Abstract
Introduction:
Direct antiviral agents (DAAs) have dramatically changed the landscape of liver diseases associated with chronic hepatitis C virus (HCV) infection. However, limited data are available on the antiviral effect of sofosbuvir (SOF) + velpatasvir (VEL) ± ribavirin (RBV), SOF + VEL + voxilaprevir (VOX), and glecaprevir (GLE) + pibrentasvir (PIB) in treating patients infected with HCV GT3 in a real-world setting.
Methods:
Using the EMBASE, PubMed, and Cochrane Library databases, articles were screened from 1 January 2016 to 1 June 2024. The sustained virologic response (SVR) rates were analyzed using the Freeman–Tukey double arcsine transformation in a random-effects model in R4.1.0 software.
Results:
We recruited 3,177 patients with HCV GT3 in 19 studies from 9 countries. The pooled SVR12/24 rate of the three evaluated regimens was 94.00% (95% CI: 90.87-96.59%). Furthermore, the SVR rate was 83.81% (95% CI: 75.70-90.62%) in patients receiving SOF+VEL+VOX; 94.98% (95% CI: 92.02-97.33%) in patients receiving SOF+VEL ± RBV; and 96.96% (95% CI: 93.20-99.45%) in patients receiving GLE+PIB. The pooled SVR12/24 rate of the three regimens was 95.70% (95% CI: 91.74-98.58%) and 90.50% (95% CI: 83.50-95.90%) in non-cirrhotic and cirrhotic patients, respectively. The pooled SVR rate was 96.79% (95% CI: 93.37-99.13%) and 88.41% (95% CI: 82.67-93.22%) in treatment-naive and treatment-experienced patients, respectively.
Conclusion:
SOF+VEL ± RBV, GLE+PIB, and SOF+VEL+VOX had good antiviral effectiveness for chronic HCV-GT3 infection in real-world settings. Factors such as cirrhosis and treatment experience, especially previous DAA treatment failure, may influence the SVR rate.
Introduction
Approximately 58 million individuals have been infected by the hepatitis C virus (HCV) in the world and 290,000 patients died from diseases associated with HCV in 2019 (1). A worldwide health sector strategy to eliminate HCV by 2030 was proposed by the World Health Organization(WHO)6 years ago (2). The sustained virologic response (SVR) has been improved significantly with the clinical application of direct-acting antivirals (DAAs) in recent years (3–9). It is reported that SVR rates of different DAAs are variable depending on the HCV genotype (GT), especially genotype 3 (10–12). Significant progress in the inhibition of HCV replication has been achieved by using new drug regimens and drug combinations such as sofosbuvir (SOF) + velpatasvir (VEL) ± ribavirin (RBV) in 2016 and SOF + VEL + voxilaprevir (VOX) and glecaprevir (GLE) + pibrentasvir (PIB) in 2017, which were approved by the European Medicine Agency (EMA) or the United States Food and Drug Administration (FDA) (13).
The antiviral effectiveness of DAAs may be decreased in a real-world setting because of poor compliance and the population diversity of patients (14–17). There is a dearth of analysis on the antiviral effectiveness of SOF+VEL ± RBV, SOF+VEL+VOX, and GLE+PIB in a real-world setting.
Thus, to evaluate the pooled SVR rate against HCV-GT3 infection in a real-world setting, we systematically searched and analyzed the latest data on SOF+VEL ± RBV, SOF+VEL+VOX, and GLE+PIB.
Methods
Literature search method
Using EMBASE, PubMed, and Cochrane Library, studies were searched for from 1 January 2016 to 1 June 2024 using the following terms: (“Epclusa” OR “velpatasvir” AND “sofosbuvir”) OR (“Mavyret” OR “pibrentasvir” AND “glecaprevir”) OR (“Vosevi” OR “voxilaprevir” AND “velpatasvir” AND “sofosbuvir”).
Inclusion and exclusion criteria
Two independent researchers screened the abstracts and titles of potentially eligible publications. A full-text review of the selected articles was then performed for advanced selection in accordance with the criteria for inclusion and exclusion. Objections were discussed and resolved with a third party. The criteria for inclusion were: subject (patients infected with HCV GT3 chronically); intervention (SOF+VEL± RBV, GLE+PIB, or SOF+VEL+VOX); primary outcome (SVR rate after 8-24 weeks); and study design (real-world study). The following exclusion criteria were used: A) inaccessibility of valid data on HCV-GT3; B) assessing fewer than 10 cases; and C) meta-analyses, summaries, or case reports.
Data extraction
Two authors independently extracted the data. Data on the demographics, SVR12, therapy duration, average HCV RNA concentration at baseline, drug dosage, treatment regimen, and virological failure were extracted using standardized forms from the articles.
Data analysis
The SVR rates were analyzed using the Freeman–Tukey double arcsine transformation in a random-effects model. Furthermore, Egger’s test assessed publication bias, and the data analysis was conducted using R4.4.1 software. The output results do not involve ethical issues, and the research was exempted from ethical review by the Ethics Committee.
Results
Main characteristics of the populations and studies
Overall, 3,177 HCV GT3-infected individuals were recruited in the 19 included articles, selected from a total of 3443 articles step by step as shown in Figure 1. These studies were conducted in eight countries: Italy (n=4), the USA (n=4), China (n=3), Germany (n=2), Japan (n=2), Denmark (n=1), Myanmar (n=1), Spain (n=1), and the UK(n=1). Of the studies, 42.11% were on GLE+PIB (8/19), 36.84% on SOF+VEL ± RBV (7/19), and 21.05% on SOF+VEL+VOX (4/19), as presented in Figure 2.
Figure 1

A flowchart depicting the article selection process.
