metastatic nonfunctioning pancreatic neuroendocrine tumors a SEER based study. Medicine (2021) 100(27): e26347. doi: 10.1097/MD.0000000000026347
17
Bodelon C Anderson GL Rossing MA Chlebowski RT Ochs-Balcom HM Vaughan TL . Hormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal women. Cancer Prev Res (Phila). (2011) 4(6):840–50. doi: 10.1158/1940-6207.CAPR-10-0389
18
Wu H Xu L Chen J Hu J Yu S Hu G et al . Association of estrogen receptor beta variants and serum levels of estradiol with risk of colorectal cancer: A case control study. BMC Cancer. (2012) 12:276. doi: 10.1186/1471-2407-12-276
19
Rossi RE Massironi S Conte D Peracchi M . Therapy for metastatic pancreatic neuroendocrine tumors. Ann Trans Med (2014) 2(1):8–. doi: 10.3978/j.issn.2305-5839.2013.03.01
20
Pavel M Baudin E Couvelard A Krenning E Öberg K Steinmüller T et al . ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. (2012) 95(2):157–76. doi: 10.1159/000335597
21
Kulke MH Anthony LB Bushnell DL de Herder WW Goldsmith SJ Klimstra DS et al . NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. (2010) 39(6):735–52. doi: 10.1097/MPA.0b013e3181ebb168
22
Riccardi F Rizzo M Festino L Ambrosio F Molino C Uomo G et al . Therapy innovation for the treatment of pancreatic neuroendocrine tumors. Expert Opin Ther Targets. (2012) 16:S91–S102. doi: 10.1517/14728222.2012.665880
23
Nelles JL Joseph SA Konety BR . The impact of marriage on bladder cancer mortality. Urol Oncol (2009) 27(3):263–7. doi: 10.1016/j.urolonc.2008.04.016
24
Li Y Zhu MX Qi SH . Marital status and survival in patients with renal cell carcinoma. Med (Baltimore). (2018) 97(16):e0385. doi: 10.1097/MD.0000000000010385
25
Wang YH Lin Y Xue L Wang JH Chen MH Chen J . Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: A single-institution analysis (1995-2012) in south China. BMC Endocr Disord (2012) 12:30. doi: 101186/1472-6823-12-30
26
Cienfuegos JA Rotellar F Salguero J Ruiz-Canela M Núñez Córdoba JM Sola I et al . A single institution's 21-year experience with surgically resected pancreatic neuroendocrine tumors: an analysis of survival and prognostic factors. Rev Esp Enferm Dig. (2016) 108(11):689–96. doi: 10.17235/reed.2016.4323/2016
27
Nanno Y Toyama H Otani K Asari S Goto T Terai S et al . Microscopic venous invasion in patients with pancreatic neuroendocrine tumor as a potential predictor of postoperative recurrence. Pancreatology. (2016) 16(5):882–7. doi: 10.1016/j.pan.2016.06.008
Summary
Keywords
nomogram, cancer-specific survival, pancreatic, neuroendocrine tumors, prognosis
Citation
Huang G, Song W, Zhang Y, Ren B, Lv Y and Liu K (2023) Construction and validation of a nomogram for patients with pancreatic neuroendocrine tumors: A population study of 5,927 patients. Front. Gastroenterol. 1:1088133. doi: 10.3389/fgstr.2022.1088133
Received
03 November 2022
Accepted
22 December 2022
Published
10 January 2023
Volume
1 - 2022
Edited by
Marianna Arvanitakis, Université libre de Bruxelles, Belgium
Reviewed by
Caiming Xu, Dalian Medical University, China; Zhijun Zhou, University of Oklahoma Health Sciences Center, United States
Updates
Copyright
© 2023 Huang, Song, Zhang, Ren, Lv and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang Liu, kangliu@xjtu.edu.cn
This article was submitted to Gastroenterology and Cancer, a section of the journal Frontiers in Gastroenterology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.