Figure 2
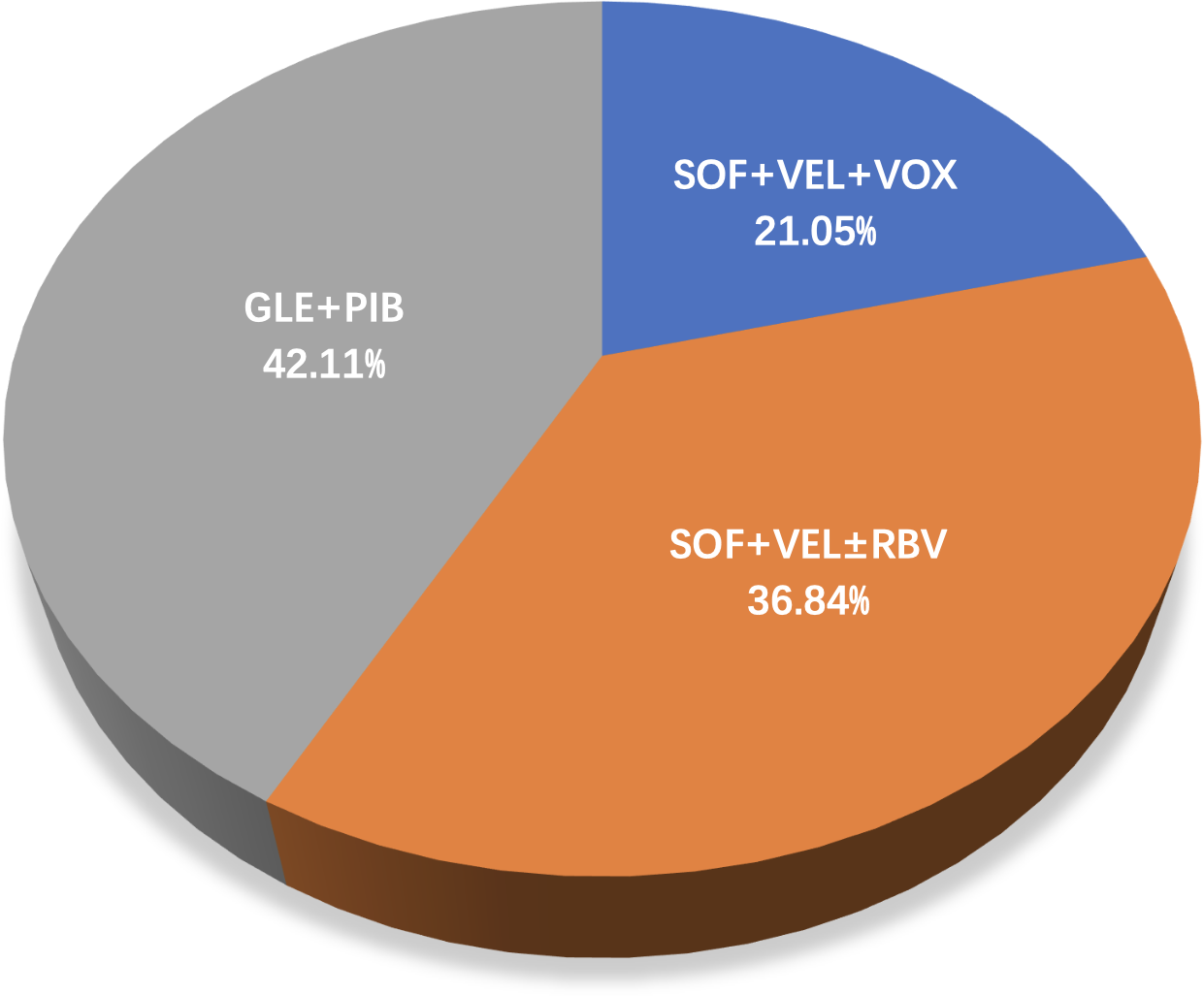
A pie chart showing the distribution of three regimens.
The patients’ clinical and demographic characteristics are shown in Table 1. The stages of liver disease in the patients infected by genotype 3 HCV included in these real-world studies ranged from hepatitis, advanced fibrosis, and compensated cirrhosis to decompensated cirrhosis. Some patients had at least one of the following refractory comorbidities: HIV/HBV coinfection, hepatocellular carcinoma (HCC), diabetes, history of liver transplantation, renal failure, alcohol abuse, intravenous drug use, and history of previous DAA treatment failure.
Table 1
| Author, Year | Region | Sample size | Pre-treatment HCV RNA (log10 IU/mL) | Age (years) | Male, No. (%) | Demographic characteristics | Regimen, duration (weeks) | SVR12/24, No. | Virological failure* |
|---|---|---|---|---|---|---|---|---|---|
| Mangia et al., 2019 (18) | Italy | 205 | 2.53 ± 4.3 | 52.9 | 175 (85.3) | 72.8% treatment naïve; 54.1% transient elastography results >20KPa; 18.5% alcohol abuse; 17.6% with diabetes; 14.6% past intravenous drug use; 9.8% HIV positive; 5.3% with HCC; all with cirrhosis. | SOF+VEL, 12 | 200 | 5 |
| Llaneras et al., 2019 (19) | Spain | 30 | NA | NA | NA | 43.3% with cirrhosis; all treated with DCV and SOF or LDV or VEL. | SOF+VEL+VOX, 12 | 24 | 6 |
| Hlaing et al., 2019 (20) | Myanmar | 83 | NA | NA | NA | 67.6% with cirrhosis or advanced fibrosis; 6.16% treatment-experienced: Peg-IFN or DAA-based. | SOF+VEL ± RBV, 12-24 | 75 | 8 |
| Degasperi et al., 2019 (21) | Italy | 42 | NA | NA | NA | All failed with OBV/PTV-r + DSV, or SOF and LDV or VEL | SOF+VEL+VOX ± RBV, 12 | 33 | 3 |
| D’Ambrosio et al., 2019 (22) | Italy | 68 | NA | NA | NA | NA | GLE+PIB, 8-16 | 66 | 2 |
| Berg et al., 2019 (23) | Germany | 176 | NA | NA | NA | Non-cirrhotic and treatment-naïve patients treated for 8 weeks, 12 were cirrhotic and treatment-naïve, 16 were treatment-experienced. | GLE+PIB, 8-16 | 174 | NA |
| Belperio et al., 2019 (24) | USA | 1735 | 6.05 | 58.95 | 1662 (95.8) | 26.5% with cirrhosis; 9.7% treatment experienced: SOF/LDV ± RBV, PegIFN+RBV, SOF+ PegIFN+RBV, SOF+RBV;2.42% with HCC. | SOF+VEL ± RBV, 12-24 | 1573 | NA |
| Belperio et al., 2019 (25) | USA | 45 | 6.1 | 59.7 | NA | 51.1% with cirrhosis; 37.8% with diabetes; 4.44% with HCC; 2.22% with HIV coinfected; all treated with DCV and SOF or LDV or VEL. | SOF+VEL+VOX, 12 | 42 | NA |
| Felden et al., 2018 (26) | Germany | 222 | NA | NA | NA | 26.6% with cirrhosis; 24.8% treatment-experienced: Peg-IFN ± RBV or DAA-based; 8.56% with HIV coinfected. | SOF+VEL ± RBV, 12 | 213 | NA |
| Tao et al., 2018 (27) | China | 21 | 6.04 | 37.38 | 13 (59.3) | 23.8% with cirrhosis. | SOF+VEL, 12-24 | 21 | 0 |
| Mangia et al., 2019 (28) | Italy | 204 | NA | NA | NA | 57.4% with F3-F4 stage; 43.14% PWID; 42.6% with F0-F2; 12.25% with ribavirin. | SOF+VEL ± RBV, 12 | 198 | NA |
| Toyoda et al., 2020 (29) | Japan | 14 | 6.5 | 46 | 6 (42.9) | 42.9% with cirrhosis; 28.6% with HCC; 21.4% DAA-experienced: DAA-based. | GLE+PIB, 12 | 13 | 1 |
| Nozaki et al., 2020 (30) | Japan | 20 | NA | 54 | 9 (45.0) | 75.0% treatment-naïve; 30.0% with cirrhosis; 30.0% with HCC; 25.0% treated by SOF/RBV or DCV/ASV. | GLE+PIB, 12 | 16 | 4 |
| Smith et al., 2021 (31) | UK | 62 | 6.4 | 57 | 53 (85.5) | 59.67% with cirrhosis; 14.5% with prior liver transplant;12.9% with HCC; all with previous DAA treatment failure. | SOF+VEL+VOX, 8-24 | 50 | NA |
| Chen et al., 2021 (32) | China Taiwan | 86 | NA | NA | NA | 87.21% treatment-naïve; 79.07% without cirrhosis;20.93% with cirrhosis; 12.79% treatment-experienced. | GLE+PIB, 8-16 | 82 | NA |
| Curry et al., 2021 (33) | USA | 57 | NA | 43 | 22 (38.6) | 23% CKD Stage 1-3; 5% CKD Stage 4-5;all treatment-naïve and non-cirrhotic. | GLE+PIB, 8 | 57 | 0 |
| Solomon et al., 2022 (34) | USA | 80 | NA | 43 | NA | All treatment-naïve. | SOF+VEL, 12 | 73 | NA |
| Madsen et al., 2022 (35) | Denmark | 11 | NA | NA | NA | All treatment-naïve and without significant liver fibrosis. | GLE+PIB, 4-8 | 10 | NA |
| Chang et al., 2021 (36) | China Taiwan | 11 | NA | NA | NA | All treatment-naïve. | GLE+PIB, 8 | 11 | NA |
The demographic characteristics of the patients.
* denotes a relapse or breakthrough or undefined virological failure.
Pooled SVR rate for all cases
The pooled SVR12/24 rate for cases that received SOF+VEL+VOX, SOF+VEL ± RBV, and GLE+PIB was 94.00% (95% CI: 90.87-96.59%) (Figure 3). In addition, the SVR12/24 rate was 83.81% (95% CI: 75.70-90.62%) in cases that received SOF+VEL+VOX, 94.98% (95% CI: 92.02-97.33%) in cases that received SOF+VEL ± RBV, and 96.96% (95% CI: 93.20-99.45%) in cases that received GLE+PIB.
Figure 3

The forest plots of the pooled SVR rates for all patients.
Stratification assessment of non-cirrhotic cases
The subgroup assessment of non-cirrhotic cases showed that the pooled SVR12/24 rate was 95.70% (95% CI: 91.74-98.58%) in cases that received GLE+PIB, SOF+VEL+VOX, and SOF+VEL ± RBV (Figure 4A). Moreover, the SVR12/24 rate was 95.91% (95% CI: 83.38-100%) in cases that received GLE+PIB, 92.68% (95% CI: 81.43-99.44%) in cases that received SOF+VEL+VOX, and 94.61% (95% CI: 89.78-98.00%) in cases that received SOF+VEL ± RBV.
Figure 4
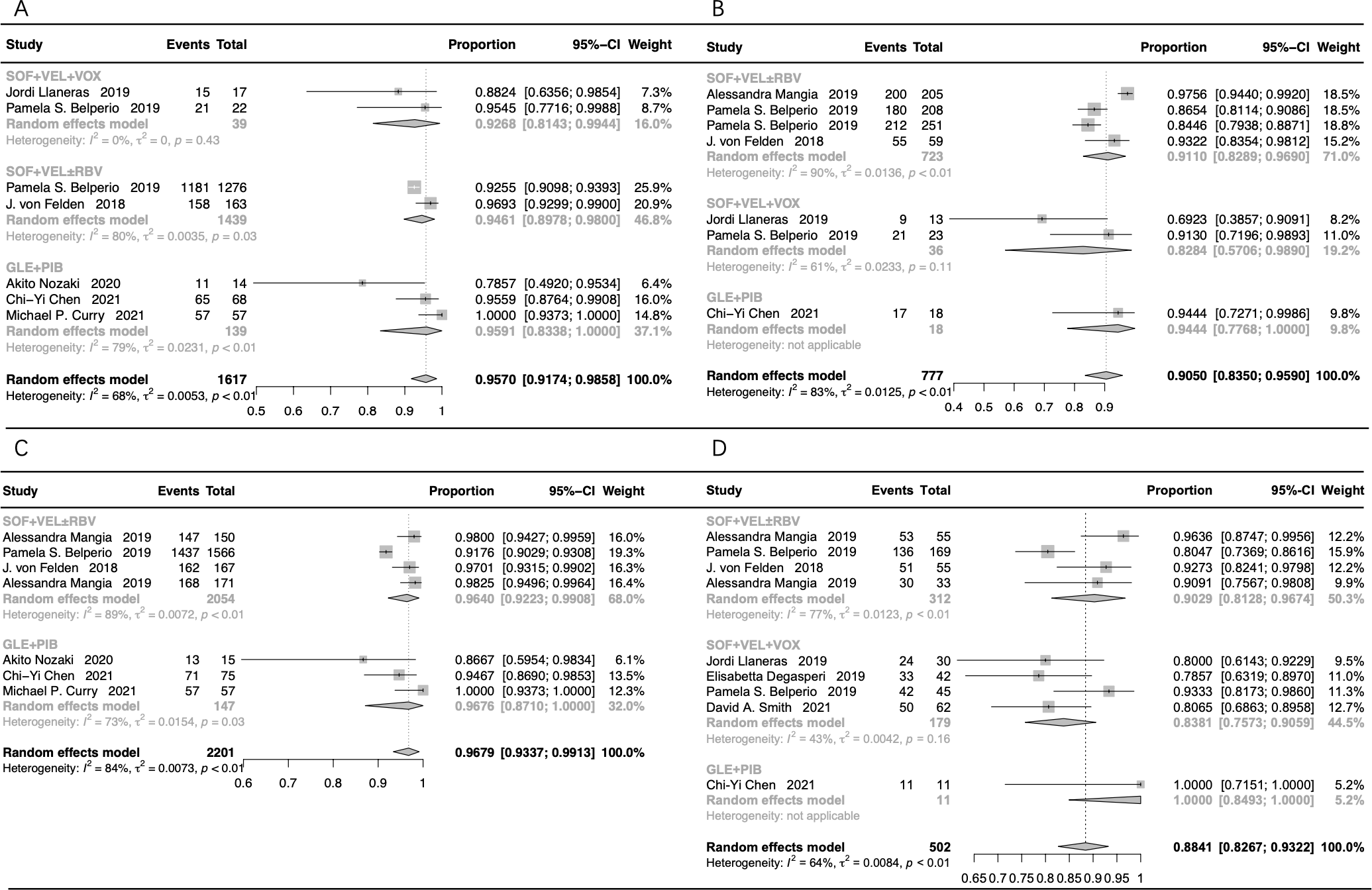
The forest plots of the stratification analysis. (A) The forest plots of SVR rates in non-cirrhotic patients; (B) the forest plots of SVR rates in cirrhotic patients; (C) the forest plots of SVR rates in treatment-naive patients; (D) the forest plots of SVR rates in treatment-experienced patients.
Stratification assessment of cirrhotic cases
The pooled SVR12/24 rate was 90.50% (95% CI: 83.50-95.90%) in cirrhotic patients treated with GLE+PIB, SOF+VEL+VOX, and SOF+VEL ± RBV (Figure 4B). The SVR12/24 rate was 91.10% (95% CI: 82.89-96.90%) in cases that received SOF+VEL ± RBV, 94.44% (95% CI: 77.68-100%) in cases that received GLE+PIB, and 82.84% (95% CI: 57.06-98.90%) in cases that received SOF+VEL+VOX.
Stratification assessment of treatment-naive cases
The subgroup assessment of the treatment-naive cases showed that the pooled SVR12/24 rate in cases that received GLE+PIB and SOF+VEL ± RBV was 96.79% (95% CI: 93.37-99.13%), as presented in Figure 4C. In addition, the SVR rate in cases that received GLE+PIB and SOF+VEL ± RBV was 96.76% (95% CI: 87.10-100%) and 96.40% (95% CI: 92.23-99.08%), respectively.
Stratification assessment of treatment-experienced cases
The pooled SVR12/24 rate for the treatment-experienced cases with HCV-GT3 infection that received SOF+VEL+VOX, SOF+VEL ± RBV, and GLE+PIB was 88.41% (95% CI: 82.67-93.22%), as presented in Figure 4D. Furthermore, the corresponding SVR12/24 rate was 83.81% (95% CI: 75.73-90.59%), 90.29% (95% CI: 81.28-96.74%), and 100% (95% CI: 84.93-100%), respectively.
Risk of bias and quality assessment
Detailed data on all genotypes instead of genotype 3 HCV patients in the majority of included articles were available, as shown in Table 1. The risk of bias due to missing data was moderate or high, as presented in Figure 5. The Egger’s test showed no significant publication bias (t=0.51, DF=17, P=0.6150).
Figure 5
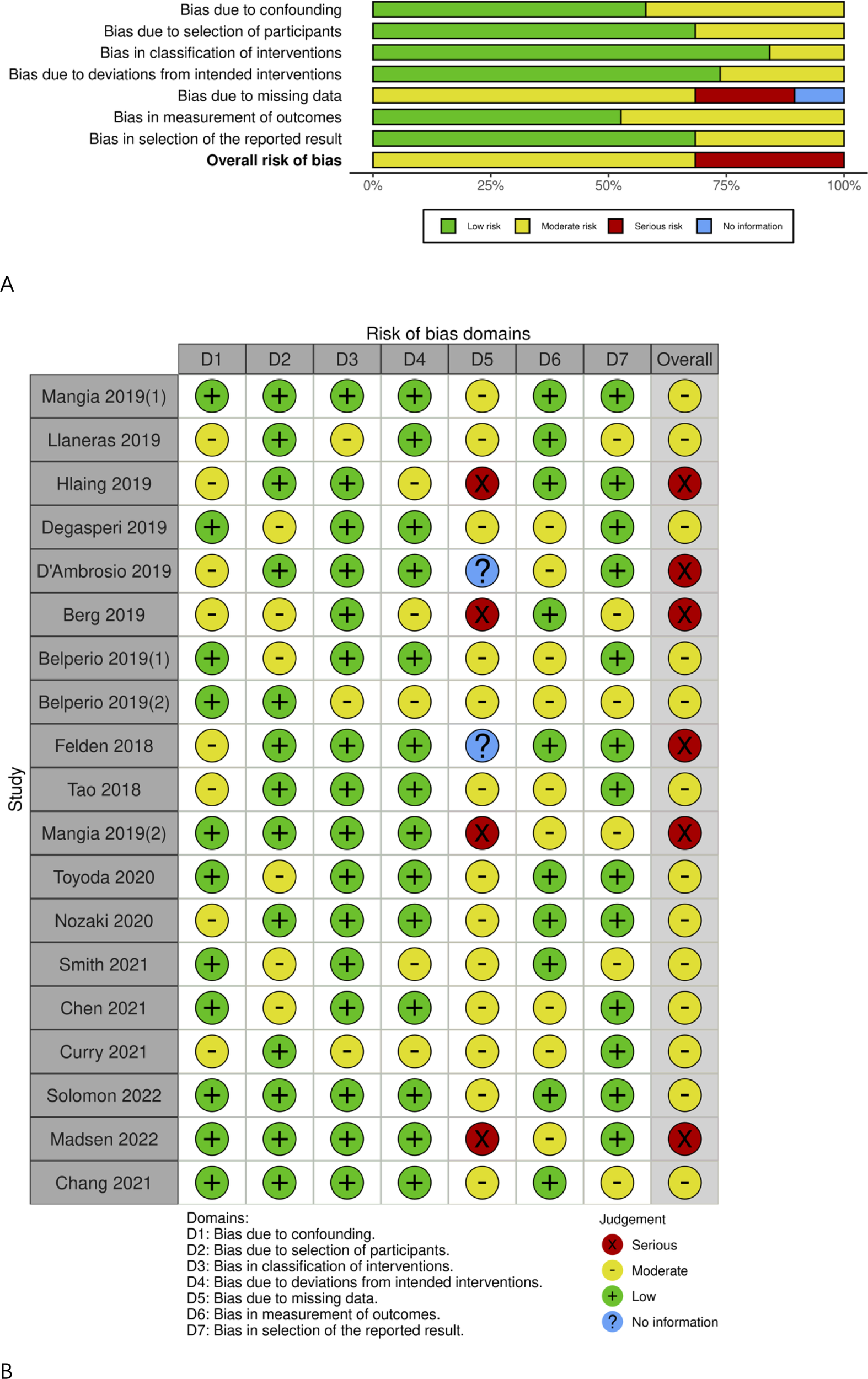
Risk of bias graph. (A) Risk of bias item among studies; (B) Risk of bias item in each study.
Discussion
With the approval of the direct-acting antivirals, the landscape of HCV treatment has significantly changed since 2015. New regimens and their combinations have been researched to resolve difficulties through antiviral therapy.
The pooled SVR12 rate of 3,177 HCV-GT3 patients who received SOF+VEL ± RBV, GLE+PIB, and SOF+VEL+VOX was 94.00% in the meta-analysis of rates in real-world settings. Patients with decompensated cirrhosis, prior DAA treatment-failure, HBV/HCV or HCV/HIV coinfection, chronic kidney disease, HCC, or a prior liver transplant who were considered difficult to treat were involved. The SVR12 rate was 90.50% (n=694/777) and 95.70% (n=1508/1617) in the patients with and without cirrhosis, respectively, and 88.41% (n=430/502) and 96.79% (n=2055/2201) in the treatment-experienced and treatment-naïve patients, respectively. A decrease of approximately 5.20% in the SVR rate in the patients with cirrhosis and an 8.38% decrease in the treatment-experienced patients were observed. Thus, the fibrosis stage and history of antiviral treatment might significantly impact the antiviral effectiveness. Furthermore, the fibrosis stage and particularly treatment history may significantly affect the SVR rates.
In the ASTRAL-3 study reported by Foster et al. (37), the SVR12 rate was 97% (n=191/197) and 91% (n=73/80) in HCV GT3 patients without cirrhosis and with compensated cirrhosis, respectively, and 97% (n=200/206) and 90% (n=64/71) in treatment-naïve and treatment-experienced patients, respectively. A retrospective study on patients who had compensated cirrhosis or advanced fibrosis had an SVR12 rate of 95% (n=145/153) in GT3 patients (38). Through subgroup analysis, the SVR12 rate of the treatment-experienced patients prescribed SOF+VEL ± RBV was 90.29% (n=270/312), which was lower than that of the treatment-naive patients (96.40%, n=1914/2054). Similar decreases were observed in the subgroup populations that received SOF+VEL+VOX. The SVR12 rate of patients with cirrhosis treated with SOF+VEL ± RBV was 91.10% (n=647/723), which was lower than that of patients without cirrhosis (94.61%, n=1339/1439), as well as that those that received GLE+PIB and SOF+VEL+VOX.
High SVR rates of patients with GLE+PIB have been reported in registration trials in recent years, ranging between 95% and 100% (39). The effect of HCV genotype, fibrosis stage, history of antiviral treatment, HCC, and advanced chronic kidney disease (CKD) on the efficacy of GLE+PIB seemed to be limited because of the excellent SVR rate (30). In the present analysis, the SVR12 rate of GT3 patients treated with GLE+PIB was 96.96% (n=429/448) in real-world settings. When analyzing a subgroup of patients with cirrhosis and treatment experience, the SVR rate results did not fluctuate significantly, mirroring those reported in previous trials.
Belperio et al. reported that among 13 GT3 patients with prior SOF/VEL exposure, the SVR rates were 100% (n=6/6) in those without cirrhosis and 71.4% (n=5/7) in those with cirrhosis. Thus, they considered that cirrhosis occurring with prior SOF/VEL exposure may augment the risk of relapse rather than cirrhosis alone (25). All the GT3 patients enrolled in the four studies of this meta-analysis that received SOF+VEL+VOX were treatment-experienced with SOF/LDV, OBV/PTV-r+DSV, SOF/VEL, or SOF/DCV. The pooled SVR12 rate was 83.81% (n=149/179) in the patients with and without cirrhosis. The SVR rate of the patients with cirrhosis and prior DAA exposure was 82.84% (n=30/36) and 92.68% (n=36/39), respectively, compared to the patients with prior DAA exposure but without cirrhosis. A decrease of 9.84% showed that the GT3 patients with cirrhosis and prior DAA failure were a more difficult-to-treat cohort.
Previous studies indicated that genotype 3 HCV with variants such as A30K, L31M, and Y93H of NS5A was refractory (40–42). Zeuzem et al. (40) reported that in the ENDURANCE-3 trial, GT3 patients with the A30K mutation at baseline had a lower SVR12 rate. However, most patients achieved SVR regardless of the A30K variant. In the ASTRAL-3 study, an SVR rate of 84% in patients with Y93H substitution compared to that of 97% in patients without the substitution was attained from patients who received SOF/VEL (37). Sarrazin et al. (43) reported that SVR12 rates were similar in patients with/without NS3 and/or NS5A resistance-associated variants (RASs) and patients with/without VOX- or VEL-specific RASs who received SOF + VEL + VOX for 12 weeks. Seven articles in this meta-analysis (19, 21, 22, 25, 26, 30, 31) completed the RAVs test, concluding that RASs may not be associated with a lower SVR rate. Nozaki et al. (30) indicated that the effect of RASs on therapeutic results was limited because of the 99.1% overall SVR12 rate. It may not be necessary to test for RASs before treatment because of the high SVR rates in patients completing therapy.
The limitations of this meta-analysis included high heterogeneity in the baseline characteristics and clinical features of the patients, along with a small number of patients who received SOF+VEL+VOX. More studies are needed in order to analyze the real-world antiviral effectiveness of DAAs in chronic HCV GT3-infected patients.
Conclusions
In conclusion, SOF+VEL ± RBV, GLE+PIB, and SOF+VEL+VOX had good antiviral effectiveness for chronic HCV-GT3 infection in real-world settings. Factors such as cirrhosis and treatment experience, especially previous DAA treatment failure, may influence the SVR rate.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LZ: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JL: Methodology, Writing – review & editing. YZ: Methodology, Writing – original draft. SJ: Data curation, Investigation, Writing – review & editing. HX: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by National Key R&D Program of China (2021YFC2301801); Capital’s Funds for Health Improvement and Research of China (CFH 2020-1-2171); The Digestive Medical Coordinated Development Center of Beijing Hospitals Authority under Grant No.XXT26.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Organization WH . Hepatitis C (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (Accessed July 27, 2021).
2
Organization WH . Global Health Sector Strategy on Viral Hepatitis 2016-2021: Towards Ending Viral Hepatitis. Geneva: WHO Document and Production Services (2016) p. 1–56.
3
Lawitz E Freilich B Link J German P Mo H Han L et al . A phase 1, randomized, dose-ranging study of Gs-5816, a once-daily Ns5a inhibitor, in patients with genotype 1-4 hepatitis C virus. J Viral Hepat. (2015) 22:1011–9. doi: 10.1111/jvh.12435
4
Feld JJ Jacobson IM Hezode C Asselah T Ruane PJ Gruener N et al . Sofosbuvir and velpatasvir for Hcv genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. (2015) 373:2599–607. doi: 10.1056/NEJMoa1512610
5
Kwo PY Poordad F Asatryan A Wang S Wyles DL Hassanein T et al . Glecaprevir and pibrentasvir yield high response rates in patients with Hcv genotype 1-6 without cirrhosis. J Hepatol. (2017) 67:263–71. doi: 10.1016/j.jhep.2017.03.039
6
Forns X Lee SS Valdes J Lens S Ghalib R Aguilar H et al . Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (Expedition-1): A single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. (2017) 17:1062–8. doi: 10.1016/S1473-3099(17)30496-6
7
Lawitz E Reau N Hinestrosa F Rabinovitz M Schiff E Sheikh A et al . Efficacy of sofosbuvir, velpatasvir, and Gs-9857 in patients with genotype 1 hepatitis C virus infection in an open-label, phase 2 trial. Gastroenterology. (2016) 151:893–901.e1. doi: 10.1053/j.gastro.2016.07.039
8
Gane EJ Kowdley KV Pound D Stedman CA Davis M Etzkorn K et al . Efficacy of sofosbuvir, velpatasvir, and Gs-9857 in patients with hepatitis C virus genotype 2, 3, 4, or 6 infections in an open-label, phase 2 trial. Gastroenterology. (2016) 151:902–9. doi: 10.1053/j.gastro.2016.07.038
9
Jacobson IM Lawitz E Gane EJ Willems BE Ruane PJ Nahass RG et al . Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic Hcv infection: 2 phase 3 randomized trials. Gastroenterology. (2017) 153:113–22. doi: 10.1053/j.gastro.2017.03.047
10
Gane E Lawitz E Pugatch D Papatheodoridis G Brau N Brown A et al . Glecaprevir and pibrentasvir in patients with Hcv and severe renal impairment. N Engl J Med. (2017) 377:1448–55. doi: 10.1056/NEJMoa1704053
11
Gane EJ Schwabe C Hyland RH Yang Y Svarovskaia E Stamm LM et al . Efficacy of the combination of sofosbuvir, velpatasvir, and the ns3/4a protease inhibitor gs-9857 in treatment-naive or previously treated patients with hepatitis C virus genotype 1 or 3 infections. Gastroenterology. (2016) 151:448–56.e1. doi: 10.1053/j.gastro.2016.05.021
12
Zhuang L Li J Zhang Y Ji S Li Y Zhao Y et al . Real-world effectiveness of direct-acting antiviral regimens against hepatitis C virus (Hcv) genotype 3 infection: A systematic review and meta-analysis. Ann Hepatol. (2021) 23:100268. doi: 10.1016/j.aohep.2020.09.012
13
Smolders EJ Jansen AME Ter Horst PGJ Rockstroh J Back DJ Burger DM . Viral hepatitis C therapy: pharmacokinetic and pharmacodynamic considerations: A 2019 update. Clin Pharmacokinet. (2019) 58:1237–63. doi: 10.1007/s40262-019-00774-0
14
Tacke F Gunther R Buggisch P Klinker H Schober A John C et al . Treatment of Hcv genotype 2 with sofosbuvir and ribavirin results in lower sustained virological response rates in real life than expected from clinical trials. Liver Int. (2017) 37:205–11. doi: 10.1111/liv.13206
15
Backus LI Belperio PS Shahoumian TA Loomis TP Mole LA . Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans. Aliment Pharmacol Ther. (2015) 42:559–73. doi: 10.1111/apt.13300
16
Buggisch P Vermehren J Mauss S Gunther R Schott E Pathil A et al . Real-world effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J Hepatol. (2018) 68:663–71. doi: 10.1016/j.jhep.2017.11.009
17
Wedemeyer H Craxi A Zuckerman E Dieterich D Flisiak R Roberts SK et al . Real-world effectiveness of ombitasvir/paritaprevir/ritonavir+/-dasabuvir+/-ribavirin in patients with hepatitis C virus genotype 1 or 4 infection: A meta-analysis. J Viral Hepat. (2017) 24:936–43. doi: 10.1111/jvh.12722
18
Mangia A Cenderello G Copetti M Verucchi G Piazzolla V Lorusso C et al . Svr12 higher than 97% in Gt3 cirrhotic patients with evidence of portal hypertension treated with Sof/Vel without ribavirin: A nation-wide cohort study. Cells. (2019) 8(4). doi: 10.3390/cells8040313
19
Llaneras J Riveiro-Barciela M Lens S Diago M Cachero A Garcia-Samaniego J et al . Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with daas. J Hepatol. (2019) 71:666–72. doi: 10.1016/j.jhep.2019.06.002
20
Hlaing NKT Nangia G Tun KT Lin S Maung MZ Myint KT et al . High sustained virologic response in genotypes 3 and 6 with generic Ns5a inhibitor and sofosbuvir regimens in chronic Hcv in Myanmar. J Viral Hepat. (2019) 26:1186–99. doi: 10.1111/jvh.13133
21
Degasperi E Spinetti A Lombardi A Landonio S Rossi MC Pasulo L et al . Real-life effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous daa failure. J Hepatol. (2019) 71:1106–15. doi: 10.1016/j.jhep.2019.07.020
22
D’Ambrosio R Pasulo L Puoti M Vinci M Schiavini M Lazzaroni S et al . Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol. (2019) 70:379–87. doi: 10.1016/j.jhep.2018.11.011
23
Berg T Naumann U Stoehr A Sick C John C Teuber G et al . Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C infection: data from the German hepatitis C-registry. Aliment Pharmacol Ther. (2019) 49:1052–9. doi: 10.1111/apt.15222
24
Belperio PS Shahoumian TA Loomis TP Mole LA Backus LI . Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. (2019) 70:15–23. doi: 10.1016/j.jhep.2018.09.018
25
Belperio PS Shahoumian TA Loomis TP Backus LI . Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir in 573 direct-acting antiviral experienced hepatitis C patients. J Viral Hepat. (2019) 26:980–90. doi: 10.1111/jvh.13115
26
von Felden J Vermehren J Ingiliz P Mauss S Lutz T Simon KG et al . High efficacy of sofosbuvir/velpatasvir and impact of baseline resistance-associated substitutions in hepatitis C genotype 3 infection. Aliment Pharmacol Ther. (2018) 47:1288–95. doi: 10.1111/apt.14592
27
Tao YC Deng R Wang ML Lv DD Yuan M Wang YH et al . Satisfactory virological response and fibrosis improvement of sofosbuvir-based regimens for Chinese patients with hepatitis C virus genotype 3 infection: results of a real-world cohort study. Virol J. (2018) 15:150. doi: 10.1186/s12985-018-1066-8
28
Mangia A Piazzolla V Giannelli A Visaggi E Minerva N Palmieri V et al . Svr12 rates higher than 99% after sofosbuvir/velpatasvir combination in Hcv infected patients with F0-F1 fibrosis stage: A real world experience. PloS One. (2019) 14(5). doi: 10.1371/journal.pone.0215783
29
Toyoda H Atsukawa M Watanabe T Nakamuta M Uojima H Nozaki A et al . Real-world experience of 12-week direct-acting antiviral regimen of glecaprevir and pibrentasvir in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. (2020) 35:855–61. doi: 10.1111/jgh.14874
30
Nozaki A Atsukawa M Kondo C Toyoda H Chuma M Nakamuta M et al . The effectiveness and safety of glecaprevir/pibrentasvir in chronic hepatitis C patients with refractory factors in the real world: A comprehensive analysis of a prospective multicenter study. Hepatol Int. (2020) 14:225–38. doi: 10.1007/s12072-020-10019-z
31
Smith DA Bradshaw D Mbisa JL Manso CF Bibby DF Singer JB et al . Real world Sof/Vel/Vox retreatment outcomes and viral resistance analysis for Hcv patients with prior failure to daa therapy. J Viral Hepatitis. (2021) 28(9). doi: 10.1111/jvh.13549
32
Chen CY Huang CF Cheng PN Tseng KC Lo CC Kuo HT et al . Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world nationwide hepatitis C virus registry programme in Taiwan. Liver Int. (2021) 41:1265–77. doi: 10.1111/liv.14849
33
Curry MP Kort J Marx S Strezewski J Wick N Flamm SL et al . Real-world effectiveness of 8-week glecaprevir/pibrentasvir (G/P) for treatment naive, noncirrhotic patients with Hcv infection in the trio network. GastroHep. (2020) 2:64–71. doi: 10.1002/ygh2.388
34
Solomon SS Wagner-Cardoso S Smeaton L Sowah LA Wimbish C Robbins G et al . A minimal monitoring approach for the treatment of hepatitis C virus infection (Actg A5360 [Minmon]): A phase 4, open-label, single-arm trial. Lancet Gastroenterol Hepatol. (2022) 7:307–17. doi: 10.1016/s2468-1253(21)00397-6
35
Madsen LW Christensen PB Hansen JF Røge BT Holm DK Dröse S et al . Four weeks treatment with glecaprevir/pibrentasvir + Ribavirin-a randomized controlled clinical trial. Viruses. (2022) 14(3). doi: 10.3390/v14030614
36
Chang KC Tung SY Wei KL Shen CH Hsieh YY Chen WM et al . Real-world efficacy and safety of pangenotypic direct-acting antivirals against hepatitis C virus infection in Taiwan. Sci Rep. (2021) 11:13543. doi: 10.1038/s41598-021-93095-x
37
Foster GR Afdhal N Roberts SK Bräu N Gane EJ Pianko S et al . Sofosbuvir and velpatasvir for hcv genotype 2 and 3 infection. N Engl J Med. (2015) 373:2608–17. doi: 10.1056/NEJMoa1512612
38
Asselah T Bourgeois S Pianko S Zeuzem S Sulkowski M Foster GR et al . Sofosbuvir/velpatasvir in patients with hepatitis C virus genotypes 1-6 and compensated cirrhosis or advanced fibrosis. Liver Int. (2018) 38:443–50. doi: 10.1111/liv.13534
39
Gane E Poordad F Wang S Asatryan A Kwo PY Lalezari J et al . High efficacy of abt-493 and Abt-530 treatment in patients with Hcv genotype 1 or 3 infection and compensated cirrhosis. Gastroenterology. (2016) 151:651–9.e1. doi: 10.1053/j.gastro.2016.07.020
40
Zeuzem S Foster GR Wang S Asatryan A Gane E Feld JJ et al . Glecaprevir-pibrentasvir for 8 or 12 weeks in hcv genotype 1 or 3 infection. N Engl J Med. (2018) 378:354–69. doi: 10.1056/NEJMoa1702417
41
Wyles D Poordad F Wang S Alric L Felizarta F Kwo PY et al . Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology. (2018) 67:514–23. doi: 10.1002/hep.29541
42
Smith D Magri A Bonsall D Ip CLC Trebes A Brown A et al . Resistance analysis of genotype 3 hepatitis C virus indicates subtypes inherently resistant to nonstructural protein 5a inhibitors. Hepatology. (2019) 69:1861–72. doi: 10.1002/hep.29837
43
Sarrazin C Cooper CL Manns MP Reddy KR Kowdley KV Roberts SK et al . No impact of resistance-associated substitutions on the efficacy of sofosbuvir, velpatasvir, and voxilaprevir for 12 Weeks in Hcv Daa-experienced patients. J Hepatol. (2018) 69:1221–30. doi: 10.1016/j.jhep.2018.07.023
Summary
Keywords
real-world effectiveness, sofosbuvir/velpatasvir, glecaprevir/pibrentasvir, sofosbuvir/velpatasvir/voxilaprevir, genotype 3 HCV
Citation
Zhuang L, Li J, Zhang Y, Ji S and Xing H (2025) Real-world effectiveness of sofosbuvir/velpatasvir, glecaprevir/pibrentasvir, and sofosbuvir/velpatasvir/voxilaprevir against genotype 3 hepatitis C virus infection: a systematic review and meta-analysis. Front. Gastroenterol. 4:1511150. doi: 10.3389/fgstr.2025.1511150
Received
14 October 2024
Accepted
08 January 2025
Published
12 February 2025
Volume
4 - 2025
Edited by
Ana Sandoval-Rodriguez, University of Guadalajara, Mexico
Reviewed by
Amir Sultan Seid, Addis Ababa University, Ethiopia
Tadashi Ikegami, Tokyo Medical University, Japan
Updates
Copyright
© 2025 Zhuang, Li, Zhang, Ji and Xing.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huichun Xing, huichunxing@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.